Summary
Epidermal growth factor receptor (EGFR) ligands undergo a complex series of processing events during their maturation to active signaling proteins. Like its mammalian homologs, the predominant Drosophila EGFR ligand Spitz is produced as a transmembrane pro-protein. In the secretory pathway, Spitz is cleaved within its transmembrane domain to release the extracellular signaling domain. This domain is modified with an N-terminal palmitate group that tethers it to the plasma membrane. We found that the pro-protein can reach the cell surface in the absence of proteolysis, but that it fails to activate the EGFR. To address why the transmembrane pro-protein is inactive, whereas membrane association through the palmitate group promotes activity, we generated a panel of chimeric constructs containing the Spitz extracellular region fused to exogenous transmembrane proteins. Although the orientation of the EGF domain and its distance from the plasma membrane varies in these chimeras, they are all active in vivo. Thus, tethering Spitz to the membrane via a transmembrane domain at either terminus does not prevent activity. Conversely, removing the N-terminal palmitate group from the C-terminally tethered pro-protein does not render it active. Furthermore, we show that the Spitz transmembrane pro-protein can activate the EGFR in a tissue culture assay, indicating that its failure to signal in vivo is not due to structural features. In polarized imaginal disc cells, unprocessed Spitz pro-protein localizes to apical puncta, whereas the active chimeric Spitz constructs are basolaterally localized. Taken together, our data support the model that localized trafficking of the pro-protein restricts its ability to activate the receptor in polarized tissues.
Key words: Palmitoylation, Rhomboid, Imaginal disc, Spitz, EGFR
Introduction
The epidermal growth factor receptor (EGFR) family of transmembrane tyrosine kinase receptors is involved in diverse developmental programs, and dysregulation of EGFR signaling drives numerous human cancers (Hynes and Lane, 2005). Binding of an extracellular ligand to the EGFR induces a cascade of phosphorylation events that ultimately results in changes in gene expression (Jorissen et al., 2003). Mammals possess seven canonical EGFR ligands, all of which share a conserved domain organization including at least one receptor-binding EGF domain, a transmembrane domain and a C-terminal intracellular domain (Schneider and Wolf, 2009). Cleavage by metalloproteinases in the juxtamembrane region produces diffusible growth factors that are capable of paracrine pathway activation (Singh and Harris, 2005). Mammalian EGFR ligands have also been observed to induce juxtacrine pathway activation by binding to their receptors in their transmembrane pro-protein forms (Anklesaria et al., 1990; Brachmann et al., 1989; Wong et al., 1989). Juxtacrine and paracrine activation of the receptor can result in different outcomes, suggesting that regulation of ligand cleavage can determine the consequences of pathway activity (Iwamoto et al., 1999; Pan et al., 2002; Prince et al., 2010; Singh et al., 2004; Takemura et al., 1997).
Drosophila melanogaster has proven to be a valuable model for studying EGFR signaling. The EGFR pathway is conserved in Drosophila, but it has much less complexity and redundancy than the mammalian system, with only four ligands, one receptor and a single downstream signal transduction pathway (Shilo, 2005). Three of the ligands, Spitz (Spi), Keren (Krn) and Gurken (Grk), resemble the canonical mammalian ligands and are produced as transmembrane pro-proteins, whereas Vein (Vn) resembles the non-canonical mammalian neuregulin ligands and lacks a transmembrane domain (Schnepp et al., 1996). Spi is required for numerous processes throughout development (Rutledge et al., 1992), acting redundantly with Krn in the eye, ovary and gut (Brown et al., 2007; Jiang and Edgar, 2009; McDonald et al., 2006; Xu et al., 2011), whereas Grk expression is limited to the germline (Neuman-Silberberg and Schüpbach, 1993).
Spi is synthesized as a type I transmembrane pro-protein (membrane Spi, mSpi) (Schweitzer et al., 1995). Transport of mSpi out of the endoplasmic reticulum (ER) requires the transmembrane chaperone Star (Lee et al., 2001; Tsruya et al., 2002). In later secretory compartments, Spi is cleaved in its transmembrane domain by the multi-pass serine protease Rhomboid (Rho) (Strisovsky et al., 2009; Urban et al., 2001) and palmitoylated at its ultimate N-terminal cysteine by the membrane bound O-acyltransferase Rasp (Miura et al., 2006) (Fig. 1A). Both post-translational modifications are necessary for Spi to activate the EGFR in vivo (Lee et al., 2001; Miura et al., 2006; Tsruya et al., 2002). As spi and rasp are ubiquitously expressed, the expression pattern of rho determines the location of Spi activity. Rho and Rasp appear to act independently; a form of Spi that is truncated at the Rho cleavage site and, therefore, lacks the transmembrane domain and cytoplasmic C-terminus (secreted Spi, sSpi) (Schweitzer et al., 1995), is palmitoylated normally, and a non-palmitoylatable form of Spi pro-protein (mSpiCS) is cleaved (Miura et al., 2006).
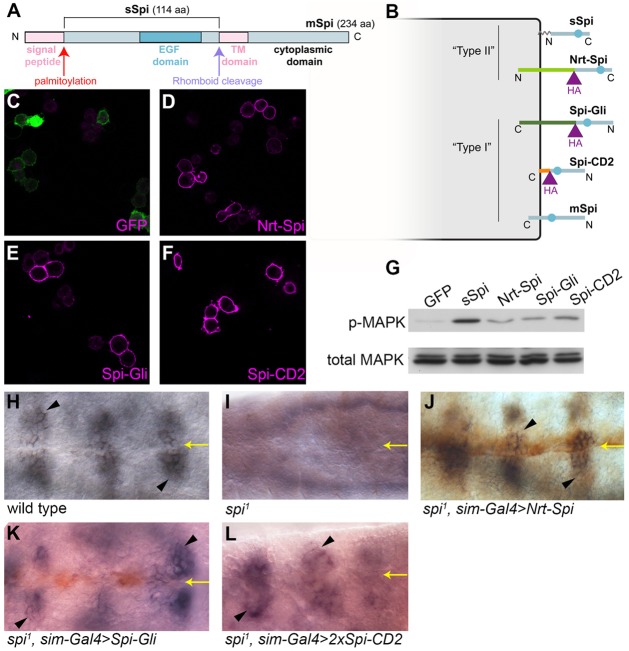
Fig. 1.
Transmembrane Spi chimeras are trafficked to the cell surface and can activate the EGFR in vitro. (A) Full-length mSpi pro-protein has its receptor-binding EGF domain located close to the transmembrane (TM) domain. Removal of the signal peptide and cleavage in the transmembrane domain by Rho generates active sSpi, which is palmitoylated at its N-terminus. (B) Chimeric transmembrane proteins were created by fusing sSpi to Nrt (light green), Gli (dark green) or the transmembrane domain of CD2 (orange). All three chimeras have an HA tag (purple). The EGF domain is represented by a blue circle. (C–F) S2R+ cells expressing Nrt–Spi (D), Spi–Gli (E), Spi–CD2 (F) or control cells expressing only intracellular GFP (C) were stained for HA (magenta, D–F) or GFP (magenta, C) in the absence of detergent. GFP fluorescence (green) is visible in C. (G) S2 cells expressing intracellular GFP, sSpi, Nrt–Spi, Spi–Gli or Spi-CD2 were co-cultured with EGFR-expressing D2F cells and assayed by western blotting for MAPK phosphorylation (p-MAPK) and total MAPK levels. (H–L) FasIII expression (purple, arrowheads) in the embryonic ventral ectoderm (H) was lost in spi zygotic mutant embryos (I) and was rescued by expression of Nrt–Spi (J), Spi–Gli (K) or two copies of Spi–CD2 (L)(stained with anti-HA antibody in brown) driven in the ventral midline cells by sim-GAL4. Yellow arrows indicate the ventral midline.
Spi, like the mammalian EGFR ligands, has been suggested to act as a diffusible paracrine factor. Spi is secreted into the medium when expressed in cultured cells (Lee et al., 2001; Schweitzer et al., 1995), and can act at a distance of three to four cell diameters in vivo (Freeman et al., 1992; Golembo et al., 1996a). Palmitoylation results in Spi having a strong membrane association and has been proposed to concentrate it on the surface of secreting cells (Miura et al., 2006), restricting its range of action. Although the Spi pro-protein and the cleaved ligand are both membrane-associated, only the latter is thought to signal. Our results confirm previous reports that, in contrast to mammalian EGFR ligands, Spi is incapable of signaling in its transmembrane pro-protein form (Freeman, 1996; Pickup and Banerjee, 1999; Schweitzer et al., 1995). We therefore sought to understand the mechanism by which post-translational processing controls Spi activity. We used a panel of chimeric proteins to show that structural features of the extracellular domain do not prevent signaling by the pro-protein mSpi, and found that mSpi is in fact capable of activating the receptor in tissue culture. Our data suggest that signaling by mSpi is limited in vivo by polarized trafficking in epithelial cells. A similar trafficking mechanism has been proposed to underlie the basolateral secretion of Hedgehog (Hh) (Callejo et al., 2011), suggesting that this is a common route for the secretion of palmitoylated proteins.
Results
Membrane-tethered forms of Spi can activate the EGFR
Although Spi was originally thought to be a soluble diffusible ligand (Schweitzer et al., 1995), our previous results suggested that palmitoylation at the N-terminal cysteine residue tethers Spi to the cell membrane, restricting its diffusion (Miura et al., 2006). If the palmitate group directly inserts into the plasma membrane, this would place the N-terminus of Spi proximal to the membrane (Fig. 1A,B). In order to understand why the extracellular domain of Spi (sSpi) is active when tethered to the membrane by a palmitate group but not when anchored to the membrane by the transmembrane domain of the pro-protein, we first tested the effect of substituting an exogenous transmembrane domain for the palmitate group. We fused the transmembrane protein Neurotactin (Nrt) to the N-terminus of the extracellular domain of Spi, replacing the palmitoylation site, to generate the chimeric protein Nrt–Spi. Because Nrt is a type II transmembrane protein (de la Escalera et al., 1990; Hortsch et al., 1990), the Nrt–Spi chimera would have its N-terminus proximal to the membrane and its C-terminus distal, reproducing the predicted orientation of wild-type palmitoylated Spi (Fig. 1B). Unlike cytoplasmic GFP, HA-tagged Nrt–Spi was detectable on the plasma membrane of cultured S2R+ cells stained in the absence of detergent, indicating that the chimeric protein is trafficked to the cell surface (Fig. 1C,D). When S2 cells expressing Nrt–Spi were co-cultured with EGFR-expressing cells, the receptor was activated, leading to MAPK phosphorylation (Fig. 1G). Furthermore, Nrt–Spi was able to substitute for Spi in vivo. Expression of Fasciclin III (Fas III) in the embryonic ventral ectoderm requires Spi expression at the ventral midline (Golembo et al., 1996a). When Nrt-–Spi was expressed at the ventral midline using the single-minded (sim)-GAL4 driver in spi mutant embryos, Fas III expression was restored (Fig. 1H–J). Substitution of the palmitate group by an N-terminal transmembrane domain thus allows Spi activity.
Unlike Nrt–Spi, the transmembrane Spi precursor (mSpi) is unable to signal in vivo until it is cleaved by Rho (Golembo et al., 1996a; Lee et al., 2001, see Fig. 3). mSpi differs from Nrt–Spi in the orientation of the extracellular domain of Spi with respect to the cell membrane. To assess whether orientation is important for Spi activity, we generated the chimeric construct Spi–Gli, in which the extracellular domain of Spi is fused at its C-terminus to the type I transmembrane protein Gliotactin (Gli; Fig. 1B) and palmitoylation is prevented by mutating the N-terminal cysteine to serine (Miura et al., 2006). Aside from the difference in orientation, the extracellular domains of Gli and Nrt are similar in size and sequence (Auld et al., 1995). Like Nrt–Spi, Spi–Gli is transported to the plasma membrane of S2R+ cells, activates the EGFR in the co-culture assay and rescues Fas III expression in spi mutant embryos (Fig. 1E,G,K). Thus, Spi can activate the EGFR when it is tethered to the membrane in either the type I or the type II orientation.
Fig. 3.
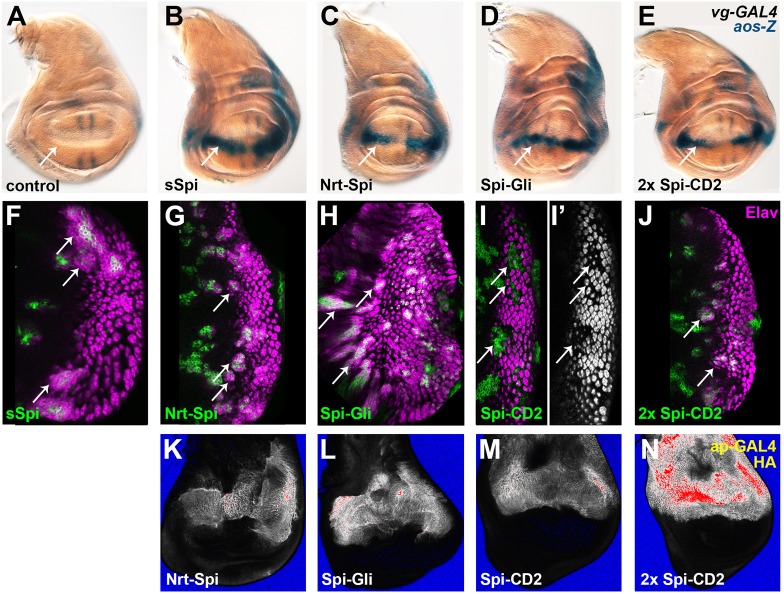
The mSpi pro-protein does not activate the EGFR in vivo. (A) Palmitoylation of mSpi would result in membrane association at both termini, whereas mSpiCS cannot be palmitoylated and remains free at its N-terminus. mSpiAG and mSpiCSAG contain mutations that prevent cleavage by Rho (purple asterisks) (Strisovsky et al., 2009). (B,C) Neither mSpi (B) nor mSpiCS (C) induced ectopic aos-lacZ (aos-Z) expression at the dorsal-ventral midline (arrows) when coexpressed with Star in the wing disc using vg-GAL4. (D–G) Flip-out clones (marked with GFP, green) expressing mSpi (D), mSpiCS (E), mSpiAG (F) or mSpiCSAG (G), together with Star, did not recruit ectopic photoreceptors (Elav, magenta, shown alone in D′, E′, F′ and G′). (H–I) Mitotic clones (arrows) mutant for rasp (H) failed to differentiate photoreceptors R1–R7 (marked with Elav in red, shown alone in H′), although differentiation of the founder photoreceptor R8 (marked with Senseless in blue) was normal. Clones mutant for ru and rho in addition to rasp showed a similar loss of photoreceptor differentiation (I,I′).
In the Nrt–Spi and Spi–Gli chimeras, a large extracellular domain separates the receptor-binding EGF domain from the membrane, whereas in mSpi the EGF domain is located close to the membrane. To test whether the distance between the EGF domain and the membrane is important, we constructed the chimera Spi–CD2, in which the extracellular domain of Spi carrying the palmitoylation-site mutation is fused at its C-terminus to the transmembrane domain of the rat type I cell surface protein CD2 (Fig. 1B). Like the other chimeras, Spi–CD2 localized to the plasma membrane, activated the receptor in cultured cells and rescued Fas III expression (Fig. 1F,G,L), indicating that separation of the EGF domain from the membrane is not essential for Spi signaling. However, all the chimeric forms of Spi were weaker activators of MAPK phosphorylation in S2 cells than constitutively secreted and palmitoylated sSpi (Fig. 1G).
In vivo, overexpression of sSpi stimulates strong EGFR activation, and palmitoylation is essential for its activity (Miura et al., 2006). To compare the activities of our transmembrane Spi chimeras in vivo, we assayed their ability to activate the EGFR in larval imaginal discs. Expression of sSpi, Nrt–Spi, Spi–Gli or Spi–CD2 at the dorsal-ventral margin of the wing imaginal disc using the vestigial (vg)-GAL4 driver induced ectopic expression of argos (aos), a transcriptional target of the EGFR pathway (Golembo et al., 1996b, Fig. 2A–E). Another transcriptional target of the pathway, kekkon1 (Ghiglione et al., 1999), was also ectopically expressed in response to expression of each of the three chimeras in the wing disc (supplementary material Fig. S1). However, Spi–CD2 appeared to be a weaker activator of the pathway, as two copies of the Spi–CD2 transgene were required to activate aos and kekkon1 to the same extent as a single copy of the other chimeras. In the eye imaginal disc, ectopic expression of sSpi results in the recruitment of extra photoreceptors (Freeman, 1996), which can be detected by the neuronal marker Elav (Fig. 2F). Each of the three transmembrane chimeras induced ectopic photoreceptor differentiation when expressed in clones in the eye disc (Fig. 2G–J). Spi–CD2 again appeared to be a weaker activator of the pathway, as two copies of Spi–CD2 were required to induce ectopic photoreceptor formation (Fig. 2J). Expression of a single Spi–CD2 transgene resulted in loss of R1–R7 photoreceptors (Fig. 2I), a common consequence of weak pathway activation that is probably due to induction of Aos, a feedback inhibitor of the pathway (Golembo et al., 1996b; Lesokhin et al., 1999; Miura et al., 2006). Cell surface expression of Spi–CD2 was comparable to the other chimeric proteins in the wing disc (Fig. 2K–N), suggesting that its weaker activity is due to the proximity of the EGF domain to the membrane. Taken together, our tissue culture and in vivo data demonstrate that Spi does not require palmitoylation for its activity when it is tethered by a transmembrane domain. Moreover, its activity is not critically dependent on either the orientation or the distance of the EGF domain from the cell membrane.
Fig. 2.
Transmembrane Spi chimeras activate the EGFR in vivo. (A–E) Wing imaginal discs expressing sSpi (B), Nrt–Spi (C), Spi–Gli (D) or two copies of Spi–CD2 (E) at the dorsal-ventral midline driven by vg-GAL4 showed ectopic aos-lacZ expression (blue, arrows) compared to control discs (A). (F–J) GFP-marked spi mutant clones (green) expressing sSpi (F), Nrt–Spi (G), Spi–Gli (H) or two copies of Spi–CD2 (J) in eye imaginal discs recruited ectopic photoreceptors (stained with Elav in magenta, arrows). Clones expressing only one copy of Spi–CD2 (I) displayed loss of photoreceptors (arrows; Elav channel shown in white in I′). (K–N) Nrt–Spi (K), Spi–Gli (L) and Spi–CD2 (M) were expressed on the cell surface at similar levels. Expressing two copies of Spi–CD2 (N) resulted in much higher protein levels. Discs were processed in parallel by extracellular immunofluorescent staining with anti-HA antibody (pixel intensity heat map) following expression with apterous-GAL4. Images were taken with identical confocal settings.
The Spi pro-protein does not activate the EGFR in vivo, even when it is not palmitoylated
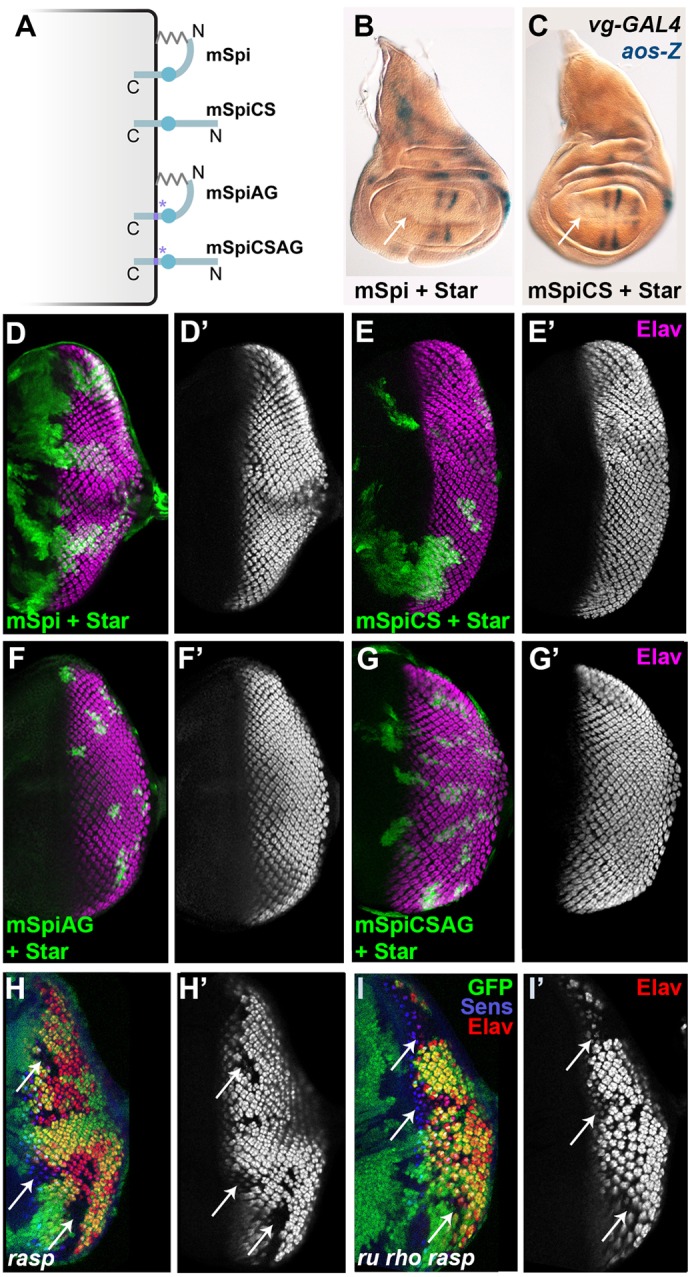
Although overexpression of sSpi strongly activated the EGFR in vivo, overexpression of the full-length pro-protein (mSpi) had no effect, even when its chaperone Star was coexpressed (Fig. 3B,D). These results are in agreement with previous reports showing that mSpi is only active in cells expressing endogenous Rho (Freeman, 1996; Pickup and Banerjee, 1999; Schweitzer et al., 1995). We confirmed that an unprocessed form of mSpi carrying mutations that disrupt the Rho cleavage site (mSpiAG) is also inactive (Fig. 3F) (Strisovsky et al., 2009). In contrast, Spi–CD2, which resembles mSpi in its type I orientation and short extracellular domain, is able to activate the EGFR. Because Spi–CD2 lacks the N-terminal palmitoylation site, we hypothesized that the presence of membrane tethers at both the N-terminus and C-terminus of mSpi might promote a conformation that is unfavorable for signaling (Fig. 3A). To test whether unpalmitoylated mSpi can activate the EGFR, we ectopically expressed the palmitoylation site mutant form mSpiCS in imaginal discs. Neither mSpiCS nor uncleavable and unpalmitoylatable mSpiCSAG induced ectopic aos expression or ectopic photoreceptor recruitment when coexpressed with Star (Fig. 3C,E,G), indicating that unpalmitoylated mSpi remains inactive.
In the eye disc, clones of cells lacking the Spi acyltransferase Rasp show reduced differentiation of photoreceptors R1–R7 (Fig. 3H) (Miura et al., 2006). In rasp mutant clones, the endogenous Spi pro-protein is processed by Rho and a second protease specific to the eye disc, Roughoid (Ru) (Wasserman et al., 2000), but fails to be palmitoylated (Miura et al., 2006). We tested whether preventing the cleavage and release of unpalmitoylated Spi by removing ru and rho function from rasp mutant clones would restore photoreceptor differentiation. We observed no rescue of photoreceptor differentiation in ru rho rasp triple mutant clones, confirming that the endogenous uncleaved and unpalmitoylated pro-protein is inactive (Fig. 3I). Therefore, the inability of mSpi to activate the EGFR is independent of its palmitoylation.
The Spi pro-protein accumulates on the cell surface and activates the EGFR in tissue culture
The failure of mSpi to activate the EGFR in vivo led us to consider the possibility that mSpi is not trafficked to the plasma membrane. In order to visualize only protein on the cell surface, we expressed Flag-tagged mSpi in cultured S2 cells and stained with anti-Flag antibody under non-permeabilizing conditions. We were able to detect mSpi at the cell surface only in the presence of co-expressed Star, which allows its export from the ER (Fig. 4A,B). The same was true for mSpiCS, the palmitoylation site mutant (Fig. 4C,D). To verify that cell surface staining represented full-length Spi pro-protein and not Spi processed by Rho and Rasp, we repeated the experiment using the mSpi variants lacking the Rho cleavage site (mSpiAG) (Strisovsky et al., 2009) and both the cleavage and palmitoylation sites (mSpiCSAG). These proteins were also detectable on the cell surface, demonstrating that the mSpi pro-protein can be trafficked to the plasma membrane in cultured cells (Fig. 4E–H).
Fig. 4.
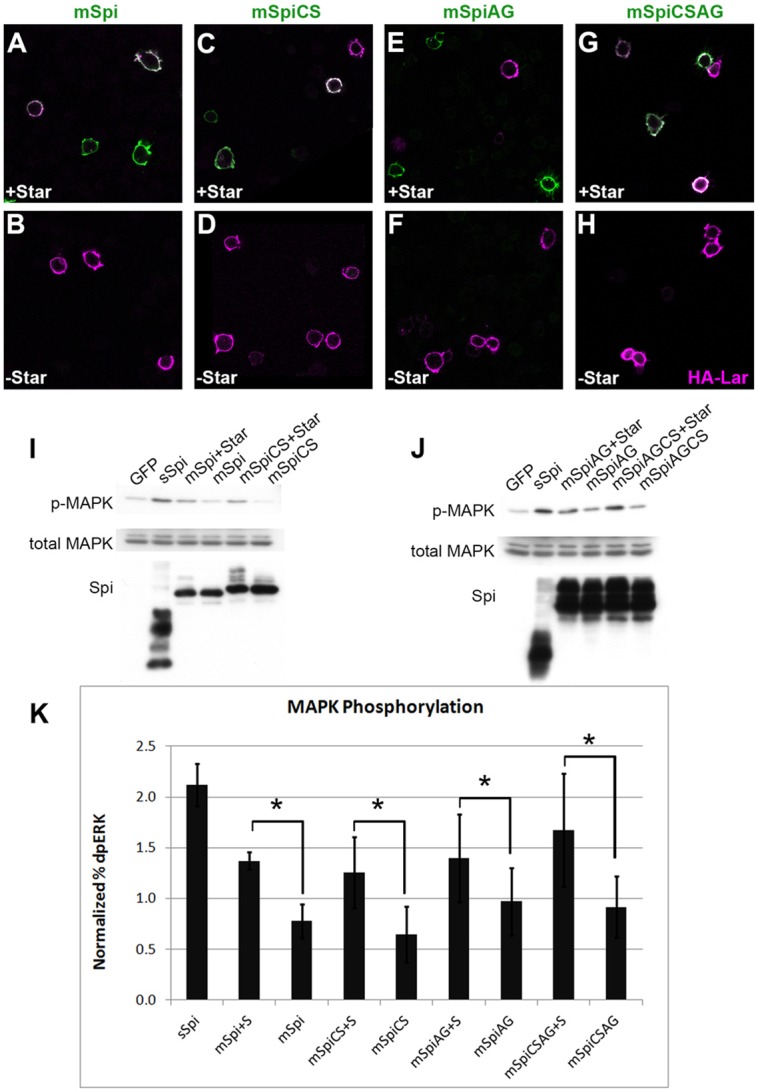
mSpi pro-protein activates the EGFR in vitro. (A–H) Flag-tagged mSpi (A,B), mSpiCS (C,D), mSpiAG (E,F) or mSpiCSAG (G,H) was detected on the surface of S2 cells by anti-Flag staining (green) in the absence of detergent when co-expressed with Star (A,C,E,G). In the absence of Star, no cell-surface mSpi was detected (B,D,F,H). Staining for the transmembrane protein HA–Lar (magenta) marks the plasma membrane. (I–K) Co-culture of S2 cells expressing mSpi, mSpiCS, mSpiAG or mSpiCSAG with EGFR-expressing D2F cells resulted in MAPK phosphorylation. Quantification was performed using Li-Cor Odyssey (K). For each condition, the phospho-MAPK level was divided by total MAPK level (% dpERK) and this value was normalized to the percentage dpERK obtained for control D2F cells co-cultured with S2 cells expressing intracellular GFP alone in that experiment. The mean±s.e.m. of three experiments is shown. sSpi-expressing cells induced more than a twofold increase in phospho-MAPK in co-cultured D2F cells compared to GFP-expressing cells. Each mSpi construct co-expressed with Star induced about a 1.5-fold increase in phospho-MAPK compared to GFP, whereas mSpi constructs without co-expressed Star did not increase phospho-MAPK above the level observed with GFP. *P<0.05 between the percentage dpERK induced by each Spi construct when co-expressed with Star compared to the percentage dpERK induced when expressed without Star (asterisks) as determined by paired Student's t-tests.
Surprisingly, when mSpi-expressing S2 cells were co-cultured with EGFR-expressing cells, the EGFR was activated and MAPK phosphorylation was induced (Fig. 4I–K). This effect was dependent on co-expression of Star, confirming that the observed activity was due to binding of mSpi to EGFR at the cell surface. Mutant forms of mSpi lacking the palmitoylation site, the Rhomboid cleavage site or both, were likewise capable of activating EGFR in a Star-dependent manner (Fig. 4I–K). These data reveal that the unprocessed mSpi protein is able to activate the EGFR in cultured cells.
Localization at the cell membrane correlates with signaling activity
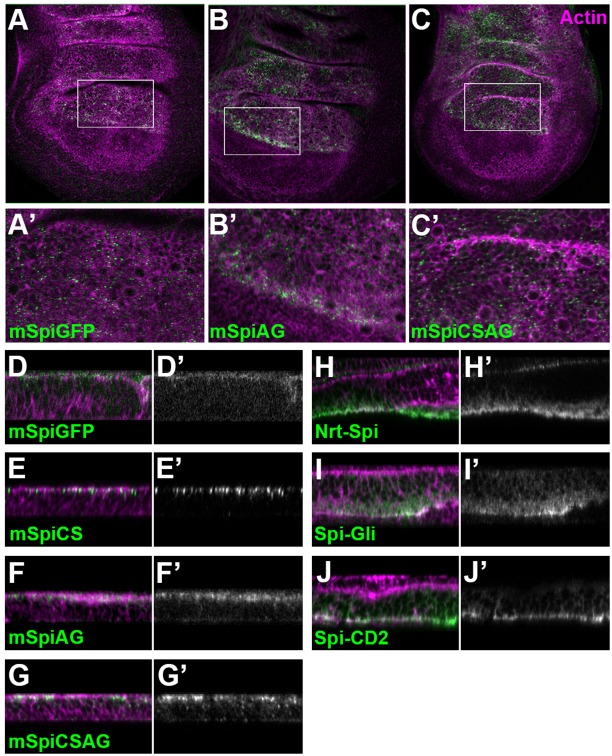
The results above suggest that mSpi is inherently capable of activating the EGFR, but is prevented from doing so in vivo. In order to investigate the nature of this inhibition, we used an extracellular immunofluorescent staining protocol to detect cell surface GFP- or Flag-tagged mSpi and its uncleavable and/or unpalmitoylatable variants in wing discs expressing these molecules together with Star in the dorsal compartment under the control of apterous (ap)-GAL4 (Strigini and Cohen, 2000). We were able to detect all the tagged forms of mSpi extracellularly, confirming that mSpi can reach the cell surface in vivo (Fig. 5A–G). mSpi staining was punctate (Fig. 5A–C) and localized to the apical region of the plasma membrane in imaginal disc cells (Fig. 5D–G). In contrast, the active Spi chimeras were confined to the basolateral domain (Fig. 5H–J). These data suggest that productive signaling occurs in the basolateral domain, and that localization of mSpi to the apical domain could render it inactive in vivo.
Fig. 5.
mSpi localizes to apical puncta at the cell surface in vivo. Imaginal discs were stained for extracellular antigen (green, shown alone in D′, E′, F′, G′, H′, I′, J′) and counterstained following fixation and permeabilization with TRITC–phalloidin (magenta) to mark cell membranes. mSpi–GFP (anti-GFP; A,D), mSpiAG (anti-Flag; B,F), mSpiCSAG (anti-Flag; C,G) and mSpiCS (anti-Flag; E) were detected on the cell surface in apical puncta. In contrast, Nrt–Spi (H), Spi–Gli (I) and Spi–CD2 (J), all detected with anti-HA antibody, were observed basolaterally.
The Spi cytoplasmic domain is not sufficient for apical localization and inactivation
Despite their similar structures and almost identical extracellular domains, Spi–CD2 and the unpalmitoylated pro-protein mSpiCS show different activities and apical-basal localization. To test whether the cytoplasmic domain of mSpi controls its trafficking and inhibits its signaling ability, we used it to replace the cytoplasmic domain of Spi–Gli, creating Spi–Gli–Spi (Fig. 6A). In comparison with Spi-Gli, which was a strong activator of the pathway, Spi–Gli–Spi only weakly activated the EGFR in vivo. Spi–Gli–Spi induced weak ectopic aos-lacZ expression in the wing imaginal disc (Fig. 6F), and caused loss of R1–R7 photoreceptors in the eye disc, consistent with aos induction by weak EGFR activation (Fig. 6H). Nevertheless, when coexpressed with Star, Spi–Gli–Spi was able to strongly activate the pathway, inducing strong ectopic aos-lacZ expression (Fig. 6G) and ectopic photoreceptor differentiation (Fig. 6I). Thus, adding the Spi cytoplasmic domain partially inhibits the activity of Spi–Gli, but this inhibition can be overcome by the presence of Star, suggesting that its effect is to sequester Spi–Gli–Spi in the ER (Lee et al., 2001). Consistent with this hypothesis, we observed greater cell surface expression of Spi–Gli–Spi in the presence of coexpressed Star (Fig. 6B,C), and Spi–Gli activity was not enhanced by Star (Fig. 6D,E). With or without Star coexpression, Spi–Gli–Spi localized basolaterally (Fig. 6B,C), consistent with its ability to activate EGFR. Thus the cytoplasmic domain of Spi is not sufficient to drive its apical localization.
Fig. 6.
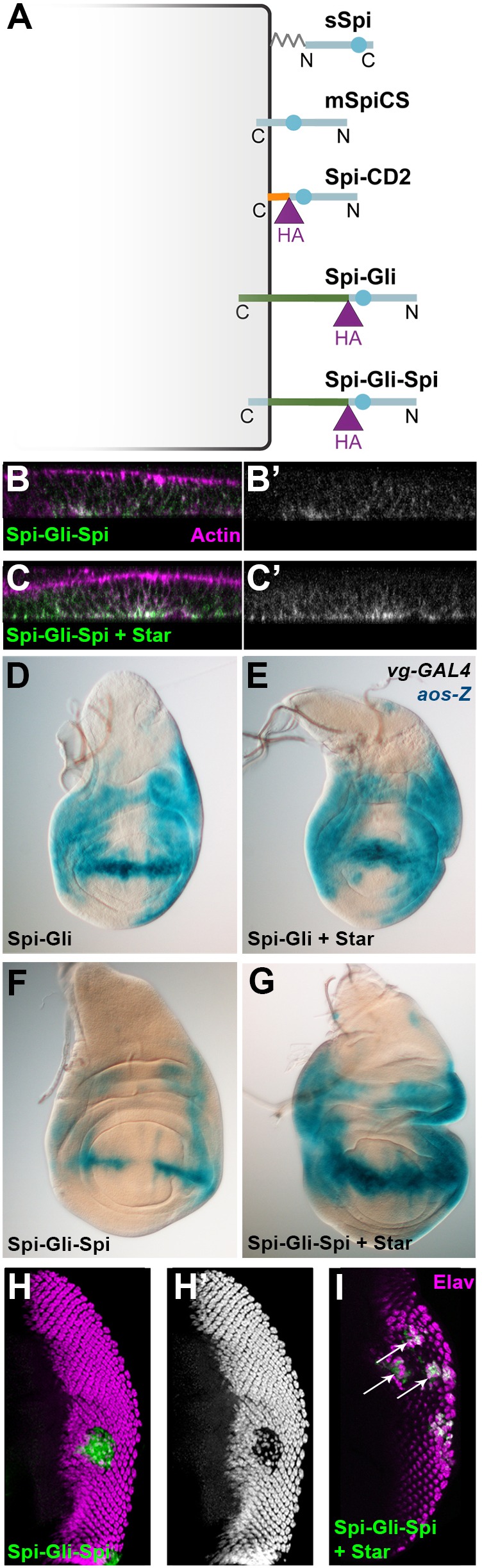
Spi–Gli–Spi activity depends on Star. (A) The Gliotactin cytoplasmic domain in Spi–Gli was replaced with the Spi cytoplasmic domain to generate Spi–Gli–Spi. (B,C) Spi–Gli–Spi, detected with anti-HA extracellular staining (green, white in B′ and C′; TRITC–phalloidin shown in magenta) localized basolaterally in the absence (B) and presence (C) of co-expressed Star, but its cell surface expression was greater when co-expressed with Star. B and C were imaged in parallel with identical confocal settings. (D–G) Both Spi-Gli (D–E) and Spi-Gli-Spi (F–G) induced ectopic aos-lacZ in wing imaginal discs when expressed with vg-GAL4 either alone (D,F) or with co-expressed Star (E,G), but for Spi–Gli–Spi the induction was stronger when it was co-expressed with Star. D–G were stained and imaged in parallel. (H,I) Flip-out clones (green) expressing Spi–Gli–Spi and Star (I) induced ectopic photoreceptor recruitment (marked with Elav in magenta), whereas clones expressing Spi–Gli–Spi without Star caused photoreceptor loss (H, Elav shown alone in H′).
Discussion
EGFR ligands are diffusible paracrine molecules that are produced by cleavage of transmembrane pro-proteins. Some pro-proteins are also capable of activating the receptor, but they often elicit different responses from the processed ligands. We investigated why the Drosophila EGFR ligand Spi is inactive in its pro-protein form (mSpi), even though the active processed form (sSpi) is associated with the membrane through a palmitate adduct. Our results show that sSpi is capable of activating the receptor when tethered to the membrane by an exogenous transmembrane domain in either orientation. Increased spacing between the receptor-binding EGF domain and the membrane appears favorable but not essential for signaling. The mSpi pro-protein is trafficked to the cell surface and can activate the EGFR in cultured cells, but not in vivo. mSpi appears largely confined to the apical domain of imaginal disc cells and is observed in puncta, whereas active Spi forms are uniformly distributed in the basolateral domain (Fig. 7A). The intracellular domain alone is not sufficient to direct a Spi chimera to the apical surface, suggesting a possible role for the transmembrane domain in this process. Taken together, our results suggest that the mSpi pro-protein is structurally capable of signaling, but is prevented from activating the EGFR in vivo by directed trafficking.
Fig. 7.
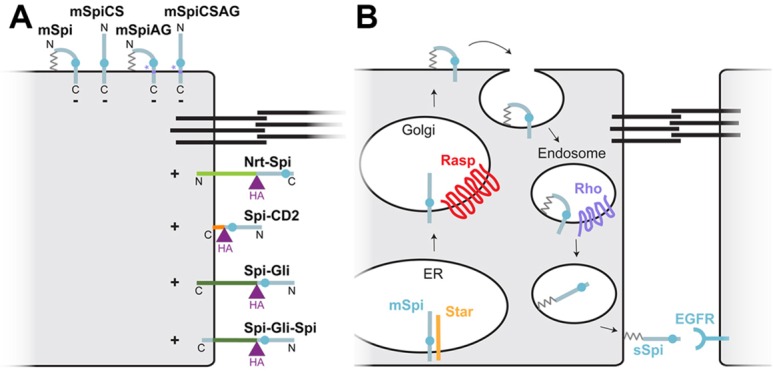
Models for Spi trafficking and activation. (A) Cartoon representing the localization and activity of all the Spi variants tested. Black lines indicate the adherens junctions; mSpi, mSpiCS, mSpiAG and mSpiCSAG localize to the apical surface and are inactive (−), whereas Nrt–Spi, Spi–CD2, Spi–Gli and Spi–Gli–Spi localize to the basolateral surface and are active (+). (B) A model for Spi trafficking. mSpi is exported from the ER with the help of the chaperone protein Star. It is palmitoylated by Rasp in the Golgi and trafficked to the apical cell surface. It might be endocytosed from this location, cleaved in late endosomes by the protease Rho and secreted basolaterally. The palmitate group would tether sSpi to the basolateral plasma membrane.
Determinants of mSpi localization
Although Spi–CD2 and mSpi differ only in their transmembrane and cytoplasmic domains, Spi–CD2 localizes to the basolateral surface of epithelial cells and activates the EGFR in vivo, whereas mSpi does not. Therefore, we expected the Spi cytoplasmic domain to contain sequences that control localization and signaling. The cytoplasmic domain of TGF-α is sufficient for basolateral trafficking in MDCK cells (Dempsey et al., 2003), and the transmembrane and cytoplasmic domains of human EGF prevent it from engaging in juxtacrine signaling (Dong et al., 2005). Indeed, replacing the cytoplasmic domain of the strong activator Spi–Gli with the mSpi cytoplasmic domain significantly weakens its signaling capacity. Nonetheless, Spi–Gli–Spi is localized basolaterally, and coexpression of Star with Spi–Gli–Spi restores strong signaling activity, suggesting that the inhibitory effect of the cytoplasmic domain is due to ER retention. Previous studies have shown roles for both the cytoplasmic and lumenal domains of mSpi in mediating Star dependence (Lee et al., 2001; Reich and Shilo, 2002). Thus, the Spi cytoplasmic domain is not sufficient to drive apical trafficking or prevent signaling. The Spi transmembrane domain might contribute to apical trafficking, or the Gliotactin transmembrane and extracellular domains, which promote basolateral trafficking (Schulte et al., 2003), might override the Spi trafficking signal in Spi-Gli-Spi.
Sorting of receptor and ligand to apical and basolateral domains
Although the mSpi pro-protein is inactive in vivo, we found that it is capable of activating EGFR in cultured S2 cells. One possible explanation for this difference is that S2 cells, unlike epithelial imaginal disc cells, lack polarity. In wing disc cells, the active Spi chimeras localize to a different membrane domain than the inactive pro-protein, potentially allowing them preferential access to the receptor. Previous studies have reported an apical localization of Drosophila EGFR in some tissues (Alvarado et al., 2004; Zak and Shilo, 1992); however, our results strongly suggest that, at least in the imaginal discs, active signaling can occur in the basolateral domain. We have observed extracellular EGFR staining throughout the apical-basal axis in wing imaginal discs (data not shown).
Spatial segregation of the ligand and receptor has been demonstrated in cultured human airway epithelia, where the EGFR ligand heregulin α (also known as neuregulin-1) is apically localized and its receptors are restricted basolaterally. In this context, signaling is prevented when the epithelial sheet is intact, and only upon wounding can the ligand access the receptor, thereby initiating the signaling that induces cell proliferation for wound repair (Vermeer et al., 2003). In contrast, localization of the C. elegans EGFR homolog LET-23 to the basolateral surface of vulval precursor cells is necessary to allow its interaction with LIN-3 ligand secreted from the anchor cell (Stetak et al., 2006) (Kaech et al., 1998). Our study suggests that localization of the ligand as well as the receptor regulates EGFR signaling in vivo. A recent study of Spi production by socket cells in mechanosensory organs reached a similar conclusion; polarized protrusions from the basolateral surface of socket cells appear to deliver Spi to the prospective bract cell (Peng et al., 2012).
A striking feature of mSpi localization is its appearance in puncta detectable with an extracellular staining protocol (Strigini and Cohen, 2000). One possibility is that these puncta represent aggregates at the cell surface, similar to those observed for another palmitoylated ligand, Hh (Vyas et al., 2008). Alternatively, they might represent endocytosing vesicles trapped at the cell surface by the cold temperature of the staining protocol. Rapid endocytosis of the pro-protein could prevent the protein from residing at the cell surface long enough to engage and activate the receptor. Wg receptors have been reported to undergo endocytosis preferentially from the apical domain (Marois et al., 2006). In addition, Hh is thought to undergo a recycling process in which it is first secreted apically from producing cells, then endocytosed and shuttled to the basolateral domain by Dispatched. Hh released from the basolateral domain associates with Interference hedgehog, Dally-like protein and filopodia to signal over a long range (Callejo et al., 2011). It is possible that a similar mechanism limits release of functional Spi to the basolateral domain. A role for endocytosis in Spi release has already been proposed based on the colocalization of Rho with endosomal markers and the requirement for the recycling endosome component Rab11 for secretion of functional Spi from photoreceptors (Tsruya et al., 2007; Yogev et al., 2010). Our results suggest a model wherein mSpi is exported to the apical surface and rapidly endocytosed, processed by Rho in the endocytic pathway, and subsequently recycled to the basolateral domain (Fig. 7B). This model predicts that endocytosis would promote release of the active ligand, and might also prevent signaling by the pro-protein. Alternatively, mSpi might be exported from the Golgi to Rho-containing endosomes and localized directly to the basolateral surface. In this case, the presence of mSpi on the apical plasma membrane would result from its inappropriate accumulation when overexpressed in cells that lack Rho.
Pro-protein signaling
The signaling ability of mammalian EGFR ligand pro-proteins has been controversial. Studies in vitro showed that a TGF-α pro-protein carrying mutations in the metalloproteinase cleavage site could signal, but in vivo, mutation of the metalloproteinase itself appeared to recapitulate loss of TGF-α (Brachmann et al., 1989; Peschon et al., 1998; Sanderson et al., 2006; Wong et al., 1989). In recent years, mounting evidence has been provided in support of pro-protein signaling, both by TGF-α and by HB-EGF, which has opposing effects on cell survival in its membrane-bound and secreted forms (Pan et al., 2002; Prince et al., 2010; Shi et al., 2000; Singh et al., 2007; Yang et al., 2000). Some pro-proteins, such as amphiregulin, might be released on exosomes, allowing them to act at a distance (Higginbotham et al., 2011). However, EGF appears to signal only in its soluble cleaved form (Dong et al., 2005; Dong et al., 1999). Pro-protein signaling often induces different outcomes from signaling by the mature ligands, making ligand processing a critical step in determining pathway effects.
We found that mSpi can signal in a tissue culture assay but not in imaginal discs. This seems to be the result of trafficking to different regions of the cell surface in polarized cells. Notably, sSpi is a stronger activator of the pathway than mSpi in S2 cells (Fig. 4), indicating that the pro-protein and processed ligand are not equivalent even in this context. Our model suggests that pro-proteins are not inherently inactive and that certain mammalian contexts might have evolved to utilize the pro-proteins as active signaling molecules, perhaps by altering their localization or retention on the cell surface.
Materials and Methods
Drosophila stocks
Drosophila stocks used were: aosW11, kek101433 and ap-GAL4 (Bloomington Drosophila Stock Center, Bloomington, IN, USA), UAS-sSpi, UAS-Rasp-HA, UAS-Star, UAS-rho, FRT40 spiSC1, FRT2A raspT802, UAS-mSpi, and UAS-mSpiCS (Miura et al., 2006), vg-GAL4 (Simmonds et al., 1995) and sim-GAL4 (Golembo et al., 1996a). The triple ru rho rasp mutant stock was made by recombining FRT2A raspT802 with ru1 rho7M43 h th st cu sr e ca (Bloomington Drosophila Stock Center). Transgenic lines were made by Genetic Services Inc. (Cambridge, MA, USA) or GenetiVision (Houston, TX, USA).
MARCM clones were made using hs-FLP, UAS-GFP; FRT40, tub-GAL80; tub-GAL4/TM6B. Flip-out clones were made using actin>y+>GAL4, UAS-GFP; hs-FLP, MKRS/TM6B or actin>CD2>GAL4, hs-FLP; UAS-GFP/TM6B.
Molecular biology
The neurotactin coding region was amplified from genomic DNA using primers 5′Nrt_EcoRI 5′-TTCTCGCAGAATTCTCTAAACGAT-3′ and 3′Nrt_BsiWIHA 5′-CGTACGGGCGTAATCTGGCACATCATACGGGTAGCGCGCATACCGCGGCACAAT-3′ and cloned into pUASt-sSpiCS (Miura et al., 2006) using EcoRI and BsiWI to make UAS-Nrt-Spi.
sSpiCS, which contains the signal peptide, has the cysteine residue following the signal peptide mutated to serine and is truncated at the Rho cleavage site (Miura et al., 2006), was cloned into Gateway pENTR/D-TOPO (Invitrogen/Life Technologies, Grand Island, NY, USA) using primers Spi up TOPO 5′-CACCATGCATTCCACAATGAGTGTACAAC-3′ and Spi down TOPO 5′-CATATGCCGGTAAAGCTTGGCGTAATCTGGCACATCATACGGGTACGGCCTCTTGGGCAGGTAAG-3′. The downstream primer introduced an HA tag as well as HindIII and NdeI sites. Gliotactin was amplified from cDNA RE15719 (Drosophila Genomics Resource Center, Bloomington, IN, USA) using primers Gli up 5′-ATGGGCAAGCTTCGGCCAGGCGTTGACTACCA-3′ and Gli down 5′-CGGGTACATATGTCACCGACTGCTGCCCGAGACACTG-3′ and cloned into pENTR-sSpiCS using HindIII and NdeI. Spi–Gli was then recombined into pTWG (DGRC) to make UAS-Spi-Gli.
The transmembrane domain from rat CD2 was amplified from cDNA (Invitrogen I.M.A.G.E. clone 7374969) using primers CD2 up 5′-ATGGGCAAGCTTGTCAACTGTCCAGAGAAA-3′ and CD2 down 5′-CGGGTACATATGTCATTTTATCTCCAGCTCTTC-3′ and cloned into pENTR-sSpiCS using HindIII and NdeI. Spi-CD2 was then recombined into pTWG (DGRC) to make UAS-Spi-CD2.
pUASt-mSpi-Flag was a gift from B. Shilo (Tsruya et al., 2002). Flag-tagged mSpiAG was a gift from M. Freeman (Strisovsky et al., 2009). mSpiAG-Flag was subcloned into pUASt using BglII and KpnI sites introduced by the following primers: mSpi_FBglII 5′-GCATAGATCTGCCGATACGCTACGCCAAAAAG-3′ and mSpi_RKpn 5′-GCATGGTACCCGCTCACAGCTTGTTGCTGCGT-3′. A Flag tag was introduced into the BsiWI site of pUASt-mSpiCS by annealing the following oligos: Bsi_FlagF 5′-GTACGGATTACAAGGATGACGATGACAAGC-3′ and Bsi_FlagR 5′-GTACGCTTGTCATCGTCATCCTTGTAATCC-3′. The CS mutation was introduced into pUASt-mSpiAG using overlapping PCR products made with the following primers: mSpi_FBglII 5′-GCATAGAT\CTGCCGATACGCTACGCCAAAAAG-3′ and mSpiCS_XhoIR 5′-GTCCGTACGGCTGCTCGAGGCCTCGACGTGCCAC-3′, mSpiCS_XhoIF 5′-GTGGCACGTCGAGGCCTCGAGCAGCCGTACGGAC-3′ and mSpiCS_SapI 5′-GTGCCGGACATGGACGAGGAGA-3′. The mutated fragment was cloned into pUASt-mSpiAG using the BglII and SapI sites.
UAS-Spi-Gli-Spi was cloned by overlap PCR, using the primers Spi-Gli-Kpn5′ 5′-AGAAGGTACCCGTTTATATGTACGTAC-3′ and Spi-Gli-overlap3′ 5′-AGCCCGCTGCTCGAAGCGCCACATGATGCAGCAGATGAC-3′ to amplify a region of Spi-Gli ending in the transmembrane domain, and the primers Spi-Gli-overlap5′ 5′-GTCATCTGCTGCATCATGTGGCGCTTCGAGCAGCGGGCT-3′ and Spi-AscI3′ 5′-ATTCGGCGCGCCGCATGCGGTAGGTAAATAGGAGTTATC-3′ to amplify the cytoplasmic domain of Spi and introduce an AscI site. The two PCR products were mixed and amplified with Spi-Gli-Kpn5′ and Spi-AscI3′ to generate a product that was cloned into the KpnI and AscI sites of UAS-Spi-Gli.
Tissue culture and phospho-MAPK assay
S2 and S2R+ cells were maintained in Schneider's Drosophila medium (Invitrogen/Life Technologies) supplemented with 10% fetal calf serum and penicillin-streptomycin. All UAS constructs were co-transfected with actin-Gal4 using Effectene Transfection Reagent (Qiagen, Germantown, MD, USA). Constructs used for transfection were UAS-GAP-GFP (Ritzenthaler et al., 2000), UAS-sSpi (Miura et al., 2006), UAS-mSpi-Flag (Tsruya et al., 2002), UAS-mSpiCS-Flag, UAS-mSpiAG-Flag, UAS-mSpiCSAG-Flag, pRM-Star-Myc (a gift from M. Freeman; Lee et al., 2001), UAS-Nrt-Spi, UAS-Spi-Gli, UAS-Spi-CD2 and UAS-Spi-Gli-Spi.
For the phospho-MAPK assay, on day 1, S2 cells stably expressing the EGFR (D2F, (Schweitzer et al., 1995)) were plated and S2 cells were plated and transfected with pRM-Star-Myc, actin-GAL4 and a UAS-Spi construct. On day 2, transfected cells were treated with 700 µM CuSO4 to induce Star expression. On day 3, EGFR expression was induced in the D2F cells with 60 µM CuSO4. After 3 hours, cells were collected, washed in PBS, resuspended in half the volume of Schneider's medium, and co-cultured for 1–3 hours with rocking at room temperature. Co-cultures were resuspended in RIPA buffer and immediately centrifuged, diluted into Laemmli buffer, boiled for 5 minutes and frozen.
Antibody staining and Western blot
Chamber slides (LabTek, Electron Microscopy Sciences, Hatfield, PA, USA) were treated with poly-L-lysine overnight. Cultured cells were stained under non-permeabilizing conditions in the absence of detergent as follows. Cells were allowed 30 minutes to adhere following plating. Cells were fixed in 4% formaldehyde-PBS, washed for 15 minutes to 1 hour in PBS, and stained overnight in primary antibody in PBS at 4°C. Cells were washed in PBS, stained for 2 hours in secondary antibody and imaged.
Tissues were stained using previously described protocols (Lee and Treisman, 2001). Extracellular staining was performed following the protocol of Strigini and Cohen (Strigini and Cohen, 2000). Imaginal discs were dissected in complete Schneider's medium and incubated for 30–60 minutes in primary antibody diluted in complete Schneider's medium with rocking at 4°C. Discs were rinsed in cold PBS and fixed at 4°C in 4% formaldehyde for 30 minutes, washed in PBS plus 0.1% Triton X-100, incubated with secondary antibody, and counterstained with TRITC–phalloidin (Sigma, St Louis, MO, USA). Discs were mounted in Fluoromount G (Southern Biotech, Birmingham, AL, USA). All fluorescence images were taken with a Zeiss LSM510 Confocal (Carl Zeiss, Oberkochen, Germany). X-gal staining was performed as described previously (Lee and Treisman, 2001).
Primary antibodies for immunostaining were: rat anti-HA 3F10 (1∶100 for standard immunofluorescence, 1∶30 for extracellular staining, Roche, Basel, Switzerland), mouse anti-Flag M2 (1∶500 for standard immunofluorescence, 1∶150 for extracellular staining; Sigma), rat anti-Elav (1∶100) and mouse anti-Fasciclin III (1∶1) (Developmental Studies Hybridoma Bank, Iowa City, IA, USA), guinea pig anti-Senseless (1∶1000; Nolo et al., 2000), rabbit anti-β–galactosidase (1∶5000, Cappel, MP Biomedicals, Santa Ana, CA, USA), mouse anti-GFP (1∶16 for extracellular staining, Santa Cruz Biotechnology, Dallas, TX, USA), mouse anti-EGFR E2906 (1∶50 for extracellular staining, Sigma-Aldrich, St. Louis, MO) or rabbit anti-EGFR (1∶500)(Rodrigues et al., 2005). Secondary antibodies were from Jackson Immunoresearch (1∶200, Westgrove, PA, USA) or Molecular Probes (1∶1000). Embryo stainings were developed with DAB (Sigma) and Vectastain Elite ABC System (Vector Laboratories, Burlingame, CA, USA).
Western blots were performed as described previously (Miura et al., 2006), blocked in 5% evaporated milk, and developed either with ECL (Thermo Fisher Scientific, Rockford, IL, USA) or with Odyssey (Li-Cor, Lincoln, NE, USA). Quantification was performed using Odyssey. Primary antibodies for western blotting were: mouse anti-diphosphorylated-ERK (1∶2000, Sigma), rabbit anti-MAPK (1∶20,000, Sigma), rat anti-Spi (1∶50) (Developmental Studies Hybridoma Bank), mouse anti-HA (1∶1000; Covance, Princeton, NJ, USA). Secondary antibodies were HRP-conjugated antibodies from Jackson Immunoresearch (1∶4000) or Infrared-conjugated antibodies from Li-Cor (1∶10,000).
Supplementary Material
Acknowledgments
We thank Nick Baker, Hugo Bellen, Matthew Freeman, Justin Kumar, Mark Lemmon, Daniel Marenda, Benny Shilo, the Bloomington Drosophila Stock Center, the Drosophila Genomics Resource Center, and the Developmental Studies Hybridoma Bank for fly stocks and reagents. We are grateful to Ruth Lehmann for the use of her confocal microscope. The manuscript was improved by the critical comments of Sergio Astigarraga, Cheuk Hei Ho, Kevin Legent, Justine Oyallon, and Annabelle Suisse.
Footnotes
Author contributions
J.S. performed the majority of the experiments. H.H.L. performed tissue culture experiments shown in Fig. 4. E.M. performed staining shown in Fig. 6. J.E.T. generated the Spi-Gli-Spi construct. J.S. and J.E.T. designed the experiments and wrote the manuscript.
Funding
This work was supported by the March of Dimes Birth Defects Foundation; and the National Institutes of Health [grant number EY13777]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.131169/-/DC1
References
- Alvarado D., Rice A. H., Duffy J. B. (2004). Knockouts of Kekkon1 define sequence elements essential for Drosophila epidermal growth factor receptor inhibition. Genetics 166, 201–211 10.1534/genetics.166.1.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anklesaria P., Teixidó J., Laiho M., Pierce J. H., Greenberger J. S., Massagué J. (1990). Cell-cell adhesion mediated by binding of membrane-anchored transforming growth factor alpha to epidermal growth factor receptors promotes cell proliferation. Proc. Natl. Acad. Sci. USA 87, 3289–3293 10.1073/pnas.87.9.3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld V. J., Fetter R. D., Broadie K., Goodman C. S. (1995). Gliotactin, a novel transmembrane protein on peripheral glia, is required to form the blood-nerve barrier in Drosophila. Cell 81, 757–767 10.1016/0092-8674(95)90537-5 [DOI] [PubMed] [Google Scholar]
- Brachmann R., Lindquist P. B., Nagashima M., Kohr W., Lipari T., Napier M., Derynck R. (1989). Transmembrane TGF-alpha precursors activate EGF/TGF-alpha receptors. Cell 56, 691–700 10.1016/0092-8674(89)90591-6 [DOI] [PubMed] [Google Scholar]
- Brown K. E., Kerr M., Freeman M. (2007). The EGFR ligands Spitz and Keren act cooperatively in the Drosophila eye. Dev. Biol. 307, 105–113 10.1016/j.ydbio.2007.04.025 [DOI] [PubMed] [Google Scholar]
- Callejo A., Bilioni A., Mollica E., Gorfinkiel N., Andrés G., Ibáñez C., Torroja C., Doglio L., Sierra J., Guerrero I. (2011). Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proc. Natl. Acad. Sci. USA 108, 12591–12598 10.1073/pnas.1106881108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Escalera S., Bockamp E. O., Moya F., Piovant M., Jiménez F. (1990). Characterization and gene cloning of neurotactin, a Drosophila transmembrane protein related to cholinesterases. EMBO J. 9, 3593–3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey P. J., Meise K. S., Coffey R. J. (2003). Basolateral sorting of transforming growth factor-alpha precursor in polarized epithelial cells: characterization of cytoplasmic domain determinants. Exp. Cell Res. 285, 159–174 10.1016/S0014-4827(03)00035-1 [DOI] [PubMed] [Google Scholar]
- Dong J., Opresko L. K., Dempsey P. J., Lauffenburger D. A., Coffey R. J., Wiley H. S. (1999). Metalloprotease-mediated ligand release regulates autocrine signaling through the epidermal growth factor receptor. Proc. Natl. Acad. Sci. USA 96, 6235–6240 10.1073/pnas.96.11.6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Opresko L. K., Chrisler W., Orr G., Quesenberry R. D., Lauffenburger D. A., Wiley H. S. (2005). The membrane-anchoring domain of epidermal growth factor receptor ligands dictates their ability to operate in juxtacrine mode. Mol. Biol. Cell 16, 2984–2998 10.1091/mbc.E04-11-0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. (1996). Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell 87, 651–660 10.1016/S0092-8674(00)81385-9 [DOI] [PubMed] [Google Scholar]
- Freeman M., Klämbt C., Goodman C. S., Rubin G. M. (1992). The argos gene encodes a diffusible factor that regulates cell fate decisions in the Drosophila eye. Cell 69, 963–975 10.1016/0092-8674(92)90615-J [DOI] [PubMed] [Google Scholar]
- Ghiglione C., Carraway K. L., III, Amundadottir L. T., Boswell R. E., Perrimon N., Duffy J. B. (1999). The transmembrane molecule kekkon 1 acts in a feedback loop to negatively regulate the activity of the Drosophila EGF receptor during oogenesis. Cell 96, 847–856 10.1016/S0092-8674(00)80594-2 [DOI] [PubMed] [Google Scholar]
- Golembo M., Raz E., Shilo B. Z. (1996a). The Drosophila embryonic midline is the site of Spitz processing, and induces activation of the EGF receptor in the ventral ectoderm. Development 122, 3363–3370 [DOI] [PubMed] [Google Scholar]
- Golembo M., Schweitzer R., Freeman M., Shilo B. Z. (1996b). Argos transcription is induced by the Drosophila EGF receptor pathway to form an inhibitory feedback loop. Development 122, 223–230 [DOI] [PubMed] [Google Scholar]
- Higginbotham J. N., Demory Beckler M., Gephart J. D., Franklin J. L., Bogatcheva G., Kremers G. J., Piston D. W., Ayers G. D., McConnell R. E., Tyska M. J. et al. (2011). Amphiregulin exosomes increase cancer cell invasion. Curr. Biol. 21, 779–786 10.1016/j.cub.2011.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortsch M., Patel N. H., Bieber A. J., Traquina Z. R., Goodman C. S. (1990). Drosophila neurotactin, a surface glycoprotein with homology to serine esterases, is dynamically expressed during embryogenesis. Development 110, 1327–1340 [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Lane H. A. (2005). ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer 5, 341–354 10.1038/nrc1609 [DOI] [PubMed] [Google Scholar]
- Iwamoto R., Handa K., Mekada E. (1999). Contact-dependent growth inhibition and apoptosis of epidermal growth factor (EGF) receptor-expressing cells by the membrane-anchored form of heparin-binding EGF-like growth factor. J. Biol. Chem. 274, 25906–25912 10.1074/jbc.274.36.25906 [DOI] [PubMed] [Google Scholar]
- Jiang H., Edgar B. A. (2009). EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development 136, 483–493 10.1242/dev.026955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorissen R. N., Walker F., Pouliot N., Garrett T. P., Ward C. W., Burgess A. W. (2003). Epidermal growth factor receptor: mechanisms of activation and signalling. Exp. Cell Res. 284, 31–53 10.1016/S0014-4827(02)00098-8 [DOI] [PubMed] [Google Scholar]
- Kaech S. M., Whitfield C. W., Kim S. K. (1998). The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell 94, 761–771 10.1016/S0092-8674(00)81735-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. D., Treisman J. E. (2001). Sightless has homology to transmembrane acyltransferases and is required to generate active Hedgehog protein. Curr. Biol. 11, 1147–1152 10.1016/S0960-9822(01)00323-2 [DOI] [PubMed] [Google Scholar]
- Lee J. R., Urban S., Garvey C. F., Freeman M. (2001). Regulated intracellular ligand transport and proteolysis control EGF signal activation in Drosophila. Cell 107, 161–171 10.1016/S0092-8674(01)00526-8 [DOI] [PubMed] [Google Scholar]
- Lesokhin A. M., Yu S. Y., Katz J., Baker N. E. (1999). Several levels of EGF receptor signaling during photoreceptor specification in wild-type, Ellipse, and null mutant Drosophila. Dev. Biol. 205, 129–144 10.1006/dbio.1998.9121 [DOI] [PubMed] [Google Scholar]
- Marois E., Mahmoud A., Eaton S. (2006). The endocytic pathway and formation of the Wingless morphogen gradient. Development 133, 307–317 10.1242/dev.02197 [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Pinheiro E. M., Kadlec L., Schüpbach T., Montell D. J. (2006). Multiple EGFR ligands participate in guiding migrating border cells. Dev. Biol. 296, 94–103 10.1016/j.ydbio.2006.04.438 [DOI] [PubMed] [Google Scholar]
- Miura G. I., Buglino J., Alvarado D., Lemmon M. A., Resh M. D., Treisman J. E. (2006). Palmitoylation of the EGFR ligand Spitz by Rasp increases Spitz activity by restricting its diffusion. Dev. Cell 10, 167–176 10.1016/j.devcel.2005.11.017 [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg F. S., Schüpbach T. (1993). The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell 75, 165–174 [PubMed] [Google Scholar]
- Nolo R., Abbott L. A., Bellen H. J. (2000). Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102, 349–362 10.1016/S0092-8674(00)00040-4 [DOI] [PubMed] [Google Scholar]
- Pan B., Sengoku K., Goishi K., Takuma N., Yamashita T., Wada K., Ishikawa M. (2002). The soluble and membrane-anchored forms of heparin-binding epidermal growth factor-like growth factor appear to play opposing roles in the survival and apoptosis of human luteinized granulosa cells. Mol. Hum. Reprod. 8, 734–741 10.1093/molehr/8.8.734 [DOI] [PubMed] [Google Scholar]
- Peng Y., Han C., Axelrod J. D. (2012). Planar polarized protrusions break the symmetry of EGFR signaling during Drosophila bract cell fate induction. Dev. Cell 23, 507–518 10.1016/j.devcel.2012.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon J. J., Slack J. L., Reddy P., Stocking K. L., Sunnarborg S. W., Lee D. C., Russell W. E., Castner B. J., Johnson R. S., Fitzner J. N. et al. (1998). An essential role for ectodomain shedding in mammalian development. Science 282, 1281–1284 10.1126/science.282.5392.1281 [DOI] [PubMed] [Google Scholar]
- Pickup A. T., Banerjee U. (1999). The role of star in the production of an activated ligand for the EGF receptor signaling pathway. Dev. Biol. 205, 254–259 10.1006/dbio.1998.9119 [DOI] [PubMed] [Google Scholar]
- Prince R. N., Schreiter E. R., Zou P., Wiley H. S., Ting A. Y., Lee R. T., Lauffenburger D. A. (2010). The heparin-binding domain of HB-EGF mediates localization to sites of cell-cell contact and prevents HB-EGF proteolytic release. J. Cell Sci. 123, 2308–2318 10.1242/jcs.058321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich A., Shilo B. Z. (2002). Keren, a new ligand of the Drosophila epidermal growth factor receptor, undergoes two modes of cleavage. EMBO J. 21, 4287–4296 10.1093/emboj/cdf439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzenthaler S., Suzuki E., Chiba A. (2000). Postsynaptic filopodia in muscle cells interact with innervating motoneuron axons. Nat. Neurosci. 3, 1012–1017 10.1038/79833 [DOI] [PubMed] [Google Scholar]
- Rodrigues A. B., Werner E., Moses K. (2005). Genetic and biochemical analysis of the role of Egfr in the morphogenetic furrow of the developing Drosophila eye. Development 132, 4697–4707 10.1242/dev.02058 [DOI] [PubMed] [Google Scholar]
- Rutledge B. J., Zhang K., Bier E., Jan Y. N., Perrimon N. (1992). The Drosophila spitz gene encodes a putative EGF-like growth factor involved in dorsal-ventral axis formation and neurogenesis. Genes Dev. 6, 1503–1517 10.1101/gad.6.8.1503 [DOI] [PubMed] [Google Scholar]
- Sanderson M. P., Dempsey P. J., Dunbar A. J. (2006). Control of ErbB signaling through metalloprotease mediated ectodomain shedding of EGF-like factors. Growth Factors 24, 121–136 10.1080/08977190600634373 [DOI] [PubMed] [Google Scholar]
- Schneider M. R., Wolf E. (2009). The epidermal growth factor receptor ligands at a glance. J. Cell. Physiol. 218, 460–466 10.1002/jcp.21635 [DOI] [PubMed] [Google Scholar]
- Schnepp B., Grumbling G., Donaldson T., Simcox A. (1996). Vein is a novel component in the Drosophila epidermal growth factor receptor pathway with similarity to the neuregulins. Genes Dev. 10, 2302–2313 10.1101/gad.10.18.2302 [DOI] [PubMed] [Google Scholar]
- Schulte J., Tepass U., Auld V. J. (2003). Gliotactin, a novel marker of tricellular junctions, is necessary for septate junction development in Drosophila. J. Cell Biol. 161, 991–1000 10.1083/jcb.200303192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer R., Shaharabany M., Seger R., Shilo B. Z. (1995). Secreted Spitz triggers the DER signaling pathway and is a limiting component in embryonic ventral ectoderm determination. Genes Dev. 9, 1518–1529 10.1101/gad.9.12.1518 [DOI] [PubMed] [Google Scholar]
- Shi W., Fan H., Shum L., Derynck R. (2000). The tetraspanin CD9 associates with transmembrane TGF-alpha and regulates TGF-alpha-induced EGF receptor activation and cell proliferation. J. Cell Biol. 148, 591–602 10.1083/jcb.148.3.591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo B. Z. (2005). Regulating the dynamics of EGF receptor signaling in space and time. Development 132, 4017–4027 10.1242/dev.02006 [DOI] [PubMed] [Google Scholar]
- Simmonds A. J., Brook W. J., Cohen S. M., Bell J. B. (1995). Distinguishable functions for engrailed and invected in anterior-posterior patterning in the Drosophila wing. Nature 376, 424–427 10.1038/376424a0 [DOI] [PubMed] [Google Scholar]
- Singh A. B., Harris R. C. (2005). Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell. Signal. 17, 1183–1193 10.1016/j.cellsig.2005.03.026 [DOI] [PubMed] [Google Scholar]
- Singh A. B., Tsukada T., Zent R., Harris R. C. (2004). Membrane-associated HB-EGF modulates HGF-induced cellular responses in MDCK cells. J. Cell Sci. 117, 1365–1379 10.1242/jcs.01037 [DOI] [PubMed] [Google Scholar]
- Singh A. B., Sugimoto K., Dhawan P., Harris R. C. (2007). Juxtacrine activation of EGFR regulates claudin expression and increases transepithelial resistance. Am. J. Physiol. 293, C1660–C1668 10.1152/ajpcell.00274.2007 [DOI] [PubMed] [Google Scholar]
- Stetak A., Hoier E. F., Croce A., Cassata G., Di Fiore P. P., Hajnal A. (2006). Cell fate-specific regulation of EGF receptor trafficking during Caenorhabditis elegans vulval development. EMBO J. 25, 2347–2357 10.1038/sj.emboj.7601137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini M., Cohen S. M. (2000). Wingless gradient formation in the Drosophila wing. Curr. Biol. 10, 293–300 10.1016/S0960-9822(00)00378-X [DOI] [PubMed] [Google Scholar]
- Strisovsky K., Sharpe H. J., Freeman M. (2009). Sequence-specific intramembrane proteolysis: identification of a recognition motif in rhomboid substrates. Mol. Cell 36, 1048–1059 10.1016/j.molcel.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura T., Kondo S., Homma T., Sakai M., Harris R. C. (1997). The membrane-bound form of heparin-binding epidermal growth factor-like growth factor promotes survival of cultured renal epithelial cells. J. Biol. Chem. 272, 31036–31042 10.1074/jbc.272.49.31036 [DOI] [PubMed] [Google Scholar]
- Tsruya R., Schlesinger A., Reich A., Gabay L., Sapir A., Shilo B. Z. (2002). Intracellular trafficking by Star regulates cleavage of the Drosophila EGF receptor ligand Spitz. Genes Dev. 16, 222–234 10.1101/gad.214202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsruya R., Wojtalla A., Carmon S., Yogev S., Reich A., Bibi E., Merdes G., Schejter E., Shilo B. Z. (2007). Rhomboid cleaves Star to regulate the levels of secreted Spitz. EMBO J. 26, 1211–1220 10.1038/sj.emboj.7601581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S., Lee J. R., Freeman M. (2001). Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell 107, 173–182 10.1016/S0092-8674(01)00525-6 [DOI] [PubMed] [Google Scholar]
- Vermeer P. D., Einwalter L. A., Moninger T. O., Rokhlina T., Kern J. A., Zabner J., Welsh M. J. (2003). Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 422, 322–326 10.1038/nature01440 [DOI] [PubMed] [Google Scholar]
- Vyas N., Goswami D., Manonmani A., Sharma P., Ranganath H. A., VijayRaghavan K., Shashidhara L. S., Sowdhamini R., Mayor S. (2008). Nanoscale organization of hedgehog is essential for long-range signaling. Cell 133, 1214–1227 10.1016/j.cell.2008.05.026 [DOI] [PubMed] [Google Scholar]
- Wasserman J. D., Urban S., Freeman M. (2000). A family of rhomboid-like genes: Drosophila rhomboid-1 and roughoid/rhomboid-3 cooperate to activate EGF receptor signaling. Genes Dev. 14, 1651–1663 [PMC free article] [PubMed] [Google Scholar]
- Wong S. T., Winchell L. F., McCune B. K., Earp H. S., Teixidó J., Massagué J., Herman B., Lee D. C. (1989). The TGF-alpha precursor expressed on the cell surface binds to the EGF receptor on adjacent cells, leading to signal transduction. Cell 56, 495–506 10.1016/0092-8674(89)90252-3 [DOI] [PubMed] [Google Scholar]
- Xu N., Wang S. Q., Tan D., Gao Y., Lin G., Xi R. (2011). EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev. Biol. 354, 31–43 10.1016/j.ydbio.2011.03.018 [DOI] [PubMed] [Google Scholar]
- Yang H., Jiang D., Li W., Liang J., Gentry L. E., Brattain M. G. (2000). Defective cleavage of membrane bound TGFalpha leads to enhanced activation of the EGF receptor in malignant cells. Oncogene 19, 1901–1914 10.1038/sj.onc.1203513 [DOI] [PubMed] [Google Scholar]
- Yogev S., Schejter E. D., Shilo B. Z. (2010). Polarized secretion of Drosophila EGFR ligand from photoreceptor neurons is controlled by ER localization of the ligand-processing machinery. PLoS Biol. 8, e1000505 10.1371/journal.pbio.1000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak N. B., Shilo B. Z. (1992). Localization of DER and the pattern of cell divisions in wild-type and Ellipse eye imaginal discs. Dev. Biol. 149, 448–456 10.1016/0012-1606(92)90299-V [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.