SUMMARY
Developing T cells express diverse antigen receptors whose specificities are not pre-matched to the foreign antigens they eventually encounter. Past experiments have revealed that thymocytes must productively signal in response to self-antigens to mature and enter the peripheral T cell pool (positive selection), but how this process enhances effective mature T cell responses to foreign antigen is not fully understood. Here we have documented an unsuspected connection between thymic recognition events and foreign antigen-driven T cell responses. We find that the strength of self-reactivity is a clone-specific property unexpectedly directly related to the strength of T cell receptor (TCR) binding to presented foreign antigen. T cells with receptors showing stronger interaction with self dominate in responses to infections and accumulate in aging individuals, revealing that positive selection contributes to effective immunity by skewing the mature TCR repertoire towards highly effective recognition of pathogens that pose a danger to the host.
INTRODUCTION
CD4+ and CD8+ T cells are crucial effector cells whose response to infection requires recognition of pathogen-derived peptides bound to molecules encoded by the Major Histocompatibility Complex (pMHC). The relevant pMHC antigen receptors are clone-specific heterodimeric molecules (αβTCRs) whose chains are derived via quasirandom somatic recombination of gene segments and non-templated nucleotide addition (Davis and Bjorkman, 1988). This mechanism generates a population of immature T cells collectively capable of recognizing an enormous diversity of pMHC combinations, including clones with potentially harmful self-reactivity. To purge such dangerous cells, immature T cells are tested for self-responsiveness in the thymus. Strong signaling upon self-recognition results in removal of these cells from the mature repertoire (negative selection). Rather surprisingly, thymocyte survival and maturation requires productive signaling in response to self-antigens, but at a lower intensity than that leading to deletion (positive selection) (Morris and Allen, 2012).
While the necessity of negative selection is easily appreciated, the benefit of positive selection to the immune system is not yet fully understood, although several hypotheses have been proposed. The earliest concept was that positive selection adapts the T cell repertoire to the specific allelic forms of MHC gene products expressed by the host (“MHC restriction”) (Bevan, 1977). However, structural data show that contacts between TCR and MHC are mainly mediated by the CDR1 and CDR2 regions of the Vα and Vβ segments of the TCR, which are germline encoded (Garboczi et al., 1996; Reinherz et al., 1999), and, unlike what the MHC restriction hypothesis would predict, concordant variations between Vα and Vβ abundance and MHC haplotype have rarely been observed (Garcia et al., 2009; Marrack et al., 2008; Okada and Weissman, 1989; Rock et al., 1994). Indeed, residues in CDR1 and 2 have been described that contact conserved amino acids present in different MHC alleles. Some CDR2 residues are conserved across different species, suggesting that the specificity of the TCR for MHC was evolutionarily selected (Marrack et al., 2008; Scott-Browne et al., 2011). It is the variable region of the TCR, CDR3, that primarily contacts presented peptide and dictates the strength of recognition (Jorgensen et al., 1992), consistent with evidence that the peptides presented in the thymus substantially impact the diversity of the TCR repertoire (Barton and Rudensky, 1999; Germain, 1990; Grubin et al., 1997; Hogquist et al., 1993; Nikolic-Zugic and Bevan, 1990).
More recently, two other possible roles for self-pMHC in the generation of T cell responses to foreign antigen have been described. Interactions of naïve T cells with self-pMHC result in the partial tyrosine phosphorylation of the TCR ζ chain, polarize components of the signaling apparatus, and sustain T cell sensitivity to foreign antigen (Stefanova et al., 2002). In addition, specific self-pMHCs can act as co-agonists that augment the activation of T cells by agonist pMHC (Krogsgaard et al., 2005). In some instances, the co-agonist self-pMHC are the same as those responsible for positive selection in the thymus (Ebert et al., 2009; Lo et al., 2009), although the in vivo relevance of these findings remains controversial (Krogsgaard et al., 2005; Ma et al., 2008; Sporri and Reis e Sousa, 2002).
One straightforward hypothesis that has not been addressed is simply that TCRs able to bind self-pMHC well (but below the negative selection threshold) also bind especially well to foreign pMHC and hence, that positive selection ensures that T cells most useful for host defense against pathogens are selected from a diverse initial repertoire to populate the peripheral T cell pool. A direct relationship between self and foreign antigen binding is not evident in available structural data on pMHC recognition by TCR, but this would provide such a powerful explanation for the utility of positive selection that we decided to test the possibility experimentally. Here we report that indeed, there is such a direct relationship that can be seen using methods that avoid the complications of possible self-ligand co-agonist function or persistent self-recognition by peripheral T cells in vivo. Quantitative studies showed that repertoire binding to self and foreign pMHC is strongly skewed during positive selection towards higher affinity cells and that the latter predominate in response to diverse pathogens and among memory cells in mice and humans. These findings have implications not only for understanding basic T cell biology, but suggest ways to probe and manipulate the polyclonal T cell pool to facilitate immune function or limit autoimmunity.
RESULTS
Naïve CD4+ T cell CD5 expression reflects the clonotype-specific strength of TCR self-recognition
To determine whether there is a relationship between the self-reactivity of CD4+ T cells and their binding of agonist pMHC, we required methods for assessing the strength of self-recognition on both a clone-specific and population basis. Examining TCR self-binding affinities directly was impractical, given the technical problems of detecting the very low affinity of TCRs for self-pMHC and the difficulty of doing so for all individual naïve CD4+ T cells. Instead, we took advantage of studies suggesting that the expression of CD5, a negative regulator of TCR signaling, is proportional to TCR signaling strength during immature T cell selection in the thymus (Azzam et al., 1998). The maintenance of CD5 expression on CD4+ and CD8+ T cells requires contact with self-pMHC (Mandl et al., 2012; Smith et al., 2001) and is correlated with the extent of self-pMHC driven lymphopenia-induced proliferation (Kassiotis et al., 2003; Kieper et al., 2004; Smith et al., 2001). These data suggested the possibility that the steady-state CD5 expression on peripheral naïve CD4+ T cells might also reflect proximal TCR signal strength in response to self-ligands.
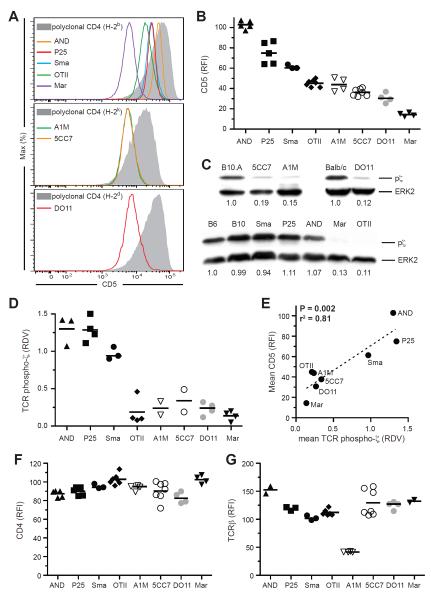
To examine this issue, we first quantified CD5 on naïve T cells from 8 distinct CD4+ TCR transgenic (Tg) Rag1−/− or Rag2−/− mice (Fig. 1). Each TCR Tg T cell population had a specific, reproducible surface amount of CD5 that, when comparing the various Tg cell populations, covered a wide range from low (Marilyn) to high (AND) (Fig. 1, A and B). The narrow distribution of CD5 expression on cells from a given Tg was in contrast to the broad distribution seen with polyclonal CD4+ T cells (Fig. 1A). We next quantified steady-state TCR subthreshold signaling due to interaction with self ligands by measuring partial TCR ζ chain phosphorylation (pζ) by immunoblot, finding that the amount of pζ varied widely among the distinct clonal populations examined (Fig. 1, C and D). There was a significant correlation between measures of pζ and CD5 for the different TCR Tg CD4+ T cells (Fig. 1E), suggesting that CD5 expression reflects a clonotype-specific heterogeneity in TCR self-reactivity that is dependent on the strength of interaction with self-pMHC. In contrast, there was no correlation between pζ and either CD4, TCRβ, CD127, CD122, Thy, CD45, CD69, CD25, CD62L, or CD2 (Fig. 1, F and G; fig. S1). Consistent with partial ζ phosphorylation being dependent on interaction with self-pMHC, pζ amounts were unchanged in germ-free mice (fig. S2A).
Figure 1. TCR transgenic CD4+ T cell CD5 expression reflects the clone-specific strength of self-reactivity.
(A and B) CD5 surface expression on naïve peripheral CD4+ T cells from TCR Tg mice relative to polyclonal CD4+ T cells from mice of the respective background. Representative plots, (A), data from 2–4 independent experiments, (B). (C and D) Immunoblot for tyrosine phosphorylation of the TCR ζ chain and total ERK-2 in purified peripheral CD4+ T cells. Representative blots with relative densitometry values (RDV) given below each lane (p-ζ/ERK2 ratio) relative to CD4+ polyclonal cells, (C); data from 2–4 independent experiments, (D). (E) Correlation between mean CD5 relative fluorescent intensity (RFI) and mean pζ chain RDV for each TCR Tg strain. (F and G) CD4 (F) and TCRβ (G) surface expression on naïve CD4+ T cells from TCR Tg mice relative to polyclonal CD4+ T cells from mice of the respective background.
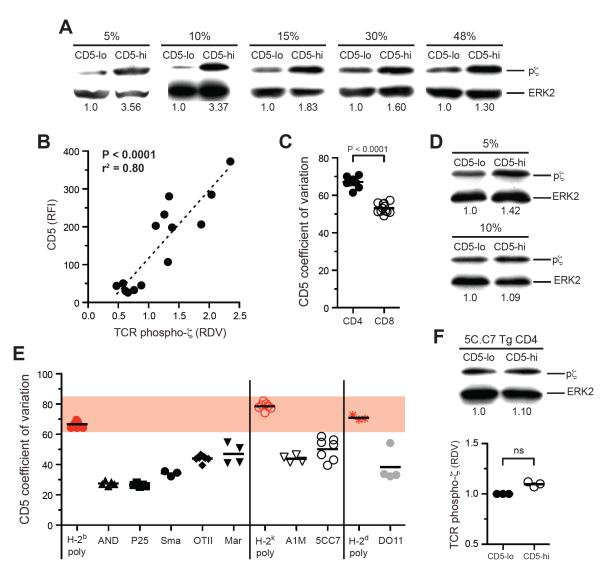
To determine whether CD5 is a marker for clonotype-specific TCR self-reactivity within a polyclonal CD4+ T cell repertoire, we sorted T cells based on high or low CD5 expression and measured pζ in sorted cells (Fig. 2 and fig. S2B). The more separate the sorted CD5hi and CD5lo populations, the greater the difference seen in pζ amounts (Fig. 2A). The difference was maximal (>3 fold) comparing the 5% CD5 brightest and dimmest naïve CD4+ T cells, but was observed even when the population was divided in two based on CD5 expression (Fig. 2A). Binding of CD5 antibody to CD4+ T cells did not impact pζ, nor did it affect the responsiveness of T cells to agonist peptide in vitro (fig. S2C). As with TCR Tg CD4+ T cells, we found a highly significant linear relationship between the proximal TCR signal strength measured by pζ and the expression of CD5, but not of TCRβ or CD4, on sorted polyclonal CD4+ T cells (Fig. 2B and fig. S2B). The positive correlation between CD5 and pζ amounts might be unexpected given the reported inhibitory role of CD5 on TCR signaling (Azzam et al., 1998) and a simplistic model in which CD5 mediates perfect feedback control to normalize the functional pMHC signaling response of all T cell clones. These data make clear that any negative regulatory role of CD5, while it may blunt the response of highly reactive T cells, does not equalize TCR subthreshold signal strength among CD4+ T cells.
Figure 2. Polyclonal CD4+ T cells show a broad range of self-reactivity that is correlated with CD5 expression.
(A, D, and F) Immunoblot analysis for tyrosine phosphorylation of the TCR ζ chain and total ERK2 in naïve polyclonal CD5hi and CD5lo CD4+ T cells, (A); polyclonal CD8+ T cells, (D); and 5C.C7 TCR Tg CD4+ T cells, (F). RDV relative to the CD5lo population is given below each lane. Sort stringencies are for the 10% highest or lowest populations unless specified otherwise. Representative data from 2–3 experiments are shown. (B) Correlation between CD5 RFI and phospho-ζ RDV for CD4+ T cells sorted on CD5 expression. RDVs are normalized relative to unsorted CD4+ polyclonal cells. Data are from 5 independent experiments. (C and D) CD5 coefficient of variation for polyclonal CD4+ and CD8+ T cells, (C); polyclonal or TCR Tg CD4+ T cells (E). ns, not significant.
For polyclonal CD8+ T cells the CD5 distribution was significantly narrower than for CD4+ T cells (Fig. 2C) and differences in pζ amounts were less pronounced among CD8+ T cells sorted for CD5 expression (Fig. 2D). CD8 coreceptor expression is tuned according to self-affinity on CD8+ T cells, which may act to reduce the effective breadth in TCR reactivity for self-pMHC on CD8+ T cells (Park et al., 2007). Consistent with this, we found an inverse relationship between CD8 and CD5 expression on CD8+ T cells (fig. S2D). In contrast, on CD4+ T cells we saw no such equalizing role for the CD4 coreceptor (Fig. 1F). As would be expected if CD5 reflects clonotypic heterogeneity in self-reactivity, for all mouse strains tested the CD5 coefficient of variation was greater among polyclonal CD4+ T cells than for any individual TCR Tg CD4+ T cell population (Fig. 2E). In contrast to polyclonal cells, we could not detect a significant difference in pζ between the highest and lowest 10% CD5 expressors among monoclonal 5C.C7 or other TCR Tg cells (Fig. 2F and data not shown). Together, these data suggest that CD5 is a surface marker on naïve CD4+ T cells whose variable expression in a polyclonal T cell population reflects heterogeneity in the strength of individual antigen receptor reactivity to non-agonist self ligands.
An unexpected direct relationship between TCR self reactivity and strength of binding to agonist peptide-MHC
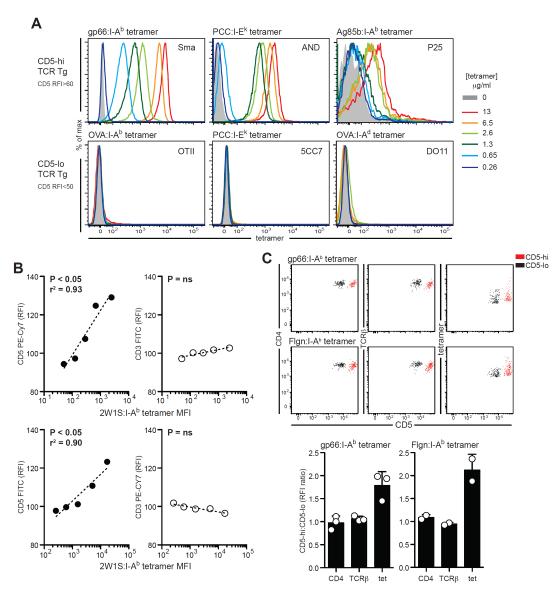
Given the diversity among TCR Tg T cells in self-reactivity and our ability to track this parameter using CD5 expression, we next asked whether there were also differences in the binding strength of these TCR to agonist foreign pMHC antigen, which contributes to clonal dominance during foreign antigen responses (Busch and Pamer, 1999; Malherbe et al., 2004; Price et al., 2005; Savage et al., 1999). To this end, we used pMHC-II tetramers, which are antigen-based reagents able to stain only cells with TCRs whose binding strength is above a particular threshold (Sabatino et al., 2011). TCRs with a low binding strength often dissociate faster from pMHC tetramers and therefore binding can be partly or completely absent at the time of post-wash analysis (Busch and Pamer, 1999; Crawford et al., 1998; Laugel et al., 2007; Yee et al., 1999). Using this method to evaluate foreign antigen recognition rather than functional response assays avoids the possible confounding effects of self-ligands contributing as co-agonists to the measured response. OT-II, 5C.C7, and DO11.10 TCR Tg T cells, all low in CD5 surface expression, did not show staining with their specific tetramer at 13μg/ml or less. In contrast, SMARTA, AND, and P25 TCR Tg T cells, which are high CD5 expressors, had detectable pMHC-II tetramer staining under these conditions (Fig. 3A). These data are consistent with results in the literature - 2D2 TCR Tg T cells do not stain with specific MOG:I-Ab pMHC-II tetramers (Sabatino et al., 2011) and have low CD5 and pζ (data not shown).
Figure 3. CD4+ T cell self-reactivity is positively correlated with TCR strength of binding to foreign antigen.
(A) Dose-response of binding of specific pMHC tetramers to TCR Tg CD4+ T cells. Data are representative of 3–4 independent experiments. (B) Naïve CD4+ T cells sorted from pre-immune mice using 2W1S:I-Ab tetramer binding were gated by tetramer staining intensity after concatenation of data from 5–7 mice and analyzed for CD3 and CD5 expression using flow cytometry. Correlation between tetramer MFI and CD3 or CD5 RFI is shown (top). Results were unchanged when fluorochromes were inverted (bottom). Data are representative of 3 independent experiments. (C) CD4, TCRβ and pMHC-tetramer staining are shown as representative dot plots (top) or the RFI ratio between the brightest and dimmest 15% CD5hi and CD5lo cells sorted from naïve polyclonal CD4+ T cells pooled from 20 mice (bottom). Bars show mean±s.d; ns, not significant.
At much higher concentrations, tetramer binding was observed with OT-II, DO11.10, and 5C.C7 Tg cells (fig. S3A), indicating that the absence of tetramer binding at lower concentrations was not due to non-functional reagents. Furthermore, omitting the wash step, or acquiring the fluorescent signal of cells immediately after washing, enhanced the staining of the CD5lo TCR Tg 5C.C7 and OT-II cells (fig. S3B), suggesting the absence of tetramer binding for CD5lo TCR Tg cells was attributable to a faster off-rate. The tetramer staining conditions used did not lead to T cell activation as measured by CD69 expression or to changes in CD5 expression (data not shown). The PCC:I-Ek tetramer that failed to stain 5C.C7 Tg T cells was identical to the tetramer used to successfully stain AND TCR Tg T cells at the same concentrations (Fig. 3A). An MCC:I-Ek tetramer does stain 5C.C7 Tg T cells (Savage et al., 1999), but the staining was nonetheless still of lower intensity than AND cells exposed to the same tetramer concentration (fig. S3C). A tetramer dissociation assay showed that CD5lo 5C.C7 cells had a faster dissociation rate from MCC:I-Ek than CD5hi AND cells (fig. S3D). Together, these data allowed us to separate our panel of TCR Tg mice into two groups: CD5hi clones that showed good tetramer binding and CD5lo clones that showed poor tetramer binding, suggesting a possible relationship between strength of TCR self-pMHC reactivity and their specific foreign pMHC binding strength.
To ask whether there is indeed a direct relationship between the strength of self and foreign antigen recognition, we assessed the relationship between CD5 expression and tetramer staining in a pool of naïve polyclonal CD4+ T cells. In this case, all the antigen-reactive cells above a certain affinity threshold for the pMHC employed would be stained by the same reagent. Using a magnetic bead enrichment method, we examined a population of 2W1S:I-Ab–specific CD4+ T cells, for which there are ~200 precursor cells in a pre-immune mouse (Moon et al., 2007). Concatenation of staining data from 5–7 mice yielded ~1000 tetramer+ events, allowing us to divide these into equal event gates according to tetramer staining intensity and examine both CD5 and CD3 expression on cells within each gate (fig. S3E). We found a significant positive correlation between CD5 and 2W1S tetramer staining intensity but not between CD3 and 2W1S tetramer staining intensity (Fig. 3B). Furthermore, inverting the fluorophores used for CD3 and CD5 staining gave the same result (Fig. 3B). As only the 2W1S-specific CD4+ T cell precursor frequency was large enough to perform such a correlation analysis with available MHC-II tetramer reagents, we investigated whether the relationship between agonist pMHC binding and CD5 expression was present with other specific pre-immune CD4+ T cell cohorts using a modified methodology. We sorted polyclonal naïve CD4+ T cells from 20 mice for the 15% CD5 highest and 15% dimmest and then stained the sorted populations with either of two distinct foreign antigen tetramers, LCMV GP66–77:I-Ab and Flgn456–475:I-Ab. As for 2W1S-specific CD4+ T cells, the more self-reactive CD5hi CD4+ T cells had a greater tetramer staining intensity with each reagent compared with the less self-reactive CD5lo CD4+ T cells (Fig. 3C). This difference could not be attributed to differences in CD4 or TCRβ expression (Fig. 3C).
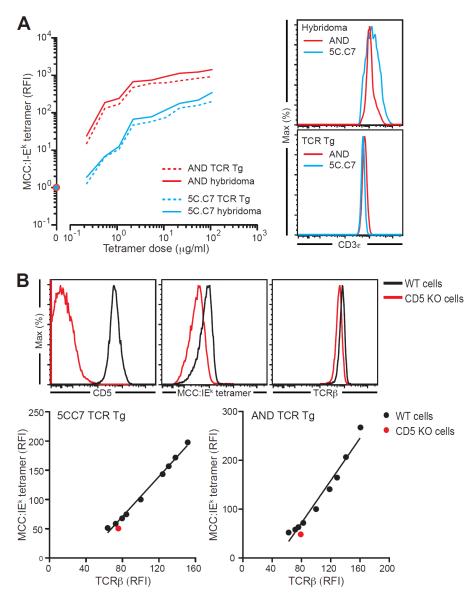
TCR polarization results from active recognition of self ligands by CD4+ T cells in the lymphoid microenvironment (Stefanova et al., 2002). TCR clustering in response to tonic self-engagement could facilitate effective multivalent tetramer binding and explain the differential staining of CD5lo and CD5hi cells, rather than such differences arising from the biophysical ligand-binding properties of the TCR recognition domain. To examine this possibility, we tested whether T cells maintained in the absence of self-ligands show the differences in tetramer binding seen for freshly isolated naïve T cells. We generated hybridomas bearing the 5C.C7 (CD5lo) and the AND (CD5hi) TCRs and tested them for the ability to bind MCC:I-Ek tetramer. Despite growing autonomously in absence of self-pMHC, 5C.C7 and AND hybridomas showed the same difference in tetramer binding as fresh TCR Tg cells, demonstrating that TCR-intrinsic biophysical properties determine the tetramer staining differences observed (Fig. 4A). This difference was not a result of lower TCR expression by the 5C.C7 hybridoma, as CD3ε expression was similar to that of the AND hybridoma (Fig. 4A).
Figure 4. Relationship between CD5 and TCR agonist binding strength is not explained by factors aside from TCR specificity.
(A) T cell hybridomas derived from 5C.C7 and AND TCR Tg T cells were expanded in culture 2 weeks after hybrid selection, and their ability to bind to MCC:IEk determined. Left panel: binding curves for fresh TCR transgenic cells (solid lines) versus hybridomas (dashed lines) bearing the AND or 5C.C7 TCR, calculated relative to a no-tetramer control (circles). Right panels: CD3ε expression of these cells. (B) TCRβ and MCC:IEk staining was performed on wild type or Cd5−/− 5C.C7 or AND TCR Tg lymph node cells. Upper panel: example staining for CD5, TCRβ and MCC:IEk in one 5C.C7 Cd5+/+ or 5C.C7 Cd5−/− mouse. Both tetramer staining and TCRβ expression was lower in Cd5−/− CD4+ T cells. Lower panel: lymph node CD4+CD3+ populations from 5C.C7 or AND mice were electronically divided into subpopulations based on TCRβ expression using FlowJo and the MCC:IEk MFI was calculated for each subpopulation. Graphs show the correlation between these two parameters in the subpopulations of wild type cells (black) and the bulk transgenic population obtained from the Cd5−/− mice (red). The lower tetramer binding of Cd5−/− CD4+ T cells is completely explained by lower TCRβ expression and is not due to the absence of CD5 expression. Results are representative of 2– 3 independent experiments.
We also considered the possibility that CD5 was directly involved in pMHC binding and assessed tetramer binding of Cd5−/− TCR Tg CD4+ T cells. Both TCRβ and tetramer staining was diminished on Cd5−/− compared to wild type AND and 5C.C7 cells (Fig. 4B). However, reduced TCRβ expression in Cd5−/− cells entirely explained the reduction in tetramer staining. Therefore, consistent with studies that showed that the activity of CD5 as a negative regulator of TCR signaling does not require its extracellular domain (Bhandoola et al., 2002), CD5 played no direct role in agonist pMHC binding (Fig. 4B).
Heterogeneity in TCR self-reactivity results in the differential contribution of CD4+ T cells to foreign antigen responses
If this relationship between self and foreign antigen recognition represents a key aspect of functional T cell biology, we would expect variation in self-reactivity to influence the response of polyclonal T cell responses to infectious agents. We predicted that naïve CD4+ T cells on the high end of the scale of self-reactivity would contribute more to foreign antigen responses in vivo, although the difference would not be expected to be large because less useful cells have already been filtered out by thymic `neglect'. Previous studies describing the outgrowth of T cells with greater affinity for agonist pMHC have relied on identifying specific cells using tetramers (Busch and Pamer, 1999; Malherbe et al., 2004; Price et al., 2005; Savage et al., 1999), which may miss a substantial number of T cells contributing to the response (Sabatino et al., 2011). Therefore, we sorted the 15–20% CD5 brightest and dimmest cells from two naïve polyclonal CD4+ T cell populations distinguishable by a congenic marker, mixed the sorted cells in a ~1:1 ratio, and adoptively transferred them to recipient mice. Recipients were infected 1–3 days later with either lymphocytic choriomeningitis virus Armstrong (LCMV), ΔActA Listeria monocytogenes expressing OVA-2W1S (LM), or an OVA323–339 expressing Influenza A virus (Fig. 5, A and B). Transferred sorted cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) to track responding cell division. In all three infections, the recovery of cells that underwent extensive pathogen-induced cell division and survived at the time of assay was greater for CD5hi CD4+ T cells than for CD5lo CD4+ T cells (Fig. 5C). This distinct in vivo responsiveness could not be attributed to intrinsic differences in the proliferative capacity of CD5lo and CD5hi cells. First, the response of these cells to polyclonal stimulation in vitro was similar (fig. S4A). Second, this differential response was only seen upon TCR stimulation with cognate pMHC, as in vivo administration of anti-CD3 and anti-CD28 following adoptive transfer of both CD5hi and CD5lo CD4+ cells into the same recipients resulted in equivalent expansion and recovery (Fig. 5D). Nor can the greater antigen responsiveness of the CD5hi population be attributed to differences in responsiveness to IL-7 or survival rates. In the absence of any pathogen challenge, we detected no differences in the in vivo survival or homeostatic proliferation of CD5hi versus CD5lo naïve CD4+ T cells on day 4 or day 43 following adoptive transfer to lymphoreplete recipients (fig. S4B–D). These findings are consistent with our conclusion that CD5hi CD4+ T cells have a greater predictable reactivity with foreign ligands than CD5lo CD4+ T cells and with published data documenting that mature peripheral T cells with stronger TCR binding of foreign pMHC are enriched after infection or immunization (Busch and Pamer, 1999; Malherbe et al., 2004; Price et al., 2005; Savage et al., 1999).
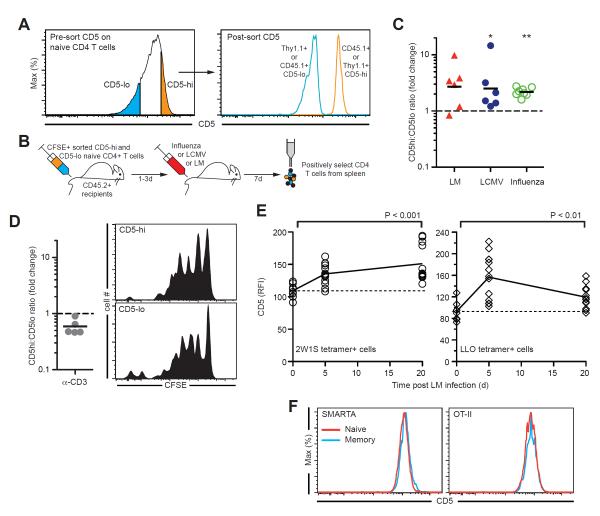
Figure 5. CD5hi T cells with the greatest reactivity for self-antigens dominate the response to diverse foreign antigens.
(A and B) Experimental setup: congenically marked 15–20% CD5lo or CD5hi naïve CD4+ T cells were sorted, labeled with CFSE, mixed in a ~1:1 ratio and adoptively transferred to CD45.2+ recipients that were then infected with Listeria monocytogenes (LM), Influenza A virus or LCMV. (C) Fold change in CD5hi:CD5lo ratio in divided (CFSElo) versus undivided (CFSEhi) transferred cells 7 days after infection. Each triangle or circle represents an individual mouse, lines denote means. Data summarized from 2–3 independent experiments. (D) Setup as in (A), but recipients were given anti-CD3 and anti-CD28 i.v.. The fold change in CD5hi:CD5lo ratio in divided versus undivided transferred cells and representative CFSE profiles 4 days after antibody administration are shown. (E) CD5 expression on antigen-specific CD4+ T cells identified using tetramers (2W1S:IAb, left panel; LLO:IAb, right panel) on days 0, 5, or 21 following LM infection. Each symbol represents an individual mouse and lines denote means. (F) CD5 expression on naïve (CD44lo) and memory (CD44hi) SMARTA and OT-II cells from TCR Tg mice. *P<0.05, **P<0.01.
As an additional test of whether CD5hi cells predominate in a polyclonal immune response to a pathogen and persist after its clearance, we infected mice with LM and followed CD5 expression of CD4+ T cells specific for 2W1S:I-Ab or for a peptide derived from Listeriolysin O (LLO). We found that in both antigen-specific T cell populations, mean CD5 expression was increased at the peak of the CD4+ T cell response to LM (d5) and remained higher among specific memory CD4+ T cells after pathogen clearance (d21) (Fig. 5E). Given that CD5 expression of CD4+ T cells for a given TCR clonotype was unchanged by the transition from naïve to memory (Fig. 5F), our data suggest that of the antigen-specific cells originally present in the naïve pool, CD5hi cells are indeed contributing more to an antigen response than CD5lo cells following LM infection (Fig. 5E). Thus, these in vivo results further validate our striking finding that there is a direct relationship between the strength of self-recognition of a TCR and its strength of binding to foreign antigen.
Positive selection in the thymus skews the TCR repertoire towards the higher end of self-reactivity
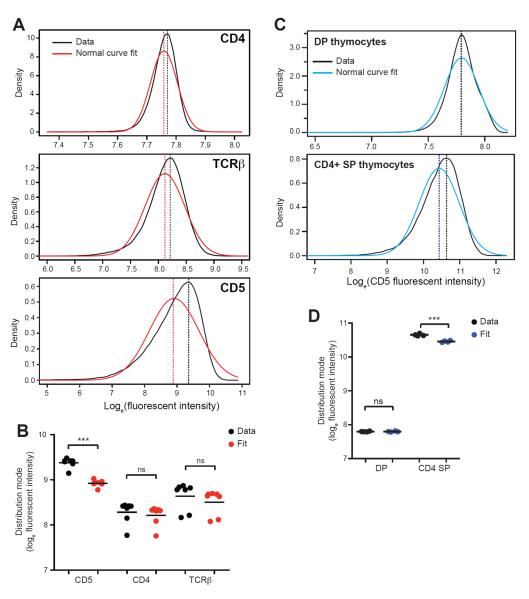
Given these findings, we asked whether thymic positive selection has a preference for TCRs binding self more avidly by examining the distribution of CD5 expression on naïve CD4+ T cells. Most cellular proteins are log-normally distributed within a cell population due to stochasticity in gene expression (Niepel et al., 2009). We therefore asked whether the distribution of CD4, TCRβ, and CD5 followed this pattern, log-transforming fluorescent intensity data measured on naïve CD4+ T cell populations from 7 individual mice and fitting Gaussian curves to the data. As expected, the distribution of CD4 and TCRβ were well approximated by normal curves with no significant difference between the mode of the best normal fit and that of the data (Fig. 6A and 6B). In contrast, the distribution of CD5 deviated significantly from being log-normal, showing an enrichment for CD5hi cells (Fig. 6A and 6B). This skew was only apparent among polyclonal CD4+ T cell populations and not among monoclonal TCR Tg CD4+ T cells and thus was not a property of how CD5 expression is regulated in a population of cells expressing the same TCR (Fig. S5).
Figure 6. Thymic selection skews the naïve CD4+ T cell repertoire towards cells with greater self-sensitivity.
(A – D) A normal distribution was fit to the natural log-transformed distribution of CD4, TCRβ, or CD5 fluorescent intensity measured in individual mice. The mode of the best normal fit and of the experimental data were compared to assess for a skew in the distribution of the fluorescent intensity data. (A and C) Representative data with the best normal fit are shown for peripheral CD4+ T cells, (A) and double positive, CD3lo CD4+CD8+ (DP) or single positive (SP) thymocytes, (C). Dashed lines indicate distribution mode. (B and D) Mode of fluorescent intensity data or best normal fit to the data is shown for peripheral CD4+ T cells, (B) and DP or SP thymocytes, (D). Circles represent modes from data or fit of individual animals, lines denote means. **P<0.01, ***P<0.001; ns = not significant.
If positive selection is operating to place the most foreign antigen-reactive cells in the peripheral repertoire, we would expect this skew in the distribution of self-reactivity among naïve CD4+ T cells to arise following positive selection in the thymus. We therefore examined CD5 expression by double positive (DP) thymocytes that have not yet undergone selection and single positive CD4+ thymocytes that have been successfully selected and found that the right-skewed CD5 distribution only became apparent among SP thymocytes (Fig. 6C and 6D). Thus, positive selection exhibits a preference for the maturation of TCRs that have a greater reactivity for self (but are within the permissible range of TCR affinities constrained by negative selection on the high end).
Antigen selection further skews the post-immune TCR repertoire and impacts the naïve TCR repertoire in adults
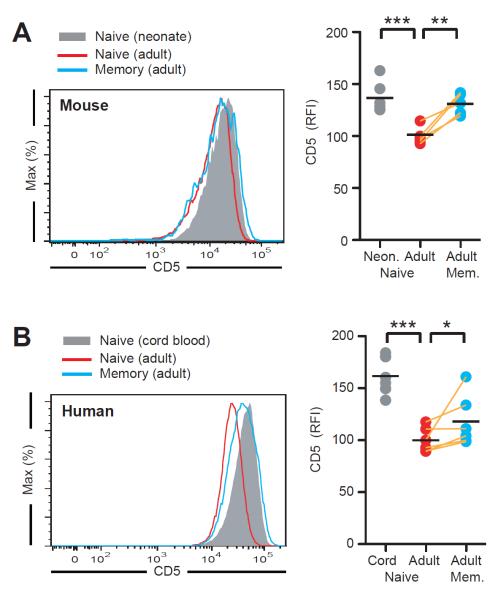
Given our data showing that upon infection, CD5hi cell outperform CD5lo cells, we anticipated that with age, more CD5hi than CD5lo naïve T cells would be recruited into the memory pool. As the thymic supply of naïve T cells declines, the mean CD5 expression of the naïve T cell population should decrease while that of memory T cells should be higher. To investigate this hypothesis, we compared CD5 expression by naïve CD4+ T cells from neonatal and adult mice. Indeed, we found that mean CD5 expression was significantly lower in adult CD4+ naïve T cells as compared to those of neonates. Furthermore, among adults, memory CD4+ T cells had a greater mean CD5 expression as compared to naïve CD4+ T cells (Fig. 7A). Strikingly, we observed the same pattern for CD5 expression on naïve CD4+ T cells from cord blood vs. adult humans, (Fig. 7B), thus indicating that CD5 may also be associated with strength of self-pMHC reactivity on human T cells. In addition, as in mice, we found that in human adults, CD5 expression was significantly greater on memory as compared to naïve CD4+ T cells (Fig. 7B).
Figure 7. Antigen selection further skews the post-immune TCR repertoire and impacts the naïve TCR repertoire in adults.
(A) CD5 expression on naïve cells of day 5 neonates (n=6) and naïve or memory cells of day 40 adult mice (n=5). (B) CD5 expression on naïve CD4+ T cells (CD45RO− CD27+) from human cord blood (n=10) and naïve or memory (CD45RO+ CD27+) CD4+ T cells from peripheral blood of healthy donors (n=6). *P<0.05, **P<0.01, ***P<0.001.
DISCUSSION
Although the process of positive selection occupies a central place in immunological thinking and was described 35 years ago (Bevan, 1977; Zinkernagel et al., 1978), why selection on self-pMHC is an effective way to construct a T cell repertoire able to anticipate the unpredictable foreign antigens that will be encountered has remained incompletely understood. The findings we present here provide compelling evidence that there is a direct relationship between the strength of binding of a TCR to self-pMHC and to agonist pMHC on a per T cell basis, thus providing an unsuspected connection between thymic developmental events and foreign antigen-driven responses. We reached this conclusion by first establishing in TCR Tg and polyclonal naïve CD4+ T cell populations that CD5 expression reflected the clone-specific strength of TCR reactivity with self-pMHC, based on the correlation of CD5 with partial pζ in these T cells. Using CD5 as a measure of self-reactivity, we then documented a direct relationship between self-ligand binding strength and foreign pMHC tetramer binding in the absence of any confounding effects of self-ligands in the assay. The establishment of a CD5 – pζ correlation also allowed us, for the first time, to assess the heterogeneity in self-reactivity present within a polyclonal pool of T cells, to examine the distribution of this heterogeneity, and to assess the function of cells with distinct self-reactivities with respect to pathogen responsiveness. We found that positive selection biases the peripheral CD4+ TCR repertoire towards cells with greater self-reactivity, yielding a larger fraction of mature cells better able to respond to foreign antigen.
Because the key conclusion of this study relies on use of tetramer binding and its relationship with self-ligand-induced pζ amounts, it was critical to assess whether explanations other than a direct TCR-based relationship between self- and foreign antigen reactivity could explain these data. We and others have found no evidence that CD5 directly affects pMHC binding (Bhandoola et al., 2002). T cell hybridomas cultured in the absence of interaction with self-pMHC retained the differences in tetramer binding seen for primary monoclonal T cells with the same TCRs, arguing against the notion that in vivo self-ligand induced TCR clustering could explain the connection between CD5 and tetramer staining intensity. Finally, by assessing TCR affinity for agonist pMHC using tetramers, we could also exclude that self-pMHC was contributing to agonist pMHC binding through a feedback process dependent on signaling. It has previously been shown by some groups (Ebert et al., 2009; Krogsgaard et al., 2005; Lo et al., 2009), although not by others (Sporri and Reis e Sousa, 2002), that endogenous pMHC can augment signaling in T cells in response to agonist-pMHC. However, because we measured agonist pMHC binding strength independently of self-pMHC using tetramers, the co-agonist action of self-pMHC cannot explain the relationship between self and agonist TCR binding that we detect.
In terms of the better response of CD5hi cells in vivo to infectious agents, neither we nor others (Cho et al., 2010) have found any relationship between CD5 and expression of the cytokine receptors CD25, CD127, or CD122 on naïve CD4+ T cells (unlike for CD8+ T cells) and thus there is no evidence that would suggest the differential outgrowth of CD5hi cells during infection is a consequence of greater sensitivity to cytokines. Nor is this behavior explained by differences in survival between CD5lo and CD5hi cells. Our results also cannot be explained by intrinsic differences in the proliferative capacity of CD5hi and CD5lo cells, given equivalent responses to anti-CD3 and CD28 stimulation in vivo.
It is thus the TCR itself that appears to lie at the heart of the relationship between self and agonist binding strength as well as the variation in pathogen response. Yet neither available structural information, nor previous studies examining MHC restriction or the role of self in responses of T cells to foreign antigen, predicts a direct relationship between self-reactivity and affinity for agonist (Garcia et al., 2009; Germain, 1990; Krogsgaard et al., 2005; Nikolic-Zugic and Bevan, 1990; Stefanova et al., 2002), making the existence of such a relationship a surprising finding. The lack of information on the specific self-ligands that positively select the majority of T cell clones makes experiments to investigate the molecular basis for this relationship challenging. For the few positively selecting self peptides that have been described for particular monoclonal TCRs, there is no evidence that sequence resemblance to agonist peptides beyond key residues anchoring the peptide to the MHC binding groove is a necessary feature of selecting peptides (Berg et al., 1999; Ebert et al., 2009; Lo et al., 2009; Santori et al., 2002; Sasada et al., 2001). It may be that the positioning of the epitopic residues recognized by the TCR CDR3 regions with respect to these anchor sites plays an important role in the self-foreign binding relationship we report here (Garcia et al., 2009; Germain, 1990) or the ability of TCRs to specifically interact with structurally different ligands in the same general orientation but with slightly different strategies (Colf et al., 2007; Felix et al., 2007) account for these observations. In either case, our findings argue for revisiting the issue of how pMHC recognition occurs and what structural elements of the TCR and pMHC might support the unanticipated ability of T cells selected on self to bind well to foreign peptides bound to the same MHC molecule.
Given that all naïve CD4+ T cells in an individual have passed the extensive filtering process in the thymus (Scollay et al., 1980), it is remarkable that within the permissible range of self-reactivity present following thymic selection we could nonetheless detect a greater contribution of CD5hi CD4+ T cells to an anti-pathogen response. The modest (~2–10 fold) difference in contribution between CD5hi and CD5lo cells is expected if positive selection indeed acts to optimize the TCR repertoire in the way that we propose. It would not be doing so very effectively if CD5lo cells were largely useless in responding to foreign antigen. In fact, consistent with recent studies using assays that are able to detect antigen-specific but tetramer-staining negative CD4+ T cells (Sabatino et al., 2011), our data similarly indicates that CD5lo cells do contribute to responses against infectious agents. Yet our data are also in agreement with studies that have described an increase in the frequency of stronger agonist binding TCRs in the course of immune responses (Busch and Pamer, 1999; Malherbe et al., 2004; Price et al., 2005; Savage et al., 1999).
Given this dominance of CD5hi TCRs in in vivo immune responses, it might have been expected that most TCR Tg CD4+ T cells derived from actively immunized mice would have greater CD5 expression than the mean of a naïve polyclonal population. However, the TCR α and β genes used for the generation of these transgenic animals primarily come from T cells repeatedly stimulated in vitro with high amounts of agonist ligand, which leads to the preferential activation-induced cell death of the most avid T cells in the population (Kruisbeek, 2001; Lenardo et al., 1999; Ryan et al., 2005). Therefore, this method of obtaining monoclonal TCRs may have biased the available population of Tg TCRs towards those with low – intermediate agonist pMHC binding strength. Indeed, the use of CD5 expression as a means of identifying CD4+ T cells that dominate an antigen-specific response could be a useful tool for the selection of more avid monoclonal TCR populations.
Compensation for lower TCR avidity by elevated CD8 coreceptor expression prevented us from conducting an adequate analysis of the CD8+ T cell population for the relationship we document here for CD4+ T cells. Our results nonetheless provide an intuitive explanation for the effectiveness of thymic positive selection in predicting the unknown and generating a useful T cell repertoire with which to combat infection in absence of any `knowledge' of what foreign antigens will be encountered. If the capacity to bind self-pMHC is a direct predictor of affinity for foreign agonist, it is easy to see why the TCR testing strategy of positive selection is successful, because it ensures the preferential maturation of TCR clonotypes that have the greatest capacity to respond to foreign antigen. While this may also result in the T cell repertoire being closer to the threshold at which overt responses to self-pMHC are possible, having a system that is poised at this fine balance may be beneficial if this will provide a sufficient pool of diverse T cells capable of effectively responding to pathogenic invasion when antigen presentation is low. This poising at the overt self-reactivity border may explain the many additional factors needed to ensure that peripheral T cells do not cross the activation threshold in response to self. It will be important to investigate further whether CD5 expression provides sufficient information on the strength of self- and foreign antigen reactivity of particular T cells to make use of this approach for clinical purposes. The availability of a surface marker to predict which cells will be better effectors during polyclonal immune responses could prove of substantial value in guiding development of immunomodulatory therapeutics, either to reduce pathogenic autoimmune and allograft rejection responses or to improve immunity against tumors or infectious agents.
EXPERIMENTAL PROCEDURES
Mice
Animal housing, care and research were in accordance with the Guide for the Care and Use of Laboratory animals and all procedures performed were approved by the NIAID Animal Care and Use Committee. Mouse strains and sources are listed in Supplementary Experimental Procedures.
Cell surface and tetramer staining
Lymphoid cells from lymph nodes and/or spleen were collected, washed in PBS 2mM EDTA + 5% FCS, Fc-receptors were blocked with 2.4G2 (BD Biosciences), and antibodies used for surface staining. Cells were acquired on a LSRII (BD Biosciences). Tetramer staining was performed as previously described (Moon et al., 2007; Savage et al., 1999) at 37°C for 1hr (except for 2W1S:I-Ab, which was done at 25°C), in buffer containing 0.1% sodium azide to reduce the effects of TCR-MHC internalization. Flow cytometric data were analyzed using FlowJo (Tree Star). Reagents used are listed in Supplementary Experimental Procedures.
Hybridoma preparation
T cell hybridomas were re-derived from 5C.C7 or AND TCR transgenic cells as previously described (Kruisbeek, 2001). Two weeks after drug selection, viable cells were expanded and used in tetramer binding assays at room temperature, as described above.
Cell sorting
Spleen and lymph node cells were pooled and pre-enriched for CD4+ T cells by negative selection using EasySep (StemCell Technologies). The enriched fraction was stained with antibodies for CD8 (53–6.7), CD5 (53–7.3), B220 (RA3-6B2), and CD44 (IM7) (eBiosciences). CD8−B220−CD44low cells were sorted into CD5hi and CD5low populations with the required stringency indicated in each experiment. Sorts were performed using a FACSAria (BD Bioscience).
Immunoblot analysis
To quantify partial tyrosine phosphorylation of the TCR ζ chain and ERK-2, fresh CD4+ T cells were obtained by negative selection (MACS, Miltenyi), or by sorting CD4+ T cell fractions as described. Cells were lysed in buffer containing 1% NP–40 (Pierce) with protease and phosphatase inhibitors and immunoblotting performed as described (Dorfman et al., 2000). Densitometry was done using ImageJ software (NIH).
Tetramer-based enrichment and analysis
Lymph node and spleen cells from individual mice were collected and a 2W1S:I-Ab tetramer pull-down was done as described (Moon et al., 2007). For the CD5 versus tetramer staining intensity correlation analysis, bound fractions obtained from individual mice were electronically concatenated in FlowJo. Tetramer stainings were done using the following panel, unless otherwise described in the figure legend: B220, CD11b, CD11c, Ly6-G, F4/80, NK1.1 (dump channel) eFluor450; CD4 BD Horizon V500; TCRβ or CD3 FITC; CD8 PercP-eFluor710; CD44 Alexa Fluor 700; CD5 PE-Cy5. Naïve tetramer+ cells were defined as Dump−TCRβ+CD4+CD8−CD44lowTetramer+ cells (fig. S3E).
Adoptive transfers and in vivo stimulations or infections
Congenically marked Thy1.1+ CD5hi and CD45.1+ CD5lo sorted cells (or sorted cells with the congenic markers inverted relative to CD5 expression) were CFSE labeled, mixed at an approximately 1:1 ratio, and 4–6×106 cells injected into 2 – 4 month old C57BL/6 recipients. Recipients were infected 1–3 days later with either 2×105 PFU LCMV Armstrong, 1×107 CFU ΔActA 2W1S-OVA Listeria monocytogenes, or 0.6 LD50 Influenza H1N1 PR8 virus expressing OVA323. Alternatively, on the same day as the adoptive transfer, recipients were given 10μg anti-CD3 and 5μg anti-CD28 i.v. (BD Bioscience). Day 7 after infection or day 4 after antibody administration, spleens were harvested, CD4+ T cell positively selected by MACS, and CFSE dilution in the transferred cells was analyzed by FACS. To assess whether there were differences in division of CD5hi versus CD5lo T cells following infection for each individual mouse, results were expressed as the fold change in CD5hi:CD5low ratio in the population that diluted CFSE (divided cells) versus the CFSEhi cells (undivided cells).
Analysis of CD5 changes during aging and infection
C57BL/6 mice were infected with 1×107 CFU ΔActA 2W1S-OVA LM. Mice were euthanized at the peak of CD4+ T cell expansion (day 5) or after pathogen clearance (day 21). Non-infected mice were used as controls to obtain naïve antigen-specific T cells. In all cases, lymph nodes and spleens were harvested and 2W1S or LLO-specific CD4+ T cells were enriched as described. All samples (naïve, d5 and d21) were prepared and acquired on the same day. CD5 expression was also determined on CD4+ T cells from the spleen and lymph nodes of neonate (5 day old) and adult (8 week old) sex-matched mice.
Analysis of CD5 distribution skew
Fluorescent intensity data for CD4, TCRβ and CD5 on individual peripheral naïve polyclonal CD4+ T cells from a total of 7 mice (from 2 independent experiments) and for CD5 on individual CD3lo, CD4+ CD8+ double positive and CD3+CD4+ single positive polyclonal thymocytes from a total of 4 mice (from 2 independent experiments) or from AND or 5C.C7 TCR Tg mice were exported from FlowJo. Because of the large number of events, even small deviations from normality would be significant when using a normality test. Instead, the datasets were aggregated and the skew of the distribution of the data was assessed by: (1) fitting a Gaussian distribution to the histograms of either TCR, CD4 or CD5 expression after log-transforming this data, using the fitdist routine in the fitdistrplus package in the statistical software R Project, version 2.10.1 (Ihaka and Gentleman, 1996), (2) taking the mode of the best Gaussian fit and the mode of the actual data, (3) performing an ANOVA to assess whether the mode of the best Gaussian fit is significantly different from the mode of the data across multiple mice for TCR, CD4, and CD5 fluorescent intensity. A significant difference between the mode of the data and of the best normal fit indicated a skew in the actual data and a significant deviation from normality.
Human cell analyses
Human adult peripheral blood mononuclear cells (PBMC) and cord blood samples were kindly provided by D. van Baarle (University Medical Center Utrecht, The Netherlands). Cord blood was obtained from healthy full-term neonates directly after delivery and adult blood was obtained by vena-puncture from blood bank donors visiting the University Medical Center Utrecht, The Netherlands. Mononuclear cells were obtained by Ficoll-Paque density gradient centrifugation and cryopreserved until further use. Written informed consent was obtained from all participants or their legal guardians in agreement with the Helsinki Declaration of 1975, revised in 1983. Cryopreserved PBMC were thawed and 1×106 cells incubated with anti-CD5 (UCHT2) and anti-CD4 (RPA-T4) (Biolegend), CD27 (CLB-CD27/1, 9F4) (Sanquin Reagents), anti-CD8 (RPA-T8) and anti-CD45RO (UCHL1) (BD Biosciences) and CD3 (OKT3) (eBioscience). Cells were stained and acquired as described.
Statistical analyses
We analyzed data using GraphPad, Prism or R Project version 2.10.1 (Ihaka and Gentleman, 1996). A criterion of P < 0.05 was used in all statistical analyses. Coefficient of variation analyses (robust CV) of flow cytometric data was performed using FlowJo.
Supplementary Material
HIGHLIGHTS
Direct relationship between strength of self-reactivity and foreign pMHC recognition
CD4+ T cells with stronger self-reactivity contribute more to anti-pathogen responses
Preferential inclusion of TCRs with greater self-reactivity into T cell repertoire
TCR repertoire selection based on self-reactivity optimizes foreign antigen responses
ACKNOWLEDGEMENTS
The authors would like to thank J. Edwards for performing the CD5 sorts; N. Subramanian for assistance with immunoblots; M. Levy and A. Yates for statistical advice; Y. Belkaid for providing germ-free mice; P. Love for providing AND CD5−/− mice. M.K. Jenkins and M. Pepper for providing L. monocytogenes ΔActA, tetramer reagents and assistance with the tetramer bead enrichment methodology; the NIH Tetramer Facility for providing pMHC tetramer reagents; G. Punkosdy and R. Ahmed for providing LCMV-Armstrong; P. Thomas and P. Doherty for providing the initial stock of Influenza A virus; D. van Baarle for providing human blood samples and reagents; N. Nanlohy for providing assistance with human experiments. We are grateful to I. Stefanova and M. Jenkins for discussion and technical advice, J.D. Ashwell, G.I. Germain, M.J. Lenardo, A. Poholek, and C. Reis e Sousa for their critical review of the manuscript, and members of the Lymphocyte Biology Section for intellectual support and critical discussions.
This work was supported by the Intramural Research Program of NIAID, NIH, DHHS, and also by the Netherlands Organization for Scientific Research (N.V.) and by the NIH Office of AIDS Research (J.N.M.). J.P.M. is a Pew Latin American Fellow in the Biomedical Sciences.
Footnotes
AUTHOR CONTRIBUTIONS
JPM, JNM, NV, and RNG designed the study. JNM, JPM, and NV conducted all experiments. All authors contributed to interpreting the data and writing the paper. The order of the first three authors is arbitrary and based on alphabetical order and in no way should be considered to reflect differences in experimental or intellectual contributions – these authors played equal and critical roles in all aspects of the research reported here.
REFERENCES
- Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton GM, Rudensky AY. Requirement for diverse, low-abundance peptides in positive selection of T cells. Science. 1999;283:67–70. doi: 10.1126/science.283.5398.67. [DOI] [PubMed] [Google Scholar]
- Berg RE, Princiotta MF, Irion S, Moticka JA, Dahl KR, Staerz UD. Positive selection of an H2-M3 restricted T cell receptor. Immunity. 1999;11:33–43. doi: 10.1016/s1074-7613(00)80079-5. [DOI] [PubMed] [Google Scholar]
- Bevan MJ. In a radiation chimaera, host H-2 antigens determine immune responsiveness of donor cytotoxic cells. Nature. 1977;269:417–418. doi: 10.4049/jimmunol.176.1.677. [DOI] [PubMed] [Google Scholar]
- Bhandoola A, Bosselut R, Yu Q, Cowan ML, Feigenbaum L, Love PE, Singer A. CD5-mediated inhibition of TCR signaling during intrathymic selection and development does not require the CD5 extracellular domain. Eur. J. Immunol. 2002;32:1811–1817. doi: 10.1002/1521-4141(200206)32:6<1811::AID-IMMU1811>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J. Exp. Med. 1999;189:701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Kim HO, Surh CD, Sprent J. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity. 2010;32:214–226. doi: 10.1016/j.immuni.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colf LA, Bankovich AJ, Hanick NA, Bowerman NA, Jones LL, Kranz DM, Garcia KC. How a single T cell receptor recognizes both self and foreign MHC. Cell. 2007;129:135–146. doi: 10.1016/j.cell.2007.01.048. [DOI] [PubMed] [Google Scholar]
- Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Dorfman JR, Stefanova I, Yasutomo K, Germain RN. CD4+ T cell survival is not directly linked to self-MHC-induced TCR signaling. Nat. Immunol. 2000;1:329–335. doi: 10.1038/79783. [DOI] [PubMed] [Google Scholar]
- Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat. Immunol. 2009;10:1162–1169. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix NJ, Donermeyer DL, Horvath S, Walters JJ, Gross ML, Suri A, Allen PM. Alloreactive T cells respond specifically to multiple distinct peptide-MHC complexes. Nat. Immunol. 2007;8:388–397. doi: 10.1038/ni1446. [DOI] [PubMed] [Google Scholar]
- Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- Garcia KC, Adams JJ, Feng D, Ely LK. The molecular basis of TCR germline bias for MHC is surprisingly simple. Nat. Immunol. 2009;10:143–147. doi: 10.1038/ni.f.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain RN. Immunology. Making a molecular match. Nature. 1990;344:19–22. doi: 10.1038/344019a0. [DOI] [PubMed] [Google Scholar]
- Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Gavin MA, Bevan MJ. Positive selection of CD8+ T cells induced by major histocompatibility complex binding peptides in fetal thymic organ culture. J. Exp. Med. 1993;177:1469–1473. doi: 10.1084/jem.177.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R. R: a language for data analysis and graphics. J. Comput. Graph. Stat. 1996;5:299–314. [Google Scholar]
- Jorgensen JL, Esser U, Fazekas de St Groth B, Reay PA, Davis MM. Mapping T-cell receptor-peptide contacts by variant peptide immunization of single-chain transgenics. Nature. 1992;355:224–230. doi: 10.1038/355224a0. [DOI] [PubMed] [Google Scholar]
- Kassiotis G, Zamoyska R, Stockinger B. Involvement of avidity for major histocompatibility complex in homeostasis of naive and memory T cells. J. Exp. Med. 2003;197:1007–1016. doi: 10.1084/jem.20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. J. Immunol. 2004;172:40–44. doi: 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- Kruisbeek AM. Production of mouse T cell hybridomas. Curr Protoc Immunol. 2001;Chapter 3(Unit 3):14. doi: 10.1002/0471142735.im0314s24. [DOI] [PubMed] [Google Scholar]
- Laugel B, van den Berg HA, Gostick E, Cole DK, Wooldridge L, Boulter J, Milicic A, Price DA, Sewell AK. Different T cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties. J. Biol. Chem. 2007;282:23799–23810. doi: 10.1074/jbc.M700976200. [DOI] [PubMed] [Google Scholar]
- Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- Lo WL, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nat. Immunol. 2009;10:1155–1161. doi: 10.1038/ni.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Sharp KA, Janmey PA, Finkel TH. Surface-anchored monomeric agonist pMHCs alone trigger TCR with high sensitivity. PLoS Biol. 2008;6:e43. doi: 10.1371/journal.pbio.0060043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe L, Hausl C, Teyton L, McHeyzer-Williams MG. Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity. 2004;21:669–679. doi: 10.1016/j.immuni.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Mandl JN, Liou R, Klauschen F, Vrisekoop N, Monteiro JP, Yates AJ, Huang AY, Germain RN. Unexpected differences between naive CD4 and CD8 T cells in their lymph node transit dynamics and interactions with self-MHC ligands. 2012. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P, Scott-Browne JP, Dai S, Gapin L, Kappler JW. Evolutionarily conserved amino acids that control TCR-MHC interaction. Annu. Rev. Immunol. 2008;26:171–203. doi: 10.1146/annurev.immunol.26.021607.090421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat Immunol. 2012;13:121–128. doi: 10.1038/ni.2190. [DOI] [PubMed] [Google Scholar]
- Niepel M, Spencer SL, Sorger PK. Non-genetic cell-to-cell variability and the consequences for pharmacology. Curr. Opin. Chem. Biol. 2009;13:556–561. doi: 10.1016/j.cbpa.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic-Zugic J, Bevan MJ. Role of self-peptides in positively selecting the T-cell repertoire. Nature. 1990;344:65–67. doi: 10.1038/344065a0. [DOI] [PubMed] [Google Scholar]
- Okada CY, Weissman IL. Relative V beta transcript levels in thymus and peripheral lymphoid tissues from various mouse strains. Inverse correlation of I-E and Mls expression with relative abundance of several V beta transcripts in peripheral lymphoid tissues. J Exp Med. 1989;169:1703–1719. doi: 10.1084/jem.169.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, Erman B, Liu X, Ellmeier W, Bosselut R, et al. `Coreceptor tuning': cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat. Immunol. 2007;8:1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, Koup RA, Migueles SA, Gostick E, Wooldridge L, et al. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J. Exp. Med. 2005;202:1349–1361. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz EL, Tan K, Tang L, Kern P, Liu J, Xiong Y, Hussey RE, Smolyar A, Hare B, Zhang R, et al. The crystal structure of a T cell receptor in complex with peptide and MHC class II. Science. 1999;286:1913–1921. doi: 10.1126/science.286.5446.1913. [DOI] [PubMed] [Google Scholar]
- Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KR, McCue D, Anderton SM. Fas-mediated death and sensory adaptation limit the pathogenic potential of autoreactive T cells after strong antigenic stimulation. Journal of leukocyte biology. 2005;78:43–50. doi: 10.1189/jlb.0205059. [DOI] [PubMed] [Google Scholar]
- Sabatino JJ, Jr., Huang J, Zhu C, Evavold BD. High prevalence of low affinity peptide-MHC II tetramer-negative effectors during polyclonal CD4+ T cell responses. J. Exp. Med. 2011;208:81–90. doi: 10.1084/jem.20101574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santori FR, Kieper WC, Brown SM, Lu Y, Neubert TA, Johnson KL, Naylor S, Vukmanovic S, Hogquist KA, Jameson SC. Rare, structurally homologous self-peptides promote thymocyte positive selection. Immunity. 2002;17:131–142. doi: 10.1016/s1074-7613(02)00361-8. [DOI] [PubMed] [Google Scholar]
- Sasada T, Ghendler Y, Neveu JM, Lane WS, Reinherz EL. A naturally processed mitochondrial self-peptide in complex with thymic MHC molecules functions as a selecting ligand for a viral-specific T cell receptor. J. Exp. Med. 2001;194:883–892. doi: 10.1084/jem.194.7.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- Scott-Browne JP, Crawford F, Young MH, Kappler JW, Marrack P, Gapin L. Evolutionarily conserved features contribute to alphabeta T cell receptor specificity. Immunity. 2011;35:526–535. doi: 10.1016/j.immuni.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Seddon B, Purbhoo MA, Zamoyska R, Fisher AG, Merkenschlager M. Sensory adaptation in naive peripheral CD4 T cells. J. Exp. Med. 2001;194:1253–1261. doi: 10.1084/jem.194.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporri R, Reis e Sousa C. Self peptide/MHC class I complexes have a negligible effect on the response of some CD8+ T cells to foreign antigen. Eur. J. Immunol. 2002;32:3161–3170. doi: 10.1002/1521-4141(200211)32:11<3161::AID-IMMU3161>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J. Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- Zinkernagel RM, Callahan GN, Klein J, Dennert G. Cytotoxic T cells learn specificity for self H-2 during differentiation in the thymus. Nature. 1978;271:251–253. doi: 10.1038/271251a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.