Abstract
Antibodies that inhibit Plasmodium falciparum invasion of erythrocytes form an important component of human immunity against malaria, but key target antigens are largely unknown. Phenotypic variation by P. falciparum mediates the evasion of inhibitory antibodies, contributing to the capacity of P. falciparum to cause repeat and chronic infections. However, antigens involved in mediating immune evasion have not been defined and studies of the function of human antibodies are limited. In this study, we used novel approaches to determine the importance of P. falciparum erythrocyte-binding antigens (EBAs), which are important invasion ligands, as targets of human invasion inhibitory antibodies and define their role in contributing to immune evasion through variation in function. We evaluated the invasion-inhibitory activity of acquired antibodies from malaria-exposed children and adults from Kenya using P. falciparum with disruption of genes encoding EBA140, EBA175, and EBA181, either individually or combined as EBA140/EBA175 or EBA175/EBA181 double knock-outs. Our findings provide important new evidence that variation in the expression and function of the EBAs plays an important role in evasion of acquired antibodies and that a substantial amount of phenotypic diversity results from variation in expression of different EBAs that contributes to immune evasion by P. falciparum. All three EBAs were identified as important targets of naturally-acquired inhibitory antibodies demonstrated by differential inhibition of parental parasites greater than EBA knockout lines. This knowledge will help to advance malaria vaccine development and suggests that multiple invasion ligands need to be targeted to overcome the capacity of P. falciparum for immune evasion.
Keywords: EBA, immunity, malaria, PfRh, Plasmodium falciparum
Introduction
Malaria due to Plasmodium falciparum is a major cause of morbidity and mortality globally, with up to one million deaths each year (1). Malaria disease develops during the blood-stage of infection, when the merozoite form of the parasite invades erythrocytes and replicates inside them. After repeated exposure to P. falciparum infection, natural immunity is acquired that appears to prevent clinical symptoms by controlling blood-stage parasite replication (2, 3). This provides a strong rationale that the development of an effective malaria vaccine is achievable (4). Antibodies are an important component of acquired human immunity against malaria (5), and key targets of these antibodies include antigens expressed by merozoites (4). Antibodies that target merozoite antigens are believed to be important in mediating both acquired immunity and immunity generated by candidate blood-stage vaccines (6-9) and function, in part, by directly inhibiting invasion of erythrocytes (6, 7, 10, 11). However, there is a limited understanding of the targets of functionally important human antibodies and very few studies on these responses. P. falciparum can cause repeated and chronic infections due to its capacity for immune evasion, which has significant implications for vaccine development. However, the molecular basis for evasion of immune responses targeting merozoite antigens is unclear.
Merozoites can use different pathways, defined by receptor-ligand interactions, for invasion of erythrocytes and recent studies have suggested that this capacity for phenotypic variation contributes to immune evasion by P. falciparum (12). Using different parasite clones that varied only in their invasion phenotype, it was shown that changes in invasion pathways used by the merozoite influenced the susceptibility of P. falciparum to human invasion-inhibitory antibodies (12). The molecular basis for this immune evasion remains undefined, however, the use of alternate invasion pathways appears to primarily result from variation in the expression and/or use of members of two invasion ligand families, the erythrocyte binding antigens (EBAs) and P. falciparum reticulocyte-binding homologues (PfRh) (13-29). These protein families play essential roles in invasion, but the degree of functional redundancy among them means that not all ligands are required for invasion. Diversity in invasion phenotypes and variation in the expression and use of the EBA and PfRh proteins and has been demonstrated among clinical isolates in different populations (14, 22, 30-32) and using defined laboratory-adapted clones of P. falciparum (25, 28, 33, 34).
The EBAs are located in the micronemes and include EBA175, EBA140 (also known as BAEBL), EBA181 (also known as JESEBL), and EBL1 (35-37). The PfRh proteins are located in the rhoptries and include PfRh1, 2a, 2b, 4 and 5 (23, 25, 38-40). Additional members of these families, EBA165 and PfRh3, occur as pseudogenes (25, 41, 42). Invasion phenotypes can be broadly classified into two main pathways: i) sialic acid (SA)-dependent invasion, demonstrated by poor invasion of neuraminidase-treated erythrocytes (neuraminidase cleaves SA on the erythrocyte surface), and ii) SA-independent invasion, demonstrated by efficient invasion of neuraminidase-treated erythrocytes. SA-dependent (neuraminidase-sensitive) invasion involves the EBAs, and PfRh1 (15, 17-19, 23, 24, 28, 43, 44). EBA175 and EBA140 bind to the erythrocyte surface molecules glycophorin A (43-45) and C (19), respectively. EBA181 binds to SA on the erythrocyte surface and to band 4.1 protein (18, 46). EBL1 appears only to be expressed by some isolates, and can bind glycophorin B (37). PfRh1 binds SA residues on erythrocytes, but the specific receptor is unknown (23, 28). PfRh2 and PfRh4 are important in SA-independent invasion (17, 25, 33), but PfRh2 may also play a role in SA-dependent invasion (47-49). The two forms of PfRh2 are identical for approximately 80% of the N-terminal region (17). The receptor for PfRh2 is unknown but PfRh4 binds to complement receptor 1 on the surface of erythrocytes (34). PfRh5 was recently shown to bind to the erythrocyte protein basigin (50) and is thought to be essential for invasion, but unlikely to play a role in phenotypic variation (51).
The EBAs and PfRh ligands are considered promising vaccine candidates due to their important functional roles; however, their importance as targets of acquired protective responses in humans has not been established, and the significance of the variation in EBA and PfRh expression for immune evasion is unknown. Antibodies are acquired to the EBAs and PfRh proteins through natural exposure (12, 52-55), and antibodies to EBA175, EBA140, EBA181, PfRh2, and PfRh4 (53-57) were found to be strongly associated with protective immunity in prospective longitudinal studies of children. Immunization of experimental animals with recombinant EBAs and PfRh proteins can generate antibodies that inhibit erythrocyte invasion in vitro (19, 58-64).
Dissecting the importance of specific antigens as targets of inhibitory antibodies in humans is challenging because antibodies to multiple antigens are co-acquired through natural exposure, making it difficult to attribute inhibitory activity to specific antigens. Additionally, comparing the inhibitory activity of human antibodies to isolates with different invasion phenotypes is complicated by other differences between isolates, such as polymorphisms in specific antigens that can influence the inhibitory activity of antibodies (65, 66). In this study, we used new approaches to understand the significance of the EBAs in immune evasion and as targets of immunity. To do this, we tested the ability of acquired antibodies from malaria-exposed African children and adults to inhibit invasion of P. falciparum isolates that had targeted disruption of EBA175, EBA140, or EBA181, or isolates with disruption of two EBAs in combination, compared to parental parasites. Changes in the susceptibility to human inhibitory antibodies resulting from disrupted EBA expression would point to the importance of phenotypic variation in EBA function in contributing to immune evasion and the importance of the EBAs as targets of inhibitory antibodies. Additionally, we evaluated the presence of antibodies specific to EBA and PfRh ligands in the study population. The findings from these studies are significant for advancing our understanding of immune evasion by P. falciparum, and have major implications for the malaria vaccine design and development.
Materials and Methods
Invasion inhibition assays
All sera were dialyzed to remove non-specific inhibitors, run in duplicates, 1/10 dilution in two separate assays with synchronized (sorbitol used every second day for 2 weeks before starting the assay) P. falciparum 3D7wt, 3D7ΔEBA175, 3D7ΔEBA140, 3D7ΔEBA181, 3D7ΔEBA175/181, 3D7ΔEBA140/175, W2mefwt, and W2mefΔEBA140 parasites (67). For a subset of samples, the inhibitory effect of treated samples was confirmed by immunoglobulin purified from the same samples (67). Rabbit antibodies to MSP119 were kindly provided by Brendan Crabb (68). Samples from non-exposed donors were used as negative and polyclonal anti-AMA1 antibodies as positive controls in all assays. Samples were tested with all the different lines in parallel in the same experiments. A difference in invasion between the lines of >25% in invasion was designated as the cut-off for differential inhibition on the basis that this was greater than the variance seen in the assays, and that a difference of >25% was likely to be biologically significant; in repeated testing of non-immune control samples in our assays, two standard deviation from the mean was 10-15%. We have previously demonstrated that these assays have a high level of reproducibility (11, 67, 69). Preadsorption of treated sera against erythrocytes did not alter their inhibitory activity. A selection of sera was also tested for antibodies to the surface of uninfected erythrocytes maintained in culture (70); there was very little reactivity against normal erythrocytes and there was no relationship between antibody binding to erythrocytes and invasion inhibitory activity.
Antibodies to recombinant proteins by ELISA
Standard ELISAs were performed (12). For each serum, the absorbance from wells containing GST only was deducted. Recombinant proteins used were EBA140 RIII-V 3D7 allele (aa746-1045) (19), EBA175 RIII-V W2mef and 3D7 alleles (aa 761-1271) (71), EBA181 RIII-V 3D7 allele (aa755-1339) (18), PfRh4 3D7 allele (aa1160-1370) (25) and PfRh2 3D7 allele (aa2027-2533) (17), as well as schizont extract (3D7 allele) (12).
Study population and sera samples
Sera samples from 18 adults (47 (18-81) years, 22.2% male, 16.7% P. falciparum positive) and 53 children (median age (range) 8 (2-12) years, 60.4% male, 49.1% P. falciparum positive) were randomly selected from a community-based cross-sectional survey of residents in Ngerenya in the Kilifi District, Kenya, in September 1998, which immediately preceded a period of increased malaria transmission (71). We also obtained 31 samples from children in Ngerenya (median age 5 (0-8) years, 41.9% male, 12.9% P. falciparum positive) collected in May 2003, at the start of the rainy season associated with higher malaria transmission. These samples were selected from a larger cohort based on their inhibitory activity against 3D7wt. Ngerenya is an area with transmission of malaria mainly during two annual rainy seasons (May and October/November). We also used randomly selected sera from 28 adult, anonymous blood donors collected in May 2004 in Kilifi, and as controls non-exposed adult residents in Australia/UK (n=40). Ethical approval was obtained from the Ethics Committee of the Kenya Medical Research Institute, Nairobi, Kenya and from the Walter and Eliza Hall Institute Ethics Committee, Melbourne, Australia, and Alfred Hospital Human Research and Ethics Committee (for the Burnet Institute). All samples were obtained after written informed consent.
Statistical analysis
Differences in invasion between knock-out and 3D7wt were assessed using paired t-tests separately for each population sample set. The association of invasion with age and parasitaemic status was assessed using t-tests or Mann-Whitney U test where appropriate. Differences in the frequency of high and low invasion inhibitors with the sample set was assessed using chi-squared or Fisher’s exact tests where appropriate. The correlation between IgG responses to EBAs/PfRhs and invasion inhibition results was assessed by Spearman’s rank correlation using all ELISA values. SPSS (for Windows Rel 16.0. 2007. Chicago:SPSS Inc) was used for statistical analysis.
Results
Phenotypic variation and evasion of inhibitory antibodies
To study the function of the EBA proteins in phenotypic variation for immune escape and as targets of human inhibitory antibodies, we used 3D7 parasite lines that had targeted disruption of single EBA genes (EBA140, EBA175, or EBA181) and 3D7 parasites with disruption of two EBA genes: EBA140/EBA175, or EBA175/EBA181 (61). Previously, transcriptional analysis of these lines revealed significant up-regulation in PfRh4 gene expression in 3D7ΔEBA175, 3D7ΔEBA140/175 and 3D7ΔEBA175/181 compared to the 3D7 parental line (61). Otherwise, there were no significant changes in merozoite gene expression. In 3D7ΔEBA140, there were no changes in transcriptional levels of these genes compared to 3D7 parental parasites.
Human serum antibodies were tested for their ability to inhibit invasion of parasites with targeted disruption of EBA protein expression versus parental parasites. Differential inhibition by acquired antibodies of parasites that differ only in the presence or absence of a specific EBA protein would suggest it plays a role in immune evasion and in defining the inhibitory or antigenic phenotype of parasites. Serum antibodies were tested from individuals residing in a malaria-endemic region of Kenya for inhibitory activity of merozoite invasion. Four sets of samples were used to represent different ages and levels of exposure to malaria. Samples from children and adults were collected at the same time in the Ngerenya community in 1998. Samples from Ngerenya children were also collected at a later time when malaria transmission was reduced (2003), and also from adults around the Kilifi township in 2004. For these studies, differential inhibition was regarded as significant if there was a difference of at least 25% in the extent of inhibition between the comparison lines (12).
Testing human antibodies in invasion inhibition assays, we found that the disruption of EBAs had a major effect on the susceptibility of parasites to inhibitory antibodies when comparing inhibition of the EBA knock-out lines versus the parental parasites. This was seen among all samples sets examined (Table I; Fig. 1 and 2). The effect was greatest with 3D7ΔEBA140 and 3D7ΔEBA175 for which 84% of samples (for both lines) gave differential inhibition of knock-out versus parental parasites. Interestingly, for 3D7ΔEBA140 most of the samples with differential inhibition showed greater inhibition of parental parasites compared to the knock-out parasites in all sample sets, suggesting that antibodies were directed against EBA140, and that absence of EBA140 function led to escape from inhibitory antibodies for most samples. Reflecting this, the mean inhibition of 3D7ΔEBA140 by all samples was significantly lower (p<0.05) than 3D7 (Fig. 2). In contrast, for 3D7ΔEBA175 there was a very different phenotype with significantly greater (p<0.05) mean inhibition of the knock-out compared to parental parasites by all study samples, and a substantial proportion of samples inhibited 3D7ΔEBA175 greater than 3D7 parental parasites. This suggests that the phenotypic change induced by loss of EBA175 function led to greater antibody inhibitory activity against the knock-out parasite, possibly due to antibodies targeting ligands that were replacing the function of EBA175, such as PfRh proteins. Lack of EBA181 function also had a significant effect on susceptibility to inhibitory antibodies, with 40% of samples showing differential inhibition of 3D7ΔEBA181 compared to 3D7wt. Most samples showed greater inhibition of the knock-out compared to the parental parasites, as was seen with 3D7ΔEBA175, but this effect was not as great as that seen with 3D7ΔEBA175; mean inhibition of 3D7ΔEBA181 by all samples was significantly greater (p<0.05) than 3D7wt. Comparing the pattern of inhibitory activity against the different lines by individual samples suggests there is also substantial diversity in the repertoire of responses seen among individuals. Individual samples varied in the extent and direction of inhibition of mutant versus parental parasite lines (Fig. 3; Supplementary Material, Fig. S1).
TABLE I.
Proportion of individuals with differential invasion inhibition of 3D7wt and 3D7 knock-out lines.
| ΔEBA140 | ΔEBA175 | ΔEBA181 | ΔEBA140/175 | ΔEBA175/181 | |
|---|---|---|---|---|---|
| Inhibition of 3D7wt>3D7-KO line1 | |||||
| All Samples (n=130) | 107 (82.3) | 13 (13.1)* | 7 (5.4) | 21 (16.2) | 27 (20.8) |
| Kilifi Adults 2006 (n=28) | 23 (82.1) | N/A* | 4 (14.3) | 5 (17.9) | 3 (10.7) |
| Ngerenya Adults 1998 (n=18) | 13 (72.2) | 3 (16.7)* | 0 (0) | 3 (16.7) | 2 (11.1) |
| Ngerenya Children 1998 (n=53) | 45 (84.9) | 9 (17.0)* | 2 (3.8) | 11 (20.8) | 21 (39.6) |
| Ngerenya Children 2003 (n=31) | 26 (83.9) | 1 (3.2) | 1 (3.2) | 2 (6.5) | 1 (3.2) |
|
| |||||
| Inhibition of 3D7-KO line > 3D7wt2 | |||||
| All Samples (n=130) | 2 (1.5) | 7 (70.7)* | 45 (34.6) | 28 (21.5) | 15 (11.5) |
| Kilifi Adults 2006 (n=28) | 2 (7.1) | N/A* | 0 (0) | 6 (21.4) | 3 (10.7) |
| Ngerenya Adults 1998 (n=18) | 0 (0) | 7 (43.8)* | 4 (22.2) | 7 (38.9) | 2 (11.1) |
| Ngerenya Children 1998 (n=53) | 0 (0) | 31 (59.6)* | 23 (43.4) | 6 (11.3) | 4 (7.5) |
| Ngerenya Children 2003 (n=31) | 0 (0) | 13 (41.9) | 18 (58.1) | 9 (29) | 6 (19.4) |
Values show the number of samples (n=130) with differential inhibition and the total number of samples tested (proportion %).
Results show the proportion of samples that inhibited the in vitro invasion of 3D7wt greater than the corresponding 3D7 knock-out line.
Results show the proportion of samples that inhibited the in vitro invasion of the 3D7 knock-out line greater than 3D7wt.
sample numbers were n=99 (all samples), N/A samples not available (Kilifi Adults 2006), n=16 (Ng Adults 1998), n=52 (Ng Children 1998) due to limited sample availability.
FIGURE 1. Differential inhibition of P. falciparum lines by serum antibodies from malaria-exposed Kenyan individuals.
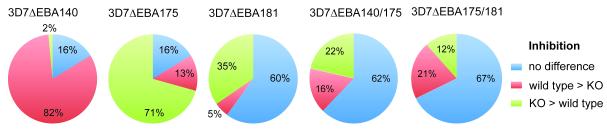
Results show the proportion of samples (n=130) that differentially inhibited the invasion of 3D7wt parasites compared to 3D7 lines with disruption of specific EBA genes. Differential inhibition was considered significant if there was >25% difference in the level of inhibition between two parasite lines; ‘no difference’ was regarded as <25% difference in the level of inhibition (indicated in blue). The proportion of samples that inhibited the knock-out line more than the 3D7wt line is shown in green; the proportion of samples that inhibited 3D7wt more than the knock-out line is shown in red.
FIGURE 2. Mean of invasion inhibition by serum antibodies from Kenyan donors.
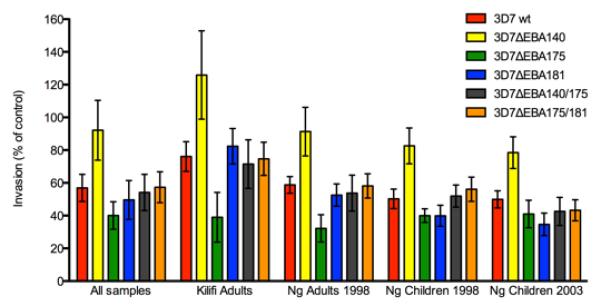
Serum samples were tested against P. falciparum 3D7wt or 3D7 lines with disruption of EBA175, EBA140, or EBA181, both EBA140 and EBA175, or both EBA175 and EBA181. Results are shown for all samples tested, or grouped for each of the different sample sets used. Values are expressed relative to control samples from non-exposed donors. Values represent means and error bars show one standard deviation. Differences in mean invasion of the EBA knockout lines was significantly different (p<0.05) compared to 3D7wt parasites for all lines and samples subsets, except for 3D7ΔEBA175/140 with analysis of all samples, and Ngerenya adults and children 1998; 3D7ΔEBA181 for analysis of Ngerenya adults and children 1998; and 3D7ΔEBA140/175 for analysis of Ngereyan adults 1998.
FIGURE 3. Inhibition of different P. falciparum lines by serum antibodies from malaria-exposed Kenyan individuals.
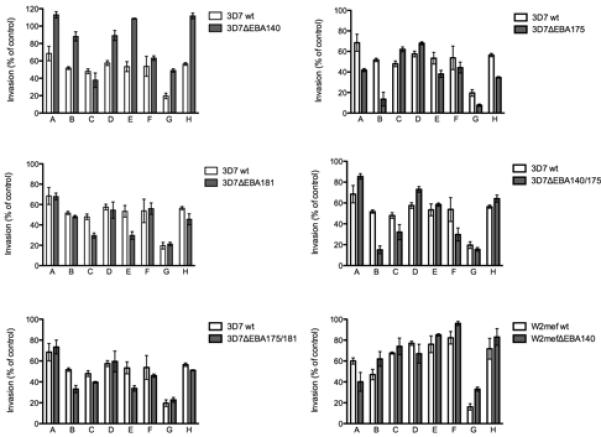
Results show the effect of serum antibodies (from individuals A-H) on the invasion of the parental parasite line versus the knock-out line. Samples shown were selected to demonstrate representative examples of the inhibitory activities observed in the study. Values are expressed as percentage of invasion relative to non-exposed donors. All samples were tested in duplicate in two separate assays and values represent mean ± range.
Prior studies have shown that the function and binding affinity of EBA140 can vary substantially between different isolates, which is associated with polymorphisms in EBA140 (19). Therefore, we investigated whether the genetic background of the parasite influenced the activity of human inhibitory antibodies to EBA140 by testing human antibodies for differential inhibition of the W2mef parasite with disruption of EBA140. In the W2mef isolate, EBA140 displays lower erythrocyte-binding activity and appears less important in invasion compared to its role in the 3D7 isolate. Reflective of this functional difference, deletion of EBA140 in the W2mef isolate had a much less marked effect on antibody activity than seen in 3D7. Only 1.5% of the samples we tested differentially inhibited W2mefΔEBA140 versus W2mef parental parasites, and the mean±SD level of invasion in the presence of serum antibodies was very similar for the two isolates (77%±18% in W2mefΔEBA140 and 73±16% for W2mefwt). This is consistent with EBA140 being less important in invasion in W2mef versus 3D7 isolates, and indicates that the genetic background of the parasite line influences the importance of EBA140 as an antibody target and for phenotypic variation to mediate immune evasion.
These findings demonstrate that changes in the expression of EBA175, EBA140, or EBA181 substantially impact on the antigenic properties of merozoite invasion and the susceptibility to acquired inhibitory antibodies, and that there are differences in the antigenic properties of the different EBA knockout parasites. As controls in these studies, we tested the mutant and parental parasite lines for inhibition by antibodies raised against MSP1-19, the expression of which does not vary between isolates, or by heparin (1 mg/ml), which specifically inhibits erythrocyte invasion (72). The level of inhibition was very similar between the parental and different knock-out lines (supplementary material, Fig. S2). Furthermore, we included serum antibodies from malaria-naïve donors and they gave no differential inhibition of the different parasite lines, in contrast to the findings using samples from Kenyan donors. There were no consistent associations between active parasitemia in donors and the level of invasion inhibition by samples. However, few individuals were parasitemic at the time of sample collection and the study was not designed to detect this association.
Inhibition of parasites with deletion of two EBAs
We next tested the impact of disruption of two different EBAs (double knock-out parasites) on inhibitory antibodies compared to 3D7 parental parasites (parasite lines 3D7ΔEBA140/175 and 3D7ΔEBA175/181; Fig. 1-3). Differential inhibition of the two double knock-out isolates compared to parental parasites was seen in a substantial proportion of samples, and in all sample sets; overall 38% and 32% of samples showed differential inhibition of 3D7ΔEBA140/175 and 3D7ΔEBA175/181, respectively (Fig. 1; Table I). For the double knock-out parasite lines, there was little overall difference in the median inhibition of the parental versus knock-out parasites by all samples (Fig. 2) because there were similar numbers of samples that gave greater or lower inhibition of the knock-out parasites compared to parental. These results suggest that disruption of two EBA genes led to substantial changes in the susceptibility to inhibitory antibodies; however, it did not result in a more pronounced phenotypic change compared to single EBA knock-outs.
To determine whether the antigenic phenotypes of the single and double KO lines were different, we tested samples for differential inhibition of the various mutant parasite lines (Fig. 4). A large proportion of samples (65%) gave differential inhibition of 3D7ΔEBA140 compared to 3D7ΔEBA140/175 double knockout, clearly indicating that the antigenic properties of these two lines and the pattern of susceptibility to inhibitory antibodies were very different. There was also significant differential inhibition of 3D7ΔEBA181 versus 3D7ΔEBA175/181 (21% of samples), suggesting the phenotypes of these isolates differ. In contrast, there was little differential inhibition of 3D7ΔEBA175 versus either of the double knockout lines, suggesting that the antigenic properties and phenotypes of these lines are similar. Overall, the 3D7ΔEBA175/181 and 3D7ΔEBA175/140 double knockouts appear to be more similar to the EBA175 single knockout in their antigenic properties, whereas the phenotypes of the 3D7ΔEBA140 and 3D7ΔEBA181 appear to be distinct from the other knock-out lines and from parental parasites.
FIGURE 4. Differential invasion inhibition by human antibodies of P. falciparum lines with single versus double gene knock-outs.
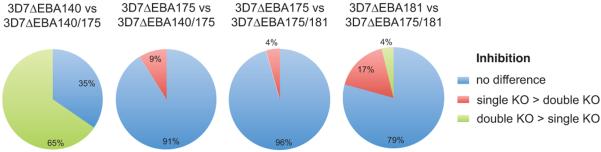
Results show the proportion of serum antibody samples (n=130) that differentially inhibited the invasion of 3D7 parasites with a single EBA knockout compared to 3D7 lines with disruption of two different EBAs (double knockouts). In red is the proportion of samples that inhibited the in vitro invasion of the 3D7 single knockout parasite line greater than the 3D7 double knock-out line, and in green is the proportion of samples that inhibited the invasion of 3D7 double knock-out parasite line more than the 3D7 single knock-out line. ‘No difference’ means <25% difference in inhibition (indicated in blue).
Relatedness between antibody responses and antigenic differences between parasites
To better understand the acquisition of immunity and antigenic differences between the different EBA-knockout parasite lines, we examined the relationship between inhibitory antibodies to the different isolates. We first examined whether samples that showed less inhibition of one EBA knock-out line also showed less inhibition of other EBA knock-out parasites, compared to parental parasites (Table II). These analyses suggested that inhibitory antibody responses to different isolates were not strongly related. For example, of the many samples that showed less inhibition of 3D7ΔEBA140 compared to 3D7wt parasites, only a small proportion showed less inhibition of 3D7ΔEBA175 (16.2% of all samples), 3D7ΔEBA181 (3.1%), 3D7ΔEBA140/175 (15.4%) or 3D7ΔEBA175/181 (19.2%) compared to parental parasites. We next determined whether samples that showed greater inhibition of one EBA knock-out line also gave greater inhibition of other EBA knock-out parasites, compared to the parental line (Table II). Again, these inhibitory antibody responses were not highly related between isolates. For example, of the many samples that inhibited 3D7ΔEBA175 more than 3D7wt parasites, only a small proportion showed greater inhibition of 3D7ΔEBA181 (19.1% of all samples), 3D7ΔEBA140/175 (11.8%), or 3D7ΔEBA175/181 (4.4%), compared to parental parasites; no samples showed this pattern of inhibition for both 3D7ΔEBA175 and 3D7ΔEBA140 parasites.
TABLE II.
Relatedness between inhibitory antibodies: proportion of individuals showing differential invasion inhibition of two knock-out lines compared to 3D7wt.
| ΔEBA140 | ΔEBA175 | ΔEBA181 | ΔEBA140/175 | |
|---|---|---|---|---|
|
| ||||
| n (%) | n (%) | n (%) | n (%) | |
| Inhibition of 3D7wt>3D7-KOline1 | ||||
| ΔEBA175 | 11 (16.2) | |||
| ΔEBA181 | 4 (3.1) | 1 (1.5) | ||
| ΔEBA140/175 | 20 (15.4) | 2 (2.9) | 4 (3.1) | |
| ΔEBA175/181 | 25 (19.2) | 4 (5.9) | 3 (2.3) | 10 (7.7) |
|
| ||||
| Inhibition of 3D7-KOline>3D7wt2 | ||||
| ΔEBA175 | 0 (0) | |||
| ΔEBA181 | 0 (0) | 13 (19.1) | ||
| ΔEBA140/175 | 2 (1.5) | 8 (11.8) | 11 (8.5) | |
| ΔEBA175/181 | 0 (0) | 3 (4.4) | 5 (3.8) | 9 (6.9) |
Values represent number of samples and percent of total samples in brackets (percentages calculated with sample numbers of n=130 with the exception of 3D7ΔEBA175 where percentages represent n=68 (Ngerenya 1998 samples only)).
Samples that showed inhibition of the in vitro invasion of 3D7wt that was greater than the corresponding 3D7 knock-out line, for two different knock-out line comparisons.
Samples that showed inhibition of the in vitro invasion of a 3D7 knock-out line that was greater than 3D7wt, for two different knockout line comparisons.
Overall, these analyses suggest that inhibitory antibody responses were not highly related to each other in the study samples and that each of the EBA single knock-outs led to distinct antigenic or inhibitory phenotypes, as defined by the susceptibility to inhibitory antibodies. However, as noted earlier, the inhibitory phenotypes and antigenic properties of the 3D7ΔEBA175/181 and 3D7ΔEBA175/140 double knockouts appeared similar to 3D7ΔEBA175. Overall, these analyses further support the concept that variation in the use or expression of the EBA proteins, which is observed among clinical isolates, creates a substantial amount of phenotypic diversity that alters susceptibility to human antibodies and contributes to immune escape.
Inhibitory antibodies target SA-dependent and SA-independent invasion pathways
We next aimed to understand the extent to which invasion-inhibitory antibodies target ligands of SA-dependent and SA-independent invasion pathways, and the EBAs more specifically. The parasite line 3D7 invades erythrocytes using ligands of both the SA-dependent (including EBA175, EBA140, and EBA181) and SA-independent (such as PfRh2 and PfRh4) pathways.
Reduced invasion inhibition by serum samples of the EBA knock-out lines compared to parental parasites suggests the presence of antibodies to the EBA proteins (and possibly other ligands of SA-dependent invasion). A large proportion of samples (82%) showed greater inhibition of the parental parasites compared to the 3D7ΔEBA140 parasites, suggesting that inhibitory antibodies target EBA140. The 3D7ΔEBA140 showed significantly less inhibition compared to 3D7wt in all four sample sets: Kilifi adults, Ngerenya 1998 adults, Ngerenya 1998 children, and Ngerenya 2003 children (Fig. 1-2, p ≤ 0.001 for all differences). With other EBA knock-out parasite lines, there were fewer samples with this effect; 13% inhibited 3D7wt more than 3D7ΔEBA175 parasites, and only 5% inhibited 3D7wt parasites more than the 3D7ΔEBA181. This might suggest that EBA175 and EBA181 are less important targets of inhibitory antibodies in 3D7, or that a change in invasion phenotype may occur with the loss of EBA175 or EBA181 that leads to a greater susceptibility to other inhibitory antibodies that target ligands replacing the function of EBA175 or EBA181. For the double knock-outs, 16% and 21% inhibited the parental more than 3D7ΔEBA140/175 and 3D7ΔEBA175/181, respectively, further supporting the conclusion that some individuals have inhibitory antibodies to the EBAs, and possibly other ligands of SA-dependent invasion.
Inhibition of the EBA knock-out lines more than the parental parasites suggest that inhibitory antibodies may also target ligands of SA-independent invasion, such as PfRh2 and PfRh4, particularly when comparing the double knock-outs to parental parasites. This pattern of greater inhibition of knockout versus parental parasites was seen for 71% and 35% of samples for 3D7ΔEBA175 and 3D7ΔEBA181, respectively, but was uncommon for 3D7ΔEBA140 (1.5% of samples). Greater inhibition of 3D7ΔEBA140/175 and 3D7ΔEBA175/181 compared to parental parasites was seen among 11% and 22% of samples (Table I). Disruption of EBA genes, particularly EBA175, leads to a greater reliance on SA-independent ligands for efficient invasion of erythrocytes (25, 59). Therefore, greater inhibition of knockout lines compared to parental parasites suggest inhibitory antibodies target these ligands, and previous studies have shown that affinity-purified human antibodies to PfRh4 can inhibit SA-independent invasion (54); however, further studies are needed to understand the importance of ligands of SA-independent invasion as targets of human inhibitory antibodies.
Presence of antibodies to EBA and PfRh invasion ligands
Differential inhibition by human antibodies of EBA knock-out parasites compared to parental parasites suggests that the EBA and PfRh ligands are important targets of antibodies. To assess this, we measured IgG responses to recombinant EBA and PfRh antigens by ELISA in the different sample sets (Fig. 5). Results confirm that antibodies to EBA175, EBA140, and EBA181 are common in the study population although antibodies to EBA181 were lower in the Ngerenya 2003 children. Antibodies to PfRh2 and PfRh4, as representative examples of SA-independent invasion ligands, were also common, although the prevalence of antibodies to PfRh4 was more variable across the samples sets than PfRh2. In general, adults showed higher levels of antibodies compared to children’s samples. There was little or no reactivity of sera from malaria-naïve individuals. Antibody levels to different EBA and PfRH proteins were also significantly positively correlated with each other, suggesting the co-acquisition of antibodies to multiple invasion ligands following P. falciparum exposure. Significant correlations were seen between antibodies to different EBAs (range of rs values 0.63 to 0.78; p<0.01 for all), PfRh2 and PfRh4 (rs=0.58; p<0.01), and between EBAs and PfRh proteins (range of rs values 0.47 to 0.73; p<0.01 for all).
FIGURE. 5. Antibodies against recombinant EBA and PfRh proteins measured by ELISA in Kenyan individuals.
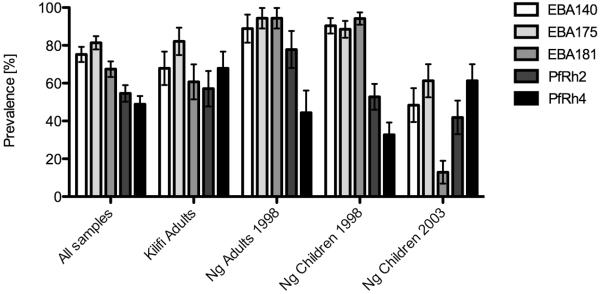
Results show the prevalence of antibodies to recombinant EBA and PfRh proteins measured by ELISA among the different sample sets included in the study. Error bars represent standard error of a proportion. Antibody reactivity among malaria-naïve donors was negligible.
We also examined the relationship between antibodies to EBA and PfRh ligands and differential invasion inhibition of parasites. Considering the correlation between antibodies to different invasion ligands by ELISA, the ability to dissect the relationship between any antigen-specific response by ELISA and specific invasion inhibitory activity was limited. Greater inhibition of 3D7 parental parasites than 3D7ΔEBA140 suggested the presence of inhibitory antibodies targeting EBA140. Consistent with this, there was a significant correlation, of moderate strength, between antibodies to EBA140 by ELISA and the extent of differential inhibition of 3D7 parental parasites compared to 3D7ΔEBA140 (rs=0.27; p<0.05). There was a similar finding for EBA181, with antibodies to EBA181 by ELISA being significantly correlated with the extent of differential inhibition of 3D7 parental parasites compared to 3D7ΔEBA181 (rs=0.32; p<0.05). This suggests that antibodies measured by ELISA to these recombinant proteins are broadly reflective of functional inhibitory activity, but are not strongly predictive of ligand-specific inhibitory activity of serum antibodies. Few samples showed differential inhibition of 3D7 parental parasites greater than 3D7ΔEBA175 (Figure 1); to the contrary, most samples gave greater inhibition of 3D7ΔEBA175 compared to 3D7 parental, suggesting the presence of inhibitory antibodies targeting ligands of SA-independent invasion, which includes PfRh2 and PfRh4, because disruption of EBA175 leads to a greater reliance on SA-independent ligands for invasion (25, 59). Consistent with this, antibodies to PfRh2 were significantly correlated with greater inhibition of 3D7ΔEBA175 parasites compared to 3D7 parental parasites (rs=0.42; p<0.01), especially in the Ngerenya 2003 children’s samples (rs=0.53, p<0.01). This was consistent with data showing that disruption of EBA175 leads to a greater reliance on ligands of SA-independent invasion, such as PfRh2 (17, 24, 25, 73). There was also a correlation between antibodies to PfRh2 and inhibition of the double knockout parasites 3D7ΔEBA140/175 (r=0.47, p<0.01 in Ngerenya children 2003; r=0.29, p<0.05 in Ngerenya children 1998, r=0.76 p<0.001 in Ngerenya 1998 adults) and 3D7ΔEBA175/181 (r=0.45, p<0.001 in Ngerenya children 1998, r=0.50 p<0.1 in Ngerenya 1998 adults) compared to 3D7 parental, consistent with these parasites also being more reliant on ligands of SA-independent invasion.
Discussion
The identification of major targets of protective immune responses and understanding mechanisms of immune evasion that enable chronic and repeated infections over time are key issues in malaria immunity and vaccine development. In this study we aimed to determine whether all of the EBA ligands are important targets of invasion inhibitory antibodies, and whether variation in the expression/use of EBAs contributes to immune evasion. To achieve this we used genetically modified parasites with targeted disruption of EBA protein expression, individually or in combination, in assays that measure human invasion inhibitory antibodies. The advantage of assays employing live parasites is that the antigens are correctly folded and presented in their native context, and antibodies are measured to all epitopes presented on the protein rather than assessing antibodies only to specific domains or regions as is regularly done in ELISA assays.
Our striking findings that disrupting the function of individual EBAs dramatically alters the susceptibility to human inhibitory antibodies provide important new insights into the molecular basis of evasion of human immune responses by P. falciparum. A substantial proportion of serum samples showed differential inhibition of parasites with disruption of EBA expression compared to parental parasites, indicating that changes in antigenic properties occur with changes in EBA function. Disruption of each of EBA140, EBA175, and EBA181 led to major changes in susceptibility to inhibitory antibodies indicating that all three EBAs can contribute to immune evasion through variation in their function. Furthermore, our findings strongly suggest that each of the three knock-out lines and the parental parasites are antigenically-distinct from each other, as defined by the inhibitory profile against our panel of human antibodies. This indicates that there is a significant level of antigenic diversity that could contribute to immune evasion. Differences in the inhibitory activity of antibodies against genetically different isolates have been long known and believed to be important in immune evasion (11, 70, 71, 74) but the underlying molecular mechanisms have remained unclear, in part due to a lack of tools to dissect specific responses. Our findings illustrate the value of translating new molecular approaches to studies of clinical immunology.
Substantial polymorphism is present in the erythrocyte binding region of EBA175, but is less marked in EBA140 or EBA181. The significance of these polymorphisms in EBA175 for evasion of inhibitory antibodies has not been clearly established. Studies using antibodies raised in rabbits suggest that polymorphisms in the binding region of EBA175 do not have a major impact on the invasion inhibitory activity of antibodies (76, 77). However, we did demonstrate here that the genetic background of the parasite influences the importance of EBA140 as an antibody target and its role in phenotypic variation to mediate immune evasion. While EBA140 appeared to be an important target of inhibitory antibodies with the 3D7 isolate, it appeared much less important in the W2mef isolate. This is consistent with previous studies suggesting that EBA140 is less important in invasion with the W2mef isolate. The W2mef EBA140 polymorphic variant has a reduced erythrocyte binding activity compared to 3D7 EBA140 even though the expression of protein appears similar between isolates (75). The EBA140 sequence differs in three amino acids between 3D7 and W2mef, (in the F1 domain). Furthermore, antibodies to EBA140 generated in rabbits gave much greater inhibition of invasion with 3D7 parasites than W2mef parasites (19, 75), consistent with our findings here using human antibodies, and indicating that EBA140 has a less prominent role in the invasion of the W2mef isolate.
The differential inhibition of parental parasites more than knockout lines that we observed indicates that all three EBAs appear to be targets of human inhibitory antibodies. A majority of samples showed reduced inhibition of 3D7ΔEBA140 compared to 3D7wt, suggesting inhibitory antibodies to EBA140 are prominent in this population. It has been shown previously that deletion of the EBA140 gene does not lead to any significant changes in levels of mRNA for EBA175, EBA181, PfRh2, PfRh4 (61), indicating that the differential inhibition seen is not due to that action of antibodies against other EBAs or PfRhs.
Fewer samples demonstrated reduced inhibition of 3D7ΔEBA175 compared to 3D7wt, reflecting the presence of inhibitory antibodies to EBA175 in the population. It is possible that EBA175 is a less important target of inhibitory antibodies with the 3D7 parasite line we used, compared to EBA140. Alternatively, the loss of EBA175 may lead to a wider change in the invasion phenotype and lead to a greater reliance on other invasion ligands, such as EBA140 or PfRh ligands that are prominent targets of inhibitory antibodies. The importance of the EBAs as targets of inhibitory antibodies was supported by results showing that antibodies to recombinant EBAs by ELISA were highly prevalent in the population, and there were some significant correlations between antibodies to recombinant EBA140 and EBA181 proteins and differential inhibition of parental versus knockout parasite lines. However, our results suggest that measuring antibodies to recombinant proteins by ELISA cannot be relied upon as a proxy for functional antibody activity. Future studies with full-length or native proteins may help understand the relationship between antibody levels versus function; however, standard immunoassays cannot account for antibody affinity and epitope specificity that are likely to be important for functional activity. This highlights the value of using functional assays to complement data obtained from standard immunoassays.
Our findings also suggest that ligands of SA-independent invasion, such as PfRh2 and PfRh4, are important targets of inhibitory antibodies. This was demonstrated by greater inhibition by human antibodies of 3D7ΔEBA175 (or EBA175/140 and EBA175/181 double knockouts) versus 3D7wt parasites. Compared to 3D7wt, 3D7ΔEBA175 has increased expression of PfRh4 (61) and has a neuramindase-resistant invasion phenotype (59), suggesting a greater reliance on ligands of SA-independent invasion compared to parental parasites. In contrast, PfRh4 expression was unchanged in 3D7ΔEBA140, which had less inhibition of by human antibodies compared to parental parasites. Consistent with this conclusion, acquired antibodies were reactive to recombinant PfRh2 and PfRh4 in the population, as measured by ELISA, and the level of antibodies to PfRh2 were significantly correlated with differential inhibition of 3D7ΔEBA140/175 or 3D7ΔEBA175 compared to 3D7wt parasites. Recent studies have reported that antibodies to PfRh2 and PfRh4 are associated with protective immunity, and that affinity purified antibodies to PfRh4 from malaria-exposed donors can inhibit invasion at physiologically relevant concentrations (53, 54). Many individuals may have functional antibodies to both EBAs and PfRh ligands, which may explain why we did not observe significant differential inhibition of 3D7ΔEBA175 or 3D7ΔEBA140/175 compared to 3D7wt among some samples.
The EBAs are considered attractive vaccine candidates because of their important biological role in invasion, and their relatively limited level of polymorphism (4). Our results suggest that the amount of antigenic diversity at the level of erythrocyte invasion, mediated by variation in the use/expression of the EBAs and PfRhs, is substantial, and this strongly suggests that a vaccine based on any of the EBAs would require the inclusion of multiple antigens to overcome the parasite’s capacity for immune evasion, such as a combination of EBA and PfRh ligands (12). While most research on the EBAs has focussed on EBA175 as a potential subunit vaccine candidate, our results highlight EBA140 as an important target of inhibitory antibodies and this antigen needs to be further studied as a potential vaccine candidate. Recent reports suggest that vaccine-induced antibodies raised in rabbits against PfRh5, or its binding partner, can inhibit multiple parasite lines, and may represent attractive vaccine candidates (51, 78). However, the significance of these antigens as targets of acquired human immunity is not known.
In conclusion, we have provided new insights into the molecular basis for evasion of human immune responses by P. falciparum, providing evidence that variation in the function/expression of the EBAs contributes to immune evasion and that the EBAs are important targets of human invasion-inhibitory antibodies. The importance of the EBAs supports their development as promising vaccine candidates; however, the demonstration here of their role in immune evasion through phenotypic variation suggests that vaccines would need to target multiple invasion ligands in order to counter parasite immune evasion and be highly effective. Therefore, our results have important implications for malaria vaccine development and understanding human immunity against malaria.
Supplementary Material
Acknowledgements
We thank M. Mosobo, T. Mwangi and B. Lowe for assistance with samples. We are grateful to the participants in the study. Erythrocytes were provided by The Red Cross Blood Bank, Melbourne. This manuscript is published with the permission of the Director, Kenya Medical Research Institute.
Grant support: This work was supported by the National Health and Medical Research Council, Australia (JB, AC, FM, FF; and IRIIS scheme), the Australia-India Strategic Research Fund of the Australian Government (JB, AC), Australia Research Council (JB), the Wellcome Trust, UK (076059, 092741 KM, JB), Wenner-Gren Fellow, MSB, Svenska Läkaresällskapet, and Sida (KP), The Miller Fellowship of the Walter and Eliza Hall Institute (JB), and Operational Infrastructure Support grants, Victoria, Australia.
Abbreviations
- Pf
Plasmodium falciparum
- EBA
erythrocyte-binding antigen
- PfRh
Plasmodium falciparum reticulocyte-binding homologue
- wt
wild-type
- SA
sialic acid
Footnotes
Disclosures The authors have no financial conflicts of interest.
REFERENCES
- 1.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 2.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nature immunology. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 3.Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, Gilson PR, Murphy VJ, Anders RF, Mueller I, Beeson JG. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009;77:1165–1174. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards JS, Beeson JG. The future for blood-stage vaccines against malaria. Immunol Cell Biol. 2009;87:377–390. doi: 10.1038/icb.2009.27. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S, McGregor IA, Carrington SC. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 6.Brown GV, Anders RF, Mitchell GF, Heywood PF. Target antigens of purified human immunoglobulins which inhibit growth of Plasmodium falciparum in vitro. Nature. 1982;297:591–593. doi: 10.1038/297591a0. [DOI] [PubMed] [Google Scholar]
- 7.Cohen S, Butcher GA, Crandall RB. Action of malarial antibody in vitro. Nature. 1969;223:368–371. doi: 10.1038/223368a0. [DOI] [PubMed] [Google Scholar]
- 8.Wipasa J, Elliott S, Xu H, Good MF. Immunity to asexual blood stage malaria and vaccine approaches. Immunol Cell Biol. 2002;80:401–414. doi: 10.1046/j.1440-1711.2002.01107.x. [DOI] [PubMed] [Google Scholar]
- 9.Osier FH, Fegan G, Polley SD, Murungi L, Verra F, Tetteh KK, Lowe B, Mwangi T, Bull PC, Thomas AW, Cavanagh DR, McBride JS, Lanar DE, Mackinnon MJ, Conway DJ, Marsh K. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun. 2008;76:2240–2248. doi: 10.1128/IAI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodder AN, Crewther PE, Anders RF. Specificity of the protective antibody response to apical membrane antigen 1. Infect Immun. 2001;69:3286–3294. doi: 10.1128/IAI.69.5.3286-3294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCallum FJ, Persson KE, Mugyenyi CK, Fowkes FJ, Simpson JA, Richards JS, Williams TN, Marsh K, Beeson JG. Acquisition of growth-inhibitory antibodies against blood-stage Plasmodium falciparum. PLoS One. 2008;3:e3571. doi: 10.1371/journal.pone.0003571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persson KE, McCallum FJ, Reiling L, Lister NA, Stubbs J, Cowman AF, Marsh K, Beeson JG. Variation in use of erythrocyte invasion pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J Clin Invest. 2008;118:342–351. doi: 10.1172/JCI32138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baum J, Thomas AW, Conway DJ. Evidence for diversifying selection on erythrocyte-binding antigens of Plasmodium falciparum and P. vivax. Genetics. 2003b;163:1327–1336. doi: 10.1093/genetics/163.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bei AK, Membi CD, Rayner JC, Mubi M, Ngasala B, Sultan AA, Premji Z, Duraisingh MT. Variant merozoite protein expression is associated with erythrocyte invasion phenotypes in Plasmodium falciparum isolates from Tanzania. Mol Biochem Parasitol. 2007;153:66–71. doi: 10.1016/j.molbiopara.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Camus D, Hadley TJ. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science. 1985;230:553–556. doi: 10.1126/science.3901257. [DOI] [PubMed] [Google Scholar]
- 16.Dolan SA, Miller LH, Wellems TE. Evidence for a switching mechanism in the invasion of erythrocytes by Plasmodium falciparum. J Clin Invest. 1990;86:618–624. doi: 10.1172/JCI114753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duraisingh MT, Triglia T, Ralph SA, Rayner JC, Barnwell JW, McFadden GI, Cowman AF. Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. EMBO J. 2003b;22:1047–1057. doi: 10.1093/emboj/cdg096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilberger TW, Thompson JK, Triglia T, Good RT, Duraisingh MT, Cowman AF. A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J Biol Chem. 2003;278:14480–14486. doi: 10.1074/jbc.M211446200. [DOI] [PubMed] [Google Scholar]
- 19.Maier AG, Duraisingh MT, Reeder JC, Patel SS, Kazura JW, Zimmerman PA, Cowman AF. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat Med. 2003;9:87–92. doi: 10.1038/nm807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer DC, Kaneko O, Hudson-Taylor DE, Reid ME, Miller LH. Characterization of a Plasmodium falciparum erythrocyte-binding protein paralogous to EBA-175. Proc Natl Acad Sci U S A. 2001;98:5222–5227. doi: 10.1073/pnas.081075398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nery S, Deans AM, Mosobo M, Marsh K, Rowe JA, Conway DJ. Expression of Plasmodium falciparum genes involved in erythrocyte invasion varies among isolates cultured directly from patients. Mol Biochem Parasitol. 2006;149:208–215. doi: 10.1016/j.molbiopara.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okoyeh JN, Pillai CR, Chitnis CE. Plasmodium falciparum field isolates commonly use erythrocyte invasion pathways that are independent of sialic acid residues of glycophorin A. Infect Immun. 1999;67:5784–5791. doi: 10.1128/iai.67.11.5784-5791.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rayner JC, Vargas-Serrato E, Huber CS, Galinski MR, Barnwell JW. A Plasmodium falciparum homologue of Plasmodium vivax reticulocyte binding protein (PvRBP1) defines a trypsin-resistant erythrocyte invasion pathway. J Exp Med. 2001;194:1571–1581. doi: 10.1084/jem.194.11.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed MB, Caruana SR, Batchelor AH, Thompson JK, Crabb BS, Cowman AF. Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid-independent pathway of invasion. Proc Natl Acad Sci U S A. 2000;97:7509–7514. doi: 10.1073/pnas.97.13.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stubbs J, Simpson KM, Triglia T, Plouffe D, Tonkin CJ, Duraisingh MT, Maier AG, Winzeler EA, Cowman AF. Molecular mechanism for switching of P. falciparum invasion pathways into human erythrocytes. Science. 2005;309:1384–1387. doi: 10.1126/science.1115257. [DOI] [PubMed] [Google Scholar]
- 26.Tham WH, Healer J, Cowman AF. Erythrocyte and reticulocyte binding-like proteins of Plasmodium falciparum. Trends Parasitol. 2012;28:23–30. doi: 10.1016/j.pt.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Thompson JK, Triglia T, Reed MB, Cowman AF. A novel ligand from Plasmodium falciparum that binds to a sialic acid-containing receptor on the surface of human erythrocytes. Mol Microbiol. 2001;41:47–58. doi: 10.1046/j.1365-2958.2001.02484.x. [DOI] [PubMed] [Google Scholar]
- 28.Triglia T, Duraisingh MT, Good RT, Cowman AF. Reticulocyte-binding protein homologue 1 is required for sialic acid-dependent invasion into human erythrocytes by Plasmodium falciparum. Mol Microbiol. 2005;55:162–174. doi: 10.1111/j.1365-2958.2004.04388.x. [DOI] [PubMed] [Google Scholar]
- 29.Triglia T, Thompson J, Caruana SR, Delorenzi M, Speed T, Cowman AF. Identification of proteins from Plasmodium falciparum that are homologous to reticulocyte binding proteins in Plasmodium vivax. Infect Immun. 2001a;69:1084–1092. doi: 10.1128/IAI.69.2.1084-1092.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Escobar N, Amambua-Ngwa A, Walther M, Okebe J, Ebonyi A, Conway DJ. Erythrocyte invasion and merozoite ligand gene expression in severe and mild Plasmodium falciparum malaria. J Infect Dis. 2010;201:444–452. doi: 10.1086/649902. [DOI] [PubMed] [Google Scholar]
- 31.Lobo CA, de Frazao K, Rodriguez M, Reid M, Zalis M, Lustigman S. Invasion profiles of Brazilian field isolates of Plasmodium falciparum: phenotypic and genotypic analyses. Infect Immun. 2004;72:5886–5891. doi: 10.1128/IAI.72.10.5886-5891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baum J, Pinder M, Conway DJ. Erythrocyte invasion phenotypes of Plasmodium falciparum in The Gambia. Infect Immun. 2003a;71:1856–1863. doi: 10.1128/IAI.71.4.1856-1863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaur D, Furuya T, Mu J, Jiang LB, Su XZ, Miller LH. Upregulation of expression of the reticulocyte homology gene 4 in the Plasmodium falciparum clone Dd2 is associated with a switch in the erythrocyte invasion pathway. Mol Biochem Parasitol. 2006;145:205–215. doi: 10.1016/j.molbiopara.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Tham WH, Wilson DW, Lopaticki S, Schmidt CQ, Tetteh-Quarcoo PB, Barlow PN, Richard D, Corbin JE, Beeson JG, Cowman AF. Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand. Proc Natl Acad Sci U S A. 2011;107:17327–17332. doi: 10.1073/pnas.1008151107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson DS, Wellems TE. EBL-1, a putative erythrocyte binding protein of Plasmodium falciparum, maps within a favored linkage group in two genetic crosses. Mol Biochem Parasitol. 2000;105:105–113. doi: 10.1016/s0166-6851(99)00173-5. [DOI] [PubMed] [Google Scholar]
- 36.Sim BK, Toyoshima T, Haynes JD, Aikawa M. Localization of the 175-kilodalton erythrocyte binding antigen in micronemes of Plasmodium falciparum merozoites. Mol Biochem Parasitol. 1992;51:157–159. doi: 10.1016/0166-6851(92)90211-2. [DOI] [PubMed] [Google Scholar]
- 37.Mayer DC, Cofie J, Jiang L, Hartl DL, Tracy E, Kabat J, Mendoza LH, Miller LH. Glycophorin B is the erythrocyte receptor of Plasmodium falciparum erythrocyte-binding ligand, EBL-1. Proc Natl Acad Sci U S A. 2009;106:5348–5352. doi: 10.1073/pnas.0900878106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baum J, Tonkin CJ, Paul AS, Rug M, Smith BJ, Gould SB, Richard D, Pollard TD, Cowman AF. A malaria parasite formin regulates actin polymerization and localizes to the parasite-erythrocyte moving junction during invasion. Cell Host Microbe. 2008;3:188–198. doi: 10.1016/j.chom.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Hayton K, Gaur D, Liu A, Takahashi J, Henschen B, Singh S, Lambert L, Furuya T, Bouttenot R, Doll M, Nawaz F, Mu J, Jiang L, Miller LH, Wellems TE. Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of Plasmodium falciparum invasion. Cell Host Microbe. 2008;4:40–51. doi: 10.1016/j.chom.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rayner JC, Galinski MR, Ingravallo P, Barnwell JW. Two Plasmodium falciparum genes express merozoite proteins that are related to Plasmodium vivax and Plasmodium yoelii adhesive proteins involved in host cell selection and invasion. Proc Natl Acad Sci U S A. 2000;97:9648–9653. doi: 10.1073/pnas.160469097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor HM, Grainger M, Holder AA. Variation in the expression of a Plasmodium falciparum protein family implicated in erythrocyte invasion. Infect Immun. 2002;70:5779–5789. doi: 10.1128/IAI.70.10.5779-5789.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Triglia T, Thompson JK, Cowman AF. An EBA175 homologue which is transcribed but not translated in erythrocytic stages of Plasmodium falciparum. Mol Biochem Parasitol. 2001b;116:55–63. doi: 10.1016/s0166-6851(01)00303-6. [DOI] [PubMed] [Google Scholar]
- 43.Orlandi PA, Klotz FW, Haynes JD. A malaria invasion receptor, the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum recognizes the terminal Neu5Ac(alpha 2-3)Gal-sequences of glycophorin A. J Cell Biol. 1992;116:901–909. doi: 10.1083/jcb.116.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sim BK, Chitnis CE, Wasniowska K, Hadley TJ, Miller LH. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994b;264:1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 45.Sim BK, Carter JM, Deal CD, Holland C, Haynes JD, Gross M. Plasmodium falciparum: further characterization of a functionally active region of the merozoite invasion ligand EBA-175. Exp Parasitol. 1994a;78:259–268. doi: 10.1006/expr.1994.1027. [DOI] [PubMed] [Google Scholar]
- 46.Lanzillotti R, Coetzer TL. The 10 kDa domain of human erythrocyte protein 4.1 binds the Plasmodium falciparum EBA-181 protein. Malar J. 2006;5:100. doi: 10.1186/1475-2875-5-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gunalan K, Gao X, Liew KJ, Preiser PR. Differences in erythrocyte receptor specificity of different parts of the Plasmodium falciparum reticulocyte binding protein homologue 2a. Infect Immun. 2011;79:3421–3430. doi: 10.1128/IAI.00201-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahar T, Reddy KS, Bharadwaj M, Pandey AK, Singh S, Chitnis CE, Gaur D. Plasmodium falciparum reticulocyte binding-like homologue protein 2 (PfRH2) is a key adhesive molecule involved in erythrocyte invasion. PLoS One. 2011;6:e17102. doi: 10.1371/journal.pone.0017102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Triglia T, Chen L, Lopaticki S, Dekiwadia C, Riglar DT, Hodder AN, Ralph SA, Baum J, Cowman AF. Plasmodium falciparum merozoite invasion is inhibited by antibodies that target the PfRh2a and b binding domains. PLoS pathogens. 2011;7:e1002075. doi: 10.1371/journal.ppat.1002075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, Uchikawa M, Mboup S, Ndir O, Kwiatkowski DP, Duraisingh MT, Rayner JC, Wright GJ. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Douglas AD, Williams AR, Illingworth JJ, Kamuyu G, Biswas S, Goodman AL, Wyllie DH, Crosnier C, Miura K, Wright GJ, Long CA, Osier FH, Marsh K, Turner AV, Hill AV, Draper SJ. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nature communications. 2011b;2:601. doi: 10.1038/ncomms1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okenu DM, Riley EM, Bickle QD, Agomo PU, Barbosa A, Daugherty JR, Lanar DE, Conway DJ. Analysis of human antibodies to erythrocyte binding antigen 175 of Plasmodium falciparum. Infect Immun. 2000;68:5559–5566. doi: 10.1128/iai.68.10.5559-5566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reiling L, Richards JS, Fowkes FJ, Barry AE, Triglia T, Chokejindachai W, Michon P, Tavul L, Siba PM, Cowman AF, Mueller I, Beeson JG. Evidence That the Erythrocyte Invasion Ligand PfRh2 is a Target of Protective Immunity against Plasmodium falciparum Malaria. J Immunol. 2010;185:6157–67. doi: 10.4049/jimmunol.1001555. [DOI] [PubMed] [Google Scholar]
- 54.Reiling L, Richards JS, Fowkes FJ, Wilson DW, Chokejindachai W, Barry AE, Tham WH, Stubbs J, Langer C, Donelson J, Michon P, Tavul L, Crabb BS, Siba PM, Cowman AF, Mueller I, Beeson JG. The Plasmodium falciparum erythrocyte invasion ligand Pfrh4 as a target of functional and protective human antibodies against malaria. PLoS One. 2012;7:e45253. doi: 10.1371/journal.pone.0045253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richards JS, Stanisic DI, Fowkes FJ, Tavul L, Dabod E, Thompson JK, Kumar S, Chitnis CE, Narum DL, Michon P, Siba PM, Cowman AF, Mueller I, Beeson JG. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis. 2010;51:e50–60. doi: 10.1086/656413. [DOI] [PubMed] [Google Scholar]
- 56.Dobano C, Quelhas D, Quinto L, Puyol L, Serra-Casas E, Mayor A, Nhampossa T, Macete E, Aide P, Mandomando I, Sanz S, Puniya SK, Singh B, Gupta P, Bhattacharya A, Chauhan VS, Aponte JJ, Chitnis CE, Alonso PL, Menendez C. Age-dependent IgG subclass responses to Plasmodium falciparum EBA-175 are differentially associated with incidence of malaria in Mozambican children. Clinical and vaccine immunology : CVI. 2012;19:157–166. doi: 10.1128/CVI.05523-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCarra MB, Ayodo G, Sumba PO, Kazura JW, Moormann AM, Narum DL, John CC. Antibodies to Plasmodium falciparum erythrocyte-binding antigen-175 are associated with protection from clinical malaria. The Pediatric infectious disease journal. 2011;30:1037–1042. doi: 10.1097/INF.0b013e31822d1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Douglas AD, Andrews L, Draper SJ, Bojang K, Milligan P, Gilbert SC, Imoukhuede EB, Hill AV. Substantially reduced pre-patent parasite multiplication rates are associated with naturally acquired immunity to Plasmodium falciparum. J Infect Dis. 2011a;203:1337–1340. doi: 10.1093/infdis/jir033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duraisingh MT, Maier AG, Triglia T, Cowman AF. Erythrocyte-binding antigen 175 mediates invasion in Plasmodium falciparum utilizing sialic acid-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2003a;100:4796–4801. doi: 10.1073/pnas.0730883100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao X, Yeo KP, Aw SS, Kuss C, Iyer JK, Genesan S, Rajamanonmani R, Lescar J, Bozdech Z, Preiser PR. Antibodies targeting the PfRH1 binding domain inhibit invasion of Plasmodium falciparum merozoites. PLoS pathogens. 2008;4:e1000104. doi: 10.1371/journal.ppat.1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lopaticki S, Maier A, Thompson J, Wilson D, Tham W, Triglia T, Gout A, Speed T, Beeson J, Healer J, Cowman A. Reticulocyte and erythrocyte binding-like proteins function cooperatively in invasion of human erythrocytes by malaria parasites. Infect Immun. 2011;79:1107–1117. doi: 10.1128/IAI.01021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Narum DL, Haynes JD, Fuhrmann S, Moch K, Liang H, Hoffman SL, Sim BK. Antibodies against the Plasmodium falciparum receptor binding domain of EBA-175 block invasion pathways that do not involve sialic acids. Infect Immun. 2000;68:1964–1966. doi: 10.1128/iai.68.4.1964-1966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pandey KC, Singh S, Pattnaik P, Pillai CR, Pillai U, Lynn A, Jain SK, Chitnis CE. Bacterially expressed and refolded receptor binding domain of Plasmodium falciparum EBA-175 elicits invasion inhibitory antibodies. Mol Biochem Parasitol. 2002;123:23–33. doi: 10.1016/s0166-6851(02)00122-6. [DOI] [PubMed] [Google Scholar]
- 64.Tham WH, Wilson DW, Reiling L, Chen L, Beeson JG, Cowman AF. Antibodies to reticulocyte binding protein-like homologue 4 inhibit invasion of Plasmodium falciparum into human erythrocytes. Infect Immun. 2009;77:2427–2435. doi: 10.1128/IAI.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Healer J, Murphy V, Hodder AN, Masciantonio R, Gemmill AW, Anders RF, Cowman AF, Batchelor A. Allelic polymorphisms in apical membrane antigen-1 are responsible for evasion of antibody-mediated inhibition in Plasmodium falciparum. Mol Microbiol. 2004;52:159–168. doi: 10.1111/j.1365-2958.2003.03974.x. [DOI] [PubMed] [Google Scholar]
- 66.F Fluck C, Smith T, Beck HP, Irion A, Betuela I, Alpers MP, Anders R, Saul A, Genton B, Felger I. Strain-specific humoral response to a polymorphic malaria vaccine. Infect Immun. 2004;72:6300–6305. doi: 10.1128/IAI.72.11.6300-6305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Persson KE, Lee CT, Marsh K, Beeson JG. Development and optimization of high-throughput methods to measure Plasmodium falciparum-specific growth inhibitory antibodies. J Clin Microbiol. 2006;44:1665–1673. doi: 10.1128/JCM.44.5.1665-1673.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drew DR, O’Donnell RA, Smith BJ, Crabb BS. A common cross-species function for the double epidermal growth factor-like modules of the highly divergent plasmodium surface proteins MSP-1 and MSP-8. J Biol Chem. 2004;279:20147–20153. doi: 10.1074/jbc.M401114200. [DOI] [PubMed] [Google Scholar]
- 69.Wilson DW, Fowkes FJI, Gilson PR, Elliott SR, tavul L, Michon P, dabod E, Siba PM, Mueller I, Crabb BS, Beeson JG. Quantifying the importance of MSP1-19 as a target of growth-inhibitory and protective antibodies against Plasmodium falciparum in humans. Plos One. 6(11):e27705. doi: 10.1371/journal.pone.0027705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beeson JG, Mann EJ, Elliott SR, Lema VM, Tadesse E, Molyneux ME, Brown GV, Rogerson SJ. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes and adhesion inhibitory antibodies are associated with placental malaria and have overlapping and distinct targets. J Infect Dis. 2004;189:540–551. doi: 10.1086/381186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis. 2005;191:1932–1939. doi: 10.1086/430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boyle MJ, Richards JS, Gilson PR, Chai W, Beeson JG. Interactions with heparin-like molecules during erythrocyte invasion by Plasmodium falciparum merozoites. Blood. 2010;115:4559–4568. doi: 10.1182/blood-2009-09-243725. [DOI] [PubMed] [Google Scholar]
- 73.Baum J, Maier AG, Good RT, Simpson KM, Cowman AF. Invasion by P. falciparum merozoites suggests a hierarchy of molecular interactions. PLoS pathogens. 2005;1:e37. doi: 10.1371/journal.ppat.0010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson RJ, Phillips RS. Method to test inhibitory antibodies in human sera to wild populations of Plasmodium falciparum. Nature. 1976;263:132–134. doi: 10.1038/263132a0. [DOI] [PubMed] [Google Scholar]
- 75.Maier AG, Baum J, Smith B, Conway DJ, Cowman AF. Polymorphisms in erythrocyte binding antigens 140 and 181 affect function and binding but not receptor specificity in Plasmodium falciparum. Infection and Immunity. 2009;77:1689–1699. doi: 10.1128/IAI.01331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang L, Lopez-Barragan MJ, Jiang H, Mu J, Gaur D, Zhao K, Felsenfeld G, Miller LH. Epigenetic control of the variable expression of a Plasmodium falciparum receptor protein for erythrocyte invasion. Proc Natl Acad Sci U S A. 2011;107:2224–2229. doi: 10.1073/pnas.0913396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mamillapalli A, Pattnaik P, Sharma M, Sharma SK, Tyagi PK, Joshi H, Chitnis CE. Sequence polymorphisms in the receptor-binding domain of Plasmodium falciparum EBA-175: implications for malaria vaccine development. Mol Biochem Parasitol. 2006;146:120–123. doi: 10.1016/j.molbiopara.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 78.Chen L, Lopaticki S, Riglar DT, Dekiwadia C, Uboldi AD, Tham WH, O’Neill MT, Richard D, Baum J, Ralph SA, Cowman AF. An EGF-like protein forms a complex with PfRh5 and is required for invasion of human erythrocytes by Plasmodium falciparum. PLoS pathogens. 2011;7:e1002199. doi: 10.1371/journal.ppat.1002199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


