Abstract
We report the clinical, biochemical, and molecular characterization of a patient with a novel defect of cholesterol biosynthesis. This patient presented with a complex phenotype, including multiple congenital anomalies, mental retardation, and liver disease. In the patient’s plasma and cells, we found increased levels of lathosterol. The biosynthesis of cholesterol in the patient’s fibroblasts was defective, showing a block in the conversion of lathosterol into 7-dehydrocholesterol. The activity of 3β-hydroxysteroid-Δ5-desaturase (SC5D), the enzyme involved in this reaction, was deficient in the patient’s fibroblasts. Sequence analysis of the SC5D gene in the patient’s DNA, showing the presence of two missense mutations (R29Q and G211D), confirmed that the patient is affected by a novel defect of cholesterol biosynthesis.
Human malformations are usually caused by mutations in genes that regulate embryonic patterning. Recently, inborn errors of cholesterol biosynthesis emerged as another cause of human malformations. Progress in this field was marked by the identification of the metabolic and molecular bases of well-known clinical entities, formerly classified as dysmorphogenetic syndromes, and by the redefinition of these disorders as inborn errors of metabolism (Opitz 1999; Kelley and Hennekam 2000). The prototype for this group of disorders is Smith-Lemli-Opitz (SLO) syndrome (MIM 270400), in which mental retardation is associated with major malformations that involve virtually all organs and systems. This disease, caused by 7-dehydrocholesterol reductase deficiency (Irons et al. 1993; Fitzky et al. 1998; Wassif et al. 1998; Waterham et al. 1998), is characterized by high blood levels of specific metabolites of postsqualene cholesterol biosynthesis. The identification of the metabolic and molecular bases of SLO syndrome led to the discovery of other defects of cholesterol biosynthesis. At present, recognized disorders of this biochemical pathway include X-linked dominant chondrodysplasia punctata (CDPX2 [MIM 302960]; also known as “Conradi-Hunermann syndrome”), caused by sterol Δ8,Δ7 isomerase deficiency (Braverman et al. 1999; Kelley et al. 1999); X-linked dominant congenital hemidysplasia with ichthyosis and limb defects (CHILD) syndrome (MIM 308050), caused by defective sterol C4 demethylase (Konig et al. 2000); and autosomal recessive desmosterolosis (MIM 602398), caused by 3β-hydroxysterol Δ24-reductase deficiency (Waterham et al. 2001). Defects of cholesterol biosynthesis have been proposed in autosomal recessive Greenberg dysplasia (MIM 215140), which is likely due to sterol C14 reductase deficiency (Kelley 2000), and in FGFR2-negative patients with Antley-Bixler syndrome (MIM 207410), which is likely due to defective lanosterol 14-α-demethylase (Kelley et al. 2002).
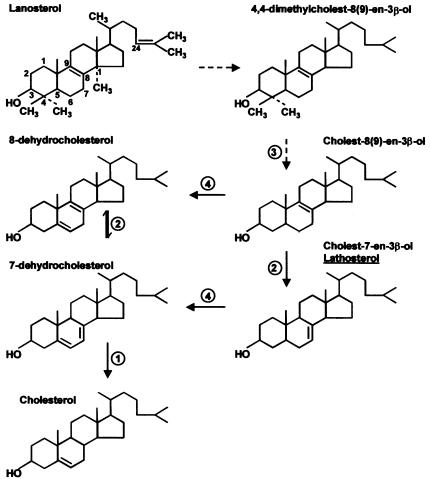
The cholesterol biosynthesis pathway in mammals is complex, involving ⩾19 enzymatic steps, beginning with the condensation of acetyl-CoA to form mevalonate, which in turn is converted into squalene. In the postsqualene pathway, lanosterol originates from the cyclization of squalene. Cholesterol is produced from lanosterol through several multienzymatic reactions, including two demethylations, an isomerization, a desaturation, and three double-bond reductions (fig. 1). It is possible that each step in this pathway can be defective and involved in human disease. A challenge for clinicians and molecular biologists is to identify “candidate” syndromes for or patients with this group of disorders.
Figure 1.
Cholesterol biosynthetic pathway—schematic representation of the main 27-carbon sterol intermediates derived from 30-carbon lanosterol. Enzymatic defects associated with the distal Kandutsch-Russell pathway have already been described in human and mouse. Desaturation of the C5 bond may occur at two different steps in the pathway: 7-dehydrocholesterol is generated from the action of C5 sterol desaturase on lathosterol (cholest-7-en-3β-ol); this enzyme also acts on zymostenol (cholest-8[9]-en-3β-ol), generating 8-dehydrocholesterol. The enzymatic steps shown are as follows: (1) 3β-hydroxysteroid-Δ7-reductase (7-dehydrocholesterol reductase; E.C. 1.3.1.21), defective in SLO syndrome; (2) 3β-hydroxysteroid-Δ8,Δ7-isomerase (zymostenol isomerase; E.C. 5.3.3.5) defective in CDPX2 and tattered mouse; (3) C3 sterol dehydrogenase (NSDHL) defective in bare patches mouse and CHILD syndrome; and (4) SC5D.
We have observed a proband with a complex phenotype, including multiple congenital anomalies, mental retardation, and liver disease, who was affected by a new defect of cholesterol biosynthesis. The proband was born to healthy nonconsanguineous parents. Her mother previously had two pregnancies: The first ended with the abortion of a female fetus at 22 wk gestation, because of multiple malformations that were revealed by a routine ultrasound scan and included microcephaly, lumbosacral myelomeningocele, hexadactyly of hands and feet, and bilateral clubfoot; a standard karyotype of fetal amniocytes was normal (46,XX). The second sibling was an unaffected male child.
The proband’s mother's pregnancy was characterized by poor maternal weight gain (4,000 g) and gestational hypertension. Maternal serology for Toxoplasma, cytomegalovirus, herpesvirus, and rubella was negative. The proband was born at 42 wk gestation, by spontaneous delivery. Birth weight was 3,000 g (10th–25th percentile), length was 48 cm (10th percentile) and occipitofrontal circumference was 30 cm (<5th percentile).
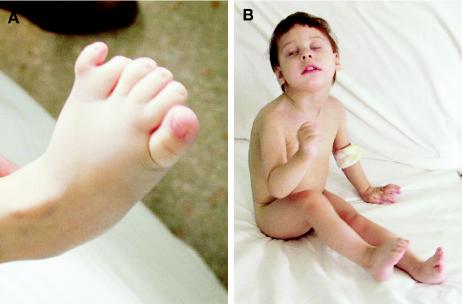
Neonatal jaundice was treated with phototherapy. Physical examination at birth revealed dysmorphic features, including severe microcephaly, receding forehead, anteverted nares, micrognathia, prominent upper lip, high arched palate, postaxial hexadactyly of the left foot (fig. 2A), and syndactyly between the 2nd–4th toes and between the 5th toe and the extra digit. External genitalia were normal. Axial hypotonia and a fracture of the right clavicle were also present. Neither ichthyosis nor chondrodysplasia punctata were detectable. Biochemical signs of abnormal liver function were detected during the 1st year of life (aspartate aminotransferase 262–310 U/liter, alanine aminotransferase 188–218 U/liter, γ-glutamyl transpeptidase 539–639 U/liter, alkaline phosphatase 1,362–1,731 U/liter, total bilirubin 2.47 mg/dl, and conjugated bilirubin 1.73 mg/dl). Viral infections, immune diseases, and cystic fibrosis were excluded. Blood cholesterol was 130 mg/dl. A standard karyotype and metabolic screening tests were normal. A liver biopsy, performed at age 14 mo, showed intrahepatic cholestasis with irregular bile ducts and cholangiolitis with mild granulocyte infiltration and diffuse expression in periportal hepatocytes of biliary cytokeratin; no signs of intracellular lipid storage were seen. Treatment with oral ursodeoxycholic acid was started.
Figure 2.
Lathosterolosis phenotype. A, Postaxial hexadactyly of the left foot, with syndactyly between the 2nd–4th toes and between the 5th toe and the extra digit. B, Proband at age 3 years. Facial dysmorphisms include microcephaly, micrognathia, bilateral epicanthus, broad nasal bridge with anteverted nares, long philtrum, thin lips with prominent upper lip, and ogival palate.
Severe psychomotor delay became increasingly evident with age. Social smiling and interactions were extremely poor. At age 2 years, the facial dysmorphism had gradually changed, resembling that of SLO syndrome (fig. 2B). An ultrasound scan of the abdomen showed a bilobate gallbladder and mild left pyelectasis, and an x-ray survey of the skeleton showed a cleft of the 8th thoracic vertebra. Conductive deafness was found at the auditory evoked potentials. Brain magnetic resonance imaging was normal.
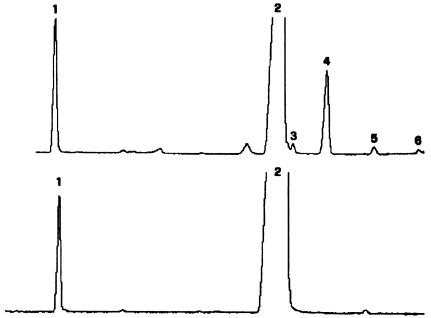
At age 2 years, the proband was screened for SLO syndrome, by use of a validated gas chromatography/mass spectrometry (GC/MS) method (Corso et al. 2002) for plasma sterol profile. The analysis showed no 7-dehydrocholesterol, whereas a peak corresponding to cholest-7-en-3β-ol (lathosterol) (fig. 3) and representing 6.69% of total plasma sterols was clearly detectable. Trace amounts of lathosterol precursors—tentatively identified as cholest-8(9)-en-3β-ol, 4-methylcholest-8(9)-en-3β-ol, and 4,4-dimethylcholest-8(9)-en-3β-ol—were also found (table 1). Sterol profile was performed in control plasma and cells, for comparison. In the analysis, possible interference due to cholestasis and ursodeoxycholic acid treatment was excluded by the examination, under the same conditions, of two patients affected by Alagille syndrome (MIM 118450), two cholestatic patients treated with ursodeoxycholic acid, and two patients who had received liver transplants and were treated with ursodeoxycholic acid, as well as by the repetition of the GC/MS sterol profile in the proband’s plasma after discontinuation of therapy for 2 wk.
Figure 3.
Sterol profiles in proband with lathosterolosis. Typical GC/MS analysis of plasma from the proband (top) and from an unaffected control individual (bottom). The compounds definitively identified are as follows: (1) internal standard (5α-cholestane); (2) cholesterol; (3) zymostenol (cholest-8[9]-en-3β-ol); (4) lathosterol (cholest-7-en-3β-ol). The compounds tentatively identified are as follows: (5) 4-methylcholest-8[9]-en-3β-ol; and (6) 4,4-dimethylcholest-8[9]-en-3β-ol.
Table 1.
GC/MS Sterol Determination in Plasma and Cells[Note]
|
Mean (SD) Levels of(%) |
|||||
| Cholesterol | Cholest-8(9)-en-3β-ol | Lathosterol | 4-Methylcholest-8(9)-en-3β-ol | 4,4-Dimethylcholest-8(9)-en-3β-ol | |
| Proband: | |||||
| Total plasma sterols (n = 3) | 89.97 (1.98) | 1.16 (.3) | 6.69 (1.21) | .87 (.19) | .42 (.24) |
| Free plasma sterols (n = 3) | 87.37 (4.81) | 1.04 (.3) | 10.32 (4.19) | .99 (.1) | .2 (.32) |
| Red cell sterols (n = 3) | 87.54 (4.22) | .69 (.45) | 10.62 (3.17) | .76 (.43) | .1 (.22) |
| Fibroblast sterols (n = 5) | 86.88 (5.74) | .63 (.41) | 12.49 (5.35) | nd | nd |
| Father: | |||||
| Total plasma sterols | 99.64 | nd | .36 | nd | nd |
| Mother: | |||||
| Total plasma sterols | 99.77 | nd | .23 | nd | nd |
| Brother: | |||||
| Total plasma sterols | 99.57 | nd | .43 | nd | nd |
| Control individuals: | |||||
| Total plasma sterols (n = 20) | 100 | nd | nd | nd | nd |
| Red cell sterols (n = 20) | 100 | nd | nd | nd | nd |
| Fibroblast sterols (n = 10)a | 100 | nd | nd | nd | nd |
Note.— nd = Below the detection limit (<0.05 μg/liter); n indicates the number of determinations.
Cultured in delipidated medium.
Sterol profiling of red cell membranes and fibroblasts, cultured in delipidated medium for 15 d, resulted in the same abnormal pattern (table 1). A slight increase of lathosterol was found in the parents’ plasma (table 1) and red cell membranes (not shown).
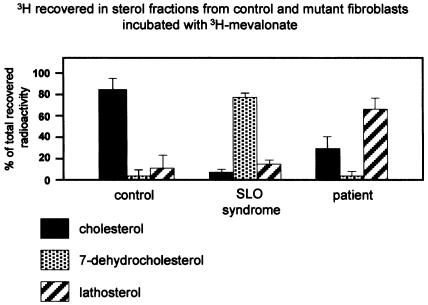
Cholesterol biosynthesis in the proband’s fibroblasts was studied according to the method of Honda et al. (1995), and the results were compared to those obtained in control and SLO syndrome cell lines. Fibroblasts were cultured in delipidated medium for 3 d; 3H-mevalonate, an early intermediate of cholesterol biosynthesis, was then added to the medium; and, after 3 d, fibroblasts were harvested and extracted with a chloroform/metanol (2/1 v/v) mixture. The extracted sterols were separated by AgNO3 silica thin-layer chromatography (TLC), with a chloroform/acetone (96.5/3.5 v/v) mixture as solvent. When cholesterol and lathosterol were not sufficiently resolved, a second TLC separation was run, with a diethyl ether/hexane (5/1 v/v) mixture as solvent. The spots corresponding to cholesterol, lathosterol, and 7-dehydrocholesterol were scraped, and radioactivity was measured. The proband’s cells accumulated lathosterol (66.1% of the recovered radioactivity), whereas SLO syndrome fibroblasts showed accumulation of 7-dehydrocholesterol (78.2%). In control fibroblasts, 3H-mevalonate was normally converted to cholesterol (84.0%) (fig. 4). These results suggested a block in cholesterol biosynthesis at the level of 3β-hydroxysteroid-Δ5-desaturase (SC5D [E.C. 1.3.3.2]; also known as “lathosterol dehydrogenase”). SC5D is an enzyme that catalyzes a dehydrogenation reaction introducing a C5-C6 double bond into lathosterol.
Figure 4.
Cholesterol biosynthesis in control, lathosterolosis, and SLO fibroblasts. The percentage of recovered radioactivity is reported (mean ± SD of three different experiments). In control fibroblasts, 84.0%±18% of the incorporated radioactivity was recovered in the cholesterol fraction; in SLO fibroblasts, 78.2%±1.6% of the radioactivity was present as 7-dehydrocholesterol, and 14.0%±1.8% of the radioactivity was present as lathosterol. In the proband, of the total radioactivity, cholesterol represented 30.0%±11.0%, 7-dehydrocholesterol represented 3.4%±2.9%, and lathosterol represented 66.1%±10%.
We measured SC5D enzymatic activity in fibroblasts cultured in delipidated medium (Kelley 1995), according to the method described by Taton et al. (2000). In brief, fibroblast extracts containing 200 μg protein in 0.1 M phosphate buffer (pH 7.5) with 3 mM reduced glutathione and 20% glycerol were incubated for 90 min at 37°C with 3H-lathosterol emulsified in Tween 80 (1.5 g/liter), with the addition of 500 μM reduced nicotinamide adenine dinucleotide. The incubation volume was 200 μl. The reaction was stopped with 6% KOH in ethanol and was extracted with hexane. The different sterols were separated on an AgNO3 silica TLC, as indicated above. The spots corresponding to the separated sterols were scraped, were extracted with hexane, and were counted on a β-counter. The SC5D activity in the proband’s fibroblasts was defective in four separate experiments (10±12 pmol/mg protein/min), as compared to that of control cell lines (104±27 pmol/mg protein/min).
Cloning and sequencing of genes that encode SC5D have been reported in a number of organisms, such as Saccharomyces cerevisiae, Arabidopsis thaliana, Candida glabrata, and C. albicans (Arthington et al. 1991; Geber et al. 1995; Gachotte et al. 1996; Miyazaki et al. 1999). The human SC5D cDNA was cloned from a human brain library as a sequence homologous to yeast enzyme, complementing SC5D-deficient yeast mutant cells (Matsushima et al. 1996). The human SC5D gene is composed of five exons (Sugawara et al. 2001) and maps to 11q23.3 (Nishi et al. 2000; Sugawara et al. 2001). Hydropathy analysis of SC5D suggests that the enzyme is an integral membrane protein with four to five membrane-spanning regions (Kyte et al. 1982).
We looked for mutations in the SC5D gene in the proband described herein. Sense and antisense oligonucleotide primers, corresponding to intronic sequences flanking the SC5D coding exons, were synthesized and were used to amplify exons and their intronic junctions of genomic DNA from the proband and her relatives. Amplified DNA products were checked on agarose gel and were then sequenced bidirectionally on an ABI 377 automated DNA sequencer.
We found two missense mutations in the proband’s DNA, a 88G→A transversion that changes a basic amino acid (arginine) into a neutral amino acid (glutamine) at codon 29 in exon 1 (R29Q) and a 632 G→A transversion that changes a glycine into an aspartic acid at codon 211 in exon 4 (G211D). Each parent was heterozygous for one of the two mutations (R29Q, in the mother, and G211D, in the father), indicating that the proband is a compound heterozygote and suggesting that the disease has an autosomal recessive pattern of inheritance. The proband’s unaffected brother was heterozygous for the R29Q mutation. To exclude the possibility that the mutations found are common polymorphisms among the general population, we analyzed, by denaturing high-performance liquid chromatography, PCR-amplified products that corresponded to exons 1 and 4, in 50 unaffected subjects, using the Wave DNA-fragment–analysis system (Transgenomic). The mutations were not present in the 100 alleles screened.
Cholesterol biosynthesis defects, characterized by the accumulation of specific metabolic precursors in blood and tissues, have been increasingly implicated in human malformation syndromes. The proband described herein represents the first example of a new entry in this group of disorders. The profiling, by GC/MS, of plasma and cell sterols in the proband and studies of cholesterol biosynthesis in cultured fibroblasts suggested a metabolic block in the conversion of lathosterol into 7-dehydrocholesterol. SC5D deficiency in the proband was confirmed by direct enzymatic assay in fibroblasts.
Mutational analysis in the proband’s DNA showed two missense mutations of the SC5D gene. The finding of defective SC5D in fibroblasts strongly suggests that these mutations affect SC5D catalytic activity. One of the mutations (G224D) changes an amino acid, close to one of three histidine-rich regions of the SC5D protein, that is highly conserved throughout phylogenesis. These histidine-rich motifs are found in an important class of integral membrane enzymes that includes desaturases, hydroxylases, acetylenases, and epoxygenases. Histidine-rich motifs are conserved among mammals, yeasts, and plants and are likely involved in the formation of a presumed catalytic Fe center, where oxygen activation and substrate oxidation occur (Shanklin et al. 1994). In A. thaliana, site-directed mutagenesis of these residues abolished enzyme activity (Taton et al. 2000). Mutations in this region are expected to affect enzyme activity. The other mutation found is in a nonconserved residue located outside the membrane, as predicted by hydropathy analysis, and changes a strong basic amino acid into a neutral residue.
Although there is no formal proof that the metabolic derangement is causative of the phenotype observed in the proband whom we studied, similarities with other known defects of cholesterol biosynthesis make this hypothesis very likely. The appearance of the proband and the presence of hexadactyly and liver disease (occasionally reported in patients with SLO syndrome) are particularly indicative in this respect. Lathosterol levels observed in the proband described herein (13.04±2.65 mg/dl) are comparable to 7-dehydrocholesterol levels observed in patients with SLO syndrome (13.8±6.4 mg/dl, in 11 patients analyzed in our laboratory). It is interesting that an enzymatic block immediately upstream from 7-dehydrocholesterol reductase, as observed in the proband, results in a phenotype more similar to that of SLO syndrome than to the phenotypes observed in defects that involve earlier steps of cholesterol biosynthesis. Additional evidence that the phenotype observed in the proband is the result of SC5D deficiency derives from the recent development of a murine model of SC5D deficiency, showing perinatal lethality, micrognathia, intrauterine growth retardation, palatoschisis, short limbs, syndactyly, postaxial hexadactyly, and hepatomegaly (Krakowiak et al. 2001). The severe phenotype observed in the knockout mouse is likely due to a complete disruption of the SC5D gene. In the proband described herein, we found normal plasma cholesterol levels and reduced synthesis of cholesterol in fibroblasts incubated with 3H-mevalonate (30% of recovered radioactivity), suggesting residual SC5D catalytic activity.
The relationships between abnormal cholesterol metabolism and disturbed morphogenesis have been extensively studied in recent years, bringing to the attention of geneticists and dysmorphologists a new research field, “metabolic morphogenesis” (Kelley 1998). An increasing number of studies has implicated cholesterol in the hedgehog embryonic-signaling pathway (Opitz 1999; Kelley et al. 2000). It has been shown that covalent attachment of cholesterol to Sonic hedgehog (Shh) is essential for the posttranslational autoprocessing of Shh into the mature and active N-terminal polypeptide—for the correct localization of this peptide in membranes and for the interactions with the sterol-sensing domain in Patched-1 and -2 (Villavivencio et al. 2000), two transmembrane receptor proteins that regulate signal transduction. Mutations of genes that encode other Shh pathway members acting downstream from signal transduction are also implicated in human disease and are associated with birth defects and cancer (Villavivencio et al. 2000).
The hypothesis that malformations in SLO syndrome and in related defects stem from abnormal functioning of the Shh pathway is attractive; however, several points need further investigation. It has been questioned whether cholesterol deficiency or increased levels of intermediate sterols are responsible for the abnormal functioning of the pathway (Kelley 1998) in SLO syndrome. Several lines of evidence support the role that cholesterol deficiency plays in causing malformations. The normal plasma cholesterol found in the proband described herein should be considered carefully, since it might not reflect cholesterol concentration in rapidly growing cells during the embryonic period.
In conclusion, our data implicate SC5D deficiency in human disease and extend the range of cholesterol biosynthesis defects that cause disturbed morphogenesis. The precise definition of the phenotype that results from SC5D deficiency has an impact on clinical practice, since it may provide additional indications for biochemical and molecular screening of patients with unexplained malformations and since it allows prenatal diagnosis of this disease. A precise clinical characterization of patients also enables the improvement of our understanding of the interactions between specific metabolic blocks and Shh and related proteins.
Acknowledgments
We thank Prof. P. Vajro (Department of Pediatrics, Federico II University, Naples) for providing samples, for sterol profiling, from patients with Alagille syndrome and chronic liver disease, and we thank Prof. G. S. Tint (Veterans Affairs Medical Center, East Orange, N.J.) for kindly providing us with 3H-lathosterol. This work was supported, in part, by Telethon Foundation grant E.790 (to G.P.), Ministero Università e Ricerca Scientifica e Tecnologica grants 9806183096_001 and MM06182533_001 (to G.A.) and MM06182533_005 (to A.D.R.), and Consiglio Nazionale delle Ricerche grant 99.02448.04 (to A.D.R.).
Electronic-Database Information
Accession numbers and the URL for data presented herein are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for SLO syndrome [MIM 270400], CDPX2 [MIM 302960], CHILD syndrome [MIM 308050], desmosterolosis [MIM 602398], Greenberg dysplasia [MIM 215140], Antley-Bixler syndrome [MIM 207410], and Alagille syndrome [MIM 118450])
References
- Arthington B, Bennett LG, Skatrud PL, Guynn CJ, Barbuch RJ, Ulbright CE, Bard M (1991) Cloning, disruption and sequence of the gene encoding yeast C-5 sterol desaturase. Gene 102:39–44 [DOI] [PubMed] [Google Scholar]
- Braverman N, Lin P, Moebius FF, Obie C, Moser A, Glossmann H, Wilcox WR, Rimoin DL, Smith M, Kratz L, Kelley RI, Valle D (1999) Mutations in the gene encoding 3β-hydroxysteroid-Δ8,Δ7-isomerase cause X-linked dominant Conradi-Hunermann syndrome. Nat Genet 22:291–294 [DOI] [PubMed] [Google Scholar]
- Corso G, Rossi M, De Brasi D, Rossi I, Dello Russo A (2002) Effects of sample storage on 7- and 8-dehydrocholesterol levels analysed on whole blood spots by gas chromatography-mass spectrometry-selected ion monitoring. J Chromatogr B Analyt Technol Biomed Life Sci 766:365–370 [DOI] [PubMed] [Google Scholar]
- Fitzky BU, Witsch-Baumgartner M, Erdel M, Lee JN, Paik YK, Glossmann H, Utermann G, Moebius FF (1998) Mutations in the Δ7-sterol reductase gene in patients with the Smith-Lemli-Opitz syndrome. Proc Natl Acad Sci USA 95:8181–8186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachotte D, Husselstein T, Bard M, Lacroute F, Benveniste P (1996) Isolation and characterization of an Arabidopsis thaliana cDNA encoding a Δ7-sterol-C-5-desaturase by functional complementation of a defective yeast mutant. Plant J 9:391–398 [DOI] [PubMed] [Google Scholar]
- Geber A, Hitchcock CA, Swartz JE, Pullen FS, Marsden KE, Kwon-Chung KJ, Bennett JE (1995) Deletion of the Candida glabrata ERG3 and ERG11 genes: effect on cell viability, cell growth, sterol composition, and antifungal susceptibility. Antimicrob Agents Chemother 39:2708–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A, Tint GS, Salen G, Batta AK, Chen T, Shefer S (1995) Defective conversion of 7-dehydrocholesterol to cholesterol in cultured skin fibroblasts from Smith-Lemli-Opitz syndrome homozygotes. J Lipid Res 36:1595–1601 [PubMed] [Google Scholar]
- Irons M, Elias ER, Salen G, Tint GS, Batta A (1993) Defective cholesterol biosynthesis in Smith-Lemli-Opitz syndrome. Lancet 341:1414 [DOI] [PubMed] [Google Scholar]
- Kelley RI (1995) Diagnosis of Smith-Lemli-Opitz syndrome by gas chromatography/mass spectrometry of 7-dehydrocholesterol in plasma, amniotic fluid and cultured skin fibroblasts. Clin Chim Acta 236:45–58 [DOI] [PubMed] [Google Scholar]
- ——— (1998) RSH/Smith-Lemli-Opitz syndrome: mutations and metabolic morphogenesis. Am J Hum Genet 63:322–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ——— (2000) Inborn errors of cholesterol biosynthesis. Adv Pediatr 47:1–53 [PubMed] [Google Scholar]
- Kelley RI, Hennekam RCM (2000) The Smith-Lemli-Opitz syndrome. J Med Genet 37:321–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley RI, Kratz LE, Rivka LG, Netzloff MI, Wolf LM, Jabs EW (2002) Abnormal sterol metabolism in a patient with Antley-Bixler syndrome and ambiguous genitalia. Am J Med Genet 110:95–102 [DOI] [PubMed] [Google Scholar]
- Kelley RI, Wilcox WG, Smith M, Kratz LE, Moser A, Rimoin DS (1999) Abnormal sterol metabolism in patients with Conradi-Hunermann-Happle syndrome and sporadic lethal chondrodysplasia punctata. Am J Med Genet 83:213–219 [DOI] [PubMed] [Google Scholar]
- Konig A, Happle R, Bornholdt D, Engel H, Grzeschik KH (2000) Mutations in the NSDHL gene, encoding a 3-β-hydroxysteroid dehydrogenase, cause CHILD syndrome. Am J Med Genet 90:339–346 [PubMed] [Google Scholar]
- Krakowiak PA, Wassif CA, Kratz L, Vied DA, Kelley RI, Porter RD (2001) Lathosterol oxidase disruption: a new inborn error of cholesterol biosynthesis. Am J Hum Genet Suppl 69:190 [Google Scholar]
- Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132 [DOI] [PubMed] [Google Scholar]
- Matsushima M, Inazawa J, Takahashi E, Suzumori K, Nakamura Y (1996) Molecular cloning and mapping of a human cDNA (SC5DL) encoding a protein homologous to fungal sterol-C5-desaturase. Cytogenet Cell Genet 74:252–254 [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Geber A, Miyazaki H, Falconer D, Parkinson T, Hitchcock C, Grimberg B, Nyswaner K, Bennett JE (1999) Cloning, sequencing, expression and allelic sequence diversity of ERG3 (C-5 sterol desaturase gene) in Candida albicans. Gene 236:43–51 [DOI] [PubMed] [Google Scholar]
- Nishi S, Nishino H, Ishibashi T (2000) cDNA cloning of the mammalian sterol C5-desaturase and the expression in yeast mutant. Biochim Biophys Acta 1490:106–108 [DOI] [PubMed] [Google Scholar]
- Opitz JM (1999) RSH (so-called Smith-Lemli-Opitz) syndrome. Curr Opin Pediatr 11:353–362 [DOI] [PubMed] [Google Scholar]
- Shanklin J, Whittle E, Fox BG (1994) Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry 33:12787–12794 [DOI] [PubMed] [Google Scholar]
- Sugawara T, Fujimoto Y, Ishibashi T (2001) Molecular cloning and structural analysis of human sterol C5 desaturase. Biochim Biophys Acta 1533:277–284 [DOI] [PubMed] [Google Scholar]
- Taton M, Husselstein T, Benveniste P, Rahier A (2000) Role of highly conserved residues in the reaction catalyzed by recombinant Δ7-sterol-C5(6)-desaturase studied by site-directed mutagenesis. Biochemistry 39:701–711 [DOI] [PubMed] [Google Scholar]
- Villavivencio EH, Walterhouse DO, Iannaccone P (2000) The Sonic hedgehog–Patched–Gli pathway in human development and disease. Am J Hum Genet 67:1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassif CA, Maslen C, Kachilele-Linjewile S, Lin D, Linck LM, Connor WE, Steiner RD, Porter FD (1998) Mutations in the human sterol Δ7-reductase gene at 11q12-13 cause Smith-Lemli-Opitz syndrome. Am J Hum Genet 63:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Koster J, Romeijn GJ, Hennekam RC, Vreken P, Andersson HC, FitzPatrick DR, Kelley RI, Wanders RJ (2001) Mutations in the 3β-hydroxysterol Δ24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am J Hum Genet 69:685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Wijburg FA, Hennekam RC, Vreken P, Poll-The BT, Dorland L, Duran M, Jira PE, Smeitink JA, Wevers RA, Wanders RJ (1998) Smith-Lemli-Opitz syndrome is caused by mutations in the 7-dehydrocholesterol reductase gene. Am J Hum Genet 63:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]