Significance
Efficient and scalable catalysts that mediate the interconversion of electrical and chemical energy are essential to the future world economy. Central to this challenge are robust systems that can split water into its elements, hydrogen and oxygen, with minimal cost. In this article, we describe the discovery and characterization of an efficient, single-site homogeneous water oxidation catalyst based on a cationic cobalt-porphyrin system. Important roles of the buffer base in this catalysis are also described. Our results shed light on the design of next-generation water oxidation catalysts using earth-abundant transition metals and redox active ligand systems.
Keywords: electrocatalysis, oxygen, photosynthesis, high valent, single site
Abstract
A series of cationic cobalt porphyrins was found to catalyze electrochemical water oxidation to O2 efficiently at room temperature in neutral aqueous solution. Co–5,10,15,20-tetrakis-(1,3-dimethylimidazolium-2-yl)porphyrin, with a highly electron-deficient meso-dimethylimidazolium porphyrin, was the most effective catalyst. The O2 formation rate was 170 nmol⋅cm−2⋅min−1 (kobs = 1.4 × 103 s−1) with a Faradaic efficiency near 90%. Mechanistic investigations indicate the generation of a CoIV-O porphyrin cation radical as the reactive oxidant, which has accumulated two oxidizing equivalents above the CoIII resting state of the catalyst. The buffer base in solution was shown to play several critical roles during the catalysis by facilitating both redox-coupled proton transfer processes leading to the reactive oxidant and subsequent O–O bond formation. More basic buffer anions led to lower catalytic onset potentials, extending below 1 V. This homogeneous cobalt-porphyrin system was shown to be robust under active catalytic conditions, showing negligible decomposition over hours of operation. Added EDTA or ion exchange resin caused no catalyst poisoning, indicating that cobalt ions were not released from the porphyrin macrocycle during catalysis. Likewise, surface analysis by energy dispersive X-ray spectroscopy of the working electrodes showed no deposition of heterogeneous cobalt films. Taken together, the results indicate that Co–5,10,15,20-tetrakis-(1,3-dimethylimidazolium-2-yl)porphyrin is an efficient, homogeneous, single-site water oxidation catalyst.
Water oxidation is a key step in photosynthesis that efficiently harvests and stores solar energy (1). The oxidation of H2O to O2 is a four-electron, four-proton process in which O–O bond formation is the key chemical step. In photosystem II, these proton-coupled electron transfers (PCETs) occur via a tyrosine at the Mn4Ca oxygen-evolving complex (2). An important thermodynamic aspect of photosynthesis is the efficient conversion of photonic energy to electrical potential, thus providing 99% of the driving force required to convert CO2 to carbohydrates (Eqs. 1 and 2):
and
The development of synthetic catalysts that can mediate water oxidation under mild conditions with a minimal energy cost has become an appealing challenge for chemists (3–7). Among various approaches, homogeneous molecular catalysts have shown attractive features such as controllable redox properties, ease of investigating reaction mechanisms, and strategies for the characterization of reactive intermediates (8, 9). Recently, such efforts have resulted in the development of a significant number of systems based on single-site and multinuclear transition metal complexes including Mn, Fe, Co, Cu, Ru, and Ir (9–15). Examples of cobalt-based molecular catalysts include a cobalt phthalocyanine (16), a cobalt “hangman” corrole (17), multipyridine cobalt complexes (18, 19), a dinuclear Co-peroxo species (20), and most recently, Co-porphyrins (21). Determining if these molecular complexes retain their homogenous nature during catalysis or merely act as precursors of truly active heterogeneous species such as films and nanoparticles has proven to be problematic (8, 22, 23). This scenario is especially critical for Co-based molecular catalysts because heterogeneous CoOx species are highly active, so that even small amounts of this surface oxide layer could contribute significantly to the catalytic activity (24–27). Further, very little detailed mechanistic information for these systems is available to date, and the assignment of the key oxidants responsible for oxidizing a water molecule has been challenging (28). Here we describe mechanistic studies of a series of water-soluble, cationic cobalt porphyrins that effectively catalyze water oxidation electrochemically under mild conditions at neutral pH. The homogeneity of these catalysts was confirmed by a variety of techniques including electrochemical, spectroscopic, and surface analysis that exclude the alternative formation of heterogeneous cobalt oxide films. Our results indicate the generation of a reactive, high-valent CoIV-porphyrin cation radical that has accumulated two oxidizing equivalents above the resting CoIII catalyst. Further, we demonstrate a critical role for the buffer base that accepts a proton during the O–O bond-formation event.
Results
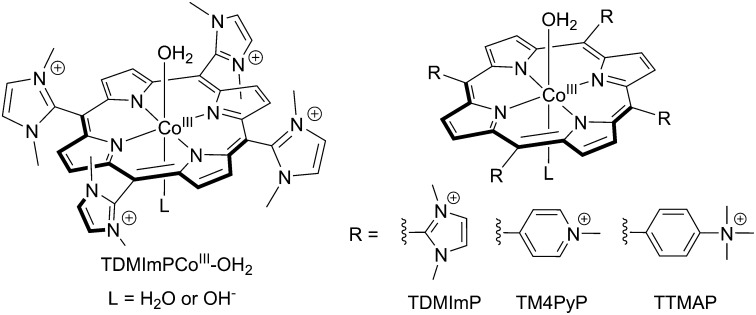
The cobalt-porphyrin precursor, Co(II)–5,10,15,20-tetrakis-(1,3-dimethylimidazolium-2-yl) porphyrin tetrachloride (CoII-TDMImP; Scheme 1) was prepared by metallation of the corresponding free base (29) and characterized by UV–vis, EPR, and mass spectroscopies. The UV–vis spectrum of CoII-TDMImP showed a Soret band at 407 nm and two Q bands at 527 nm and 558 nm (SI Appendix, Fig. S1). The high-resolution electrospray mass spectrum exhibited an ion at a mass-to-charge ratio (m/z) of 186.82 (SI Appendix, Fig. S2), with the mass and isotope distribution patterns matching the calculated value (m/z = 186.82) for the quaternary ion [CoII-TDMImP]4+. The continuous-wave X-band EPR spectrum of CoII-TDMImP showed a resonance at geff = 2.30 with partially resolved hyperfine splitting due to the nuclear spin of 59Co (I = 7/2) (SI Appendix, Fig. S3, solid), indicating a low-spin CoII center (S = 1/2). This signal is distinct from that of the free Co2+ ion measured under identical conditions (SI Appendix, Fig. S3, dotted line), which showed broad features at geff = 5.56 and 3.13 (30). Therefore, the amount of any free Co2+ salt as an impurity in the sample is very small. The 1-e− electrochemical oxidation of CoII-TDMImP at an applied potential of 300 mV generated CoIII-TDMImP, the active form of the catalyst (described below), which showed red-shifted optical features at 419 nm (Soret band), 540 nm, and 575 nm (Q bands). The 1H NMR spectrum (SI Appendix, Fig. S4) clearly indicated that CoIII-TDMImP is a diamagnetic species, as expected, with sharp resonances assignable to the porphyrin β-pyrrole protons, the imidazolium C4 and C5 protons, and the methyl protons at δ = 9.34, 8.12, and 3.67 ppm, respectively. The X-band EPR spectrum showed that CoIII-TDMImP was EPR silent (SI Appendix, Fig. S3, dashed), with negligible CoII species present. Taken together, these results indicate that both CoII-TDMImP and CoIII-TDMImP had been prepared in high purity (>99%). There was no evidence of Na–Pi buffer binding to either CoII-TDMImP or CoIII-TDMImP at pH 7 (SI Appendix, Fig. S5).
Scheme 1.
Water-soluble cobalt porphyrins studied as water oxidation catalysts in this work.
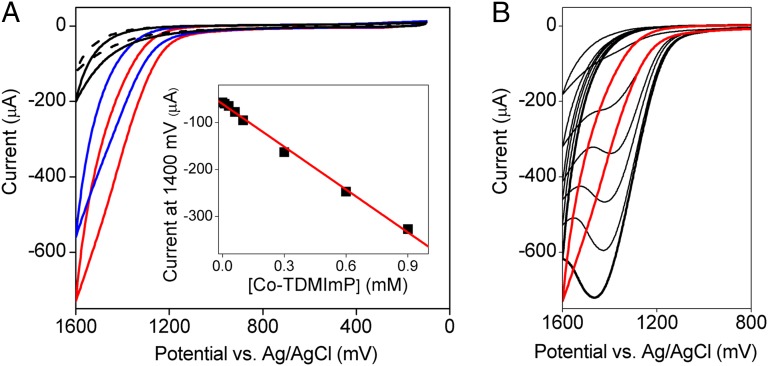
A strong catalytic current with an onset potential of ∼1.2 V was observed in the cyclic voltammogram (CV) of 1 mM CoII-TDMImP carried out in 0.2 M aqueous sodium phosphate (Na–Pi) at pH 7 (Fig. 1A, red trace). This feature, which was absent in scans of solutions containing buffer alone (Fig. 1A, black trace), showed a strong, linear dependence on the concentration of catalyst (Fig. 1A, Inset). CoII-TDMImP (0.5 mM, pH 7) was then subjected to controlled potential bulk electrolysis at 1.30 V using an indium tin oxide (ITO) working electrode, while monitoring O2 evolution with a Clark electrode. The rate constant (kobs) of the O2 formation was determined to be 1.4 × 103 s−1, which is ∼18-fold larger than that reported by Berlinguette and coworkers (19). [kobs was determined using the equation icat/idiff = 2.24n(RTkobs/Fν)1/2, where icat is the catalytic current, idiff is the diffusional current of the CoIII/II couple, n = 4, and ν is the scan rate. A plot of icat/idiff vs. ν1/2 gives a linear relationship with a slope of 2.24n(RTkobs/F)1/2, where kobs can be calculated.] The faradaic yield for oxygen production determined by these measurements for this Co-porphyrin was 85–90% (SI Appendix, Fig. S6).
Fig. 1.
WOC by Co-TDMImP and Co(NO3)2. (A) CVs of buffer background (black solid), 1 mM Co-TDMImP in H2O (red) and in D2O (blue), and clean buffer using the working electrode removed from 1 mM Co-TDMImP–containing solution after 20 CV scans (black dashed). (A, Inset) Plot of the catalytic current for Co-TDMImP at 1.40 V (Ag/AgCl) vs. [Co-TDMImP]. The red line represents the best linear fit. (B) CVs of 1 mM Co-TDMImP (red trace) and Co(NO3)2 (black traces) at concentrations (from top to bottom) of 0.01 mM, 0.05 mM, 0.1 mM, 0.13 mM, 0.17 mM, and 0.2 mM. Other conditions include glassy carbon working electrode, 0.2 M Na–Pi buffer, pH 7, 22 °C, and scan rate 100 mV⋅s−1.
Sustained electrocatalytic oxygen evolution was observed for several hours without significant loss of the catalytic current (SI Appendix, Fig. S7). Inspection of the well-defined UV–vis spectrum during and after the electrolysis indicated that loss of the Co-porphyrin chromophore was insignificant and that the catalyst inventory remained predominantly CoIII-TDMImP (>98%) throughout (SI Appendix, Fig. S8). The catalytic current decreased with increasing scan rate over a range of scan rates from 50 to 500 mV⋅s−1 (SI Appendix, Fig. S9). This result indicates a rate-limiting chemical step, likely O–O bond formation, that precedes subsequent peroxide oxidation to O2 on the electrode surface (31). The CV of Co-TDMImP taken in D2O showed a significantly smaller catalytic current (Fig. 1A, blue trace) compared with that obtained in H2O, indicative of a solvent isotope effect [kinetic isotope effect (KIE) or equilibrium isotope effect (EIE)] of 2.8 [the KIE was calculated according to KIE = kcat,H2O/kcat,D2O = (icat,H2O/icat,D2O)2; see ref. 31].
We performed a number of controls to assess the possible formation of heterogeneous metal oxide films. First, the stability of the catalyst was probed under active water oxidation catalysis (WOC) reaction conditions by monitoring the characteristic visible spectrum of CoIII-TDMImP (Q bands at 540 and 575 nm). As can be seen in SI Appendix, Fig. S8, there was negligible decomposition (<2%) over a period of 4 h. We found that 1 mM Co-TDMImP produced a catalytic current similar to that of a heterogeneous cobalt oxide film derived from 0.2 mM Co(NO3)2 (Fig. 1B). Accordingly, at least 20% decomposition of Co-TDMImP would have been required to generate the observed current, which is completely inconsistent with this spectral monitoring. Likewise, linear sweep voltammetry showed that the catalytic activity of Co-TDMImP remained constant over this time (SI Appendix, Fig. S10). We next designed experiments using additives that would scavenge any free Co2+ ions present in the reaction medium. As illustrated in the SI Appendix, Fig. S11A, the addition of 0.25 mM EDTA completely suppressed the WOC activity of 0.2 mM Co(NO3)2. Further, no cobalt oxide film was observed to form on the electrode surface after multiple CV scans, whereas cobalt oxide was readily detected without added EDTA. In sharp contrast, the catalytic current observed for 1 mM Co-TDMImP retained its full intensity in the presence of 0.25 mM EDTA. These results indicate that there was no significant release of free Co2+ ions into the solution during catalysis in the presence of Co-TDMImP. This result also excludes the possibility that trace cobalt impurities in Co-TDMImP contribute to the observed WOC activity. Similar results were obtained using Chelex resin in a three-phase test. The addition of Chelex beads drastically reduced the observed catalytic current of 0.2 mM Co(NO3)2 sevenfold by sequestering free Co2+ ions (SI Appendix, Fig. S11B). However, the presence of Chelex beads had no effect on the observed WOC current with Co-TDMImP over the period of the measurement.
The onset potentials obtained using Co-TDMImP and Co(NO3)2 and the appearance of the catalytic waves are completely different (Fig. 1B). Further, there was no detectable lag phase in the development of catalytic current for the homogeneous cobalt-porphyrin system. When the working electrodes (both glassy carbon and ITO) were removed from the Co-porphyrin solutions after multiple CV scans or bulk electrolysis and used in clean buffers after gentle rinsing, no significant anodic current was observed relative to a freshly prepared electrode (Fig. 1A and SI Appendix, Fig. S7). Inspection of the surface morphology and composition of the glassy carbon electrodes by environmental scanning electron microscope (ESEM) and energy dispersive X-ray spectroscopy (EDX) analysis after multiple CV runs in Co-TDMImP solution with a concentration of even 5 mM showed clean surfaces with no sign of any heterogeneous cobalt phase (SI Appendix, Figs. S12 and S13). By contrast, an obvious cobalt-containing heterogeneous film formed on the electrode surface after scans in the same buffer solution containing as little as 0.1 mM Co(NO3)2. Accordingly, the release of any free Co2+ ions from Co-TDMImP must be less than 2% and not nearly enough to account for the large WOC current.
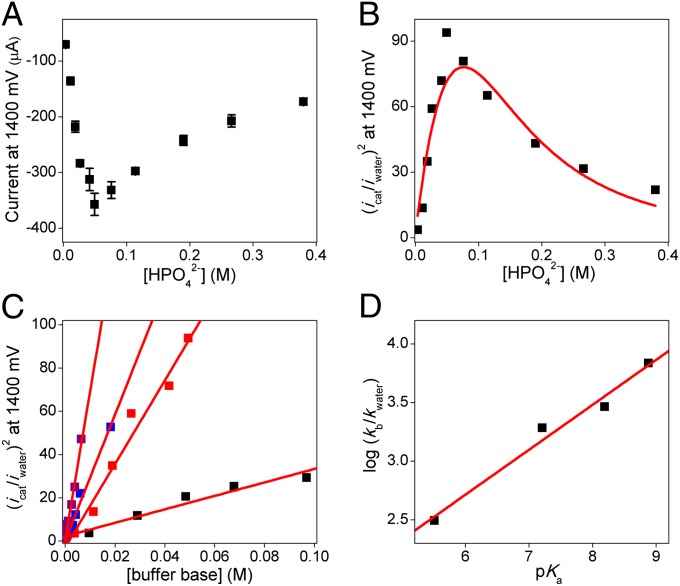
Several experiments were performed to examine the effects of the buffer concentration on the catalytic water oxidation activity. As shown in Fig. 2 and SI Appendix, Fig. S14, no catalytic activity was observed in unbuffered H2O solution (pH ∼7). However, a significant enhancement of the fixed-potential catalytic current, icat, was observed with increasing concentrations of HPO42– buffer up to 60 mM. Care was taken during these measurements to maintain the ionic strength of the solution at 0.1 M with NaNO3. Interestingly, a decrease of the catalytic current was observed when the concentration of the buffer base was increased beyond 60 mM (Fig. 3A). This observation suggests the presence of an inhibition pathway at high buffer concentrations. Besides Na–Pi (pKa = 7.21), Co-TDMImP also catalyzed water oxidation at pH 7 with a variety of buffers, including phthalate (pKa = 5.51), bicarbonate (pKa = 6.37), n-butylphosphonate (pKa = 8.19), and tert-butylphosphonate (pKa = 8.88) (Figs. 2C and 3A).
Fig. 2.
Roles of the buffer anion in WOC. (A) Plot of the catalytic current for Co-TDMImP measured at 1.4 V vs. [HPO42–]. (B) Plot of (icat/iwater)2 at 1.4 V vs. [HPO42–] and the best fit according to SI Appendix, Eq. S3 (red). (C) Plot of (icat/iwater)2 at 1.4 V vs. the buffer dianion concentration; phthalate, black; Na–Pi, red; n-butylphosphonate, blue; tert-butylphosphonate, purple. (D) Plot of log (kb/kwater) vs. pKa of the buffer used and best linear fits (red). Other conditions include room temperature, scan rate 100 mV⋅s−1, and pH 7.
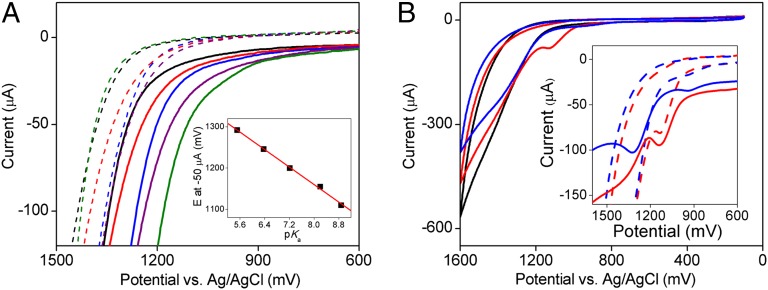
Fig. 3.
Effect of the buffer anion on WOC onset potential and comparison of several cationic cobalt porphyrin WOC catalysts. (A) CVs of 1 mM Co-TDMImP in phthalate (black), bicarbonate (red), Na–Pi (blue), n-butylphosphonate (purple), and tert-butylphosphonate (green) buffers. The solid and dashed lines represent forward and backward scans, respectively. (A, Inset) Plot of the potential measured at –50 μA as a function of pKa of the buffer species. The red line represents the best linear fit with a slope of –54 mV.pKa–1. (B) CVs of 1 mM Co-TDMImP (black), Co-TM4PyP (red), and Co-TTMAP (blue). (B, Inset) Amplified CVs (dashed lines) and SWVs (solid lines) of Co-TM4PyP (red) and Co-TTMAP (blue) showing the 600–1,400 mV range. Other conditions include room temperature, scan rate 100 mV⋅s−1, and pH 7.
No redox processes associated with the oxidation of CoIII or the porphyrin were observed by either CV or square wave voltammetry (SWV) for Co-TDMImP, presumably because any species more oxidized than CoIII are short lived. To gain more mechanistic insight, we compared the behavior of Co-TDMImP with two other water-soluble cationic Co-porphyrins, Co–5,10,15,20-tetrakis-(N-methylpyridinium-4-yl)porphyrin (TM4PyP) (32) and Co–5,10,15,20-tetrakis-(N,N,N-trimethylanilinium-4-yl)porphyrin (TTMAP) (Scheme 1). As shown in Fig. 3B, Co-TM4PyP and Co-TTMAP also catalyzed water oxidation under these conditions, but with diminished efficiency. The catalytic activity, as illustrated by the intensity of the catalytic current at a fixed potential (e.g., 1.50 V), decreased in the order of Co-TDMImP > Co-TM4PyP > Co-TTMAP. Accordingly, the reactive species generated in solutions of Co-TM4PyP and Co-TTMAP are apparently less potent oxidants of H2O than that of Co-TDMImP. Notably, anodic waves were observed at 1,130 mV and 920 mV in the CVs of Co-TM4PyP and Co-TTMAP, respectively (Fig. 3B, Inset).
To illuminate the redox properties of the porphyrin ring, we examined the electrochemical behavior of the corresponding GaIII-porphyrins. A single oxidative process was observed at 1,140 mV and 930 mV for GaIII-TM4PyP and GaIII-TTMAP, respectively, both by CV and SWV (SI Appendix, Fig. S15). For GaIII-TDMImP, a broad anodic feature was observed at ∼1,380 mV. These oxidative waves were insensitive to the pH of the solution. Bulk electrolytic oxidation of these GaIII porphyrins afforded a species with an absorption feature centered in a range of 650–660 nm, typical of a porphyrin radical cation (33, 34). Furthermore, the oxidation potential of GaIII-TM4PyP (1,140 mV) is similar to that of (O)FeIV-TM4PyP, which generates the known oxoiron porphyrin cation radical (34, 35).
An additional oxidative wave beyond the oxidation of the porphyrin was observed at 1,320 mV for Co-TTMAP by SWV (Fig. 3B, Inset). This feature was not elicited in the CV, as it lies under the catalytic current, indicating that this wave represents the generation of the reactive oxidant that mediates H2O oxidation catalysis. Moreover, similar to Co-TDMImP, a dependence of the onset potential on the pKa of the buffer base was also observed (SI Appendix, Fig. S16). The second oxidation of Co-TM4PyP was less resolved but still evident in the SWV (Fig. 3B, Inset), whereas this feature could not be observed for Co-TDMImP. Presumably, the oxidized species of the latter two Co-porphyrins are too reactive to accumulate.
Discussion
The results show that the electron-deficient cobalt porphyrin, Co-TDMImP, is an efficient catalyst for the electrochemical oxidation of water to oxygen. The onset potential of ∼1.2 V (Fig. 1A) compares favorably with values reported for other cobalt-based catalysts, whereas the catalytic current appears to be significantly greater than those of other systems (17, 25). The observed O2 formation rate, 170 nmol⋅cm−2⋅min−1, is much higher than that of a recently described iridium-based homogeneous catalyst (36), and the 85–90% faradaic yield for oxygen production indicates nearly exclusive 4-e− oxidation of H2O (SI Appendix, Fig. S6).
Several lines of evidence show that this is clearly a homogeneous system. In particular, the stability of the resting CoIII complex over hours of measurement and the lack of any effects on catalytic efficiency by either cobalt ion sequestration with EDTA or a three-phase test with Chelex resin show that free cobalt ions are not contributing to the observed WOC activity. This conclusion is further confirmed by the lack of any sign of heterogeneous surface films on the electrodes. Moreover, indications from the scan-rate dependence that O–O bond formation is rate limiting and the strictly linear dependence of catalytic current on catalyst concentration argue that Co-TDMImP is a single-site, molecular catalyst.
The titration of two acidic protons in CoIII(OH2)2-TDMImP (pKa = 5.1 and 9.4; SI Appendix, Fig. S1) and the measured CoIII/II potential at 250 mV (ΔE = 100 mV) vs. Ag/AgCl reference (SI Appendix, Figs. S5 and S17) indicate that the resting state of the Co-TDMImP catalyst at pH 7 is H2O–CoIII–OH. The CoIII/II redox potential (250 mV; SI Appendix, Fig. S17) of Co-TDMImP is higher than that of Co-TM4PyP and Co-TTMAP; however, it is still too low for the exchange labile CoII state to be involved in catalysis. This conclusion could explain why Co-TDMImP does not lose cobalt ions during catalysis.
The cyclic voltammetry and square wave electrochemical results for the family of cobalt and gallium porphyrins studied indicate that porphyrin ring oxidation was occurring at potentials at or below the onset potential for water oxidation. This behavior was most clearly evident for GaIII- and CoIII-TM4PyP, which showed ring oxidations at 1.14 and 1.13 V, respectively. Bulk spectroelectrochemical analysis of GaIII-TM4PyP showed a typical, broad, porphyrin radical cation absorbance in the visible spectrum ∼660 nm.
These experimental findings strongly indicate that the observed anodic features ∼1 V vs. Ag/AgCl represent ligand oxidations to the corresponding porphyrin radical cations. No other oxidation waves were observed for the GaIII porphyrins, indicating that the formation of porphyrin dications is not likely. More importantly, oxidation potentials of GaIII-TM4PyP and GaIII-TTMAP are almost identical to those observed for CoIII-TM4PyP and CoIII-TTMAP (Fig. 3B and SI Appendix, Fig. S15). Therefore, we conclude that the oxidative wave found in CVs of these Co-porphyrins also represents the oxidation of CoIII porphyrin to the CoIII porphyrin radical cation (+P-CoIII–OH). Because the observed ring oxidations occurred before the onset of the WOC catalytic current, the +P-CoIII–OH is not the reactive oxidant in this system. The second anodic oxidation, beyond that of the porphyrin ring, which was observed at 1,320 mV for Co-TTMAP by SWV (Fig. 3B, Inset), appeared to coincide with water oxidation. We suggest that this process represents a 1-e− oxidation of the +P-CoIII–OH coupled with the loss of one proton to the buffer anion to generate a CoIV–O porphyrin radical cation (+P-CoIV–O). Thus, the formally cobalt(V) species that is catalytically competent for WOC has accumulated two oxidation equivalents above CoIII. Subsequent, rate-limiting O–O bond formation at a single cobalt site is supported by the scan-rate dependence of the catalytic current and the linear dependence of the catalytic current on the catalyst concentration.
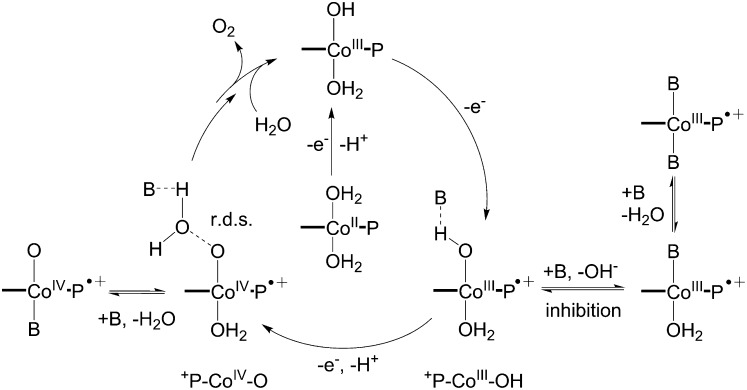
Considering the results above, we propose a mechanism for Co-porphyrin-mediated water oxidation as shown in Scheme 2. The generation of the reactive oxidant, +P-CoIV–O, requires the transfer of two electrons and one proton from the resting (aqua)CoIII–OH complex. The first oxidation, which is pH independent, would generate +P-CoIII–OH, which then must undergo a second oxidation at the cobalt center, with loss of a single proton. The key O–O bond formation step involves nucleophilic addition of H2O to +P-CoIV–O to generate a presumed Co-hydroperoxo or peroxo intermediate that is further oxidized to produce O2. Recently, a CoIV–O–containing unit was characterized in a catalytic H2O oxidation system mediated by a heterogeneous cobalt oxide catalyst (37). Likewise, a CoIV-corrole cation radical was proposed as the active species by Nocera and coworkers for the hangman systems (17). This hypothetical intermediate has been investigated by Lai et al. using density function theory (DFT) computational methods (38). This work indicated that O–O bond formation in an oxoCoIV-corrole cation radical was the most exothermic reaction with the lowest activation barrier (3.6 kcal/mol) among a number of intermediates studied. The data presented here provide experimental support for such a pathway in the cobalt-porphyrin platform. The single-site aspect of the WOC cycle in Scheme 2 and the critical role of porphyrin ring oxidation contrast to the cobalt-oxyl dimerization pathway were recently suggested by Sakai and coworkers for a photocatalytic cobalt-porphyrin water oxidation that used a peroxide as a sacrificial oxidant (21).
Scheme 2.
Proposed mechanism for water oxidation catalyzed by Co-porphyrins involving two one-electron oxidations of CoIII to +P-CoIII–OH and +P-CoIV–O. Roles for the buffer anion (B) include serving as a base to assist proton transfer and inhibition of the catalyst through coordination.
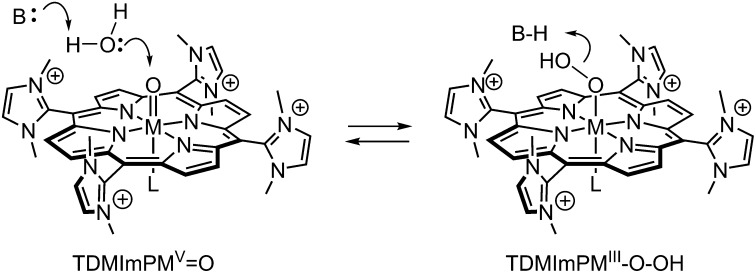
The nucleophilic addition of water to a doubly oxidized cobalt-oxo intermediate is formally the microscopic reverse of the conversion of the manganese analog HOO-MnIIITDMImP to trans-dioxoMnVTDMImP that we have recently described (Scheme 3) (39, 40). Here, both the hydroperoxo–manganese(III) complex and the oxo–manganese(V) complex were spectroscopically observed and the kinetics of the process could be analyzed in detail. Significantly, the activation enthalpy of the O–O bond cleavage event was determined to be only 4.2 kcal/mol despite the large-scale reorganization that seems to be required for such a transformation. Likewise, reaction of chloride ion with trans-dioxo-MnVTDMImP to form hypochlorite via an analogous O–Cl bond formation was shown to be fast and reversible (41, 42). Redox potential determinations and Nernst equation considerations showed that the potential of this MnV/MnIII porphyrin system was poised only 100–200 mV below the hydrogen peroxide/water couple. Thus, the high potentials of the electron-deficient TDMImP porphyrin ring oxidation and that expected for the CoIV/CoIII couple have apparently crossed that threshold.
Scheme 3.
Proposed pathways for O–O bond formation and cleavage catalyzed by cobalt and manganese metalloporphyrins bearing the electron withdrawing TDMImP ligand.
An important aspect of the current work is the prominent effect of the buffer anion on cobalt WOC. No water oxidation was observed in the absence of the buffer in this aqueous medium at constant ionic strength. For such a base-assisted process, the overall O–O bond formation rate (kcat) can be expressed by the sum of the rate in unbuffered solution (kwater) and the rate contributed by the addition of buffer base (kb[B]), as shown in Eq. 3 (31, 43). Thus, a plot of (icat/iwater)2 as a function of the buffer base concentration [B] should give a linear correlation with an intercept close to 1 and a slope equal to kb/kwater (Eq. 4). Indeed, this prediction was observed experimentally (Fig. 2B, red filled squares), indicating that one equivalent of buffer base is involved in the rate-limiting O–O bond formation step:
and
As shown in Fig. 2C, the (icat/iwater)2 value showed a linear dependence on the concentration of each buffer base. Notably, a Brønsted relationship could be discerned between log (kb/kwater) and the pKa of the buffer used (Fig. 2D). The slope of 0.38 is a further indication that there is a significant component of proton transfer from the catalyst to the buffer anion in the transition state for the rate-limiting O–O bond formation step (44). We were able to fit the observed biphasic behavior of WOC activity over the entire range of buffer concentrations studied for the four buffers used (SI Appendix, Fig. S18 and Table S1) by assuming that multiple equilibria are involved in the inhibition pathway (SI Appendix, Data Fitting for Details). Interactions of both the –OH ligand on +P–CoIII–OH and a H2O ligand with a buffer anion are necessary to get the best fit. The latter could occur at either the CoIII or CoIV porphyrin cation radical stage (Scheme 2).
The nature of the buffer also affected the onset potential of WOC. As shown in Fig. 3A, the onset potential at pH 7 decreased significantly in solutions containing more basic buffer anions; the lowest onset potential was found in the tert-butylphosphonate buffer, which has the highest pKa among the five buffers used. Importantly, a plot of the potential measured at a fixed current (–50 μA) as a function of pKa of the buffer used gave a linear correlation with a slope of –54 mV⋅pKa–1, indicating clearly that the generation of the reactive oxidant that effects WOC requires the removal of one proton from the precursor (43). In this PCET process (45), the electron transfer (ET) part appears to become more favorable, as illustrated by the negative shift of the onset potential, when the PT component is facilitated by the presence of a more basic buffer anion.
The data indicate that the buffer base must play multiple roles in the catalytic cycle (Scheme 2), including (i) acting as a proton acceptor in the conversion of the +P–CoIII–O–H intermediate to the doubly oxidized +P-CoIV–O precursor of WOC; (ii) acting as a base to deprotonate water coupled with the rate-limiting O–O bond formation step, consistent with a KIE (or EIE) value of 2.8 measured in H2O vs. in D2O; and (iii) inhibiting catalytic water oxidation activity at high buffer concentrations. Buffer anion catalysis of the O–O bond formation step and buffer inhibition has been reported for a homogeneous, single-site ruthenium water oxidation catalyst system developed by Meyer, et al. (31), and it has been postulated that the pendant carboxylate moiety of the cobalt hangman corrole acts as a general base to assist the O–O bond formation by mediating the transfer of a proton from a water molecule (17, 38).
In summary, water-soluble cationic Co-porphyrins are effective homogeneous water oxidation electrocatalysts that function at neutral pH and a modest overpotential. Co-TDMImP, which was the best catalyst, was robust during catalysis and was not a precursor of a heterogeneous cobalt oxide film. Mechanistic studies indicate that a high-valent Co-porphyrin, presumably a CoIV–O porphyrin radical cation, is the reactive oxidant responsible for attacking a H2O molecule to form the O–O bond. Accordingly, the catalysis proceeds through a CoV/III cycle that would guarantee high activity, while minimizing catalyst decomposition by avoiding the exchange labile CoII state. Co-TDMImP, the most electron-deficient complex having the highest redox potential, was the best catalyst among the three Co-porphyrins studied. Accordingly, the reactive oxidant has a higher oxidation potential than those of Co-TM4PyP and Co-TTMAP, providing a more thermodynamic driving force for water oxidation. The buffer base has been demonstrated to play multiple, critical roles during catalysis.
The dramatic effect of the buffer pKa on the WOC onset potential is worthy of special note. Significantly, water oxidation began near 1 V with the most basic phosphonate buffer anion. This observation is very informative and suggests that a WOC catalyst with properly positioned internal phosphonates could provide the necessary proton transfer catalysis without the inhibitory effects observed at high buffer concentrations. These considerations are informing our continuing studies of next-generation WOC catalysts using earth-abundant transition metals.
Supplementary Material
Acknowledgments
We thank the Princeton Institute for the Science and Technology of Materials and Dr. Shiyou Xu for the help of ESEM and EDX analyses. Support of this research by the National Science Foundation (CHE-1148597) is gratefully acknowledged. Electrocatalysis was supported by the Center for Catalytic Hydrocarbon Functionalization (CCHF), an Energy Frontier Research Center, US Department of Energy, Office of Science, Basic Energy Sciences, under Award DE-SC0001298.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315383110/-/DCSupplemental.
References
- 1.Barber J. Photosynthetic energy conversion: Natural and artificial. Chem Soc Rev. 2009;38(1):185–196. doi: 10.1039/b802262n. [DOI] [PubMed] [Google Scholar]
- 2.McEvoy JP, Brudvig GW. Water-splitting chemistry of photosystem II. Chem Rev. 2006;106(11):4455–4483. doi: 10.1021/cr0204294. [DOI] [PubMed] [Google Scholar]
- 3.Concepcion JJ, House RL, Papanikolas JM, Meyer TJ. Chemical approaches to artificial photosynthesis. Proc Natl Acad Sci USA. 2012;109(39):15560–15564. doi: 10.1073/pnas.1212254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faunce TA, et al. Energy and environment policy case for a global project on artificial photosynthesis. Energy Environ Sci. 2013;6(3):695–698. [Google Scholar]
- 5.Faunce T, et al. Artificial photosynthesis as a frontier technology for energy sustainability. Energy Environ Sci. 2013;6(4):1074–1076. [Google Scholar]
- 6.Dau H, et al. The mechanism of water oxidation: From electrolysis via homogeneous to biological catalysis. Chemcatchem. 2010;2(7):724–761. [Google Scholar]
- 7.Helm ML, Stewart MP, Bullock RM, DuBois MR, DuBois DL. A synthetic nickel electrocatalyst with a turnover frequency above 100,000 s⁻¹ for H₂ production. Science. 2011;333(6044):863–866. doi: 10.1126/science.1205864. [DOI] [PubMed] [Google Scholar]
- 8.Artero V, Fontecave M. Solar fuels generation and molecular systems: Is it homogeneous or heterogeneous catalysis? Chem Soc Rev. 2013;42(6):2338–2356. doi: 10.1039/c2cs35334b. [DOI] [PubMed] [Google Scholar]
- 9.Wasylenko DJ, Palmer RD, Berlinguette CP. Homogeneous water oxidation catalysts containing a single metal site. Chem Commun (Camb) 2013;49(3):218–227. doi: 10.1039/c2cc35632e. [DOI] [PubMed] [Google Scholar]
- 10.Sartorel A, Bonchio M, Campagna S, Scandola F. Tetrametallic molecular catalysts for photochemical water oxidation. Chem Soc Rev. 2013;42(6):2262–2280. doi: 10.1039/c2cs35287g. [DOI] [PubMed] [Google Scholar]
- 11.Hetterscheid DGH, Reek JNH. Mononuclear water oxidation catalysts. Angew Chem Int Ed Engl. 2012;51(39):9740–9747. doi: 10.1002/anie.201202948. [DOI] [PubMed] [Google Scholar]
- 12.Concepcion JJ, et al. Making oxygen with ruthenium complexes. Acc Chem Res. 2009;42(12):1954–1965. doi: 10.1021/ar9001526. [DOI] [PubMed] [Google Scholar]
- 13.Ellis WC, McDaniel ND, Bernhard S, Collins TJ. Fast water oxidation using iron. J Am Chem Soc. 2010;132(32):10990–10991. doi: 10.1021/ja104766z. [DOI] [PubMed] [Google Scholar]
- 14.Fillol JL, et al. Efficient water oxidation catalysts based on readily available iron coordination complexes. Nat Chem. 2011;3(10):807–813. doi: 10.1038/nchem.1140. [DOI] [PubMed] [Google Scholar]
- 15.Codolà Z, et al. Electronic effects on single-site iron catalysts for water oxidation. Chemistry. 2013;19(25):8042–8047. doi: 10.1002/chem.201301112. [DOI] [PubMed] [Google Scholar]
- 16.Abe T, et al. An organic photoelectrode working in the water phase: Visible-light-induced dioxygen evolution by a perylene derivative/cobalt phthalocyanine bilayer. Angew Chem Int Ed Engl. 2006;45(17):2778–2781. doi: 10.1002/anie.200504454. [DOI] [PubMed] [Google Scholar]
- 17.Dogutan DK, McGuire R, Jr, Nocera DG. Electocatalytic water oxidation by cobalt(III) hangman β-octafluoro corroles. J Am Chem Soc. 2011;133(24):9178–9180. doi: 10.1021/ja202138m. [DOI] [PubMed] [Google Scholar]
- 18.Leung C-F, et al. A cobalt(II) quaterpyridine complex as a visible light-driven catalyst for both water oxidation and reduction. Energy Environ Sci. 2012;5(7):7903–7907. [Google Scholar]
- 19.Wasylenko DJ, Ganesamoorthy C, Borau-Garcia J, Berlinguette CP. Electrochemical evidence for catalytic water oxidation mediated by a high-valent cobalt complex. Chem Commun (Camb) 2011;47(14):4249–4251. doi: 10.1039/c0cc05522k. [DOI] [PubMed] [Google Scholar]
- 20.Rigsby ML, et al. Cobalt analogs of Ru-based water oxidation catalysts: Overcoming thermodynamic instability and kinetic lability to achieve electrocatalytic O2 evolution. Chem Sci. 2012;3(10):3058–3062. [Google Scholar]
- 21.Nakazono T, Parent AR, Sakai K. Cobalt porphyrins as homogeneous catalysts for water oxidation. Chem Commun (Camb) 2013;49(56):6325–6327. doi: 10.1039/c3cc43031f. [DOI] [PubMed] [Google Scholar]
- 22.Crabtree RH. Resolving heterogeneity problems and impurity artifacts in operationally homogeneous transition metal catalysts. Chem Rev. 2012;112(3):1536–1554. doi: 10.1021/cr2002905. [DOI] [PubMed] [Google Scholar]
- 23.Widegren JA, Finke RG. A review of the problem of distinguishing true homogeneous catalysis from soluble or other metal-particle heterogeneous catalysis under reducing conditions. J Mol Catal A. 2003;198(1-2):317–341. [Google Scholar]
- 24.Stracke JJ, Finke RG. Electrocatalytic water oxidation beginning with the cobalt polyoxometalate [Co4(H2O)2(PW9O34)2]10-: Identification of heterogeneous CoOx as the dominant catalyst. J Am Chem Soc. 2011;133(38):14872–14875. doi: 10.1021/ja205569j. [DOI] [PubMed] [Google Scholar]
- 25.Wasylenko DJ, Palmer RD, Schott E, Berlinguette CP. Interrogation of electrocatalytic water oxidation mediated by a cobalt complex. Chem Commun (Camb) 2012;48(15):2107–2109. doi: 10.1039/c2cc16674g. [DOI] [PubMed] [Google Scholar]
- 26.Natali M, et al. Is [Co4(H2O)2(α-PW9O34)2](10-) a genuine molecular catalyst in photochemical water oxidation? Answers from time-resolved hole scavenging experiments. Chem Commun (Camb) 2012;48(70):8808–8810. doi: 10.1039/c2cc34804g. [DOI] [PubMed] [Google Scholar]
- 27.Hong D, et al. Water-soluble mononuclear cobalt complexes with organic ligands acting as precatalysts for efficient photocatalytic water oxidation. Energy Environ Sci. 2012;5(6):7606–7616. [Google Scholar]
- 28.Artero V, Chavarot-Kerlidou M, Fontecave M. Splitting water with cobalt. Angew Chem Int Ed Engl. 2011;50(32):7238–7266. doi: 10.1002/anie.201007987. [DOI] [PubMed] [Google Scholar]
- 29.Tjahjono DH, Akutsu T, Yoshioka N, Inoue H. Cationic porphyrins bearing diazolium rings: Synthesis and their interaction with calf thymus DNA. Biochim Biophys Acta. 1999;1472(1-2):333–343. doi: 10.1016/s0304-4165(99)00139-7. [DOI] [PubMed] [Google Scholar]
- 30.McAlpin JG, et al. EPR evidence for Co(IV) species produced during water oxidation at neutral pH. J Am Chem Soc. 2010;132(20):6882–6883. doi: 10.1021/ja1013344. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, et al. Concerted O atom-proton transfer in the O-O bond forming step in water oxidation. Proc Natl Acad Sci USA. 2010;107(16):7225–7229. doi: 10.1073/pnas.1001132107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kellett RM, Spiro TG. Cobalt (I) porphyrin catalysis of hydrogen production from water. Inorg Chem. 1985;24(15):2373–2377. [Google Scholar]
- 33.Groves JT, Haushalter RC, Nakamura M, Nemo TE, Evans BJ. High-valent iron-porphyrin complexes related to peroxidase and cytochrome P450. J Am Chem Soc. 1981;103(10):2884–2886. [Google Scholar]
- 34.Bell SR, Groves JT. A highly reactive p450 model compound I. J Am Chem Soc. 2009;131(28):9640–9641. doi: 10.1021/ja903394s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lei J, Ju H, Ikeda O. Catalytic oxidation of nitric oxide and nitrite mediated by water-soluble high-valent iron porphyrins at an ITO electrode. J Electroanal Chem. 2004;567(2):331–338. [Google Scholar]
- 36.Schley ND, et al. Distinguishing homogeneous from heterogeneous catalysis in electrode-driven water oxidation with molecular iridium complexes. J Am Chem Soc. 2011;133(27):10473–10481. doi: 10.1021/ja2004522. [DOI] [PubMed] [Google Scholar]
- 37.Surendranath Y, Kanan MW, Nocera DG. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J Am Chem Soc. 2010;132(46):16501–16509. doi: 10.1021/ja106102b. [DOI] [PubMed] [Google Scholar]
- 38.Lai WZ, et al. Why is cobalt the best transition metal in transition-metal hangman corroles for O-O bond formation during water oxidation? J Phys Chem Lett. 2012;3(17):2315–2319. doi: 10.1021/jz3008535. [DOI] [PubMed] [Google Scholar]
- 39.Jin N, Lahaye DE, Groves JT. A “push-pull” mechanism for heterolytic O-O bond cleavage in hydroperoxo manganese porphyrins. Inorg Chem. 2010;49(24):11516–11524. doi: 10.1021/ic1015274. [DOI] [PubMed] [Google Scholar]
- 40.Lahaye D, Groves JT. Modeling the haloperoxidases: Reversible oxygen atom transfer between bromide ion and an oxo-Mn(V) porphyrin. J Inorg Biochem. 2007;101(11-12):1786–1797. doi: 10.1016/j.jinorgbio.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Umile TP, Groves JT. Catalytic generation of chlorine dioxide from chlorite using a water-soluble manganese porphyrin. Angew Chem Int Ed Engl. 2011;50(3):695–698. doi: 10.1002/anie.201004482. [DOI] [PubMed] [Google Scholar]
- 42.Umile TP, Wang D, Groves JT. Dissection of the mechanism of manganese porphyrin-catalyzed chlorine dioxide generation. Inorg Chem. 2011;50(20):10353–10362. doi: 10.1021/ic201430v. [DOI] [PubMed] [Google Scholar]
- 43.Bard AJ, Faulkner LR. Electrode reactions with coupled homogeneous chemical reactions. In: Harris D, editor. Electrochemical Methods: Fundamentals and Applications. Hoboken, NJ: Wiley; 2001. pp. 471–534. [Google Scholar]
- 44.Ansyln EV, Dougherty DA. Modern Physical Organic Chemistry. University Science Books, Sausalito, CA; 2006. [Google Scholar]
- 45.Weinberg DR, et al. Proton-coupled electron transfer. Chem Rev. 2012;112(7):4016–4093. doi: 10.1021/cr200177j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.