Abstract
Objectives
To test the hypothesis that right bundle branch block (RBBB) patients have larger scar size than left bundle branch block (LBBB) patients.
Background
A proximal septal perforating branch of the left anterior descending (LAD) coronary artery most commonly perfuses the right bundle branch and left anterior fascicle, but not the left posterior fascicle. Thus, proximal LAD occlusions should cause RBBB, not LBBB.
Methods
We performed electrocardiograms and magnetic resonance imaging for scar quantification in 233 patients with left ventricular ejection fraction (LVEF) ≤35% receiving primary prevention implantable defibrillators (ICD cohort). Scar size and location were compared among RBBB, LBBB, non-specific LV conduction delay (LVCD) and QRS <120 ms patients. A second cohort of 20 hypertrophic cardiomyopathy patients undergoing alcohol septal ablation was studied to determine whether controlled infarction in a proximal LAD septal perforator caused RBBB or LBBB.
Results
In the ICD cohort, LVEF was similar between RBBB and LBBB patients (24.9% vs. 25.0%, p=0.98), however RBBB patients had significantly larger scar size (24.0% vs. 6.5%, p<0.0001). Patients with LVCD or QRS <120 ms had intermediate scar size (12.9% vs.14.4%). Those with RBBB (compared to LBBB) were more likely to have ischemic heart disease (79% vs. 29%, p<0.0001). In the alcohol septal ablation cohort, 15/20 patients (75%) developed RBBB, but no patients developed LBBB.
Conclusions
In patients with LVEF<35%, RBBB is associated with significantly greater scar size than LBBB and occlusion of a proximal LAD septal perforator causes RBBB. In contrast, LBBB is most commonly caused by nonischemic pathologies.
Keywords: Right bundle branch block, left bundle branch block, myocardial infarction, ischemic heart disease, nonischemic cardiomyopathy
Introduction
A conventional clinical concept is that new onset left or right bundle branch block (LBBB or RBBB) that occurs with acute myocardial infarction (MI) is associated with massive MIs (1-3). However, the relation between coronary artery and bundle branch anatomy suggests that very different relationships should exist between MI location and size with bundle branch block type. A prior necropsy study demonstrated that the proximal left anterior descending coronary artery (LAD) septal perforators perfuse the right bundle branch and the anterior fascicle of the left bundle branch in 90% of cases, while the right coronary artery (via the AV-nodal artery) perfuses the posterior fascicle of the left bundle branch in 90% of cases (4). There is dual blood supply to each of these fascicles in 40-50% of cases (4). This means that proximal LAD occlusions could cause RBBB and/or left anterior fascicular block. However, both proximal LAD and right coronary artery occlusions would be required typically for MIs to cause LBBB. Instead, as originally described by Lenègre (5), histopathology studies demonstrated that the disruption of the left bundle branch almost always occurs at its junction with the main bundle, often with histological findings of fibrosis or sclerosis with occasional calcification (5-7). Furthermore, at this location, the left bundle branch can be compressed between connective tissue of the central fibrous body and the base of the ventricular septum (5-7), especially when subjected to mechanical strain from a hypertrophied or dilated left ventricle (LV).
In chronic cardiomyopathy, prolonged QRS duration is associated with increased mortality (8), however, the cause of the association likely differs depending on the conduction type. In LBBB, the large delay between activation of the interventricular septum and LV free wall leads to dyssynchronous and inefficient LV contraction (9). In RBBB, activation of the LV is normal; however, increased mortality may be due to the association of RBBB and large anteroseptal MIs that portend poor prognosis.
Cardiac magnetic resonance (CMR) with late gadolinium enhancement (LGE) is the gold standard for identifying the location of and quantifying the amount of myocardial scar caused by prior MI or nonischemic causes of cardiomyopathy (10). In a cohort of chronic ischemic and nonischemic cardiomyopathy patients, we used CMR to test the hypothesis that RBBB patients have a larger scar burden than LBBB patients due to a higher prevalence of proximal LAD MIs and less frequent nonischemic pathologies. As a proof-of-concept group, we also studied the ECG characteristics of a cohort of patients with the obstructive form of hypertrophic cardiomyopathy who underwent percutaneous alcohol septal ablation of the proximal septal perforator and could thus serve as a controlled model of proximal anteroseptal infarction.
Methods
ICD Cohort Population
Patients referred clinically to Johns Hopkins Medical Institutions for ICD placement for primary prevention of sudden cardiac death were prospectively enrolled between November, 2003 and December, 2010 (11). The population has been described previously (11-14). All patients had to have left ventricular ejection fraction ≤35%, coronary angiography, no other indications for ICD placement, and no contraindications to CMR. Patients were classified as “nonischemic” if they had no history of MI or revascularization and no evidence of coronary artery stenoses >50% of two or more epicardial vessels or left main or proximal left anterior descending coronary artery stenosis >50%. Other patients were classified as “ischemic”. All MIs had occurred >1 month prior to enrollment. This study protocol was approved by the Johns Hopkins Institutional Review Board. All patients gave written informed consent.
Hypertrophic Cardiomyopathy Alcohol Septal Ablation Cohort
Patients with hypertrophic cardiomyopathy referred clinically to Johns Hopkins Medical Institutions for percutaneous alcohol septal ablation were enrolled between December, 2000 and November, 2005 as part of a single-center prospective cohort of CMR before and after septal ablation. This study protocol was approved by the Johns Hopkins Institutional Review Board. All patients gave written informed consent.
CMR Acquisition and Analysis in ICD Cohort
Patients underwent cine and CMR-LGE imaging using a 1.5-Tesla scanner (GE Signa CV/I or Siemens Avanto). Image analysis was performed using custom research software CINEtool (GE Healthcare, WI, USA). Cine images were used to measure ejection fraction and volumes and LGE images were used to measure total scar size for the entire LV. The LGE area was outlined and pixels with signal intensity >50% of the maximum within the LGE area were labeled as scar “core” (12,14). A region of normal myocardium without artifacts was then selected and the peak signal intensity within the normal myocardium was determined. Myocardium with signal intensity >peak remote signal intensity but <50% of maximal signal intensity within the LGE region was labeled “gray” zone to represent the heterogeneous peri-scar zone (12,14). Total scar size was expressed as core +1/2 gray zone as a percent of total LV mass (14).
Scar location was determined using the American Heart Association 17-segment model of the LV (15). Patients with ischemic cardiomyopathy were grouped into those having infarct with an LAD, RCA and/or left circumflex infarction pattern (15). Patients with non-ischemic cardiomyopathy were grouped into 6 patterns: no scar present, scar confined to the mid-wall myocardium, scar confined to the epicardium, scar confined to the endocardium, transmural scar and scar at the right ventricular insertion points based on previously described patterns (16). Scar transmural involvement in each of the 17 segments was graded on a 0-4 point scale as described previously (0 for 0% hyperenhancement, 1 for 1-25% hyperenhancement, 2 for 26-50% hyperenhancement, 3 for 51-75% hyperenhancement and 4 for 76-100% hyperenhancement) (17).
ECG Analysis for Both Cohorts
ECGs at the time of CMR were analyzed according to previously specified criteria (14,18,19):
LBBB – QRS duration ≥140 ms (men) or ≥130 ms (women), QS or rS in V1 and V2 with mid QRS notching/slurring in two of the leads I, aVL, V1, V2, V5 or V6;
RBBB – QRS duration ≥120 ms with rR’ or qR in V1 and a wide S wave in lead I (patients with left axis deviation (−180 to −45 degrees) were classified as left anterior fascicular block + RBBB;
Non-specific LV Conduction Delay (LVCD) – QRS duration ≥120 ms and QS or rS in V1, but not meeting LBBB or RBBB criteria; and
QRS duration <120 ms.
Statistical Analysis
Variables following Gaussian distributions were compared by ECG conduction type with parametric measures (two-sample t-test, one-way ANOVA) while those not following Gaussian distributions were analyzed non-parametrically (Wilcoxon rank sum, non-parametric one-way ANOVA). Categorical variables were evaluated by Chi-square. Analysis was performed separately comparing RBBB vs. LBBB patients and comparing all four ECG conduction types. P-values <0.05 were considered significant.
Results
Of the 235 primary prevention ICD patients available for analysis, two were excluded because only ventricular paced ECGs were available. The remaining 233 had a mean age of 57 years, were 77% male, had a mean LV ejection fraction of 27%, had 56% ischemic etiology and had a distribution of New York Heart Association heart failure classes of: 25% Class I, 41% Class II and 34% Class III. There were no differences in age, gender, ethnicity, heart failure class or LV ejection fraction between RBBB and LBBB patients (Table 1). However, there were significant differences in scar size, anatomic scar location and prevalence of ischemic vs. nonischemic cardiomyopathy. In addition, there was a trend toward larger LV end diastolic volume in LBBB patients.
Table 1. Baseline Characteristics of ICD Cohort.
|
RBBB
(n=19) |
LBBB
(n=45) |
LVCD
(n=35) |
QRSd<120
(n=134) |
P-Value
(RBBB vs. LBBB) |
|
|---|---|---|---|---|---|
| Age (yrs) | 59.1 ± 7.8 | 59.7 ± 11.4 | 64.2 ± 12.6 | 54.6 ± 12.2 | 0.85 |
| Female | 14 (74%) | 29 (64%) | 27 (77%) | 109 (81%) | 0.48 |
| LVEF (%) | 24.9 ± 8.7 | 25.0 ± 9 | 26.1 ± 8.9 | 28.3 ± 8.9 | 0.98 |
| Ischemic | 15 (79%) | 13 (29%) | 25 (71%) | 78 (58%) | <0.0001 * |
| QRS Duration (ms) | 150.3 ± 16.8 | 162.2 ± 20.3 | 130.7 ± 14.1 | 98.3 ± 10.7 | 0.028 * |
| Ethnicity | 0.27 | ||||
| Caucasian | 15 (79%) | 34 (76%) | 27 (77%) | 86 (64%) | |
| African American | 3 (16%) | 10 (22%) | 8 (23%) | 42 (31%) | |
| Other | 1 (5%) | 0 (0%) | 0 (0%) | 5 (4%) | |
| NYHA Class | 0.095* | ||||
| I | 4 (21%) | 2 (4%) | 9 (26%) | 43 (32%) | |
| II | 7 (37%) | 16 (36%) | 4 (11%) | 68 (51%) | |
| III | 8 (42%) | 27 (60%) | 22 (63%) | 23 (17%) | |
| LVEDV/BSA (m1/m2) | 114.9 ± 34.4 | 137.2 ± 50.0 | 131.4 ± 43.1 | 115.6 ± 36.3 | 0.081* |
| LVESV/BSA (m1/m2) | 89.0 ± 37.3 | 105.9 ± 51.7 | 98.8 ± 40.5 | 84.8 ± 34.6 | 0.20* |
| LV Mass/BSA (m1/m2) | 73.5 ± 22.5 | 82.1 ± 32.6 | 78.9 ± 27 | 70.2 ± 20.8 | 0.30* |
indicates p<0.05 for groupwise comparison among 4 groups
Right vs. Left Bundle Branch Block
Figure 1 shows the CMR-LGE image of a RBBB patient with ischemic cardiomyopathy and a large anteroseptal scar involving 45% of the LV, while Figure 2 shows the CMR-LGE of a LBBB patient with nonischemic cardiomyopathy and no scar. As a group, the mean scar size in RBBB patients was significantly greater than in LBBB patients (24.0 vs. 6.5%, p<0.0001), while patients with nonspecific LVCD and QRS duration <120 ms had intermediate scar size (12.9 and 14.4%, respectively) (Table 2). Of note, while analysis in this study was performed with the pre-specified strict LBBB criteria (14,18,20), defining LBBB with conventional LBBB criteria (QRS ≥120 ms, with QS or rS V1) still resulted in a large difference in scar size between LBBB (9.3±10.2% LV) and RBBB (24.0±15.7% LV) patients (p<0.0001).
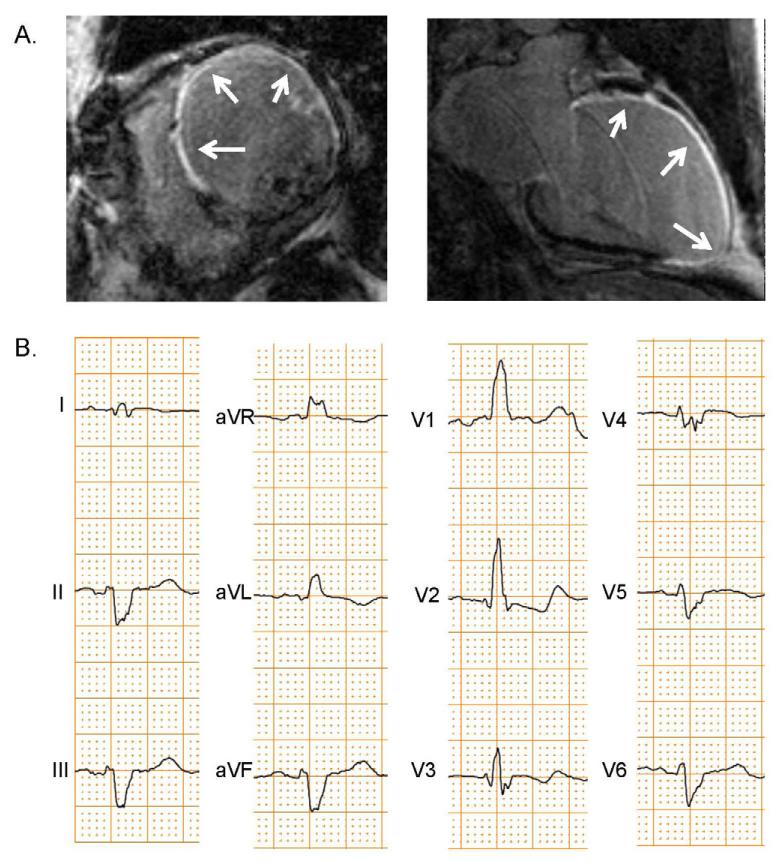
Figure 1. CMR-LGE and ECG of RBBB Patient with Ischemic Cardiomyopathy and Large Anteroseptal Scar.
This patient had extensive scarring (arrows) by CMR-LGE (A) from a prior proximal LAD occlusion. The patient’s ECG (B) showed a RBBB with left anterior fascicular block.
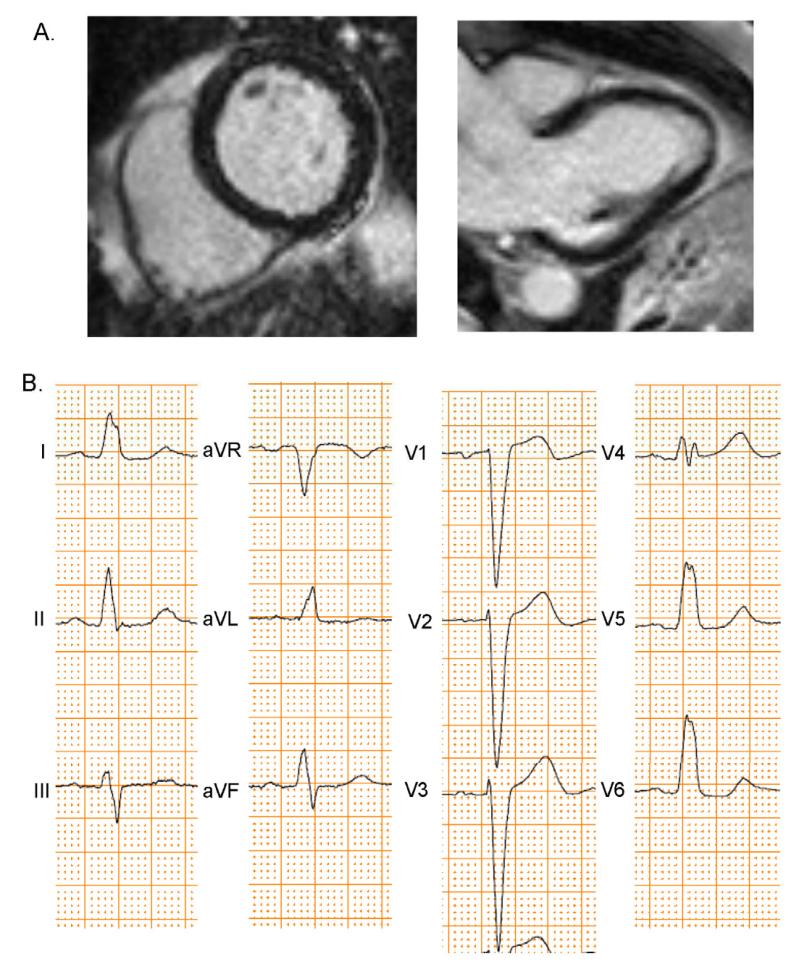
Figure 2. CMR-LGE and ECG of LBBB Patient.
This patient had nonischemic cardiomyopathy and no scar present by CMR-LGE (A). The patient’s ECG (B) showed LBBB.
Table 2. CMR-LGE Scar Size of ICD Cohort.
| RBBB | LBBB | LVCD | QRSd<120 | P-Value (RBBB vs. LBBB) |
|
|---|---|---|---|---|---|
| All | N=19 | N=45 | N=35 | N=134 | |
| Scar Size (% LV) | 24.0 ± 15.1 | 6.5 ± 9.4 | 12.9 ± 10 | 14.4 ± 13.7 | <0.0001 * |
| Scar Core (%LV) | 17.8 ± 11.1 | 4.3 ± 6.1 | 9.4 ± 7.1 | 10.6 ± 10.4 | <0.0001 * |
| Scar Gray (%LV) | 12.3 ± 9.2 | 4.2 ± 7.0 | 7 ± 6.4 | 7.6 ± 7.9 | <0.0001 * |
| Ischemic (N) | N=15 | N=13 | N=25 | N=77 | |
| Scar Size (% LV) | 28.5 ± 13.7 | 14.7 ± 9.7 | 17.4 ± 8.2 | 22.5 ± 11 | 0.006 * |
| Scar Core (%LV) | 21.0 ± 10.2 | 10.0 ± 6.2 | 12.6 ± 5.7 | 16.7 ± 8.8 | 0.002 * |
| Scar Gray (%LV) | 14.7 ± 8.6 | 9.0 ± 7.3 | 9.5 ± 6.0 | 11.6 ± 7.1 | 0.072 |
| Nonischemic (N) | N=4 | N=32 | N=10 | N=57 | |
| Scar Size (% LV) | 7.3 ± 4.7 | 3.2 ± 7.1 | 1.8 ± 1.9 | 3.4 ± 8.1 | 0.27 |
| Scar Core (%LV) | 5.7 ± 3.4 | 2.0 ± 4.3 | 1.4 ± 1.6 | 2.3 ± 5.4 | 0.110 |
| Scar Gray (%LV) | 3.1 ± 4.5 | 2.3 ± 6.0 | 0.8 ± 0.8 | 2.2 ± 5.4 | 0.80 |
indicates p<0.05 for groupwise comparison among 4 groups
The difference in scar size was partially explained by a higher prevalence of ischemic cardiomyopathy in RBBB compared to LBBB patients (79% vs. 29%, p<0.0001). However, among ischemic cardiomyopathy patients, a larger scar size persisted amongst those with RBBB vs. LBBB (28.5% vs. 14.7%, p=0.006). The scar distribution in each of the 17 segments is shown in polar plots in Figure 3. The right bundle branch runs approximately through segment 8 (mid anteroseptal wall), which was the segment with the highest transmural scar grade amongst the RBBB patients, and was significantly higher than the mid anteroseptal scar grade (measure of scar transmural involvement) in LBBB patients (2.47 vs. 0.89, p=0.001).
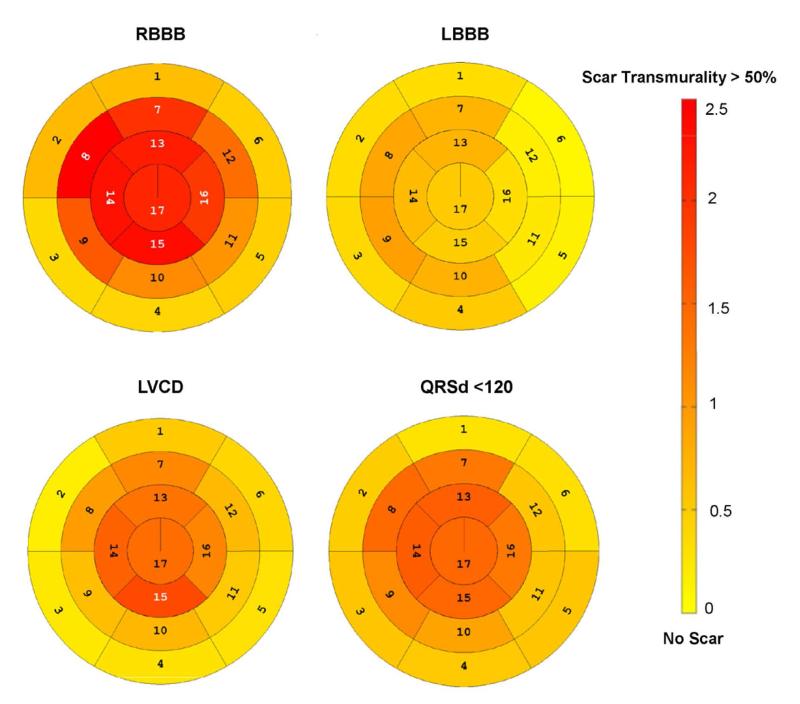
Figure 3. Segmental Scar Distribution in the ICD Cohort.
Polar plots show the average transmural scar extent within each of the 17 AHA defined myocardial segments graded on a 0-4 point scale (0 for 0% hyperenhancement, 1 for 1-25% hyperenhancement, 2 for 26-50% hyperenhancement, 3 for 51-75% hyperenhancement and 4 for 76-100% hyperenhancement) (17). The figure is scaled from no scar (light yellow) to a transmural scar grade of 2.5 (dark red). Note that the right bundle branch runs through segment 8 (mid anteroseptal), which had the highest scar grade of any segment in RBBB patients.
When considering only ischemic patients, 11 of 15 (73%) RBBB patients had scar consistent with LAD occlusions (two of whom also had scar in a second territory) and the average scar size was 34.8% of the LV (range 19%-50% LV) for LAD-only scar. Of the 4 other ischemic RBBB patients, 2 were RCA-territory scar, 1 was a left circumflex scar and 1 was not a typical ischemic pattern (scar near right ventricular insertion points). In contrast, among the 13 ischemic LBBB patients there were 3 with scar patterns consistent with LAD+RCA, 6 LAD-only, 2 RCA only and 2 left circumflex-only. Prior necropsy studies have suggested that both LAD and RCA infarcts would usually be required for MI to be the sole cause of a LBBB, and it is unlikely for an LAD-only MI to cause a LBBB. Among the 6 LAD-only LBBB patients, only 1 of them had a large scar (35% LV), while the remainder had scar sizes not more than 16% of the LV (range 5% to 16%). This suggests that in at least 5 of these 6 cases, the LAD scar was not likely the direct cause of the LBBB, but rather the LBBB developed due to stress and strain on the left bundle fibers associated with LV dilatation and progressive cardiomyopathy. Indeed, there was a trend toward larger LV end diastolic volume (corrected for body surface area) in LBBB vs. RBBB patients overall (137.2 vs. 114.9 ml/m2, p=0.081).
Among nonischemic patients, 0 of 4 RBBB patients had no scar, while 20 of 32 (63%) LBBB patients had no scar (p=0.017 for comparison). There was no significant difference in the scar patterns (mid-wall, epicardial, endocardial, transmural vs. right ventricular insertion) in RBBB vs. LBBB patients.
Alcohol Septal Ablation
QRS duration was normal in all hypertrophic cardiomyopathy patients prior to the septal ablation. Subsequent to the alcohol septal ablation of a proximal LAD septal perforator, 15 of 20 (75%) developed RBBB, while no patients developed LBBB. Figure 4 shows the ECGs of a patient before and after alcohol septal ablation that developed RBBB. The patients’ CMR-LGE image shows a focal area of LGE in the basal septum representing the area of necrosis causing RBBB.
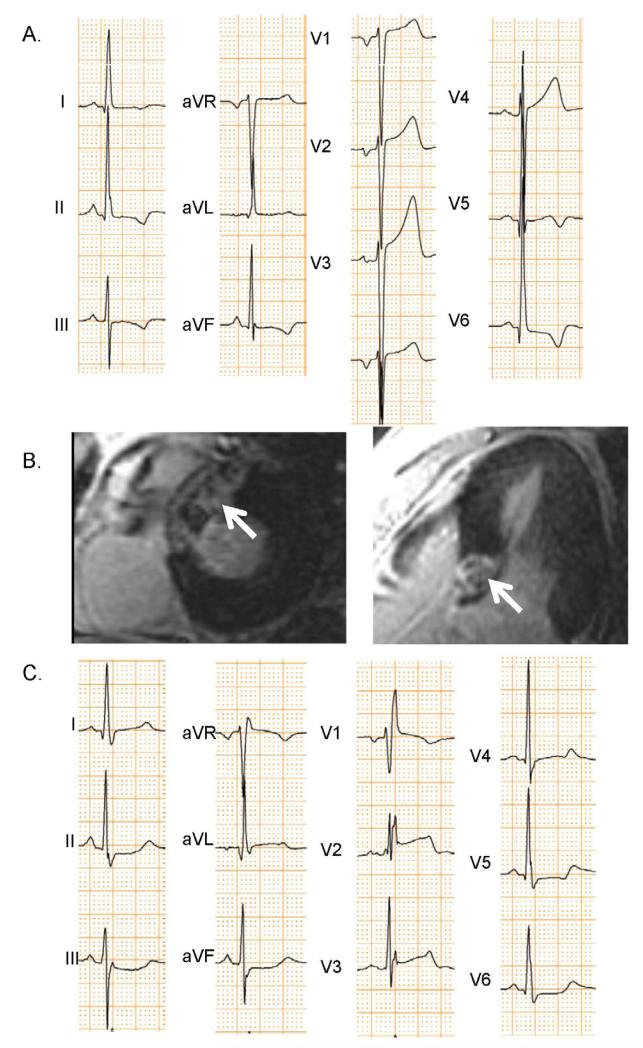
Figure 4. ECGs and CMR-LGE of Patient Developing RBBB after Alcohol Septal Ablation.
This hypertrophic cardiomyopathy patient had a baseline ECG (A) showing left ventricular hypertrophy, but not bundle branch block. Alcohol septal ablation was performed in a proximal LAD septal perforator leading to necrosis highlighted by arrows in the CMR-LGE image (B). Post septal ablation ECGs (C) showed that RBBB developed.
Among those who developed RBBB, 3 of 15 also developed left anterior fascicular block. QRS duration increased by 42±11 ms among those developing RBBB (p<0.0001 compared to baseline), while there was no significant change in QRS duration among those that did not develop RBBB (p=0.24). No patients developed left anterior fascicular block without RBBB.
Discussion
Our study supports the premise that RBBB has a strong association with large anteroseptal scar in cardiomyopathy patients, and occlusion of a proximal LAD septal perforator causes RBBB. In contrast, the majority of LBBB patients have nonischemic cardiomyopathies and, even amongst those with ischemic cardiomyopathy, LBBB patients have significantly lower overall scar burden than RBBB, while LVCD and QRS duration patients have intermediate scar sizes. These results oppose the conventional clinical concept that LBBB is caused by massive septal infarction. Instead, LBBB is more likely caused by a combination of sclerosis and fibrosis combined with mechanical strain on the left bundle fibers near the left bundle junction with the main bundle, where the conduction fibers are sandwiched between the connective tissue of the central fibrous body and base of the ventricular septum (5-7). However, it is possible that these observations are an artifact of survival bias. The relationship between proximal LAD occlusions and RBBB (but not LBBB) was further confirmed by our cohort with hypertrophic cardiomyopathy in whom alcohol ablation of a proximal LAD septal perforator led to RBBB in 75% of patients, but not to LBBB. This evidence that LAD occlusions cause RBBB, but not LBBB, has important potential clinical implications for primary prevention ICD and CRT patients, such as those in this study, but also potentially for acute MI patients.
CRT studies performed in the past decade were limited by the lack of distinction between ventricular block types. CRT trials enrolled patients with QRS duration ≥120 ms (without distinction between conduction types) and showed that CRT reduced heart failure symptoms, heart failure hospitalization and mortality (21-26). However, more recent analysis has suggested that the benefit from CRT is driven by LBBB patients (26-28), especially in the Multicenter Automated Defibrillator Implantation Trial that enrolled New York Heart Association Class I and II patients (27). In that trial, neither RBBB patients nor nonspecific LV conduction delay patients benefited from CRT. Furthermore, large outcomes studies of Medicare patients demonstrated that, among CRT recipients, mortality is highest with RBBB, intermediate with LVCD and lowest with LBBB (29,30). CRT likely benefits LBBB patients most because RBBB and LVCD patients have normal Purkinje activation of the LV, but poor prognosis with RBBB may also be due to RBBB association with large scar burden, as demonstrated in the present study.
Our results have potential implications for acute MI patients, as well. Prior studies have described the association between new onset LBBB and occlusion of the proximal LAD artery and large MI size (2). However, this was based on remote studies using Q waves in V1-V3 to determine anteroseptal MI location and creatine kinase measured infarct size (1). Recent studies using CMR-LGE have demonstrated that in LBBB, large R waves (not Q waves) in leads V1-V3 represent anteroseptal MI (14,18). As discussed previously, postmortem studies support the observation that proximal LAD septal perforators most commonly perfuse the right bundle branch and the anterior half of left bundle branch, while the RCA (via the AV-nodal artery) most commonly perfuses the posterior half of the left bundle branch (4). Thus, proximal LAD occlusions can cause RBBB and/or left anterior fascicular block, however, both proximal LAD and RCA occlusions would typically be required for infarcts to be the cause of LBBB. In addition, some patients diagnosed with LBBB by conventional ECG criteria do not have activation consistent with LBBB and the recently proposed strict LBBB criteria (14,18,20) used in the present should be assessed in future studies in patients presenting with symptoms of acute coronary syndrome.
Our data from the hypertrophic cardiomyopathy patients undergoing septal ablation of a proximal LAD septal perforator support this concept as 15 of 20 patients developed RBBB (with 3 developing left anterior fascicular block in addition to RBBB), while no patients developed LBBB. This is consistent with prior studies (31,32). Qin et al. reported that 62% of patients developed RBBB after septal ablation, while 6% developed LBBB (31). It is possible that the limited number of patients supposedly developing LBBB did not actually develop LBBB, but rather left anterior fascicular block that caused the patients to meet conventional ECG criteria for LBBB, but they would not have met strict criteria for LBBB used in this study (20).
Limitations
All patients enrolled in the ICD cohort portion of this study had ejection fraction ≤35% and met criteria for primary prevention ICDs. Thus, the results cannot necessarily be extrapolated outside of this population. While we comment on how our findings might translate to patients presenting with bundle branch block and acute MI, this should be interpreted with caution and requires further study. Although the sub-group comparison of ischemic patients still demonstrates smaller scar in the LBBB group, this may be an artifact of survival bias; specifically, that patients with large anterior MIs who develop or have LBBB are less likely to survive long enough to qualify for an ICD. Studying hypertrophic cardiomyopathy patients undergoing alcohol septal ablation of a proximal LAD septal perforator did allow us to investigate the direct effect of proximal LAD occlusion in an acute setting; however, directed alcohol infusion down a septal perforator may not be representative of naturally occurring LAD coronary occlusions.
Conclusions
In a chronic cardiomyopathy cohort, RBBB is associated with ischemic cardiomyopathy and large anteroseptal scar, while LBBB is associated with nonischemic etiologies. The large myocardial scar in RBBB patients may explain why they have even worse mortality than non-specific LV conduction delay patients receiving CRT. This study highlights that the LAD coronary artery branches perfuse the RBBB and left anterior fascicle, but not the entire left bundle branch. Future clinical prognostic studies should specifically distinguish between RBBB and LBBB patients rather than consider them homogeneously. Assessment of scar burden as another risk factor may also be of value.
Acknowledgments
We thank research coordinators Barbara Butcher, Jeannette Walker, Sanaz Norgard and Angela Steinberg; and magnetic resonance technologist, Terry Frank for their efforts. We are also grateful to the patients for their participation. Dr. Tomaselli is the Michel Mirowski Professor of Medicine. Dr. Robert Weiss is the Clarence Doodeman Professor of Cardiology.
Financial Support: This project was supported in part by the FDA’s Critical Path Initiative, the Donald W. Reynolds Cardiovascular Research Center at Johns Hopkins University and the National Heart, Lung, Blood Institute, NIH (HL103812 to KCW, HL91062 to GFT, and HL61912 to RGW).
Relationship with Industry: Use of the custom research software tool, Cinetool, was obtained through a research agreement between Dr. Wu and GE Healthcare, WI, USA. Dr. Wu receives modest royalties for the licensing rights to use the gray zone methodology described in this article.
Abbreviation List
- CMR-LGE
cardiac magnetic resonance late gadolinium enhancement
- CRT
cardiac resynchronization therapy
- ICD
implantable cardioverter-defibrillator
- LAD
left anterior descending coronary artery
- LV
left ventricle/ventricular
- LVCD
left ventricular conduction delay
- LBBB
left bundle branch block
- MI
myocardial infarction
- RBBB
right bundle branch block
- RCA
right coronary artery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the U.S. Department of Health and Human Services.
Other authors do not have any conflicts of interest to report.
References
- 1.Opolski G, Kraska T, Ostrzycki A, Zielinski T, Korewicki J. The effect of infarct size on atrioventricular and intraventricular conduction disturbances in acute myocardial infarction. Int J Cardiol. 1986;10:141–7. doi: 10.1016/0167-5273(86)90222-6. [DOI] [PubMed] [Google Scholar]
- 2.Sgarbossa EB, Pinski SL, Barbagelata A, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators. N Engl J Med. 1996;334:481–7. doi: 10.1056/NEJM199602223340801. [DOI] [PubMed] [Google Scholar]
- 3.Neeland IJ, Kontos MC, de Lemos JA. Evolving considerations in the management of patients with left bundle branch block and suspected myocardial infarction. J Am Coll Cardiol. 2012;60:96–105. doi: 10.1016/j.jacc.2012.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frink RJ, James TN. Normal blood supply to the human His bundle and proximal bundle branches. Circulation. 1973;47:8–18. doi: 10.1161/01.cir.47.1.8. [DOI] [PubMed] [Google Scholar]
- 5.Lenegre J. Contribution à L’etude des Blocs de Branche. JB Bailliere et Fils; Paris: 1958. [Google Scholar]
- 6.Lev M, Unger PN, Rosen KM, Bharati S. The anatomic substrate of complete left bundle branch block. Circulation. 1974;50:479–86. doi: 10.1161/01.cir.50.3.479. [DOI] [PubMed] [Google Scholar]
- 7.Sugiura M, Okada R, Okawa S, Shimada H. Pathohistological studies on the conduction system in 8 cases of complete left bundle branch block. Japanese Heart Journal. 1970;11:5–16. doi: 10.1536/ihj.11.5. [DOI] [PubMed] [Google Scholar]
- 8.Cygankiewicz I, Gillespie J, Zareba W, et al. Predictors of long-term mortality in Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) patients with implantable cardioverter-defibrillators. Heart Rhythm. 2009;6:468–73. doi: 10.1016/j.hrthm.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Kass DA. An epidemic of dyssynchrony: but what does it mean? J Am Coll Cardiol. 2008;51:12–7. doi: 10.1016/j.jacc.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu KC, Gerstenblith G, Guallar E, et al. Combined cardiac magnetic resonance imaging and C-reactive protein levels identify a cohort at low risk for defibrillator firings and death. Circ Cardiovasc Imaging. 2012;5:178–86. doi: 10.1161/CIRCIMAGING.111.968024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–14. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strauss DG, Selvester RH, Lima JA, et al. ECG quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: correlation with cardiac magnetic resonance and arrhythmogenesis. Circ Arrhythm Electrophysiol. 2008;1:327–36. doi: 10.1161/CIRCEP.108.798660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 16.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–74. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 17.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–53. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 18.Strauss DG, Selvester RH. The QRS complex--a biomarker that “images” the heart: QRS scores to quantify myocardial scar in the presence of normal and abnormal ventricular conduction. J Electrocardiol. 2009;42:85–96. doi: 10.1016/j.jelectrocard.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Loring Z, Chelliah S, Selvester RH, Wagner G, Strauss DG. A detailed guide for quantification of myocardial scar with the Selvester QRS score in the presence of electrocardiogram confounders. J Electrocardiol. 2011;44 doi: 10.1016/j.jelectrocard.2011.06.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. 2011;107:927–34. doi: 10.1016/j.amjcard.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 22.Young JB, Abraham WT, Smith AL, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–94. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 23.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 24.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 25.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 26.Tang AS, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–95. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 27.Zareba W, Klein H, Cygankiewicz I, et al. Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) Circulation. 2011;123:1061–72. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 28.Sipahi I, Chou JC, Hyden M, Rowland DY, Simon DI, Fang JC. Effect of QRS morphology on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. Am Heart J. 2012;163:260–7. e3. doi: 10.1016/j.ahj.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilchick KC, Kamath S, Dimarco JP, Stukenborg GJ. Bundle-Branch Block Morphology and Other Predictors of Outcome After Cardiac Resynchronization Therapy in Medicare Patients. Circulation. 2010;122:2022–2030. doi: 10.1161/CIRCULATIONAHA.110.956011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loring Z, Caños DA, Selzman K, et al. Left Bundle Branch Block Predicts Better Survival in Women than Men Receiving Cardiac Resynchronization Therapy: Long Term Follow-Up of 145,000 Patients. J Am Coll Cardiol HF. 2013;1:237–44. doi: 10.1016/j.jchf.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Qin JX, Shiota T, Lever HM, et al. Conduction system abnormalities in patients with obstructive hypertrophic cardiomyopathy following septal reduction interventions. Am J Cardiol. 2004;93:171–5. doi: 10.1016/j.amjcard.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal S, Tuzcu EM, Desai MY, et al. Updated meta-analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;55:823–34. doi: 10.1016/j.jacc.2009.09.047. [DOI] [PubMed] [Google Scholar]