Abstract
Male Zucker diabetic fatty (ZDF) rats were used to study effects of oral administration of interferon tau (IFNT) in reducing obesity. Eighteen ZDF rats (28 days of age) were assigned randomly to receive 0, 4 or 8 μg IFNT/kg body weight (BW) per day (n=6/group) for 8 weeks. Water consumption was measured every two days. Food intake and BW were recorded weekly. Energy expenditure in 4-, 6-, 8-, and 10-week-old rats was determined using indirect calorimetry. Starting at 7 weeks of age, urinary glucose and ketone bodies were tested daily. Rates of glucose and oleate oxidation in liver, brown adipose tissue, and abdominal adipose tissue, leucine catabolism in skeletal muscle, and lipolysis in white and brown adipose tissues were greater for rats treated with 8 μg IFNT/kg BW/day in comparison with control rats. Treatment with 8 μg IFNT/kg BW/day increased heat production, reduced BW gain and adiposity, ameliorated fatty liver syndrome, delayed the onset of diabetes, and decreased concentrations of glucose, free fatty acids, triacylglycerol, cholesterol, and branched-chain amino acids in plasma, compared to control rats. Oral administration of 8 μg IFNT/kg BW/day ameliorated oxidative stress in skeletal muscle, liver and adipose tissue, as indicated by decreased ratios of oxidized glutathione to reduced glutathione and increased concentrations of the antioxidant tetrahydrobiopterin. These results indicate that IFNT stimulates oxidation of energy substrates and reduces obesity in ZDF rats and may have broad important implications for preventing and treating obesity-related diseases in mammals.
Keywords: Obesity, interferon tau, diabetes, energy expenditure, tissue oxidation
1. Introduction
Obesity is rapidly becoming an epidemic worldwide in both developed and underdeveloped nations (1). This disease results from a chronic imbalance between food energy intake and whole-body energy expenditure, as well as from inflammation and oxidative stress of certain tissues (2). Obesity is a growing public health concern and results in adverse health outcomes, including insulin resistance, type II diabetes mellitus, obstructive sleep apnea, osteoarthritis, stroke, hypertension and certain types of cancer, such as colon and breast cancers (3). In developed nations, such as the United States, poor diet and lack of physical activity are associated with over 400,000 deaths per year, including those attributable to diabetes (4), while obesity-related deaths are also on the rise. In 2003, approximately $75 billion was spent in the United States to treat obesity-related medical conditions (5) and those costs increased in 2010 to $123 billion (6). In 2004, the Center for Disease Control and Prevention ranked obesity as the number one health risk facing the United States. Also, according to the World Health Organization, overweight and obesity are causing more deaths worldwide than deaths related to underweight, and are also “the fifth in leading risks of global death” (7). Therefore, it is essential to develop effective preventive and therapeutic interventions to reduce the prevalence of obesity and related mortality and morbidity.
Several factors are contributing to the rise in obesity worldwide, including the rate of energy intake being far greater than energy expenditure, living a sedentary lifestyle, genetic predisposition, consumption of high fat diets, and inflammation (8,9). As a chronic syndrome, obesity is a risk factor for both insulin resistance and type II diabetes mellitus, with obesity-induced inflammation playing a major role in insulin resistance and the development of diabetes (10). Obesity is often accompanied by inflammation in white adipose tissue (WAT) and liver due to increases in inflammatory cells (10). Inflammation has also been linked to oxidative stress leading to obesity and insulin resistance (8). Therefore, in addition to understanding the role of inflammation in the causal pathway of obesity, it is imperative to identify new means to reduce inflammation and oxidative stress in obese subjects.
Ovine interferon tau (IFNT) was discovered for its regulatory role in the reproductive cycle of sheep (11,12). IFNT possesses stable acid properties as well as antiviral, antiproliferative and immunomodulatory activities (12–16). IFNT is a member of the type-I interferon family but, unlike interferons alpha and beta, is considered non-toxic even at high concentrations (15). Therefore, IFNT can be administered intravenously to animals (13–15). Importantly, oral administration of IFNT has been used to treat inflammatory autoimmune diseases such as multiple sclerosis in NZW mice (16) and to inhibit autoimmune diabetes in NOD mice (a type-I diabetic animal model; 17). Having recognized the link between inflammation and obesity and the anti-inflammatory properties of IFNT (18), we determined the effect of IFNT on the onset of diabetes, oxidation of glucose and fatty acids in skeletal muscle, liver and WAT, energy expenditure, body mass, and tissue weights in Zucker diabetic fatty (ZDF) rats, an animal model of type-II diabetes mellitus with defects in leptin receptors (19,20).
2. Materials and Methods
2.1. ZDF Rats
Male ZDF rats (23 days of age) were obtained from Charles River and fed a Purina 5008 diet throughout the study. The diet contained 23.5% crude protein, 6.0% fat, 34.9% starch, 2.6% sucrose, 0.5% glucose plus fructose, 6.8% minerals, 3.8% fiber and 17,364 kJ (4,150 kcal) gross energy/kg (21). The rats were housed in a temperature- and humidity-controlled facility on a 12h light:12h dark cycle, with the dark period being between 8:00 PM and 8:00 AM, and the light period being between 8:00 AM and 8:00 PM. This study was approved by the Texas A&M University Animal Care and Use Committee.
2.2. IFNT treatment, biochemical and physiological measurements, and tissue collection
At 28 days of age, ZDF rats were assigned randomly to receive IFNT in drinking water (distilled and deionized H2O), which provided 0, 4 or 8 μg IFNT/kg body weight (BW) per day. There were 6 rats per treatment group. Recombinant ovine IFNT was produced using the Pichia pastoris expression system as described by VanHeeke (22). The drinking water was changed and water consumption was measured every 2 days. Concentrations of IFNT in the drinking water were adjusted according to the volume of water consumption to provide the desired dosages. Over the 8-week treatment period, concentrations of IFNT in the drinking water averaged 0, 13.4 and 26.8 ng/ml, respectively, for rats receiving 0, 4 or 8 μg IFNT/kg BW/day. Oral administration of IFNT was chosen because previous studies revealed that oral delivery of IFNT inhibited onset of autoimmune diabetes in NOD mice (17) and multiple sclerosis in NZW mice (16). Food intake and body weights were recorded weekly. Every 4 weeks, blood samples (100 μl) were obtained, for analysis of glucose and amino acids, from the tail vein of rats that had been food-deprived for 5 h, using a microhematocrit tube (23). Starting at 7 weeks of age (approximately one week before the usual onset of diabetes in ZDF rats), urinary glucose and ketone bodies were tested daily using Keto-Diastix® (Bayer Corporation, Elkhart, IN). The onset of diabetes in rats was diagnosed by positive tests of urinary glucose and ketone bodies for two consecutive days and was confirmed by analysis of glucose concentrations in the plasma of blood from the animal’s tail vein obtained on the same days as described by Wu (23).
Three days before measurements of O2 consumption and CO2 production by rats, they were placed in their assigned metabolic cages (1 rat/cage) for 4 h each day to acclimatize them to the cages. On the day of measurement, individual rats were placed in their metabolic cages for 1 h before any measurement was taken. Energy expenditure was measured when rats were 4, 6, and 8 weeks of age (weeks 0, 2, and 4 of the IFNT treatment period, respectively) by placing them individually, between 9:00 and 11:00 AM, in a computer-controlled Oxymas instrument (an open circuit calorimeter; Columbus Instruments, Ohio, USA). Gas analyzers were calibrated using a standard gas mixture containing known concentrations of CO2 (0.50%), O2 (20.5%), and N2 (79%). One rat from each treatment group was simultaneously analyzed for variables of energy expenditure. Energy expenditure in individual ZDF rats was also measured at 10 weeks of age (week 6 of the IFNT treatment) beginning at 2:00 PM for a 24 h period. During this 24h period, rats had free access to food and their respective treatments in drinking water. The following variables related to energy expenditure were measured: O2 consumption (L/h/kg BW), CO2 production (L/h/kg BW), respiratory quotient (O2 consumption/CO2 production), and heat production (kcal/kg BW/h).
At the end of the 8-week treatment, rectal temperatures of rats were recorded using a thermometer for rats. Then they were euthanized in the fed state at 8:00 AM using CO2, immediately followed by collection of cardiac blood samples, liver, gastrocnemius muscle, brown adipose, and WAT (21). These tissues were used immediately to determine the oxidation of glucose, leucine and oleate, as described below. The remaining tissues were frozen in liquid nitrogen and stored at −80°C until analyzed for glutathione [both reduced and oxidized forms (24)]; and tetrahydrobiopterin (25) as indicators of oxidative stress, as well as glycogen and total triacylglycerols (24) as indicators of fatty liver syndrome. Free fatty acids, total triacylglycerols, cholesterol, insulin, leptin, and adiponectin in plasma were analyzed using assay kits as described by (21).
2.3. Measurements of glucose, leucine and oleate oxidation as well as glycerol release
Tissues were cut into small and thin pieces (~10 mg/piece with ~10 mm in thickness). In previous studies, we found that oxidation of glucose and oleic acid in skeletal muscle, liver and adipose tissue of ZDF rats was linear with respect to time during a 2-h incubation period [21]. The tissue samples (~ 60 mg) were rinsed with oxygenated (95% O2/5% CO2) Krebs bicarbonate buffer (pH 7.4) and then incubated at 37°C for 2 h in 2 ml of oxygenated (95% O2/5% CO2) Krebs bicarbonate buffer (pH 7.4) containing 2.5 ng/ml insulin, 20 mM D-glucose, 2 mM leucine, and 2 mM oleate. The medium also contained 0.1 μCi D-[U-14C]glucose, [1-14C]leucine, or [1-14C]oleic acid to study oxidation of glucose, leucine and oleic acid, respectively (21,26). These concentrations of glucose, leucine and oleate were chosen to mimic plasma levels of glucose (18 to 25 mM), branched-chain amino acids (1 to 1.5 mM) and FFA (1.5–2 mM) in ZDF rats (21,27). In all experiments, the medium incubated in duplicate without tissues was included as a blank. At the end of the 2h incubation period, 0.2 mL of 1.5 mol/L HClO4 was added through a rubber stopper into the medium, and 14CO2 was collected in 0.2 mL NCS-II (Amersham) for determination of radioactivity (28). The specific activities of D-[U-14C]glucose and [1-14C]oleate in the incubation medium were used to calculate the rate of production of 14CO2. Neutralized medium was analyzed enzymatically for glycerol using a fluorometric method (21).
2.4. Statistical analysis
Results are expressed as means ± SEM. The energy expenditure variables, VO2 (L/h/kg BW), VCO2 (L/h/kg BW), RQ and heat expenditure per kg BW (kcal/kg BW/h) were analyzed using linear mixed effects models in the study following log transformations (29). An unstructured variance covariance matrix was specified for the variance covariance matrix. The unstructured covariance matrix allowed capture of the correlations that exist between the repeated energy expenditure variables for each animal (29). We tested for effects of treatment, time, and treatment × time interactions over the 24h period of observation of energy expenditure. The inclusion of time in the model allowed us to assess the impact of time of observation on energy expenditure. In addition to assessing the overall treatment effect over the 24-h period of observation, we also determined the hourly differences among treatments using ANOVA models. The analyses were performed using PROC MIXED of SAS 9.2 (SAS Institute, Cary NC). Linear mixed effects models were also used to analyze for effects of treatment on body weight, food intake and water intake. The covariates included in these models were treatment and age (weeks) of the rat. ANOVA models were also used to analyze for effects of treatment on oxidization of glucose, leucine and oleic acid, as well as on concentrations of metabolites in plasma and tissues (30). Differences among treatment groups were determined using the Student-Newman-Keuls multiple comparison test. A probability value of 0.05 was used to determine statistical significance of the covariates included in the model. When an interaction term was found to be statistically significant, multiple comparisons were done to better understand the differences among treatments (30).
3. Results
3.1. Food and water intakes, BW of rats, and onset of diabetes
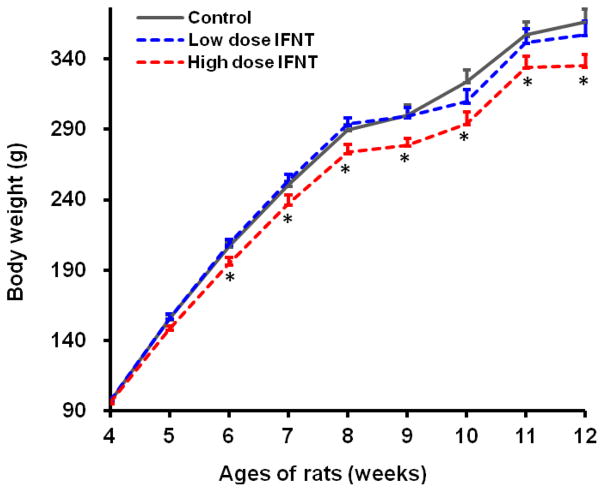
Food intake, water consumption, and body temperature (indicated by rectal temperature) of rats were not affected (P > 0.05) by oral administration of 4 and 8 μg IFNT/kg BW/day (Table 1). At 4 weeks of age (the beginning of IFNT treatment), the BW of rats did not differ (P > 0.05) among the three groups. Figure 1 illustrates changes in BW of rats during the 8-week period of oral administration of IFNT. Statistical differences (P < 0.05) in BW between rats treated with 8 μg IFNT/kg BW/day (high dose IFNT) and the control or 4 μg IFNT/kg BW/day (low dose IFNT) group were detected beginning at 3 weeks after initiation of IFNT administration. At the end of the 8-week study, rats treated with 8 μg IFNT/kg BW/day had 6.2% lower BW (P < 0.05) as compared with rats in the control group (Table 1). Oral administration of 8 μg IFNT/kg BW/day reduced (P < 0.05) BW gain over the 8-week period by 11% and delayed (P < 0.05) the onset of diabetes by 8.4 days, in comparison with the control group. Oral administration of 4 μg IFNT/kg BW/day to ZDF rats did not affect (P > 0.05) either BW or age at onset of diabetes, as compared to control rats (Table 1).
Table 1.
Effects of oral administration of IFNT on white adipose tissue, brown adipose tissue, liver, body-weight gains, and rectal temperature in ZDF rats
| Variable | Oral IFNT dose (μg/kg BW/day)
|
||
|---|---|---|---|
| 0 | 4 | 8 | |
| Initial body weight at week 4, g | 96.9 ± 1.6 | 97.2 ± 1.9 | 95.9 ± 1.3 |
| Final body weight at week 12, g | 366.4 ± 8.9a | 357.4 ± 11.9a | 335.3 ± 7.5b |
| Weight gain between weeks 4 and 12, g | 269.5 ± 4.2a | 260.2 ± 3.5a | 239.4 ± 4.5b |
| Food intake, g/kg BW/day | 92.6 ± 5.1 | 92.1 ± 4.7 | 91.8 ± 5.3 |
| Water consumption, ml/kg BW/day | 307 ± 21 | 314 ± 19 | 309 ± 25 |
| Age at onset of diabetes, days | 56.1 ± 0.4b | 56.0 ± 0.5b | 64.5 ± 3.2a |
| Rectal temperature, °C | 97.4 ± 0.07 | 97.5 ± 0.05 | 97.5 ± 0.06 |
Data are means ± SEM, n = 6 per treatment.
Tissue weights were determined in 12-week-old ZDF rats. Values within a row with different superscript letters differ (P < 0.05), as analyzed by one-way ANOVA.
Figure 1.
Body weights (BW) of ZDF rats during the 8-week period of IFNT treatment. Beginning at 4 weeks of age, male ZDF rats received oral administration of 0, 4 or 8 μg IFNT/kg BW/day until 12 weeks of age. Data are means ± SEM, n=6. P values were as follows: 0.007 for IFNT treatment effect; 0.011 for the high IFNT dose vs the control; < 0.0001 for the effect of age. No IFNT treatment × age interaction was detected (P = 0.240). * Statistical differences (P < 0.05) in BW between rats treated with 8 μg IFNT/kg BW/day and the control or 4 μg IFNT/kg BW/day group were detected beginning at 3 weeks after initiation of IFNT administration (i.e., when rats were 6 to 12 weeks of age).
3.2. Tissue weights of ZDF rats
Compared with the control group, oral administration of 8 μg IFNT/kg BW/day reduced (P < 0.05) retroperitoneal (abdominal), inguinal (subcutaneous), mesenteric WAT, and liver weights by 37%, 44%, 41%, and 22%, respectively, while increasing (P < 0.05) brown adipose tissue (BAT) in the interscapular region by 46% (Table 1). Abdominal adipose tissue, mesenteric adipose tissue, and BAT weights did not differ (P > 0.05) between the 0 and 4 μg IFNT/kg BW/day groups. However, inguinal (subcutaneous) WAT and liver weight were 18% and 12% lower (P < 0.05) in the 4 μg IFNT/kg BW/day group than in control rats. Treatment with 4 or 8 μg IFNT/kg BW/day for 8 weeks did not affect weights of skeletal muscle or other tissues (Table 2).
Table 2.
Effects of IFNT treatment on tissue weights of 12-week-old ZDF rats
| Tissue | Oral IFNT dose (μg/kg BW/day)
|
||
|---|---|---|---|
| 0 | 4 | 8 | |
| Abdominal fat, g | 10.8 ± 0.33a | 10.9 ± 0.72a | 6.81 ± 0.72b |
| Inguinal fat, g | 9.09 ± 0.35a | 7.43 ± 0.47b | 5.09 ± 0.49c |
| Mesenteric fat, g | 3.62 ± 0.14a | 3.53 ± 0.33a | 2.12 ± 0.14b |
| Brown adipose tissue1, g | 1.06 ± 0.12b | 1.09 ± 0.11b | 1.55 ± 0.18a |
| Liver, g | 23.6 ± 0.67a | 20.7 ± 0.30b | 18.3 ± 0.46c |
| Gastrocnemius muscle | 1.32 ± 0.05 | 1.34 ± 0.04 | 1.38 ± 0.05 |
| EDL muscle, mg | 112 ± 4 | 110 ± 4 | 110 ± 3 |
| Soleus muscle, mg | 118 ± 3 | 115 ± 4 | 115 ± 3 |
| Heart | 1.08 ± 0.03 | 1.18 ± 0.07 | 1.02 ± 0.03 |
| Lung | 1.39 ± 0.10 | 1.26 ± 0.02 | 1.34 ± 0.09 |
| Spleen | 0.54 ± 0.02 | 0.51 ± 0.02 | 0.50 ± 0.02 |
| Thymus | 0.24 ± 0.03 | 0.24 ± 0.03 | 0.25 ± 0.02 |
| Kidney | 1.54 ± 0.05 | 1.69 ± 0.05 | 1.41 ± 0.05 |
| Small Intestine | 9.81 ± 0.15 | 9.61 ± 0.37 | 9.14 ± 0.47 |
| Pancreas | 0.94 ± 0.09 | 0.97 ± 0.07 | 0.94 ± 0.10 |
| Testes | 2.84 ± 0.06 | 2.71 ± 0.04 | 2.74 ± 0.03 |
| Brain | 1.66 ± 0.05 | 1.68 ± 0.04 | 1.62 ± 0.05 |
| Large Intestine | 3.80 ± 0.23 | 3.41 ± 0.11 | 3.52 ± 0.17 |
| Stomach | 2.00 ± 0.11 | 1.93 ± 0.06 | 1.97 ± 0.13 |
Values, expressed as g, are means ± SEM, n = 6. Tissue weights were determined in 12-week-old ZDF rats. EDL = extensor digitorum.
Obtained from the interscapular region.
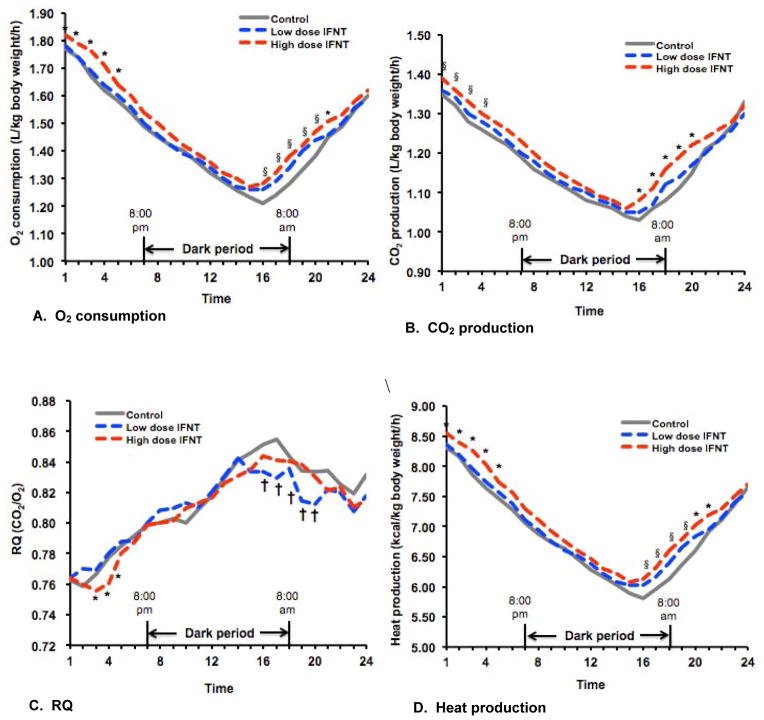
3.3. Energy expenditure
Energy expenditure in ZDF rats was increased (P < 0.05) in 6- and 8-week-old ZDF rats treated with 8 μg IFNT/kg BW/day, compared with control rats and the low-dose IFNT group (Table 3). Over a 24h period, O2 consumption, CO2 production, respiratory quotient, and heat production in 10-week-old ZDF rats in the control, low-dose IFNT (4 μg IFNT/kg BW/day), and high-dose IFNT (8 μg IFNT/kg BW/day) groups changed (P < 0.001) with time of the day (Figure 2). Overall, the rate of energy expenditure over the entire 24h period was greater (P < 0.05) for rats in the high-dose IFNT group than in the control and low-dose IFNT groups (Table 4). Differences in heat production between the high-dose IFNT group and the other two groups of rats at time points 1, 2, 3, 4, 5, 20, and 21 (corresponding to 2:00 – 3:00 PM, 3:00 – 4:00 PM, 4:00 – 5:00 PM, 5:00 – 6:00 PM, 6:00 – 7:00 PM, 9:00 – 10:00 AM, 10:00 – 11:00 AM, respectively) were detected (P < 0.05) (Figure 2). At time points 16, 17, 18 and 19 (corresponding to 5:00 – 6:00 AM, 6:00 – 7:00 AM, 7:00 – 8:00 AM, and 8:00 – 9:00 AM, respectively).
Table 3.
Effects of oral administration of IFNT on energy expenditure in 4, 6 and 8-week-old ZDF rats
| Variable | Oral IFNT dose (μg/kg BW/day)
|
||
|---|---|---|---|
| 0 | 4 | 8 | |
| 4-week-old rats | |||
| O2 consumption, L/h/kg BW | 2.04 ± 0.03 | 2.02 ± 0.03 | 2.05 ± 0.04 |
| CO2 production, L/h/kg BW | 1.78 ± 0.02 | 1.75 ± 0.03 | 1.79 ± 0.03 |
| Respiratory quotient (RQ) | 0.873 ± 0.003 | 0.868 ± 0.004 | 0.875 ± 0.004 |
| Heat production, kcal/kg BW/h | 9.84 ± 0.14 | 9.74 ± 0.13 | 9.89 ± 0.15 |
| 6-week-old rats | |||
| O2 consumption, L/h/kg BW | 1.80 ± 0.03b | 1.83 ± 0.02b | 1.92 ± 0.03a |
| CO2 production, L/h/kg BW | 1.53 ± 0.03b | 1.51 ± 0.02b | 1.70 ± 0.02a |
| Respiratory quotient (RQ) | 0.852 ± 0.004b | 0.828 ± 0.003c | 0.886 ± 0.003a |
| Heat production, kcal/kg BW/h | 8.55 ± 0.13b | 8.73 ± 0.10b | 9.30 ± 0.12a |
| 8-week-old rats | |||
| O2 consumption, L/h/kg BW | 1.68 ± 0.02 | 1.70 ± 0.03 | 1.77 ± 0.02 |
| CO2 production, L/h/kg BW | 1.26 ± 0.01b | 1.29 ± 0.02ab | 1.36 ± 0.02a |
| Respiratory quotient (RQ) | 0.754 ± 0.003 | 0.761 ± 0.004 | 0.769 ± 0.004 |
| Heat production, kcal/kg BW/h | 7.87 ± 0.08b | 7.99 ± 0.11b | 8.34 ± 0.10a |
Data are means ± SEM, n = 6 per treatment. Energy expenditure was determined between 9:00 and 11:00 AM in 4, 6, and 8-week-old ZDF rats.
Values within a row with different superscript letters differ (P < 0.05), as analyzed by one-way ANOVA.
Figure 2.
Energy expenditure in 10-week-old ZDF rats over a 24-h period. Rats were placed in individual metabolic cages beginning at 2:00 PM. The dark period was between 8:00 PM and 8:00 AM, and the light period was between 8:00 AM and 8:00 PM next day. During the entire period of measurement of energy expenditure, rats had free access to food and drinking water providing 0, 4 or 8 μg IFNT/kg BW/day. Hourly values for O2 consumption (Panel A), CO2 production (Panel B), respiratory quotient (CO2/O2, vol/vol; RQ) (Panel C), and heat production (Panel D) were calculated for 24 time points (one-hour interval/time point), with time 1 being 2:00 – 3:00 PM and time 24 being 1:00 – 2:00 PM next day. Data are hourly means for 6 rats per treatment, with SEM values for each time point being < 1.9% of the mean for O2 consumption, < 1.6% of the mean for CO2 production, < 0.5% of the mean for respiratory quotient, and < 1.5% of the mean for heat production. For O2 consumption or CO2 production, the pooled SEM values were 0.02, 0.02, and 0.03 L/kg BW/h, respectively, for ZDF rats receiving 0, 4 and 8 μg/kg BW/day. For respiratory quotient, the pooled SEM value was 0.003 for each group. For heat production, the pooled SEM values were 0.11, 0.10, and 0.12 kcal/kg BW/h, respectively, for ZDF rats receiving 0, 4 and 8 μg/kg BW/day. P values were 0.035 for IFNT treatment effect and < 0.0001 for time of the day. No IFNT treatment × time interaction was detected (P = 0.518). * P < 0.05 for the high-dose IFNT group vs the control and low-dose IFNT groups, § P < 0.05 for the high-dose IFNT group vs the control, and † P < 0.05 for the low-dose IFNT group vs the control, as analyzed by one-way ANOVA and the Student-Newman-Keuls multiple comparison.
Table 4.
Effects of oral administration of IFNT on 24-h energy expenditure in 10-week-old ZDF rats
| Oral IFNT dose (μg/kg BW/day)
|
|||
|---|---|---|---|
| 0 | 4 | 8 | |
| O2 consumption (L/h/kg BW) | 1.44 ± 0.01b | 1.46 ± 0.01b | 1.50 ± 0.01a |
| CO2 production (L/h/kg BW) | 1.17 ± 0.01 | 1.16 ± 0.01 | 1.19 ± 0.01 |
| Respiratory quotient (CO2/O2, v/v) | 0.815 ± 0.001a | 0.796 ± 0.001b | 0.794 ± 0.001b |
| Heat production (kcal/kg BW/h) | 6.85 ± 0.04b | 6.92 ± 0.05b | 7.12 ± 0.04a |
Data are the hourly means ± SEM, n = 6 per treatment. Energy expenditure was determined over a 24-h period in 10-week-old ZDF rats, during which food and drinking water containing the respective doses of IFNT were provided.
Values within a row with different superscript letters differ (P < 0.05), as analyzed by one-way ANOVA.
3.4. Oxidation of energy substrates and glycerol release
Compared with the control and the low-dose IFNT groups, 8 μg IFNT/kg BW/day increased the rates of oxidation of glucose and oleic acid by the liver (P < 0.05) and BAT (P < 0.001) of ZDF rats (Table 5). There were no differences in rates of glucose or oleic acid oxidation by any tissue between the control and 4 μg IFNT/kg BW/day groups (P>0.05). The rates of net transamination and oxidative decarboxylation of leucine in skeletal muscle were higher (P < 0.05) in rats receiving the high IFNT dose as compared with the control and low-dose IFNT groups (Table 6). IFNT did not affect leucine degradation in other tissues (P > 0.05). Rates of glycerol release from BAT, WAT and skeletal muscle of rats receiving 8 μg IFNT/kg BW/day were higher (P < 0.05) in comparison with the control and low-dose IFNT groups (Table 5).
Table 5.
Effects of oral administration of IFNT on oxidation of glucose and oleic acid and on lipolysis in tissues of ZDF rats
| Tissue | Oral IFNT dose (μg/kg BW/day)
|
||
|---|---|---|---|
| 0 | 4 | 8 | |
| Glucose oxidation (nmol CO2/mg tissue/2 h) | |||
| Liver | 0.13 ± 0.01b | 0.13 ± 0.01b | 0.18 ± 0.01a |
| Gastrocnemius muscle | 0.15 ± 0.01b | 0.16 ± 0.01b | 0.20 ± 0.01a |
| Brown adipose tissue | 0.30 ± 0.01b | 0.32 ± 0.02b | 0.62 ± 0.03a |
| Abdominal adipose tissue | 0.14 ± 0.00b | 0.14 ± 0.01b | 0.19 ± 0.01a |
| Oleate Oxidation (nmol CO2/mg tissue/2 h) | |||
| Liver | 0.17 ± 0.01b | 0.18 ± 0.01b | 0.23 ± 0.01a |
| Gastrocnemius muscle | 0.30 ± 0.01b | 0.31 ± 0.01b | 0.39 ± 0.01a |
| Brown adipose tissue | 0.20 ± 0.01b | 0.20 ± 0.01b | 0.26 ± 0.01a |
| Abdominal adipose tissue | 0.015 ± 0.001 | 0.016 ± 0.001 | 0.016 ± 0.001 |
| Glycerol release (nmol/mg tissue/2 h) | |||
| Gastrocnemius muscle | 0.26 ± 0.02b | 0.27 ± 0.02b | 0.34 ± 0.02a |
| Brown adipose tissue | 1.04 ± 0.05b | 1.12 ± 0.07b | 1.36 ± 0.06a |
| Abdominal adipose tissue | 2.63 ± 0.14b | 2.75 ± 0.15b | 3.41 ± 0.19a |
Data are the means ± SEM, n = 6 per treatment. Tissue weights were determined in 12-week-old ZDF rats.
Values within a row with different superscript letters differ (P < 0.05), as analyzed by one-way ANOVA.
Table 6.
Effects of oral administration of IFNT on leucine oxidative decarboxylation and net leucine transamination in tissues of ZDF rats
| Tissue | Oral IFNT dose (μg/kg BW/day)
|
||
|---|---|---|---|
| 0 | 4 | 8 | |
| Leucine oxidative decarboxylation (a) | |||
| Liver | 0.048 ± 0.004 | 0.050 ± 0.005 | 0.051 ± 0.004 |
| Gastrocnemius muscle | 0.36 ± 0.02b | 0.34 ± 0.01b | 0.46 ± 0.01a |
| Brown adipose tissue | 0.72 ± 0.04 | 0.69 ± 0.04 | 0.68 ± 0.04 |
| Abdominal adipose tissue | 0.27 ± 0.01 | 0.28 ± 0.02 | 0.27 ± 0.02 |
| Net release of α-ketoisocaproate (b) | |||
| Liver | 0.011 ± 0.003 | 0.010 ± 0.003 | 0.011 ± 0.003 |
| Gastrocnemius muscle | 0.10 ± 0.01b | 0.12 ± 0.02b | 0.17 ± 0.01a |
| Brown adipose tissue | 0.94 ± 0.06 | 0.92 ± 0.07 | 0.95 ± 0.08 |
| Abdominal adipose tissue | 0.11 ± 0.02 | 0.10 ± 0.01 | 0.12 ± 0.02 |
| Net transamination of leucine (a + b) | |||
| Liver | 0.059 ± 0.005 | 0.060 ± 0.006 | 0.062 ± 0.006 |
| Gastrocnemius muscle | 0.46 ± 0.03b | 0.47 ± 0.02b | 0.63 ± 0.02a |
| Brown adipose tissue | 1.66 ± 0.09 | 1.61 ± 0.06 | 1.63 ± 0.11 |
| Abdominal adipose tissue | 0.38 ± 0.02 | 0.37 ± 0.02 | 0.39 ± 0.03 |
Values, expressed as nmol/mg tissue/2 h, are the means ± SEM, n = 6 per treatment.
Values within a row with different superscript letters differ (P < 0.05), as analyzed by one-way ANOVA.
3.5. Concentrations of amino acids, glucose, lipids, and hormones in plasma
The ZDF rats treated with 8 μg IFNT/kg BW/day had increased (P < 0.05) concentrations of arginine and reduced (P < 0.05) concentrations of branched-chain amino acids (BCAA) in plasma (Table 7), as compared with the control and low-dose IFNT groups. Concentrations of glucose, free fatty acids, triacylglycerols, and cholesterol were lower (P < 0.05) in plasma of ZDF rats treated with 8 μg IFNT/kg BW/day than for the other two treatment groups. Compared with the control and 4 μg IFNT/kg BW/day treatment groups, 8 μg IFNT/kg BW/day reduced (P < 0.05) concentrations of leptin in plasma, but had no effect on concentrations of insulin or adiponectin (Table 7).
Table 7.
Effects of oral administration of IFNT on concentrations of amino acids, glucose, lipids and hormones in the plasma of ZDF rats
| Metabolites or hormones in plasma | Oral IFNT dose (μg/kg BW/day)
|
||
|---|---|---|---|
| 0 | 4 | 8 | |
| Arginine, μM | 110 ± 4b | 115 ± 5b | 149 ± 6a |
| Valine, μM | 219 ± 9a | 201 ± 7a | 172 ± 6b |
| Isoleucine, μM | 208 ± 8a | 206 ± 7a | 178 ± 6b |
| Leucine, μM | 245 ± 10a | 233 ± 9a | 196 ± 8b |
| Glucose, mM | 24.5 ± 0.3a | 23.8 ± 0.4a | 21.9 ± 0.4b |
| Free fatty acids, mM | 1.60 ± 0.06a | 1.53 ± 0.05a | 1.34 ± 0.05b |
| Triacylglycerol, mM | 6.05 ± 0.13a | 5.90 ± 0.27a | 5.17 ± 0.11b |
| Total cholesterol, mM | 5.18 ± 0.23a | 4.94 ± 0.24a | 4.23 ± 0.13b |
| Insulin, pM | 307 ± 10 | 294 ± 11 | 301 ± 7 |
| Adiponectin, mg/L | 2.78 ± 0.08 | 2.94 ± 0.09 | 2.64 ± 0.13 |
| Leptin, μg/L | 19.6 ± 1.2b | 19.3 ± 1.0b | 13.7 ± 0.9a |
Plasma samples were obtained from 12-week-old rats. Values are the means ± SEM, n = 6 per treatment.
Values within a row sharing with superscript letters differ (P < 0.05), as analyzed by one-way ANOVA.
3.6. Concentrations of glutathione, tetrahydrobiopterin, glycogen and lipids in tissues
Compared with the control and the low-dose IFNT groups, 8 μg IFNT/kg BW/day increased (P < 0.05) concentrations of reduced glutathione and tetrahydrobiopterin in liver, WAT, and skeletal muscle, while decreasing (P < 0.05) the concentrations of oxidized glutathione, the ratio of oxidized glutathione to reduced glutathione, and concentrations of glycogen in those tissues (Table 8). In the liver, the concentrations of oxidized glutathione and the ratio of oxidized glutathione to reduced glutathione were lower (P < 0.05), but concentrations of tetrahydrobiopterin were higher (P < 0.05), in ZDF rats treated with 4 μg IFNT/kg BW/day than in the control group. IFNT treatment reduced concentrations of total lipids (primarily triacylglycerol) in liver and skeletal muscle, but had no effect on concentration of total lipids in WAT (Table 8).
Table 8.
Effects of oral administration of IFNT on concentrations of glutathione, tetrahydrobiopterin, glycogen, and triacylglycerols in tissues of ZDF rats
| Tissue | Oral IFNT dose (μg/kg BW/day)
|
||
|---|---|---|---|
| 0 | 4 | 8 | |
| Reduced glutathione (GSH, μmol/g wet tissue) | |||
| Liver | 4.10 ± 0.14b | 4.26 ± 0.11b | 4.84 ± 0.13a |
| Gastrocnemius muscle | 1.30 ± 0.06b | 1.35 ± 0.04b | 1.63 ± 0.05a |
| Abdominal adipose tissue | 0.46 ± 0.02b | 0.47 ± 0.01b | 0.55 ± 0.02a |
| Oxidized glutathione (GSSG, nmol/g tissue) | |||
| Liver | 509 ± 22a | 443 ± 16b | 387 ± 14c |
| Gastrocnemius muscle | 227 ± 6a | 214 ± 10a | 184 ± 5b |
| Abdominal adipose tissue | 119 ± 6a | 112 ± 5a | 94 ± 4b |
| Ratio of GSH to GSSG (μmol/μmol) | |||
| Liver | 0.125 ± 0.006a | 0.104 ± 0.005b | 0.080 ± 0.004c |
| Gastrocnemius muscle | 0.177 ± 0.010a | 0.158 ± 0.006a | 0.114 ± 0.005b |
| Abdominal adipose tissue | 0.265 ± 0.017a | 0.241 ± 0.014a | 0.173 ± 0.009b |
| Tetrahydrobiopterin (nmol/g wet tissue) | |||
| Liver | 2.25 ± 0.07c | 2.59 ± 0.09b | 3.04 ± 0.12a |
| Gastrocnemius muscle | 0.118 ± 0.005b | 0.125 ± 0.006b | 0.159 ± 0.008a |
| Abdominal adipose tissue | 0.295 ± 0.022b | 0.304 ± 0.015b | 0.374 ± 0.020a |
| Glycogen (mg/g wet tissue) | |||
| Liver | 49.5 ± 1.2a | 43.7 ± 1.0b | 37.0 ± 0.8c |
| Gastrocnemius muscle | 17.0 ± 0.5a | 16.1 ± 0.5a | 14.0 ± 0.3b |
| Abdominal adipose tissue | 1.82 ± 0.08a | 1.69 ± 0.07a | 1.42 ± 0.05b |
| Lipids (mg/g wet tissue for liver and muscle; or % for WAT) | |||
| Liver | 54.7 ± 1.4a | 48.3 ± 1.1b | 40.5 ± 0.9c |
| Gastrocnemius muscle | 23.2 ± 1.2a | 21.9 ± 0.9a | 17.6 ± 0.7b |
| Abdominal adipose tissue | 81.7 ± 0.7 | 81.2 ± 0.6 | 81.5 ± 0.6 |
Values are the means ± SEM, n = 6 per treatment.
Values within a row with different superscript letters differ (P < 0.05), as analyzed by one-way ANOVA. WAT, white adipose tissue.
4. Discussion
The ZDF rats are genetically obese animals used widely as a model to study type-II diabetes mellitus (19–21,31). The male rats become hyperglycemic by 8 weeks of age with elevated glucose and lipids in blood throughout their life span (32,33). Studies with ZDF rats have generated important information about mechanisms responsible for fatty liver syndrome, hyperglycemia, dyslipidemia, insulin resistance, and stroke in diabetic animals (21–22,31). Because these rats were severely obese, rates of oxidation of glucose and fatty acids in their skeletal muscle were relatively low (Table 5). Major findings from the present study are that IFNT treatment: (1) increased rates of glucose and fatty acid oxidation and lipolysis in skeletal muscle, liver and WAT and rates of BCAA catabolism in skeletal muscle; (2) increased the mass and metabolic activity of BAT; (3) increased whole-body energy expenditure; (4) decreased adiposity and ameliorated fatty liver syndrome; and (5) improved overall metabolic profiles.
There was no difference in food intake or water consumption among the ZDF rats in the control, low-dose IFNT (4 μg/kg BW/day) and high-dose IFNT (8 μg/kg BW/day) groups (Table 1), indicating a lack of any adverse effects of IFNT at the doses used. Despite the similar intake of dietary energy, rats on the high-dose IFNT treatment had lower BW when compared to the rats on the control and low-dose IFNT groups (Figure 1). In addition to reducing BW, the weights of abdominal, inguinal, and mesenteric WAT as well as the liver were also reduced for the rats on the high dose IFNT treatment when compared to the controls, without affecting the weights of skeletal muscle and other tissues (Tables 1 and 2). These results indicate a selective effect of IFNT to reduce WAT and the fatty liver mass in ZDF rats. Consistent with this finding, IFNT treatment increased the oxidation of both glucose and oleic acid in skeletal muscle, liver and WAT (Table 4), and decreased concentrations of glycogen and total lipids in liver and skeletal muscle (Table 8). Our findings provide an explanation for the observation that IFNT increased the metabolic rate and reduced adiposity in ZDF rats. This effect of IFNT on skeletal muscle, liver and WAT was similar to that of arginine (21). Interestingly, IFNT treatment also increased circulating levels of arginine in these animals possibly by inhibiting its utilization by multiple cells and tissues, including macrophages, intestine, and kidney via the arginase pathway. IFNT may also enhance expression of genes that promote mitochondrial oxidation of energy substrates in skeletal muscle, liver, and WAT. Future studies are necessary to test these hypotheses.
Another very important observation from this study was the effect of IFNT on increasing the mass and metabolic activity of BAT. Thus, oral administration of IFNT is a promising treatment for reducing BW and white fat, while increasing BAT in obese subjects. BAT is distinct from WAT anatomically, histologically and biochemically (34). While WAT is diffusely distributed throughout the body, BAT exists in discrete lobes (primarily in the intrascapular, peri-renal, and neck regions) in many species, including humans, rats, and sheep (35). Within the first month of postnatal life, BAT depots are drastically reduced in infants in response to environmental changes. A physiologically significant amount of BAT remains present in adults (36), but its presence is highly variable (37), possibly depending on ambient temperatures and nutritional status. Evidence from both genetic and cell culture studies indicate that peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is the master regulator of expression of uncoupling protein-1 (a biomarker for brown adipose tissue) and mitochondrial biogenesis and, therefore, BAT development (38). We postulate that IFNT stimulates expression of both PGC-1α and uncoupling protein-1 in BAT, but further studies are needed to test this hypothesis.
Elevated concentrations of BCAA in plasma of obese animals (20) and humans (39) is linked to development of insulin resistance in the whole body (40). We found that oral administration of 8 μg IFNT/kg BW/day reduced circulating levels of all three BCAA in ZDF rats (Table 6). It is possible that IFNT increases mitochondrial function and activity in skeletal muscle to stimulate BCAA degradation. In support of this view, the rate of leucine transamination, which occurs in both mitochondria and in the cytoplasm, and the rate of leucine oxidative decarboxylation in mitochondria, were increased in skeletal muscle of IFNT-treated ZDF rats (Table 6). Our results provide the first evidence that IFNT affects amino acid metabolism in animals.
Obesity has been linked to inflammation and oxidative stress in tissues such as the liver and WAT (41). Inflammation results from increased production of inflammatory cytokines by immune cells infiltrating WAT and exacerbates an imbalance between oxidative stress and the endogenous pool of antioxidants in obese subjects (10). It is possible that the IFNT exerts its promising therapeutic effects by suppressing the production of inflammatory cytokines in skeletal muscle, abdominal WAT, and liver of obese rats while reducing oxidative stress and increasing BAT. This would be consistent with a known anti-inflammatory effect of IFNT on the conceptus (12–14), which may result from increased concentrations of tetrahydrobiopterin [a potent antioxidant (42)] and a reduced ratio of oxidized glutathione to reduced glutathione [a sensitive indicator of oxidative stress (43)] in liver, skeletal muscle, and WAT (Table 7). Improvement of antioxidative capacity in skeletal muscle may promote oxidation of glucose, thereby delaying the onset of diabetes in ZDF rats (Table 1).
A salient observation from this study was that effects of IFNT on whole-body energy expenditure were dependent on the time of day (Table 3). Our results confirm prior reports of diurnal patterns of energy expenditure in genetically diabetic obese rats (44,45) and in streptozotocin-induced diabetic rats (46). Another study also found diurnal changes in energy expenditure at 8 weeks of age, but not at 24 weeks of age after the manifestation of type-II diabetes mellitus (44). Ichikawa et al. (44) concluded that the change in the diurnal pattern of energy expenditure is associated with the progression of insulin resistance and diabetes in Otsuka Long Evans Tokushima Fatty (OLETF) rats and not simply age. It is possible that IFNT influences expression of key biological-clock genes that are related to energy metabolism. In this regard, it is noteworthy that PGC-1α, a transcriptional coactivator that regulates energy metabolism, is rhythmically expressed in the liver and skeletal muscle of mice (47–49) and that an increase in its expression is associated with a reduction of adiposity in ZDF rats in response to dietary supplementation with arginine (21). PGC-1α stimulates the expression of clock genes, notably Bmal1 (Arntl) and Rev-erbalpha (Nr1d1), through coactivation of the retinoid-related orphan receptor (ROR) family, thereby integrating effect of the mammalian biological clock and energy metabolism (48).
5. Conclusion
Oral administration of 8 μg IFNT/kg BW/day reduced oxidative stress and increased oxidation of energy substrates to CO2 in skeletal muscle, liver and WAT, while enhancing BAT mass and metabolic activity, stimulating whole-body energy expenditure, and improving metabolic profiles in ZDF rats. IFNT treatment also ameliorates fatty liver syndrome in ZDF rats and reduces WAT mass in multiple depots. Future studies are warranted to elucidate the underlying mechanisms. IFNT may be an effective agent to prevent and treat obesity in mammals, including humans and companion animals.
Acknowledgments
We thank Katherine Kelly for technical assistance. This work was supported by grants from the AHA (10GRNT4480020 and 11GRNT7930004) and NIH (R21 HL094689 and R37-CA057030). C.D. Tekwe was supported by a postdoctoral training grant from the National Cancer Institute (R25T - 090301).
Footnotes
Author contributions: F. W. B., C. J. M., and G. W. designed the research; C. D. T., J. L., K. Y., R. R., X. L., S. D., and G. W. performed the experiments; C. D. T., R. J. C., F. W. B. and G. W. analyzed the data; and C. D. T., C. J. M., F. W. B. and G. W. wrote the paper.
The authors declare no conflicts of interest.
References
- 1.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 2.Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem. 2006;17:571–588. doi: 10.1016/j.jnutbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Abelson P, Kennedy D. The obesity epidemic. Science. 2004;304:1413. doi: 10.1126/science.304.5676.1413. [DOI] [PubMed] [Google Scholar]
- 4.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein EA, Fielborn IC, Wang G. State-level estimates of annual medical expenditures attributable to obesity. Obesity Res. 2004;12:18–24. doi: 10.1038/oby.2004.4. [DOI] [PubMed] [Google Scholar]
- 6.Endocrine Society. 2010 ObesityinAmerica.org.
- 7.WHO. 2012 http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 8.Fernandez-Sanchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales- González A, et al. Inflammation, oxidative stress and obesity. Int J Mol Sci. 2011;12:3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 10.Dandona P, Aljada A, Bandyopadhay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Martal J, Lacroix MC, Loudes C, Saunier M, Winterberger-Torres S. Trophoblastin, an antiluteolytic protein present in early pregnancy in sheep. J Reprod Fertil. 1979;56:63–73. doi: 10.1530/jrf.0.0560063. [DOI] [PubMed] [Google Scholar]
- 12.Bazer FW, Johnson HM. Type I conceptus interferons: maternal recognition of pregnancy signals and potential therapeutic agents. Am J Reprod Immun. 1991;26:19–22. doi: 10.1111/j.1600-0897.1991.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 13.Pontzer CH, Torres BA, Vallet JL, Bazer FW, Johnson HM. Antiviral activity of the pregnancy recognition hormone ovine trophoblast protein-1. Biochem Biophy Res Commun. 1988;152:801–807. doi: 10.1016/s0006-291x(88)80109-8. [DOI] [PubMed] [Google Scholar]
- 14.Pontzer CH, Bazer FW, Johnson HM. Antiproliferative activity of a pregnancy recognition hormone, ovine trophoblast protein-1. Cancer Res. 1991;51:5304–5307. [PubMed] [Google Scholar]
- 15.Soos JM, Subramaniam PS, Hobeika AC, Schiffenbauer J, Johnson HM. The IFN pregnancy recognition hormone IFN τ blocks both developmental and superantigen reaction of experimental allergic encephalomyelitis without associated toxicity. J Immunol. 1995;155:2747–2753. [PubMed] [Google Scholar]
- 16.Soos JM, Mujtaba MG, Subramaniam PS, Streit WJ, Johnson HM. Oral feeding of interferon τ can prevent acute forms of experimental allergic encephalomyelitis. J Neuroimmunol. 1997;75:43–50. doi: 10.1016/s0165-5728(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 17.Sobel DO, Ahvazi B, Amjad F, Mitnaul L, Pontzer C. Interferon-tau inhibits the development of diabetes in NOD mice. Autoimmunity. 2008;41:543–553. doi: 10.1080/08916930802194195. [DOI] [PubMed] [Google Scholar]
- 18.Bazer FW, Kim J, Song G, Ka H, Tekwe CD, Wu G. Select nutrients, progesterone, and interferon tau affect conceptus metabolism and development. Ann NY Acad Sci. 2012;1271:88–96. doi: 10.1111/j.1749-6632.2012.06741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruth MR, Taylor CG, Zahradka P, Field CJ. Abnormal immune responses in fa/fa Zucker rats and effects of feeding conjugated linoleic acid. Obesity (Silver Spring) 2008;16:1770–1779. doi: 10.1038/oby.2008.266. [DOI] [PubMed] [Google Scholar]
- 20.Wijekoon EP, Hall B, Ratnam S, Brosnan ME, Zeisel SH, Brosnan JT. Homocysteine metabolism in ZDF (type 2) diabetic rats. Diabetes. 2005;54:3245–3251. doi: 10.2337/diabetes.54.11.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, et al. Dietary L-arginine supplementation reduces fat mass in Zucker Diabetic Fatty rats. J Nutr. 2005;135:714–721. doi: 10.1093/jn/135.4.714. [DOI] [PubMed] [Google Scholar]
- 22.VanHeeke G, Ott TL, Strauss A, Ammaturo D, Bazer FB. High yield expression and secretion of the pregnancy recognition hormone ovine interferon-τ by Pichia pastoris. J Interferon Res. 1996;16:119–126. doi: 10.1089/jir.1996.16.119. [DOI] [PubMed] [Google Scholar]
- 23.Wu G. Nitric oxide synthesis and the effect of aminoguanidine and NG- monomethyl-L-arginine on the onset of diabetes in the spontaneously diabetic BB rat. Diabetes. 1995;44:360–364. doi: 10.2337/diab.44.3.360. [DOI] [PubMed] [Google Scholar]
- 24.Jobgen WJ, Meininger CJ, Jobgen SC, Li P, Lee MJ, et al. Dietary L- arginine supplementation reduces white-fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr. 2009;139:230–237. doi: 10.3945/jn.108.096362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meininger CJ, Wu G. Regulation of endothelial cell proliferation by nitric oxide. Methods Enzymol. 2002;352:280–295. doi: 10.1016/s0076-6879(02)52026-7. [DOI] [PubMed] [Google Scholar]
- 26.Wu G, Thompson JR. Ketone bodies inhibit leucine degradation in chick skeletal muscle. Int J Biochem. 1987;19:937–943. doi: 10.1016/0020-711x(87)90175-3. [DOI] [PubMed] [Google Scholar]
- 27.Wu G, Collins JK, Perkins-Veazie P, Siddiq M, Dolan KD, et al. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J Nutr. 2007;137:2680–2685. doi: 10.1093/jn/137.12.2680. [DOI] [PubMed] [Google Scholar]
- 28.Wu G, Field CJ, Marliss EB. Glucose and glutamine metabolism in rat macrophages: enhanced glycolysis and unaltered glutaminolysis in spontaneously diabetic BB rats. Biochim Biophys Acta. 1991;1115:166–173. doi: 10.1016/0304-4165(91)90026-d. [DOI] [PubMed] [Google Scholar]
- 29.Littel R, Milliken G, Stroup W. System for Mixed Models. SAS Institute; Cary, N.C: 1996. [Google Scholar]
- 30.Wei JW, Carroll RJ, Harden KK, Wu G. Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids. 2012;42:2031–2035. doi: 10.1007/s00726-011-0924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kakuma T, Lee Y, Higa M, Wang ZW, Pan W, et al. Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Proc Natl Acad Sci USA. 2000;97:8536–8541. doi: 10.1073/pnas.97.15.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson RG, Little LA, Neel MA. Zucker diabetic fatty rats as a model for non-insulin-dependent diabetes mellitus. ILAR J. 1990;32(3) doi: 10.1093/ilar.32.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Yorek MA. Treatment of Zucker diabetic fatty rats with AVE7688 improves vascular and neural dysfunction. Diabetes Obes Metab. 2009;11:223–233. doi: 10.1111/j.1463-1326.2008.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satterfield MC, Wu G. Growth and development of brown adipose tissue: significance and nutritional regulation. Front Biosci. 2011;16:1589–1608. doi: 10.2741/3807. [DOI] [PubMed] [Google Scholar]
- 35.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 36.Yoneshiro S, Aita S, Matsushita M, Kameya T, Nakada K, et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 2011;19:13–16. doi: 10.1038/oby.2010.105. [DOI] [PubMed] [Google Scholar]
- 37.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 38.Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010;11:257–262. doi: 10.1016/j.cmet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marliss EB, Chevalier S, Gougeon R, Morais JA, Lamarche M, et al. Elevations of plasma methylarginines in obesity and ageing are related to insulin sensitivity and rates of protein turnover. Diabetologia. 2006;49:351–359. doi: 10.1007/s00125-005-0066-6. [DOI] [PubMed] [Google Scholar]
- 40.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71:332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 42.Shi W, Meininger CJ, Haynes TE, Hatakeyama K, Wu G. Regulation of tetrahydrobiopterin synthesis and bioavailability in endothelial cells. Cell Biochem Biophys. 2004;41:415–433. doi: 10.1385/CBB:41:3:415. [DOI] [PubMed] [Google Scholar]
- 43.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 44.Ichikawa M, Miyasaka K, Fujita Y, Shimazoe T, Funakoshi A. Disappearance of diurnal rhytm of energy expenditure in genetically diabetic obese rats. Jpn J Physiol. 1998;48:211–214. doi: 10.2170/jjphysiol.48.211. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida Y, Ichikawa M, Ohta M, Kanai S, Kobayash M, et al. A peroxisome proliferator-activated receptor gamma agonist influenced daily profile of energy expenditure in genetically obese diabetic rats. Jpn J Pharmacol. 2002;88:279–284. doi: 10.1254/jjp.88.279. [DOI] [PubMed] [Google Scholar]
- 46.Ichikawa M, Kanai S, Ichimani Y, Funakoshi A, Miyasaka K. The diurnal rhythm of energy expenditure differs between obese and glucose intolerant rats and Streptozotocin-induced diabetic rats. J Nutr. 2000;130:2562–2567. doi: 10.1093/jn/130.10.2562. [DOI] [PubMed] [Google Scholar]
- 47.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC- 1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 48.Li S, Lin JD. Molecular control of circadian metabolic rhythms. J Appl Physiol. 2009;107:1959–1964. doi: 10.1152/japplphysiol.00467.2009. [DOI] [PubMed] [Google Scholar]
- 49.Dai ZL, Wu ZL, Yang Y, Wang JJ, Satterfield MC, et al. Nitric oxide and energy metabolism in mammals. BioFactors. 2013 doi: 10.1002/biof.1099. [DOI] [PubMed] [Google Scholar]