Summary
Active segregation of essential organelles is required for successful cell division. The essential budding yeast myosin-V, Myo2, actively segregates most organelles along polarized actin cables[1, 2]. The mechanism of mitochondrial segregation remains controversial, with movement driven by actin polymerization[3–5], by association with transported cortical ER[6, 7], and direct transport by Myo2[8–11], proposed as models. Two non-essential proteins, Mmr1 and the Rab-GTPase Ypt11, bind Myo2 and have been implicated in mitochondrial inheritance, although their specific roles are also contended[7, 11]. We generated myo2sens mutations that exhibit no overt phenotype, but render MMR1 essential and have compromised Ypt11 binding. We then isolated myo2sens mmr1ts conditional mutants and determined that they have a specific and severe defect in active mitochondrial inheritance, revealing mitochondrial transport by Myo2 as an essential function. ypt11Δ mmr1ts cells also have conditional defects in growth and active transport of mitochondria into the bud, both of which are suppressed by artificially forcing mitochondrial inheritance. At the restrictive temperature, cells defective in mitochondrial inheritance give rise to dead buds that go through cytokinesis normally, showing no evidence of a proposed cell cycle mitochondrial inheritance checkpoint[12]. Thus, active mitochondrial inheritance is an essential process and a function of Myo2 that requires either Mmr1 or Ypt11.
Results and Discussion
Isolation of conditional myo2sens mmr1ts strains
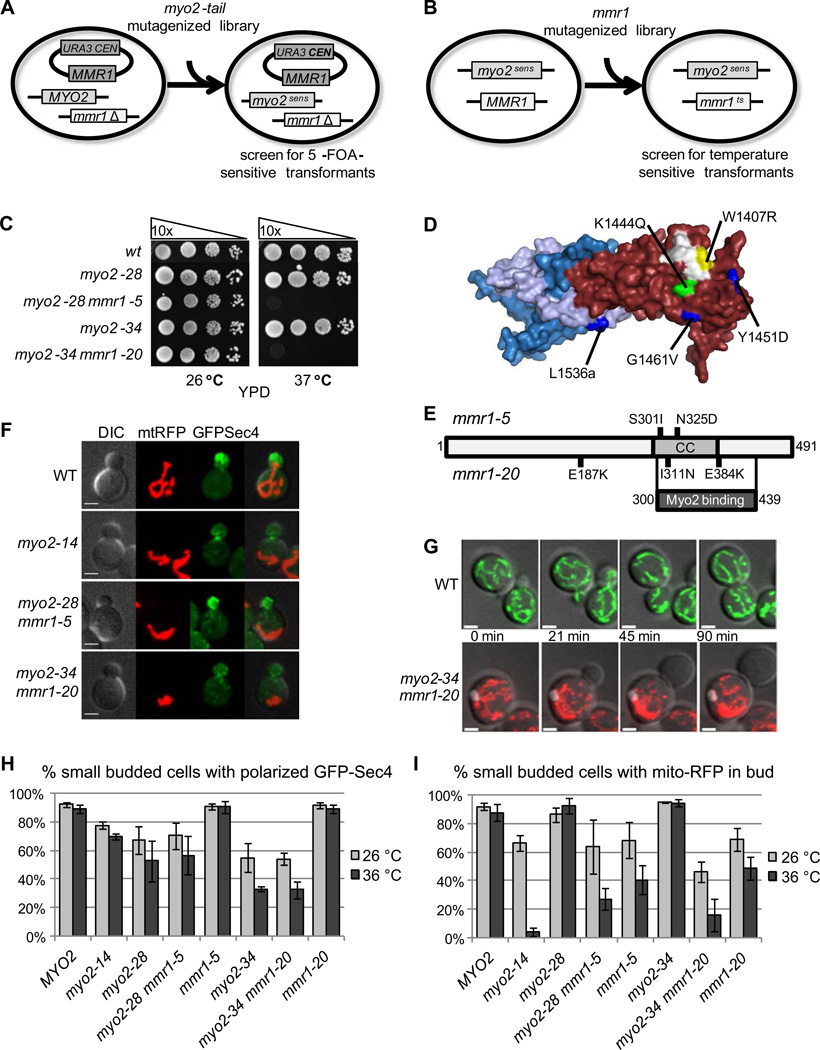
We have previously described conditional mutations in the cargo-binding tail domain of Myo2 that result in failure to transport secretory vesicles to sites of growth at restrictive temperature[13]. In preliminary experiments, we found that one of these mutants, myo2-14, shows synthetic lethality with mmr1Δ and its growth defect can be suppressed by introduction of a second copy of MMR1. These data suggested a genetic approach for the isolation of conditional alleles of MMR1 to determine the function of Mmr1. First, we generated new sensitizing alleles of MYO2 (myo2sens) that confer no growth phenotype, but render Myo2 essential in the absence of MMR1 (Fig. 1A, S1A), and are fully complemented by MYO2 and MMR1 (Fig. S1B). Second, we used the myo2sens alleles to generate temperature-sensitive conditional mutations in MMR1 (mmr1ts) (Fig. 1B). Interestingly, of the twenty five mmr1ts candidates sequenced, all except one contained mutations in the Myo2-binding C-terminal region of Mmr1 [8]. Two mutants were selected for further study: myo2-28 mmr1-5 and myo2-34 mmr1-20.
Figure 1. Mmr1 functions in mitochondrial inheritance.
(A,B) Diagrams of the two step mutagenesis scheme for generating myo2sens mmr1ts alleles. (C) myo2-28 mmr1-5 and myo2-34 mmr1-20 strains are temperature sensitive. Strains were grown overnight in YPD at 26 °C, adjusted to OD600 of 1.0, serially 10-fold diluted, spotted on YPD plates and incubated at 26 °C and 37 °C for 3 days. (D) Surface diagram of the Myo2-tail structure showing mutations present in the myo2-14 (blue), myo2-28 (green), and myo2-34 (yellow) alleles. Domain I is in blue (aa1152-1309) and lilac (aa1527-1574), Domain II is in red (aa1310-1526), and the Rab binding site is in white. (E) Schematic of Mmr1, indicating mutations present in mmr1-5 and mmr1-20 alleles, and the Myo2 binding domain of Mmr1. (F) myo2sens mmr1ts strains have defects in mitochondrial inheritance. Cells expressing GFP-Sec4 and mito-RFP from plasmids were grown to logarithmic phase in SD-ura-leu, incubated at 36 °C for 1.5 h, and analyzed by confocal fluorescence microscopy at 36 °C. Bar, 2 µm. (G) Wildtype cells expressing mito-GFP and myo2-34 mmr1-20 cells expressing mito-RFP were grown to logarithmic phase in SD-ura-leu at 26 °C, mixed together, fixed to glass dishes and analyzed by time-resolved 3D confocal fluorescence microscopy at 37 °C. Images correspond to stills from movies S1A and S1C, and minutes since start of bud formation are indicated. Bar, 2 µm. (H,I) Cells were grown to logarithmic phase in SD-ura-leu, incubated at 26 °C or 36 °C for 1.5 h, and analyzed by confocal fluorescence microscopy at 26 °C or 36 °C. Cells carrying small buds were scored for GFP-Sec4 polarization in H and for presence of mito-RFP in the bud in I. Quantifications are mean values from three independent experiments (n = 100), and error bars indicate standard deviations.
myo2-28 MMR1 and myo2-34 MMR1 parent strains grow well at all temperatures, whereas myo2-28 mmr1-5 and myo2-34 mmr1-20 do not grow at or above 35° (Fig. 1C), a defect complemented by expression of either MYO2 or MMR1 (Fig. S1C). The temperature sensitivity is not due to degradation of Myo2 at elevated temperatures (Fig. S1D). Both myo2-28 and myo2-34 have mutations on the surface of domain II of the Myo2-tail in the vicinity of the Rab-binding site (K1444Q and W1407R, respectively) (Fig. 1D), which is distant from the Mmr1 binding site on domain I [14]. The myo2-14 allele has three mutations, two point mutations resulting in amino acid changes Y1451D and G1461V in domain II, and an amber mutation at position 1536 resulting in a 39 residue C-terminal truncation removing part of a loop and helix extending from domain II that embraces domain I (Fig. 1D). The mmr1-5 and mmr1-20 alleles have two and three mutations, respectively (mmr1-5: S301I, N325D and mmr1-20: E187K, I311N, E384K), notably each containing two mutations in the Myo2-binding region [8] (Fig. 1E).
The myo2sens mmr1ts mutations cause a conditional defect in mitochondrial inheritance but not in polarizing secretory vesicles
As transport of secretory vesicles into the bud is the only known essential function of Myo2 [13, 15], we visualized the extent of secretory vesicle polarization using the marker GFP-Sec4. Since Mmr1 has previously been implicated in mitochondrial inheritance [8, 9], we also used a mitochondrially localized (mito)-RFP to analyze mitochondrial distribution and morphology [16].
GFP-Sec4 is polarized in small budded wildtype cells at 26 °C and after 1.5 hours at 36 °C (Fig. 1F,H). Cells harboring the myo2-14, myo2-28 or myo2-34 alleles alone have a slight to moderate defect in vesicle polarization at 36° C, and the mmr1-5 and mmr1-20 alleles alone are unaffected. Importantly, in myo2-28 mmr1-5 or myo2-34 mmr1-20 mutants, the presence of the mmr1ts mutation has no further deleterious effect on polarization of GFP-Sec4. Thus, Myo2 has an essential function distinct from transport of secretory vesicles, and this new function involves Mmr1.
Robust mitochondrial inheritance is seen in wildtype cells at both temperatures when small buds (Fig.1F,I) or large buds (Fig. S1E,G) are examined. Mitochondrial inheritance is unaffected in strains containing either myo2sens alleles alone (Figs. 1I, S1F,G), but is compromised after 1.5h at the restrictive temperature in the myo2-14 mutant, and in both myo2sens mmr1ts mutants (Figs. 1F,I, S1E,G). These inheritance defects become increasingly pronounced upon prolonged incubation, accounting for the temperature-sensitive growth phenotypes.
In wildtype cells, mitochondria move into the bud soon after its formation (Fig. 1G, Movie S1A). In strains defective in mitochondrial inheritance, mitochondria retain their tubular morphology, but remain clumped in the mother cell, and fail to move into the bud at 36 °C (Fig. 1G, Movie S1B,C). No mitochondrial movement into the bud was seen in 100 of 109 cell divisions for myo2-14 and 66 of 76 for myo2-34 mmr1-20, demonstrating a defect in active transport rather than tethering at the bud tip. No defects in ER segregation were seen (Fig. S1H).
Thus, Myo2 performs an essential function in addition to polarizing secretory vesicles that involves active transport of mitochondria, a process that requires Mmr1 in the sensitized myo2sens strains. Many of the myo2sens alleles are also partially defective in polarizing secretory vesicles, indicating a mechanistic relationship between transport of secretory vesicles and mitochondrial inheritance. Indeed, the location of the myo2sens mutations in the vicinity of the binding site for the Rab protein Sec4, which is part of the receptor for Myo2 on secretory vesicles[17], may explain this partial defect.
Overexpression of either Ypt11 or Mmr1 enhances mitochondrial segregation
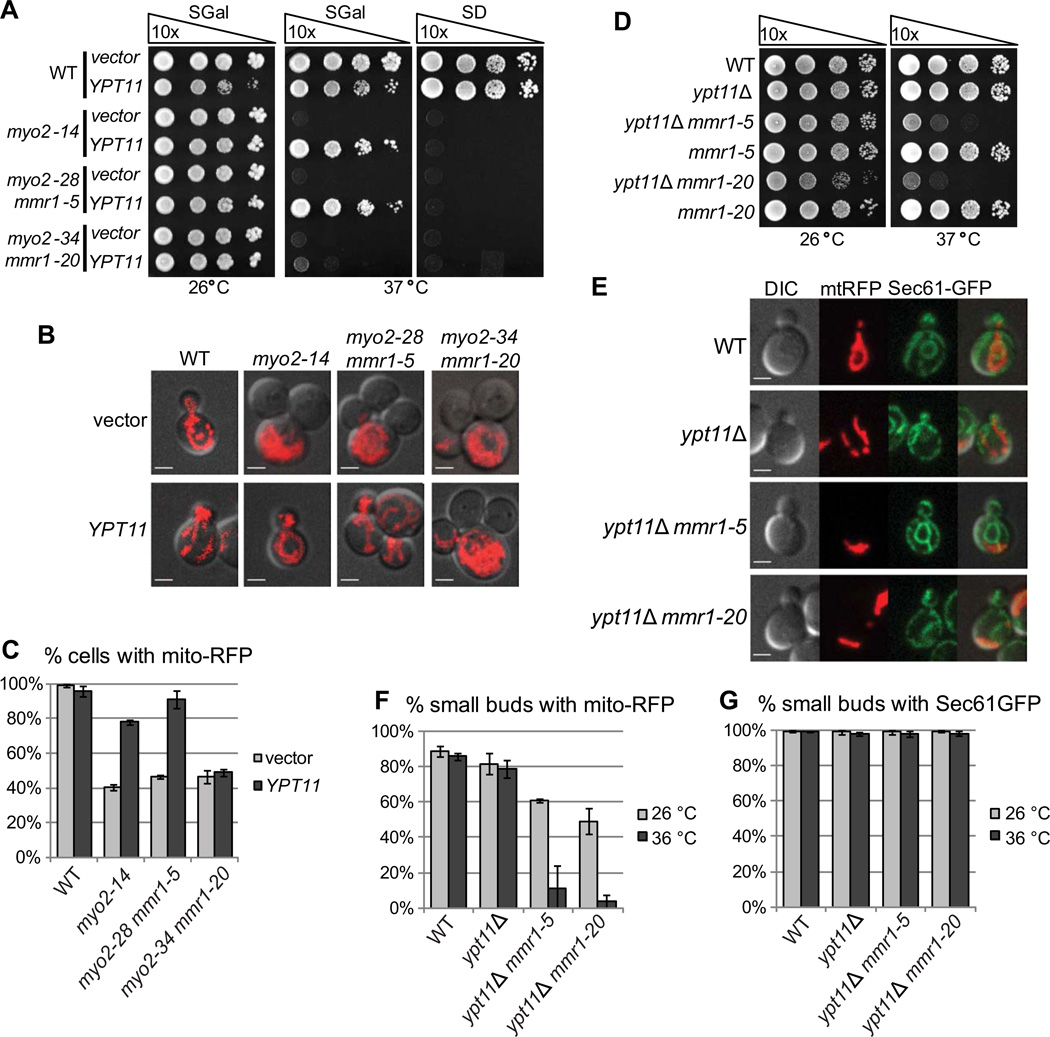
The Rab protein Ypt11 has been suggested to be either directly involved in Myo2-dependent transport of mitochondria into the bud [11], or necessary for transport of endoplasmic reticulum into the bud to tether mitochondria [7]. We explored whether the temperature sensitivity and mitochondrial segregation defect seen in our myo2sens mmr1ts strains could be suppressed by elevating Ypt11 levels. Overexpression of YPT11 suppresses the temperature sensitive growth defect and rescues mitochondrial inheritance after 5 hours at 37 °C in myo2-14 and myo2-28 mmr1-5, but not myo2-34 mmr1-20 mutants (Fig. 2A–C). As reported [9, 18], overexpression of YPT11 in wildtype cells results in hyperpolarization of mitochondria to the bud (Fig 2B). The inability of Ypt11 over-expression to suppress myo2-34 mmr1-20 is likely due to a more severe defect in the ability of the Myo2-34 tail to bind Ypt11, as discussed below, an interpretation that is consistent with its stronger defect in polarization of GFP-Sec4.
Figure 2. Ypt11 functions in mitochondrial inheritance.
(A) Overexpression of Ypt11 suppresses temperature sensitive growth defect in myo2-14 and myo2-28 mmr1-5, but not myo2-34 mmr1-20 strains. Cells transformed with a plasmid carrying PGAL-YPT11 or an empty vector were grown in SRaff-leu overnight, adjusted to OD600 of 1.0, serially 10-fold diluted, spotted on SD-leu and SRaffGal-leu plates and grown at 26 °C and 37 °C for 3 days. (B) Overexpression of Ypt11 rescues mitochondrial inheritance. Cells transformed with a plasmid carrying PGAL-YPT11 or an empty vector and expressing mito-RFP from the chromosome were grown in SRaffGal-ura-leu overnight, shifted to 36 °C for 5 hours, and analyzed by confocal fluorescence microscopy at 36 °C. Bar, 2 µm. (C) Cells were grown and analyzed as in B and scored for presence of mitochondria. Quantifications are mean values from three independent experiments (n = 300), and error bars indicate standard deviations. (D) ypt11Δ mmr1-5 and ypt11Δ mmr1-20 strains are temperature sensitive. Strains were grown overnight in YPD at 26 °C, adjusted to OD600 of 1.0, serially 10-fold diluted, spotted on YPD plates and incubated at 26 °C and 37 °C for 3 days. (E) ypt11Δ mmr1-5 and ypt11Δ mmr1-20 strains have defects in mitochondrial inheritance. Cells expressing Sec61-GFP from a plasmid and mito-RFP from the chromosome were grown to logarithmic phase in SD-ura-leu, incubated at 36 °C for 1.5 h, and analyzed by confocal fluorescence microscopy at 36 °C. Bar, 2 µm. (F,G) Cells were grown to logarithmic phase in SD-ura-leu, incubated at 26 °C or 36 °C for 1.5 h, and analyzed by confocal fluorescence microscopy at 26 °C or 36 °C. Cells carrying small buds were scored for presence of mito-RFP in the bud in F and for presence of Sec61-GFP in the bud in G. Quantifications are mean values from three independent experiments (n = 100), and error bars indicate standard deviations.
We also tested the effect of MMR1 overexpression from the GAL1 promoter in the myo2-14 strain. As reported [8, 9, 18], MMR1 overexpression in wildtype cells also results in accumulation of mitochondria in the bud (Fig. S2C). In the myo2-14 strain, overexpression of MMR1 suppresses the temperature sensitive growth defect at 37 °C and restores mitochondrial distribution to near wildtype levels (Fig. S2B–D). These results, and the location of residues mutated in Myo2-14 (Fig. 1D), support a model in which Myo2-14 is partially compromised in binding both Mmr1 and Ypt11. Notably, overexpression of either YPT11 or MMR1 has no effect on levels of GFPSec4 polarization (Fig. S2A,E).
ypt11Δ mmr1ts mutants are temperature sensitive due to defect in mitochondrial inheritance
Our results so far suggest that Ypt11 and Mmr1 can each contribute to mitochondrial inheritance, and that mmr1ts alleles have compromised Mmr1 function at restrictive temperature. Since ypt11Δ shows synthetic lethality with mmr1Δ [8, 9], we combined ypt11Δ with mmr1ts alleles in a wildtype MYO2 background. Whereas the ypt11Δ, mmr1-5, and mmr1-20 strains grow like wildtype, ypt11Δ mmr1-5 and ypt11Δ mmr1-20 each have a conditional growth defect (Fig. 2D) that can be complemented by either YPT11 or MMR1 (Fig. S2F). Both double mutants have severe defects in mitochondrial inheritance in small and large budded cells (Fig. 2E,F, S2G,H). No active mitochondrial transport into the bud was seen n 99 of 112 cell divisions recorded in live cell movies at 37° C in ypt11Δ mmr1-20 cells, again indicating a defect in active transport rather than bud capture (Movie S1D). Moreover, no defects were observed in segregation of the endoplasmic reticulum into the bud (Fig. 2E,G), which contradicts the model in which ER segregation depends on Ypt11 and mitochondria are tethered to the ER by Mmr1[7]. No defects in polarization of secretory vesicles were observed (Fig. S2I).
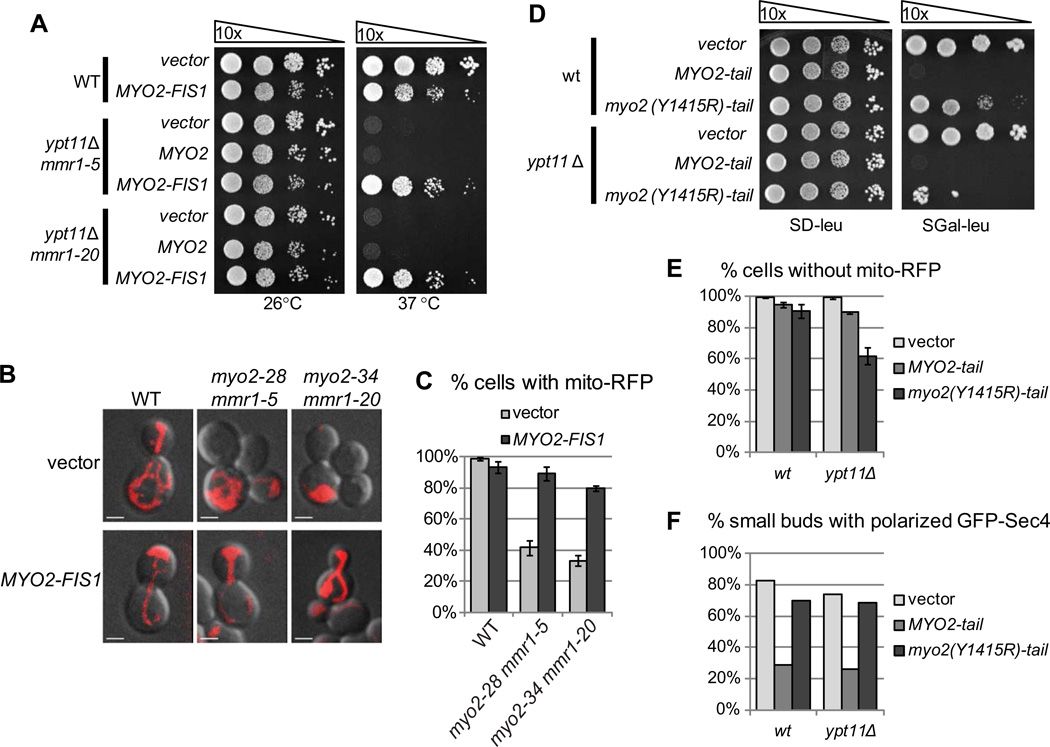
ypt11Δ mmr1ts mutants have both temperature sensitive growth and mitochondrial inheritance defects. To test if these defects are related, we examined whether tethering Myo2 directly to mitochondria rescues growth by circumventing the need for Myo2 receptors and forcing mitochondrial inheritance. To this end, we expressed the MYO2-FIS1 construct[10, 11], in which the Myo2 tail domain is replaced with the membrane anchor of the mitochondrial protein Fis1. Expression of this construct alters mitochondrial distribution slightly in wildtype cells, but does not affect growth or levels of mitochondrial inheritance (Fig. 3A–C). Both growth and mitochondrial inheritance is restored in the ypt11Δ mmr1-5 and ypt11Δ mmr1-20 strains by expression of MYO2-FIS1 (Fig. 3A–C). Therefore, the essential function compromised in the ypt11Δ mmr1ts strains is in the active transport of mitochondria into the bud.
Figure 3. Active mitochondrial inheritance by Myo2 is essential for cell viability.
(A) MYO2-FIS1 chimera rescues growth in ypt11Δ mmr1ts mutants. Cells transformed with a CEN LEU2 plasmid carrying MYO2, MYO2-FIS1, or an empty vector were grown overnight in SD-ura-leu at 26 °C, adjusted to OD600 of 1.0, serially 10-fold diluted, spotted on SD-ura-leu plates and grown at 26 °C or 37 °C for 3 days. (B) MYO2-FIS1 chimera rescues mitochondrial inheritance in ypt11Δ mmr1ts mutants. Cells transformed with a CEN LEU2 plasmid carrying MYO2-FIS1 or an empty vector and expressing mito-RFP from the chromosome were grown to logarithmic phase in SD-ura-leu, incubated at 36 °C for 5 h, and analyzed by confocal fluorescence microscopy at 36 °C. Bar, 2 µm. (C) Cells were grown and analyzed as in B and scored for presence of mitochondria. Quantifications are mean values from three independent experiments (n = 300), and error bars indicate standard deviations. (D) Overexpression of the Myo2(Y1415R)-tail is toxic in a ypt11Δ, but not wildtype, strain. Cells transformed with a plasmid carrying PGAL-MYO2-tail, PGALMYO2(Y1415R)-tail or an empty vector were grown in SRaff-leu overnight, adjusted to OD600 of 1.0, serially 10-fold diluted, spotted on SD-leu and SGal-leu plates and grown at 26 °C 3 days. (E) Overexpression of the Myo2(Y1415R)-tail in a ypt11Δ strain causes a mitochondrial inheritance defect. Strains shown in D and expressing mito-RFP from the chromosome were grown to logarithmic phase in SRaff-ura-leu, induced with galactose for 6 h, analyzed by confocal fluorescence microscopy at 26 °C, and cells were scored for presence of mitochondria. Quantifications are mean values from three independent experiments (n = 300), and error bars indicate standard deviations. (F) Overexpression of the Myo2(Y1415R)-tail does not affect GFP-Sec4 polarization. Strains shown in E and expressing GFP-Sec4 from a plasmid were grown to logarithmic phase in SRaff-ura-leu, induced with galactose for 1.5 h, analyzed by confocal fluorescence microscopy at 26 °C, and scored for GFP-Sec4 polarization.
This approach was possible because in our S288C strain background, ypt11Δ and mmr1Δ show synthetic lethality. In the W303 background, the ypt11Δ mmr1Δ deletion is viable, but the triple ypt11Δ mmr1Δ gem1Δ deletion is lethal, implying that Gem1 also contributes to mitochondrial inheritance [16, 18]. Recently, Gem1 was found to associate with and regulate the ER-mitochondrial encounter structure (ERMES), a site of association between the ER and mitochondria [19]. While earlier work had shown that defects in the ERMES complex affect both mitochondrial morphology and inheritance [4], mitochondrial inheritance defects in some ERMES mutants are a secondary consequence of the morphological defect [20]. The precise contribution of Gem1 to mitochondrial inheritance in W303 cells remains to be elucidated.
Our finding that either Mmr1 or Ypt11 can mediate mitochondrial transport by Myo2 challenges the report by Fortsch et al. that Ypt11, but not Mmr1, acts with Myo2 to transport mitochondria [10, 11]. Their conclusion was based on the findings that the myo2(Q1233R,L1301P) mutant is synthetic lethal with ypt11Δ, but has no synthetic interaction with mmr1Δ. In light of our data and the identification of Q1233 and L1301 residues as part of the Mmr1 binding site on the Myo2 tail [14], we suggest that the myo2(Q1233R,L1301P) mutant is dependent on Ypt11 for mitochondrial inheritance because it is unable to bind Mmr1.
Direct involvement of the Myo2 tail in mitochondrial inheritance
The model thus far indicates that mitochondrial inheritance is actively mediated by Myo2 and requires either Ypt11 or Mmr1. Since both Ypt11 and Mmr1 interact with the Myo2 tail [8, 9], the model predicts that the myo2sens alleles should compromise the ability of Myo2 to bind Ypt11, and that the mmr1ts alleles inhibit binding of Myo2 to Mmr1, while myo2-14 compromises binding to both Mmr1 and Ypt11. This prediction is complicated by the fact that the Rab proteins Ypt11 and Sec4 bind to an overlapping region on the Myo2-tail [14, 17], so mutations in Myo2 that completely abrogate Ypt11 interactions would be lethal due to the requirement for Sec4-binding for secretory vesicle transport.
We used two-hybrid analysis to examine the interaction of Mmr1 with the Myo2 tails (Fig. S3). As predicted, the Myo2-14 tail is severely compromised in its interaction with Mmr1, making it dependent on Ypt11 for mitochondrial inheritance, whereas the Myo2-28 and Myo2-34 tails show wildtype binding, and the mmr1-5 allele abrogates all interaction with Myo2. For unknown reasons the two-hybrid interaction of the Myo2 tail with Ypt11 is much more robust than with Sec4. While a defect in the interaction of the Myo2-14 tail with Ypt11 is not evident, it is clearly compromised in its ability to interact with the Rab Sec4, thus accounting for its conditional phenotype in mitochondrial inheritance and partial defect in GFPSec4 polarization. The Myo2-34 tail is compromised in binding Ypt11, thereby explaining its dependence on Mmr1 for viability and our findings that findings that overexpression of YPT11 does not rescue the defects of the myo2-34 mmr1-20 strain. Myo2-28 has a modest defect in binding Ypt11, which is sufficient to render Mmr1 essential.
Transport of mitochondria into the bud by Myo2 via Mmr1 and Ypt11 requires an association of Mmr1 and Ypt11 with mitochondria. Mmr1 has been reported to localize to mitochondria [8], but Ypt11 has been reported to localize to the endoplasmic reticulum [7, 21] or the Golgi apparatus [22]. However, it was recently shown that Ypt11 mislocalizes when it is over-expressed, and endogenous Ypt11 is indeed localized to mitochondria and contributes to mitochondrial inheritance [23].
Over-expression of the Myo2 tail is lethal, most likely by titrating out receptors needed for polarized delivery of secretory vesicles [13, 17, 24]. Indeed, the lethal over-expression of the wildtype Myo2 tail results in depolarization of GFPSec4 in both wildtype and ypt11Δ strains (Fig, 3D,F). However, over-expression of the Myo2 tail harboring the Y1415R mutation, which abolishes interaction with Rab proteins including Sec4 and Ypt11 [14, 17, 25], is not lethal and does not affect GFPSec4 polarization, but results in a mild mitochondrial inheritance defect in wildtype cells (Fig. 3D,E). If mitochondrial inheritance is essential, and requires the interaction of Ypt11 or Mmr1 with the Myo2 tail, expression of the Myo2(Y1415R) tail in a ypt11Δ strain should generate a mitochondrial inheritance defect by titrating Mmr1, and lead to a growth defect. Indeed, expression of the mutant tail in ypt11Δ cells is toxic and induces a mitochondrial inheritance defect, but has no effect on GFP-Sec4 polarization (Fig. 3D–F). This shows that interfering with the ability of Myo2 to bind mitochondria and transport them into the bud is lethal.
Loss of mitochondrial inheritance does not inhibit cytokinesis and leads to formation of multiple buds lacking mitochondria
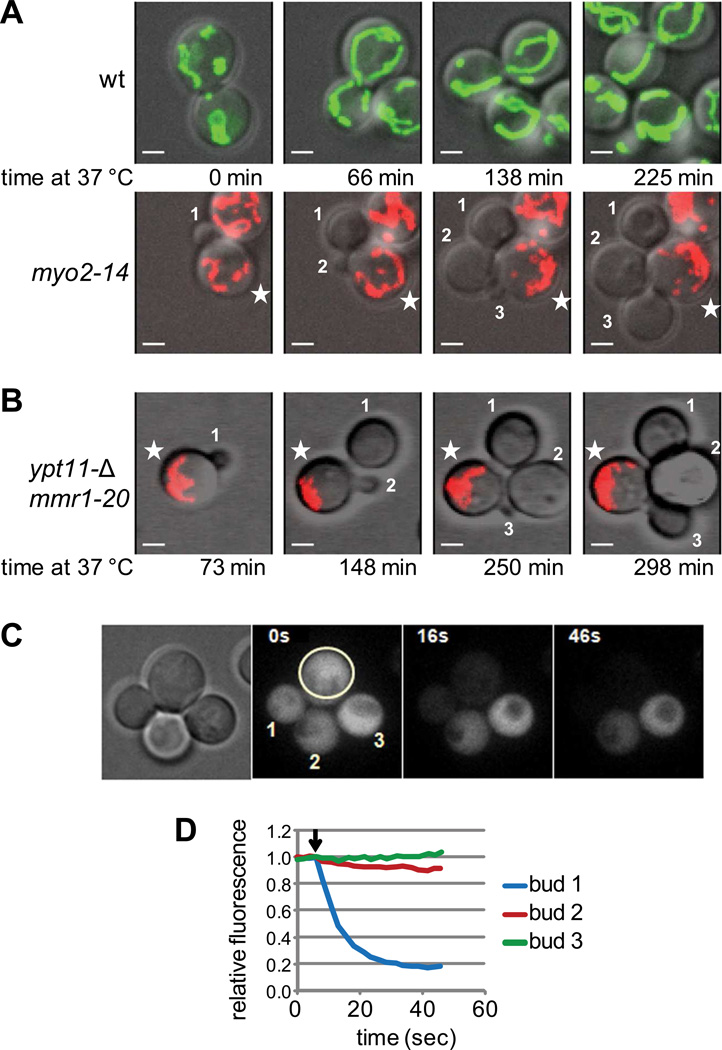
A checkpoint to delay cytokinesis in daughter cells lacking mitochondria has been observed in studies with ERMES mutants that affect mitochondrial morphology and inheritance[12]. To explore this in our system where mitochondrial morphology is unaffected, we mixed wildtype cells expressing mito-GFP with myo2-14 cells expressing mito-RFP and imaged over time at the restrictive temperature. Strikingly, myo2-14 cells budded multiple times even when none of the daughter buds received mitochondria, whereas mitochondrial inheritance was normal in wildtype cells (Fig. 4A, Movies S2A,B). Multiple budding cycles were tracked and the time from initial bud formation to the emergence of the next bud determined. We found no difference in the average budding time at 37 °C, with 83±31 minutes for wildtype cells, and 83±28 minutes for myo2-14 cells. The large error is due to a lot of scatter in the data, likely caused by buds growing outside the focal plane. Formation of multiple buds lacking mitochondria was also observed in ypt11Δ mmr1-20 cells (Fig. 4B, Movie S2C).
Figure 4. Cells defective in mitochondrial inheritance form multiple buds lacking mitochondria.
(A) Wildtype cells expressing mito-GFP and myo2-14 cells expressing mito-RFP were grown to logarithmic phase in SD-ura, mixed, attached to a glass-bottom dish and imaged at 37 °C for 5 hours. Images correspond to stills from movies S2A and S2B, and time at 37 °C is indicated. Star indicates mother cell, daughter cells are numbered in order they appear. Bar, 2 µm. Budding time of 29 cells divisions quantified for wildtype cells, 30 for myo2-14 cells. (B) ypt11Δ mmr1-20 cells expressing mito-RFP were grown and analyzed as in A. Images correspond to stills from movie S1C. (C) myo2-14 cells transformed with a plasmid carrying PGALGFP and expressing mitoRFP from the chromosome (not shown) were grown to logarithmic phase in SRaff-leu, shifted to 36 °C and induced with galactose for 4 hours, and analyzed by confocal fluorescence microscopy at 36 °C. The mother cell (circled) was continuously photobleached, and fluorescence in each of the daughter cells was measured and quantified in (D).
To determine if myo2-14 cells undergo cytokinesis before they initiate growth of a new bud, soluble GFP was expressed from the GAL1 promoter in myo2-14 cells and continually bleached in a mother cell with multiple buds. In the representative example shown in Fig. 4C and quantified in Fig. 4D, fluorescence in only one of the three buds is bleached, whereas the other two buds remain unaffected, indicating that two of the three buds lacking mitochondria have undergone cytokinesis. In each of the seventeen “multi-budded” cells examined in this manner, fluorescence was bleached in at most one bud. This shows that buds without mitochondria can undergo cytokinesis, and do so before emergence of the next bud. Whether the difference in cytokinesis between our studies and those of Garcia-Rodriguez et al. is due to the altered mitochondrial morphology in mdm10Δ and mmm1Δ cells remains to be determined. The insurance of having redundant pathways may explain why cells have evolved without the need for a mitochondrial inheritance checkpoint.
In this study we have provided strong evidence that mitochondrial inheritance is an active and essential function of Myo2 and requires either Mmr1 or Ypt11. Perhaps surprisingly, we find no evidence for a mitochondrial inheritance cell cycle checkpoint. The isolation of ypt11Δ mmr1ts mutants in which mitochondrial inheritance is conditionally defective in the presence of wildtype Myo2 provides a useful system in which to study the inheritance of this essential organelle.
Experimental Procedures
See Supplementary Material
Supplementary Material
Highlights.
Active mitochondrial segregation is an essential process independent of ER segregation
Mitochondrial segregation is an essential specific function of myosin-V, Myo2
Active segregation depends on association of Ypt11 or Mmr1 with Myo2
Without mitochondrial segregation, cells continue to divide giving rise to dead cells
Acknowledgements
We are grateful to James Young, who first uncovered a relationship between Myo2 and Mmr1 in the Bretscher lab. We thank S. Emr and J. Shaw for providing constructs used in this study, and C. Fromme, T. Fox, and members of the Bretscher lab for comments on the manuscript. This work was supported by PHS grant GM039066 to AB and NIH NRSA Fellowship F32GM097999 to IC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pruyne D, Legesse-Miller A, Gao L, Dong Y, Bretscher A. Mechanisms of polarized growth and organelle segregation in yeast. Annu Rev Cell Dev Biol. 2004;20:559–591. doi: 10.1146/annurev.cellbio.20.010403.103108. [DOI] [PubMed] [Google Scholar]
- 2.Weisman LS. Organelles on the move: insights from yeast vacuole inheritance. Nat Rev Mol Cell Biol. 2006;7:243–252. doi: 10.1038/nrm1892. [DOI] [PubMed] [Google Scholar]
- 3.Boldogh I, Vojtov N, Karmon S, Pon LA. Interaction between mitochondria and the actin cytoskeleton in budding yeast requires two integral mitochondrial outer membrane proteins, Mmm1p and Mdm10p. The Journal of cell biology. 1998;141:1371–1381. doi: 10.1083/jcb.141.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boldogh IR, Nowakowski DW, Yang HC, Chung H, Karmon S, Royes P, Pon LA. A protein complex containing Mdm10p, Mdm12p, and Mmm1p links mitochondrial membranes and DNA to the cytoskeleton-based segregation machinery. Molecular biology of the cell. 2003;14:4618–4627. doi: 10.1091/mbc.E03-04-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boldogh IR, Yang HC, Nowakowski WD, Karmon SL, Hays LG, Yates JR, 3rd, Pon LA. Arp2/3 complex and actin dynamics are required for actin-based mitochondrial motility in yeast. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3162–3167. doi: 10.1073/pnas.051494698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boldogh IR, Ramcharan SL, Yang HC, Pon LA. A type V myosin (Myo2p) and a Rab-like G-protein (Ypt11p) are required for retention of newly inherited mitochondria in yeast cells during cell division. Molecular biology of the cell. 2004;15:3994–4002. doi: 10.1091/mbc.E04-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swayne TC, Zhou C, Boldogh IR, Charalel JK, McFaline-Figueroa JR, Thoms S, Yang C, Leung G, McInnes J, Erdmann R, et al. Role for cER and Mmr1p in anchorage of mitochondria at sites of polarized surface growth in budding yeast. Current biology : CB. 2011;21:1994–1999. doi: 10.1016/j.cub.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itoh T, Toh EA, Matsui Y. Mmr1p is a mitochondrial factor for Myo2p-dependent inheritance of mitochondria in the budding yeast. The EMBO journal. 2004;23:2520–2530. doi: 10.1038/sj.emboj.7600271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itoh T, Watabe A, Toh EA, Matsui Y. Complex formation with Ypt11p, a rab-type small GTPase, is essential to facilitate the function of Myo2p, a class V myosin, in mitochondrial distribution in Saccharomyces cerevisiae. Mol Cell Biol. 2002;22:7744–7757. doi: 10.1128/MCB.22.22.7744-7757.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altmann K, Frank M, Neumann D, Jakobs S, Westermann B. The class V myosin motor protein, Myo2, plays a major role in mitochondrial motility in Saccharomyces cerevisiae. The Journal of cell biology. 2008;181:119–130. doi: 10.1083/jcb.200709099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortsch J, Hummel E, Krist M, Westermann B. The myosin-related motor protein Myo2 is an essential mediator of bud-directed mitochondrial movement in yeast. The Journal of cell biology. 2011;194:473–488. doi: 10.1083/jcb.201012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Rodriguez LJ, Crider DG, Gay AC, Salanueva IJ, Boldogh IR, Pon LA. Mitochondrial inheritance is required for MEN-regulated cytokinesis in budding yeast. Current biology : CB. 2009;19:1730–1735. doi: 10.1016/j.cub.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schott D, Ho J, Pruyne D, Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. The Journal of cell biology. 1999;147:791–808. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eves PT, Jin Y, Brunner M, Weisman LS. Overlap of cargo binding sites on myosin V coordinates the inheritance of diverse cargoes. The Journal of cell biology. 2012;198:69–85. doi: 10.1083/jcb.201201024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schott DH, Collins RN, Bretscher A. Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. The Journal of cell biology. 2002;156:35–39. doi: 10.1083/jcb.200110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frederick RL, McCaffery JM, Cunningham KW, Okamoto K, Shaw JM. Yeast Miro GTPase, Gem1p, regulates mitochondrial morphology via a novel pathway. The Journal of cell biology. 2004;167:87–98. doi: 10.1083/jcb.200405100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santiago-Tirado FH, Legesse-Miller A, Schott D, Bretscher A. PI4P and Rab inputs collaborate in myosin-V-dependent transport of secretory compartments in yeast. Developmental cell. 2011;20:47–59. doi: 10.1016/j.devcel.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frederick RL, Okamoto K, Shaw JM. Multiple pathways influence mitochondrial inheritance in budding yeast. Genetics. 2008;178:825–837. doi: 10.1534/genetics.107.083055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornmann B, Osman C, Walter P. The conserved GTPase Gem1 regulates endoplasmic reticulum-mitochondria connections. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14151–14156. doi: 10.1073/pnas.1111314108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen TT, Lewandowska A, Choi JY, Markgraf DF, Junker M, Bilgin M, Ejsing CS, Voelker DR, Rapoport TA, Shaw JM. Gem1 and ERMES do not directly affect phosphatidylserine transport from ER to mitochondria or mitochondrial inheritance. Traffic. 2012;13:880–890. doi: 10.1111/j.1600-0854.2012.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buvelot Frei S, Rahl PB, Nussbaum M, Briggs BJ, Calero M, Janeczko S, Regan AD, Chen CZ, Barral Y, Whittaker GR, et al. Bioinformatic and comparative localization of Rab proteins reveals functional insights into the uncharacterized GTPases Ypt10p and Ypt11p. Molecular and cellular biology. 2006;26:7299–7317. doi: 10.1128/MCB.02405-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arai S, Noda Y, Kainuma S, Wada I, Yoda K. Ypt11 functions in bud-directed transport of the Golgi by linking Myo2 to the coatomer subunit Ret2. Curr Biol. 2008;18:987–991. doi: 10.1016/j.cub.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Lewandowska A, Macfarlane J, Shaw JM. Mitochondrial association, protein phosphorylation and degradation regulate the availability of the active Rab GTPase, Ypt11, for mitochondrial inheritance. Molecular biology of the cell. 2013 doi: 10.1091/mbc.E12-12-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karpova TS, Reck-Peterson SL, Elkind NB, Mooseker MS, Novick PJ, Cooper JA. Role of actin and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae. Molecular biology of the cell. 2000;11:1727–1737. doi: 10.1091/mbc.11.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casavola EC, Catucci A, Bielli P, Di Pentima A, Porcu G, Pennestri M, Cicero DO, Ragnini-Wilson A. Ypt32p and Mlc1p bind within the vesicle binding region of the class V myosin Myo2p globular tail domain. Mol Microbiol. 2008;67:1051–1066. doi: 10.1111/j.1365-2958.2008.06106.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.