Abstract
Objectives
The aim of this review is to examine the evidence for a functional cholinergic system operating within the periodontium and determine the evidence for its role in periodontal immunity.
Introduction
Acetylcholine can influence the immune system via the ‘cholinergic anti-inflammatory pathway’. This pathway is mediated by the vagus nerve which releases acetylcholine to interact with the α7 subunit of the nicotinic acetylcholine receptor (α7nAChR) on proximate immuno-regulatory cells. Activation of the α7nAChR on these cells leads to down-regulated expression of pro-inflammatory mediators and thus regulates localised inflammatory responses. The role of the vagus nerve in periodontal pathophysiology is currently unknown. However, non-neuronal cells can also release acetylcholine and express the α7nAChR; these include keratinocytes, fibroblasts, T cells, B cells and macrophages. Therefore, by both autocrine and paracrine methods non-neuronal acetylcholine can also be hypothesised to modulate the localised immune response.
Methods
A Pubmed database search was performed for studies providing evidence for a functional cholinergic system operating in the periodontium. In addition, literature on the role of the ‘cholinergic anti-inflammatory pathway’ in modulating the immune response was extrapolated to hypothesise that similar mechanisms of immune regulation occur within the periodontium.
Conclusion
The evidence suggests a functional nonneuronal ‘cholinergic anti-inflammatory pathway’ may operate in the periodontium and that this may be targeted therapeutically to treat periodontal disease.
Keywords: Periodontal disease, Acetylcholine, Alpha 7 nicotinic receptor, Inflammation
Introduction
Periodontal disease (PD) is a chronic, destructive, inflammatory disease which left untreated leads to tooth mobility, loss of dental function and ultimately tooth loss. PD is an important clinical problem which affects an estimate of 8–15 % of the UK population [1]. A widely accepted view is that although periodontal disease is initiated by the pathogenic microflora of the subgingival plaque the host response plays an essential role in disease pathogenesis. In an attempt to remove the pathogenic microflora of the periodontium the host mounts an immune response which in susceptible individuals becomes dysregulated. Instead of clearing the pathogenic threat the dysregulated immune response leads to bystander damage such as the breakdown of connective tissue and bone loss [2]. Although the pathogenesis of periodontal disease has yet to be fully elucidated; key cellular and molecular mechanisms have been identified which contribute to the tissue destruction and bone loss which present clinically. In response to threat, immune-competent oral keratinocytes and resident macrophages become excessively activated and orchestrate the infiltration of neutrophils and other inflammatory cells into the oral mucosal tissue. As part of this process increased angiogenesis occurs which helps support the inflammatory infiltrate. If these inflammatory processes are not effectively regulated it can lead to the creation of a hyper-inflammatory environment characterised by excessive production of immuno-modulatory mediators including; cytokines, chemokines, prostanoids, antimicrobials and enzymes. Many of these mediators, such as the matrix metalloproteinases (MMPs) and antimicrobial peptides play direct roles in localised tissue destruction. In addition, many inflammatory mediators regulate the processes of bone remodelling. Indeed a hyper-inflammatory environment can perturb the balance between bone formation (osteoblastogenesis) and bone resorption (osteoclastogenesis) leading to loss of the alveolar bone which supports the teeth. Therefore the prominent role of the inflammatory response in periodontal disease pathogenesis suggests that host response modulation may provide novel therapeutic interventions for treatment [3].
Recently, it has become clear that Acetylcholine (ACh) is an important regulator of immune responses. Exogenous ACh has been shown to bind to the appropriate receptors on immune and tissue cells involved in inflammatory processes and modulate the expression of key inflammatory mediators [4]. As ACh is an important neurotransmitter, the vagus nerve was demonstrated to be a major source of free ACh. However, there are many parts of the body which are far removed from the influence of the vagus nerve. Indeed, to date there is little evidence that indicates that the vagus nerve directly influences the periodontium. The vagus nerve however is not the only source of free ACh. In fact, there is now overwhelming evidence that cells out with neuronal networks synthesize, store and release ACh [5]. In addition, all components of the cholinergic system; including ACh and/or the synthesizing enzyme, choline acetyltransferase (ChAT), the enzyme responsible for its breakdown, Acetylcholinesterase (AChE) and receptors to which ACh binds (nicotinic and muscarinic) have been demonstrated in the vast majority of non-neuronal human cells [6]. These include immune-competent epithelial cells such as keratinocytes and fibroblasts, as well as cells of the immune system such as monocytes/macrophages, B cells and T cells. Therefore, non-neuronally derived ACh has also been implicated to play a role in regulating localised immune responses.
In this review we will examine the evidence for a functional cholinergic system in the periodontium. In addition, we will describe the potential cytotransmitter function of non-neuronal ACh and the role it plays in regulating the immune response. Using evidence from the literature derived from studies of other chronic inflammatory conditions we will also speculate a role for ACh in modulating the pathogenesis of periodontal disease and examine the potential of cholinergic based therapies in order to treat the condition.
Acetylcholine and its receptors
ACh is widely distributed in prokaryotic and eukaryotic cells. To date, despite its ubiquitous expression, research into the biological role of ACh has primarily focused on its neurotransmitter function. However, as early as 1963 Whittaker stated that: ‘acetylcholine occurs in non-nervous tissues and is widely distributed in nature to suggest a non-nervous function of it’ [7]. Thus, the biological role of ACh in humans has had to be revised to accommodate two roles: neuronal ACh acting as a neurotransmitter to mediate rapid communication between neurons and effector cells and non-neuronal ACh acting as a local signalling molecule involved in the regulation of cellular phenotype [8].
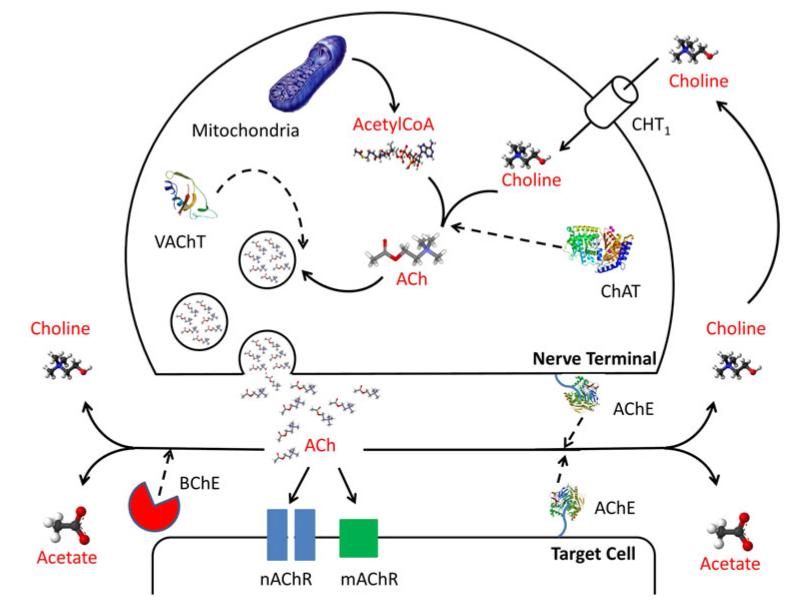
Knowledge of the synthesis, storage, metabolism and actions of ACh has been derived mostly from studies of the mammalian nervous system [9] and our current understanding is demonstrated in Fig. 1. In brief, ACh is synthesized predominantly by choline acetyltransferase (ChAT) from choline and acetyl coenzyme A (AcCoA). AcCoA is the major product of carbohydrate, protein and lipid catabolism in aerobic organisms and is thus present in more or less all cells. Choline on the other hand originates from the intracellular breakdown of choline containing phospholipids or from the uptake of extracellular choline via lowor high-affinity choline transporter 1 (CHT1). At nerve terminals, ACh is taken up by the vesicular acetylcholine transporter (VAChT) and stored in characteristic small, optically clear vesicles. ACh is then released from nerve terminals by exocytosis. Release studies using the human placenta as a model of the non-neuronal cholinergic system, due to the fact it lacks nervous innervation, have also demonstrated that non-neuronal ACh is actively released despite the tissue being less well endowed with cholinergic vesicles. However, the mechanism of release is hypothesised to be different to neuronal ACh due to the fact that the majority of non-neuronal cells cannot generate action potentials or open voltage regulated calcium channels which trigger exocytosis [10]. Indeed, in the placental model of non-neuronal ACh release it has been found that ACh leaves cells via organic cation transporters (OCTs) [11]. OCTs are members of the solute carrier family of transporters responsible for the influx and efflux of organic cations, such as ACh, across cell membranes. There are 37 known members of the OCT family and they are ubiquitously expressed in humans, although the evidence, from the placental model of non-neuronal ACh, suggests that OCT1 and OCT3 are the favoured subtype for involvement in ACh release [11]. Unlike a typical hormone, ACh is rapidly hydrolyzed upon its release predominantly by the enzyme acetylcholinesterase (AChE), with a small contribution from butyrylcholinesterase (BChE), into choline and acetate [6], therefore limiting the efficacies of ACh to cells in close proximity to its site of synthesis and release (Fig. 1).
Fig. 1.
Diagram demonstrating the mechanism of acetylcholine synthesis, release, action and breakdown at a cholinergic nerve terminal. ACh Acetylcholine, ChAT choline acetyltransferase, CHT1 highaffinity choline transporter-1, VAChT vesicular ACh transporter, AChE acetylcholinesterase, BChE butyrylcholinesterase, mAChR muscarinic receptors and nAChR nicotinic receptors (Adapted from Kummer et al. [72])
Upon release, ACh acts on either muscarinic or nicotinic receptors (mAChR and nAChR, respectively). The metabotropic actions of ACh are mediated by activation of the muscarinic receptor family. These receptors are seven transmembrane glycoproteins encoded by intronless genes; and comprise five distinct subtypes, denoted as muscarinic receptor type 1–5 (M1–M5). Activation of mAChRs is relatively slow and depending on the subtypes involved causes alterations in cellular levels of phospholipase C, inositol trisphosphate, cyclic AMP (cAMP) and free calcium [12]. Upon activation, mAChRs couple to heterotrimeric guanine nucleotide binding proteins (G proteins) to regulate second messengers and ion channel activities [8]. Nicotinic acetylcholine receptors (nAChRs) on the other hand are classic representatives of a large superfamily of ligand gated ion channel proteins. The nAChRs are fast ionotropic cationic receptors, which mediate the influx of Na+ and Ca2+ as well as the efflux of K+ ions. The increase in intracellular Ca2+ that arises from nAChR activation can activate calcium sensitive signal transduction protein kinases such as adenylyl cyclase (AC), protein kinase A (PKA), protein kinase C (PKC), Ca2+ calmodulin dependent protein kinase (CaMK) and phosphatidylinositol 3-kinase (PI3K). In turn, these phosphorylate downstream targets, such as extracellular signal regulated mitogen activated protein kinase (ERK), which leads to the activation of transcription factors such as the cAMP response element-binding protein (CREB) and nuclear factor kappa light chain enhancer of activated B cells (NF-κB). In turn, activation of these transcription factors then modulates gene expression and leads to phenotypical changes in the cell. To date, 13 nAChR subunits have been cloned in humans; seven α like subunits (α2, α3, α4, α5, α6, α7, α9 and α10) and 6 non-α subunits (β2, β3, β4, δ, ε and γ). The α2–α6, β2–β 4, δ, ε and γ subunits can all form hetero-oligomeric receptors, whereas the α7 and α9 subunits can only form homo-oligomeric receptors. To date however the exact signalling mechanism(s) mediating downstream effects of many of the nAChRs are not yet completely understood [13].
The cholinergic system of the periodontium
The influence of neuronally derived ACh on periodontal tissues is currently unknown. However, oral epithelial cells have been demonstrated to express components of a functional non-neuronal cholinergic system. Therefore, epithelially derived ACh can act in both an autocrine and paracrine manner to regulate periodontal tissue function. The epithelial cells lining the human attached gingiva have been shown to express the ACh synthesising enzyme, ChAT, and two molecular forms of the degrading enzyme, AChE [14]. In addition, although the literature is sparse on the subject, there is pharmacological evidence to show that oral epithelial cells express OCTs [15]. The demonstration of these components in human oral epithelium is evidence that cells of the periodontium can synthesise and release ACh. Indeed, free ACh has been detected in oral keratinocyte homogenates and culture supernatants [16] as well as gingival tissue samples [17]. In addition, oral keratinocytes and fibroblasts express both nicotinic (nAChR) and muscarinic (mAChR) ACh receptors. The α3, α5, α7, α9, β2 and β4 nAChR receptor subunits [14] and the muscarinic type 2 (M2), type 3 (M3), type 4 (M4) and type 5 (M5) receptors [18] are all expressed in human gingival keratinocytes. Furthermore, although the ACh receptor profile of oral fibroblasts has yet to be fully elucidated, periodontal ligament fibroblasts are known to express the α7nAChR [19] and pharmacological evidence indicates that gingival fibroblasts express the M2 and M3 receptor [20, 21].
Research into the biology of ACh within the periodontium has mainly centred on its role in the regulation of oral keratinocyte growth and differentiation. Indeed it has been demonstrated that tobacco products act upon nAChRs and can alter cell cycle progression and lead to squamatisation of oral keratinocytes and squamous cell carcinoma [22]. Interestingly, alterations to oral keratinocyte cell cycle progression and differentiation to the squamous cell phenotype was found to coincide with increased expression of nAChR subunits [22, 23]. Furthermore activation of the nAChRs was shown to play a central role in the oral keratinocytes response to tobacco products via an intracellular signalling pathway which increased expression and nuclear translocation of both the signal transducer and activator of transcription 3 (STAT-3) and NF-κB transcription factors; which play a role in the control of expression of genes involved in mediating cell growth and apoptosis [22–24].
These findings present strong functional evidence for a non-neuronal cholinergic system operating within the periodontium. Although to date the research only suggests an important role for ACh in regulating cellular growth, death and differentiation. Non-neuronal ACh is however a multifunctional cytotransmitter involved in numerous cellular processes including modulating gene expression, cellular proliferation, cytoskeletal organization, cell–cell contact (tight and gap junctions, desmosomes), locomotion, migration, ciliary activity, electrical activity, secretion and absorption. In addition, non-neuronal ACh also plays a role in the control of unspecific and specific immune functions [6]. It is therefore interesting to speculate a similar role for non-neuronal ACh in the periodontal environment.
The cholinergic anti-inflammatory pathway
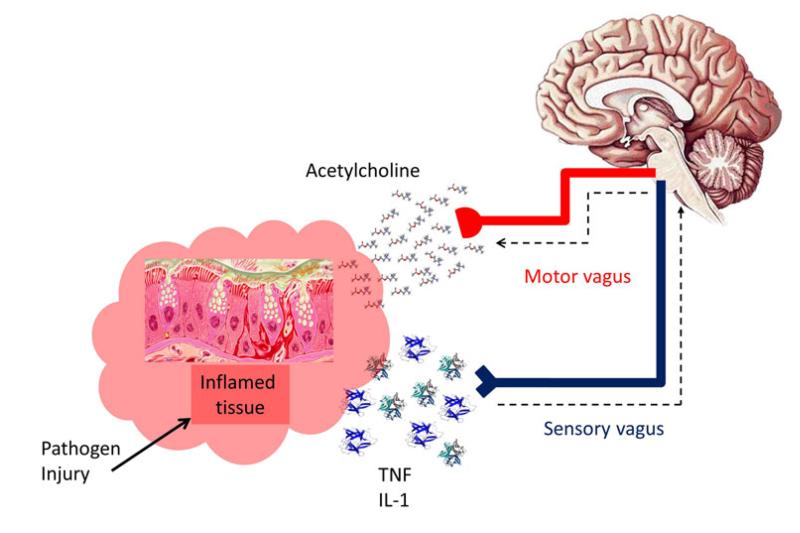
Recently the alpha7 nicotinic receptor (α7AChR) has received a great deal of attention from immunologists due its crucial role in regulating the ‘cholinergic anti-inflammatory pathway’. The discovery of the ‘cholinergic antiinflammatory pathway’ was a major breakthrough and was described as the ‘missing link in neuroimmunomodulation’ [25]. The pathway was first postulated after pioneering work by the group of Kevin Tracey who demonstrated that systemic, hepatic and splenic Tumour Necrosis Factor alpha (TNF-α) production and symptoms of endotoxemia were exacerbated in rodents which had undergone a vagotomy and reversed in animals subjected to electrical stimulation of the cervical vagal nerve [26]. The pathway is initiated by hallmarks of inflammation activating the afferent vagus nerve and relaying information warning of the occurring inflammation to the brain. Subsequent efferent vagal nerve activity then leads to an increase in ACh release within the inflamed peripheral tissues lying proximal to the nerve. The free ACh then binds to the α7AChRs on immune competent cells and regulates localised inflammatory processes. It is therefore the efferent arm of the vagal nerve reflex which is actually termed the ‘cholinergic anti-inflammatory pathway’. The cholinergic anti-inflammatory pathway is therefore important in ensuring the appropriate degree of immune system activation for the perceived threat (Fig. 2).
Fig. 2.
Diagram demonstrating the basic principles of the ‘cholinergic anti inflammatory pathway’. Cytokines such as those of the TNF and IL-1 family released from inflamed tissue activate the sensory (afferent) vagus nerve relaying information of the occurring inflammation to the brain. Subsequent (motor) efferent vagal nerve activity then leads to ACh release within the inflamed peripheral tissues lying proximal to the nerve. The free ACh then binds to the α7AChRs on immune competent cells and down regulates localised inflammation (Adapted from Pavlov et al. [25])
The ‘cholinergic anti-inflammatory pathway’ has been the subject of extensive research over the last 10 years. Studies have demonstrated that vagus nerve stimulation can attenuate the systemic inflammatory response to endotoxin [26]. Furthermore, studies using α7nAChR-deficient (α7nAChR−/−) animals and pharmacological α7nAChR agonists/antagonists have demonstrated the importance of this receptor in modulating the ‘cholinergic anti-inflammatory pathway’ and controlling unrestrained inflammation in response to pathogens. For example, α7nAChR−/− mice were found to be hypersensitive to bacterial lipopolysaccharide (LPS) and exhibited an exaggerated production of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 [27]. Indeed, using α-bungarotoxin, an inhibitor of the α7nAChR, activation of this receptor was demonstrated to be critical for the suppression of TNF-α release in response to LPS in mice [27]. In addition, α7nAChR−/− mice, upon treatment with LPS or upon infection with Escherichia coli (E. coli), were shown to develop more severe lung injury and to have more mortality than corresponding α7nAChR+/+ mice in a model of sepsis induced lung injury [28]. It has also been shown that nicotine, an α7nAChR agonist, attenuates serum levels of the key mediator of lethal systemic inflammation, high mobility group box protein 1 (HMGB1), and therefore improves survival in mouse models of disease [29].
Targeting the ‘cholinergic anti-inflammatory pathway’ has been the subject of investigation for potential therapeutics to treat chronic inflammatory conditions such as Crohn’s disease, psoriasis, rheumatoid arthritis, asthma, sepsis and diabetes [30]. An anti-inflammatory role for ACh in arthritis was suggested using both vagal nerve stimulation and cholinergic agonists. Vagal nerve stimulation was shown to suppress the development of collagen-induced arthritis (CIA) in a rat model and this coincided with a decrease in serum levels of TNF-α and in turn a reduction in osteoclasts within the joint. This suggested an indirect role for cholinergic control of bone destruction [31]. Furthermore, mice that were subjected to a cervical vagotomy and induction of arthritis with type II collagen demonstrated that clinical arthritis was exacerbated by vagotomy and ameliorated by nicotine administration [32,33]. In fact, the mice that were administered nicotine showed inhibited bone degradation and reduced TNF-α expression, while mice that were administered a specific α7nAChR agonist (AR-R17779) demonstrated a total amelioration of clinical arthritis and significantly reduced synovial inflammation accompanied by a reduction of TNF-α levels in both plasma and synovial tissue [33]. As the agonist AR-R17779 could not pass the blood brain barrier the authors hypothesized that its effects occurred on α7nAChRs proximal to the site of inflammation. Indeed, expression of the α7nAChR was later observed on the intimal lining of the synovium and in cultured fibroblast-like synoviocytes (FLS) [34, 35]. Furthermore, in vitro studies showed that stimulation of the α7nAChR on FLS suppresses the expression of IL-6 and several chemokines, including CXCL8 (IL-8) [36]. These results suggest a strong involvement of the localised α7nAChR in the regulation of inflammation in the synovium and therefore further studies were performed in vivo using α7nAChR−/− mice. In a model of CIA, α7nAChR−/− mice exhibited significant increases in incidences and severity of arthritis as well as increased synovial inflammation and joint destruction compared to wild type litter mates. This exacerbation was found to coincide with elevated systemic levels of proinflammatory cytokines, an enhanced Th1 profile and elevated TNF-α production from spleen cells [37]. In contrast, using a similar animal model, the lack of α7nAChR was actually found to suppress the development of CIA [38]. However, this study was performed over a 14 day period whilst the previous study was performed over a 44 day period which is more representative of the acute phase of the disease. Interestingly though, this second study demonstrated that the α7nAChR plays a role in the adaptive immune response, in addition to localised innate immune responses, in arthritis as in the α7nAChR−/− animals a decreased T cell content and proliferation was observed in both the spleen and lymph nodes [38].
The evidence therefore suggests that ACh and the α7nAChR can play a key role in the pathogenesis of chronic inflammatory diseases such as rheumatoid arthritis. It is known that there are remarkable similarities in the pathogenesis of periodontal disease and rheumatoid arthritis; in particular with regard to a poorly modulated inflammatory response resulting in tissue injury and bone loss. Therefore it is interesting to speculate that similar mechanisms may operate within the periodontium to prevent an unrestrained inflammatory response.
Cholinergic regulation of periodontal disease
The role of ACh in modulating inflammation, coupled with the presence of the α7nAChR on resident and infiltrating immune-competent cells of the periodontium allows us to speculate that cholinergic modulation of the oral immune response may occur. However, the influence of the vagal nerve on the oral cavity is a matter of debate. Indeed whether the vagal nerve can regulate the periodontal immune response is unknown at present. Undoubtedly, the vagal nerve plays an important role as a sensory and nociceptive system to communicate the activation state of the immune system to the brain. However, it is still questionable whether the anti-inflammatory effect on immune cells is mediated directly by vagally released neuronal ACh [39]. Indeed, it has to be considered that the enzyme responsible for the breakdown of ACh, AChE, is extremely effective and stops neuronal ACh from migrating far from its source. Therefore, for the mechanism to be effective the immune cells need to be in close proximity to the source of neuronal ACh. Although some immune cells do show close membrane apposition with neuronal elements, for example, in the area postrema [40], this is not always the case. Indeed there is little evidence to suggest that there is close apposition of immune cells with neuronal elements within the periodontium. Therefore, the question remains as to how a vagally mediated efferent response on immune cells is migrated through periodontal tissue which are not in close proximity to nerve fibers? Indeed, although a functional relationship between inflammatory disease pathogenesis and the ‘cholinergic anti-inflammatory pathway’ was confirmed when it was discovered that a decrease in vagal nerve tone (reflecting parasympathetic activity) occurs in patients with rheumatoid arthritis [41]. The actual response of the isolated inflammatory cells from these patients to cholinergic agonists ex vivo was found to be mediated by mechanisms that were independent of vagus nerve activity [42]. The evidence therefore suggested that non-neuronally derived ACh played a role in regulating localised inflammation. The α7nAChR is widely expressed by cell types found in the periodontium and infiltrating immune cells with defined roles in the pathogenesis of periodontal disease; including monocytes and macrophages, B cells and T cells. Indeed, T cells and monocytes have been demonstrated to contain high levels of ACh [43]. This therefore suggests it has both autocrine and paracrine functions in these cell types and indeed the potential for a localised cholinergic anti-inflammatory regulatory circuit.
At present, there is no direct evidence for a role of ACh in regulating the immune response of oral epithelial cells. However, ACh (via the α7nAChR) has been demonstrated to play a role in modulating the immune response of various other immune-competent epithelial cells. In airway epithelial cells of cystic fibrosis patient’s the activation of the α7nAChR was shown to inhibit Toll like receptor 2 (TLR-2) mediated IL-8 expression [44]. In addition, activation of the α7nAChR was demonstrated to inhibit TNF-α induced IL-8 expression in human colon epithelial cells [45] and nicotine was also shown to reduce TNF-α expression in HBE16 airway epithelial cells, mainly through the α7nAChR influencing the NF-κB pathway [46]. As both oral keratinocytes and fibroblasts have been shown to express the α7nAChR it could be hypothesised that a similar immunomodulatory phenomenon occurs in these cells types. Indeed preliminary data has shown that a specific α7nAChR agonist (PHA-543613) can inhibit the Porphyromonas gingivalis (P. gingivalis) induced expression of IL-8 by the oral keratinocyte cell line; OKF6/TERT2 (Zoheir et al. unpublished observations). This therefore suggests that ACh may play a role in modulating neutrophil chemotaxis within the periodontium; although further research is required to confirm this.
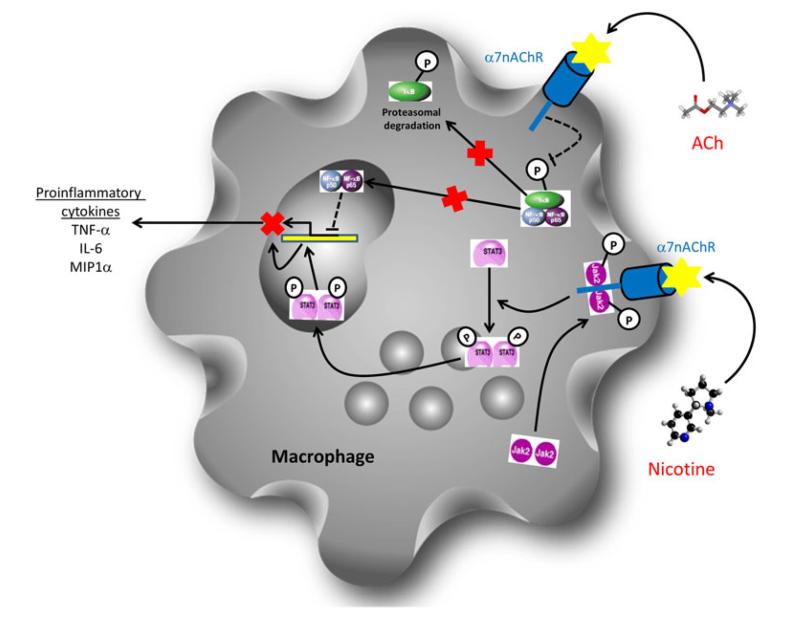
Macrophages act as sentinels of the periodontal immune system alerting effectors of both the innate and adaptive immune systems to pathogenic threats. Macrophages dictate the innate immune response through the release of pro-inflammatory mediators and direct phagocytosis of invading pathogens. In addition, macrophages are antigen presenting cells and can present phagocytosed constituents of invading pathogens on the cell surface to dictate the adaptive immune response. In vitro studies have demonstrated a role for the α7nAChR in modulating the immune response of macrophages. In addition, it is a well established fact that macrophages release ACh as a method to communicate their activation state. Macrophages/monocytes have also been shown to express the α7nAChR [47] and its activation demonstrated to mediate signalling pathways leading to down regulated NF-κB nuclear translocation and the suppression of transcription of proinflammatory cytokines. Indeed, treatment of LPS activated human peripheral monocytes with low doses of nicotine caused inhibition of TNF-α and macrophage inflammatory protein 1 alpha (MIP-1α) expression. These effects were mediated through the α7nAChR which led to the suppressed phosphorylation of the Inhibitor of kappa B α (IκBα) and in turn inhibited the transcriptional activity of NF-κB [48] (Fig. 3). Furthermore, studies in peritoneal macrophages showed that activation of the α7nAChR can also stimulate the JAK-2/STAT-3 pathway both in vitro and in vivo. Activation of the STAT-3 cascade by the ligation of nicotine to the α7nAChR led to inhibition of proinflammatory cytokine release as STAT-3 is a negative regulator of the inflammatory response [49] (Fig. 2). Interestingly, STAT-3 activation is required for the antiinflammatory action of IL-10 via the IL-10 receptor and studies have shown that activation of the α7nAChR may only induce specific down regulation of pro-inflammatory cytokines as it did not prevent the constitutive release of the anti-inflammatory; IL-10 [49].
Fig. 3.
Diagram demonstrating the two major mechanisms by which activation of the α7nAChR can inhibit expression and release of pro-inflammatory cytokines by macrophages. Activation of the α7nAChR has been demonstrated to inhibit the expression of pro-inflammatory cytokines. Activation of the α7nAChR inhibits the phosphorylation of Inhibitor of Kappa B (IκB) by the Inhibitor of Kappa B kinase (IKK). This in turn inhibits the dissociation of IκB from the active p50/p65 NF-κB complex and prevents translocation of the active NF-κB transcription factor into the nucleus and therefore inhibits expression of pro-inflammatory cytokines. Activation of the α7nAChR has also been shown to lead to the formation of a heterodimeric protein complex with phosphorylated Janus Kinase 2 (Jak2). The active JAK2 then activates the signal transducer and activator of transcription 3 (STAT3). The active STAT3 molecule can then translocate to the nucleus where it acts as a negative regulator of pro-inflammatory cytokine expression
As well as playing a role in modulating the innate immune response there is a multitude of evidence to suggest that ACh plays vital roles in modulating adaptive immunity. Although the role the adaptive immune response plays in periodontal disease is still not fully understood there is evidence that it is important in disease progression as mature IgG secreting B cells are often associated with established periodontal lesions. In addition, there is often a predominance of Th2 cells and Th2 cytokines associated with periodontal lesions and antibodies against periodontal pathogens can be detected in lesions as well as serum, saliva and gingival crevicular fluid (GCF) of periodontal disease patients. T cells are a major source of the detectable levels of ACh found in blood and T cells express the α7nAChR [43]. In human and murine T cells, α7nAChR mRNA expression is elevated upon antigenic and mitogenic stimulation [50]. Recent studies have shown that activation of the α7nAChR on lymphocytes modulates the Th1/Th2 dynamic by inhibiting production of Th1 cytokines and IL-17, elevating IL-10 expression and also increasing the expression of the prototypic Th2 cytokine, IL-4, by NF-κB mediated signal pathways [51]. In addition, activation of the T cell α7nAChR plays a direct role in modulating T cell proliferation and has been demonstrated to induce upregulated expression of the trans-acting T cell specific transcription factor GATA-3 (GATA-3), a pivotal Th2 transcription factor, but down regulate expression of T cell specific T-box transcription factor (T-bet), a pivotal Th1 transcription factor [52]. Therefore data suggests that ACh plays a major role in skewing the Th lineage development from Th1 and Th17 to Th2. Furthermore, the α7nAChR has been demonstrated to be present on CD4+CD25+ regulatory T cells (Tregs) and activation was found to enhance the regulatory capacity of these cells by inducing upregulated expression of forkhead box protein 3 (FoxP3) and the cytotoxic T lymphocyte associated antigen 4(CTLA-4), while decreasing secretion of IL-2. Therefore, the evidence suggests that the α7nAChR may be a critical regulator of the immunosuppressive functions of Tregs [53]. The additional importance of α7nAChR+ T cell populations in regulating the suppressive effect of ACh was demonstrated recently by the discovery of a unique ACh-producing (ChAT+), memory phenotype (CD4+ CD44high CD26Low) T cell population in the spleen of mice that was found to be integral to the vagal nerve dictated ‘cholinergic anti-inflammatory pathway’ [54]. Indeed, although it has been established that many immune cells can produce ACh, this report is the first to demonstrate that innate immune cell derived ACh and not neuronal ACh plays an integral role in controlling innate immune responses in vivo.
As well as modulating the T cell response the α7nAChR has also been demonstrated to have a direct effect on T cell signalling. Evidence has shown that the α7nAChR on T cells interacts with CD3ξ, the mitogenic portion of the T cell receptor complex. Indeed both CD4+ and CD8+ T cells demonstrated expression of the α7nAChR after in vitro TCR/CD3 crosslinking [55]. This, coupled with the fact that T cells are a major source of ACh suggests that the α7nAChR may play a major role in modulating T cell function [56].
The role of the α7nAChR on B cell populations is to date relatively unknown. B cells express the α7nAChR [57] and it has been demonstrated to play a role in cellular proliferation and activation via a CD40 dependant pathway [58]. In addition, levels of α7nAChR expression have been shown to be elevated in mature B cells and it has been suggested that the α7nAChR can negatively control CD40-mediated B lymphocyte proliferation without affecting affect the IgM–IgG class switch or memory B cell activation [59]. Indeed, in vitro, the α7nAChR has been demonstrated to be recruited to the immune synapse between human T and B cells and inhibit the binding of CD40 to the CD40 ligand and therefore inhibit B lymphocyte activation [60]. These data therefore suggest that ACh is an additional modulator of B lymphocyte activation.
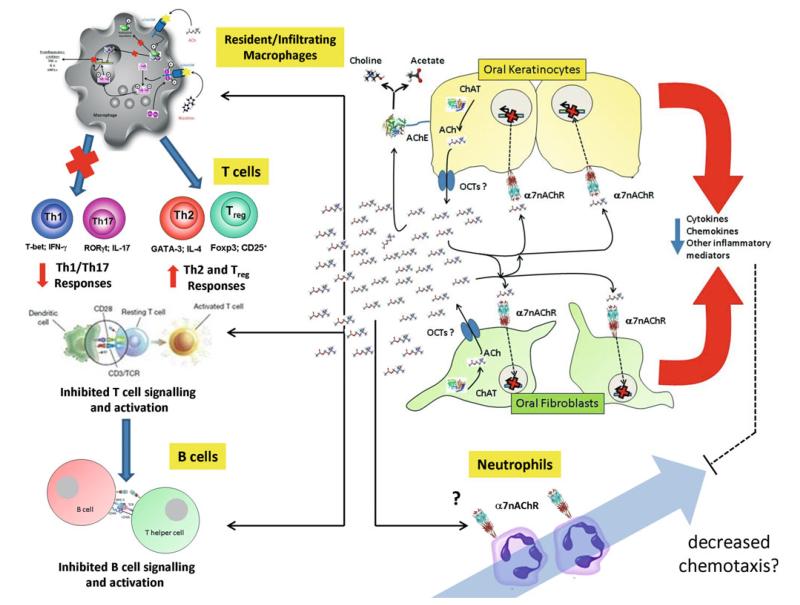
The literature suggests that ACh and the α7nAChR play key roles in modulating both the innate and adaptive immune response. Indeed, ACh has been demonstrated to modulate the immune response of a multitude of cell types involved in the pathogenesis of periodontal disease. Therefore, combined with the fact that all the components of a non-neuronal ACh system are present in the cells of the periodontium, it is reasonable to hypothesise that a non-neuronal ACh driven cholinergic anti-inflammatory mechanism may operate within the periodontium and function to promote resolution of inflammation and promote healing. Figure 4 demonstrates the potential roles for ACh and the α7nAChR in modulating the periodontal immune response. Although research in this field is at present in its infancy it is still interesting to speculate that this pathway can be targeted therapeutically to modulate periodontal disease pathogenesis.
Fig. 4.
The proposed mechanism of non-neuronal acetylcholine production by oral epithelial cells and its hypothesised influence on the pathogenesis of periodontal disease. Both oral keratinocytes and fibroblasts are known to express ChAT and therefore produce ACh. The exact mechanism of release however is unknown but there is tentative evidence for the expression of the organic cation transporters (OCTs) in the oral epithelium. In addition, oral keratinocytes are known to express the ACh degrading enzyme, AChE and therefore can recycle choline. However, there is no evidence as yet to describe the expression of a functional CHT1. Once released, ACh can act in both an autocrine and paracrine manner on the α7nAChR present on oral keratinocytes and fibroblasts and downregulate the expression of pro-inflammatory mediators. This therefore directly down regulates the localised innate inflammatory response. In reducing the pro-inflammatory nature of the cytokine milieu, ACh can also indirectly influence the progression of inflammation as a reduction in pro-inflammatory signals will lead to decreased invasion of innate immune cells such as macrophages and neutrophils into the periodontium. Although neutrophils have been found to express the α7nAChR, there is little data on the direct influence of ACh on the neutrophil response. In contrast, ACh can directly influence resident or invading macrophages which again express the α7nAChR and activation of which also leads to downregulated expression of pro-inflammatory mediators by these cells. Furthermore, ACh via macrophages has been shown to influence the T cell paradigm and inhibit the expression of Th1 and Th17 promoting cytokines and therefore promote a Th2 and Treg response. In addition, the α7nAChR has been found to be closely associated with the CD3ξ mitogenic portion of the T cell receptor on T cells and therefore ACh may play a direct role in influencing T cell signalling. Likewise, expression of the α7nAChR has been shown to be elevated in mature B cells and it has been suggested that the α7nAChR can negatively control CD40-mediated B lymphocyte proliferation
Nicotine and periodontal disease
There is overwhelming evidence that the use of tobacco products is associated with increased incidence of periodontal disease and poor response to periodontal therapy. However, while smokers are more susceptible to developing inflammatory periodontal diseases, smoking masks overt signs of gingival inflammation [61]. Therefore the relationship between nicotine, the host immune response and periodontal disease pathogenesis is complex and to date still not well understood.
It is now well established that oral epithelial cells express a variety of nAChRs [14, 62] and the evidence indicates that direct activation of nAChRs plays a major role in the pathogenesis of nicotine-related periodontitis. Indeed, nicotine activation of nAChRs was found to enhance both IL-1β and P. gingivalis LPS induced IL-8 release from gingival epithelial cell lines [62]. This therefore suggests a direct pro-inflammatory role for nicotine in periodontal disease pathogenesis. Indeed, in a ligature induced rat model of periodontal disease nicotine was shown to upregulate expression of IL-1β in periodontal ligament cells. In addition, α-bungarotoxin (a specific α7nAChR antagonist) was shown to partially inhibit this phenomenon [19]. This therefore suggests a role for the α7nAChR in promoting inflammation; which is contradictory to the literature in other inflammatory diseases. However, it must be also considered that α-bungarotoxin also acts upon the α3-coupled nAChRs and indeed these receptors have been found to be the major candidates for mediating the deleterious effects of tobacco products in other tissues [63]. In contrast to the study by Kashiwagi et al., activation of nAChRs has actually been shown to have a suppressive effect on periodontal immunity. Indeed, although Breivik et al. [64] showed that nicotine enhanced bone loss in a rat model of ligature induced periodontitis, the authors did observe a smaller increase in circulating levels of pro-inflammatory cytokines after intra-peritoneal injection of LPS in the animals treated with nicotine. This led the authors to therefore hypothesise that nicotine enhances susceptibility to periodontitis through suppression of protective immune responses via the ‘cholinergic anti-inflammatory pathway’. This study is therefore in agreement with the current accepted view of nicotine-induced periodontal disease pathogenesis.
Conclusions
Cholinergic regulation of oral inflammation has the potential to offer new therapeutic targets for the treatment of periodontal disease. However, at present, much more research is required into the mechanisms by which cells of the periodontium synthesise, release and respond to ACh. In addition, there is an urgent need to gain an understanding of the nervous innervation of periodontal tissues, specifically the presence and role (if any) of cholinergic nerve fibres. This will help us begin to determine the mechanisms by which ACh modulates the immune response of resident and invading periodontal immune-competent cells and understand how to therapeutically target this system. To target the cholinergic pathway to treat periodontal disease then careful consideration must be taken as to the compounds utilised. Many studies described have demonstrated a role for nicotine in activating the α7nAChR and modulating inflammation in vivo. However, the cellular effects of nicotine in the human periodontium or in animal models of periodontal disease have not been fully elucidated at present. Indeed, the literature to date demonstrates a detrimental effect for nicotine on periodontal disease pathogenesis. Although it is hypothesised to be due to immune suppression enhancing susceptibility to pathogenic microflora of plaque [64] it is possible that nicotine directly enhances pro-inflammatory response and enhances progression to a chronic inflammatory state [62]. Indeed, it is important to stress that nicotine is pharmacologically non-specific and also has toxic side effects. In this review we have not discussed the potential roles of the other members of the nAChR family and the mAChRs in modulating inflammation. Indeed, there is evidence to suggest that different nAChRs may play differing roles in the inflammatory response [60] and furthermore there is a host of evidence to suggest that in fact mAChRs play an opposing role to the α7nAChR and actually have pro-inflammatory roles [65]. Therefore as many resident and transient cells of the periodontium express a repertoire of both nAChRs and mAChRs it can hypothesised using pharmacologically non specific compounds such as nicotine may elicit complex and detrimental effects in vivo. Therefore when considering potential therapeutics to target the cholinergic anti-inflammatory pathway then it is important that they exhibit specificity for the α7nAChR. In recent years a number of α7nAChR specific agonist compounds have been investigated in the literature as potential therapeutics for treatment of inflammatory diseases and have shown successful outcomes when applied to in vivo animal models (Table 1). Therefore, investigation into the use of these compounds as therapeutics to treat periodontal disease may be a desirable subject for future investigation.
Table 1.
Specific α7nAChR pharmaceutical agonists investigated in the literature for their in vivo therapeutic effects on the pathogenesis of a number of inflammatory conditions
| α7nAChR specific agonist |
Animal model |
Inflammatory condition |
Outcome | Reference |
|---|---|---|---|---|
| GTS-21 | Mouse | Endotoxemia/ sepsis |
Improved survival coinciding with decreased serum levels of TNF-α and HMGB1 | [66] |
| Mouse | Pancreatitis | Lower pancreatic inflammation with decreased plasma levels of IL-6 and amylase | [67] | |
| PNU-282987 | Mouse | Acute lung injury | Decreased excess lung water and lung vascular permeability with reduced TNF-α and MIP2 in BAL | [68] |
| AR-R17779 | Mouse | Rheumatoid arthritis |
Ameliorated clinical arthritis and reduced synovial inflammation with a reduction of TNF-α levels | [33] |
| Mouse | Intestinal inflammation |
Prevents postoperative ileus characterised by reduced inflammatory cell recruitment | [69] | |
| DMPP | Mouse | Asthma | Reduced airway inflammation with reduced IgE and number of eosinophils in BAL | [70] |
| TC-7020 | Mouse | Type 2 diabetes | Reduced weight gain, glucose and glycated hemoglobin levels and lowered plasma levels of TNF-α | [71] |
Although host response modulation offers exciting potential for the development of therapeutics to treat periodontal disease we must still consider that the pathogenic bacteria of plaque are major players in disease pathogenesis. Therefore, when considering any immuno-modulatory strategy to treat periodontal disease the need for good oral hygiene and antimicrobial strategies must not be neglected. Therefore, it may be interesting to speculate that the use of specific α7nAChR agonists as a complement to a good oral hygiene regime and in conjunction with current antimicrobial therapies, rather than a stand alone treatment, might be more efficacious in the treatment of periodontal disease.
References
- 1.Kelly MSJ, Nuttall N, et al. The condition of supporting structures. In: Walker A, Cooper I, editors. Adult dental health survey. Oral health in the United Kingdom. vol. 2000. Stationary office Books; London: 1998. pp. 123–146. [Google Scholar]
- 2.Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79:1585–91. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- 3.Preshaw PM. Host response modulation in periodontics. Periodontology. 2000;2008(48):92–110. doi: 10.1111/j.1600-0757.2008.00252.x. [DOI] [PubMed] [Google Scholar]
- 4.Fujii T, Takada-Takatori Y, Kawashima K. Basic and clinical aspects of non-neuronal acetylcholine: expression of an independent, non-neuronal cholinergic system in lymphocytes and its clinical significance in immunotherapy. J Pharmacol Sci. 2008;106:186–92. doi: 10.1254/jphs.fm0070109. [DOI] [PubMed] [Google Scholar]
- 5.Klapproth H, Reinheimer T, Metzen J, et al. Non-neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:515–23. doi: 10.1007/pl00004977. [DOI] [PubMed] [Google Scholar]
- 6.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–71. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.VP W. Identification of acetylcholine and related esters of biological origin. In: Eichler O, Farah A, Koelle GB, editors. Handbuch Der Experimentellen Pharmakologie. vol. 15. 1963. pp. 1–39. [Google Scholar]
- 8.Wessler I, Kilbinger H, Bittinger F, Unger R, Kirkpatrick CJ. The non-neuronal cholinergic system in humans: expression, function and pathophysiology. Life Sci. 2003;72:2055–61. doi: 10.1016/s0024-3205(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 9.Kawashima K, Fujii T. Basic and clinical aspects of non-neuronal acetylcholine: overview of non-neuronal cholinergic systems and their biological significance. J Pharmacol Sci. 2008;106:167–73. doi: 10.1254/jphs.fm0070073. [DOI] [PubMed] [Google Scholar]
- 10.Wessler I, Roth E, Schwarze S, et al. Release of non-neuronal acetylcholine from the human placenta: difference to neuronal acetylcholine. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:205–12. doi: 10.1007/s002100100445. [DOI] [PubMed] [Google Scholar]
- 11.Wessler I, Roth E, Deutsch C, et al. Release of non-neuronal acetylcholine from the isolated human placenta is mediated by organic cation transporters. Br J Pharmacol. 2001;134:951–6. doi: 10.1038/sj.bjp.0704335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishii M, Kurachi Y. Muscarinic acetylcholine receptors. Curr Pharm Des. 2006;12:3573–81. doi: 10.2174/138161206778522056. [DOI] [PubMed] [Google Scholar]
- 13.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen VT, Hall LL, Gallacher G, et al. Choline acetyltransferase, acetylcholinesterase, and nicotinic acetylcholine receptors of human gingival and esophageal epithelia. J Dent Res. 2000;79:939–49. doi: 10.1177/00220345000790040901. [DOI] [PubMed] [Google Scholar]
- 15.Brayton JJ, Yang Q, Nakkula RJ, Walters JD. An in vitro model of ciprofloxacin and minocycline transport by oral epithelial cells. J Periodontol. 2002;73:1267–72. doi: 10.1902/jop.2002.73.11.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grando SA, Kist DA, Qi M, Dahl MV. Human keratinocytes synthesize, secrete, and degrade acetylcholine. J Invest Dermatol. 1993;101:32–6. doi: 10.1111/1523-1747.ep12358588. [DOI] [PubMed] [Google Scholar]
- 17.Rajeswari KR, Satyanarayana M. Cholinergic components in human gingiva in healthy and inflamed states. Indian J Dent Res. 1990;2:166–9. [PubMed] [Google Scholar]
- 18.Arredondo J, Hall LL, Ndoye A, Chernyavsky AI, Jolkovsky DL, Grando SA. Muscarinic acetylcholine receptors regulating cell cycle progression are expressed in human gingival keratinocytes. J Periodontal Res. 2003;38:79–89. doi: 10.1034/j.1600-0765.2003.01006.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang XJ, Liu YF, Wang QY, et al. Functional expression of alpha 7 nicotinic acetylcholine receptors in human periodontal ligament fibroblasts and rat periodontal tissues. Cell Tissue Res. 2010;340:347–55. doi: 10.1007/s00441-010-0949-9. [DOI] [PubMed] [Google Scholar]
- 20.Thangjam GS, Kondaiah P. Regulation of oxidative-stress responsive genes by arecoline in human keratinocytes. J Periodontal Res. 2009;44:673–82. doi: 10.1111/j.1600-0765.2008.01176.x. [DOI] [PubMed] [Google Scholar]
- 21.Thangjam GS, Agarwal P, Khan I, et al. Transglutaminase-2 regulation by arecoline in gingival fibroblasts. J Dent Res. 2009;88:170–5. doi: 10.1177/0022034508329633. [DOI] [PubMed] [Google Scholar]
- 22.Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocytes. FASEB J. 2006;20:2093–101. doi: 10.1096/fj.06-6191com. [DOI] [PubMed] [Google Scholar]
- 23.Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: acceleration of sequential expression of alpha5 and alpha7 nicotinic receptor subunits in oral keratinocytes exposed to cigarette smoke. FASEB J. 2008;22:1356–68. doi: 10.1096/fj.07-9965.com. [DOI] [PubMed] [Google Scholar]
- 24.Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: alterations of the NF-kappaB expression and activity downstream of alpha7 nicotinic receptor in oral keratinocytes. Life Sci. 2007;80:2191–4. doi: 10.1016/j.lfs.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125–34. [PMC free article] [PubMed] [Google Scholar]
- 26.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 28.Su X, Matthay MA, Malik AB. Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing Gram-negative sepsis-induced acute lung inflammatory injury. J Immunol. 2010;184:401–10. doi: 10.4049/jimmunol.0901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–21. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 30.Bencherif M, Lippiello PM, Lucas R, Marrero MB. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol Life Sci. 2011;68:931–49. doi: 10.1007/s00018-010-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang P, Han D, Tang T, Zhang X, Dai K. Inhibition of the development of collagen-induced arthritis in Wistar rats through vagus nerve suspension: a 3-month observation. Inflamm Res. 2008;57:322–8. doi: 10.1007/s00011-008-8070-1. [DOI] [PubMed] [Google Scholar]
- 32.Li T, Zuo X, Zhou Y, et al. The vagus nerve and nicotinic receptors involve inhibition of HMGB1 release and early pro-inflammatory cytokines function in collagen-induced arthritis. J Clin Immunol. 2010;30:213–20. doi: 10.1007/s10875-009-9346-0. [DOI] [PubMed] [Google Scholar]
- 33.van Maanen MA, Lebre MC, van der Poll T, et al. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009;60:114–22. doi: 10.1002/art.24177. [DOI] [PubMed] [Google Scholar]
- 34.Waldburger JM, Boyle DL, Pavlov VA, Tracey KJ, Firestein GS. Acetylcholine regulation of synoviocyte cytokine expression by the alpha7 nicotinic receptor. Arthritis Rheum. 2008;58:3439–49. doi: 10.1002/art.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westman M, Engstrom M, Catrina AI, Lampa J. Cell specific synovial expression of nicotinic alpha 7 acetylcholine receptor in rheumatoid arthritis and psoriatic arthritis. Scand J Immunol. 2009;70:136–40. doi: 10.1111/j.1365-3083.2009.02266.x. [DOI] [PubMed] [Google Scholar]
- 36.van Maanen MA, Stoof SP, van der Zanden EP, et al. The alpha7 nicotinic acetylcholine receptor on fibroblast-like synoviocytes and in synovial tissue from rheumatoid arthritis patients: a possible role for a key neurotransmitter in synovial inflammation. Arthritis Rheum. 2009;60:1272–81. doi: 10.1002/art.24470. [DOI] [PubMed] [Google Scholar]
- 37.van Maanen MA, Stoof SP, Larosa GJ, Vervoordeldonk MJ, Tak PP. Role of the cholinergic nervous system in rheumatoid arthritis: aggravation of arthritis in nicotinic acetylcholine receptor alpha7 subunit gene knockout mice. Ann Rheum Dis. 2010;69:1717–23. doi: 10.1136/ard.2009.118554. [DOI] [PubMed] [Google Scholar]
- 38.Westman M, Saha S, Morshed M, Lampa J. Lack of acetylcholine nicotine alpha 7 receptor suppresses development of collagen-induced arthritis and adaptive immunity. Clin Exp Immunol. 2010;162(1):62–67. doi: 10.1111/j.1365-2249.2010.04204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–45. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goehler LE, Erisir A, Gaykema RP. Neural-immune interface in the rat area postrema. Neuroscience. 2006;140:1415–34. doi: 10.1016/j.neuroscience.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 41.Stojanovich L, Milovanovich B, de Luka SR, et al. Cardiovascular autonomic dysfunction in systemic lupus, rheumatoid arthritis, primary Sjogren syndrome and other autoimmune diseases. Lupus. 2007;16:181–5. doi: 10.1177/0961203306076223. [DOI] [PubMed] [Google Scholar]
- 42.Bruchfeld A, Goldstein RS, Chavan S, et al. Whole blood cytokine attenuation by cholinergic agonists ex vivo and relationship to vagus nerve activity in rheumatoid arthritis. J Intern Med. 2010;268:94–101. doi: 10.1111/j.1365-2796.2010.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neumann S, Razen M, Habermehl P, et al. The non-neuronal cholinergic system in peripheral blood cells: effects of nicotinic and muscarinic receptor antagonists on phagocytosis, respiratory burst and migration. Life Sci. 2007;80:2361–4. doi: 10.1016/j.lfs.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Greene CM, Ramsay H, Wells RJ, O’Neill SJ, McElvaney NG. Inhibition of Toll-like receptor 2-mediated interleukin-8 production in Cystic Fibrosis airway epithelial cells via the alpha7-nicotinic acetylcholine receptor. Mediators Inflamm. 2010;2010:423241. doi: 10.1155/2010/423241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Summers AE, Whelan CJ, Parsons ME. Nicotinic acetylcholine receptor subunits and receptor activity in the epithelial cell line HT29. Life Sci. 2003;72:2091–4. doi: 10.1016/s0024-3205(03)00089-4. [DOI] [PubMed] [Google Scholar]
- 46.Li Q, Zhou XD, Kolosov VP, Perelman JM. Nicotine reduces TNF-alpha expression through a alpha7 nAChR/MyD88/NF-kB pathway in HBE16 airway epithelial cells. Cell Physiol Biochem. 2011;27:605–12. doi: 10.1159/000329982. [DOI] [PubMed] [Google Scholar]
- 47.Toyabe S, Iiai T, Fukuda M, et al. Identification of nicotinic acetylcholine receptors on lymphocytes in the periphery as well as thymus in mice. Immunology. 1997;92:201–5. doi: 10.1046/j.1365-2567.1997.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshikawa H, Kurokawa M, Ozaki N, et al. Nicotine inhibits the production of pro-inflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin Exp Immunol. 2006;146:116–23. doi: 10.1111/j.1365-2249.2006.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Jonge WJ, van der Zanden EP, The FO, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–51. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 50.Chernyavsky AI, Arredondo J, Galitovskiy V, Qian J, Grando SA. Structure and function of the nicotinic arm of acetylcholine regulatory axis in human leukemic T cells. Int J Immunopathol Pharmacol. 2009;22:461–72. doi: 10.1177/039463200902200223. [DOI] [PubMed] [Google Scholar]
- 51.Galitovskiy V, Qian J, Chernyavsky AI, et al. Cytokine-induced alterations of alpha7 nicotinic receptor in colonic CD4 T cells mediate dichotomous response to nicotine in murine models of Th1/Th17- versus Th2-mediated colitis. J Immunol. 2011;187:2677–87. doi: 10.4049/jimmunol.1002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nizri E, Irony-Tur-Sinai M, Lory O, Orr-Urtreger A, Lavi E, Brenner T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J Immunol. 2009;183:6681–8. doi: 10.4049/jimmunol.0902212. [DOI] [PubMed] [Google Scholar]
- 53.Wang DW, Zhou RB, Yao YM, et al. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine increases suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. J Pharmacol Exp Ther. 2010;335:553–61. doi: 10.1124/jpet.110.169961. [DOI] [PubMed] [Google Scholar]
- 54.Rosas-Ballina M, Olofsson PS, Ochani M, et al. Acetylcholinesynthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qian J, Galitovskiy V, Chernyavsky AI, Marchenko S, Grando SA. Plasticity of the murine spleen T-cell cholinergic receptors and their role in in vitro differentiation of naive CD4 T cells toward the Th1, Th2 and Th17 lineages. Genes Immun. 2011;12:222–30. doi: 10.1038/gene.2010.72. [DOI] [PubMed] [Google Scholar]
- 56.Karimi K, Bienenstock J, Wang L, Forsythe P. The vagus nerve modulates CD4+ T cell activity. Brain Behav Immun. 2010;24:316–23. doi: 10.1016/j.bbi.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 57.Skok MV, Kalashnik EN, Koval LN, et al. Functional nicotinic acetylcholine receptors are expressed in B lymphocyte-derived cell lines. Mol Pharmacol. 2003;64:885–9. doi: 10.1124/mol.64.4.885. [DOI] [PubMed] [Google Scholar]
- 58.Skok MV, Grailhe R, Agenes F, Changeux JP. The role of nicotinic receptors in B-lymphocyte development and activation. Life Sci. 2007;80:2334–6. doi: 10.1016/j.lfs.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Koval LM, Yu Lykhmus O, Omelchenko DM, Komisarenko SV, Skok MV. The role of alpha7 nicotinic acetylcholine receptors in B lymphocyte activation. Ukr Biokhim Zh. 2009;81:5–11. [PubMed] [Google Scholar]
- 60.Koval L, Lykhmus O, Zhmak M, et al. Differential involvement of alpha4beta2, alpha7 and alpha9alpha10 nicotinic acetylcholine receptors in B lymphocyte activation in vitro. Int J Biochem Cell Biol. 2011;43:516–24. doi: 10.1016/j.biocel.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Scott DA, Singer DL. Suppression of overt gingival inflammation in tobacco smokers clinical and mechanistic considerations. Int J Dent Hygiene. 2004;2:104–10. doi: 10.1111/j.1601-5037.2004.00079.x. [DOI] [PubMed] [Google Scholar]
- 62.Kashiwagi Y, Yanagita M, Kojima Y, et al. Nicotine up-regulates IL-8 expression in human gingival epithelial cells following stimulation with IL-1β or P gingivalis lipopolysaccharide via nicotinic acetylcholine receptor signaling. Arch Oral Biol. 2011;57(5):483–90. doi: 10.1016/j.archoralbio.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Arredondo J, Chernyavsky AI, Marubio LM, et al. Receptormediated tobacco toxicity: regulation of gene expression through alpha3beta2 nicotinic receptor in oral epithelial cells. Am J Pathol. 2005;166(2):597–613. doi: 10.1016/s0002-9440(10)62281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Breivik T, Gundersen Y, Gjermo P, von Horsten S, Opstad PK. Nicotinic acetylcholine receptor activation mediates nicotineinduced enhancement of experimental periodontitis. J Periodontal Res. 2009;44:297–304. doi: 10.1111/j.1600-0765.2009.01223.x. [DOI] [PubMed] [Google Scholar]
- 65.Razani-Boroujerdi S, Behl M, Hahn FF, Pena-Philippides JC, Hutt J, Sopori ML. Role of muscarinic receptors in the regulation of immune and inflammatory responses. J Neuroimmunol. 2008;194:83–8. doi: 10.1016/j.jneuroim.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pavlov VA, Ochani M, Yang LH, et al. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 2007;35:1139–44. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 67.van Westerloo DJ, Giebelen IA, Florquin S, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–30. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 68.Su X, Lee JW, Matthay ZA, et al. Activation of the alpha7 nAChR reduces acid-induced acute lung injury in mice and rats. Am J Respir Cell Mol Biol. 2007;37:186–92. doi: 10.1165/rcmb.2006-0240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.The FO, Boeckxstaens GE, Snoek SA, et al. Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology. 2007;133:1219–28. doi: 10.1053/j.gastro.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 70.Blanchet MR, Israel-Assayag E, Cormier Y. Modulation of airway inflammation and resistance in mice by a nicotinic receptor agonist. Eur Respir J. 2005;26:21–7. doi: 10.1183/09031936.05.00116104. [DOI] [PubMed] [Google Scholar]
- 71.Marrero MB, Lucas R, Salet C, et al. An alpha7 nicotinic acetylcholine receptor-selective agonist reduces weight gain and metabolic changes in a mouse model of diabetes. J Pharmacol Exp Ther. 2010;332:173–80. doi: 10.1124/jpet.109.154633. [DOI] [PubMed] [Google Scholar]
- 72.Kummer W, Lips KS, Pfeil U. The epithelial cholinergic system of the airways. Histochem Cell Biol. 2008;130:219–34. doi: 10.1007/s00418-008-0455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]