Abstract
Although metal ions are involved in a myriad of biological processes, a non-invasive means of detecting free metal ions in a deep tissue remains a formidable challenge. We present an approach for specifically sensing the presence of Ca2+ in which the amplification strategy of chemical exchange saturation transfer (CEST) is combined with the broad range in chemical shifts found in 19F NMR to obtain MR images of Ca2+. We exploit the chemical shift change (Δω) of 19F upon binding of Ca2+ to the difluoro derivative of [1,2,-bis(o-aminophenoxy) ethane-N,N,-N′,N′, tetra-acetic acid] (5F-BAPTA), by RF labeling at the bound-19F frequency, ω[Ca-5F-BAPTA], and detecting the label transfer to the free-19F frequency, ω5F-BAPTA. Through the substrate binding kinetics we were able to amplify the signal of Ca2+ onto free 5F-BAPTA and thus indirectly detect low Ca2+ concentrations with high sensitivity.
Metal ions play a crucial role in a myriad of biological processes, and the ability to monitor real-time changes in metal ion levels is essential for understanding a variety of physiological events. Ca2+ has garnered interest due to its involvement in many cellular functions and signaling pathways.1 Currently, imaging dynamic changes in Ca2+ levels is restricted to fluorescence-based methodologies,2,3 which are limited by low tissue penetration and therefore restrict in vivo Ca2+ imaging in deep tissues. Recent advances in the field of molecular magnetic resonance imaging (MRI) has lead to the development of new strategies in the design and synthesis of responsive contrast agents for detecting biologically relevant metal ions. Lanthanide-based complexes4–7 and modified superparamagnetic iron oxide8,9 nanoparticles have been developed for Ca2+ sensing using MRI. 1,2-bis(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA), was proposed by Tsien10 as a Ca2+ indicator, and later, its difluoro-derivative, 5F-BAPTA, showed large 19F NMR chemical shifts upon chelating divalent cations.11 The high selectivity of the binding of 5F-BAPTA to Ca2+ compared to Mg2+, and the high resolution in the 19F-NMR spectra have been exploited for intracellular Ca2+ detection in vitro and in vivo.11–13 However, MR spectroscopy (MRS)-based approaches rely on observation of the 19F resonance of the Ca-5F-BAPTA complex for Ca2+ detection resulting in limited spatial resolution due to sensitivity considerations. One alternative, suggested by Kuchel and co-workers,14 is the possibility to transfer magnetization between bound Ca2+ and free 5F-BAPTA during NMR experiments.
Chemical exchange saturation transfer (CEST) is a widely used MRI contrast mechanism in which a dynamic exchange process between radiofrequency labeled protons and bulk water is exploited for contrast enhancement, and has been used for many applications in molecular and cellular MRI.15–22 We employ a saturation transfer approach that couples 19F- and CEST-MRI for sensing the presence of Ca2+ or Mg2+ through their substrate binding kinetics, which we have termed ion CEST (iCEST). Using RF labeling at the bound ion [Ca-5F-BAPTA] 19F frequency and detection of label transfer to the free 5F-BAPTA 19F frequency (0 ppm), we are able to amplify the signal of bound Ca2+ by a factor of 100. We demonstrate that the resulting Z-spectra display supreme sensitivity to bound Ca2+ over other M2+ cations.
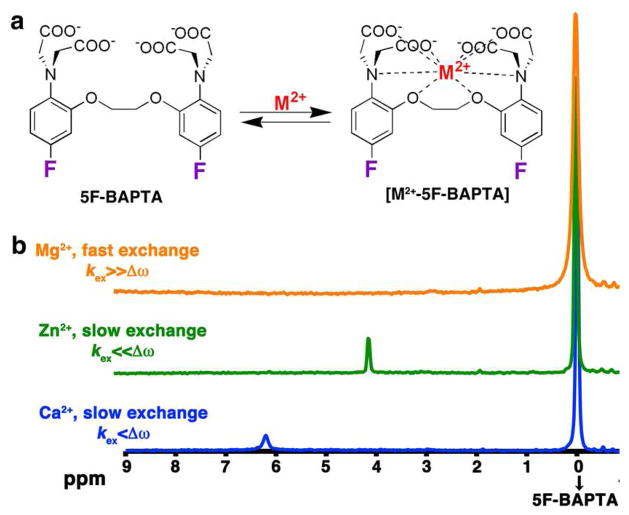
Figure 1a illustrates the dynamic exchange process between free 5F-BAPTA and its complex with M2+, [M2+-5F-BAPTA]. Upon M2+ binding, there is a 19F chemical shift change (Δω) for 5F-BAPTA. If the exchange rate (kex) between M2+-bound and free 5F-BAPTA is fast on the NMR time scale (Δω≪kex), no peak can be resolved as is demonstrated in Figure 1b for Mg2+.
Figure 1.
M2+ binding 5F-BAPTA. a) Schematic depiction of the dynamic exchange process between free 5F-BAPTA and bound [M2+-5F-BAPTA]. b) 19F NMR spectra (470 MHz) of 5F-BAPTA in the presence of Mg2+ (orange), Zn2+ (green), or Ca2+ (blue).
When the kex is sufficiently slow at the field strength used, a well-defined peak is observed for the [M2+-5F-BAPTA] resonance as is shown for Zn2+ (green, Δω≫kex) and Ca2+ (blue, Δω>kex). As was previously reported, the observed Δω’s are typical and unique for each ion that is complexed by 5F-BAPTA and ranges from a few ppm in the cases of Ca2+, Zn2+, Ba2+, Sr2+, Cd2+, Pb2+ and others to tens of ppm upon binding of Fe2+, Co2+ and Ni2+.11,23 The dissociation constant (Kd) of [M2+-5F-BAPTA] is different for each M2+, and as a result so is the kex for the process in Figure 1a.24,38 The Zn2+-5F-BAPTA peak (Figure 1b, green, 4.1 ppm) is sharper than that of Ca2+-5F-BAPTA (Figure 1b, blue, 6.2 ppm), which is correlated with their reported differences in Kd.23,38 Note that increasing the temperature from 25°C to 37°C (Figure S3) or the addition of high concentrations of fast exchanging ions such as K+ and Mg2+ (Figure S4) lead to an upfield shift of the free 5F-BAPTA resonance at the 19F-NMR spectrum.
The 19F-iCEST properties of 5F-BAPTA in the presence of Ca2+ (slow-to-intermediate kex), Zn2+ (very slow kex) and Mg2+ (fast kex) were determined on a 16.4 T MRI scanner and are summarized in Figure 2 for two different pH values, i.e. 7.2 (Figure 2a–c) and 6.4 (Figure 2d–f). A pronounce saturation transfer contrast was detected in the Ca2+ containing solutions (Figure 2a,d) but not in the Zn2+ or Mg2+ containing solutions (Figure 2b,e or Figure 2c,f, respectively). Importantly, a broad asymmetry is observed at very high fractional Mg2+ concentrations (Figure S5b, χ(5F-BAPTA/Mg)=50:1), which peaks at ~1.8 ppm, a frequency much lower than Ca2+ (Figure S5a). For faster ion exchange processes between free 5F-BAPTA and bound M2+-5F-BAPTA, such as the exchange observed for Mg2+ (Figure S5b), other CEST imaging methods, such as frequency-labeled exchange (FLEX), may be considered to improve the detection of these ions.36–37 Interestingly, the Δω between [Ca-5F-BAPTA] and free 5F-BAPTA was found to be dependent on pH (Figures 2, 3, S1, S2 and S6 and Table S1), but the kex between [Ca-5F-BAPTA] and 5F-BAPTA was preserved for all examined pH values as determined by Bloch simulations (190±10 s−1, Figures 2 and S1).25
Figure 2.

iCEST characteristics: 19F-iCEST Z-spectra of solutions containing 10 mM of 5F-BAPTA and 50 μM of M2+ (M2+: Ca2+, blue; Zn2+, green; or Mg2+, orange) in 40 mM Hepes buffer with the pH of the solutions adjusted to 7.2 (a–c) or 6.4 (d–f). Dots represent the raw experimental data. For Ca2+, lines represent Bloch simulations (two pool model) and arrows point to the frequency of the [Ca2+-5F-BAPTA] complex.
Figure 3.

Imaging Ca2+ with iCEST. 1H-MRI, 19F-MRI, and iCEST (Δω=6.2 or 5.0 ppm) of M2+ solutions with pH values of 7.2 or 6.4. Each tube contains 10 mM of 5F-BAPTA and 50 μM of M2+. Small water tubes (shown on 1H-MRI) were included to determine the orientation of the samples.
These results are in a good agreement with a previous report showing that the binding of Ca2+ was unaffected at pH 6–811 using 19F-MRS. 19F-NMR spectra collected with an internal reference revealed that upon pH change, the frequency of the free 5F-BAPTA shifts but not the frequency of bound M2+-5F-BAPTA (Figure S2). The T2 values of 5F-BAPTA are also sensitive to pH as can be seen by the broadening in the Z-spectra (Figures 2, S1 and Table S1). The T2-value changes seem to be dependent on 5F-BAPTA protonation and not kex-dependent based on the observation that the same Z-spectra line widths were found for solutions containing Mg2+ (Δω≪kex) and Zn2+ (Δω≫kex). Figure 3 shows MR images of the samples that have been used in this study, i.e., 10 mM of 5F-BAPTA and 50 μM of M2+. As expected no difference in MR contrast was observed between the samples when using conventional 1H-MRI or 19F-MRI.
However, contrary to the Mg2+- or Zn2+-containing samples, which did not generate iCEST contrast at this concentration, a large iCEST contrast was detected for the Ca2+ containing sample when a saturation pulse (B1=3.6 μT/2000 ms) was applied at the appropriate frequency offset of the [Ca2+-5F-BAPTA] complex, i.e., Δω=6.2 ppm (pH=7.2) and Δω=5.0 ppm (pH=6.4). Figure S6 shows the dependence of Δω on pH, with Δω ranging from 2.1 ppm to 6.2 ppm for pH values of 5.6 to 7.2. In addition, iCEST images were acquired for solutions containing mixtures of Ca2+ and Mg2+ (50 μM Ca2+, 200 μM Mg2+) and Ca2+ and Zn2+ (50μM Ca2+, 50 μM Zn2+) with 10 mM BAPTA at pH 7.2. The iCEST contrast produced by the Ca2+ was still significant (~22%) at Δω=6 ppm for all mixtures (Figure S5). Although high Mg2+ concentrations generate iCEST contrast at Δω=1.8 ppm (Figure S5a–b) the larger Δω, smaller kex of [Ca-5F-BAPTA] and its much higher iCEST contrast makes this approach better for Ca2+ sensing (Figure S5b, amplification factor = ×10 for Mg2+, ×100 for Ca2+).
To evaluate the sensitivity of our suggested approach we examined the iCEST contrast at different ratios of Ca2+ to 5F-BAPTA (χCa, Figures 4a and S7). As clearly shown in Figure S7, Ca2+ is easily detected with iCEST MRI at χCa=1:1000, where ~11% contrast is observed in the Z-spectrum for this phantom. The same amplification was obtained when 0.5 mM 5F-BAPTA was used to detect 500 nM Ca2+ (Figure 4b), showing the potential of iCEST to sense low Ca2+ concentrations.
Figure 4.
Ca2+ sensing using iCEST. a) χCa vs. MTR plot. b) Detection of 500 nM Ca2+ in the presence of 0.5 mM of 5F-BAPTA. Inset depicts 19F-MRI of the sample with an overlaid iCEST image. Lines represent Bloch simulations. Error bars represent the inter-voxel standard deviations.
In this study, we show for the first time that spatial information of Ca2+ and Mg2+ levels can be obtained using amplification of the sensitivity by iCEST with 5F-BAPTA as the ion indicator. One advantage of using 5F-BAPTA as an MRI responsive agent for detecting metal ions over 1H- MRI26 or 129Xe-MRI27 based probes is that no attachment of a contrast enhancer is required. The 19F atoms serve on the chelates as the responsive group as well as contrast generators. Hyperpolarized 129Xe-CEST (hyperCEST)28–30 was the first example of non-1H CEST-MR imaging, although on a gas bubbled into solution instead of solute such as BAPTA. Earlier heteronuclear NMR experiments using magnetization transfer protocols have allowed detection of exchange between two pools of nuclear spins in MRS studies.14,31,32
Our study shows the potential of exploiting the iCEST concept using 19F-MRI, as 1:2000 concentration ratios are amplified to 1:20 changes in 19F signal (Figures 4a and S7), i.e., an amplification factor of ~100 for a kex of 190 s−1. In addition to the advantages of using 19F-MRI, i.e., high γ, 100% natural isotopic abundance, and negligible amount of 19F in soft tissues, 33,34,39 the large range of 19F chemical shifts (about 20 times that of 1H35) and the sensitivity of 19F Δω to the details of the local environment is an ad- vantage for iCEST-based applications. One obstacle of the iCEST approach would be the detectability level of the free 19F-agent. This could be surmounted by collecting high-resolution 1H-MR images, which provide spatial information, and reducing the resolution for iCEST to allow localized detectability of the 19F-based agent with improved SNR39 (see SI for detectability discussion). Using paramagnetic 1H CEST probes7 for detecting Ca2+ will allow better spatial resolution and higher SNR compared to iCEST, but also will have a worse sensitivity for detecting low Ca2+ concentrations. In the iCEST approach, the signal from low concentration solutes [Ca2+-5F-BAPTA] is amplified through saturation transfer onto the signal of the high concentration free 5F-BAPTA. Since this contrast is dependent on χCa, lower concentrations of Ca2+ can be detected through simply reducing the free 5F-BAPTA concentrations, when these concentrations are NMR detect-able.39 This is an advantage of the iCEST approach, since this feature is not available for 1H CEST, which is based on water. Finally, the unique Δω found for each [M2+-5F-BAPTA] and the diversity of the obtained kex may be exploited for multi-ion MR imaging approaches in which each ion generates iCEST contrast with an identifiable amplitude and Δω. This concept was shown for different exchangeable protons in 1H-CEST and termed multi-color imaging.17
In conclusion, we have developed a new approach for sensing metal ions with spatial information using MRI, in which the amplification strategy of CEST is combined with the Δω specificity in 19F frequency. The outlined principles can be further extended for designing new iCEST agents to detect other ions.
Supplementary Material
Acknowledgments
Supported by MSCRFII-0161-00, R01EB012590, R01EB015031, R01EB015032, MSCRFF-0103-00.
Footnotes
Supporting Information file includes experimental methods, discussions and simulations. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Clapham DE. Cell. 2007;131:1047. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Mank M, Griesbeck O. Chem Rev. 2008;108:1550. doi: 10.1021/cr078213v. [DOI] [PubMed] [Google Scholar]
- 3.Tsien RY. Annu Rev Neurosci. 1989;12:227. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- 4.Angelovski G, Fouskova P, Mamedov I, Canals S, Toth E, Logothetis NK. Chembiochem. 2008;9:1729. doi: 10.1002/cbic.200800165. [DOI] [PubMed] [Google Scholar]
- 5.Dhingra K, Fouskova P, Angelovski G, Maier ME, Logothetis NK, Toth E. J Biol Inorg Chem. 2008;13:35. doi: 10.1007/s00775-007-0296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li WH, Fraser SE, Meade TJ. J Am Chem Soc. 1999;121:1413. [Google Scholar]
- 7.Angelovski G, Chauvin T, Pohmann R, Logothetis NK, Toth E. Bioorg Med Chem. 2011;19:1097. doi: 10.1016/j.bmc.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Atanasijevic T, Shusteff M, Fam P, Jasanoff A. Proc Natl Acad Sci U S A. 2006;103:14707. doi: 10.1073/pnas.0606749103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taktak S, Weissleder R, Josephson L. Langmuir. 2008;24:7596. doi: 10.1021/la8006298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsien RY. Biochemistry. 1980;19:2396. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- 11.Smith GA, Hesketh RT, Metcalfe JC, Feeney J, Morris PG. Proc Natl Acad Sci U S A. 1983;80:7178. doi: 10.1073/pnas.80.23.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marban E, Kitakaze M, Kusuoka H, Porterfield JK, Yue DT, Chacko VP. Proc Natl Acad Sci U S A. 1987;84:6005. doi: 10.1073/pnas.84.16.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson SA, Song SK, Ackerman JJ, Hotchkiss RS. J Neurochem. 1999;72:2617. doi: 10.1046/j.1471-4159.1999.0722617.x. [DOI] [PubMed] [Google Scholar]
- 14.Gilboa H, Chapman BE, Kuchel PW. NMR Biomed. 1994;7:330. doi: 10.1002/nbm.1940070707. [DOI] [PubMed] [Google Scholar]
- 15.Bar-Shir A, Liu G, Liang Y, Yadav NN, McMahon MT, Walczak P, Nimmagadda S, Pomper MG, Tallman KA, Greenberg MM, van Zijl PC, Bulte JW, Gilad AA. J Am Chem Soc. 2013;135:1617. doi: 10.1021/ja312353e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratnakar SJ, Viswanathan S, Kovacs Z, Jindal AK, Green KN, Sherry AD. J Am Chem Soc. 2012;134:5798. doi: 10.1021/ja211601k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G, Moake M, Har-el YE, Long CM, Chan KW, Cardona A, Jamil M, Walczak P, Gilad AA, Sgouros G, van Zijl PC, Bulte JW, McMahon MT. Magn Reson Med. 2012;67:1106. doi: 10.1002/mrm.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longo DL, Busato A, Lanzardo S, Antico F, Aime S. Magn Reson Med. 2012 doi: 10.1002/mrm.24513. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Sheth VR, Liu G, Pagel MD. Contrast Media Mol Imaging. 2011;6:219. doi: 10.1002/cmmi.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aime S, Carrera C, Delli Castelli D, Geninatti Crich S, Terreno E. Angew Chem Int Ed Engl. 2005;44:1813. doi: 10.1002/anie.200462566. [DOI] [PubMed] [Google Scholar]
- 21.Chan KW, Liu G, Song X, Kim H, Yu T, Arifin DR, Gilad AA, Hanes J, Walczak P, van Zijl PC, Bulte JW, McMahon MT. Nat Mat. 2013;12:268. doi: 10.1038/nmat3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu G, Song X, Chan KWY, McMahon MT. NMR Biomed. 2013;26:810. doi: 10.1002/nbm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirschenlohr HL, Grace AA, Vandenberg JI, Metcalfe JC, Smith GA. Biochem J. 2000;346:385. [PMC free article] [PubMed] [Google Scholar]
- 24.Csermely P, Sandor P, Radics L, Somogyi J. Biochem Biophys Res Commun. 1989;165:838. doi: 10.1016/s0006-291x(89)80042-7. [DOI] [PubMed] [Google Scholar]
- 25.McMahon MT, Gilad AA, Zhou J, Sun PZ, Bulte JW, van Zijl PC. Magn Reson Med. 2006;55:836. doi: 10.1002/mrm.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Que EL, Chang CJ. Chem Soc Rev. 2010;39:51. doi: 10.1039/b914348n. [DOI] [PubMed] [Google Scholar]
- 27.Kotera N, Tassali N, Leonce E, Boutin C, Berthault P, Brotin T, Dutasta JP, Delacour L, Traore T, Buisson DA, Taran F, Coudert S, Rousseau B. Angew Chem Int Ed. 2012;51:4100. doi: 10.1002/anie.201109194. [DOI] [PubMed] [Google Scholar]
- 28.Schroder L, Lowery TJ, Hilty C, Wemmer DE, Pines A. Science. 2006;314:446. doi: 10.1126/science.1131847. [DOI] [PubMed] [Google Scholar]
- 29.Seward GK, Bai YB, Khan NS, Dmochowski IJ. Chem Sci. 2011;2:1103. doi: 10.1039/C1SC00041A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotera N, Tassali N, Leonce E, Boutin C, Berthault P, Brotin T, Dutasta JP, Delacour L, Traore T, Buisson DA, Taran F, Coudert S, Rousseau B. Angew Chem Int Ed. 2012;51:4100. doi: 10.1002/anie.201109194. [DOI] [PubMed] [Google Scholar]
- 31.Kupriyanov VV, Balaban RS, Lyulina NV, Steinschneider A, Saks VA. Biochim Biophys Acta. 1990;1020:290. doi: 10.1016/0005-2728(90)90160-6. [DOI] [PubMed] [Google Scholar]
- 32.Alger JR, Shulman RG. Q Rev Biophys. 1984;17:83. doi: 10.1017/s0033583500005266. [DOI] [PubMed] [Google Scholar]
- 33.Bulte JWM. Nat Biotechnol. 2005;23:945. doi: 10.1038/nbt0805-945. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz-Cabello J, Barnett BP, Bottomley PA, Bulte JWM. NMR Biomed. 2011;24:114. doi: 10.1002/nbm.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brey WS, Brey ML. Encyclopedia of Magn Reson. Chichester: John Wiley & Sons, Ltd; 2007. p. 2063. [Google Scholar]
- 36.Friedman JI, McMahon MT, Stivers JT, Van Zijl PC. J Am Chem Soc. 2010;132:1813. doi: 10.1021/ja909001q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Zijl PC, Yadav NN. Magn Reson Med. 2011;65:927. doi: 10.1002/mrm.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schanne FA, Dowd TL, Gupta RK, Rosen JF. Environ Health Persp. 1990;84:99. doi: 10.1289/ehp.908499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahrens ET, Zhong J. NMR Biomed. 2013;26:860. doi: 10.1002/nbm.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.