Abstract
C4 photosynthesis has independently evolved from the ancestral C3 pathway in at least 60 plant lineages, but, as with other complex traits, how it evolved is unclear. Here we show that the polyphyletic appearance of C4 photosynthesis is associated with diverse and flexible evolutionary paths that group into four major trajectories. We conducted a meta-analysis of 18 lineages containing species that use C3, C4, or intermediate C3–C4 forms of photosynthesis to parameterise a 16-dimensional phenotypic landscape. We then developed and experimentally verified a novel Bayesian approach based on a hidden Markov model that predicts how the C4 phenotype evolved. The alternative evolutionary histories underlying the appearance of C4 photosynthesis were determined by ancestral lineage and initial phenotypic alterations unrelated to photosynthesis. We conclude that the order of C4 trait acquisition is flexible and driven by non-photosynthetic drivers. This flexibility will have facilitated the convergent evolution of this complex trait.
DOI: http://dx.doi.org/10.7554/eLife.00961.001
Research organism: Other
eLife digest
Plants rely on carbon for their growth and survival: in a process called photosynthesis, they use energy from sunlight to convert carbon dioxide and water into carbohydrates and oxygen gas. The chemical reactions that make up photosynthesis are powered by a chain of enzymes, and plants must ensure that these enzymes—which are in the leaves of the plant—are supplied with enough carbon dioxide and water. Carbon dioxide from the atmosphere enters plants through pores in their leaves, but water must be carried up the plant from the roots.
The type of photosynthesis used by about 90% of flowering plant species—including tomatoes and rice—is called C3 photosynthesis. The first step in this process begins with an enzyme called RuBisCO, which reacts with carbon dioxide and a substance called RuBP to form molecules that contain three carbon atoms (hence the name C3 photosynthesis).
In a hot climate, however, a plant can lose a lot of water through the pores in its leaves: closing these pores allows the plant to retain water, but this also reduces the supply of carbon dioxide. Under these circumstances this causes problems because RuBisCO uses oxygen to break down RuBP, instead of creating sugars, when carbon dioxide is not readily available. To prevent this process, which wastes a lot of energy and resources, some plants—including maize, sugar cane and many other agricultural staples—have evolved an alternative process called C4 photosynthesis. Although it is more complex than C3 photosynthesis, and required many changes to be made to the structure of leaves, C4 photosynthesis has evolved on more than 60 different occasions.
In C4 plants, the mesophyll—the region that is associated with the capture of carbon dioxide by RuBisCO in C3 plants—contains high levels of an alternative enzyme called PEPC that converts carbon dioxide molecules into an acid that contains four carbon atoms. To avoid carbon dioxide being captured by both enzymes, C4 plants evolved to relocate RuBisCO from the mesophyll to a second set of cells in an airtight structure known as the bundle sheath. The four-carbon acids produced by PEPC diffuse to the cells in the bundle sheath, where they are broken down into carbon dioxide molecules, and photosynthesis then proceeds as normal. This process allows photosynthesis to continue when the level of carbon dioxide in the leave is low because the plant has closed its pores to retain water.
Since C4 plants grow faster than C3 plants, and also require less water, plant biologists would like to introduce certain C4 traits into C3 crop plants. To help with this process, Williams, Johnston et al. have used computational methods to explore how C4 photosynthesis evolved from ancestral C3 plants. This involved investigating the prevalence of 16 traits that are common to C4 plants in a total of 73 species that undergo C3 or C4 photosynthesis (including 37 species that possess characteristics of both C3 and C4).
Williams, Johnston et al. then went on to produce a new mathematical model that represents evolutionary processes as pathways across a multi-dimensional “landscape”. The model shows that traits can be acquired in various orders, and that C4 photosynthesis evolved through a number of independent pathways. Some traits that evolved early in the transitions to C4 photosynthesis influenced how evolution proceeded, providing “foundations” upon which further changes evolved.
Interestingly, the structure of the leaf itself appeared to change before any of the photosynthetic enzymes changed. This led Williams, Johnston et al. to conclude that climate change—in particular, the declines in carbon dioxide levels that occurred in prehistoric times—was probably not responsible for the original evolution of C4 photosynthesis. Nevertheless, these results could help with efforts to adapt important C3 crop plants to on-going changes in our climate.
Introduction
The convergent evolution of complex traits is surprisingly common, with examples including camera-like eyes of cephalopods, vertebrates, and cnidaria (Kozmik et al., 2008), mimicry in invertebrates and vertebrates (Santos et al., 2003; Wilson et al., 2012) and the different photosynthetic machineries of plants (Sage et al., 2011a). While the polyphyletic origin of simple traits (Hill et al., 2006; Steiner et al., 2009) is underpinned by flexibility in the underlying molecular mechanisms, the extent to which this applies to complex traits is less clear. C4 photosynthesis is both highly complex, involving alterations to leaf anatomy, cellular ultrastructure, and photosynthetic metabolism, and also convergent, being found in at least 60 independent lineages of angiosperms (Sage et al., 2011a). As the emergence of the entire C4 phenotype cannot be comprehensively explored experimentally, C4 photosynthesis is an ideal system for the mathematical modelling of complex trait evolution as transitions on an underlying phenotype landscape. Furthermore, understanding the evolutionary events that have generated C4 photosynthesis on many independent occasions has the potential to inform approaches being undertaken to engineer C4 photosynthesis into C3 crop species (Hibberd et al., 2008).
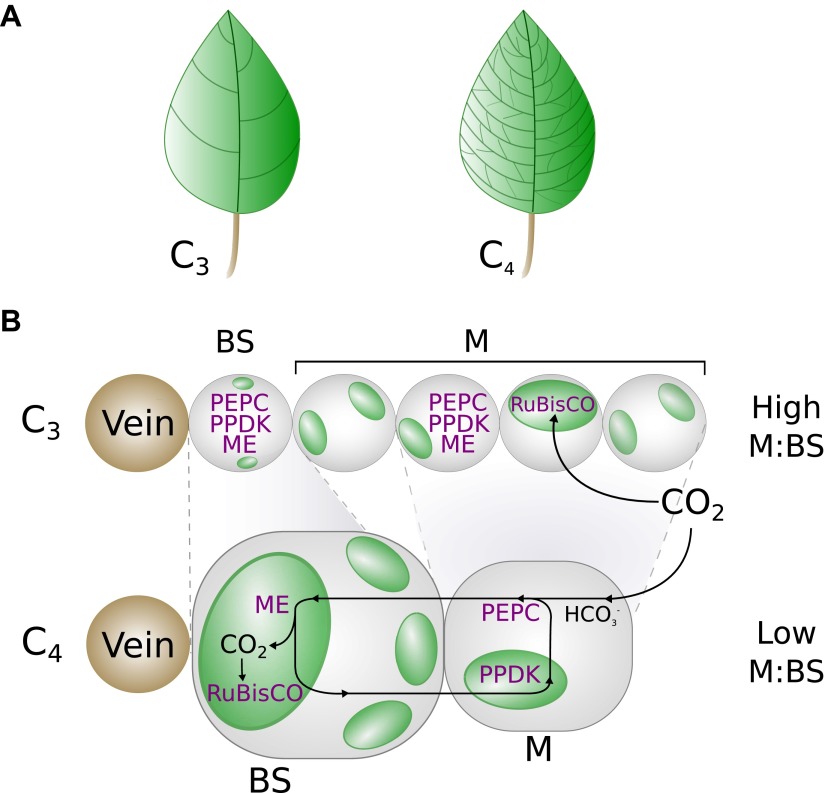
The C4 pathway is estimated to have first evolved between 32 and 25 million years ago (Christin et al., 2011b) in response to multiple ecological drivers, including decreasing atmospheric CO2 concentration (Vicentini et al., 2008). C4 species have since radiated to represent the most productive crops and native vegetation on the planet because modifications to their leaves increase the efficiency of photosynthesis in the sub-tropics and tropics (Edwards et al., 2010). In C4 plants, photosynthetic efficiency is improved compared with C3 species because significant alterations to leaf anatomy, cell biology and biochemistry lead to higher concentrations of CO2 around the primary carboxylase RuBisCO Slack and Hatch, 1967; Langdale, 2011). The morphology of C4 leaves is typically modified into so-called Kranz anatomy that consists of repeating units of vein, bundle sheath (BS) and mesophyll (M) cells (Hattersley, 1984; Langdale, 2011) (Figure 1—figure supplement 1). Photosynthetic metabolism becomes modified and compartmentalised between the M and BS, with M cells lacking RuBisCO but instead containing high activities of the alternate carboxylase PEPC to generate C4 acids. The diffusion of these acids followed by their decarboxylation in BS cells around RuBisCO increases CO2 supply and therefore photosynthetic efficiency (Zhu et al., 2008). C4 acids are decarboxylated by at least one of three enzymes within BS cells: NADP- or NAD-dependent malic enzymes (NADP-ME or NAD-ME respectively), or phosphoenolpyruvate carboxykinase (PCK) (Hatch et al., 1975). Specific lineages of C4 species have typically been classified into one of three sub-types, based on the activity of these decarboxylases, as well as anatomical and cellular traits that consistently correlate with each other (Furbank, 2011).
The genetic mechanisms underlying the evolution of cell-specific gene expression associated with the separation of photosynthetic metabolism between M and BS cells involve both alterations to cis-elements and trans-acting factors (Akyildiz et al., 2007; Brown et al., 2011; Kajala et al., 2012; Williams et al., 2012). Phylogenetically independent lineages of C4 plants have co-opted homologous mechanisms to generate cell specificity (Brown et al., 2011) as well as the altered allosteric regulation of C4 enzymes (Christin et al., 2007) indicating that parallel evolution underpins at least part of the convergent C4 syndrome. However, while a substantial amount of work has addressed the molecular alterations that generate the biochemical differences between C3 and C4 plants (Williams et al., 2012) much less is known about the order and flexibility with which phenotypic traits important for C4 photosynthesis are acquired (Sage et al., 2012). Clues to this question exist in the form of C3–C4 intermediates, species exhibiting characteristics of both C3 or C4 photosynthesis, such as the activity or localisation of C4 cycle enzymes (Hattersley and Stone, 1986), the possession of one or more anatomical or cellular adaptations associated with C4 photosynthesis (Moore et al., 1987), or combinations of both (e.g., Kennedy et al., 1980; Kotayeva et al., 2010). To address these unknown aspects of C4 evolutionary history, we combined the concept of considering evolutionary paths as stochastic processes on complex adaptive landscapes (Wright, 1932; Gavrilets, 1997) with the analysis of extant C3–C4 intermediate species to develop a predictive model of how the full C4 phenotype evolved.
Results
A meta-analysis of photosynthetic phenotypes
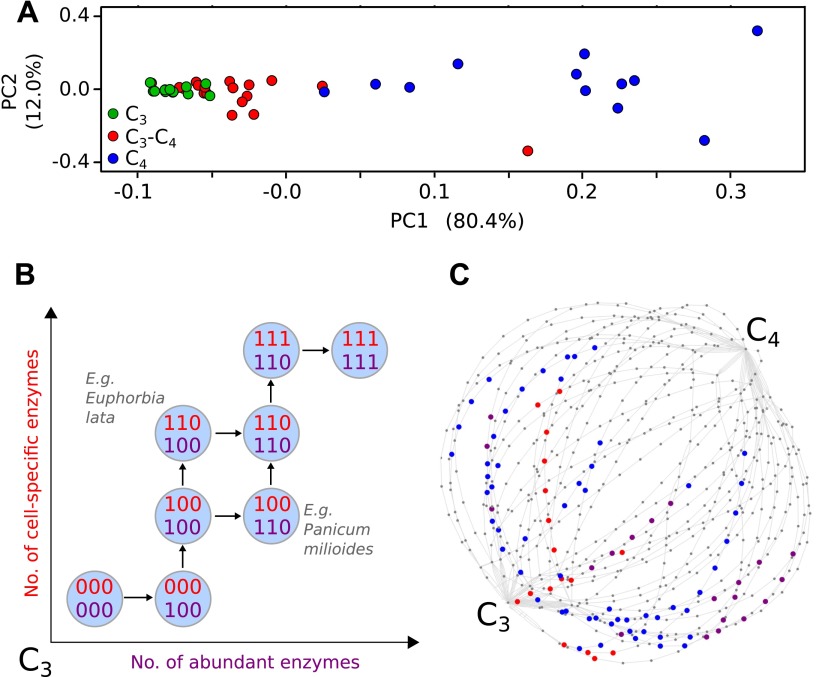
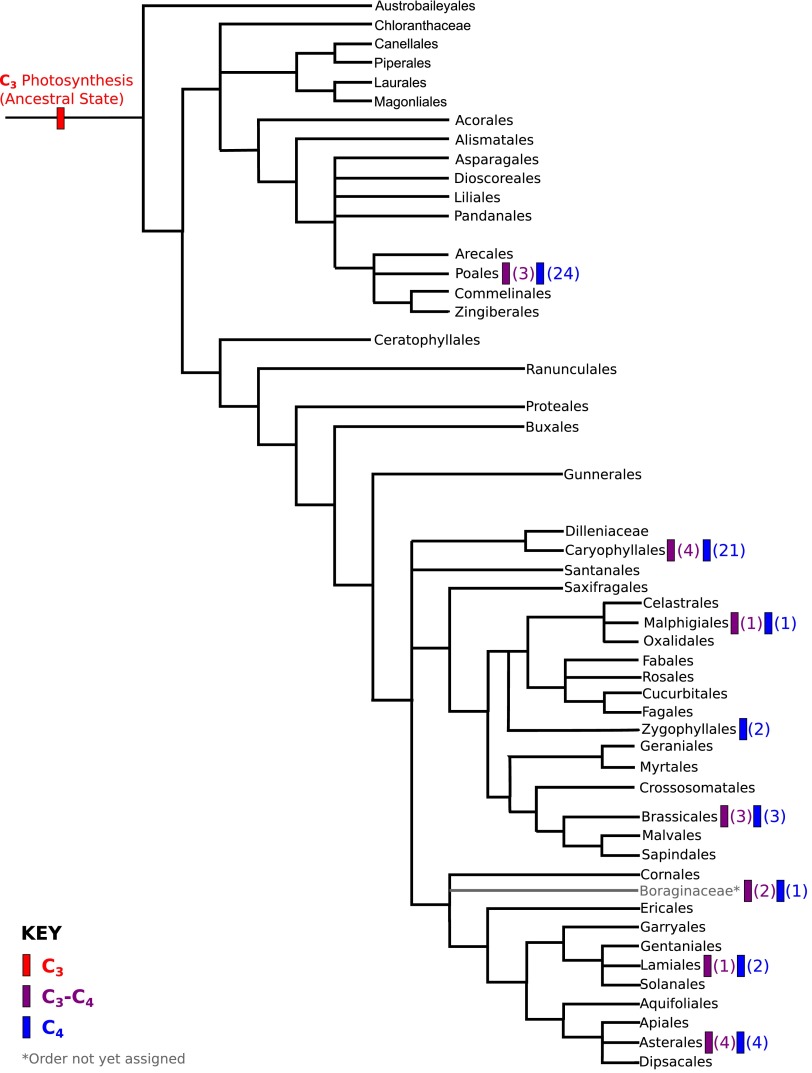
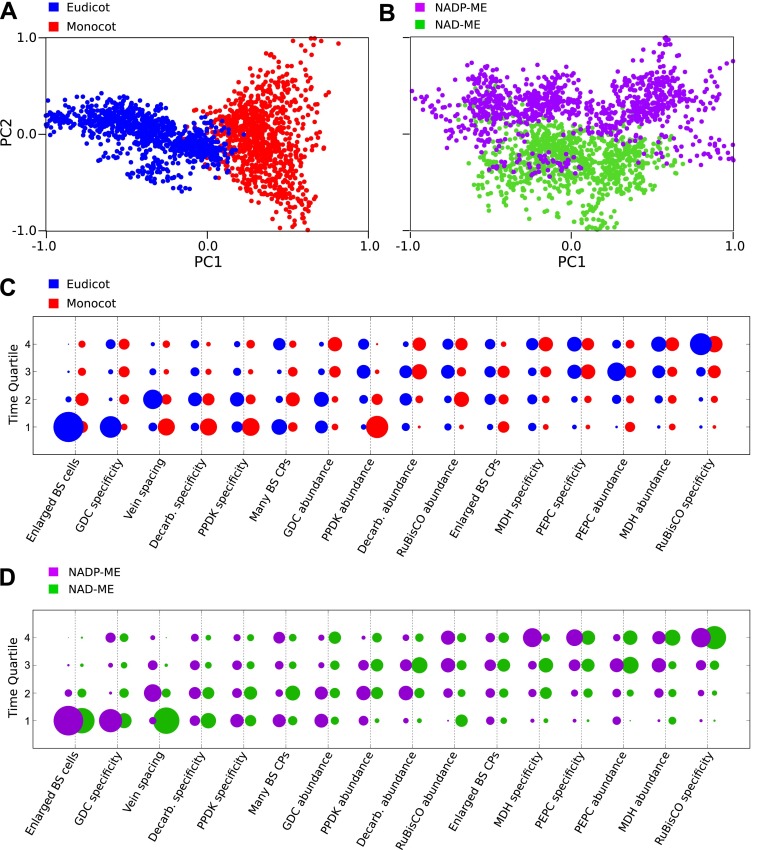
To parameterise the phenotypic landscape underlying photosynthetic phenotypes, data was consolidated from 43 studies encompassing 18 C3, 18 C4, and 37 C3–C4 intermediate species from 22 genera (Table 1). These C3–C4 species are from 18 independent lineages likely representing 18 distinct evolutionary origins of C3–C4 intermediacy (Sage et al., 2011a) (Figure 1—figure supplement 2). These studies were used to quantify 16 biochemical, anatomical, and cellular characteristics associated with C4 photosynthesis (Figure 1—source data 1). Principal components analysis (PCA) was performed to confirm the phenotypic intermediacy of the C3–C4 species (Figure 1A). This result, the sister-group relationships of C3–C4 species with congeneric C4 clades (McKown et al., 2005; Vogan et al., 2007; Christin et al., 2011a; Sage et al., 2011a; Khoshravesh et al., 2012) and the prevalence of extant C3–C4 species in genera with the most recent origins of C4 photosynthesis (Christin et al., 2011b) all support the notion that C3–C4 species represent phenotypic states through which transitions to C4 photosynthesis could occur. The combined traits of C3–C4 intermediate species therefore represent samples from across the space of phenotypes connecting C3 to C4 photosynthesis (Figure 1B). Within our meta-analysis data, C3–C4 phenotypes were available for 33 eudicot and 4 monocot species. 16 and 17 of these species have extant congeneric relatives performing NADP-ME or NAD-ME sub-type C4 photosynthesis respectively. No C3–C4 relatives of PCK sub-type C4 species are known (Sage et al., 2011a). Our meta-analysis therefore encompassed a variety of taxonomic lineages, as well as representing close relatives of known phenotypic variants performing C4 photosynthesis.
Table 1.
Summary of C3–C4 lineages assessed
| Family | Species | References* |
|---|---|---|
| Amaranthaceae | Alternanthera ficoides (C3–C4) | Rajendrudu et al. (1986) |
| Alternanthera tenella (C3–C4) | Devi and Raghavendra (1993) | |
| Alternanthera pungens (C4) | Devi et al. (1995) | |
| Asteraceae | Flaveria cronquistii (C3) | |
| Flavera pringlei (C3) | ||
| Flaveria robusta (C3) | ||
| Flaveria angustifolia (C3–C4) | ||
| Flaveria anomala (C3–C4) | Ku et al. (1983) | |
| Flaveria chloraefolia (C3–C4) | Holaday et al. (1984) | |
| Flaveria floridana (C3–C4) | Adams et al. (1986) | |
| Flaveria linearis (C3–C4) | Brown and Hattersley (1989) | |
| Flaveria oppositifolia (C3–C4) | Ku et al. (1991) | |
| Flaveria ramosissima (C3–C4) | Rosche et al. (1994) | |
| Flaveria sonorensis (C3–C4) | Casati et al. (1999) | |
| Flaveria brownie (C3–C4) | McKown et al. (2005) | |
| Flaveria vaginata (C3–C4) | McKown and Dengler (2007) | |
| Flaveria pubescens (C3–C4) | Gowik et al. (2011) | |
| Flaveria australasica (C4) | ||
| Flaveria bidentis (C4) | ||
| Flaveria kochiana (C4) | ||
| Flaveria trinervia (C4) | ||
| Parthenium incanum (C3) | Moore et al. (1987) | |
| Parthenium hysterophorus (C3–C4) | Devi and Raghavendra (1993) | |
| Boraginaceae | Heliotropium europaeum (C3) | |
| Heliotropium calcicola (C3) | Vogan et al. (2007) | |
| Heliotropium convolvulaceum (C3–C4) | Muhaidat et al. (2011) | |
| Heliotropium greggii (C3–C4) | ||
| Heliotropium polyphyllum (C4) | ||
| Brassicaceae | Moricandia foetida (C3) | Holaday et al. (1981) |
| Moricandia arvensis (C3–C4) | Rawsthorne et al. (1988) | |
| Moricandia spinosa (C3–C4) | Beebe and Evert (1990) | |
| Moricandia nitens (C3–C4) | Rawsthorne et al. (1998) | |
| Raphanus sativus (C3) | Ueno et al. (2003) | |
| Diplotaxis muralis (C3–C4) | Ueno et al. (2006) | |
| Diplotaxis tenuifolia (C3–C4) | ||
| Chenopodiaceae | Salsola oreophila (C3) | P’yankov et al. (1997) |
| Salsola arbusculiformis (C3–C4) | Voznesenskaya et al. (2001) | |
| Salsola arbuscula (C4) | ||
| Cleomaceae | Cleome spinosa (C3) | Voznesenskaya et al. (2007) |
| Cleome paradoxa (C3–C4) | Koteyeva et al. (2010) | |
| Cleome gynandra (C4) | ||
| Cyperaceae | Eleocharis acuta (C3) | Bruhl and Perry (1995) |
| Eleocharis acicularis (C3–C4) | Keeley (1999) | |
| Eleocharis tetragona (C4) | ||
| Euphorbiaceae | Euphorbia angusta (C3) | |
| Euphorbia acuta (C3–C4) | Sage et al. (2011b) | |
| Euphorbia lata (C3–C4) | ||
| Euphorbia mesembryanthemifolia (C4) | ||
| Molluginaceae | Mollugo tenella (C3) | |
| Mollugo verticillata (C3–C4) | Sayre et al. (1979) | |
| Mollugo naudicalis (C3–C4) | Kennedy et al. (1980) | |
| Mollugo pentaphylla (C3–C4) | Christin et al. (2011a) | |
| Mollugo cerviana (C4) | ||
| Poaceae | Avena sativa (C3) | Slack and Hatch (1967) |
| Neurachne tenuifolia (C3) | Hattersley and Stone (1986) | |
| Neurachne minor (C3–C4) | Brown and Hattersley (1989) | |
| Neurachne munroi (C4) | ||
| Panicum bisculatum (C3) | Goldstein et al. (1976) | |
| Panicum hians (C3–C4) | Ku et al. (1976) | |
| Panicum milioides (C3–C4) | Ku and Edwards (1978) | |
| Panicum miliaceum (C4) | Rathnam and Chollet (1978) | |
| Rathnam and Chollet (1979) | ||
| Holaday and Black (1981) | ||
| Hattersley (1984) | ||
| Saccharum officinarum (C4) | Slack and Hatch (1967) | |
| Sorghum bicolor (C4) | Slack and Hatch (1967) | |
| Triticum aestivum (C3) | Slack and Hatch (1967) | |
| Zea mays (C4) | Slack and Hatch (1967) | |
| Portulaceae | Sesuvium portulacastrum (C3) | |
| Portulaca cryptopetala (C3–C4) | Voznesenskaya et al. (2010) | |
| Portulaca oleracea (C4) | ||
| Scrophularaceae | Anticharis kaokoensis (C3) | Khoshravesh et al. (2012) |
| Anticharis ebracteata (C3–C4) | ||
| Anticharis imbricate (C3–C4) | ||
| Anticharis namibensis (C3–C4) | ||
| Anticharis glandulosa (C4) |
The family, species, photosynthetic type and original study are listed. In total, 16 characteristics relating to C4 photosynthesis were extracted from 43 studies encompassing 18 C3, 18 C4, and 37 C3–C4 intermediate species.
References apply to all species within each genus.
Figure 1. Evolutionary paths to C4 phenotype space modelled from a meta-analysis of C3–C4 phenotypes.
Principal component analysis (PCA) on data for the activity of five C4 cycle enzymes confirms the intermediacy of C3–C4 species between C3 and C4 phenotype spaces (A). Each C4 trait was considered absent in C3 species and present in C4 species, with previously studied C3–C4 intermediate species representing samples from across the phenotype space (B). With a dataset of 16 phenotypic traits, a 16-dimensional space was defined. (C) A 2D representation of 50 pathways across this space. The phenotypes of multiple C3–C4 species were used to identify pathways compatible with individual species (e.g., Alternanthera ficoides [red nodes] and Parthenium hysterophorus [blue nodes]), and pathways compatible with the phenotypes of multiple species (purple nodes).
DOI: http://dx.doi.org/10.7554/eLife.00961.004
Figure 1—figure supplement 1. A graphical representation of key phenotypic changes distinguishing C3 and C4 leaves.
Figure 1—figure supplement 2. Phylogenetic distribution of C4 and C3–C4 lineages across the angiosperm phylogeny.
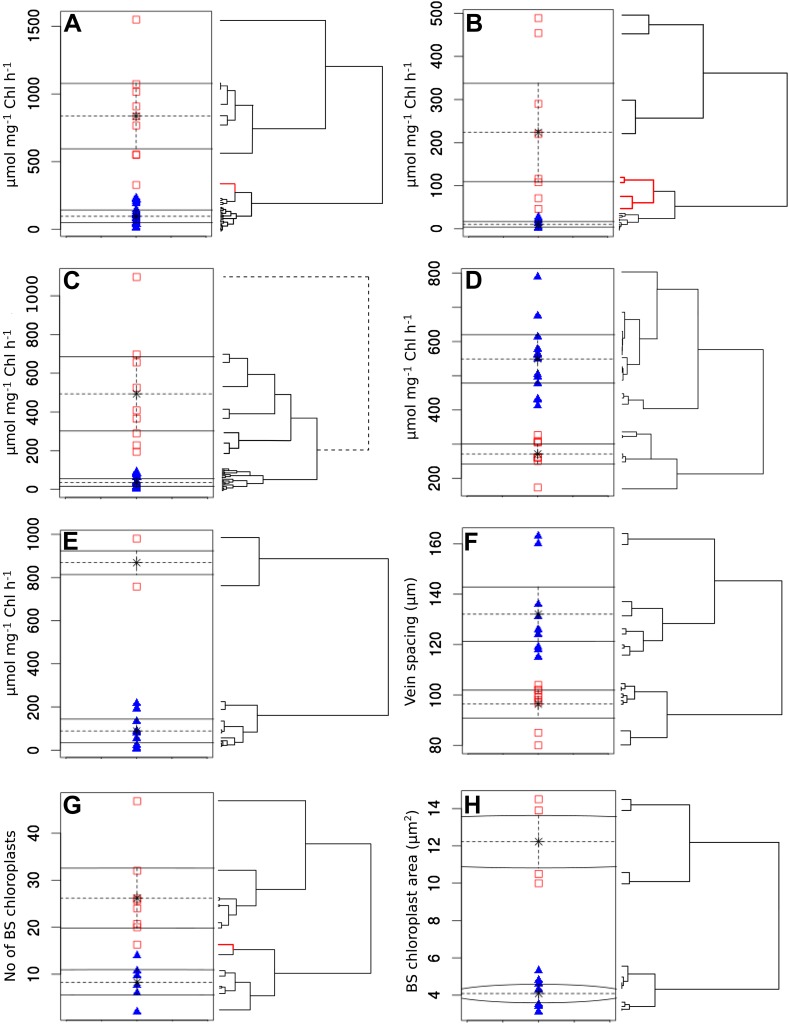
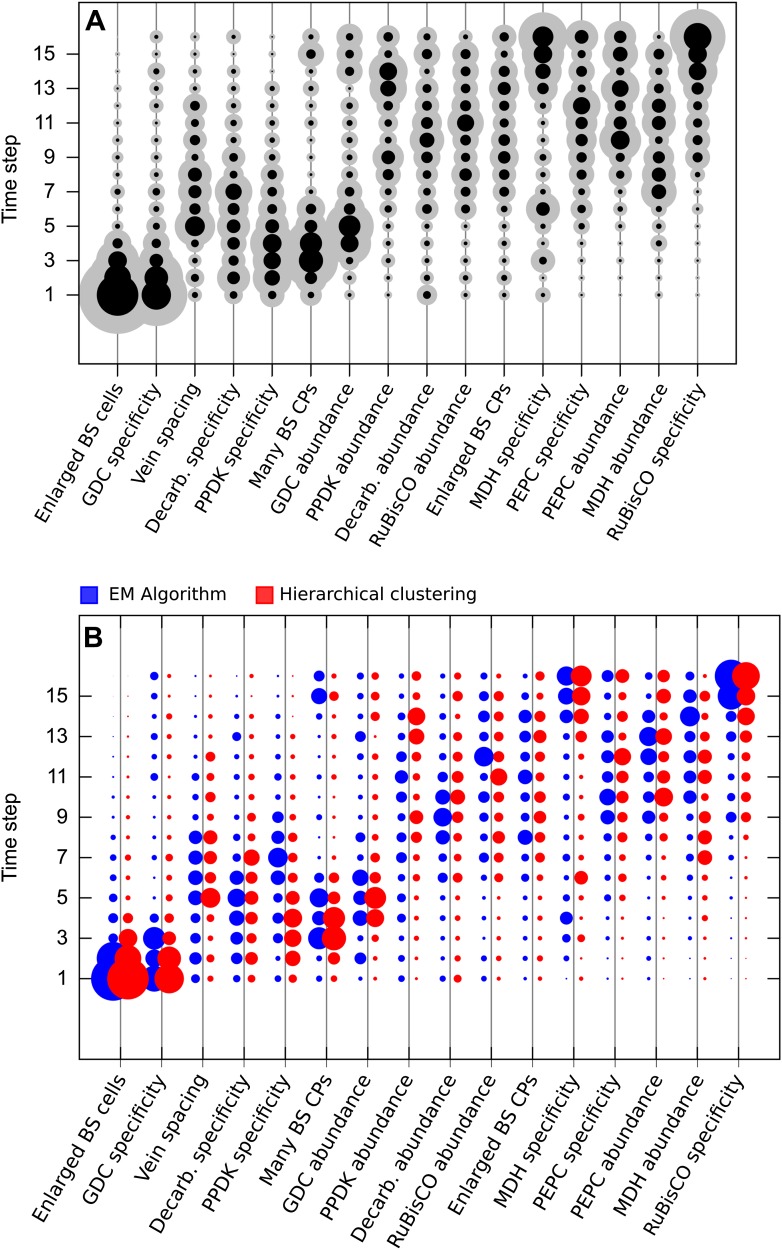
Figure 1—figure supplement 3. Clustering quantitative traits by EM algorithm and hierarchical clustering.
Figure 1—figure supplement 4. Illustration of the principle by which evolutionary pathways emit intermediate signals.
We defined each C4 trait as either being absent (0) or present (1). For quantitative traits the expectation-maximization (EM) algorithm and hierarchical clustering were used to impartially assign binary scores (Figure 1—figure supplement 3). This generated a 16-bit string for each of the species (Figure 1—source data 1), with a presence or absence score for each of the traits included in our meta-analysis. This defined a 16-dimensional phenotype space with 216 (65,536) nodes corresponding to all possible combinations of presence (1) and absence (0) scores for each characteristic.
A novel Bayesian approach for predicting evolutionary trajectories
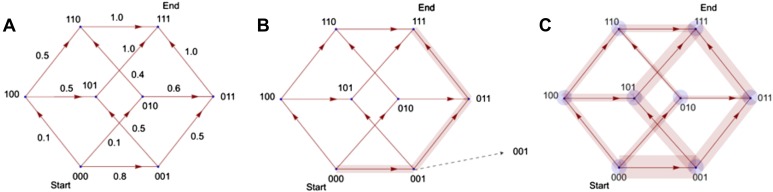
Many existing methods of inference for evolutionary trajectories rely on phylogenetic information or assumptions about the fitness landscape underlying evolutionary dynamics (Weinreich et al., 2005; Lobkovsky et al., 2011; Mooers and Heard, 2013). In convergent evolution, these properties are not always known, as convergent lineages may be genetically distant and associated with poor phylogenetic reconstructions. In addition, the selective pressures experienced by each may be different and dynamic. We therefore consider the convergent evolution of C4 fundamentally as the acquisition of the key phenotypic traits identified through our meta-analysis (Figure 1B). The process of acquisition of these traits can be pictured as a path on the 16-dimensional hypercube (Figure 1C), from the node labelled with all 0’s (the C3 phenotype, with no C4 characteristics) to the node labelled with all 1’s (the C4 phenotype, with all C4 characteristics).
The phenotypic landscape underlying the evolution of C4 photosynthesis was then modelled as a transition network, with weighted edges describing the probability of transitions occurring between two phenotypic states (two nodes on the hypercube, Figure 1—figure supplement 4). Observed intermediate points were then used to constrain the structure of these phenotypic landscapes. To do this, we developed inferential machinery based on the framework of Hidden Markov Models (HMMs) (Rabiner, 1989) (Figure 1—figure supplement 4) and simulated an ensemble of Markov chains on trial transition networks. Each of these chains represents a possible evolutionary pathway from C3 to C4, and passes through several intermediate phenotypic states. The likelihood of observing intermediate states with characteristics compatible with the biologically observed data on C3–C4 intermediates was recorded for the set of paths supported on each trial network. A Bayesian MCMC procedure was used to sample from the set of networks most compatible with the meta-analysis dataset, and thus most likely to represent the underlying dynamics of C4 evolution. The order in which phenotypic characteristics were acquired was recorded for paths on each network compatible with the C3–C4 species data, and posterior probability distributions (given uninformative priors) for the time-ordered acquisition of each C4 trait were generated. For further information and mathematical details, see ‘Methods’.
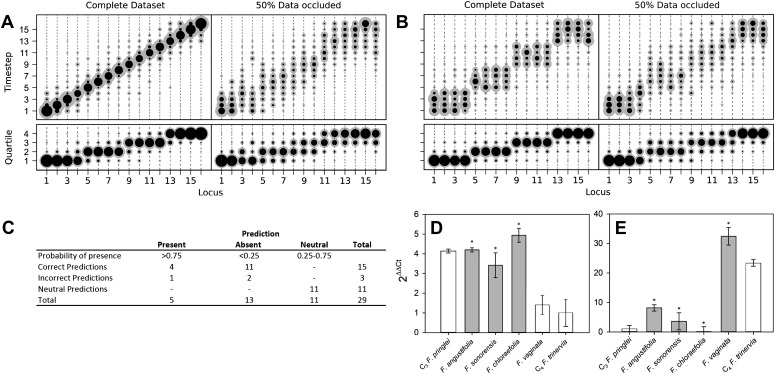
To model the evolutionary paths generating C4 without requiring additional dimensionality, we imposed that only one C4 trait may be acquired at a time, and loss of acquired C4 traits was forbidden. To test if we were nevertheless able to detect traits acquired simultaneously in evolution, we tested our approach on artificial positive control datasets containing intermediate nodes representing a stepwise evolutionary sequence of events (Figure 2A) and an evolutionary pathway in which four traits are acquired simultaneously at a time (Figure 2B). Our approach clearly assigned equal acquisition probabilities to traits whose timing was linked in the underlying dataset, even when 50% of the data was occluded (Figure 2B). These data are consistent with this approach detecting the simultaneous acquisition of traits in evolution, even though single-trait acquisitions are simulated.
Figure 2. Verifying a novel Bayesian approach for predicting evolutionary trajectories.
(A and B) Datasets were obtained from an artificially constructed diagonal dynamic matrix (A), and a diagonal matrix with linked timing of locus acquisitions (B). The single, diagonal evolutionary trajectory was clearly replicated in both examples, over a time-scale of 16 individual steps, or four coarse-grained quartiles. We subjected these artificial datasets to our inferential machinery with fully characterised artificial species, and with 50% of data occluded in order to replicate the proportion of missing data from our C3–C4 dataset. (C) When applied to our meta-analysis of C3–C4 data, predictions were generated for every trait missing from the biological dataset. We tested this predictive machinery by generating 29 artificial datasets, each missing one data point, and comparing the presence/absence of the trait as predicted by our approach with the experimental data from the original study. (D and E) Quantitative real-time PCR (qPCR) was used to verify the predicted phenotypes of four C3–C4 species. The abundance RbcS (D) and MDH (E) transcripts were determined from six Flaveria species. White bars represent phenotypes already determined by other studies, grey bars those that were predicted by the model and asterisks denote intermediate species phenotypes correctly predicted by our approach (Error bars indicate SEM, N = 3).
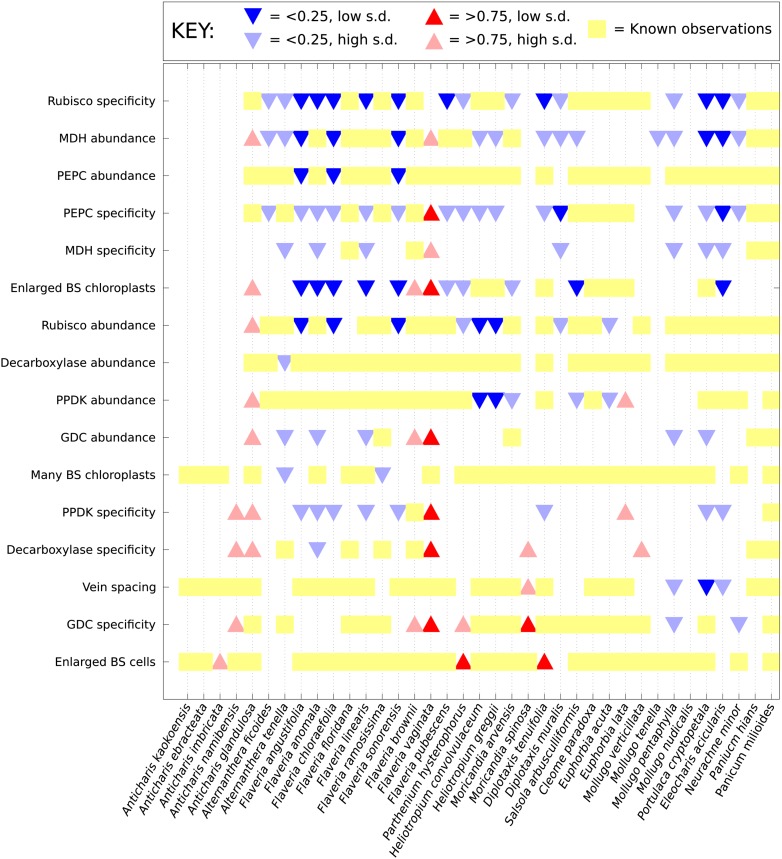
Figure 2—figure supplement 1. Computational prediction of C3–C4 intermediate phenotypes.
Verifying prediction accuracy
The presence and absence of unknown phenotypes were predicted by recording all phenotypes encountered along a set of simulated evolutionary trajectories that were compatible with the data from a given species (Figure 1—figure supplement 4), and calculating the posterior distribution of the proportion of these phenotypes with the value 1 for the unknown trait. If the mean of this distribution was <25% or >75%, and that value fell outside one standard deviation of the mean, the missing trait was assigned a strong prediction of absence or presence. To comprehensively test the accuracy of our predictive machinery, we generated 29 occluded datasets, consisting of the original full dataset with one randomly chosen data point removed. The predicted phenotype of each missing trait was then compared with the known phenotype published in the original study. For 29 occluded traits 18 were strongly predicted to be present or absent, and the remaining 11 predictions were neutral. Of the 18 strongly predicted traits (i.e., <25% or >75% probability), 15 were correct, with only one false positive and two false negative predictions (Figure 2C). The approach therefore assigns neutral predictions much more frequently than false positive or false negative predictions, suggesting that its outputs are highly conservative, and thus unlikely to produce artefacts. Predictions were generated for phenotypes that have not yet been described in C3–C4 species (Figure 2—figure supplement 1). Quantitative real-time PCR experimentally verified a subset of these, relating to abundance of C4 enzymes not previously measured (Figure 2D–E). We also found that the model was able to successfully infer evolutionary dynamics in artificially constructed datasets (Figure 2A–B). Taken together, these prediction and verification studies illustrate that our approach robustly identifies key features of C4 evolution.
A high-resolution model for the evolutionary events generating C4
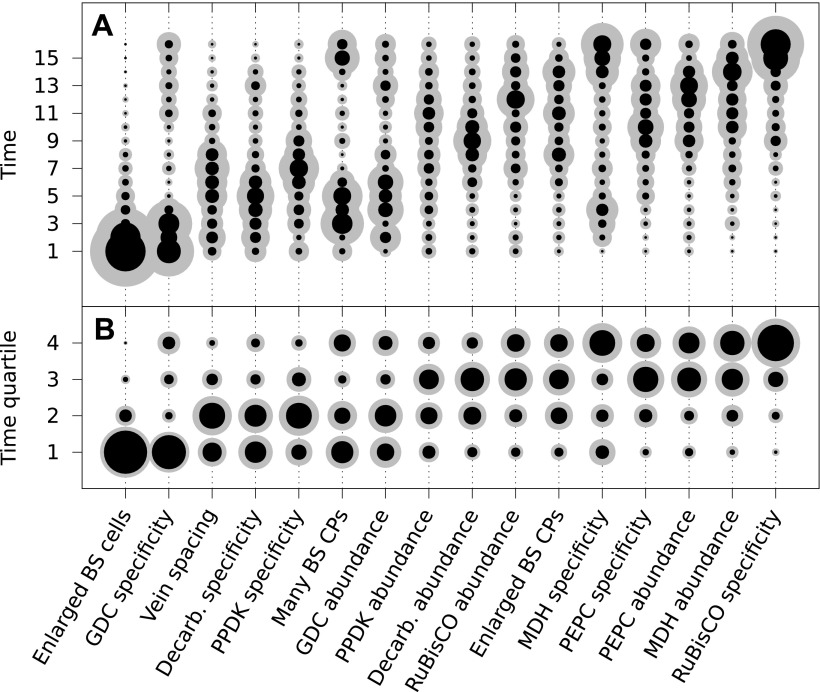
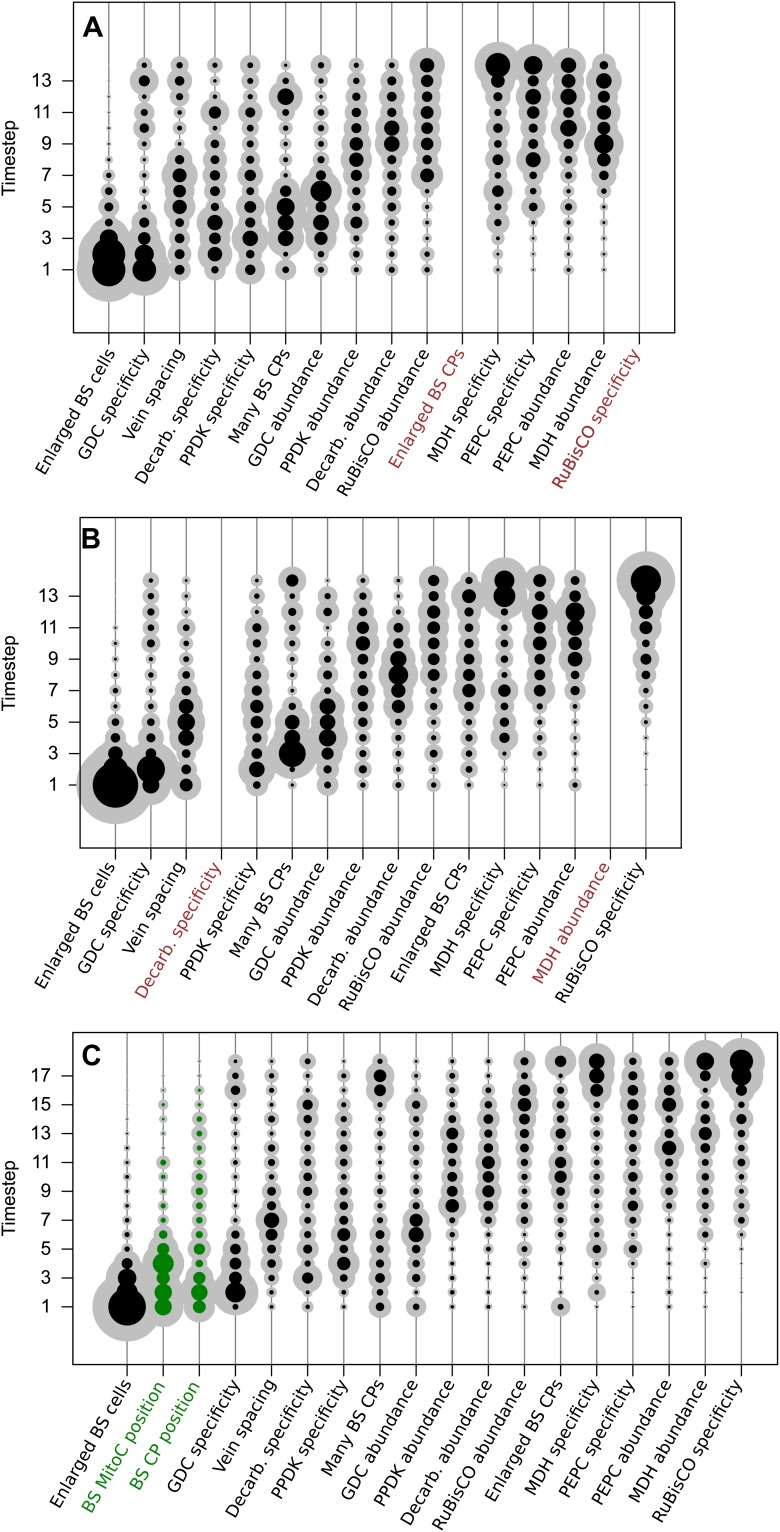
The posterior probability distributions for the acquisition time of each phenotypic trait were combined to produce an objective, computationally generated blueprint for the order of evolutionary events generating C4 photosynthesis (Figure 3). These results were consistent with previous work on subsets of C4 lineages that proposed the BS-specificity of GDC occurs prior to the evolution of C4 metabolism (Hylton et al., 1988; Rawsthorne et al., 1988; Devi et al., 1995; Sage et al., 2012), and loss of RuBisCO from M cells occurs late (Cheng et al., 1988; Khoshravesh et al., 2012), but also provided higher resolution insight into the order of events generating C4 metabolism. Alterations to leaf anatomy as well as cell-specificity and increased abundance of multiple C4 cycle enzymes were predicted to evolve prior to any alteration to the primary C3 and C4 photosynthetic enzymes RuBisCO and phosphoenolpyruvate carboxylase (PEPC) (Figure 3).
Figure 3. The mean ordering of phenotypic changes generating C4 photosynthesis.
EM-clustered data from C3–C4 intermediate species were used to generate posterior probability distributions for the timing of the acquisition of C4 traits in sixteen evolutionary steps (A) or four quartiles (B). Circle diameter denotes the mean posterior probability of a trait being acquired at each step in C4 evolution (the Bayes estimator for the acquisition probability). Halos denote the standard deviation of the posterior. The 16 traits are ordered from left to right by their probability of being acquired early to late in C4 evolution. Abbreviations: bundle sheath (BS), glycine decarboxylase (GDC), chloroplasts (CPs), decarboxylase (Decarb.), pyruvate, orthophosphate dikinase (PPDK), malate dehydrogenase (MDH), phosphoenolpyruvate carboxylase (PEPC).
Figure 3—figure supplement 1. Results obtained using data clustered by hierarchical clustering.
Figure 3—figure supplement 2. Adding or removing traits does not affect the predicted order of evolutionary events.
Figure 3—figure supplement 3. Probabilities of C4 traits being acquired simultaneously.
There was also strong evidence for enlargement of BS cells as an early innovation in most C4 lineages (Figure 3), consistent with the suggestion that this was an ancestral state within C3 ancestors of C4 grass lineages and that this contributed to the high number of C4 origins within this family (Christin et al., 2013; Griffiths et al., 2013). The compartmentation of PEPC into M cells and its increased abundance compared with C3 leaves was predicted to occur at similar times, but for all other C4 enzymes the evolution of increased abundance and cellular compartmentation were clearly separated by the acquisition of other traits (Figure 3). This result is consistent with molecular analysis of genes encoding C4 enzymes that indicates cell-specificity and increased expression are mediated by different cis-elements (Akyildiz et al., 2007; Kajala et al., 2012; Wiludda et al., 2012).
Two approaches were taken to verify that these conclusions are robust and accurately reflect biological data. First, the analysis was repeated using scores for presence or absence of traits that were assigned by hierarchical clustering, as opposed to using the EM algorithm (Figure 3—figure supplement 1A). Although hierarchical clustering generated differences in the scoring of a small number of traits, the predicted evolutionary trajectories were not affected, producing highly similar results (Figure 3—figure supplement 1B). Second, we introduced structural changes to the phenotype space, by both adding and subtracting traits from the analysis (Figure 3—figure supplement 2). Removing two independent pairs of traits from the analysis did not affect the predicted timing of the remaining 14 traits (Figure 3—figure supplement 2A–B). However, increased standard deviations were observed in some cases (e.g., for the probabilities of acquiring enlarged BS cells, or decreased vein spacing) likely a consequence of using fewer data. To test if the addition of data might also affect the results, we performed an analysis with two additional traits included (Figure 3—figure supplement 2C). We selected two traits that have been widely observed in C3–C4 species, the centripetal positioning of mitochondria and the centrifugal or centripetal position of chloroplasts within BS cells (Sage et al., 2012). Despite the widespread occurrence of these traits, their functional importance remains unclear (Sage et al., 2012). Consistent with observations made from several genera, we predict that these cellular alterations are acquired early in the evolution of C4 photosynthesis (Hylton et al., 1988; McKown and Dengler, 2007; Muhaidat et al., 2011; Sage et al., 2011b). Importantly, including these additional early traits in the analysis did not alter the predicted order of the original 16 traits. Together, these analyses did not alter our main conclusions, suggesting that they are robust.
The order of C4 trait evolution is flexible
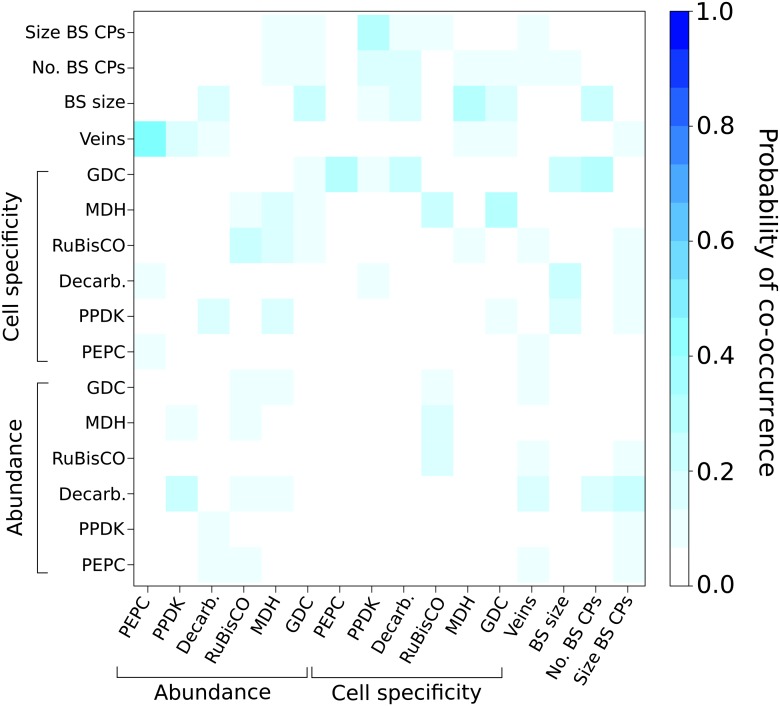
In addition to the likely order of evolutionary events generating C4 photosynthesis, the number of molecular alterations required is also unknown. We therefore aimed to test if multiple traits were predicted to evolve with linked timing, and therefore likely mediated by a single underlying mechanism. To achieve this, we performed a contingency analysis by considering trajectories across phenotype space beginning with a given initial acquisition step. In this analysis, the starting genome had one of the 16 traits acquired and the rest absent, and the contingency of the subsequent trajectory upon the initial step was recorded. This approach was designed to test if acquiring one C4 trait increased the probability of subsequently acquiring other traits, thus detecting if the evolution of multiple traits is linked by underlying mechanisms. Inflexible linkage between multiple traits was detected in artificial positive control datasets (Figure 2B) but not in the C3–C4 dataset (Figure 3—figure supplement 3). This result suggests that the order of C4 trait acquisition is flexible. Multiple origins of C4 may therefore have been facilitated by this flexibility in the evolutionary pathways connecting C3 and C4 phenotypes.
C4 evolved via multiple distinct evolutionary trajectories
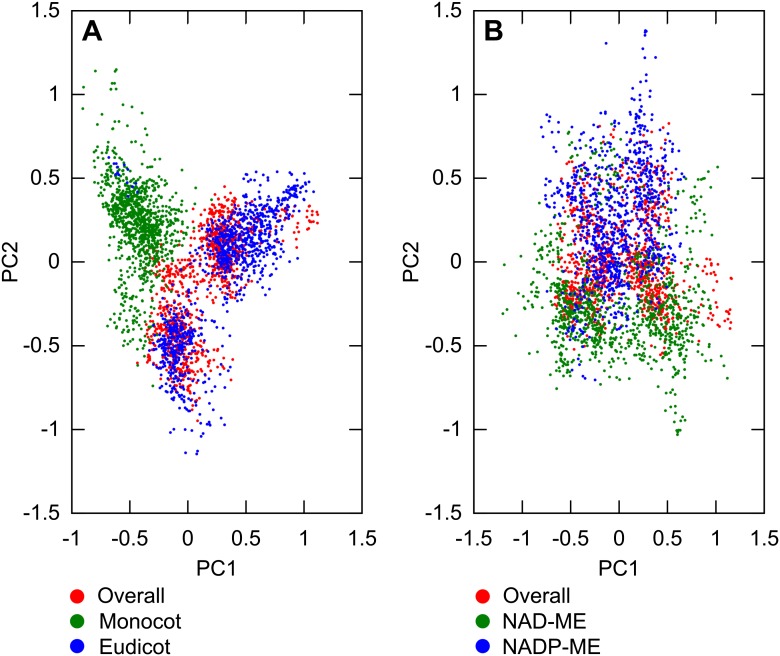
Our Bayesian analysis strongly indicates that there are multiple evolutionary pathways by which C4 traits are acquired by all lineages of C4 plants. First, no single sequence of acquisitions was capable of producing intermediate phenotypes compatible with all observations (‘Methods’). Second, several traits such as compartmentation of GDC into BS and the increased number of chloroplasts in the BS clearly displayed bimodal probability distributions for their acquisition (Figure 3). This bimodality is indicative of multiple distinct pathways to C4 photosynthesis that acquire traits at earlier or later times. To investigate factors underlying this bimodality, we inferred evolutionary pathways generating the C4 leaf using data from monocot and eudicot lineages, or from lineages using NAD malic enzyme (NAD–ME) or NADP malic enzyme (NADP-ME) as their primary C4 acid decarboxylase. PCA on the entire set of inferred transition networks for monocot and dicot subsets revealed distinct separation (Figure 4A), suggesting that the topology of the evolutionary landscape surrounding C4 is largely different for these two anciently diverged taxa. Performing this PCA including networks that were inferred from the full data set (with both lineages) confirmed that this separation is a robust result and involves posterior variation on a comparable scale to that of the full set of possible networks (Figure 4—figure supplement 1). Analysis of the posterior probabilities of the mean pathways representing either monocots or dicots revealed that this separation is the result of differences in the timing of events generating both anatomical and biochemical traits (Figure 4C). We propose that the ancient divergence of the monocot and eudicot clades constrained the evolution of C4 photosynthesis to broadly different evolutionary pathways in each.
Figure 4. Differences in the evolutionary events generating different C4 sub-types and distantly related taxa.
Principal component analysis (PCA) on the entire landscape of transition probabilities using only monocot and eudicot data (A) and data from NADP-ME and NAD-ME sub-type lineages (B) shows broad differences between the evolutionary pathways generating C4 in each taxon. Monocots and eudicots differ in the predicted timing of events generating C4 anatomy and biochemistry (C), whereas NADP-ME and NAD-ME lineages differ primarily in the evolution of decreased vein spacing and greater numbers of chloroplasts in BS cells (D).
Figure 4—figure supplement 1. Variation between lineages compared to variance of overall dataset.
There was more overlap between the landscapes generating NAD–ME and NADP-ME species (Figure 4B), likely reflecting the convergent origins of NAD–ME and NADP-ME sub-types (Furbank, 2011; Sage et al., 2011a). Despite the traditional definition of these lineages on the basis of biochemical differences, we detected differences in the timing of their anatomical evolution (Figure 4D). For example, in NAD–ME lineages, increased vein density was predicted to be acquired early in C4 evolution, while in NADP-ME species this trait showed a broadly different trajectory (Figure 4D). The proliferation of chloroplasts in the BS was also acquired with different timings between the two sub-types. The alternative evolutionary pathways generating the NADP-ME and NAD–ME subtypes were therefore defined by differences in the timing of anatomical and cellular traits that are predicted to precede the majority of biochemical alterations (Figure 3, Figure 4D). We therefore conclude that these distinct sub-types evolved as a consequence of alternative evolutionary histories in response to non-photosynthetic pressures. Furthermore, we propose that early evolutionary events determined the downstream phenotypes of C4 sub-types by restricting lineages to independent pathways across phenotype space.
Discussion
A novel Bayesian technique for inferring stochastic trajectories
The adaptive landscape metaphor has provided a powerful conceptual framework within which evolutionary transitions can be modelled (Gavrilets, 1997; Whibley et al., 2006; Lobkovsky et al., 2011). However, the majority of complex biological traits provide numerous challenges in utilising such an approach, including missing phenotypic data, incomplete phylogenetic information and in the case of convergent evolution, variable ancestral states. Here we report the development of a novel, predictive Bayesian approach that is able to infer likely evolutionary trajectories connecting phenotypes from sparsely sampled, highly stochastic data. With this model, we provided insights into the evolution of one of the most complex traits to have arisen in multiple lineages: C4 photosynthesis. However, as our approach is not dependent on detailed phylogenetic inference, we propose that it could be used to model the evolution of other complex traits, such as those in the fossil record, which are also currently limited by the fragmented nature of data available (Kidwell and Holland, 2002). Our approach is also not limited by the time-scale over which predicted trajectories occur. As a result, it may be useful in inferring pathways underlying stochastic processes occurring over much shorter timescales, such as disease or tumour progression, or the differentiation of cell types.
C4 evolution was initiated by non-photosynthetic drivers
A central hypothesis for the ecological drivers of C4 evolution is that declining CO2 concentration in the Oligocene decreased the rate of carboxylation by RuBisCO, creating a strong pressure to evolve alternative photosynthetic strategies (Christin et al., 2008; Vicentini et al., 2008). According to this hypothesis, alterations to the localisation and abundance of the primary carboxylases PEPC and RuBisCO would be expected to occur early in the evolutionary trajectories generating C4. Conversely, our data suggest that alterations to anatomy and cell biology were predicted to precede the majority of biochemical alterations, and that other enzymes of the C4 pathway are recruited prior to PEPC and RuBisCO (Figure 3). These enzymes, such as PPDK and C4 acid decarboxylases, function in processes not related to photosynthesis within leaves of C3 plants (Aubry et al., 2011), so the early changes to abundance and localisation of these enzymes within C4 lineages may have been driven by non-photosynthetic pressures. A recent in silico study also predicts that changes to photorespiratory metabolism and GDC in BS cells evolved prior to the C4 pathway (Heckman et al., 2013). Our model predicts that BS-specificity of GDC was acquired early in C4 evolution for the majority of lineages. However, we also note that the predicted timing of GDC BS-specificity is bimodal in our analysis (Figure 3), and not predicted to be acquired early in monocot lineages (Figure 4C). These results suggest that this is not a feature of C4 evolution to have occurred repeatedly in all lineages.
Recent evidence from physiological and ecological studies has identified a number of additional environmental pressures that may have driven the evolution and radiation of C4 lineages, including high evaporative demands (Osborne and Sack, 2012) and increased fire frequency (Edwards et al., 2010). Increased BS volume and vein density have been proposed as likely adaptations to improve leaf hydraulics under drought (Osborne and Sack, 2012; Griffiths et al. 2013), but nothing is known about how early recruitment of GDC, PPDK, and C4 acid decarboxylases (Figure 3) may relate to these pressures. A better understanding of the mechanisms underlying the recruitment of these enzymes (Brown et al., 2011; Kajala et al., 2012; Wiludda et al., 2012) may help identify the key molecular events facilitating C4 evolution.
Our data also suggest that modifications to leaf development drove the evolution of diverse C4 sub-types. For example, we find that differences in the timing of events altering leaf vascular development and BS chloroplast division occur prior to the appearance of the alternative evolutionary pathways generating the NADP-ME and NAD-ME biochemical sub-types (Figure 4D). These traits are predicted to evolve prior to any alterations to the C4 acid decarboxylase enzymes that traditionally define these sub-types (Furbank, 2011). As an homologous mechanism has been shown to regulate the cell-specificity of gene expression in both NADP-ME and NAD-ME gene families in independent lineages (Brown et al., 2011), it is unlikely that mechanisms underlying the recruitment of these enzymes drove the evolution of distinct sub-types. We therefore conclude that these different sub-types evolved as a consequence of alternative evolutionary histories in leaf development, rather than biochemical or photosynthetic pressures. This may explain why differences in the carboxylation efficiency or photosynthetic performance of different C4 sub-types have never been detected (Furbank, 2011), making the adaptive significance of different decarboxylation mechanisms difficult to explain. Instead, we propose that early evolutionary events determined the downstream phenotypes of C4 sub-types by restricting lineages to independent pathways across phenotype space. The numerous differences in leaf development and cell biology between C4 sub-types (Furbank, 2011) may provide clues as to which developmental changes underlie subsequent differences in metabolic evolution.
Convergent evolution was facilitated by flexibility in evolutionary trajectories
C4 photosynthesis provides an excellent example of how independent lineages with a wide range of ancestral phenotypes can converge upon similar complex traits. Several studies on more simple traits have demonstrated that convergence upon a phenotype can be specified by diverse genotypes, and thus non-homologous molecular mechanisms in independent lineages (Wittkopp et al., 2003; Hill et al., 2006; Steiner et al., 2009). Taken together, our data also indicate that flexibility in the viable series of evolutionary events has also facilitated the convergence of this highly complex trait. First, we show that at least four distinct evolutionary trajectories underlie the evolution of C4 lineages (Figure 4). Second, we find no evidence for inflexible linkage between the predicted timing of distinct C4 traits (Figure 3—figure supplement 1). This diversity in viable pathways also helps explain why C4 has been accessible to such a wide variety of species and not limited to a smaller subset of the angiosperm phylogeny. A recent model for the evolution of the biochemistry associated with the C4 leaf also found that C4 photosynthesis was accessible from any surrounding point of a fitness landscape (Heckman et al., 2013). Our study of C4 anatomy, biochemistry, and cell biology also suggests the C4 phenotype is accessible from multiple trajectories. Encouragingly, the trajectories predicted by Heckman et al. (2013) were found to pass through phenotypes of C3–C4 species, despite the fact that these species were not used to parameterise the evolutionary landscape. As different mechanisms generate increased abundance and cell-specificity for the majority C4 enzymes in independent C4 lineages (reviewed in Langdale, 2011; Williams et al., 2012), it is likely that mechanistic diversity underlies the multiple evolutionary pathways generating C4 photosynthesis and may be a key factor in facilitating the convergent evolution of complex traits. This may benefit efforts to recapitulate the acquisition of C4 photosynthesis through the genetic engineering of C3 species (Hibberd et al., 2008), expanding the molecular toolbox available to establish C4 traits in distinct phenotypic backgrounds.
Methods
Biological data from C4 intermediates
Data from eighteen C3, seventeen C4, and thirty-seven C3–C4 species were consolidated from 43 studies that have examined the phenotypic characteristics of C3–C4 species since their discovery in 1974 (Table 1). Values for sixteen of the most widely studied C3 characteristics were recorded for each intermediate species, as well as congeneric C3 and C4 relatives where available. The majority of data on enzyme abundance and the number and size of bundle sheath (BS) chloroplasts were obtained from studies employing the same methodology and were thus cross-comparable (e.g., Goldstein et al., 1976; Ku et al., 1976; Ku and Edwards, 1978; Sayre et al., 1979; Holaday et al., 1981; Holaday and Black, 1981; Ku et al., 1983; Adams et al., 1986; Rajendrudu et al., 1986; Ku et al., 1991; Devi and Raghavendra, 1993; Bruhl and Perry, 1995; Casati et al., 1999; Keeley, 1999). These cross-comparable quantitative data were partitioned into presence absence scores using two clustering techniques, the expectation-maximisation (EM) algorithm and hierarchical clustering (Figure 1—figure supplement 3). EM clustering was performed using a one-dimensional mixture model with two assigned components (e.g., presence and absence clusters), allowing for variable variance between the two components of the model, and variable population size between the two components. Hierarchical clustering was performed using a complete-linkage agglomerative approach, partitioning clusters by maximum distance according to a Euclidean distance metric. This approach identifies clusters with common variance, thus contrasting with the clusters of variable variance identifiable by EM.
For quantitative data not comparable with other studies (e.g., Ku and Edwards, 1978; Rathnam and Chollet, 1978; Rathnam and Chollet, 1979; Holaday et al., 1984; Brown and Hattersley, 1989; Beebe and Evert, 1990; P’yankov et al., 1997; Gowik et al., 2011), values obtained for intermediate species were scored as 1 or 0 if they were closer to the values for the respective C4 or C3 controls used in the original study. For qualitative abundance data from immunoblots (e.g., Rosche et al., 1994; Voznesenskaya et al., 2007; Voznesenskaya, 2010), relative band intensity was measured using ImageJ software (Abramoff et al., 2004) and abundance was scored as 1 or 0 if the band intensity value was closer to the C4 or C3 control respectively. For qualitative cell-specificity data from immunolocalisations (e.g. Voznesenskaya et al., 2001; Ueno et al., 2003, 2006; Muhaidat et al., 2011), a presence score was only assigned if the enzyme appeared completely absent from either mesophyll (M) or BS cells. We represent the phenotypic properties of each intermediate species as a string of L = 16 numbers (Figure 1—source data 1). We will refer to these strings as phenotype strings of L loci, with each locus describing data on the corresponding phenotypic trait. In a given locus, 0 denotes the absence of a C4 trait, 1 denotes the presence of a C4 trait, and 2 denotes missing data.
Principal component analysis (PCA)
PCA was performed on five variables for C4 cycle enzyme activity, with missing values estimated using the EM algorithm for PCA as described by Roweis (1998).
Model transition networks
The fundamental element underlying our analysis is a transition network P, consisting of a directed graph with 2L = 65,536 nodes, and the weight of the edge Pij denoting the probability of a transition occurring from node i to node j. Each node corresponds to a possible phenotype: we labeled nodes with labels li so that li was the binary representation of the phenotype at node i, and Pij takes on the specific meaning of the probability of a transition from phenotype li to phenotype lj. We made several restrictions on the structure of P. We allowed only transitions that change a given phenotype at one locus, so Pij = 0 if H (li,lj) ≠ 1, where H (b1,b2) is the Hamming distance between bitstrings b1 and b2. Transitions that changed loci with value 1 to value 0 (steps back towards the C3 state) were forbidden, so Pij = 0 if H (li,l0) > H (lj,l0), where l0 is the phenotypic string containing only zeroes. We assume that the possibility of events involving backwards steps, and multiple simultaneous trait acquisitions constitute second-order effects which will not strongly influence the inferred evolutionary dynamics.
Evolutionary trajectories
Given the transition network P, we modelled the evolutionary trajectories that may give rise to C4 photosynthesis through the picture of a discrete analogue to a Brownian bridge, that is as a stochastic process on P with constrained start and end positions (Revuz and Yor, 1999). We enforced the start state of the process to be (the phenotype string of all zeroes) and the end state, through the imposed structure of P, to be (the string of all ones). The dynamics of the process between these points consisted of L steps, with a phenotypic trait being acquired at each step, and a step from node i to node j occurring with probability Pij.
Sampling intermediates
As many evolutionary trajectories may lead to the acquisition of the required phenotypic traits, we considered an ensemble of evolutionary trajectories for each transition network. Each member of this ensemble is started at and allowed to step across the network according to probabilities P.
To compare the dynamics of a given transition network to the properties of observed biological intermediates, we pictured this ensemble of trajectories as a modification of a hidden Markov model (HMM [Rabiner, 1989]). At each timestep in each individual trajectory, the process may with some probability emit a signal to the observer, with that signal being simply li, the label of the node at which the process currently resides. Over an ensemble of trajectories, a set of randomly emitted signals is thus built up (Figure 1—figure supplement 4).
We define a compatibility function between two strings as
| (1) |
| (2) |
C(s,t) thus returns 1 if a signal comprising string s could be responsible for observation t once some of the loci within s have been obscured: signal s is compatible with observation t.
Likelihood of observing biological data
We wish to compute the likelihood of observing biological data B given a transition network P. Under our model, this likelihood is calculated by considering the compatibility of randomly emitted signals from processes supported by P with the observed data B. We write
| (3) |
Here, is the probability of specific trajectory x arising on network P, is the probability that trajectory x emits signal s, and C(s,Bi) gives the compatibility of signal s with intermediate state Bi. The term within the product operator thus describes the probability that evolutionary dynamics on network P give rise to a signal that is compatible with species Bi, with the overall likelihood being the product of this probability over all observed species.
Simulation
The uniform and random nature of signal emission means that is a constant if signal s can be emitted from trajectory x, and zero otherwise. Our simulation approach only produces signals which can be emitted from the trajectory under consideration, so will always take the same constant value (which depends on the probability of signal emissions). As we will be considering ratios of network likelihoods and will not be concerned with absolute likelihoods we will ignore this term henceforth. For each network P we simulate an ensemble of Nchain trajectories and, for each node encountered throughout this ensemble, we record compatibilities with each of the biologically observed intermediates. We sum these compatibilities over the ensemble, obtaining . A network that does not encounter any node compatible with a particular intermediate will thus be assigned zero likelihood; networks that encounter compatible nodes many times will be assigned high likelihoods.
For each transition network, we simulated Nchain = 2 × 104 individual trajectories running from C3 to C4. This value was chosen after preliminary investigations to analyse the ability of trajectory ensembles to broadly sample available paths on networks.
Bayesian MCMC over compatible networks
Given uninformative prior knowledge about the evolutionary dynamics leading to C4 photosynthesis (specifically, our prior involves each possible transition from a given node being assigned equal probability), we aimed to build a posterior distribution over a suitable description of the evolutionary dynamics. We represented the dynamics supported on a network P through a matrix π, where πi,n describes the probability that acquisition of trait i occurs at the nth step in an evolutionary trajectory. The values of matrix πi,n were built up from sampling over the ensemble of trajectories simulated on P.
We used Bayesian MCMC to sample networks based on their associated likelihood values (Wasserman, 2004). At each iteration, we perturbed the transition probability of the current network P a small amount (see below) to yield a new trial network P’. We calculated and accepted P as the new network if , where r was taken from . For practical reasons we implemented this scheme using log-likelihoods.
The perturbations we applied to transition probabilities are Normally distributed in logarithmic space: for each edge wij we used . To show that this scheme obeys detailed balance, consider two states and , for simplicity described by a one-dimensional scalar quantity. Consider the proposed move from A when Δ is the result of the random draw. This proposal is if , implying that . The probability of proposing move is thus , and the probability of proposing is . By the symmetry of the Normal distribution, these two probabilities are equal.
We started each MCMC run with a randomly initialised transition matrix. We allowed 2 × 104 burn-in steps then sampled over a further 2 × 105 steps. The value σ = 0.1 was chosen for the perturbation kernel. These values were chosen through an initial investigation to analyse the convergence of MCMC runs under different parameterisations. We performed 40 MCMC runs for each experiment and confirmed that the resulting posterior distributions had converged and yielded consistent results.
Summary dynamics matrices
We report the posterior distributions inferred from sampling compatible networks as above. In the coarse-grained time representation, we use , summing over sets of ordinals of size 4.
We used the transition network P, rather than a more coarse-grained representation of the evolutionary dynamics (e.g., the summary matrices π), as the fundamental element within our simulations so as not to discard possible details that would be lost in a coarse-grained approach – for example, the presence of multiple distinct pathways, which may be averaged over in a summary matrix.
Proofs of principle
To verify our approach, we constructed artificial data sets, consisting of sets of strings in which phenotypic traits were acquired in a single ordering. Specifically, πi,n = δi,n, so the first step always resulted in acquiring the first trait, and so on. To test the approach in a pleiotropic setting, where multiple traits were acquired simultaneously, we also constructed data sets where traits were acquired at only four timesteps, each corresponding to the simultaneous acquisition of four traits. We subjected these datasets to our inferential machinery with all data intact, and with 50% of data points occluded, to determine the sensitivity and robustness of our approach (Figure 2A–B). The approach accurately determines the ordering of events in both the bare and occluded cases and assigns very similar posterior probability distributions to the ordering of those traits acquired simultaneously.
Comparing the evolution of multiple C4 sub-types
To compare the pathways generating C4 in monocots and eudicots, and in NADP-ME and NAD-ME sub-type lineages, we performed inference on two data sets: B1 and B2, each comprising phenotype measurements from one of the groups of interest. We reported the posteriors on the resulting summary dynamics as before, and for the principal components analysis (PCA) we sampled 103 summary dynamic matrices πi,n from the inferred posterior distribution during the Bayesian MCMC procedure, and performed PCA on these sampled matrices.
Predictions
When a simulated chain encountered a phenotypic node compatible with a given biological intermediate, the values of traits corresponding to missing data in the biological data were recorded. These recorded values, sampled over the sampled set of networks, allowed us to place probabilities on the values of biologically unobserved traits inferred from the encounters of compatible dynamics with the corresponding phenotypic possibilities. For example, if 70% of paths on network P pass through point 101 and 30% pass through point 001, we infer a 70% probability that the missing trait in biological intermediate 201 takes the value 1. Predictions were presented if the inferred probability of a ‘1’ value was >75% (predicting a ‘1’) or <25% (predicting a ‘0’). If one of these inequalities held and the limiting value fell outside one standard deviation of the inferred probability (i.e., for mean μ and standard deviation σ, μ > 0.75 and μ − σ > 0.75 [predicting a ‘1’] or μ > 0.25 and μ + σ < 0.25 [predicting a ‘0’]), the prediction was presented as ‘strict’.
Acquisition ordering and evidence against a single pathway
We used a dynamic programming approach to explore whether a deterministic sequence of events, with a trait Tn always being acquired at timestep n (), was compatible with the biological data. Performing an exhaustive search over sequences of single transitions that were compatible with the observed data, we identified several such sequences that accounted for all but one trait acquisition, but no single sequence exists that accounts for all the data.
Contingent trait acquisition
To explore the possibility of multiple traits being acquired simultaneously, we tracked acquisition probabilities for later traits given that a certain trait was acquired first. This tracking was performed over all sampled compatible networks, building up ‘contingent’ acquisition tables γ with the i, j th element given by . If a pair of traits i and j were acquired simultaneously, we would expect γij and γji to both be higher than expected in the non-contingent case (as j should always appear to be immediately acquired after i and vice versa).
Quantitative real-time PCR (qPCR)
RNA was extracted from mature leaves of six Flaveria species as part of the One Thousand Plants Consortium (www.onekp.com), using the hot acid phenol protocol as described by Johnson et al. (2012) (protocol no. 12). cDNA was synthesised from 0.5 µg RNA using Superscript II (Life Technologies, Glasgow, U.K.) following manufacturer’s instructions. An oligo dT primer (Roche, Basel, Switzerland) was used to selectively transcribe polyadenylated transcripts. To each RNA sample, 1 fmol GUS transcript was added for use as an exogenous control or ‘RNA spike’, against which measured transcript abundance was normalised as described by Smith et al. (2003).
qPCR was performed as described by Bustin (2000) using the DNA-binding marker SYBR Green (Sigma Aldrich, St. Louis, MO) according to manufacturer’s instructions. Primers were designed using cDNA sequences for Flaveria species available at Genbank (http://www.ncbi.nlm.nih.gov/genbank) and synthesised by Life Technologies. Amplification was performed using a Rotor-Gene Q instrument (Qiagen, Hilden, Germany), using the following cycling parameters: 94°C for 2 min, followed by 40 cycles at 94°C for 20 s, 60°C for 30 s, 72°C for 30 s, followed by a 5 min incubation at 72°C. Relative transcript abundance was calculated as described by Livak and Schmittgen (2001).
Acknowledgements
We thank S Kelly, JA Langdale, H Griffiths, and N Jones for advice.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding Information
This paper was supported by the following grants:
Biotechnology and Biological Sciences Research Council to Ben P Williams, Iain G Johnston, Julian M Hibberd.
International Rice Research Institute to Sarah Covshoff.
Additional information
Competing interests
The authors declare that no competing interests exist.
Author contributions
BPW, Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article.
IGJ, Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article.
SC, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article.
JMH, Conception and design, Analysis and interpretation of data, Drafting or revising the article.
References
- Abramoff MD, Magalhaes PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics Int 11:36–42 [Google Scholar]
- Adams CA, Leung F, Sun SSM. 1986. Molecular properties of phosphoenolpyruvate carboxylase from C3, C3-C4 intermediate, and C4 Flaveria species. Planta 167:218–25. 10.1007/BF00391418 [DOI] [PubMed] [Google Scholar]
- Akyildiz M, Gowik U, Engelmann S, Koczor M, Streubel M, Westhoff P. 2007. Evolution and function of a cis-regulatory module for mesophyll-specific gene expression in the C4 dicot Flaveria trinervia. Plant Cell 19:3391–402. 10.1105/tpc.107.053322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry S, Brown NJ, Hibberd JM. 2011. The role of proteins in C3 plants prior to their recruitment into the C4 pathway. J Exp Bot 62:3049–59. 10.1093/jxb/err012 [DOI] [PubMed] [Google Scholar]
- Beebe DU, Evert RF. 1990. The morphology and anatomy of the leaf of Moricandia arvensis (L.) DC. (Brassicaceae). Bot Gaz 151:184–203. 10.1086/337818 [DOI] [Google Scholar]
- Brown NJ, Newell CA, Stanley S, Chen JE, Perrin AJ, Kajala K, et al. 2011. Independent and parallel recruitment of preexisting mechanisms underlying C4 photosynthesis. Science 331:1436–9. 10.1126/science.1201248 [DOI] [PubMed] [Google Scholar]
- Brown RH, Hattersley PW. 1989. Leaf anatomy of C3-C4 species as related to evolution of C4 photosynthesis. Plant Physiol 91:1543–50. 10.1104/pp.91.4.1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhl JJ, Perry S. 1995. Photosynthetic pathway-related ultrastructure of C3, C4 and C3-like C3-C4 intermediate sedges (Cyperaceae), with special reference to Eleocharis. Aust J Plant Physiol 22:521. 10.1071/PP9950521 [DOI] [Google Scholar]
- Bustin SA. 2000. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 25:169–93. 10.1677/jme.0.0250169 [DOI] [PubMed] [Google Scholar]
- Casati P, Fresco AG, Andreo CS, Drincovich MF. 1999. An intermediate form of NADP-malic enzyme from the C3-C4 intermediate species Flaveria floridana. Plant Science 147:101–9. 10.1016/S0168-9452(99)00101-6 [DOI] [Google Scholar]
- Cheng SH, Moore BD, Edwards GE, Ku MS. 1988. Photosynthesis in Flaveria brownii, a C4-like species: leaf anatomy, characteristics of CO2 exchange, compartmentation of photosynthetic enzymes, and metabolism of CO2. Plant Physiol 87:867–73. 10.1104/pp.87.4.867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin P-A, Osborne CP, Chatelet DS, Columbus JT, Besnard G, Hodkinson TR, et al. 2013. Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proc Natl Acad Sci USA 110:1381–6. 10.1073/pnas.1216777110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin P-A, Osborne CP, Sage RF, Arakaki M, Edwards EJ. 2011b. C4 eudicots are not younger than C4 monocots. J Exp Bot 62:3171–81. 10.1093/jxb/err041 [DOI] [PubMed] [Google Scholar]
- Christin P-A, Sage TL, Edwards EJ, Ogburn RM, Khoshravesh R, Sage RF. 2011a. Complex evolutionary transitions and the significance of C3-C4 intermediate forms of photosynthesis in Molluginaceae. Evolution 65:643–60. 10.1111/j.1558-5646.2010.01168.x [DOI] [PubMed] [Google Scholar]
- Christin P, Besnard G, Samaritani E, Duvall M, Hodkinson T, Savolainen V, et al. 2008. Oligocene CO2 decline promoted C4 photosynthesis in grasses. Curr Biol 18:37–43. 10.1016/j.cub.2007.11.058 [DOI] [PubMed] [Google Scholar]
- Christin P, Salamin N, Savolainen V, Duvall M, Besnard G. 2007. C4 photosynthesis evolved in grasses via parallel adaptive genetic changes. Curr Biol 17:1241–7. 10.1016/j.cub.2007.06.036 [DOI] [PubMed] [Google Scholar]
- Devi MT, Raghavendra AS. 1993. Partial reduction in activities of photorespiratory enzymes in C3-C4 intermediates of Alternanthera and Parthenium. J Exp Bot 44:779–84. 10.1093/jxb/44.4.779 [DOI] [Google Scholar]
- Devi MT, Rajagopalan AV, Raghavendra AS. 1995. Predominant localization of mitochondria enriched with glycine-decarboxylating enzymes in bundle sheath cells of Alternanthera tenella, a C3-C4 intermediate species. Plant Cell Environ 18:589–94. 10.1111/j.1365-3040.1995.tb00559.x [DOI] [Google Scholar]
- Edwards EJ, Osborne CP, Stromberg CAE, Smith SA, C4 grasses consortium 2010. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328:587–91. 10.1126/science.1177216 [DOI] [PubMed] [Google Scholar]
- Furbank RT. 2011. Evolution of the C4 photosynthetic mechanism: are there really three C4 acid decarboxylation types? J Exp Bot 62:3103–8. 10.1093/jxb/err080 [DOI] [PubMed] [Google Scholar]
- Gavrilets S. 1997. Evolution and speciation on holey adaptive landscapes. Trends Ecol Evol 12:307–12. 10.1016/S0169-5347(97)01098-7 [DOI] [PubMed] [Google Scholar]
- Goldstein L, Ray T, Kestler D, Mayne B, Brown R, Black C. 1976. Biochemical characterization of panicum species which are intermediate between C3 and C4 photosynthesis plants. Plant Sci 6:85–90. 10.1016/0304-4211(76)90140-1 [DOI] [Google Scholar]
- Gowik U, Brautigam A, Weber KL, Weber APM, Westhoff P. 2011. Evolution of C4 photosynthesis in the genus Flaveria: how many and which genes does it take to make C4? Plant Cell 23:2087–105. 10.1105/tpc.111.086264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths H, Weller G, Toy LFM, Dennis RJ. 2013. You’re so vein: bundle sheath physiology, phylogeny and evolution in C3 and C4 plants. Plant Cell Environ 36:249–61. 10.1111/j.1365-3040.2012.02585.x [DOI] [PubMed] [Google Scholar]
- Hatch MD, Kagawa T, Craig S. 1975. Subdivision of C4 pathway species based on differing C4 acid decarboxylating systems and ultrastructural features. Aust J Plant Physiol 2:111–28. 10.1071/PP9750111 [DOI] [Google Scholar]
- Hattersley PW. 1984. Characterization of C4 type leaf anatomy in grasses (Poaceae). Mesophyll: bundle sheath area ratios. Ann Bot 53:163–80 [Google Scholar]
- Hattersley PW, Stone NE. 1986. Photosynthetic enzyme activities in the C3-C4 intermediate Neurachne minor. Aust J Plant Physiol 13:399. 10.1071/PP9860399 [DOI] [Google Scholar]
- Heckman D, Schulze S, Denton A, Gowik U, Westhoff P, Weber APM, et al. 2013. Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell 153:1579–88. 10.1016/j.cell.2013.04.058 [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Sheehy JE, Langdale JA. 2008. Using C4 photosynthesis to increase the yield of rice-rationale and feasibility. Curr Opin Plant Biol 11:228–31. 10.1016/j.pbi.2007.11.002 [DOI] [PubMed] [Google Scholar]
- Hill RC, Egydio de Carvalho C, Salogiannis J, Schlager B, Pilgrim D, Haag ES. 2006. Genetic flexibility in the convergent evolution of hermaphroditism in Caenorhabditis nematodes. Dev Cell 10:531–8. 10.1016/j.devcel.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Holaday AS, Black CC. 1981. Comparative characterization of phosphoenolpyruvate carboxylase in C3, C4, and C3-C4 intermediate Panicum species. Plant Physiol 67:330–4. 10.1104/pp.67.2.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaday AS, Lee KW, Chollet R. 1984. C3-C4 Intermediate species in the genus Flaveria: leaf anatomy, ultrastructure, and the effect of O2 on the CO2 compensation concentration. Planta 160:25–32. 10.1007/BF00392462 [DOI] [PubMed] [Google Scholar]
- Holaday AS, Shieh Y-J, Lee KW, Chollet R. 1981. Anatomical, ultrastructural and enzymic studies of leaves of Moricandia arvensis, a C3-C4 intermediate species. Biochim Biophys Acta 637:334–41. 10.1016/0005-2728(81)90172-9 [DOI] [Google Scholar]
- Hylton CM, Rawsthorne S, Smith AM, Jones DA, Woolhouse HW. 1988. Glycine decarboxylase is confined to the bundle-sheath cells of leaves of C3-C4 intermediate species. Planta 175:452–9. 10.1007/BF00393064 [DOI] [PubMed] [Google Scholar]
- Johnson MTJ, Carpenter EJ, Tian Z, Bruskiewich R, Burris JN, Carrigan CT, et al. 2012. Evaluating methods for isolating total RNA and predicting the success of sequencing phylogenetically diverse plant transcriptomes. PLOS ONE 7:e50226. 10.1371/journal.pone.0050226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajala K, Brown NJ, Williams BP, Borrill P, Taylor LE, Hibberd JM. 2012. Multiple Arabidopsis genes primed for recruitment into C4 photosynthesis. Plant J 69:47–56. 10.1111/j.1365-313X.2011.04769.x [DOI] [PubMed] [Google Scholar]
- Keeley JE. 1999. Photosynthetic pathway diversity in a seasonal pool community. Funct Ecol 13:106–18. 10.1046/j.1365-2435.1999.00294.x [DOI] [Google Scholar]
- Kennedy RA, Eastburn JL, Jensen KG. 1980. C3-C4 Photosynthesis in the genus Mollugo: structure, physiology and evolution of intermediate characteristics. Am J Bot 67:1207–17. 10.2307/2442363 [DOI] [Google Scholar]
- Khoshravesh R, Hossein A, Sage TL, Nordenstam B, Sage RF. 2012. Phylogeny and photosynthetic pathway distribution in Anticharis Endl. (Scrophulariaceae). J Exp Bot 63:5645–58. 10.1093/jxb/ers218 [DOI] [PubMed] [Google Scholar]
- Kidwell SM, Holland SM. 2002. The quality of the fossil record: implications for evolutionary analyses. Ann Rev Ecol Syst 33:561–88. 10.1146/annurev.ecolsys.33.030602.152151 [DOI] [Google Scholar]
- Koteyeva NK, Voznesenskaya EV, Roalson EH, Edwards GE. 2010. Diversity in forms of C4 in the genus Cleome (Cleomaceae). Ann Bot 107:269–83. 10.1093/aob/mcq239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozmik Z, Ruzickova J, Jonasova K, Matsumoto Y, Vopalensky P, Kozmikova I, et al. 2008. Assembly of the cnidarian camera-type eye from vertebrate-like components. Proc Natl Acad Sci USA 105:8989–93. 10.1073/pnas.0800388105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku MSB, Edwards GE. 1978. Photosynthetic efficiency of Panicum hians and Panicum milioides in relation to C3 and C4 plants. PlantCell Physiol 19:665–75 [Google Scholar]
- Ku MSB, Edwards GE, Kanai R. 1976. Distribution of enzymes related to C3 and C4 pathway of photosynthesis between mesophyll and bundle sheath cells of Panicum hians and Panicum milioides. Plant Cell Physiol 17:615–20 [Google Scholar]
- Ku MSB, Monson RK, Littlejohn RO, Nakamoto H, Fisher DB, Edwards GE. 1983. Photosynthetic characteristics of C3-C4 intermediate Flaveria species1: I. leaf anatomy, photosynthetic responses to O2 and CO2, and activities of key enzymes in the C3 and C4 Pathways. Plant Physiol 71:944–8. 10.1104/pp.71.4.944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku MSB, Wu J, Dai Z, Scott RA, Chu C, Edwards GE. 1991. Photosynthetic and photorespiratory characteristics of Flaveria species. Plant Physiol 96:518–28. 10.1104/pp.96.2.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdale JA. 2011. C4 cycles: past, present, and future research on C4 photosynthesis. Plant Cell 23:3879–92. 10.1105/tpc.111.092098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) method. Methods 25:402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lobkovsky AE, Wolf YI, Koonin EV. 2011. Predictability of evolutionary trajectories in fitness landscapes. PLOS Comput Biol 7:e1002302. 10.1371/journal.pcbi.1002302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown AD, Dengler NG. 2007. Key innovations in the evolution of Kranz anatomy and C4 vein pattern in Flaveria (Asteraceae). Am J Bot 94:382–99. 10.3732/ajb.94.3.382 [DOI] [PubMed] [Google Scholar]
- McKown AD, Moncalvo J-M, Dengler NG. 2005. Phylogeny of Flaveria (Asteraceae) and inference of C4 photosynthesis evolution. Am J Bot 92:1911–28. 10.3732/ajb.92.11.1911 [DOI] [PubMed] [Google Scholar]
- Mooers AO, Heard SB. 2013. Inferring evolutionary process from phylogenetic tree shape. Q Rev Biol 72:31–54. 10.1086/419657 [DOI] [Google Scholar]
- Moore BD, Franceschi VR, Cheng S-H, Wu J, Ku MSB. 1987. Photosynthetic characteristics of the C3-C4 intermediate Parthenium hysterophorus. Plant Physiol 85:978–83. 10.1104/pp.85.4.978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhaidat R, Sage TL, Frohlich MW, Dengler NG, Sage RF. 2011. Characterization of C3-C4 intermediate species in the genus Heliotropium L. (Boraginaceae): anatomy, ultrastructure and enzyme activity. Plant Cell Environ 34:1723–36. 10.1111/j.1365-3040.2011.02367.x [DOI] [PubMed] [Google Scholar]
- Osborne CP, Sack L. 2012. Evolution of C4 plants: a new hypothesis for an interaction of CO2 and water relations mediated by plant hydraulics. Philos Trans R Soc Lond B Biol Sci 367:583–600. 10.1098/rstb.2011.0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- P’yankov VI, Voznesenskaya EV, Kondratschuk AV, Black CC. 1997. A comparative anatomical and biochemical analysis in Salsola (Chenopodiaceae) species with and without a Kranz type leaf anatomy: a possible reversion of C4 to C3 photosynthesis. Am J Bot 84:597. [PubMed] [Google Scholar]
- Rabiner L. 1989. A tutorial on hidden Markov models and selected applications in speech recognition. Proc IEEE 77:257–86. 10.1109/5.18626 [DOI] [Google Scholar]
- Rajendrudu G, Prasad JSR, Das VSR. 1986. C3-C4 intermediate species in Alternanthera (Amaranthaceae)1: leaf anatomy, CO2 compensation point, net CO2 exchange and activities of photosynthetic enzymes. Plant Physiol 80:409–14. 10.1104/pp.80.2.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnam CKM, Chollet R. 1978. CO2 donation by malate and aspartate reduces photorespiration in Panicum milioides, A C3-C4 intermediate species. Biochem Biophys Res Comm 85:801–8. 10.1016/0006-291X(78)91233-0 [DOI] [PubMed] [Google Scholar]
- Rathnam CKM, Chollet R. 1979. Photosynthetic carbon metabolism in Panicum milioides, a C3-C4 intermediate species: evidence for a limited C4 dicarboxylic acid pathway of photosynthesis. Biochim Biophys Acta 548:500–19. 10.1016/0005-2728(79)90061-6 [DOI] [PubMed] [Google Scholar]
- Rawsthorne S, Hylton CM, Smith AM, Woolhouse HW. 1988. Photorespiratory metabolism and immunogold localization of photorespiratory enzymes in leaves of C3 and C3-C4 intermediate species of Moricandia. Planta 173:298–308. 10.1007/BF00401016 [DOI] [PubMed] [Google Scholar]
- Rawsthorne S, Morgan CL, O’Neill CM, Hylton CM, Jones DA, Frean ML. 1998. Cellular expression pattern of the glycine decarboxylase P protein in leaves of an intergeneric hybrid between the C3-C4 intermediate species Moricandia nitens and the C3 species Brassica napus. Theoret App Genet 96:922–7. 10.1007/s001220050821 [DOI] [Google Scholar]
- Revuz D, Yor M. 1999. Continuous Martingales and Brownian Motion. Springer [Google Scholar]
- Rosche E, Streubel M, Westhoff P. 1994. Primary structure of the photosynthetic pyruvate orthophosphate dikinase of the C3 plant Flaveria pringlei and expression analysis of pyruvate orthophosphate dikinase sequences in C3, C3-C4 and C4 Flaveria species. Plant Mol Biol 26:763–9. 10.1007/BF00013761 [DOI] [PubMed] [Google Scholar]
- Roweis S. 1998. Em algorithms for PCA and SPCA. Advances in Neural Information Processing Systems. Cambridge, MA: MIT Press; p. 626–32 [Google Scholar]
- Sage RF, Christin P-A, Edwards EJ. 2011a. The C4 plant lineages of planet Earth. J Exp Bot 62:3155–69. 10.1093/jxb/err048 [DOI] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. 2012. Photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol 63:19–47. 10.1146/annurev-arplant-042811-105511 [DOI] [PubMed] [Google Scholar]
- Sage TL, Sage RF, Vogan PJ, Rahman B, Johnson DC, Oakley JC, et al. 2011b. The occurrence of C2 photosynthesis in Euphorbia subgenus Chamaesyce (Euphorbiaceae). J Exp Bot 62:3183–95. 10.1093/jxb/err059 [DOI] [PubMed] [Google Scholar]
- Santos JC, Coloma LA, Cannatella DC. 2003. Multiple, recurring origins of aposematism and diet specialization in poison frogs. Proc Natl Acad Sci USA 100:12792–7. 10.1073/pnas.2133521100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre RT, Kennedy RA, Pringnitz DJ. 1979. Photosynthetic enzyme activities and localization in Mollugo verticillata populations differing in the levels of C3 and C4 cycle operation. Plant Physiol 64:293–9. 10.1104/pp.64.2.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack CR, Hatch MD. 1967. Comparative studies on the activity of carboxylases and other enzymes in relation to the new pathway of photosynthetic carbon dioxide fixation in tropical grasses. Biochem J 103:660–5. 10.1042/bj1030660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RD, Brown B, Ikonomi P, Schechter AN. 2003. Exogenous reference RNA for normalization of real-time quantitative PCR. BioTechniques 34:88–91 [DOI] [PubMed] [Google Scholar]
- Steiner CC, Römpler H, Boettger LM, Schöneberg T, Hoekstra HE. 2009. The genetic basis of phenotypic convergence in beach mice: similar pigment patterns but different genes. Mol Biol Evol 26:35–45. 10.1093/molbev/msn218 [DOI] [PubMed] [Google Scholar]
- Ueno O, Bang SW, Wada Y, Kondo A, Ishihara K, Kaneko Y, et al. 2003. Structural and biochemical dissection of photorespiration in hybrids differing in genome constitution between Diplotaxis tenuifolia (C3-C4) and Radish (C3). Plant Physiol 132:1550–9. 10.1104/pp.103.021329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno O, Wada Y, Wakai M, Bang SW. 2006. Evidence from photosynthetic characteristics for the hybrid origin of Diplotaxis muralis from a C3-C4 intermediate and a C3 species. Plant Biol 8:253–9. 10.1055/s-2005-873050 [DOI] [PubMed] [Google Scholar]
- Vicentini A, Barber JC, Aliscioni SS, Giussani LM, Kellogg EA. 2008. The age of the grasses and clusters of origins of C4 photosynthesis. Glob Change Biol 14:2963–77. 10.1111/j.1365-2486.2008.01688.x [DOI] [Google Scholar]
- Vogan PJ, Frohlich MW, Sage RF. 2007. The functional significance of C3-C4 intermediate traits in Heliotropium L. (Boraginaceae): gas exchange perspectives. Plant Cell Environ 30:1337–45. 10.1111/j.1365-3040.2007.01706.x [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Artyusheva EG, Franceschi VR, Pyankov VI, Kiirats O, Ku M, et al. 2001. Salsola arbusculiformis, a C3-C4 Intermediate in Salsoleae (Chenopodiaceae). Ann Bot 88:337–48. 10.1006/anbo.2001.1457 [DOI] [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Chuong SDX, Ivanova AN, Barroca J, Craven LA, et al. 2007. Physiological, anatomical and biochemical characterisation of photosynthetic types in genus Cleome (Cleomaceae). Funct Plant Biol 34:247. 10.1071/FP06287 [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Edwards GE, Ocampo G. 2010. Revealing diversity in structural and biochemical forms of C4 photosynthesis and a C3-C4 intermediate in genus Portulaca L. (Portulacaceae). J Exp Bot 61:3647–62. 10.1093/jxb/erq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman L. 2004. All of statistics: a concise course in statistical inference. Springer [Google Scholar]
- Weinreich DM, Watson RA, Chao L. 2005. Sign epistasis and genetic constraint on evolutionary trajectories. Evolution 59:1165–74. 10.1111/j.0014-3820.2005.tb01768.x [DOI] [PubMed] [Google Scholar]
- Whibley AC, Langlade NB, Andalo C, Hanna AI, Bangham A, Thébaud C, et al. 2006. Evolutionary paths underlying flower color variation in Antirrhinum. Science 313:963–6. 10.1126/science.1129161 [DOI] [PubMed] [Google Scholar]
- Williams BP, Aubry S, Hibberd JM. 2012. Molecular evolution of genes recruited into C4 photosynthesis. Trends Plant Sci 17:213–20. 10.1016/j.tplants.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Wilson JS, Williams KA, Forister ML, von Dohlen CD, Pitts JP. 2012. Repeated evolution in overlapping mimicry rings among North American velvet ants. Nat Commun 3:1272. 10.1038/ncomms2275 [DOI] [PubMed] [Google Scholar]
- Wiludda C, Schulze S, Gowik U, Engelmann S, Koczor M, Streubel M, et al. 2012. Regulation of the photorespiratory GLDPA gene in C4 Flaveria: an intricate interplay of transcriptional and post-transcriptional processes. Plant Cell 24:137–51. 10.1105/tpc.111.093872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkopp PJ, Williams BL, Selegue JE, Carroll SB. 2003. Drosophila pigmentation evolution: divergent genotypes underlying convergent phenotypes. Proc Natl Acad Sci USA 100:1808–13. 10.1073/pnas.0336368100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. 1932. The roles of mutation, inbreeding, crossbreeding and selection in evolution. Proc Sixth Int Congr Genet 1:356–66 [Google Scholar]
- Zhu X-G, Long SP, Ort DR. 2008. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr Opin Biotechnol 19:153–9. 10.1016/j.copbio.2008.02.004 [DOI] [PubMed] [Google Scholar]