Abstract
Myocardial development is regulated by an elegantly choreographed ensemble of signaling events mediated by a multitude of intermediates that take a variety of forms. Cellular differentiation and maturation are a subset of vertically integrated processes that extend over several spatial and temporal scales to create a well-defined collective of cells that are able to function cooperatively and reliably at the organ level. Early efforts to understand the molecular mechanisms of cardiomyocyte fate determination focused primarily on genetic and chemical mediators of this process. However, increasing evidence suggests that mechanical interactions between the extracellular matrix (ECM) and cell surface receptors as well as physical interactions between neighboring cells play important roles in regulating the signaling pathways controlling the developmental processes of the heart. Interdisciplinary efforts have made it apparent that the influence of the ECM on cellular behavior occurs through a multitude of physical mechanisms, such as ECM boundary conditions, elasticity, and the propagation of mechanical signals to intracellular compartments, such as the nucleus. In addition to experimental studies, a number of mathematical models have been developed that attempt to capture the interplay between cells and their local microenvironment and the influence these interactions have on cellular self-assembly and functional behavior. Nevertheless, many questions remain unanswered concerning the mechanism through which physical interactions between cardiomyocytes and their environment are translated into biochemical cellular responses and how these signaling modalities can be utilized in vitro to fabricate myocardial tissue constructs from stem cell-derived cardiomyocytes that more faithfully represent their in vivo counterpart. These studies represent a broad effort to characterize biological form as a conduit for information transfer that spans the nanometer length scale of proteins to the meter length scale of the patient and may yield new insights into the contribution of mechanotransduction into heart development and disease.
Keywords: Heart, Cardiomyocyte, Mechanotransduction, Sarcomeregenesis, ECM
1 Introduction
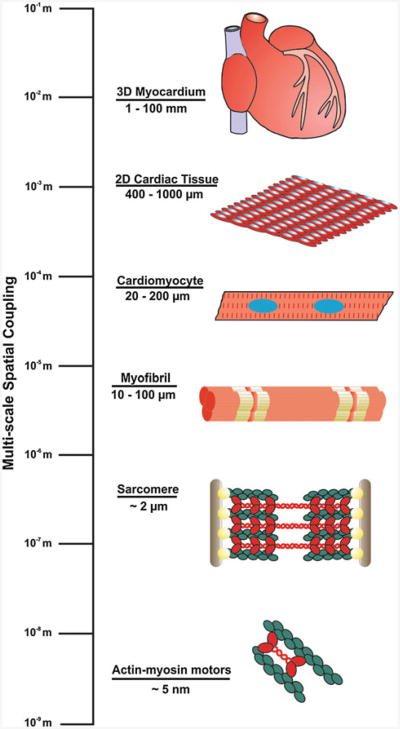
A vexing issue in cardiac development is the presence of a three-dimensional structural and functional hierarchy that spans several orders of spatial magnitude from the centimeter length scale of the myocardium to the nanometer length scale of actomyosin motors (Fig. 1). One possible mechanism through which architectural and temporal synchrony is maintained during cardiac organogenesis is the propagation of mechanical forces, encoding multi-scale information, from super-cellular, cellular, and sub-cellular networks that are physically connected throughout the heart. Simplified, qualitative models of cellular development often overlook the importance of mechanical cues and the bi-directional flow of information between cells and their local microenvironment during tissue formation (Ingber 1993). Physical forces transmitted between cells and the extracellular matrix (ECM), as well as between neighboring cells, could prove vital to the emergent form and function of the healthy myocardium during cardiac morphogenesis. These mechanical cues may be an essential component of a larger biological network that integrates chemical and mechanical signals to drive nascent cells to adopt relevant phenotypes based on contextual information encoded in the local microenvironment (Parker and Ingber 2007).
Fig. 1.
Spatial scaling of the functional components of the heart The functional components of the myocardium demonstrate a hierarchical relationship that spans several orders of spatial magnitude, from the nanometer length scale of the proteins comprising actomyosin cross-bridges to the millimeter length sheets of laminar myocardial tissue that make up the muscular walls of the heart chambers. (adapted from Chien et al. 2008)
It is now well accepted that epigenetic factors, such as mechanical forces, play a fundamental role in regulating organ development (Ingber 2006a,b). The physical properties of the local microenvironment and the contractile activity of cells influence the developmental processes that take place during embryogenesis (Wozniak and Chen 2009). Individual cells sense external mechanical cues primarily through interactions with the ECM via integrin binding and from neighboring cells through intercellular junctions (Chen et al. 2004). Mechanical tension in the cytoskeleton arising from intercellular junctions and ECM adhesions has been shown experimentally to contribute to epithelial branching (Jamora et al. 2003) and angiogenesis during lung development (Moore et al. 2005; Ingber 2002). Mechanical forces also underlie morphological changes that occur in the heart during development, wherein alterations to cardiomyocyte shape and spatial organization arising from actin cytoskeletal dynamics influence looping of the embryonic heart tube, for example. (Latacha et al. 2005; Taber 2001; Itasaki et al. 1991). In the post-natal myocardium, forces endured during the contraction cycle are postulated to contribute to development and remodeling through cardiomyocyte hypertrophy, in which changes to myofibril and ECM architecture can lead to either adaptive or maladaptive growth (Jacot et al. 2010; McCain and Parker 2011). This review will examine the contributing role of mechanical forces to cellular development within the heart and explore the potential benefits of mathematical models for capturing the involvement of post-translational cytoskeletal dynamics in recapitulating the development of the myocardium in vitro. Knowledge gained from these models could provide valuable insight for designing custom ECM micro-environments that allow the construction of functional, patient-relevant engineered tissues from ES- and iPS-derived cardiomyocytes.
2 Mechanical cues during cardiac development
During formation of the heart, the cardiac mesoderm arises from the primitive streak as a product of temporally synchronized Wnt, BMP, and activin/Nodal signaling events that occur in a spatially asymmetric manner (Evans et al. 2010). In turn, this left–right asymmetrical patterning results in activation of distinct gene expression profiles, differential proliferation, and cardiomyocyte shape changes that are responsible for regionalized myocardial lineage specification and the morphological transition from a linear tube to the four-chambered structure that the heart ultimately adopts (Evans et al. 2010; Taber et al. 1995; Damon et al. 2009). Looping of the embryonic heart tube is primarily the product of localized changes in cardiomyocyte morphology that have been shown to arise from intrinsic remodeling of the actin cytoskeleton and may also play a role in activating the regional changes in proliferation and gene expression observed in subsequent phases of cardiac development (Srivastava and Olson 2000; Price et al. 1996; Taber et al. 1995). Post-natal cardiomyocyte growth and development occurs through a process known as ‘hypertrophy’ that is mediated by signaling events that are activated by both biochemical and biomechanical stimuli (Heineke and Molkentin 2006). Cardiac hypertrophy is initiated by hemodynamic load and characterized by increased cardiomyocyte size and the expression of genes that are believed to act as a compensatory mechanism to normalize ventricular afterload (Frey and Olson 2003; Sheehy et al. 2009). In order to recognize and respond to changes in systolic wall stress, the cells comprising the myocardium must possess a communications pathway that allows external physical cues to activate intracellular signaling cascades.
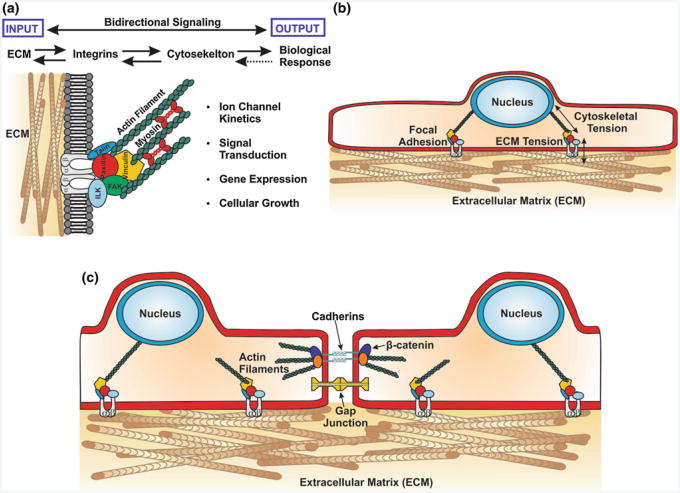
The cytoskeleton is the primary conduit for mechanically encoded information in the cell (Alenghat et al. 2002), regulating cell shape (Singhvi et al. 1994) and influencing migration, cellular function and homeostasis (Chen et al. 1997; Bray et al. 2008; Parker et al. 2002). Transmembrane integrin receptors provide a direct mechanical linkage between the ECM and the cytoskeleton within the cell through which external physical forces may influence intra-cellular processes (Fig. 2a) (Schwartz et al. 1995). Over the course of development, the heart undergoes coordinated changes in ECM composition and expression of α- and β-integrin isoforms that specifically recognize various ECM components (Price et al. 1992; Ross and Borg 2001; Terracio et al. 1991). In the fetal stage, expression levels of fibronectin and α5β1 integrin receptors are elevated relative to expression levels observed in the adult myocardium (Farhadian et al. 1995; Samuel et al. 1994; Carver et al. 1994). This differential expression of fibronectin and β1 integrins has been associated with the proliferation and spreading that is observed in pre-natal cardiomyocytes, but absent post-partum (Hilenski et al. 1992; Hornberger et al. 2000). Variations in β1 integrin splice isoforms also distinguish the embryonic and adult myocardium. Embryonic cardiomyocytes have been shown to primarily express the β1A isoform, while adult cardiomyocytes preferentially express the β1D variant (Belkin et al. 1996; van der Flier et al. 1997; de Melker and Sonnenberg 1999). Furthermore, ablation of β1 integrin expression in the ventricular myocytes of transgenic mice revealed that disruption of normal integrin function resulted in pathological fibrotic remodeling, increased susceptibility to dilated cardiomyopathy, and perinatal mortality (Keller et al. 2001; Shai et al. 2002). Taken together, this relationship between the shift in integrin isoform expression and ECM composition suggests that the integrin-ECM interface may serve as a means for the cells of the nascent heart to recognize and respond to the ever changing mechanical forces that are present during cardiac organogenesis.
Fig. 2.
Bi-directional signaling interfaces in the simplified myocardium a External mechanical cues are transmitted from the ECM to intracellular compartments via transmembrane integrin receptors that physically link it to the cytoskeleton. These mechanical signals elicit a number of biological responses ranging from ion channel activity to programmed cell death, and in turn these biological processes can feed information back to the extracellular space via the same mechanical pathway. b Transmembrane integrin receptors form a direct physical linkage between the ECM and the cytoskeleton through focal adhesions that provides a conduit for transmitting mechanical signals directly to intracellular compartments, such as the nucleus. c In addition to mechanotransduction through the integrin-ECM interface, cardiomyocytes also respond to mechanical signals from neighboring cells through intercellular junctions and direct transmembrane ligand-receptor interactions
3 Signaling through the integrin–ECM interface
Transmembrane integrin receptors form a direct mechanical linkage between the ECM and the cytoskeleton (Wang et al. 1993) and serve as the primary conduit of bi-directional signaling between cells and the ECM (Matthews et al. 2006; Meyer et al. 2000) despite the fact that they lack intrinsic kinase activity (Schwartz et al. 1995). Mechanical forces are transmitted across the integrin–ECM interface to the cytoskeleton, where they activate mechanosensitive signal transducers, such as focal adhesion kinase (FAK), that are able to translate the mechanical cue into a biochemical response (Burridge and Chrzanowska-Wodnicka 1996; Schwartz and Ginsberg 2002; Samarel 2005). This mode of information transmission has been shown to activate a variety of chemical signaling pathways, including the Rho kinase, PI3K, ILK, Src, ERK, and MAP kinase pathways that modulate transcriptional activity and direct important cellular activities, such as cell cycle entry and the induction of apoptosis (Schwartz et al. 1995; Ingber 2006a; Chen et al. 1997; Discher et al. 2009; Fletcher and Mullins 2010). Many of these signaling intermediates are immobilized on the cytoskeleton, particularly at the Z-discs in cardiomyocytes, and are thus subject to mechanical perturbations that may modulate their activity and translocation to cellular compartments, such as the nucleus (Wang et al. 2009; Gjorevski and Nelson 2009; Dahl et al. 2008). Cardiomyocytes express a protein known as melusin that flanks sarcomeric α-actinin at the Z lines and interacts with the cytoplasmic domain of β1 integrins (Brancaccio et al. 1999). Melusin has been implicated as an important sensor of myocardial wall stress in murine knockout studies that showed a specific attenuation of glycogen kinase 3β signaling in melusin-null hearts (Brancaccio et al. 2003). Another Z-disc protein that is widely regarded to serve as a mechano-sensor is muscle LIM protein (MLP), which is believed to transduce mechanical signals via the calcineurin-NFAT pathway to activate the hypertrophic response in cardiomyocytes (Knoll et al. 2002; Heineke et al. 2005). Titin, a component of the sarcomere that regulates diastolic tension, possesses a C-terminal kinase domain that has been implicated in cardiomyocyte strain sensing (Puchner et al. 2008). It has been shown that this catalytic domain is involved in regulating the activity of the muscle-specific transcriptional co-activators MuRF2 and four-and-a-half-LIM-domain (FHL) through changes in titin conformation (Sheikh et al. 2008; Lange et al. 2005, 2002). Evidence suggests that MLP stabilizes the interaction between T-cap and titin at the Z-disc, providing an interface through which mechanical forces can be transmitted between the ECM and titin to initiate signaling events at the titin catalytic domain in response to hemodynamic load (Kruger and Linke 2009; Boateng et al. 2007; Linke 2008).
An intriguing alternative signaling paradigm is the transduction of mechanical signals through the ECM-cytoskeletal network to structures deep within the cytoplasm, such as the nucleus (Fig. 2b), where they can alter enzymatic activity or gene expression by modulating nuclear shape or physically deforming genomic structures within the nuclear compartment (Maniotis et al. 1997a,b). This hypothesis is supported by in situ PCR measurements taken from osteoblasts that revealed cell shape-dependent alterations in nuclear morphology resulted in differential regulation of osteocalcin expression, suggesting that cytoskeletal tension directly impacted transcriptional activity (Thomas et al. 2002). Given the kinetic nature of the myocardium, and observations that cardiomyocyte nuclei reversibly deform during each contraction cycle (Bray et al. 2009), the possibility exists that mechanical effects on nuclear morphology may influence the expression of genes in cardiomyocytes as well.
Experimental data suggest that individual filaments of the cytoskeleton bear tensile and compressive loads and give rise to a mechanical network under isometric tension that propagates physical signals throughout the cell at a velocity far exceeding the limits of chemical diffusion (Brangwynne et al. 2008; Wang et al. 1993, 2009; Ingber 1997). Neonatal rat ventricular myocytes (NRVMs) cultured on micro-contact printed ECM substrates that imposed an anisotropic morphology and organization possessed elongated nuclei that demonstrated a high degree of mutual alignment across the tissue constructs and underwent dynamic deformation during contraction (Bray et al. 2009). Genes encoding proteins involved in tissue remodeling processes have been found to be susceptible to changes in cellular morphology induced as a consequence of direct perturbation of cytoskeletal structure with actin and microtubule disrupting agents, such as cytochalasin D and colchicine (Samarakoon and Higgins 2002). Studies of the link between cytoskeletal dynamics, motility, and gene expression during myocardial development revealed that myocardin-related transcription factors (MRTFs) are physically bound to globular actin monomers until they are incorporated into actin filaments. Upon release from actin monomers, the MRTFs are free to translocate to the nucleus, where they interact with the transcription factor serum response factor (SRF) to promote the expression of genes under its control (Olson and Nordheim 2010). This relationship between actin cytoskeletal assembly and regulation of cardiac gene expression by MRTF-SRF has been shown to be mediated through a Rho-dependent mechanism that may provide a feed-forward loop for driving the expression of genes necessary for myofibrillogenesis during myocardial development and in response to hypertrophic stimuli (Kuwahara et al. 2005, 2007).
Many genetic markers of vascular smooth muscle-specific differentiation code for proteins associated with contractility, giving rise to a potential role for Rho-dependent changes in smooth muscle contractility in regulating smooth muscle gene expression during vascular development (Mack et al. 2001). Experiments conducted on capillary network formation by human microvascular endothelial cells in vitro and retinal angiogenesis in vivo using the Rho inhibitor p190RhoGAP revealed that Rho-induced changes in cytoskeletal architecture regulated angiogenesis by modulating the activities of two antagonistic transcription factors, TFII-1, and GATA2, that govern expression of the VEGF receptor in a manner that was sensitive to ECM elasticity (Mammoto et al. 2009). Dynamic assembly and disassembly of cytoskeletal elements generates directed forces that perturb cell shape and guide the organization of cellular components (Alenghat et al. 2002; Fletcher and Mullins 2010). The evidence from these studies supports the notion that mechanical force-balance influences cellular behavior by modulating gene expression activity and may be an important factor for regulating cell fate decisions during co-development of the heart and circulatory system.
4 Signaling through intercellular junctions
In addition to force transmission across the integrin–ECM interface, cells also receive biomechanical input from their neighbors via intercellular junctions and through direct transmembrane ligand–receptor interactions (Fig. 2c) (Simpson et al. 1993; Kresh and Chopra 2011). Cytoskeletal tension arising from actomyosin crossbridges plays a key role in the formation and maintenance of intercellular junctions during cardiac tissue development (Peters et al. 1994; Niessen et al. 2011; Miyake et al. 2006). In vitro studies have shown that the magnitude of tractional forces transmitted through the cytoskeletons of adjacent endothelial cells across adherens junctions was correlated with the size and strength of these junctions (Liu et al. 2010). The phenotype and spatial organization of nascent cells during tissue formation require the coordinated regulation of gene expression and precise interactions between neighboring cells. Interactions that typically target transmembrane Notch receptors and the Wnt signaling intermediates localize to adherens junctions (Nelson and Nusse 2004a; Gessert and Kuhl 2010). The activity of the Notch and Wnt/β-catenin signaling pathways has been found to have reciprocal effects in cardiac progenitor cells during embryonic development of the heart (Androutsellis-Theotokis et al. 2006; Boni et al. 2008; Nelson and Nusse 2004b; Ueno et al. 2007). In addition, canonical and noncanonical Wnt signaling have differential, stage-dependent effects on cardiomyocyte maturation during normal heart development (Gessert and Kuhl 2010; Rao and Kuhl 2010). Notch1 signaling promotes differentiation of cardiac progenitor cells and negatively regulates the activity of β-catenin. On the other hand, activation of β-catenin by the canonical Wnt pathway inhibits differentiation by negatively regulating cardiac transcription factors and instead promotes proliferation of cardiac progenitor cells (Kwon et al. 2009). In addition, Notch1 activation in cardiac progenitor cells gives rise to a population of Nkx2.5 expressing transit amplifying myocytes that are believed to mediate post-natal growth of the myocardium (Boni et al. 2008).
Notch and Wnt/β-catenin signaling pathways also play a role in regulating the incidence of endothelial-mesenchymal transformation (EMT) during valve development in a potentially mechanosensitive manner (Combs and Yutzey 2009; Yang et al. 2008). Formation of the heart valves during the early stages of heart development is traditionally considered to be a product of VEGF signaling, but it coincides with increased fluid shear stress and mechanical strain (Hove et al. 2003; Miller 2011; Santhanakrishnan and Miller 2011). In vitro studies of engineered valve endothelial cells showed that the incidence of EMT in these constructs was not only enhanced by the application of chronic cyclic stretch, but also that the magnitude of applied strain initiated EMT via different pathways (Balachandran et al. 2011). A mechanical load of 10% strain initiated EMT via TGF-β1, while wnt/β-catenin signaling was implicated at 20% strain, providing evidence that cells not only respond to external mechanical cues, but can also distinguish loads of different magnitudes. Furthermore, a myofibroblast phenotype emerged concomitantly with an increased incidence of EMT in valve endothelial cells cultured on an ECM substrate that imposed an anisotropic cellular organization and subjected to orthogonal, 20% cyclic strain. Break down of adherens junctions is a hallmark of EMT, along with the expression of factors that inhibit endothelial genes (Arciniegas et al. 2007), suggesting that the magnitude of tension transmitted across intercellular junctions may serve as a mediator of phenotype switching during cardiac valve morphogenesis.
The assembly of gap junction channels in cardiomyocytes has been shown experimentally to be closely tied to the formation of adherens junctions (Saffitz and Kleber 2004), in which N-cadherin and connexin 43 share a temporal relationship in their expression and spatial co-localization during adherens junction formation (Samarel 2005). Immunolabeling studies conducted on embryonic, adolescent, and adult rat hearts revealed that connexin 43 gap junctionrelated immunoreactivity could be observed in the ventricles as early as 10 days post-conception, and incorporation of connexin 43 gap junctions into the costameres of ventricular myocytes proceeded well into adolescence (Gourdie et al. 1992). Dual voltage clamp measurements taken on pairs of NRVMs with pre-defined aspect ratios revealed that intercellular conductance increased with the volume of observed connexin 43 immunosignal as cellular aspect ratio increased (McCain et al. 2012). Further, mechanical forces acting on myocytes during contraction in vivo and pulsatile stretch in vitro were found to cause a dramatic increase in the expression of connexin 43 and a concomitant increase in conduction velocity due to increased electrical coupling between myocytes (Saffitz and Kleber 2004). Given the relationship between cell alignment and impulse propagation velocity in cardiac tissues (Bursac et al. 2002; Chung et al. 2007), mechanical forces transmitted between neighboring cardiomyocytes could be important for establishing and maintaining the anisotropic conduction pattern necessary for proper functioning of the heart.
5 Effects of the mechanical environment on cardiomyocyte form and function
When considering the cellular microenvironment, the traditional paradigm has been that of the diffusion of soluble mitogens in autocrine and paracrine signaling through the interstitial space. Over the last 10–15 years, an interdisciplinary group of cell biologists and engineers have postulated an alternative and supplementary vision of the cellular microenvironment as a mechanical network of cells coupled by the protein polymer network of the extracellular matrix that propagates information, encoded as mechanical forces, between cells at data rates that far exceed that of chemical diffusion. In the embryonic heart, evolving from a pulsatile tube to a cyclically contracting 4-chambered structure, mechanical forces are imposed on maturing cardiomyocytes that vary in their magnitude and frequency over the course of development (Taber 2001; Jacot et al. 2010). Regional alterations in cell shape have been observed and postulated to contribute to the overall morphology that emerges during heart development (Manasek and Monroe 1972; Taber et al. 1995). The in vitro cellular microenvironment can be engineered to force isolated cells to adopt the morphology and configuration of their native tissues so that researchers can study the contributions of tissue geometry to functionality (Pijnappels et al. 2008; Bursac et al. 2002; Geisse et al. 2009; Fu et al. 2010; Parker and Ingber 2007). Interactions between cardiomyocytes and the ECM cause changes in cell shape that direct actin filament orientation, sarcomere organization, and myofibrillogenesis in vitro (Bray et al. 2008). Studies of cultured cardiovascular cells have shown that shape also influences a number of functional properties, such as voltage-gated ion currents, calcium dynamics, and contractility, suggesting that it is an important parameter to consider when designing engineered tissue constructs (Walsh and Parks 2002; Pong et al. 2011; Yin et al. 2004; Alford et al. 2011).
Phenomena, such as durotaxis, haveleadmany researchers to postulate that the physical properties of the cellular micro-environment can influence cell phenotype and tissue morphogenesis (Georges and Janmey 2005;Pelham and Wang 1997). Experiments conducted on stem cells encapsulated in RGD-modified matrices suggest a role for cellular tractional forces generated through interactions between the ECM and specific integrin isoforms in guiding lineage commitment (Huebsch et al. 2010; Guilak et al. 2009). In addition, studies of embryonic cardiomyocytes reveal that changes in matrix rigidity associated with normal heart development and fibrotic ECM remodeling reminiscent of that observed after myocardial infarction dramatically affect rhythmic contraction of the cells (Engler et al. 2008; Tobita et al. 2002). Isolated cardiomyocytes cultured on poly-acrylamide gels with tunable elastic moduli have repeatedly demonstrated increased contractile activity and myofibril organization when the stiffness of their underlying substrate resembles that measured for the healthy native myocardium (approximately 20kPa), as opposed to culturing them on substrates with higher or lower elastic moduli (Bajaj et al. 2010; Bhana et al. 2010; Engler et al. 2008; Jacot et al. 2008). In the case of marrow-derived mesenchymal stem cells (MSCs), studies have shown that culturing naïve MSCs on elastic substrates with a modulus of approximately 10 kPa specifically induced a myogenic phenotype (Engler et al. 2006). It is believed that cells sense the mechanical stiffness of the ECM through tractional forces generated by cross-bridge interactions between actin and myosin II. In vitro experiments utilizing inhibitors of non-muscle myosin II isoforms lend support to this hypothesis, demonstrating that cytoskeletal tension plays a role in mediating mechano-sensation between focal adhesions and the ECM (Zajac and Discher 2008). Force measurements collected on various types of cells cultured on elastomeric substrates revealed a linear relationship between apparent focal adhesion size and cellular force generation that was dependent upon actomyosin interactions (Balaban et al. 2001). While myocardial contractile activity is not considered to play a role in the early stages of heart development (Manasek and Monroe 1972; Taber 2001; Latacha et al. 2005), there is evidence in zebrafish models that hemodynamic load does alter cardiomyocyte shape, leading to aberrations in ventricular morphogenesis (Hove et al. 2003; Auman et al. 2007). Together, these findings demonstrate a clear role for the physical micro-environment in myocardial development that researchers may potentially harness in the creation of artificial tissue constructs.
Cardiomyocytes are constantly subjected to physical stretching as a result of the contractile activity of the heart, presenting the possibility that the motion of the myocardium may activate mechano-sensitive signaling pathways that affect the characteristics of cardiac cells (Trepat et al. 2007). Pulsatile stretch has been shown experimentally to promote parallel alignment of NRVMs and induce mechanotransductive signaling events through the β1 integrin–ECM interface that were responsible for the up-regulation of N-cadherin and connexin 43 expression via activation of FAK and the ERK1/2 pathway (Shanker et al. 2005; Salameh et al. 2010; Yamada et al. 2005). Concomitant with the enhanced expression of connexin 43, cyclic stretch was also found to increase propagation velocity in NRVM monolayers (Zhuang et al. 2000). Gene expression measurements taken on NRVM cultures exposed to pulsatile stretch show that the mechanical stimulus gives rise to a hypertrophic phenotype with expression of the “fetal gene program” and myosin heavy chain isoform switching that are hallmarks of cardiac hypertrophy (Frank et al. 2008; Gopalan et al. 2003; Cadre et al. 1998). All together, these studies reveal that the mechanical properties of the cellular microenvironment do contribute to the functional maturation of the myocardium and that there is a need for computational tools that will allow researchers to construct effective strategies for harnessing these mechanical cues to optimize engineered cardiac tissue self-assembly and contractile performance.
6 Computational modeling of mechanotransduction in cellular self-organization
The working myocardium is comprised of rectangular myocytes organized into a laminar arrangement that serves to optimize cooperative sarcomeric force output. It is postulated that the interplay between geometric cues encoded in the ECM and cardiomyocyte cytoskeletal dynamics confers these morphological characteristics that are vital to the proper functioning of the heart. Refinements in our understanding of cellular mechanobiology, facilitated in large part by advances in soft lithography, have given rise to mathematical models of the phenomena and parameters involved in mechano-sensation that may contribute to myocardial tissue development. The most pivotal of these experimental methodologies was the development of in vitro techniques to regulate ECM composition and geometry (Mrksich et al. 1997, 1996) that have made it possible to engineer cells whose morphology and function are amenable to computational modeling, thus closing the loop between theory and experiment. This is important, because one poorly studied problem in cell biology is the post-translational self-assembly and self-organization of multimeric intracellular protein structures, such as the cytoskeleton.
A variety of models have been reported (summarized in Table 1) that examine the role of cell architecture and molecular motors in the self-assembly of the cytoskeletal network. A simple phenomenological model has been proposed that predicts the distribution of bound integrin complexes and was validated in a square fibroblast cell (Novak et al. 2004). This model explains possible mechanisms behind the higher concentration of focal adhesions (FA) at the edges and corners of cells (Table 1, column 2). While this simple phenomenological model lacks the features to predict fiber distributions in more complex cell shapes, it provides insight into actin cytoskeletal dynamics without taking into account the mechanical interactions between the substrate, integrins, and the cytoskeleton. Finite element models that include these interactions can replicate myofibril distributions in several epithelial and fibroblast cell shapes (Deshpande et al. 2006, 2008). A chemo-mechanical model that explores the FA complex formation without modeling the cell as a whole produces a detailed prediction of both stresses and strains in the FA (Paszek et al. 2009) (Table 1, column 3). These models predict the simple assembly of the actin network or integrin clustering in the vicinity of the focal adhesion in cells with simple cytoskeletal architecture. However, the assembly of myofibrils in cardiac myocytes represents a more challenging phenomenon to model due to its heightened complexity.
Table 1. Contrasting features of a selection of myofibrillogenesis computation models.
| Model property | Novak et al. (2004) | Deshpande et al. (2006, 2008) | Paszek et al. (2009) | Grosberg et al. (2011) |
|---|---|---|---|---|
| Model type | Phenomeno-logical | Finite element/solid mechanics | Chemo-mechanical | Phenomeno-logical |
| Model compared to cell type | NIH3T3 fibroblasts (Brock et al. 2003) | Retinal pigment epithelial human cells or fibroblast cells | No specific cell type | Neonatal rat ventricular myocytes |
| Integrins have a free and bound state (or high/low affinity) | Yes | Yes | Yes | Yes |
| Focal adhesions more stable with greater force | Yes | Yes | Yes | Yes |
| Myofibrils/stress fibers | Yes | Ye s | No | Yes |
| included in model Fiber length-force dependence | No | Not explicitly | No | Yes |
| Differentiating between pre-myofibril and nascent myofibril formation | No | No | N/A | Yes |
| Myofibril actively mutually align | No | No | N/A | Yes |
| Testing boundary conditions/symmetry breaking | No | No | No | Yes |
| Detailed model of solid mechanics of the interface | No | Yes | Yes | No |
| Substrate mechanical load | No | Yes, but not the deformation of the substrate | Yes and Detailed, i.e. both stress and strain—deformation | Yes, but very simply (only traction) |
| Computationally complex | No | Yes | Yes | No |
Cardiomyocytes, like skeletal muscle, are striated myocytes that utilize the actin cytoskeleton as a scaffold for the clustering and assembly of other proteins in sarcomeres, aligned serially in a contractile structure known as the myofibril. To date, most models of myofibrillogenesis are qualitative and give detailed descriptions of how sarcomeric proteins sequentially appear in the sarcomere. Most computational models of cytoskeletal self- assembly focused on the architecture of the actin cytoskeleton and were not designed to recapitulate the rather specialized case of striated muscle, where the maturation from pre-myofibril to nascent myofibrils and their active mutual alignment in cardiomyocytes represent an important developmental step in the spatiotemporal coordination of rhythmic, cellular contraction and ordered cardiac myocyte shortening.
We reported a model that describes the kinetics of myofibrillogenesis in a cardiomyocyte subjected to ECM boundary conditions that impose a particular shape on the cell as the product of cooperative interaction between focal adhesions and premyofibrils, along with parallel bundling of nascent myofibrils (Grosberg et al. 2011) (Table 1, column 4). This model, borrowing from Novak et al.'s model of focal adhesion distribution, was tested with in vitro experiments conducted on engineered NRVMs to gauge its effectiveness in predicting the architecture of the myofibrillar network in response to extracellular boundary conditions, i.e., cell shape. Unlike previous models, ours accounted for the increased force produced by longer myofibrils and their active parallel coupling. As a result, our model predicted the steady-state localization of focal adhesions. Additionally, our model showed that, in the absence of symmetry breaking boundary conditions, the myofibrillar network requires a longer period of time to self-organize into a steady-state configuration. Thus, the cell never polarizes unless parallel coupling of the myofibrils is appropriately accounted for.
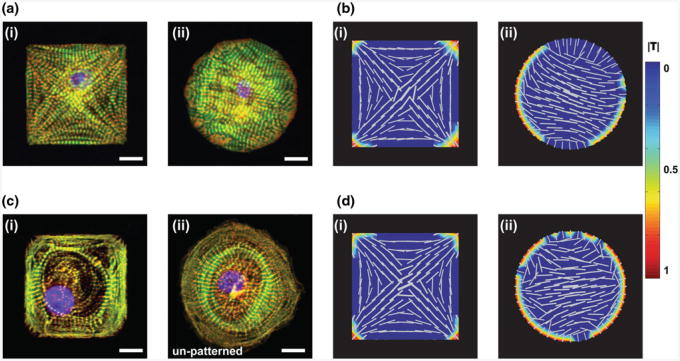
For example, a neonatal mouse ventricular myocyte seeded on a micropatterned square fibronectin island in culture will reliably self-organize to have a distinct myofibrillar network, with the myofibrils aligned along the diagonals of the square, anchored at the corners by focal adhesions [Fig. 3a(i)] (Bray et al. 2008; Grosberg et al. 2011; Parker et al. 2008). Further, when seeded on FN islands with homogenous boundary conditions, the myocytes can potentially polarize in an arbitrary direction [Fig. 3a(ii)] (Parker et al. 2008; Bray et al. 2008; Grosberg et al. 2011). Using a combination of in vitro and in silico methods, we show that, in the presence of mutual alignment of myofibrils, it is possible to predict the polarization of cells on islands with both homogenous and non-homogenous boundary conditions for cardiomyocytes from multiple species (Fig. 3b) (Grosberg et al. 2011).
Fig. 3.
Comparison of myofibrillogenesis observed in primary and stem cell-derived cardiomyocytes in vitro with in silico simulations a NMVM on patterned FN at day 3 after seeding (i) square, (ii) circle; b Computational model of a (i) square and (ii) circular cell with myofibril mutual alignment turned on showing polarization in both cell shapes; c human iPS-derived cardiomyocytes on (i) patterned FN square, and (ii) isotropic FN; d Computational model of a (i) square and (ii) circular cell with myofibril mutual alignment turned off showing polarization only in the cell type with non-homogenous boundary conditions; (a, c) scale bar = 10μm; (b, d)color bar shows normalized traction stress (|T|) see Grosberg et al. (2011) for details on the model
Commercially available human iPS-derived cardiomyocytes (hiPS-CM) do not polarize on a square FN island, but instead the myofibrils take on a circular architecture with no single direction [Fig. 3c(i)]. Furthermore, these cells, when seeded on isotropic FN, often take on a pin-wheel circular myofibril architecture [Fig. 3c(ii)]. The pin-wheel architecture is reminiscent of the non-polarized circular cell produced by the model with mutual parallel coupling of the myofibrils turned off (Fig. 3d). However, the current model is unable to predict the other features lacking in the hiPS-CMs because these cells do not respond to non-homogeneous boundary conditions in the same manner as the in silico or primary cardiomyocytes [Fig. 3b(i), d(i)]. This example illustrates the gap in knowledge between our current observations of stem cell differentiation and maturation in vitro and the state of the art in computational modeling of post-translational cellular development. It is intended to elucidate the potential role of cellular mechano-modeling in understanding developmental cell biology.
In addition to mathematical descriptions of single cell in vitro model systems, researchers have also begun to develop computation simulations of tissue- and organ-scale in vivo myocardial environments to guide the development of engineered tissues for therapeutic applications (Goktepe et al. 2010). A multi-scale mathematical model of strain-driven eccentric growth and stress-driven concentric growth of the myocardium during ischemic injury has been reported that allows researchers to explore the effects of local changes in stress/strain distribution caused by fibrosis on cardiac function (Latimer et al. 2003). These models suggest a foundation for quantitative descriptions of the role that mechanical forces play in the self-assembly of myocardial cells into contractile tissues and serve to guide researchers in developing microenvironments that give rise to engineered myocardial tissues that can be used to accurately simulate the function of the heart in vitro. However, none of these models have been applied to stem cell-derived cardiomyocytes, and as the unique physiology of these cells pose new challenges to our understanding of cellular self-assembly, it is essential that the modeling community provides support by creating new mathematical descriptions of these phenomena.
7 Summary
Physical microenvironmental cues are believed to be fundamental to proper myocardial development and may be a necessary component to experimental strategies for constructing accurate heart tissue analogs from stem cell-derived cardiomyocytes in the laboratory. Mechanical signals are received by cells through integrin-ECM interactions and intercellular junctions, where they are transmitted across the cytoskeleton to intracellular relays that activate intracellular biochemical processes. To fully realize the impact of mechanical cues on cardiac development, further research is necessary to tease apart the contributions of soluble cytokines and physical perturbations to myocardial lineage specification and determine how to incorporate appropriate mechanical cues into existing in vitro model systems. While the details of mechanotransduction remain to be fully elucidated, researchers have developed a basic understanding of the role that the cytoskeleton plays in this signaling modality and are beginning to use this information to create custom ECM microenvironments that utilize the integrin-ECM interface and intercellular junctions to modulate engineered myocardial tissue form and function. However, these engineered tissues are typically much less sophisticated than their in vivo counterpart, consisting of two-dimensional monolayers of a homotypic cell population cultured on a matrix comprised of a single type of ECM protein. To better recapitulate the native myocardium, it will be necessary to develop new methods for fabricating 3-dimensional tissue constructs with the same diversity in ECM protein composition and cellular phenotypes. Furthermore, mathematical models are emerging that quantitatively describe the effects of mechanical cues on cellular processes, such as myofibrillogenesis and contractility. Refinement of these mathematical models to capture the complex interplay between large populations of heterotypic cells interacting in a three-dimensional matrix, combined with experimental techniques to recapitulate the in vivo microenvironment, may provide researchers with the necessary tools to fabricate artificial myocardial tissue constructs with equivalent structural and functional characteristics to natural heart tissue and replace costly animal models of cardiotoxicity with cheaper, human-relevant in vitro alternatives.
Acknowledgments
We are grateful to Yvonne Aratyn for providing images of immunostained human iPS-derived cardiomyocytes. We also gratefully acknowledge funding from the National Institutes of Health under award numbers NIH 1 R01 HL079126, NIH/NINDS 1 U01 NS073474-01, and NIH/NHLBI 1 U01 HL100408-02.
Contributor Information
Sean P. Sheehy, Disease Biophysics Group, Wyss Institute for Biologically Inspired Engineering, School of Engineering and Applied Sciences, Harvard University, Pierce Hall Rm. 321, 29 Oxford St., Cambridge, MA 02138, USA
Anna Grosberg, Disease Biophysics Group, Wyss Institute for Biologically Inspired Engineering, School of Engineering and Applied Sciences, Harvard University, Pierce Hall Rm. 321, 29 Oxford St., Cambridge, MA 02138, USA.
Kevin Kit Parker, Email: kkparker@seas.harvard.edu, Disease Biophysics Group, Wyss Institute for Biologically Inspired Engineering, School of Engineering and Applied Sciences, Harvard University, Pierce Hall Rm. 321, 29 Oxford St., Cambridge, MA 02138, USA.
References
- Alenghat FJ, Ingber DE, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE. 2002;2002(119):pe6. doi: 10.1126/stke.2002.119.pe6. [DOI] [PubMed] [Google Scholar]
- Alford PW, Nesmith AP, Seywerd JN, Grosberg A, Parker KK. Vascular smooth muscle contractility depends on cell shape. Integr Biol (Camb) 2011;3(11):1063–1070. doi: 10.1039/c1ib00061f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RDG. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442(7104):823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L1–L8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- Auman HJ, Coleman H, Riley HE, Olale F, Tsai HJ, Yelon D. Functional modulation of cardiac form through regionally confined cell shape changes. PLoS Biol. 2007;5(3):e53. doi: 10.1371/journal.pbio.0050053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj P, Tang X, Saif TA, Bashir R. Stiffness of the substrate influences the phenotype of embryonic chicken cardiac myocytes. J Biomed Mater Res A. 2010;95(4):1261–1269. doi: 10.1002/jbm.a.32951. [DOI] [PubMed] [Google Scholar]
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3(5):466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- Balachandran K, Alford PW, Wylie-Sears J, Goss JA, Grosberg A, Bischoff J, Aikawa E, Levine RA, Parker KK. Cyclic strain induces dual-mode endothelial-mesenchymal transformation of the cardiac valve. Proc Natl Acad Sci USA. 2011;108(50):19943–19948. doi: 10.1073/pnas.1106954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkin AM, Zhidkova NI, Balzac F, Altruda F, Tomatis D, Maier A, Tarone G, Koteliansky VE, Burridge K. Beta 1D integrin displaces the beta 1A isoform in striated muscles: localization at junctional structures and signaling potential in nonmuscle cells. J Cell Biol. 1996;132(1–2):211–226. doi: 10.1083/jcb.132.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhana B, Iyer RK, Chen WLK, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng. 2010;105(6):1148–1160. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- Boateng SY, Belin RJ, Geenen DL, Margulies KB, Martin JL, Hoshijima M, de Tombe PP, Russell B. Cardiac dysfunction and heart failure are associated with abnormalities in the subcellular distribution and amounts of oligomeric muscle LIM protein. Am J Physiol Heart Circ Physiol. 2007;292(1):H259–H269. doi: 10.1152/ajpheart.00766.2006. [DOI] [PubMed] [Google Scholar]
- Boni A, Urbanek K, Nascimbene A, Hosoda T, Zheng H, Delucchi F, Amano K, Gonzalez A, Vitale S, Ojaimi C, Rizzi R, Bolli R, Yutzey KE, Rota M, Kajstura J, Anversa P, Leri A. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci USA. 2008;105(40):15529–15534. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Brancaccio M, Fratta L, Notte A, Hirsch E, Poulet R, Guazzone S, De Acetis M, Vecchione C, Marino G, Altruda F, Silengo L, Tarone G, Lembo G. Melusin, a muscle-specific integrin beta1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nat Med. 2003;9(1):68–75. doi: 10.1038/nm805. [DOI] [PubMed] [Google Scholar]
- Brancaccio M, Guazzone S, Menini N, Sibona E, Hirsch E, De Andrea M, Rocchi M, Altruda F, Tarone G, Silengo L. Melusin is a new muscle-specific interactor for beta(1) integrin cytoplasmic domain. J Biol Chem. 1999;274(41):29282–29288. doi: 10.1074/jbc.274.41.29282. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Koenderink GH, Mackintosh FC, Weitz DA. Nonequilibrium microtubule fluctuations in a model cytoskeleton. Phys Rev Lett. 2008;100(11):118104. doi: 10.1103/PhysRevLett.100.118104. [DOI] [PubMed] [Google Scholar]
- Bray MA, Sheehy SP, Parker KK. Sarcomere alignment is regulated by myocyte shape. Cell Motil Cytoskeleton. 2008;65(8):641–651. doi: 10.1002/cm.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray MAP, Adams WJ, Geisse NA, Feinberg AW, Sheehy SP, Parker KK. Nuclear morphology and deformation in engineered cardiac myocytes and tissues. Biomaterials. 2009;31(19):5143–5150. doi: 10.1016/j.biomaterials.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock A, Chang E, Ho CC, LeDuc P, Jiang XY, Whitesides GM, Ingber DE. Geometric determinants of directional cell motility revealed using microcontact printing. Langmuir. 2003;19(5):1611–1617. doi: 10.1021/la026394k. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Bursac N, Parker KK, Iravanian S, Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy: a model for functional electrophysiological studies of cardiac muscle. Circ Res. 2002;91(12):e45–e54. doi: 10.1161/01.res.0000047530.88338.eb. [DOI] [PubMed] [Google Scholar]
- Cadre BM, Qi M, Eble DM, Shannon TR, Bers DM, Samarel AM. Cyclic stretch down-regulates calcium transporter gene expression in neonatal rat ventricular myocytes. J Mol Cell Cardiol. 1998;30(11):2247–2259. doi: 10.1006/jmcc.1998.0788. [DOI] [PubMed] [Google Scholar]
- Carver W, Price RL, Raso DS, Terracio L, Borg TK. Distribution of beta-1 integrin in the developing rat heart. J Histochem Cytochem. 1994;42(2):167–175. doi: 10.1177/42.2.8288862. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chen CS, Tan J, Tien J. Mechanotransduction at cell-matrix and cell-cell contacts. Annu Rev Biomed Eng. 2004;6:275–302. doi: 10.1146/annurev.bioeng.6.040803.140040. [DOI] [PubMed] [Google Scholar]
- Chien KR, Domian IJ, Parker KK. Cardiogenesis and the complex biology of regenerative cardiovascular medicine. Science. 2008;322(5907):1494–1497. doi: 10.1126/science.1163267. [DOI] [PubMed] [Google Scholar]
- Chung CY, Bien H, Entcheva E. The role of cardiac tissue alignment in modulating electrical function. J Cardiovasc Electrophysiol. 2007;18(12):1323–1329. doi: 10.1111/j.1540-8167.2007.00959.x. [DOI] [PubMed] [Google Scholar]
- Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105(5):408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl KN, Ribeiro AJS, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ Res. 2008;102(11):1307–1318. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damon BJ, Remond MC, Bigelow MR, Trusk TC, Xie W, Perucchio R, Sedmera D, Denslow S, Thompson RP. Patterns of muscular strain in the embryonic heart wall. Dev Dyn. 2009;238(6):1535–1546. doi: 10.1002/dvdy.21958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melker AA, Sonnenberg A. Integrins: alternative splicing as a mechanism to regulate ligand binding and integrin signaling events. Bioessays. 1999;21(6):499–509. doi: 10.1002/(SICI)1521-1878(199906)21:6<499::AID-BIES6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Deshpande VS, McMeeking RM, Evans AG. A bio-chemomechanical model for cell contractility. Proc Natl Acad Sci USA. 2006;103(45):14015–14020. doi: 10.1073/pnas.0605837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande VS, Mrksich M, McMeeking RM, Evans AG. A biomechanical model for coupling cell contractility with focal adhesion formation. J Mech Phys Solids. 2008;56(4):1484–1510. doi: 10.1016/j.jmps.2007.08.006. [DOI] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324(5935):1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121(22):3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Evans SM, Yelon D, Conlon FL, Kirby ML. Myocardial lineage development. Circ Res. 2010;107(12):1428–1444. doi: 10.1161/CIRCRESAHA.110.227405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadian F, Contard F, Corbier A, Barrieux A, Rappaport L, Samuel JL. Fibronectin expression during physiological and pathological cardiac growth. J Mol Cell Cardiol. 1995;27(4):981–990. doi: 10.1016/0022-2828(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Fletcher DA, Mullins D. Cell mechanics and the cytoskeleton. Nature. 2010;463(7280):485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D, Kuhn C, Brors B, Hanselmann C, Ludde M, Katus HA, Frey N. Gene expression pattern in biomechanically stretched cardiomyocytes: evidence for a stretch-specific gene program. Hypertension. 2008;51(2):309–318. doi: 10.1161/HYPERTENSIONAHA.107.098046. [DOI] [PubMed] [Google Scholar]
- Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7(9):733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisse NA, Sheehy SP, Parker KK. Control of myocyte remodeling in vitro with engineered substrates. In Vitro Cell Dev Biol Anim. 2009;45(7):343–350. doi: 10.1007/s11626-009-9182-9. [DOI] [PubMed] [Google Scholar]
- Georges PC, Janmey PA. Cell type-specific response to growth on soft materials. J Appl Physiol. 2005;98(4):1547–1553. doi: 10.1152/japplphysiol.01121.2004. [DOI] [PubMed] [Google Scholar]
- Gessert S, Kuhl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ Res. 2010;107(2):186–199. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- Gjorevski N, Nelson CM. Bidirectional extracellular matrix signaling during tissue morphogenesis. Cytokine Growth Factor Rev. 2009;20(5-6):459–465. doi: 10.1016/j.cytogfr.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goktepe S, Abilez OJ, Parker KK, Kuhl E. A multiscale model for eccentric and concentric cardiac growth through sarcomerogenesis. J Theor Biol. 2010;265(3):433–442. doi: 10.1016/j.jtbi.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Gopalan SM, Flaim C, Bhatia SN, Hoshijima M, Knoell R, Chien KR, Omens JH, McCulloch AD. Anisotropic stretch-induced hypertrophy in neonatal ventricular myocytes micropatterned on deformable elastomers. Biotechnol Bioeng. 2003;81(5):578–587. doi: 10.1002/bit.10506. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Green CR, Severs NJ, Thompson RP. Immunolabelling patterns of gap junction connexins in the developing and mature rat heart. Anat Embryol (Berl) 1992;185(4):363–378. doi: 10.1007/BF00188548. [DOI] [PubMed] [Google Scholar]
- Grosberg A, Kuo PL, Guo CL, Geisse NA, Bray MA, Adams WJ, Sheehy SP, Parker KK. Self-organization of muscle cell structure and function. PLoS Comput Biol. 2011;7(2):e1001088. doi: 10.1371/journal.pcbi.1001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5(1):17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7(8):589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- Heineke J, Ruetten H, Willenbockel C, Gross SC, Naguib M, Schaefer A, Kempf T, Hilfiker-Kleiner D, Caroni P, Kraft T, Kaiser RA, Molkentin JD, Drexler H, Wollert KC. Attenuation of cardiac remodeling after myocardial infarction by muscle LIM protein-calcineurin signaling at the sarcomeric Z-disc. Proc Natl Acad Sci USA. 2005;102(5):1655–1660. doi: 10.1073/pnas.0405488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilenski LL, Ma XH, Vinson N, Terracio L, Borg TK. The role of beta 1 integrin in spreading and myofibrillogenesis in neonatal rat cardiomyocytes in vitro. Cell Motil Cytoskeleton. 1992;21(2):87–100. doi: 10.1002/cm.970210202. [DOI] [PubMed] [Google Scholar]
- Hornberger LK, Singhroy S, Cavalle-Garrido T, Tsang W, Keeley F, Rabinovitch M. Synthesis of extracellular matrix and adhesion through beta(1) integrins are critical for fetal ventricular myocyte proliferation. Circ Res. 2000;87(6):508–515. doi: 10.1161/01.res.87.6.508. [DOI] [PubMed] [Google Scholar]
- Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421(6919):172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9(6):518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE. The riddle of morphogenesis: a question of solution chemistry or molecular cell engineering. Cell. 1993;75(7):1249–1252. doi: 10.1016/0092-8674(93)90612-t. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91(10):877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. Faseb J. 2006a;20(7):811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanical control of tissue morphogenesis during embryological development. Int J Dev Biol. 2006b;50(2–3):255–266. doi: 10.1387/ijdb.052044di. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Nakamura H, Sumida H, Yasuda M. Actin bundles on the right side in the caudal part of the heart tube play a role in dextro-looping in the embryonic chick heart. Anat Embryol (Berl) 1991;183(1):29–39. doi: 10.1007/BF00185832. [DOI] [PubMed] [Google Scholar]
- Jacot JG, Martin JC, Hunt DL. Mechanobiology of cardiomyocyte development. J Biomech. 2010;43(1):93–98. doi: 10.1016/j.jbiomech.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 2008;95(7):3479–3487. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422(6929):317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller RS, Shai SY, Babbitt CJ, Pham CG, Solaro RJ, Valencik ML, Loftus JC, Ross RS. Disruption of integrin function in the murine myocardium leads to perinatal lethality, fibrosis, and abnormal cardiac performance. Am J Pathol. 2001;158(3):1079–1090. doi: 10.1016/S0002-9440(10)64055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, Bang ML, Hayashi T, Shiga N, Yasukawa H, Schaper W, McKenna W, Yokoyama M, Schork NJ, Omens JH, McCulloch AD, Kimura A, Gregorio CC, Poller W, Schaper J, Schultheiss HP, Chien KR. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell. 2002;111(7):943–955. doi: 10.1016/s0092-8674(02)01226-6. [DOI] [PubMed] [Google Scholar]
- Kresh JY, Chopra A. Intercellular and extracellular mechanotransduction in cardiac myocytes. Pflugers Arch. 2011;462(1):75–87. doi: 10.1007/s00424-011-0954-1. [DOI] [PubMed] [Google Scholar]
- Kruger M, Linke WA. Titin-based mechanical signalling in normal and failing myocardium. J Mol Cell Cardiol. 2009;46(4):490–498. doi: 10.1016/j.yjmcc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Kuwahara K, Barrientos T, Pipes GCT, Li S, Olson EN. Musclespecific signaling mechanism that links actin dynamics to serum response factor. Mol Cell Biol. 2005;25(8):3173–3181. doi: 10.1128/MCB.25.8.3173-3181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara K, Teg Pipes GC, McAnally J, Richardson JA, Hill JA, Bassel-Duby R, Olson EN. Modulation of adverse cardiac remodeling by STARS, a mediator of MEF2 signaling and SRF activity. J Clin Invest. 2007;117(5):1324–1334. doi: 10.1172/JCI31240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Qian L, Cheng P, Nigam V, Arnold J, Srivastava D. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol. 2009;11(8):951–957. doi: 10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S, Auerbach D, McLoughlin P, Perriard E, Schafer BW, Perriard JC, Ehler E. Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2. J Cell Sci. 2002;115(Pt 24):4925–4936. doi: 10.1242/jcs.00181. [DOI] [PubMed] [Google Scholar]
- Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, Gunnarsson LG, Hughes SM, Marchand S, Sejersen T, Richard I, Edstrom L, Ehler E, Udd B, Gautel M. The kinase domain of titin controls muscle gene expression and protein turnover. Science. 2005;308(5728):1599–1603. doi: 10.1126/science.1110463. [DOI] [PubMed] [Google Scholar]
- Latacha KS, Remond MC, Ramasubramanian A, Chen AY, Elson EL, Taber LA. Role of actin polymerization in bending of the early heart tube. Dev Dyn. 2005;233(4):1272–1286. doi: 10.1002/dvdy.20488. [DOI] [PubMed] [Google Scholar]
- Latimer DC, Roth BJ, Parker KK. Analytical model for predicting mechanotransduction effects in engineered cardiac tissue. Tissue Eng. 2003;9(2):283–289. doi: 10.1089/107632703764664747. [DOI] [PubMed] [Google Scholar]
- Linke WA. Sense and stretchability: the role of titin and titinassociated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc Res. 2008;77(4):637–648. doi: 10.1016/j.cardiores.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci USA. 2010;107(22):9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack CP, Somlyo AV, Hautmann M, Somlyo AP, Owens GK. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J Biol Chem. 2001;276(1):341–347. doi: 10.1074/jbc.M005505200. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LEH, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457(7233):1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasek FJ, Burnside MB, Waterman RE. Myocardial cell shape change as a mechanism of embryonic heart looping. Dev Biol. 1972;29(4):349–371. doi: 10.1016/0012-1606(72)90077-2. [DOI] [PubMed] [Google Scholar]
- Manasek FJ, Monroe RG. Early cardiac morphogenesis is independent of function. Dev Biol. 1972;27(4):584–588. doi: 10.1016/0012-1606(72)90196-0. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Bojanowski K, Ingber DE. Mechanical continuity and reversible chromosome disassembly within intact genomes removed from living cells. J Cell Biochem. 1997;65(1):114–130. [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA. 1997;94(3):849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci. 2006;119(Pt 3):508–518. doi: 10.1242/jcs.02760. [DOI] [PubMed] [Google Scholar]
- McCain ML, Desplantez T, Geisse NA, Rothen-Rutishauser B, Oberer H, Parker KK, Kleber AG. Cell-to-cell coupling in engineered pairs of rat ventricular cardiomyocytes: relation between Cx43 immunofluorescence and intercellular electrical conductance. Am J Physiol Heart Circ Physiol. 2012;302(2):H443–H450. doi: 10.1152/ajpheart.01218.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain ML, Parker KK. Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Arch. 2011;462(1):89–104. doi: 10.1007/s00424-011-0951-4. [DOI] [PubMed] [Google Scholar]
- Meyer CJ, Alenghat FJ, Rim P, Fong JH, Fabry B, Ingber DE. Mechanical control of cyclic AMP signalling and gene transcription through integrins. Nat Cell Biol. 2000;2(9):666–668. doi: 10.1038/35023621. [DOI] [PubMed] [Google Scholar]
- Miller LA. Fluid dynamics of ventricular filling in the embryonic heart. Cell Biochem Biophys. 2011;61(1):33–45. doi: 10.1007/s12013-011-9157-9. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Inoue N, Nishimura K, Kinoshita N, Hosoya H, Yonemura S. Actomyosin tension is required for correct recruitment of adherens junction components and zonula occludens formation. Exp Cell Res. 2006;312(9):1637–1650. doi: 10.1016/j.yexcr.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Moore KA, Polte T, Huang S, Shi B, Alsberg E, Sunday ME, Ingber DE. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn. 2005;232(2):268–281. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- Mrksich M, Chen CS, Xia Y, Dike LE, Ingber DE, Whitesides GM. Controlling cell attachment on contoured surfaces with self-assembled monolayers of alkanethiolates on gold. Proc Natl Acad Sci USA. 1996;93(20):10775–10778. doi: 10.1073/pnas.93.20.10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrksich M, Dike LE, Tien J, Ingber DE, Whitesides GM. Using microcontact printing to pattern the attachmentofmammalian cells to self-assembled monolayers of alkanethiolates on transparent films of gold and silver. Exp Cell Res. 1997;235(2):305–313. doi: 10.1006/excr.1997.3668. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004a;303(5663):1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004b;303(5663):1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen CM, Leckband D, Yap AS. Tissue organization by cadherin adhesion molecules: dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol Rev. 2011;91(2):691–731. doi: 10.1152/physrev.00004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak IL, Slepchenko BM, Mogilner A, Loew LM. Cooperativity between cell contractility and adhesion. Phys Rev Lett. 2004;93(26 Pt 1):268109. doi: 10.1103/PhysRevLett.93.268109. [DOI] [PubMed] [Google Scholar]
- Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11(5):353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. Faseb J. 2002;16(10):1195–1204. doi: 10.1096/fj.02-0038com. [DOI] [PubMed] [Google Scholar]
- Parker KK, Ingber DE. Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering. Philos Trans R Soc Lond B Biol Sci. 2007;362(1484):1267–1279. doi: 10.1098/rstb.2007.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KK, Tan J, Chen CS, Tung L. Myofibrillar architecture in engineered cardiac myocytes. Circ Res. 2008;103(4):340–342. doi: 10.1161/CIRCRESAHA.108.182469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Boettiger D, Weaver VM, Hammer DA. Integrin clustering is driven by mechanical resistance from the glycocalyx and the substrate. PLoS Comput Biol. 2009;5(12) doi: 10.1371/journal.pcbi.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham RJ, Jr, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94(25):13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters NS, Severs NJ, Rothery SM, Lincoln C, Yacoub MH, Green CR. Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation. 1994;90(2):713–725. doi: 10.1161/01.cir.90.2.713. [DOI] [PubMed] [Google Scholar]
- Pijnappels DA, Schalij MJ, Ramkisoensing AA, van Tuyn J, de Vries AAF, van der Laarse A, Ypey DL, Atsma DE. Forced alignment of mesenchymal stem cells undergoing cardiomyogenic differentiation affects functional integration with cardiomyocyte cultures. Circ Res. 2008;103(2):167–176. doi: 10.1161/CIRCRESAHA.108.176131. [DOI] [PubMed] [Google Scholar]
- Pong T, Adams WJ, Bray MA, Feinberg AW, Sheehy SP, Werdich AA, Parker KK. Hierarchical architecture influences calcium dynamics in engineered cardiac muscle. Exp Biol Med (Maywood) 2011;236(3):366–373. doi: 10.1258/ebm.2010.010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RL, Chintanowonges C, Shiraishi I, Borg TK, Terracio L. Local and regional variations in myofibrillar patterns in looping rat hearts. Anat Rec. 1996;245(1):83–93. doi: 10.1002/(SICI)1097-0185(199605)245:1<83::AID-AR13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Price RL, Nakagawa M, Terracio L, Borg TK. Ultrastructural localization of laminin on in vivo embryonic, neonatal, and adult rat cardiac myocytes and in early rat embryos raised in wholeembryo culture. J Histochem Cytochem. 1992;40(9):1373–1381. doi: 10.1177/40.9.1506674. [DOI] [PubMed] [Google Scholar]
- Puchner EM, Alexandrovich A, Kho AL, Hensen U, Schafer LV, Brand-meier B, Grater F, Grubmuller H, Gaub HE, Gautel M. Mechanoenzymatics of titin kinase. Proc Natl Acad Sci USA. 2008;105(36):13385–13390. doi: 10.1073/pnas.0805034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106(12):1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- Ross RS, Borg TK. Integrins and the myocardium. Circ Res. 2001;88(11):1112–1119. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- Saffitz JE. Dependence of electrical coupling on mechanical coupling in cardiac myocytes: insights gained from cardiomyopathies caused by defects in cell-cell connections. Ann N Y Acad Sci. 2005;1047:336–344. doi: 10.1196/annals.1341.030. [DOI] [PubMed] [Google Scholar]
- Saffitz JE, Kleber AG. Effects of mechanical forces and mediators of hypertrophy on remodeling of gap junctions in the heart. Circ Res. 2004;94(5):585–591. doi: 10.1161/01.RES.0000121575.34653.50. [DOI] [PubMed] [Google Scholar]
- Salameh A, Wustmann A, Karl S, Blanke K, Apel D, Rojas-Gomez D, Franke H, Mohr FW, Janousek J, Dhein S. Cyclic mechanical stretch induces cardiomyocyte orientation and polarization of the gap junction protein connexin43. Circ Res. 2010;106(10):1592–1602. doi: 10.1161/CIRCRESAHA.109.214429. [DOI] [PubMed] [Google Scholar]
- Samarakoon R, Higgins PJ. MEK/ERK pathway mediates cellshape-dependent plasminogen activator inhibitor type 1 gene expression upon drug-induced disruption of the microfilament and microtubule networks. J Cell Sci. 2002;115(Pt 15):3093–3103. doi: 10.1242/jcs.115.15.3093. [DOI] [PubMed] [Google Scholar]
- Samarel AM. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am J Physiol Heart Circ Physiol. 2005;289(6):H2291–H2301. doi: 10.1152/ajpheart.00749.2005. [DOI] [PubMed] [Google Scholar]
- Samuel JL, Farhadian F, Sabri A, Marotte F, Robert V, Rappaport L. Expression of fibronectin during rat fetal and postnatal development: an in situ hybridisation and immunohistochemical study. Cardiovasc Res. 1994;28(11):1653–1661. doi: 10.1093/cvr/28.11.1653. [DOI] [PubMed] [Google Scholar]
- Santhanakrishnan A, Miller LA. Fluid dynamics of heart development. Cell Biochem Biophys. 2011;61(1):1–22. doi: 10.1007/s12013-011-9158-8. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4(4):E65–E68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90(4):458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- Shanker AJ, Yamada K, Green KG, Yamada KA, Saffitz JE. Matrix-protein-specific regulation of Cx43 expression in cardiac myocytes subjected to mechanical load. Circ Res. 2005;96(5):558–566. doi: 10.1161/01.RES.0000158964.42008.a2. [DOI] [PubMed] [Google Scholar]
- Sheehy SP, Huang S, Parker KK. Time-warped comparison of geneexpression in adaptive and maladaptive cardiac hypertrophy. Circ Cardiovasc Genet. 2009;2(2):116–124. doi: 10.1161/CIRCGENETICS.108.806935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh F, Raskin A, Chu PH, Lange S, Domenighetti AA, Zheng M, Liang X, Zhang T, Yajima T, Gu Y, Dalton ND, Mahata SK, Dorn GW, Heller-Brown J, Peterson KL, Omens JH, McCulloch AD, Chen J. An FHL1-containing complex within the cardiomyocyte sarcomeremediates hypertrophic biomechanical stress responses in mice. J Clin Invest. 2008;118(12):3870–3880. doi: 10.1172/JCI34472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DG, Decker ML, Clark WA, Decker RS. Contractile activity and cell-cell contact regulate myofibrillar organization in cultured cardiac myocytes. J Cell Biol. 1993;123(2):323–336. doi: 10.1083/jcb.123.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, White-sides GM, Ingber DE. Engineering cell shape and function. Science. 1994;264(5159):696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature. 2000;407(6801):221–226. doi: 10.1038/35025190. [DOI] [PubMed] [Google Scholar]
- Taber LA. Biomechanics of cardiovascular development. Annu Rev Biomed Eng. 2001;3:1–25. doi: 10.1146/annurev.bioeng.3.1.1. [DOI] [PubMed] [Google Scholar]
- Taber LA, Lin IE, Clark EB. Mechanics of cardiac looping. Dev Dyn. 1995;203(1):42–50. doi: 10.1002/aja.1002030105. [DOI] [PubMed] [Google Scholar]
- Terracio L, Rubin K, Gullberg D, Balog E, Carver W, Jyring R, Borg TK. Expression of collagen binding integrins during cardiac development and hypertrophy. Circ Res. 1991;68(3):734–744. doi: 10.1161/01.res.68.3.734. [DOI] [PubMed] [Google Scholar]
- Thomas CH, Collier JH, Sfeir CS, Healy KE. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc Natl Acad Sci USA. 2002;99(4):1972–1977. doi: 10.1073/pnas.032668799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobita K, Schroder EA, Tinney JP, Garrison JB, Keller BB. Regional passive ventricular stress-strain relations during development of altered loads in chick embryo. Am J Physiol Heart Circ Physiol. 2002;282(6):H2386–H2396. doi: 10.1152/ajpheart.00879.2001. [DOI] [PubMed] [Google Scholar]
- Trepat X, Deng L, An SS, Navajas D, Tschumperlin DJ, Gerthoffer WT, Butler JP, Fredberg JJ. Universal physical responses to stretch in the living cell. Nature. 2007;447(7144):592–595. doi: 10.1038/nature05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci USA. 2007;104(23):9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier A, Gaspar AC, Thorsteinsdottir S, Baudoin C, Groeneveld E, Mummery CL, Sonnenberg A. Spatial and temporal expression of the beta1D integrin during mouse development. Dev Dyn. 1997;210(4):472–486. doi: 10.1002/(SICI)1097-0177(199712)210:4<472::AID-AJA10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Walsh KB, Parks GE. Changes in cardiac myocyte morphology alter the properties of voltage-gated ion channels. Cardiovasc Res. 2002;55(1):64–75. doi: 10.1016/s0008-6363(02)00403-0. [DOI] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10(1):75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10(1):34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Green KG, Samarel AM, Saffitz JE. Distinct pathways regulate expression ofcardiac electrical and mechanical junction proteins in response to stretch. Circ Res. 2005;97(4):346–353. doi: 10.1161/01.RES.0000178788.76568.8a. [DOI] [PubMed] [Google Scholar]
- Yang JH, Wylie-Sears J, Bischoff J. Opposing actions of Notch1 and VEGF in post-natal cardiac valve endothelial cells. Biochem Biophys Res Commun. 2008;374(3):512–516. doi: 10.1016/j.bbrc.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Bien H, Entcheva E. Scaffold topography alters intracellular calcium dynamics in cultured cardiomyocyte networks. Am J Physiol Heart Circ Physiol. 2004;287(3):H1276–H1285. doi: 10.1152/ajpheart.01120.2003. [DOI] [PubMed] [Google Scholar]
- Zajac AL, Discher DE. Cell differentiation through tissue elasticity-coupled, myosin-driven remodeling. Curr Opin Cell Biol. 2008;20(6):609–615. doi: 10.1016/j.ceb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Yamada KA, Saffitz JE, Kleber AG. Pulsatile stretch remodels cell-to-cell communication in cultured myocytes. Circ Res. 2000;87(4):316–322. doi: 10.1161/01.res.87.4.316. [DOI] [PubMed] [Google Scholar]