Abstract
Objective
A new image analysis method called the Spatial Phantom Evaluation of Cellular Thermal Response in Layers (SPECTRL) is presented for assessing spatial viability response to nanoparticle enhanced photothermal therapy in tissue representative phantoms.
Materials and Methods
Sodium alginate phantoms seeded with MDA-MB-231 breast cancer cells and single walled nanohorns were laser irradiated with an ytterbium fiber laser at a wavelength of 1064 nm and irradiance of 3.8 watts/cm2 for 10–80 seconds. SPECTRL quantitatively assessed and correlated 3D viability with spatiotemporal temperature.
Results and Conclusions
Based on this analysis, kill and transition zones increased from 3.7 mm3 and 13 mm3 respectively to 44.5 mm3 and 44.3 mm3 as duration was increased from 10–80 seconds. SPECTRL provides a quantitative tool for measuring precise spatial treatment regions, providing information necessary to tailor therapy protocols.
Keywords: Cancer, Nanohorns, Photothermal, Spatial, Viability
Introduction
Cancer is currently the second leading cause of death in the United States, and is responsible for almost 1 out of every 4 deaths [1]. The inability of conventional cancer treatments (i.e. chemotherapy and radiation) to effectively treat malignant cancers which cannot be easily removed via surgical resection such as those in the brain, pancreas, and liver has led researchers to develop new cancer therapies such as irreversible electroporation [2], magnetic hyperthermia therapy [3], and photothermal therapy [4–6]. Specifically, photothermal therapy utilizes heat generated by absorbed light in the targeted tumor to produce therapeutic effects. This treatment method can provide a minimally invasive and potentially more effective treatment alternative to conventional surgical resection of cancerous tissue [7]. Photothermal therapy can also be used in conjunction with photo-absorptive nanoparticles which increase heat generation and could be used as drug delivery platforms [4, 8]. Nanoparticles, such as Single Walled Carbon Nanohorns (SWNHs), enhance laser light absorption to facilitate local temperature elevation and cancer cell death [4, 9, 10]. SWNHs have been shown to be effective exogenous chromophores in tissue [11] with low cytotoxicity to cells exposed for periods of up to 24 hours [4]. Previous studies have used this effect to successfully eliminate flank tumors from mice [7]. SWNHs also can act as versatile functionalization platforms for drug delivery [12, 13]. These factors make SWNHs an attractive multi-modal tool for enhancing the effectiveness of photothermal therapy. However, previous tumor models used to evaluate nanoparticle mediated photothermal therapies frequently either do not accurately represent the biological and heat transfer properties of tissue, or use quantitative spatial viability measurements necessary to assess treatment margins precisely [7, 10, 14, 15].
Tumor Models
Evaluation of photothermal therapy is most commonly conducted using biological models such as two-dimensional (2D) in vitro cell cultures [4, 9, 16] and in vivo animal models [2, 7, 14]. While these tissue models have provided useful information regarding the relationship between laser and nanoparticle parameters with temperature elevation, they have many limitations. For example, cells in 2D culture lack the diffusional gradients, cell morphology, cell-extracellular matrix (ECM) interactions, and mechanical stresses experienced by cells in vivo, and are therefore not representative of 3D tissues [17, 18]. In addition, 2D cell cultures submerged in media transfer absorbed heat through convection rather than 3D conduction; resulting in asymmetric heat distributions and thermally driven fluid flow [19]. While in vivo animal models are the most physiologically relevant models available, they pose significant challenges to detailed viability measurements.
Viability Measurement
Because biological tissue has a high degree of optical scattering, fluorescence imaging cannot be used in vivo to measure viability deep within tissue [20, 21]. Instead, viability response to photothermal therapy in in vivo models is typically evaluated by measuring changes in tumor size and mouse survival length with and without treatment [7, 14, 22]. These macroscopic viability measurements neglect cellular level viability changes. Other measurement methods such as Terminal deoxynucleotidyl transferase (TUNEL) assays provide microscopic evaluation of viability, but neglect spatial viability distributions by averaging viability over a small area [23]. Histological methods can provide spatial viability information, but are limited by the ease with which quantitative viability information may be obtained, and the area over which these measurements may be conducted. Consequently, there is currently no method for rapid and quantitative evaluation of spatial viability response to tumor treatment in vivo. There are several quantitative viability measurement methods available in 2D in vitro cell cultures, such as metabolic activity assays (MTT) [23, 24], metabolic ATP (adenosine triphosphate) detection [25], membrane permeability assays such as Trypan blue exclusion [5], and calcein AM, a dye permeable to live cell membranes which becomes activated only in the presence of functional esterase [9]. Metabolic assays are limited because they are indirect measurements of viability, and measure overall rather than individual cellular function [23, 24]. Membrane exclusion dyes and fluorescent viability assays are able to assess viability in individual cells, but are typically sampled in specific areas to extrapolate the viability across an entire sample, or to measure the viability in cell suspensions [4, 5]. Recent methods developed by our group have been able to use fluorescent and bright field viability assays to determine spatial viability in 2D cell cultures, but were limited because thermal convective currents present in 2D cell cultures which do not accurately represent photothermal heating experienced in tissue [19]. Therefore, there is a need for 3D tissue models that are more physiologically and thermally representative of in vivo conditions than 2D cultures and permit measurements of viability and temperature difficult to obtain in in vivo models.
3D In VitroTissue Phantom Representativeness and Viability Measurements
Recently researchers have transitioned to using three-dimensional (3D) in vitro tumor models to study cancer physiology and determine response to treatments. 3D in vitro tumor models (also called phantoms), such as hydrogels and electro-spun scaffolds, mimic biological tissue allowing them to engage in cell-cell and cell-matrix interactions such as integrin ligation, protein expression, ECM mediated clustering, and intracellular tension not previously afforded in 2D cell culture [17, 26–30]. In addition, typical 3D phantoms composed of collagen hydrogels or sodium alginate mimic the thermal conductivity and near infrared (NIR) optical absorption of tissue which are important when modeling photothermal therapy [31–33]. Groups investigating the use of sodium alginate as a versatile tissue phantom have been able to modify the formulation and methodology of creating phantoms in order to generate specific porosity [34], and compressive and shear mechanical properties[35]. This suggests that sodium alginate phantoms can be designed to mimic the mechanical properties of specific tissues. However, this study holds the mechanical properties of the phantoms constant, in order to only evaluate the impact increasing durations of laser exposure. However, while many groups have developed 3D in vitro tumor models to study drug penetration and gene expression [36, 37] there has been little use of 3D tissue phantoms to evaluate viability response to photothermal therapy [38–40]. Methods commonly used to measure viability in 3D tissue phantoms are similar to those used in 2D cell cultures in which dyed or fluorescently stained cells enable viability measurements as a function of either depth through optical focus and destructive scaffold sectioning, or short range cell migration [41–43]. However these methods do not measure the 3D spatial viability distributions, treatment margins, or the damage dealt to surrounding healthy tissue which are critical to understanding the effectiveness of photothermal therapy [43, 44]. The ability to measure 3D treatment margins resulting from photothermal therapy would provide valuable information needed to selectively treat cancerous tissue while minimizing damage to surrounding healthy tissue.
Objectives
The goal of this work is to establish a methodology to quantitatively assess 3D viability in response to nanoparticle mediated photothermal therapy in a 3D in vitro tumor model. To demonstrate the capability of this method, 3D tumor phantoms were constructed using biocompatible sodium alginate hydrogels seeded with MDA-MB-231 breast cancer cells. Single-walled carbon nanohorns (SWNH) were added to the tumor phantoms as photo-absorbers due to previously published work demonstrating their capability to enhance the effectiveness of photothermal therapy [4]. Sodium alginate was chosen as the 3D tumor phantom material due to its biological compatibility, ease of use, optical transparency, ability to absorb light in the near NIR, and thermal conductivity which is similar to tissue, as well as its thermal stability up to 100°C [45–48]. The tumor phantoms were laser irradiated with varying exposure durations and cell viability was evaluated in three-dimensions using a custom LabVIEW image processing algorithm. This algorithm measured both the viability and spatial location of cells in three dimensions by imaging at multiple focal depths within the phantom. The temperature was recorded in real-time during laser irradiation to correlate viability loss to spatial temperature distribution and exposure. The combination of digital image analysis of fluorescently stained image mosaics (small images tiled together to create a larger image) [19] with the use of 3D tissue models was investigated. The resulting 3D viability information provided detailed kill and transition zone boundaries which could help clinicians precisely plan photothermal therapy.
Methodology
Tumor Phantom Fabrication
Sodium alginate was used to fabricate 3D tumor phantoms as previously described [47, 49]. Briefly, a stock sodium alginate solution of 3.6 % w/v was created by mixing sodium alginate with deionized water and subsequently filtering the solution with a 0.22 micron filter for sterilization. Next, the alginate solution was mixed with SWNHs at a final concentration of 0.085 mg/ml in deionized water and 0.0425 mg/ml Pluronic to aid in SWNH suspension, based on previous work with these concentrations [4, 19]. Next, MDA-MB-231 breast cancer cells were trypsinized, mixed with Dulbecco’s Modified Eagle Medium (DMEM) cell culture media (Life Technologies, Grand Island NY), centrifuged, and the supernatant was removed twice in to neautralize the trypsin effects. The alginate/SWNH suspension was then mixed with media resulting in a final concentration of 1.5×105 cells per ml. The final phantom solution was then put into a cylindrical mold (33 mm in diameter, 1.5 mm thick) and cross-linked using a 100 mmol calcium chloride solution. The solution was cross-linked at 37°C for 18 minutes, rinsed 3x with PBS in order to remove excess CaCl2, and then placed in cell media immediately before laser treatment. The dimensions of these phantoms are similar to previously used three dimensional tumor models [50], and the mass transfer capabilities of 3.6 % w/v sodium alginate are sufficient to allow complete media and stain penetration to the interior of the phantom [51].
Tumor Phantom Photothermal Treatment
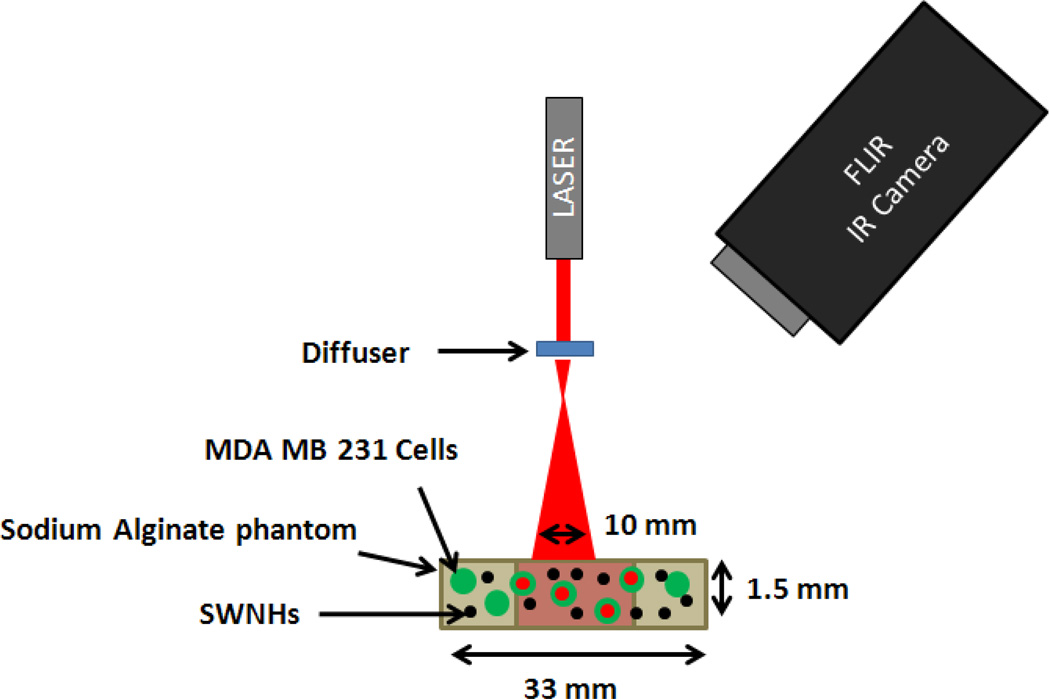
Phantoms were placed in a six well dish and maintained at 23°C prior to photothermal treatments. Figure 1 illustrates the physical setup used to irradiate phantoms. Phantoms were irradiated with a continuous wave, ytterbium fiber laser, YLD-5-1064-LP (IPG Photonics) at a wavelength of 1064 nm with an irradiance of 3.8 watts/cm2 over a 10 mm diameter area. These laser parameters were selected due to effective therapeutic response previously demonstrated in vivo [7, 9]. A diffusing lens distributed laser irradiation over a 10 mm diameter to avoid ablation of the phantom. During irradiation, the phantom was imaged with an FLIR A40 Thermovision infra-red (IR) camera every 2 seconds to measure the surface temperature distribution across the phantom. In this experiment, phantoms were irradiated for the following durations: 10, 20, 40, 60, and 80 seconds (n=3). After irradiation, the phantoms were placed in an incubator for 24 hours before viability was measured. To measure percent viability, live cells were stained with a mixture of 4 µM calcein AM in cell media for 30 min. Phantoms were then rinsed with fresh media, and dead cells were stained with 1.5 mM propidium iodide for 15 minutes. Samples were then placed in fresh cell culture media and imaged with a Leica DMI-6000 B fluorescent microscope. A 5x objective was used to image the cells within the phantom over a 10×10 grid (26.4×19.8 mm2 area) at each of four to twelve focal depths 500 to 125 µm apart respectively to determine overall 3D spatial viability as explained below.
Figure 1.
Diagram of phantom irradiation experimental setup using a ytterbium fiber laser with a wavelength of 1064 nm and irradiance of 3.8 W/cm2. The FLIR camera records the surface temperature during irradiation at two second intervals to create a spatiotemporal thermal input for later analysis.
SPECTRL Viability Mapping Algorithm
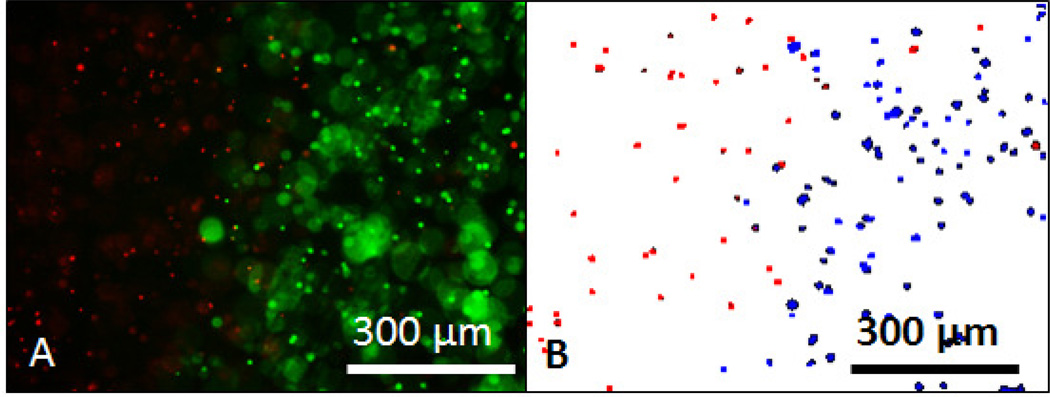
A custom designed digital method called the Spatial Phantom Evaluation of Cellular Thermal Response in Layers (SPECTRL) was used to measure 3D cell viability in the tumor phantoms. SPECTRL is adapted from our previously described digital method of spatial cell viability measurement in 2D cell culture [19], and has been expanded in this work to analyze the spatial viability of multiple focal depths within a tissue phantom. The steps taken in the SPECTRL method are as follows: first, fluorescent images of cells stained with a propidium iodide and calcein AM viability assay were taken with a Leica DMI-6000 B fluorescent microscope in a 10×10 grid pattern. In each image, dead cells were visible as red dots and live cells were visible as green dots (Figure 2, A). Second, the red and green components of each image were separated isolating the fluorescent signals from calcein AM (live) and propidium iodide (dead) cells. Third, areas containing cells were separated from non-uniform backgrounds using a Prewitt edge detection algorithm. The resulting grayscale images were then applied with a pixel intensity threshold, converting the grayscale images of both the live and dead cells into binary representations. Since out of focus cells are both larger and fainter than normal cells, they can be removed by using pixel and size thresholds. The pixel and size thresholds were set to filter out all pixels below an intensity of 40 on a scale of 0 to 255, and of the pixels above 40 remove any remaining clusters outside a size threshold range of 25 and 170 pixels. These thresholds are based on size distributions of cells from previous studies in which thresholds were set to separate in-focus from out of focus cells. Fourth, the resulting binary image, called a digital viability representation (DVR), encompasses all the particles identified as cells, with dead cells displayed as red dots and live cells displayed as blue dots (Figure 2, B). The XY location, focal depth, and viability state of each cell is recorded. Finally, both the binary and fluorescent images were digitally stitched together to create single larger images of the entire area imaged at each focal depth, called image mosaics.
Figure 2.
The SPECTRL digital image analysis process. A) Combined fluorescent image of dead cells stained with propidium iodide (red) and live cells stained with calcein AM (green) within a phantom treated with laser irradiation. Out of focus cells are much larger and slightly fainter than in focus cells. B) Binary representation of the fluorescent image created by SPECTRL with dead cells (red) and live cells (blue) with out of focus cells removed.
3D Viability Data Analysis
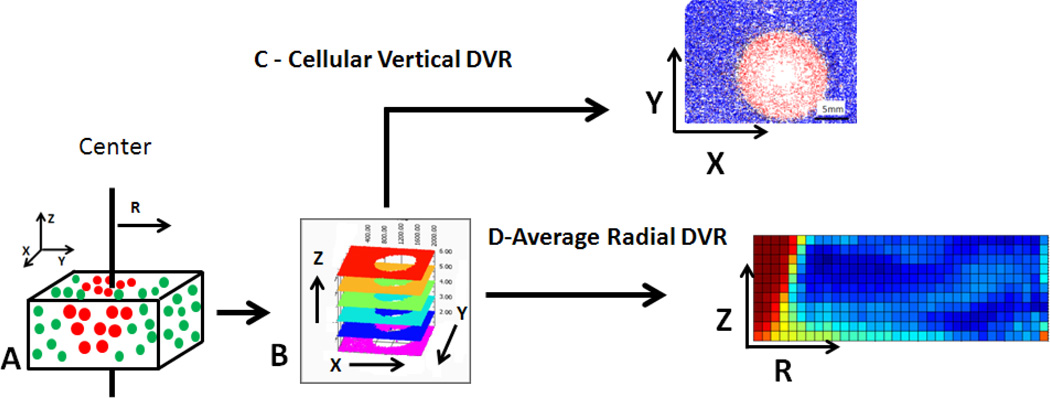
After fluorescent images of tissue phantoms (Figure 3, A) were converted into digital viability representation (DVRs), they were arranged by X, Y, and Z location into a layered DVR (Figure 3, B). The 3D viability data from the layered DVR can be presented in two ways. The first is a top down 2D viability analysis, called a vertical DVR, which allows for the X–Y components of spatial viability to be analyzed (Figure 3, C). The second analysis method, called a radial DVR, examines the radial viability distribution as a function of depth into the phantom (Z-R) (Figure 3, D). This radial analysis assumes vertically axissymmetric temperature and viability response data and allows analysis of depth of treatment. In both analysis methods viability reduction caused by photothermal treatment was separated from variability in cell viability from individual batches through normalization. Viability measured in untreated regions of phantoms was on average 84%, with approximately +/−10% variability between individual phantoms.
Figure 3.
Illustration of analysis structure. A) 3D Tissue phantoms stained with fluorescent viability dyes are imaged with a tiling microscope at varying focal depths. Measurements are made in reference to depth through the phantom (Z axis), lateral position (X and Y axis), as well as radial distance from the center of irradiation (R axis). Fluorescent images from each focal depth are converted into B) a layered DVR, representing 3D viability. This 3D viability is analyzed C) top down, resulting in a 2D vertical DVR along the×and y axis and D) from the side, creating a radial DVR of average viability as a function of depth into the phantom (z-axis) and radial distance from the center of the dish at which irradiation occurred (r-axis).
Two-Dimensional (X–Y) Viability Analysis: Vertical DVR
After acquiring both viability and temperature data from each tissue phantom, 2D temperature data acquired by the FLIR thermal camera was averaged per pixel over time, generating the average thermal dose per pixel of the IR camera image. Viability data was geometrically scaled to match temperature dimensions. This was done by assigning each temperature pixel a viability value based on the number of live and dead cells in the region. Viability and temperature data were spatially correlated by aligning the point of maximum temperature with the center of irradiation at the site of maximum viability loss in the vertical DVR. The spatial temperature/viability data generated by this analysis was used to determine both the average radial viability, measured from the center of treatment, and average radial temperature, measured from the point of maximum temperature.
Three-Dimensional (Z-R) Viability Analysis: Radial DVR
In order to quantitatively compare the effect of increasing laser irradiation duration through the thickness of the samples, SPECTRL output can be analyzed through a radial DVR (Figure 3, D). These reconstructions were created by using the cell positions and viabilities measured at each focal depth, averaged in concentric rings similar to the overall average radial viability. These per-layer radial viability plots were then arranged by depth, and the plotted points were represented as colors, from red (0% normalized viability) to blue (100% normalized viability). The resulting plot is a representation of the average radial viability per depth of the entire 3D structure, in 2D. For qualitative comparison of the accuracy of these reconstructions, 3D digital radial viability plots, such as those presented in figure 3, D were mirrored at the radial center, creating digital average viability cross sections. These cross sections were then compared with physical cross sections of phantoms imaged using the microscope.
Determination of Kill Zone and Transition Zone
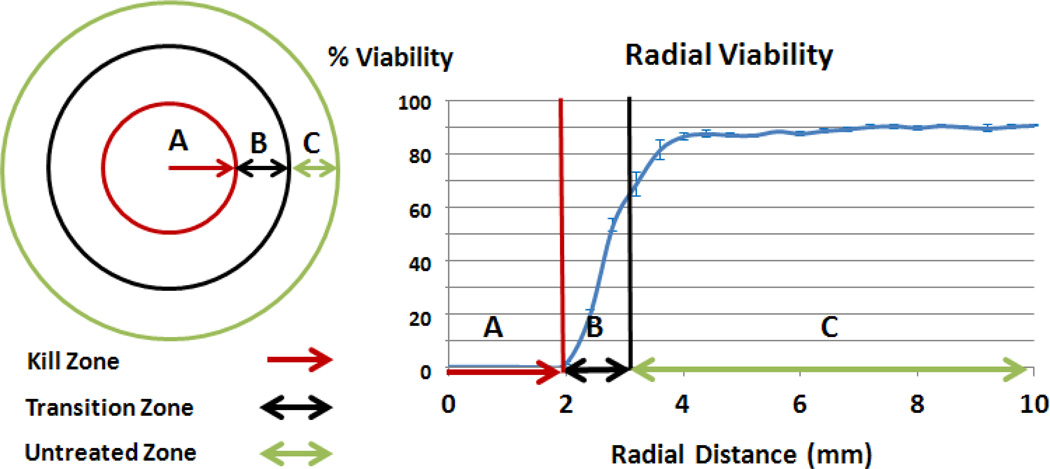
In order to determine the effectiveness of photothermal therapy in vitro at a given irradiation duration, both the areas of the kill zone and the transition zone were calculated from SPECTRL digital analysis of fluorescence images. The kill zone was defined as the region with viability less than 1%. The transition zone was defined as the region outside the kill zone, which has undergone significant (quantitatively defined subsequently), but not complete, cellular damage due to treatment. The viability in individual images within the untreated region had a standard deviation of 16.6%. Therefore, to ensure that viability losses recorded were caused by thermal damage resulting from treatment, a threshold of two standard deviations below the normalized mean, or 100 − 2*16.6 = 66.6%, was set as the viability cutoff between treated and untreated regions. This ensured that a maximum of 5% of normal viability fluctuation was attributed to photothermal loss. The resulting viability range for the transition zone was therefore between 1% and 66.6%. Untreated viability was the viability in the untreated region of the phantom where viability is greater than 66.6%. These regions are displayed visually with a top down depiction of a phantom, and a sample radial viability graph (figure 4). The kill zone and transition zone are metrics for determining the area of tissue that could be treated effectively (i.e. near 100% cell death), and what area of healthy tissue would suffer undesired injury. In the top down vertical DVR analysis, both the kill zone and transition zone were measured in terms of radius (mm) and projected volume (mm3).
Figure 4.
Illustration displaying the radial measurement of the size of the kill zone (completely treated region, A, red arrow), transition zone (region of partially damaged tissue, B, double black arrow), and the untreated viability (untreated region, C, double green arrow). Error bars indicate +/− one standard deviation.
From these radial measurements, the projected volume of both the kill and transition zones were calculated using the following equations:
| Eq 1. |
| Eq 2. |
The variable r represents the radius of the kill zone, and h is the thickness of the phantom, or 1.5 mm. This allowed for comparison between the calculated volumetric kill and transition zones using 2D analysis which ignore variation in viability between focal depths, and the measurements of kill and transition volume from 3D analysis which takes these differences into consideration.
Results
2D Representation of Viability Response Data
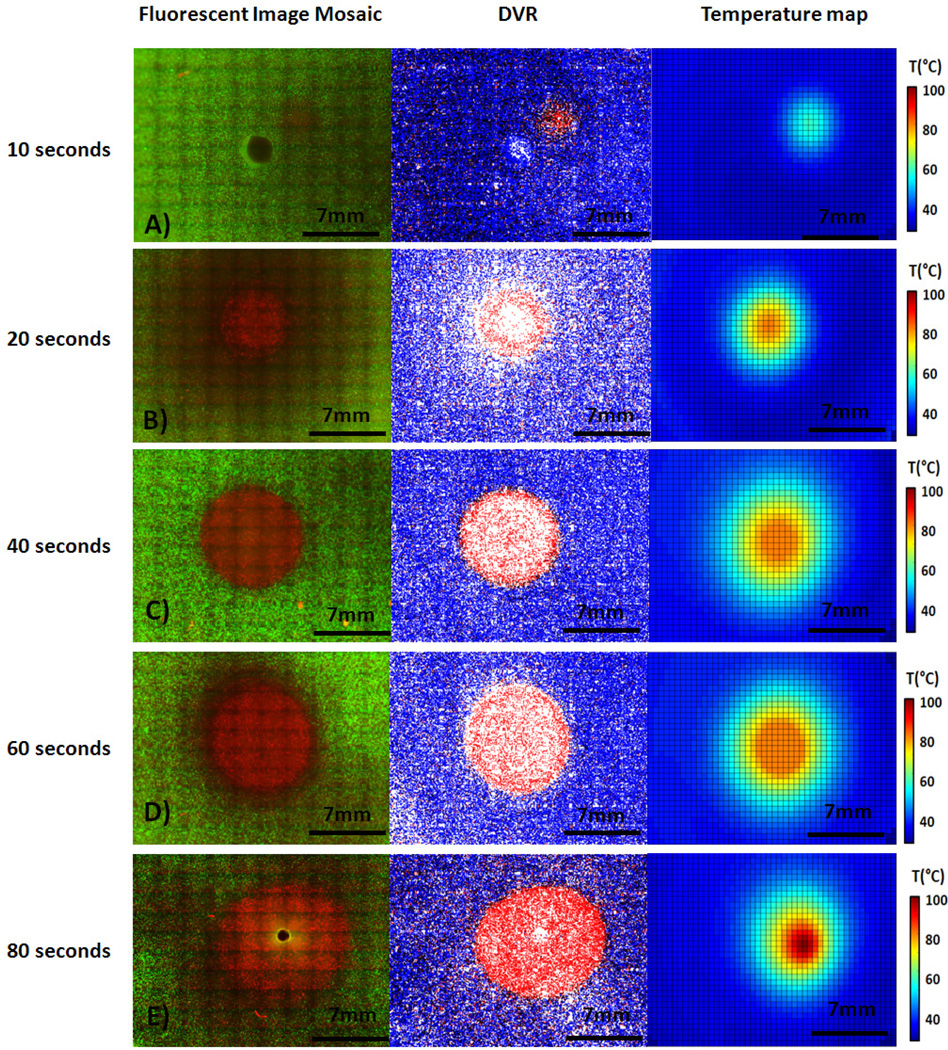
Figure 5 displays fluorescent image mosaics (left) compared with corresponding DVRs (center) and temperature maps (right) at each laser duration measured. Fluorescent image mosaics are composed of individual fluorescence images taken throughout the 1.5 mm thick phantom and cover an area of 26 mm2 (figure 5, A–E left). These mosaics reveal increasing levels of cellular damage (visible as areas of PI red stained cells) surrounded by areas that have not undergone significant cellular damage (areas of calcein AM green stained cells). Both the kill zone area (area of dead cells only) and treatment zone (area of reduced cell viability) increased with greater laser irradiation duration. A live-dead analysis was performed by the digital image analysis program SPECTRL, and a vertical DVR was created from SPECTRL output. This DVR shows the XY location of live and dead cells as blue and red dots respectively (figure 5, A–E center). Figure 5, A–E right displays corresponding temperature maps which indicate that the temperature rises over successively larger areas as laser irradiation duration increases. Viability is visibly reduced in a relatively small zone at the lowest durations (10 sec) of laser exposure, as is indicated by the presence of propidium iodide (red) staining at the center of treatment (figure 5, A). The area of viability reduction increases from a 1.75 mm radius at 10 seconds to approximately 4.5 mm radius at 80 seconds (figure 5, A–E). At the highest durations of laser exposure, the laser also began to melt the phantom. This melting is undesirable from a clinical and experimental standpoint, and serves as a probable upper bound for a useful combination of laser fluence, laser duration, and nanoparticle concentration (figure 5, E).
Figure 5.
Fluorescent image mosaic of treated area in phantom with propidium iodide (dead stain) is shown in red, calcien AM (live stain) is shown in green (Left), vertical DVR output, constructed from the fluorescent images (Center), and thermal image collected by the IR camera during irradiation (Right) for samples treated with 10, 20, 40, 60, and 80 seconds of laser irradiation (A–E, respectively).
Radial Temperature and Viability in 3D tumor phantoms via photothermal heating
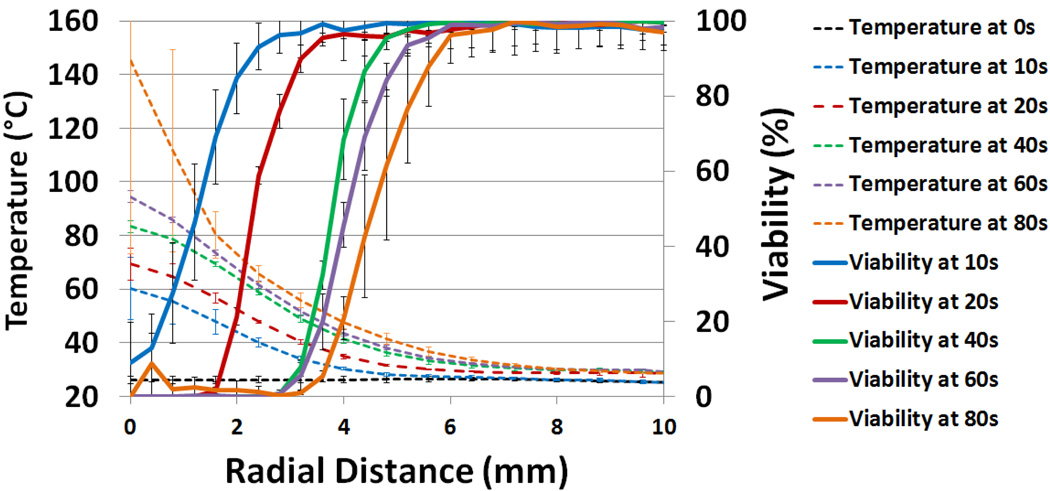
Figure 6 displays both viability (solid lines) and temperature (dashed lines) as a function of radial distance from the beam center of irradiation and laser irradiation duration. IR camera images taken during laser irradiation of tissue phantoms reveal rapid temperature increases at the center of irradiation, as well as constant heat conduction outward from the center. The 10 second irradiation tests resulted in a temperature increase of 30°C at the center, while the 60 second tests showed a 65°C increase. Additionally, the figure illustrates temperature increases which are highest at the beam center of irradiation, resulting in lesser increases in temperature radially outwards over time. This increasing temperature surpasses 42°C, the previously identified temperature thresholds to cause cellular damage, in radially increasing areas [52–54]. For example after 10 seconds of irradiation an area with a diameter of 4 mm exceeded 42°C, while after 60 seconds of irradiation an area with a diameter of 8.8 mm reached temperatures higher than 42°C (figure 6). Temperature variation between phantoms remained low (average standard deviation = 1.26 °C), indicating low variation in the SWNH concentration and distribution between phantoms. This consistency persisted up to durations of 60 seconds of laser irradiation. Between 60 and 80 seconds of laser irradiation, the temperature at the center of several phantoms exceeded 100°C causing the phantom to melt. This resulted in a high degree of temperature variability between samples.
Figure 6.
Normalized percent viability (solid lines) and temperature (dashed lines) as a function of radial distance for varying durations of laser exposure. Error bars indicate +/− one standard deviation.
Figure 6 displays viability (solid lines) as a function of both radial distance from the beam center and laser irradiation duration. Radial viability was calculated by averaging radial viability over all focal depths. The maximum radial viability of each set of phantoms exposed to the same laser irradiation duration was normalized to 100% for comparison (figure 6, right vertical axis). The viability data shows a strong correlation between increasing laser irradiation duration, and expansion of area with reduced viability. Several conclusions may be drawn from the combination of radial viability and temperature data. First, in all irradiation durations longer than 10 seconds, the radius of each kill zone very closely correlates with the point where the radial temperature achieves 55°C, suggesting the lethal temperature threshold for this phantom system is not duration dependent (figure 6). Conversely, the temperature which correlates with the outer edge of the transition zone (66% viability) decreases with increasing duration of irradiation. Figure 6 shows that at 10 seconds, the 66% viability benchmark was achieved at 48°C. At longer durations, the same benchmark was achieved at 45°C, 42°C, 41°C, and 40°C, at 20, 40, 60, and 80 seconds, respectively. This suggests that the temperature required to cause a transition zone with laser irradiation is duration dependent, and that lower durations require higher temperatures. The nonzero viability observed at 80 seconds was caused by regions of burned phantom which auto-fluoresce at the emission wavelength of calcein AM (515 nm). This induced auto-fluorescence may be identified by SPECTRL as live cells, causing a false positive reading. This identifiable error is low, (<10%) but indicates that this system may read the viability of auto-fluorescent or burned tissue incorrectly.
Determination of Kill, Transition, and Treatment Zones
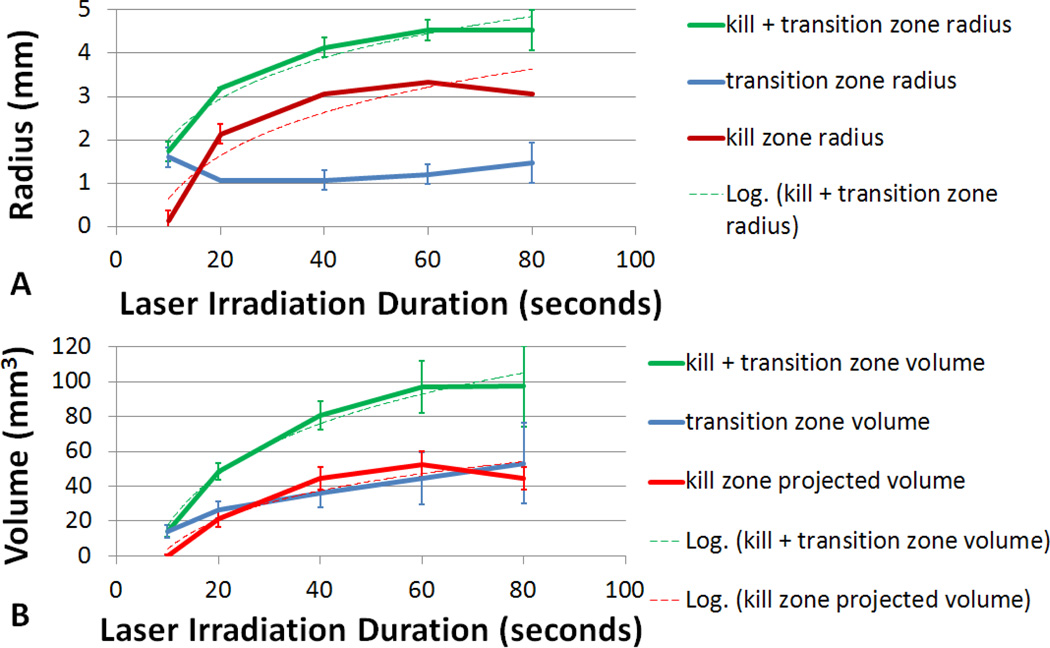
Figure 7 shows kill and transition zone size resulting from varying durations of laser exposure calculated from the vertical DVR analysis. The kill zone and transition zones expanded with increasing laser duration. This expansion over time is caused by thermal energy conducting outward, killing cells further away from the center as irradiation duration increases. A best fit logarithmic curve to the radial increase in kill zone size with an R2 value of 0.85 was found, where radius(kill zone) = 1.44*ln(t)−2.68, where t is time in seconds. This resulted in an average increase of 0.036 mm/s over the first 80 seconds of irradiation. The kill zone radius size experienced exponential decay plateauing at approximately a 3.3 mm radius at 60 seconds, suggesting that there may be an upper limit to the maximum size of the kill zone. In addition, the radius of the transition zone remained fairly constant at an average of 1.28 mm throughout the variety of laser durations while increases in the kill zone radius decreased over time (figure 7 A). The result is a treatment zone (kill + transition zone) which increased at a decaying rate, and plateaus at 4.53 mm. However the variation in the volume of the transition and treatment zones increased with longer laser duration. This suggests that multiple treatments utilizing shorter duration laser pulses could allow for greater control over treatment boundaries. In addition, variability of kill zone radial size between samples was extremely small (on average less than +/−0.05 mm); indicating that precise control over kill zone boundaries using nanoparticle mediated photothermal therapy is possible.
Figure 7.
Kill, transition, and kill + transition zone volumes calculated from vertical DVR analysis. A) Radial size and B) volume of the kill zone and transition zone as a function of irradiation duration. Projected volume is based on vertical DVR analysis and assumed phantom thickness of 1.5mm. Error bars indicate +/− one standard deviation.
These radial increases in kill and transition zone over time equate to increasing projected kill and transition zone volumes using the equations described in the methodology (figure 7, B). The kill zone volume increased logarithmically, plateauing at 52.5mm3 at 80 seconds. The kill zone volume can be approximated with a logarithmic equation volume(kill zone)=24.19*ln(t) – 51.56, which has a R2 fit of 0.899. The volume of the transition zone increased linearly at approximately 0.55mm3/s. This results in a combined kill and transition zone volume which plateaus at 97.5mm3 and may be approximated with the logarithmic curve volume(kill + transition) = 17.87*ln(t)−27.55 with an R2 fit value of 0.987. Again, the fact that the kill + transition zone volume reach a steady-state level suggests that using laser irradiation of 3.8 watts/cm2 and a SWNH concentration of 0.085 mg/ml results in a maximum treatment volume with increasing time.
3D Viability Analysis through the Thickness of Tumor Phantoms
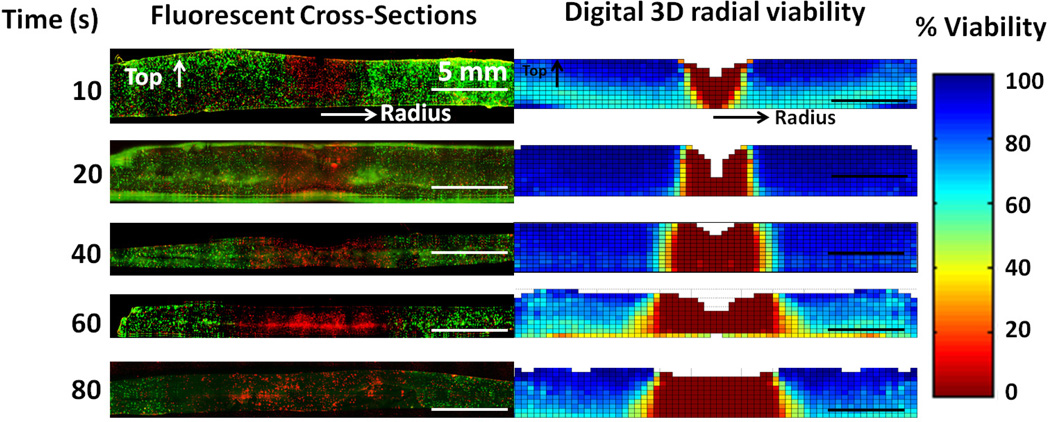
Figure 8 left column shows cross-sectional images of individual phantoms on the microscope after 10, 20, 40, and 60 seconds of irradiation, which mirror the digitally constructed viability measurements created by measuring viability at each focal depth and averaging the viability at each radial point (right column, figure 8). Irradiation was directed from the top down, and radial distance from the beam center of irradiation increases horizontally from the center. The kill zone is visible in the fluorescent cross sections as the region at the center which is almost entirely stained red with propidium iodide, while the untreated region is visible as cells stained primarily with calcein AM (green). The kill zone (viability less than 1%) is visible in the radial DVR as red, the untreated region (viability greater than 66%), is visible as teal and blue regions and the transition zone (viability greater than 1% and lower than 66%) is visible in orange and yellow. The similarity between the physical cross sections and digital viability maps show how it is possible to re-create 3D viability within solid tissue nondestructively by analyzing images taken at sequential focal depths. In addition, the digital viability images illustrate how short duration laser irradiation results in a cone-shaped viability loss with the widest part of the kill zone at the top where the laser initially contacts the phantom (figure 8, 10 seconds). Additional temperature measurements indicate that after 10 seconds of irradiation the center of the top of the phantom reaches an average temperature of 5°C hotter than the center of the bottom of the phantom, and this temperature gradient decreases with increasing laser irradiation duration (data not shown). At 20 seconds of laser irradiation the kill zone became increasingly uniform from top to bottom (figure 8, 20 seconds). At 40 seconds of irradiation the kill zone had expanded radially outward, and had a slightly larger kill zone at the bottom of the phantom which could be caused by convective cooling at the top surface of the phantom. This trapezoid shaped kill zone continues to expand at the bottom of the phantom from 40 to 80 seconds of irradiation, resulting in a kill zone which is 0.8 mm in diameter larger at the bottom than the top, and a transition zone which is 5.6 mm in diameter larger at the bottom compared to the top. This may be caused by a variety of factors including greater convective cooling at the top of the phantom which could result in higher temperatures and lower viability at the bottom of the phantom. Figure 8 also illustrates phantom material lost at the top surface directly under laser irradiation is detectable as an absence of data shown as white, or absent pixels. This could be caused by a number of factors, including phantom dehydration and physical ablation resulting from laser treatment.
Figure 8.
Demonstration and validation of 3D viability measurement. Left Column: Fluorescently imaged physical cross sections of tissue phantom with live cells stained green with calcein AM and dead cells stained red with propidium iodide. Right Column: Corresponding average radial viability measurement from non-destructive imaging mirrored and scaled to match the fluorescent cross section. Fluorescent images are contrast enhanced to improve visibility.
Quantitative 3D Kill, Transition, and Treatment Zones
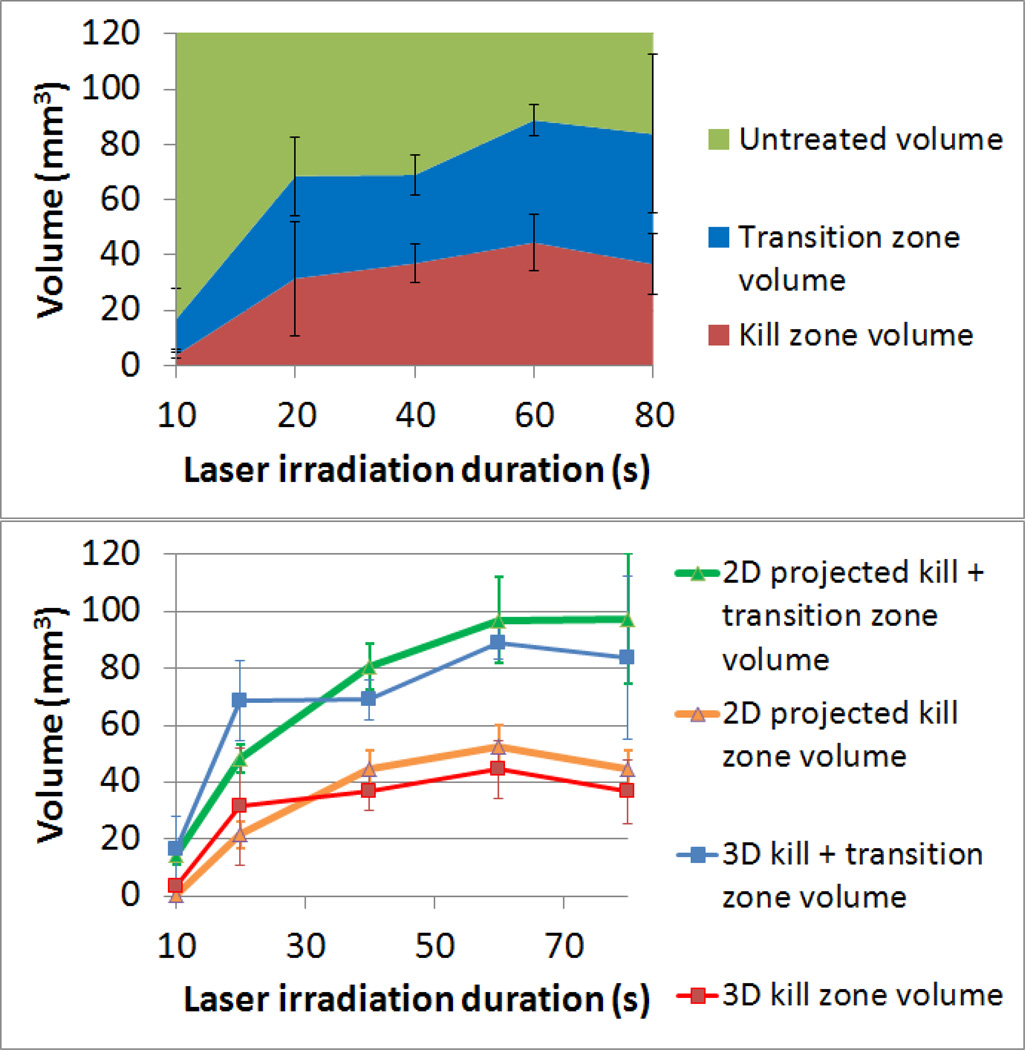
Quantitative analysis of 3D viability data was conducted to determine the volume of kill zone, transition zone, and non-treated tissue shown in figure 9. This figure demonstrates how the volume of photothermally treated tissue increases with longer irradiation duration. From 10 to 60 seconds the kill zone increases from 3.7 mm3 to 44.5 mm3, while the transition region increases from 13 mm3 to 44.3 mm3. The slope of the volume of the combined transition and kill zones approximately follows a logarithmic curve:volume(kill + transition) = 30.65 In(t) – 441.675, where t is the duration of laser irradiation in seconds. Figure 9 A demonstrates both the volume of treatment, and the variability of samples. The average standard deviation for the kill and transition zone volume are 9.95 mm3 and 13.35 mm3 respectively.
Figure 9.
Volume of kill and transition zones and comparison between projected 2D analysis and measured 3D analysis. A) Volumetric measurement of kill zone, transition zone, and untreated volume within a 6 mm radius from the center of irradiation as a function of duration of laser exposure. B) Comparison of 2D projected kill and transition zone volumes and 3D measurements of kill and transition zone volumes. Error bars indicate +/− one standard deviation.
Comparison of 2D Projection and 3D Measurements of Kill and Transition Volume
Differences between the 2D projected kill and transition volumes and measured 3D kill and transition volumes are displayed in figure 9, B. When compared with 3D analysis, the 2D analysis which adds cells across all focal depths and takes a viability measurement of that group slightly underestimates both the kill zone and transition zone volume for durations between 10 and 20 seconds. This is caused by high viability at the bottom of the phantom during short irradiation durations which positively biases the 2D viability analysis. High viability at the bottom of the phantom (side facing away from the laser) is detected by 3D analysis, while lowering the projected kill zone volume with 2D analysis. In addition, 2D projected kill and transition volumes are slightly larger than the volumes measured using 3D analysis for irradiation durations greater than 20 seconds, again caused by uneven viability distributions top to bottom. In addition the variability resulting from the two measurement methods is distinct; standard deviations are 5.13 mm3 and 10.93 mm3 for the 2D kill and transition projection volumes and 9.95 mm3 and 13.35 mm3 for the 3D kill and transition zone volumes respectively. Because 2D analysis averages viability across all focal depths, viability variations in the Z direction are not considered. This leads to an underestimation of the viability variation within the phantom. This variation is detected in 3D analysis, making 3D analysis a more accurate measurement of both treatment volume and variation within samples.
Discussion
Previous work has demonstrated the ability of photothermal therapy to kill cancer cells using nanoparticles such as single walled carbon nanotubes (SWNTs) [55–57], multi-walled carbon nanotubes (MWNTs) [7, 58–60], SWNHs [4, 61], and gold nanoshells [62–64]. However, these experiments frequently utilize cell monolayers which do not have the same heat transfer characteristics or 3D structure as live tissue. 3D tissue models have the capacity to dramatically improve the clinical relevance and detail of information provided by in vitro studies compared to 2D cell culture [18]. This study demonstrates how phantoms incorporating cells and SWNHs provide the ability to create a 3D thermally representative environment in which the 3D viability can be quantified in response to varying treatment parameters. This work has also demonstrated the ability of the SPECTRL method to determine the influence of irradiation duration on 2D and 3D temperature and viability response in 3D tissue phantoms. This is important for measuring the variability of viability response to photothermal parameters, determining the relationship between thermal exposure and viability response, and identifying the size of the transition zone surrounding the kill zone. It is probable that spatial viability measurements of transition zones resulting from photothermal therapy have not been made because the spatial precision of tumor treatment is irrelevant in 2D cell monolayers and unimportant in mouse flank tumors where excess thermal damage is unlikely to harm vital organs [4, 7, 9, 14]. Understanding the volume of the kill and transition zones is critical for planning tumor treatment where damage to healthy tissue surrounding a tumor is undesirable, such as in brain and pancreatic cancer [65, 66].
The radial temperature profile observed in figure 5 correlates with the viability response which is symmetric to the radial beam profile. This treatment profile is more representative of the symmetric kill zones observed after laser photothermal heating of tissue [67, 68] as compared to the asymmetric temperature and kill zones typically observed using cell monolayers culture models [19]. The treatment zone also corresponds with the shape of light propagation predicted by Monte Carlo simulations [9, 69].
The concentration of SWNHs used was selected because previous studies have demonstrated that it is high enough to cause large temperature increases in combination with laser irradiation, while remaining fully in suspension [4, 19]. The laser fluence used in this study, 3.8 watts/cm2, compares closely with fluences of NIR lasers used in other in vivo nanoparticle mediated photothermal therapy studies (3 and 4 watts/cm2 respectively) [7, 9]. However the temperature increases of 70°C in 60 seconds observed in this study are significantly higher than the temperature increases of 15 and 50 degrees in 60 seconds observed in those same in vivo studies. This could be caused by a number of factors such as the uncertainty of nanoparticle concentration within the mouse tumor, variation between nanoparticle types, and cooling in in vivo models due to blood circulation. The laser fluence used in this study is also significantly lower (3.8 as compared to 40 watts/cm2) than a previous in vitro cell monolayer study conducted by our group, despite resulting in higher temperature increases (68°C vs. 30°C rise in 60 seconds) [4]. This is likely due to evaporative heat losses, as well as higher thermal convection and conduction present in liquid media cell cultures.
The SPECTRL digital viability assessment used in the current study is similar to the digital image analysis method previously developed by our group to assess viability response to photothermal therapy in 2D cell monolayers which was verified by quantitative comparison with manual viability counts [4]. The strong resemblance between the images of the fluorescence viability assay and the vertical DVRs in figure 5 qualitatively supports the accuracy of the SPECTRL method. The spatial information provided by this method allows for additional conclusions to be drawn regarding the relationship between the treatment parameters and the viability response. For example, the volume of intersection between the laser beam and tissue phantom can be approximated using the laser beam diameter (10 mm) and phantom thickness (1.5 mm). These dimensions create a volumetric intersection of approximately 118 mm3, which is similar in magnitude to the total treatment volume measured, and suggests that the treatment volume may be partially dependent on laser beam diameter. In addition, the transition and kill zones expanded symmetrically away from the irradiation center at a logarithmic rate, suggesting that treatment zone volume increases could be predicted with reasonable accuracy. This study also demonstrates that for the laser and nanoparticle parameters used, the kill and transition zones both increase at very similar rates, suggesting that in order to achieve a high kill zone to transition zone ratio it may be necessary to use a different set of laser or nanoparticle parameters, such as a pulsed laser, higher fluence, or more concentrated nanoparticle delivery.
In addition to providing information about the kill and transition zone volumes, the combined temperature and viability data in figure 6 establish the minimum temperature thresholds necessary to induce cellular damage (40°C+). This finding is similar to studies which assert that temperatures in excess of 42°C must be reached to initiate cell damage [52–54]. The viability and temperature distributions also provide minimum combined temperature and exposure durations in order to achieve total cell death, such as 55°C for 80 seconds (figure 6). These temperature thresholds could be used to compare the effects of varying laser and nanoparticle parameters on cells, and therefore evaluate different methods of delivering photothermal therapy.
The accuracy of viability measurements was also verified through qualitative comparison of reconstructed 3D viability with fluorescently imaged cross sections of treated phantom. The viability response as a function of depth in the phantom supports previous research indicating that reducing irradiation duration, potentially through the use of pulsed lasers may result in shallow treatment, and that longer duration of laser irradiation can result in cell death deep within tissue [7, 70]. Therefore varying irradiation duration may be useful in planning treatment or targeting tumors on the surface or deeply embedded in tissue. In addition the comparison between 2D and 3D measurements reveal how measurements ignoring variation within the third dimension of tissue may make measurement errors in both the magnitude and variation of viability response.
Conclusion
In this study a new method of digitally measuring viability in tissue phantoms was introduced, demonstrated, and qualitatively evaluated for its ability to determine 3D viability response to photothermal therapy. While 3D tissue phantoms do not reproduce physiological features such as blood flow or other heterogeneities found within tissue, they may serve as important sources of photothermal therapy efficacy data difficult to obtain in vivo. Precise control over nanoparticle concentration, tissue geometry, and thermal conditions make the use of sodium alginate phantoms an appealing medium for assessing the effectiveness of photothermal therapies. In the future the ability to make detailed measurements of cell viability within 3D tissue phantoms could be expanded beyond photothermal therapy to include the study of cell migration, growth, and other responses to spatially distributed stimuli. The combination of representative 3D cellular environments with clinically relevant 3D viability responses makes SPECTRL analysis a valuable method of evaluating photothermal therapy.
Future Perspective
In the future the ability to make detailed measurements of cell viability within 3D tissue phantoms could be expanded beyond photothermal therapy to include the study of cell migration, growth, and other responses to spatially distributed stimuli. The combination of representative 3D cellular environments with clinically relevant spatial viability measurements makes SPECTRL analysis a valuable method for evaluating tumor response to photothermal therapy alone or in combination with nanoparticles.
Summary.
Nanoparticle-mediated photothermal therapy is being investigated as a method for treating tumors which cannot be effectively treated with resection and chemotherapy.
Evaluating the precision of photothermal therapy requires spatial viability measurements.
This paper develops and tests the capability of the digital viability analysis method called SPECTRL to determine the 3D viability within tissue phantoms.
The kill and transition zones expanded with increasing laser irradiation duration, and were of comparable size. The transition zone ranged from 46% to 78% of the total treatment volume.
This method provides a valuable method for measuring the 3D spatial viability effects of photothermal therapy alone or in combination with nanoparticles.
Acknowledgments
Funding was provided by the National Science Foundation Grants 0731108, 0933571, and 0955072, National Institute of Health Grants 1 R21 CA135230-01, R21 CA156078, and RO1CA128428, and an Institute for Critical Technology and Applied Science (ICTAS, Virginia Tech) Grant. A portion of this research was conducted at the Center for Nanophase Materials Sciences, which is sponsored by Oak Ridge National Laboratory by the Office of Basic Energy Sciences, U.S. Department of Energy. SWNHs were synthesized under research sponsored by the Materials Sciences and Engineering Division, Office of Basic Energy Sciences, U.S. Department of Energy.
References
- 1.Society AC. Cancer Facts & Figures 2012. 2012 [Google Scholar]
- 2.Neal RE, Rossmeisl JH, Garcia PA, Lanz OI, Henao-Guerrero N, Davalos RV. Successful Treatment of a Large Soft Tissue Sarcoma With Irreversible Electroporation. Journal of Clinical Oncology. 2011;29(13):e372–e377. doi: 10.1200/JCO.2010.33.0902. [DOI] [PubMed] [Google Scholar]
- 3.Hergt R, Dutz S, Müller R, Zeisberger M. Magnetic particle hyperthermia: nanoparticle magnetism and materials development for cancer therapy. Journal of Physics: Condensed Matter. 2006;18(38):S2919. [Google Scholar]
- 4.Whitney JR, Sarkar S, Zhang J, et al. Single walled carbon nanohorns as photothermal cancer agents. Lasers in Surgery and Medicine. 2011;43(1):43–51. doi: 10.1002/lsm.21025. [DOI] [PubMed] [Google Scholar]
- 5.Torti SV, Byrne F, Whelan O, et al. Thermal ablation therapeutics based on CNx multi-walled nanotubes. Int J Nanomed. 2007;2(4):707–714. [PMC free article] [PubMed] [Google Scholar]
- 6.Loo C, Lowery A, Halas N, West J, Drezek R. Immunotargeted Nanoshells for Integrated Cancer Imaging and Therapy. Nano letters. 2005;5(4):709–711. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- 7.Burke A, Ding X, Singh R, et al. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proceedings of the National Academy of Sciences. 2009;106(31):12897–12902. doi: 10.1073/pnas.0905195106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ajima K, Murakami T, Mizoguchi Y, et al. Enhancement of In Vivo Anticancer Effects of Cisplatin by Incorporation Inside Single-Wall Carbon Nanohorns. ACS Nano. 2008;2(10):2057–2064. doi: 10.1021/nn800395t. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch LR, Stafford RJ, Bankson JA, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proceedings of the National Academy of Sciences. 2003;100(23):13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, Xing D, Ou Z, Wu B, Resasco DE, Chen WR. Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. Journal of Biomedical Optics. 2009;14(2):021009–021009. doi: 10.1117/1.3078803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hood RL, Carswell W, Rodgers A, et al. Spatially controlled photothermal heating of bladder tissue through single-walled carbon nanohorns delivered with a fiberoptic microneedle device. Lasers in Medical Science. 2012:1–8. doi: 10.1007/s10103-012-1202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajima K, Yudasaka M, Murakami T, Maigné A, Shiba K, Iijima S. Carbon Nanohorns as Anticancer Drug Carriers. Molecular Pharmaceutics. 2005;2(6):475–480. doi: 10.1021/mp0500566. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Yudasaka M, Kouraba S, Sekido M, Yamamoto Y, Iijima S. Single wall carbon nanohorn as a drug carrier for controlled release. Chemical Physics Letters. 2008;461(4–6):189–192. [Google Scholar]
- 14.O'neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Letters. 2004;209(2):171–176. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Huang XH, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. Journal of the American Chemical Society. 2006;128(6):2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 16.Shafiee H, Garcia PA, Davalos RV. A Preliminary Study to Delineate Irreversible Electroporation From Thermal Damage Using the Arrhenius Equation. Journal of Biomechanical Engineering. 2009;131(7):074509. doi: 10.1115/1.3143027. [DOI] [PubMed] [Google Scholar]
- 17.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7(3):211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 18.Yamada KM, Cukierman E. Modeling Tissue Morphogenesis and Cancer in 3D. Cell. 2007;130(4):601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Whitney AR Jon R, Harvie Erica, Carswell William F, Torti Suzy, Puretzky Alex a, Rouleau Christopher M, Geohegan David B, Rylander Christopher G, Rylander Marissa N. Spatial and temporal measurements of temperature and cell viability in response to nanoparticle-mediated photothermal therapy. Nanomedicine (London, England) 2012:1–14. doi: 10.2217/nnm.12.66. [DOI] [PubMed] [Google Scholar]
- 20.Anderson RR, Parrish JA. The Optics of Human Skin. J Investig Dermatol. 1981;77(1):13–19. doi: 10.1111/1523-1747.ep12479191. [DOI] [PubMed] [Google Scholar]
- 21.Van Dyke T, Jacks T. Cancer Modeling in the Modern Era: Progress and Challenges. Cell. 2002;108(2):135–144. doi: 10.1016/s0092-8674(02)00621-9. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Murakami T, Ajima K, et al. Fabrication of ZnPc/protein nanohorns for double photodynamic and hyperthermic cancer phototherapy. Proceedings of the National Academy of Sciences. 2008;105(39):14773–14778. doi: 10.1073/pnas.0801349105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F, Xing D, Ou Z, Wu B, Resasco DE, Chen WR. Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. Journal of Biomedical Optics. 2009;14(2):021009. doi: 10.1117/1.3078803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang HC, Rege K, Heys JJ. Spatiotemporal Temperature Distribution and Cancer Cell Death in Response to Extracellular Hyperthermia Induced by Gold Nanorods. ACS Nano. 4(5):2892–2900. doi: 10.1021/nn901884d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krupka TM, Dremann D, Exner AA. Time and Dose Dependence of Pluronic Bioactivity in Hyperthermia-Induced Tumor Cell Death. Exp. Biol. Med. 2009;234(1):95–104. doi: 10.3181/0807-RM-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peyton SR, Kim PD, Ghajar CM, Seliktar D, Putnam AJ. The effects of matrix stiffness and RhoA on the phenotypic plasticity of smooth muscle cells in a 3-D biosynthetic hydrogel system. Biomaterials. 2008;29(17):2597–2607. doi: 10.1016/j.biomaterials.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proceedings of the National Academy of Sciences. 1994;91(26):12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieminski AL, Hebbel RP, Gooch KJ. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Experimental Cell Research. 2004;297(2):574–584. doi: 10.1016/j.yexcr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 29.Szot CS, Buchanan CF, Rylander MN, Freeman JW. Cancer cells cultured within collagen I hydrogels exhibit an in vivo solid tumor phenotype. Bioengineering Conference (NEBEC), 2011 IEEE 37th Annual Northeast. 2011 [Google Scholar]
- 30.Sung JH, Shuler ML. A micro cell culture analog ([small micro]CCA) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab on a Chip. 2009;9(10):1385–1394. doi: 10.1039/b901377f. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Kim YL, Backman V. Development of a bioengineered tissue model and its application in the investigation of the depth selectivity of polarization gating. Appl. Opt. 2005;44(12):2288–2299. doi: 10.1364/ao.44.002288. [DOI] [PubMed] [Google Scholar]
- 32.Taroni P, Comelli D, Pifferi A, Torricelli A, Cubeddu R. Absorption of collagen: effects on the estimate of breast composition and related diagnostic implications. Journal of Biomedical Optics. 2007;12(1):014021. doi: 10.1117/1.2699170. [DOI] [PubMed] [Google Scholar]
- 33.Sarkar S, Gurjarpadhye AA, Rylander CG, Rylander MN. Optical properties of breast tumor phantoms containing carbon nanotubes and nanohorns. Journal of Biomedical Optics. 2011;16(5):051304. doi: 10.1117/1.3574762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 35.Leroux MA, Guilak F, Setton LA. Compressive and shear properties of alginate gel: Effects of sodium ions and alginate concentration. Journal of Biomedical Materials Research. 1999;47(1):46–53. doi: 10.1002/(sici)1097-4636(199910)47:1<46::aid-jbm6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 36.Ong S-M, Zhao Z, Arooz T, et al. Engineering a scaffold-free 3D tumor model for in vitro drug penetration studies. Biomaterials. 2010;31(6):1180–1190. doi: 10.1016/j.biomaterials.2009.10.049. [DOI] [PubMed] [Google Scholar]
- 37.Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro—A growing case for three-dimensional (3D) culture systems. Seminars in Cancer Biology. 2005;15(5):405–412. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Szot CS, Buchanan CF, Freeman JW, Rylander MN. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials. 2011;32(31):7905–7912. doi: 10.1016/j.biomaterials.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischbach C, Chen R, Matsumoto T, et al. Engineering tumors with 3D scaffolds. Nat Meth. 2007;4(10):855–860. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 40.Liu VG, Cowan TM, Jeong SW, Jacques SL, Lemley EC, Chen WR. Selective Photothermal Interaction Using an 805-nm Diode Laser and Indocyanine Green in Gel Phantom and Chicken Breast Tissue. Lasers in Medical Science. 2002;17(4):272–279. doi: 10.1007/s101030200040. [DOI] [PubMed] [Google Scholar]
- 41.Garcia Y, Collighan R, Griffin M, Pandit A. Assessment of cell viability in a three-dimensional enzymatically cross-linked collagen scaffold. Journal of Materials Science: Materials in Medicine. 2007;18(10):1991–2001. doi: 10.1007/s10856-007-3091-9. [DOI] [PubMed] [Google Scholar]
- 42.Bryant SJ, Anseth KS. The effects of scaffold thickness on tissue engineered cartilage in photocrosslinked poly(ethylene oxide) hydrogels. Biomaterials. 2001;22(6):619–626. doi: 10.1016/s0142-9612(00)00225-8. [DOI] [PubMed] [Google Scholar]
- 43.Whited BM, Whitney JR, Hofmann MC, Xu Y, Rylander MN. Pre-osteoblast infiltration and differentiation in highly porous apatite-coated PLLA electrospun scaffolds. Biomaterials. 32(9):2294–2304. doi: 10.1016/j.biomaterials.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Gantenbein-Ritter B, Potier E, Zeiter S, Van Der Werf M, Sprecher CM, Ito K. Accuracy of Three Techniques to Determine Cell Viability in 3D Tissues or Scaffolds. Tissue Engineering Part C: Methods. 2008;14(4):353–358. doi: 10.1089/ten.tec.2008.0313. [DOI] [PubMed] [Google Scholar]
- 45.Sarkar S, Fisher J, Rylander C, Rylander MN. Photothermal Response of Tissue Phantoms Containing Multi-Walled Carbon Nanotubes. Journal of Biomechanical Engineering. 2010;132(4):044505. doi: 10.1115/1.3212100. [DOI] [PubMed] [Google Scholar]
- 46.Lal S, Clare SE, Halas NJ. Nanoshell-Enabled Photothermal Cancer Therapy: Impending Clinical Impact. Accounts of Chemical Research. 2008;41(12):1842–1851. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 47.Kupchik HZ, Langer RS, Haberern C, El Deriny S, O'brien M. A new method for the three-dimensional in vitro growth of human cancer cells. Experimental Cell Research. 1983;147(2):454–460. doi: 10.1016/0014-4827(83)90228-8. [DOI] [PubMed] [Google Scholar]
- 48.Oates CG, Ledward DA. Studies on the effect of heat on alginates. Food Hydrocolloids. 1990;4(3):215–220. [Google Scholar]
- 49.Sarkar S, Zimmermann K, Leng W, et al. Measurement of the Thermal Conductivity of Carbon Nanotube–Tissue Phantom Composites with the Hot Wire Probe Method. Annals of Biomedical Engineering. 2011;39(6):1745–1758. doi: 10.1007/s10439-011-0268-7. [DOI] [PubMed] [Google Scholar]
- 50.Scott Verbridge NWC, Zheng Ying, Brooks Daniel, Stroock Abraham, Fischbach Claudia. Oxygen Controlled Three-Dimensional Cultures to Analyze Tumor Angiogenesis. Tissue Engineering Part A. 2010;16(7):2133–2141. doi: 10.1089/ten.tea.2009.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luchetti #160F, et al. Hyperthermia triggers apoptosis and affects cell adhesiveness in human neuroblastoma cells. University of Murcia, Murcia, ESPAGNE; 2003. p. 18. [DOI] [PubMed] [Google Scholar]
- 52.Thomsen S, Pearce JA. Thermal Damage and Rate Processes in Biologic Tissues. In: Welch AJ, Gemert MJC, editors. Optical Thermal Response of Laser Irradiated Tissue. Netherlands: Springer; 2011. pp. 487–549. [Google Scholar]
- 53.Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. International Journal of Radiation Oncology*Biology*Physics. 1984;10(6):787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 54.Moroz P, Jones SK, Gray BN. Status of hyperthermia in the treatment of advanced liver cancer. Journal of Surgical Oncology. 2001;77(4):259–269. doi: 10.1002/jso.1106. [DOI] [PubMed] [Google Scholar]
- 55.Shao N, Lu S, Wickerstrom E, Panchapakesan B. Integrated Molecular Targeting of IGF1R and HER2 surface receptors and destruction of breast cancer cells using single wall carbon nanotubes. Nanotechnology. 2007;18:9. [Google Scholar]
- 56.Zhou F, Xing D, Ou Z, Wu B, Resasco D, Chen W. Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. Journal of Biomedical Optics. 2009;14(2):021009–021001. 021009–021007. doi: 10.1117/1.3078803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong Shi Kam N, O'connel M, Wisdom J, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci USA. 2005;102(33):11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarkar S, Fisher J, Rylander CG, Rylander MN. Photothermal Response of Tissue Phantoms Containing Multi-walled Carbon Nanotubes. Journal of Biomechanical Engineering. 2010;132(4):044505–044505. doi: 10.1115/1.3212100. [DOI] [PubMed] [Google Scholar]
- 59.Torti S, Byrne F, Whelan O, Levi N, Ucer B, Schmid M, Torti F, Akman S, Liu J, Ajayan P, Nalamau O, Carroll D. Thermal ablation therapeutics based on CNx multi-walled nanotubes. International Journal of Nanomedicine. 2007;2(4):707–771. [PMC free article] [PubMed] [Google Scholar]
- 60.Fisher J, Sarkar S, Buchanan C, Szot C, Whitney J, Hatcher H, Torti S, Rylander C, Rylander MN. Photothermal Response of Human and Murine Cancer Cells to Multiwalled Carbon Nanotubes after Laser Irradiation. Cancer Research. 2010;70(23):1–10. doi: 10.1158/0008-5472.CAN-10-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miyako E, et al. Near-infrared laser-triggered carbon nanohorns for selective elimination of microbes. Nanotechnology. 2007;18(47):475103. [Google Scholar]
- 62.O’neal D, Hirsch L, Halas N, Payne J, West J. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Letters. 2004;209:171–176. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Zharov V, Letfullin R, Galitovskaya E. Microbubbles overlapping mode for laser killing of cancer cells with absorbing nanoparticle clusters. Journal of Physics D: Applied Physics. 2005;38:2571–2581. [Google Scholar]
- 64.Loo C, Lowery a, Halas N, West J, Drezek R. Immunotargeted Nanoshells for Integrated Cancer Imaging and Therapy. Nano Letters. 2005;5(4):709–711. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- 65.Anzai Y, Lufkin R, Desalles A, Hamilton DR, Farahani K, Black KL. Preliminary experience with MR-guided thermal ablation of brain tumors. American Journal of Neuroradiology. 1995;16(1):39–48. [PMC free article] [PubMed] [Google Scholar]
- 66.Dobelbower RRJ. Current radiotherapeutic approaches to pancreatic cancer. Journal Name: Cancer (Philadelphia); (United States) 1981:1729–1733. doi: 10.1002/1097-0142(19810315)47:6+<1729::aid-cncr2820471445>3.0.co;2-l. Journal Volume: 47:6, Medium: X; Size: Pages: [DOI] [PubMed] [Google Scholar]
- 67.Beccaria K, Canney MS, Carpentier AC. Magnetic Resonance-Guided Laser Interstitial Thermal Therapy for Brain Tumors. In: Beccaria K, editor. Tumors of the Central Nervous System. Volume 5. Netherlands: Springer; 2012. pp. 173–185. [Google Scholar]
- 68.Breen M, Breen M, Butts K, Chen L, Saidel G, Wilson D. MRI-guided Thermal Ablation Therapy: Model and Parameter Estimates to Predict Cell Death from MR Thermometry Images. Annals of Biomedical Engineering. 2007;35(8):1391–1403. doi: 10.1007/s10439-007-9300-3. [DOI] [PubMed] [Google Scholar]
- 69.Flock ST, Wilson BC, Patterson MS. Monte Carlo modeling of light propagation in highly scattering tissues. II. Comparison with measurements in phantoms. Biomedical Engineering, IEEE Transactions on. 1989;36(12):1169–1173. doi: 10.1109/10.42107. [DOI] [PubMed] [Google Scholar]
- 70.Jacques SL. Role of tissue optics and pulse duration on tissue effects during high-power laser irradiation. Appl. Opt. 1993;32(13):2447–2454. doi: 10.1364/AO.32.002447. [DOI] [PubMed] [Google Scholar]