Abstract
Reports of age-related changes to medial prefrontal cortex (mPFC) activity during socio-cognitive tasks have shown both age-equivalence and under recruitment. Emotion work illustrates selective mPFC response dependent on valence, such that negative emotional images evoke increased ventral mPFC activity for younger adults, while older adults recruit ventral mPFC more for positive material. By testing whether this differential age-related response toward valenced material is also present for the social task of forming impressions, we may begin to understand inconsistencies regarding when age differences are present vs. absent in the literature. Using fMRI, participants intentionally formed impressions of positive and negative face-behavior pairs in anticipation of a memory task. Extending previous findings to a social task, valence-based reversals were present in dorsal and ventral mPFC, and posterior cingulate cortex. Younger adults elicited increased activity when forming negative impressions, while older adults had more recruitment when forming positive impressions. This suggests an age-related shift toward emphasizing positive social information may be reflected in the recruitment of regions supporting forming impressions. Overall, the results indicate an age-related shift in neural response to socio-cognitive stimuli that is valence dependent rather than a general age-related reduction in activity, in part informing prior inconsistencies within the literature.
Keywords: aging, medial prefrontal cortex, impression formation, valence
Throughout life people constantly form and use impressions to guide their social interactions and decisions. Neuroimaging work illustrates that medial prefrontal cortex (mPFC)–especially dorsal mPFC (dmPFC) —supports this process (Ma, Vandekerckhove, VanOverwalle, Seurinck, & Fias, 2011; J. Mitchell, Macrae, & Banaji, 2004). Potential age differences in mPFC function are relatively underexplored, which is surprising since forming impressions is essential throughout life. Studying these potential age-related changes within regions supporting impression formation may yield intriguing results, given that older adults are particularly susceptible to fraud and deception (Rabiner, Brown, & O’Keeffe, 2004), which potentially indicates that they are impaired in processing person information. However, the few studies addressing potential age-related functional changes to mPFC that could inform this idea have shown patterns of both equivalent activity among younger and older adults (Beadle, Yoon, & Gutchess, 2012; Cassidy, Shih, & Gutchess, 2012; Gutchess, Kensinger, & Schacter, 2007) and under recruitment of mPFC in older adults relative to young (Gutchess, Kensinger, & Schacter, 2010; K. Mitchell et al., 2009; Moran, Jolly, & Mitchell, 2012) during socio-cognitive tasks (e.g., self-referencing and theory of mind), making it difficult to pinpoint the locus of age-related changes in recruitment of mPFC activity and related social cognition regions.
Decreased mPFC activity among older adults relative to young, however, may not necessarily reflect overall functional decline with age. One possible reason for reported under recruitment of mPFC in older adults may be due to changes in how social stimuli are processed. In both behavioral (Isaacowitz, Wadlinger, Goren, & Wilson, 2006; Mather & Carstensen, 2005; Spaniol, Voss, & Grady, 2008) and neuroimaging (Addis, Leclerc, Muscatell, & Kensinger, 2010; Leclerc & Kensinger, 2008, 2010; Mather et al., 2004) research, younger and older adults have displayed different responses to valenced stimuli, with younger adults displaying biases toward negative information, and older adults for positive material. Socioemotional Selectivity Theory (Carstensen, Isaacowitz, & Charles, 1999) suggests that with the perception that time is limited in one’s life, individuals emphasize socioemotional rather than knowledge acquisition goals. This changing emphasis promotes emotion regulation, or the extent to which one exerts control over emotional experiences. In turn, research shows that older adults may optimize their emotional experiences by emphasizing positive information, and this shift may have positive implications for social experiences and well-being (Carstensen, Pasupathi, Mayr, & Nesselroade, 2000). For example, older adults have reported fewer negative experiences than younger adults (Gross et al., 1997), participating more than younger adults in personally fulfilling activities, like spending time with close family (Fung, Carstensen, & Lutz, 1999). Age-related functional changes towards the processing of valenced social material would be consistent with the evidence for these different valence-based biases in younger and older adults. Thus, the valence of incoming social information may influence mPFC recruitment as a function of age.
Evidence of a “positivity bias” in aging has also been reflected in neural activity. When viewing emotional images, age-related valence-based reversals have emerged in ventral mPFC (vmPFC) (Leclerc & Kensinger, 2008, 2010). While younger adults activate vmPFC more for negative over positive images, the reverse bias is apparent with age, with older adults activating vmPFC more for positive than negative emotional material. A similar pattern of activity has been shown in the amygdala (Mather et al., 2004), demonstrating that an age-related shift in neural activity may occur in multiple regions involved in emotional processing. Thus, as individuals emphasize more positive socioemotional information with age, the brain regions supporting these social and emotional processes may also become more responsive to this content.
Based on the “positivity bias” literature, age-related functional under recruitment of mPFC may reflect processing differences in stimulus content across age rather than a generalized functional impairment in older adults. An open question regards whether the age-related positivity biases extend from emotional to social tasks. While the nature of social interactions may contain intrinsically emotional information (Kensinger & Gutchess, in press), recent work posits that while emotional information is processed relatively automatically due to its biological relevance, the processing of social information may be more resource intensive (Sakaki, Niki, & Mather, 2012). Thus, while the age-related positivity biases that are elicited in emotional tasks involving non-human but valenced stimuli (e.g., a gun or diamond ring) may be attributed to a relatively automatic affective response to stimuli, it is unclear whether those biases extend to a higher order social task. While still containing emotional information, social tasks may require more controlled utilization of resources (e.g., thinking about meeting someone for the first time).
An extension of this age-related positivity bias to a socio-cognitive task would provide a possible explanation for the general inconsistency of reported age-related mPFC function in the social domain. Moran and colleagues (2012), for instance, found decreased dmPFC activity in older adults during moral permissibility ratings (e.g., rating the permissibility of putting poison in another person’s coffee by accident or on purpose). That investigation, however, used stimulus materials that were only negative or neutral in valence. In contrast with this finding, other work has found that younger and older adults similarly recruit mPFC when more socioemotionally meaningful aspects of stimuli are explicitly involved, such as when emphasizing potential relationships that involve the participant (Cassidy et al., 2012), or asking participants to make self-relevant judgments (Gutchess et al., 2007). If older adults emphasize negative information less than the young, then decreased dmPFC function might be expected in tasks that primarily utilize negative stimuli, possibly representing less task engagement. If, however, the age-related shift from negativity toward positivity biases demonstrated in the emotion literature is evident in socio-cognitive tasks, an age-related shift toward positivity biases may be reflected in neural recruitment during a social task. Impression formation engages both dmPFC and vmPFC, among other regions (Ma et al., 2011). Because mPFC is consistently recruited in tasks during which individuals form impressions from face-behavior pairs (Cassidy et al., 2012; Gilron & Gutchess, 2012; J. Mitchell et al., 2004; J. Mitchell, Macrae, & Banaji, 2005), studying impression formation might be particularly well suited to highlight age-related valence-based reversals in both vmPFC activity, as previously shown during emotional tasks (Leclerc & Kensinger, 2008, 2010), and in dmPFC, a region whose recruitment may be more unique to socio-cognitive tasks (Van Overwalle, 2009). Evidence of these biases within the neural regions supporting impression formation may indicate that age-related valence-based reversals may not only be elicited during simple affective processes, but also may manifest for higher-order social cognitive tasks.
While we predict age-related reversals in activity using valenced information in vmPFC and dmPFC, it’s also possible that these patterns of activity will exist in other brain regions supporting impression formation, similar to how valence-based reversals in the processing of emotional material have also been evidenced in the amygdala (Mather et al., 2004). Recent work has demonstrated that amygdala and posterior cingulate cortex (PCC) subserve different aspects of impression formation from the more general person processing supported by the dmPFC, and specifically, separating evaluation-relevant from irrelevant behavioral information about new people (Schiller, Freeman, Mitchell, Uleman, & Phelps, 2009). Other work suggests that age differences in PCC activity may depend on whether the context in which information is presented appeals to an age group’s social goals (Cassidy et al., 2012), consistent with Socioemotional Selectivity Theory. We predicted that, similar to vmPFC and dmPFC, age-related valence-based reversals in neural activity might be present in PCC and amygdala during impression formation
To summarize, we investigated age differences in mPFC recruitment during impression formation of positive and negative materials to determine whether age-related valence-based reversals in activity extend from simple affective tasks to higher-order social processing tasks. We expected that valence-based reversals would occur in vmPFC and dmPFC, and also expected the same patterns of activity to occur in the amygdala and PCC. More specifically, we predicted that negative over positive person information would engage these regions in younger adults, while older adults would recruit these regions more for positive over negative material.
Method
Participants
Eighteen older (66-87 years old, 12 females; M = 75.56, SD = 7.76) and 19 younger adults (19-35 years old, 11 females; M = 24.32, SD = 4.61) recruited from Brandeis University and the surrounding community participated, and provided written informed consent. The Brandeis University and Partners Healthcare institutional review boards approved this study. All older adults had MMSE scores > 26 (Folstein, Folstein, & McHugh, 1975) (M = 29.06, SD = 1.11), and were characterized on cognitive measures to ensure comparability to other older adult samples in the literature. Young (M = 15.55, SD = 1.59) and older (M = 15.42, SD = 2.33) adults did not differ in years of education, t(35) = 0.21, p = 0.84. Older adults (M = 36.94, SD = 3.08) had higher vocabulary scores (Shipley, 1986) than the young (M = 33.63, SD = 3.30), t(35) = 3.15, p = 0.003, whereas the young showed faster processing speed (M = 79.74, SD = 15.31) than the older adults (M = 54.94, SD = 8.65), t(35) = 6.02, p < 0.001, as measured by digit-comparison (Hedden et al., 2002) and letter-number sequencing scores (Wechsler, 1997) (young: M = 11.53, SD = 2.84; older adults: M = 9.44, SD = 2.53) (t(35) = 2.35, p = 0.02).
Stimuli
168 images of Caucasian faces (evenly distributed across male/female and four age groups [18-29, 30-49, 50-69, and 70-94]) with neutral expressions were drawn from the Productive Aging Laboratory face stimuli database (Minear & Park, 2004) as well as 168 unique behavioral sentences (Somerville, Wig, Whalen, & Kelley, 2006) rated on valence, served as stimuli in this experiment. Equal numbers (56 each) of positive (e.g., “This person is a loyal friend.”), negative (e.g., “This person has a violent temper.”), and neutral (e.g., “This person uses blue pens.”) behaviors were selected from the original dataset of 185 behaviors based on valence ratings. The ratings were obtained for a previous study (exact instructions can be found in Somerville et al., 2006) using a 9-point scale (−4 (very negative) to 4 (very positive)). To choose the 168 behaviors for the study, the full set of 185 behaviors was sorted in order by average valence rating. The 56 most negatively rated behaviors were selected as “negative,” the 56 most positively rated behaviors were selected as “positive,” and the 56 behaviors closest to the middle in ratings were selected as “neutral.” Assignment of faces to the valence conditions was counterbalanced across participants so that the faces appeared in each of the three conditions, totaling three versions of the task. Equal numbers of behaviors in the three valence conditions appeared across the age and gender groups. The stimuli were split into two blocks of 84 face-behavior pairs each, and the age/gender/valence distribution was equivalent between both blocks.
Procedure
Prior to scanning, participants practiced the encoding (i.e., impression formation) and retrieval (i.e., memory) tasks on a laptop. Participants were told they would form impressions of individuals based on face-behavior pairs, and that they should focus on how they felt about each face-behavior pair as if meeting this person for the first time. Participants also knew they would be tested on their memory for each person’s exact behavior, and also for the valence (e.g. positive, negative, neutral) of that behavior, intended to elicit intentional encoding. All stimuli were presented with E-Prime software (Psychology Software Tools, Pittsburgh, PA).
Participants formed impressions based on face-behavior pairs (Figure 1), presented one at a time for 5000 msec each. A prompt indicating valence (“positive, “negative,” or “neutral”) appeared below the behavior for the last 3500 msec of each trial. Participants were told this indicated how most people felt about this behavior. Participants were instructed to press “1” once after reading both pieces of information, so that one button press was required per trial, to maintain attention while in the scanner. Trials were interspersed with variable fixations ranging from 0 to 12,500 msec to introduce jitter into the event-related design (Dale & Buckner, 1997). Fixation intervals were optimized using Optseq (http://surfer.nmr.mgh.harvard.edu/opt-seq). Positive, negative, and neutral face-behavior pairs were randomly distributed across six functional runs with 28 face-behavior pairs in each run. Runs lasted 4.67 minutes (112 TRs), for a total of approximately 28 minutes of functional scans.
Figure 1.
Examples of positive and negative face-behavior stimuli.
After three encoding runs, participants completed two self-paced retrieval tasks (non-scanned, but completed inside the scanner), followed by another set of three encoding and two non-scanned and self-paced retrieval runs (completed outside the scanner). During retrieval, participants saw every face from the study phase and had to separately endorse the valence of each person’s behavior, as well as each person’s exact behavior (choosing a target among three valence-matched lures). Near-chance memory performance in both younger and older participants (i.e., 12 of 19 younger and 4 of 18 older adults participants’ memory performance was above chance for all conditions) prevented a thorough examination of encoding-specific neural response, and the memory data will not be considered further.
Image Acquisition and Analysis
Functional scans were collected with a Siemens Trio 1.5T whole-body scanner, using an echo-planar imaging sequence (TR=2500msec, TE=40msec, FOV=200mm, flip angle=90) to acquire 33 AC/PC oriented slices 3.0mm thick with a 10% skip. Voxel-wise data was collected in 3.1mm in 3.1mm by 3.2mm resolution. Stimuli were back projected onto a screen, and viewed through a mirror attached to the headcoil. High-resolution anatomical images were acquired with a multi-planar rapidly acquired gradient echo (MP-RAGE) sequence (128 slices, 1.33mm thick, TR=2730 msec, TE=3.39 msec).
Preprocessing and analyses of functional data were conducted in SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK). Images were slice-time corrected, realigned to correct for motion, normalized to the MNI (Montreal Neurological Institute) template, and smoothed using a 6-mm FWHM isotropic Gaussian kernel. For each participant, each event (whether forming impressions of positive, negative, or neutral face-behavior pairs) was convolved with a canonical hemodynamic response function. Estimates for each participant were included in a group level analysis, treating participants as a random effect. A 2 (Age Group: Younger, Older) × 3 (Valence: Positive, Negative, Neutral) ANOVA model was created to explore any effects of valence during impression formation differing by age group. We used a whole-brain analysis with an uncorrected threshold of p < 0.001 and an extent threshold of 5 voxels (comparable to other aging-related fMRI work (Duarte, Henson, & Graham, 2008; Dulas & Duarte, 2011)) to assess potential age-related valence-reversals in neural activity. To do this, we investigated the age by valence interaction during the formation of positive versus negative impressions (Positive > Negative, Old > Young). Locations of peak activation on the cortical surface were identified using SPM8, and Brodmann areas were obtained with MRIcron (Rorden & Brett, 2000). Based on the whole-brain analysis, we chose to further characterize the pattern of valence effects based on regions previous implicated in the impression formation literature by extracting parameter estimates from significant clusters within mPFC, PCC, and the amygdala, and plotting them, where relevant. We also used a whole-brain analysis to examine age differences on valenced (positive and negative) versus neutral impressions (Positive + Negative > Neutral, Old > Young). There were no significant effects within our regions of interest when comparing against neutral items, thus the neutral condition will not be discussed further.
Results
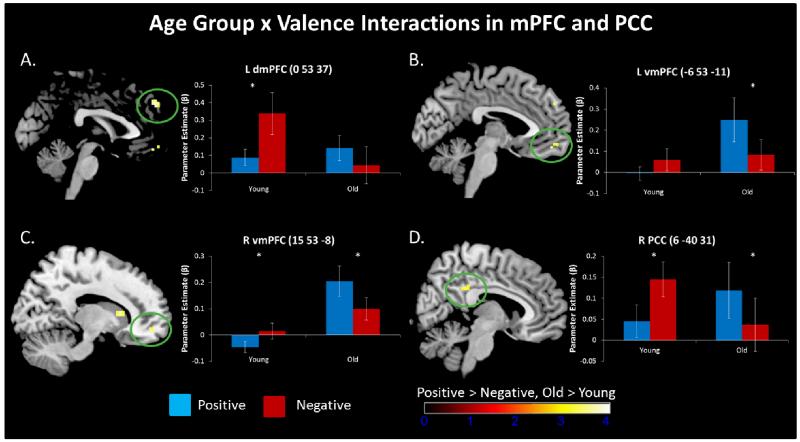
To investigate age-related changes in response to valence, we contrasted younger and older adults’ activations while forming positive or negative impressions of others based on the face-behavior pairs (Table 1). Based on SPM contrasts, age group by valence interactions emerged in left dmPFC and bilateral vmPFC. Follow-up statistical tests on these clusters’ parameter estimates, to characterize the nature of the interaction, revealed that age-related valence-based reversals in neural activity extended to dmPFC recruitment during impression formation, as evidenced by an age group by valence interaction in left dmPFC, F(1, 35) = 14.20, p = 0.001, ηp2 = 0.29 (Figure 2a). Specifically, younger adults recruited left dmPFC more for negative than positive impressions, t(18) = 4.03, p = 0.001, whereas older adults did not differ across valences, t(17) = 1.43, p = 0.17 (although there was a visual trend for greater dmPFC activity for positive over negative impressions). The interaction in the left vmPFC, F(1, 35) = 9.71, p < 0.005, ηp2 = 0.22 (Figure 2b), was characterized by greater activity for positive than negative impressions in the older adults, t(17) = 2.96, p = 0.01. Departing from previous findings using emotional stimuli (Leclerc & Kensinger, 2008, 2010), however, younger adults did not show valence differences in this region, t(18) = 1.32, p = 0.20. There was no main effect of age in left dmPFC and left vmPFC activity when collapsed across valence, Fs < 2.37, ps > 0.13. Unlike left dmPFC and vmPFC, older adults showed enhanced activity relative to younger adults in right vmPFC, F(1, 35) = 10.98, p = 0.002, ηp2 = 0.24. Activity across the age groups again varied by valence, F(1, 35) = 12.20, p = 0.001, ηp2 = 0.26 (Figure 2c). Characterizing this interaction, older adults recruited right vmPFC more when forming positive over negative impressions t(17) = 2.71, p = 0.02, while younger adults had enhanced engagement when forming negative over positive impressions, t(18) = 2.17, p = 0.04, consistent with previous findings in emotional stimuli (Leclerc & Kensinger, 2008, 2010).
Table 1. Age differences in response to forming impressions of positive versus negative face-behavior pairs.
| Age (Younger, Older) × Valence (Positive, Negative) Interactions | ||||||
|---|---|---|---|---|---|---|
| Region | BA | k | Activation peak (x, y, z) | t-value | ||
| L dorsomedial prefrontal cortex | 9 | 12 | 0 | 53 | 37 | 3.76 |
| L ventromedial prefrontal cortex | 11 | 7 | −6 | 53 | −11 | 3.59 |
| R ventromedial prefrontal cortex | 11 | 8 | 15 | 53 | −8 | 3.74 |
| L middle orbitofrontal gyrus | 47 | 43 | −33 | 50 | −5 | 4.09 |
| L ventromedial prefrontal cortex | 11 | −27 | 56 | −5 | 3.43 | |
| L parahippocampal gyrus | 35 | 6 | −15 | −19 | −20 | 4.37 |
| R posterior cingulate gyrus | 23 | 5 | 6 | −40 | 31 | 3.68 |
| R hippocampus/amygdala | 18 | 27 | −10 | −8 | 3.76 | |
| L inferior frontal cortex | 48 | 12 | −42 | 11 | 19 | 4.31 |
| L middle temporal gyrus | 21 | 22 | −60 | −34 | −2 | 4.10 |
| L middle temporal gyrus | 21 | −54 | −34 | −8 | 3.78 | |
| L anterior cingulate gyrus | 11 | 19 | −9 | 38 | −2 | 4.03 |
| R caudate | 26 | 15 | 17 | 10 | 3.79 | |
| R middle frontal gyrus | 47/10 | 28 | 27 | 47 | 7 | 3.74 |
| R superior temporal sulcus | 21 | 7 | 54 | −31 | 1 | 3.53 |
| L hippocampus | 7 | −33 | −10 | −20 | 3.50 | |
| R superior temporal sulcus | 22 | 7 | 57 | −4 | −11 | 3.44 |
Note: The data show regions emerging in the contrast with an overall threshold of p < 0.001 and an extent threshold of 5 voxels. Regions listed without a cluster size are subsumed by the larger cluster listed directly above. mPFC regions are listed first due to a priori hypotheses. Other regions are listed from highest to lowest t-value. L = Left; R = right; k = cluster size
Figure 2.
Age differences in response to forming impressions of positive and negative face-behavior pairs in mPFC and PCC are depicted on a standard brain in MNI space (uncorrected threshold of p < 0.001, 5 contiguous voxels). ROI bar graphs characterize activatio n maps, reflecting peak activation in each brain region categorized by age group and valence plotted in arbitrary units. Valence reversals in neural response across age were found in left dmPFC (A), left vmPFC (B), right vmPFC (C), and right PCC. Error bars represent standard error. *p < 0.05
Based on SPM contrasts, a significant age by valence interaction was also evident in a cluster overlapping a region in the right hippocampus, extending into the amygdala, F(1, 35) = 12.22, p = 0.001, ηp2 = 0.26. Using parameter estimates extracted from this cluster to characterize the interaction, we saw that similar to the interactions in mPFC, older adults showed enhanced activity when forming positive over negative impressions t(17) = 2.69, p = 0.02, while younger adults showed increased engagement for negative over positive impressions, t(18) = 2.12, p = 0.04. There was no main effect of age within this cluster, F(1, 35) = 0.28, p = 0.60. A significant age by valence interaction was also found in a small cluster within right PCC, F(1, 35) = 15.93, p < 0.001, ηp2 = 0.31 (Figure 2d). Older adults recruited right PCC more when forming positive over negative impressions t(17) = 2.68, p = 0.02, while younger adults had enhanced engagement when forming negative over positive impressions, t(18) = 2.97, p = 0.01. Overall activity within this PCC cluster did not vary by age, F(1, 35) = 0.06, p = 0.81. Other regions showing significant age by valence interactions are reported in Table 1.
Discussion
Neuroimaging studies of emotional processing have demonstrated age differences in neural recruitment depending on the valence of incoming emotional material. Within vmPFC (Leclerc & Kensinger, 2008, 2010) and the amygdala (Mather et al., 2004) younger adults have increased activity toward negative over positive information, while older adults elicit enhanced recruitment for positive over negative material. These findings may be indicative of an age-related “positivity effect” in information processing (Carstensen & Mikels, 2005), potentially showing that rather than overall reduced activity with age, some neural regions may become more responsive to positive relative to negative materials (Mather et al., 2004). While emotion work evidencing age-related valence-based reversals in vmPFC activity (Leclerc & Kensinger, 2008, 2010) may reflect automatic processing due to the biological relevance of emotional stimuli, evaluating social stimuli (e.g., forming impressions) may require more complex cognitive computations (Sakaki et al., 2012). Through evidence for an age-related valence-based reversal in mPFC function during the social cognitive task of impression formation, which involves deriving meaning from human behaviors versus basic affective reactions, we suggest that these valence-based biases exist for complex social processes. Showing that older adults may activate mPFC more in the presence of positive versus negative social information may offer a potential explanation for inconsistencies within age-related mPFC function, which have reported age-equivalent activity (Beadle et al., 2012; Cassidy et al., 2012; Gutchess et al., 2007) and under recruitment (Gutchess et al., 2010; Moran et al., 2012) with age, but have not all utilized both positive and negative stimuli. Critically, we find that with aging, dmPFC and vmPFC can be robustly recruited during socio-cognitive tasks particularly in the presence of positive versus negative social material, indicating a marked shift with aging in the circumstances under which these regions are engaged. This demonstrates that age differences in recruitment may not only exist for basic affective responses to emotional images, but may importantly extend to higher order social processes, influencing our interactions with others (Ambady & Rosenthal, 1992). Not all social tasks involve the presence of both positive and negative information and can compare mPFC response across valence, potentially giving rise to inconsistent reports in the literature of age-related mPFC function. An age-related shift towards greater mPFC response toward positive over negative person information may critically impact the interpretation of aging data from tasks measuring response from negative stimuli (e.g., task 1, Moran et al., 2012). If older adults’ prioritization of positive social information is reflected in biased mPFC activity, older adults may be expected to have deficits in neural activity relative to young in tasks focusing on negative material, especially if the lack of a positive condition limits the ability to compare activity across valence. Such results must be interpreted cautiously, as extant age-related deficits in activity may be more complicated given the broad literature describing the presence of a potential “positivity bias” with age (Reed & Carstensen, 2012).
Notably, the relationship between valence and age in dmPFC appeared to be largely driven by younger adults’ negativity biases (Murphy & Isaacowitz, 2008; Skowronski & Carlston, 1989), whereas in vmPFC, this relationship was driven by older adults’ positivity biases. Although these relationships are consistent with behavioral evidence showing a negativity bias within younger adults for a number of social domains, including impression formation and moral judgment (Baumeister, Bratslavsky, Finkenauer, & Vohs, 2001), and more emphasis on positive and emotionally meaningful material (Carstensen & Mikels, 2005) with increasing age, it may be that younger and older adults approached impression formation in the current task using different strategies. Biases engaging a region in response to valenced information may be particularly strong within an age group depending on the strategy deployed. Supporting this idea, previous work illustrates that that people recruit vmPFC when thinking about similar others or themselves, as in self-reflective (Jenkins & Mitchell, 2011; Johnson et al., 2002) or self-referencing tasks (Kelley et al., 2002; Macrae, Moran, Heatherton, Banfield, & Kelley, 2004). Thinking about more distant others, however, largely recruits dmPFC, such as when we watch strangers’ social interactions (Iacoboni et al., 2004), or form impressions of abstract others (Gilron & Gutchess, 2012).
This dissociation in dorsal and ventral mPFC activity when mentalizing about close and distant others has been documented within the same study (J. Mitchell, Macrae, & Banaji, 2006), suggesting that in some situations, perceivers use information about themselves to make social evaluations. If older adults engage in more self-reflection than young when forming impressions, their positivity biases may be best reflected by increased vmPFC activity, similar to work illustrating that making judgments about own-versus other-age individuals results in enhanced vmPFC activity in both younger and older adults (Ebner et al., 2011; Ebner et al., 2013). In contrast, if younger adults approach the task in the traditionally more abstract way, such that they are acquiring impressions of strangers, their emphasis on negative information during impression formation might be best represented within dmPFC activity. These possibilities would be consistent with Socioemotional Selectivity Theory (Carstensen et al., 1999), in that older adults may use self-reflection to enhance their socioemotional experience, while younger adults may attempt to form as many impressions as possible for their knowledge acquisition goals. To test this intriguing possibility, future research may want to consider older and younger adults’ mPFC recruitment when mentalizing about similar and dissimilar others within the same task. It may be that older adults’ mPFC activity is most preserved relative to young when orienting to person information in a self-reflective way. Another way to test this idea could be to assess age differences in mPFC activity by comparing activity during impression formation when focusing on a potential personal connection to an individual versus treating an individual as a hypothetical other who will never be met. Such an experiment would assess whether age-related valence-based biases can be represented in either dorsal or ventral mPFC depending on how individuals orient to a task.
Recent work examining age differences in recognizing facial expressions (Ebner, Johnson, & Fischer, 2012; Keightley, Chiew, Winocur, & Grady, 2007) may highlight another potential mechanism for why older adults’ positivity bias was driven by vmPFC response, and younger adults’ negativity bias was driven by dmPFC engagement. Both younger and older adults show similar enhanced vmPFC engagement, known to be associated with processing happy faces (Keightley et al., 2007), in response to viewing positive versus negative expressions (Ebner et al., 2012). This potentially reflects vmPFC’s role in representing the rewarding nature of positive stimuli (O’Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001). It could be that an age-related positivity bias, reflected by vmPFC activity, represents reward value for positive information leading to first impressions, similar to work showing that older adults are more likely to learn by seeking gains while younger adults learn by avoiding losses (Denburg, Recknor, Bechara, & Tranel, 2006). In contrast, both younger and older adults show similarly increased dmPFC response toward processing negative over positive facial expressions (Ebner et al., 2012), which may reflect the increased complexity of social processes (Van Overwalle, 2009) necessary to identify and derive meaning from negative stimuli. It could be that a negativity bias driven by dmPFC activity among younger adults represents a prioritization of processing complex material during impression formation in the pursuit of knowledge-related goals.
These differences may also help explain why, divergent with previous work in finding age-based reversals (Leclerc & Kensinger, 2008, 2010), only older adults showed a valence-based bias in left vmPFC, while consistent with prior work, both age groups showed valence-based biases in right vmPFC. It could be that valence-based biases reflected in vmPFC activity present themselves bilaterally in older adults given that they may have used multiple task strategies (e.g., reward processing of positive social information and self-reflection) known to involve this region relative to younger adults. To clarify the mechanism behind this dissociation, future work could directly compare younger and older adults’ activity while they use different task strategies. This would allow for the assessment of the conditions under which mPFC activity during impression formation is preserved with age. For instance, it could be that older adults have a positivity bias in vmPFC activity for person information because that information is inherently rewarding. If, however, older adults anticipate a reward for remembering a subset of positive and negative impressions, a positivity bias will be eliminated, as both positive and negative behaviors will have explicit reward value. Moreover, by comparing age differences in mPFC response when using isolated (e.g., reward anticipation) or combined (e.g., reward anticipation and self-reflection) task strategies during impression formation, we can highlight the processes that may underlie valence-based biases in bilateral recruitment of vmPFC in older adults, but unilateral vmPFC and dmPFC activity in young.
Despite evidence of age-related valence-based reversals in mPFC in the current task, overall activity collapsed across valence was similar for younger and older adults, and in the case of right vmPFC, appeared to be enhanced in older adults relative to young. While speculative, our results may illustrate the important role of personal meaningfulness (i.e., the extent to which information can be connected or related to oneself) in mPFC activation among older adults. Interestingly, one difference between tasks demonstrating age-equivalence (Beadle et al., 2012; Cassidy et al., 2012; Gutchess et al., 2007) and under recruitment (Moran et al., 2012) in activations is that tasks eliciting age-equivalent activity require participants to place themselves within the task. It could be that positive and negative valences may motivate younger and older adults differently, leading to enhanced activity among older adults under conditions in which they find information more personally meaningful (i.e. when incoming information is positive), and thus prioritize information processing. Future research may consider explicitly manipulating the personal meaningfulness of social tasks when assessing age differences in mPFC response to valenced material.
Although age-related shifts in neural response to positive and negative social information seem to exist, these results may not fully account for why some studies find preserved mPFC function and some do not. For example, older and younger adults may have dissimilar internal goals (e.g., processing socioemotional information versus acquiring knowledge) when performing a task, leading to age differences in neural activity. Additionally, depending on task difficulty or processing demands, older adults may show mPFC functional impairment relative to younger adults regardless of the effects of valence. For instance, although both age groups similarly activate mPFC when self-referencing (Gutchess et al., 2007), they may show different patterns of activity in the mPFC in the more difficult task of subsequently remembering self-versus other-related information (Gutchess et al., 2010). Supporting this idea, age-equivalent activation of mentalizing circuitry has been observed during a fairly easy mentalizing task allowing for near-ceiling performance levels among younger and older adults (Castelli et al., 2010), whereas for more taxing mentalizing tasks where age-related performance differences exist, older adults exhibited impaired dmPFC function (Moran et al., 2012). Although age-related valence-based reversals in vmPFC function in emotion processing have been seen in implicit (Leclerc & Kensinger, 2008) and more challenging explicit (Leclerc & Kensinger, 2010) tasks, more research is needed to determine how the age-related reversals elicited in the current task would translate to more difficult socio-cognitive tasks requiring controlled processing.
Although an age-related shift toward emphasizing positive over negative information may be protective in some respects (e.g., maintaining positive affect given the more negative aspects of healthy aging, like physical decline), it may have potentially detrimental consequences for social functioning. Federal government reports (Lormel, 2001; Telemarketing fraud against older Americans, 2007) suggest that placing more weight on positive information, and in turn having increasingly positive responses to others, may in part explain older adults’ elevated susceptibility to fraud compared to younger adults. Supporting this idea, older adults perceive negative facial cues (e.g. untrustworthy-looking faces) more positively than younger adults (Castle et al., 2012; Zebrowitz, Franklin, Hillman, & Boc, 2013), and do not display enhanced left anterior insula response to untrustworthy over trustworthy-looking faces like younger adults (Castle et al., 2012). This suggests a potentially weaker response among older adults to implicit negative social cues derived from facial characteristics. Evidence of age differences in neural response toward valenced behaviors could possibly inform why older adults are more vulnerable to fraud than younger adults. The current study suggests that older adults may show increased activity toward explicitly positive relative to negative behaviors, rather than solely attenuated response to negative behaviors, compared to negativity biases in younger adults. In combination, these effects may skew older adults’ perception of potentially deceptive individuals and situations, providing the conditions under which older adults are most likely to fall victim to fraud. It would be interesting to test if these biases not only exist when processing valenced social material, but also for when remembering this information. It may be that older adults’ pattern of greater mPFC activity for positive over negative information extends to the successfully encoding of positive over negative information. Although poor performance on the memory component of the current study prevents an investigation of this idea, it remains an important topic warranting further research in order to generate a more comprehensive theory behind age differences in fraud vulnerability.
An age-related reversal in neural activity toward valenced information was also present in right PCC. Previous work (Schiller et al., 2009) indicates that while dmPFC activity supports the processing of person information, PCC and amygdala engagement may more specifically contribute to separating evaluation-relevant from irrelevant information. Although not primarily designed to assess the valuation of person information, our results may provide preliminary evidence that valence-based reversals reflect how individuals value valenced information with age, providing an exciting avenue for future research. One cluster did extend into the amygdala, although peak activity did not occur within the region. Further, our coordinates in that region were more dorsal than those previously specified (Schiller et al., 2009), limiting our ability to speculate on the region’s contribution to valence-based age differences in neural activity during impression formation. A study design exploring how individuals value valenced information may also be helpful to demonstrate that this reversal can be represented more specifically in the amygdala. For instance, a study could examine how individuals update their impressions based on incoming positive and negative material. If more value is assigned to positive over negative material with age, we might expect older adults to have greater amygdala activity when positively updating impressions, while younger adults might have greater activity when negatively updating impressions. Alternatively, similar to work finding intact striatal and insular activity in response to monetary gain, but not loss, anticipation with age (Samanez-Larkin et al., 2007), older adults may show intact amygdala activity in anticipation of positive versus negative social interactions if positive social information is more valued overall in healthy aging.
Although not considered for the presented analyses, an important caveat of the present work is that participants anticipated a later test of impression memory. Although our task elicited mPFC activity similar to other impression formation tasks (Cassidy et al., 2012; Gilron & Gutchess, 2012), awareness of a memory task may have changed the strategies used to form impressions, making impression formation less spontaneous than simple evaluation or judgment tasks (Uleman, 1999). Our instructions were more akin to intentional impression formation tasks, in which individuals are instructed to form impressions (Uleman, 1999). Notably, however, both spontaneous and intentional impressions can evoke with mPFC activity (Ma et al., 2011); the idea that age-related valence-based reversals in mPFC activity occurred even though participants intentionally formed impressions for a later memory test potentially speaks to the robustness of this finding. However, demonstrating whether this reversal in neural activity is present during more traditional spontaneous impression formation tasks is an important step to better connect this finding to behavioral work on impression formation.
In conclusion, consistent with evidence of a “positivity bias” in aging (Carstensen & Mikels, 2005), we demonstrate that the age-related valence-based reversals present in vmPFC during emotional processing extend to dmPFC, and persist in vmPFC, when forming impressions, showing that age differences in mPFC activity exist for higher order social processes, like interpreting person information. In addition, these age-related reversals in neural activity extend to the amygdala and PCC, other regions implicated in impression formation. These findings may in part resolve prior inconsistency within the literature on the effects of aging on mPFC function by highlighting the important influence of valence on mPFC recruitment.
Acknowledgments
This work was supported by the National Institute on Aging grant R21 AG032382 (to A.H.G.), a National Science Foundation graduate fellowship (to B.S.C.), and by the National Institutes on Health grant T32 AG000204-21 (supporting E.D.L.) This research was carried out at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41RR14075, a P41 Regional Resource supported by the Biomedical Technology Program of the National Center for Research Resources (NCRR), National Institutes of Health. We thank Sarah Huff, Chad Nussinow, and Rebekah Lafontant for research assistance.
References
- Addis DR, Leclerc CM, Muscatell K, Kensinger EA. There are age-related changes in neural connectivity during the encoding of positive, but not negative, information. Cortex. 2010;46(4):425–433. doi: 10.1016/j.cortex.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambady N, Rosenthal R. Thin slices of expressive behavior as predictors of interpersonal consequences: a meta-analysis. Psychological Bulletin. 1992;111(2):256–274. [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5:323–370. [Google Scholar]
- Beadle JN, Yoon C, Gutchess AH. Age-related neural differences in affiliation and isolation. Cognitive, Affective, and Behavioral Neuroscience. 2012;12(2):269–279. doi: 10.3758/s13415-012-0085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: a theory of socioemotional selectivity. American Psychologist. 1999;54(3):165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA. At the intersection of emotion and cognition. Current Directions in Psychological Science. 2005;14(3):117–121. [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult lifespan. Journal of Personality and Social Psychology. 2000;79:644–655. [PubMed] [Google Scholar]
- Cassidy BS, Shih J, Gutchess AH. Age-related changes to the neural correlates of social evaluation. Social Neuroscience. 2012;7(6):552–564. doi: 10.1080/17470919.2012.674057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli I, Baglio F, Blasi V, Alberoni M, Falini A, Liverta-Sempio O, Marchetti A. Effects of aging on mindreading ability through the eyes: An fMRI study. Neuropsychologia. 2010;48(9):2586–2594. doi: 10.1016/j.neuropsychologia.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Castle E, Eisenberger NI, Seeman TE, Moons WG, Boggero IA, Grinblatt MS, Taylor SE. Neural and behavioral bases of age differences in perceptions of trust. Proceedings of the National Academy of Sciences. 2012;109(51):20848–20852. doi: 10.1073/pnas.1218518109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Human Brain Mapping. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Recknor EC, Bechara A, Tranel D. Psychophysiological anticipation of positive outcomes promotes advantageous decision-making in normal older persons. International Journal of Psychophysiology. 2006;61(1):19–25. doi: 10.1016/j.ijpsycho.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Duarte A, Henson RNA, Graham KS. The effects of aging on the neural correlates of subjective and objective recollection. Cerebral Cortex. 2008;18:2169–2180. doi: 10.1093/cercor/bhm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. The effects of aging on material-independent and material-dependent neural correlates of contextual binding. Neuroimage. 2011;57:1191–1204. doi: 10.1016/j.neuroimage.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Ebner NC, Gluth S, Johnson MR, Raye CL, Mitchell KJ, Johnson MK. Medial prefrontal activity when thinking about others depends on their age. Neurocase: The Neural Basis of Cognition. 2011;17(3):2011. doi: 10.1080/13554794.2010.536953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Johnson MK, Fischer H. Neural mechanisms of reading facial emotions in young and older adults. Frontiers in Psychology. 2012;3(223) doi: 10.3389/fpsyg.2012.00223. doi: 10.3389/fpsyg.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Johnson MR, Rieckmann A, Durbin KA, Johnson MK, Fischer H. Processing own-age vs other-age faces: Neuro-behavioral correlates and effects of emotion. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fung HH, Carstensen LL, Lutz AM. Influence of time on social preferences: implications for lifespan development. Psychology and Aging. 1999;14(595-604) doi: 10.1037//0882-7974.14.4.595. [DOI] [PubMed] [Google Scholar]
- Gilron R, Gutchess AH. Remembering first impressions: Effects of intentionality and diagnosticity of subsequent memory. Cognitive, Affective, and Behavioral Neuroscience. 12(1):85–98. doi: 10.3758/s13415-011-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Carstensen LL, Pasupathi M, Tsai J, Gotestam-Skorpen C, Hsu AYC. Emotion and aging: Experience, expression, and control. Psychology and Aging. 1997;12(4):590–599. doi: 10.1037//0882-7974.12.4.590. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter, Daniel L. Functional neuroimaging of self-referential encoding with age. Neuropsychologia. 2010;48:211–219. doi: 10.1016/j.neuropsychologia.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Aging, self-referencing, and medial prefrontal cortex. Social Neuroscience. 2007;2(2):117–133. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- Hedden T, Park DC, Nisbett R, Ji LJ, Jing Q, Jiao S. Cultural variation in verbal versus spatial neuropsychological function across the lifespan. Neuropsychology. 2002;16:65–73. doi: 10.1037//0894-4105.16.1.65. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Lieberman MD, Knowlton BJ, Molnar-Szakacs I, Moritz M, Throop CJ, Fiske AP. Watching social interactions produces dorsomedial prefrotal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage. 2004;21(3):1167–1173. doi: 10.1016/j.neuroimage.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye-tracking study. Psychology and Aging. 2006;21(1):40–48. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP. Medial prefrontal cortex subserves diverse forms of self-reflection. Social Neuroscience. 2011;6(3):211–218. doi: 10.1080/17470919.2010.507948. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125(8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Keightley ML, Chiew KS, Winocur G, Grady CL. Age-related differences in brain activity underlying identification of emotional expressions. Social, Cognitive, & Affective Neuroscience. 2007;2:292–302. doi: 10.1093/scan/nsm024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self?: An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Gutchess AH, editors. Memory for emotional and social information in adulthood and old age. Blackwell; Wiley: in press. [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cognitive, Affective, and Behavioral Neuroscience. 2008;8(2):153–164. doi: 10.3758/cabn.8.2.153. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related valence-based reversal in recruitment of medial prefrontal cortex on a visual search task. Social Neuroscience. 2010;5(5-6):560–576. doi: 10.1080/17470910903512296. [DOI] [PubMed] [Google Scholar]
- Lormel DM. Federal Bureau of Investigation. Fraud Against the Elderly. 2001 Retrieved from www.fbi.gov/news/testimony/fraud-against-the-elderly. [Google Scholar]
- Ma N, Vandekerckhove M, VanOverwalle F, Seurinck R, Fias W. Spontaneous and intentional trait inferences recruit a common mentalizing network to a different degree: Spontaneous inferences activate only its core areas. Social Neuroscience. 2011;6(2):123–138. doi: 10.1080/17470919.2010.485884. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefronal activity predics memory for self. Cerebral Cortex. 2004;14(6):647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner KN, Carstensen LL. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15(4):259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9(10):496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behavior Research Methods, Instruments, & Computers. 2004;36(4):630–633. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Encoding-Specific Effects of Social Cognition on the Neural Correlates of Subsequent Memory. Journal of Neuroscience. 2004;24(21):4912–4917. doi: 10.1523/JNEUROSCI.0481-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Forming impressions of people versus inanimate objects: social-cognitive processing in the medial prefrontal cortex. Neuroimage. 2005;26(1):251–257. doi: 10.1016/j.neuroimage.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Ebner NC, Tubridy SM, Frankel H, Johnson MK. Age-group differences in medial cortex activity associated with thinking about self-relevant agendas. Psychology and Aging. 2009;24(2):438–449. doi: 10.1037/a0015181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Jolly E, Mitchell JP. Social-cognitive deficits in normal aging. Journal of Neuroscience. 2012;32(16):5553–5561. doi: 10.1523/JNEUROSCI.5511-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy NA, Isaacowitz DM. Preferences for emotional information in older and younger adults: A meta-analysis of memory and attention tasks. Psychology and Aging. 2008;23(2):263–286. doi: 10.1037/0882-7974.23.2.263. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representation in the human orbitofrontal cortex. Nature Reviews Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Rabiner DJ, Brown D, O’Keeffe J. Financial exploitation of older persons: policy issues and reccommendations for addressing them. Journal of Elder Abuse & Neglect. 2004;16(1):65–84. [Google Scholar]
- Reed AE, Carstensen LL. The theory behind the age-related positivity effect. Frontiers in Psychology. 2012;3(339) doi: 10.3389/fpsyg.2012.00339. doi: 10.3389/fpsyg.2012.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioral Neurology. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Sakaki M, Niki K, Mather M. Beyond arousal and valence: The importance of the biological versus social relevance of emotional stimuli. Cognitive, Affective, and Behavioral Neuroscience. 2012;12(1):115–139. doi: 10.3758/s13415-011-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SEB, Khanna K, Nielsen L, Carstensen LL, Knutson B. Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience. 2007;10:787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA. A neural mechanism of first impressions. Nature Neuroscience. 2009;12:508–514. doi: 10.1038/nn.2278. [DOI] [PubMed] [Google Scholar]
- Shipley WC. Shipley Institute of Living Scale. Western Psychological Services; Los Angeles: 1986. [Google Scholar]
- Skowronski JJ, Carlston DE. Negativity and extremity biases in impression formation: a review of explanations. Psychological Bulletin. 1989;105(1):131–142. [Google Scholar]
- Somerville LH, Wig GS, Whalen PJ, Kelley WM. Dissociable Medial Temporal Lobe Contributions to Social Memory. Journal of Cognitive Neuroscience. 2006;18(8):1253–1265. doi: 10.1162/jocn.2006.18.8.1253. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Voss A, Grady CL. Aging and emotional memory: Cognitive mechanisms underlying the positivity effect. Psychology and Aging. 2008;23(4):859–872. doi: 10.1037/a0014218. [DOI] [PubMed] [Google Scholar]
- Telemarketing fraud against older Americans. 2007 Federal Trade Commission Retrieved from www.ftc.gove/reports/Fraud/fraudcon.shtm. [Google Scholar]
- Uleman JS. Spontaneous versus intentional inferences in impression formation. In: Chaiken S, Trope Y, editors. Dual-process theories in social psychology. Guilford Press; New York, NY US: 1999. pp. 141–160. [Google Scholar]
- Van Overwalle F. Social cognition and the brain: A meta-analysis. Human Brain Mapping. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler memory scale. Psychological corporation; San Antonio: 1997. [Google Scholar]
- Zebrowitz LA, Franklin RG, Hillman S, Boc H. Older and younger adults’ first impressions from faces: Similar in agreement but different in positivity. Psychology and Aging. 2013;28(1) doi: 10.1037/a0030927. [DOI] [PMC free article] [PubMed] [Google Scholar]