The vast majority of the electroencephalographic (EEG) activities that are examined during clinical EEG interpretation lie below 30 Hz. Studies have shown some time ago that discharges up to 120 Hz could be seen in intracerebral EEG studies at seizure onset (Fisher et al., 1992). Several reports also indicated that activity between 40 and 100 Hz was common during infantile spasms and could even be recorded from the scalp (Kobayashi et al., 2004; Akiyama et al., 2005). Several experimental and human studies from the University of California, Los Angeles (UCLA) group (Bragin et al., 1999; Staba et al., 2002, 2004; Engel et al., 2009 for review) have demonstrated that activity at frequencies from 100–500 Hz could be recorded with microelectrodes placed in mesial temporal structures and that some of these activities were characteristic of the region of seizure onset. Their work differentiated the “ripples,” brief runs of waves with a frequency of 100–200 Hz, which appear to represent normal physiological activity, from “fast ripples,” in the 200–500 Hz frequency range, present mostly in the seizure onset region. It was shown in experimental animals that the region generating these high frequency oscillations (HFOs) were very small, extending a few hundred microns at most (Bragin et al., 2002).
These interesting findings raised the question of the presence of such high-frequency activity in the intracerebral EEG at first, and possibly in the scalp EEG or magnetoencephalogram (MEG). The difference between a microelectrode and an EEG electrode is the surface of metal in contact with the brain. Given the presumed size of the generator (a few hundred microns) and the size of most EEG electrodes (several square millimeters) it appears unlikely that EEG electrodes could record ripples and fast ripples. A series of studies were performed at the Montreal Neurological Institute with EEG electrodes that are a little smaller (1 mm2) than most commercially available electrodes (4 mm2) but still hundreds of time larger than microelectrodes. These studies demonstrated that high-frequency activity up to 500 Hz can be recorded with EEG electrodes and that this activity is linked with the region generating seizures.
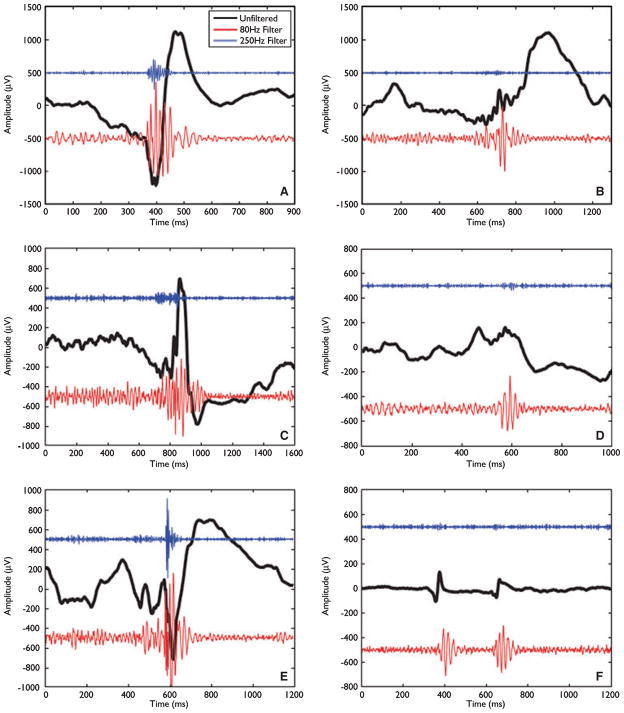
The first study demonstrated HFOs during epileptic seizures (Jirsch et al., 2006). High frequency activity, as brief oscillations or as prolonged discharges, was found most often in the electrodes where the seizure started and rarely in regions to which it propagated. Patients for whom the seizure onset had been missed (for instance if clinical signs preceded EEG changes) did not show HFOs. It was then demonstrated that interictal HFOs were also relatively frequent in the ripple and in fast ripples (FR) frequency bands (Urrestarazu et al., 2007). These oscillations occurred in three situations: (1) on top of traditional EEG spikes (as was most often the case for FRs recorded with micro-electrodes); (2) totally independently of spikes; (3) as a result of filtering a sharp spike. In both studies, HFOs were found in neocortical regions as well as in mesial temporal lobe structures. Examples of HFOs are shown in Fig. 1.
Figure 1.
Examples of high-frequency oscillations (HFOs) occurring at the same time as spikes (A,C,E, and possibly F) and outside of spikes (B,D). The black trace is the original electroencephalography (EEG). The red and blue traces result from high-pass filtering at 80 and 250 Hz, respectively. The amplitude for these traces has been multiplied by 10 to facilitate viewing. From Bagshaw et al. (2009).
Epilepsia © ILAE
It is known that the zone of interictal spiking and the seizure-onset zone are related to each other but the relationship is not always tight. By examining the spatial relationship between the seizure-onset zone, the region in which interictal spiking takes place, and the region in which HFOs are found, we could determine that HFOs have a tighter correspondence than interictal spikes with the seizure-onset zone (Jacobs et al., 2008). HFOs, therefore, appear to be a better candidate for a biomarker of ictogenesis than spikes. The importance of HFOs was further underlined when it was found that, in patients with lesions, HFOs were more closely coupled with the region of seizure onset than with the lesion, which is sometimes but not always the source of the seizures (Jacobs et al., 2009a).
If there is a good spatial correspondence between the region of seizure onset and HFOs, one can naturally wonder if there is also temporal coupling. A natural question is whether HFOs become more frequent as a seizure approaches. Khosravani et al. (2009) found that HFOs often increased in the few seconds immediately preceding a seizure. This study also demonstrated that HFOs could be recorded from commercially available subdural electrodes, with a surface contact of 4 mm2. When examining fluctuations in HFOs in the 15-, 5-, and 1-min intervals preceding seizure occurrence, Jacobs et al. (2009b) did not find any systematic change. These two studies indicate that if there is a change in HFOs prior to seizures, it is only immediately prior to their occurrence.
Looking at HFO fluctuations at the time scale of days in relation to seizure occurrence and to changing antiepileptic medication, Zijlmans et al. (2009) demonstrated that medication reduction results directly in an increase in the rate of HFO occurrence. Seizures, on the other hand, when they occurred in a context of stable medication, did not cause any change in HFO rates. This is in contrast to interictal spikes (Gotman & Marciani, 1985; Gotman & Koffler, 1989; Spencer et al., 2008), for which medication reduction does not result directly in an increased rate; seizures, however, are followed by increased spiking (this explains the often seen increase in spiking after medication withdrawal, as seizures occur more often and mediate the increase in spiking). Comparing HFOs and spikes, one, therefore, concludes that HFOs behave more like seizures than spikes do in the context of changing medication levels. Therefore, they may be a better marker of disease activity than spikes.
In conclusion, examining the EEG between 100 and 500 Hz may add clinically useful information to the classical interpretation of intracerebral EEGs. It is unlikely that such high-frequency activity can be recorded from the scalp or with MEG because the generator regions appear too small. However, this is worth investigating because the same reason had been invoked earlier for the unlikely possibility to record HFOs with intracerebral EEG electrodes, and was proven wrong. It is important to note that the skull does not filter out high frequencies (Oostendorp et al., 2000); it only makes their recording less likely by virtue of the distance it creates between a small generator and the scalp electrode, and because of its resistivity that attenuates an already small activity.
Footnotes
Disclosure
The author was a major shareholder of Stellate.
References
- Akiyama T, Otsubo H, Ochi A, Ishiguro T, Kadokura G, Ramachandrannair R, Weiss SK, Rutka JT, Carter Snead O., 3rd Focal cortical high-frequency oscillations trigger epileptic spasms: confirmation by digital video subdural EEG. Clin Neurophysiol. 2005;116:2819–2825. doi: 10.1016/j.clinph.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J. Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia. 2009;50:617–628. doi: 10.1111/j.1528-1167.2008.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern W. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid – treated rats with chronic seizures. Epilepsia. 1999;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Webber WR, Lesser RP, Arroyo S, Uematsu S. High-frequency EEG activity at the start of seizures. J Clin Neurophysiol. 1992;9:441–448. doi: 10.1097/00004691-199207010-00012. [DOI] [PubMed] [Google Scholar]
- Gotman J, Marciani MG. Electroencephalographic spiking activity, drug levels, and seizure occurrence in epileptic patients. Ann Neurol. 1985;17:597–603. doi: 10.1002/ana.410170612. [DOI] [PubMed] [Google Scholar]
- Gotman J, Koffler DJ. Interictal spiking increases after seizures but does not after decrease in medication. Electroencephalogr Clin Neurophysiol. 1989;72:7–15. doi: 10.1016/0013-4694(89)90026-6. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Châtillon CE, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009a;132:1022–1037. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Zelmann R, Jirsch J, Chander R, Dubeau CE, Gotman J. High frequency oscillations (80–500 Hz) in the preictal period in patients with focal seizures. Epilepsia. 2009b Mar 21; doi: 10.1111/j.1528-1167.2009.02067.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Khosravani H, Mehrotra N, Rigby M, Hader WJ, Pinnegar CR, Pillay N, Wiebe S, Federico P. Spatial localization and time-dependant changes of electrographic high frequency oscillations in human temporal lobe epilepsy. Epilepsia. 2009;50:605–616. doi: 10.1111/j.1528-1167.2008.01761.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Oka M, Akiyama T, Inoue T, Abiru K, Ogino T, Yoshinaga H, Ohtsuka Y, Oka E. Very fast rhythmic activity on scalp EEG associated with epileptic spasms. Epilepsia. 2004;45:488–496. doi: 10.1111/j.0013-9580.2004.45703.x. [DOI] [PubMed] [Google Scholar]
- Oostendorp TF, Delbeke J, Stegeman DF. The conductivity of the human skull: results of in vivo and in vitro measurements. IEEE Trans Biomed Eng. 2000;47:1487–1492. doi: 10.1109/TBME.2000.880100. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Goncharova II, Duckrow RB, Novotny EJ, Zaveri HP. Interictal spikes on intracranial recording: behavior, physiology, and implications. Epilepsia. 2008;49:1881–1892. doi: 10.1111/j.1528-1167.2008.01641.x. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J., Jr High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130:2354–2366. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72:979–986. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]