Abstract
Mesenchymal stem cell (MSC) therapy offers the potential to promote recovery after myocardial infarction (MI). However, therapeutic efficacy may be limited by poor survival and retention of transplanted cells. A combination of gene and cell therapy has the capacity to prevent donor cell death and augment the reparative and regenerative effects of cell transfer. The present study investigates the effect of exogenous heat shock protein 27 (Hsp27) expression in MSCs in an in vitro model of ischemia and in an in vivo rat MI model and aims to determine if this could enhance the therapeutic benefit associated with cell delivery. Hsp27 overexpression by lentivirus vector modification resulted in increased MSC survival in vitro and in vivo. Furthermore, decreased apoptosis in the infarcted tissue and improved cardiac function was observed in the Hsp27 group, enhancing the therapeutic effect of MSCs. Together, these data demonstrate that ex vivo genetic modification—specifically Hsp27 overexpression—offers the possibility of enhancing the efficacy of MSC therapy in MI.
McGinley and colleagues demonstrate that mesenchymal stem cells (MSCs) can be rendered resistant to ischemia and hypoxia in vitro through lentivirus-mediated overexpression of heat shock protein 27 (Hsp27). In a rat model of myocardial ischemia, Hsp27-modified MSCs exhibit improved engraftment compared with unmodified cells with associated benefits in cardiac function.
Introduction
Stem cell transplantation has emerged as a novel therapy for the treatment of cardiac dysfunction. Given the increasing prevalence of cardiovascular disease in modern society and limited treatments, which target symptom alleviation rather than restoration of lost function, tailor-made adult stem cell transplantation (or cellular cardiomyoplasty) has been heralded as the next generation of regenerative therapeutics. This approach aims to repair and regenerate the myocardium and offers the potential to significantly improve outcomes in patients with cardiovascular disease. To date, the exact mechanism by which stem cells mediate such repair remains unclear, but it is possible that transplanted cells either directly replace functional cardiomyocytes or else encourage healing in existing host tissue via paracrine signaling effects. Despite the ambiguity surrounding the mechanism, mesenchymal stem cells (MSCs) have been shown to have therapeutic value, with preclinical studies reporting positive outcomes (Nagaya et al., 2004; Amado et al., 2005; Valina et al., 2007). With respect to human clinical trials, results to date suggest that adult stem cell therapy is safe and potentially efficacious (Perin et al., 2003; Wollert et al., 2004; Assmus et al., 2006; Schachinger et al., 2006a,b), although most studies have been performed using unfractionated marrow samples. However, functional benefit after cell transfer has been described as modest and short-lived in nature (George, 2010). This may be attributed in part to poor survival and retention of transplanted cells, which poses a major limitation to successful cell therapy for cardiac repair. Limited rates of transplanted cell survival have been described in several preclinical studies, with reports that 99% of cells are lost within 4 days of transfer to uninjured mouse hearts ( Zhang et al., 2001). It is not surprising that such high levels of cell death also occur in myocardial infarction (MI) models, as transplanted cells are exposed to a toxic, proinflammatory infarct microenvironment that is not conducive to cell survival and/or engraftment. Cell death by both apoptosis and necrosis has been implicated because of ischemia and associated hypoxia and oxidative stress, as well as proapoptotic factors and inflammatory response (Elsasser et al., 2001). Consequently, stem cell transplantation approaches to restore damaged myocardium are limited by the failure to supply large enough quantities of surviving cells that may be required for a therapeutic effect. Protection of MSCs against cell death and apoptosis after transplantation into an infarct is therefore critical for successful stem cell therapy.
Gene therapy may enhance the reparative effects and functional benefit of stem cell therapy in several areas, including cell survival, migration, homing, engraftment, and paracrine factor production. The vector transduction capacity of MSCs allows for a potentially cumulative therapeutic effect in cardiac tissue regeneration. In addition to the direct cellular effects of MSCs, including secretion of paracrine factors and possible differentiation, expression of functional transgenes from within the MSC and secretion into the surrounding injured tissue may have positive effects on damaged tissues. Several strategies have led to improved stem cell survival within the infarct zone and have also demonstrated significant increases in cardiac function compared with unmodified cells, in particular, MSC modification to express Akt, Bcl-2, SDF-1, and CXCR4 (Mangi et al., 2003; Noiseux et al., 2006; Li et al., 2007; Zhang et al., 2007; Cheng et al., 2008). Heat shock protein 27 (Hsp27), a member of the small heat shock proteins, is ubiquitously expressed at low levels in unstressed cells. During the cellular stress response, elevated Hsp27 expression has been associated with enhanced cell survival in response to apoptotic stimuli, facilitated by cytoprotective effects (Mehlen et al., 1996; Samali and Cotter, 1996)—including its role as a molecular chaperone (Hartl and Hayer-Hartl, 2002), inhibition of caspase activation mechanisms (Bruey et al., 2000; Charette et al., 2000), and maintenance of cytoskeletal integrity (Lavoie et al., 1993; Perng et al., 1999; Hino et al., 2000). Additionally, Hsp27 has been demonstrated to have both antioxidant and anti-inflammatory actions as well as the ability to activate the prosurvival protein Akt (Mehlen et al., 1996). The present study involves the genetic modification of MSCs to enhance their posttransplantation survival and regenerative effects in cardiac repair. We hypothesize that transduced MSCs overexpressing prosurvival genes may possess an increased resistance to ischemic conditions, thereby improving their survival and engraftment in injured tissue. Specifically, we investigated the effect of exogenous Hsp27 overexpression on MSC survival in an in vitro model of ischemia. In addition, we assessed the effect of Hsp27 on MSC survival and persistence in a rat model of MI, as well as any cumulative benefit of this genetic modification on MSC therapeutic efficacy.
Materials and Methods
All reagents utilized were from Sigma-Aldrich unless otherwise stated.
Lentivirus production and titration
The human HSPB1 gene encoding Hsp27 was cloned into the lentiviral expression plasmid pWPT using Mlu1 and Sal1 restriction sites. Second-generation VSV-G pseudotyped lentiviral vector, rHIV-pWPT-EF1-α-GFP-W, and production plasmids were provided by Didier Trono (Lausanne, Switzerland). Vectors were produced by standard transient transfection of a three-plasmid system into producer cells. Briefly, packaging plasmid ps-PAX2.2 (Addgene plasmid 12260), envelope plasmid pMD2.G (Addgene plasmid 12259), and either pWPT-GFP (Addgene plasmid 12255) or pWPT-Hsp27 expression plasmids were transfected into HEK293 cells using Jet PEI transfection reagent (Polyplus Transfection Inc.) according to manufacturers' instructions. Culture medium was replaced 16 hr posttransfection. Vector-containing supernatants were collected 48 and 72 hr posttransfection, filtered, pooled, and concentrated by ultracentrifugation at 27,000×g for 3 hr. Lentivirus vector titer was determined by a quantitative real-time polymerase chain reaction (PCR)-based method to detect stably integrated virus sequences (copy number) in target HeLa cells and was expressed as transducing units per milliliter.
Rat MSC isolation, expansion, and characterization
All procedures involving animals were performed in accordance with the ethics regulations of the National University of Ireland, Galway. MSCs were isolated from the bone marrow of 8–12-week-old male Sprague Dawley rats (Harlan Laboratories) as previously described (Scutt and Bertram, 1999; Neuhuber et al., 2004, 2008). Briefly, after euthanization, marrow was flushed from femoral and tibial compartments with complete rat MSC (rMSC) growth medium, which consisted of 44.5% α-MEM (Gibco, Invitrogen) and 44.5% F12-Ham (Gibco, Invitrogen), supplemented with 10% fetal bovine serum (FBS; Gibco, Invitrogen), 100 U/ml penicillin G, and 100 μg/ml streptomycin sulfate (Gibco, Invitrogen). Recovered suspensions were pooled, counted, and plated at a density of 1.2×106 cells/cm2. Nonadherent cells were removed after 3 days and cells were re-fed with complete rMSC medium, with additional medium changes every 3–4 days. After approximately 8 days or when cell cultures reached confluence, cells were detached with 0.25% trypsin/1 mM EDTA solution and re-plated at 5.7×103 cells/cm2, with subsequent passage when they again reached confluence. The ability to differentiate down the adipogenic, osteogenic, and chondrogenic lineages, following isolation procedures described, is routinely tested inhouse. According to criteria recommended by the International Society for Cellular Therapy, an MSC deemed acceptable for laboratory-based investigations and preclinical studies is defined by its adherence to plastic, differentiation capacity, and cell surface markers (CD105, CD73, CD90 positive and CD34, CD45, CD14 or CD11b, CD79a or CD19, and HLA-DR negative). Isolated MSCs were routinely characterized inhouse by flow cytometry for their cell surface markers, as previously described (McMahon et al., 2006; Hoare et al., 2010).
MSC transduction and transgene expression analysis
MSCs were seeded at 1×104 cells/cm2, grown overnight, and transduced with lentiviral vectors rHIV-pWPT-EF1-α-Hsp27-W or rHIV-pWPT-EF1-α-GFP-W at a multiplicity of infection of 100 for all experiments. MSCs were harvested for analysis, at the earliest, 96 hr posttransduction and transgene expression was measured by immunofluorescent staining and Western blot. For Western blot analysis, early passage (P1) cells were transduced at a multiplicity of infection of 100 and expanded to P4, collecting samples at each passage and also after cryopreservation and storage in liquid nitrogen. Membranes were probed with a mouse monoclonal antibody against Hsp27 diluted 1:2,000 (Stressgen, Enzo Life Sciences) and ECL antimouse horseradish peroxidase (HRP)-linked as secondary antibody (Amersham Biosciences, GE Healthcare), and developed using SuperSignal West Pico Chemiluminescent Substrate solution (Pierce, Thermo Scientific). House-keeping β-Actin protein was detected by a monoclonal mouse anti-β-actin HRP-conjugated antibody diluted 1:5,000 (Sigma). Immunofluorescence staining was performed in eight-well chamber slides 72 hr posttransduction. Briefly, cells were fixed in 4% paraformaldehyde (PFA), permeabilized in 0.1% Triton X-100, blocked in 5% FBS, and incubated with a mouse primary antibody against Hsp27 diluted 1:50 (Stressgen, Enzo Life Sciences), followed by a fluorescein isothiocyanate-conjugated secondary antibody. Slides were mounted with Vectashield HardSet Fluorescent Mounting Medium containing DAPI (Vector Laboratories).
In vitro models of ischemia
To mimic the in vivo scenario of ischemia, MSCs were exposed to conditions of hypoxia (PaO2 0.5% plus complete Dulbecco's modified Eagle's medium [DMEM]), ischemia (PaO2 0.5% in serum and glucose-free DMEM), and complete O2 and glucose deprivation by inhibition of glycolysis (ischemia+2-deoxyglucose) in an in vivo 400 hypoxia chamber (Ruskinn Technologies). 2-Deoxyglucose (2DG) is a glycolytic inhibitor that inhibits hexokinase phosphorylation of glucose. Addition of this inhibitor provided an in vitro model of complete glucose deprivation. For this series of experiments, MSCs were seeded and transduced under normal culture conditions until 72 hr posttransduction when cells were optimally expressing transgene. The appropriate medium for each condition (hypoxia, ischemia, ischemia+2DG) was placed in the hypoxia chamber for a minimum of 3 hr to deplete the oxygen levels to the required 0.5%.
In vitro assessment of viability and apoptosis
MSC viability was determined by standard MTT assay as previously described (Mosmann, 1983). Apoptosis levels were assessed by identification of apoptotic nuclei by DAPI staining of MSCs. Percentage apoptotic cell number was determined by counting total nuclei and apoptotic nuclei per field of 10 random fields. Caspase activation was also examined by a quantitative caspase-3-like enzyme activity assay using Ac-DEVD-AFC substrate (Biomol, Enzo Life Sciences) and staurosporine (STS; 500 nM for 12 hr) as a positive control.
Adipogenesis differentiation assay
MSCs (both transduced and nontransduced) were seeded at 2×104 cells/cm2. Once cells had reached confluence, adipogenic differentiation was induced by three 72 hr cycles of adipogenic induction medium (containing 1 μM dexamethasone, 10 μg/ml insulin, 200 μM indomethacin, 500 μM 3-isobutyl-1-methyl-Xanthine, 100 U/ml penicillin, 100 μg/ml streptomycin, 10% FBS, and 5% rabbit serum in high-glucose DMEM). After each round of induction, cells were maintained in maintenance medium for 24 hr (10 μg/ml insulin in complete high-glucose medium) and for 5–7 days after final induction. Cells were fixed in 10% formalin and differentiated cells were identified by Oil Red O stain for lipid vacuoles. Images of 10 random fields for each well were acquired and differentiated cells containing stained lipid vacuoles per microscope field were counted. Oil Red O stain was also quantified by extraction of the stain into 99% isopropanol and absorbance measurement on a Wallac Victor plate reader (PerkinElmer) at 490 nm. Noninduced MSCs, maintained in normal growth medium, were included as negative controls for the adipogenesis assay.
MI and intramyocardial injection of cells
All in vivo experiments were performed in female Sprague Dawley rats weighing approximately 200 g—all animals used in the study were identical in terms of strain, age, and weight. Surgeries were performed by one blinded experienced operator, in a consistent manner. MI was induced by complete ligation of the left anterior descending (LAD) coronary artery, which was placed at the same level in each animal. Permanent occlusion of the LAD has been previously described in Sprague Dawley rats and produces important tissue necrosis and early inflammatory responses that are required for analysis of therapeutic intervention. For analysis of cardiac function, two-dimensional and M-Mode echocardiography was carried out before surgery (baseline), 1 day postinfarct (preintervention), and 28 days after stem cell delivery (postintervention), as detailed below. MSCs were prepared for injection 24 hr postinfarction, whereby 4×106 MSCs were suspended in 100 μl of serum-free and antibiotic-free F-12 HAM medium (Gibco, Invitrogen). Cell suspensions were mixed thoroughly and drawn into sterile syringes, and 4×106 cells were injected at five points surrounding the border zone of the infarct (5×20 μl per injection) using a 25-gauge needle. Animals were euthanized by CO2 asphyxiation at either 7 or 28 days±1 day after stem cell injection, and hearts were harvested. For engraftment analysis, samples were washed in phosphate-buffered saline and stored dry at −20°C. For histological analysis, samples were stored in buffered formalin at +4°C.
Echocardiography analyses
Functional recovery and possible benefit of cell intervention was assessed by transthoracic echocardiography. Echocardiography studies were performed by an experienced investigator, blinded to treatment allocation, within a week of initial surgery, 24 hr after treatment, and again 28 days after treatment. Images were obtained on a GE Systems Vivid5 ultrasound machine (GE Medical Systems) with a 10 Hz probe. Rats were anesthetized with 2–3% isoflurane, and the transducer was placed on the chest wall where short-axis views at the midventricular level were obtained. M-mode tracings were recorded at the same midventricular level. Three images per view were recorded, and parameters were calculated from three consecutive cardiac cycles (i.e., in triplicate), and analysis was performed using EchoPAC software. The following parameters were calculated: interventricular septal dimension in diastole (IVSd), left ventricular end diastolic dimension (LVEDD), left ventricular posterior wall dimension in diastole (LVPWd), interventricular septal dimension in systole (IVSs), left ventricular end systolic dimension (LVESD), and left ventricular posterior wall dimension in systole (LVPWs). Percentage fractional shortening was calculated as follows: (LVEDD − LVESD)/LVEDD×100. Percentage ejection fraction was calculated as follows: (LVEDD3 − LVESD3)/LVEDD3×100. Relative wall thickness was calculated as follows: 2LVPWd/LVEDD. Animals were included in the study if their ejection fraction decreased by >15% from baseline.
Genomic DNA extraction and determination of MSC engraftment levels
Engraftment of transplanted MSCs in infarcted hearts was assessed by detection of male DNA (injected cells) in the recipient female heart by sex-mismatch PCR. A standard curve for the SRY gene (male DNA) was constructed, and quantitative real-time PCR was performed to determine the presence of transplanted MSCs in isolated DNA. A total concentration of 200 ng of DNA per reaction (2 μl) was analyzed for the presence of SRY gene sequences. Unknown quantity of male DNA in sample female heart DNA was calculated by conventional standard curve methods. The following male-specific primer sequences for SRY gene intronic sequences were used: SRY sense, CGA AGG GTT AAA GTG CCA CAG; SRY antisense, GGT TGT TGT CCC ATT GCA GC. Primers against the single-copy AGXT gene were used as housekeeping controls: AGXT sense, CCC CAA CAC ACC TCA CTT CT; AGXT antisense, AGA ACC TAG CCC AGA GCA CA.
Histology
Harvested hearts were fixed and stored in buffered formalin at +4°C until required for sectioning. Fixed whole hearts were transversely cut into even 2 mm sections using a Vibratome (Leica Microsystems). After overnight processing, hearts were embedded in paraffin wax and sections cut (5 μm) on a rotary microtome (Leica Microsystems). Sections were deparaffinized and rehydrated before Masson's trichrome staining and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay.
Analysis of infarct size
The Masson's trichrome histological staining method was used to assess the size of myocardial infarct 28 days postintervention. Percentage infarct area versus healthy area was determined, and data were expressed as percentage infarct per treatment group.
TUNEL assay
TUNEL staining was performed to detect and quantify apoptosis levels at single cell level, in tissue sections of infarcted hearts. Midpoint sections of infarcted tissue were stained for apoptosis using a standard TUNEL labeling assay kit (Roche Diagnostics Corporation). Briefly, slides were pretreated with proteinase K, and incubated in TdT enzyme and fluorescein-conjugated nucleotide substrate for 1 hr at 37°C. Slides were mounted in Vectashield hard-set mounting medium containing DAPI, and images acquired by fluorescence microscopy. Assay controls (negative-intact myocordium, positive DNase treated) are shown in Supplementary Fig. S1 (Supplementary Data are available online at www.liebertonline.com/hum). Percentage apoptosis was determined by quantification of total TUNEL-positive number (green) and total cell number (DAPI-stained cells, blue) from five random microscope fields per section using a custom-written MATLAB code for image analysis.
Statistical analysis
Statistical analyses were carried out on data sets using SigmaStat for Windows, version 3.5 (Systat). One-way analysis of variance (ANOVA) was used to determine statistical significance between groups, followed by multiple paired comparisons for normally distributed data (Tukey test). Nonnormally distributed data were analyzed by ANOVA on ranks using either Dunn's test or Kruskal–Wallis test. Paired t-tests were used for pairwise comparisons. Values of p<0.05 were considered statistically significant (*p<0.05, **p<0.001).
Results
Lentiviral vector-mediated Hsp27 transgene expression in MSCs
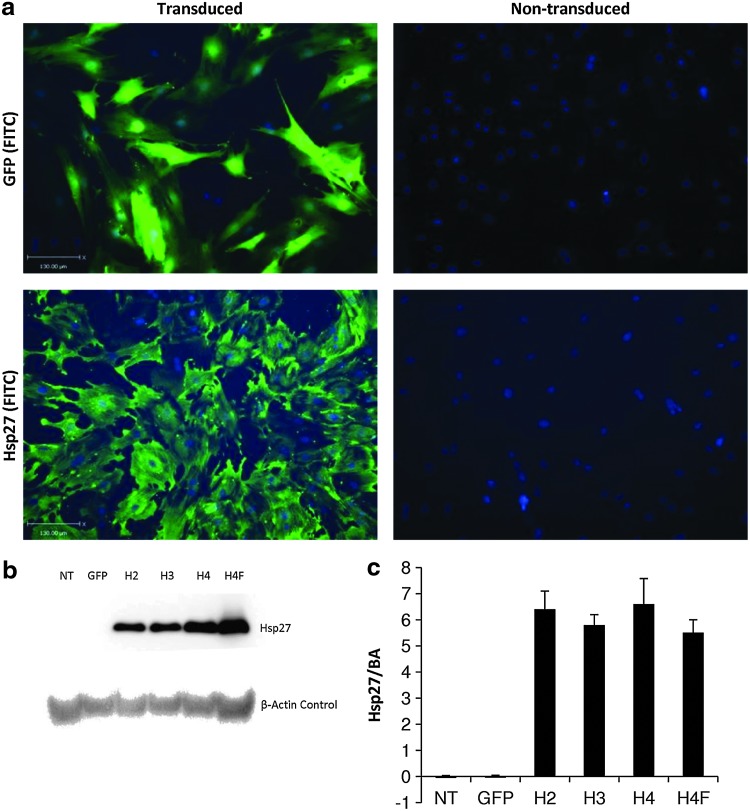
Immunocytochemical and immunoblot analyses demonstrated high levels of Hsp27 protein expression in transduced compared with nontransduced control MSCs (Fig. 1). It was also demonstrated that neither expansion nor cryopreservation of transduced cell populations negatively affected Hsp27 protein expression (Fig. 1b and c). Hsp27 expression was observed neither in nontransduced rMSC samples nor in control GFP vector-transduced samples.
FIG. 1.
Transgene expression in lentivirus vector-transduced rMSCs. rMSCs (P1) were transduced with rHIV-pWPT-EF1-α-Hsp27-W vector at a multiplicity of infection of 100 and Hsp27 transgene expression was analyzed. Unmodified and rHIV-pWPT-EF1-α-GFP-W vector-transduced rMSCs were used as controls. (a) Immunostaining of transduced and nontransduced rMSCs. Lower-panel images show cells that were stained Hsp27 protein (left transduced and right nontransduced). (b) Western blot analysis of rMSCs transduced with lentiviral vector showing Hsp27 protein bands at 27 kDa and β-actin control at 42 kDa. NT, nontransduced; H2/3/4, modified MSCs overexpressing Hsp27 at passage number 2/3/4; H4F, P4 MSCs overexpressing Hsp27 after cryopreservation. (c) Densitometry analysis of Western blot. Data are shown as mean+SD (n=3) or as representative images (scale bars 130 μm), of three independent experiments. Hsp27, heat shock protein 27; rMSCs, rat mesenchymal stem cells.
Hsp27 overexpression in MSCs and in vitro ischemia
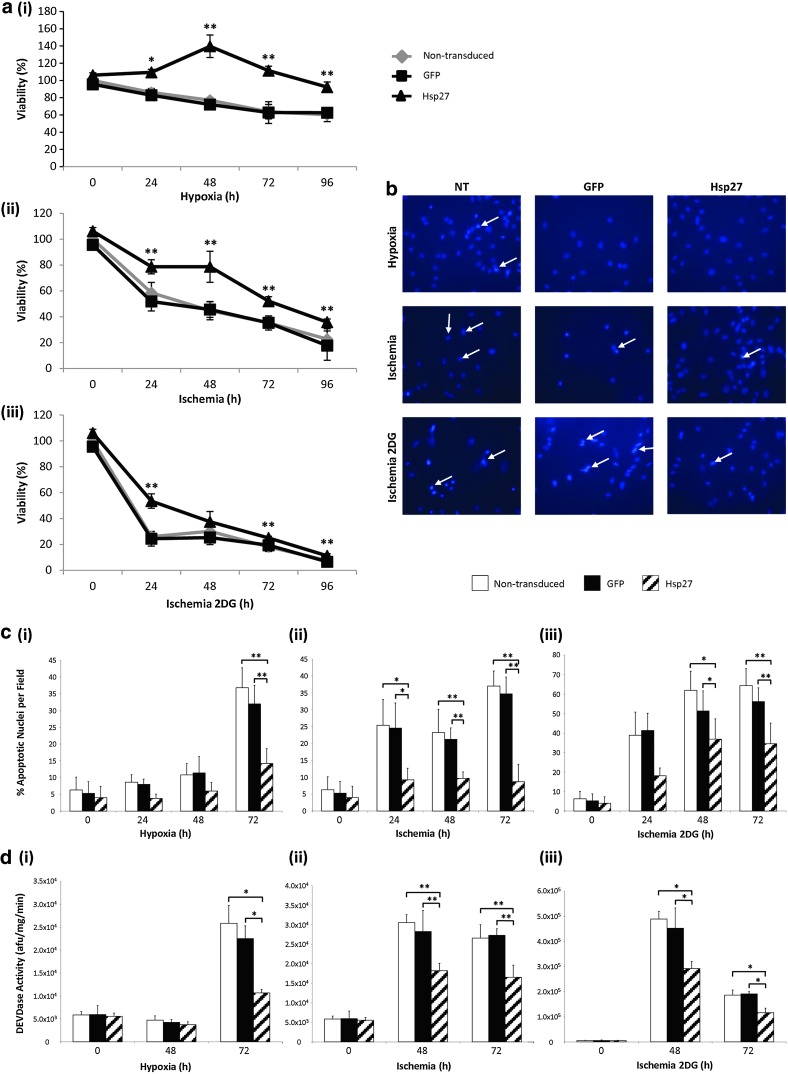
To assess any protective effect of Hsp27 overexpression, MSCs were exposed to hypoxia, ischemia, and ischemia with inhibition of glycolysis (2DG). In terms of cell survival, Hsp27 expression significantly increased viability compared with nontransduced and GFP control MSCs at all hypoxia and ischemia (Fig. 2a) exposure times (*p<0.05, **p<0.001). Addition of 2DG to the ischemia protocol exacerbated the observed effects on MSC viability and, although the stress of ischemia+2DG seemed to have a potent deleterious effect on the MSCs, this effect was limited by overexpression of Hsp27 (Fig. 2a-iii), where viability was significantly increased compared with controls at 24, 72, and 96 hr (**p<0.001). Viability of control GFP-MSCs remained similar to that of nontransduced MSCs, proving that any observed effects were as a result of transgene overexpression and not because of effects of the lentiviral vector per se. The ability of Hsp27 to protect against ischemia-induced apoptosis was also examined by nuclear morphology analysis and caspase-3 activity assay. Hsp27 overexpression significantly reduced the occurrence of apoptotic nuclei compared with controls at 72 hr of hypoxic conditions (Fig. 2b and c, **p<0.001) and in ischemic conditions at all measured time points (Fig. 2b and c, *p<0.05, **p<0.001). Similarly, in ischemia+2DG conditions, there was a significant decrease in apoptotic nuclei in the Hsp27-MSC group (Fig. 2b and c) at the latest time point of 72 hr (*p<0.05). Caspase-3 activity measurement was also performed to further analyze ischemia-induced apoptosis and any protective benefit of Hsp27 overexpression (Fig. 2d). High levels of caspase activity were induced by all three treatment conditions. This induction was ameliorated in Hsp27-MSCs after 72 hr hypoxia compared with controls (*p<0.05). Reduced caspase activity was also observed in Hsp27-MSCs at both 48 and 72 hr of ischemia compared with controls (**p<0.001). Caspase-3 induced by ischemia+2DG reached its maximum activity at 48 hr, after which levels decreased by almost half. At both time points examined, Hsp27-MSCs demonstrated a significant reduction in caspase activity compared with controls (*p<0.05).
FIG. 2.
Enhanced MSC survival by lentiviral vector modification in hypoxia, ischemia and ischemia with glycolysis inhibition (complete glucose deprivation). (a) Percentage rMSC viability as determined by MTT assay after exposure to (i) hypoxia, (ii) ischemia, and (iii) ischemia with glycolysis inhibition (*p<0.05 and **p<0.001, Hsp27-MSCs vs. nontransduced MSCs/GFP-MSCs). (b) DAPI staining of MSCs after 72 hr hypoxia, ischemia, and ischemia with glycolysis inhibition. (c) Percentage apoptotic nuclei per microscope field (n=10) at 72 hr (i) hypoxia, (ii) ischemia, and (iii) ischemia with glycolysis inhibition (*p<0.05 and **p<0.001, Hsp27-MSCs vs. nontransduced MSCs/GFP-MSCs). (d) Caspase-3 activity in MSCs after 48 and 72 hr (i) hypoxia, (ii) ischemia, and (iii) ischemia with glycolysis inhibition (*p<0.05 and **p<0.001, Hsp27-MSCs vs. nontransduced MSCs/GFP-MSCs). Data are shown as mean+SD (n=3) or as representative images of three independent experiments.
Hsp27 overexpression and MSC differentiation in ischemia
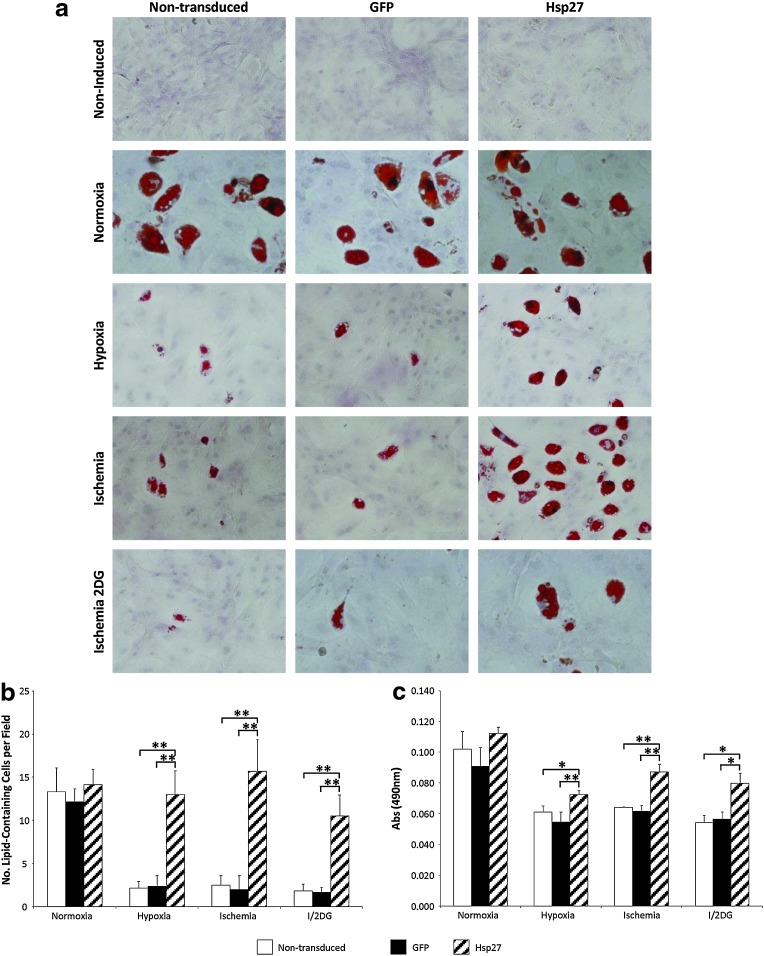
In normal oxygen conditions (21% PaO2), adipogenic differentiation levels were comparable between groups, demonstrating that there was no inhibitory effect of transduction with the lentivirus vector and subsequent GFP or Hsp27 expression in MSCs. When exposed to hypoxia, ischemia, and ischemia+2DG, MSCs differentiated with a reduced efficiency. However, Hsp27 overexpression preserved the adipogenic differentiation capacity of MSCs, in terms of both numbers of differentiated cells (**p<0.001 vs. controls) and lipid accumulation levels (*p<0.05, **p<0.001 vs. controls) in all three treatment conditions (Fig. 3b and c).
FIG. 3.
Enhanced MSC differentiation by lentiviral vector modification in hypoxia and ischemia. (a) Oil Red O staining for lipid vacuoles after rMSC adipogenic differentiation. There was no negative effect of lentivirus vector transduction and overexpression of GFP or Hsp27 on MSC adipogenic differentiation capacity. (b) Numbers of lipid-containing cells per microscope field (n=10) (**p<0.001, Hsp27-MSCs vs. nontransduced MSCs/GFP-MSCs). (c) Oil Red O quantity (*p<0.05 and **p<0.001, Hsp27-MSCs vs. nontransduced MSCs/GFP-MSCs). Data are shown as mean+SD (n=3) or as representative images of three independent experiments.
Hsp27-MSC engraftment in the infarcted heart
To validate the protective effect of Hsp27 that was observed in vitro, the survival of transplanted MSCs in a rat permanent ligation MI model of acute ischemia was assessed by quantitative PCR for the male SRY gene (Fig. 4a). Previous studies utilizing this method have shown that Y-chromosomes from nonviable cells are removed and degraded by macrophages or other mechanisms within 24 hr (Muller-Ehmsen et al., 2002), indicating that only male DNA from viable transplanted cells is detectable. The same study detected decreasing levels of transplanted cells over time, with a relative rate of 1% survival in the longer-term. Consistent with this, in the present study, very low levels of donor MSCs were detected at 7 days and even lower at 28 days. No significant differences were observed between control GFP-MSCs and unmodified MSCs (data not shown). Increased numbers of transplanted MSCs were detected in the Hsp27 group compared with unmodified MSCs at both time points assessed. At the earlier time point of 7 days, considerably higher levels of transplanted cells were detected (Fig. 4a-iii) with a 10-fold increase in MSC retention compared with control MSCs (*p<0.001). At 28 days postintervention, detectable levels of MSCs were low, with an approximate 1.8-fold increase (*p<0.05) in the Hsp27-MSC group (Fig. 4a-iv).
FIG. 4.
Detection of transplanted MSCs in infarcted heart and assessment of post-MI cardiac function. (a) Y-chromosome SRY detection in whole-heart sample tissue in MSC and Hsp27-MSC treatment groups. Representative standard curve (i) and Rn versus cycle (ii) used for qPCR analysis of SRY sequences in DNA extracted from a whole male heart (serial dilutions in female DNA). SRY detected at (iii) 7 days postintervention (**p<0.001, Hsp27-MSCs vs. nontransduced MSCs) and (iv) 28 days postintervention (*p<0.05, Hsp27-MSCs vs. nontransduced MSCs). (b) Left panels: cardiac function at day zero, before MI (T1), post-MI, preintervention (T2), and at endpoint 28 days post-MI (T3), as determined by echocardiography analysis of (i) left ventricular ejection fraction (LVEF), (ii) fractional shortening (FS), (iii) LV end-diastolic dimension (LVEDD), and (iv) relative wall thickness (RWT). (c) Right panels: fold change at 28 days compared with directly post-MI (before intervention) of (i) LVEF, (ii) FS, (iii) LVEDD, and (iv) RWT (**p<0.001, Hsp27-MSC vs. indicated groups; *p<0.05, Hsp27-MSC vs. indicated groups). Data are presented as mean+SD (n=20 rats MSC-Hsp27 group; n=10 rats per control group). MI, myocardial infarction; qPCR, quantitative real-time polymerase chain reaction.
Post-MI ventricular dysfunction and fibrosis (infarct size)
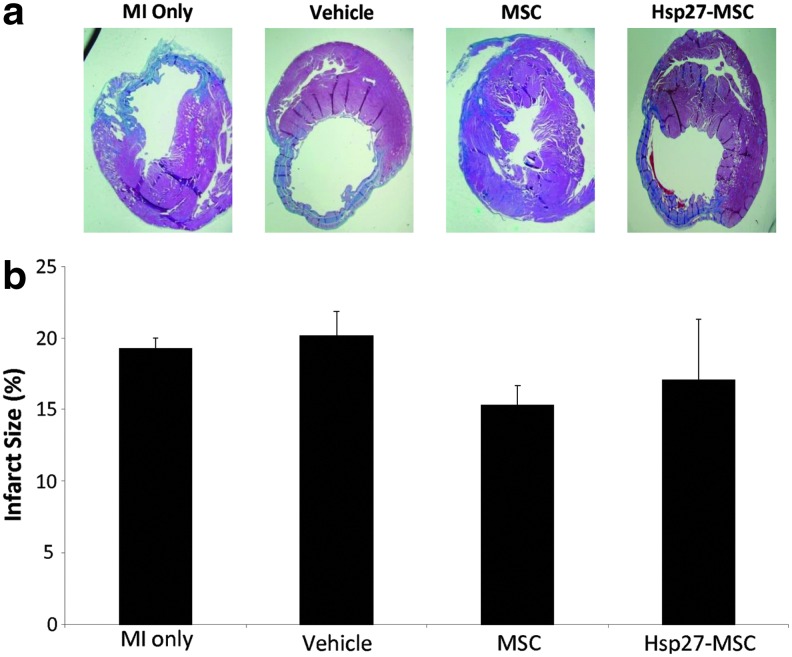
Echocardiography studies were performed to ascertain any treatment benefit and possible return of cardiac function post-MI (Fig. 4b). For all parameters measured, there were no differences between groups at baseline (T1) or directly after MI induction (T2), demonstrating that there was no significant variability at baseline or in the extent of the infarction after induction. At the endpoint of 28 days, a significant improvement in LV ejection fraction was observed in the Hsp27-MSC group (Fig. 4b-i, **p<0.001 vs. MI and vehicle groups, *p<0.05 vs. MSC group). In terms of fractional shortening and relative wall thickness, there was no significant difference between the groups (Fig. 4b-ii and 4b-iv)). Left ventricular end-diastolic dimension (LVEDD), a sensitive marker of left ventricular remodeling, was decreased in the cell treatment groups compared with control groups (Fig. 4b-iii). This reduction in LVEDD in the Hsp27-MSC group was significant compared with all other groups at 28 days (*p<0.05), indicative of positive remodeling post-MI. These significant improvements in LVEF and LVEDD at 28 days may be clearly observed when expressed as fold change compared with post-MI (Fig. 4c-i and 4c-iii). Examination of Masson's trichrome-stained tissue sections showed thinning of the ventricular wall, modest myocardial fibrosis, and significant infarction (representative images 28 days post-MI; Fig. 5a). Although the infarct size was reduced post-MSC treatment, this was not statistically significant. Furthermore, in contrast to the results for cardiac function, lenti-Hsp27-modified MSCs did not reduce infarct size more than MSCs alone (Fig. 5b). GFP-MSCs were included as a control for MSC transduction, and again no differences were observed between this group and unmodified MSCs (data not shown).
FIG. 5.
Infarct size analysis 28 days post-MSC administration. Hearts were harvested 28 days after cell intervention and cut into 5 μm sections, and Masson's trichrome staining was performed on 15–18 sections per group. Experimental groups: MI only, vehicle, unmodified MSCs, and Hsp27-MSCs. (a) Masson's trichrome-stained sections showing infarcted area (blue) and healthy tissue (red). (b) Percentage infarct area versus healthy area was determined for each section and expressed as percentage infarct per treatment group. Data are presented as representative images or as mean+SD (n=6 rats per group).
Hsp27-MSCs and infarct border zone apoptosis
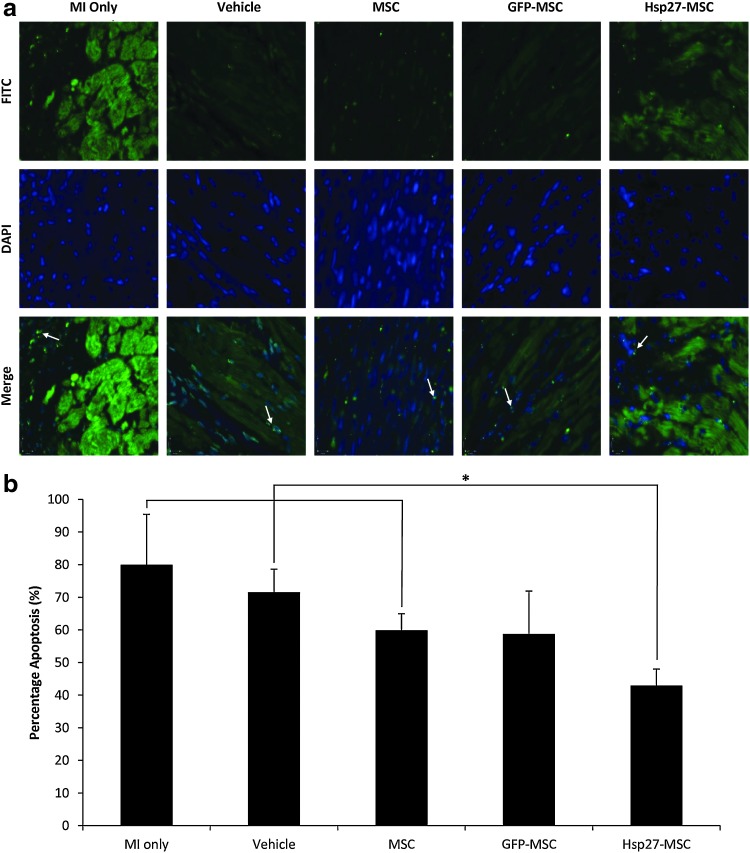
Apoptosis in the infarct border zones was assessed by TUNEL assay 28 days posttreatment (Fig. 6). As expected, high numbers of TUNEL-positive cells indicative of DNA fragmentation and apoptosis were present. Quantitative image analysis showed percentage apoptosis ranging from 40% to 80% (Fig. 6b). Here, a significant reduction in apoptosis and increase in viable cells was observed in all of the cell treatment groups compared with control MI-only and vehicle-only groups (*p<0.05). Furthermore, the Hsp27-MSC group exhibited significantly decreased apoptosis levels of 42% compared with levels of ∼60% of nontransduced MSC (*p<0.05) and GFP-MSC (p=0.073).
FIG. 6.
Hsp27-MSCs reduce apoptosis in infarct border zone (BZ). Apoptosis was detected in the ischemic border zone by TUNEL staining of 5 μm midpoint tissue sections 28 days post-MI. Experimental groups: MI only, vehicle, unmodified MSCs, and Hsp27-MSCs. (a) TUNEL stain (green, top panel), nuclear DAPI stain (blue, middle panel), and merged images (lower panel). Typical apoptotic bodies are evident (examples marked with arrows) and show an intense fluorescence by TUNEL assay, indicative of massive DNA fragmentation. (b) Quantitative analyses of TUNEL-positive cells (*p<0.05, Hsp27-MSC group vs. MI only, vehicle only, and MSC control groups). Data are presented as representative images or as mean+SD (n=5–7 animals per group, 10–15 random fields per animal). TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling.
Discussion
Stem cell transplantation is increasingly recognized as a potential strategy to salvage damaged myocardium and to support endogenous repair of cardiac tissue. To date, the transplantation of MSCs in animal models of MI has significantly improved LV function (Tomita et al., 1999; Toma et al., 2002; Kudo et al., 2003; Amado et al., 2005; Dai et al., 2005; Silva et al., 2005; Grauss et al., 2008). Overall, the result has been modest and somewhat inconsistent. The therapeutic effect and resulting outcome of cell transplantation are dependent on the capacity of the donor cells to survive in the target damaged tissue (Zhang et al., 2001; Robey et al., 2008; Shujia et al., 2008). Post-MI ischemia, scar tissue and inflammation make a less-than-ideal environment for cell survival. A combination of gene and cell therapy provides an opportunity to enhance the beneficial effects of MSC-based therapies (McGinley et al., 2011). Genetic modification of MSCs before transplantation has been shown in several instances to support increased survival capacity and reparative function (Mangi et al., 2003; Tang et al., 2005; Cheng et al., 2008). Heat-shock treatment and subsequent upregulation of Hsps has been shown to improve the survival of several transplanted cell types, including skeletal myoblasts and human embryonic stem cell-derived and neonatal cardiomyocytes (Suzuki et al., 2000; Zhang et al., 2001; Laflamme et al., 2007). This cytoprotective effect has been harnessed by way of ex vivo modification of donor cells to overexpress individual Hsps (Chang et al., 2009; Wang et al., 2009). Although one study has reported that Hsp27 has a protective effect against ischemia/reperfusion injury in vivo (Hollander et al., 2004), the current study used lentivirus as a means of genetic modification and the model used was one of complete vascular occlusion.
The present study utilized a lentivirus vector to modify MSCs to overexpress the therapeutic gene Hsp27. Previous studies in our laboratory have shown that lentivirus vectors effectively and efficiently transduce MSCs and do not seem to have any deleterious effect on the ability of the cells to differentiate in vitro ( McMahon et al., 2006; McGinley et al., 2011). While adipogenesis may not be a major factor to be considered in the context of MSC-mediated repair in conditions of hypoxia and ischemia, and cardiac myogenesis is a more clinically relevant event and would be an ideal model, to the best of our knowledge, definitive methodology has not yet been developed to allow MSC differentiation into this lineage. This is a point to be noted in interpretation of these results. However, based on the published literature, the proposed principle mechanism of action for MSC transplantation to the heart is paracrine in nature and not dependent on myogenic differentiation. Given the integrative nature of lentiviral vector transduction, induced gene expression in MSCs is persistent over time, which may potentially maintain numbers of surviving transplanted cells over longer periods. Our data demonstrate appreciable levels of Hsp27 transgene expression in early passage MSCs mediated by a lentiviral vector, with no apparent reduction in expression levels upon cell expansion or cryopreservation. This is significant from a logistical point of view as much less virus vector is required when a smaller number of cells can be transduced and expanded to the numbers necessary for transplantation. This may not be feasible with nonintegrating gene therapy vectors because of the transient nature of transgene expression.
We report that Hsp27 overexpression had a robust protective effect on MSCs in models of ischemia and its components in vitro. Lentivirus vector-mediated Hsp27 overexpression consistently enhanced survival and prevented caspase-3-induced apoptosis, in oxygen-, serum-, and glucose-deprived MSCs, across all time points examined. The potent prosurvival effects of Hsp27 expression on MSCs exposed to ischemia and its associated conditions in vitro have, to our knowledge, not been documented previously. Reduction of caspase activity alone may be sufficient to explain the action of Hsp27 overexpression in MSCs, but the numerous other functions of Hsp27 do not preclude other prosurvival mechanisms.
The effect of transplantation of MSCs and genetically modified MSCs to a rat model of MI was next studied. As previously reported, transplantation of MSCs was associated with a beneficial effect. In terms of survival of transplanted cells, overexpression of Hsp27 significantly increased MSC persistence in the ischemic heart compared with unmodified MSCs. This was associated with reduced rates of apoptosis in the infarct border zone and improved cardiac function as assessed by left ventricular ejection fraction and left ventricular end-diastolic dimension, but not with a reduction in myocardial infarct size. Immunohistological analyses of Hsp27 expression in engrafted MSCs and further experiments into the mechanism of Hsp27-mediated cytoprotection and improved cardiac function will undoubtedly further strengthen the interpretation of our observed results and are the next logical steps in exploring the potential therapeutic value of induced Hsp27 expression.
From these data, it seems that increased cell survival and retention may have been sufficient to promote a functional effect. However, it is unlikely that the positive effects are caused by transdifferentiation of MSCs and regeneration of myocardium, since a significant reduction in infarct size was not observed. A possible hypothesis is that a paracrine mechanism is responsible for the observed cardioprotective effects, whereby additional therapeutic factors are secreted by Hsp27-MSCs. These secreted factors could include various cytokines, growth factors, antiapoptotic molecules, or combinations capable of producing a protective milieu. Other investigators, including Mirotsou et al., have shown that MSCs produce and secrete a wide variety of factors and have suggested secreted frizzled related protein 2 (SFRP2) as the key mediator of the efficacy observed with Akt-MSCs. SFRP2 solely has the ability to prevent ischemic-related cardiomyocyte injury by upregulation of cellular and nuclear beta-catenin levels, thereby imitating the canonical Wnt signaling antiapoptotic pathway (Mirotsou et al., 2007). Whether Hsp27-MSCs may have similar changes in their secretome remains undefined by the present study. A recent in vitro study reported that Hsp20 (a small heat shock protein also known as alpha-crystallin) overexpression in MSCs produced increased secretions of VEGF, FGF-2, and IGF-1 in response to hypoxia (Wang et al., 2009); this may also be the case with Hsp27. Clearly, definition of the mechanisms involved in the enhanced effects of Hsp27-MSCs and further study are required for identification of paracrine mediators.
Overall, this study supports the use of MSCs as candidates for cell delivery to the injured myocardium. Gene therapy approaches toward cytoprotection represents an appealing strategy to enhance current MSC-based therapies for the treatment of MI and ischemia. We have identified Hsp27 as a potent cytoprotective agent that may prove efficacious by preventing MSC death in ischemic conditions, thereby promoting repair and regeneration in injured areas. Future analysis of the exact nature and mechanism of Hsp27-MSC-mediated repair may well have important implications for the future development of therapy for MI.
Supplementary Material
Acknowledgments
The authors thank Xizhe Chen, Charles McHale, and Georgina Shaw (Regenerative Medicine Institute) for technical assistance, and Julia Raykin (Georgia Institute of Technology) for MATLAB image processing. This work was funded by Grant 08/CE/B1436 from Science Foundation Ireland.
Author Disclosure Statement
T.O.B. has the following competing financial interests: Procure—founder and equity holder (no funding received); Pfizer, Novartis, and Merck Ltd.—received education grants; Medtronic—received research grants. The other authors declare that they have no competing interests.
References
- Amado L.C., et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmus B., et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N. Engl. J. Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- Bruey J.M., et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell. Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Chang W., et al. Mesenchymal stem cells pretreated with delivered Hph-1-Hsp70 protein are protected from hypoxia-mediated cell death and rescue heart functions from myocardial injury. Stem Cells. 2009;27:2283–2292. doi: 10.1002/stem.153. [DOI] [PubMed] [Google Scholar]
- Charette S.J., et al. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol. Cell Biol. 2000;20:7602–7612. doi: 10.1128/mcb.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol. Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- Dai W., et al. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- Elsasser A., et al. The role of apoptosis in myocardial ischemia: a critical appraisal. Basic Res. Cardiol. 2001;96:219–226. doi: 10.1007/s003950170052. [DOI] [PubMed] [Google Scholar]
- George J.C. Stem cell therapy in acute myocardial infarction: a review of clinical trials. Transl. Res. 2010;155:10–19. doi: 10.1016/j.trsl.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Grauss R.W., et al. Forced myocardin expression enhances the therapeutic effect of human mesenchymal stem cells after transplantation in ischemic mouse hearts. Stem Cells. 2008;26:1083–1093. doi: 10.1634/stemcells.2007-0523. [DOI] [PubMed] [Google Scholar]
- Hartl F.U. Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Hino M., et al. Small heat shock protein 27 (HSP27) associates with tubulin/microtubules in HeLa cells. Biochem. Biophys. Res. Commun. 2000;271:164–169. doi: 10.1006/bbrc.2000.2553. [DOI] [PubMed] [Google Scholar]
- Hoare M., et al. Enhanced lipoplex-mediated gene expression in mesenchymal stem cells using reiterated nuclear localization sequence peptides. J. Gene Med. 2010;12:207–218. doi: 10.1002/jgm.1426. [DOI] [PubMed] [Google Scholar]
- Hollander J.M., et al. Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation. 2004;110:3544–3552. doi: 10.1161/01.CIR.0000148825.99184.50. [DOI] [PubMed] [Google Scholar]
- Kudo M., et al. Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J. Mol. Cell. Cardiol. 2003;35:1113–1119. doi: 10.1016/s0022-2828(03)00211-6. [DOI] [PubMed] [Google Scholar]
- Laflamme M.A., et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- Lavoie J.N., et al. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J. Biol. Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- Li W., et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- Mangi A.A., et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- McGinley L., et al. Lentiviral vector mediated modification of mesenchymal stem cells & enhanced survival in an in vitro model of ischaemia. Stem Cell Res. Ther. 2011;2:12. doi: 10.1186/scrt53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon J.M., et al. Gene transfer into rat mesenchymal stem cells: a comparative study of viral and nonviral vectors. Stem Cells Dev. 2006;15:87–96. doi: 10.1089/scd.2006.15.87. [DOI] [PubMed] [Google Scholar]
- Mehlen P. Schulze-Osthoff K. Arrigo A.P. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J. Biol. Chem. 1996;271:16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- Mirotsou M., et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc. Natl. Acad. Sci. USA. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Muller-Ehmsen J., et al. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J. Mol. Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- Nagaya N., et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2670–H2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- Neuhuber B., et al. Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype. J. Neurosci. Res. 2004;77:192–204. doi: 10.1002/jnr.20147. [DOI] [PubMed] [Google Scholar]
- Neuhuber B., et al. Effects of plating density and culture time on bone marrow stromal cell characteristics. Exp. Hematol. 2008;36:1176–1185. doi: 10.1016/j.exphem.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiseux N., et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol. Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Perin E.C., et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- Perng M.D., et al. Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J. Cell Sci. 1999;112:2099–2112. doi: 10.1242/jcs.112.13.2099. [DOI] [PubMed] [Google Scholar]
- Robey T.E., et al. Systems approaches to preventing transplanted cell death in cardiac repair. J. Mol. Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samali A. Cotter T.G. Heat shock proteins increase resistance to apoptosis. Exp. Cell Res. 1996;223:163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- Schachinger V., et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur. Heart J. 2006a;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- Schachinger V., et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N. Engl. J. Med. 2006b;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- Scutt A. Bertram P. Basic fibroblast growth factor in the presence of dexamethasone stimulates colony formation, expansion, and osteoblastic differentiation by rat bone marrow stromal cells. Calcif. Tissue Int. 1999;64:69–77. doi: 10.1007/s002239900581. [DOI] [PubMed] [Google Scholar]
- Shujia J., et al. Stable therapeutic effects of mesenchymal stem cell-based multiple gene delivery for cardiac repair. Cardiovasc. Res. 2008;77:525–533. doi: 10.1093/cvr/cvm077. [DOI] [PubMed] [Google Scholar]
- Silva G.V., et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- Suzuki K., et al. Heat shock treatment enhances graft cell survival in skeletal myoblast transplantation to the heart. Circulation. 2000;102:III216–III221. doi: 10.1161/01.cir.102.suppl_3.iii-216. [DOI] [PubMed] [Google Scholar]
- Tang Y.L., et al. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J. Am. Coll. Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- Toma C., et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- Tomita S., et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II247–II256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- Valina C., et al. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur. Heart J. 2007;28:2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- Wang X., et al. Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells. 2009;27:3021–3031. doi: 10.1002/stem.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert K.C., et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- Zhang M., et al. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J. Mol. Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- Zhang M., et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.