Abstract
Objectives
A multiplex real-time polymerase chain reaction (RT-PCR) method was developed for the identification of three Vibrio species: Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus.
Methods
Specific primers and probes targeting the hlyA, tlh, and vvhA genes were selected and used for multiplex real-time PCR to confirm the identification of V. cholerae, V. parahaemolyticus, and V. vulnificus, respectively. This method was applied to screen Vibrio species from environmental samples and combining it with a culture-based method, its effectiveness was evaluated in comparison with culture-based methods alone.
Results
Specific PCR fragments were obtained from isolates belonging to the target species, indicating a high specificity of this multiplex real-time PCR. No cross-reactivity with the assay was observed between the tested bacteria. The sensitivity of the multiplex real-time PCR was found to have a lower limit of 104 colony-forming units/reaction for all three Vibrio species. The combination strategy raised the isolation ratio of all three Vibrio species 1.26- to 2.75-fold.
Conclusion
This assay provides a rapid, sensitive, and specific technique to detect these three Vibrio species in the environment.
Keywords: TaqMan probe multiplex real-time PCR, Vibrio cholerae, Vibrio parahaemolyticus, Vibrio vulnificus
1. Introduction
Vibrio is a genus of Gram-negative bacteria that possess a curved rod shape and naturally inhabits aquatic environments worldwide [1–5]. Within the genus Vibrio, several species are known to be important human pathogens [6–8]. Among these, Vibrio cholerae, Vibrio vulnificus, and Vibrio parahaemolyticus are the major pathogenic Vibrio species. V. cholerae and V. parahaemolyticus, contracted through consumption of contaminated seafood and seawater, can cause gastroenteritis whereas V. vulnificus can cause septicemia by exposure of an open wound to seawater or consumption of contaminated seafood [9–11]. In Korea especially, owing to the common practice of raw seafood consumption, gastroenteritis caused by infection with V. parahaemolyticus and septicemia caused by infection with V. vulnificus occur frequently.
Because cases of infection by Vibrio spp. are commonly found in coastal areas, it is prudent to investigate the distribution of the pathogenic Vibrio spp. in the coastal environment of Korea. However, the isolation of bacteria using conventional microbiological method is a time-consuming and laborious process that presents potential chance for error resulting in nondetection of Vibrio spp. present in environmental samples.
Real-time PCR is a rapid, sensitive, and highly specific technique. In many reports, real-time PCR has been used to detect various human and animal pathogens [12–19].
In the present study, we developed a simple multiplex PCR method based on TaqMan real-time PCR to detect V. parahaemolyticus, V. vulnificus, and V. cholerae in a single PCR reaction. In this assay, we selected specific primers and probes targeting the hemolysin genes of three Vibrio spp. for their identification. The hlyA, tlh, and vvhA genes are species-specific markers for V. cholerae, V. parahaemolyticus, and V. vulnificus, respectively [20–23].
This PCR method allowed for quick and easy isolation of three Vibrio species from environmental samples. Owing to its sensitivity, accuracy, and the potential increase in isolation rate in conjunction with other isolation methods, such as the use of chromogenic selective media, the method provides a rapid and effective detection tool for research and diagnostics.
2. Materials and Methods
2.1. Bacterial strains and DNA extraction
A total of 56 strains from the Korean Centers for Diseases Control and Prevention strain collection were used in this study (Table 1). These included five reference strains and 51 clinical and environmental strains. All strains were cultured on tryptic soy agar (Becton Dickinson and Company, Sparks, MD, USA) with overnight incubation at 37 °C.
Table 1.
Strains tested for assay specificity
| Species (No. isolates) | Gene target |
||
|---|---|---|---|
| hlyA | tlh | vvhA | |
| Vibrio cholerae (10) | + | – | – |
| Vibrio parahaemolyticus (10) | – | + | – |
| Vibrio vulnificus (10) | – | – | + |
| Vibrio mimicus (2) | – | – | – |
| Vibrio alginolyticus (1) | – | – | – |
| ETEC ATCC 43896 | – | – | – |
| ETEC (5) | – | – | – |
| EHEC ATCC 43895 | – | – | – |
| EAEC (3) | – | – | – |
| Shigella spp. (2) | – | – | – |
| Shigella flexneri (3) | – | – | – |
| Shigella sonnei (2) | – | – | – |
| Salmonella spp. (2) | – | – | – |
| Salmonella enteritidis (2) | – | – | – |
| Salmonella typhimurium (2) | – | – | – |
EAEC = enteroaggregative Escherichia coli; EHEC = enterohemorrhagic Escherichia coli; ETEC = enterotoxigenic Escherichia coli.
Genomic DNA extraction was performed by boiling. Briefly, one colony was suspended in 200 μL of sterile distilled water and boiled for 10 minutes to lyse the bacteria. After boiling, the suspension was centrifuged for 2 minutes at 14,000 rpm to sediment the cell debris. The supernatant was collected and used as a template for PCR.
2.2. Primer and TaqMan probe design
Suitable primers and probes for the multiplex TaqMan real-time PCR (amplifying the hlyA, tlh, and vvhA genes of V. cholerae, V. parahaemolyticus, and V. vulnificus, respectively) were designed using Primer Express (Applied Biosystems, Foster City CA, USA) and were synthesized by Applied Biosystems (Table 2). Each set of primers was used to amplify its respective target gene from the purified genomic DNA of three Vibrio species reference strains (V. cholera ATCC 14033, V. parahaemolyticus ATCC 17802, and V. vulnificus ATCC 27562). All PCR products were verified by confirming their expected sizes via electrophoresis in a 3% Nusieve 3:1 agarose gel Lonza Group Ltd., Basel, Switzerland.
Table 2.
Target genes, primers, and probes used for multiplex real-time PCR detection of the three Vibrio spp
| Vibrio spp. | Primer or probe sequence (5′ to 3′) | Target gene | Amplicon size (bp) | |
|---|---|---|---|---|
| V. cholerae | VCF | GCGTTGGGAGTGGCGTAA | hlyA | 57 |
| VCR | GGACTCGCCGCTGTAGACA | |||
| VCP | FAM-AGCACAGATGAATTGACCAMG-BNHQ | |||
| V. parahaemolyticus | VPF | AACCGTGGCGTTCCAGAA | tlh | 58p |
| VPR | CCGTCAAACGAATCAGTGCTT | |||
| VPP | VIC-TGAAAGCGGATTATGC-MGB | |||
| V. vulnificus | VVF | GATCGTTGTTTGACCGTAAACG | vvhA | 79p |
| VVR | TGCTAAGTTCGCACCACACTGT | |||
| VVP | NED-CAAAACGCTCACAGTCG-MGB | |||
F = forward primer; P = probe; R = reverse primer.
2.3. Multiplex TaqMan real-time PCR for detection of V. cholerae, V. parahaemolyticus, and V. vulnificus
The real-time PCR reactions were performed on a Roche LightCycler 480 (Roche Diagnostics Ltd., Penzberg, Germany). Typical reactions contained 10 μL of 2× probes Master (Roche Diagnostics Ltd.), 90 nM each of the hlyA, tlh, and vvhA forward and reverse primers, 200 nM of the TaqMan MGB probes for hlyA, tlh, and vvhA (Table 2), and 1 uL of DNA template in a total volume of 20 μL. The cycling conditions were as follows: 2 minutes at 50 °C and 5 minutes at 95 °C, followed by 40 cycles, each consisting of 15 seconds at 95 °C and 1 minute at 60 °C. Data were analyzed using the LightCycler480 software (Roche Diagnostics Ltd.). PCR amplification was detected directly by monitoring the increase in fluorescence of each dye-labeled probe. Samples with cycle threshold (Ct) value above 35 were considered as negative.
2.4. Specificity and sensitivity of detection by multiplex TaqMan real-time PCR
The specificity of each set of primers and probes for its respective target gene was determined by PCR amplification of the purified genomic DNA from 33 Vibrio spp. strains and 23 other bacterial strains.
To identify the lower limit of detection and generate standard curves, 10-fold serial dilutions from 101 to 107 colony-forming units (CFU)/mL of each reference strain were used. Reference strains were cultured in tryptic soy broth media at 37 °C to yield 109 CFU/mL. Bacterial growth was monitored by observation of the absorbance at 600 nm using a spectrophotometer (GeneQuant pro; Amersham Pharmacia Biotech Inc., Cambridge, UK). Ten-fold serial dilutions of bacteria in physiological saline, from 101 to 107 CFU/mL, were used to generate templates for multiplex real-time PCR. To determine the number of cells in the samples, 100 μL of each 10-fold serial dilution was spread on a trypticase soy agar plate and incubated at 37 °C for 16 hours, and the grown bacterial colonies were counted. The standard curves were determined by plotting the Ct values against the log CFU/reaction. Amplification efficiency was calculated using the equation E=10[−1/slope] − 1.
2.5. Application of multiplex real-time PCR as a screening method
Prior to the isolation of Vibrio species strains from the samples, real-time PCR was performed to detect the presence of V. cholerae, V. parahaemolyticus, and V. vulnificus in the samples. From May 2007 to December 2007, 2729 marine environmental samples (seawater, sediments, and plankton etc.) were collected from 11 Korean coastal areas. Ten milliliters or 10 g of the samples was added to 90 mL of alkaline peptone water (1% peptone, 1% NaCl, pH 8.4) and incubated 6–8 hours at 37 °C. After enrichment of the samples, a 1 mL portion of alkaline peptone water was removed, and the total DNA extracted by the boiling method previously described. The extracted DNA was used as a template for multiplex real-time PCR, as described above.
A loopful of each enrichment culture was also streaked onto thiosulfate citrate bile salts sucrose (TCBS; Beckton Dickinson and Company) agar plates and incubated for 18–20 hours at 37 °C. After incubation, candidate colonies from the plate corresponding to the PCR positive culture were replica plated onto CHROMagar Vibrio (CV; CHROMagar, Paris, France) and TCBS agar plates. By comparing the growth on the TCBS and CV agar plates, a candidate species could be identified for each colony: a colony that was green on TCBS and blue on CV was thought to be V. vulnificus; a colony that was green on TCBS and mauve on CV was thought to be V. parahaemolyticus; and a colony that was yellow on TCBS and blue on CV was thought to be V. cholerae. The colonies were further identified biochemically using an API 20E identification kit (bioMérieux, Inc., Hazelwood, MO, USA).
3. Results
3.1. Specificity and sensitivity of detection
The primers and probes were specifically designed to identify the hemolysin genes present in V. cholerae, V. parahaemolyticus, and V. vulnificus: the hlyA, tlh, and vvhA genes, respectively. PCR amplification with these primers yielded amplicons of the expected molecular weights (57 bp for hlyA, 58 bp for tlh, and 79 bp for vvhA; Figure 1, Table 2).
Figure 1.
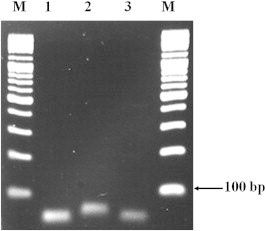
The molecular size of polymerase chain reaction (PCR) fragments amplified with species-specific primers. Agarose gel electrophoresis showing the results from a PCR amplification of genomic DNA from three Vibrio spp. Lane M: 100 bp DNA ladder; Lane 1, Lane 2, and Lane 3: amplified DNA using oligonucleotide primers specific for the hlyA, vvhA, and tlh genes showing specific bands of DNA of the expected sizes of 57 bp, 79 bp, and 58 bp, respectively.
The specificity of the primers and probes against 33 Vibrio spp. strains and 23 other bacterial strains was tested by multiplex real-time PCR. Specificity was confirmed by PCR amplification of the hemolysin genes of V. cholerae, V. parahaemolyticus, and V. vulnificus, respectively, but no amplification was detected for any other Vibrio spp. or non-Vibrio spp. (Table 1).
The aim of this study was to develop and evaluate a method for the simultaneous detection of V. cholerae, V. parahaemolyticus, and V. vulnificus using multiplex real-time PCR in a single sample. During development of the assay, no cross-reactivity between the tested bacteria was observed.
To determine the detection limit of the multiplex real-time PCR and to establish a standard curve for quantification, DNA extracted from 10-fold serial dilutions of three Vibrio spp. at final concentration of 101 – 107 CFU/reaction was analyzed by real-time PCR. Standard curves were constructed for three Vibrio spp. using a single target DNA in the mixture of three primer sets and three fluorescent probes. When only one Vibrio species target was amplified by the multiplex real-time PCR assay, the detection limits were 103 CFU/reaction. The amplification efficiencies were 98% for V. cholerae, 97% for both V. parahaemolyticus, and V. vulnificus, resulting in highly accurate standard curves (Figure 2).
Figure 2.
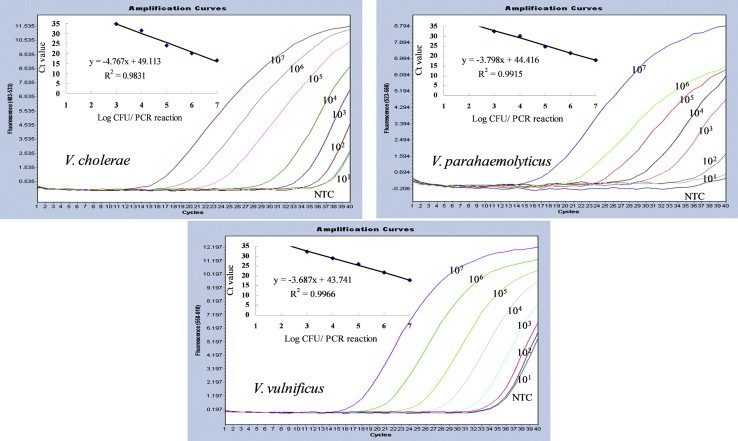
Multiplex real-time polymerase chain reaction (PCR) amplification plots and standard curves of single target genes for Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus, respectively. Standard curves were plotted for the log cell number of bacteria versus the number of cycles required to reach Ct. Samples were derived from DNA extracted from 10-fold dilutions of cells at concentrations of 101 – 107 colony-forming units/reaction. Although all samples were assayed in duplicate, only a single replicate is displayed for each sample for clarity. The equations of the lines obtained for V. cholerae, V. parahaemolyticus, and V. vulnificus were y = –4.767x + 49.113 (R2 = 0.9831), y = –3.798x + 44.416 (R2 = 0.9915), and y = –3.678x + 43.741 (R2 = 0.9966), respectively.
Next, simultaneous detection of three Vibrio species was attempted using a mixture of DNA from the same three Vibrio species. As a consequence, the detection limit decreased by 10-fold (104 CFU/reaction) for all three Vibrio spp. However, the amplification efficiency was similar to the 98% achieved with a single target for all three Vibrio spp., indicating that this assay effectively quantified each target (Figure 3).
Figure 3.
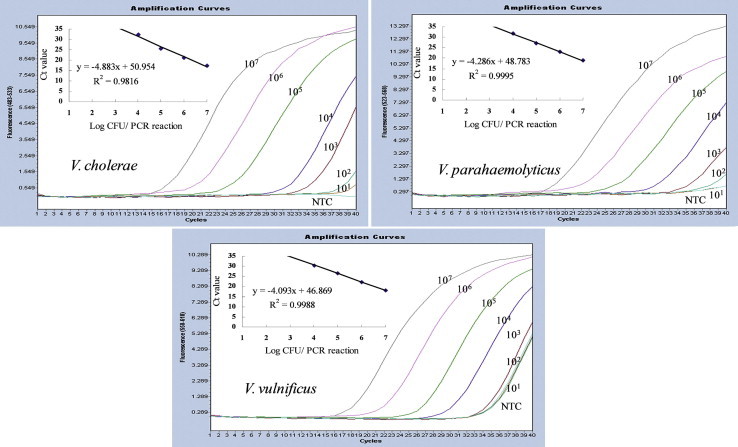
Multiplex real-time polymerase chain reaction (PCR) amplification plots and standard curves of three target genes for Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus, respectively. Standard curves were plotted for the log cell number of bacteria versus the number of cycles required to reach Ct. Samples were derived from DNA extracted from 10-fold dilutions of cells at concentrations of 101 – 107 colony-forming units/reaction. Although all samples were assayed in duplicate, only a single replicate is displayed for each sample for clarity. The equations of the lines obtained for V. cholerae, V. parahaemolyticus, and V. vulnificus were y = –4.883x + 50.954 (R2 = 0.9816), y = –4.286x + 48.783 (R2 = 0.9995), and y = –4.093x + 46.869 (R2 = 0.9988), respectively.
3.2. Application of real-time multiplex PCR as a screening method
The multiplex real-time PCR assay developed in this study was used to screen samples from a pilot surveillance study of marine Vibrio in Korea. Of the 2729 marine environmental samples tested by the multiplex real-time PCR assay, 2085 (64.7%) were positive for the tlh gene, 621 (19.3%) were positive for the vvhA gene, and 330 (10.3%) were positive for the hlyA gene (Table 3).
Table 3.
Test results for the multiplex real-time PCR assay and isolation of Vibrio spp. from environmental samples
| Vibrio spp. | No. positive (%) |
||
|---|---|---|---|
| 2007 |
2006 |
||
| RT-PCR (n = 2,729) | Isolates (n = 2,769) | Isolates (n = 5,445) | |
| V. cholerae | 330 (10.3) | 228 (5.6) | 213 (3.3) |
| V. parahaemolyticus | 2085 (64.7) | 1501 (37) | 1893 (29.3) |
| V. vulnificus | 621 (19.3) | 180 (4.4) | 106 (1.6) |
Data are presented as n (%).
Isolation of the three Vibrio spp. was attempted from samples that had tested positive in the multiplex real-time PCR assay. The most prominent Vibrio species, V. parahaemolyticus, was isolated from 1501 (37%) of the 2769 samples, whereas V. cholerae and V. vulnificus were found in 228 (5.6%) and 180 (4.4%) of the samples, respectively. V. parahaemolyticus was the most frequently detected of the three Vibrio species using both culture-based methods and multiplex real-time PCR.
The same surveillance project was performed in 2006, during the same seasonal period from May to December, prior to the development of multiplex PCR screening. In that study, V. parahaemolyticus was isolated from 1893 (29.3%) of the 5445 samples, followed by V. cholerae from 213 (3.3%), and V. vulnificus from 106 (1.6%; Table 3).
4. Discussion
With climate change, the threat of huge outbreaks of disease caused by Vibrio species such as V. cholerae is increasing. Consequently, the importance of surveillance of pathogenic Vibrio species present in the environment is also growing [24]. However, isolation of specific Vibrio species from the environment is not easy due to the difficulty associated with discriminating between Vibrio species on selective media. Additionally, in culture-based methods, colony confirmation is usually carried out using a panel of biochemical tests. In a previous study, O’Hara et al reported that the API 20E identification kit possessed an accuracy >90% for V. parahaemolyticus identification, but it was able to identify only 50% and 60% of V. cholerae and V. vulnificus strains, respectively [25]. Because commercial bacterial identification systems are used for clinical isolates and a comprehensive evaluation of the ability of these systems to identify bacteria accurately from environmental samples has not been performed, commercial systems may misidentify Vibrio spp. as other bacterial species [26–28]. The phenotypic variability of environmental strains and the close relationship among Vibrio spp. account for the failure to identify Vibrio variants accurately. It has been reported that some V. cholerae strains are unable to ferment sucrose [29]. In 2004–2006, the V. cholerae variants isolated from four outbreaks in Taiwan were misidentified as V. mimicus and V. alginolyticus using an API 20E identification kit. However, these strains were correctly identified by several molecular techniques, including PCR [30].
For these reasons, many molecular biological tools have been developed to detect Vibrio species more easily, and most of these are PCR-based methods [15,20,31]. These PCR methods are usually employed to detect only one species, and, even when multiplex PCR assays are used to detect multiple species, they are performed using conventional PCR methods, which have limited sensitivity and are time consuming [32,33].
In the present study, we developed and tested a TaqMan probe-based multiplex real-time PCR assay to detect V. cholerae, V. vulnificus, and V. parahaemolyticus in environmental samples after enrichment. Because this multiplex real-time PCR utilizes three fluorescent probes simultaneously in a single PCR reaction, the speed at which three Vibrio spp. can be detected is greatly increased. This assay provides results within approximately 3 hours of enrichment because there is no need for postamplification analysis, such as agarose gel electrophoresis, for confirmation of real-time detection. In addition, the threshold cycles affords a further advantage of semiquantitation.
We applied this multiplex real-time PCR method to screen marine environmental samples and performed culture-based isolation for the PCR-positive samples only. We compared the effectiveness of the combination of this multiplex real-time PCR and culture-based methods (samples collected in 2007) to culture-based methods alone (samples collected in 2006). The isolation ratios of V. cholerae, V. vulnificus, and V. parahaemolyticus were higher with the combination method than with the culture-based methods alone. The isolation ratio of V. vulnificus using combination methods was 2.75-fold higher than that of culture-based methods alone. The combination methods provided a higher isolation ratio than that of culture-based methods alone because we could neglect the samples that were PCR-negative and focus on which species to isolate from each of the PCR-positive samples.
In conclusion, we developed a multiplex real-time PCR assay for the simultaneous detection of V. cholerae, V. parahaemolyticus, and V. vulnificus in a single reaction. The multiplex real-time PCR provides a rapid, sensitive, and highly specific means for the detection of three Vibrio spp. from environmental and clinical samples. This technique might be useful to detect the three pathogenic Vibrio spp. during mass outbreaks and sporadic cases of vibriosis, as well as in contaminated seafood and wastewater. It could facilitate monitoring of pathogenic Vibrio contamination, thereby improving hygiene.
Acknowledgments
This research was supported by a grant from the Korea Centers for Disease Control and Prevention.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Blackwell K.D., Oliver J.D. The ecology of Vibrio vulnificus, Vibrio cholerae, and Vibrio parahaemolyticus in North Carolina estuaries. J Microbiol. 2008 Apr;46(2):146–153. doi: 10.1007/s12275-007-0216-2. [DOI] [PubMed] [Google Scholar]
- 2.Eiler A., Gonzalez-Rey C., Allen S., Bertilsson S. Growth response of Vibrio cholerae and other Vibrio spp. to cyanobacterial dissolved organic matter and temperature in brackish water. FEMS Microbiol Ecol. 2007 Jun;60(3):411–418. doi: 10.1111/j.1574-6941.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 3.Oliver J.D., Warner R.A., Cleland D.R. Distribution of Vibrio vulnificus and other lactose-fermenting vibrios in the marine environment. Appl Environ Microbiol. 1983 Mar;45(3):985–998. doi: 10.1128/aem.45.3.985-998.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamplin M., Rodrick G.E., Blake N.J., Cuba T. Isolation and characterization of Vibrio vulnificus from two Florida estuaries. Appl Environ Microbiol. 1982 Dec;44(6):1466–1470. doi: 10.1128/aem.44.6.1466-1470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright A.C., Hill R.T., Johnson J.A., Roghman M.C., Colwell R.R., Morris J.G., Jr. Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl Environ Microbiol. 1996 Feb;62(2):717–724. doi: 10.1128/aem.62.2.717-724.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty S., Nair G.B., Shinoda S. Pathogenic vibrios in the natural aquatic environment. Rev Environ Health. 1997 Apr–Jun;12(2):63–80. doi: 10.1515/reveh.1997.12.2.63. [DOI] [PubMed] [Google Scholar]
- 7.Hoge C.W., Watsky D., Peeler R.N., Libonati J.P., Israel E., Morris J.G., Jr. Epidemiology and spectrum of Vibrio infections in a Chesapeake Bay community. J Infect Dis. 1989 Dec;160(6):985–993. doi: 10.1093/infdis/160.6.985. [DOI] [PubMed] [Google Scholar]
- 8.Tarr C.L., Patel J.S., Puhr N.D., Sowers E.G., Bopp C.A., Strockbine N.A. Identification of Vibrio isolates by a multiplex PCR assay and rpoB sequence determination. J Clin Microbiol. 2007 Jan;45(1):134–140. doi: 10.1128/JCM.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin J.B., DePaola A., Bopp C.A. Outbreak of Vibrio parahaemolyticus gastroenteritis associated with Alaskan oysters. N Engl J Med. 2005 Oct;353(14):1463–1470. doi: 10.1056/NEJMoa051594. [DOI] [PubMed] [Google Scholar]
- 10.Morris J.G., Jr. Cholera and other types of vibriosis: a story of human pandemics and oysters on the half shell. Clin Infect Dis. 2003 Jul;37(2):272–280. doi: 10.1086/375600. [DOI] [PubMed] [Google Scholar]
- 11.Powell J.L. Vibrio species. Clin Lab Med. 1999 Sep;19(3):537–552. vi. [PubMed] [Google Scholar]
- 12.Gubala A.J. Multiplex real-time PCR detection of Vibrio cholerae. J Microbiol Methods. 2006 May;65(2):278–293. doi: 10.1016/j.mimet.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Halse T.A., Musser K.A., Limberger R.J. A multiplexed real-time PCR assay for rapid detection of Chlamydia trachomatis and identification of serovar L-2, the major cause of lymphogranuloma venereum in New York. Mol Cell Probes. 2006 Oct;20(5):290–297. doi: 10.1016/j.mcp.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 14.McDonough E.A., Barrozo C.P., Russell K.L., Metzgar D. A multiplex PCR for detection of Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, and Bordetella pertussis in clinical specimens. Mol Cell Probes. 2005 Oct;19(5):314–322. doi: 10.1016/j.mcp.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Panicker G., Bej A.K. Real-time PCR detection of Vibrio vulnificus in oysters: comparison of oligonucleotide primers and probes targeting vvhA. Appl Environ Microbiol. 2005 Oct;71(10):5702–5709. doi: 10.1128/AEM.71.10.5702-5709.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qvarnstrom Y., Visvesvara G.S., Sriram R., da Silva A.J. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J Clin Microbiol. 2006 Oct;44(10):3589–3595. doi: 10.1128/JCM.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi H., Hara-Kudo Y., Miyasaka J., Kumagai S., Konuma H. Development of a quantitative real-time polymerase chain reaction targeted to the toxR for detection of Vibrio vulnificus. J Microbiol Methods. 2005 Apr;61(1):77–85. doi: 10.1016/j.mimet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Ward L.N., Bej A.K. Detection of Vibrio parahaemolyticus in shellfish by use of multiplexed real-time PCR with TaqMan fluorescent probes. Appl Environ Microbiol. 2006 Mar;72(3):2031–2042. doi: 10.1128/AEM.72.3.2031-2042.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf S., Williamson W.M., Hewitt J. Sensitive multiplex real-time reverse transcription-PCR assay for the detection of human and animal noroviruses in clinical and environmental samples. Appl Environ Microbiol. 2007 Sep;73(17):5464–5470. doi: 10.1128/AEM.00572-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyon W.J. TaqMan PCR for detection of Vibrio cholerae O1, O139, non-O1, and non-O139 in pure cultures, raw oysters, and synthetic seawater. Appl Environ Microbiol. 2001 Oct;67(10):4685–4693. doi: 10.1128/AEM.67.10.4685-4693.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordstrom J.L., Vickery M.C., Blackstone G.M., Murray S.L., DePaola A. Development of a multiplex real-time PCR assay with an internal amplification control for the detection of total and pathogenic Vibrio parahaemolyticus bacteria in oysters. Appl Environ Microbiol. 2007 Sep;73(18):5840–5847. doi: 10.1128/AEM.00460-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright A.C., Morris J.G., Jr., Maneval D.R., Jr., Richardson K., Kaper J.B. Cloning of the cytotoxin-hemolysin gene of Vibrio vulnificus. Infect Immun. 1985 Dec;50(3):922–924. doi: 10.1128/iai.50.3.922-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z.H., Lou Y.L., Lu Y.Y., Yan J. Development of quantitative real-time polymerase chain reaction for the detection of Vibrio vulnificus based on hemolysin (vvhA) coding system. Biomed Environ Sci. 2008 Aug;21(4):296–301. doi: 10.1016/S0895-3988(08)60045-8. [DOI] [PubMed] [Google Scholar]
- 24.Emch M., Feldacker C., Islam M.S., Ali M. Seasonality of cholera from 1974 to 2005: a review of global patterns. Int J Health Geogr. 2008 Jun;7:31. doi: 10.1186/1476-072X-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Hara C.M., Sowers E.G., Bopp C.A., Duda S.B., Strockbine N.A. Accuracy of six commercially available systems for identification of members of the family Vibrionaceae. J Clin Microbiol. 2003 Dec;41(12):5654–5659. doi: 10.1128/JCM.41.12.5654-5659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Croci L., Suffredini E., Cozzi L. Comparison of different biochemical and molecular methods for the identification of Vibrio parahaemolyticus. J Appl Microbiol. 2007 Jan;102(1):229–237. doi: 10.1111/j.1365-2672.2006.03046.x. [DOI] [PubMed] [Google Scholar]
- 27.MacDonell M.T., Singleton F.L., Hood M.A. Diluent composition for use of API 20E in characterizing marine and estuarine bacteria. Appl Environ Microbiol. 1982 Aug;44(2):423–427. doi: 10.1128/aem.44.2.423-427.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez-Urtaza J., Lozano-Leon A., Viña-Feas A., de Novoa J., Garcia-Martin O. Differences in the API 20E biochemical patterns of clinical and environmental Vibrio parahaemolyticus isolates. FEMS Microbiol Lett. 2006 Feb;255(1):75–81. doi: 10.1111/j.1574-6968.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- 29.Ansaruzzaman M., Rahman M., Kibriya A.K., Bhuiyan N.A., Islam M.S., Albert M.J. Isolation of sucrose late-fermenting and nonfermenting variants of Vibrio cholerae O139 Bengal: implications for diagnosis of cholera. J Clin Microbiol. 1995 May;33(5):1339–1340. doi: 10.1128/jcm.33.5.1339-1340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei S.W., Chern L.L., Wu Y.C., Wang Y.L., Lin C.M., Chiou C.S. Foodborne disease outbreaks caused by sucrose-nonfermenting and beta-galactosidase-deficient variants of Vibrio cholerae. Int J Food Microbiol. 2008 Feb;122(1–2):148–155. doi: 10.1016/j.ijfoodmicro.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 31.Panicker G., Myers M.L., Bej A.K. Rapid detection of Vibrio vulnificus in shellfish and Gulf of Mexico water by real-time PCR. Appl Environ Microbiol. 2004 Jan;70(1):498–507. doi: 10.1128/AEM.70.1.498-507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aridgides L.J., Doblin M.A., Berke T., Dobbs F.C., Matson D.O., Drake L.A. Multiplex PCR allows simultaneous detection of pathogens in ships' ballast water. Mar Pollut Bull. 2004 Jun;48(11–12):1096–1101. doi: 10.1016/j.marpolbul.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Bauer A., Rørvik L.M. A novel multiplex PCR for the identification of Vibrio parahaemolyticus, Vibrio cholerae and Vibrio vulnificus. Lett Appl Microbiol. 2007 Oct;45(4):371–375. doi: 10.1111/j.1472-765X.2007.02195.x. [DOI] [PubMed] [Google Scholar]


