Abstract
Numerous studies have demonstrated that targeting Ag to Fc receptors (FcR) on APCs can enhance humoral and cellular immunity. However, studies are lacking that examine both the use of FcR-targeting in generating immune protection against infectious agents and the use of FcRs in the induction of mucosal immunity. Francisella tularensis is a category A intracellular mucosal pathogen. Thus, intense efforts are underway to develop a vaccine against this organism. We hypothesized that protection against mucosal infection with F. tularensis would be significantly enhanced by targeting inactivated F. tularensis live vaccine strain (iFt) to FcRs at mucosal sites, via intranasal immunization with mAb-iFt complexes. These studies demonstrate for the first time that: 1) FcR-targeted immunogen enhances immunogen-specific IgA production and protection against subsequent infection in an IgA-dependent manner, 2) FcγR and neonatal FcR are crucial to this protection, and 3) inactivated F. tularensis, when targeted to FcRs, enhances protection against the highly virulent SchuS4 strain of F. tularensis, a category A biothreat agent. In summary, these studies show for the first time the use of FcRs as a highly effective vaccination strategy against a highly virulent mucosal intracellular pathogen.
Numerous studies have demonstrated that targeting Ag to Fc receptors (FcR),3 including FcγRs, on APCs can enhance humoral and cellular immunity (1–13). FcγRs are classified based on their molecular weight, IgG-Fc-binding affinities, IgG subclass-binding specificity, and cellular distribution. Three subtypes of FcγRs have been described in mice and humans: FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16). FcγRI is a high-affinity FcγR, which binds monomeric and multimeric IgG. It is also an activating receptor and contains an ITAM in its cytoplasmic domain. FcγRIIA, an FcγRII allotype, is a low-affinity FcγR that binds multimeric IgG only and is also an activating FcγR. In contrast, FcγRIIB, also an FcγRII allotype, is a low-affinity FcγR that binds multimeric IgG only, but it expresses an ITIM in its cytoplasmic domain. As a result, FcγRIIB, the only FcγR expressed on B cells, is unique among FcγRs, in that it is the only FcγR with the ability to inhibit B lymphocyte activation and thus Ab production (14, 15). Importantly, recent studies have demonstrated that enhanced immune responses can be generated with mAb-Ag complexes despite mAb-Ag interaction with FcγRIIB. These studies indicate that while such interactions limit the degree of enhancement, they do not prevent it (16). Furthermore, when targeting Ag to FcγRs, adjuvant is not required, and both cellular and humoral immunity can be enhanced (1, 4, 10). Thus, targeting Ag to FcγRs makes it possible to generate protection against both extracellular and intracellular pathogens in the absence of such adjuvants. For example, in the absence of alum, tetanus toxoid (TT)-C fragment-Fc fusion protein was superior to the commercial vaccine (TT plus alum) at inducing TT-specific Ab in vivo (17). Additionally, when mice were immunized with the TT-C fragment-Fc fusion protein and then challenged with tetanus toxin, they were protected. Additionally, studies targeting “weak Ag” to human FcγRI (hFcγRI) using hFcγRI transgenic mice also demonstrated that Ag-specific Ab responses could be enhanced as a consequence of targeting Ag to FcγRs (8). Furthermore, FcR targeting of immunogens is not limited to FcγR. Studies targeting Ag to receptors for IgA and IgE have also demonstrated that these receptors can serve as targets for generating enhanced immune responses (5, 13, 18). However, there is a general lack of studies that examine the use of FcR targeting in generating immune protection against infectious agents, and in particular there is a paucity of studies aimed at defining the role of FcRs in the generation of mucosal immunity.
With regard to pulmonary immunity, at issue in this report, both the neonatal FcR (FcRn) and FcγRs could play a significant role in generating immune protection. In fact, recent work has suggested that FcRn is present in adult lungs of mice and humans, and that it can transport FcRn-targeted Ag to FcR-bearing APCs within mucosal lymphoid tissues (19–22). Furthermore, FcγRs, which have been demonstrated to mediate enhanced immune responses in vitro and in vivo (1–13), are present on APCs at mucosal sites. Specifically, FcγRI is constitutively expressed on macrophages and dendritic cells (23–7), while FcγRII and FcγRIII are constitutively expressed on a number of cell types, including APCs. Based on the above, and in particular on the demonstrated ability of FcR-targeted Ag to enhance cellular and humoral immunity (1–3), we hypothesized that protection against mucosal infection with Francisella tularensis would be significantly enhanced by targeting inactivated F. tularensis live vaccine strain (LVS) (iFt) to FcRs at mucosal sites via intranasal (i.n.) immunization with mAb-iFt complexes.
F. tularensis is a Gram-negative intracellular mucosal pathogen, and it has been designated as a category A biothreat agent based, in part, on the fact a single organism can be lethal when inhaled (28). Both cellular and humoral immunity have now been shown to mediate varying levels of protection against infection with this organism, but no approved vaccine is currently available (28–33). Therefore, these studies are designed to determine whether iFt, when targeted to FcRs in the form of mAb-iFt at a mucosal site, would enhance protection against challenge with this infectious agent. Importantly, results of these studies fill a significant gap in our knowledge regarding the use of FcR-targeted Ag as an immunogen, while establishing FcR targeting as an alternative and viable vaccine strategy for enhancing mucosal immunity to both intracellular and extracellular pathogens.
Materials and Methods
Mice
BALB/c and C57BL/6 mice were procured from Taconic Farms. IgA−/− mice with a C57BL/6 × 129 background were bred at Albany Medical College (Albany, NY). The B6.129×1-Fcrgttm1Dcr/Dcr (FcRn−/−) and (C57BL6 × 129) F1 hybrids were obtained from The Jackson Laboratory, while FcγR−/− mice on C57BL/6 × 129 background were procured from Taconic Farms. All mice were housed in the Animal Resources Facility at Albany Medical College. Mice were provided with ad lib water and food during the course of each experiment. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee.
Cells
RAW 264.7 cells are a FcγRI/FcγRIIB-expressing mouse myeloid cell line (American Type Culture Collection). Cells were grown in DMEM with 4.5 g/L glucose and L-glutamine without sodium pyruvate (Mediatech) containing 10% FBS (HyClone) and 1 mg/ml gentamicin (Life Technologies).
Generation of immunogen (iFt and mAb-iFt complexes)
GFP-expressing F. tularensis LVS organisms were provided by Dr. Mats Forsman (Swedish Defense Research Agency, Stockholm). GFP-expressing organisms were used to facilitate the monitoring of iFt vs mAb-iFt binding to myeloid FcRs. To inactivate these organisms, 1 × 1010 CFU/ml live GFP-expressing F. tularensis LVS were incubated in 1 ml of sterile PBS (Mediatech) containing 2% paraformaldehyde (Sigma-Aldrich) for 2 h at room temperature. Fixed bacteria (iFt) were then washed with sterile PBS three times. Importantly, while very resistant to fixation, GFP fluorescence may be reduced as a result (34). However, as indicated in subsequent studies, GFP fluorescence was maintained despite fixation. Furthermore, any reduction in fluorescence is most likely uniformly distributed, and the ability to detect differential binding of iFt vs mAb-iFt to myeloid cells was not affected. Inactivation was verified by culturing a 100-µl sample (1 × 109 CFU) of the iFt on chocolate agar plates (Fisher Scientific) for 10 days. To make mAb-iFt, mouse IgG2a anti-F. tularensis LPS mAb (Fitzgerald Industries International) was added at a final concentration of 1, 5, 10, and 30 µg/ml to iFt in PBS (1 × 109 CFU/ml) and then incubated overnight on a rocker at 4°C. Following overnight incubation, the mAb-iFt complexes were washed three times with sterile PBS and then resuspended in 1 ml of PBS.
Analysis of iFt and mAb-iFt binding to Fc receptors
Binding of mAb-iFt to FcγR-bearing RAW cells was visualized by flow cytometry. RAW cells were washed three times with PBS/BSA/azide. Next, 5 × 105 cells in 20 µl PBS containing 0.02% azide (Sigma-Aldrich) was added to each well of a 96-well plate, followed by 40 µl (4 × 107 CFU) of mAb-complexed or noncomplexed iFt. The cells were then incubated for ∼1 h on a rocker at 4°C and washed three times with 200 µl/well cold PBS/BSA/azide, followed by the addition of 200 µl of 2% paraformaldehyde in PBS. The samples were analyzed on a FACSCanto flow cytometer (BD Biosciences).
Immunization and challenge experiments
Mice were divided into three groups consisting of 5–6 mice/group, 8–12 wk of age. Each mouse was administered i.n. with either 20 µl of PBS (vehicle), 2 × 107 CFU of iFt, or 2 × 107 CFU of iFt in the form of mAb-iFt. Before immunization, mice were bled and serum was isolated and tested for the presence of F. tularensis-specific Ab. Unless otherwise indicated, mice were immunized on day 0 and boosted on day 21. Immunized mice were then challenged on day 35 i.n. with 2 × 104 CFU of F. tularensis LVS or ∼20 CFU of F. tularensis SchuS4, and they were subsequently monitored for survival for at least 21 days. Exact CFU administered were also verified by culturing and counting the inoculum subsequent to challenge.
Quantification of bacterial burden
Following immunization and challenge, mice were euthanized at various intervals, and tissues such as lung, liver, and spleen were collected aseptically in PBS containing a protease inhibitor mixture (1 tablet in 10 ml sterile PBS) (Roche Diagnostics) and subjected to mechanical homogenization using a MiniBeadbeater-8 (BioSpec Products). Supernatants were then diluted 10-fold in sterile saline, and 10 µl of each dilution was spotted onto chocolate agar plates in duplicate and incubated at 37°C for 2 days. The number of colonies on the plates were counted and expressed as log10 CFU/ml for the respective tissue. The remaining tissue homogenate was spun at 14,000 × g for 20 min, and the clarified supernatant was stored at −20°C for cytokine analysis.
In vivo cytokine production
Tissue homogenates were obtained as indicated above for measuring bacterial burdens. Additionally, however, tissue homogenates were then spun at 14,000 × g for 10 s in a microcentrifuge (Eppendorf) to remove tissue debris. Mouse inflammation and Th1/Th2 cytometric bead array (CBA) kits (BD Pharmingen) were then used for the simultaneous measurement of multiple cytokines in tissue homogenates. Data were acquired on a FACSArray instrument (BD Immunocytometry Systems) and analyzed using CBA software version 1.0.1 (BD Immunocytometry Systems).
In vitro cytokine production
Spleen cells were harvested from PBS, iFt, or mAb-iFt immunized mice and cultured as previously described, with some modification (1). Briefly, to the individual wells of a 24-well plate, 1 ml of RPMI 1640 plus 10% FBS containing 4 × 106 spleen cells was added. Then, to appropriate wells, 100 µl of 4 × 106 CFU iFt in the above culture medium was added. As a negative control, 100 µl of RPMI 1640 plus 10% FBS without iFt was added. Spleen cell cultures were then placed in a humidity chamber at 37°C, 5% CO2, and supernatants were harvested at 3, 5, or 7 days of incubation. Supernatants were then analyzed for cytokine production using the CBA assay as indicated above.
Histological evaluation
Following immunization and challenge, mice were euthanized at various intervals, and tissues including lung, liver, and spleen were collected aseptically in 10% neutral-buffered formalin (Richard-Allan Scientific) and embedded in paraffin (Richard-Allan Scientific). Paraffin-embedded sections (3 µm) were stained with H&E (Richard-Allan Scientific), and disease severity was characterized based on cellular infiltration and pathological findings in the various tissues.
Measurement of F. tularensis-specific Ab production
Anti-F. tularensis Ab production in response to immunization with mAb-iFt vs iFt was measured by ELISA. Briefly, ELISA plates (Nalge Nunc International) were coated with 100 µl of F. tularensis LVS (5 × 107 CFU/ml) in carbonate buffer (4.3 g/L sodium bicarbonate (Sigma-Aldrich) and 5.3 g/L sodium carbonate (Sigma-Aldrich) (at pH 9.4) for 2 h at 37°C and then used immediately or incubated overnight at 4°C. The plates were washed with PBS containing 0.05% Tween 20 (Fisher Scientific) and blocked for 2 h with 200 µl of PBS containing 10% BSA (Baxter Healthcare Corporation). Serial 2-fold dilutions of sera (starting with 1/50) and bronchoalveolar lavage (BAL) fluid (starting with 1/1) were added to the plates (100 µl/well) and incubated for 90 min at 37°C. After washing, biotin-conjugated goat anti-mouse Abs specific for IgG or IgA (Caltag) were added and incubated for 1 h at 37°C. Plates were washed and then 100 µl/well of streptavidin conjugated to HRP (Caltag) was added. The plates were incubated for 20 min followed by addition of 100 µl of TMB peroxidase substrate (KPL). After 20 min, the reaction was stopped using 1.8 N sulfuric acid (Fischer Scientific), and the plates were read at 650 nm using a kinetic microplate reader (Molecular Devices). BSA-coated wells were used as specificity controls for F. tularensis LVS.
Statistical analysis
To compare survival curves, Kaplan-Meier log-rank analyses were used. Statistical data for bacterial burden and cytokine analysis were generated using ANOVA on day 7, which is the peak of infection. Ab titers were assessed by using the unpaired two-tailed t test. GraphPad Prism 4 provided the software for the statistical analysis.
Results
Enhanced protection against F. tularensis LVS challenge following i.n. immunization with mAb-iFt complexes
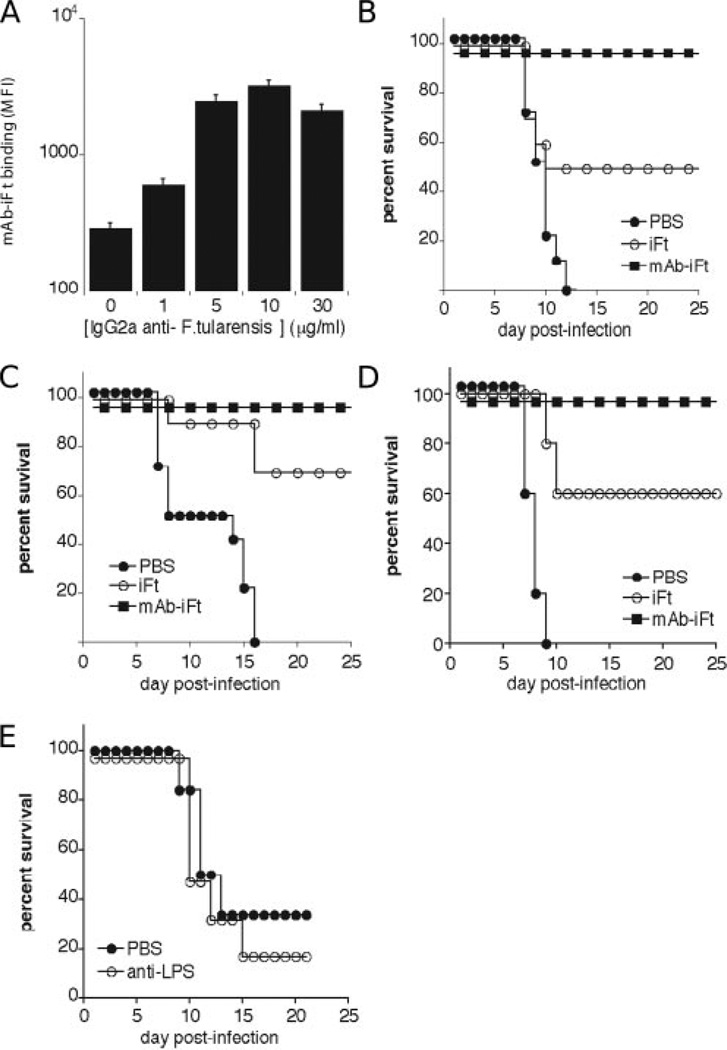
Based on studies demonstrating the generation of FcR-enhanced humoral and cellular immune responses, when targeting Ag to FcRs (1, 2, 4, 5, 7, 10–13, 35, 36), we hypothesized that targeting inactivated F. tularensis LVS (iFt) to FcR at a mucosal site would enhance protection against F. tularensis challenge. To test this hypothesis, mice were immunized i.n. with either vehicle (PBS), iFt, or mAb-iFt complexes and subsequently challenged with F. tularensis LVS. To create mAb-iFt complexes, iFt was combined with a mouse IgG2a anti-F. tularensis LPS mAb as described in Materials and Methods. To confirm successful mAb-iFt complex formation, mAb-iFt complexes were then incubated with an FcR-bearing mouse myeloid cell line (RAW), and binding of mAb-iFt vs iFt to these cells was monitored by flow cytometry. As would be expected for mAb-iFt complexes, enhanced binding of mAb-iFt was observed when compared with noncomplexed iFt (Fig. 1A). Furthermore, the level of enhanced mAb-iFt binding varied with mAb/iFt ratio, with submaximal binding occurring when mAb-iFt complexes were made with 1 µg/ml mAb, and maximal binding occurring at 10 µg/ml mAb. Since published data suggest that anti-LPS Ab production dominates the anti-F. tularensis LVS response and may contribute to protection (37–39), we initially chose to use mAb-iFt complexes made with 1 µg/ml mAb (Fig. 1A), thereby minimizing the potential interference of excess anti-LPS mAb in the generation of anti-LPS responses. When using the latter mAb-iFt complex as an i.n. immunogen for either BALB/c or C57BL/6 strains of mice, and subsequently challenging these mice with F. tularensis LVS at 4 × LD50 14 days postimmunization, we observed significantly better protection (100%) compared with iFt (between 50 and 65%) (Fig. 1, B and C). Similar results were also obtained in C57BL/6 mice following the same immunization regimen, but challenging with F. tularensis LVS 30 days postimmunization (Fig. 1D). Importantly, the latter, in particular, implies that immune memory is generated following mAb-iFt immunization.
FIGURE 1.
IgG2a mAb-iFt complexes enhance both iFt binding to FcR-bearing mouse myeloid cells and protection against i.n. challenge with F. tularensis LVS., A, IgG2a mAb-iFt was made as described in Materials and Methods. Briefly, mouse IgG2a anti-F. tularensis LPS mAb was incubated with formaldehyde-fixed GFP-expressing F. tularensis LVS (iFt), and the complex was then washed to remove free IgG2a mAb. The concentration of mAb added to iFt to form complexes was varied, as indicated in the x-axis. IgG2a mAb-iFt or iFt was then combined with RAW cells at 4°C for 1–2 h, unbound iFt was washed away, samples were formaldehyde-fixed, and flow cytometry was subsequently conducted to measure iFt binding to RAW cells. Data are expressed as mean fluorescence intensity (MFI) ± SD. Similar results were obtained in three separate experiments when using either mouse (RAW) or human (U937) FcR-expressing myeloid cells. Challenge studies were conducted as follows: BALB/c (B) and C57BL/6 (C and D) mice were divided into three groups and immunized i.n. with either 20 µl of vehicle (PBS), 20 µl of 2 × 107 inactivated organisms (iFt), or 20 µl of 2 × 107 inactivated organisms with mAb attached (mAb-iFt). Mice were immunized on day 0 and boosted on day 21. Mice were then challenged i.n. on day 35 (B and C) or on day 50 (D) with 2 × 104 CFU (4 × LD50) of live F. tularensis LVS and subsequently monitored for 21 days for survival. Survival curves are presented. The survival data in both B and C represent combined data from two separate experiments (a combined total of 10–12 mice/group). The p values of the iFt groups vs the mAb-iFt groups in B–D were <0.05., E, Two groups of 5 mice each were administered with either PBS or anti-LPS mAb (5 µg) on day 0. Both groups were then challenged with 8000 CFU of F. tularensis LVS on day 14 postimmunization and their survival was monitored. The p value between the PBS- and anti-LPS-immunized groups was >0.1, indicating a lack of significant difference.
To exclude the possibility that the enhanced protection observed was due to the administration of anti-LPS mAb, independent of the presence of iFt, anti-LPS mAb was administered in an amount substantially higher than that present in mAb-iFt complexes, in which free (unbound) anti-LPS mAb is removed before use. Additionally, mice were challenged with 8,000 CFU F. tularensis LVS, below that used in prior challenge studies (20,000 CFU). Despite providing these more favorable conditions for the observation of anti-LPS-mediated protection, most anti-LPS-immunized mice succumbed to infection, with PBS-immunized mice actually appearing slightly more resistant to challenge than the former (Fig. 1E). Further evidence of mAb-iFt-enhanced protection was provided when examining bacterial burden following i.n. immunization with mAb-iFt vs iFt, and subsequent challenge with F. tularensis LVS. Although results showed no difference in bacterial burden in the lungs of mAb-iFt- and iFt-immunized mice at 1 and 3 days postchallenge, a 10–20-fold reduction in bacterial burden was detected in mAb-iFt- vs iFt-immunized mice by day 7 (Fig. 2A). Importantly, it was not until day 7 postchallenge that PBS-and iFt-immunized mice began to die (Fig. 1). Similar differences in bacterial burden were apparent in liver and spleen as early as day 3 (Fig. 2, B and C, respectively), with bacteria being cleared from all the organs of the surviving mice by day 21 (data not shown.).
FIGURE 2.
Bacterial burden is reduced in mAb-iFt-immunized mice compared with that of iFt-immunized mice. BALB/c mice were immunized as described in Fig. 1. Bacterial burden studies were then conducted as described in Materials and Methods. Briefly, lung, liver, and spleen were harvested at 1, 3, 7, and 14 days postchallenge and bacterial counts conducted. Each point represents the average of 3 mice sacrificed per time point ± SD. On day 7, mAb-iFt-treated mice had significantly lower bacterial burden in tissues than did iFt-treated mice (p< 0.001), as indicated by **. On day 14, all PBS-treated mice were dead, as indicated by a plus sign (+) on the x-axis. These data are representative of two independent experiments.
Consistent with the observed increase in survival noted above, as well as the reduction of bacterial burden in the lungs, liver, and spleen when immunizing with mAb-iFt, a reduction in inflammation in the same tissues was also apparent (Fig. 3). Specifically, in lung sections (×29) from PBS- and iFt-immunized groups (Fig. 3, A and D), alveolar macrophage and some neutrophils (cellular unfiltrate (CI)) were intermingled around the peribronchial region (PB). Alveolar septa were thickened and showed increased numbers of mononuclear cells. Some inflammatory cells were also seen in alveoli and air passages (AV). In contrast, the air passages and alveoli in sections from mAb-iFt-immunized mice were clear (Fig. 3G) and only mild congestion was observed. The infiltration of neutrophils was minimal. In liver sections (×10) from the PBS group (Fig. 3B), venous and sinusoidal congestion (V/SC) was observed, as well as widely distributed clusters of hepatocyte degeneration (HD), with pyknotic nuclei. The latter were surrounded by clusters of mononuclear cells and neutrophils (CI). Results were similar in the iFt group (Fig. 3E). In contrast, in the mAb-iFt group there were minimal inflammatory changes other than congestion of blood vessels (Fig. 3H). In spleen sections (×4) from PBS and iFt groups there was mild to moderate expansion of red pulp (RP) (Fig. 3, C and F). The germinal centers (GC) were reduced in size with infiltration of neutrophils (CI). In spleen sections from the mAb-iFt group, there was a normal distribution of red pulp and white pulp (WP) (Fig. 3I). However, in the case of some white pulp, there was infiltration of small numbers of neutrophils.
FIGURE 3.
Inflammation in the lungs, liver, and spleen of mAb-iFt-immunized mice is reduced as compared with that of iFt-immunized mice. BALB/c mice were immunized and challenged as indicated in Fig. 1. Tissues were then harvested 5 days postchallenge, fixed with paraformalde-hyde, paraffin-embedded, sectioned, and stained with H&E., J, K, and L, Tissues taken from normal (unimmunized and unchallenged) mice. These data are representative of two independent experiments. PB, peribronchial region; AV, alveoli and air passage; HD, hepatocyte degeneration; V/SC, venous and sinusoidal congestion; RP, red pulp; WP, white pulp; GC, germinal center; CI, cellular infiltrate.
Finally, levels of inflammatory cytokines within the lungs of immunized and challenged mice were measured. Consistent with the reduced inflammation in mAb-iFt-immunized mice noted above, IL-6, CCL2, IFN-γ, and TNF-α levels were also reduced in the lungs, livers, and spleens of mice immunized with mAb-iFt vs iFt. (Fig. 4). Thus, based on four separate parameters used to evaluate protection (survival, bacterial burden, tissue inflammation, and inflammatory cytokine production), enhanced protection against F. tularensis infection is observed when immunizing i.n. with mAb-iFt complexes.
FIGURE 4.
Inflammatory cytokine levels are reduced in the lungs of mAb-iFt- vs iFt-immunized mice. IL-6, CCL2, IFN-γ, and TNF-α were measured after immunization and challenge, as described in Fig. 1. Specifically, mice were immunized i.n. with mAb-iFt vs iFt on day 0, boosted on day 21, and then challenged on day 35 with 2 × 104 CFU of live F. tularensis LVS. Mice were then sacrificed at varying time points postchallenge (A–D) or on day 7 postchallenge (E–H), at which point the lungs, spleen, and livers were obtained and homogenized, and then supernatants from the homogenized tissues were analyzed by CBA analysis. Data represent the average of 4–6 BALB/c mice/treatment group ± SD. *p< 0.05; **p< 0.01. On day 7, IL-6, CCL2, and TNF-α levels in tissues from mAb-iFt-treated mice were significantly lower than those of iFt-treated mice (p< 0.05). These data are representative of two independent experiments.
Involvement of FcR in mAb-iFt-enhanced protection against F. tularensis LVS challenge
To confirm FcR involvement in mAb-iFt binding observed in Fig. 1A, several different approaches were used. First, RAW cells were preincubated with heat-aggregated mouse IgG to block FcγRs before addition of mAb-iFt complex. As indicated in Fig. 5A, 10 µg/ml heat-aggregated mouse IgG was sufficient to block 100% of mAb-iFt binding to RAW cells. Additionally, mAb-iFt complexes made using F(ab′)2 mAb failed to bind to these same cells, indicating a requirement for the Fc portion of the mAb (Fig. 5B). In an effort to define the specific Fc receptor types (I or II/III) involved in mAb-iFt binding to RAW cells, the latter were preincubated with monomeric IgG2a or F(ab′)2 2.4G2 mAb. Only FcγRI, the high-affinity FcγR, binds monomeric IgG, and thus monomeric IgG2a can compete with immune complexes for binding to FcγRI. The mAb 2.4G2 is specific for the IgG binding site of mouse FcγRII/III, and it can thus block immune complex binding to mouse FcγRII/III. To determine the optimal concentrations of these reagents for use in the blocking experiments, binding curves were first generated (Fig. 5C), and three blocking concentrations (high, medium, and low) were selected. As indicated in Fig. 5D, heat-aggregated mouse IgG and monomeric IgG2a almost completely blocked mAb-iFt binding to RAW cells at the highest concentrations used (Fig. 5C).
FIGURE 5.
Binding of mAb-iFt complexes to RAW cells is Fc/FcR dependent. In this case, mAb-iFt complexes were made using 1 µg/ml mAb only., A, Before addition of mAb-iFt to RAW cells, heat-aggregated mouse IgG was added at varying concentrations as indicated in the x-axis and incubated for 1 h at 4°C. The binding of mAb-iFt to RAW cells was then measured as indicated in Fig. 1A. B, Binding of mAb-iFt complexes generated using whole vs F(ab′)2 mAb was measured as in Fig. 1A. C, Binding curves for rat anti-mouse FcγRII/III 2.4G2 mAb and monomeric (nonspecific) mouse IgG2a to RAW cells were generated. RAW cells were incubated for 1–2 h at 4°C with varying concentrations of 2.4G2 mAb or nonspecific mouse IgG2a, as indicated in the x-axis. This was followed by three washes and addition of anti-rat or anti-mouse IgG (H+L)-specific mAb labeled with FITC, respectively., D, Heat-aggregated mouse IgG, 2.4G2 mAb, or nonspecific mouse IgG2a was added to cells before the addition of complexes and incubated for 1 h at 4°C, as in Fig. 5A. Three separate concentrations of each were used, based on binding curves generated for each in Fig. 4C, in the cases of 2.4G2 and IgG2a. Aggregated IgG concentrations used were 0.3 µg/ml (low), 3 µg/ml (medium), and 30 µg/ml (high); 2.4G2 concentrations used were 0.03 µg/ml (low), 0.3 µg/ml (medium), and 3 µg/ml (high); and nonspecific IgG2a concentrations used were 0.1 µg/ml (low), 1 µg/ml (medium), and 10 µg/ml (high). Blocking data are expressed as percentage blocking by heat-aggregated IgG, 2.4G2, or nonspecific IgG2a. Specifically, percentage blocking was calculated based on the reduction of the mean fluorescence intensity (MFI) following Ab addition to the RAW cells, as compared with the MFI levels in the absence of blocking Ab. Similar blocking results were obtained in three separate experiments.
In contrast, no blocking with 2.4G2 was observed in three separate experiments, even at saturating concentrations. To provide further evidence of FcR involvement in vivo, and to exclude a role for aggregate formation, which we did observe in the generation of mAb-iFt complexes, BALB/c and C57BL/6 mice were immunized with F(ab′)2 mAb-iFt vs mAb-iFt and iFt and subsequently challenged with F. tularensis LVS. In both cases, when immunizing with F(ab′)2 mAb-iFt, the level of protection was reduced to that of noncomplexed iFt (Fig. 6, A and B). Furthermore, knockout mice lacking either FcγRI/III (Fig. 6C) or FcRn (Fig. 6D) were not protected by iFt or mAb-iFt immunization. Thus, the above F(ab′)2 data strongly support a role for FcγRs in mAb-iFt-enhanced protection at the time of immunization, while the data using FcγRI/III or FcRn knockout mice support a role for FcγRI/III and FcRn in protection generated by both iFt and mAb-iFt. In the latter case, whether the critical role of these specific receptors includes their usage during immunization with mAb-iFt itself is less clear and will require further investigation.
FIGURE 6.
Enhanced protection against F. tularensis challenge, mediated by mAb-iFt in vivo, is Fc/FcR dependent. Challenge studies were conducted as described in Fig. 1. BALB/c (A) and C57BL/6 (B) mice (6 mice/group) were immunized with either PBS, iFt, mAb-iFt, or F(ab′)2 mAb-iFt and subsequently challenged, as described in Fig. 1. C, Either WT or FcγR−/− mice (6 mice/group) were immunized with PBS, iFt, or mAb-iFt and challenged, as described in Fig. 1. D, Either WT or FcRn−/− mice (6 mice/group) were immunized with PBS, iFt, or mAb-iFt and challenged, as described in Fig. 1. The survival of both genetically deficient mAb-iFt immunized mice (C and D) was significantly lower compared with their wild-type counterparts (p< 0.01). These data are representative of two independent experiments.
Enhanced F. tularensis-specific IgA production and IFN-γ secretion following mAb-iFt immunization correlates with, and is required for, protection against F. tularensis LVS challenge
Humoral immune responses have now been shown to play a role in resolving both extracellular and intracellular infections (40–45). Additionally, numerous studies have shown that targeting Ag to FcRs in vivo enhances Ag-specific IgG production (1, 8, 10). Furthermore, recent studies by the authors of this report indicate that Ab, including IgA, can play a significant role in protection against F. tularensis infection (32, 33). IgA is the predominant Ab found at mucosal sites, and thus enhancement of its production provides one possible means of enhancing protection against mucosal infection. Consistent with the latter, when mAb-iFt complexes, which generate 100% protection against F. tularensis LVS challenge, were used as immunogen, F. tularensis-specific IgA production was significantly enhanced in the BAL fluid and sera of both BALB/c and C57BL/6 mice above that of iFt (Fig. 7, A and B). Similarly, F. tularensis-specific IgA levels were also higher in mice receiving iFt, which provides 50–65% protection, compared with those immunized with PBS, which provides no protection (Fig. 7, A and B). In contrast to IgA levels, F. tularensis-specific IgG levels were significantly reduced when immunizing with mAb-iFt vs iFt (Fig. 7, C and D). Additionally, lack of IgA resulted in loss of protection, as the IgA−/− mice were not protected by mAb-iFt complexes or iFt (Fig. 8A). A similar result was also observed in the case of IFN-γ−/− mice, also indicating the importance of this type I cytokine in resolving infection with LVS (Fig. 8B). Furthermore, splenocytes from C57BL/6 mice immunized with either PBS, iFt, or mAb-iFt, and then cultured with fixed LVS, exhibited increased production of IFN-γ in the supernatants of iFt- and mAb-iFt-immunized mice, with the levels being 3-fold higher in splenocytes derived from the mAb-iFt- vs iFt-immunized group (Fig. 8C). In summary, these data indicate a clear correlation between enhanced F. tularensis-specific IgA and IFN-γ production following mAb-iFt immunization, as well as subsequent protection against a lethal F. tularensis LVS infection.
FIGURE 7.
Immunization with mAb-iFt results in enhanced production of F. tularensis-specific IgA and reduced F. tularensis-specific IgG production in mAb-iFt- vs iFt-immunized mice. BALB/c mice were divided into three groups (4–6 mice/group) and immunized i.n. with 20 µl of PBS, 20 µl of 2 × 107 organisms (iFt), or 20 µl of 2 × 107 organisms (mAb-iFt). Mice were immunized on day 0 and boosted on day 21, as in challenge studies in Fig. 1. BAL fluid and serum were then collected at 35 days and tested for the presence of anti-F. tularensis IgA and IgG by ELISA, as described in Materials and Methods. Ab data represent the mean of 4–6 mice ± SD. *p< 0.05; **p< 0.01. The levels of IgA were significantly higher in the BAL fluid and sera of the mAb-iFt- vs iFt-treated group (p< 0.01 and p< 0.05, respectively). These data are representative of two independent experiments.
FIGURE 8.
Enhanced protection against F. tularensis challenge, mediated by mAb-iFt in vivo, is IgA and IFN-γ dependent. IgA−/− (A) and IFN-γ−/− mice (B) (6–8 mice/group) were immunized with either PBS, iFt, or mAb-iFt and subsequently challenged, as described in Fig. 1. C57BL/6 × 129 mice were used for controls. In both cases the survival of the genetically deficient mAb-iFt-immunized mice was significantly lower compared with their immunized controls (p< 0.01)., C, Wild-type mice (5/group) were immunized with either PBS, iFt, or mAb-iFt. On day 35, splenocytes were obtained and cultured in the presence of fixed LVS. The IFN-7 levels in the supernatants were measured by CBA assay at days 3, 5, and 7. Cytokine levels in the mAb-iFt immunized group supernatants were significantly higher compared with those of the iFt group (p< 0.01), as indicated by **. Data in A and C are representative of two independent experiments.
Immunization with mAb-iFt generates protection against the highly virulent F. tularensis SchuS4 strain
Finally, we wanted to determine whether targeting iFt to FcR in the form of mAb-iFt administered i.n. would enhance protection against the highly virulent form of F. tularensis, SchuS4. In this case, mice were challenged with 10–20 × LD50 to increase the significance of any protection observed. When immunizing C57BL/6 mice with mAb-iFt made from F. tularensis LVS, we observed between 40 and 50% protection against SchuS4 challenge, as opposed to immunization with PBS or iFt, which provided no protection (Fig. 9). Thus, targeting iFt to FcRs not only represents a potential mechanism for stimulating protection against more traditional pathogens of moderate virulence, but also the highly virulent strain of F. tularensis, SchuS4.
FIGURE 9.
Protection against mucosal F. tularensis SchuS4 challenge is also enhanced in mice immunized with mAb-iFt vs iFt. C57BL/6 mice were divided into three groups and immunized i.n. with 20 µl of PBS, 20 µl of 2 × 107 organisms (iFt), or 20 µl of 2 × 107 organisms (mAb-iFt). In the latter case, mAb-iFt was made using 5 µg/ml mAb. Mice were then immunized on days 0, 14, and 28. Mice were then challenged on day 42 i.n. with 18 CFU (A) or 23 CFU (B) (∼10–20 × LD50) of live F. tularensis SchuS4 and subsequently monitored for survival. Survival curves are presented., A and B represent two independent experiments. Each experiment contained 6 mice/group. The increased survival of the mAb-iFt group over that of the PBS and iFt group was significant in A and B (p< 0.01 and p< 0.05, respectively).
Evidence for the involvement of immune memory in mAb-iFt-induced protection
In order for FcR targeting to be a successful a vaccine strategy, it will be important to demonstrate that immunization with mAb-iFt generates immune memory. Ultimately, to accomplish this fully, a number of long-term experiments will be required to determine the length of protection generated by mAb-iFt. It is also likely that further optimization of this strategy will be possible in the interim. However, we do address this issue in a series of short-term experiments. Specifically, data are provided in Fig. 1D demonstrating that 100% protection against F. tularensis LVS challenge is maintained following immunization with mAb-iFt, even when the challenge occurs 30 days after mAb-iFt immunization. Additionally, anti-F. tularensis-specific IgA production, an adaptive immune response in which immune memory plays an integral role, is enhanced following mAb-iFt immunization and is required for the protection observed (Figs. 7 and 8). Furthermore, survival following a single immunization with mAb-iFt and subsequent F. tularensis SchuS4 challenge (14 days postimmunization) was not observed (Fig. 10A). This latter experiment not only indicates that the induction of innate effector cells 14 days after mAb-iFt immunization is not responsible for the protection observed, but also that booster immunization with mAb-iFt and the likely induction of an amnestic/recall response are required. Lastly, an experiment in which mice were challenged 42 days postimmunization with 40 CFU of F. tularensis SchuS4 also demonstrated increased survival in the mAb-iFt-immunized group, versus no survival in the PBS-and iFt-immunized groups (Fig. 10B). While the level of survival in this particular case was low (20%), the observation is consistent with a similar F. tularensis LVS challenge experiment (Fig. 1D). Additionally, the lower level of survival observed could be attributed, in part, to the higher challenge dose (20–40 × LD50 vs 10–20 × LD50 in Fig. 9). Also, given the high challenge dose used in Fig. 10B, it is mathematically unlikely that the survival observed in Fig. 10B was due to a failure to receive a lethal dose, which is, in the case of SchuS4, 1–2 organisms. This contention is also supported by the data in Fig. 10A showing no survival following a 9 CFU challenge of mice receiving a single mAb-iFt immunization, as well as by 100% death of the PBS- and iFt-immunized groups in Fig. 10B.
FIGURE 10.
Evidence for immune memory involvement in mAb-iFt-mediated protection. C57BL/6 mice were divided into three groups and immunized i.n. with 20 µl of PBS, 20 µl of 2 × 107 organisms (iFt), or 20 µl of 2 × 107 organisms (mAb-iFt). As in Fig. 9, mAb-iFt was made using 5 µg/ml mAb., A, Mice were immunized on day 0 and then challenged with 9 CFU of F. tularensis SchuS4 14 days after primary immunization and then monitored for survival., B, Mice were immunized as above on days 0, 14, and 28 and then challenged on day 70 i.n. with 40 CFU (∼20–40 × LD50) of live F. tularensis SchuS4, and subsequently monitored for survival. Survival curves are presented. For the mAb-iFt group vs PBS and iFt groups in B, p< 0.1.
Discussion
Numerous studies have demonstrated that targeting Ag to FcRs enhances both humoral and cellular immune responses (1–13). However, it has not been demonstrated that this same strategy can be used to enhance protection against challenge with an infectious agent. Furthermore, very little is known about the potential use of FcR targeting as a vaccine strategy at mucosal sites. In this regard, we now demonstrate for the first time that immunogen can be targeted to FcRs at a mucosal site, thereby generating enhanced protection against an infectious agent. In particular, these studies focused on the intracellular biothreat agent, F. tularensis (46). A number of parameters were used as evidence to support the conclusion that FcR targeting of immunogen, in the form of inactivated F. tularensis LVS (iFt), is a viable strategy for enhancing protection against this organism. First, a significant increase in survival of two different strains of challenged wild-type mice was observed when iFt was administered complexed with mAb vs noncomplexed iFt (Fig. 1). This was accompanied by a 10-fold decrease in bacterial burden (Fig. 2), a reduction in tissue inflammation (Fig. 3), and reduced production of inflammatory cytokines (Fig. 4).
The use of mAb complexed to iFt to target iFt to FcR was chosen due to the lack of an identified protective Ag for F. tularensis (28, 31), as well as to a general lack of knowledge regarding which specific FcR might be involved in immune responses at mucosal sites. Thus, it was necessary to choose a targeting strategy with broad specificity for multiple FcRs, including FcRn. With regard to the latter, recent data suggest that FcRn plays a role in Ag delivery to mucosal lymphoid tissue at mucosal sites (19–22). This, combined with the fact that FcγRs are expressed on APCs at mucosal sites, strongly favors the potential involvement of both FcγRs and FcRn. Therefore, mouse IgG2a anti-F. tularensis mAb was chosen to generate mAb-iFt complexes. The mouse IgG2a isotype, when in multimeric form, binds all three (low- and high-affinity) mouse FcγR, as well as mouse FcRn (15, 26). To prove FcR involvement in the observed protection noted above, several different approaches were taken.
We first confirmed FcR involvement in mAb-iFt binding to a mouse myeloid cell line expressing FcRs in vitro. In this case, if FcRs play a role in mAb-iFt binding to RAW cells, preincubation of these cells with sufficient amounts of heat-aggregated IgG should result in FcγR saturation and failure of mAb-iFt to bind. This was in fact the case (Fig. 5A). Furthermore, when mAb-iFt complexes were generated, which lacked the IgG2a Fc domain, mAb-iFt binding to RAW cells was eliminated (Fig. 5B). To determine the relative roles of FcγRI and FcγRII/III in this binding, RAW cells were preincubated with saturating concentrations of monomeric (nonspecific) IgG2a, which binds only to high-affinity FcγRI or to F(ab′)2 mAb 2.4G2, which blocks IgG binding to FcγRII/III (47). In the case of IgG2a, saturation of FcγRI completely blocked mAb-iFt binding, while 2.4G2 had no effect on mAb-iFt binding. However, despite the latter result, it is unlikely that FcγRII/III is not involved in mAb-iFt binding, given its proven propensity to bind immune complexes (15, 26). It is more likely that despite FcγRII/III blocking with 2.4G2 mAb, binding of the mAb-iFt complexes to the high-affinity FcγRI is sufficient to maintain mAb-iFt binding levels. Furthermore, these data also suggest that FcγRI interaction is critical to maintain mAb-iFt binding to RAW cells.
Despite the above in vitro data indicating FcR involvement in mAb-iFt binding to RAW cells, this does not prove FcR involvement in vivo. Thus, a number of additional in vivo experiments were conducted to determine which FcRs play an important role in the observed mAb-iFt-mediated protection (Fig. 1). If FcRs are involved, then removal of the IgG2a Fc domain from the mAb-iFt complex should eliminate the protective effect of this complex. In fact, this was the case. When mice were immunized with F(ab′)2 mAb-iFt vs iFt, the level of protection induced in the former case was equivalent to that of noncomplexed iFt (Fig. 6, A and B). Furthermore, mice lacking either FcγRI/III or FcRn were not protected by immunization with mAb-iFt or iFt. The failure of F(ab′)2 mAb-iFt to enhance protection above that of iFt strongly suggests that FcγRs are involved at the time of immunization. However, in the studies using FcγR and FcRn knockout mice, it is difficult to determine at what stage these FcRs are critical. In fact, they may play a significant role both during and after immunization. In either case, this is the first time that a role for FcRn in the protection of adult mice against mucosal infection has been demonstrated. The latter is also consistent with studies demonstrating the expression of FcRn in adult tissues (19–22), as well as the ability of this receptor to deliver Fc-Ag fusion protein to mucosal lymphoid tissue. Similarly, substantial data suggest that FcγRI, when targeted on APCs using FcγRI-specific Ag fusion proteins, can enhance humoral and cellular immunity (1, 4, 6, 11, 48).
With regard to the mechanism of protection, increased anti-F. tularensis IgA levels were correlated with increased protection. Specifically, when immunizing with iFt, a F. tularensis-specific Ab titer of 5 was achieved in the BAL fluid of immunized mice, and ∼50% protection from F. tularensis LVS challenge was observed (Figs. 1 and 7). Following mAb-iFt immunization, the anti-F. tularensis IgA titer in the BAL fluid of immunized mice rose to 30, accompanied by 100% protection (Figs. 1 and 7). Additionally, in the absence of IgA, no protection was observed (Fig. 8). Because the above analysis requires that mice be sacrificed, it was not possible to perform this analysis with challenged mice. However, the immunization schedule in Ab titer experiments was identical with that of challenge experiments, and BAL fluid and sera were collected on day 35, the same day mice were challenged in protection experiments. The idea that increased F. tularensis-specific IgA production plays a role in the observed protection is also supported by recent studies demonstrating a requirement for IgA in protection against F. tularensis LVS i.n. challenge, following immunization with inactivated organisms (32), as well as by the growing literature now supporting the premise that Ab can mediate protection against intracellular pathogens (40–45).
In contrast to F. tularensis-specific IgA levels, F. tularensis-specific IgG levels dropped significantly following immunization with mAb-iFt complexes vs iFt. This implies that IgG levels subsequent to challenge are not a critical factor in mAb-iFt-mediated protection (Fig. 7), yet FcγRs are critical for protection (Fig. 6). Specifically, in the case of mAb-iFt immunization, challenge data using F(ab′)2-mAb-iFt as immunogen strongly suggest that FcγRs play a significant role immediately following mAb-iFt immunization, most likely via the enhancement of iFt presentation by APCs (3, 4, 11). However, data using FcγRI/III or FcRn knockout mice also indicate important roles for FcγRI/III or FcRn in resolving infection, independent of the immunogen itself (iFt vs mAb-iFt). In the case of FcγRs, this is consistent with previous observations supporting a role for FcγRs in protecting mice against F. tularensis LVS challenge in the presence of Abs (33). These data are also important in that while IgG production is suppressed, IgA production is enhanced (Fig. 7). There are several possible explanations for the latter, which are now being actively investigated. First, reduced inflammation as a result of mAb-iFt immunization may reduce IgG leakage into the lungs, leading to the lower IgG levels observed. However, suppressed IgG production in the serum is also observed (Fig. 7D). Alternatively, immune complex interaction with FcγRIIB on B cells has been shown to negatively regulate Ab responses, and this is the more likely explanation (49). In fact, preliminary data, which have been obtained but are not presented in this manuscript, suggest that FcγRIIB is involved. The question, however, remains as to why a similar reduction in IgA levels is not seen when immunizing with mAb-iFt. This suggests a disconnection between FcγRIIB-mediated inhibition of IgG vs IgA production. In fact, there have been no studies examining the effect of FcγRIIB interaction with Ag-Ab complexes on IgA production. A third potential explanation involves the production of TGF-β. Studies have demonstrated that the secretion of TGF-β can differentially regulate IgA vs IgG production, suppressing the latter while not effecting or enhancing IgA production (50, 51). Thus, investigations are now underway to address each of these possibilities.
Finally, we wanted to determine whether protection against challenge with the highly virulent F. tularensis SchuS4 strain could be achieved by immunization with mAb-iFt complexes. It is currently thought that inactivated organisms are incapable of generating protection against F. tularensis SchuS4. In fact, our initial studies using mAb-iFt complexes made with 1 µg/ml mAb (Fig. 1), as well as the use of a prime then single boost schedule, identical with that used to protect against F. tularensis LVS, failed to produce protection against F. tularensis SchuS4 challenge (data not shown). Thus, we evaluated a series of different mAb-iFt mixtures/complexes and also increased the number of booster immunizations. When mAb-iFt complexes were made using 5 µg/ml mAb (Fig. 1A), and the primary immunization was followed by two booster immunizations, significant protection was observed, ranging between 40 and 50% (Fig. 9). Two potential factors may have played a role in the improved results. As evidenced in Fig. 1A, use of 5 µg/ml mAb in mAb-iFt complex generation enhances mAb-iFt binding to FcγR-bearing cells. This, combined with an additional boost, likely increases the overall recall response to challenge. Note also that this protection was observed at challenge doses between 18 and 40 CFU of F. tularensis SchuS4, well above the 1 CFU, which is nearly 100% fatal when administered i.n. Despite this success, it will be important to test a variety of conditions for mAb-iFt generation, as well as varied immunization schedules, to determine whether this protection can be improved. Furthermore, although we have demonstrated that targeting immunogen to FcRs i.n. enhances protection against subsequent infectious disease challenge, significant additional study will be required to determine the length of time such protection lasts, and whether sterilizing immunity is ultimately achieved. Note, however, that studies presented in this manuscript do suggest that a recall response is involved in protection to both F. tularensis LVS and SchS4 (Figs. 1D, 7, and 10). Furthermore, sterile immunity was observed in two SchuS4 experiments conducted (data not presented). However, further study is warranted in this case, given that recent studies immunizing with attenuated organisms have indicated that F. tularensis can remain in protected animals for extended periods of time (52).
In summary, these are the first studies to demonstrate that: 1) FcR-targeted immunogen enhances immunogen-specific IgA production and protection against subsequent infection in an IgA-dependent manner, 2) both FcγR and FcRn are crucial to this protection, and 3) inactivated F. tularensis, when targeted to FcRs, enhances protection against the highly virulent SchuS4 strain of F. tularensis, a category A biothreat agent. Furthermore, these studies raise important questions regarding the role of FcγRIIB (or lack thereof) in the regulation of IgA vs IgG production.
Acknowledgments
We thank the Immunology Core at Albany Medical College as well as the Microbiology/BSL3 Core at Albany Medical College for significant contributions to this work.
Footnotes
These studies were funded by a grant from the National Institutes of Health (P01 AI056320).
Abbreviations used in this paper: FcR, Fc, receptor; BAL, bronchoalveolar lavage; CBA, cytometric bead array; hFcγRI, human FcγRI; FcRn, neonatal FcR; iFt, inactivated F. tularensis live vaccine strain; i.n., intranasal; LVS, live vaccine strain; TT, tetanus toxoid.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Adamova E, Walsh MC, Gosselin DR, Hale K, Preissler MT, Graziano RF, Gosselin EJ. Enhanced antigen-specific antibody and cytokine responses when targeting antigen to human Fcγ receptor type I using an anti-human Fcγ receptor type I-streptavidin fusion protein in an adjuvant-free system. Immunol. Invest. 2005;34:417–429. doi: 10.1080/08820130500265372. [DOI] [PubMed] [Google Scholar]
- 2.Rafiq K, Bergtold A, Clynes R. Immune complex-mediated antigen presentation induces tumor immunity. J. Clin. Invest. 2002;110:71–79. doi: 10.1172/JCI15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C, Goldstein J, Graziano RF, He J, O’Shea JK, Deo Y, Guyre PM. F(c)γRI-targeted fusion proteins result in efficient presentation by human monocytes of antigenic and antagonist T cell epitopes. J. Clin. Invest. 1996;98:2001–2007. doi: 10.1172/JCI119004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guyre PM, Graziano RF, Goldstein J, Wallace PK, Morganelli PM, Wardwell K, Howell AL. Increased potency of Fc-receptor-targeted antigens. Cancer Immunol. Immunother. 1997;45:146–148. doi: 10.1007/s002620050418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heyman B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu. Rev. Immunol. 2000;18:709–737. doi: 10.1146/annurev.immunol.18.1.709. [DOI] [PubMed] [Google Scholar]
- 6.Walsh MC, Banas JA, Mudzinski SP, Preissler MT, Graziano RF, Gosselin EJ. A two-component modular approach for enhancing T-cell activation utilizing a unique anti-FcγRI-streptavidin construct and microspheres coated with biotinylated-antigen. Biomol. Eng. 2003;20:21–33. doi: 10.1016/s1389-0344(02)00089-8. [DOI] [PubMed] [Google Scholar]
- 7.Jelley-Gibbs DM, Plitnick LM, Gosselin EJ. Differences in IgG subclass do not effect immune complex-enhanced T cell activation despite differential binding to antigen presenting cells. Hum. Immunol. 1999;60:469–478. doi: 10.1016/s0198-8859(99)00023-3. [DOI] [PubMed] [Google Scholar]
- 8.Keler T, Guyre PM, Vitale LA, Sundarapandiyan K, van De Winkel JG, Deo YM, Graziano RF. Targeting weak antigens to CD64 elicits potent humoral responses in human CD64 transgenic mice. J. Immunol. 2000;165:6738–6742. doi: 10.4049/jimmunol.165.12.6738. [DOI] [PubMed] [Google Scholar]
- 9.Snider DP, Kaubisch A, Segal DM. Enhanced antigen immunogenicity induced by bispecific antibodies. J. Exp. Med. 1990;171:1957–1963. doi: 10.1084/jem.171.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heijnen IA, van Vugt MJ, Fanger NA, Graziano RF, de Wit TP, Hofhuis FM, Guyre PM, Capel PJ, Verbeek JS, van de Winkel JG. Antigen targeting to myeloid-specific human FcγRI/CD64 triggers enhanced antibody responses in transgenic mice. J. Clin. Invest. 1996;97:331–338. doi: 10.1172/JCI118420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gosselin EJ, Wardwell K, Gosselin DR, Alter N, Fisher JL, Guyre PM. Enhanced antigen presentation using human Fcγ receptor (monocyte/macrophage)-specific immunogens. J. Immunol. 1992;149:3477–3481. [PubMed] [Google Scholar]
- 12.Amigorena S, Bonnerot C. Fc receptors for IgG and antigen presentation on MHC class I and class II molecules. Semin. Immunol. 1999;11:385–390. doi: 10.1006/smim.1999.0196. [DOI] [PubMed] [Google Scholar]
- 13.Shen L, van Egmond M, Siemasko K, Gao H, Wade T, Lang ML, Clark M, van De Winkel JG, Wade WF. Presentation of ovalbumin internalized via the immunoglobulin-A Fc receptor is enhanced through Fc receptor γ-chain signaling. Blood. 2001;97:205–213. doi: 10.1182/blood.v97.1.205. [DOI] [PubMed] [Google Scholar]
- 14.Daeron M. Fc receptor biology. Annu. Rev. Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 15.Gessner JE, Heiken H, Tamm A, Schmidt RE. The IgG Fc receptor family. Ann. Hematol. 1998;76:231–248. doi: 10.1007/s002770050396. [DOI] [PubMed] [Google Scholar]
- 16.Getahun A, Dahlstrom J, Wernersson S, Heyman B. IgG2a–mediated enhancement of antibody and T cell responses and its relation to inhibitory and activating Fcγ receptors. J. Immunol. 2004;172:5269–5276. doi: 10.4049/jimmunol.172.9.5269. [DOI] [PubMed] [Google Scholar]
- 17.Chargelegue D, Drake PM, Obregon P, Prada A, Fairweather N, Ma JK. Highly immunogenic and protective recombinant vaccine candidate expressed in transgenic plants. Infect. Immun. 2005;73:5915–5922. doi: 10.1128/IAI.73.9.5915-5922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corthesy B. Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J. Immunol. 2007;178:27–32. doi: 10.4049/jimmunol.178.1.27. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J. Clin. Invest. 2006;116:2142–2151. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, Lencer WI. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J. Exp. Med. 2002;196:303–310. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bitonti AJ, Dumont JA, Low SC, Peters RT, Kropp KE, Palombella VJ, Stattel JM, Lu Y, Tan CA, Song JJ, et al. Pulmonary delivery of an erythropoietin Fc fusion protein in non-human primates through an immunoglobulin transport pathway. Proc. Natl. Acad. Sci. USA. 2004;101:9763–9768. doi: 10.1073/pnas.0403235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanger NA, Voigtlaender D, Liu C, Swink S, Wardwell K, Fisher J, Graziano RF, Pfefferkorn LC, Guyre PM. Characterization of expression, cytokine regulation, and effector function of the high affinity IgG receptor FcγRI (CD64) expressed on human blood dendritic cells. J. Immunol. 1997;158:3090–3098. [PubMed] [Google Scholar]
- 24.Guyre PM, Graziano RF, Vance BA, Morganelli PM, Fanger MW. Monoclonal antibodies that bind to distinct epitopes on FcγRI are able to trigger receptor function. J. Immunol. 1989;143:1650–1655. [PubMed] [Google Scholar]
- 25.Graziano RF, Tempest PR, White P, Keler T, Deo Y, Ghebremariam H, Coleman K, Pfefferkorn LC, Fanger MW, Guyre PM. Construction and characterization of a humanized anti-γ-Ig receptor type I (FcγRI) monoclonal antibody. J. Immunol. 1995;155:4996–5002. [PubMed] [Google Scholar]
- 26.van de Winkel JG, Anderson CL. Biology of human immunoglobulin G Fc receptors. J. Leukocyte Biol. 1991;49:511–524. doi: 10.1002/jlb.49.5.511. [DOI] [PubMed] [Google Scholar]
- 27.Guyre CA, Keler T, Swink SL, Vitale LA, Graziano RF, Fanger MW. Receptor modulation by FcγRI-specific fusion proteins is dependent on receptor number and modified by IgG. J. Immunol. 2001;167:6303–6311. doi: 10.4049/jimmunol.167.11.6303. [DOI] [PubMed] [Google Scholar]
- 28.Conlan JW, Oyston P. Vaccines against Francisella tularensis. Ann. NY Acad. Sci. 2007;1105:325–350. doi: 10.1196/annals.1409.012. [DOI] [PubMed] [Google Scholar]
- 29.McLendon MK, Apicella MA, Allen LA. Francisella tularensis: taxonomy, genetics, and immunopathogenesis of a potential agent of biowarfare. Annu. Rev. Microbiol. 2006;60:167–185. doi: 10.1146/annurev.micro.60.080805.142126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isherwood KE, Titball RW, Davies DH, Felgner PL, Morrow WJ. Vaccination strategies for Francisella tularensis. Adv. Drug Deliv. Rev. 2005;57:1403–1414. doi: 10.1016/j.addr.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 31.Conlan JW. Vaccines against Francisella tularensis: past, present and future. Expert Rev. Vaccines. 2004;3:307–314. doi: 10.1586/14760584.3.3.307. [DOI] [PubMed] [Google Scholar]
- 32.Baron SD, Singh R, Metzger DW. Inactivated Francisella tularensis live vaccine strain protects against respiratory tularemia by intranasal vaccination in an immunoglobulin A-dependent fashion. Infect. Immun. 2007;75:2152–2162. doi: 10.1128/IAI.01606-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J. Immunol. 2007;179:532–539. doi: 10.4049/jimmunol.179.1.532. [DOI] [PubMed] [Google Scholar]
- 34.Brazelton TR, Blau HM. Optimizing techniques for tracking transplanted stem cells in vivo. Stem Cells. 2005;23:1251–1265. doi: 10.1634/stemcells.2005-0149. [DOI] [PubMed] [Google Scholar]
- 35.Celis E, Zurawski VR, Jr, Chang TW. Regulation of T-cell function by antibodies: enhancement of the response of human T-cell clones to hepatitis B surface antigen by antigen-specific monoclonal antibodies. Proc. Natl. Acad. Sci. USA. 1984;81:6846–6850. doi: 10.1073/pnas.81.21.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore T, Ekworomadu CO, Eko FO, MacMillan L, Ramey K, Ananaba GA, Patrickson JW, Nagappan PR, Lyn D, Black CM, Igietseme JU. Fc receptor-mediated antibody regulation of T cell immunity against intracellular pathogens. J. Infect. Dis. 2003;188:617–624. doi: 10.1086/377134. [DOI] [PubMed] [Google Scholar]
- 37.Conlan JW, Shen H, Webb A, Perry MB. Mice vaccinated with the O-antigen of Francisella tularensis LVS lipopolysaccharide conjugated to bovine serum albumin develop varying degrees of protective immunity against systemic or aerosol challenge with virulent type A and type B strains of the pathogen. Vaccine. 2002;20:3465–3471. doi: 10.1016/s0264-410x(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 38.Dreisbach VC, Cowley S, Elkins KL. Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and γ interferon. Infect. Immun. 2000;68:1988–1996. doi: 10.1128/iai.68.4.1988-1996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fulop M, Mastroeni P, Green M, Titball RW. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001;19:4465–4472. doi: 10.1016/s0264-410x(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 40.Yager E, Bitsaktsis C, Nandi B, McBride JW, Winslow G. Essential role for humoral immunity during Ehrlichia infection in immunocompetent mice. Infect. Immun. 2005;73:8009–8016. doi: 10.1128/IAI.73.12.8009-8016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casadevall A. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 1998;6:102–107. doi: 10.1016/s0966-842x(98)01208-6. [DOI] [PubMed] [Google Scholar]
- 42.Edelson BT, Unanue ER. Intracellular antibody neutralizes Listeria growth. Immunity. 2001;14:503–512. doi: 10.1016/s1074-7613(01)00139-x. [DOI] [PubMed] [Google Scholar]
- 43.Feng HM, Whitworth T, Olano JP, Popov VL, Walker DH. Fc-dependent polyclonal antibodies and antibodies to outer membrane proteins A and B, but not to lipopolysaccharide, protect SCID mice against fatal Rickettsia conorii infection. Infect. Immun. 2004;72:2222–2228. doi: 10.1128/IAI.72.4.2222-2228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li JS, Yager E, Reilly M, Freeman C, Reddy GR, Reilly AA, Chu FK, Winslow GM. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 2001;166:1855–1862. doi: 10.4049/jimmunol.166.3.1855. [DOI] [PubMed] [Google Scholar]
- 45.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar typhimurium. Infect. Immun. 2000;68:3344–3348. doi: 10.1128/iai.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clin. Microbiol. Rev. 2002;15:631–634. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heyman B. Inhibition of IgG-mediated immunosuppression by a monoclonal anti-Fc receptor antibody. Scand. J. Immunol. 1989;29:121–126. doi: 10.1111/j.1365-3083.1989.tb01106.x. [DOI] [PubMed] [Google Scholar]
- 48.Wallace PK, Tsang KY, Goldstein J, Correale P, Jarry TM, Schlom J, Guyre PM, Ernstoff MS, Fanger MW. Exogenous antigen targeted to FcγRI on myeloid cells is presented in association with MHC class I. J. Immunol. Methods. 2001;248:183–194. doi: 10.1016/s0022-1759(00)00351-3. [DOI] [PubMed] [Google Scholar]
- 49.Fridman WH. Regulation of B-cell activation and antigen presentation by Fc receptors. Curr. Opin. Immunol. 1993;5:355–360. doi: 10.1016/0952-7915(93)90053-u. [DOI] [PubMed] [Google Scholar]
- 50.Twigg HL, III, Spain BA, Soliman DM, Bowen LK, Heidler KM, Wilkes DS. Impaired IgG production in the lungs of HIV-infected individuals. Cell. Immunol. 1996;170:127–133. doi: 10.1006/cimm.1996.0142. [DOI] [PubMed] [Google Scholar]
- 51.Goodrich ME, McGee DW. Regulation of mucosal B cell immunoglobulin secretion by intestinal epithelial cell-derived cytokines. Cytokine. 1998;10:948–955. doi: 10.1006/cyto.1998.0385. [DOI] [PubMed] [Google Scholar]
- 52.Wu TH, Hutt JA, Garrison KA, Berliba LS, Zhou Y, Lyons CR. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect. Immun. 2005;73:2644–2654. doi: 10.1128/IAI.73.5.2644-2654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]