Abstract
Poor maternal zinc status has been associated with foetal loss, congenital malformations, intrauterine growth retardation, reduced birth weight, prolonged labour and preterm or post-term deliveries. A meta-analysis completed in 2007 showed that maternal zinc supplementation resulted in a small but significant reduction in preterm birth. The purposes of this analysis are to update that previous review and expand the scope of assessment to include maternal, infant and child health outcomes. Electronic searches were carried out to identify peer-reviewed, randomised controlled trials where daily zinc supplementation was given for at least one trimester of pregnancy. The co-authors applied the study selection criteria, assessed trial quality and abstracted data. A total of 20 independent intervention trials involving more than 11 000 births were identified. The 20 trials took place across five continents between 1977 and 2008. Most studies assessed the zinc effect against a background of other micronutrient supplements, but five were placebo-controlled trials of zinc alone. The provided dose of supplemental zinc ranged from 5 to 50 mg/day. Only the risk of preterm birth reached statistical significance (summary relative risk 0.86 [95% confidence interval 0.75, 0.99]). There was no evidence that supplemental zinc affected any parameter of foetal growth (risk of low birth weight, birth weight, length at birth or head circumference at birth). Six of the 20 trials were graded as high quality. The evidence that maternal zinc supplementation lowers the risk of preterm birth was graded low; evidence for a positive effect on other foetal outcomes was graded as very low. The effect of zinc supplementation on preterm birth, if causal, might reflect a reduction in maternal infection, a primary cause of prematurity. While further study would be needed to explore this possibility in detail, the overall public health benefit of zinc supplementation in pregnancy appears limited.
Keywords: birth weight, pregnancy, prematurity, supplementation, zinc
Zinc plays an important role in many biological functions including protein synthesis, cellular division and nucleic acid metabolism.1 Although severe zinc deficiency is relatively rare in human populations, mild to moderate depletion appears to be quite prevalent. Zinc intake data suggest that the risk of deficiencies is high. Using a model that related reported zinc intakes of pregnant women to the recommended intake, Caulfield estimated that 82% of the pregnant women worldwide have inadequate zinc intakes.2
Studies in rats, mice, pigs and ewes show that severe zinc deficiency increases foetal death due to spontaneous abortions or multiple congenital anomalies.3 Every organ system is affected; malformations of the heart, lungs, brain, urogenital system and skeletal system are especially common. These malformations seem to stem from an abnormal synthesis of nucleic acids and protein, impaired cellular growth and morphogenesis, abnormal tubulin polymerisation, chromosomal defects and excessive lipid peroxidation of cellular membranes. Animal studies show that maternal zinc deficiency has long-term effects on the growth, immunity and metabolic status of the surviving offspring.4,5 For example, maternal zinc depletion reduced the offspring’s immune function, a condition that persisted for three generations.6
In humans, women with acrodermatitis enteropathica, an inherited defect in zinc absorption causing severe deficiencies, have poor pregnancy outcomes; foetal losses and congenital malformations are common.7 Poor markers of maternal zinc status have been linked to poor pregnancy outcomes in women without acrodermatitis entheropathica. For example, low maternal serum or leucocytic zinc concentrations has been associated with prolonged labour, preterm labour, postpartum haemorrhage, post-term deliveries, small-for-gestational-age babies, intra-uterine growth retardation or reduced birth weight in some,3,8–10 but not all, studies.11,12 Lack of sensitive biomarkers of zinc status or the presence of other underlying nutritional problems affecting pregnancy outcomes may account for the divergent findings. The additional zinc need for human pregnancies estimated from the zinc concentration and the weight of tissues gained is about 100 mg (1540 mmol).13 The additional daily need during the last half of pregnancy when foetal growth is most rapid is about 0.6 mg/day (9.2 mmol/day). Studies of maternal zinc intakes fail to show an increased intake during pregnancy, but the methods for assessing food intake are likely to be too imprecise to detect this small difference.3 It is unclear if homeostatic adjustments in zinc absorption or excretion occurs during pregnancy to improve zinc retention for foetal growth. In pregnant rats, zinc absorption increased almost twofold during the last 3 days of pregnancy to meet foetal needs at that stage of pregnancy.14 Similar changes have not been observed in pregnant women possibly because the relative foetal zinc demand in humans is much less than that of in rats with multiple offspring.3
Recent estimates of about 0.5 million maternal and child deaths annually due to zinc deficiency has raised the concern about the adequacy of zinc intakes among pregnant women in developing countries.15 These women frequently subsist on diets lacking zinc-rich animal source foods. Instead, cereals that are high in phytate, which limits zinc absorption, are the primary source of zinc. Maternal plasma zinc concentrations tend to be reduced slightly either because of marginal intakes or because of chronic infections that reduces plasma zinc concentrations.16 Lower plasma zinc concentrations could reduce placental zinc transport and the foetal zinc supply. Based on these observations, United Nations Childrens Fund (UNICEF) recommended the use of multiple micronutrient supplements including zinc by all pregnant women in developing countries.17 Because supplemental zinc significantly improves the weight and height gain in growing children, it was assumed that maternal zinc supplementation would improve foetal growth.
During the past 30 years, numerous randomised controlled trials of zinc supplementation have been performed. In 2007, a systematic review and meta-analysis of 17 of these trials was published.18 This analysis updates that previous review and expands the scope of assessment to include maternal, infant and child health outcomes.
Methods
Systematic literature search
Electronic searches were conducted through the MEDLINE, Cochrane Library, BIOSIS, WHOLIS, PAHO and LILACS databases in April 2011 seeking original peer-reviewed descriptions of maternal zinc supplementation trials. Medical Subject Heading terminology was used in MEDLINE with an analogous search adapted for each database (Web Supplement 1). Search terms were in English with no restrictions on the language or date of publication. Prior to reviewing citations, the following inclusion criteria were declared: randomised controlled trial in humans; daily zinc supplementation of at least one trimester duration lasting up to delivery; comparison group receiving the identical supplements (save for zinc) in at least one arm of the trial; peer-reviewed; and at least one of six outcomes reported: low birth weight, preterm birth, neonatal growth morbidity or mortality, childhood growth morbidity or mortality, maternal nutritional status and maternal growth morbidity or mortality. Additionally excluded were letters, conference proceedings, reviews, case series, commentary and citations lacking an English or Portuguese version of the full-text document.
Two reviewers (JK, BC) independently assessed fulltext copies of titles and abstracts marked as potentially relevant. The citation listings of these publications as well as review articles were searched by hand for relevant citations not captured by the electronic search. Publications were included if they matched the predetermined criteria. A consensus was later reached regarding any citation selected by only one reviewer.
Data abstraction
Study attributes and results were abstracted to standardised forms (available upon request). Quantitative information was recorded as reported, or when necessary, calculated from values in the tables or text. Stratified results were pooled when possible to estimate a population-level effect. For cluster-randomised trials, adjustment for intra-cluster correlation was either achieved by the published study or approximated. When multiple publications drew results from an identical set of participants (e.g. the results of a single trial reported across several articles), any outcome was recorded only once for each independent trial, selecting the estimate drawn from the largest population (e.g. the total trial vs. a subset).
Meta-analysis
Across the six broad outcomes of interest, meta-analysis was deemed appropriate for relevant events reported from a minimum of five independent study populations using comparable or convertible measures and definitions (e.g. kilograms or cumulative incidence). Other relevant outcome events derived from multiple independent studies were compiled for a separate qualitative synthesis. Three binary outcomes (low birth weight, defined as <2500 g at birth; preterm birth, defined as <37 weeks of gestation; and small-for-gestational age, defined by each study’s criteria) and four continuous outcomes measured at birth (weight, length, gestational age and head circumference) met criteria for meta-analysis.
For binary outcomes, overall and subgroup Mantel-Haenszel fixed-effects19 summary relative risks (sRR) were calculated using statistical software (Stata IC version 10.1, StataCorp, College Station, TX, USA). Ninety-five per cent confidence intervals (CI) were calculated by the method of Shore20 (Shore corrected 95% CI) whenever this adjustment resulted in more conservative (wider) intervals. The sensitivity of the sRR to the exclusion of individual studies was assessed, and subgroup analysis was performed to explore sources of heterogeneity. An overall DerSimonian-Laird random-effects21 sRR was also calculated whenever considerable heterogeneity was suggested by an I2 statistic in excess of 50% (where I2 equals the Cochrane Q heterogeneity statistic minus degrees of freedom, divided by the Q statistic, then converted to a percentage). For continuous outcomes, a fixed-effects summary mean difference (sMD) was calculated using the general variance inverse-weighting method19 to compare mean outcomes in the zinc treated group to the comparison arm. Sensitivity and subgroup analyses were repeated as was done for the sRR.
Funnel plots served as a visual means for assessing any disproportionate representation of study results according to strength and precision.22 A Begg adjusted rank correlation test23 formally tested for any trend of increasing association strength with reducing precision. Such an effect could represent the preferential publication of statistically significant positive results,24 which could bias summary measures. In the event of a significant trend, the rank-based data-augmentation technique of Duval and Tweedie25 was used to generate an augmented summary measure for comparison under the hypothetical scenario that association measures of similarly low precision but opposite direction had also been reported in the literature.
Quality of evidence determination
The overall quality and relevance of the available data was assessed according to procedures described by the Child Health Epidemiology Reference Group (CHERG),26 based on earlier criteria of the Grade Working Group.27,28 Quality determination was organised by health outcome, with four possible levels of evidence: high, moderate, low or very low, as detailed elsewhere.26 The primary objective of quality determination was not to judge to the intrinsic scientific merit of each study, but to weigh the totality of published evidence for a beneficial effect of zinc supplementation in pregnancy on specific outcomes. As such, factors negatively impacting overall quality grades not only included the risk of bias in individual studies, but also failure to show a positive effect of the intervention, a lack of consistency (extensive heterogeneity) across studies or poor precision of the effect estimate. Briefly, both authors independently assessed each eligible trial for possible bias related to the GRADE criteria, and assigned an individual quality grade. The overall quality of evidence organised by outcome was then determined based on the magnitude, consistency and generalisability of the pooled estimate, as well as limitations identified in reviewing the individual studies.
Results
Systematic literature search
The electronic search generated 1438 hits across six databases, representing 941 non-duplicate citations. Of these, 85 citations were examined as full-text copies and 55 deemed to meet inclusion criteria for review covering 20 independent intervention trials.11,29–82 More than 11 000 births were recorded among trial participants. A manual search of the citations listings of these publications as well as previous review articles yielded two additional titles,12,83 which were both later excluded. A flow diagram is provided to summarise the search process (Figure 1) in accordance with CHERG recommendations.26 Among the identified studies that actually featured zinc supplementation in pregnancy, notable exclusions were a study of zinc in the context of supplemental food,84 a case series of 20 women with uncertain peer-review,9 a study in which supplementation was not carried through to the end of pregnancy,85 a letter to the editor,86 a study of night-blind women with brief follow-up,87 a study published in Chinese language journals with uncertain peer-review88,89 and a non-randomised intervention in which only 10 individuals were provided zinc.90 Also excluded were any reports of short-term changes in biochemical indicators of maternal status that did not also assess those indicators later in pregnancy. Of the 55 included publications, seven42,43,45,51,58,63,66 are not represented in any tables or summary measures below because these studies only reported results for a subset of trial participants already included in other publications.
Figure 1.
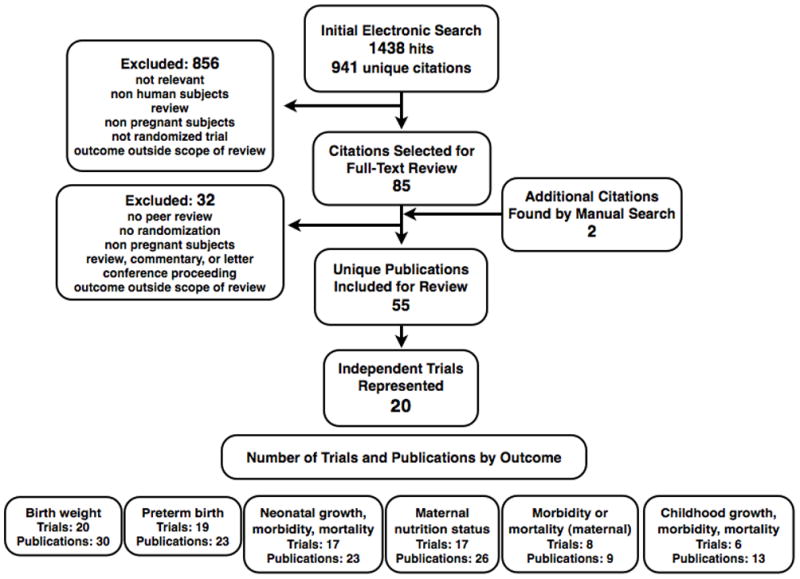
Flow diagram for identifying studies of zinc supplementation during pregnancy.
Qualitative summary
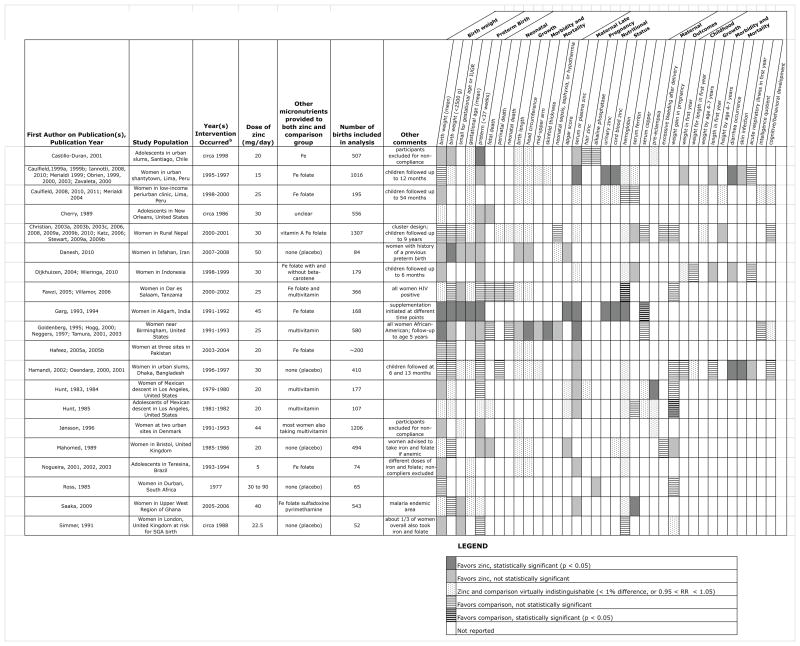
Figure 2 summarises the characteristics and selected findings of the maternal zinc supplementation trials across the six broad outcomes of interest for review. Only outcomes reported by multiple studies were included in the table. Various obstetric outcomes such as breech presentation, caesarean section, placental weight and pregnancy complications were beyond the scope of this review. The more detailed forms used in data abstraction are available upon request.
Figure 2.
Overall characteristics and qualitative summary of included trials of zinc supplementation in pregnancy for select infant, child, and maternal outcomes.
mg, milligrams; g, grams; IUGR, intrauterine growth retardation; Fe, iron; HIV, human immunodeficiency virus; RR, relative risk; SGA, small for gestational age.
aStatistically significant results favouring the intervention are shown in dark gray, and those favouring the comparison group in dark hatches. Several obstetric outcomes as well as any outcomes originating from just one trial are not included.
bFor some trials the year the intervention occurred was estimated from the publication date.
The 20 included trials took place across five continents in countries ranging from low to high income. The earliest included trial was implemented in 1977,32 and the most recent was completed in 2008.79 Longterm follow-up studies of existing cohorts might continue, as one study82 was published within weeks of the electronic search. Most studies assessed the action of zinc as an augmentative agent against a background of other micronutrient supplements, although five were placebo-controlled trials of zinc alone.32–34,46,50,52,79 Daily doses of elemental zinc ranged from 5 to more than 50 mg. Only six trials30,31,35,49,52,73 described at what time of day participants were instructed to take the provided supplement, although all of these recommended within 2 h of a meal.
Relatively few statistically significant findings were reported, with trial results commonly showing little difference in the zinc supplemented group vs. the comparison group. Three trials35–37,39,44,51,59 did consistently report favourable health outcomes with zinc supplementation across multiple outcomes. Across all trials, the most consistently favourable outcome for zinc supplementation was an increase in maternal serum zinc status in late gestation. All studies evaluated birth outcomes, although fewer achieved follow-up into childhood. Notably, there did appear to be a trend towards decreased diarrhoea occurrence at 6–13 months of age with prenatal zinc, but this outcome was only assessed in three trials.50,80,81
Quality assessment
The individual trials were graded for quality of evidence according to previously determined criteria26 (Web Supplement 2). Six trials were graded as providing high-quality evidence.33,40–43,45–47,50,52,54–56,58,62,65–67,69–71,73–76,78,80 When considering overall quality of evidence of a beneficial outcome, risk of preterm birth received a grade of ‘low’, while the other six outcomes assessed by meta-analysis were graded as ‘very low’ (Table 1).
Table 1.
Summary of Meta-Analysis Estimates, Sorted by Outcome
| Overall Consistency | Summary of Findings | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Number of trials | Heterogeneity (quantitative) | Direction and statistical significance of results | Generalizability to resource poor settings | Heterogeneity of the intervention | Number of births | Statistical method | Pooled estimate (95% CI) |
| Risk of preterm birth (<37 weeks): Overall quality of evidence grade= low | |||||||
| 16 | Q = 20.3, p = 0.16; I2 = 26% | 10 trials favored zinc; 2 of these statistically significant | 11 trials conducted in low or middle income countries | Daily zinc dose from 15 to 50 mg; augmentative and placebo-controlled trials | 7818 (963 preterm) | Mantel-Haenszel fixed-effects relative risk; Shore corrected 95% CI | 0.86 (0.75, 0.99) |
| Risk of low birth weight (<2500 grams): Overall quality of evidence grade= very low | |||||||
| 11 | Q = 16.0, p = 0.10; I2 = 37% | 4 trials favored zinc; 2 of these statistically significant | 8 trials conducted in low or middle income countries | Daily zinc dose from 15 to 50 mg; augmentative and placebo-controlled trials | 5614 (937 low birth weight) | Mantel-Haenszel fixed-effects relative risk; Shore corrected 95% CI | 1.06 (0.91, 1.23) |
| Risk of small for gestational age birth (as defined by individual authors): Overall quality of evidence grade= very low | |||||||
| 5 | Q = 9.8, p = 0.04; I2 = 59% | 2 trials favored zinc; 1 of these statistically significant | 4 trials conducted in low or middle income countries | Daily zinc dose from 25 to 45 mg; augmentative and placebo-controlled trials | 3441 (1155 SGA) | Mantel-Haenszel fixed-effects relative risk; Shore corrected 95% CI | 1.03 (0.91, 1.17) |
| Mean difference in birth weight (grams): Overall quality of evidence grade= very low | |||||||
| 20 | Q = 77.4, p < 0.005; I2 = 75% | 11 trials favored zinc; 2 of these statistically significant | 13 trials conducted in low or middle income countries | Daily zinc dose from 5 to >50 mg; augmentative and placebo-controlled trials | 8138 | Inverse-variance weighted fixed-effects mean difference | 13 g (−9, 35) |
| Mean difference in length at birth (centimeters): Overall quality of evidence grade= very low | |||||||
| 12 | Q = 16.0, p = 0.14; I2 = 31% | 6 trials favored zinc; 1 of these statistically significant | 9 trials conducted in low or middle income countries | Daily zinc dose from 5 to 50 mg; augmentative and placebo-controlled trials | 6285 | Inverse-variance weighted fixed-effects mean difference | −0.1 cm (−0.3, 0.0) |
| Mean difference in gestational age at birth (weeks): Overall quality of evidence grade= very low | |||||||
| 12 | Q = 12.0, p = 0.37; I2 = 8% | 7 trials favored zinc; 1 of these statistically significant | 11 trials conducted in low or middle income countries | Daily zinc dose from 5 to >50 mg; augmentative and placebo-controlled trials | 5273 | Inverse-variance weighted fixed-effects mean difference | 0.1 cm (−0.1, 0.2) |
| Mean difference in head circumference at birth (centimeters): Overall quality of evidence grade= very low | |||||||
| 11 | Q = 21.0, p = 0.02; I2 = 52% | 4 trials favored zinc; 2 of these statistically significant | 9 trials conducted in low or middle income countries | Daily zinc dose from 5 to 50 mg; augmentative and placebo-controlled trials | 5065 | Inverse-variance weighted fixed-effects mean difference | 0.0 cm (−0.1, 0.1) |
Table format adapted from Walker, Fischer-Walker, Bryce et al., International Journal of Epidemiology, 2010;39:i21–i31 and Cochrane Review Manager “Data and Analysis” pre-formatted table.
95% CI = Ninety-five percent confidence interval
mg = milligrams
g = grams
cm = centimeters
SGA = Small for gestational age
Q = Cochrane’s heterogeneity statistic
I2 = 100% × (Q − degrees of freedom)/Q
Quantitative measures of heterogeneity: large Q, small p, large I2 all indicate increased heterogeneity
All included results were derived from randomized controlled trials.
For binary outcomes, sRR < 1 favors zinc intervention (unfavorable outcome less likely).
For continuous outcomes, sMD > 0 favors zinc intervention (larger, more developed infants).
Number of births and number of events were estimated for one trial.
All summary estimates were calculated by fixed effects meta-analysis. Random effects estimates are provided in the text when appropriate.
Quantitative summary
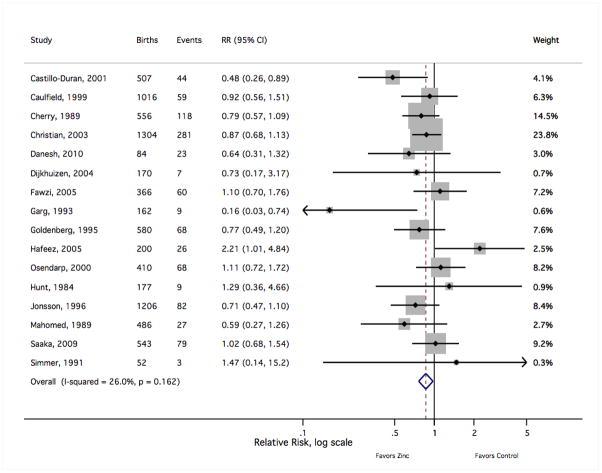
Meta-analysis results are summarised in Table 1. Of the seven outcomes reported by at least five independent trials, only the risk of preterm birth reached nominal statistical significance (sRR 0.86 [Shore corrected 95% CI 0.75, 0.99]), suggesting a modest reduction in the frequency of preterm delivery with maternal zinc supplementation (Figure 3). The sRR was not sensitive to the exclusion of any individual study result, limited to a range from 0.84 to 0.88 with the removal of any single finding. There was not strong evidence of an effect on the risk of low birth weight (1.06 [0.91, 1.23]) (Figure 4). Removal of the most heavily weighted finding54 did not qualitatively alter the sRR (0.99 [0.81, 1.22]), nor did the overall fixed-effects sRR differ greatly from the random-effects estimate (0.98 [0.81, 1.19]). Similarly, the pooled risk of a small-for-gestational age birth showed little difference with zinc supplementation (1.03 [0.91, 1.17]) (Figure 5). This estimate was bound within a range from 0.98 to 1.06 with the exclusion of any single result, and the random-effects estimate (0.99 [0.83, 1.18]) closely resembled the fixed-effects measure.
Figure 3.
The forest plot graphically depicts the individual results included in meta-analysis. Sizes of the boxes are proportional to the weight assigned in calculating the fixed-effects sRR, where weight was assigned inversely to variance. Results to the left of relative risk = 1 indicate a lower risk of preterm birth with zinc supplementation. Events indicate the number of births delivered preterm (gestational age < 37 weeks). RR, relative risk; CI, confidence interval.
Figure 4.
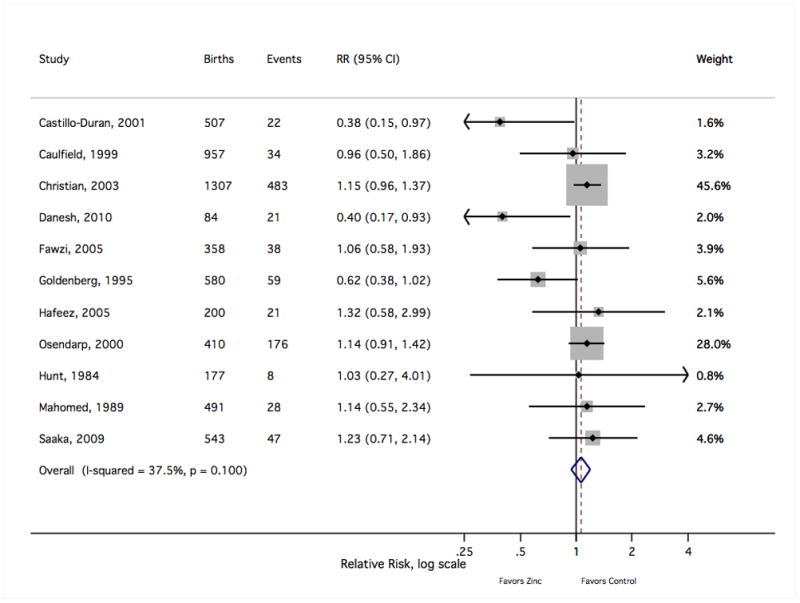
The forest plot graphically depicts the individual results included in meta-analysis. Sizes of the boxes are proportional to the weight assigned in calculating the fixed-effects sRR, where weight was assigned inversely to variance. Results to the left of relative risk = 1 indicate a lower risk of low birth weight with zinc supplementation. Events indicate the number of low weight births (<2500 grams). RR, relative risk; CI, confidence interval.
Figure 5.
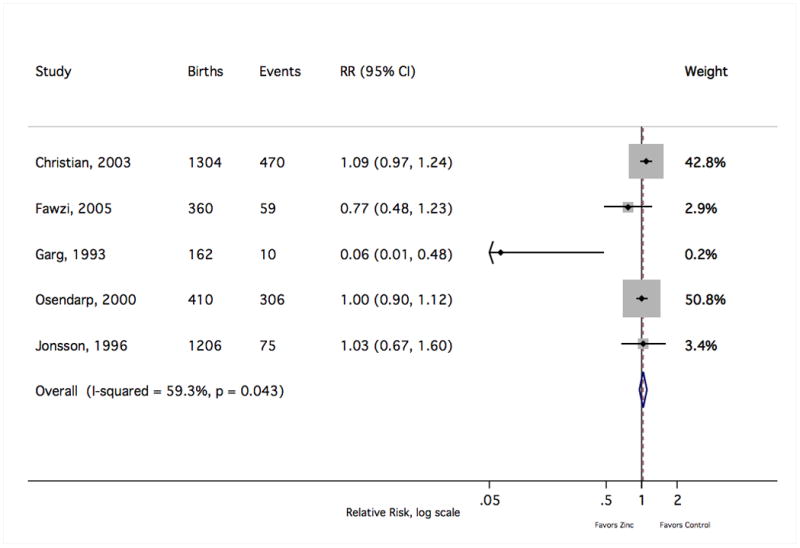
The forest plot graphically depicts the individual results included in meta-analysis. Sizes of the boxes are proportional to the weight assigned in calculating the fixed-effects sRR, where weight was assigned inversely to variance. Results to the left of relative risk = 1 indicate a lower risk of a SGA birth with zinc supplementation. Events indicate the number of SGA births. RR, relative risk; CI, confidence interval.
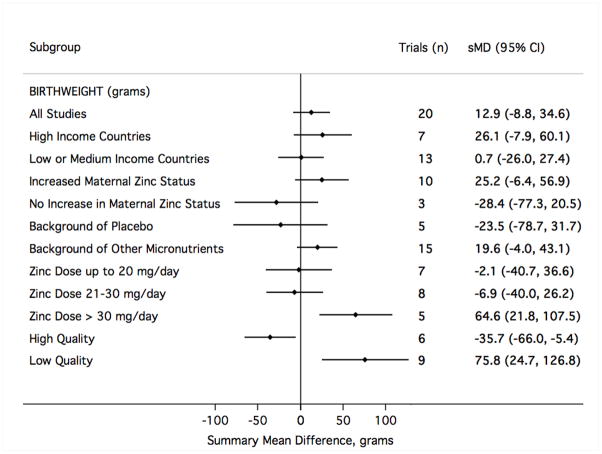
There was no evidence to support a meaningful zinc supplementation effect on mean birth weight, length at birth, gestational age at birth or head circumference at birth (Table 1). The pooled mean difference in birth weight suggested a slight increase for the zinc group over the comparison group (sMD 13 g [95% CI −9, 35]) that did not reach statistical significance despite including data from approximately 8000 births (Figure 6). The exclusion of one result35 was sufficient to drop the sMD within 2 g of the null value (2 g [−20, 24]). That relatively small trial,35 which was given a low quality grade, accounted for less than 2% of the weight in calculating the sMD but contributed to nearly three-fourths of the value of the Cochrane Q heterogeneity statistic. That study was also an outlier in considering the risk of preterm birth (Figure 3) and the risk of small-for-gestational age delivery (Figure 5). Notably, this trial delivered one of the largest doses of supplemental zinc (45 g/day) and was the only study to report a statistically significant reduction in copper status with zinc supplementation relative to controls.36 Summary measures for the mean differences at birth in length (Figure 7), gestational age (Figure 8) and head circumference (Figure 9) all centred near zero and were by and large insensitive to the exclusion of individual results.
Figure 6.
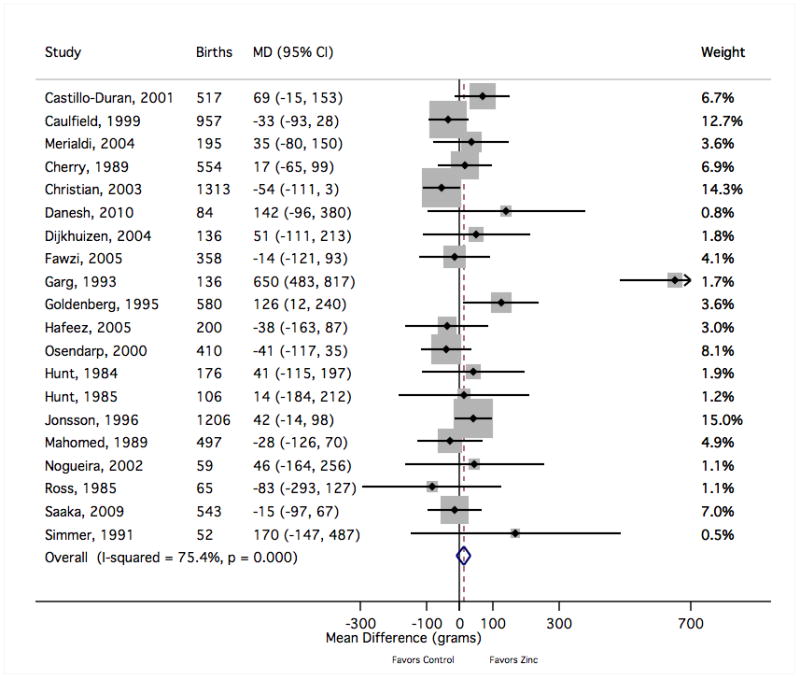
The forest plot graphically depicts the individual results included in meta-analysis. Sizes of the boxes are proportional to the weight assigned in calculating the fixed-effects sMD, where weight was assigned inversely to variance. Results to the right of mean difference = 0 indicate greater mean birth weight with zinc supplementation (in grams). MD, mean difference; CI, confidence interval.
Figure 7.
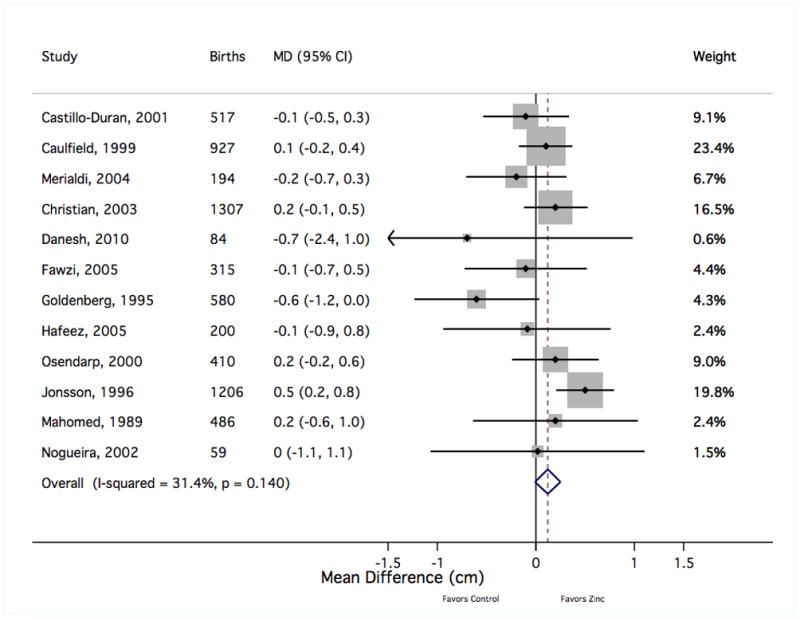
The forest plot graphically depicts the individual results included in meta-analysis. Sizes of the boxes are proportional to the weight assigned in calculating the fixed-effects sMD, where weight was assigned inversely to variance. Results to the right of mean difference = 0 indicate greater mean birth length with zinc supplementation (in centimeters). MD, mean difference; CI, confidence interval.
Figure 8.
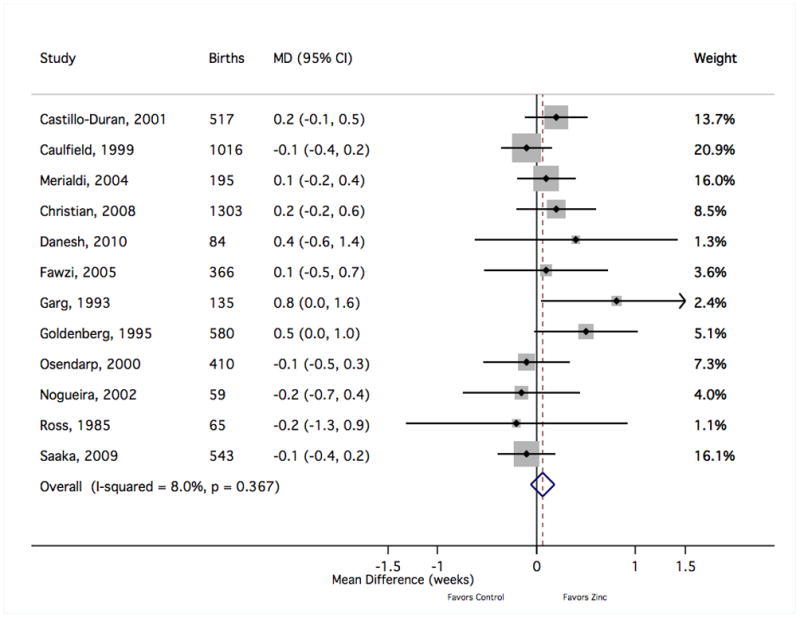
The forest plot graphically depicts the individual results included in meta-analysis. Sizes of the boxes are proportional to the weight assigned in calculating the fixed-effects sMD, where weight was assigned inversely to variance. Results to the right of mean difference = 0 indicate greater mean gestational age with zinc supplementation (in weeks). MD, mean difference; CI, confidence interval.
Figure 9.
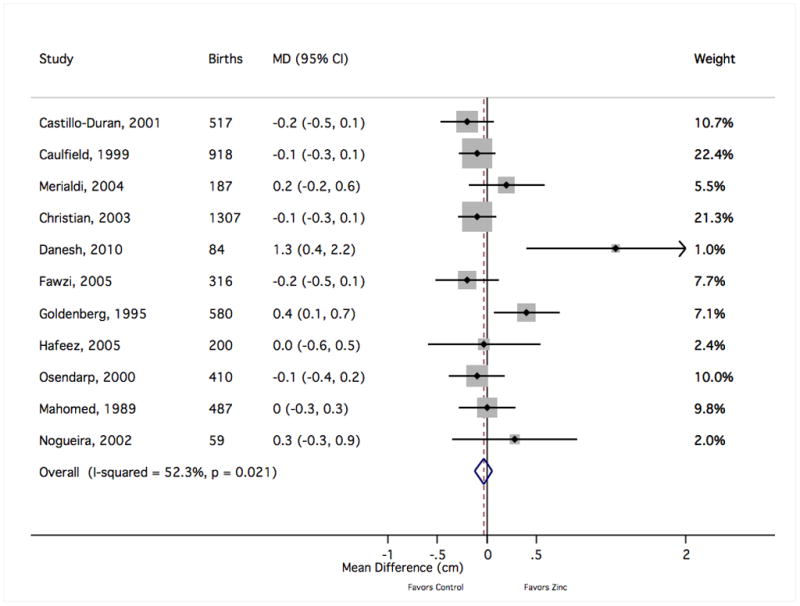
The forest plot graphically depicts the individual results included in meta-analysis. Sizes of the boxes are proportional to the weight assigned in calculating the fixed-effects sMD, where weight was assigned inversely to variance. Results to the right of mean difference = 0 indicate greater mean head circumference with zinc supplementation (in centimeters). MD, mean difference; CI, confidence interval.
Visual inspection of funnel plots22 (Web Supplement 3) did not indicate a disproportionate representation of study results according to strength and precision for any of the above outcomes. A Begg test23 indicated a statistically significant trend towards results favouring zinc supplementation with decreasing precision only for head circumference at birth (continuity corrected P = 0.02). However, for the three summary measures that were altered under a trim and fill procedure,25 none of these augmented estimates differed greatly from those obtained from observed values: weight at birth (−12 g [−32, 8]), gestational age at birth (0.0 weeks [−0.1, 0.1]) or head circumference at birth (−0.1 cm [−0.2, 0.0]). Although the estimated summary mean difference in birth weight changed direction, it did not gain statistical significance.
Subgroup analysis
For each of the outcomes considered in meta-analysis, the summary findings based only on those trials graded as low quality pointed to a more beneficial effect of zinc than those graded as high quality (Figures 10–12). There was not a strong signal that the impact of zinc supplementation differed greatly by any of the three other subgroups considered: national income, whether a trial also reported an increase in maternal blood zinc status, or by the nature of the comparison group (placebo or multiple micronutrients) (Figures 10–12). There was a slight indication that above doses of 30mg/day of supplemental zinc, there was an increase in birth weight (based on five studies32,35,38,73,79) and head circumference at birth (based on a single study79). However, this finding did not hold for any other outcome.
Figure 10.
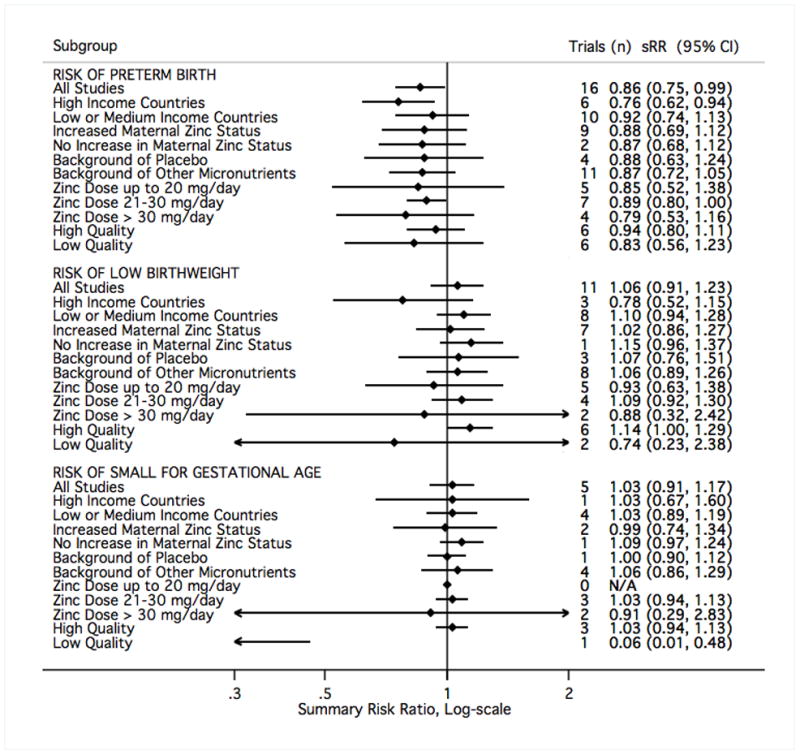
The study results contributing to meta-analysis were divided into groups based on study characteristics (left), and fixed-effects sRR were calculated accordingly. Results to the left of relative risk = 1 indicate a beneficial effect of zinc supplementation. sRR, summary relative risk; CI, confidence interval.
Figure 12.
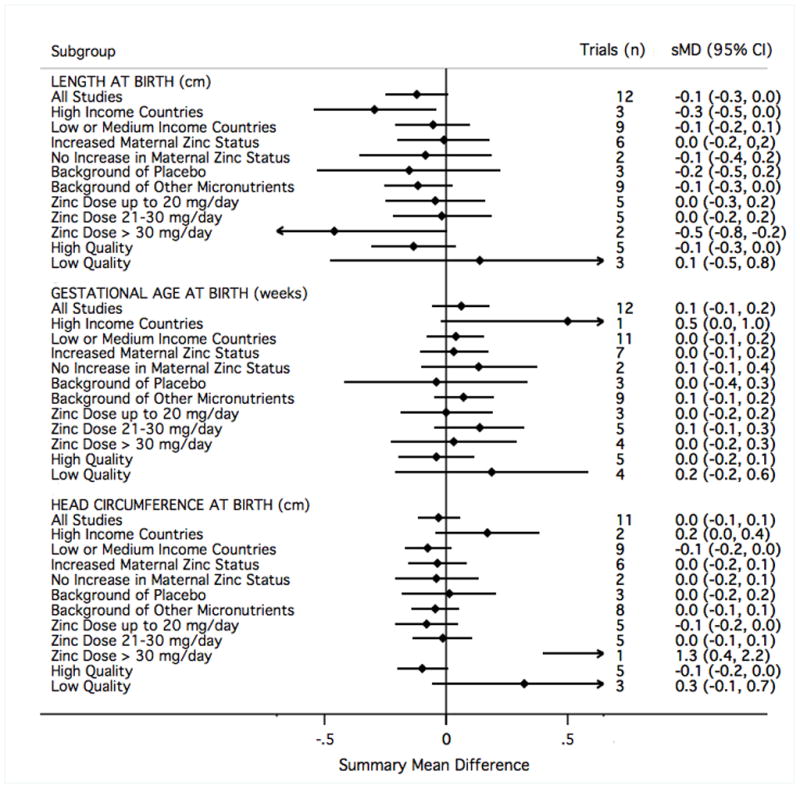
The study results contributing to meta-analysis were divided into groups based on study characteristics (left), and fixed-effects sMD were calculated accordingly. Results to the right of mean difference = 0 indicate greater length (in centimeters), gestational age (in weeks), or head circumference (in centimeters) at birth.
Comments
Our findings of the effects of zinc supplementation for improving pregnancy and infant outcomes agrees with those published by Mahomed and co-workers in 2007.18 Although we draw conclusions from a larger and not entirely overlapping set of trials, the overall impression regarding the effect of supplemental zinc was unchanged. Using data from 16 trials including 7818 births (963 preterm), we estimated a reduction in the risk of a preterm birth with zinc supplementation (sRR 0.86 [95% CI 0.75, 0.99]). We found no effect, however, on mean gestational age. This may be because there were fewer studies of gestational age than of preterm birth (12 vs. 16 trials), and the measurement errors for determining gestational age may be greater than that classifying the birth as preterm. Several different methods can be used to assess gestational age and most require considerable judgement by the evaluator.91 Whereas classifying a birth as preterm is a binary decision that is easier to make, especially if the infant is very preterm. We found no evidence that the effect on preterm birth was affected by zinc dose. One group79 studied the effect of supplemental zinc (50 mg/day) on the risk of preterm birth in a cohort of women who had a previous preterm delivery. There was a suggestion that supplemental zinc reduced prematurity in this high-risk group, but the sample size (42 women/group) was relatively small.
The steady increase in the incidence of preterm birth during the past decade is troubling. Infants born preterm are at greater risk for mortality and a variety of health and developmental problems.91 Thus, if reflective of a true causal effect, a 14% reduction in preterm birth with zinc supplementation, as estimated from these trials, would be of major public health importance. Several plausible explanations for the positive effect of zinc on preterm births exist. Zinc deficiency alters circulating levels of a number of hormones associated with the onset of labour. For example, lower levels of serum progesterone and prolactin concentrations in zinc-deficient ewes was associated with preterm deliveries.92 Also, systemic and intrauterine infections are a major cause of preterm birth.91 Zinc is essential for normal immune function.1 Zinc supplementation may reduce the incidence or the severity of maternal infections that, in turn, lower the risk of preterm birth. It has been reported that iron supplementation interferes with zinc absorption in pregnancy.1,45 However, we did not observe substantial differences when the analysis was restricted only to those trials providing supplemental zinc alone vs. only those that provided zinc along with iron and other micronutrients (Figures 10–12).
In concordance with previously reported findings,18 zinc supplementation did not appear to significantly improve birth weight, birth length or head circumference (Table 1). The overall mean difference in birth weight slightly favoured the intervention; however, this estimate was relatively sensitive to the inclusion of one or two particular study results, which likely influenced the differences observed in subgroup analysis (Figure 11). Despite the imprecision surrounding this summary estimate, it is unlikely that additional trials would be sufficient to shift the balance in favour of a clinically and statistically significant effect of zinc on foetal growth. Many investigators have found a relationship between maternal zinc status and birth weight in observational studies.3,8–10 The inability to show a consistent growth effect in intervention trials could be related to the challenges, such as participant compliance, inherent in improving zinc status in field settings. Nonetheless, there was no indication of a stronger effect when summary results excluded those trials that failed to demonstrate an improvement in maternal serum zinc concentrations (Figures 10–12). Alternatively, supplementation might only be effective among those suffering from zinc deficiency, and therefore, population-level effects might not capture improvements among this subgroup. While data were not available to examine this possibility directly, we did not observe stronger effects in low and middle-income countries, where zinc deficiency is likely to be common.2
Figure 11.
The study results contributing to meta-analysis were divided into groups based on study characteristics (left), and fixed-effects sMD were calculated accordingly. Results to the right of mean difference = 0 indicate greater mean birth weight with zinc supplementation (in grams). sMD, summary mean difference; CI, confidence interval.
The technique of meta-analysis has been the subject of criticism,93 particularly when summary estimates are considered without a detailed exploration of sources of heterogeneity. These concerns are valid, and we agree that meta-analysis is problematic if intentioned to precisely estimate causal effects. Because of heterogeneity in study designs, study populations and the context of the interventions, CI derived from meta-analysis are artificially narrow. That said, meta-analysis is an extremely useful tool for summarising the results of existing intervention trials in a systematic and objective way, with strengths that outnumber weaknesses.94 While the precision of our numeric estimates may be overstated, we believe that the general impression of our findings – that previous trials have shown little to no impact of maternal zinc supplementation on foetal growth but have suggested a modest reduction in the risk of preterm birth that cannot yet be confirmed – is not driven by inherent bias in our quantitative methods.
Meta-analysis, or any standard literature review for that matter, is necessarily limited to the published evidence. Therefore, any tendency of investigators or journals to selectively publish statistically significant positive results could yield a bias when findings are taken in aggregate. While this type of bias has been well described,95 we did not observe an overwhelming trend towards positive results with decreasing statistical precision of individual trial findings (Web Supplement 3). We did detect a stronger zinc effect when pooling studies graded as low quality vs. those graded as high quality (Figures 10–12). Because the quality grades are subjective, firm conclusions cannot be drawn. Yet, this suggests possible preferential publication of methodologically weaker studies with positive results. Again, despite the prospect of bias in quantitative estimates, our general conclusions were not swayed towards remarkably positive findings.
The results of our meta-analysis and that of Mahomed and co-workers18 suggest that prenatal zinc supplementation does not effect foetal growth. One group measured foetal bone growth by ultrasound during gestation.96 Of the four measurements made, only femur diaphysis length was greater in foetuses of mothers receiving supplemental zinc. However, this outcome differs from the effects of supplemental zinc on growth in pre-pubertal children. A metaanalysis of 33 studies showed a highly significant positive effect on both height and weight increments with greater responses seen in children with low weight-for-age or height-for-age z scores.97 These findings suggest that the impact of zinc, per se, on growth in utero is less than that in a young child. It is unclear why this difference exists. Possibly, zinc is prioritised towards developing the immune system and other organs or tissue functions during the in utero period instead of skeletal growth. Or, maybe the effect of maternal nutrition on the foetal growth trajectory is established before conception or early in pregnancy prior to the usual initiation of zinc supplementation. These findings suggest, however, that stunting is more likely to be improved by providing zinc supplements to the child rather than to the mother during pregnancy.98
The small effects of supplemental zinc on pregnancy outcomes suggest a need to compare the effects of delivering nutrients through supplements vs. other methods, such as food. Early studies showed that improving the quality, or nutrient density, of the mother’s diet dramatically improved pregnancy outcomes.99–101 The use of prenatal multiple micronutrient supplements became common in the 1960s to prevent iron deficiency. A recent systematic review found that multiple micronutrient supplements did not reduce maternal anaemia or infant mortality when compared with iron-folate supplementation alone, but did report a statistically significant 9% reduction in the risk of small-for-gestational age births when pooling data across 14 studies in developing countries.102 The relative impacts of supplements vs. food on pregnancy outcomes merits further attention.
Strong evidence exists that zinc supplements improve the prognosis of children being treated for diarrhoea.98 Of the studies we reviewed, three trials reported a trend towards a decreased incidence of diarrhoea between 6 and 13 months of age with prenatal supplemental zinc.46,80,81 The effect on acute diarrhoea was stronger than that for episodes of persistent diarrhoea. Possibly, prenatal zinc supplementation improved the infant’s immune function, which would be consistent with our hypothesis that maternal zinc supplement is prioritised towards the development of immunity rather than growth in the foetus. Previous research suggests that development of the fetal nervous system in utero is influenced by maternal zinc status. For example, the offspring of rhesus monkeys deprived of zinc during the third trimester were not as active and they explored less than control infants.16 Morphological examinations showed that the structure and migration of neuronal cell types were altered in these offspring. In a study of Egyptian women, Kirskey and co-workers noted a positive association between maternal zinc status and newborn behaviour.103 However, prenatal zinc supplementation had no effect in neurocognitive development in Bangladeshi infants at 13 months of age, US children at 5 years of age or Peruvian children at 4.5 years of age.52,59,77 Neurocognitive development was evaluated in three trials reviewed by us.52,59,61 The three groups measured different end-points at different time points and reported three different findings. One group found that the mental and psychomotor development was worse in the zinc group compared with the controls at 13 months of age.52 Another reported an improvement in foetal neurobehaviour as measured by foetal heart rate,61 and the third group found no effects on differential abilities, visual or auditory sequential memory scores, gross motor scale and grooved pegboard scores in the children at 5 years of age.59
Of the 20 studies we evaluated, five groups measured postnatal outcomes –Bangladesh,50,52 Indonesia,60 Nepal,69 Peru70,80 and the US.59 Postnatal growth was evaluated more than any other outcome. Recent discoveries show that the interaction between our genes and their environment in utero has health longterm effects. This phenomenon is called developmental programming. Given the role of zinc in regulating DNA synthesis and gene expression, the foetal zinc environment likely influences the developmental programming of that individual. Some evidence for this association exists. Over 30 years ago, Beach and co-workers showed that maternal zinc deficiency adversely affected immune function in the offspring, which persisted for the next three generations.6 Also, Jou and co-workers recently reported that the offspring of pregnant rats fed diets marginally low in zinc gained excessive weight post-natally and had impaired insulin sensitivity.5 These epigenetic changes are thought to be due to shifts in DNA and histone methylation. It has been known since 1985 that zinc deficiency decreases methylation of those nuclear components.104 In the future, longer-term studies are needed to assess the impact of prenatal zinc supplementation on the health of the child. Neuro-cognitive and immunological function should be assessed in addition to growth.
Given the logistical and financial barriers in completing long-term follow-up of intervention trial participants, it is not surprising that less evidence is available on the impact of maternal zinc supplementation on child health than on foetal growth and preterm birth. The suggestion of a reduction in diarrhoea occurrence among infants merits further attention, although from the trials examining impacts on childhood growth, cognitive development and respiratory illness, little evidence of a benefit has emerged (Figure 2). Future work may be directed towards further assessment of functional outcomes that become apparent during childhood or later in life.
In aggregate, results published to date suggest no harm but uncertain benefits with maternal zinc supplementation in pregnancy. While an imperfect global estimator of causal effects, meta-analysis provides a quantitative summary that is unlikely to obscure a strong benefit merely through bias in our quantitative methods. The specifics of the population and implementation protocol should always be considered when anticipating the impacts, although we did not detect striking patterns when considering subgroups of studies separated by national income, dose of zinc provided or the simultaneous provision of other micronutrients. The suggestion that maternal zinc supplementation might reduce the occurrence of preterm birth or the frequency of diarrhoea in childhood is notable and encouraging, as even small reductions in these events would be significant public health achievements. The decisions to undertake further research or to initiate zinc-based interventions, however, must be made within the context of costs, feasibility and the presence of other potential interventions that might offer a greater probability of success. With much to be learned about the mechanism of action and possible lingering effects into childhood, further studies of maternal zinc supplementation might provide new insight. Yet, balanced with considerable evidence of no improvement in foetal growth, it appears unlikely that zinc supplementation in pregnancy will play a leading role in future advances in maternal and child health.
Supplementary Material
Acknowledgments
Dr Chaffee receives support from a dental scientist training grant (F30) from the National Institute of Health, Bethesda, Maryland (DE022208-01).
Footnotes
Conflicts of interests
The authors report that they have no conflicts of interest related to this review.
References
- 1.King JC, Cousins RJ. Zinc. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. 10. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. pp. 271–285. [Google Scholar]
- 2.Caulfield LE, Zavaleta N, Shankar AH, Merialdi M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. American Journal of Clinical Nutrition. 1998;68:499S–508S. doi: 10.1093/ajcn/68.2.499S. [DOI] [PubMed] [Google Scholar]
- 3.King JC. Determinants of maternal zinc status during pregnancy. American Journal of Clinical Nutrition. 2000;71:1334S–1343S. doi: 10.1093/ajcn/71.5.1334s. [DOI] [PubMed] [Google Scholar]
- 4.McArdle HJ, Andersen HS, Jones H, Gambling L. Fetal programming: causes and consequences as revealed by studies of dietary manipulation in rats – a review. Placenta. 2006;27 (Suppl A):S56–S60. doi: 10.1016/j.placenta.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Jou MY, Philipps AF, Lonnerdal B. Maternal zinc deficiency in rats affects growth and glucose metabolism in the offspring by inducing insulin resistance postnatally. Journal of Nutrition. 2010;140:1621–1627. doi: 10.3945/jn.109.119677. [DOI] [PubMed] [Google Scholar]
- 6.Beach RS, Gershwin ME, Hurley LS. Persistent immunological consequences of gestation zinc deprivation. American Journal of Clinical Nutrition. 1983;38:579–590. doi: 10.1093/ajcn/38.4.579. [DOI] [PubMed] [Google Scholar]
- 7.Hambidge KM, Neldner KH, Walravens PA. Zinc, acrodermatitis enteropathica, and congenital malformations. Lancet. 1975;1:577–578. doi: 10.1016/s0140-6736(75)91601-3. [DOI] [PubMed] [Google Scholar]
- 8.Prema K, Ramalagshmi B, Neelakumari S. Serum copper and zinc in pregnancy. Indian Medical Research. 1980;71:547–553. [PubMed] [Google Scholar]
- 9.Jameson S. Effects of zinc deficiency in human reproduction. Acta Medica Scandinavica. 1976;593:3–89. [PubMed] [Google Scholar]
- 10.Simmer K, Thompson RPH. Maternal zinc and intrauterine growth retardation. Clinical Science. 1985;68:395–399. doi: 10.1042/cs0680395. [DOI] [PubMed] [Google Scholar]
- 11.Cherry FF, Sandstead HH, Rojas P, Johnson LK, Batson KH, Wang XB. Adolescent pregnancy: associations among body weight, zinc nutriture, and pregnancy outcome. American Journal of Clinical Nutrition. 1989;50:945–954. doi: 10.1093/ajcn/50.5.945. [DOI] [PubMed] [Google Scholar]
- 12.Kynast G, Saling E. Effect of oral zinc application during pregnancy. Gynecologic and Obstetric Investigation. 1986;21:117–123. doi: 10.1159/000298940. [DOI] [PubMed] [Google Scholar]
- 13.Swanson CA, King JC. Zinc and pregnancy outcome. American Journal of Clinical Nutrition. 1987;46:763–771. doi: 10.1093/ajcn/46.5.763. [DOI] [PubMed] [Google Scholar]
- 14.Davies NT, Williams RB. The effect of pregnancy and lactation on the absorption of zinc and lysine by the rat duodenum in situ. British Journal of Nutrition. 1977;38:417–423. doi: 10.1079/bjn19770106. [DOI] [PubMed] [Google Scholar]
- 15.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 16.Uriu-Adams JY, Keen CL. Zinc and reproduction: effects of zinc deficiency on prenatal and early postnatal development. Birth Defects Research Part B, Developmental and Reproductive Toxicology. 2010;89:313–325. doi: 10.1002/bdrb.20264. [DOI] [PubMed] [Google Scholar]
- 17.Ramakrishnan U. Nutrition and low birthweight: from research to practice. American Journal of Clinical Nutrition. 2004;79:17–21. doi: 10.1093/ajcn/79.1.17. [DOI] [PubMed] [Google Scholar]
- 18.Mahomed K, Bhutta Z, Middleton P. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database of Systematic Reviews. 2007;(2):CD000230. doi: 10.1002/14651858.CD000230.pub3. [DOI] [PubMed] [Google Scholar]
- 19.Petitti D. Meta-analysis, Decision Analysis, and Cost Effectiveness Analysis. Oxford: Oxford University Press; 1994. pp. 108–111. [Google Scholar]
- 20.Shore R, Gardner M, Pannett B. Ethylene oxide: an assessment of the epidemiological evidence on carcinogenicity. British Journal of Industrial Medicine. 1993;50:971–997. doi: 10.1136/oem.50.11.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed) 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg C, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 24.Light R, Pillemer D. Summing Up: the Science of Reviewing Research. Cambridge, MA: Harvard University Press; 1984. pp. 65–67. [Google Scholar]
- 25.Duval S, Tweedie R. Trim and fill: a simple funnelplot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 26.Walker N, Fischer-Walker C, Bryce J, Bahl R, Cousens S. Standards for CHERG reviews of intervention effects on child survival. International Journal of Epidemiology. 2010;39 (Suppl 1):i21–i31. doi: 10.1093/ije/dyq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grade Working Group. Grading quality of evidence and strength of recommendations. BMJ (Clinical Research Ed) 2008;328:1490–1498. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyatt GH, Oxman AD, Kunz R, Jaeschke R, Helfand M, Liberati A, Vist GE, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Research Ed) 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt IF, Murphy NJ, Cleaver AE, Faraji B, Swendseid ME, Coulson AH, et al. Zinc supplementation during pregnancy: zinc concentration of serum and hair from low-income women of Mexican descent. American Journal of Clinical Nutrition. 1983;37:572–582. doi: 10.1093/ajcn/37.4.572. [DOI] [PubMed] [Google Scholar]
- 30.Hunt IF, Murphy NJ, Cleaver AE, Faraji B, Swendseid ME, Coulson AH, et al. Zinc supplementation during pregnancy: effects of selected blood constituents and on progress and outcome of pregnancy in low-income women of Mexican descent. American Journal of Clinical Nutrition. 1984;40:508–521. doi: 10.1093/ajcn/40.3.508. [DOI] [PubMed] [Google Scholar]
- 31.Hunt IF, Murphy NJ, Cleaver AE, Faraji B, Swendseid ME, Browdy BL, et al. Zinc supplementation during pregnancy in low-income teenagers of Mexican descent: effects on selected blood constituents and on progress and outcome of pregnancy. American Journal of Clinical Nutrition. 1985;42:815–828. doi: 10.1093/ajcn/42.5.815. [DOI] [PubMed] [Google Scholar]
- 32.Ross SM, Nel E, Naeye RL. Differing effects of low and high bulk maternal dietary supplements during pregnancy. Early Human Development. 1985;10:295–302. doi: 10.1016/0378-3782(85)90061-1. [DOI] [PubMed] [Google Scholar]
- 33.Mahomed K, James DK, Golding J, McCabe R. Zinc supplementation during pregnancy: a double blind randomised controlled trial. BMJ (Clinical Research Ed) 1989;299:826–830. doi: 10.1136/bmj.299.6703.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmer K, Lort-Phillips L, James C, Thompson RP. A double-blind trial of zinc supplementation in pregnancy. European Journal of Clinical Nutrition. 1991;45:139–144. [PubMed] [Google Scholar]
- 35.Garg HK, Singhal KC, Arshad Z. A study of the effect of oral zinc supplementation during pregnancy on pregnancy outcome. Indian Journal of Physiology and Pharmacology. 1993;37:276–284. [PubMed] [Google Scholar]
- 36.Garg HK, Singhal KC, Arshad Z. Effect of oral zinc supplementation on copper and haemoglobin levels in pregnant women. Indian Journal of Physiology and Pharmacology. 1994;38:272–276. [PubMed] [Google Scholar]
- 37.Goldenberg RL, Tamura T, Neggers Y, Copper RL, Johnston KE, DuBard MB, et al. The effect of zinc supplementation on pregnancy outcome. Journal of the American Medical Association. 1995;274:463–468. doi: 10.1001/jama.1995.03530060037030. [DOI] [PubMed] [Google Scholar]
- 38.Jonsson B, Hauge B, Larsen MF, Hald F. Zinc supplementation during pregnancy: a double blind randomised controlled trial. Acta Obstetricia et Gynecologica Scandinavica. 1996;75:725–729. doi: 10.3109/00016349609065735. [DOI] [PubMed] [Google Scholar]
- 39.Neggers YH, Goldenberg RL, Tamura T, Johnston KE, Copper RL, DuBard M. Plasma and erythrocyte zinc concentrations and their relationship to dietary zinc intake and zinc supplementation during pregnancy in low-income African-American women. Journal of the American Dietetic Association. 1997;11:1269–1274. doi: 10.1016/s0002-8223(97)00304-0. [DOI] [PubMed] [Google Scholar]
- 40.Caulfield LE, Zavaleta N, Figueroa A, Leon Z. Maternal zinc supplementation does not affect size at birth or pregnancy duration in Peru. Journal of Nutrition. 1999;129:1563–1568. doi: 10.1093/jn/129.8.1563. [DOI] [PubMed] [Google Scholar]
- 41.Caulfield L, Zavaleta N, Figueroa A. Adding zinc to prenatal iron and folate supplements improves maternal and neonatal zinc status in a Peruvian population. American Journal of Clinical Nutrition. 1999;69:1257–1263. doi: 10.1093/ajcn/69.6.1257. [DOI] [PubMed] [Google Scholar]
- 42.Merialdi M, Caulfield L, Zavaleta N, Figueroa A, DiPietro JA. Adding zinc to prenatal iron and folate tablets improves fetal neurobehavioral development. American Journal of Obstetrics and Gynecology. 1999;180:483–490. doi: 10.1016/s0002-9378(99)70236-x. [DOI] [PubMed] [Google Scholar]
- 43.O’Brien KO, Zavaleta N, Caulfield LE, Yang DX, Abrams SA. Influence of prenatal iron and zinc supplements on supplemental iron absorption, red blood cell iron incorporation, and iron status in pregnant Peruvian women. American Journal of Clinical Nutrition. 1999;69:509–515. doi: 10.1093/ajcn/69.3.509. [DOI] [PubMed] [Google Scholar]
- 44.Hogg BB, Tamura T, Johnston KE, DuBard MA, Goldenberg RL. Second-trimester plasma homocysteine levels and pregnancy-induced hypertension, preeclampsia, and intrauterine growth restriction. American Journal of Obstetrics and Gynecology. 2000;183:805–809. doi: 10.1067/mob.2000.109044. [DOI] [PubMed] [Google Scholar]
- 45.O’Brien KO, Zavaleta N, Caulfield LE, Wen J, Abrams SA. Prenatal iron supplements impair zinc absorption in pregnant Peruvian women. Journal of Nutrition. 2000;130:2251–2255. doi: 10.1093/jn/130.9.2251. [DOI] [PubMed] [Google Scholar]
- 46.Osendarp SJ, van Raaij JM, Arifeen SE, Wahed M, Baqui AH, Fuchs GJ. A randomized, placebo-controlled trial of the effect of zinc supplementation during pregnancy on pregnancy outcome in Bangladeshi urban poor. American Journal of Clinical Nutrition. 2000;71:114–119. doi: 10.1093/ajcn/71.1.114. [DOI] [PubMed] [Google Scholar]
- 47.Zavaleta N, Caulfield LE, Garcia T. Changes in iron status during pregnancy in peruvian women receiving prenatal iron and folic acid supplements with or without zinc. American Journal of Clinical Nutrition. 2000;71:956–961. doi: 10.1093/ajcn/71.4.956. [DOI] [PubMed] [Google Scholar]
- 48.Castillo-Duran C, Marin VB, Alcazar LS, Iturralde H, Ruz MO. Controlled trial of zinc supplementation in Chilean pregnant adolescents. Nutrition Research. 2001;21:715–724. [Google Scholar]
- 49.Nogueira NN, Marreiro DN, Parente JV, Cozzolino SM. Utilization of different iron concentrations on pregnant adolescents also supplemented with zinc and folate. Archivos Latinoamericanos de Nutrición. 2001;51:225–229. (in Portuguese) [PubMed] [Google Scholar]
- 50.Osendarp SJ, van Raaij JM, Darmstadt GL, Baqui AH, Hautvast JG, Fuchs GJ. Zinc supplementation during pregnancy and effects on growth and morbidity in low birthweight infants: a randomised placebo controlled trial. Lancet. 2001;357:1080–1085. doi: 10.1016/s0140-6736(00)04260-4. [DOI] [PubMed] [Google Scholar]
- 51.Tamura T, Olin KL, Goldenberg RL, Johnston KE, Dubard MB, Keen CL. Plasma extracellular superoxide dismutase activity in healthy pregnant women is not influenced by zinc supplementation. Biological Trace Element Research. 2001;80:107–113. doi: 10.1385/BTER:80:2:107. [DOI] [PubMed] [Google Scholar]
- 52.Hamadani JD, Fuchs GJ, Osendarp SJ, Huda SN, Grantham-McGregor SM. Zinc supplementation during pregnancy and effects on mental development and behaviour of infants: a follow-up study. Lancet. 2002;360:290–294. doi: 10.1016/S0140-6736(02)09551-X. [DOI] [PubMed] [Google Scholar]
- 53.Nogueira NN, Macedo AS, Parente JV, Cozzolino SM. Nutritional profile of newborns of adolescent mothers supplemented with iron, in different concentrations, zinc and folic acid. Revista de Nutrição. 2002;15:193–200. (in Portuguese) [Google Scholar]
- 54.Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Shrestha SR, et al. Effects of alternative maternal micronutrient supplements on low birthweight in rural Nepal: double blind randomised community trial. BMJ (Clinical Research Ed) 2003;326:571. doi: 10.1136/bmj.326.7389.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christian P, Shrestha J, LeClerq SC, Khatry SK, Jiang T, Wagner T, et al. Supplementation with micronutrients in addition to iron and folic acid does not further improve the hematologic status of pregnant women in rural Nepal. Journal of Nutrition. 2003;133:3492–3498. doi: 10.1093/jn/133.11.3492. [DOI] [PubMed] [Google Scholar]
- 56.Christian P, West KP, Jr, Khatry SK, LeClerq SC, Pradhan EK, Katz J, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster-randomized trial in Nepal. American Journal of Clinical Nutrition. 2003;78:1194–1202. doi: 10.1093/ajcn/78.6.1194. [DOI] [PubMed] [Google Scholar]
- 57.Nogueira NN, Parente JV, Cozzolino SM. Changes in plasma zinc and folic acid concentrations in pregnant adolescents submitted to different supplementation regimens. Cadernos de Saúde Pública. 2003;19:155–160. doi: 10.1590/s0102-311x2003000100017. (in Portuguese) [DOI] [PubMed] [Google Scholar]
- 58.O’Brien KO, Zavaleta N, Abrams SA, Caulfield LE. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. American Journal of Clinical Nutrition. 2003;77:924–930. doi: 10.1093/ajcn/77.4.924. [DOI] [PubMed] [Google Scholar]
- 59.Tamura T, Goldenberg RL, Ramey SL, Nelson KG, Chapman VR. Effect of zinc supplementation of pregnant women on the mental and psychomotor development of their children at 5 y of age. American Journal of Clinical Nutrition. 2003;77:1512–1516. doi: 10.1093/ajcn/77.6.1512. [DOI] [PubMed] [Google Scholar]
- 60.Dijkhuizen MA, Wieringa FT, West CE, Muhilal Zinc plus beta-carotene supplementation of pregnant women is superior to beta-carotene supplementation alone in improving vitamin A status in both mothers and infants. American Journal of Clinical Nutrition. 2004;80:1299–1307. doi: 10.1093/ajcn/80.5.1299. [DOI] [PubMed] [Google Scholar]
- 61.Merialdi M, Caulfield LE, Zavaleta N, Figueroa A, Dominici F, Dipietro JA. Randomized controlled trial of prenatal zinc supplementation and the development of fetal heart rate. American Journal of Obstetrics and Gynecology. 2004;190:1106–1112. doi: 10.1016/j.ajog.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 62.Fawzi W, Villamor E, Msamanga G, Antelman G, Aboud S, Urassa W, et al. Trial of zinc supplements in relation to pregnancy outcomes, hematologic indicators, and T cell counts among HIV-1-infected women in Tanzania. American Journal of Clinical Nutrition. 2005;81:161–167. doi: 10.1093/ajcn/81.1.161. [DOI] [PubMed] [Google Scholar]
- 63.Hafeez A, Mahmood G, Hassan M, Batool T, Hayat H, Mazhar F, et al. Serum zinc levels and effects of oral supplementation in pregnant women. Journal of College of Physicians and Surgeons Pakistan. 2005;15:612–615. [PubMed] [Google Scholar]
- 64.Hafeez A, Mehmood G, Mazhar F. Oral zinc supplementation in pregnant women and its effect on birthweight: a randomised controlled trial. Archives of Disease in Childhood Fetal and Neonatal Edition. 2005;90:F170–F171. doi: 10.1136/adc.2004.063008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christian P, Jiang T, Khatry SK, LeClerq SC, Shrestha SR, West KP., Jr Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. American Journal of Clinical Nutrition. 2006;83:788–794. doi: 10.1093/ajcn/83.4.788. [DOI] [PubMed] [Google Scholar]
- 66.Katz J, Christian P, Dominici F, Zeger SL. Treatment effects of maternal micronutrient supplementation vary by percentiles of the birthweight distribution in rural Nepal. Journal of Nutrition. 2006;136:1389–1394. doi: 10.1093/jn/136.5.1389. [DOI] [PubMed] [Google Scholar]
- 67.Villamor E, Aboud S, Koulinska IN, Kupka R, Urassa W, Chaplin B, et al. Zinc supplementation to HIV-1-infected pregnant women: effects on maternal anthropometry, viral load, and early mother-to-child transmission. European Journal of Clinical Nutrition. 2006;60:862–869. doi: 10.1038/sj.ejcn.1602391. [DOI] [PubMed] [Google Scholar]
- 68.Caulfield LE, Donangelo CM, Chen P, Junco J, Merialdi M, Zavaleta N. Red blood cell metallothionein as an indicator of zinc status during pregnancy. Nutrition. 2008;24:1081–1087. doi: 10.1016/j.nut.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christian P, Darmstadt GL, Wu L, Khatry SK, Leclerq SC, Katz J, et al. The effect of maternal micronutrient supplementation on early neonatal morbidity in rural Nepal: a randomised, controlled, community trial. Archives of Disease in Childhood. 2008;93:660–664. doi: 10.1136/adc.2006.114009. [DOI] [PubMed] [Google Scholar]
- 70.Iannotti LL, Zavaleta N, Leon Z, Shankar AH, Caulfield LE. Maternal zinc supplementation and growth in Peruvian infants. American Journal of Clinical Nutrition. 2008;88:154–160. doi: 10.1093/ajcn/88.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Christian P, Khatry SK, LeClerq SC, Dali SM. Effects of prenatal micronutrient supplementation on complications of labor and delivery and puerperal morbidity in rural Nepal. International Journal of Gynaecology and Obstetrics. 2009;106:3–7. doi: 10.1016/j.ijgo.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 72.Christian P, Stewart CP, LeClerq SC, Wu L, Katz J, West KP, Jr, et al. Antenatal and postnatal iron supplementation and childhood mortality in rural Nepal: a prospective follow-up in a randomized, controlled community trial. American Journal of Epidemiology. 2009;170:1127–1136. doi: 10.1093/aje/kwp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saaka M, Oosthuizen J, Beatty S. Effect of prenatal zinc supplementation on birthweight. Journal of Health, Population, and Nutrition. 2009;27:619–631. doi: 10.3329/jhpn.v27i5.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saaka M, Oosthuizen J, Beatty S. Effect of joint iron and zinc supplementation on malarial infection and anaemia. East African Journal of Public Health. 2009;6:55–62. doi: 10.4314/eajph.v6i1.45748. [DOI] [PubMed] [Google Scholar]
- 75.Stewart CP, Christian P, LeClerq SC, West KP, Jr, Khatry SK. Antenatal supplementation with folic acid + iron + zinc improves linear growth and reduces peripheral adiposity in school-age children in rural Nepal. American Journal of Clinical Nutrition. 2009;90:132–140. doi: 10.3945/ajcn.2008.27368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stewart CP, Christian P, Schulze KJ, Leclerq SC, West KP, Jr, Khatry SK. Antenatal micronutrient supplementation reduces metabolic syndrome in 6- to 8-year-old children in rural Nepal. Journal of Nutrition. 2009;139:1575–1581. doi: 10.3945/jn.109.106666. [DOI] [PubMed] [Google Scholar]
- 77.Caulfield LE, Putnick DL, Zavaleta N, Lazarte F, Albornoz C, Chen P, et al. Maternal gestational zinc supplementation does not influence multiple aspects of child development at 54 mo of age in Peru. American Journal of Clinical Nutrition. 2010;92:130–136. doi: 10.3945/ajcn.2010.29407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Christian P, Murray-Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, et al. Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. Journal of the American Medical Association. 2010;304:2716–2723. doi: 10.1001/jama.2010.1861. [DOI] [PubMed] [Google Scholar]
- 79.Danesh A, Janghorbani M, Mohammad HB. Effects of zinc supplementation during pregnancy on pregnancy outcome in women with history of preterm delivery: a double-blind randomized, placebo-controlled trial. Journal of Maternal-fetal and Neonatal Medicine. 2010;23:403–408. doi: 10.1080/14767050903165214. [DOI] [PubMed] [Google Scholar]
- 80.Iannotti LL, Zavaleta N, Leon Z, Huasquiche C, Shankar AH, Caulfield LE. Maternal zinc supplementation reduces diarrheal morbidity in Peruvian infants. Journal of Pediatrics. 2010;156:960–964. doi: 10.1016/j.jpeds.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 81.Wieringa FT, Dijkhuizen MA, Muhilal, Van der Meer JW. Maternal micronutrient supplementation with zinc and beta-carotene affects morbidity and immune function of infants during the first 6 months of life. European Journal of Clinical Nutrition. 2010;64:1072–1079. doi: 10.1038/ejcn.2010.115. [DOI] [PubMed] [Google Scholar]
- 82.Caulfield LE, Zavaleta N, Chen P, Lazarte F, Albornoz C, Putnick DL, et al. Maternal zinc supplementation during pregnancy affects autonomic function of Peruvian children assessed at 54 months of age. Journal of Nutrition. 2011;141:327–332. doi: 10.3945/jn.110.128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hakimi M, Dibley M, Surjono A, Nurdiati D. Impact of vitamin A and zinc supplementation on puerperal sepsis: a randomized controlled trial in rural Indonesia. In: Nurdiati D, editor. Nutrition and reproductive health in central Java, Indonesia; an epidemiological approach (PhD thesis) Umea, Sweden: Umea University Medical Dissertations; 2001. [Google Scholar]
- 84.An H, Yin S, Xu Q. Effects of supplementing calcium, iron and zinc on the fetus development and growth during pregnancy. Zhonghua Yu Fang Yi Xue Za Zhi. 2001;35:370–373. (in Chinese) [PubMed] [Google Scholar]
- 85.Mahmoudian A, Khademloo M. The effect of simultaneous administration of zinc sulfate and ferrous sulfate in the treatment of anemic pregnant women. Journal of Research in Medical Sciences. 2005;10:205–209. [Google Scholar]
- 86.Robertson JS, Heywood B, Atkinson SM. Zinc supplementation during pregnancy. Journal of Public Health Medicine. 1991;13:227–229. doi: 10.1093/oxfordjournals.pubmed.a042625. [DOI] [PubMed] [Google Scholar]
- 87.Christian P, Khatry SK, Yamini S, Stallings R, LeClerq SC, Shrestha SR, et al. Zinc supplementation might potentiate the effect of vitamin A in restoring night vision in pregnant Nepalese women. American Journal of Clinical Nutrition. 2001;73:1045–1051. doi: 10.1093/ajcn/73.6.1045. [DOI] [PubMed] [Google Scholar]
- 88.Xie L, Pan J, Chen X. Zinc supplementation to rural Chinese pregnant women and pregnancy outcome. Acta Nutrimenta Sinica. 1999;21:65–73. (in Chinese) [Google Scholar]
- 89.Yuan W, Geng G, Chen A, Wu J, Zhang Z, Gao E. Effects of zinc supplementation of rural pregnant women on the growth of offspring in early childhood. Fudan University Journal of Medical Sciences. 2004;5:496–501. (in Chinese) [Google Scholar]
- 90.Hambidge KM, Krebs NF, Jacobs MA, Favier A, Guyette L, Ikle DN. Zinc nutritional status during pregnancy: a longitudinal study. American Journal of Clinical Nutrition. 1983;37:429–442. doi: 10.1093/ajcn/37.3.429. [DOI] [PubMed] [Google Scholar]
- 91.Institute of Medicine. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 92.Apgar J. Zinc and reproduction: an update. Journal of Nutritional Biochemistry. 1992;3:266–278. [Google Scholar]
- 93.Greenland S. Invited commentary: a critical look at some popular meta-analytic methods. American Journal of Epidemiology. 1994;140:290–296. doi: 10.1093/oxfordjournals.aje.a117248. [DOI] [PubMed] [Google Scholar]
- 94.Lau J, Ioannidis J, Schmid C. Summing up evidence: one answer is not always enough. Lancet. 1998;351:123–127. doi: 10.1016/S0140-6736(97)08468-7. [DOI] [PubMed] [Google Scholar]
- 95.Hopewell S, Loudon K, Clarke M, Oxman A, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews. 2009;(1):MR000006. doi: 10.1002/14651858.MR000006.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Merialdi M, Caulfield LE, Zavaleta N, Figueroa A, Costigan KA, Dominici F, et al. Randomized controlled trial of prenatal zinc supplementation and fetal bone growth. American Journal of Clinical Nutrition. 2004;79:826–830. doi: 10.1093/ajcn/79.5.826. [DOI] [PubMed] [Google Scholar]
- 97.Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition. 2002;75:1062–1071. doi: 10.1093/ajcn/75.6.1062. [DOI] [PubMed] [Google Scholar]
- 98.Shrimpton R, Gross R, Darnton-Hill I, Young M. Zinc deficiency: what are the most appropriate interventions? BMJ (Clinical Research Ed) 2005;330:347–349. doi: 10.1136/bmj.330.7487.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burke BS. Nutrition and its relationship to the complications of pregnancy and the survival of the infant. American Journal of Public Health and the Nation’s Health. 1945;35:334–339. doi: 10.2105/ajph.35.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ebbs JH, Tisdall FF, Scott ML. Dietary study of Toronto school teachers. Canadian Medical Association Journal. 1944;50:208–213. [PMC free article] [PubMed] [Google Scholar]
- 101.Higgins AC. Nutritional status and the outcome of pregnancy. Journal of the Canadian Dietetic Association. 1976;37:17–35. [Google Scholar]
- 102.Haider BA, Yakoob MY, Bhutta ZA. Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes. BMC Public Health. 2011;11:S3–S19. doi: 10.1186/1471-2458-11-S3-S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kirksey A, Rahmanifar A, Wachs TD, McCabe GP, Bassily NS, Bishry Z, et al. Determinants of pregnancy outcome and newborn behavior of a semirural Egyptian population. American Journal of Clinical Nutrition. 1991;54:657–667. doi: 10.1093/ajcn/54.4.657. [DOI] [PubMed] [Google Scholar]
- 104.Wallwork JC, Duerre JA. Effect of zinc deficiency on methionine metabolism, methylation reactions and protein synthesis in isolated perfused rat liver. Journal of Nutrition. 1985;115:252–262. doi: 10.1093/jn/115.2.252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.