Abstract
Background
Prostate-specific antigen (PSA) testing is recommended every 6 to 12 months for the first 5 years following radical prostatectomy as a means to detect potential disease recurrence. Despite substantial research on factors affecting treatment decisions, recurrence, and mortality, little is known about whether men receive guideline-concordant surveillance testing or whether receipt varies by year of diagnosis, time since treatment, or other individual characteristics.
Methods
Surveillance testing following radical prostatectomy among elderly men was examined using Surveillance, Epidemiology, and End Results cancer registry data linked to Medicare claims. Multivariate logistic regression was used to examine the effect of demographic, tumor, and county-level characteristics on the odds of receiving surveillance testing within a given one-year period following treatment.
Results
Overall, receipt of surveillance testing was high, with 96% of men receiving at least one test the first year after treatment and approximately 80% receiving at least one test in the fifth year after treatment. Odds of not receiving a test declined with time since treatment. Non-married men, men with less advanced disease, and non-Hispanic Blacks and Hispanics had higher odds of not receiving a surveillance test. Year of diagnosis did not affect the receipt of surveillance tests.
Conclusion
Most men receive guideline-concordant surveillance PSA testing after prostatectomy, although evidence of a racial disparity between non-Hispanic Whites and some minority groups exists. The decline in surveillance over time suggests the need for well-designed long-term surveillance plans following radical prostatectomy.
Keywords: prostatic neoplasms, prostate-specific antigen, practice guidelines as topic, prostatectomy, population surveillance
Introduction
Prostate cancer survivors comprise the largest proportion of male cancer survivors (41%).1 Among men included in a large, national registry biopsy-proven prostate cancer cases, nearly half received radical prostatectomy, making it the most common form of curative treatment overall and in all age groups except men ages 75 and older.2 Approximately one-quarter to one-third of patients treated with radical prostatectomy for clinically localized prostate cancer will experience disease recurrence.3,4 Detectable or rising prostate-specific antigen (PSA) levels after treatment are often the first indicator of recurrent disease, and early diagnosis can facilitate potentially curative salvage therapy initiation.5 PSA surveillance is a cornerstone of prostate cancer survivorship care, because patients with biochemical recurrence often have no associated symptoms. If left untreated, biochemical recurrence can progress to metastatic disease, with a median time from detectable PSA to distant metastasis of 8 years.6
PSA values over time, tumor characteristics, and time since treatment in part predict local versus distant recurrence and help determine the choice of secondary therapy, especially for patients initially receiving radical prostatectomy.6,7 Patients receiving salvage radiation therapy after biochemical recurrence appear to have a survival benefit compared to patients who do not.8 The effectiveness of salvage radiation in achieving disease control appears to be greatest among patients who receive it at lower PSA levels, typically shortly after detection of recurrent disease.7 PSA surveillance may be especially important in groups of men facing documented cancer treatment, recurrence, and survival disparities, particularly African-American men.9,10 Therefore, appropriate post-treatment PSA surveillance is essential for all men who receive radical prostatectomy.
Since 1997 the National Comprehensive Cancer Network (NCCN) Guidelines have recommended PSA testing every 6 months (revised to 6-12 months in 2007) over the first 5 years following initial treatment and annually thereafter for men receiving potentially curative initial therapy.11 However, little research exists to document PSA surveillance following radical prostatectomy, particularly in contrast to post-treatment surveillance for other common malignancies.12 The only study measuring PSA surveillance testing patterns found that 22-29% of men did not receive surveillance in any given year after diagnosis, and 45% received at least 1 test each year during a 9-year follow-up period; however, the data came from a small, community-based cohort diagnosed more than 20 years ago and preceded NCCN guideline establishment.13 Therefore, it is timely to document PSA surveillance patterns in men treated with radical prostatectomy for clinically localized prostate cancer and to identify groups at risk for not receiving guideline-concordant surveillance.
Methods
We used data from the entire linked Surveillance, Epidemiology, and End Results (SEER)-Medicare database14 for men diagnosed with prostate cancer between 1998 and 2007 (n=371,133). We determined eligibility using inclusion criteria detailed in Figure 1 that have been used in previous studies of prostate cancer using SEER-Medicare data.15,16 The sample was further refined by focusing on men with tumors receiving a pT2-pT3N0M0 (organ confined [pT2] to extraprostatic extension [pT3], no regional lymph node metastases [N0], no distant metastasis [M0])17 pathological classification and who received radical prostatectomy within 180 days of diagnosis. Men who received adjuvant radiation therapy within 180 days of surgery were included18 because radiation therapy in these cases is considered to be part of initial curative treatment rather than a response to disease recurrence. Men receiving any type of neoadjuvant therapy or secondary treatment in the form of salvage radiation therapy (i.e., initiated more than 180 days after surgery), hormone therapy, or chemotherapy in the year following surgery were excluded as these therapies may indicate that radical prostatectomy was not fully effective in achieving disease control.
Figure 1.
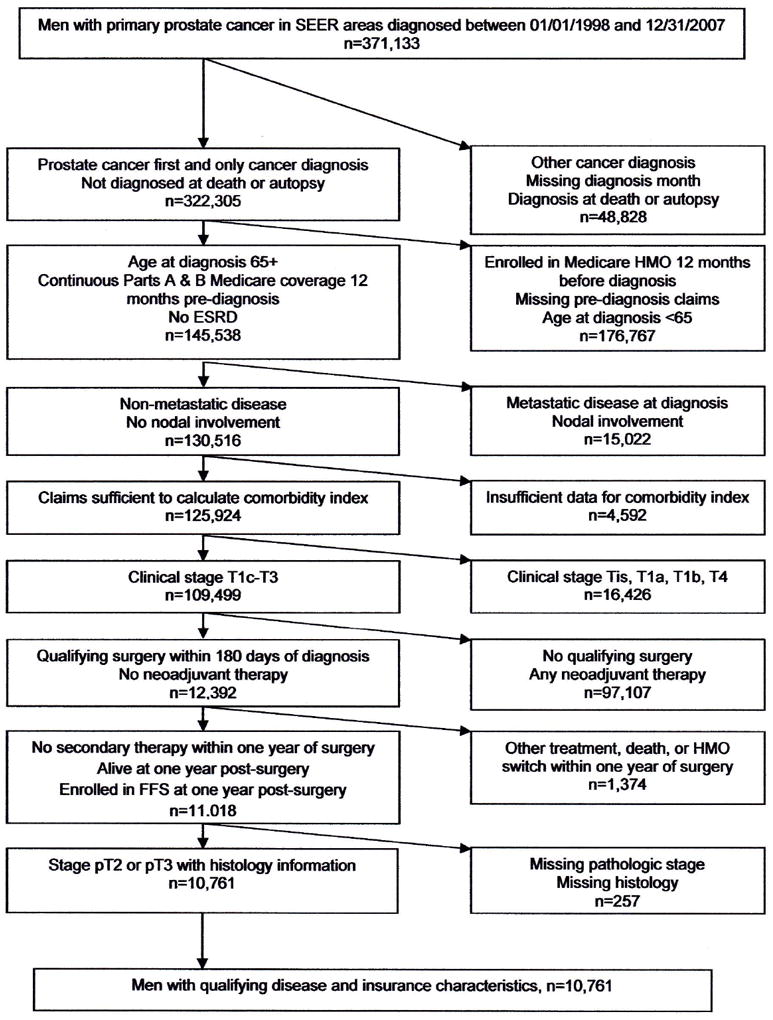
Sample Counts for Included and Excluded Observations
Figure shows the number of men excluded at each stage in the sample creation process. SEER: Surveillance, Epidemiology, and End Results; HMO: Health maintenance organization; ESRD: End-stage renal disease; FFS: Fee-for-service.
The final sample consisted of 10,761 men. The unit of observation was the person-year, resulting in a total of 47,042 observations. Partial years of data were excluded, and men were excluded from the analysis at the time of censoring.
Study Outcome Measure
The primary measure of PSA surveillance testing was a binary variable indicating whether a patient received at least one PSA test during a given year following initial treatment. The surveillance period began 60 days after prostatectomy (or the final radiation therapy treatment for men receiving adjuvant radiation therapy) and continued to the SEER date of death, initiation of secondary therapy (salvage radiation therapy, hormonal therapy, or chemotherapy), a switch from Medicare FFS to a health maintenance organization (HMO) plan, or December 31, 2009. Years without any Medicare claims were included as years without a PSA test, as all men were continuously enrolled in Medicare during the time they were eligible for inclusion. The clinical nature of post-treatment monitoring PSA testing typically ordered by a patient’s provider in the course of clinical care supports that PSA tests received by men in our sample are well-captured in Medicare claims.
PSA surveillance tests were identified in Medicare claims by Healthcare Common Procedure Coding System codes (84152, 84153, 84154, and G0103), and initial and secondary therapies were identified using SEER treatment variables and Medicare claims as described in previous studies.16
Key Independent and Control Variables
The key independent variable was time elapsed since completion of initial treatment, measured as an indicator of whether the observation captured the first, second, third, fourth, fifth, or sixth or later year. Individual-level measures of age, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic and other/unknown), marital status, tumor characteristics, co-morbidities, and Medicare state buy-in at diagnosis were included as control variables. Tumor characteristics included indicators of pathologic tumor classification (pT2 or pT3) and tumor differentiation (combined Gleason score <= 7 vs. >7). PSA value at diagnosis and specific Gleason scores were used in a model restricted to men diagnosed in 2003 and later, but the results were similar to those presented here. We chose to use the general measure of tumor differentiation available throughout the sample period to increase the average length of follow-up time.
The prostate cancer-specific condition weights of the National Cancer Institute Combined Comorbidity Index measured comorbidities at diagnosis.19 Medicare state buy-in at diagnosis has been used in previous studies to identify low-income individuals dually-eligible for Medicare and Medicaid, however, a recent study cast doubt on the adequacy of the buy-in indicator to appropriately identify all dually-eligible individuals.20 Although imperfect, it is the only control available for individual socioeconomic status.
Continuous, linear county-level measures of population density,21 persistent poverty,22 racial isolation,23 social capital,24 and Medicare HMO penetration21 were included in the model to control for access to care, community-level social support, and local practice patterns. These measures each capture different contextual factors and are not highly correlated with one another.
Statistical Analysis
Differences in characteristics of men who received at least one annual test for up to the first 5 years of surveillance and men who went at least one year without a PSA test were evaluated using t-tests and chi-square tests. PSA test receipt was recorded for every year the patient was in the sample, which may have been fewer than 5 years for some men. Specific differences in test receipt by diagnosis year and race were summarized graphically. We estimated a logistic regression model, using a generalized estimating equations framework, to evaluate the influence of covariates on not receiving a PSA test in a given year. We modeled the likelihood of not receiving a PSA test for ease in interpreting the estimated odds ratios. Model specification and error term correlation structure were evaluated using Wald test statistics25 and the quasi-likelihood under the independence model information criterion (QIC).26 Individual coefficients are reported as odds ratios (ORs), and statistical significance was determined by examining the estimated test statistics, using an alpha of .05 and two-sided statistical testing.25 All models were estimated using robust standard errors.
We compiled and analyzed the data in SAS, version 9.1 (SAS, Cary, NC) and Stata, version 10 (StataCorp, College Station, TX), respectively. The Office of Human Research Ethics at the University of North Carolina at Chapel Hill approved this research.
Results
Overall, men received an average of 2.0 (standard deviation [SD] = 1.0) PSA tests per year after treatment, but nearly 25% went at least one year without a test during the first 5 years after treatment (including only the years in which they were eligible for inclusion in the sample, which may have been less than 5). Table 1 presents characteristics of the study sample stratified by men who received at least one test each year for the first 5 years after treatment and those men who did not. Non-Hispanic White men, men diagnosed and treated at younger ages, married men, and men with pathological T3 classified disease were more likely to receive at least one annual test during the first 5 years after treatment than their referent groups, respectively
Table 1.
Sample Characteristics for Men Receiving at Least One PSA Test per Year versus Men with at Least One Year with no PSA Test, During the First 5 Years of Surveillance
| Overall % or mean (SD) | At least one annual test | One or more years with no test | p-value† | |
|---|---|---|---|---|
| Number of observations | 10,761 | 8,155 | 2,606 | |
|
| ||||
| Age at diagnosis (in years) | 69.5 (3.1) | 69.5 (3.0) | 69.6 (3.2) | .0285 |
|
| ||||
| Age by category (%) | .0592 | |||
| 65-69 | 57.3 | 58.0 | 55.3 | |
| 70-74 | 35.6 | 35.1 | 37.0 | |
| 75 + | 7.1 | 7.0 | 7.7 | |
|
| ||||
| Married at diagnosis (%) | 82.0 | 82.8 | 79.5 | .0002 |
|
| ||||
| State buy-in at diagnosis (%) | 6.4 | 5.7 | 8.7 | <.0001 |
|
| ||||
| Tumor Histology (%) | .0029 | |||
| Well/moderately differentiated | 84.5 | 85.1 | 82.6 | |
| Poorly differentiated | 15.5 | 14.9 | 17.4 | |
|
| ||||
| Pathologic classification T2 (%) | 82.3 | 81.3 | 85.4 | <.0001 |
|
| ||||
| NCI Comorbidity Index at diagnosis | 0.10 (0.25) | 0.10 (0.25) | 0.11 (0.26) | .3199 |
|
| ||||
| NCI Comorbidity Index by category (%) | .4855 | |||
| 0 | 78.3 | 78.5 | 77.8 | |
| >0 | 21.7 | 21.5 | 22.2 | |
|
| ||||
| Race (%) | .0065 | |||
| Non-Hispanic White | 83.3 | 83.8 | 81.6 | |
| Non-Hispanic Black | 5.8 | 5.6 | 6.5 | |
| Hispanic | 6.1 | 5.7 | 7.3 | |
| Other/Unknown | 4.8 | 4.9 | 4.6 | |
|
| ||||
| Year of diagnosis (%) | <.0001 | |||
| 1998 | 4.3 | 3.7 | 6.3 | |
| 1999 | 4.5 | 3.9 | 6.5 | |
| 2000 | 7.8 | 7.1 | 9.9 | |
| 2001 | 9.5 | 8.4 | 13.1 | |
| 2002 | 10.5 | 9.3 | 14.2 | |
| 2003 | 10.7 | 9.7 | 14.1 | |
| 2004 | 12.4 | 12.2 | 12.9 | |
| 2005 | 11.8 | 12.5 | 9.7 | |
| 2006 | 12.8 | 14.4 | 7.9 | |
| 2007 | 15.6 | 18.9 | 5.4 | |
|
| ||||
| Years in sample | 4.5 (2.7) | 4.1 (2.6) | 5.7 (2.4) | <.0001 |
p-values obtained using t-tests and chi-square tests and apply to differences in percentages or means across columns 3 and 4.
Note: PSA: Prostate-specific antigen; NCI: National Cancer Institute; SD: Standard deviation
Regardless of diagnosis year, almost all men received at least one PSA test in the first year after treatment, ranging from 94% for men diagnosed in 1999 to 97% for men diagnosed in 2005 (p = .0026) (Figure 2). However, over time post-treatment, the percentage of men receiving at least one test fell significantly (p < 0.0001 for the percentage of men receiving a test in year 1 versus the percentage of men receiving a test in year 5). By 5 years after treatment, there is no significant difference in test receipt by year of diagnosis; the percentage ranges from 80% for men diagnosed in 1999 to 81% for men diagnosed in 2003. Using a testing schedule of every 6 months rather than 1 year, we found that approximately 55% of men received the recommended surveillance in the fifth year after treatment.
Figure 2.
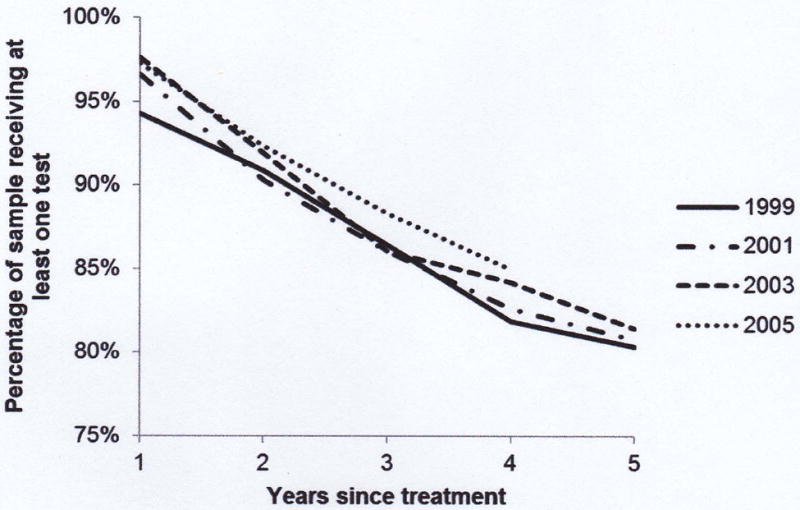
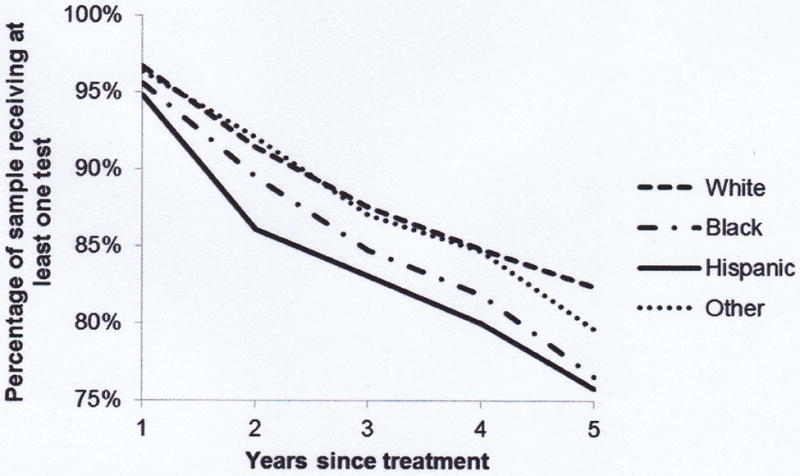
- Selected Year of Diagnosis
- Race/ethnicity
Figures show the percentage of the sample receiving at least one prostate-specific antigen test in each year following initial treatment. In Figure 2(a) the sample is separated by year of diagnosis (for selected years) and in Figure 2(b) the sample is divided by race/ethnicity.
By race, non-Hispanic Whites and men of other/unknown race had consistently higher rates of annual test receipt in the years following treatment than non-Hispanic Blacks and Hispanics (Figure 2), although all 4 racial groups begin with high rates of test receipt. The largest gap in the first year following treatment is observed between non-Hispanic Whites (96.7%) and Hispanics (94.7%) (p = .0119). The gap between races widens as time from treatment increases. By 5 years post-treatment, the test receipt rate among non-Hispanic Whites is highest, at 82.3% and is 6.5-percentage points higher than the lowest testing rate (Hispanics, 75.8%) (p = .0039). A large difference was also observed between non-Hispanic Whites and non-Hispanic Blacks at 5 years post-treatment (82.3% versus 76.5%) (p = .0196).
Results from the multivariate regression model mirror those found in the bivariate analyses (Table 2). Men who were not married, had state buy-in at diagnosis, and who were diagnosed at older ages had higher odds of not receiving at least one PSA test in a given year. Men with pT2 tumors had 1.20 higher odds (95% confidence interval [CI] = 1.07-1.35) of not receiving at least one PSA test in a year than men with pT3 tumors. Tumor differentiation and comorbidities at diagnosis were not found to be significant predictors of the odds of not receiving at least one test in a given year. Men of other/unknown race had odds of test receipt equivalent to non-Hispanic Whites, whereas non-Hispanic Blacks and Hispanics had significantly higher odds for not receiving a test than non-Hispanic Whites (OR = 1.29 with 95% CI = 1.06-1.56 for non-Hispanic Black and OR = 1.32 with 95% CI = 1.10-1.59 for Hispanic).
Table 2.
Logistic Regression Results for No Receipt of 1 PSA Surveillance Test During a 1-year Interval
| OR (p-value) | 95% CI | |
|---|---|---|
| Years since treatment (1 is reference) | ||
| 2 | 2.77 (<.001) | 2.47-3.10 |
| 3 | 4.26 (<.001) | 3.78-4.79 |
| 4 | 5.45 (<.001) | 4.82-6.16 |
| 5 | 6.49 (<.001) | 5.71-7.37 |
| 6 or more | 9.67 (<.001) | 8.55-10.95 |
| Age at diagnosis | 1.02 (.004) | 1.01-1.03 |
|
| ||
| Not married at diagnosis | 1.21 (<.001) | 1.09-1.34 |
|
| ||
| State buy-in at diagnosis | 1.47 (<.001) | 1.24-1.75 |
|
| ||
| Tumor poorly differentiated | 1.06 (.328) | 0.95-1.18 |
|
| ||
| Pathologic classification T2 | 1.20 (.002) | 1.07-1.35 |
|
| ||
| NCI Comorbidity Index at Diagnosis | 1.06 (.450) | 0.91-1.25 |
|
| ||
| Race (Non-Hispanic White is reference) | ||
| Non-Hispanic Black | 1.29 (.011) | 1.06-1.56 |
| Hispanic | 1.32 (.003) | 1.10-1.59 |
| Other/Unknown | 1.01 (.960) | 0.77-1.32 |
|
| ||
| Person-year observations | 47,033 | |
| Men | 10,496 | |
| QIC | 34,713 | |
Note: OR: Odds ratio; CI: Confidence interval; PSA: Prostate-specific antigen; NCI: National Cancer Institute; QIC: Quasi-likelihood under the independence model information criterion. Robust standard errors used. Model also included controls for county characteristics and year of diagnosis.
Discussion
PSA measurement remains the cornerstone of monitoring for post-treatment prostate cancer recurrence.27 Despite the public health burden of prostate cancer and the pivotal nature of this testing in survivorship care, little is known about patterns of adherence to relevant recommendations in real-world patients. Overall, we found that most men received post-treatment surveillance PSA tests in line with guideline recommendations. With an average of two tests per year during the entire observation period, most men met the recommended surveillance schedule of a PSA test every 6 months. During the study period, the NCCN Guidelines changed the recommended surveillance interval from every 6 months to every 6 to 12 months for the first five years after treatment. By this revised schedule, approximately 80% of men received surveillance in accordance with guidelines in the fifth year past treatment, regardless of year of diagnosis. By a strict adherence definition of a test every 6 months (which was recommended under the NCCN Guidelines from 1998 to 2007), approximately 55% of men received the recommended surveillance in the fifth year after treatment.
By far, the most important factor influencing whether a man receives a PSA test is time elapsed since treatment. Test receipt drops significantly each year for the first 5 years after treatment. This finding is troubling given that most prostate cancer recurrences generally occur during the first 5 years after local therapy,6 and 25%-30% of the men in the sample men could be expected to experience PSA recurrence.3 The majority of men at 5 years from treatment do, however, receive at least one test during the year.
The observed rate of surveillance test receipt is high compared to those observed in studies focused on post-treatment surveillance in breast and colorectal cancers.28,29 Despite the low surveillance rates reported in these studies, any differences in post-treatment surveillance between prostate cancer survivors and breast and colorectal cancer survivors should not be overstated as PSA testing is logistically simpler, less invasive, and less costly than either mammography or colonoscopy.
Although time elapsed since treatment dominates the results, there are other interesting findings. Non-Hispanic Blacks and Hispanics have higher odds of not receiving a test than non-Hispanic Whites and men of other/unknown race, which is in accord with previously reported racial differences in prostate cancer treatment and mortality.9,10 The difference in test receipt between non-Hispanic Blacks and non-Hispanic Whites is in line with the results reported in the only other study to focus on post-treatment PSA receipt.13 Although the racial disparity results in no way suggest that differences in surveillance lead to differences in mortality, they do suggest that the difference in surveillance by race may be clinically significant as well as statistically significant. Given that previous research has demonstrated a racial disparity in prostate cancer overall survival among Medicare surgery patients,10 the link between surveillance and outcomes in minority prostate cancer patients is a topic worthy of future investigation.
This study is limited by the use of claims data to identify PSA testing, as claims provide no information on test motivation. That is, there is no way to distinguish between men receiving multiple tests to follow up on previous test results and men receiving multiple tests due to lack of communication across providers. Furthermore, the results of the PSA tests are not available in these data, which limits the ability to draw conclusions regarding the frequency of abnormal (in this context, detectable) PSA results and any actions that might be indicated on the basis of those results.
The limitation of the sample to men in all SEER cancer registries with Medicare FFS insurance limits the generalizability of results to the entire prostate cancer population or to the entire Medicare population. However, the SEER-Medicare sample represents the nation’s elderly population well,30 and the national enrollment in Medicare managed care plans was relatively low during our study period (13-19%),31 so our results are applicable to the majority of men covered by Medicare during this time period. The results of this study apply only to the portion of prostate cancer patients and survivors who receive radical prostatectomy soon after diagnosis. As the majority of the men included in the SEER-Medicare dataset with qualifying disease characteristics did not meet the surgical inclusion criteria for this study, the group of men to whom our results can be generalized is relatively small. Future research should investigate whether the same testing patterns are observed in men treated with radiation therapy or active surveillance. Additionally, findings of this study may not apply to younger men who are not covered by Medicare; these men may face a different set of competing health risks and experience different treatment patterns.32 Finally, given the relatively long natural history of clinically localized prostate cancer, with a median of 8 years from the time of PSA recurrence after treatment to the development of metastatic disease,6 future study in cohorts with long-term follow-up is needed to ascertain the relationship between post-treatment PSA surveillance, secondary treatment with salvage therapy, and metastasis-free, disease-specific, and overall survival. Surveillance for early detection of recurrent cancer is predicated on the fundamental assumption that effective salvage treatment may alter the natural history of disease progression.8 Evidence supporting this assumption could justify the consideration of post-treatment surveillance as a measure of cancer care quality.
Our primary finding is that most men receive surveillance PSA testing concordant with current NCCN Guideline recommendations following radical prostatectomy. Nevertheless, adherence rates are not uniformly high, and, perhaps more importantly, test receipt declines as time from treatment increases, a result robust across model specifications, patient groups, and testing intervals. Our results suggest that one way to improve test receipt may be to focus on creating educational interventions underscoring the rationale for follow-up strategies that span many years following treatment and to highlight the significance of long-term follow-up as part of a survivorship care plan. Although there were some differences in test receipt across racial groups, individual characteristics, and tumor stage at diagnosis, the magnitude of the odds ratios associated with these factors compared to the odds ratios associated with time intervals from treatment suggests that decreasing these disparities may not be the most efficient strategy to increase overall long-term surveillance. Therefore, emphasizing the importance of disease surveillance through regular PSA testing to all patients and providers is crucial to high-quality long-term care as patients make the transition from cancer patient to cancer survivor.
Acknowledgments
This research was funded by R25 CA11633901, 1KL2RR025746. The Integrated Cancer Information and Surveillance System (ICISS), UNC Lineberger Comprehensive Cancer Center with funding provided by the University Cancer Research Fund via the State of North Carolina supported portions of this work.
Footnotes
No financial disclosure by authors.
Contributor Information
Laurel Clayton Trantham, Health Policy and Management, Gillings School of Global Public Health, University of North Carolina at Chapel Hill (UNC-CH).
Matthew E. Nielsen, Division of Urology, Department of Surgery, University of North Carolina at Chapel Hill, UNC Lineberger Comprehensive Cancer Center.
Lee R. Mobley, Institute of Public Health, Georgia State University.
Stephanie B. Wheeler, Health Policy and Management, Gillings School of Global Public Health, UNC-CH.
William R. Carpenter, Health Policy and Management, Gillings School of Global Public Health, UNC-CH, UNC Lineberger Comprehensive Cancer Center.
Andrea K. Biddle, Health Policy and Management, Gillings School of Global Public Health, UNC-CH.
References
- 1.Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Natl Academy Pr; 2006. [Google Scholar]
- 2.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010 Mar 1;28(7):1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001 Aug;28(3):555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 4.Bianco FJ, Jr, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”) Urology. 2005 Nov;66(5 Suppl):83–94. doi: 10.1016/j.urology.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 5.Ciezki JP, Reddy CA, Stephenson AJ, et al. The importance of serum prostate-specific antigen testing frequency in assessing biochemical and clinical failure after prostate cancer treatment. Urology. 2010 Feb;75(2):467–471. doi: 10.1016/j.urology.2009.08.051. [DOI] [PubMed] [Google Scholar]
- 6.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. Jama. 1999 May 5;281(17):1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 7.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007 May 20;25(15):2035–2041. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs. observation in men with biochemical recurrence after radical prostatectomy. Jama. 2008 Jun 18;299(23):2760–2769. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jemal A, Ward E, Wu X, Martin HJ, McLaughlin CC, Thun MJ. Geographic patterns of prostate cancer mortality and variations in access to medical care in the United States. Cancer Epidemiol Biomarkers Prev. 2005 Mar;14(3):590–595. doi: 10.1158/1055-9965.EPI-04-0522. [DOI] [PubMed] [Google Scholar]
- 10.Godley PA, Schenck AP, Amamoo MA, et al. Racial differences in mortality among Medicare recipients after treatment for localized prostate cancer. J Natl Cancer Inst. 2003 Nov 19;95(22):1702–1710. doi: 10.1093/jnci/djg094. [DOI] [PubMed] [Google Scholar]
- 11.Mohler J, Babaian RJ, Bahnson RR, Boston B. NCCN Practice Guidelines in Oncology: Prostate Cancer. 2007 v 2 ed2007. [Google Scholar]
- 12.Salloum RG, Hornbrook MC, Fishman PA, Ritzwoller DP, O’Keeffe Rossetti MC, Elston Lafata J. Adherence to surveillance care guidelines after breast and colorectal cancer treatment with curative intent. Cancer. 2012 Mar 20; doi: 10.1002/cncr.27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeliadt SB, Penson DF, Albertsen PC, Concato J, Etzioni RD. Race independently predicts prostate specific antigen testing frequency following a prostate carcinoma diagnosis. Cancer. 2003 Aug 1;98(3):496–503. doi: 10.1002/cncr.11492. [DOI] [PubMed] [Google Scholar]
- 14.Ambs A, Warren JL, Bellizzi KM, Topor M, Haffer SC, Clauser SB. Overview of the SEER--Medicare Health Outcomes Survey linked dataset. Health Care Financ Rev. 2008 Summer;29(4):5–21. [PMC free article] [PubMed] [Google Scholar]
- 15.Carpenter WR, Howard DL, Taylor YJ, Ross LE, Wobker SE, Godley PA. Racial differences in PSA screening interval and stage at diagnosis. Cancer Causes Control. 2010 Mar 24; doi: 10.1007/s10552-010-9535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen J, Schoenbach V, Kaufman J, et al. Racial differences in clinical progression among Medicare recipients after treatment for localized prostate cancer (United States) Cancer Causes Control. 2006 Aug;17(6):803–811. doi: 10.1007/s10552-006-0017-7. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. Prostate AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2010. Prostate. [Google Scholar]
- 18.Mohler J, Amling CL, Bhanson RR. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Version 1, 2010. National Comprehensive Cancer Network; 2010. [DOI] [PubMed] [Google Scholar]
- 19.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007 Aug;17(8):584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Koroukian SM, Dahman B, Copeland G, Bradley CJ. The utility of the state buy-in variable in the Medicare denominator file to identify dually eligible Medicare-Medicaid beneficiaries: a validation study. Health Serv Res. 2010 Feb;45(1):265–282. doi: 10.1111/j.1475-6773.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.RTI International. [December 18, 2011];Spatial impact factor data 2011; Version 4. https://rtispatialdata.rti.org.
- 22.Economic Research Service. Persistent poverty counties, 1970-2000. [December 18, 2011];2004 http://www.ers.usda.gov/briefing/rurality/typology/maps/poverty.htm.
- 23.Massey DS, Denton NA. The dimensions of residential segregation. Social Forces. 1988;67(2):281–315. [Google Scholar]
- 24.Rupasingha A, Goetz SJ. US county-level social capital data, 1990-2005. [December 18, 2011];2008 http://nercrd.psu.edu/Social_Capital/index.html.
- 25.Cameron A, Trivedi P. Microeconomics Using Stata. College Station, TX: Stata Press; 2009. [Google Scholar]
- 26.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001 Mar;57(1):120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 27.Partin AW, Oesterling JE. The clinical usefulness of prostate specific antigen: update 1994. J Urol. 1994 Nov;152(5 Pt 1):1358–1368. doi: 10.1016/s0022-5347(17)32422-9. [DOI] [PubMed] [Google Scholar]
- 28.Salz T, Weinberger M, Ayanian JZ, et al. Variation in use of surveillance colonoscopy among colorectal cancer survivors in the United States. BMC Health Serv Res. 2010;10:256. doi: 10.1186/1472-6963-10-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carcaise-Edinboro P, Bradley CJ, Dahman B. Surveillance mammography for Medicaid/Medicare breast cancer patients. J Cancer Surviv. 2010 Mar;4(1):59–66. doi: 10.1007/s11764-009-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002 Aug;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 31.Kaiser Family Foundation. Medicare Advantage Fact Sheet. Publication #2052-162012:2. [Google Scholar]
- 32.Hamilton AS, Albertsen PC, Johnson TK, et al. Trends in the treatment of localized prostate cancer using supplemented cancer registry data. BJU Int. 2011 Feb;107(4):576–584. doi: 10.1111/j.1464-410X.2010.09514.x. [DOI] [PubMed] [Google Scholar]


