Abstract
Background
Aspirin-exacerbated respiratory disease (AERD) is distinguished from aspirin tolerant asthma/chronic sinusitis in large part by an exuberant infiltration of eosinophils that are characterized by their over-expression of metabolic pathways that drive the constitutive and aspirin-induced secretion of cysteinyl leukotrienes (CysLTs).
Objective
We defined the inflammatory milieu that in part drives CysLT overproduction and, in particular, the role of interferon (IFN)-γ in the differentiation of eosinophils.
Methods
qPCR was performed for Th1 and Th2 signature cytokines on control, chronic hyperplastic eosinophilic sinusitis (CHES), and AERD tissue and their cellular source was determined. The influence of interferon (IFN)-γ on maturation, differentiation, and functionality of eosinophils derived from hematopoietic stem cells was determined.
Results
Gene expression analysis revealed that both aspirin tolerant and AERD tissue display a Th2 cytokine signature, however AERD was distinguished from CHES by the prominent expression of IFN-γ. Intracellular and immunohistochemical cytokine staining revealed that the major source of these cytokines were the eosinophils themselves. IFN-γ promoted the maturation of eosinophil progenitors as measured by increased mRNA and surface expression of CCR3 and Siglec-8. Additionally, IFN-γ increased expression of genes involved in leukotriene synthesis that led to increased secretion of cysteinyl leukotrienes. IFN-γ-matured eosinophil progenitors were also primed as demonstrated by their enhanced degranulation.
Conclusions
High IFN-γ levels distinguish AERD from aspirin tolerant asthma and underlie the robust constitutive and aspirin-induced secretion of CysLTs that characterize this disorder.
Keywords: aspirin exacerbated respiratory disease, aspirin tolerant asthma, chronic sinusitis, cytokines, eosinophils, interferon-γ, nasal polyps
Introduction
Aspirin-exacerbated respiratory disease (AERD) is a disease of the upper (chronic sinusitis/nasal polyposis (NP)) and – usually, but not always – the lower (asthma) respiratory tract, that is distinguished from aspirin tolerant asthma/chronic sinusitis by the pathognomonic sensitivity to aspirin and other non-selective inhibitors of cyclooxygenase (COX)-1. AERD comprises as many as 20% of adult-onset asthmatics and up to 30% of adult asthmatics with NPs 1, 2. There are several features of AERD that make it clear that this is a unique disorder (endotype) and not just a “variant” of aspirin tolerant asthma/chronic sinusitis. AERD patients are distinguished from those who are aspirin tolerant through a distinctive natural history, including the almost universal tendency to develop de novo in adulthood in patients not having a previous history of asthma or allergies 1, 3. The usual absence of sensitization to inhalant allergens in AERD suggests that, when present, allergies are just the coincidental presence of this common disorder. AERD can further be distinguished from aspirin tolerant disease via the presence of markedly increased circulating and tissue eosinophilia. For example, in published studies from our group 4, 5 and others 6, 7, NPs from AERD subjects expressed ~3-fold greater numbers of eosinophils or eosinophil-cationic protein (ECP) compared to aspirin tolerant eosinophilic sinus disease (the condition termed chronic hyperplastic eosinophilic sinusitis (CHES)). Similarly, when asthma is present, endobronchial biopsy tissue expresses ~5-fold greater numbers of eosinophils in AERD 8. However, the most impressive feature that distinguishes these conditions is that AERD patients display particularly robust aspirin-induced but also constitutive over-production of cysteinyl leukotrienes (CysLTs) secondary to the over-expression of the CysLT synthesis pathway; in particular the rate-limiting enzyme leukotriene (LT) C4 synthase 5, 7, 9. Reflecting the elevated expression of LT receptors, AERD patients display hyperreactivity to these lipid mediators 10–12. As a result, AERD is uniquely therapeutically responsive to leukotriene modifiers 13.
Elucidation of the unique histological and inflammatory mediators for each asthma/sinusitis endotype is required to further understand and ultimately to better treat these diseases. However, little is understood regarding the immunological basis for AERD. Eosinophilia categorically reflects a cytokine-dependent process. We speculated that aspirin tolerant asthma/CHES and AERD could be distinguished based on their patterns of cytokine expression. Asthma and nasal polyposis are, in general, characterized by a Th2 cytokine signature with prominent expression of interleukin (IL)-4, IL-13, GM-CSF, and especially, IL-5 and other features conducive to an eosinophilic infiltrate including expression of eosinophil-targeting chemokines 6, 14–19. However, these studies have not fully delineated unique patterns of expression of these mediators in aspirin tolerant asthma/CS and AERD to address the basis for their differentiation. The current studies were therefore performed to assess the cytokine profile and cellular source of these cytokines in NP tissue from subjects with CHES or AERD. In particular, we were able to demonstrate the ability of IFN-γ, that is over-expressed in AERD, to drive the distinctive feature that characterizes that condition – specifically the robust increased expression of metabolic pathways driving CysLT production.
Methods
Subjects
Nasal polyp tissue was obtained from subjects referred to the University of Virginia Health System for sinus surgery under a protocol approved by the University of Virginia Institutional Review Board. Exclusion criteria included the presence of cystic fibrosis, allergic fungal sinusitis, sinonasal tumor or carcinoma, or an immunodeficiency. Control tissue was harvested from the sinus cavities of patients undergoing surgery that required access to their paranasal sinuses for reasons other than chronic sinusitis (e.g. orbital decompression, cerebrospinal fluid leak repair, or transphenoidal pituitary surgery). Immediately following removal, tissue specimens were transported to the research laboratory for processing and analysis. Depending upon the quantity of tissue available, specimens were divided and used for subsets of the various experimental procedures outlined below. NPs were obtained from 64 subjects including 43 with CHES and 21 with AERD and, additionally, control tissue was obtained from 12 subjects. Eosinophilic sinusitis was histologically defined as described below. AERD was diagnosed based upon clinical criteria and was defined by the presence of a compelling history involving a hypersensitivity reaction within 2–3 hrs of ingestion of either aspirin or another non-steroidal anti-inflammatory drug. Diagnosis of AERD was confirmed in 12/21 subjects who underwent a subsequent aspirin challenge/desensitization procedure after sinus surgery.
Histological evaluation for diagnosis of CHES
CHES was distinguished from idiopathic non-eosinophilic nasal polyposis via histological evaluation as we have previously described 4. Briefly, polyp tissue was fixed in 4% paraformaldehyde, paraffin embedded, sectioned, and hematoxylin-eosin (H&E) stained by the Histology Core Laboratory of the University of Virginia. Sections were examined under 400× magnification in a blinded fashion and eosinophils were counted in 10 random sections for each sample with the final number being the average number of cells per 10 high-powered fields. CHES was distinguished by the presence of ≥5 eosinophils/hpf.
Quantitative real-time polymerase chain reaction (qPCR)
qPCR was performed on NP tissue for IL-4, IL-5, IL-13, IFN-γ, and on newly differentiated eosinophils for ECP, CysLT1, CysLT2, and P2Y12 receptors, Siglec-8, and leukotriene C4 synthase (LTC4S). For the NP studies, polyps were minced and digested with Accutase (Innovative Cell Technologies, San Diego, CA) for 1 hr at 37 °C and the leukocyte-containing fraction collected by passing the cell suspension through a 70 mm nylon mesh strainer (BD Falcon, Bedford, MA). Total RNA was extracted using TRI® reagent (Sigma, St Louis, MO). Conversion of mRNA to cDNA was performed using a Taqman Reverse Transcription kit (Roche, Branchburg, NJ). Briefly, 200 ng of RNA were added to each reaction along with oligo dT primers, 5.5 mM MgCl2, 2 mM dNTPs, RNasin, and reverse transcriptase. Reactions went through 10 min at 25° C, 30 min at 48° C and 5 min at 95° C in a Bio-Rad iCycler thermocycler (Bio-Rad; San Jose, CA). The PCR mix consisted of iQ sybr-green supermix (Bio-Rad), cDNA, and 200µM of each primer. Data were analyzed as the change in CT of each cytokine transcript in comparison to either EF1α or β-actin (as appropriate based upon concordance of their CT with that of the gene of interest). Primers for β-actin, CysLT1 receptor (R), CysLT2R, IL-4, IL-5, IL-13, and IFN-γ were as we have previously described 20, 21 and those for EF1α, P2Y12, ECP, LTC4S, and Siglec-8 were purchased (SABiosciences; Frederick, MD).
Flow cytometry of NP-derived leukocytes and intracellular cytokine staining
To identify the cellular source for cytokine expression, flow cytometry with intracellular cytokine staining was performed. Polyps were processed as described above after which red blood cells were lysed by resuspending the cellular pellet in lysis buffer. Cells were subsequently incubated with 50 µl of mouse IgG (1 mg/ml; Lampire Biologicals, Pipersville, PA) to block non-specific binding. Cells were incubated with the pan-leukocyte marker CD45 (PE Cy5 conjugate; BD Biosciences, San Jose, CA) along with either anti-CD4, -CD8, or -CCR3 antibodies (PE conjugate; BD Biosciences). Intracellular staining was performed by addition of 100 µl of Solution A from the Invitrogen Fix & Perm® kit for 10 min at room temperature (Grand Island, NY). The cells were washed and 100 µl of Solution B was added along with 50 µl of blocking solution and 5 µl of appropriate antibody (APC conjugate; anti-IL-4, IL-5 or IFN-γ; BD Biosciences). Flow cytometry was performed on a Becton Dickinson FACSCalibur machine equipped with CellQuest software version 5.2 (BD Biosciences). The flow cytometer was restricted to analyses of the live gate by forward and side scatter characteristics and data subsequently analyzed for the CD45+ population. Data were analyzed using FlowJo version 6.4.1 (Tree Star Inc., Ashland, OR).
Imagestream® Cytometry
Flow cytometry using the Imagestream® (Amnis Corp., Seattle, WA) cytometer combines elements of flow cytometry with confocal microscopy. As with conventional flow cytometry, cells are stained with different fluorophores and passed through the machine in a single cell suspension. Unlike conventional flow cytometry, a picture of each cell is taken as it passes by the detector. In addition to staining for cell surface markers, a nuclear dye is added which allowed us through observer visualization to confirm cellular identification suggested by surface marker staining. This is of particular use for surface markers that are expressed on multiple cell types. Two color surface staining for CD45 and CCR3 was performed as described for standard flow cytometry, after which the cells were permeabilized and the nuclei stained with DAPI (Sigma).
Immunohistochemical staining
Samples were deparaffinized and rehydrated. Antigen retrieval was performed by heating sections for 15 min at 37° C with proteinase K (0.6 unit/ml) in TE buffer with 0.5% triton X-100. Slides were washed in tris buffered saline and blocked using 1% bovine albumin and 10% mouse serum in tris buffered saline for 2 hrs at room temperature. Specific staining for IFN-γ was performed using an APC-conjugated mouse anti-human IFN-γ antibody (1:250; BD Pharmingen) for 2 hrs and compared with results obtained with an APC-labeled isotype control rabbit IgG antibody (BD Pharmingen). Nuclei were stained with 100 ng/ml DAPI (4’, 6-diamidino-2-phenylindole, Sigma) for 30 min. Samples were aqueous mounted with VectaMount AQ (Vector Laboratories, Burlington, CA) and analyzed using a Zeiss AxioImager Z2 equipped with Apotome for optical sectioning (Zeiss, Thornwood, NY).
Eosinophil Differentiation
We investigated the ability of cytokines associated with aspirin tolerant disease and AERD to modulate expression of LTC4S by eosinophils. Eosinophils were enriched from peripheral blood by Ficoll-Hypaque (Sigma) density centrifugation followed by dextran sedimentation and hypotonic lysis. Eosinophils were enriched from granulocytes using negative magnetic affinity column purification (CD16−; Miltenyi Biotec) and were greater than 99% pure as measured by flow cytometry 20. Eosinophils were incubated with IL-3, IL-4, IL-5, GM-CSF, and IFN-γ alone, or in various combinations (all cytokines used at 10 ng/ml; BD Biosciences). At the end of varying time periods (2 hrs – 7 days) mRNA was extracted and LTC4S transcripts quantified as described.
Eosinophil progenitor activation
Peripheral blood mononuclear cells (PBMCs) were isolated through Ficoll-Hypaque (Sigma) density centrifugation from blood obtained from 24 healthy volunteers (again, not all studies could be performed on all subjects). CD34+ cells were enriched from PBMCs using positive magnetic affinity column purification (CD34+; Miltenyi Biotec) and eosinophil progenitors were derived using the technique of Hudson et al. 22 by culturing purified CD34+ cells in complete medium (RPMI1640 and 10% FBS) supplemented with stem cell factor (SCF; 25 ng/ml; BD Biosciences), thymopoietin (TPO; 25 ng/ml; R&D System, Minneapolis, MN), Fms-like tyrosine kinase 3 (Flt3) ligand (25 ng/ml; BD Biosciences), IL-3 (25 ng/ml; BD Biosciences) and IL-5 (25 ng/ml; BD Biosciences) with or without IFN-γ (20 ng/ml; BD Bioscience) for 3 days and then cultured for an additional 3 weeks with just the IL-3 and IL-5 (again ±IFN-γ). Cells were washed and fresh media and cytokines were applied weekly.
Influence of IFN-γ on generation of mature eosinophils
Eosinophils newly differentiated from progenitors with or without IFN-γ were evaluated for eosinophil maturation by flow cytometry and histochemistry. Samples were cytospun and stained (H&E). Flow cytometry was performed for expression of the markers of mature eosinophils, PE-conjugated anti-CCR3 and Siglec-8, and for mature basophils using APC-conjugated CD203c (Miltenyi Biotec, Auburn, CA) and PE-conjugated FcεRI (eBiosicence; SanDiego, CA) along with relevant control antibodies. Anti-Siglec-8 was a gift from Dr. Bruce Bochner and was conjugated with AlexaFluor 488 (Invitrogen, Grand Island, NY). qPCR was performed as described above for ECP, Siglec-8, CysLT1, CysLT2, and P2Y12 receptors, and LTC4S.
Functional behavior of newly differentiated eosinophils
Eosinophils differentiated with or without IFN-γ were either left in a resting state or stimulated with the combination of calcium ionophore A23187 (2 µg/ml; Sigma) and phorbol myristate acetate (PMA; 100 ng/ml; Fisher) for 15 min. Supernatants were collected and assayed by enzyme immunoassay (EIA) for eosinophil-derived neurotoxin (EDN; MBL International, Woburn, MA) and CysLTs (Assay Designs, Inc., Ann Arbor, MI) according to the manufacturer’s directions.
Statistical analyses
Data were contrasted between paired samples by independent t-tests with or without equal variances where appropriate using SPSS 17.0 software (Cary, NC).
Results
qPCR cytokine profile of CHES and AERD
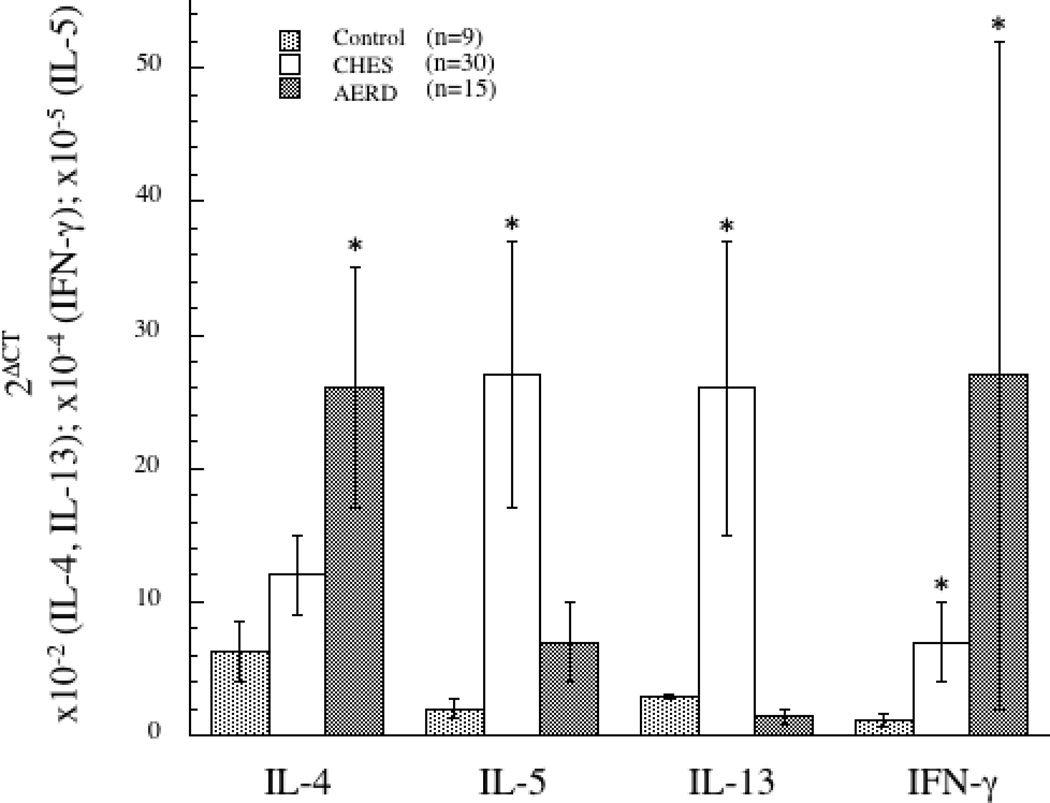
Our initial interest was to identify unique features of the cytokine profile that might contribute to the distinct phenotypes of aspirin tolerant chronic sinusitis (CHES) and AERD. Nasal polyps from patients with CHES (n=30) or AERD (n=15) and control sinus tissue (n=9) were utilized for the qPCR component of these studies. Quantitative PCR indicated that CHES, as previously reported 6, 16, 23, was characterized as having a prominent Th2 cytokine profile, indicated by increased expression of IL-4, IL-5 and IL-13 as compared to control tissue (Figure 1) with the increase for IL-5 and IL-13 achieving statistical significance. In contrast, AERD polyps had significantly elevated levels of IL-4 mRNA (p<0.05). However, the more striking feature was their robust expression of IFN-γ (p<0.05), a cytokine traditionally more ascribed to a Th1 signature (Figure 1). IL-5 levels were also elevated in AERD when compared to control tissue (p<0.05), although not as high as in CHES and surprisingly, IL-13 levels were actually decreased in AERD compared to controls.
Figure 1. Th1/Th2 cytokine signature in control, CHES, and AERD sinus tissue by qPCR.
Tissue samples were homogenized following surgical removal and RNA isolated. Transcript levels for IL-4, IL-5, IL-13 and IFN-γ were quantified using PCR with sybr-green detection. Data (mean±SEM) reflect relative expression of each gene in comparison to β-actin (2ΔCT). Control samples (n=9) are depicted in black bars, CHES (n=30) in grey bars and AERD (n=15) in white bars. *p<0.05 as compared to control.
Intracellular cytokine staining of nasal polyp leukocytes
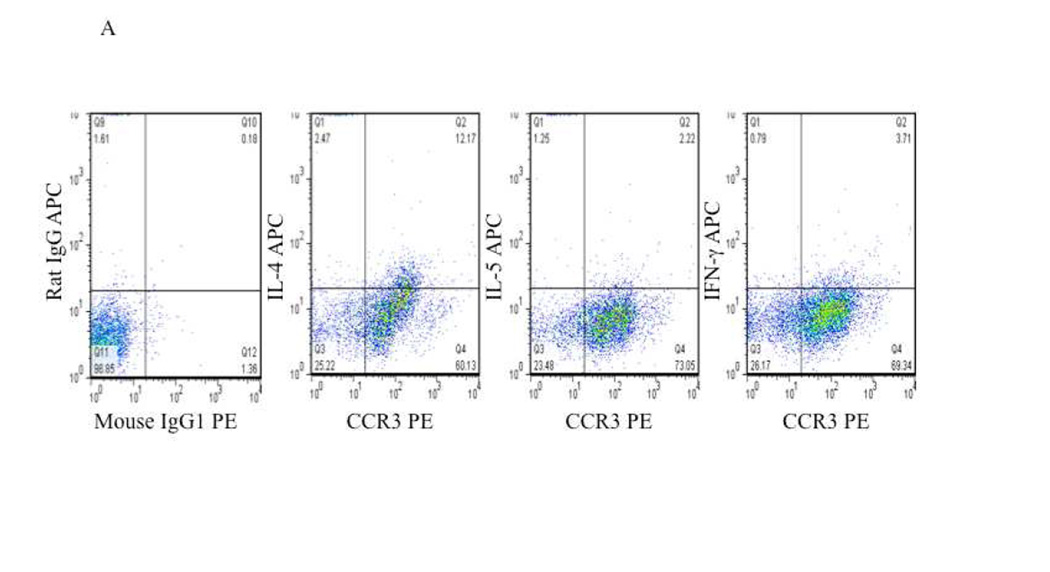
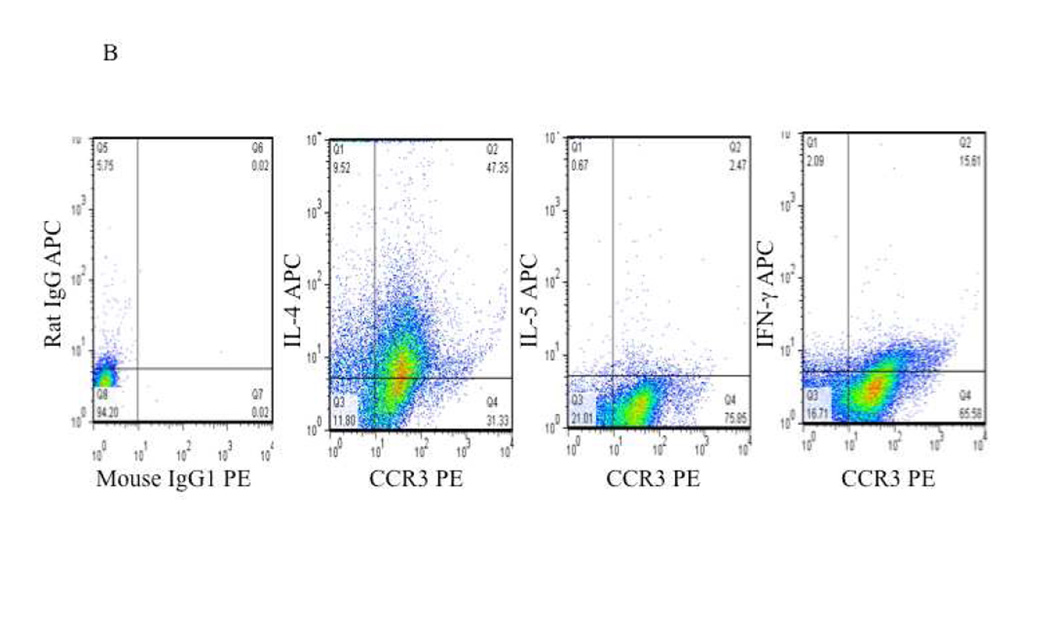
To confirm the post-translational expression of these cytokines and to define their cellular source, we performed intracellular cytokine staining for IL-4, IL-5 and IFN-γ. As a percentage, very few T cells (CD4+ or CD8+) were observed within the nasal polyp samples (data not shown) and, consistent with numerous reports regarding the histological appearance of polyps 4, 7, 8, most of the CD45+ leukocytes within both CHES and AERD polyps were eosinophils (CCR3+) (Figures 2A and 2B). Within the constraints imposed by the sensitivity of intracellular cytokine staining, only modest cytokine staining was observed amongst the CD45+CCR3− cells. In contrast, the majority of IL-4 and IFN-γ staining was observed in eosinophils reflecting, in contrast to lymphocytes, the constitutive intracellular accumulation of these cytokines in eosinophil granules 24 (Figures 2A and 2B). Also consistent with the modest production of IL-5 by eosinophils, intracellular cytokine staining for this cytokine was not observed. In CHES and AERD, IL-4 was similar and was detected in concentrations significantly above background staining in 30.6±5.9% and 33.1±10.4% of the eosinophils, respectively (Table I). In contrast, in AERD, the percentage of eosinophils expressing IFN-γ was nearly twice that of CHES (27.5±10.4% vs 18.1±3.4%).
Figure 2. Intracellular cytokine staining of CCR3 positive eosinophils in CHES and AERD.
Nasal polyps were digested and cells were separated from collagenous support material. Cells were stained with appropriate markers and analyzed by flow cytometry. Initial gating was performed to include only CD45+ cells. This was followed by extracellular staining for CCR3, CD4, CD8, or CD19 and intracellular staining for cytokines. A. Representative staining of CHES nasal polyp cells that are CCR3+ showing intracellular levels of IL-4, IL-5 and IFN-γ. B. Representative staining of AERD nasal polyp cells that are CCR3+ showing intracellular levels of IL-4, IL-5 and IFN-γ.
Table I.
Percentage eosinophil intracellular cytokine expression
| CHES | AERD | |
|---|---|---|
| IL-4 | 30.6±5.9 | 33.1±10.4 |
| IFN-γ | 18.1±3.4 | 27.5±10.4 |
CHES n=17; AERD n=5
Imagestream flow cytometry and immunofluorescent staining of nasal polyps
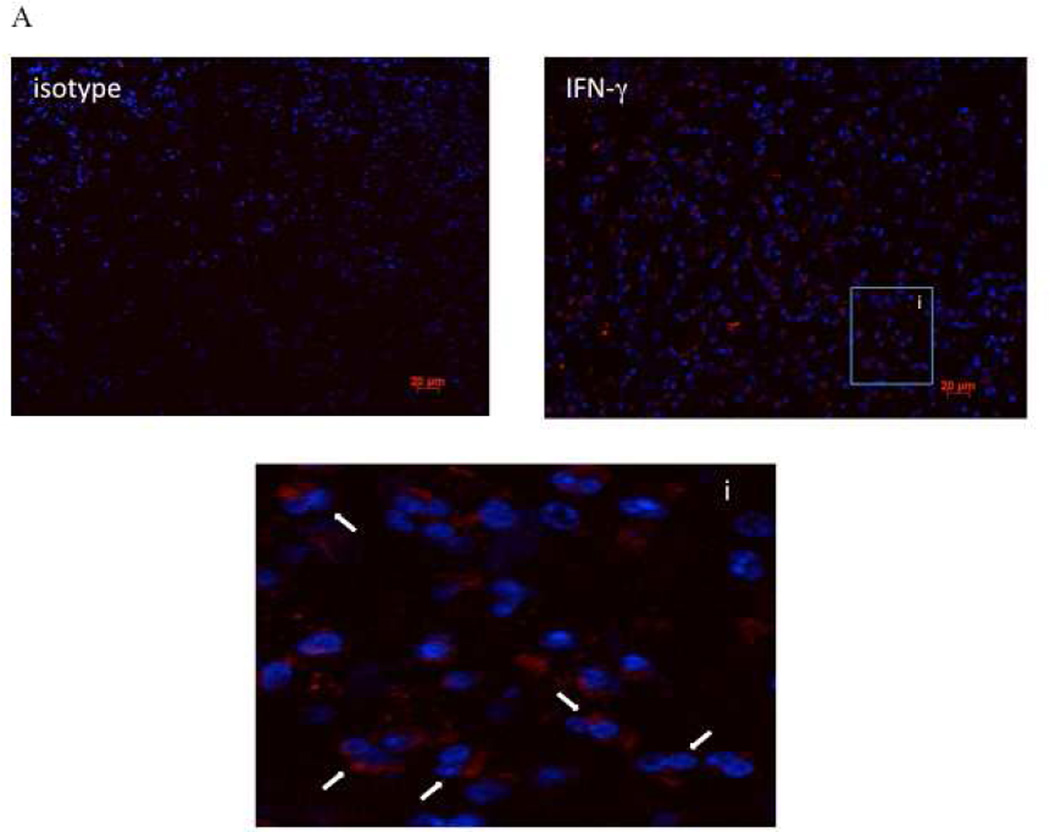
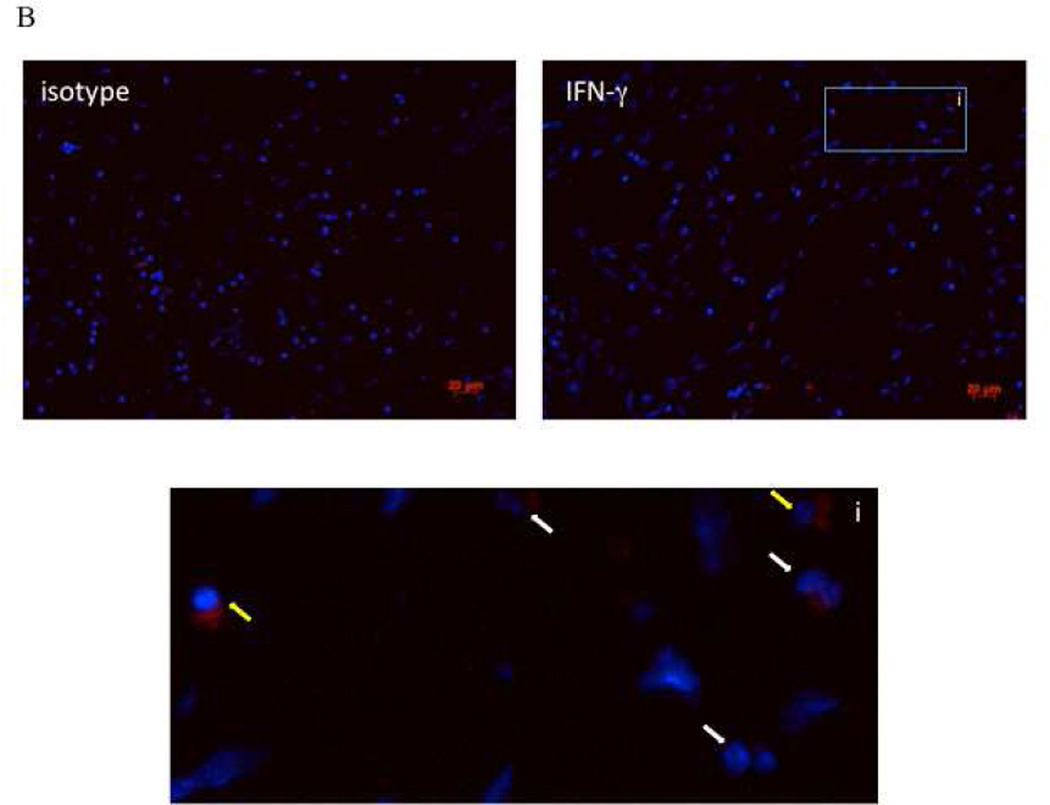
As CCR3 can be expressed on other cells besides eosinophils including lymphocytes and basophils, we performed imagestream flow cytometry of the CD45+CCR3+ cells to examine their appearance. Consistent with the recognition that eosinophils comprise the overwhelmingly predominant leukocyte in both CHES and AERD, >99% of the CD45+CCR3+ population were eosinophils as determined by their bi-lobed nucleus and robust granularity (Figure 1S online supplement). In order to verify that the IFN-γ staining observed in the flow cytometry was specific to the eosinophils, immunofluorescence staining on paraffin embedded sections from AERD and CHES subjects was performed. >90% of eosinophils from the AERD subjects were positive for IFN-γ with intense staining observed in most cells (Figure 3A). In contrast, in the CHES samples <50% of eosinophils did not stain positive for IFN-γ or were weakly positive (Figure 3B). Additionally, in the CHES samples, mononuclear cells could also be detected that were positive for IFN-γ which was not seen in the AERD subjects.
Figure 3. Immunofluorescence for IFN-γ in AERD and CHES polyps.
Sections from paraffin-embedded nasal polyps from AERD (3A) and CHES (3B) subjects were stained with an APC-labeled antibody directed against IFN-γ and counterstained with DAPI to display nuclei. Each grouping displays an isotype control, specific IFN-γ staining and an enlarged region to show specific cells. In these figures, red represents IFN-γ staining, blue is nuclear staining, i=insert, white arrows show eosinophils, and yellow arrows show mononuclear cells.
Influence of IFN-γ on eosinophil differentiation
We next began to investigate the mechanism of how IFN-γ could contribute to the unique phenotypic features of AERD. As noted, AERD is characterized by strikingly elevated eosinophil numbers in the nasal polyp and, these eosinophils are characterized by their over-expression of LTC4S 4, 7, 8. Initially, we investigated the ability of IL-1, TNF-α, IL-3, IL-4, IL-5, GM-CSF, and IFN-γ to induce LTC4S transcripts in eosinophils. Perhaps reflecting their terminal differentiation status, no influence of any of these cytokines either alone or in various combinations was observed (data not shown).
Influence of IFN-γ on eosinophilopoiesis
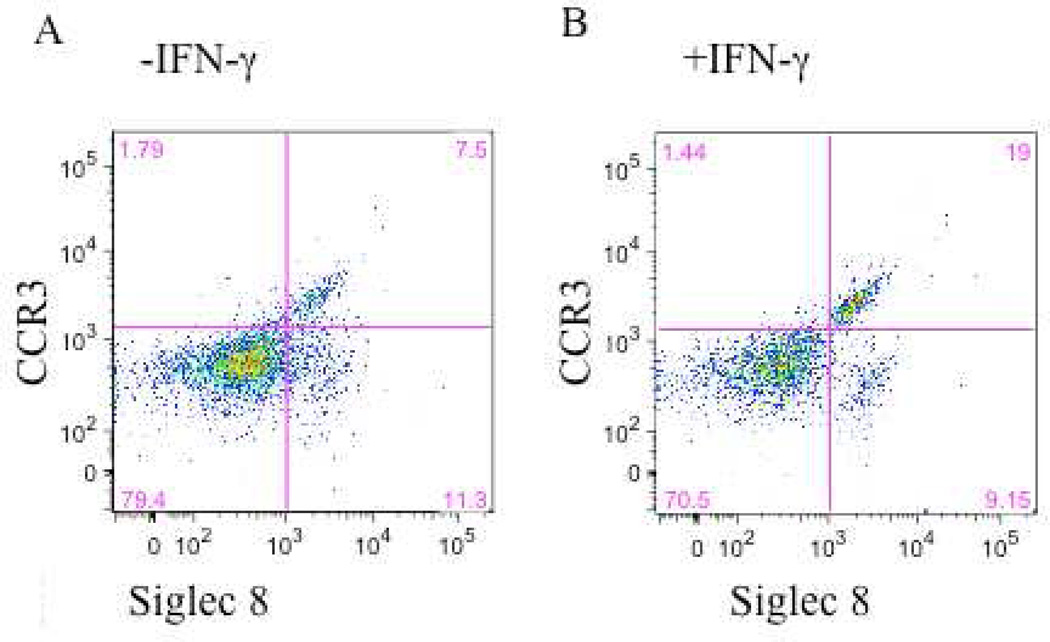
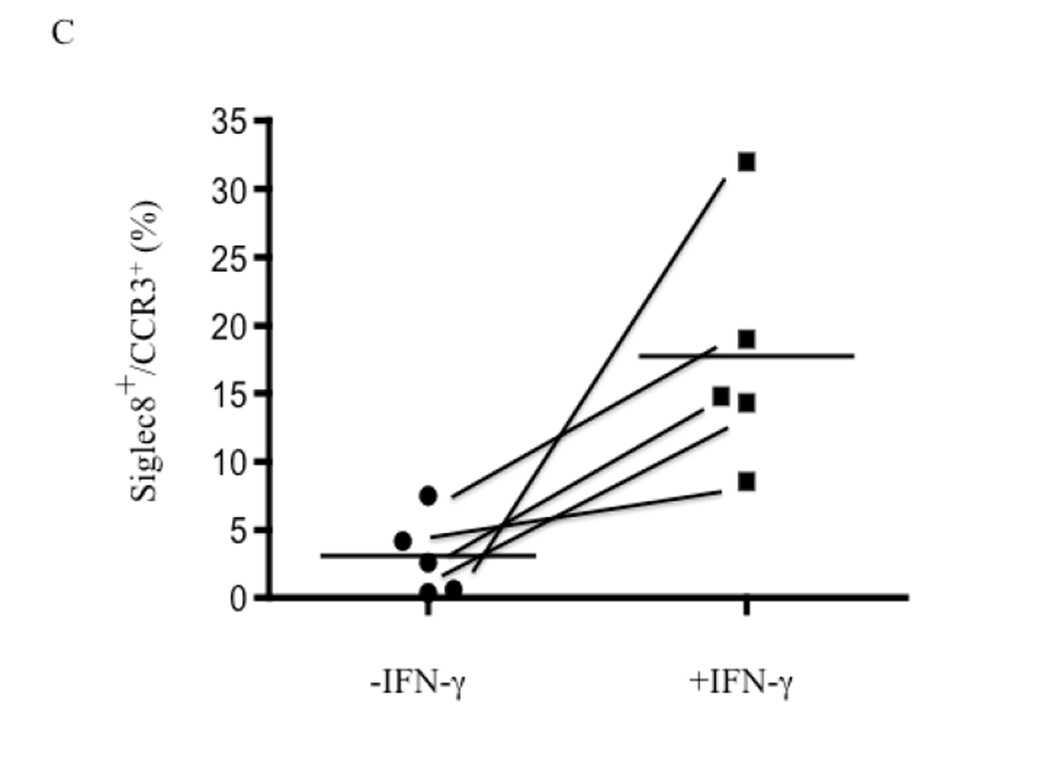
We therefore hypothesized that IFN-γ would primarily act by contributing to the development and differentiation of eosinophil progenitors. Eosinophils were derived by culturing CD34+ enriched cells with Flt3L, TPO, SCP, IL-3 and IL-5 with or without the additional presence of IFN-γ for 3 days, followed by just IL-3 and IL-5 (±IFN-γ) for an additional 3 weeks. Similar to previous reports 25, we observed a decrease in total cell numbers when the CD34+ cells were cultured in the presence of IFN-γ (1.33±.21×106 without IFN-γ compared to 0.56±.17×106 with IFN-γ (mean±SEM of 10 experiments; p<0.01)). While overall cell numbers were decreased, this reflected the enhanced terminal differentiation of eosinophils in the additional presence of IFN-γ. A portion of the cells were removed and examined by H&E staining. Along with a preponderance of apoptotic bodies, all of the viable cells had the appearance of granulocytes at varying stages of maturation including fully mature cells with readily apparent bi-lobed nuclei (Figure 2S online supplement). We next examined these CD34+-derived cells by flow cytometry for markers of mature eosinophils, specifically for their surface expression of CCR3 and Siglec-8, with the flow cytometer gated exclusively on the viable population (representative data are displayed in Figures 4A and 4B and summarized in Figure 4C). Dual CCR3+Siglec-8+ expression was observed on a significantly greater percent of viable cells grown in the presence of IFN-γ (17.73±3.94%) compared to those without IFN-γ (3.06±1.31%; mean±SEM of 5 experiments; p<0.03). To exclude the possibility that the dual CCR3+Siglec-8+ cells were basophils, flow cytometry was used to examine surface expression of CD203c and FcεRI on CD34+ cells differentiated in the presence or absence of IFN-γ. Less than 3% of the cells stained dual positive for both markers indicating an absence of basophils (Fig 3S online supplement).
Figure 4. IFN-γ potentiation of expression of markers for mature eosinophils.
CD34+-enriched hematopoietic stem cells were cultured for 3 days with SCF, TPO, Flt3L, IL-3, and IL-5 after which they were cultured for 3 additional weeks with just IL-3 and IL-5 with or without the additional presence of IFN-γ. Media was changed weekly. Maturation status was assessed by flow cytometry for CCR3 and Siglec-8. A. Cells matured with IL-5 and IL-3 only. B. Cells matured in the additional presence of IFN-γ. C. Summary data for all 5 samples.
Influence of IFN-γ on eosinophil-associated gene expression
Expression of mRNA for the eosinophil maturation marker Siglec-8 was significantly increased (2.88-fold; p<0.05) when progenitors were co-incubated with IFN-γ (Table II). In contrast, no further increase in ECP gene expression was observed in cells exposed to IFN-γ (reflecting that more mature cells decrease their expression of transcripts for these preformed mediators 22, 26). As noted, the distinctive feature of AERD in comparison to aspirin tolerant disease is the marked overexpression of cysteinyl leukotrienes and their receptors 5, 7, 9–12. Our previous work indicated that IFN-γ increases CysLT receptor expression on circulating eosinophils 20. Based on those results, we measured gene expression for leukotriene receptors (CysLT1, CysLT2, and P2Y12) and for LTC4S in eosinophil progenitors stimulated with IFN-γ. As with mature eosinophils, IFN-γ resulted in a modest 1.46-fold increase in CysLT1 receptor expression (p<0.05). We also observed a slight increase in the newly described CysLT receptor, P2Y12 (2.11-fold). Under these conditions, no further increase was observed for the CysLT2 receptor. Most importantly, however, LTC4S expression was significantly increased following IFN-γ stimulation (2.38-fold; p<0.05). In contrast, no further increase in LTC4S was observed in the additional presence of IL-4 (data not shown).
Table II.
IFN-γ influence on gene expression in newly differentiated eosinophils
| Siglec-8 | ECP | CysLT1R | CysLT2R | P2Y12 | LTC4S | |
|---|---|---|---|---|---|---|
| −IFN-γ | .0063±.0017 | .109±.030 | .052±.024 | .00059±.00031 | .0068±.0021 | .033±.014 |
| +IFN- γ | .018±.006 | .093±.018 | .076±.027 | .00053±.00012 | .0144±.0042 | .080±.021 |
| fold increase | 2.88* | .85 | 1.46* | .90 | 2.11* | 2.38* |
qPCR data reflect relative expression in comparison to house-keeping gene (EF1α) with data reflecting 2ΔΔCT (n=12).
Fold increased induced by IFN-γ in comparison to IL-5 and IL-3 alone
p<0.05compared to without IFN-γ
Influence of IFN-γ on eosinophil activation
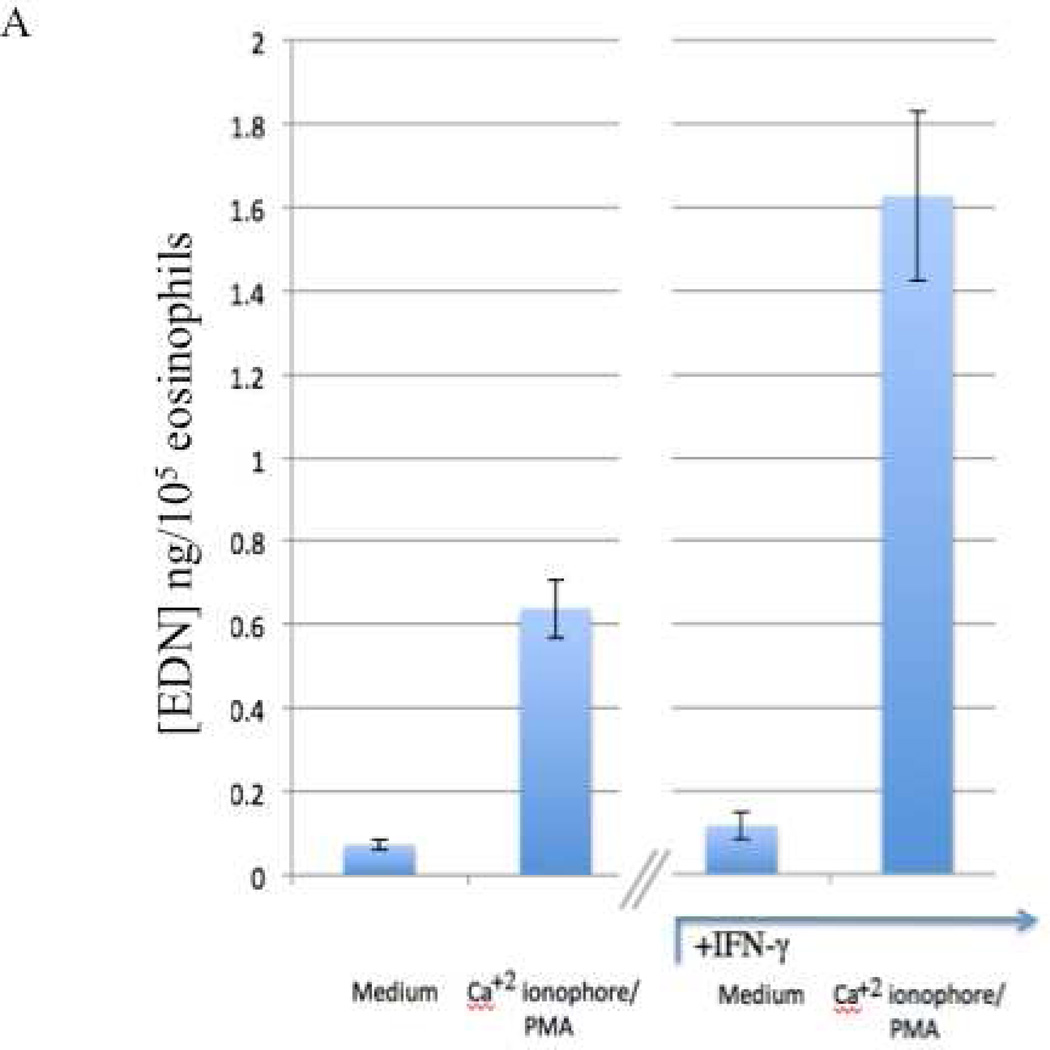
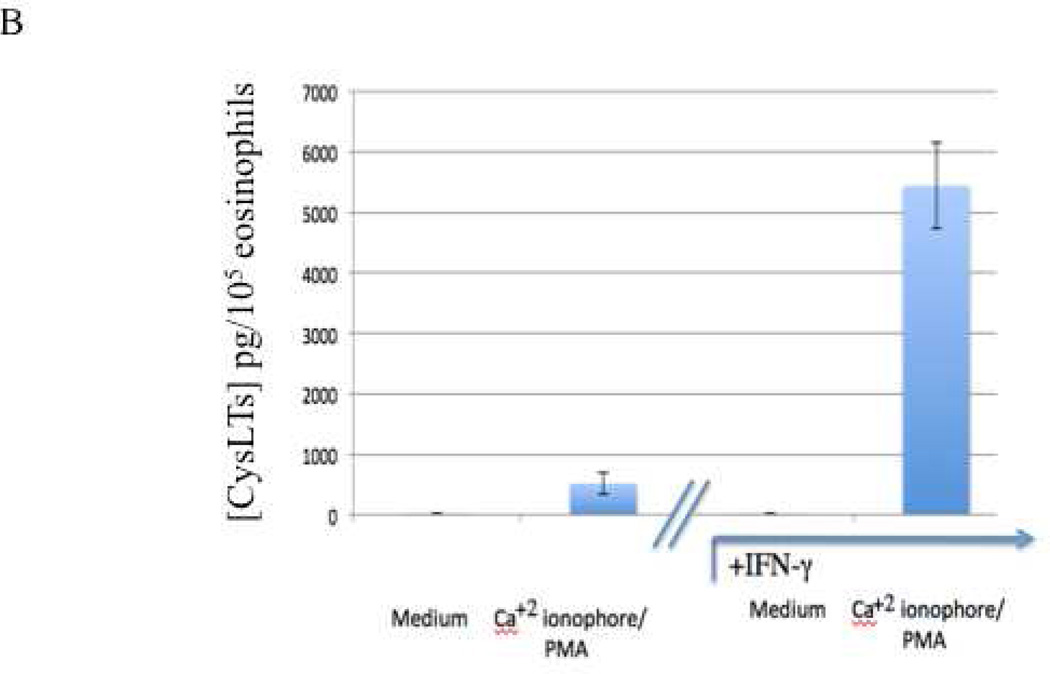
Finally, we investigated the influence of IFN-γ on the differentiation and priming of viable eosinophils as their capacity to degranulate and to secrete CysLTs (the mean±SEM of 10 and 7 results, respectively are displayed in Figures 5A and 5B). The presence of IFN-γ induced a significantly enhanced capacity to degranulate (EDN) (p<0.001) and to secrete CysLTs (p=0.02).
Figure 5. Enhanced secretion by eosinophils differentiated in the additional presence of IFN-γ.
Eosinophils matured from CD34+ progenitors, as described, were activated with or without calcium ionophore and PMA for 20 minutes. Supernatants were collected and A. EDN and B. CysLT levels quantified.
Discussion
Many features of aspirin-tolerant asthma and AERD lead to the conclusion that these are distinct disorders and not different presentations of a spectrum of the same underlying process. Besides the eponymous feature of aspirin intolerance, this includes their distinct clinical presentations and natural histories. Aspirin tolerant asthma usually presents in childhood and adolescence in allergic subjects. As noted, AERD most commonly presents in the 3rd or 4th decades of life and the frequency with which it develops in non-allergic subjects suggests that, when present, allergies are merely the coincidental presence of this common disorder 2, 3. Their natural histories are distinguished by the refractoriness of the nasal polyposis in AERD to treatment 27 and the greater tendency of AERD to lead to irreversible loss of lung function 28, 29. These disorders are especially distinguished by their inflammatory component. One of the striking features of AERD is the virtually diagnostic upregulation of LTC4S in both the lungs and NPs 5, 7, 8, largely within eosinophils, and this upregulation leads to a constitutive overproduction of CysLTs and promotes the surge in CysLT production that occurs after ingestion of aspirin 9. AERD patients also display over-responsiveness to CysLTs 10 reflecting the over-expression of CysLT receptors 11, 12 including perhaps specific LTE4 receptors 30–32. Previously, we found that even though both CHES and AERD are characterized by eosinophilic infiltration, as with lung tissue 8, the numbers of eosinophils infiltrating the NPs of AERD patients were dramatically higher 4. Eosinophilia is a cytokine-dependent process and many facets of eosinophil function are further regulated by cytokines. This prompted us to query whether differences in cytokine expression might underlie these distinct presentations.
Not surprisingly, given the prominent eosinophilia, the presence of a Th2 cytokine signature in NPs has previously been well established 6, 14–19. These studies have not, however, fully addressed the distinct signature of AERD as compared to CHES. Initially, we screened for cytokine expression utilizing qPCR of NP (or control sinus) tissue. We confirmed the variable expression of Th2 cytokines in both diseases (Figure 1) and also demonstrated that eosinophils themselves were an important source for these cytokines (Figures 2, 3 and Table I) although other cells, such as innate lymphoid type 2 cells (ILC2 cells), likely contribute to the Th2 cytokine expression. Eosinophils can secrete >30 cytokines and chemokines when stimulated 33 and, unlike other lymphocytes, where these factors are synthesized and immediately secreted, eosinophils store pre-formed cytokines that can be rapidly released from granules 24, 34. In interpreting these qPCR results it is important to note the limitations posed by performing these studies on heterogeneous tissue and that these results may thereby be skewed by the distinct cellular makeup of CHES and AERD polyps. For example, most of the AERD mRNA would have been derived from eosinophils whereas CHES represents a more diffuse distribution of leukocytes and, as a percent, a greater contribution of the mRNA would have come from stromal tissue. Within these constraints, the more robust expression of IL-4 in AERD in comparison especially to IL-5 likely represents the physiology of eosinophil cytokine transcript expression and specifically that IL-5 is not an important eosinophil-derived cytokine. It is also worth considering that the high IL-5/IL-13 and low IL-4 signature in CHES is consistent with the pattern of cytokines observed in ILC2 cells, an increasingly recognized source of these cytokines in allergic disorders, and a cell type that is specifically expressed in NPs 35, 36.
However, the most striking feature of these studies was the elevated expression of IFN-γ mRNA and protein in AERD (Figures 1 and 3), consistent with recognition that this cytokine can be expressed by eosinophils in substantial amounts 24, 33, 37. One previous study investigated cytokine expression in “non-allergic” sinusitis, a cohort within which AERD subjects are likely to be over-represented, and reported similar high levels of IFN-γ expression 23. Van Zele et al. previously described high IFN-γ levels in chronic sinusitis tissue, but not nasal polyps, although their study was not designed to investigate subjects with AERD 38. We did not identify T lymphocytes as an important source for these cytokines. This does not rule out a role for T cell-derived cytokines in these disorders, as it remains quite plausible that the relevant T cells orchestrating the inflammation within NPs might reside in sinonasal-associated lymphatic tissue or other lymphoid structures. Interestingly, the Th1/Th2 balance we observed in NP eosinophils in AERD is consistent with a prior report that detected higher levels of IFN-γ in circulating CD8+ cells compared to aspirin-tolerant controls 39 a pattern that decreased following aspirin desensitization.
IFN-γ, despite its primary association with the Th1 cytokine signature, is present and contributes to the severity of asthma and other allergic inflammatory disorders 37. Given the capacity of IFN-γ to block IgE class switch recombination, it is possible that this co-expression of IFN-γ might contribute to the absence of allergy (despite the shared expression of IL-4).
We speculated that the primary function of IFN-γ would be to contribute to the distinct inflammatory features of AERD, specifically the hypereosinophilia and the overexpression of CysLTs and their receptors 10–12, 40. It is known that an IFN-γ-induced transcription factor (Icsbp) can be critical for regulating the development of eosinophils 41. Eosinophil and basophil progenitors or colony-forming units (Eo/B CFU) are bone marrow-derived mononuclear cells that express CD34 and IL-5 receptors that are capable of responding to appropriate cytokine signals to differentiate into mature eosinophils and basophils 42. Given that Eo/B CFU are increased in the blood and bone marrow of asthmatic patients and have also been identified in NP tissue 43, 44, we hypothesized that IFN-γ would synergize with IL-5 to enhance eosinophil maturation in AERD. As previously reported 25, we observed a decrease in the overall cell numbers when the CD34+ cells were cultured in the presence of IFN-γ. Despite the lower cell numbers, our studies demonstrate that the addition of IFN-γ significantly accelerated the terminal maturation of eosinophils as evinced by their surface expression of CCR3 and Siglec-8 (Figures 4 and 5) and increased their mRNA expression of Siglec-8 (Table II). Consistent with a role in driving the CysLT over-responsiveness observed in AERD 45 and, consistent with our previous studies performed with mature eosinophils 20, we also demonstrated modest increases in expression of transcripts for CysLT1 and a putative third CysLT receptor, P2Y12 (Table II) 30, 31.
Over-expression of LTC4S underlies the generation of CysLTs in AERD and this has been ascribed to the eosinophil 8. To date, only one study has shown modulation of LTC4S gene expression and that was by IL-4 in mast cells 46. No basis for the increase in eosinophil LTC4S in AERD has been demonstrated. Our studies failed to demonstrate an ability of numerous innate or adaptive cytokines including IL-3, IL-4, IL-5, GM-CSF, IL-1, TNF-α, or even IFN-γ to modulate LTC4S expression on circulating eosinophils, perhaps consistent with their terminal differentiation status (data not shown). In contrast, our data do demonstrate a statistically significant increase in IFN-γ-driven LTC4S expression in eosinophil progenitors (Table II). Most importantly, this translated into increased capacity of these newly differentiated eosinophils to secrete CysLTs and release EDN (Figures 5A and 5B). In contrast to its effect on mast cells 46, this capacity did not extend to IL-4 (data not shown). Thus, IFN-γ at present uniquely carries the capacity to drive this disease-defining characteristic of AERD.
In summary, our studies demonstrate that while both aspirin tolerant disease (CHES) and AERD have components of Th2 disorders, AERD is distinguished by the prominent expression of IFN-γ. Interestingly, much of the cytokine expression, including IFN-γ, was derived from the eosinophils themselves. Eosinophil-derived IFN-γ synergizes with IL-3 and IL-5 to drive maturation of eosinophils and these eosinophils display an upregulated capacity to not only respond to CysLTs but – comprising the most important diagnostic feature of AERD – also to secrete cysteinyl leukotrienes.
Supplementary Material
Clinical Implications.
High IFN-γ in AERD may explain the absence of IgE-mediated allergies associated with the disease and increased cysteinyl leukotriene synthesis driven by IFN-γ underlies why leukotriene modifiers are partially effective in treatment of this disease.
Acknowledgements
We would like to thank Dr. Bruce Bochner for kindly providing us with the Siglec-8 antibody we used for flow cytometry and Dr. Steven Ackerman for helpful conversations on differentiating eosinophils from CD34+ progenitor cells.
Supported by NIH grants R01-AI47737 and P01-AI50989
Abbreviations
- AERD
aspirin exacerbated respiratory disease
- CFU
colony forming unit
- CHES
chronic hyperplastic eosinophilic sinusitis
- COX
cyclooxygenase
- CS
chronic sinusitis
- CysLT
cysteinyl leukotriene
- DAPI
4’, 6-diamidino-2-phenylindole
- ECP
eosinophil cationic protein
- EIA
enzyme-lined immunoassay
- Eo/B
eosinophil and basophil progenitors
- Flt3L
Fms-like tyrosine kinase 3 ligand
- H&E
hematoxylin-eosin
- IFN
interferon
- Icsbp
IFN consensus binding protein
- IL
interleukin
- ILC2
innate lymphoid type 2 cells
- LT
leukotriene
- LTC4S
leukotriene C4 synthase
- NP
nasal polyps
- PG
prostaglandin
- SCF
stem cell factor
- Th
T helper
- TNF
tumor necrosis factor
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Szczeklik A, Sanak M. Molecular mechanisms in aspirin-induced asthma. ACI International. 2000;12/4:171–176. [Google Scholar]
- 2.Vally H, Taylor ML, Thompson PJ. The prevalence of aspirin intolerant asthma (AIA) in Australian asthmatic patients. Thorax. 2002;57:569–574. doi: 10.1136/thorax.57.7.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szczeklik A, Nizankowska E. Clinical features and diagnosis of aspirin induced asthma. Thorax. 2000;55:S42–S44. doi: 10.1136/thorax.55.suppl_2.S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payne SC, Early SB, Huyett P, Han JK, Borish L, Steinke JW. Evidence for distinct histologic profile of nasal polyps with and without eosinophilia. The Laryngoscope. 2011;121:2262–2267. doi: 10.1002/lary.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinke JW, Bradley D, Arango P, Crouse CD, Frierson H, Kountakis SE, et al. Cytseinyl leukotriene expression in chronic hyperplastic sinusitis-nasal polyposis: Importance to eosinophilia and asthma. J Allergy Clin Immunol. 2003;111:342–349. doi: 10.1067/mai.2003.67. [DOI] [PubMed] [Google Scholar]
- 6.Bachert C, Wagenmann M, Hauser U, Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997;99:837–842. doi: 10.1016/s0091-6749(97)80019-x. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Novo CA, Watelet JB, Claeys C, van Cauwenberge P, Bachert C. Prostaglandin, leukotiene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol. 2005;115:1189–1196. doi: 10.1016/j.jaci.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Cowburn AS, Sladek K, Soja J, Adamek L, Nizankowska E, Szczeklik A, et al. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J Clin Invest. 1998;101:834–846. doi: 10.1172/JCI620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christie PE, Tagari P, Ford-Hutchinson AW, Charlesson S, Chee P, Arm JP, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143:1025–1029. doi: 10.1164/ajrccm/143.5_Pt_1.1025. [DOI] [PubMed] [Google Scholar]
- 10.Arm JP, O'Hickey S, Spur BW, Lee TH. Airway responsiveness to histamine and leukotriene E4 in subjects with aspirin-induced asthma. Am Rev Respir Dis. 1989;140:148–153. doi: 10.1164/ajrccm/140.1.148. [DOI] [PubMed] [Google Scholar]
- 11.Sousa AR, Parikh A, Scadding G, Corrigan CJ, Lee TH. Leukotriene-receptor expression on nasal mucosal inflammatory cells in asprin-sensitive rhinosinusitis. N Engl J Med. 2002;347:1493–1499. doi: 10.1056/NEJMoa013508. [DOI] [PubMed] [Google Scholar]
- 12.Corrigan C, Mallett K, Ying S, Roberts D, Parikh A, Scadding G, et al. Expression of the cysteinyl leukotriene receptors cysLT(1) and cysLT(2) in aspirin-sensitive and aspirin-tolerant chronic rhinosinusitis. J Allergy Clin Immunol. 2005;115:316–322. doi: 10.1016/j.jaci.2004.10.051. [DOI] [PubMed] [Google Scholar]
- 13.Dahlen B, Nizankowska E, Szczeklik A. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Critc Care Med. 1998;157:1187–1194. doi: 10.1164/ajrccm.157.4.9707089. [DOI] [PubMed] [Google Scholar]
- 14.Bachert C, Gevaert P, van Cauwenberge P. Nasal polyposis- A new concept on the formation of polyps. ACI International. 1999;11:130–135. [Google Scholar]
- 15.Minshall EM, Cameron L, Lavigne F, Leung DYM, Hamilos D, Garcia-Zepada A, et al. Eotaxin mRNA and protein expression in chronic sinusitis and allergen-induced nasal responses in seasonal allergic rhinitis. Amer J Resp Cell Mol Biol. 1997;17:683–690. doi: 10.1165/ajrcmb.17.6.2865. [DOI] [PubMed] [Google Scholar]
- 16.Hamilos DL, Leung DYM, Huston DP, Kamil A, Wood R, Hamid Q. GM-CSF, IL-5, and RANTES immunoreactivity and mRNA expression in chronic hyperplastic sinusitis with nasal polyposis. Clin Exp Allergy. 1998;28:1145–1152. doi: 10.1046/j.1365-2222.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- 17.Kamil A, Ghaffar O, Lavigne F, Taha R, Renzi PM, Hamid Q. Comparison of inflammatory cell profile and Th2 cytokine expression in the ethmoid sinuses, maxillary sinuses, and turbinates of atopic subjects with chronic sinusitis. Otolaryngol Head Neck Surg. 1998;18:804–809. doi: 10.1016/S0194-5998(98)70273-6. [DOI] [PubMed] [Google Scholar]
- 18.Riechelmann H, Deutschle T, Rozsasi A, Keck T, Polzehl D, Burner H. Nasal biomarker profiles in acute and chronic rhinosinusitis. Clin Exp Allergy. 2005;35:1186–1191. doi: 10.1111/j.1365-2222.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 19.Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121:1435–1441. doi: 10.1016/j.jaci.2008.02.018. 41 e1–3. [DOI] [PubMed] [Google Scholar]
- 20.Early SB, Barekzi E, Negri J, Hise K, Borish L, Steinke JW. Concordant modulation of cysteinyl leukotriene receptor expression by IL-4 and IFN-γ on peripheral immune cells. Am J Respir Cell Mol Biol. 2007;36:715–720. doi: 10.1165/rcmb.2006-0252OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Payne SC, Han JK, Huyett P, Negri J, Krophf EZ, Borish L, et al. Microarray analysis of distinct gene transcription profiles in non-eosinophilic chronic sinusitis with nasal polyps. Am J Rhinol. 2008;22:568–581. doi: 10.2500/ajr.2008.22.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudson SA, Herrmann H, Du J, Cox P, Haddad el B, Butler B, et al. Developmental, malignancy-related, and cross-species analysis of eosinophil, mast cell, and basophil siglec-8 expression. Journal of clinical immunology. 2011;31:1045–1053. doi: 10.1007/s10875-011-9589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilos DL, Leung DYM, Wood R, Cunningham L, Bean DK, Yasruel Z, et al. Evidence for distinct cytokine expression in allergic versus nonallergic chronic sinusitis. J Allergy Clin Immunol. 1995;96:537–544. doi: 10.1016/s0091-6749(95)70298-9. [DOI] [PubMed] [Google Scholar]
- 24.Spencer LA, Szela CT, Perez SAC, Kirchhoffer CL, Neves JS, Radke AL, et al. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Bruin AM, Buitenhuis M, van der Sluijs KF, van Gisbergen KP, Boon L, Nolte MA. Eosinophil differentiation in the bone marrow is inhibited by T cell-derived IFN-gamma. Blood. 2010;116:2559–2569. doi: 10.1182/blood-2009-12-261339. [DOI] [PubMed] [Google Scholar]
- 26.Boyce JA, Friend D, Matsumoto R, Austen KF, Owen WF. Differentiation in vitro of hybrid eosinophil/basophile granulocytes: autocrine function of an eosinophil intermediate. J Exp Med. 1995;182:49–57. doi: 10.1084/jem.182.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berges-γimeno MP, Simon RA, Stevenson DD. The natural history and clinical characteristics of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2002;89:474–478. doi: 10.1016/S1081-1206(10)62084-4. [DOI] [PubMed] [Google Scholar]
- 28.ten Brinke A, Grootendorst DC, Schmidt JT, de Bruine FT, van Buchem MA, Sterk PJ, et al. Chronic sinusitis in severe asthma is related to sputum eosinophilia. J Allergy Clin Immunol. 2002;109:621–626. doi: 10.1067/mai.2002.122458. [DOI] [PubMed] [Google Scholar]
- 29.Mascia K, Haselkorn T, Deniz YM, Miller DP, Bleecker ER, Borish L. Aspirin sensitivity and severity of asthma: evidence for irreversible airway obstruction in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2005;116:970–975. doi: 10.1016/j.jaci.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 30.Nonaka Y, Hiramoto T, Fujita N. Identification of endogenous surrogate ligands for human P2Y12 receptors by in silico and in vitro methods. Biochem Biophys Res Commun. 2005;337:281–288. doi: 10.1016/j.bbrc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 31.Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, et al. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med. 2009;206:2543–2555. doi: 10.1084/jem.20091240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maekawa A, Kanaoka Y, Xing W, Austen KF. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc Natl Acad Sci U S A. 2008;105:16695–16700. doi: 10.1073/pnas.0808993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akuthota P, Xenakis JJ, Weller PF. Eosinophils: Offenders or General Bystanders in Allergic Airway Disease and Pulmonary Immunity? J Innate Immun. 2011;3:113–119. doi: 10.1159/000323433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer LA, Melo RCN, Perez SAC, Bafford SP, Dvorak AM, Weller PF. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc Natl Acad Sci USA. 2006;103:3333–3338. doi: 10.1073/pnas.0508946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature immunology. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 36.Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. Journal of immunology. 2012;188:703–713. doi: 10.4049/jimmunol.1101270. [DOI] [PubMed] [Google Scholar]
- 37.Kanda A, Driss V, Hornez N, Abdallah M, Roumier T, Abboud G, et al. Eosinophil-derived IFN-γ induces airway hyperresponsiveness and lung inflammation in the absence of lymphocytes. J Allergy Clin Immunol. 2009;124:573–582. doi: 10.1016/j.jaci.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 38.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 39.Shome GP, Tarbox J, Shearer M, Kennedy R. Cytokine expression in peripheral blood lymphocytes before and after aspirin desensitization in aspirin-exacerbated respiratory disease. Allergy Asthma Proc. 2007;28:706–710. doi: 10.2500/aap.2007.28.3052. [DOI] [PubMed] [Google Scholar]
- 40.Adamjee J, Suh YJ, Park HS, Choi JH, Penrose JF, Lam BK, et al. Expression of 5-lipoxygenase and cyclooxygenase pathway enzymes in nasal polyps of patients with aspirin-intolerant asthma. J Pathol. 2006;209:392–399. doi: 10.1002/path.1979. [DOI] [PubMed] [Google Scholar]
- 41.Milanovic M, Terszowski G, Struck D, Liesenfeld O, Carstanjen D. IFN consensus sequence binding protein (Icsbp) is critical for eosinophil development. J Immunol. 2008;181:5045–5053. doi: 10.4049/jimmunol.181.7.5045. [DOI] [PubMed] [Google Scholar]
- 42.Denburg JA, Sehmi R, Saito H, Pil-Seob J, Inman MD, O'Bryne PM. Systemic aspects of allergic disease: bone marrow responses. J Allergy Clin Immunol. 2000;106:S242–S246. doi: 10.1067/mai.2000.110156. [DOI] [PubMed] [Google Scholar]
- 43.Kim YK, Uno M, Hamilos DL, Beck L, Bochner B, Schleimer RP, et al. Immunolocalization of CD34 in nasal polyp. Effect of topical corticosteroids. Am J Respir Cell Mol Biol. 1999;20:388–397. doi: 10.1165/ajrcmb.20.3.3060. [DOI] [PubMed] [Google Scholar]
- 44.Denburg JA. Haemopoietic mechanisms in nasal polyposis and asthma. Thorax. 2000;55:S24–S25. doi: 10.1136/thorax.55.suppl_2.S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujii M, Tanaka H, Abe S. Interferon-gamma up-regulates expression of cysteinyl leukotriene type 2 receptors on eosinophils in asthmatic patients. Chest. 2005;128:3148–3155. doi: 10.1378/chest.128.5.3148. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh FH, Lam BK, Penrose JF, Austen KF, Boyce JA. T helper cell type 2 cytokines coordinately regulate immunoglobulin E-dependent cysteinyl leukotriene production by human cord blood-derived mast cells: profound induction of leukotriene C4 synthase expression by interleukin 4. J Exp Med. 2001;193:123–133. doi: 10.1084/jem.193.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.