Abstract
The past decade has seen tremendous developments in novel cancer therapies, through targeting of tumor cell-intrinsic pathways whose activity is linked to genetic alterations, as well as the targeting of tumor cell-extrinsic factors such as growth factors. Furthermore, immunotherapies are entering the clinic at an unprecedented speed following the demonstration that T cells can efficiently reject tumors and that their anti-tumor activity can be enhanced with antibodies against immune regulatory molecules (checkpoints blockade). Current immunotherapy strategies include monoclonal antibodies against tumor cells or immune regulatory molecules, cell-based therapies such as adoptive transfer of ex vivo activated T cells and natural killer (NK) cells, and cancer vaccines. Herein, we discuss the immunological basis for therapeutic cancer vaccines and how the current understanding of dendritic cell (DC) and T cell biology might enable development of next-generation curative therapies for patients with cancer.
Introduction
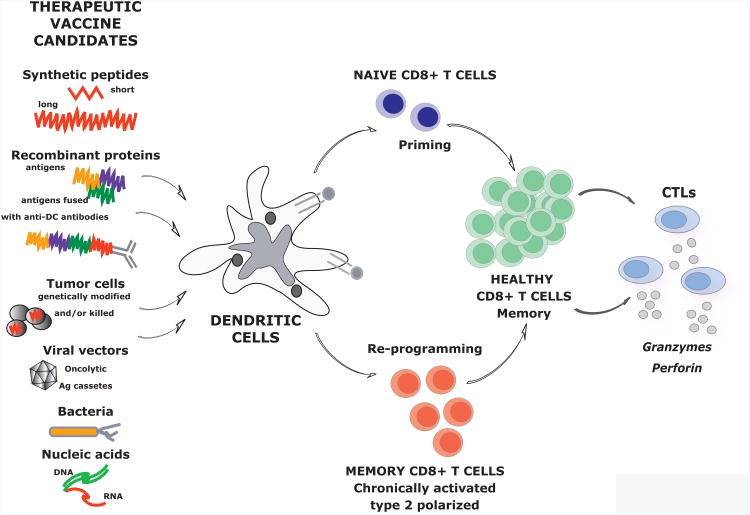
Vaccination represents one of the most effective methods to prevent disease (Finn and Edwards, 2009; Nabel, 2013; Subbarao et al., 2006). Preventive vaccines are designed to block the spread of infection and their activity correlates with the induction of specific antibodies and long-lived memory B cells (Pulendran and Ahmed, 2011). Cellular immunity can also be induced, especially with vaccines composed of attenuated microbes (Pulendran and Ahmed, 2011). On the other hand, therapeutic vaccines are designed to eliminate the cause of a given disease, e.g. elimination of cancer cells or virally-infected cells, and to treat the disease. Their activity is mostly dependent on antigen-specific CD8+ T cell educated to generate cytotoxic T lymphocytes (CTLs) that reject cancer or infected cells. Ideally, therapeutic vaccines should both prime naive T cells and modulate existing memory T cells, i.e., induce a transition from non-protective CD8+ T cells to healthy CD8+ T cells able to yield effective CTLs (Figure 1). Indeed, cancer is a chronic disease and as such it is associated with skewed T cell memory, for example, chronically activated CD8+ T cells that express programmed cell death 1 (PD-1) and are anergic (Freeman et al., 2006). In addition, vaccination should lead to generation of long-lived memory CD8+ T cells that will act to prevent relapse (Figure 1).
Figure 1. Therapeutic vaccines act via dendritic cells to generate protective CD8+T cell immunity.
Therapeutic vaccines are designed to elicit cellular immunity. In this goal, they are expected to prime new T cells as well as induce a transition from chronically activated non-protective CD8+ T cells to healthy CD8+ T cells able to i) generate cytotoxic T lymphocytes (CTLs) that reject cancer and ii) provide long-lived memory CD8+ T cells able to rapidly generate new effector T cells secreting cytotoxic molecules thereby preventing relapse. Numerous approaches to therapeutic vaccines that are being pursued are illustrated. Their common denominator is the action via DCs either randomly or specific targeting.
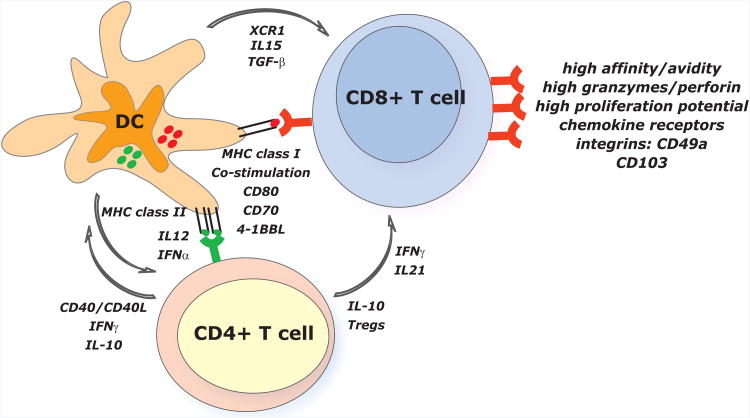
The numerous clinical studies assessing therapeutic vaccination in cancer during the past two decades have helped us define the desired properties of vaccine-elicited CD8+ T cells associated with rejection of cancer (Appay et al., 2008). These include: i) high T cell receptor (TCR) affinity and high T cell avidity for peptide MHC (pMHC) complexes expressed on tumor cells (Appay et al., 2008); ii) high amounts of granzymes and perforin (Appay et al., 2008); iii) expression of surface molecules that allow T cell trafficking into the tumor [e.g. CXCR3 (Mullins et al., 2004)] and persistence in the tumor site [e.g. the integrins CD103 (Le Floc'h et al., 2007) and CD49a (Sandoval et al., 2013a)]; and iv) high expression of costimulatory [e.g. CD137 (Wilcox et al., 2002)] or low expression of inhibitory [ e.g. Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) (Peggs et al., 2009) or PD-1 (Freeman et al., 2006)] molecules. The components of the immune system necessary for the induction of such CD8+ T cells include: i) the presentation of antigen by appropriate antigen presenting cells (APCs) (Joffre et al., 2012; Lizee et al., 2012); and ii) the generation of CD4+ T cells producing cytokines helping CD8+ T cell proliferation and differentiation, for example IL-21 (Spolski and Leonard, 2008) (Figure 2).
Figure 2. Dendritic cells play a central role in vaccination.
The desired properties of vaccine-elicited CD8+ T cells include: i) high TCR affinity and high T cell avidity; ii) high levels of granzymes and perforin; iii) trafficking into the tumor and persistence in the tumor site; and iv) high proliferation potential. Naïve CD8+ T cells initiate a CTL differentiation program upon encounter with DCs presenting tumor-derived peptides via MHC class I. This is supported by co-stimulation mediated by CD80, CD70 and 4-1BB and by DC-derived cytokines such IL-15. XCR1 chemokine secreted by DCs facilitates the interaction with naïve CD8+ T cells. TGFβ expressed by DCs is critical for CD8+ T cells to express CD103 and acquire mucosal phenotype. CD8+ T cell differentiation, especially generation of memory, is dependent on the quality of CD4+ T cell help. The latter one is partially dependent on the IL-12 secreted by DCs. CD4+ T cells producing IFNγ and/or IL-21 can help CD8+ T cell expansion and differentiation. Tregs might play a critical role during the selection of high-avidity CD8+ T cells. This might be ascribed to the cross-talk between DCs and CD4+ T cells where CD4+ T cells control DC functions. There, Tregs can suppress DCs via IL-10 production and also regulate the production of chemokines, thereby limiting the interactions between DCs and low-avidity T cells. CD4+ T cells can also provide DC maturation signals via CD40.
Numerous avenues of therapeutic vaccination against cancer are currently pursued (Finn, 2008). Searching the term “cancer vaccines” in clinicaltrials.gov yields 1307 clinical studies (as of July 2013), with 152 in Phase III clinical trials and 591 in Phase II clinical trials, which highlights the clinical activity in the field. A common feature among these studies, and a critical step in vaccination, is the efficient presentation of cancer antigens to T cells (Figure 2). Because DC are the most efficient antigen presenting cells (Banchereau and Steinman, 1998), exploiting their diversity, in terms of subsets as well as plasticity, is likely to yield improved therapeutic vaccines.
DCs are an essential component of vaccination through their capacity to capture, process, and present antigens to T cells (Banchereau and Steinman, 1998). While immature DCs in peripheral tissues efficiently capture antigens (Mellman and Steinman, 2001), antigen presentation usually results in immune tolerance because of the lack of costimulatory molecules (Steinman et al., 2003; Tarbell et al., 2007). Induction of immune tolerance occurs through various mechanisms including T cell deletion and expansion of regulatory T cells (Treg) (Steinman et al., 2003; Tarbell et al., 2007). Activated (mature), antigen-loaded DCs initiate the differentiation of antigen-specific T cells into effector T cells displaying unique functions and cytokine profiles. DC maturation is associated with a wide variety of cellular changes including: i) decreased antigen-capture activity; ii) increased expression of surface MHC class II molecules and costimulatory molecules; iii) acquisition of chemokine receptors e.g.CCR7, which guide their migration (Trombetta and Mellman, 2005); and iv) the ability to secrete different cytokines e.g. interleukin-12 (IL-12) that control T cell differentiation. It is now accepted that vaccine adjuvants act by inducing DC maturation (Steinman and Banchereau, 2007). Vaccines can also reach lymph-node resident DCs directly through the lymphatics (Itano et al., 2003). Recent years brought about an increased understanding of DC biology, the existence of distinct DC subsets with specific functions as well as distinct molecular mechanism that DCs use to regulate the immune response. Hereunder, we will discuss how this progress can be harnessed for improved vaccination against cancer.
Human DC subsets
Human DCs in the steady state were first studied in whole blood and skin. Three cell surface markers characterize blood DCs: CD303, expressed on plasmacytoid DCs (pDCs), and CD1c and CD141, both expressed on circulating DCs (Dzionek et al., 2000; Dzionek et al., 2001; MacDonald et al., 2002). Both CD1c+ and CD141+ DCs can produce IL-12, thereby enabling the generation of Interferon-γ (IFN-γ)-secreting type 1 CD4+ T (Th1) cells, and the priming of naive CD8+ T cells (Meixlsperger et al., 2013; Schlitzer et al., 2013). Both CD1c+ and CD141+ DCs, isolated from blood or tissues, are able to cross-present long peptides of melanoma-tissue-derived antigen (MART-1) to T cell lines (Segura et al., 2012) and acquire viral antigens and drive antiviral effector CD8+ T cell responses (Yu et al., 2013). However, they also display unique features. CD141+CD1c− DCs, the human counterpart of mouse CD8α+ DCs, produce very large amounts of IFN-α upon recognition of synthetic double-stranded RNA (dsRNA) (Meixlsperger et al., 2013) and, when activated with poly I:C, efficiently cross-prime CD8+ T cells (Bachem et al., 2010; Crozat et al., 2010; Haniffa et al., 2012; Jongbloed et al., 2010; Lauterbach et al., 2010; Mittag et al., 2011; Poulin et al., 2010). CD1c+ DCs from both blood and lungs are uniquely able to drive the differentiation of CD103+CD8+ mucosal T cells with high retention capacity in the lung (Yu et al., 2013).
Studies of human cutaneous DCs demonstrated their phenotypic and functional heterogeneity (Klechevsky et al., 2008; Nestle et al., 2009; Joffre et al., 2012). In particular, LCs specialize in priming CD8+ T cell immunity whereas interstitial/dermal (CD14+) DCs promote humoral immunity (Klechevsky et al., 2008). The efficiency of LCs in priming naïve CD8+ T can be partially explained by their ability to produce IL-15 (Banchereau et al., 2012a; Romano et al., 2012) and/or upregulate CD70 (van der Aar et al., 2011). Interstitial DCs can either act directly on B cells (Dubois et al., 1997) or prime CD4+ T cells to differentiate into T follicular helper (Tfh) cells that help B cell differentiation in germinal centers (GCs) (Crotty S., 2011). They induce the differentiation of Tfh through the production of IL-12 (Schmitt et al., 2013). Interstitial DCs can generate type 2 CD8+ T cells (Tc2) producing low amounts of Granzyme A and displaying poor CTL functions, a property that can be inhibited by blocking ILT4 (Banchereau et al., 2012b). Thus, vaccines that target interstitial DCs might raise good antibody responses but poor CD8+ T cell immunity.
DCs express numerous non-clonal pattern recognition receptors (PRRs), which permit sensing and transmission of danger signals to adaptive immunity. PRRs include membrane C-type lectins and Toll-like receptors (TLRs), and cytoplasmic NOD-like receptors (NLRs) and DNA/RNA sensors (Barber, 2011; Desmet and Ishii, 2012). These receptors allow DCs to sense pathogens, apoptotic and necrotic cells, and stressed cell products, for example extruded DNA (Caielli et al., 2012). Herein, we will only discuss a few examples of these recognition mechanisms to illustrate how these DC properties can be harnessed to generate more efficient cancer vaccines. Interested readers can find more in-depth discussion in recent reviews (Coffman et al., 2010; Desmet and Ishii, 2012; Latz et al. 2013).
Nucleic acid detection can lead to the production of protective type I IFN via endosomal or cytoplasmic sensors (Barber, 2011; Desmet and Ishii, 2012; Zhang et al., 2011a; Zhang et al., 2011b). This offers a venue for development of potent vaccine adjuvants generating high levels of type I IFN, such as poly I:C binding TLR3 and cytoplasmic sensors, Imiquimod binding TLR7 and CpG oligonucleotides binding TLR9 (Coffman et al., 2010). Some lectins harbor signaling motifs in their cytoplasmic regions that deliver activation signals when engaged by ligands expressed on necrotic cells (Sancho and Reis e Sousa, 2013). For example, macrophage-inducible C-type lectin (MINCLE) detects nuclear ribonucleoproteins released from damaged cells (Sancho and Reis e Sousa, 2013), whereas CLEC9A, expressed uniquely on CD141+ DCs, detects actin exposed on necrotic cells (Ahrens et al., 2012; Zhang et al., 2012), thereby facilitating cross-presentation of necrotic cell antigens (Sancho et al., 2009). DCs also express inflammasome components that regulate the release of caspase activation-dependent cytokines, including IL-1β, IL-18 and high-mobility group box 1 (HMGB1) (Latz et al., 2013). Inflammasome activation in DCs can occur through recognition of microbial ligands such as flagellin or through indirect mechanisms resulting from the phagocytosis of particles, including alum, uric acid, and biodegradable particles that are currently being tested as vaccine adjuvants (Coffman et al., 2010; Latz et al, 2013). Activation of the inflammasome also plays a very important role in response to cancer therapy via so-called “immunogenic cancer cell death” (Kroemer et al., 2013). There, certain types of anti-cancer chemotherapy drugs such as anthracyclines or oxaliplatin can induce immunogenic cancer cell death, which is characterized by secretion of HMGB1 from dying cells, which engages TLR4 on DCs (Kroemer et al., 2013). As DCs simultaneously capture dying cancer cells, this signal facilitates cancer antigen processing and presentation by DC to T cells (Kroemer et al., 2013). This in turn plays an important role in boosting anti-cancer immunity via endogenous vaccination. Indeed, the absence of HMGB1 expression by dying tumor cells compromises DC-dependent T-cell priming by tumor-associated antigens (Yamazaki et al., 2013). Exploiting these unique molecular pathways for antigen delivery and DC activation represents another way of harnessing DCs for vaccination.
Dendritic cell-based vaccines
DCs can be exploited for vaccination against cancer through various means including: 1) non-targeted peptide/protein and nucleic acids-based vaccines captured by DCs in vivo, 2) vaccines composed of antigens directly coupled to anti-DC-antibodies, or 3) vaccines composed of ex vivo generated DCs that are loaded with antigens. We will discuss selected examples of current therapeutic vaccination approaches to illustrate these key concepts. All these approaches are assessed in ongoing clinical trials.
Non-targeted vaccines
Vaccines composed of short 9-10 amino-acids (aa) long peptides, with or without adjuvants demonstrated that MHC class I-restricted antigen-specific CD8+ T cell immunity can be mounted in patients with metastatic disease (Boon et al., 2006; Rosenberg et al., 1998; Speiser et al., 2008). The clinical success was however limited (Rosenberg et al., 2005), possibly because of the lack of CD4+ T cell help which we now know is necessary for the generation of potent CTLs and long-lived memory CD8+ T cells (Janssen et al., 2005; Filipazzi et al., 2012). Long synthetic peptides of ∼ 25-50aa have the advantage of potentially inducing broad immunity with both CD8+ T cell and CD4+ T cell responses against multiple epitopes (Quakkelaar and Melief, 2012). Vaccination of 20 patients with high-grade vulvar intraepithelial neoplasia with a long peptide covering the two oncogenic proteins E6 and E7 of high-risk human papilloma virus type 16 (HPV16), led to complete regression of all lesions and eradication of virus in 9 individuals (Kenter et al., 2009). A high ratio of vaccine antigen-specific effector T cells to CD4+CD25+Foxp3+ Treg cells was predictive of clinical benefit (Welters et al., 2010). Vaccination of patients suffering from recurrent ovarian cancer with long peptides covering p53 led to expansion of p53-specific CD4+ T cells in blood and tumor (Leffers et al., 2009). However, no impact on the clinical course of the disease was observed (Leffers et al., 2009). The lack of clinical responses might be explained by the domination of the immune response to vaccine antigens by CD4+ T cells that secreted type 2 (IL-4 and IL-5) rather then IFN-γ. Indeed, Type 2 CD4+ T cells might not be protective against cancer. Durable expansion of p53-specific type 2 CD4+ T cells was also observed in patients with colorectal cancer (Speetjens et al., 2009). Combining the long p53 peptide vaccine with IFN-α resulted in increased expansion of antigen-specific IFN-γ–secreting CD4+ T cells, though the impact on clinical efficacy remains to be established (Zeestraten et al., 2013). These results further illustrate the challenges that the re-programming of preexisting T cell memory represents, and the need to identify vaccines that will enable priming of a new T cell repertoire.
With the advances of proteomics, vaccines can now be prepared with peptides representing antigens identified from patients' tumors. The peptides are combined with granulocyte-macrophage colony stimulating factor (GM-CSF) to attract and activate DCs, and low dose of cyclophosphamide to control Tregs. This regimen led to immune responses that were associated with clinical responses (Walter et al., 2012). While it is difficult to assess which component of this therapy accounted for good immune and clinical efficacy, shifting from shared tumor antigens common to many patients to patient-specific neo-antigens may enable to efficiently activate an available T cell repertoire against which Tregs might not have developed. The concept of patient-specific vaccines was initiated more than two decades ago with idiotype vaccines in lymphoma (Kwak et al., 1992) where tumor idiotypic determinants were conjugated to the immune carrier keyhole limpet hemocyanin (KLH) (Kwak et al., 1992). A Phase III trial in patients with lymphoma showed that such a vaccine combined with GM-CSF can lead to significant prolongation of disease-free survival (Schuster et al., 2011).
Peptide-protein vaccines are poorly immunogenic by themselves unless adjuvants are added to generate robust anti-tumor immune responses. Many adjuvants are currently under evaluation as constituents of cancer vaccines (Dubensky and Reed, 2010). These include agonists of various TLRs such as TLR3 (poly I:C), TLR4 (monophosphoryl lipid A; MPL), TLR5 (flagellin), TLR7 [Aldara® (Imiquimod)], TLR7-TLR8 (Resiquimod), and TLR9 (CpG) (Dubensky and Reed, 2010). Combinations of adjuvants targeting different pathways might synergize to generate more potent immune responses as their combination can activate DCs in a synergistic fashion (Coffman et al., 2010). A promising candidate is GlaxoSmithKline's (GSK) AS15 adjuvant system, which incorporates monophosphoryl lipid A (MPL) that acts via TLR4, the saponin QS-21, and CpG oligonucleotides that act via TLR9 (Cluff, 2010). Vaccines composed of recombinant MAGE-A3 protein and AS15 elicited specific immune responses and clinical activity in both a Phase II study in patients with metastatic melanoma (NCT00086866) and a Phase II study in patients with resected non-small cell lung cancer (NSCLC) (NCT00290355) (Brichard and Lejeune, 2007). Phase III trial are currently ongoing in two settings: 1) in patients with resectable regionally advanced melanoma (DERMA Phase III trial, NCT00796445) (Kirkwood, 2011); and 2) in patients with MAGE-A3 expressing NSCLC with minimal residual disease post-surgery (NCT00480025). Clearly, a better understanding of DC biology will provide a fertile ground for discovery of novel adjuvants.
DCs are also engaged in response to complex vaccine preparations such as GVAX® tumor cell based vaccines where cancer cells are genetically modified to express GM-CSF which attracts and activates DCs (Le et al., 2010). Such GVAX vaccines have shown some immune and clinical activity in pancreatic cancer (Thomas et al., 2004; Lutz et al., 2011) and other types of solid tumors (Dranoff, 2002). Another vaccine platform is based on recombinant Listeria monocytogenes(Lm), an intracellular bacterium that targets DCs in vivo and utilizes both class I and II antigen-processing pathways (Brockstedt et al., 2004; Le et al., 2012). The live mutant Lm-based vaccine that expresses mesothelin elicits mesothelin-specific T cells in mice and humans (Le et al., 2012). Engineered viruses can ferry selected antigens as well as co-stimulation cassettes (Larocca and Schlom, 2011). A randomized Phase II trial with a poxvirus-based vaccine expressing prostate-specific antigen (PSA) (PROSTVAC) and TRICOM (CD54, CD58 and CD80) carried out in men with metastatic prostate cancer showed an improved overall survival (8.5 months) (Kantoff et al., 2010b). Another strategy is based on intratumoral delivery of oncolytic viruses, i.e., viruses that preferentially infect and kill cancer cells. These can be modified to express GM-CSF to attract DCs and lymphocytes at the lysed tumor site (Russell et al., 2012). A phase II study of GM-CSF-oncolytic herpes virus in patients with stage IIIc/IV melanoma indicated durable regression in both injected and non-injected lesions suggesting systemic effect (Senzer et al., 2009). The recent data from randomized prospective phase III clinical trial, showed tumor regression lasting at least 6 months in 16% of patients treated with the recombinant virus. Only 2% of patients treated with GM-CSF in the control arm showed such response (OPTiM, Oncovex Pivotal Trial in Melanoma, Amgen website). A formal analysis of the trial is expected later this year. Viral vectors to deliver antigens to DCs, either directly by encoded genes or indirectly via tumor lysis, is an attractive strategy as it mimics the natural way of infection and generation of protective immunity. Yet, the immunogenicity of these vectors might prevent their efficacy upon boosting, therefore calling for prime-boost strategies where a second vector is used for boosting the specific immune response. This strategy is currently developed in the context of HIV vaccines (both preventive and therapeutic) and could be applied to cancer in case of success.
Vaccination with ex vivo generated DCs
DC can be generated ex vivo, loaded with different forms of antigens, activated and injected in patients (Palucka and Banchereau, 2012). Clinical studies from the past 15 years have analyzed: i) different DC vaccine preparations; ii) different DC activators; iii) different forms of antigen preparations from short peptides to complex whole tumor cell hybrids; iv) different routes of DC injection. These studies were initially performed as single treatments but combination studies are now being assessed with agents such as systemic adjuvants, for example poly I:C (Aarntzen et al., 2008; Kalinski et al., 2013; Palucka and Banchereau, 2012; Schuler, 2010). These studies concluded that DC-based vaccines are safe and can induce the expansion of circulating CD4+ T cells and CD8+ T cells that are specific for tumor antigens. While objective clinical responses have been observed in certain patients, there is a discrepancy between the blood immune response and the rate of clinical responses, as we will later discuss. The clinical response takes time to build up but remissions can be long-lasting. The US Food and Drug Administration (FDA) has approved, for the treatment of metastatic prostate cancer, Sipuleucel-T, a cellular product composed of enriched blood APC cultured with a fusion protein of prostatic acid phosphatase (PAP) and GM-CSF. Treatment with Sipuleucel-T resulted in a ∼4-month-prolonged median survival in patients with prostate cancer (Kantoff et al., 2010a). Another subsets of blood DCs, plasmacytoid DCs, which represent the main source of type I IFN upon viral infection have also been assessed as the basis for cancer vaccines (Liu, 2005; Tel et al., 2013). Some patients with metastatic melanoma, who have been vaccinated with activated pDCs loaded with tumor antigen-peptides, showed antigen-specific CD4+ and CD8+ T cell responses (Tel et al., 2013).
While considerable progresses have been made over the years, additional studies are required to fully reveal the potential immunotherapeutic impact of ex vivo generated DCs. Most studies have been performed in late stage patients who display strong immunosuppression mechanisms, for example Tregs that counteract the induction of effective immunity to vaccine antigens. Nevertheless, there are two ongoing phase III trials assessing in comparative studies clinical efficacy of monocyte-derived ex vivo generated DC vaccines. One trial is testing DC vaccine in patients with newly diagnosed brain tumor (glioblastoma) following surgery as add-on to the standard of care combining radiation and chemotherapy (NCT00045968; Northwest Therapeutics). The DCs are loaded with autologous tumor lysate. The second trial is testing DC vaccine in patients with advanced kidney cancer (renal carcinoma) as add-on to targeted therapy with Sunitinib, a receptor tyrosine kinase inhibitor (NCT01582672; ADAPT trial, Argos Therapeutics). The DCs are loaded with autologous tumor RNA. The three common features of these two trials are 1. Vaccination of patients with resected tumors, and thus lower tumor burden, 2. Vaccination in combination with other therapy; and 3. Loading DCs with autologous tumor preparations. Time will show whether the promising phase II data observed with these vaccines will be confirmed in phase III.
In vivo DC targeting
Pioneering studies from Ralph Steinman and Michel Nussenzweig demonstrated the principle of targeting antigens to DCs in vivo through the coupling of antigens to antibodies specific to DC surface receptors such as DEC205 or DCIR (Bonifaz et al., 2002; Hawiger et al., 2001; Soares et al., 2007a). Importantly, in the absence of adjuvants, targeting antigens to DEC205+ DCs in vivo induces antigen-specific tolerance (Hawiger et al., 2001), which can be used as treatment against autoimmune diseases such as type I diabetes (Steinman, 2012). Administration of these complex vaccines with DC-activators such as TLR3, TLR7-8, or CD40 agonists enables the maturation of DCs and thus the establishment of immunity rather than tolerance (Steinman, 2012). The induced immunity was shown to be protective in a number of diseases including various infections (malaria, HIV) and cancer (Steinman, 2012; Tacken and Figdor, 2011). DC targeting-based vaccination studies in non-human primates demonstrated robust T cell immunity in prime-boost design with HIV gag-DEC205 targeting vaccine (Flynn et al., 2011).
Currently, numerous in vitro and in vivo studies in human and mice are focused on developing DC-targeting vaccines. For example, targeting antigens through the DC surface lectinss DCIR (Klechevsky et al., 2010; Meyer-Wentrup et al., 2009), DC-SIGN (Dakappagari et al., 2006), Dectin 1 (Ni et al. 2010), CLEC9A (Sancho et al., 2008) and Langerin (Flacher et al., 2009), results in humoral and cellular responses including both CD4+ and CD8+ T cells. As observed in the original studies with DEC205, the presence or absence of adjuvants has profound impact on immune responses. Thus, in the absence of adjuvants, injection of antigens coupled to antibodies against CLEC9A results in strong antibody responses, which are linked to the generation of Tfh cells (Caminschi et al., 2012). It also results in priming of Tregs (Joffre et al., 2010) but not CD8+ T cell immunity despite the capture and the cross-presentation of targeted antigens by CD8α+ DCs (Sancho et al., 2008). This can be skewed by the addition of adjuvants, for example poly I:C, at which point targeting of antigen to DCs via CLEC9A results in potent and robust anti-tumor CD4+ and CD8+ T cell immunity (Sancho et al., 2008; Joffre et al., 2010). In vivo studies in mice comparing immunogenicity of HIV antigens linked with antibodies to Langerin/CD207, DEC205/CD205, and CLEC9A receptors, along with anti-CD40 antibody to induce DC activation induced comparable levels of gag-specific Th1 and CD8+ T cells (Idoyaga et al., 2011). These target molecules are expressed by CD8α+ DCs and the responses were more robust than those obtained by targeting gag to CD8α- DCs via DCIR (Idoyaga et al., 2011). Thus, when the appropriate DC subset is targeted with a vaccine antigen with appropriate adjuvants, several different receptors expressed by that subset are able to initiate T cell immunity.
However, different DC receptors can deliver different signals to the same DC leading to distinct types of immune responses. For example, targeting antigens to DC-ASGPR, in the absence of adjuvants, favors the generation of antigen-specific IL-10 secreting CD4+ T cells with regulatory properties both in vitro in the human and in vivo in non-human primates (Li et al., 2012). Targeting the same DC population with antibodies to LOX-1 results in Th1 responses (Li 2012). Furthermore, targeting different human DC receptors revealed the importance of the antigen internalization into either early or late endosomes (Chatterjee et al., 2012). Thus, in human BDCA1+ and monocyte-derived DCs, antibodies to CD40 and mannose receptor targeted antigens to early endosomes, whereas antibodies to DEC205 targeted antigens primarily to late compartments. The receptor that was least efficient at internalization, CD40, turns out to be the most efficient at cross-presentation because it promotes limited intra-endosomal degradation (Chatterjee et al., 2012). Similarly, the targeting of different DC receptors generates quantitatively and qualitatively different T cell responses in vivo in mice (Dudziak et al., 2007; Soares et al., 2007b). There, unlike CD8α+ DCs that express DEC205, CD8α- DCs, which express 33D1 antigen, are specialized for presentation of targeted antigen on major histocompatibility complex (MHC) class II. This difference in antigen processing was shown to be intrinsic to the DC subsets and associated with increased expression of proteins involved in MHC processing (Dudziak et al., 2007). Thus, it will be essential to refine the understanding of DC biology to guide the processing of targeted antigen and subsequent presentation resulting in CD8+ T cell immunity.
CD8+ T cell immunity
Therapeutic vaccination aims at expanding high avidity CD8+ T cells that can differentiate into CTLs able to kill cancer cells, and can generate long-lived memory CD8+T cells. This could be accomplished either through the priming of naïve T cells or the re-programming of memory T cells that differentiated earlier in an environment not conducive to the generation of potent cytotoxic T cells (Figure 1). Naïve CD8+ T cells differentiate into CTLs in lymphoid organs upon encounter with DCs presenting tumor-derived peptides (Bousso and Robey, 2003) (Figure 2) in the context of co-stimulation through CD80 (Chen et al., 1992), CD70 and 4-1BB (Shuford et al., 1997) as well as DC-derived cytokines such as IL-12 and IL-15 (Araki et al., 2010; Waldmann, 2006; Zhang and Bevan, 2011). The priming of the new repertoire of T cells might be critical for clinical success. Studies with adoptive T cell transfer showed that effector cells derived from naive CD8+ T cells expressed higher CD27 and retained longer telomeres, suggesting a greater proliferative potential (Hinrichs et al., 2011; Klebanoff et al., 2012).
Circulating memory CD8+ T cells include both central memory and effector cells that circulate between secondary lymphoid organs and peripheral tissues. A third category i.e., tissue-resident memory T cells has been recently identified (Jiang et al., 2012; Mueller et al., 2012) and shown to be superior to circulating memory T cells at providing rapid long-term protection against re-infection (Gebhardt et al., 2009; Jiang et al., 2012). CD103 (αE7) integrin allows peripheral CD8+ T cell retention in epithelial compartments (Sheridan and Lefrancois, 2011). In the context of cancer, the expression CD103 by CTLs facilitate their adherence to cancer cells expressing E-cadherin eventually leading to tumor cell lysis and rejection (Le Floc'h et al., 2007). Indeed, for mucosal cancer vaccines, the homing to and retention of CD8+ T cells in the mucosa is critical for efficacy (Sandoval et al., 2013b). In this context, the growth of orthotopic head and neck or lung cancers can be inhibited by a cancer vaccine provided it is administered by the intranasal mucosal route but not the intramuscular route (Sandoval et al., 2013b). This is explained by the induction through intranasal vaccination of mucosal CD8+ T cells expressing the mucosal integrin CD49a, the expression of which is essential for cancer vaccine efficacy (Sandoval et al., 2013b). The critical role of tissue DCs in imprinting the trafficking patterns of elicited T cells explains the critical role of the route of immunization (Mullins et al., 2003; Sheasley-O'neill S et al., 2007) (Mora et al., 2003). The current challenge is to find out how to control T cell differentiation and trafficking in patients.
Designing tomorrow's therapeutic cancer vaccines
The challenge for next generation vaccines is to resolve the discrepancy between the immune and clinical efficacy measured by the rate of cancer rejection. We will summarize herein the three key aspects which, when combined, can bring the resolution to this challenge: 1. The quality of vaccine-elicited CD8+ T cell immunity; 2. The quality of vaccine-elicited CD4+ T cells; and 3. The barriers that vaccine-elicited CD8+ T cells must confront to access and reject cancer.
As discussed at the beginning of this review, adoptive T cell transfer and cancer vaccine studies yielded a better understanding of what constitutes a potent anti-tumor CD8+ T cell immunity. Thus, next generation DC vaccines need to be based on those DC subsets that are best equipped to elicit CD8+ T cells that fulfill these criteria. For example, targeting cancer antigens to CD141+ DCs would allow generation of highly potent CTLs. On the other hand, targeting the antigen to CD1c+ DCs would allow expansion of CD103+CD8+ T memory T cells able to reside in the tissue.
CD4+ T cells regulate CD8+ T cell immunity both in the priming and effector phase. For example, Tregs can inhibit the effector functions of CD8+ T cells thereby preventing tumor rejection (Tanchot et al., 2012). However, Tregs also play a critical role during the priming by promoting the selection of high avidity CD8+ T cells (Pace et al., 2012). Although mostly helping tumor rejection, Th1 cells might contribute to tumor escape via secretion of IFNγ that triggers expression of PDL-1 in tissues, thus providing an off-signal to effector CD8+ T cells (Sharpe et al., 2007). Th17 cells (Dong, 2008) exert either pro- or anti-tumor activity depending on the tissue environment in which they reside (reviewed in (Wei et al., 2012)). Indeed, IL-17 can synergize with IFNγ to induce tumor cells to secrete CXCL9 and CXCL10, which attract cytotoxic CD8+ T cells (Wei et al., 2012). Thus, it will be now critically important to unravel molecular factors governing CD4+ T cell programming and differentiation and DC molecules that can control such factors. Again, the functional specialization amongst human DC subsets can be harnessed here. Indeed, as we discussed above CD14+ DCs are able to prime Tfh. Meanwhile, LCs prime Th2 cells (Klechevsky et al., 2008); and CD1c+ but not CD141+ DCs are molecularly equipped to generate Th17 responses in human (Schlitzer A, et al. 2013). This knowledge can be applied to design of next generation vaccines to direct the differentiation of antigen-specific CD4+ T cells to a desired phenotype and function.
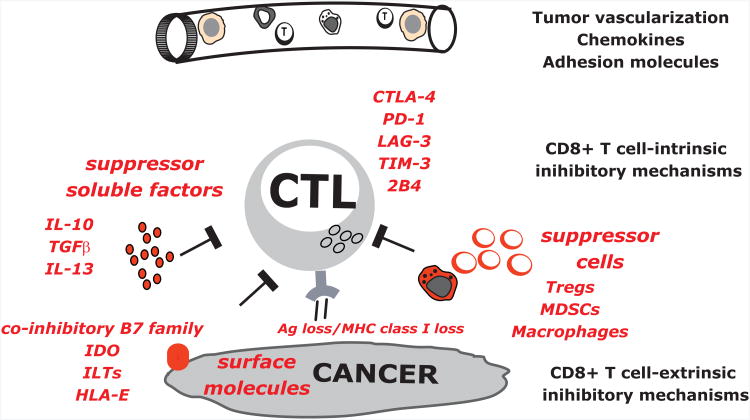
Last but not least, once elicited, CD8+ T cells must confront numerous barriers including: i) intrinsic regulators for example CD28-CTLA-4, PD1-PDL1, and ILTs (Pardoll, 2012) as well as extrinsic regulators cells such as Tregs (Fehervari and Sakaguchi, 2004) or myeloid-derived suppressor cells (MDSCs) (Gabrilovich and Nagaraj, 2009); ii) a corrupted tumor microenvironment with pro-tumor inflammation (Coussens et al., 2013; Klebanoff et al., 2011); iii) antigen loss and immune evasion of tumor targets (Klebanoff et al., 2011); and iv) tissue specific alterations such as fatty cells in breast cancer or desmofibrosis in pancreatic cancer stroma (Figure 3). Defining strategies to bypass these obstacles is the object of intense studies to improve the clinical efficacy of vaccination via DCs. A logical approach to address these issues is in the combination of DC vaccine candidates with agents that target different pathways. For example, checkpoint inhibitors such as antagonists to CTLA-4 or PD-1 might offset inhibitor signals (Figure 3) (see companion article by Topalian and Pardoll). The combination of GVAX with anti-CTLA4 antibody (Ipilimumab) has proven to be safe (van den Eertwegh et al., 2012) and pre-clinical models show increased effector CD8+ T cells and enhanced tumor-antigen directed CTL function (Wada et al., 2013).
Figure 3. The barriers for CD8+ T cell-mediated tumor rejection.
The next generation vaccines must confront and address numerous barriers that CD8+ T cells face including: i) T cells access to the tumor site; ii) T cell intrinsic regulators, for example CD28-CTLA-4, PD1-PDL1; iii) T cell extrinsic regulators such as suppressor cells: Tregs, MDSCs or pro-tumor macrophages; tumor secreted suppressive factors including IL-10; and suppressive surface molecules including co-inhibitory molecules from B7 family.
We foresee tomorrow's vaccines as based on anti-DC antibodies which, thanks to progresses in antibody engineering, can be made into polyvalent vaccines targeting distinct yet specific DC subsets to trigger an ideal composite anti-cancer immune response. Such vaccines will also carry DC activators as well as immunomodulatory molecules to neutralize inhibitory signals as for example anti-PDL-1. This will keep us busy for a while.
Conclusions
We have come a long way since the first clinical trial with ex vivo DCs that was launched in 1996 (Hsu et al, 1996) in our understanding of the main problem: what is needed to elicit therapeutic immunity when cancer escapes the natural barrier of protective immunity. The considerable progress made in the understanding of the biology of DCs and effector and regulatory T cells open avenues for the development of new and novel vaccine strategies. Progresses in “omics” will enable linking genetic alterations with the type of immune response. Novel protocols will be tailored to the patient-specific mutation (Schreiber et al., 2011) and immune alterations the patients display. Thus, there has never been a more exciting time for working on cancer vaccines.
Acknowledgments
Dedicated to patients and volunteers who participated in our studies and clinical trials. We thank Drs Robert Coffman, Hideki Ueno and Romain Banchereau for critical reading of the manuscript. We thank former and current members of BIIR for their contributions, in particular: Hideki Ueno, MD, PhD; Joseph Fay, MD; Sangkon Oh, PhD; Virginia Pascual, MD; Lee Roberts, PhD; and Gerard Zurawski, PhD. Supported by the NIH (P01 CA084514, U19 AIO57234, R01 CA089440 and CA078846), the Dana Foundation, the Susan Komen Foundation, the Baylor Health Care System; the Baylor Health Care System Foundation, the ANRS and the INSERM. KP holds the Michael A. Ramsay Chair for Cancer Immunology Research. Due to space limitations we could cite only a part of rich literature relevant to this topic.
Literature
- Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, Schreibelt G, Mus R, De Wilt JH, Haanen JB, et al. Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res. 2013;73:19–29. doi: 10.1158/0008-5472.CAN-12-1127. [DOI] [PubMed] [Google Scholar]
- Aarntzen EH, Figdor CG, Adema GJ, Punt CJ, de Vries IJ. Dendritic cell vaccination and immune monitoring. Cancer Immunol Immunother. 2008;57:1559–1568. doi: 10.1007/s00262-008-0553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens S, Zelenay S, Sancho D, Hanc P, Kjaer S, Feest C, Fletcher G, Durkin C, Postigo A, Skehel M, et al. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity. 2012;36:635–645. doi: 10.1016/j.immuni.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Ali H, Donovan B, Wand H, Read TR, Regan DG, Grulich AE, Fairley CK, Guy RJ. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ. 2013;346:f2032. doi: 10.1136/bmj.f2032. [DOI] [PubMed] [Google Scholar]
- Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- Araki K, Youngblood B, Ahmed R. The role of mTOR in memory CD8 T-cell differentiation. Immunol Rev. 2010;235:234–243. doi: 10.1111/j.0105-2896.2010.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Thompson-Snipes L, Zurawski S, Blanck JP, Cao Y, Clayton S, Gorvel JP, Zurawski G, Klechevsky E. The differential production of cytokines by human Langerhans cells and dermal CD14(+) DCs controls CTL priming. Blood. 2012a;119:5742–5749. doi: 10.1182/blood-2011-08-371245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Zurawski S, Thompson-Snipes L, Blanck JP, Clayton S, Munk A, Cao Y, Wang Z, Khandelwal S, Hu J, et al. Immunoglobulin-like transcript receptors on human dermal CD14+ dendritic cells act as a CD8-antagonist to control cytotoxic T cell priming. Proc Natl Acad Sci U S A. 2012b;109:18885–18890. doi: 10.1073/pnas.1205785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber GN. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr Opin Immunol. 2011;23:10–20. doi: 10.1016/j.coi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biragyn A, Tani K, Grimm MC, Weeks S, Kwak LW. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat Biotechnol. 1999;17:253–258. doi: 10.1038/6995. [DOI] [PubMed] [Google Scholar]
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- Brichard VG, Lejeune D. GSK's antigen-specific cancer immunotherapy programme: pilot results leading to Phase III clinical development. Vaccine. 2007;25(2):B61–71. doi: 10.1016/j.vaccine.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Brockstedt DG, Giedlin MA, Leong ML, Bahjat KS, Gao Y, Luckett W, Liu W, Cook DN, Portnoy DA, Dubensky TW., Jr Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci U S A. 2004;101:13832–13837. doi: 10.1073/pnas.0406035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caielli S, Banchereau J, Pascual V. Neutrophils come of age in chronic inflammation. Curr Opin Immunol. 2012;24:671–7. doi: 10.1016/j.coi.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminschi I, Vremec D, Ahmet F, Lahoud MH, Villadangos JA, Murphy KM, Heath WR, Shortman K. Antibody responses initiated by Clec9A-bearing dendritic cells in normal and Batf3(-/-) mice. Mol Immunol. 2012;50:9–17. doi: 10.1016/j.molimm.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Chatterjee B, Smed-Sorensen A, Cohn L, Chalouni C, Vandlen R, Lee BC, Widger J, Keler T, Delamarre L, Mellman I. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood. 2012;120:2011–2020. doi: 10.1182/blood-2012-01-402370. [DOI] [PubMed] [Google Scholar]
- Cluff CW. Monophosphoryl lipid A (MPL) as an adjuvant for anti-cancer vaccines: clinical results. Adv Exp Med Biol. 2010;667:111–123. doi: 10.1007/978-1-4419-1603-7_10. [DOI] [PubMed] [Google Scholar]
- Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, Vu Manh TP, Baranek T, Storset AK, Marvel J, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1283–1292. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakappagari N, Maruyama T, Renshaw M, Tacken P, Figdor C, Torensma R, Wild MA, Wu D, Bowdish K, Kretz-Rommel A. Internalizing antibodies to the C-type lectins, L-SIGN and DC-SIGN, inhibit viral glycoprotein binding and deliver antigen to human dendritic cells for the induction of T cell responses. J Immunol. 2006;176:426–440. doi: 10.4049/jimmunol.176.1.426. [DOI] [PubMed] [Google Scholar]
- de Saint-Vis B, Fugier-Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Ait-Yahia S, Banchereau J, Liu YJ, Lebecque S, Caux C. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160:1666–1676. [PubMed] [Google Scholar]
- Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. 2012;12:479–491. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- Dranoff G. GM-CSF-based cancer vaccines. Immunol Rev. 2002;188:147–154. doi: 10.1034/j.1600-065x.2002.18813.x. [DOI] [PubMed] [Google Scholar]
- Dubensky TW, Jr, Reed SG. Adjuvants for cancer vaccines. Semin Immunol. 2010;22:155–161. doi: 10.1016/j.smim.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Dubois B, Vanbervliet B, Fayette J, Massacrier C, Van Kooten C, Briere F, Banchereau J, Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J Exp Med. 1997;185:941–51. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–1217. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipazzi P, Pilla L, Mariani L, Patuzzo R, Castelli C, Camisaschi C, Maurichi A, Cova A, Rigamonti G, Giardino F, et al. Limited induction of tumor cross-reactive T cells without a measurable clinical benefit in early melanoma patients vaccinated with human leukocyte antigen class I-modified peptides. Clin Cancer Res. 2012;18:6485–6496. doi: 10.1158/1078-0432.CCR-12-1516. [DOI] [PubMed] [Google Scholar]
- Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- Finn OJ, Edwards RP. Human papillomavirus vaccine for cancer prevention. N Engl J Med. 2009;361:1899–1901. doi: 10.1056/NEJMe0907480. [DOI] [PubMed] [Google Scholar]
- Flacher V, Sparber F, Tripp CH, Romani N, Stoitzner P. Targeting of epidermal Langerhans cells with antigenic proteins: attempts to harness their properties for immunotherapy. Cancer Immunol Immunother. 2009;58:1137–1147. doi: 10.1007/s00262-008-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn BJ, Kastenmuller K, Wille-Reece U, Tomaras GD, Alam M, Lindsay RW, Salazar AM, Perdiguero B, Gomez CE, Wagner R, et al. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc Natl Acad Sci U S A. 2011;108:7131–7136. doi: 10.1073/pnas.1103869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJ. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin Cancer Res. 2011;17:2270–2280. doi: 10.1158/1078-0432.CCR-10-2888. [DOI] [PubMed] [Google Scholar]
- Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, Wang XN, Malinarich F, Malleret B, et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo K, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs CS, Borman ZA, Gattinoni L, Yu Z, Burns WR, Huang J, Klebanoff CA, Johnson LA, Kerkar SP, Yang S, et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117:808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R. Vaccination patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52–8. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- Idoyaga J, Lubkin A, Fiorese C, Lahoud MH, Caminschi I, Huang Y, Rodriguez A, Clausen BE, Park CG, Trumpfheller C, Steinman RM. Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9. A Proc Natl Acad Sci U S A. 2011;108:2384–9. doi: 10.1073/pnas.1019547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski P, Muthuswamy R, Urban J. Dendritic cells in cancer immunotherapy: vaccines and combination immunotherapies. Expert Rev Vaccines. 2013;12:285–295. doi: 10.1586/erv.13.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandalaft LE, Powell DJ, Jr, Chiang CL, Tanyi J, Kim S, Bosch M, Montone K, Mick R, Levine BL, Torigian DA, et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology. 2013;2:e22664. doi: 10.4161/onci.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010a;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, et al. Overall Survival Analysis of a Phase II Randomized Controlled Trial of a Poxviral-Based PSA-Targeted Immunotherapy in Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2010b;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- Khan S, Bijker MS, Weterings JJ, Tanke HJ, Adema GJ, van Hall T, Drijfhout JW, Melief CJ, Overkleeft HS, van der Marel GA, et al. Distinct uptake mechanisms but similar intracellular processing of two different toll-like receptor ligand-peptide conjugates in dendritic cells. J Biol Chem. 2007;282:21145–21159. doi: 10.1074/jbc.M701705200. [DOI] [PubMed] [Google Scholar]
- Kirkwood J. A Phase III Study to Test the Benefit of a New Kind of Anti-cancer Treatment in Patients With Melanoma, After Surgical Removal of Their Tumor. J Clin Oncol. 2011;29 supplement [Google Scholar]
- Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev. 2011;239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother. 2012;35:651–660. doi: 10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechevsky E, Flamar AL, Cao Y, Blanck JP, Liu M, O'Bar A, Agouna-Deciat O, Klucar P, Thompson-Snipes L, Zurawski S, et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116:1685–1697. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- Kwak LW, Campbell MJ, Czerwinski DK, Hart S, Miller RA, Levy R. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med. 1992;327:1209–1215. doi: 10.1056/NEJM199210223271705. [DOI] [PubMed] [Google Scholar]
- Larocca C, Schlom J. Viral vector-based therapeutic cancer vaccines. Cancer J. 2011;17:359–371. doi: 10.1097/PPO.0b013e3182325e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach H, Bathke B, Gilles S, Traidl-Hoffmann C, Luber CA, Fejer G, Freudenberg MA, Davey GM, Vremec D, Kallies A, et al. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J Exp Med. 2010;207:2703–2717. doi: 10.1084/jem.20092720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Dubenksy TW, Jr, Brockstedt DG. Clinical development of Listeria monocytogenes-based immunotherapies. Semin Oncol. 2012;39:311–322. doi: 10.1053/j.seminoncol.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Pardoll DM, Jaffee EM. Cellular vaccine approaches. Cancer J. 2010;16:304–310. doi: 10.1097/PPO.0b013e3181eb33d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Floc'h A, Jalil A, Vergnon I, Le Maux Chansac B, Lazar V, Bismuth G, Chouaib S, Mami-Chouaib F. Alpha E beta 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med. 2007;204:559–570. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffers N, Lambeck AJ, Gooden MJ, Hoogeboom BN, Wolf R, Hamming IE, Hepkema BG, Willemse PH, Molmans BH, Hollema H, et al. Immunization with a P53 synthetic long peptide vaccine induces P53-specific immune responses in ovarian cancer patients, a phase II trial. Int J Cancer. 2009;125:2104–2113. doi: 10.1002/ijc.24597. [DOI] [PubMed] [Google Scholar]
- Li D, Romain G, Flamar AL, Duluc D, Dullaers M, Li XH, Zurawski S, Bosquet N, Palucka AK, Le Grand R, et al. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J Exp Med. 2012;209:109–121. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Lizee G, Overwijk WW, Radvanyi L, Gao J, Sharma P, Hwu P. Harnessing the power of the immune system to target cancer. Annu Rev Med. 2012;64:71–90. doi: 10.1146/annurev-med-112311-083918. [DOI] [PubMed] [Google Scholar]
- Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–335. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- Maldonado-Lopez R, Maliszewski C, Urbain J, Moser M. Cytokines regulate the capacity of CD8α+and CD8α-dendritic cells to prime Th1/Th2 cells in vivo. J Immunol. 2001;167:4345–4350. doi: 10.4049/jimmunol.167.8.4345. [DOI] [PubMed] [Google Scholar]
- Manicassamy S, Pulendran B. Modulation of adaptive immunity with Toll-like receptors. Semin Immunol. 2009;21:185–193. doi: 10.1016/j.smim.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meixlsperger S, Leung CS, Ramer PC, Pack M, Vanoaica LD, Breton G, Pascolo S, Salazar AM, Dzionek A, Schmitz J, et al. CD141+ dendritic cells produce prominent amounts of IFN-alpha after dsRNA recognition and can be targeted via DEC-205 in humanized mice. Blood. 2013 doi: 10.1182/blood-2012-12-473413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Meyer-Wentrup F, Cambi A, Joosten B, Looman MW, de Vries IJ, Figdor CG, Adema GJ. DCIR is endocytosed into human dendritic cells and inhibits TLR8-mediated cytokine production. J Leukoc Biol. 2009;85:518–525. doi: 10.1189/jlb.0608352. [DOI] [PubMed] [Google Scholar]
- Mittag D, Proietto AI, Loudovaris T, Mannering SI, Vremec D, Shortman K, Wu L, Harrison LC. Human dendritic cell subsets from spleen and blood are similar in phenotype and function but modified by donor health status. J Immunol. 2011;186:6207–6217. doi: 10.4049/jimmunol.1002632. [DOI] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- Morse MA, Chapman R, Powderly J, Blackwell K, Keler T, Green J, Riggs R, He LZ, Ramakrishna V, Vitale L, et al. Phase I study utilizing a novel antigen-presenting cell-targeted vaccine with Toll-like receptor stimulation to induce immunity to self-antigens in cancer patients. Clin Cancer Res. 2011;17:4844–4853. doi: 10.1158/1078-0432.CCR-11-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T Cell Subsets, Migration Patterns, and Tissue Residence. Annu Rev Immunol. 2012 doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- Mullins DW, Sheasley SL, Ream RM, Bullock TN, Fu YX, Engelhard VH. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins IM, Slingluff CL, Lee JK, Garbee CF, Shu J, Anderson SG, Mayer ME, Knaus WA, Mullins DW. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res. 2004;64:7697–7701. doi: 10.1158/0008-5472.CAN-04-2059. [DOI] [PubMed] [Google Scholar]
- Nabel GJ. Designing tomorrow's vaccines. N Engl J Med. 2013;368:551–560. doi: 10.1056/NEJMra1204186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L, Gayet I, Zurawski S, Duluc D, Flamar AL, Li XH, O'Bar A, Clayton S, Palucka AK, Zurawski G, et al. Concomitant activation and antigen uptake via human dectin-1 results in potent antigen-specific CD8+ T cell responses. J Immunol. 2010;185:3504–3513. doi: 10.4049/jimmunol.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace L, Tempez A, Arnold-Schrauf C, Lemaitre F, Bousso P, Fetler L, Sparwasser T, Amigorena S. Regulatory T cells increase the avidity of primary CD8+ T cell responses and promote memory. Science. 2012;338:532–536. doi: 10.1126/science.1227049. [DOI] [PubMed] [Google Scholar]
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–25. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, Keller AM, Joffre O, Zelenay S, Nye E, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1261–1271. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293:253–256. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- Quakkelaar ED, Melief CJ. Experience with synthetic vaccines for cancer and persistent virus infections in nonhuman primates and patients. Adv Immunol. 2012;114:77–106. doi: 10.1016/B978-0-12-396548-6.00004-4. [DOI] [PubMed] [Google Scholar]
- Romano E, Cotari JW, Barreira da Silva R, Betts BC, Chung DJ, Avogadri F, Fink MJ, St Angelo ET, Mehrara B, Heller G, et al. Human Langerhans cells use an IL-15R-alpha/IL-15/pSTAT5-dependent mechanism to break T-cell tolerance against the self-differentiation tumor antigen WT1. Blood. 2012;119:5182–5190. doi: 10.1182/blood-2011-09-382200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, Royal RE, Kammula U, Restifo NP, Hughes MS, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, Hernanz-Falcon P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D, Mourao-Sa D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, Carlyle JR, Reis e Sousa C. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D, Reis e Sousa C. Sensing of cell death by myeloid C-type lectin receptors. Curr Opin Immunol. 2013;25:46–52. doi: 10.1016/j.coi.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G, et al. Mucosal imprinting of vaccine-induced CD8(+) T cells is crucial to inhibit the growth of mucosal tumors. Sci Transl Med. 2013a;5:172ra120. doi: 10.1126/scitranslmed.3004888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G, et al. Mucosal Imprinting of Vaccine-Induced CD8+ T Cells Is Crucial to Inhibit the Growth of Mucosal Tumors. Sci Transl Med. 2013b;5:172ra120. doi: 10.1126/scitranslmed.3004888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber H, Rowley JD, Rowley DA. Targeting mutations predictably. Blood. 2011;118:830–831. doi: 10.1182/blood-2011-06-357541. [DOI] [PubMed] [Google Scholar]
- Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, et al. IRF4 Transcription Factor-Dependent CD11b(+) Dendritic Cells in Human and Mouse Control Mucosal IL-17 Cytokine Responses. Immunity. 2013;38:970–83. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N, Bustamante J, Bourdery L, Bentebibel SE, Boisson-Dupuis S, Hamlin F, Tran MV, Blankenship D, Pascual V, Savino DA, et al. IL-12 receptor beta1 deficiency alters in vivo T follicular helper cell response in humans. Blood. 2013;121:3375–85. doi: 10.1182/blood-2012-08-448902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler G. Dendritic cells in cancer immunotherapy. Eur J Immunol. 2010;40:2123–2130. doi: 10.1002/eji.201040630. [DOI] [PubMed] [Google Scholar]
- Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, Winter JN, Flowers CR, Nikcevich DA, Sotomayor EM, et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol. 2011;29:2787–2794. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- Segura E, Durand M, Amigorena S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J Exp Med. 2013 doi: 10.1084/jem.20121103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura E, Valladeau-Guilemond J, Donnadieu MH, Sastre-Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med. 2012 doi: 10.1084/jem.20111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, Gonzalez R, Glaspy J, Whitman E, Harrington K, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27:5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- Sharma A, Koldovsky U, Xu S, Mick R, Roses R, Fitzpatrick E, Weinstein S, Nisenbaum H, Levine BL, Fox K, et al. HER-2 pulsed dendritic cell vaccine can eliminate HER-2 expression and impact ductal carcinoma in situ. Cancer. 2012;118:4354–4362. doi: 10.1002/cncr.26734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- Sheasley-O'neill SL, Brinkman CC, Ferguson AR, Dispenza MC, Engelhard VH. Dendritic cell immunization route determines integrin expression and lymphoid and nonlymphoid tissue distribution of CD8 T cells. J Immunol. 2007;178:1512–1522. doi: 10.4049/jimmunol.178.3.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nat Immunol. 2011;12:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, Dudziak D, Nussenzweig MC, Steinman RM. A subset of dendritic cells induces CD4+ T cells to produce IFN-{gamma} by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med. 2007a doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, Dudziak D, Nussenzweig MC, Steinman RM. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med. 2007b;204:1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speetjens FM, Kuppen PJ, Welters MJ, Essahsah F, Voet van den Brink AM, Lantrua MG, Valentijn AR, Oostendorp J, Fathers LM, Nijman HW, et al. Induction of p53-specific immunity by a p53 synthetic long peptide vaccine in patients treated for metastatic colorectal cancer. Clin Cancer Res. 2009;15:1086–1095. doi: 10.1158/1078-0432.CCR-08-2227. [DOI] [PubMed] [Google Scholar]
- Speiser DE, Baumgaertner P, Voelter V, Devevre E, Barbey C, Rufer N, Romero P. Unmodified self antigen triggers human CD8 T cells with stronger tumor reactivity than altered antigen. Proc Natl Acad Sci U S A. 2008;105:3849–3854. doi: 10.1073/pnas.0800080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- Steinman RM. Decisions About Dendritic Cells: Past, Present and Future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Subbarao K, Murphy BR, Fauci AS. Development of effective vaccines against pandemic influenza. Immunity. 2006;24:5–9. doi: 10.1016/j.immuni.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Tacken PJ, Figdor CG. Targeted antigen delivery and activation of dendritic cells in vivo: steps towards cost effective vaccines. Semin Immunol. 2011;23:12–20. doi: 10.1016/j.smim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tanchot C, Terme M, Pere H, Tran T, Benhamouda N, Strioga M, Banissi C, Galluzzi L, Kroemer G, Tartour E. Tumor-Infiltrating Regulatory T Cells: Phenotype, Role, Mechanism of Expansion In Situ and Clinical Significance. Cancer Microenviron. doi: 10.1007/s12307-012-0122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanchot C, Terme M, Pere H, Tran T, Benhamouda N, Strioga M, Banissi C, Galluzzi L, Kroemer G, Tartour E. Tumor-Infiltrating Regulatory T Cells: Phenotype, Role, Mechanism of Expansion In Situ and Clinical Significance. Cancer Microenviron. 2012 doi: 10.1007/s12307-012-0122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, Yang H, Suthanthiran M, Mojsov S, Steinman RM. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204:191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, Boerman OC, Croockewit S, Oyen WJ, van Rossum M, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063–1075. doi: 10.1158/0008-5472.CAN-12-2583. [DOI] [PubMed] [Google Scholar]
- Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, Huang LQ, Laheru DA, Goggins M, Hruban RH, Jaffee EM. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJ, van der Sluis TM, Gall HE, Harding TC, Jooss K, Lowy I, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–517. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- van der Aar AM, de Groot R, Sanchez-Hernandez M, Taanman EW, van Lier RA, Teunissen MB, de Jong EC, Kapsenberg ML. Cutting edge: virus selectively primes human langerhans cells for CD70 expression promoting CD8+ T cell responses. J Immunol. 2011;187:3488–3492. doi: 10.4049/jimmunol.1101105. [DOI] [PubMed] [Google Scholar]
- Wada S, Jackson CM, Yoshimura K, Yen HR, Getnet D, Harris TJ, Goldberg MV, Bruno TC, Grosso JF, Durham N, et al. Sequencing CTLA-4 blockade with cell-based immunotherapy for prostate cancer. J Transl Med. 2013;11:89. doi: 10.1186/1479-5876-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012 doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- Wei S, Zhao E, Kryczek I, Zou W. Th17 cells have stem cell-like features and promote long-term immunity. Oncoimmunology. 2012;1:516–519. doi: 10.4161/onci.19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welters MJ, Kenter GG, de Vos van Steenwijk PJ, Lowik MJ, Berends-van der Meer DM, Essahsah F, Stynenbosch LF, Vloon AP, Ramwadhdoebe TH, Piersma SJ, et al. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc Natl Acad Sci U S A. 2010;107:11895–11899. doi: 10.1073/pnas.1006500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox RA, Flies DB, Zhu G, Johnson AJ, Tamada K, Chapoval AI, Strome SE, Pease LR, Chen L. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H, Paul WE. Cytokines of the gamma(c) family control CD4+ T cell differentiation and function. Nat Immunol. 2012;13:1037–1044. doi: 10.1038/ni.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Hannani D, Poirier-Colame V, Ladoire S, Locher C, Sistigu A, Prada N, Adjemian S, Catani JP, Freudenberg M, et al. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 2013 doi: 10.1038/cdd.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Laird LS, Chia CY, Meckel KF, Slansky JE, Thompson JM, Jain A, Pardoll DM, Schulick RD. Live attenuated Listeria monocytogenes effectively treats hepatic colorectal cancer metastases and is strongly enhanced by depletion of regulatory T cells. Cancer Res. 2007;67:10058–10066. doi: 10.1158/0008-5472.CAN-07-0573. [DOI] [PubMed] [Google Scholar]
- Yu CI, Becker C, Wang Y, Marches F, Helft J, Leboeuf M, Anguiano E, Pourpe S, Goller K, Pascual V, et al. Human CD1c(+) Dendritic Cells Drive the Differentiation of CD103(+) CD8(+) Mucosal Effector T Cells via the Cytokine TGF-beta. Immunity. 2013;38:818–830. doi: 10.1016/j.immuni.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeestraten EC, Speetjens FM, Welters MJ, Saadatmand S, Stynenbosch LF, Jongen R, Kapiteijn E, Gelderblom H, Nijman HW, Valentijn AR, et al. Addition of interferon-alpha to the p53-SLP(R) vaccine results in increased production of interferon-gamma in vaccinated colorectal cancer patients: a phase I/II clinical trial. Int J Cancer. 2013;132:1581–1591. doi: 10.1002/ijc.27819. [DOI] [PubMed] [Google Scholar]
- Zhang JG, Czabotar PE, Policheni AN, Caminschi I, Wan SS, Kitsoulis S, Tullett KM, Robin AY, Brammananth R, van Delft MF, et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity. 2012;36:646–57. doi: 10.1016/j.immuni.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Kim T, Bao M, Facchinetti V, Jung SY, Ghaffari AA, Qin J, Cheng G, Liu YJ. DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity. 2011a;34:866–878. doi: 10.1016/j.immuni.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Yuan B, Bao M, Lu N, Kim T, Liu YJ. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol. 2011b;12:959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]