Abstract
Background
Th2 cells play a critical role in the pathogenesis of allergic asthma. Established Th2 cells have been shown to resist reprogramming into Th1 cells. The inherent stability of Th2 cells poses a significant barrier to treating allergic diseases.
Objective
We sought to understand the mechanisms by which CD4+ T cells from asthmatic patients resist the IL-27-mediated inhibition.
Methods
We isolated and cultured CD4+ T cells from both healthy individuals and allergic asthmatic patients in order to test whether IL-27 can inhibit IL-4 production by the cultured CD4+ T cells using ELISA. Culturing conditions that resulted in resistance to IL-27 were determined using both murine and human CD4+ T cell culture systems. STAT1 phosphorylation was analyzed by Western blot and flow cytometry. Suppressor of cytokine signaling (Socs) mRNA expression was measured by quantitative PCR. The small interfering RNA method was used to knockdown the expression of Socs3 mRNA.
Main Results
We demonstrated that CD4+ T cells from asthmatic patients resisted the suppression of IL-4 production mediated by IL-27. We observed that repeated exposure to Th2-inducing conditions rendered healthy human CD4+ T cells resistant to IL-27-mediated inhibition. Using an in vitro murine culture system, we further demonstrated that repeated or higher doses of IL-4 stimulation, but not IL-2 stimulation, upregulated Socs3 mRNA expression and impaired IL-27-induced STAT1 phosphorylation. The Knockdown of Socs3 mRNA expression restored IL-27-induced STAT1 phosphorylation and IL-27-mediated inhibition of IL-4-production.
Conclusions
Our findings demonstrate that differentiated Th2 cells can resist IL-27-induced reprogramming toward Th1 cells by downregulating STAT1 phosphorylation and likely explain why the CD4+ T cells of asthmatic patients are resistant to IL-27-mediated inhibition.
Keywords: asthma, Th2 stability, IL-4, STAT1 signaling, SOCS3, IL-27
INTRODUCTION
Naïve CD4+ T cells can differentiate into various helper T cell subsets, including Th1, Th2, Th9, Th17, and Th22. Although established Th cells can exhibit some plasticity, especially under certain circumstances, such as infections, Th cells that have already committed to one Th cell fate tend to down regulate their potential to differentiate into other Th cell fates. For instance, ample experimental evidence supports that committed Th1 cells silence their potential to transcribe the Il4 gene [see review 1]. Moreover, T-bet, which is important in Ifng gene transcription, 2-5 also possesses the ability to suppress Il4 gene transcription. Ectopic expression of T-bet in differentiating Th2 cells not only induces transcription of the Ifng gene but also suppresses transcription of the Il4 gene. In the absence of T-bet, mice develop spontaneous allergic airway inflammation and asthma because the Il4 gene is not silenced. 6 Others have found that certain polymorphisms in the TBX21 gene correlate with a susceptibility to developing asthma in humans. 7 Our own work shows that continuous T-bet expression is required to silence Il4 gene transcription in Th1 cells. 8
In addition to T-bet, it has been reported that IFN-γR−/−, STAT4−/−, IRF-1−/−, and IRF-2−/− mice (mice deficient in Th1 promoting factors) all demonstrated a propensity towards mounting a Th2-type immune response against pathogens that were known to elicit only a Th1 response in wild type mice. 9-13 These results clearly demonstrate that Th1-promoting factors are critical in suppressing Th2 cell differentiation.
Similarly, established Th2 cells have been shown to resist reprogramming into Th1 cells. 14, 15,16 This inherent Th2 stability poses a significant barrier to treating allergic diseases. 17 Although it has been shown that the differentiation of Th2 cells downregulates both the IL-12Rβ2 subunit and signal transducer and activator of transcription (STAT) 4 expression, rendering these Th2 cells unresponsive to IL-12, 18, 19 the mechanism of resistance to other Th1-promoting factors, such as IL-27, in committed Th2 cells has not been investigated.
IL-27 is a heterodimeric cytokine composed of Epstein-Barr virus-induced gene 3 (EBI3) and p28. It binds to the IL-27 receptor (WSX-1) and gp130 to activate STAT1. 20-22 IL-27 is a member of the IL-12 family and is primarily produced by activated dendritic cells (DCs). It has been reported that IL-27 induces Th1 cell differentiation 23 and suppresses Th17 cell differentiation. 24
IL-27 also inhibits Th2 cell differentiation. IL-27R−/− mice displayed increased Th2 production when infected with the parasites Leishmania major or Trichuris muris. 25 Transgenic expression of IL-27 suppressed Th2 responses induced by S. venezuelensis. 25, 26In vitro experiments have demonstrated that IL-27 suppressed Th2 cell differentiation independent of its ability to promote IFN-γ production. 25, 27 However, it is unclear whether IL-27 inhibits already-differentiated Th2 cells. Furthermore, despite the similarities between murine and human cytokines, we cannot assume that murine and human IL-27 will have similar Th2 inhibitory effects.
In this study, we demonstrated that human IL-27 inhibited IL-4 production by CD4+ T cells from healthy individuals, but failed to suppress IL-4 production by CD4+ T cells from asthmatic patients. We observed that repeated exposures to Th2-inducing conditions rendered healthy human CD4+ T cells resistant to IL-27-mediated inhibition of IL-4 production. Using an in vitro murine culture system, we further demonstrated that repeated or higher doses of IL-4 stimulation, but not IL-2 stimulation, upregulated Socs3 mRNA expression, which in turn impaired IL-27-induced STAT1 phosphorylation. The knockdown of Socs3 mRNA expression restored IL-27-induced STAT1 phosphorylation and IL-27-mediated inhibition of IL-4-production. We also found that levels of SOCS3 mRNA and protein, but not SOCS1 mRNA and protein, were elevated in the peripheral CD4+ T cells of allergic asthmatic patients. Together, these findings reveal a novel mechanism by which differentiated Th2 cells resist IL-27-induced reprogramming into Th1 cells.
METHODS
Human subjects
Healthy subjects with no prior history of allergic disease or with negative allergy skin tests were enrolled. Inclusion criteria included healthy males and females age 18 or older with no prior history of allergic disease (See the METHODS section in this article's Online Repository at www.jacionline.org). Mild-to-moderate allergic asthma patients were recruited through clinics of the Allergy and Immunology division at National Jewish Health (Denver, CO). The protocols (HS2619 and HS1700) have been approved by the Institutional Review Board at National Jewish Health. Additionally, the protocol (B2012-46) has been approved by the Institutional Review Board at Fudan University (See the METHODS section in this article's Online Repository at www.jacionline.org).
Human CD4+ T cell culture
Human CD4+ T cells were cultured under either neutralizing conditions or Th2-inducing conditions, as described in detail under METHODS of the Online Repository. For two-round-priming, the first-round-primed cells were washed and re-stimulated under Th2-inducing conditions in the presence or absence of rhIL-27.
Animals
C57BL/6 mice and Tbx21−/− mice on C57BL/6 background 3 were purchased from The Jackson Laboratory (Bar Harbor, ME). STAT1−/− mice on the 129 background 28 were purchased from Taconic Inc (Hudson, NY) and backcrossed to C57BL/6 background mice (three generations). All mice were maintained in the animal facility of National Jewish Health (Denver, CO). The animal protocol was approved by the Institutional Animal Care and Users Committees at National Jewish Health (Denver, CO).
Priming of murine Th cells, intracellular staining and ELISA analysis of IL-4 and IFN-γ protein
Mouse CD4+ T cells were cultured under either neutralizing conditions or Th2-inducing conditions consisting of three different concentrations of reagents as described in detail under METHODS of the Online Repository. Intracellular staining and ELISA were also described in detail under METHODS of the Online Repository.
Quantitative RT-PCR (qPCR) and western blot analysis
qPCR measurement was performed as described in detail under METHODS of the Online Repository. Primer sequences are listed in Table E1 of the Online Repository. Western blot analysis was carried out as described in detail under METHODS of the Online Repository.
E Table I.
Primers and siRNA sequences.
| Gene | 5′primer | 3′primer |
|---|---|---|
| Gp130 | GCCAGAGCTTCGAGCCATCCGG | TGGTGCTGACATCTTGCAGGGATG |
| Wsx | CAAGAAGAGGTCCCGTGCTG | TTGAGCCCAGTCCACCACAT |
| Il3r-a | AAAGCAACGGGTGACGTGCA | GTTGACGGTGATGGTGACCT |
| Il3r-b | AAGAAGGCTAGGGGACCTTC | CAGGAGCAAGGTGAGGATGA |
| Il12r-b1 | TCGTTTCGGTCGCAGCACCA | CCATGGCTGCCACTCAAGGCA |
| Il12r-b2 | CTTCT GCACC CACTC ACATT | GAGCT CTCCA TTCCA CTATG |
| Socs1 | GCAGAGAGAACTGCGGCCGTG | CTGCCACCTGGTTGCGTGCT |
| Socs2 | CGGGGTTGCCGGAGGAACAG | TCCGCAGGTTAGTCGGTCCAGC |
| Socs3 | GACCATAGGAGGCGCAGCCC | GCGGCGGGAAACTTGCTGTG |
| Socs4 | GCTCGGACAGCTCCGCTTGA | CCTGTCAGCACTTCGACTCCGAC |
| Socs5 | ACAAGCCGGGGCGTTGAGC | CCACGGCGCCAGCAATATCTGT |
| Socs6 | CGCGGCTGCAGGGTTTTCATTTCA | GGGCTGCGATGCCTCATGGGT |
| Socs7 | AGCGGTCCGGGAGCTGGATAC | GGTGTGGAGAGGTCAGGCCCC |
| human-SOCS1 | CTGGGATGCCGTGTTATTTT | TAGGAGGTGCGAGTTCAGGT |
| human-SOCS3 | GGAGTTCCTGGACCAGTACG | TTCTTGTGCTTGTGCCATGT |
| Hprt | CTCATGGACTGATTATGGACAGGAC | GCAGGTCAGCAAAGAACTTATAGCC |
| Si-control(siRNA) | UGGUUUACAUGUCGACUAA | |
| Si-Socs3(siRNA) | CAAGAGAGCTTACTACATCTA | |
| CAGTATGATGCTCCACTTTA | ||
| CAGACTTTGCACATATATTTA | ||
| AAGAAACATTTCAGTAATTTA |
Intracellular staining of STAT phosphorylation
PBMCs were stained with APC-labeled anti-Human CD294 (CRTH2) antibody. Next, the stained cells were or were not stimulated with rhIL-27 (50 ng/ml) or rhIL-4 (50 ng/ml) for 15 minutes. Cells were fixed, permeabilized, and stained as described in detail under METHODS of the Online Repository.
siRNA knock down
Naive CD4+ T cells were activated under Th2hi-inducing conditions. Twenty-fours later, cells (0.5 to 2 × 106) were collected and used for siRNA transfection as described in detail under METHODS of the Online Repository.
Statistical analysis
All of the error bars in this report represent SDs. For ELISA or qPCR analyses, the mean ± SD was derived from triplicate measurements of one experiment. Pooled data are indicated in the figure legends. The difference between two samples was analyzed with Student's t-test.
RESULTS
Human IL-27 suppresses Th2 cell differentiation independent of IFN-γ and IL-10
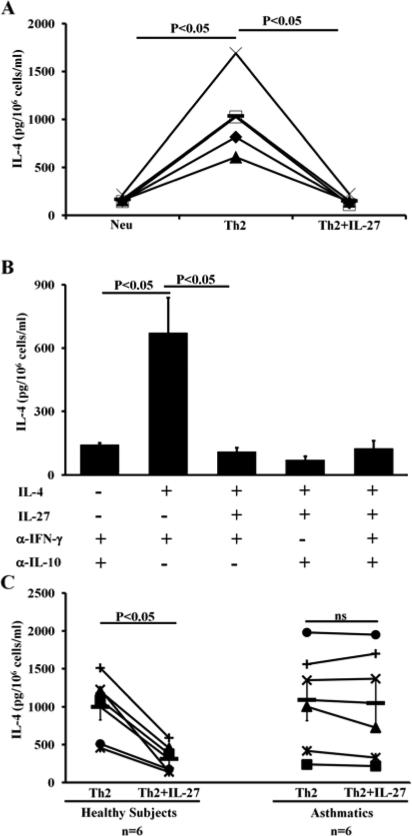
Despite the accumulated knowledge about murine IL-27 and its potential use as an effective immunotherapy for allergic diseases and asthma, there are a limited number of studies regarding human IL-27. Here, we sought to determine if human IL-27 can suppress Th2 cell differentiation in a similar fashion to its murine counterpart. We prepared CD4+ T cells from the peripheral blood of healthy individuals using Miltenyi magnetic beads. The isolated CD4+ T cells were cultured under Th2-inducing conditions in the presence or absence of human IL-27. We found that human IL-27 completely suppressed IL-4 production in human CD4+ T cells that were primed under Th2-inducing-conditions (Fig 1, A). This result demonstrates that IL-27 suppressed Th2 cell differentiation in humans.
FIG 1.
Human IL-27 suppresses Th2 cell differentiation independent of IFN-γ and IL-10. ELISA analysis of IL-4 protein produced by human CD4+ T cells primed with conditions indicated. Neu=neutralizing conditions; Th2=Th2-inducing conditions. Means ± SDs, n=4 (A). Data are representative of two independent experiments with similar results (B). Healthy volunteers (n=6), allergic asthmatic patients (n=6) (C). Each symbol represents the result from one subject (C). Ns=not significant.
Because both mouse and human IL-27 have been reported to promote IFN-γ and IL-10 production, we tested whether human IL-27 suppresses Th2 cell differentiation as a result of IFN-γ and IL-10 production. Anti-IFN-γ and/or anti-IL-10 antibody was included in our culture conditions. We showed that human IL-27 suppresses Th2 cell differentiation independent of both IFN-γ and IL-10 (Fig 1, B). Using IFN-γR−/− CD4 T cells, we observed that IL-27 suppressed Th2 cell differentiation in the absence of IFN-γ receptor (data not shown).
CD4+ T cells from asthmatic patients resist IL-27-mediated inhibition
Subsequently, we used anti-IFN-γ and anti-IL-10 antibodies in our human cell culture system to evaluate the direct effects of IL-27. We examined the effect of IL-27 on CD4+ T cells from both healthy and asthmatic subjects. We found that human IL-27 failed to suppress the differentiation of Th2 cells in allergic asthmatic patients, while it markedly inhibited the differentiation of Th2 cells in healthy controls (Fig 1, C). The data indicated that CD4+ T cells from allergic asthmatic patients resist IL-27-mediated inhibition (hereafter referred to as IL-27 resistance).
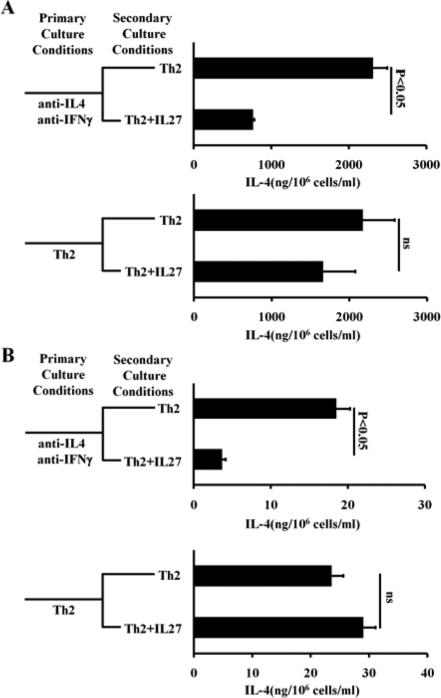
Repeated or high dose IL-4 stimulation, but not IL-2 stimulation, induces Th2 cells to resist IL-27-mediated inhibition
CD4+ T cells from healthy individuals are mostly composed of naïve CD4+ T cells, whereas CD4+ T cells from allergic asthmatic patients are predominantly Th2 cells as the result of repeated exposure to allergen stimulation. It is possible that CD4+ T cells in the peripheral blood of allergic asthmatic patients, after repeated exposure to allergen stimulation, develop a resistance to IL-27-mediated suppression. To examine this possibility, we repeatedly primed one aliquot of CD4+ T cells from healthy individuals under Th2-inducing conditions. As a control, another aliquot of CD4+ T cells from healthy individuals was first primed under neutralizing culture conditions (containing anti-IL-4 and anti-IFN-γ antibodies) and then primed under Th2-inducing conditions. We found that repeated Th2-priming enabled CD4+ T cells from healthy individuals to resist IL-27-mediated inhibition (Fig 2, A). We verified this finding with a murine Th2 differentiation system. We showed that murine IL-27 inhibited the differentiation of naïve CD4+ T cells into Th2 cells but failed to inhibit IL-4 production by already-differentiated Th2 cells (Fig 2, B). We also noted that murine IL-27 failed to inhibit IL-5 production and IL-13 production (data not shown). Thus, we concluded that repeated Th2 priming induces IL-27 resistance.
FIG 2.
Repeated exposure to Th2-inducing conditions induces IL-27 resistance. A, ELISA analysis of IL-4 produced by healthy human CD4+ T cells subjected to two rounds of priming. B, ELISA analysis of IL-4 produced by mouse CD4+ T cells primed as indicated. Error bars and statistic analysis are described in METHODS. Data represent two independent experiments with similar results.
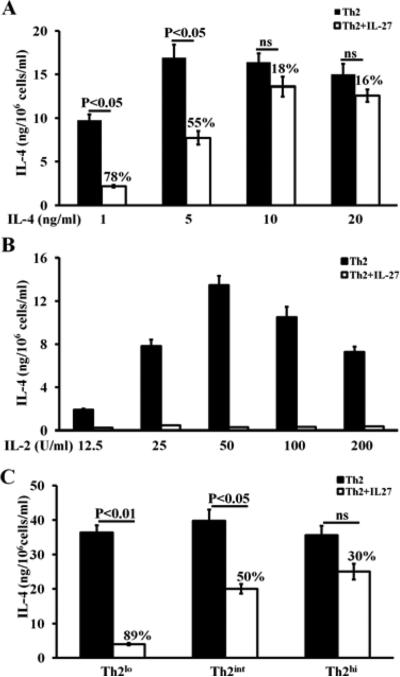
To test whether the development of IL-27 resistance depends on the specific concentration of Th2-inducing reagents, we primed naïve CD4+ T cells with varying doses of IL-4 and IL-2. IL-4 effectively primed Th2 cell differentiation, even at the lowest dose; however, IL-4 only induced IL-27 resistance at higher doses. We measured IL-27 resistance as the percent of reduction in IL-4 production where the least reduction in IL-4 production indicates the strongest resistance. We found that IL-27 resistance increased as the dose of IL-4 increased from 1 ng/ml to 20 ng/ml, i.e., the percent of reduction in IL-4 production decreased from 78% to 16% as the dose of IL-4 increased from 1 ng/ml to 20 ng/ml (Fig 3, A). IL-2 is required for Th2 cell differentiation 29. Consistent with the literature report, we observed that increasing doses of IL-2 resulted in augmented IL-4 production. Surprisingly, we found that IL-2 failed to induce IL-27 resistance, even at the highest experimental dose (Fig 3, B). Based on the above findings, we defined three Th2-inducing conditions for further experiments: 1) low concentrations of Th2-inducing reagents, described in detail under METHODS of the Online Repository (hereafter referred to as Th2lo conditions), 2) intermediate concentrations of Th2-inducing reagents (hereafter referred to as Th2int conditions), which are more equivalent to regular concentrations of Th2-inducing reagents that both we and other researchers have previously published and to those used in experiments shown in Fig 2B, and 3) high concentrations of Th2-inducing reagents (hereafter referred to as Th2hi conditions). We showed that Th2hi conditions induced the strongest IL-27 resistance (IL-27 suppressed IL-4 production by only 30%), while Th2lo conditions did not induce significant IL-27 resistance (IL-27 suppressed IL-4 production by 89%) (Fig 3, C). All three Th2-inducing conditions effectively primed IL-4 production (Fig 3, C).
FIG 3.
High doses of mouse IL-4, but not IL-2, induces IL-27 resistance after just one round of priming. ELISA analysis of IL-4 produced by mouse CD4+ T cells that were primed with increasing doses of rmIL-4 (A) and increasing doses of rhIL-2 (B) The rest of the Th2-inducing conditions are identical to the one described for the Th2int (see METHODS). C, IL-27 resistance was measured in CD4+ T cells that were primed under Th2lo, Th2int and Th2hi conditions. The percentages on the top of the white bars indicate the percentage of reduction in IL-4 production. Data are representative of four (A), or two independent experiments with similar results (B, C).
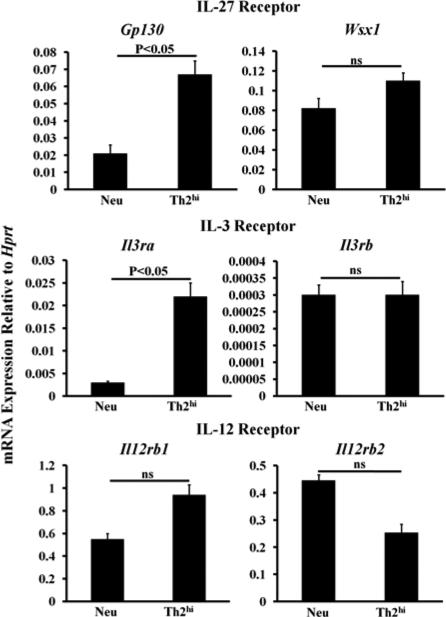
Th2-inducing reagents at high concentrations do not downregulate the IL-27 receptor
Our data do not support that downregulation of the IL-27 receptor is the mechanism by which Th2hi conditions induced IL-27 resistance. In fact, Th2hi conditions markedly upregulated Gp130 mRNA expression, a component of the IL-27 receptor that is shared by the IL-6 receptor family. Th2hi conditions moderately enhanced Wsx1 mRNA expression (E Figure 1). Th2hi conditions greatly upregulated Il3ra chain mRNA expression and did not change Il3rb chain mRNA expression (E Figure 1). The dramatic increase in the expression of the IL-3Rα chain might explain why STAT3 phosphorylation was greatly enhanced in Th2 cells primed under Th2hi conditions (Fig 4, D), a finding that is consistent with a recent report that STAT3 is activated and required for Th2 differentiation.30 Finally, Th2hi conditions slightly increased Il12rb1 mRNA expression and slightly decreased Il12rb2 mRNA expression, which is consistent with our analysis of IL-12-induced STAT4 phosphorylation shown in Fig 4, D.
FIG E1.
The Th2hi conditions-primed Th2 cells do not lack the IL-27 receptor. Naïve mouse CD4+ T cells were cultured under neutralizing conditions or Th2hi conditions for five days. RNA was prepared from the resultant cells and qPCR was performed. SDs were derived from triplicate measurements of one representative experiment. Data are representative of two independent experiments with similar results.
FIG 4.
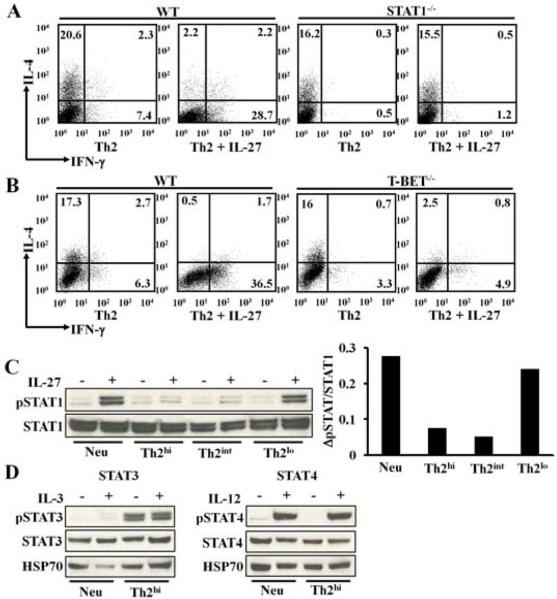
The high concentrations of Th2-inducing reagents specifically impair STAT1 phosphorylation. A-B, Intracellular staining of IL-4 and IFN-γ protein in cells primed Th2int conditions for 5 days. C, Western analysis of STAT1 phosphorylation in cultured CD4+ T cells. Densitometer measurements of ΔpSTAT1/STAT1 are shown in the right panel. ΔpSTAT1/STAT1= [pSTAT1/STAT1 in IL-27 stimulated cells] – [pSTAT1/STAT1 in not stimulated cells]. D, Western analysis of STAT3 and STAT4 phosphorylation. Data are representative of two (A, B, D) or six (C) independent experiments with similar results.
Th2-inducing reagents at high concentrations impair STAT1 signaling
Next, we tested whether Th2hi conditions impair STAT1 signaling. IL-27 has been shown to regulate IL-12 responsiveness as well as the differentiation of regulatory T cells and Th17 cells through both STAT1—dependent and –independent mechanisms 31. To determine if IL-27 depends on STAT1 to suppress Th2 cell differentiation, we primed WT or STAT1-deficient naïve CD4+ T cells under Th2int conditions in the presence or absence of IL-27. We found that in the absence of STAT1 signaling, IL-27 completely lost the ability to suppress Th2 cell differentiation (Fig 4, A), demonstrating that IL-27 entirely depends on STAT1 signaling in order to inhibit Th2 cell differentiation.
It has been demonstrated that T-bet is the key downstream target molecule of STAT1 in both promoting Ifng gene transcription and suppressing Il4 gene transcription 3, 8, 32. Surprisingly, we found that IL-27 did not use T-bet to suppress Th2 cell differentiation. We showed that in the absence of T-bet, IL-27 inhibited IL-4 expression in T-bet−/− CD4+ T cells to a degree comparable with that of the WT control even though IL-27 failed to induce IFN-γ expression in T-bet−/− CD4+ T cells (Fig 4, B).
Thus, in the IL-27 pathway, STAT1 appears to be a logical target of IL-4. We observed that Th2 cells primed under Th2hi and Th2int conditions, but not Th2lo conditions, exhibited a dramatic reduction in IL-27-induced STAT1 phosphorylation (Fig 4, C). Th2-inducing reagents at higher concentrations did not alter STAT1 protein expression in the resultant Th2 cells. On the contrary, STAT3 phosphorylation with or without IL-3 stimulation was greatly enhanced in Th2 cells primed under Th2hi conditions compared to CD4+ T cells cultured under neutralizing conditions. IL-12-induced STAT4 phosphorylation was not significantly altered in Th2 cells primed under Th2hi conditions or in CD4+ T cells primed under neutralizing conditions (Fig 4, D).
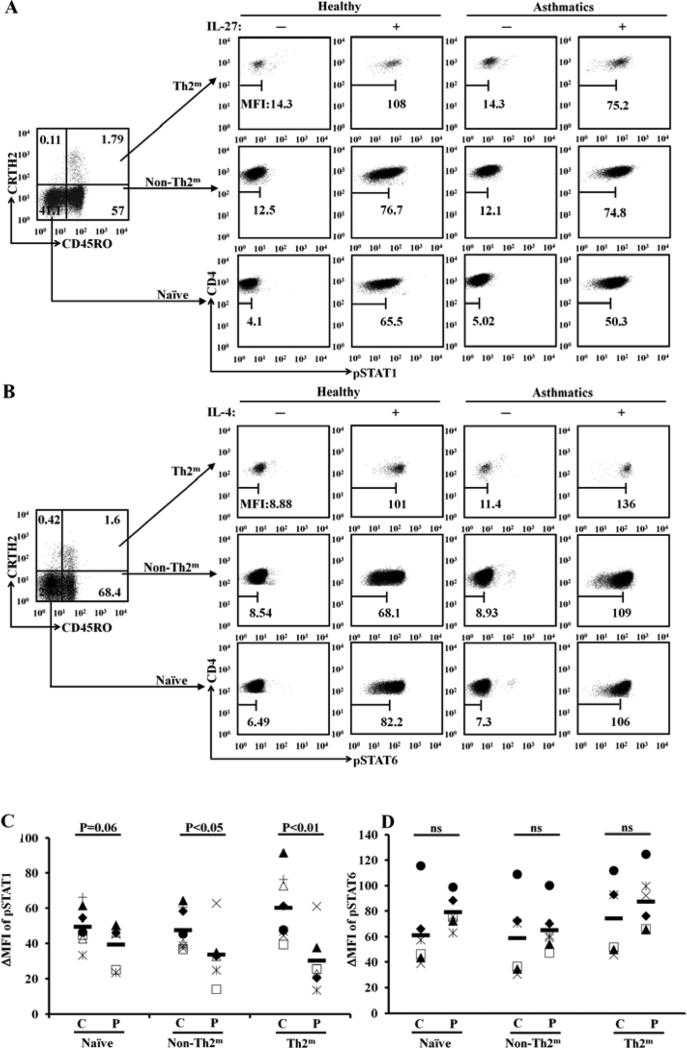
To determine whether STAT1 phosphorylation is inhibited in various CD4+ T cell populations of allergic asthmatic patients, we developed a flow cytometry-based assay. CRTH2 has been reported as an excellent marker for detecting human Th2 cells in the peripheral blood 33. We stained isolated human PMBCs with anti-human CD4, anti-human CRTH2, anti-human CD45RO, and anti-phosphorylated STAT1 (pSTAT1) antibodies. We observed three well-defined CD4+ T cell populations. We defined naïve CD4+ T cells as those cells with a phenotype of CD4+CD45RO−CRTH2−; memory Th2 cells (Th2m) as those cells with a phenotype of CD4+CD45RO+CRTH2+; and memory non-Th2 cells (non-Th2m), including memory Th1 cells and memory Th17 cells, as those cells with a phenotype of CD4+CD45RO+CRTH2− (Fig 5, A). We found that in response to IL-27 stimulation, the delta mean fluorescence intensities (ΔMFIs) of pSTAT1 staining within Th2m cells and non-Th2m cells were significantly lower in the allergic asthmatic patients than in controls (Fig 5, A and C). Interestingly, we noted that ΔMFIs of pSTAT1 staining with naïve CD4+ T cell population in the allergic asthmatic patients showed a tendency toward significant reduction compared with that in controls. We did not observe a significant difference between patients and controls in the ΔMFIs of IL-4-induced pSTAT6 staining across all three defined populations (Fig 5, B and D). These results demonstrate that STAT1 signaling is impaired in all memory Th2 cells of allergic asthmatic patients .
FIG 5.
STAT1 phosphorylation is inhibited in memory Th2 cells of allergic asthmatic patients. A, FACS analysis of STAT1 phosphorylation (pSTAT1) in various subsets of CD4+ T cells. Naïve=naïve CD4+ T cells; Th2m =memory Th2 cells; non-Th2m=memory non-Th2 cells. B, FACS analysis of pSTAT6. C, ΔMFIs of pSTAT1 in gated cell populations (P=patient, n=6; C=control, n=6). D, ΔMFIs of of pSTAT6 (P, n=6; C, n=6).
Th2-inducing reagents at high concentrations upregulate SOCS3 expression
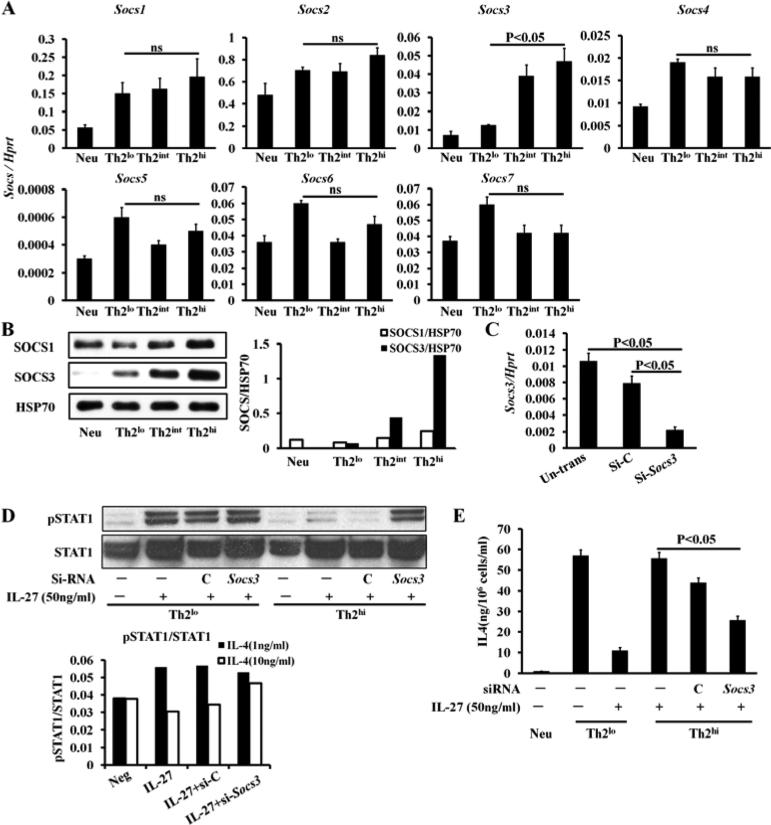
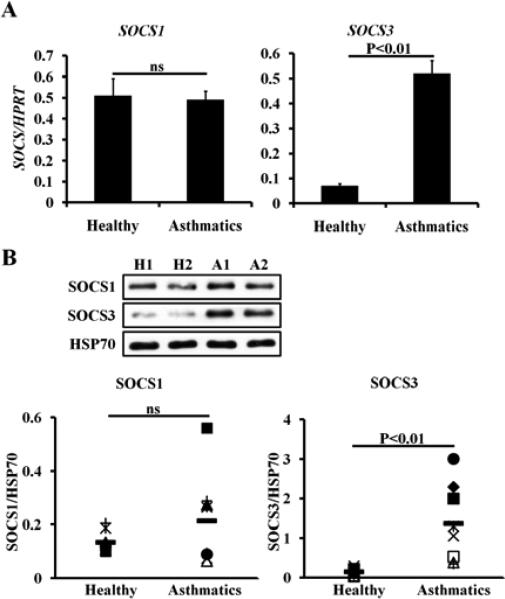
The SOCS family, which consists of seven members, has been shown to be cytokine-induced negative feedback regulators of STAT signaling. Because Th2hi conditions, but not Th2lo conditions, inhibited STAT1 phosphorylation, we compared SOCS mRNA and protein expression of all seven members in Th2 cells primed under Th2lo versus Th2hi conditions. Our data demonstrate that SOCS3 was the only member whose mRNA expression (p<0.05, Fig 6, A) and protein expression (Fig 6, B) were differentially upregulated in Th2 cells primed under Th2hi, but not Th2lo, conditions. To determine the role of SOCS3 in mediating IL-27 resistance, we knocked down expression of Socs3 mRNA (Fig 6, C) and showed that knockdown of Socs3 mRNA restored IL-27-induced STAT1 phosphorylation in Th2 cells primed under Th2hi conditions (Fig 6, D) and rendered Th2 cells, primed under Th2hi conditions, sensitive to IL-27-mediated inhibition yet again (Fig 6, E). Consistently, we found that the mRNA expression (Fig 7, A) and protein expression (Fig 7, B) of human SOCS3, but not human SOCS1, was elevated in purified peripheral CD4+ T cells from allergic asthmatic patients compared to healthy subjects.
FIG 6.
High concentrations of Th2-inducing reagents upregulate SOCS3 expression in mouse CD4+ T cells cultured for 5 days. qPCR analysis (A) and western blot analysis (B) Right panel (B) indicates densitometer measurements. HSP70=heat shock protein 70. C, SiSocs3 RNA knock down efficiency. D, Western blot analysis of pSTAT1/STAT1 in cultured CD4+ T cells. Low panel indicates densitometer measurements. E, IL-4 production by SiSocs3 knocked down in cultured CD4+ T cells. Data are representative of two independent experiments with similar results.
FIG 7.
SOCS 3 expression is elevated in CD4+ T cells of allergic asthmatic patients. A, qPCR analysis of SOCS mRNA in human CD4+ T cells. Healthy controls, n=10; allergic asthma, n=12. B, Western blot analysis. Low panel shows densitometer measurements. H=Healthy controls, n=6; A=allergic asthma, n=6.
DISCUSSION
Inherent Th2 stability poses a significant barrier in treating allergic diseases. Our work demonstrated that repeated exposure to Th2-inducing conditions renders both mouse and human CD4+ T cells resistant to IL-27. We showed that STAT1 signaling is impaired in established Th2 cells and this defect in STAT1 signaling explains why IL-27 can effectively suppress the differentiation of CD4+ T cells into Th2 cells in healthy nonatopic subjects but fails to suppress already established Th2 cells in asthmatic patients.
The mechanism by which repeated or higher doses of IL-4 stimulation induces IL-27 resistance differs from the reported mechanism that enables differentiated Th2 cells to become insensitive to IFN-γ-mediated inhibition. Donald Leung and colleagues reported that atopic conditions suppressed IFN-γ receptor expression on peripheral blood CD4+ T cells 34. Both IL-2 and IL-4 have been shown to be essential in priming CD4+ Th2 cells 35, inducing steroid resistance in Th2 cells primed with IL-4 and IL-2 36, and also inducing Th2 cells to resist IL-10 and TGFβ through a MAPK pathway 37. We also show that IL-2 enhanced Th2 priming (Fig 3). Surprisingly, we found that IL-2 did not induce IL-27 resistance.
STAT1 is required for immunity against bacteria and viruses in mice 38. Humans deficient in STAT1 die of bacterial and viral infections at an early age 39, 40. We demonstrated that IL-27, a key cytokine in generating immunity against bacterial and viral infection, also depends on STAT1 to suppress Th2 cell differentiation. In fact, STAT1 is the target for IL-4-induced resistance to IL-27. In a previous report using human CD4+ T cells from peripheral blood, the authors concluded that Th2-inducing conditions downregulate STAT1 transcription 41. We did not find significantly downregulated expression of STAT1 protein. Instead, we found that repeated or higher doses of IL-4 stimulation, but not IL-2 stimulation, caused a reduction in IL-27-induced STAT1 phosphorylation.
It has been shown that patients with mutations in the STAT1 gene are susceptible to atypical mycobacterial infection and viral infection 39, 40 and that chronic infections with Mycoplasma pneumonia 42 and viral respiratory infections are found in some severe allergic asthmatic patients.43, 44 It is conceivable that the downregulation of STAT1 phosphorylation in severe allergic asthmatic patients might render them more susceptible to certain infections. Although we have shown that STAT1 signaling was impaired in all memory CD4+ T cells of allergic asthmatic patients, an actual link between the downregulation of STAT1 phosphorylation and susceptibility to atypical mycobacterial or viral respiratory infection requires further study.
SOCS3 can be induced by a variety of cytokines in a STAT-dependent or – independent manner 45. SOCS3 is known to inhibit STAT3, which is normally activated by IL-6, insulin or prolactin 46-48. However, in our experimental system, we did not find that STAT3 or STAT4 phosphorylation was inhibited. It has been reported that IL-4 induces SOCS3 expression in B cells and Th2 cells 49, 50. It was also reported that SOCS3 is essential in maintaining Th2-type inflammation50. Kubo and colleagues showed that SOCS3 suppressed IL-12-induced STAT4 phosphorylation but not IFN-γ-induced STAT1 phosphorylation, IL-2-induced STAT5 phosphorylation or IL-4-induced STAT6 phosphorylation 50. Our work, on the other hand, demonstrated that upregulated SOCS3 in Th2 cells was associated with suppressed IL-27-induced STAT1 phosphorylation but not IL-12-induced STAT4 phosphorylation. Furthermore, our work dissected the individual component of Th2-inducing conditions that contributes to IL-27 resistance and demonstrated that higher doses of IL-4 or repeated IL-4 stimulation is necessary for SOCS3 induction. Our analysis reveals a novel mechanism that explains how Th2-induced SOCS3 expression can maintain Th2 inflammation by resisting IL-27-mediated inhibition. Since allergic asthma is associated with preexisting Th2 cells, we predict that IL-27-based therapy will be more effective in preventing rather than treating allergic diseases.
METHODS
Human subjects
Allergic asthma was defined by the presence of asthma and multiple positive skin tests or at least one positive skin test for an environmental allergen with allergic symptoms. Exclusion criteria: 1) Pregnant or lactating; 2) Subjects with other comorbid respiratory diseases; 3) Asthmatic subjects taking systemic steroids or other systemic asthma drugs (e.g. Xolair, theophylline, zileuton). Each subject donated approximately 50 ml of peripheral blood. For human SOCS1 and SOCS3 mRNA and protein expression analysis, allergic asthmatic patients and healthy controls were enrolled by Dr. Zhihong Chen, the first author who returned to Fudan University in 2011, through clinics at the Department of Respiratory Medicine of Zhangshan Hospital, Fudan University, Shanghai, China. The ages and total IgE levels of study subjects are included in Table E1 and E2.
E Table II.
Patient information
| Number | Asthmatics or not | Age | Gender | Eos(0.4-8%) | IgE(<200IU/ml) | SPTa |
|---|---|---|---|---|---|---|
| 1 | Nb | 45 | m | 2% | NDc | − |
| 2 | N | 63 | m | 5.60% | 20 | − |
| 3 | N | 35 | m | 1% | ND | − |
| 4 | N | 22 | m | 1% | ND | − |
| 5 | N | 19 | m | 1.5% | 78 | − |
| 6 | N | 44 | f | 3% | ND | − |
| 7 | N | 52 | f | 8% | 25 | − |
| 8 | N | 65 | f | 3% | ND | − |
| 9 | N | 37 | f | 3.6% | ND | − |
| 10 | N | 23 | f | 0.8% | 57 | − |
| 11 | Yd | 52 | m | 14.30% | 2236 | + |
| 12 | Y | 38 | m | 2.20% | 82 | + |
| 13 | Y | 42 | m | 1% | 291 | + |
| 14 | Y | 35 | m | 16.00% | 379 | + |
| 15 | Y | 23 | m | 7.00% | 54 | + |
| 16 | Y | 31 | m | 5.40% | 79 | + |
| 17 | Y | 52 | f | 10% | 700 | + |
| 18 | Y | 60 | f | 5.50% | 3500 | + |
| 19 | Y | 50 | f | 12.50% | 170 | + |
| 20 | Y | 20 | f | 9.00% | 377 | + |
| 21 | Y | 27 | f | 3.50% | 125 | + |
| 22 | Y | 38 | f | 2.10% | 397 | + |
| 23 | N | 28 | m | 0.2% | ND | − |
| 24 | N | 40 | f | 2% | ND | − |
| 25 | Y | 38 | m | 2.20% | 29 | + |
| 26 | Y | 23 | f | 7% | 987 | + |
skin prick test
No
not determined
Yes
Human CD4+ T cell culture and IL-4 protein measurement
Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Hypaque (Histopaque-1077; Sigma-Aldrich) gradient centrifugation. CD4+ T cells were isolated from PBMCs by magnetic bead separation (MACS) using a human CD4+ T Cell Isolation Kit (Miltenyi Biotec). We routinely obtained greater than 90% CD4+ T Cell purity. CD4+ T cells (0.25 × 106) were cultured with irradiated T cell-depleted PBMCs (1 × 106) for 5-6 days in 1 ml of Yssel's medium containing 1% human serum AB (Gemini, Cat 400-13) under either neutralizing conditions or Th2-inducing conditions. Neutralizing conditions contain 0.5 μg/ml of anti-human CD3 antibody (clone OKT3, eBioscience), 1 μg/ml of anti-human CD28 antibody (clone CD28.6, eBioscience), 50 U/ml of recombinant human (rh) IL-2 (provided by NIH AIDS Research and Reference Reagent Program), 10 μg/ml of anti-human IFN-γ antibody (clone B27, BD Bioscience), and 10 μg/ml of anti-human IL-4 antibody (clone 3007, R&D Systems). Th2-inducing conditions contain 0.5 μg/ml of anti-human CD3 antibody, 1 μg/ml of anti-human CD28 antibody, 50 U/ml of rhIL-2, 10 μg/ml of anti-human IFN-γ antibody, and 15 ng/ml of rhIL-4 (Peprotech). In some cases (as indicated in the figure), CD4+ T cells were cultured under Th2-inducing conditions in the presence of 50 ng/ml of rhIL-27, 10 μg/ml of anti-human IL-10 antibody (clone JES3-19F1, BD Bioscience) or IgG2a as an isotype control for anti-human IL-10 antibody (BD Bioscience). For preparing T cell-depleted PBMCs, CD3+ T cells were depleted using biotin-conjugated anti-human CD3 (clone UCHT1, eBioscience) and magnetic separation. T cell-depleted PBMCs were irradiated with 2500 Rad before being used to crosslink anti-CD3 and anti-CD28 antibodies as a method of activating CD4+ T cells. After a six-day culture period, the resultant cells were washed to remove cytokines that were added to prime CD4+ T cells and then stimulated overnight with PMA (50 ng/ml) and ionomycin (1 μM) to induce cytokine protein synthesis. Supernatants were collected and IL-4 protein concentration was measured by ELISA (R&D Systems).
Priming of murine Th cells and intracellular staining of IL-4 and IFN-γ protein
Purified naïve CD4+ T cells (0.2 × 106 cells) were stimulated with irradiated spleen cells (1 × 106 cells) under low concentrations of Th2-inducing reagents, hereafter referred to as the Th2lo conditions, intermediate concentrations of Th2-inducing reagents (Th2int), or high concentrations of Th2-inducing reagents (Th2hi). The Th2lo conditions contains 1 ng/ml of rmIL-4, 50 U/ml of rhIL-2 (mouse cross reactive), 1.5 μg/ml of anti-mouse CD3 antibody, and 0.5 μg/ml of anti-mouse CD28 antibody; the Th2int conditions contain 5 ng/ml of rmIL-4, 100 U/ml of rhIL-2, 3.0 μg/ml of anti-mouse CD3 antibody, and 1μg/ml of anti-mouse CD28 antibody; and the Th2hi conditions contains 10 ng/ml of rmIL-4, 150 U/ml of rhIL-2, 6 μg/ml of anti-mouse CD3 antibody, and 1.5 μg/ml of anti-mouse CD28 antibody. More detailed information on the antibodies used has been described previously. E1 Naïve CD4+ T cells were primed for five days. Intracellular staining of IL-4 and IFN-γ was carried out as described previously. E1
Quantitative RT-PCR (qPCR) and Western blot analysis
cDNAs were prepared as described previously. E1 The amount of mRNA was expressed as the amount relative to that of HPRT (relative amounts = 2−ΔCT, where ΔCT = CTSample − CTHPRT) as described previously. E2 For Western blot analysis of STAT phosphorylation, cultured cells under cultured conditions indicated or SiRNA transfected cells in the figures or figure legends were washed and starved in medium alone without FBS or any cytokines for two hours. The starved cells were incubated with rmIL-27 (50 ng/ml), rmIL-3 (50 ng/ml) or rmIL-12 (10 ng/ml) in complete RPMI for 15 minutes. For Western blot analysis of SOCS protein, CD4+ T cells, cultured as described above, were used without medium starvation or cytokine stimulation. MACS isolated human peripheral CD4+ T cells were used without culture or cultured in complete Yssel's medium supplemented with 100 U/ml of rhIL-2 overnight (no difference in SOCS mRNA and protein expression was found in uncultured versus cultured human CD4+ T cells, data not shown). Lysates were prepared and separated in 4-12% SDS-PAGE. Western blot analysis was carried out as previously described. E3 The following antibodies were used: anti-STAT1 antibody (SC592, Santa Cruz Biotechnology, Santa Cruz, CA); anti-Py-STAT1 antibody (cat# 9171, Cell Signaling, Boston, MA); anti-STAT3 (cat# 4904, Cell Signaling); anti-Py-STAT3 antibody (cat# 9145, Cell Signaling) anti-STAT4 antibody (SC486, Santa Cruz Biotechnology); anti-Py-STAT4 antibody (cat# 5267, Cell Signaling); anti-mouse/human SOCS1 (cat# 3950s, Cell Signaling); and anti-mouse/human SOCS3 (cat# 2923s, Cell Signaling). ΔpSTAT1/STAT1= [pSTAT1 band intensity/STAT1 band intensity in IL-27 stimulated cells] – [pSTAT1 band intensity /STAT1 band intensity in not stimulated cells].
Intracellular staining of STAT phosphorylation
PBMCs were stained with APC-labeled anti-Human CD294 (CRTH2) antibody. Then, the stained cells were or were not stimulated with either rhIL-27 (50 ng/ml) or rhIL-4 (50 ng/ml) for 15 minutes. Cells were fixed with 10% formadehyde for 10 minutes and permeabilized with methanol for 30 minutes on ice. The treated cells were stained with FITC-labeled anti-phosphorylated STAT1 antibody, PE-labeled anti-human CD4 antibody, and PE-Cy7-labeled anti-human CD45RO antibody. FITC-labeled anti-phosphorylated STAT1 antibody was purchased from BD Bioscience (San Jose, CA). PE-labeled anti-human CD4 antibody (clone RPA-T4), PE-Cy7-labeled anti-human CD45RO antibody (clone UCHL1), and APC-labeled anti-Human CD294 (CRTH2) antibody (clone BM16) were purchased from Biolegend (San Diego, CA). Delta mean fluorescence intensity (ΔMFI) = [MFI of pSTAT1 or pSTAT6 staining in cells stimulated with either IL-27 or IL-4] – [MFI of pSTAT1 or pSTAT6 staining in cells not stimulated with IL-27 or IL-4].
siRNA knock down
Mouse naive CD4+ T cells were activated with Th2hi conditions. Twenty-fours later, cells (0.5 to 2 × 106) were collected and used for siRNA transfection by using the Nucleofector kit (Amaxa, Lonza Ltd, USA). E2 One hundred microliters of Nucleofector T cell solution and 300 nM of CONTROL siRNA or 300 nM of pooled Socs3 siRNA were transfected into the activated CD4+ T cells using the mouse CD4+ T cell X001 transfecting program. siRNA and control siRNA were ordered from Qiagen (Valencia, CA) and sequences are listed in Table E1 of the Online Repository. Aliquots of transfected cells were cultured under either Th2lo or Th2hi conditions for additional two days for knockdown efficiency analysis using real-time PCR. Aliquots of transfected cells were further cultured for an additional four days under either Th2lo or Th2hi conditions for pSTAT1/STAT1 and IL-4 protein analysis. For pSTAT1/STAT1 analysis, the resultant cells were starved and then either stimulated or not stimulated with mIL-27 as described above in the Western blot analysis. For IL-4 protein analysis, the resultant cells were stimulated win PMA and ionomycin. The concentration of IL-4 in the supernatants of stimulated cells was measured by ELISA.
ELISA analysis
At the end of cultures, T cells were washed and stimulated with PMA (50 ng/ml) and ionomycin (1μm) at a concentration of 106 cells per 1 ml of complete medium overnight. The IL-4 protein concentration in the supernatants was measured by using commercial ELISA detection kits (BD Bioscience). Percentage of reduction in IL-4 production = [IL-4 protein produced by Th2 cells] – [IL-4 protein produced by Th2 cells treated with IL-27]/ [IL-4 protein produced by Th2 cells] × 100.
Key messages.
● CD4+ T cells from asthmatic patients resisted IL-27-mediated suppression of IL-4 production
● Repeated or high-dose IL-4 stimulation induced SOCS3 mRNA expression and impaired STAT1 phosphorylation
● Elevated Socs3 mRNA expression and impaired STAT1 phosphorylation in the CD4+ T cells of asthmatics could explain why these cells are resistant to IL-27-mediated inhibition
Acknowledgements
We thank lab members for discussion; we are grateful to J.D. Ainsworth for helping with IRB protocols; J.D. Williams for technique assistance; Michael Hubbard for his help in preparing figures; and the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NAID, NIH for the generous supply of recombinant human IL-2.
Supported by grants from the National Institutes of Health RO1 AI068083 ARRA supplement (H.H.), RO1AI083986 (H.H.), a fund provided by National Jewish Health (H.H.), and a fund provided by National Science Foundation of China (No.81270078) (Z.H.C. and C.B.).
Abbreviations used
- PBMC
Peripheral blood mononuclear cell
- SOCS
Suppressor of cytokine signaling
- STAT
Signal transducer and activator transcription
- Neu
neutralized culture conditions
- Th2lo
low-stringent Th2-inducing conditions
- Th2int
intermediate-stringent Th2-inducing conditions
- Th2hi
high-stringent Th2-inducing conditions
- Th2m
memory Th2 cells
- Non-Th2m
memory non-Th2 cells, including Th1 and Th17 cells
- siRNA
small interfering RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest: The authors declare no conflicts of interest.
References
- 1.Huang H. Suppressing allergic immune responses. Front Biosci (Elite Ed) 2011;3:864–70. doi: 10.2741/E294. [DOI] [PubMed] [Google Scholar]
- 2.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–42. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 3.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, Jankovic D, Oler AJ, Wei G, Sharma S, Hu G, et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity. 2012;37:660–73. doi: 10.1016/j.immuni.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Usui T, Preiss JC, Kanno Y, Yao ZJ, Bream JH, O'Shea JJ, et al. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med. 2006;203:755–66. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finotto S, Neurath MF, Glickman JN, Qin S, Lehr HA, Green FH, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–8. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- 7.Raby BA, Hwang ES, Van Steen K, Tantisira K, Peng S, Litonjua A, et al. T-bet polymorphisms are associated with asthma and airway hyperresponsiveness. Am J Respir Crit Care Med. 2006;173:64–70. doi: 10.1164/rccm.200503-505OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhuang Y, Huang Z, Nishida J, Brown M, Zhang L, Huang H. A continuous T-bet expression is required to silence the interleukin-4-producing potential in T helper type 1 cells. Immunology. 2009;128:34–42. doi: 10.1111/j.1365-2567.2009.03049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–7. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 10.Taki S, Sato T, Ogasawara K, Fukuda T, Sato M, Hida S, et al. Multistage regulation of Th1-type immune responses by the transcription factor IRF-1. Immunity. 1997;6:673–9. doi: 10.1016/s1074-7613(00)80443-4. [DOI] [PubMed] [Google Scholar]
- 11.Lohoff M, Ferrick D, Mittrucker HW, Duncan GS, Bischof S, Rollinghoff M, et al. Interferon regulatory factor-1 is required for a T helper 1 immune response in vivo. Immunity. 1997;6:681–9. doi: 10.1016/s1074-7613(00)80444-6. [DOI] [PubMed] [Google Scholar]
- 12.Hida S, Tadachi M, Saito T, Taki S. Negative control of basophil expansion by IRF-2 critical for the regulation of Th1/Th2 balance. Blood. 2005;106:2011–7. doi: 10.1182/blood-2005-04-1344. [DOI] [PubMed] [Google Scholar]
- 13.Fukushima A, Yamaguchi T, Ishida W, Fukata K, Udaka K, Ueno H. Mice lacking the IFN-gamma receptor or fyn develop severe experimental autoimmune uveoretinitis characterized by different immune responses. Immunogenetics. 2005;57:337–43. doi: 10.1007/s00251-005-0805-3. [DOI] [PubMed] [Google Scholar]
- 14.Hu-Li J, Huang H, Ryan J, Paul WE. In differentiated CD4+ T cells, interleukin 4 production is cytokine-autonomous, whereas interferon gamma production is cytokine-dependent. Proc Natl Acad Sci U S A. 1997;94:3189–94. doi: 10.1073/pnas.94.7.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messi M, Giacchetto I, Nagata K, Lanzavecchia A, Natoli G, Sallusto F. Memory and flexibility of cytokine gene expression as separable properties of human T(H)1 and T(H)2 lymphocytes. Nat Immunol. 2003;4:78–86. doi: 10.1038/ni872. [DOI] [PubMed] [Google Scholar]
- 16.Murphy E, Shibuya K, Hosken N, Openshaw P, Maino V, Davis K, et al. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J Exp Med. 1996;183:901–13. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber JP, Ramos HJ, Gill MA, Farrar JD. Cutting edge: Type I IFN reverses human Th2 commitment and stability by suppressing GATA3. J Immunol. 2010;185:813–7. doi: 10.4049/jimmunol.1000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–24. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usui T, Nishikomori R, Kitani A, Strober W. GATA-3 suppresses Th1 development by downregulation of Stat4 and not through effects on IL-12Rbeta2 chain or T-bet. Immunity. 2003;18:415–28. doi: 10.1016/s1074-7613(03)00057-8. [DOI] [PubMed] [Google Scholar]
- 20.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–55. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 21.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–42. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 22.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–31. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 23.Owaki T, Asakawa M, Fukai F, Mizuguchi J, Yoshimoto T. IL-27 induces Th1 differentiation via p38 MAPK/T-bet- and intercellular adhesion molecule-1/LFA-1/ERK1/2-dependent pathways. J Immunol. 2006;177:7579–87. doi: 10.4049/jimmunol.177.11.7579. [DOI] [PubMed] [Google Scholar]
- 24.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 25.Artis D, Villarino A, Silverman M, He W, Thornton EM, Mu S, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–34. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J Immunol. 2007;179:4415–23. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 27.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100:15047–52. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–42. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 29.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921–9. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stritesky GL, Muthukrishnan R, Sehra S, Goswami R, Pham D, Travers J, et al. The transcription factor STAT3 is required for T helper 2 cell development. Immunity. 2011;34:39–49. doi: 10.1016/j.immuni.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neufert C, Becker C, Wirtz S, Fantini MC, Weigmann B, Galle PR, et al. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–16. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang Y, Huang Z, Nishida J, Zhang L, Huang H. Signaling pathways that lead to the silencing of the interleukin-4-producing potential in Th1 cells. J Interferon Cytokine Res. 2009;29:399–406. doi: 10.1089/jir.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–34. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung DY, Gao PS, Grigoryev DN, Rafaels NM, Streib JE, Howell MD, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-gamma response. J Allergy Clin Immunol. 2011;127:965–73. e1–5. doi: 10.1016/j.jaci.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Immunol. 2008;181:2943–51. [PubMed] [Google Scholar]
- 36.Kam JC, Szefler SJ, Surs W, Sher ER, Leung DY. Combination IL-2 and IL-4 reduces glucocorticoid receptor-binding affinity and T cell response to glucocorticoids. J Immunol. 1993;151:3460–6. [PubMed] [Google Scholar]
- 37.Liang Q, Guo L, Gogate S, Karim Z, Hanifi A, Leung DY, et al. IL-2 and IL-4 stimulate MEK1 expression and contribute to T cell resistance against suppression by TGF-beta and IL-10 in asthma. J Immunol. 2010;185:5704–13. doi: 10.4049/jimmunol.1000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Shea JJ, Gadina M, Kanno Y. Cytokine signaling: birth of a pathway. J Immunol. 2011;187:5475–8. doi: 10.4049/jimmunol.1102913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dupuis S, Dargemont C, Fieschi C, Thomassin N, Rosenzweig S, Harris J, et al. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science. 2001;293:300–3. doi: 10.1126/science.1061154. [DOI] [PubMed] [Google Scholar]
- 40.Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet. 2003;33:388–91. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- 41.Elo LL, Jarvenpaa H, Tuomela S, Raghav S, Ahlfors H, Laurila K, et al. Genome-wide profiling of interleukin-4 and STAT6 transcription factor regulation of human Th2 cell programming. Immunity. 2010;32:852–62. doi: 10.1016/j.immuni.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Kraft M, Cassell GH, Henson JE, Watson H, Williamson J, Marmion BP, et al. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158:998–1001. doi: 10.1164/ajrccm.158.3.9711092. [DOI] [PubMed] [Google Scholar]
- 43.Dulek DE, Peebles RS., Jr. Viruses and asthma. Biochim Biophys Acta. 2011;1810:1080–90. doi: 10.1016/j.bbagen.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peebles RS, Jr., Hartert TV. Respiratory viruses and asthma. Curr Opin Pulm Med. 2000;6:10–4. doi: 10.1097/00063198-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–76. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz J, Weissenbach M, Haan S, Heinrich PC, Schaper F. SOCS3 exerts its inhibitory function on interleukin-6 signal transduction through the SHP2 recruitment site of gp130. J Biol Chem. 2000;275:12848–56. doi: 10.1074/jbc.275.17.12848. [DOI] [PubMed] [Google Scholar]
- 47.Cohney SJ, Sanden D, Cacalano NA, Yoshimura A, Mui A, Migone TS, et al. SOCS-3 is tyrosine phosphorylated in response to interleukin-2 and suppresses STAT5 phosphorylation and lymphocyte proliferation. Mol Cell Biol. 1999;19:4980–8. doi: 10.1128/mcb.19.7.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–5. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 49.Canfield S, Lee Y, Schroder A, Rothman P. Cutting edge: IL-4 induces suppressor of cytokine signaling-3 expression in B cells by a mechanism dependent on activation of p38 MAPK. J Immunol. 2005;174:2494–8. doi: 10.4049/jimmunol.174.5.2494. [DOI] [PubMed] [Google Scholar]
- 50.Seki Y, Inoue H, Nagata N, Hayashi K, Fukuyama S, Matsumoto K, et al. SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses. Nat Med. 2003;9:1047–54. doi: 10.1038/nm896. [DOI] [PubMed] [Google Scholar]
- E1.Zhuang Y, Huang Z, Nishida J, Brown M, Zhang L, Huang H. A continuous T-bet expression is required to silence the interleukin-4-producing potential in T helper type 1 cells. Immunology. 2009;128:34–42. doi: 10.1111/j.1365-2567.2009.03049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Qi X, Nishida J, Chaves L, Ohmori K, Huang H. CCAAT/enhancer-binding protein alpha (C/EBPalpha) is critical for interleukin-4 expression in response to FcepsilonRI receptor cross-linking. J Biol Chem. 2011;286:16063–73. doi: 10.1074/jbc.M110.213389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Huang Z, Xin J, Coleman J, Huang H. IFN-gamma suppresses STAT6 phosphorylation by inhibiting its recruitment to the IL-4 receptor. J Immunol. 2005;174:1332–7. doi: 10.4049/jimmunol.174.3.1332. [DOI] [PubMed] [Google Scholar]