Abstract
Recent studies on the hippocampus and the prefrontal cortex have considerably advanced our understanding of the distinct roles of these brain areas in the encoding and retrieval of memories, and of how they interact in the prolonged process by which new memories are consolidated into our permanent storehouse of knowledge. These studies have led to a new model of how the hippocampus forms and replays memories and how the prefrontal cortex engages representations of the meaningful contexts in which related memories occur, as well as how these areas interact during memory retrieval. Furthermore, they have provided new insights into how interactions between the hippocampus and prefrontal cortex support the assimilation of new memories into pre-existing networks of knowledge, called schemas, and how schemas are modified in this process as the foundation of memory consolidation.
Introduction
Our marvelous capacity to record everyday experiences and retrieve these memories much later in all manner of situations relies on two kinds of information processing. First, during learning, the brain must rapidly form an initial neural representation of the new experience. Second, the brain then must consolidate the new representation in an organization that is optimized for retrieval when cued by a stimulus that may be just distantly associated to a feature of the initial experience. An enormous amount of research has led to a general understanding of how the brain forms memory traces for a novel event. More recently substantial progress has been made in discovering how the brain organizes new memories into networks of knowledge that can be accessed flexibly in a range of circumstances. Here, we will outline some of the evidence on how the brain supports these memory processes. We will begin by outlining the brain's memory system, and then focus on two key brain structures — the hippocampus and the prefrontal cortex — that support rapid encoding of new information and consolidation and organization of memory networks.
The memory system of the brain
Consideration of the functional anatomy of the brain system that supports memory for everyday events provides preliminary insights about how the brain encodes, organizes, and retrieves memories (Figure 1) [1]. Information about objects and events that we experience, and about the places where they occur, are processed separately in the cerebral cortex. Thus, multiple sensory pathways — for vision, touch, hearing, and so on — initially process information about the identity of perceptual objects and events and their outputs, and then converge onto multimodal cortical ‘association’ areas. We will refer to these pathways as the stream of information flow for ‘what’ we remember. There is also a distinct stream of pathways involving several areas of the cerebral cortex that identify ‘where’ in space events occur. Information processed through these distinct streams is sent to the medial temporal lobe (MTL), a region critically involved in event memory. In particular, the perirhinal cortex and the lateral entorhinal area are engaged by specific object stimuli and signal the familiarity of those items, whereas the parahippocampal cortex and the medial entorhinal area are involved in processing the spatial contexts in which memorable events occur. Within the MTL, the ‘what’ and ‘where’ information streams then converge at the level of the hippocampus. As a natural consequence of this anatomical organization, the hippocampus is essential for forming cohesive memories of individual events within the context in which they occurred [2,3].
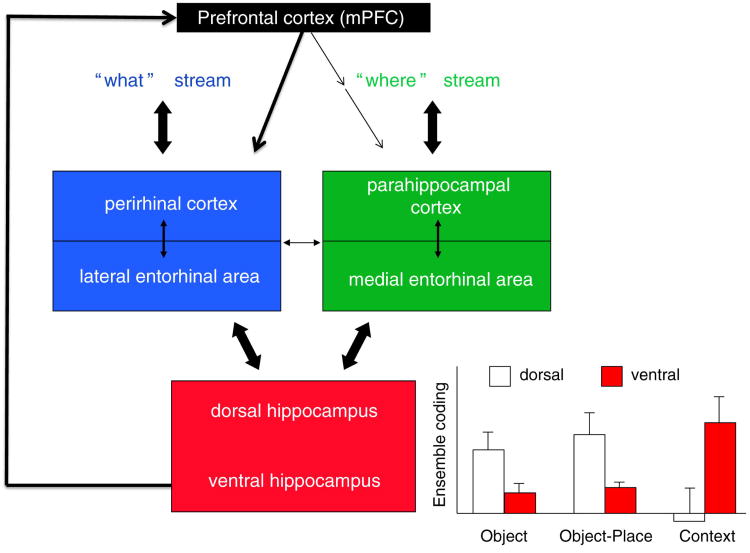
Figure 1. Pathways of information flow in between the hippocampus and prefrontal cortex.
Perceptual information about objects and events are initially processes in pathways for specific sensory modalities (vision, hearing, touch, olfaction) which project to multiple ‘association’ cortical areas (in temporal, parietal, and other cortical areas) that compose the ‘what’ stream of cortical processing that leads into perirhinal and lateral entorhinal cortex (blue). Information about ‘where’ in space events occur is processed in a separate cortical stream (including posterior parietal, retrospenial, and other cortical areas) that lead into the parahippocampal and medial entorhinal cortex (green) [1]. These streams then converge in the hippocampus. There, in the dorsal (animals) or posterior (humans) hippocampus, neural ensembles encode specific objects and the locations they occur within a context [43]. By contrast, neural ensembles in the ventral (animals) or anterior (humans) hippocampus link events within a context and strongly distinguish between different contexts. Contextual representations from the ventral/anterior hippocampus are sent directly to the medial prefrontal cortex [6], which is positioned to influence the retrieval of specific object representations via its particularly strong connections to perirhinal and lateral entorhinal cortex. (Graph adapted with permission from [43].)
Outputs of the hippocampus return to the cortical areas from which inputs arose — perirhinal–lateral entorhinal cortex, and parahippocampal–medial entorhinal cortex. These feedback pathways allow the hippocampus to support the retrieval of information about ‘what’ occurred based on a cue about ‘where’ an event occurred, and vice versa. As a consequence, hippocampal processing supports the retrieval of detailed memories that constitute strong recollective experiences in humans [1,4] and recollection-like memory in animals [5].
Furthermore, a part of the hippocampus (the ventral part in rats; anterior in humans) sends outputs to the medial prefrontal cortex (mPFC); as will be discussed below, new evidence suggests that mPFC accumulates information about the context of interrelated memories. Outputs of the mPFC are sent back to the perirhinal and lateral entorhinal cortex, by which the mPFC may bias or select the retrieval of event information in the ‘what’ stream [6]. There is also a subcortical route through the thalamus (nucleus reuniens) directly to the hippocampus, by which the prefrontal cortex could control the specificity of memory retrieval [7]. Thus, interactions between the prefrontal cortex and hippocampus may support the ability to create contextual representations that link related memories, and use these contextual representations to retrieve the memories that are appropriate within a given context.
There are many components of this brain system that make distinct contributions to its operation, but in the sections that follow we shall focus in on just two areas and their roles: the hippocampus and its critical role in rapid encoding; and the mPFC and its interactions with the hippocampus that are central to the organization, consolidation, and flexible retrieval of memories.
How does the hippocampus contribute to memory formation and consolidation?
The pioneering studies on the patient H.M. showed definitively that the hippocampus and neighboring structures of the medial temporal lobe are essential to memory [8]. H.M. suffered from severe anterograde amnesia following surgical removal of most of his MTL bilaterally in an attempt to cure his intractable epilepsy. The early observations and succeeding work on H.M. have provided compelling evidence that structures within the MTL play a selective role in declarative memory, our everyday memory for facts and events. Specifically, the hippocampus is essential for the rapid formation of new memories and for the prolonged process of consolidating newly acquired memories into our permanent storehouse of autobiographical and world knowledge in the neocortex [9–11]. The studies on H.M. inspired over a half-century of research detailing how the hippocampus and other MTL areas support memory and how they interact with areas of the neocortex in the process of consolidation [12].
At the level of neuronal networks, hippocampal principal cells in rodents — the well-known ‘place cells’ — activate as animals occupy specific locations in an environment. Furthermore, associated with the formation and retrieval of memories, these cells also encode relevant objects and behavioral events at particular places ([13,14]; reviewed in [1]). Recent studies have also revealed that, in addition to a mapping of spatial context, hippocampal neurons map the temporal organization of experiences in ensembles of ‘time cells’ that fire at sequential moments in temporally ordered episodes [15-17]. Such hippocampal time cells may be part of a mechanism underlying the essential role of the hippocampus in memory for the order of events in everyday experiences [18].
This spatial and temporal organization reflected in the response patterns of individual hippocampal neurons not only provides a coding scheme to support the rapid acquisition of individual event memories, but also allows for the mental replay of spatio-temporal sequences at later points in time. Such hippocampal replay of experiences has been hypothesized to underlie the essential role of this structure in both recollection and memory consolidation (for example [19]). Consistent with this expectation, hippocampal neural ensembles have been shown to replay place cell firing sequences during sleep and quiet waking periods following learning in rodents [20], and blocking this replay prevents subsequent memory retrieval [21].
Sequence replay is also magnified in the hippocampus before critical memory-driven responses in well-learned environments [22]. Notably, hippocampal replay is coordinated with corresponding replay events in the neocortex [19] and is accompanied by strong synchronization of neural activity between the hippocampus and prefrontal cortex [23–25]. Leading theories suggest that such coordinated hippocampal–neocortical replay is a key mechanism of memory consolidation. But before exploring in detail how interactions between hippocampus and parts of prefrontal cortex contribute to consolidation, it is important to consider evidence for how information originating in prefrontal cortex may support the retrieval of memories in the hippocampus.
Prefrontal cortex–hippocampus interactions and mechanisms of memory retrieval
While some researchers emphasize the pathway from the hippocampus to the mPFC as supporting memory consolidation (as introduced just above), there is considerable converging evidence that the prefrontal cortex contributes to memory through cognitive or strategic control over memory retrieval processes within other brain areas [26–32]. Notably, the prefrontal cortex is composed of several functionally distinct areas. Here, we will sometimes refer to prefrontal cortex in general, but often we will focus specifically on the mPFC, which appears to be particularly involved with memory retrieval and consolidation.
Patients with prefrontal damage do not have severe impairments in standard tests of event memory, but deficits resulting from prefrontal damage are apparent when memory for target information must be obtained under conditions of memory interference or distraction. For example, individuals with prefrontal damage demonstrate impaired performance on the classic A–B, A-C problem [33]: following successful learning of a set of paired associations (A-B), prefrontal patients are severely impaired in learning new associates of original elements (A-C), and the impairment is marked by intrusions of the original associations. Even when learning two lists of unrelated associations, in prefrontal patients, memory for one list is compromised by intrusions from the other, suggesting that the prefrontal cortex controls memory retrieval by selecting memories relevant to the current context and suppressing irrelevant memories [34]. What is the nature of prefrontal information processing and how does prefrontal cortex interact with the hippocampus in support of memory retrieval?
One influential theory of prefrontal cortex function proposes that prefrontal cortex–hippocampus interactions may be best understood by allusion to a railroad metaphor, in which the hippocampus is responsible for laying down new tracks, whereas the prefrontal cortex is responsible for flexibly switching between tracks [28]. In the application of this metaphor to memory, the hippocampus is viewed as forming and retrieving specific memories, while the prefrontal cortex accumulates features of related memories that compose the ‘context’ of a set of connected experiences, such as a list in which a set of words appeared, a common location where several events occur, or a common set of ongoing task rules that govern multiple memory decisions. When subsequently cued to a context, the prefrontal cortex is viewed biasing the retrieval of context-appropriate memories in the hippocampus as well as other brain areas.
In support of this view, several studies have shown that damage to mPFC impairs the ability of rats (or monkeys or humans [35]) to switch between remembering different perceptual dimensions of compound stimuli (for example [36]), to switch between remembering a ‘place’ or ‘response’ strategy for solving a spatial memory tasks [37], and to switch between remembering ‘odor’ or ‘place’ memories [38]. These findings indicate that the mPFC plays a very active role in guiding memory retrieval by using the relevant context to resolve conflicting information in the retrieval of related and competing memories.
Complementary studies on neuronal activity patterns in mPFC support the idea that the prefrontal cortex acquires representations of behavioral contexts that determine appropriate memory retrieval. Thus, neuronal ensembles in mPFC fire distinctly in different behavioral contexts [39], reset when animals are uncertain as a result of a change in contingencies [40], and make abrupt transitions between contextual representations when contingencies change [41]. Related to the strategy switching experiments described above, mPFC neuronal activity patterns were found to predict switching between remembering ‘place’ and ‘response’ strategies in the domain of spatial memory [42].
Related recent evidence suggests that the mPFC employs these contextual representations to control the retrieval of detailed memories in the hippocampus. Thus, when rats use either of two spatial contexts to guide retrieval of otherwise contradictory object–reward associations, neurons in the dorsal part of the hippocampus encode these memories as selective firing to specific objects in particular places in each spatial context [14] (Figure 1). When the mPFC is inactivated, dorsal hippocampal neurons indiscriminately retrieve both appropriate and inappropriate object memory representations [6]. This finding indicates that the hippocampus is capable of retrieving memories even in the absence of mPFC input, and the role of the mPFC is to select the appropriate memory for that context. A likely pathway by which the prefrontal cortex controls memory retrieval in the dorsal hippocampus is via prefrontal projections to the perirhinal and lateral entorhinal cortex, where object representations are processed and sent onto the hippocampus (Figure 1).
Furthermore, recent evidence suggests there is a bidirectional flow of information between the mPFC and hippocampus, wherein the events that initiate prefrontal control over memory retrieval in the hippocampus may arise in the ventral part of the hippocampus (Figure 1). Thus, in the same experimental protocol where dorsal hippocampal neurons encode specific events in particular places in each of two spatial contexts, ventral neurons gradually acquire generalized representations of all events that comprise one of the contexts and neural ensembles in the ventral hippocampus outperform those in the dorsal hippocampus in discriminating between the contexts in which events occurred ([43]; Figure 1). Consistent with this observation, functional imaging studies in humans indicate that the anterior hippocampus in humans (the human analog of ventral hippocampus in rodents) creates more general representations of the salience of events, whereas the posterior (equivalent to dorsal in rats) hippocampus distinctly represents the information content within individual events [44]. Similarly, the anterior hippocampus is differentially activated during the retrieval of the general context of memories, whereas the posterior hippocampus is differentially activated during retrieval of specific events in memories. These and several related findings have led to the recent proposal that the dorsal–ventral (posterior–anterior in humans) axis of the hippocampus may contain a topographic representation of more general features of memories [43,45].
Notably, the ventral hippocampus projects directly to the mPFC [46], providing a powerful and immediate route for hippocampal representations of meaningfully distinct spatial contexts to arrive in the prefrontal cortex. This observation, combined with findings described above, suggests a model of bidirectional hippocampus–prefrontal cortex interactions that support memory encoding and context-dependent memory retrieval (Figure 1). According to this scenario, closely related events that occur within a single context, as well as environmental cues that define the context, are processed by the ventral (in rats) and anterior (in humans) hippocampus as a collection of features and events that define the particular context where those events occur. This context-defining information is sent via the direct projections to mPFC where neural ensembles develop distinct representations that can distinguish contextual ‘rules’ during the course of learning. When subjects are subsequently put in the same context, ventral hippocampal signals carrying the contextual information are again sent directly to mPFC which then engages the appropriate rule and applies the rule to engage the context appropriate representations in the dorsal (in rats) and posterior (in humans) hippocampus, while also suppressing context inappropriate memories.
This model provides a framework for understanding the retrieval of context-appropriate hippocampal memory representations as a key process in everyday remembering. This notion of context-appropriate retrieval may also be relevant to the process of memory consolidation, to the extent that consolidation requires context-guided retrieval of previously acquired memories as a part of integrating new memories with pre-existing knowledge, as some have argued (see below) [47]. A major goal of this review is to outline how context-appropriate retrieval supports the integration of new and old memories. But, before expanding into that process, we will first consider views on the nature of memory consolidation as an integration of related memories.
Memory consolidation involves the formation of memory networks
For hundreds of years, it has been observed that brain injury can result in the loss of memories laid down during a limited period prior to the injury, leading to the idea that memories require a period of consolidation before they become immune to disruption. Historically, there have been several ideas about the nature of memory consolidation. Some have viewed the process of consolidation as a direct transfer of memories initially encoded in the hippocampus to replicates of those memories in the cortex. But there is now considerable evidence that new memories are not simply transferred to the cortex, but rather assimilated into neocortical memory networks, called ‘schemas’, through elaboration and modification of the network structure (Box 1; reviewed in [48,49]). Here, we will discuss the nature of schemas and the role of the hippocampus in schemas. In the following sections, we will suggest that schemas compose a ‘context’ of related memories, and that the prefrontal mechanisms introduced above with regard to context-guided memory retrieval also play a key role in schema development and updating.
Box 1. What is a schema?
Schemas were introduced to cognitive psychology by Piaget [51] and Bartlett [76] in their efforts to understand how new information is integrated with pre-existing knowledge. Piaget argued that humans possess structured mental representations embodied as organizations of related associations called schemas. During development, new events are interpreted in terms of existing schemas. When those events are consistent with an activated schema, they are assimilated into the mental structure. When new events challenge the existing schema, the interpretation of the events themselves may be modified to fit the schema and assimilated. Consistent with this characterization of assimilation, Bartlett's study on recall of a surreal story showed that story elements that were consistent with his subjects' culture were better remembered, and that elements inconsistent with that culture were lost or modified to fit their pre-existing conceptions. Alternatively, Piaget proposed, learning may demand that the schema itself is modified to accommodate new, conflicting information. Assimilation of new information and accommodation of schema structure continuously interact and ultimately reach an equilibration that adapts schemas to be consistent with external reality. Another key element of Piaget's theory was that organized mental structures support inferences, the ability to make logical deductions about relations between elements within schemas that are only indirectly related.
One key issue in defining a schema is the extent to which its contents include only the abstraction of common elements among assimilated events or whether a schema is any network of related events, including details that are unique to specific events. Several studies consider a schema to be the abstract ‘gist’ of knowledge derived by extraction of regularities and loss of more idiosyncratic aspects of each event. The loss of information unique to particular events could occur merely because that information is less practiced [77] or because such loss is inherent to the structure and function of semantic memory [78], consistent with the semantic transformation hypothesis of consolidation [72].
In contrast to the notion of schemas as containing only abstractions of common elements of events as characterized by the semantic transformation hypothesis, several recent approaches in cognitive neuroscience have examined the development of organized mental representations of specific experiences that contain details of individual events and share the properties of schemas. Thus, for example, prior training on scenes in a movie in humans (for example, [78]) and learning a set of flavor-place associations in rats [56] facilitates subsequent memory for details of new events that fit the existing knowledge. Furthermore, learning of small sets of overlapping associations (for example, A is associated with B, and B is associated with C) can result in the formation of a network of memories in which the features common to multiple events link indirectly related event content. These memory networks share some of the properties of schemas in that they support the ability to make inferences between indirectly related elements (A is associated with C) in both humans [47] and animals [52].
Work focused on organized mental representations has been incorporated into recent studies on memory consolidation during sleep [79,80] and has shown critical roles for the hippocampus and prefrontal cortex. In addition, it has been suggested that the phenomenon of ‘reconsolidation’, additional rounds of consolidation of previously consolidated memories following new related experiences, reflects Piaget's ‘accommodation’ of a schema to incorporate novel information [48]. Furthermore, this approach can illuminate how hippocampal and prefrontal areas interact in the development of higher order, conceptual schemas that support decision-making in novel perceptual settings [65]. These studies provide insights into the mechanisms that underlie the development of schemas and how they support memory expression in new situations.
Following the latter prominent approach in current research, here we adopt the view that a schema is any organized network of overlapping representations that has the following properties: first, new information is better remembered when it fits within a pre-existing schema; second, new information that challenges schema organization may cause modification of the existing schema or development of a new schema; and third, schemas support novel inferences between indirectly related events and their generalization to new situations.
In an early account of consolidation as schema formation and updating, McClelland et al. [50] proposed that new memories are initially represented within the hippocampus, and that during the course of consolidation they become interleaved into a network of existing related memories in the neocortex. This interleaving process incorporates new memories and typically requires modification of the pre-existing network structure to add the new memories, consistent with Piaget's [51] views on assimilation of new information and accommodation of existing schema structure to integrate the new information. While there are differing views of what constitutes a schema (see Box 1), current studies in cognitive neuroscience use simplified behavioral models of schemas wherein animal or human subjects learn a set of related memories and the development of a schema is identified by the ability to make novel judgments about items that are related indirectly within the schema structure (Box 1). These studies have shown that hippocampus plays a critical role in the interleaving of memories into a schematic organization in mice, rats, and monkeys [52–55].
In some of these studies, animals learn multiple stimulus associations that share overlapping elements. For example, in one paradigm known as associative inference (Figure 2A), animals learn to associate pairs of overlapping stimuli: for example, A is associated with B, B is associated with C. The existence of an integrated organization is demonstrated by the ability of subjects to express knowledge of relations between indirectly linked elements —in the associative inference paradigm just mentioned, that A is associated with C — a key property of schemas (Box 1). Animals with damage to the hippocampal system can learn the individual associations, but fail on the inferential expression test (Figure 2B), showing that schema development or expression depends on the hippocampus.
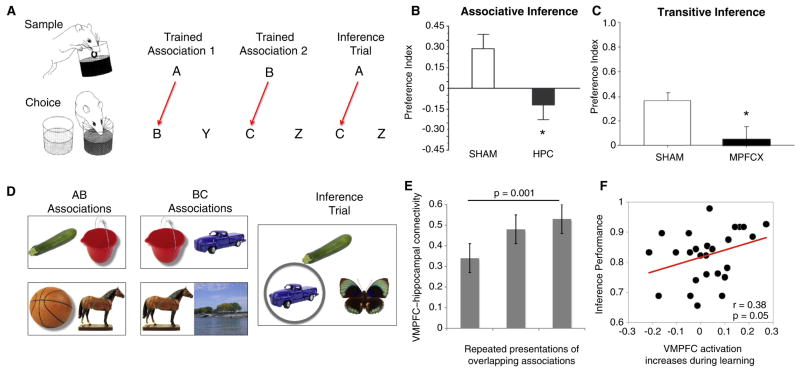
Figure 2. Hippocampal and prefrontal contributions to inference in rodents and humans.
(A) Associative inference paradigm in rodents. During the sample phase of a training trial, rats are presented with a cup containing a scented mixture of sand with a buried reward. In the choice phase, two scented choices are presented, but only one was baited with a reward. The identity of the rewarded items is dependent on the identity of the sample; for example, odor B would be the correct choice when odor A was the sample. The training associations consist of two overlapping sets of associations (A–B, B–C). During inference trials, rats must infer the relationship between A and C to determine which odor is the correct choice. (B) Rats with lesions to the hippocampus (HPC) show impaired inference [52]. (C) Lesions to the medial prefrontal cortex (MPFCX) also impair inference in a related transitive inference paradigm [60]. (D) Associative inference paradigm in humans. Individuals are trained on interleaved presentations of overlapping AB (for example., basketball-horse) and BC (horse-lake) associations during fMRI scanning and are then tested on the inferential relationships between A and C. (E) Functional interactions between ventromedial prefrontal cortex and hippocampus increase across repeated presentations of overlapping associations. (F) Increasing engagement of ventromedial prefrontal cortex during learning is related to superior inference. Panel A-B adapted with permission from [52]; panel C adapted with permission from [60]; panel D-F adapted with permission from [47].
In other experiments that examined the role of schema development and the hippocampus in memory consolidation, Tse et al. [56] trained rats to learn the locations where different flavored foods could be found in an open field. After those food-location associations were well learned (that is, a schema had been formed), new associations could be acquired very rapidly, in as little as a single trial, and retained over 24 hours. This study further demonstrated the existence of a schema for the food-location memories by showing that the acquisition of new associations was not facilitated by prior food-association learning in a different environment. Furthermore, learning food-location associations was dependent on the hippocampus, and hippocampal lesions made within three hours, but not 24 or 48 hours, of acquisition blocked retention, indicating rapid systems consolidation of new information into an existing schema dependent on the hippocampus.
The McClelland et al. [50] model and the work on associative and transitive inference, combined with the Tse et al. [56] experiment, show that memories are not acquired in isolation, but rather are interleaved with previously acquired related information, and that schema development and updating plays a central role in hippocampal-dependent memory and memory consolidation (reviewed in [48]). Next, we extend this hypothesis to incorporate the prefrontal cortex, which we will suggest plays a central role in the development and updating of schemas during consolidation.
The role of prefrontal cortex in schema development and memory consolidation
We have argued above that the prefrontal cortex plays a critical role in using context to guide the retrieval of memories, and that memory consolidation involves the hippocampus-dependent integration of new memories into existing memory networks. Here, we will link these two arguments by proposing that context-guided retrieval of the appropriate memory network, supported by the prefrontal cortex, is a key step in integrating new memories into the network.
There are currently divergent findings on the role of prefrontal cortex in memory consolidation and schema updating. Some studies have employed imaging of immediate early gene activation, which reflects neural activity, to identify brain areas engaged during the retrieval or recently and remotely acquired memories. These studies reported that the mPFC is activated during retrieval of remotely, but not recently, acquired contextual fear memories. Consistent with these findings, in the same behavioral paradigm, inactivation of medial prefrontal areas impaired retrieval of remote but not recently acquired memories [57–58], leading these investigators to suggest that mPFC comes to play a key role in organizing widespread neocortical memory representations during remote memory retrieval, much the same as the hippocampus serves this function during the consolidation period [59].
Contrary to the view that mPFC is involved only in the retrieval of remote memories, however, other studies have reported that the development of schemas into which new memories are incorporated relies on the mPFC from the outset of learning. In the transitive inference paradigm, mPFC damage slows the learning of overlapping stimulus pairs and blocks inferential expression of memories, indicating that the prefrontal cortex is critical to schema development as well as expression ([60]; Figure 2C). In studies on the food-location schema paradigm, medial prefrontal areas were strongly activated (as observed by immediate early gene activation) during new learning events that overlapped with an existing schema, and functional compromise of medial prefrontal areas impaired retrieval of both recently and remotely acquired new memories [61,62].
These findings are paralleled by a recent report [63] that, in addition to the hippocampus, the human homologue of mPFC, the ventromedial prefrontal cortex, is engaged in retrieval of both recently and remotely acquired autobiographical memories in humans. Together, the findings on rodents and humans challenge the idea that the mPFC becomes essential only at the conclusion of consolidation and instead argue that the prefrontal cortex is importantly involved during learning and expression of memories at all periods.
How might the strategic memory processing by the prefrontal cortex described above with regard to memory retrieval contribute to memory consolidation as assimilation and modification of schemas? When new experiences occur, they usually conflict in some way with pre-existing associations. For example, in the associative inference paradigm, the pre-existing association between B and A is challenged when the subject newly learns that B is now also (or instead) associated with C. In the food-location paradigm, when rats learn the places to find new flavors, the prior associations of those locations with no food must be replaced with the new flavor-place associations. So, integration of new information into a pre-existing schema goes hand-in-hand with some degree of accommodation of the schema to integrate the new information. We suggest that the strategic role of prefrontal cortex during memory formation and consolidation is the accommodation process itself, which enables conflicts between pre-existing schemas and new events to be resolved through schema modification. Such a role in conflict resolution might extend to current views on semantic transformation, if we consider that abstraction of common elements among memories can also be in service of resolving conflicts between different associations of the common elements within different memories.
Prefrontal cortex–hippocampus interactions during schema formation and expression in humans
Further insights into how the prefrontal cortex exerts its control during memory formation and consolidation can be gained by considering the relevant roles of the prefrontal cortex and the hippocampus in human neuroimaging studies that index schema generation and expression. For instance, recent work has shown that, when learning about overlapping pairs in the associative inference paradigm (Figure 2D), participants reinstate memories for prior associations (such as the A stimulus from an A–B pairing), presumably via a mechanism like hippocampal replay, while encoding new related experiences (B–C pairs), revealing the conflict between new learning and existing memories in this task [47]. The reinstatement/replay of prior events during encoding of new associations is accompanied by an increase in ventromedial prefrontal-hippocampal coupling (Figure 2E). Moreover, the degree of activation in these regions during presentation of overlapping associations predicts successful expression of inferential memories. In particular, increased recruitment of the ventromedial prefrontal cortex in the presence of increased mnemonic conflict is associated with superior inference (Figure 2F).
These findings suggest an increased need for ‘top-down’ prefrontal control of hippocampal encoding processes to resolve the conflict between existing memories and new events as they are learned. The relationship between ventromedial prefrontal activation and inference expression further suggests that, in this case, competition was resolved through the formation of an integrated memory schema that accommodated the shared relationships among A, B, and C, leading to enhanced performance on judgments about the unobserved relationship between A and C.
Other recent research has revealed that ventromedial prefrontal interactions with hippocampus are sustained during rest periods following schema formation [64], consistent with the notion that offline replay of task-evoked activation patterns in these brain regions facilitates the consolidation of newly-formed schemas. Successful expression of schemas is also associated with enhanced interaction between hippocampus and ventromedial prefrontal cortex. For example, correct performance on decisions requiring the use of a recently acquired conceptual rule is associated with increased connectivity between hippocampus and ventromedial prefrontal cortex [65]. Collectively, these human neuroimaging studies converge with rodent research to demonstrate that the hippocampus and ventromedial prefrontal cortex interact during schema formation, consolidation and expression.
Further questions
There remain several open questions regarding the time course of hippocampal and prefrontal involvement in memory. For instance, most animal and human studies have examined brain processes related to post-encoding consolidation and schema expression either immediately or at relatively short time periods after learning, and thus do not provide a strong test of the theories about the roles of the hippocampus and prefrontal cortex in these processes over time. Further cross-species research at longer timescales will be necessary to differentiate the models of consolidation presented here.
One recent study in rodents has addressed how spatial memory representations evolve over an extended period after new learning [66]. In this study, the nature of hippocampal contributions to schema updating was explored by monitoring firing patterns of multiple hippocampal neurons as rats learned new goal locations in an environment in which they already learned the locations of multiple goals. Prior to new learning, many neurons fired at multiple goals but with different subsets of these neurons active at different goals and distinct patterns of activation. These observations suggest that the hippocampal network developed a neural representation that both linked distinct goals and distinguished them within an overall scheme of the spatial environment. During new learning, some neurons began to fire as animals approached the new goals. These neurons were largely the same neurons that already fired at the original goals, and these cells exhibited activity patterns at new goals similar to those associated with the original goals, consistent with Piaget's views on a rapid assimilation of new related memories.
Learning of new goals also produced changes in the pre-existing goal related firing patterns, with some cells firing at different rates or dropping out of the representation, consistent with Piaget's notion of accommodation. Furthermore, in the days after learning, activity patterns associated with the new and original goals gradually diverged, such that the initial generalization of new goal related firing patterns was followed by a prolonged period in which new memories became distinguished within the ensemble representation. These findings support the view that consolidation involves rapid assimilation of new memories into pre-existing neural networks that require a prolonged time to accommodate relationships among new and existing memories. The role of prefrontal cortex in the updating of neural representations of schemas remains a primary goal of this line of work, and holds the promise of revealing whether it is engaged in selecting appropriate networks to update or other aspects of assimilation and accommodation that reconcile new memories with existing knowledge.
While the findings from human memory research reviewed thus far has focused primarily on the role of medial prefrontal cortex in the strategic regulation of hippocampal processing during schema formation and modification, other studies have revealed an important role for the lateral prefrontal cortex in resolving competition between memories during encoding and retrieval [67,68]. In contrast to ventromedial prefrontal cortex which is thought to resolve competition by integrating knowledge across events through schema modification, lateral prefrontal cortex has been implicated in encoding and retrieval processes that emphasize the distinctiveness of individual memories, making those memories less susceptible to interference [69–70].
A challenge moving forward is to understand whether or not the time course of lateral and medial prefrontal regions in memory formation, consolidation, and expression differs as a function of their hypothesized representational strategies that emphasize distinctive and integrated memory traces respectively. Finally, identifying functionally homologous regions of prefrontal cortex across species will also be necessary to achieve a full understanding of memory consolidation processes and their underlying neural substrates.
Towards a comprehensive understanding of consolidation
Our understanding of consolidation can be improved by considering the distinct and complementary roles of the hippocampus and prefrontal cortex during successive stages of memory processing (Table 1). During learning, the hippocampus links elements of memories (for example, A and B) to form new associations between the neocortical representations for those elements (A–B). To the extent that the new associations are unrelated to previous learning, hippocampal driven enhancement of neocortical linkages may proceed without necessary engagement of the prefrontal cortex. However, to the extent that new learning overlaps with pre-existing associations (for example, learning B–C having previously learned A–B), the prefrontal cortex (mPFC in rats; ventromedial prefrontal in humans) must reconcile the conflicts in associations of the common elements (B is associated with C and now also with A).
Table 1.
Roles of the hippocampus (HPC) and prefrontal cortex (PFC) in successive stages of memory processing.
| Learning | Consolidation | Expression | |
|---|---|---|---|
| HPC | Represent links between elements of new associations | Employ invariant representations to link overlapping associations in specific neocortical areas | Retrieve links between directly and indirectly related associations according to PFC-selected schema |
| PFC | Reconcile new associations with existing ones whose elements overlap | Create schematic organizations for multiple overlapping memories | Select correct schema for current situation |
Thus, consolidation is a process in which hippocampal networks can link indirectly related elements (A and C) via the invariant common element (B) and, guided by the prefrontal strategic control of conflicting associations to create a schema (A–B–C). During subsequent memory expression, a memory cue (“Are A and C related?”) engages prefrontal cortex to select the correct schema (A–B–C) within which the hippocampus retrieves the relevant associations (A–C via B).
Within this model, and consistent with the data reviewed here, the roles of the hippocampus and prefrontal cortex in mediating the life of a memory may not be defined solely by the age of the memory itself, but rather by how that memory relates to pre-existing knowledge and events yet to come. The key to understanding hippocampal and prefrontal contributions to memory consolidation therefore lies in understanding how these regions support the representations of new events based on the degree to which those events relate to prior knowledge. Furthermore, we suggest that a consideration of these issues explains differences in the role of interactions between the prefrontal cortex and hippocampus observed across studies of memory consolidation in different behavioral paradigms.
When new events do not overlap with pre-existing memories, initial encoding may predominantly rely on the hippocampus. Memory expression in these cases may undergo a course of consolidation characterized by a transition from reliance on the hippocampus to later reliance on prefrontal cortex (Figure 3A, green line). Thus, some rodent studies on the course of consolidation of contextual fear memories showing a lack of prefrontal involvement in the acquisition or expression of a recently acquired contextual fear memory may reflect the absence of a demand for schema modification. In a typical contextual fear paradigm, the animal would be exposed to the context for only a brief time before exposure to an aversive foot shock. Under these conditions, integration demands — and corresponding engagement of the prefrontal cortex — would be low because the animals do not have a pre-existing schema or mental model of what to expect in the new context.
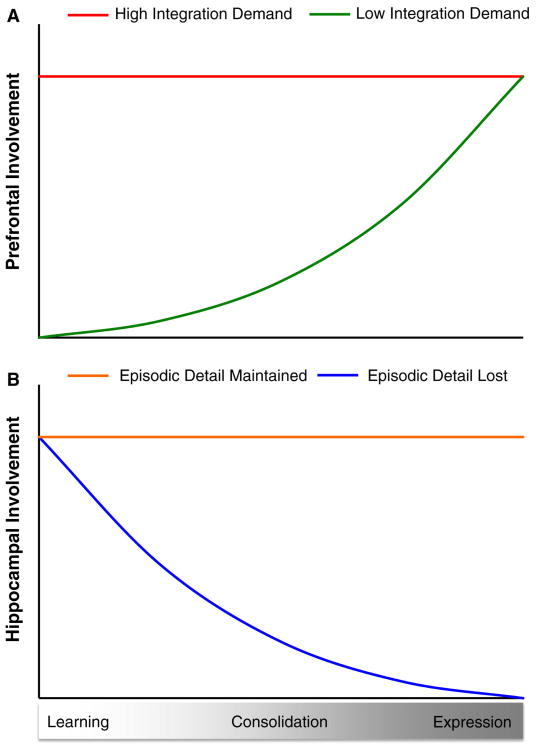
Figure 3. A synthetic view of the time course of prefrontal and hippocampal involvement in successive stages of memory processing.
(A) Our synthesis of consolidation models suggests that prefrontal involvement at different stages of memory processing depends on the degree to which an event overlaps with existing knowledge. When the demand for reconciling new events with existing memories is high during learning, prefrontal cortex is proposed to play an essential role in the reconciliation process by integrating new events into existing schemas. Converging evidence from rodents and humans indicates that prefrontal cortex continues to contribute to schema consolidation and expression after learning, both at immediate and longer time scales. Thus, events that require accessing, modifying, and consolidating schemas are proposed to require prefrontal cortex throughout all stages of memory (red line). However, when new events do not overlapping with existing knowledge, i.e., integration demands are low, there is no critical role for the prefrontal cortex in memory acquisition but an eventual reliance on this after consolidation occurs (green line). (B) The duration of hippocampal involvement in memory may rely on the extent to which episodic detail is maintained over time. For memories that retain a high degree of detail, hippocampus would play a key role during learning, consolidation, and expression (orange line). In contrast, when memories become more generic or when integration of a new event into existing schemas results in loss of episodic detail, the role of the hippocampus may be limited to the consolidation period (blue line).
The increasing reliance on prefrontal cortex with time might relate to how contextual fear gradually becomes generalized to other contexts [71], as described in a model that suggests consolidation involves the transformation of a context-specific (episodic) memory into a less detailed, more generic (semantic) memory [72]. It is notable that increasing prefrontal involvement is also observed in the case of memory for public events, which may begin as episodic memories but evolve into semantic knowledge [73]. Thus, memories that are not immediately integrated into an existing knowledge framework are not initially dependent on prefrontal regions; instead, only as they gradually become incorporated into semantic memories or schemas do they necessitate prefrontal involvement for memory expression.
In contrast, when new events must be immediately related to existing memories, such as in the associative inference and food-location schema tasks, prefrontal cortex and hippocampus are both critical to memory initial memory formation (Figure 3A, red line). Activation of a schema in medial prefrontal cortex in particular may bias hippocampal encoding, leading to the rapid integration of new information into the pre-existing cortical representations [61]. Continued medial prefrontal-hippocampal interactions during offline consolidation periods may further build connections among event memories by strengthening representations of the commonalities among events. During expression, the prefrontal cortex may critically mediate the selection of appropriate, goal-relevant information from a fully integrated and consolidated schema. Even after consolidation, the full expression of schemas may depend on continued interactions between the hippocampus and prefrontal cortex, through a constant cycle of memory updating and change.
A related issue is the phenomenon of reconsolidation, wherein a ‘reminder’ of previous experience reinitiates the consolidation process (reviewed in [48]). It is now generally accepted that one of the conditions under which reconsolidation occurs is when the reminder results in new learning. Such new learning is often in conflict with the pre-existing representation of a cue-shock association, for example, in fear conditioning where the reminder is an extinction event. Thus, as with the other examples of schema modification, the prefrontal cortex may play a key role in reconciling the reminder experience with the pre-existing memory, and the ‘reconsolidation’ process involves schema modification that is susceptible to corruption.
These ideas suggest that all memories eventually are embodied within schemas, for which prefrontal cortex plays a key role in reconciling different associations. This prefrontal-mediated reconciliation process results in modification of schemas to accommodate new related experiences. When events are relatively novel, prefrontal cortex only gradually relates the new memories to succeeding events; conversely, when new events directly relate to existing knowledge, prefrontal mediated reconciliation and integration is required at the outset of memory processing. In some situations (experimental and real life), the use of schemas emphasizes the common elements of experiences whereas in other situations, the use of schemas depends on both the unique and related elements of experiences.
What determines the duration of the consolidation period?
A final relevant consideration is the duration of the consolidation period that appears to vary considerably across species and forms of memory. We propose that the duration of involvement of the hippocampus may also be determined by the extent to which there is continued integration of new information with existing schemas, as well as the extent to which multiple aspects of unique experiences — that is, episodic detail — is maintained. Thus, consistent with the evidence of a flat retrograde amnesia gradient for episodic memories and lasting engagement of the hippocampus for autobiographical and episodic memories [74,75], the hippocampus may play a key role throughout the life of a memory (Figure 3B, orange line). Alternatively, when the integration of a new episodic memory into a schema is accompanied by loss of episodic detail (‘semantisized’), the role of the hippocampus is limited to the consolidation period (Figure 3B, blue line).
This perspective on the relationship between memory consolidation and schemas also suggests that the discrepancies in the observed duration of consolidation across species — longest in humans, intermediate in monkeys, and shortest in rodents — may result from differences in the nature of schemas across species. In rodents, schemas may be limited and relatively simplistic (such as the relationships between items in a familiar environment), resulting in a decreased tendency to process new events in relation to existing memories and more rapid consolidation relative to other species. In contrast, data from humans indicating a continued role for the hippocampus in the expression of memories for many years may result from the increased use of schemas when processing new events. Moreover, schemas in humans may be more complex requiring integration of many forms of information across events, including affective, motivational, sensory, semantic, and social information. Alternatively, continued hippocampal involvement may be required when it is beneficial to retain episode-unique features in addition to forming a schematic framework, thus accounting for observations of the continued role of the hippocampus in episodic memory. Future experiments that systematically vary the relationship between new events and existing schemas may provide an understanding of memory consolidation, including how prefrontal areas and hippocampus contribute differentially across the trajectory of consolidation.
Conclusions
Memory is a dynamic process in which representations are constantly updated to incorporate new information from the environment. Therefore, the roles of the hippocampus and component structures of the prefrontal cortex in memory consolidation and expression may not depend on the amount of time that has elapsed from when a memory was initially formed, but rather are likely to depend on the relationship between that memory and new, related events as well as the level of detail maintained over time. This perspective suggests that consolidation is therefore likely to be a complex process whose neural substrates may differ for individual memories and the history of one's experience into which they are incorporated.
Acknowledgments
This work was supported by a National Science Foundation CAREER Award (A.R.P.), Army Research Office Grant 55830-LS-YIP (A.R.P.), National Institute of Mental Health Silvio O. Conte Center for Neuroscience Research Award MH094263 (H.E.) and National Institute of Mental Health Research Grants MH52090 (H.E.) and MH100121 (A.R.P.). We thank Margaret Schlichting for comments on an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davachi L. Item, context, and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: A new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navawongse R, Eichenbaum H. Distinct pathways support rule-based memory retrieval and spatial mapping by hippocampal neurons. J Neurosci. 2013;33:1002–1013. doi: 10.1523/JNEUROSCI.3891-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu W, Sudhof T. A Neural Circuit for Memory Specificity and Generalization. Science. 2013;339:1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scoville WB, Milner B. Loss of recent memory after hippocampal lesions. J Neurol Neurosurg Psych. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corkin S. Lasting consequences of bilateral medial temporal lobectomy: Clinical course and experimental findings in H.M. Sem Neurol. 1984;4:249–259. [Google Scholar]
- 10.Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 11.Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- 12.Eichenbaum H. What H.M. taught us. J Cogn Neurosci. 2012;25:14–21. doi: 10.1162/jocn_a_00285. [DOI] [PubMed] [Google Scholar]
- 13.Wood E, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- 14.Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens. J Neurosci. 2009;29:9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naya Y, Suzuki WA. Integrating what and when across the primate medial temporal lobe. Science. 2011;333:773–776. doi: 10.1126/science.1206773. [DOI] [PubMed] [Google Scholar]
- 18.Eichenbaum H. Memory on time. Trends Cogn Sci. 2013;17:81–88. doi: 10.1016/j.tics.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer AC, Carr MF, Karlsson MP, Frank LM. Hippocampal SWR activity predicts correct decisions during initial learning of an alternation task. Neuron. 2013;77:1163–1173. doi: 10.1016/j.neuron.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 24.Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Colgin LL. Oscillations and hippocampal-prefrontal synchrony. Curr Opin Neurobiol. 2011;21:467–474. doi: 10.1016/j.conb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moscovitch M. Memory and working-with-memory: A component process model based on modules and central systems. J Cogn Neurosci. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- 27.Buckner RL, Wheeler ME. The cognitive neuroscience of remembering. Nat Rev Neurosci. 2001;2:624–634. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- 28.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 29.Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- 30.Ranganath C, Blumenfeld R. Prefrontal cortex and memory. In: Byrne J, editor. Learning & Memory: A comprehensive reference. Oxford, UK: Academic Press; 2008. pp. 261–279. [Google Scholar]
- 31.Kuhl BA, Wagner AD. Strategic control of memory. Encyc Neurosci. 2009;9:437–444. [Google Scholar]
- 32.Postle BR. Working memory as an emergent property of the mind and brain. Neurosci. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimamura AP, Jurica PJ, Mangels JA, Gershberg FB, Knight RT. Susceptibility to memory interference effects following frontal lobe damage: findings from tests of paired-associate learning. J Cogn Neurosci. 1995;7:144–152. doi: 10.1162/jocn.1995.7.2.144. [DOI] [PubMed] [Google Scholar]
- 34.Depue BE. A neuroanatomical model of prefrontal inhibitory modulation of memory retrieval. Neurosci Biobehav Rev. 2012;36:1382–1399. doi: 10.1016/j.neubiorev.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dias R, Robbins TW, Roberts AC. Dissociaton in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 36.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rich EL, Shapiro ML. Prelimbic/infralimbic inactivation impairs memory for multiple task switches, but not flexible selection of familiar tasks. J Neurosci. 2007;27:4747–4755. doi: 10.1523/JNEUROSCI.0369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ragozzino ME, Kim J, Hassert D, Minniti N, Kiang C. The contribution of the rat prelimbic-infralimbic areas to different forms of task switching. Behav Neurosci. 2003;117:1054–1065. doi: 10.1037/0735-7044.117.5.1054. [DOI] [PubMed] [Google Scholar]
- 39.Hyman JM, Ma L, Balaguer E, Durstewitz D, Seamans JK. Contextual encoding by ensembles of medial prefrontal cortex neurons. Proc Natl Acad Sci USA. 2012;109:5086–5091. doi: 10.1073/pnas.1114415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karlsson MP, Tervo DGR, Karpova AY. Network rests in medial prefrontal cortex mark the onset of behavioral uncertainty. Science. 2012;338:135–139. doi: 10.1126/science.1226518. [DOI] [PubMed] [Google Scholar]
- 41.Durstewitz D, Vittoz NM, Floresco SB, Seamons JK. Abrupt transitions between prefrontal neural ensemble states accompany behavioral transitions during rule learning. Neuron. 2010;66:438–448. doi: 10.1016/j.neuron.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 42.Rich EL, Shapiro M. Rat prefrontal cortical neurons selectively code strategy switches. J, Neurosci. 2009;29:7208–7219. doi: 10.1523/JNEUROSCI.6068-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komorowski RW, Garcia CG, Wilson A, Hattori S, Howard MW, Eichenbaum H. Ventral hippocampal neurons are shaped by experience to represent behaviorally relevant contexts. J Neurosci. 2013;33:8079–8087. doi: 10.1523/JNEUROSCI.5458-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang JC, Wagner AD, Preston AR. Content representation in the human medial temporal lobe. Cereb Cortex. 2013;23:80–96. doi: 10.1093/cercor/bhr379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Swanson LW, Wyss JM, Cowan WM. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978;181:681–715. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- 47.Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75:168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKenzie S, Eichenbaum H. Consolidation and reconsolidation: Two lives of memories? Neuron. 2011;71:224–233. doi: 10.1016/j.neuron.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang SH, Morris RG. Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol. 2010;61:49–79. doi: 10.1146/annurev.psych.093008.100523. [DOI] [PubMed] [Google Scholar]
- 50.McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psych Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 51.Piaget J. The Child's Conception of the World. Littlefield: Adams; 1929. [Google Scholar]
- 52.Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- 53.Rapp PR, Kansky MT, Eichenbaum H. Learning and memory for hierarchical relationships in the monkey: Effects of Aging. Behav Neurosci. 1996;110:887–897. doi: 10.1037//0735-7044.110.5.887. [DOI] [PubMed] [Google Scholar]
- 54.Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proc Nat Acad Sci USA. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DeVito LM, Kanter B, Eichenbaum H. The hippocampus contributes to memory expression during transitive inference in mice. Hippocampus. 2010;20:208–217. doi: 10.1002/hipo.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RG. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- 57.Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- 58.Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- 59.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 60.DeVito LM, Lykken C, Kanter BR, Eichenbaum H. Prefrontal cortex: Role in acquisition of overlapping associations and transitive inference. Learn Mem. 2010;17:161–167. doi: 10.1101/lm.1685710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, Bito H, Morris RG. Schema-dependent gene activation and memory encoding in neocortex. Science. 2011;333:891–895. doi: 10.1126/science.1205274. [DOI] [PubMed] [Google Scholar]
- 62.Wang SH, Tse D, Morris RG. Anterior cingulate cortex in schema assimilation and expression. Learn Mem. 2012;19:315–318. doi: 10.1101/lm.026336.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonnici HM, Chadwick MJ, Lutti A, Hassabis D, Weiskopf N, Maguire EA. Detecting representations of recent and remote autobiographical memories in vmMPFC and hippocampus. J Neurosci. 2012;32:16982–16991. doi: 10.1523/JNEUROSCI.2475-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Kesteren MT, Fernandez G, Norris DG, Hermans EJ. Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and post encoding rest in humans. Proc Natl Acad Sci USA. 2010;107:7550–7555. doi: 10.1073/pnas.0914892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumaran D, Summerfield JJ, Hassabis D, Maguire EA. Tracking the emergence of conceptual knowledge during human decision making. Neuron. 2009;63:889–901. doi: 10.1016/j.neuron.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McKenzie S, Robinson NTM, Herrera L, Churchill JC, Eichenbaum H. Learning causes reorganization of neuronal firing patterns to represent related experiences within a hippocampal schema. J Neurosci. 2013 doi: 10.1523/JNEUROSCI.0879-13.2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 68.Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15:219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Raposo A, Han S, Dobbins IG. Ventrolateral prefrontal cortex and self-initiatedsemantic elaboration during memory retrieval. Neuropsychologia. 2009;47:2261–2271. doi: 10.1016/j.neuropsychologia.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuhl BA, Bainbridge WA, Chun MM. Neural reactivation reveals mechanisms for updating memory. J Neurosci. 2012;32:3453–3461. doi: 10.1523/JNEUROSCI.5846-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wiltgen BJ, Zhou M, Cai K, Balaji J, Guzman Karlson M, Parivash S, Li W, Silva AJ. The hippocampus plays a selective role in the retrieval of detailed memories. Curr Biol. 2010;20:1336–1344. doi: 10.1016/j.cub.2010.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winocur G, Moscovitch M. Memory transformation and systems consolidation. J Int Neuropsychol Soc. 2011;17:766–780. doi: 10.1017/S1355617711000683. [DOI] [PubMed] [Google Scholar]
- 73.Smith CN, Squire LR. Medial temporal lobe activity during retrieval of semantic memory is related to the age of the memory. J Neurosci. 2009;29:930–938. doi: 10.1523/JNEUROSCI.4545-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 75.Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, Nadel L. Functional neuroanatomy of remote episodic, semantic and spatial memory: A unified account based on multiple trace theory. J Anat. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bartlett FC. Remembering: A Study in Experimental and Social Psychology. Cambridge University Press; 1932. [Google Scholar]
- 77.Lewis PA, Durrant SJ. Overlappingmemory replay during sleep builds cognitive schemata. Trends Cogn Neurosci. 2011;15:343–351. doi: 10.1016/j.tics.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 78.van Kesteren MT, Ruiter DJ, Fernandez G, Henson RN. How schema and novelty augment memory formation. Trends Neurosci. 2012;35:211–219. doi: 10.1016/j.tins.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 79.Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427:352–325. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- 80.Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. Human relational memory requires time and sleep. Proc Natl Acad Sci USA. 2007;104:7723–7728. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]