Hemophilia defines a group of hereditary bleeding disorders: hemophilia A (deficiency of Factor VIII, FVIII), hemophilia B (deficiency of FIX), and para-hemophilia (deficiency of FV). These result from mutations in clotting factor genes. As in the large majority of bleeding disorders (Table 1), replacement of deficient coagulation factor protein is required to prevent or reverse acute bleeding episodes. This is achieved by the administration of recombinant or plasma-derived clotting factor concentrates (PDCFC), e.g. FVIII or FIX; or inhibitor-bypassing agents such as rFVIIa or Factor VIII inhibitor bypass activity (FEIBA). Being effective at preventing and treating bleeding in patients with hemophilia, and enabling self-administration at home, PDCFC has replaced cryoprecipitate and fresh frozen plasma, thus revolutionizing hemophilia treatment in developed countries. However, cryoprecipitate and fresh frozen plasma are still used in some developing countries.
Table 1.
Management of major inherited bleeding disorders.
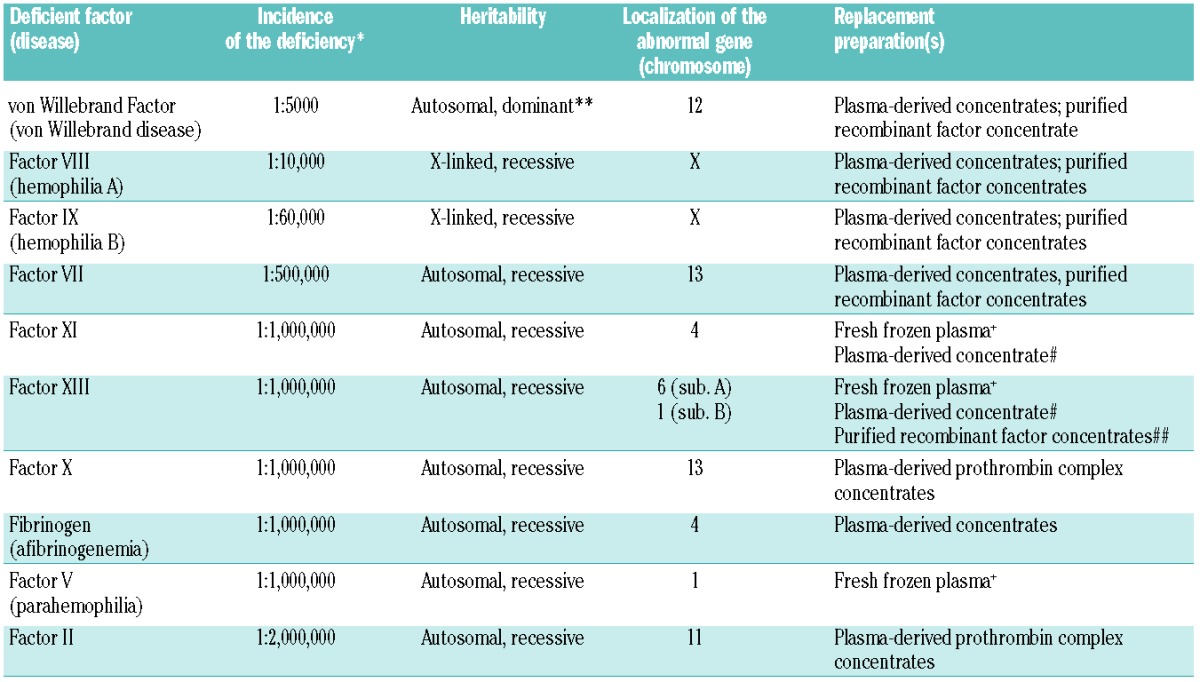
Initially, large blood donor pools were used to prepare PDCFC and pathogen screening tests were not available. As a result, infection by blood-borne pathogens was a major concern in patients with hemophilia, particularly in relation to human immunodeficiency virus (HIV) and hepatitis C virus (HCV). While the risk of hepatitis B virus (HBV) infection was reduced substantially by vaccination (prevalence of hepatitis B surface antigen among European blood donors is currently around 0.006%1), as many as 60–70% of patients with severe hemophilia were infected with HIV through contaminated FVIII and FIX,2 and over 95% of patients with severe hemophilia A were infected with HCV by contaminated plasma-derived FVIII.3 These high rates of infection were associated with significant mortality. In Italy, between 1990 and 2007, HIV and HCV were associated with 45% and 13% of hemophilia deaths, respectively.4 In Great Britain, mortality in patients with hemophilia and HIV peaked at over 10% in 1993–1996 versus 5% in 1997–1999 and 0.9% in patients with hemophilia but without HIV co-infection in 1977–1984.5 In the Netherlands, between 1985 and 2010, 17% of hemophilia deaths were related to HIV, 12% to HCV complications, and 4% to HIV-HCV co-infection.6 In the US, between 1979 and 1988, HIV-related diseases accounted for 47% of deaths in patients with hemophilia A.7
Today, the risk of contracting HBV, HIV and HCV through transfusions and blood products has almost been eliminated in developed countries. Plasma collection at specialized centers is continuously inspected by the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) prior to approving commercial use. Plasma is screened at each donation and re-screened in mini-pools with nucleic acid testing available for 5 viruses (HIV, hepatitis A virus, HBV, HCV, Parvovirus B19) and described in detail in Plasma Master Files that are approved every year by the regulatory agencies. The products are then treated with solvent/detergent, super-heated (at 80° for 3 days), pasteurized and nano-filtered. However, despite these precautions, a risk of non-enveloped virus infection persists (e.g. Parvoviruses, Picornaviruses, Circoviruses).8,9
The characteristics of the patient receiving clotting factors have changed over the years. Improved treatment quality and availability has led to increased life expectancy for patients with hemophilia. In Italy, life expectancy for people with hemophilia was 71.2 years for 2000–2007 versus 64.0 years for 1990–1997.4 In the US, between 1995 and 1998, median age of death for non-HIV-infected patients with hemophilia A was 72 years.7 Between 1992 and 2001, life expectancy of patients with hemophilia in the Netherlands was 72 years.10 Such improved survival has led to an increase in age-related clinical problems (e.g. co-morbidity) that subsequently increase risks of infection and pathogen dissemination. Factor concentrate consumption and exposure to different product batches are also increasing due to changing hemophilia treatment patterns,11 increased use of prophylaxis, higher rates of surgery in the elderly, and increased use of high-dose regimens for achieving immune tolerance in patients who develop inhibitors (approx. 30% of patients treated with PDCFC or recombinant products).12
Alongside the changing patient demographic and level of exposure to clotting factor products, the pathogenic agents of concern are also changing. Several emerging viral and non-viral pathogens have been identified, some of which exhibit blood-borne transmission, e.g. Chikungunya virus, HCV, HIV, human T-lymphotropic virus type 1, variant Creutzfeldt-Jakob disease (vCJD) prion, West Nile virus, and severe acute respiratory syndrome corona virus.13 Genetic factors and immunodeficiency may affect the risk of acquiring these infections and the clinical outcome.14,15 Environmental changes increase the likelihood of contact with, and transmission of, certain pathogens.16 Since it is not possible to screen donor blood for all emerging pathogens, systems are in place for immediate withdrawal of any products made with blood from a donor following confirmed or suspected pathogen transmission. This has major consequences for patients who depend on those products, causing as they do shortages and delay in their supply, particularly if a market is reliant on a single supplier. Such problems may be caused by: 1) procedures for identifying the infected products and batches; 2) recall of the specific batches identified; 3) testing of patients known to have been exposed; and 4) manufacture, testing and distribution of new batches. As an example, the 2010 EMA position statement on CJD and plasma-derived and urine-derived medicinal products states that there is strong evidence that vCJD is transmissible through transfusion of blood and plasma products:17 “In view of the lack of adequate information on vCJD, it is prudent to recall batches of plasma-derived medicinal products where a donor to a plasma pool subsequently develops vCJD”. Between 2011 and 2012, unconfirmed, definite or suspected vCJD was identified among blood donors in Italy. The Italian Pharmaceutical Agency (AIFA), in line with the EMA position statement, prohibited any further use of specific batches from Italian blood donors, recalled distributed batches and required that any warehouse stocks should not be distributed. Consequent product shortages meant that affected hemophilia A patients had to be switched to alternative (mostly recombinant) FVIII products.
In the 1970s, concerns regarding pathogen safety of blood and PDCFC, and an increased demand for transfusion products, led to the development of recombinant coagulation factors.18 Such products inevitably avoid the risks of blood-related pathogen transmission, and indeed there is no evidence to date of any pathogen transmission by using these products. However, there remain potential risks from reagents used in the manufacture of recombinant products; for example, cell culture media and growth factors. Reagents containing proteins of human or animal origin are required to undergo risk assessment for prions.8 Third-generation recombinant products do not contain animal or human components and, therefore, do not carry a known pathogenic transmission risk.19 While recombinant agents offer an inherently low pathogenic risk, it is important to remember that, like any manufacturing process, external contamination can occur. This was seen in 2009 when the production of recombinant gluco-cerebrosidase for patients with Gaucher’s disease was halted as a result of viral (vesivirus) contamination of the production facility (Genzyme Corporation, MA, USA). An acute shortage of this therapeutic enzyme occurred as a direct consequence.20
In spite of the wide availability of recombinant factors, PDCFC will continue to be needed. First, it is unlikely that all patients with hemophilia can be treated with recombinant products in the foreseeable future. Second, recombinant products are not available for all bleeding disorders (Table 1). Finally, economic pressures in some countries have meant that use of PDCFC is encouraged.
Despite improvements in safety of blood- and plasma-derived and recombinant coagulation factor products, these products can probably never be completely ‘safe’ in terms of pathogen risk. A pharmacovigilance system is, therefore, urgently required in the European Union to monitor long-term quality and safety of PDCFC and recombinant products.9
Acknowledgments
The authors discussed the content of this article during a meeting funded by Pfizer. Pfizer were not a part of these discussions. Minor language editing in the galley proofs was provided by Mark Davies of inScience Communications, funded by Pfizer.
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Svicher V, Cento V, Bernassola M, Neumann-Fraune M, Van Hemert F, Chen M, et al. Novel HBsAg markers tightly correlate with occult HBV infection and strongly affect HBsAg detection. Antiviral Res. 2012;93(1):86–93 [DOI] [PubMed] [Google Scholar]
- 2.Franchini M, Tagliaferri A, Mengoli C, Cruciani M. Cumulative inhibitor incidence in previously untreated patients with severe hemophilia A treated with plasma-derived versus recombinant factor VIII concentrates: a critical systematic review. Crit Rev Oncol Hematol. 2012;81(1):82–93 [DOI] [PubMed] [Google Scholar]
- 3.Goedert JJ, Chen BE, Preiss L, Aledort LM, Rosenberg PS. Reconstruction of the hepatitis C virus epidemic in the US hemophilia population, 1940–1990. Am J Epidemiol. 2007;165(12):1443–53 [DOI] [PubMed] [Google Scholar]
- 4.Tagliaferri A, Rivolta GF, Iorio A, Oliovecchio E, Mancuso ME, Morfini M, et al. Mortality and causes of death in Italian persons with haemophilia, 1990–2007. Haemophilia. 2010;16(3):437–46 [DOI] [PubMed] [Google Scholar]
- 5.Darby SC, Kan SW, Spooner RJ, Giangrande PL, Lee CA, Makris M, et al. The impact of HIV on mortality rates in the complete UK haemophilia population. AIDS. 2004;18(3):525–33 [DOI] [PubMed] [Google Scholar]
- 6.Fransen van de Putte DE, Fischer K, Pulles AE, Roosendaal G, Biesma DH, Schutgens RE, et al. Non-fatal cardiovascular disease, malignancies, and other co-morbidity in adult haemophilia patients. Thromb Res. 2012;130(2):157–62 [DOI] [PubMed] [Google Scholar]
- 7.Chorba TL, Holman RC, Clarke MJ, Evatt BL. Effects of HIV infection on age and cause of death for persons with hemophilia A in the United States. Am J Hematol. 2001;66(4):229–40 [DOI] [PubMed] [Google Scholar]
- 8.Ludlam CA, Powderly WG, Bozzette S, Diamond M, Koerper MA, Kulkarni R, et al. Clinical perspectives of emerging pathogens in bleeding disorders. Lancet. 2006;367(9506):252–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soucie JM, De Staercke C, Monahan PE, Recht M, Chitlur MB, Gruppo R, et al. Evidence for the transmission of parvovirus B19 in patients with bleeding disorders treated with plasma-derived factor concentrates in the era of nucleic acid test screening. Transfusion. 2013;53(6):1217–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plug I, Van Der Bom JG, Peters M, Mauser-Bunschoten EP, De Goede-Bolder A, Heijnen L, et al. Mortality and causes of death in patients with hemophilia, 1992–2001: a prospective cohort study. J Thromb Haemost. 2006;4(3):510–6 [DOI] [PubMed] [Google Scholar]
- 11.Trimble SR, Parker CS, Grant AM, Soucie JM, Reyes N. Assessing emerging infectious threats to blood safety for the blood disorders community. Am J Prev Med. 2010;38(4 Suppl):S468–74 [DOI] [PubMed] [Google Scholar]
- 12.Mannucci PM, Mancuso ME, Santagostino E. How we choose factor VIII to treat hemophilia. Blood. 2012;119(18):4108–14 [DOI] [PubMed] [Google Scholar]
- 13.Grillberger L, Kreil TR, Nasr S, Reiter M. Emerging trends in plasma-free manufacturing of recombinant protein therapeutics expressed in mammalian cells. Biotechnol J. 2009;4(2):186–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahlberg AS, Granfors K, Penttinen MA. HLA-B27 and host-pathogen interaction. Adv Exp Med Biol. 2009;649:235–44 [DOI] [PubMed] [Google Scholar]
- 15.Kaur P, Basu S. Transfusion-transmitted infections: existing and emerging pathogens. J Postgrad Med. 2005;51(2):146–51 [PubMed] [Google Scholar]
- 16.Zappa A, Amendola A, Romano L, Zanetti A. Emerging and re-emerging viruses in the era of globalisation. Blood Transfus. 2009;7(3):167–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Medicines Agency CHMP position statement on Creutzfeldt-Jakob disease and plasma-derived and urine-derived medicinal products. 2011; Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Position_statement/2011/06/WC500108071.pdf
- 18.Powell JS. Recombinant factor VIII in the management of hemophilia A: current use and future promise. Ther Clin Risk Manag. 2009;5(2):391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kessler CM. New perspectives in hemophilia treatment. Hematology Am Soc Hematol Educ Program. 2005:429–35 [DOI] [PubMed] [Google Scholar]
- 20.Deegan PB, Cox TM. Imiglucerase in the treatment of Gaucher disease: a history and perspective. Drug Des Devel Ther 2012;6:81–106 [DOI] [PMC free article] [PubMed] [Google Scholar]


