Abstract
Background
The purpose of the current study was to determine the sensitivity, specificity, and positive predictive value of three depression screening tools among a low-income African American population of pregnant and recently delivered women enrolled in home visitation programs in a low-income urban community.
Methods
Ninety-five women enrolled in home visitation programs—32 who were pregnant and 63 with a child <6 months comprise the study sample. Each woman completed a structured clinical interview and three depression screening tools—the Edinburgh Postnatal Depression Scale (EPDS), Center for Epidemiologic Studies Depression Scale (CES-D), and Beck Depression Inventory II (BDI-II).
Results
Over a quarter of women (28.4%) were experiencing major depression. Each screening tool was highly accurate in detecting major depression and major or minor depression among prenatal and postpartum women, with areas under the curve (AUCs) >0.90. Sensitivities of all screening tools were improved when using cutoffs lower than those considered standard by instrument developers.
Limitations
Participants were recruited from home visitation programs in an urban context which may limit generalizability to other populations of low-income African American women. Given that no women during pregnancy met criteria for minor depression, it was not possible to determine optimal prenatal cutoff scores.
Conclusions
Three depression screening tools—the EPDS, CES-D, and BDI-II—appear to be reliable and brief assessments of major and minor depression among low-income African American perinatal women. Providers using these tools should consider using lower cutoff scores to most effectively identify women in need of depression treatment.
Keywords: Postpartum depression, Screening, African American, Home visiting
1. Introduction
Perinatal depression refers to major and minor depressive episodes that occur during pregnancy or in the first year following delivery. The prevalence of major depression has been estimated at 10 to 14% during pregnancy (Gaynes et al., 2005) and 10 to 15% during the postpartum period (O"Hara & Swain, 1996), although estimates of depression during pregnancy may be conservative because certain symptoms— particularly those that are somatic—may be mistaken for signs of normal pregnancy (Altshuler et al., 2008; Yonkers et al., 2009). There is evidence that African American women, particularly those of lower socioeconomic status, may be at elevated risk for perinatal depression. Melville et al. (2010) found that nearly 19% of the African American women in their ethnically diverse sample of 1888 women met diagnostic criteria for either major or minor depression during pregnancy. An earlier study by Hobfoll et al. (1995) that interviewed women during pregnancy and in the second and third months after delivery found that 28% of low-income perinatal women were clinically depressed. Several studies have also shown that low-income African American women have higher rates of elevated depressive symptoms during the perinatal period than middle-class Caucasian women (Beck, 2001; Center for Health Statistics, 2008; Chaudron et al., 2010; Goyal et al., 2010; Luke et al.,2009; Rich-Edwards et al., 2006; Segre et al., 2007; Seguin et al., 1999a; Seguin et al., 1999b; Yonkers et al., 2009).
Depression during the perinatal period confers risk for future postpartum and non-childbirth-related depressive episodes among women (Wisner et al., 2004, 2010). Perinatal depression has also negative consequences for maternal parenting practices. Compared with women not suffering from perinatal depression, depressed women are less positive, spontaneous, and responsive with their infants (Jones et al., 2010; Murray et al., 2010; VanDoesum, et al., 2007). Mediated through these negative interactions, postpartum depression has been linked to a broad array of developmental delays among infants of depressed mothers, including social interaction difficulties, attachment insecurity, and cognitive impairments (Cogill et al., 2005; Grace et al., 2003; Hipwell et al., 2000; Murray et al., 1999; Sohr-Preston & Scaramella, 2006). Research has also shown less than optimal use of maternal infant health services among mothers with postpartum depression, including increased likelihood of (a) problem-oriented primary care visits, (b) emergency room visits, and (c) hospitalizations (Chung et al., 2004; Mandl et al., 1999; McLearn et al., 2006; Minkovitz et al., 2005).
Screening is an important initial step in identifying women at high risk for perinatal depression and securing appropriate treatment for women experiencing perinatal depression. A handful of studies have demonstrated the feasibility of conducting perinatal depression screening in primary care settings and OB/GYN clinics (Baisch et al., 2010; Chaudron et al., 2004; Georgiopoulos et al., 1999; Miller et al., 2009; Olson et al., 2005; Segre et al., 2010). However, only a small percentage of women are estimated to be screened for depression during their pregnancy or first year postpartum (Goodman &Tyer-Viola, 2010; Kelly et al., 2001; Marcus et al., 2003). For example, Marcus et al. found that 20% of pregnant women screened in obstetric clinics had elevated depressive symptom scores, yet only 13.8% of these women received any formal treatment for depression. LaRocco-Cockburn et al. (2003) found that less than one-quarter of obstetricians reported using a validated depression screening tool. Similar findings were reported from a survey of family physicians, with fewer than 20% indicating that they used a screening tool to screen mothers for perinatal depression at well-child visits (Seehusen et al., 2005).
To increase the percentage of women being screened for perinatal depression, one logical approach is to integrate screening into other settings serving pregnant and recently delivered women, such as Women, Infant, Children (WIC) clinics and home visitation programs. Found in all 50 states and operated out of various settings (e.g., county health departments, community centers, public housing), WIC clinics are charged with providing low-income women and children access to nutritious foods and information on health eating, as well as referrals to health care. Among the populations served by WIC clinics are pregnant women and breastfeeding women —defined as up to a child’s first birthday (U.S. Department of Agriculture, 2011). An estimated 500,000 women across all 50 states receive home visiting services (Gomby, 2005; Johnson, 2009), making home visiting one of the largest avenues through which perinatal women come to the attention of service providers. Home visiting programs typically enroll women prenatally with services continuing until a child reaches 2–5 years of age. Thus, they are well positioned to screen for maternal depression and provide continuity of care across the perinatal period for women and their infants. WIC and home visiting programs are also important settings for conducting perinatal depression screening given the large percentage of low-income women using these services.
Clinical (e.g., pediatric care) and community (e.g., home visitation) settings both share a need to use perinatal depression screening tools that are reliable and valid while simultaneously affordable and efficient. Although there are a variety of screening tools for depression, few studies have reported the sensitivities and specificities of the screening measures in comparison with diagnostic instruments (e.g., SCID or DIS) in minority race/ethnicity or low-income perinatal populations. Hanusa et al. (2008) compared the EPDS and the Diagnostic Interview Schedule (DIS) in a sample of 123 low-income women and found that the EPDS accurately identified women who were depressed or would experience depression in the first six months postpartum. Chaudron et al. (2010) found that the EPDS, Beck Depression Inventory II (BDI-II), and Postpartum Depression Screening Scale (PDSS) have good positive predictive validity; but they argued that traditional cutoff scores needed to be altered in their urban, low-income sample of 198 African American women enrolled in the WIC program.
Although routine screening for perinatal depression is feasible, general screening guidelines and recommendations remain controversial. For example, the American College of Obstetricians and Gynecologists, Committee on Obstetric Practice (2010) noted that there was insufficient evidence to support firm recommendations on when and how often perinatal depression screening should be conducted. Multiple studies (Chaudron et al., 2010; Gjerdingen et al., 2009; Sheeder et al.,2009; Logsdon& Myers, 2010) have reported that cultural differences as well as timing of screening influence cutoff scores for different instruments. To our knowledge, no perinatal depression screening guidelines currently exist for urban, low-income African American women. The purpose of the current study is to determine the sensitivity, specificity, and positive predictive value of three depression screening tools among a low-income African American population of pregnant and recently delivered women enrolled in home visitation programs in an urban community. A secondary objective of this study was to validate the findings from Chaudron et al. (2010) by comparing the accuracy of different screening tools with findings from their earlier study.
2. Methods
2.1. Recruitment and sample
Women were eligible for study participation if they were pregnant or had a child less than six months old. Study investigators were given the names and contact information of 146 women meeting this criterion who were enrolled in three Baltimore City home visitation programs. Of these 146 women, 109 were contacted by phone by the fieldwork interviewer. Among those contacted, 95 agreed to participate and comprise the sample for this study; four women declined study participation and 10 women agreed to participate but were unable to be located to complete the study procedures.
2.2. Study procedures
The fieldwork interviewer, a licensed clinical social worker (LCSW-C), scheduled a time to meet with each study participant to administer the three screening tools and clinical interview. All interviews took place at the home visiting program office or client’s home except for three which took place at a neighborhood library. Interviews were completed between january 2008 and January 2009. Prior to administering the screening tools and clinical interview, informed consent was obtained. All screening and clinical interview questions were read aloud, as were additional questions obtaining demographic information. The three screening tools were conducted in the order listed below with the clinical interview conducted immediately afterward on the same day. Women were paid $30 cash for study participation. Funding for this study was provided by the Thomas Wilson Sanitarium; the funder had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The Johns Hopkins University School of Medicine Institutional Review Board approved this study.
2.3. Measures
2.3.1. Demographic information
We collected demographic information on maternal age, marital status, number of previous live births, race/ethnicity, level of education, and employment status. We also collected information on number of weeks pregnant for women who had not yet delivered and child age (in months) for women who had delivered.
2.3.2. Screening tools
2.3.2.1. Center for Epidemiologic Studies Depression Scale (CES-D)
The CES-D is a 20-item scale developed as an epidemiological measure of depression for community samples (Radloff, 1977). It has been used to assess depression in perinatal samples both in the pre- and postnatal periods (e.g., Beeghly et al., 2003; Orr et al., 2002; Tandon et al., 2005). It asks respondents to indicate how many days during the last week they experienced a variety of depressive symptoms. Scores range from 0 to 60 with a cutoff of ≥16 recommended for detection of moderate depressive symptomatology and ≥ 24 for detection of severe symptomatology (Diaz et al., 2007; Heilemann et al., 2004; Marcus et al., 2003), although some studies have used higher cutoff scores to signal severe symptomatology in low-income African American perinatal samples (Orr et al., 2002; Phillips et al., 2010). The internal consistency of the scale is good; in the current sample, the scale had an alpha coefficient of 0.86, which is consistent with other studies conducted with perinatal samples (Beeghly et al., 2003; Norr et al., 2003).
2.3.2.2. Edinburgh Postnatal Depression Scale (EPDS)
The EPDS is a 10-item scale initially developed for assessment of depression in postpartum women (Cox et al., 1987), although it has been widely used to assess depression in pregnant women as well (American College of Obstetricians and Gynecologists, Committee on Obstetric Practice, 2010; Goodman & Tyer-Viola, 2010). It asks respondents the frequency of predominantly cognitive and affective symptoms experienced in the past seven days, purposefully omitting the somatic symptoms commonly associated with the perinatal period. Scores range from 0 to 30. A cutoff score of ≥10 is recommended for detection of minor depressive disorder (MnDD) or major depressive disorder (MDD) while a cutoff of ≥13 is recommended for detection of MDD. Sensitivity and specificity of the EPDS have generally been found to be lower when screening for MDD and MnDD as opposed to only MDD (Chaudron et al., 2010). The EPDS has demonstrated good reliability across a variety of samples, including low-income women (Boyd et al., 2005), and used with African American perinatal samples (e.g., Yonkers et al., 2001). In this sample, the alpha coefficient was 0.80.
2.3.2.3. Beck Depression Inventory II (BDI-II)
The BDI-II is a 21-item scale that was developed to correspond to DSM-IV criteria for depressive disorder (Beck, 1996). Respondents are asked the frequency of their depressive symptoms within the past two weeks. Scores on the BDI-II range from 0 to 63, with cutoffs of ≥14 suggestive of mild depression and ≥20 suggestive of moderate depression (Beck, 1996). The BDI-II has demonstrated good concurrent validity with other postpartum depression screening scales and has demonstrated excellent reliability in other studies with perinatal populations (Boyd et al., 2005), and used with African American perinatal women (e.g., Chaudron et al., 2010). The alpha coefficient for the BDI-II was 0.90 in this study.
2.3.3. Structured clinical interview
2.3.3.1. Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders Axis I Disorders, Non-Patient Edition (SCID-I/NP)
We used the non-patient version of the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (SCID-I/NP) to assess DSM-IV criteria for MDD and MnDD (First et al., 2002). The SCID-I/NP is a semi-structured interview developed to assess DSM-IV axis I disorders in adults, including MDD and MnDD. The fieldwork interviewer conducting the SCID received training on the administration and scoring of the SCID prior to the study. For the present study, we considered the SCID diagnosis to be the criterion standard for MDD and MnDD diagnosis to which the CES-D, EPDS, and BDI-II were compared.
2.4. Statistical analyses
Differences in demographic characteristics between women with and without SCID-diagnosed depression were assessed using t-tests and chi-square tests for continuous and categorical variables, respectively. The accuracy of the screening tools was evaluated using receiver operating characteristic (ROC) analysis. ROC curves, which plot the sensitivity versus 1 minus the specificity of a measure, were constructed and the areas under the ROC curves (AUC) were calculated for the whole sample and for each perinatal group for each screening tool. The AUC, which ranges from 0 to 1.0, indicates the accuracy of a measure. A ROC curve with an AUC >0.5 suggests that the measure identifies cases better than by chance and an AUC >0.8 is generally considered to suggest an accurate test. The closer the AUC is to 1.0, the more accurate the test. Chi-square tests were used to compare the AUCs of each screening tool; as noted in Chaudron et al. (2010), because all study participants completed all three screening tools each participants’ results were correlated. Accordingly, our chi-squared analyses took into consideration within-subject correlations (Delong et al., 1988). Sensitivity and specificity for standard cutoff scores for each tool were calculated using the SCID diagnosis as the gold standard. Optimal cutoff scores for the screening tools were calculated using the ROC curves.
3. Results
3.1. Sample characteristics
Table 1 summarizes the demographic characteristics of the entire sample as well as the prenatal and postpartum subgroups. The average age of study participants was 24.4 years. All study participants were African American, with most women unmarried and not employed. About half of the participants had obtained at least a high school degree or GED. This was the first child for one-quarter of the postpartum women while nearly 60% of prenatal women were pregnant with their first child. Among pregnant women, the average weeks gestation was 31; for postpartum women, infants were an average age of 8.2 weeks.
Table 1.
Demographic characteristics of study participants, overall and by perinatal group.
| Variable | Overall (n = 95) |
Prenatal (n = 32) |
Postpartum (n = 63) |
|---|---|---|---|
| Age (mean, SD) | 24.4 (5.8) | 23.8 (5.9) | 24.7 (5.7) |
| African American race (%) | 100 | 100 | 100 |
| Single marital status( %) | 87 | 97 | 83 |
| Primiparous (%) | 37 | 59 | 25 |
| Working part- or full-time (%) | 20 | 6 | 27 |
| Education: high school diploma/GED or greater (%) | 57 | 53 | 59 |
| Average weeks gestation (prenatal women only) (mean, SD) | 31 (7.3) | 31 (7.3) | – |
| Average age of child in weeks (postpartum women only) (mean, SD) | 8.2(3.1) | – | 8.2(3.1) |
Table 2 presents the number and proportion of perinatal women who met criteria for MDD and MnDD based on clinician diagnosis with the SCID. Due to the relatively low incidence of MnDD (approximately 5%), the remainder of the results and discussion will focus exclusively on two groups of perinatal women: those with MDD (28.4%) and those with MDD or MnDD (33.7%).
Table 2.
Number and proportion of perinatal women with SCID depression diagnoses overall and by perinatal group.
| Group | n(%) |
||
|---|---|---|---|
| MDD | MnDD | MDD/MnDD | |
| A11(N = 95) | 27 (28.4) | 5 (5.3) | 32 (33.7) |
| Prenatal (n = 32) | 7 (21.9) | 0 (0.0) | 7 (21.9) |
| Postpartum (n = 63) | 20 (31.8) | 5 (7.9) | 25 (39.7) |
Abbreviations: MDD, major depressive disorder; MnDD, minor depressive disorder.
3.2. ROC curves for screening tools
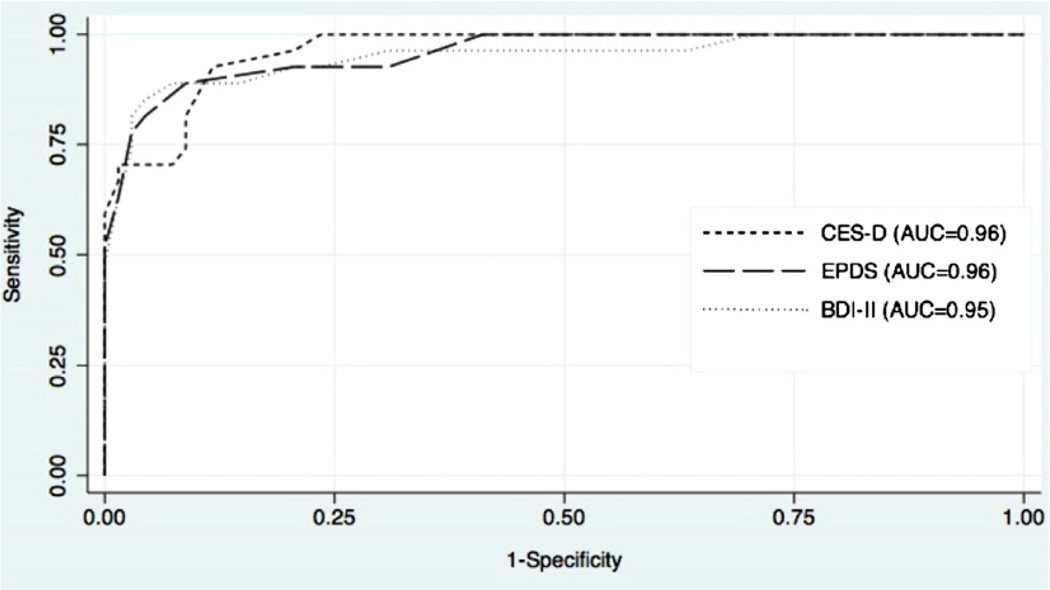
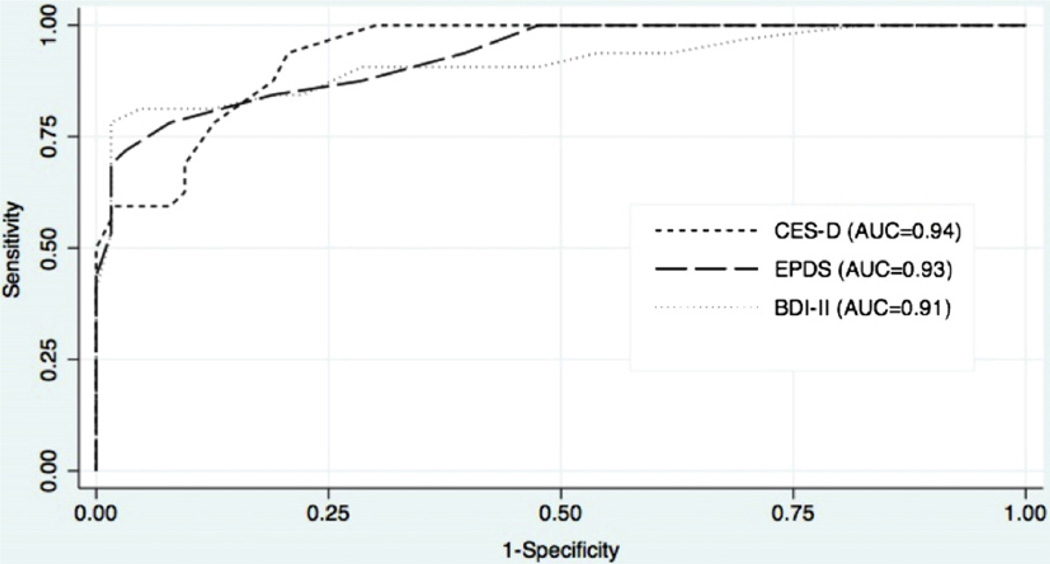
The results of the ROC analysis are presented graphically in Figs. 1 and 2. For the full sample including all prenatal and postpartum women, each tool performed extremely well for both MDD (Fig. 1) and MDD/MnDD (Fig. 2), with AUCs ≥ 0.9. In addition, all three screening tools were highly accurate when used in the prenatal or postpartum period; comparison of AUCs revealed no statistically significant differences between the screening tools (Table 3). There were no statistically significant differences in the AUCs for the screening tools for the overall sample, prenatal group, or postpartum group; this was true when comparing the AUCs for detecting MDD and MDD/MnDD (Table 3).
Fig. 1.
ROC curves for MDD.
Fig. 2.
ROC curves for MDD/MnDD.
Table 3.
Accuracy of each screening tool and comparison of screening tools for perinatal women overall and by perinatal group.
| Group | MDD |
MDD/MnDD |
||||
|---|---|---|---|---|---|---|
| AUC (95% CI) | Group χ2 | p | AUC (95% CI) | Group χ2 | p | |
| A11(N = 95) | ||||||
| BDI-II | 0.95 (0.89–1.00) | 0.28 | 0.87 | 0.91 (0.84–0.98) | 0.50 | 0.78 |
| CES-D | 0.96 (0.93–0.99) | 0.94 (0.89–0.98) | ||||
| EPDS | 0.96 (0.92–1.00) | 0.93 (0.88–0.98) | ||||
| Prenatal (n = 32) | ||||||
| BDI-II | 0.96 (0.88–1.00) | 1.06 | 0.59 | 0.96 (0.88–1.00) | 1.06 | 0.59 |
| CES-D | 0.95 (0.88–1.00) | 0.95 (0.88–1.00) | ||||
| EPDS | 0.93 (0.83–1.00) | 0.93 (0.83–1.00) | ||||
| Postpartum (n = 63) | ||||||
| BDI-II | 0.95 (0.88–1.00) | 0.29 | 0.88 | 0.90 (0.82–0.99) | 0.49 | 0.78 |
| CES-D | 0.97 (0.93–1.00) | 0.93 (0.88–0.99) | ||||
| EPDS | 0.97 (0.92–1.00) | 0.92 (0.86–0.99) | ||||
Abbreviations: MDD, major depressive disorder; MnDD, minor depressive disorder; ROC, receiver operator characteristic; AUC, area under the curve; BDI-II, Beck Depression Inventory II; CES-D, Center for Epidemiological Studies Depression Scale; EPDS, Edinburgh Postnatal Depression Scale.
3.3. Sensitivity and specificity of screening tools
Sensitivity and specificity were calculated for each of the screening tools according to standard cutoff scores. For all three screening tools, cutoffs for the prenatal period, the postpartum period, and the perinatal period overall that corresponded to the optional sensitivities and specificities are presented in Table 4. For the BDI-II, the optimal overall cutoff scores for MDD and MDD/MnDD were lower than the commonly recommended cutoff scores. For the CES-D, the optimal overall cutoff score was lower for MDD but was higher for MDD/MnDD than the standard cutoff scores. The optimal cutoff score on the EPDS for MDD/MnDD was consistent with the standard cutoff score prescribed but was slightly lower for the standard cutoff used to detect MDD.
Table 4.
Sensitivity, specificity, and optimal cutoff scores.
| Standard |
Optimal overall |
Optimal prenatal |
Optimal postpartum |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cutoff score |
Sensitivity % |
Specificity % |
Cutoff score |
Sensitivity % |
Specificity % |
Cutoff score |
Sensitivity % |
Specificity % |
Cutoff score |
Sensitivity % |
Specificity % |
|
| BDI-II | ||||||||||||
| MDD | ≥ 20 | 48.2 | 100.0 | ≥ 14 | 88.9 | 92.7 | ≥ 13 | 85.7 | 80.0 | ≥ 14 | 90.0 | 90.7 |
| MDD/MnDD | ≥ 14 | 81.3 | 95.2 | ≥ 12 | 84.4 | 81.0 | – | – | – | ≥ 12 | 84.0 | 84.2 |
| CES-D | ||||||||||||
| MDD | ≥ 24 | 70.4 | 92.7 | ≥ 21 | 92.6 | 88.2 | ≥ 22 | 85.7 | 88.0 | ≥ 21 | 90.0 | 88.4 |
| MDD/MnDD | ≥ 16 | 100.0 | 65.1 | ≥ 20 | 87.5 | 81.0 | – | – | – | ≥ 20 | 84.0 | 81.6 |
| EPDS | ||||||||||||
| MDD | ≥ 13 | 81.5 | 95.6 | ≥ 11 | 88.9 | 91.2 | ≥ 10 | 85.7 | 84.0 | ≥ 11 | 85.0 | 93.0 |
| MDD/MnDD | ≥ 10 | 84.4 | 81.0 | ≥ 10 | 84.4 | 81.0 | – | – | – | ≥ 10 | 84.0 | 79.0 |
Abbreviations: MDD, major depressive disorder; MnDD, minor depressive disorder; ROC, receiver operator characteristic; AUC, area under the curve; BDI, Beck Depression Inventory; CES-D, Center for Epidemiological Studies Depression Scale; EPDS, Edinburgh Postnatal Depression Scale.
4. Discussion
The purpose of the present study was to determine the accuracy of three depression screening tools in a perinatal sample of low-income African American women enrolled in home visitation programs. Similar to other studies that have conducted diagnostic interviews with low-income African American women (Hobfoll et al., 1995; Melville et al., 2010), we found that major depression was common, with just over 25% of the women we interviewed exhibiting major depression. This finding combined with the deleterious effects associated with perinatal depression on both mother and child underscores the need to identify screening tools and appropriate cutoff scores that can quickly and effectively detect which low-income African American women are suffering from major depression.
Our results revealed that all three screening tools—the EPDS, CES-D, and BDI-II—were quite accurate in detecting major depression. Each tool also performed well in assessing women with major or minor depression, with sensitivity and specificities similar to those seen when assessing only major depression. Moreover, we found that each tool performed equally well when identifying major or minor depression among prenatal and postpartum women. Our findings that multiple screening tools work equally well for assessing depressive symptoms in low-income African American women build on previous work conducted by Chaudron et al. (2010). Similar to the present study, Chaudron et al. (2010) found that the EPDS and BDI-II were highly accurate in detecting major or minor depression.
The present study also mirrored the findings of Chaudron et al. (2010) suggesting that the optimal cutoffs for detecting perinatal depression among each screening tool may be lower than generally recommended (i.e., standard) cutoffs. All three screening tools had decreased sensitivity when using standard cutoffs, which may lead to missing women who are in the midst of a depressive episode. The discrepancy between standard and optimal cutoffs was most pronounced with the BDI-II and CES-D suggesting that these two tools—neither of which were developed explicitly for a perinatal population—may require lower cutoffs when used with perinatal women. It should be noted that the optimal cutoffs did lower the specificity of each screening tool. Given that these screening tools are typically used by practitioners to identify women in need of further assessment for depression, this over-identification of women may result in additional, unnecessary diagnostic work on the part of health providers. However, we believe that that the greatly increased sensitivities of each screening tool associated with the optimal cutoffs generated by this study outweigh this potential provider burden.
4.1. Study limitations and strengths
There are several characteristics of our sample that are strengths of this study. First, this study is among the first to examine the accuracy of perinatal depression screening tools with low-income African American women (Bennett et al., 2008; Chaudron et al., 2010; Gjerdingen et al., 2009). It is also the first to our knowledge to recruit such a population from home visitation programs—a setting in which a sizable number of perinatal women enroll in each year. Moreover, we are aware of only one previous study (Chaudron et al., 2010) that has compared the accuracy of multiple depression screening tools among low-income African American women. The present study also examined the accuracy of multiple screening tools among pregnant women as well as postpartum—an important extension of Chaudron et al.’s (2010) study with low-income African American that focused solely on postpartum women. Establishing the accuracy of depression screening tools for prenatal women is an important addition, as early treatment can avert the pernicious effects depression can have on the development of healthy attachment relationships once the baby is born.
There are several limitations that should be noted. First, the women enrolled in this study were recruited from three home visitation programs in an urban context which may limit the generalizability of these findings to other populations of low-income African American women. Second, it was not possible to determine optimal prenatal cutoffs for each screening tool for identifying major or minor depression due to the absence of women who met criteria for minor depression during pregnancy. Future studies with larger numbers of prenatal enrollees are necessary to establish whether optimal prenatal cutoffs are lower than standard cutoffs for different screening tools, as was true when establishing cutoffs for major depression.
4.2. Clinical relevance
Every year in America over 400,000 babies are born to depressed mothers making perinatal depression the most under-diagnosed obstetric complication (Earls et al., 2010). It is well established that postpartum depression leads to wide array of negative outcomes both for the individual as well as society (National Research Council and Institute of Medicine, 2009). The pernicious effects of a mother’s depression on their infant’s development are evident in the short term–including increased risk for disruptions in breastfeeding, bonding and attachment (Earls et al., 2010; Grace et al., 2003; Lovejoy et al., 2000)—as well as an increased risk for psychopathology as the child grows older (National Research Council and Institute of Medicine, 2009). Researchers have also highlighted the association between prenatal depression and preterm births as well as poorer compliance with well-child and preventive practices—which have implications for the family as well as the health care system (Chung et al., 2004; Field et al., 2009; McLearn et al., 2006; Minkovitz et al., 2005; Phillips et al., 2010).
Despite this compelling evidence, the recommendations concerning routine depression screening from national professional organizations remain conflicted. The U.S. Preventive Services Task Force (2002) recommended routine screening in 2002; and then updated that recommendation in 2009, adding language that emphasized the need for staff supports to ensure appropriate follow-up. But the American College of Obstetricians and Gynecologists, Committee on Obstetric Practice (2010) recently concluded there is currently “insufficient evidence to support a firm recommendation for universal antepartum or postpartum screening. There are also insufficient data to recommend how often screening should be done” (p. 394). One of the ongoing obstacles to implementing effective screening in primary care settings is a lack of reliable referral sources to support the physicians who identify women in need of treatment (Gjerdingen & Yawn, 2007; U.S. Preventive Services Task Force, 2009).
The results from our study suggest that effective and low cost screening tools are available that are valid for use with low-income African American pregnant and postpartum women—whose rates of clinically significant depression are higher than the general population. They also underscore the feasibility of and need for screening women who are involved in home visitation programs. Intensive outreach, childcare, transportation are necessary to overcome barriers to depression treatment for low-income African American and Latina women identified in WIC and family planning clinics (Miranda et al., 2003). Home visiting programs may be better able to assist with the case management and follow-up that are needed to ensure that women find appropriate community-based treatments. Screening for depression in home visiting can also identify women who are at high risk for depression so that effective preventive interventions can be offered (Tandon et al.,2011).
5. Conclusions
Consistent with previous research, major depression was highly prevalent in our sample of low-income African American women. Three depression screening tools—the EPDS, CES-D, and BDI-II—all accurately detected both major depression and major and minor depression in prenatal and postpartum samples, suggesting that practitioners working with women across the perinatal period should feel comfortable using these tools. However, practitioners should consider using lower cutoff scores than those recommended by screening tool developers to most effectively identify low-income African American women in need of depression treatment.
Acknowledgments
Role of funding source
Funding for this study was provided by the Thomas Wilson Sanitarium; the funding agency had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit this paper for publication.
We would like to thank the Thomas Wilson Sanitarium for the funding to conduct this study. Shani Braxton was the licensed clinical social worker who conducted the fieldwork described in this study. We are also greatly appreciative of the support and guidance provided by the three home visiting programs from which we recruited study participants.
Footnotes
Conflict of interest
None of the authors have any conflicts of interest to report in association with the work described in this manuscript.
References
- Altshuler L, Cohen L, Vitonis A, Faraone S, Harlow B, Suri R, Frieder R, Stowe ZN. The Pregnancy Depression Scale (PDS): a screening tool for depression in pregnancy. Arch. Womens Ment Health. 2008;11:277–285. doi: 10.1007/s00737-008-0020-y. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. Committee on Obstetric Practice, 2010. Committee opinion no. 453: screening for depression during and after pregnancy. Obstet. Gynecol. 115:394. doi: 10.1097/AOG.0b013e3181d035aa. [DOI] [PubMed] [Google Scholar]
- Baisch MJ, Carey LK, Conway AE, Mounts KO. Perinatal depression: a health marketing campaign to improve screening. Nurs. Womens Health. 2010;14:20–33. doi: 10.1111/j.1751-486X.2010.01504.x. [DOI] [PubMed] [Google Scholar]
- Beck CT. A meta-analysis of predictors of postpartum depression. Nurs. Res. 1996;45:297–303. doi: 10.1097/00006199-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Beck CT. Predictors of postpartum depression: an update. Nurs. Res. 2001;50:275–285. doi: 10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Beeghly M, Olson KL, Weinberg MK, Pierre SC, Downey N, Tronick EZ. Prevalence, stability, and socio-demographic correlates of depressive symptoms in black mothers during the first 18 months postpartum. Matern. Child Health J. 2003;7:157–168. doi: 10.1023/a:1025132320321. [DOI] [PubMed] [Google Scholar]
- Bennett IM, Coco A, Coyne JC, Mitchell AJ, Nicholson J, Johnson E, Horst M, Ratcliffe S. Efficiency of a two-item pre-screen to reduce the burden of depression screening in pregnancy and postpartum: an IMPLICIT network study. J. Am. Board Fam. Med. 2008;21:317–325. doi: 10.3122/jabfm.2008.04.080048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd R, Le HN, Somberg R. Review of screening instruments for postpartum depression. Arch. Womens Ment. Health. 2005;8:141–153. doi: 10.1007/s00737-005-0096-6. [DOI] [PubMed] [Google Scholar]
- Center for Health Statistics. Preconception and interconception health status of women who recently gave birth to a live-born infant—Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 26 Reporting Areas, 2004. 2008 Website accessed at: http://www.cdc.gov/mmwr/preview/mmwrhtml/ss5610al.htm Retrieved January 18, 2011. [PubMed]
- Chaudron LH, Szilagyi PG, Kitzman HJ, Wadkins HI, Conwell Y. Detection of postpartum depressive symptoms by screening at well-child visits. Pediatrics. 2004;113:551–558. doi: 10.1542/peds.113.3.551. [DOI] [PubMed] [Google Scholar]
- Chaudron LH, Szilagyi PG, Tang W, Anson E, Talbot NL, Wadkins HI, Tu X, Wisner KL. Accuracy of depression screening tools for identifying postpartum depression among urban mothers. Pediatrics. 2010;125:609–617. doi: 10.1542/peds.2008-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, McCollum KF, Elo IT, Lee HJ, Culhane JF. Maternal depressive symptoms and infant health practices among low-income women. Pediatrics. 2004;113:523–529. doi: 10.1542/peds.113.6.e523. [DOI] [PubMed] [Google Scholar]
- Cogill S, Caplan H, Alexendra H, Robson K, Kumar R. Impact of maternal postnatal depression on cognitive development of young children. BMJ. 2005;292:1165–1167. doi: 10.1136/bmj.292.6529.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- DeLong E, DeLong DM, Clarke-Pearson D. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Diaz MA, Le HN, Cooper BA, Muñoz RF. Interpersonal factors and perinatal depressive symptomatology in a low-income Latina sample. Cult. Divers. Ethnic Minor. Psychol. 2007;13(4):328–336. doi: 10.1037/1099-9809.13.4.328. [DOI] [PubMed] [Google Scholar]
- Earls MF The Committee on Psychosocial Aspects of Child and Family Health. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032–1039. doi: 10.1542/peds.2010-2348. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Deeds O, Holder V, Schlanberg S, Kuhn C. Depressed pregnant black women have a greater incidence of prematurity and low birthweight outcomes. Infant Behav. Dev. 2009;32:10–16. doi: 10.1016/j.infbeh.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Biometrics Research. New York: New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. (SCID-I/NP) [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr K, Swinson T, Gartlhner G, Brody S, Miller WC. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid. Rep. Technol. Assess. (Summ.) 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiopoulos AM, Bryan TL, Yawn BP, Houston MS, Rummans TA, Therneau TM. Population-based screening for postpartum depression. Obstet. Gynecol. 1999;93:653–657. doi: 10.1016/s0029-7844(98)00543-2. [DOI] [PubMed] [Google Scholar]
- Gjerdingen DK, Yawn BP. Postpartum depression screening: importance, methods, barriers, and recommendations for practice. J. Am. Board Fam. Med. 2007;20(3):280–288. doi: 10.3122/jabfm.2007.03.060171. [DOI] [PubMed] [Google Scholar]
- Gjerdingen D, Crow S, McGovern P, Miner M, Center B. Postpartum depression screening at well-child visits: validity of a 2-question screen and the PHQ-9. Ann. Fam. Med. 2009;7(1):63–70. doi: 10.1370/afm.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomby D. Home Visitation in 2005: Outcomes for Children and Parents. Washington, DC: Committee for Economic Development Invest in Kids Working Group; 2005. [Google Scholar]
- Goodman JH, Tyer-Viola L. Detection, treatment, and referral of perinatal depression and anxiety by obstetrical providers. J. Womens Health. 2010;19:477–490. doi: 10.1089/jwh.2008.1352. [DOI] [PubMed] [Google Scholar]
- Goyal D, Gay C, Lee KA. How much does low socioeconomic status increase the risk of prenatal and postpartum depressive symptoms in first-time mothers? Womens Health Issues. 2010;20:96–104. doi: 10.1016/j.whi.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch. Womens Ment. Health. 2003;6:263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- Hanusa BH, Scholle SH, Haskett RF, Spadaro K, Wisner KL. Screening for depression in the postpartum period: a comparison of three instruments. J. Womens Health. 2008;17:585–596. doi: 10.1089/jwh.2006.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilemann M, Frutos L, Lee K, Kury FS. Protective strength factors, resources, and risks in relation to depressive symptoms among childbearing women of Mexican descent. Health Care Women Int. 2004;25:88–106. doi: 10.1080/07399330490253265. [DOI] [PubMed] [Google Scholar]
- Hipwell A, Goossens F, Melhuish E, Kumar R. Severe maternal psychopathology and infant-mother attachment. Dev. Psychopathol. 2000;12:157–175. doi: 10.1017/s0954579400002030. [DOI] [PubMed] [Google Scholar]
- Hobfoll SE, Ritter C, Lavin J, Hulsizer MR, Cameron RP. Depression prevalence and incidence among inner-city pregnant and postpartum women. J. Consult. Clin. Psychol. 1995;63:445–453. doi: 10.1037//0022-006x.63.3.445. [DOI] [PubMed] [Google Scholar]
- Johnson K. State-Based Home Visiting: Strengthening Programs through State Leadership. Washington, DC: National Center for Children in Poverty; 2009. [Google Scholar]
- Jones L, Scott J, Cooper C, Forty L, Smith KG, Sham P, Farmer A, McGuffin P, Craddock N, Jones I. Cognitive style, personality and vulnerability to postnatal depression. Br. J. Psychiatry. 2010;196:200–205. doi: 10.1192/bjp.bp.109.064683. [DOI] [PubMed] [Google Scholar]
- Kelly RH, Zatzick DF, Anders TF. The detection and treatment of psychiatric disorders and substance use among pregnant women cared for in obstetrics. Am. J. Psychiatry. 2001;158:213–219. doi: 10.1176/appi.ajp.158.2.213. [DOI] [PubMed] [Google Scholar]
- LaRocco-Cockburn A, Melville J, Bell M, Katon W. Depression screening attitudes and practices among obstetrician-gynecologists. Obset. Gynecol. 2003;101:892–898. doi: 10.1016/s0029-7844(03)00171-6. [DOI] [PubMed] [Google Scholar]
- Logsdon MC, Myers JA. Comparative performance of two depression screening instruments in adolescent mothers. J. Womens Health. 2010;19(6):1123–1128. doi: 10.1089/jwh.2009.1511. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E, Neuman G. Maternal depression and parenting behavior: a meta-analytic review. Clin. Psychol. Rev. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Luke S, Salihu HM, Alio AP, Mbah AK, Jeffers D, Berry EL, Mishkit VR. Risk factors for major antenatal depression among low-income African American women. J. Womens Health. 2009;18:1841–1846. doi: 10.1089/jwh.2008.1261. [DOI] [PubMed] [Google Scholar]
- Mandl KD, Tronick EZ, Brennan TA, Alpert HR, Homer CJ. Infant health care use and maternal depression. Arch. Pediatr. Adolesc. Med. 1999;153:808–813. doi: 10.1001/archpedi.153.8.808. [DOI] [PubMed] [Google Scholar]
- Marcus SM, Flynn HA, Blow FC, Barry KL. Depressive symptoms among pregnant women screened in obstetrics settings. J. Womens Health. 2003;12:373–380. doi: 10.1089/154099903765448880. [DOI] [PubMed] [Google Scholar]
- McLearn K, Minkovitz C, Strobino D, Marks E, Hou W. The timing of maternal depressive symptoms and mothers’ parenting practices with young children: implications for pediatric practice. Pediatrics. 2006;118:174–182. doi: 10.1542/peds.2005-1551. [DOI] [PubMed] [Google Scholar]
- Melville JL, Gavin A, Guo Y, Fan MY, Katon WJ. Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstet. Gynecol. 2010;116:1064–1070. doi: 10.1097/AOG.0b013e3181f60b0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L, Shade M, Vasireddy V. Beyond screening: assessment of perinatal depression in a perinatal care setting. Arch. Womens Ment. Health. 2009;12:329–334. doi: 10.1007/s00737-009-0082-5. [DOI] [PubMed] [Google Scholar]
- Minkovitz CS, Strobino D, Scharfstein D, Hou W, Miller T, Mistry KB, Swartz K. Maternal depressive symptoms and children’s receipt of health care in the first three years of life. Pediatrics. 2005;115(2):306–314. doi: 10.1542/peds.2004-0341. [DOI] [PubMed] [Google Scholar]
- Miranda J, Chung JY, Green BL, Krupnick J, Siddique J, Revicki DA, Belin T. Treating depression in predominantly low-income young minority women: a randomized controlled trial. JAMA. 2003;290:57–65. doi: 10.1001/jama.290.1.57. [DOI] [PubMed] [Google Scholar]
- Murray L, Sinclair D, Cooper P, Ducournau P, Turner P, Stein A. The socioemotional development of 5-year-old children of postnatally depressed mothers. J Child Psychol Psychiatry. 1999;40:1259–1271. [PubMed] [Google Scholar]
- Murray L, Halligan SL, Goodyer I, Herbert J. Disturbances in early parenting of depressed mothers and Cortisol secretion in offspring: a preliminary study. J. Affect. Disord. 2010;122:218–223. doi: 10.1016/j.jad.2009.06.034. [DOI] [PubMed] [Google Scholar]
- National Research Council and Institute of Medicine. Depression in Parents, Parenting and Children: Opportunities to Improve Identification, Treatment and Prevention. Washington DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- Norr KF, Crittenden KS, Lehrer EL, Reyes O, Boyd CB, Nacion KW, Watanabe K. Maternal and infant outcomes at one year for a nurse-health advocate home visiting program serving African Americans and Mexican Americans. Public Health Nurs. 2003;20:190–203. doi: 10.1046/j.0737-1209.2003.20306.x. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Swain AM. Rates and risk of postpartum depression: a meta-analysis. Int. Rev. Psychiatry. 1996;8:37–54. [Google Scholar]
- Olson A, Dietrich AJ, Prazar G, Hurley J, Tuddenham A, Hedberg V, Napinsky DA. Two approaches to maternal depression screening during well child visits. J. Dev. Behav. Peditr. 2005;26:169–176. doi: 10.1097/00004703-200506000-00002. [DOI] [PubMed] [Google Scholar]
- Orr S, James SA, Blackmore-Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African American women in Baltimore. Am. J. Epidemio. 2002;9:803–805. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- Phillips GS, Wise LA, Rich-Edwards JW, Stampfer MJ, Rosenberg L. Prepregnancy depressive symptoms and preterm birth in the Black Women’s Health Study. Ann. Epidemiol. 2010;20:8–15. doi: 10.1016/j.annepidem.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psych. Meas. 1977;1:385–401. [Google Scholar]
- Rich-Edwards JW, Kleinman K, Abrams A, Harlow BL, McLaughlin TJ, Joffe H, Gillman M. Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. J. Epidemiol. Community Health. 2006;60:221–227. doi: 10.1136/jech.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehusen DA, Baldwin LM, Runkle GP, Clark G. Are family physicians appropriately screening for postpartum depression. J. Am. Board Fam. Pract. 2005;18:104–112. doi: 10.3122/jabfm.18.2.104. [DOI] [PubMed] [Google Scholar]
- Segre LS, O’Hara MW, Arndt S, Stuart S. The prevalence of postpartum depression: the relative significance of three social status indices. Soc. Psychiatry Psychiatr. Epidemiol. 2007;42:316–321. doi: 10.1007/s00127-007-0168-1. [DOI] [PubMed] [Google Scholar]
- Segre LS, O’Hara MW, Arndt S, Beck CT. Nursing care for postpartum depression, part 1: do nurses think they should offer both screening and counseling? Am. J. Matern. Child Nurs. 2010;35:220–225. doi: 10.1097/NMC.0b013e3181dd9d81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin LS, Potvin L, St-Denis M, Loiselle J. Socio-environmental factors and postnatal depressive symptomatology: a longitudinal study. Womens Health. 1999a;29:57–69. doi: 10.1300/j013v29n01_05. [DOI] [PubMed] [Google Scholar]
- Seguin L, Potvin L, St-Denis M, Loiselle J. Depressive symptoms in the late postpartum among low socioeconomic status women. Birth. 1999b;26:157–163. doi: 10.1046/j.1523-536x.1999.00157.x. [DOI] [PubMed] [Google Scholar]
- Sheeder J, Kabir K, Stafford B. Screening for postpartum depression at well-child visits: is once enough during the first 6 months of life? Pediatrics. 2009;123:982–988. doi: 10.1542/peds.2008-1160. [DOI] [PubMed] [Google Scholar]
- Sohr-Preston SL, Scaramella LV. Implications of timing of maternal depressive symptoms for cognitive and language development during early childhood. Clin. Child Fam. Psych. Rev. 2006;9:65–83. doi: 10.1007/s10567-006-0004-2. [DOI] [PubMed] [Google Scholar]
- Tandon SD, Parillo KM, Jenkins CJ, Duggan AK. Home visitors’ recognition of and response to malleable risk factors among low-income pregnant and parenting women. Matern. Child Health. 2005;9:273–283. doi: 10.1007/s10995-005-0012-8. [DOI] [PubMed] [Google Scholar]
- Tandon SD, Perry DF, Mendelson T, Kemp K, Leis J. Preventing perinatal depression in low-income home visiting clients: a randomized controlled trial. J. Cons. Clin. Psych. 2011;79:707–712. doi: 10.1037/a0024895. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture. About WIC: WIC at a glance. 2011 Website accessed at: http://www.fns.usda.gov/wic/aboutwic/wicataglance.htm. Retrieved February 24, 2011.
- U.S. Preventive Services Task Force. Screening for Depression. Washington DC: Agency for Healthcare Research and Quality; 2002. [Google Scholar]
- U.S. Preventive Services Task Force. Screening for Depression in Adults. Washington DC: Agency for Healthcare Research and Quality; 2009. [Google Scholar]
- VanDoesum K, Hosman C, Riksen-Walraven J, Hoefnagels C. Correlates of depressed mothers’ sensitivity toward their infants: the role of maternal, child, and contextual characteristics. J. Am. Acad. Child Adol. Psych. 2007;46:747–756. doi: 10.1097/CHI.0b013e318040b272. [DOI] [PubMed] [Google Scholar]
- Wisner K, Perel J, Peindl K, Hanusa B. Timing of depression recurrence in the first year after birth. J. Affect. Disord. 2004;78:249–252. doi: 10.1016/S0165-0327(02)00305-1. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Moses-Kolko EL, Sit DK. Postpartum depression: a disorder in search of a definition. Arch. Womens Ment. Health. 2010;13:37–40. doi: 10.1007/s00737-009-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Ramin SM, Rush AJ, Navarrete CM, Carmody T, March D, Heartwell SF, Leveno KJ. Onset and persistence of postpartum depression in an inner-city maternal health clinic system. Am. J. Psychiatry. 2001;158:1856–1863. doi: 10.1176/appi.ajp.158.11.1856. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, Lockwood C. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet. Gynecol. 2009;114:703–713. doi: 10.1097/AOG.0b013e3181ba0632. [DOI] [PMC free article] [PubMed] [Google Scholar]