Abstract
Both mature BDNF and its precursor, proBDNF, play a crucial role in shaping neurons and contributing to the structural basis for neuronal connectivity. They do so in a largely opposing manner, and through differential engagement with their receptors. In this review, we will summarise the evidence that BDNF modulates neural circuit formation in vivo both within the central and peripheral nervous systems, through the control of neuronal morphology. The underlying intracellular mechanisms that translate BDNF signalling into changes of neuronal cell shape will be described. In addition, the signalling pathways that act either locally at the site of BDNF action, or over long distances to influence gene transcription will be discussed. These mechanisms begin to explain the diversity of actions that BDNF carries out on neuronal morphology.
Introduction
The development of the peripheral nervous system (PNS) includes a phase of widespread programmed cell death in order to selectively maintain appropriate connections. During this period, neurons approaching the same target compete for limited amounts of target-derived survival factors, which accounts for the selective survival of those neurons that projected to the correct target (Levi-Montalcini, 1987). Neurotrophins, such as BDNF, act as target-derived growth factors. BDNF was originally isolated from pig brain (Barde et al., 1982) and represented the second member of the neurotrophin family after NGF. Neurotrophins mediate axonal growth and neuronal survival of select subpopulations of peripheral neurons, such as cultured chick embryonic dorsal root ganglia neurons (Lindsay et al., 1985). Accordingly, BDNF null mice display reduced cell numbers in a variety of sympathetic and sensory ganglia, including the trigeminal, vestibular, dorsal root and superior cervical ganglia (Ernfors et al., 1994). BDNF has been shown to act on the nervous system in multiple ways, including promoting the differentiation, survival, growth and arborisation of neurons, as well as strengthening synaptic function.
Similar to other secreted factors, all neurotrophins, including BDNF, are initially synthesized as proneurotrophins, which are then cleaved by proteases to mature proteins of 118–120 amino acids (Chao and Bothwell, 2002). The prodomain of neurotrophins is essential for the correct folding of the mature ligand and for its targeting to the secretory pathway. In fact, a frequent human polymorphism within the BDNF prodomain, Val66Met, interferes with BDNF sorting to the regulated secretory pathway, and consequently leads to a reduced level of activity-dependent secretion of BDNF without affecting constitutive release (Chen et al., 2004; Egan et al., 2003). This is thought to be due to a disrupted interaction with the sorting receptor sortilin, which interacts with BDNF in a region encompassing the Val66Met substitution. Accordingly, downregulation of sortilin leads to a decrease in BDNF secretion from the regulated pathway, without affecting constitutive secretion (Chen et al., 2005). A separate study identified the carboxypeptidase E binding motif I16E18I105D106 within the mature domain of BDNF, and mutation of the acidic residues within this motif, or loss of carboxypeptidase E, also led to missorting of BDNF into the constitutive secretory pathway (Lou et al., 2005).
The prodomain of neurotrophins acts not only to aid in the folding and secretion of the mature neurotrophin, but the precursor can also act as an active signalling molecule itself. Intriguingly, proneurotrophins display largely opposing effects compared to their mature counterparts (Fig. 1) (Lu et al., 2005). The possible secretion of proBDNF was initially controversial (Matsumoto et al., 2008; Yang et al., 2009) due to detection issues, but a function for endogenous proBDNF during development is now established. For example, in contrast to the survival-promoting effects of target-derived mature BDNF, proBDNF can play an active role in developmental motor neuron programmed cell death and motor axon pruning of rejected connections (Je et al., 2012; Taylor et al., 2012). In addition, abundant evidence exists to show that proNGF is upregulated in a variety of injury and neurodegenerative paradigms, and both proneurotrophins can contribute to neuronal cell death (Friedman, 2010; Lee et al., 2001b; Teng et al., 2005; Teng et al., 2010).
Fig. 1. Both BDNF and its precursor, proBDNF are biologically active.
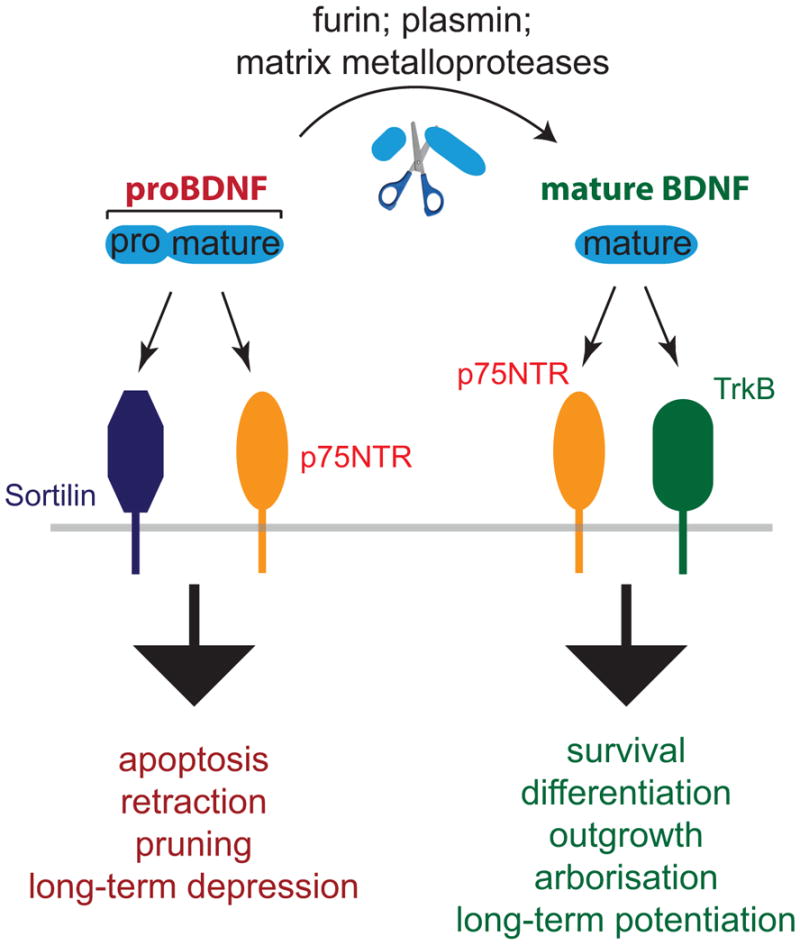
Mature BDNF is generated through elimination of the prodomain from the precursor, either intra- or extracellularly, and signals through TrkB to mediate neuronal growth and survival. Unprocessed proBDNF is also biologically active, signalling through p75NTR and Sortilin or related Vps10p family members to mediate process pruning and apoptosis.
The opposing actions of mature and proBDNF are mediated through differential receptor engagement. Mature BDNF preferentially binds and activates the receptor tyrosine kinase, TrkB. Activity-dependent release of synaptic BDNF acting on TrkB receptors results in enhanced synaptic transmission and synaptic plasticity (Nagappan and Lu, 2005; Park and Poo, 2013). In addition, BDNF can bind to the p75 pan-neurotrophin receptor (p75NTR), a member of the tumour necrosis factor (TNF) family of transmembrane receptors. Like Fas and TNF receptor, p75NTR possesses a canonical death domain in its C-terminus. The precursor form proBDNF binds with high affinity to p75NTR, and also engages sortilin and other members of the Vps10p family of sorting receptors, which are transmembrane proteins involved in protein trafficking (Fig. 1). Thus, the cellular responses to pro- and mature neurotrophins can either engage Trk receptors, p75NTR and sortilin family members (Teng et al., 2010) in different combinations.
In this chapter, we will describe the actions of the precursor and the mature forms of BDNF upon neuronal morphology. Specifically, we will discuss short-range responses to growth factors that modulate neuronal shape locally, together with signalling cascades that are relayed back to the soma, which utilize different Trk, p75NTR and sortilin family members.
Building the underlying scaffold - BDNF action on axonal and dendritic growth is context dependent
The activities of neurotrophins extend well beyond neuronal survival and death, and include the initiation of molecular mechanisms underlying neuronal growth and arborisation, as well as the strengthening of synaptic transmission (Park and Poo, 2013). The overlapping expression of multiple neurotrophin receptors and their cognate ligands allows for the creation and remodelling of multiple modes of connectivity, a process that extends into adulthood. A major mechanism is through the modulation of neuronal morphology (Fig. 2). BDNF and its precursor can signal through TrkB and p75NTR differentially to promote neural circuit formation. TrkB is widely expressed throughout the central nervous system (CNS), as well as in select populations of the PNS, such as parasympathetic and subpopulations of sensory neurons. The p75NTR protein, on the other hand, is expressed in most PNS neurons, but expression within the CNS is restricted to early development. Later on, p75NTR expression can be upregulated following injury, inflammation or traumatic circumstances (Lee et al., 2001a). Accordingly, genetic deletion of p75NTR in mice interferes with developmental axon pruning (Park et al., 2010; Singh et al., 2008) and ameliorates cell death after injury or damage (Harrington et al., 2004).
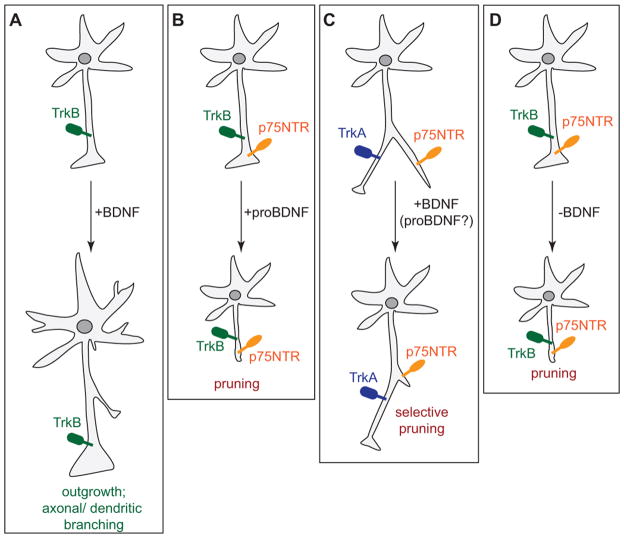
Fig. 2. Different modes of BDNF action on neuronal growth.
(A) BDNF signals through TrkB to enhance axonal and dendritic growth and arborisation. (B) The BDNF precursor proBDNF has an opposing effect. It signals through p75NTR to decrease neurite outgrowth and initiate growth cone collapse. (C) In absence of TrkB, BDNF activates p75NTR to induce axonal pruning. (D) Sudden withdrawal of BDNF not only stops enhancing outgrowth, but also leads to dying back of axons.
BDNF is best described for its ability to promote neuronal growth and as an activity dependent gene. A consequence is that the underlying structure allows a neuron to connect with multiple targets and to form elaborate networks affected by BDNF (Fig. 2A). For example, early experiments showed that treatment of organotypic slices of the ferret visual cortex with BDNF greatly enhances the dendritic complexity of layer 4 pyramidal neurons (McAllister et al., 1995). In addition, manipulating BDNF levels in the optic tectum of living Xenopus laevis tadpoles results in rapid changes in axonal complexity in retinal ganglion cells. Raising BDNF levels by supplementing exogenous BDNF leads to an increase in axonal arbour formation, while reducing BDNF by injecting blocking antibodies results in a decrease in complexity (Cohen-Cory and Fraser, 1995). In line with these experiments, targeted deletion of BDNF in mice from differentiated excitatory CNS neurons leads to neuronal growth defects. In this model, deficits in dendritic growth and spine density were observed most prominently in the striatum and to a lesser degree also in the hippocampus (Rauskolb et al., 2010).
These observations indicate that alterations in the amounts of BDNF can result in different morphological outcomes. Interestingly, raising retinal BDNF levels in X. laevis tadpoles has an opposing effect to that of raising tectal BDNF, in that retinal BDNF inhibits dendritic complexity of retinal ganglion cells, without affecting axonal arborisation (Lom et al., 2002). The fact that retinal ganglion cells thus respond differentially to BDNF depending on whether the signal was initiated at the post- or presynaptic site suggests that the location of signal initiation is crucial. This might be due to either differential expression of alternative receptors for BDNF, or to differential engagement of downstream signalling components as a result of distinct intracellular availability.
The mechanisms that foster process retraction and degeneration have been of considerable interest (Kaplan and Miller, 2003; Luo and O’Leary, 2005). A prominent example of differential BDNF action is on the degeneration of axons, which has been shown to take place in sympathetic neurons through binding to p75NTR (Singh et al., 2008). BDNF-induced axonal pruning in mouse superior cervical ganglion neurons requires activity-dependent secretion of BDNF. In this scenario, BDNF engages p75NTR in the absence of TrkB receptor expression to mediate developmental axon pruning (Fig. 2C). Specifically, activity-dependent BDNF synthesis by appropriately connected superior cervical ganglion axons has been postulated to initiate the degeneration of neighbouring, inactive axons via activation of p75NTR (Singh et al., 2008).
Although these studies show that BDNF binding to p75NTR exerts a negative effect upon sympathetic neurons in the absence of TrkB, proneurotrophins also play a role in degeneration or pruning. In dissociated chick retinal ganglion cells, axonal repulsion and reduced branching were facilitated by an interaction between p75NTR and the ephrinA ligand (Lim et al., 2008), a repellent cue. The BDNF precursor, proBDNF, was found to activate p75NTR and ephrinA5 to mediate axonal repulsion and reduce axonal arborisation (Marler et al., 2010) (Fig. 2B). Therefore, while BDNF binding to TrkB enhances retinal axon branching, proBDNF decreases the level of branching. Hence, the two forms of BDNF protein produce antagonistic results in the same population of retinal cells.
Another way that BDNF can shape neural circuits occurs after loss or sudden withdrawal of BDNF from sensory axons (Fig. 2D). A recent study demonstrated that sensory innervation of the mammary glands at early developmental stages depends upon sex hormone-dependent regulation of BDNF signalling. At E13, upregulation of gonad-derived androgen signalling in male embryos initiates mesenchymal expression of truncated TrkB (Liu et al., 2012), which sequesters the available BDNF and therefore blocks normal BDNF-TrkB signalling. Lack of growth-promoting BDNF-TrkB signalling in turn leads to degeneration of male sensory axons and ultimately to regression of mammary glands in the male embryos. Interestingly, the axonal degeneration observed in male, and not female, embryos was independent of apoptosis, arguing for a localised action. Thus, neurotrophin signalling can produce a sexually dimorphic pattern of sensory innervation leading to regression of neural circuits (Liu et al., 2012). An interesting question is whether in the absence of growth-promoting BDNF-signalling, there may be an active p75NTR-dependent pruning mechanism operating to mediate the axonal degeneration that was observed in the male embryos.
Molecular mechanisms underlying BDNF action on growth and pruning
The intracellular mechanisms that BDNF uses to alternatively promote growth or pruning of axons depend upon the extent of TrkB or p75NTR activation. These mechanisms must feed into the cytoskeletal rearrangements required for axonal and dendritic outgrowth and arborisation. In the following sections, we will summarise recent work on the mechanisms that underlie axonal growth and growth arrest.
BDNF induces collateral branch formation via a combination of local and long-range signals
The formation and maintenance of axonal branches is tightly coupled to activity- dependent events. In this regard, BDNF provides an example of a highly regulated growth factor that links to intracellular processes to change axon structure. One pathway leading to collateral branching in cortical axons requires a combination of local and long-range signalling events to locally impact on microtubule function (Jeanneteau et al., 2010). In this mechanism, BDNF-mediated TrkB activation at the distal axon was shown to induce a retrograde Erk1/2 signal, which in turn activates immediate early gene transcription in the nucleus. Among the multiple immediate early genes induced by BDNF signalling is a MAPK phosphatase, MKP-1 (DUSP1). Basal MKP-1 expression is low both in vitro and in vivo, but BDNF-Erk1/2 signalling induces a quick rise in MKP-1 protein levels, followed by rapid turnover through the ubiquitin/proteasome pathway (Brondello et al., 1999). However, in addition to initiating MKP-1 protein synthesis, continuing BDNF-Erk1/2 signalling also results in MKP-1 phosphorylation locally, which interferes with MKP-1 degradation.
One target of MKP-1 action in neurons is c-Jun N-terminal kinase (JNK). Prolonged MKP-1 activity locally shuts down JNK activity near sites of continuing BDNF signalling and thus affects the phosphorylation status of JNK substrates (Jeanneteau et al 2010), many of which are involved in cytoskeletal dynamics. Indeed, lack of JNK activity leads to decreased microtubule stability and increased microtubule dynamics in a temporally and spatially controlled manner. Therefore, MKP-1 provides a tightly regulated signalling mechanism for promoting local cytoskeletal remodelling that is required for collateral branch formation (Jeanneteau and Deinhardt, 2011; Jeanneteau et al., 2010). The action of MKP-1 downstream of BDNF provides a dual role for BDNF signalling in axonal branch formation. First, BDNF initiates immediate early gene activation. This leads to transcriptional and translational changes that have the potential to affect the morphology of the neuron. Second, long lasting BDNF-TrkB signalling leads to the stabilisation and continued activity of MKP-1, an immediate early gene product that acts proximal to the site of signal initiation (Fig. 3A). The stabilisation of MKP-1 by activity-dependent BDNF explains the ability of BDNF to act in a localized manner in neurons.
Fig. 3. Intracellular mechanisms of BDNF action on neuronal morphology.
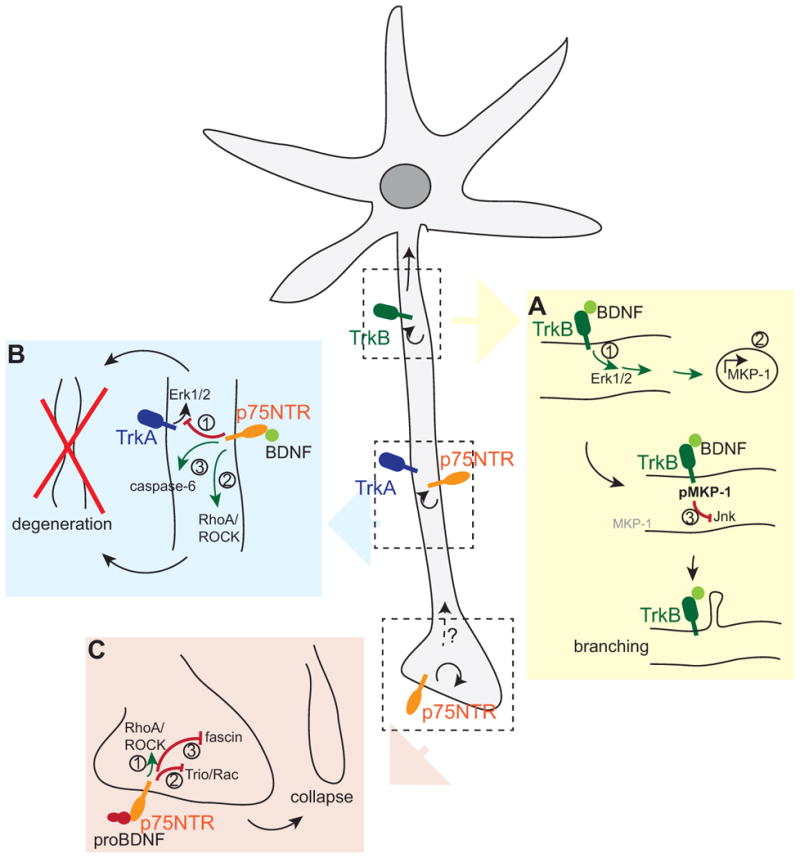
BDNF and its precursor signal locally (curved arrows) as well as over long distances (straight arrows) to modify neuronal morphology. (A) BDNF-TrkB elicit a retrograde Erk1/2 signal (1) to initiate immediate early gene expression (2). Continuing BDNF-TrkB signalling stabilises one of the immediate early genes, MKP-1, through phosphorylation and thus locally inhibits Jnk signalling (3), leading to collateral branch formation. (B) In absence of TrkB, BDNF signals through p75NTR to (1) dampen TrkA signaling, (2) activate the RhoA/ROCK pathway and (3) activate caspase-6 to initiate axonal degeneration. (C) ProBDNF signals through p75NTR to (1) activate the RhoA-ROCK pathway or inactivate (2) Rac and (3) fascin to initiate growth cone collapse.
A closely related neurotrophin, NT-3, displays another mechanism for promoting branching and growth of processes through increasing the levels of β-catenin (Kundel et al., 2009), a component of the Wnt signalling pathway and of cadherin-based adherens junctions (Valenta et al., 2012). NT-3 activates the local translation of β-catenin in hippocampal neurons, specifically in growth cones to increase growth (Kundel et al., 2009). Interestingly, NT-3 binds not only to TrkC receptors, but also to TrkB and p75NTR receptors. proNT-3 acts similarly to proBDNF in promoting cell death of sympathetic neurons (Yano et al., 2009). In addition, it is likely protein synthesis-dependent processes are regulated by mature neurotrophins to influence neuronal morphogenesis.
BDNF-p75NTR signalling induces axonal degeneration, but not cell death
In addition to axonal growth, BDNF can mediate degeneration of sympathetic axons, which express the NGF-receptor TrkA and p75NTR. Interestingly, sympathetic neurons do not express the BDNF receptor TrkB. Nevertheless, BDNF can be secreted from active axons to exclusively signal via p75NTR. The two receptors in sympathetic neurons, p75NTR and TrkA, can form high affinity binding sites for NGF depending upon the ratio of the two receptors (Chao and Hempstead, 1995; Hempstead et al., 1991). However, BDNF can act through p75NTR alone, which accumulates within the inactive, degenerating axon, where it can suppress NGF-TrkA signals (Singh et al., 2008) (Fig. 3B). This produces an alternative consequence, in which Erk1/2 activation is reduced in response to the sympathetic bona fide trophic factor, NGF. In this scenario, the BDNF-p75NTR signal is restricted to the degenerating axon, where the target-derived growth signal is suppressed. On the other hand, the surviving axon remains intact andis appropriately connected.
Another mechanism of negative control of axonal growth involves components of myelin, which are largely responsible for inhibitory signalling during regeneration (Filbin, 2003). BDNF-p75NTR signalling plays a role in myelin-induced axonal degeneration of sympathetic axons. In limiting amounts of NGF, sympathetic axons retract when grown on myelin. This is a localised phenomenon, as the degeneration is limited to the axon segment in direct contact with myelin, and does not spread to affect the entire axon. Myelin/BDNF/p75NTR signalling was shown to activate the RhoA-ROCK-cofilin pathway (Park et al 2010), which mediates axonal retraction (Fig. 3B). The Rho family of GTPases, Rho, Rac and Cdc42, are regulators of the actin cytoskeleton and directly influences neurite growth (Hall, 2005; Murakoshi et al., 2011). Interestingly, blocking the RhoA pathway inhibited axonal degeneration, but did not rescue BDNF-p75NTR/myelin-induced growth inhibition. Similarly, BDNF also led to caspase-6 activation, and blocking caspase activity rescued degeneration, but not the myelin-induced growth inhibition. Therefore, BDNF-p75NTR signalling can potentially lead to axonal degeneration via multiple pathways. In this instance, p75NTR signalling predominates over Trk tyrosine kinase activity.
ProBDNF discourages outgrowth
Proneurotrophins have been shown to inhibit neurite outgrowth and to collapse neuronal growth cones in dorsal root ganglia neurons, cortical and hippocampal neurons (Deinhardt et al., 2011; Sun et al., 2012). The growth inhibiting activities assigned to mature BDNF signalling through p75NTR may also be a consequence of the precursor to BDNF rather than mature BDNF, which promotes growth cone turning and growth. The responses of growth cones to proBDNF and mature BDNF are at least partially derived from local protein synthesis. For example, BDNF initiates phosphorylation of mRNA binding proteins such as zipcode binding protein, thus leading to local translation of β-actin to stimulate BDNF-induced growth cone turning (Sasaki et al., 2010).
Interestingly, hippocampal neurons secrete pro- rather than mature BDNF in response to activity (Pang et al, 2004), raising the possibility that a pruning signal may be initiated by the precursor of BDNF. Proneurotrophins achieve their growth-dampening action by signalling to the actin cytoskeleton. ProNGF for example signals through a complex of p75NTR and the Vps10p-domain containing sorting receptor, SorCS2, to mediate growth cone collapse in cultured hippocampal neurons (Deinhardt et al., 2011). Downstream of proNGF, the Rac activator Trio dissociates from the receptor complex, thus leading to a decrease in active Rac, which is a member of the family of Rho GTPases that normally mediates actin filament assembly and growth. In parallel, proNGF binding also leads to an inactivation of the actin filament bundling protein fascin, thus destabilising existing filaments. The combination of both proneurotrophin activites initiates a robust growth cone collapse response (Deinhardt et al., 2011) (Fig. 3C). These mechanisms are equally activated downstream of proBDNF, suggesting that in certain scenarios, proneurotrophins may substitute for each other. An alternative mechanism of proBDNF action on the actin cytoskeleton is the activation of the RhoA/ROCK/cofilin pathway downstream of p75NTR (Sun et al., 2012), which is also activated in sympathetic axon degeneration (Park et al., 2010). RhoA signalling led to a decrease in neurite outgrowth both in DRG and cortical neurons in response to proBDNF (Sun et al., 2012) (Fig. 3C). Interestingly, local axonal RhoA protein synthesis is required for growth cone collapse in response to the guidance molecule, semaphorin 3A (Wu et al., 2005), raising the possibility that local translation may also be part of the proneurotrophin response.
Building synapses – molecular mechanisms underlying BDNF-mediated filopodia and spine formation
The conversion of pro- to mature BDNF occurs through many different proteolytic mechanisms. A prominent mechanism is via the tissue plasminogen activator (tPA)/plasmin system, which has potent enzymatic activity that cleaves proBDNF to mature BDNF. It is also critically required for the late-phase long-term potentiation (L-LTP) of hippocampal synapses (Baranes et al., 1998; Pang et al., 2004). Accordingly, reduced levels of BDNF as well as the lack of the plasmin precursor, plasminogen, or its activator, tPA abolish the expression of L-LTP (Pang et al., 2004). This suggests that BDNF is released in its proform upon neuronal activity in hippocampal neurons. Interestingly, a complementary study showed that proBDNF-p75NTR signalling promotes hippocampal long-term depression (LTD) (Woo et al., 2005). However, LTD was found to be unaffected in slices prepared from mice lacking BDNF in excitatory neurons (Matsumoto et al., 2008), suggesting that the source of proBDNF might be non-neuronal. Together, these studies suggest a bidirectional control for pro- and mature BDNF in hippocampal synaptic plasticity. In fact, tPA was found to be co-released with proBDNF during a high-frequency stimulation, but not during low frequency stimulation, implying that the relative extracellular amount of pro- versus mature BDNF is controlled through the regulated secretion of converting proteases in response to neuronal activity (Nagappan et al., 2009). This allows for a spatially tightly controlled mechanism to regulate pro- versus mature BDNF action at individual synapses, and thus the precise strengthening or weakening of synaptic connections. This is at least in part achieved through changes in the underlying structure.
BDNF enhances filopodial growth and dendritic spine formation
In addition to promoting or inhibiting growth and branching of entire axons and dendrites, BDNF and proBDNF also differentially modulate finer structures, such as spines, that form the basis of connectivity in established neurons. They do so largely by signalling to the actin cytoskeleton. For example, BDNF-TrkB activates Erk1/2 signalling, which leads to the phosphorylation and thus inactivation of the actin capping protein, Eps8. This in turn disinhibits actin filament growth and thus increases the number of axonal filopodia in hippocampal neurons (Menna et al., 2009). A second study showed that BDNF/TrkB signalling enhances dendritic filopodial motility through a phosphatidylinositol–3 kinase dependent pathway (Luikart et al., 2008). Filopodia are largely thought to be the precursors of synaptic contacts, with dendritic filopodia maturing into spines and axonal filopodia maturing into presynaptic specialisations upon stabilisation. Thus, enhanced filopodial activity may amplify the number of synaptogenic contacts and therefore precede increased synaptic connectivity. Moreover, it was shown that BDNF stimulates the growth of existing dendritic spine heads, which is correlated with a strengthening of the synaptic contact. It does so by stimulating a Rac activator, the guanine nucleotide exchange factor Vav2, therefore leading to increased Rac activity. Conversely, deletion of Vav impairs spine head growth and leads to deficits in hippocampal long-term plasticity (Hale et al., 2011).
proBDNF reduces dendritic spine formation
Since an increase in active Rac downstream of BDNF leads to spine head growth in mature hippocampal cultures, and proBDNF leads to a decrease of Rac activity in young hippocampal neurons, it is tempting to speculate the proBDNF may have adverse effects on spines in a more mature system. Indeed, application of a cleavage-resistant version of proBDNF to mature hippocampal cultures leads to a decrease in spine density over the course of two to three days, while mature BDNF increases spine density in the same setting (Koshimizu et al., 2009). The decrease in spine density is accompanied by a decrease in the amplitude of synaptic currents, and requires p75NTR (Koshimizu et al., 2009). Curiously, Rac activity was increased rather than decreased downstream of proBDNF in cerebellar granule neurons, and was accompanied by activation of JNK and caspase-3 to initiate cell death (Koshimizu et al., 2010). These differences observed may either be a consequence of different cell types assessed, or of the severity of the insult to the neuron. ProBDNF has been found to be capable of initiating apoptosis in peripheral neurons (Teng et al., 2005), and proNGF induces cell death in the hippocampus following seizures (Le and Friedman, 2012; Volosin et al., 2008). However, proneurotrophin effects upon neuronal morphology do not necessarily lead to cell death (Deinhardt et al., 2011). Additionally, in hippocampal neurons the activation of JNK and caspase-3 have been shown to function in the removal of synaptic AMPA receptors during the depression of synapses and for the generation of LTD (Li et al., 2010; Zhu et al., 2005). Therefore, in hippocampal neurons the activation of the Rac-Jnk and caspase pathways by proBDNF might affect synaptic efficacy independently of inducing apoptosis and of proBDNF’s action on synaptic structure.
Conclusions
We have discussed the roles of BDNF and its precursor in modulating neuronal connectivity through modifying the morphology of individual neurons. BDNF’s growth promoting effects have been well characterised in the past, and comprise both local activities to fine-tune the structure, such as in the case of locally activating Rac to promote spine head growth and inactivating eps8 to initiate filopodia formation. In addition, BDNF signalling also triggers a robust transcriptional response, as well as local translational events, which in turn have the potential to affect the morphology and physiology of the entire cell, including sites distant to the initial signal. Therefore, a combination of local and long-range signals may ultimately determine the cellular response, such as in the case of MKP-1 induction to promote axonal branch formation.
In contrast, the degeneration signals initiated by BDNF, such as the activation of RhoA/ROCK pathway, are largely locally restricted. For example, selectively the part of the axon growing on myelin degenerates, or the less active axon is pruned, without affecting growth in other areas of the cell. Similarly, proBDNF acts specifically on the growth cone or spine. To what extend proneurotrophins also signal over long distances to prime a whole cellular response on morphology, as they do to initiate apoptosis, remains to be investigated.
Highlights.
In this review we discuss
The opposing roles of BDNF and its precursor, proBDNF on neuronal morphology
How differential receptor engagement by BDNF influences growth vs pruning decisions
The underlying molecular mechanisms leading to morphological changes
Localised vs global changes in morphology in response to BDNF
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondello JM, Pouyssegur J, McKenzie FR. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science. 1999;286:2514–2517. doi: 10.1126/science.286.5449.2514. [DOI] [PubMed] [Google Scholar]
- Chao MV, Bothwell M. Neurotrophins: to cleave or not to cleave. Neuron. 2002;33:9–12. doi: 10.1016/s0896-6273(01)00573-6. [DOI] [PubMed] [Google Scholar]
- Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci. 2005;25:6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Kim T, Spellman DS, Mains RE, Eipper BA, Neubert TA, Chao MV, Hempstead BL. Neuronal growth cone retraction relies on proneurotrophin receptor signaling through Rac. Sci Signal. 2011;4:ra82. doi: 10.1126/scisignal.2002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Friedman WJ. Proneurotrophins, seizures, and neuronal apoptosis. Neuroscientist. 2010;16:244–252. doi: 10.1177/1073858409349903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CF, Dietz KC, Varela JA, Wood CB, Zirlin BC, Leverich LS, Greene RW, Cowan CW. Essential role for vav Guanine nucleotide exchange factors in brain-derived neurotrophic factor-induced dendritic spine growth and synapse plasticity. J Neurosci. 2011;31:12426–12436. doi: 10.1523/JNEUROSCI.0685-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci U S A. 2004;101:6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Je HS, Yang F, Ji Y, Nagappan G, Hempstead BL, Lu B. Role of pro-brain-derived neurotrophic factor (proBDNF) to mature BDNF conversion in activity-dependent competition at developing neuromuscular synapses. Proc Natl Acad Sci U S A. 2012;109:15924–15929. doi: 10.1073/pnas.1207767109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanneteau F, Deinhardt K. Fine-tuning MAPK signaling in the brain: The role of MKP-1. Commun Integr Biol. 2011;4:281–283. doi: 10.4161/cib.4.3.14766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanneteau F, Deinhardt K, Miyoshi G, Bennett AM, Chao MV. The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nat Neurosci. 2010;13:1373–1379. doi: 10.1038/nn.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Axon growth inhibition: signals from the p75 neurotrophin receptor. Nat Neurosci. 2003;6:435–436. doi: 10.1038/nn0503-435. [DOI] [PubMed] [Google Scholar]
- Koshimizu H, Hazama S, Hara T, Ogura A, Kojima M. Distinct signaling pathways of precursor BDNF and mature BDNF in cultured cerebellar granule neurons. Neurosci Lett. 2010;473:229–232. doi: 10.1016/j.neulet.2010.02.055. [DOI] [PubMed] [Google Scholar]
- Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K, Nagappan G, Zaitsev E, Hirokawa T, Tatsu Y, Ogura A, Lu B, Kojima M. Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol Brain. 2009;2:27. doi: 10.1186/1756-6606-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundel M, Jones KJ, Shin CY, Wells DG. Cytoplasmic polyadenylation element-binding protein regulates neurotrophin-3-dependent beta-catenin mRNA translation in developing hippocampal neurons. J Neurosci. 2009;29:13630–13639. doi: 10.1523/JNEUROSCI.2910-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AP, Friedman WJ. Matrix metalloproteinase-7 regulates cleavage of pro-nerve growth factor and is neuroprotective following kainic acid-induced seizures. J Neurosci. 2012;32:703–712. doi: 10.1523/JNEUROSCI.4128-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Kim AH, Khursigara G, Chao MV. The uniqueness of being a neurotrophin receptor. Curr Opin Neurobiol. 2001a;11:281–286. doi: 10.1016/s0959-4388(00)00209-9. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001b;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Li Z, Jo J, Jia JM, Lo SC, Whitcomb DJ, Jiao S, Cho K, Sheng M. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YS, McLaughlin T, Sung TC, Santiago A, Lee KF, O’Leary DD. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–758. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RM, Thoenen H, Barde YA. Placode and neural crest-derived sensory neurons are responsive at early developmental stages to brain-derived neurotrophic factor. Dev Biol. 1985;112:319–328. doi: 10.1016/0012-1606(85)90402-6. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rutlin M, Huang S, Barrick CA, Wang F, Jones KR, Tessarollo L, Ginty DD. Sexually dimorphic BDNF signaling directs sensory innervation of the mammary gland. Science. 2012;338:1357–1360. doi: 10.1126/science.1228258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lom B, Cogen J, Sanchez AL, Vu T, Cohen-Cory S. Local and target-derived brain-derived neurotrophic factor exert opposing effects on the dendritic arborization of retinal ganglion cells in vivo. J Neurosci. 2002;22:7639–7649. doi: 10.1523/JNEUROSCI.22-17-07639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase e. Neuron. 2005;45:245–255. doi: 10.1016/j.neuron.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Luikart BW, Zhang W, Wayman GA, Kwon CH, Westbrook GL, Parada LF. Neurotrophin-dependent dendritic filopodial motility: a convergence on PI3K signaling. J Neurosci. 2008;28:7006–7012. doi: 10.1523/JNEUROSCI.0195-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, O’Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- Marler KJ, Poopalasundaram S, Broom ER, Wentzel C, Drescher U. Pro-neurotrophins secreted from retinal ganglion cell axons are necessary for ephrinA-p75NTR-mediated axon guidance. Neural Dev. 2010;5:30. doi: 10.1186/1749-8104-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, Barde YA. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci. 2008;11:131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Menna E, Disanza A, Cagnoli C, Schenk U, Gelsomino G, Frittoli E, Hertzog M, Offenhauser N, Sawallisch C, Kreienkamp HJ, Gertler FB, Di Fiore PP, Scita G, Matteoli M. Eps8 regulates axonal filopodia in hippocampal neurons in response to brain-derived neurotrophic factor (BDNF) PLoS Biol. 2009;7:e1000138. doi: 10.1371/journal.pbio.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagappan G, Lu B. Activity-dependent modulation of the BDNF receptor TrkB: mechanisms and implications. Trends Neurosci. 2005;28:464–471. doi: 10.1016/j.tins.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Nagappan G, Zaitsev E, Senatorov VV, Jr, Yang J, Hempstead BL, Lu B. Control of extracellular cleavage of ProBDNF by high frequency neuronal activity. Proc Natl Acad Sci U S A. 2009;106:1267–1272. doi: 10.1073/pnas.0807322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Park KJ, Grosso CA, Aubert I, Kaplan DR, Miller FD. p75NTR-dependent, myelin-mediated axonal degeneration regulates neural connectivity in the adult brain. Nat Neurosci. 2010;13:559–566. doi: 10.1038/nn.2513. [DOI] [PubMed] [Google Scholar]
- Rauskolb S, Zagrebelsky M, Dreznjak A, Deogracias R, Matsumoto T, Wiese S, Erne B, Sendtner M, Schaeren-Wiemers N, Korte M, Barde YA. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J Neurosci. 2010;30:1739–1749. doi: 10.1523/JNEUROSCI.5100-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Welshhans K, Wen Z, Yao J, Xu M, Goshima Y, Zheng JQ, Bassell GJ. Phosphorylation of zipcode binding protein 1 is required for brain-derived neurotrophic factor signaling of local beta-actin synthesis and growth cone turning. J Neurosci. 2010;30:9349–9358. doi: 10.1523/JNEUROSCI.0499-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR, Miller FD. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci. 2008;11:649–658. doi: 10.1038/nn.2114. [DOI] [PubMed] [Google Scholar]
- Sun Y, Lim Y, Li F, Liu S, Lu JJ, Haberberger R, Zhong JH, Zhou XF. ProBDNF collapses neurite outgrowth of primary neurons by activating RhoA. PLoS One. 2012;7:e35883. doi: 10.1371/journal.pone.0035883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AR, Gifondorwa DJ, Robinson MB, Strupe JL, Prevette D, Johnson JE, Hempstead B, Oppenheim RW, Milligan CE. Motoneuron programmed cell death in response to proBDNF. Dev Neurobiol. 2012;72:699–712. doi: 10.1002/dneu.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng KK, Felice S, Kim T, Hempstead BL. Understanding proneurotrophin actions: Recent advances and challenges. Dev Neurobiol. 2010;70:350–359. doi: 10.1002/dneu.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volosin M, Trotter C, Cragnolini A, Kenchappa RS, Light M, Hempstead BL, Carter BD, Friedman WJ. Induction of proneurotrophins and activation of p75NTR-mediated apoptosis via neurotrophin receptor-interacting factor in hippocampal neurons after seizures. J Neurosci. 2008;28:9870–9879. doi: 10.1523/JNEUROSCI.2841-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, Jaffrey SR. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, Chen ZY, Mark W, Tessarollo L, Lee FS, Lu B, Hempstead BL. Neuronal release of proBDNF. Nat Neurosci. 2009;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Torkin R, Martin LA, Chao MV, Teng KK. Proneurotrophin-3 is a neuronal apoptotic ligand: evidence for retrograde-directed cell killing. J Neurosci. 2009;29:14790–14802. doi: 10.1523/JNEUROSCI.2059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Pak D, Qin Y, McCormack SG, Kim MJ, Baumgart JP, Velamoor V, Auberson YP, Osten P, van Aelst L, Sheng M, Zhu JJ. Rap2-JNK removes synaptic AMPA receptors during depotentiation. Neuron. 2005;46:905–916. doi: 10.1016/j.neuron.2005.04.037. [DOI] [PubMed] [Google Scholar]