Abstract
Fossil discoveries over the past 30 years have radically transformed traditional views of Mesozoic mammal evolution. In addition, recent research provides a more detailed account of the Cretaceous diversification of flowering plants. Here, we examine patterns of morphological disparity and functional morphology associated with diet in early mammals. Two analyses were performed: (i) an examination of diversity based on functional dental type rather than higher-level taxonomy, and (ii) a morphometric analysis of jaws, which made use of modern analogues, to assess changes in mammalian morphological and dietary disparity. Results demonstrate a decline in diversity of molar types during the mid-Cretaceous as abundances of triconodonts, symmetrodonts, docodonts and eupantotherians diminished. Multituberculates experience a turnover in functional molar types during the mid-Cretaceous and a shift towards plant-dominated diets during the late Late Cretaceous. Although therians undergo a taxonomic expansion coinciding with the angiosperm radiation, they display small body sizes and a low level of morphological disparity, suggesting an evolutionary shift favouring small insectivores. It is concluded that during the mid-Cretaceous, the period of rapid angiosperm radiation, mammals experienced both a decrease in morphological disparity and a functional shift in dietary morphology that were probably related to changing ecosystems.
Keywords: Mesozoic mammals, morphological disparity, angiosperms, geometric morphometrics, mandibles, functional morphology
1. Introduction
New fossil discoveries over the past 30 years suggest that mammals radiated morphologically and taxonomically throughout the Mesozoic era. They occupied many of the ecomorphological niches that small extant mammal species now inhabit [1], suggesting that Mesozoic mammals achieved significant diversity well before the Caenozoic era. The new wealth of Mesozoic mammal data now makes possible a reassessment of morphological disparity patterns and their possible correlations with ecological changes of the Cretaceous.
Molecular evidence places the origin of angiosperms as far back as the Late Triassic [2]. Angiosperm pollen first appears in the Valanginian–Hauterivian (139–131 Ma) fossil record [3,4], and the Barremian–earliest Aptian (131–124 Ma) has produced the earliest angiosperm macrofossils [4–7]. However, most studies on early angiosperm evolution indicate that flowering plants did not begin to become diverse or abundant until the Late Aptian or Albian, at which point they quickly spread to comprise a considerable portion of the Late Cretaceous flora. Five studies of taxonomic diversity in Cretaceous angiosperms indicate that approximately 118–90 Ma was the period in which angiosperm diversification accelerated to make them a significant component of Mesozoic floral diversity [3,8–11]. The rise of angiosperms is considered to be the most significant event of the Cretaceous terrestrial revolution (KTR), which is defined by Lloyd et al. [12] as a period approximately 125–80 Ma when clades of flowering plants, mammals, lizards, social insects and birds radiated. Angiosperms brought with them the introduction of fruit (though presumably very small and non-fleshy initially [13]), an increase in seeds and an increase in primary productivity capabilities relative to other plant types [14,15]. Insects, especially the orders Coleoptera, Hymenoptera and Lepidoptera, appear to have coevolved and radiated with angiosperms as primary pollinators [16–19].
Although taxonomically diverse by the mid-Cretaceous, angiosperms may not have been ecologically dominant until the end Cretaceous [20], by which time they were abundant in most types of environments [5,11,21], displayed increased diversity and abundance of woody floras [21], and experienced cross-clade increases in leaf vein density [15]. This study puts emphasis on the taxonomic diversification of angiosperms (approx. 118–90 Ma) and the KTR (approx. 125–80 Ma), but it should be considered that evolutionary changes in angiosperm traits during the late Late Cretaceous (83.6–66 Ma) may have had a more direct impact on mammalian dietary evolution.
The concurrent taxonomic radiations of angiosperms and extant mammalian clades in the mid-Cretaceous have been noted in previous literature on biodiversity patterns [12,22] and have been supported by molecular clock data [23–25]. This evidence suggests that mammals diversified taxonomically and morphologically in response to, and at the same time as, the flowering plant and insect radiations of the KTR.
Considering the mid-Cretaceous to be a time of mammalian diversification, however, may not be accurate. Luo [1] shows graphical evidence for a decrease in the taxonomic diversity of several major mammalian clades (e.g. eutriconodonts, docodonts and spalacotheroids) during the mid-Cretaceous, though the author does not discuss this trend. Benson et al. [26] suggest the occurrence of a taxonomic turnover of mammals during the early Late Cretaceous (100–83.6 Ma). The molecular study conducted by Meredith et al. [25] shows a much greater net diversification rate at the end of the KTR (approx. 80 Ma) than during the angiosperm radiation (approx. 118–90 Ma), and recent studies conclude that a majority of extant mammalian orders did not appear and diversify until after the K–Pg boundary [27–29]. Although Wilson et al. [30] note an increase in the dental complexity disparity of multituberculates before the K–Pg boundary, the authors conclude that the diversification did not occur until after the initial angiosperm radiation.
The correlation between angiosperms and the survival or extinction of specific mammalian clades may have had a direct causal relationship (e.g. mammalian herbivores affected by turnover in food sources), an indirect causal relationship (e.g. insectivores affected by insects that were pollinating flowers) or a coincidental one (e.g. survivorship was determined by factors such as metabolic rate that were not directly related to angiosperm diversity). By emphasizing functional traits instead of taxonomic diversity, we focus on aspects of mammalian evolution that would probably coevolve, either directly or indirectly, with changes in plant communities.
In this study, we examined how turnover in dietary morphology in Cretaceous mammals relates to angiosperm diversification. Relative diversity curves of genera belonging to nine dental functional types were tabulated. Changes in relative diversity of these functional groups represent taxon-free turnover in dietary specialization, and contrast with the standard approach of tabulating diversity by higher taxa, whose turnover may or may not have ecological significance [31]. This approach also avoids contentious phylogenetic assignments, focusing instead on features that would have been involved in ecological interactions. Geometric morphometric analysis was used to quantify morphological changes in the jaws of Mesozoic mammals that are correlated with dietary patterns by establishing a functional relationship between shape and diet based on modern mammals. Molar and jaw lengths were used as proxies to study changes in body size disparity, which has functional and physiological consequences for diet in mammals.
2. Material and methods
(a). Diversity of dental functional types
Taxonomic data and temporal ranges of Mesozoic mammals were obtained primarily from the Paleobiology Database [32], with some adjustments made based on information in primary literature and Kielan-Jaworowska et al. [33]. Data are provided in the electronic supplementary material, dataset S1. Diversity data are based purely on the number of genera in 7 Myr time bins. The occurrence at mid-interval ages for all occurrences of a genus was used for determining taxonomic ranges of the genera. Recent studies have cautioned that collection and preservation biases may account for peaks and troughs in dinosaur diversity of the Cretaceous [12,34]. As mammal diversity data are likely to be similarly affected, we measured relative rather than absolute diversity to minimize the effects of sampling error. Spindle diversity curves of figure 1 were produced using Wolfram's Mathematica and Polly's Quantitative Paleontology v. 2.0 [35].
Figure 1.
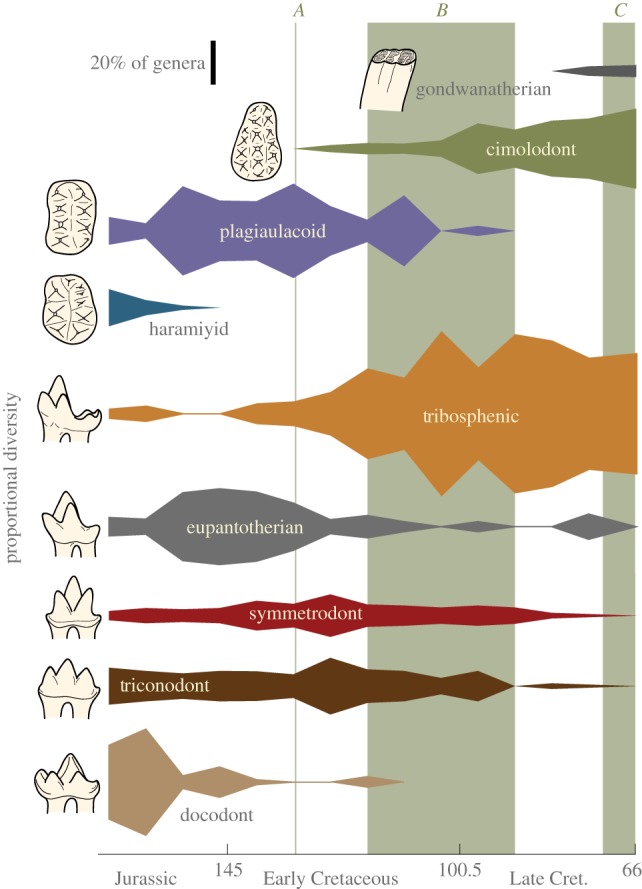
Relative (proportional) generic diversity of mammals by dental functional type. Event A represents the approximate age of the oldest angiosperm fossils [4], event B highlights the period of greatest taxonomic radiation of angiosperms [3,8–11] and event C notes the period of increased leaf vein density in angiosperms [15]. Drawings of plagiaulacoid, cimolodont and haramiyid teeth are of the occlusal surface of a left upper molar. All other drawings are lingual views of right lower molars.
Diversity data for Mesozoic mammal genera were categorized by dental functional type (see electronic supplementary material, table S1 and dataset S1), which was determined primarily by molar morphology [33] (electronic supplementary material).
(b). Geometric morphometrics and m1 lengths
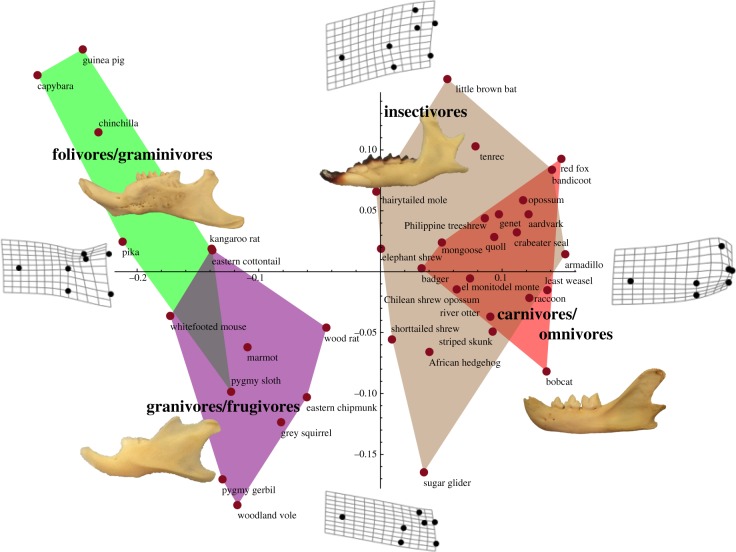
Mandibles from 39 extant mammalian species belonging to 32 families and 18 orders were studied. The buccal surfaces of mandibles from 22 species were digitally photographed at the William R. Adams Zooarchaeology Laboratory at Indiana University. In addition, 17 jaw images were obtained from peer-reviewed sources (see electronic supplementary material, table S2). See the electronic supplementary material for a discussion on the rationale behind the choice of extant species used in this study. Each extant species was categorized into one of four dietary types: (i) insectivores, (ii) flesh-eating carnivores and flesh-eating omnivores, (iii) folivores (leaf eaters) and graminivores (grass eaters), and (iv) frugivores (fruit eaters) and granivores (seed eaters) (see figure 2; electronic supplementary material, table S2). Non-flesh-eating omnivores, which include a wide range of morphologies, were designated to one of the four dietary categories based upon their primary food source.
Figure 2.
Principal component analyses for PC1 and PC2 of the lower jaws of 39 extant mammal species (red points). The polygons represent the morphospaces of the four labelled diet types (see text), with an image of a representative jaw from each category shown. Splines have been added to clarify the morphological differences along PC1 and PC2. See the electronic supplementary material, table S2, for more detailed information about the species.
Images of jaws of 87 extinct mammalian genera were collected from primary literature and Kielan-Jaworowska et al. [33] (see the electronic supplementary material, table S3). Images included original photographs and reconstructions, and only those images that showed a majority of the jaw (roughly 80% or more with at least five of seven landmarks clearly visible) were accepted. Ten genera spanned more than one epoch, and therefore their jaw images were included in each time bin in which they were present. This amounted to 97 total occurrences used for the morphospace results shown in figure 3, although only 87 distinct jaws were analysed.
Figure 3.
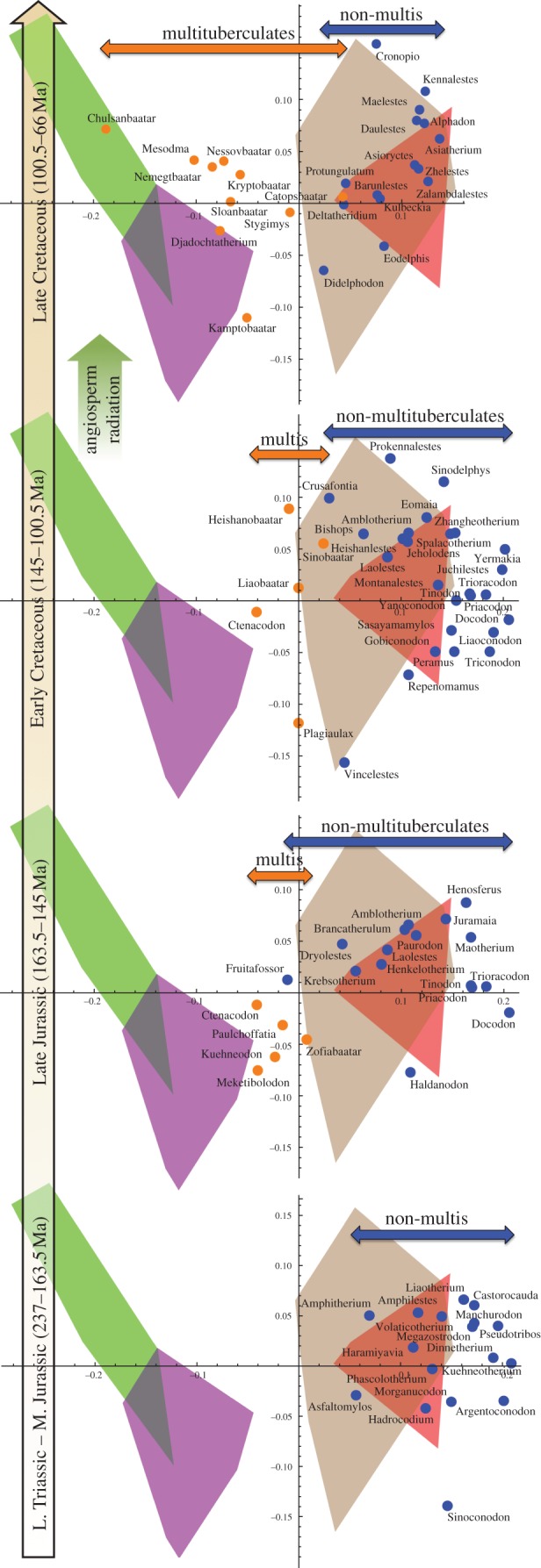
Shift in mandible shape from exclusively insectivorous and carnivorous morphologies (orange and brown polygons) in the Late Triassic–Middle Jurassic to include folivorous, granivorous and frugivorous morphologies (green and purple polygons) by the Late Cretaceous. Mesozoic mandible shapes are projected onto the first two principal component axes of the geometric morphometric space from figure 2. Orange points represent multituberculates and blue points represent non-multituberculates. The orange and blue arrows represent the ranges of the two mammal groups along PC1.
Seven jaw landmarks were chosen based on considerations of functional morphology (see the electronic supplementary material, text and figure S1). The jaw images were analysed using geometric morphometric procedures [36–38] that included a Procrustes analysis [39] and principal components analysis [38] using Wolfram's Mathematica and Polly's Morphometrics v. 8.6.4 [40]. Morphospace results for extant mammal jaws were plotted for the first principal component (PC1) and second principal component (PC2).
The PC1 and PC2 geometric morphometric analysis results of extinct mammal jaws were projected onto the morphospace created by the extant mammal jaw analysis. Projecting the data, rather than combining all data, allowed for a comparison between extinct and extant morphologies without skewing the extant data. The data for extinct species were also separated by epoch, except the Late Triassic through Middle Jurassic, which were combined into one time bin due to small sample sizes for each (figure 3). See Gradstein et al. [41] and the electronic supplementary material, figure S2, for absolute ages of time bins used in figures 3–5. The Procrustes consensus shape for each extinct mammal time bin was determined and morphological disparity was calculated as the average Procrustes distance from each shape to its mean for each time bin [42].
Figure 4.
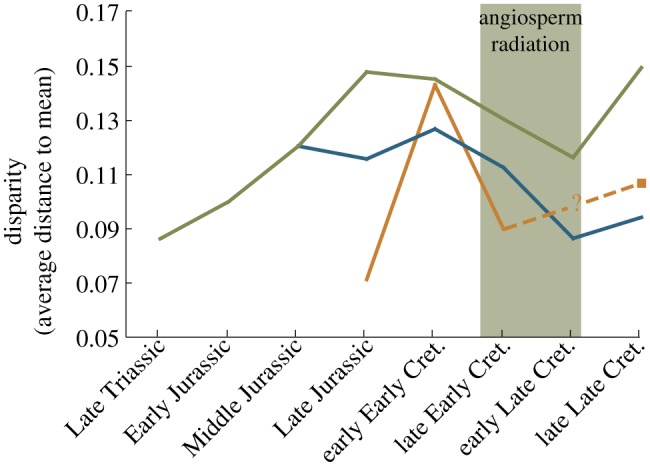
Morphological disparity of Mesozoic mammal mandible shape measured as the average Procrustes distance to the mean for each time bin (based on 87 distinct genera). Disparity was calculated for all mammal genera (green line), non-multituberculate genera (blue line) and exclusively multituberculate genera (orange line). No multituberculate value could be calculated for the early Late Cretaceous because this time period only included one multituberculate jaw.
Figure 5.
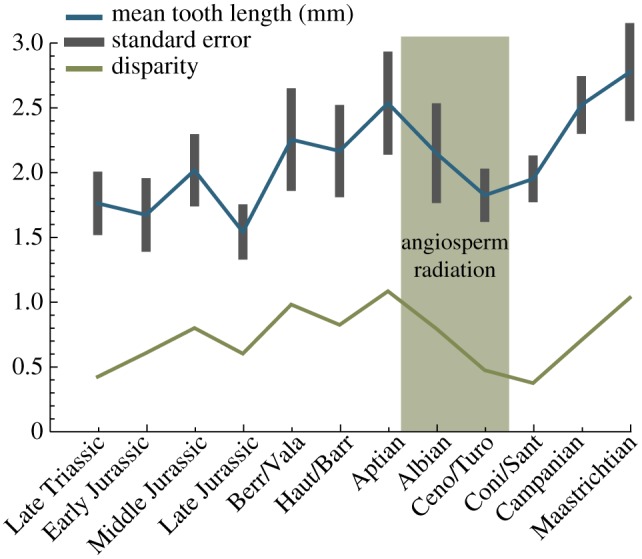
Average (mean) m1 lengths and length disparity for non-multituberculate Mesozoic mammals (based on 135 distinct genera).
Primary literature and Kielan-Jaworowska et al. [33] were used to collect m1 lengths for 135 Mesozoic mammal genera and jaw lengths for 90 Mesozoic mammal genera (see the electronic supplementary material). Disparity was calculated as the average distance between specimens and the mean for each time bin [42]. Standard errors for molar and mandible lengths were calculated from 1000 bootstrap replications in which each time bin was resampled with replacement and the mean recalculated.
3. Results
Figure 1 shows the relative diversity of dental functional groups in the late Middle Jurassic through Cretaceous. The precise timing of diversity changes in mammals is partly obscured by small sample size, poor temporal resolution, biogeographic considerations and limited numbers of localities for Cretaceous fossils [26,33]. Nevertheless, general patterns emerge. Mammals with tribosphenic molars, especially eutherians and metatherians, increased dramatically in diversity during the Cretaceous, offset by the dwindling diversity of triconodonts, symmetrodonts and eupantotherians (stem cladotherians). Multituberculata experienced a shift from plagiaulacoid to cimolodont dentition during the mid-Cretaceous.
Jaws of Mesozoic mammals were compared with jaws of modern analogue species in order to discern diet types and distinguish dietary patterns. Geometric morphometric analysis results were obtained for extant lineages and plotted for PC1 and PC2, which allowed morphospace areas to be defined for four dietary types (figure 2). There was a clear morphological distinction between carnivorous species (insectivores and carnivores/omnivores in figure 2) and herbivorous species (folivores/graminivores and granivores/frugivores in figure 2). Carnivores and flesh-eating omnivores overlapped heavily with insectivores when plotted using any of the first four principal components. Results of an ANOVA analysis indicate that variation attributed to dietary type, as defined by this study, was accounted for by the following amounts: PC1 = 38.1%, PC2 = 6.9%, PC3 = 2.3% and all other principal components are less than 1%. Therefore, location along the x-axis (PC1) appears to be the best indicator of diet type.
One notable difference between herbivore jaws and carnivore jaws is the distance between the angular process and condylar process, best discerned along the PC1 axis. The size of the coronoid process also significantly changes along PC1, decreasing in length and depth in the direction of the herbivorous morphospaces. The splines and example jaws of figure 2 display these differences.
The pattern that emerges is a decrease in diversity and partial contraction in disparity in the insectivorous/carnivorous parts of mammalian jaw morphospace during the mid-Cretaceous, followed by an expansion into herbivorous morphologies. This is seen in figure 3, which shows Mesozoic taxa grouped into four time bins projected into the extant morphospace in which the herbivorous morphologies are on the left and the insectivorous/carnivorous/flesh-eating-omnivorous morphologies are on the right. Prior to the mid-Cretaceous angiosperm radiation, most mammals fell into the right side of this space. In the Cretaceous, the diversity of non-multituberculate functional types decreased, leaving primarily the tribosphenic types, and their occupation of the morphospace contracted. By the Late Cretaceous, both insectivorous and carnivorous morphologies seem to be present. These are not clearly distinguished by jaw morphology, which is similar in these two diet types, but they can be distinguished by size. In our modern mammal sample, the mean jaw length of insectivores is significantly shorter than carnivores/omnivores (see electronic supplementary material, text and table S2). Of the Late Cretaceous genera included in the morphometric jaw analysis (figure 3), five tribosphenic genera that occur in the early Late Cretaceous fall into this insectivorous size range: Alphadon, Barunlestes, Kennalestes, Kulbeckia and Zhelestes (body size is based on tooth and jaw data, which is included with additional non-multituberculate taxa in figure 5 and the electronic supplementary material, figure S2). Their low disparity (figure 4) and small size both suggest that these tribosphenics were limited to small insectivorous ecomorphospaces during the period of the angiosperm radiation. By contrast, Late Cretaceous tribosphenics such as Didelphodon and Deltatheridium show an increase in average body size (figure 5), indicating carnivorous or omnivorous diet types. The exact timing of the Late Cretaceous shift towards herbivory by cimolodontan multituberculates is difficult to pinpoint because, unfortunately, except for Mesodma, there is a gap in the multituberculate jaw record in the early Late Cretaceous.
Morphological disparity in mandible shape, based on geometric morphometric analysis, decreased through the period of angiosperm radiation (figure 4). It should be noted that the multituberculate data are based on a relatively small sample: five jaw images from the Late Jurassic, three from the early Early Cretaceous, four from the late Early Cretaceous, one from the early Late Cretaceous (not included in figure 4) and 10 from the late Late Cretaceous. The near lack of multituberculate specimens in the early Late Cretaceous may help account for the drop in overall jaw morphological disparity during this time.
Figure 5 and the electronic supplementary material, figure S2, show changes in absolute length and length disparity (average deviation of length from the mean) for the first lower molar (m1) and mandible of non-multituberculate Mesozoic mammals. Length and length disparity also decreased through the angiosperm radiation. These results act as a proxy for body size and disparity. Multituberculates were not included within m1 and jaw length calculations, but results of Wilson et al. [30] provided body size estimates that indicated that multituberculates remained relatively small until the late Late Cretaceous, at which point body size and disparity demonstrate a notable increase.
4. Discussion
(a). Diversity of dental functional types
The results in figure 1 demonstrate a turnover of dental functional types during the Cretaceous. This observation is supported by two trends: (i) tribosphenic genera (primarily therians) appear to replace triconodonts, symmetrodonts and eupantotherians, and (ii) cimolodonts replace plagiaulacoids as the dominant multituberculates. The turnover appears to be most drastic during the time period of the angiosperm radiation (approx. 118–90 Ma) and KTR (approx. 125–80 Ma). The conclusion that there was a Cretaceous mammalian turnover are consistent with the results of Benson et al. [26], although they proposed that the turnover occurred in the early Late Cretaceous (100–83.6 Ma).
The mid-Cretaceous changes in diversity of the dental functional types can be interpreted as reflecting an overall decrease in mammalian dental disparity. The Early Cretaceous is represented by many mammalian dental functional types, including tribosphenic, eupantotherian, triconodont, symmetrodont, docodont and plagiaulacoid. The late Late Cretaceous mammalian fauna, on the other hand, was dominated by a decreased number of dental functional types, specifically tribosphenic, eupantotherian and cimolodont (figure 1). The poor fossil record of the mid-Cretaceous may be partially responsible for this decline, but the better-sampled late Late Cretaceous rocks also indicate low dental variety. This mid-Cretaceous decrease in dental disparity is also reflected by a decrease in the length and length disparity of non-multituberculate molars (figure 5).
Figure 1 shows a minor re-emergence of the eupantotherians (stem cladotherians) after the rise of the angiosperms. It should be noted that this may be the result of the biogeographic semi-isolation of South America during the Cretaceous that resulted in an endemic radiation of dryolestoids [33,43], which possessed many dental characteristics of tribosphenic molars, are closely related to therians, and are believed to have been small insectivores [33]. Therians have not been found from the Mesozoic of South America [33]. The dryolestoids may have been occupying the same ecomorphological niche in South America as was occupied elsewhere by radiating therian mammals. Viewing figure 1 with this consideration in mind adds further support to the theory that there was an overall decrease in disparity of mammals during the angiosperm radiation.
It might be assumed that therians radiated as soon as they appeared, quickly overtaking niches during the mid-Cretaceous. However, an early therian, Juramaia, was found in rocks dating back to 160 Ma [44], approximately 40 Myr prior to the therian radiation. In addition, two molecular clock studies have estimated the origin of therians to be at least as early as 190 Ma [45,46]. If therian traits (e.g. tribosphenic molars and internal development of offspring) made them more evolutionarily fit than other mammalian clades, it can be argued that they could have radiated much earlier. However, our data show that therians only experienced a substantial radiation during the time of angiosperm expansion and KTR. The concurrent timing of these radiations offers support to the hypothesis that the proliferation of flowering plants had a causal role, either direct or indirect, in the success of therians.
(b). Jaw morphology patterns
Based on jaw morphology, the Late Cretaceous multituberculates, on average, are more morphometrically similar to the modern herbivore regions of morphospace than were the earlier multituberculates (figure 3). Several of these multituberculates, including Chulsanbaatar and Nemegtbaatar, are within (or are close to) the modern folivore/graminivore morphospace, indicating a shift towards leaf-eating ecological niches. Eriksson et al. [47] show palaeobotanical evidence for an increase in seed dispersal by animals in the Late Cretaceous. The morphospace locations of genera such as Kamptobaatar and Djadochtatherium within or near the granivore/frugivore morphospace support the hypothesis that mammals were assisting early angiosperms with seed dispersal.
Wilson et al. [30] demonstrated that multituberculate dental complexity disparity, average body size and body size disparity do not increase considerably until after the initial angiosperm radiation, implying that the appearance of angiosperms did not have an immediate significant effect on multituberculate morphology and diet. Nine of the 10 Late Cretaceous multituberculates from the geometric morphometric jaw analysis in this study were exclusively from the Campanian (83.5–70.6 Ma) of the late Late Cretaceous, which is well after the initial angiosperm radiation, meaning that the morphological shift towards herbivory illustrated here could also be linked to events that occurred after the taxonomic rise in angiosperms. As postulated by Wilson et al. [30], the eventual shift towards herbivory, as indicated by increased dental complexity, may have been triggered by increases in angiosperm leaf vein density [15] and ecological diversity [11,20,21] during the late Late Cretaceous.
Gondwanatherians offer an additional piece of support for the hypothesis that mammals did not shift significantly towards herbivory until the end of the Cretaceous. These genera possess hypsodont molars, an indicator of herbivory in modern mammals. Gondwanatherians do not appear in the fossil record until the late Late Cretaceous (figure 1), the period in which angiosperms are radiating ecologically.
Interestingly, early multituberculate jaws and modern rodent jaws displayed only a moderate amount of overlap in morphospace, despite considerable morphological similarities (i.e. their jaws possess a diastema, a small coronoid process and procumbent incisors). Multituberculates incorporated a posteriorly directed movement of the mandible during jaw closure, while mandibles of modern rodents move anteriorly [48]. The muscle attachment site for the masseter superficialis of rodents resides in a more posterior position of the lower jaw compared with multituberculates [49]. The angular process of rodents is therefore much more pronounced and further separated from the condylar process. The distance between these processes appears to be a major distinguishing factor of PC1, which helps explain why multituberculates may not have moved into the morphospaces of modern herbivores to a greater extent. Further study of the functional anatomy of Mesozoic mammals and modern analogues is likely to lead to better-informed analyses of the morphological patterns of this study.
The decrease in non-multituberculate disparity in the mid-Cretaceous (figure 4) is driven by the elimination of mammals falling to the right of modern insectivores and carnivore/omnivores in the mandible morphospace shown in figure 3. Many of the genera that occupied this morphospace were eutriconodontans and symmetrodontans. These taxa possessed poorly developed angular processes and large coronoid processes (see splines of figure 2), features that imply different masticatory function than those that persisted. Therefore, the mid-Cretaceous decrease in disparity for non-multituberculates appears to reflect a loss of those jaw morphologies that dominated earlier, pre-angiosperm mammalian faunas.
Non-multituberculates of the early Late Cretaceous appear to be exclusively small insectivores, as evidenced by their locations along the PC1 axis (figure 3), a low level of jaw and tooth morphological disparity (figures 3–5), and small body sizes inferred from tooth and jaw lengths (figure 5; electronic supplementary material, figure S2), as small body size provides an obvious physical limitation for these animals to be carnivores or flesh-eating omnivores. Many of the larger, carnivorous mammals of the Mesozoic were eutriconodonts [33]. Their near extinction during the mid-Cretaceous could help to explain the absence of carnivores/flesh-eating omnivores. It might be expected that other taxa could have displaced the eutriconodonts or radiated into vacated carnivorous roles. However, this does not appear to have occurred. Relatively large Mesozoic mammals do not re-emerge until the late Late Cretaceous (figure 5), with metatherians such as Didelphodon and Eodelphis. The jaws of these large metatherians occupy similar morphospaces to large Early Cretaceous species such as Repenomamus (a known carnivore [50]), Gobiconodon and Vincelestes, providing further evidence that they expanded beyond an exclusively insectivorous diet.
Despite increases in body size and presumed diversifications of diet in the late Late Cretaceous, this time bin shows relatively low morphological disparity values for both multituberculates and non-multituberculates (figure 4). However, the morphological disparity level for all mammals increases considerably during this time. Examination of figure 3 provides the following explanation for these conflicting results: multituberculates drift away from non-multituberculates and towards herbivory, thereby increasing the overall disparity of mammals, while maintaining low within-group disparity.
(c). Why did therians survive and radiate?
Although therians appear to have been suppressed morphologically during the angiosperm radiation, they were the only mammalian group besides cimolodontans to experience a considerable taxonomic radiation during the Cretaceous [26,33] (figure 1). Possession of tribosphenic molars is one trait of therians that may have given them a competitive advantage [1,33,51]. Luo [1] states that these molars, which allowed a novel shearing and puncturing mechanism during mastication, produced a more effective means of faunivory and omnivory. Tribosphenic molars may have been ideal for consumption of insects, some orders of which were likely to have been co-evolving and radiating with angiosperms as pollinators [17–20].
According to Kemp [52], during each major turnover in synapsid fauna (i.e. pelycosaurs to dicynodonts, dicynodonts to cynodonts and cynodonts to mammals), herbivores and large carnivores do not replace other herbivores and large carnivores. Instead, small carnivores seem most successful at surviving and repopulating ecological niches. This scenario may apply to non-multituberculate mammals during the KTR, which appear to have been primarily small, insectivorous carnivores. Kemp notes that huge energy costs for small mammals may create more competition for food sources, which could also help catalyse evolutionary change through natural selection. If the ecosystem changes during the KTR acted as a stressor on mammals in general, then Kemp's hypothesis may offer an explanation as to why small, insectivorous therians emerge from the period and radiate most successfully.
Another hypothesis for the success of therians involves their method of locomotion. Evidence suggests that many early therians were scansorial or arboreal [29,44,53–55], and Shattuck & Williams [55] argue that arboreality increases longevity in mammals. Goswami et al. [54] speculate that arboreality may have experienced a strong selection pressure due to avoidance of predators or exploration of new tropical niches, concluding that the increased grasping ability and flexion required of arboreal creatures could have played a key role in future radiations of therians.
5. Conclusion
The decline in abundance of mammals possessing triconodont, symmetrodont, docodont, plagiaulacoid and eupantotherian dental functional types, coupled with the simultaneous radiation of tribosphenic therians and cimolodont multituberculates, suggests that the genera with the latter dentitions were more successful at coping with the changing ecology of the Cretaceous. The decrease in number of dental functional types also indicates an overall decrease in morphological disparity of molars. Results of a geometric morphometric analysis of jaws, supplemented with non-multituberculate tooth and jaw measurements as a proxy for body size, indicates an early Late Cretaceous dietary shift towards insectivory for non-multituberculates and a Late Cretaceous dietary shift towards herbivory for cimolodont multituberculates. It appears logical to conclude that Cretaceous mammals with plant-supplemented diets benefited from increased plant food sources (e.g. fruit and seeds), while taxa for which insects were a primary food source profited from the coevolution of pollinating insects with angiosperms. However, the story may be more complicated. Multituberculates may not have truly shifted towards herbivory until the late Late Cretaceous [30], a time in which angiosperms expanded into larger ecological roles. Non-multituberculates, which are primarily therians by the Late Cretaceous, demonstrate a decrease in morphological disparity and body size during the angiosperm radiation. A possible explanation for the therians’ taxonomic radiation and simultaneous morphological suppression may be that the clade possessed traits (e.g. small size, insectivorous diet and arboreal locomotion) ideal for the survival of ecological changes brought about by the angiosperm radiation and KTR. These considerations indicate that the ecological changes that occurred during the angiosperm radiation may have been stressful to mammals, leading to a temporary decrease in mammalian disparity.
Acknowledgements
We thank Carrie Vrabel, David Dilcher, Jackson Njau, Wesley Vermillion, Michelle Lawing, Blaire Hensley-Marschand, Allison Bormet and Richard Bykowski for discussions and constructive suggestions. The manuscript benefited greatly from recommendations offered by two anonymous reviewers. Laura Schieber provided access to material in the William R. Adams Zooarchaeology Laboratory at Indiana University.
References
- 1.Luo Z-X. 2007. Transformation and diversification in early mammal evolution. Nature 450, 1011–1019 (doi:10.1038/nature06277) [DOI] [PubMed] [Google Scholar]
- 2.Smith SA, Beaulieu JM, Donoghue MJ. 2010. An uncorrelated relaxed-clock analysis suggests an earlier origin for flowering plants. Proc. Natl Acad. Sci. USA 107, 5897–5902 (doi:10.1073/pnas.1001225107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lupia R, Lidgard S, Crane PR. 1999. Comparing palynological abundance and diversity: implications for biotic replacement during the Cretaceous angiosperm radiation. Paleobiology 25, 305–340 (doi:10.2307/2666001) [Google Scholar]
- 4.Friis EM, Pedersen KR, Crane PR. 2006. Cretaceous angiosperm flowers: innovation and evolution in plant reproduction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 232, 251–293 (doi:10.1016/j.palaeo.2005.07.006) [Google Scholar]
- 5.Hickey L, Doyle J. 1977. Early Cretaceous fossil evidence for angiosperm evolution. Bot. Rev. 43, 2–104 (doi:10.1007/BF02860849) [Google Scholar]
- 6.Sun G, Dilcher DL, Zheng S, Zhou Z. 1998. In search of the first flower: a Jurassic angiosperm, Archaefructus, from northeast China. Science 282, 1692–1695 (doi:10.1126/science.282.5394.1692) [DOI] [PubMed] [Google Scholar]
- 7.Li H. 2003. Lower Cretaceous angiosperm leaf from Wuhe in Anhui, China. Chin. Sci. Bull. 48, 611–614 (doi:10.1360/03tb9129) [Google Scholar]
- 8.Lidgard S, Crane PR. 1990. Angiosperm diversification and Cretaceous floristic trends: a comparison of palynofloras and leaf macrofloras. Paleobiology 16, 77–93 [Google Scholar]
- 9.Heimhofer U, Hochuli PA, Burla S, Dinis JML, Weissert H. 2005. Timing of Early Cretaceous angiosperm diversification and possible links to major paleoenvironmental change. Geology 33, 141–144 (doi:10.1130/G21053.1) [Google Scholar]
- 10.Butler RJ, Barrett PM, Kenrick P, Penn MG. 2009. Diversity patterns amongst herbivorous dinosaurs and plants during the Cretaceous: implications for hypotheses of dinosaur/angiosperm co-evolution. J. Evol. Biol. 22, 446–459 (doi:10.1111/j.1420-9101.2008.01680.x) [DOI] [PubMed] [Google Scholar]
- 11.Coiffard C, Gomez B, Daviero-Gomez V, Dilcher DL. 2012. Rise to dominance of angiosperm pioneers in European Cretaceous environments. Proc. Natl Acad. Sci. USA 109, 20 955–20 959 (doi:10.1073/pnas.1218633110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd GT, Davis KE, Pisani D, Tarver JE, Ruta M, Sakamoto M, Hone DWE, Jennings R, Benton MJ. 2008. Dinosaurs and the Cretaceous terrestrial revolution. Proc. R. Soc. B 275, 2483–2490 (doi:10.1098/rspb.2008.0715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dilcher DL. 2000. Toward a new synthesis: major evolutionary trends in the angiosperm fossil record. Proc. Natl Acad. Sci. USA 97, 7030–7036 (doi:10.1073/pnas.97.13.7030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyce CK, Brodribb TJ, Field TS, Zwieniecki MA. 2009. Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proc. R. Soc. B 276, 1771–1776 (doi:10.1098/rspb.2008.1919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Field TS, et al. 2011. Fossil evidence for Cretaceous escalation in angiosperm leaf vein evolution. Proc. Natl Acad. Sci. USA 108, 8363–8366 (doi:10.1073/pnas.1014456108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimaldi D. 1999. The co-radiations of pollinating insects and angiosperms in the Cretaceous. Ann. Mo. Bot. Gard. 86, 373–406 (doi:10.2307/2666181) [Google Scholar]
- 17.McKenna DD, Sequeira AS, Marvaldi AE, Farrell BD. 2009. Temporal lags and overlap in the diversification of weevils and flowering plants. Proc. Natl Acad. Sci. USA 106, 7083–7088 (doi:10.1073/pnas.0810618106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu S, Dilcher DL, Jarzen DM, Taylor DW. 2008. Early steps of angiosperm–pollinator coevolution. Proc. Natl Acad. Sci. USA 105, 240–245 (doi:10.1073/pnas.0707989105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labandeira CC, Currano ED. 2013. The fossil record of plant–insect dynamics. Annu. Rev. Earth Planet. Sci. 41, 287–311 (doi:10.1146/annurev-earth-050212-124139) [Google Scholar]
- 20.Wing SL, Boucher LD. 1998. Ecological aspects of the Cretaceous flowering plant radiation. Annu. Rev. Earth Planet. Sci. 26, 379–421 (doi:10.1146/annurev.earth.26.1.379) [Google Scholar]
- 21.Willis KJ, McElwain JC. 2002. The evolution of plants. Oxford, UK: Oxford University Press [Google Scholar]
- 22.Benton MJ. 2010. The origins of modern biodiversity on land. Phil. Trans. R. Soc. B 365, 3667–3679 (doi:10.1098/rstb.2010.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Springer MS, Murphy WJ, Eizirik E, O'Brien SJ. 2003. Placental mammal diversification and the Cretaceous–Tertiary boundary. Proc. Natl Acad. Sci. USA 100, 1056–1061 (doi:10.1073/pnas.0334222100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bininda-Emonds ORP, et al. 2007. The delayed rise of present-day mammals. Nature 446, 507–512 (doi:10.1038/nature05634) [DOI] [PubMed] [Google Scholar]
- 25.Meredith RW, et al. 2011. Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science 334, 521–524 (doi:10.1126/science.1211028) [DOI] [PubMed] [Google Scholar]
- 26.Benson RBJ, Mannion PD, Butler RJ, Upchurch P, Goswami A, Evans SE. 2012. Cretaceous tetrapod fossil record sampling and faunal turnover: implications for biogeography and the rise of modern clades. Palaeogeogr. Palaeoclimatol. Palaeoecol. 372, 88–107 (doi:10.1016/j.palaeo.2012.10.028) [Google Scholar]
- 27.Wible JR, Rougier GW, Novacek MJ, Asher RJ. 2007. Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary. Nature 447, 1003–1006 (doi:10.1038/nature05854) [DOI] [PubMed] [Google Scholar]
- 28.dos Reis M, Inoue J, Hasegawa M, Asher RJ, Donoghue PCJ, Yang Z. 2012. Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proc. R. Soc. B 279, 3491–3500 (doi:10.1098/rspb.2012.0683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Leary MA, et al. 2013. The placental mammal ancestor and the post–K–Pg radiation of placentals. Science 339, 662–667 (doi:10.1126/science.1229237) [DOI] [PubMed] [Google Scholar]
- 30.Wilson GP, Evans AR, Corfe IJ, Smits PD, Fortelius M, Jernvall J. 2012. Adaptive radiation of multituberculate mammals before the extinction of dinosaurs. Nature 483, 457–460 (doi:10.1038/nature10880) [DOI] [PubMed] [Google Scholar]
- 31.Damuth JD, Jablonski D, Harris JA, Potts R, Stucky RK, Sues HD, Weishampel DB. 1992. Taxon-free characterization of animal communities. In Terrestrial ecosystems through time: evolutionary paleoecology of terrestrial plants and animals (ed. Behrensmeyer AK.), pp. 183–203 Chicago, IL: University of Chicago Press [Google Scholar]
- 32.Alroy J. 2012. Paleobiology database. See http://paleodb.org (accessed 23 November 2012). [Google Scholar]
- 33.Kielan-Jaworowska Z, Cifelli RL, Luo Z-X. 2004. Mammals from the age of dinosaurs: origins, evolution, and structure. New York, NY: Columbia University Press [Google Scholar]
- 34.Barrett PM, McGowan AJ, Page V. 2009. Dinosaur diversity and the rock record. Proc. R. Soc. B 276, 2667–2674 (doi:10.1098/rspb.2009.0352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polly PD. 2012. Quantitative paleontology for Mathematica, version 2.0. Bloomington, IN: Department of Geological Sciences, Indiana University [Google Scholar]
- 36.Bookstein FL. 1991. Morphometric tools for landmark data. New York, NY: Cambridge University Press [Google Scholar]
- 37.Rohlf FJ, Marcus LF. 1993. A revolution in morphometrics. Trends Ecol. Evol. 8, 129–132 (doi:10.1016/0169-5347(93)90024-J) [DOI] [PubMed] [Google Scholar]
- 38.Rohlf FJ. 1998. On applications of geometric morphometrics to studies of ontogeny and phylogeny. Syst. Biol. 47, 147–158 (doi:10.1080/106351598261094) [DOI] [PubMed] [Google Scholar]
- 39.Rohlf FJ, Slice D. 1990. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Biol. 39, 40–59 (doi:10.2307/2992207) [Google Scholar]
- 40.Polly PD. 2012. Geometric morphometrics for Mathematica, version 8.6.4. Bloomington, IN: Department of Geological Sciences, Indiana University [Google Scholar]
- 41.Gradstein FM, Ogg JG, Schmitz M, Ogg G. 2012. The geologic time scale 2012. Boston, MA: Elsevier [Google Scholar]
- 42.Foote M. 1991. Morphologic and taxonomic diversity in a clade's history: the blastoid record and stochastic simulations. Contrib. Mus. of Paleontol. Univ. MI 28, 101–140 [Google Scholar]
- 43.Rougier GW, Apesteguía S, Gaetano LC. 2011. Highly specialized mammalian skulls from the Late Cretaceous of South America. Nature 479, 98–102 (doi:10.1038/nature10591) [DOI] [PubMed] [Google Scholar]
- 44.Luo Z-X, Yuan C-X, Meng Q-J, Ji Q. 2011. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature 476, 442–445 (doi:10.1038/nature10291) [DOI] [PubMed] [Google Scholar]
- 45.Woodburne MO, Rich TM, Springer MS. 2003. The evolution of tribospheny and the antiquity of mammalian clades. Mol. Phylogenet. Evol. 28, 360–385 (doi:10.1016/S1055-7903(03)00113-1) [DOI] [PubMed] [Google Scholar]
- 46.van Rheede T, Bastiaans T, Boone DN, Hedges SB, de Jong WW, Madsen O. 2006. The platypus is in its place: nuclear genes and indels confirm the sister group relation of monotremes and therians. Mol. Biol. Evol. 23, 587–597 (doi:10.1093/molbev/msj064) [DOI] [PubMed] [Google Scholar]
- 47.Eriksson O, Friis EM, Pedersen KR, Crane PR. 2000. Seed size and dispersal systems of Early Cretaceous angiosperms from Famalicao, Portugal. Int. J. Plant Sci. 161, 319–329 (doi:10.1086/314248) [DOI] [PubMed] [Google Scholar]
- 48.Gingerich PD. 1977. Patterns of evolution in the mammalian fossil record. In Patterns of evolution, as illustrated by the fossil record (ed. Hallam A.), pp. 469–500 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 49.Gambaryan PP, Kielan-Jaworowska Z. 1995. Masticatory musculature of Asian taeniolabidoid multituberculate mammals. Acta Palaeontol. Pol. 40, 45–108 [Google Scholar]
- 50.Hu Y, Meng J, Wang Y, Li C. 2005. Large Mesozoic mammals fed on young dinosaurs. Nature 433, 149–152 (doi:10.1038/nature03102) [DOI] [PubMed] [Google Scholar]
- 51.Luo Z-X, Ji Q, Wible JR, Yuan C-X. 2003. An Early Cretaceous tribosphenic mammal and metatherian evolution. Science 302, 1934–1940 (doi:10.1126/science.1090718) [DOI] [PubMed] [Google Scholar]
- 52.Kemp TS. 2005. The origin and evolution of mammals. New York, NY: Oxford University Press [Google Scholar]
- 53.Ji Q, Luo Z-X, Yuan C-X, Wible JR, Zhang J-P, Georgi JA. 2002. The earliest known eutherian mammal. Nature 416, 816–822 (doi:10.1038/416816a) [DOI] [PubMed] [Google Scholar]
- 54.Goswami A, Prasad GVR, Upchurch P, Boyer DM, Seiffert ER, Verma O, Gheerbrant E, Flynn JJ. 2011. A radiation of arboreal basal eutherian mammals beginning in the Late Cretaceous of India. Proc. Natl Acad. Sci. USA 108, 16 333–16 338 (doi:10.1073/pnas.1108723108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shattuck MR, Williams SA. 2010. Arboreality has allowed for the evolution of increased longevity in mammals. Proc. Natl Acad. Sci. USA 107, 4635–4639 (doi:10.1073/pnas.0911439107) [DOI] [PMC free article] [PubMed] [Google Scholar]