Abstract
Classic nuclear shuttling is mediated by an importin-α∙β heterodimer that binds to cargoes containing a nuclear localization signal, and shuttles most nuclear proteins immediately after their translation. Aside from this canonical mechanism, kariopheryn-βs or β-like importins operate by binding to non-canonical nuclear localization signals to mediate translocation without the assistance of importin-α. The mechanism by which these components operate is much less understood and is currently under investigation. Recently, several β-like importins have been implicated in the stimulated nuclear translocation of signaling proteins. Here, we propose that this group of importins might be responsible for the swift nuclear shuttling of many proteins following various stimuli.
Mechanisms of stimulated nuclear import
Intracellular signaling pathways transmit signals of various extracellular stimuli to their cytosolic and nuclear targets in order to induce biological responses, such as proliferation, differentiation, cell death and migration. When needed, the signals are transmitted from the cytoplasm to the nucleus via translocation of one or more components of each of the signaling pathways involved. Thus, after stimulation, a large number of signaling proteins are rapidly translocated to the nucleus to induce and regulate many nuclear processes. However, despite the importance of stimulated nuclear signaling, the mechanisms by which these components reach the nucleus upon stimulation have been elucidated only for a few signaling pathways.
Classic nuclear shuttling is mediated by an importin-α∙β complex that binds to cargoes containing a nuclear localization signal (NLS), consisting of mono- or bi-partite clusters of basic amino acids [1-3]. This importin-α∙β complex often acts as a housekeeping mechanism that shuttles most nuclear proteins immediately to the nucleus after their translation [4]. The relocalization of cargoes is followed by the dissociation of the proteins from the importins upon binding to RanGTP [5], which exports the importins back to the cytoplasm, while the cargo remains in the nucleus [6]. However, only a limited number of signaling proteins, such as NFκB [7] and ERK5 (extracellular signal-regulated kinase 5) [8-10], use this machinery for their stimulated nuclear shuttle. Aside from this canonical mechanism, importin-β [11] or similar karyopherins, termed β-like importins [12], operate by binding to non-canonical NLSs to mediate translocation without the assistance of importin-α. The mechanism by which these components operate is much less understood and is currently under investigation. Recently, several β-like importins have been implicated in the stimulated nuclear translocation of signaling proteins. Here, we propose that this group of importins might be responsible for the swift nuclear shuttling of many proteins following various stimuli.
The mechanism of ERK1/2 translocation to the nucleus
ERK1/2 are important signaling proteins that translocate to the nucleus upon stimulation. The rapid and robust activation of ERK1/2 allows the phosphorylation and modulation of the activity of more than 300 proteins, which are localized either in the cytoplasm or the nucleus [13-15]. These substrates are important for the induction and regulation of cellular processes, including proliferation, differentiation, and migration amongst others [16-19]. The sub-cellular localization of ERK1/2 plays an important role in its regulation and physiological functioning [20,21]. Interestingly, it was shown that the nuclear accumulation of ERK1/2 is important primarily for the induction of proliferation [22,23], while other ERK-dependent processes are mostly regulated by cytosolic molecules [24].
ERK1/2 localization, as well as the mechanisms that govern it, has been elucidated over the past decades. In resting cells, all components of the ERK1/2 cascade are localized primarily in the cytoplasm due to their interaction with different anchoring proteins [25-28] (see Figure 1). Upon stimulation, MEK1/2 phosphorylates ERK1/2 in their TEY motif, thereby inducing a conformational change resulting in the activation of ERK1/2 and detachment from their anchors [28]. This detachment exposes ERK1/2 to an additional phosphorylation on two Ser residues (an SPS motif) within a nine amino acid sequence, termed nuclear translocation signal (NTS) [29]. This phosphorylation can be mediated by both stimulated and constitutively active protein kinases, including protein kinase CK2 and auto-phosphorylation by active ERK1/2 [30]. The phosphorylation of the SPS motif allows it to bind importin-7, which escorts ERK1/2 molecules to the nuclear pores, inducing nuclear sliding. Once in the nucleus, RanGTP dissociates importin-7 from ERK1/2, and consequently, induces their nuclear accumulation [29]. It was also shown that ERK1/2 may interact directly with the nuclear pores, and it is possible that these direct interactions are able to facilitate the ERK1/2 translocation [31]. In addition, this process may be regulated by calcium, as a reduction in intracellular calcium concentrations was shown to induce faster nuclear shuttling [32,33].
Figure 1. Schematic representation of the mechanism of stimulated ERK1/2 translocation to the nucleus.
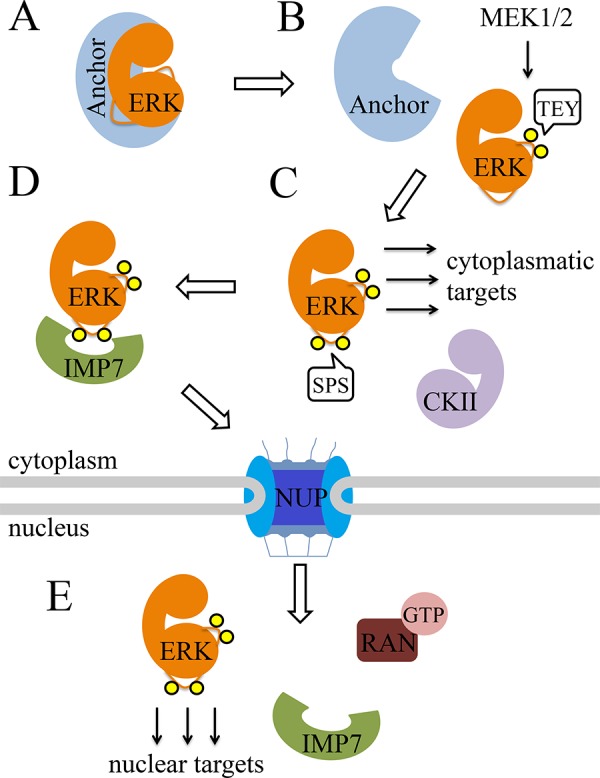
The following steps are illustrated: (A) Binding of ERK1/2 with anchor proteins in resting cells; (B) stimulation is followed by phosphorylation of the TEY motif of ERK1/2 by MEK1/2, and detachment of ERK1/2 from their anchors; (C) phosphorylation of ERK1/2 on its SPS motif by CKII. (D) Binding of phosphorylated ERK1/2 to importin-7 and nuclear sliding through the NUPs; (E) Dissociation of ERK1/2 from importin-7 by RanGTP, and nuclear accumulation of ERK1/2. For more details, see text.
Interestingly, these results in mammals were consistent with findings in Drosophila [34], where DIM-7 (the ortholog of importin-7) was identified as the carrier of D-ERK to the nucleus [35,36]. Once in the nucleus, ERK plays a critical role in the development of eyes and wings in Drosophila [37,38]. Moreover, while comparing the mechanism of nuclear translocation of components of the ERK cascade with other proteins, we established that the NTS might act as a specific stimulus-induced and importin-7-dependent nuclear translocation signal for some signaling proteins lacking an NLS. However, since many signaling proteins contain neither NLS nor NTS, it is possible that other ill-defined β-like importins participate in the stimulated translocation, using various non-canonical NLSs.
The role of β-like importins in the nuclear shuttling of signaling proteins
Although importin-α∙β complexes mediate the nuclear shuttling of a large number of proteins, it is now clear that other karyopherins are required for the translocation of the full repertoire of nuclear proteins. Such karyopherins were initially discovered as nucleoporin-binding proteins, and their homology with importin-β suggested a function in nuclear transport [39-43], which initiated their “importin” terminology [12] (see Table 1 for nomenclature). Subsequently, more dedicated studies identified at least 10 more β-like importins in mammals that share a sequence motif related to the Ran-binding site of importin-β, and can shuttle to the nucleus under various conditions [44,45]. The β-like importins known today share low overall sequence identity (10-20%), and have 19-20 helical HEAT repeats arranged into super-helical or ring-like structure [46]. Their molecular weights (90-150 kDa [46]), and isoelectric points (pI = 4.0 − 5.9 [46,47]) vary. These importins mediate the translocation of proteins into the nucleus under varying conditions, including stimulation. Here, we describe the possible involvement of the β-like importins, as well as exportin-4, in the stimulated translocation of signaling proteins.
Table 1. List of β-like importins.
| Importin | Other terminology | Examples of signaling cargos (not always stimulated shuttle) |
|---|---|---|
| Importin-2 | importin-β2, IPO2, KPNB2, MIP, MIP1, TNPO1, transportin, transportin 1, TRN, IMB2, Kapβ2, karyopherin-β2 | c-Jun [50], NPM-ALK [57], hnRNP A1 [84,85] and several mRNA binding proteins [86], EWS [87], HuR [88], c-Fos [59,60], ribosomal proteins [89]. |
| Importin-3 | Importin-3, Imp3, transportin 2, FLJ1255, KPNB2B, TRN2, Karyopherin β-2b, IPO3, TNPO2, IMB2 | HuR [88,90], hnRNP A1 [91]. |
| Importin-4 | Imp4, karyopherin-β4, Imp4b, FLJ23338, MGC131665, IPO4, IMB4, RanBP4, IMP4B | Vitamin D receptor [61], HIF1-α [51]. |
| Importin-5 | IPO5, IMB3, Pse1, Imp5, RANBP5, Kapβ3, KPNB3, MGC2068, FLJ43041, DKFZp686O1576, IMB3 | c-Jun [50], p60TRP [92], RAG-2 [93], ribosomal proteins [89]. |
| Importin-7 | IPO7, Imp7, RANBP7, FLJ14581, MGC138673 | ERK1/2 [29,35], MEK1 [29], SMAD3/4 [29,52], Egr1 [56], HIF1α [51], c-Jun [50], GR [54], Sox-2 [49], ribosomal proteins [89]. |
| Importin-8 | Imp8, IPO8, RANBP8, FLJ26580 | SMAD1/3/4 [52], NPM-ALK [57], Ago2 [58], glucocorticoid receptor [54]. |
| Importin-9 | Imp9, FLJ10402, IPO9, RANBP9, KIAA1192, DKFZp761M1547 | PR65 of PP2A [94], c-Jun [50], ARX [95], Sox-2 [49]. |
| Importin-11 | IPO11, Imp11, SLRN, RanBP11, KA120 | UbcH6 [96], UB2E2 [96]. |
| Importin-12 | Imp12, importin-12, IPO12, MTR10A, TNPO3, transportin 3, transportin 3, transportin-SR, TRN-SR, TRN-SR2, TRNSR | MLF2 [97], RBM4 [97]. |
| Importin-13 | IPO13, Imp13, KAP13, RANBP13, LGL2, KIAA0724, Karyopherin 13 | c-Jun [50], ARX [95], GR [98], Mago [99], Y14 [99]. |
| Exportin-4 | XPO4, Exp4, FLJ13046, KIAAA1721 | Sox-2 and SRY [49]. |
Modified from: Eldar and Seger Mol Cell Biol (2013), submitted.
Among the β-like importins with the highest number of identified cargoes is importin-7, which seems to utilize several mechanisms and distinct NLSs to shuttle its distinct cargoes (Table 2). In some cases, usually in non-stimulated cells, importin-7 acts in a complex with importin-β [48,49], or in parallel to importin-2 [50], importin-4 [51], and importin-8 [52]. However, importin-7 also acts by itself, mainly in stimulated translocations. Thus, importin-7 mediates the nuclear translocation of ERK1/2 described above, as well as MEK1 and SMAD3, by binding to the NTS sequences of these cargoes [29,53]. Moreover, it was reported that importin-7 is able to directly bind to a canonical NLS sequence in the glucocorticoid receptor to escort it to the nucleus upon hormonal stimulation [54]. In addition, importin-7 seems to shuttle other signaling proteins or transcription factors to the nucleus in an NTS- and canonical NLS-independent manner. Such molecules include the transcriptional regulators HIF1-α [51], c-Jun [50], several SMAD proteins [29,52,53], Sox-2 [49], HIV-1 [55], Egr-1 [56], and the oncogenic NPM-ALK [57]. However, it remains unclear whether the importin-7-mediated translocation of all these proteins is affected by stimulation, or is active merely in resting cells.
Table 2. Importin-cargo interactions of β-like importins.
| Type of interaction | Example | Stimulated |
|---|---|---|
| Monomeric, direct canonical NLS- dependent cargo binding. | Importin-7 binds the canonical NLS in glucocorticoid receptor and escorts it to the nucleus upon stimulation [54]. | Yes |
| Monomeric, direct non-canonical NLS- independent cargo binding. | Importin-7 binds to phosphorylated SPS domain of ERK1/2 upon stimulation [29]. | Yes |
| Cargo binding in complex with importin-β. Usually via canonical NLS. | Heterodimers of importin-7 ∙ β have been implicated in the nuclear accumulation of Sox-2 [49]. | No |
| Cooperation with other β-like importins. Usually bind non-canonical NLSs. | Various β-like importins cooperate in mediating nuclear translocation of c-Jun [50] and SMAD1/ 2/3 [52,55]. No direct association between them was reported. | Yes |
Although the information on other members of the family still lags behind that of importin-7, it seems that at least some of them play important roles in stimulated translocation as well. Accordingly, importin-8 was shown to induce the nuclear accumulation of Ago2 [58] and SMAD1/4 [52]. C-Jun was shown to be transported by importins 2, 5, 7, 9, and 13, that might be related, at least in part, to its stimulated nuclear accumulation [50]. Importin-2 shuttles c-Fos to the nucleus after translation [59] or upon stimulation [60], and importin-4 escorts vitamin D receptor to the nucleus upon ligand stimulation [61]. Interestingly, exportin-4, which participates mainly in nuclear export [62], has been shown to function as an importin for Sox-2, in addition to importin-β∙7 and importin-9 [49]. This makes exportin-4 a distant relative of the β-like importins (Table 1), although it is not clear whether it participates in stimulated translocations as well. In general, β-like importins are able to induce both stimulated and/or non-stimulated translocations, using at least 4 mechanisms: (i) monomeric, direct canonical NLS-dependent cargo binding; (ii) monomeric, direct non-canonical NLS-independent cargo binding; (iii) cargo binding in a complex with importin-β; and (iv) cooperation with other β-like importins (see more details in Table 2). Thus, as a group, β-like importins may play an important role in the stimulated translocation of signaling proteins and transcription factors.
Summary and future directions
There is increasing evidence that the translocation of signaling proteins into the nucleus is much more tightly regulated than it was thought just a few years ago. Aside from the NLS/importin-α∙β machinery, other mechanisms, such as passive diffusion [63,64], active transport of homodimers [64-66], direct binding to nuclear pore machinery [31,67-70], escort to the nucleus by other NLS-containing proteins [71,72] and indirect aid by the canonical machinery [64], were initially proposed for several signaling proteins. However, some of these findings were not properly verified, and later were either disputed [73-75], or found to be cell-type specific [76]. Therefore, it is worthwhile to entertain the possibility that at least some of these alleged mechanisms are, in fact, part of the wider β-like importin-dependent networks.
In addition, since dysregulation of the signaling proteins described above is involved in diseases, such as cancer and autoimmunity, it would be interesting to study the potential therapeutic implications of inhibiting their nuclear translocation. Several attempts have been made to block canonical NLS/importin-α∙β mediated nuclear translocation [77-80]. Since many proteins use this machinery to translocate to the nucleus, such inhibition might affect too many processes and may fail to develop into desired specific therapies. However, a more specific approach might be to target the non-canonical mechanism of translocation, which seems to act within a limited number of distinct proteins upon stimulation. In this direction, efforts were made to develop a blocking peptide for importin-2 [81,82]. This peptide is able to compete with natural substrates and is resistant to Ran-mediated release in the nucleus [83], therefore specifically inhibiting this process [81]. However, in order to develop strong inhibitors for a specific cargo/β-like importin complex, we need to extract precise information on the structural interaction, as well as the regulation of import. We will then be able to explore this mechanism as a new layer of therapeutic intervention.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/5/41
References
- 1.Marfori M, Mynott A, Ellis JJ, Mehdi AM, Saunders NFW, Curmi PM, Forwood JK, Bodén M, Kobe B. Molecular basis for specificity of nuclear import and prediction of nuclear localization. Biochim Biophys Acta. 2011;1813:1562–77. doi: 10.1016/j.bbamcr.2010.10.013. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/718113598
- 2.Frankel MB, Knoll LJ. The ins and outs of nuclear trafficking: unusual aspects in apicomplexan parasites. DNA Cell Biol. 2009;28:277–84. doi: 10.1089/dna.2009.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riddick G, Macara IG. A systems analysis of importin-{alpha}-{beta} mediated nuclear protein import. J Cell Biol. 2005;168:1027–38. doi: 10.1083/jcb.200409024. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/1025153
- 4.Chook YM, Süel KE. Nuclear import by karyopherin-βs: recognition and inhibition. Biochim Biophys Acta. 2011;1813:1593–606. doi: 10.1016/j.bbamcr.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/6713956
- 5.Moroianu J, Blobel G, Radu A. Nuclear protein import: Ran-GTP dissociates the karyopherin alphabeta heterodimer by displacing alpha from an overlapping binding site on beta. Proc Natl Acad Sci U S A. 1996;93:7059–62. doi: 10.1073/pnas.93.14.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/718113599
- 6.Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol. 2007;8:195–208. doi: 10.1038/nrm2114. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/718113601
- 7.Magnani M, Crinelli R, Bianchi M, Antonelli A. The ubiquitin-dependent proteolytic system and other potential targets for the modulation of nuclear factor-kB (NF-kB) Curr Drug Targets. 2000;1:387–99. doi: 10.2174/1389450003349056. [DOI] [PubMed] [Google Scholar]
- 8.Kondoh K, Terasawa K, Morimoto H, Nishida E. Regulation of nuclear translocation of extracellular signal-regulated kinase 5 by active nuclear import and export mechanisms. Mol Cell Biol. 2006;26:1679–90. doi: 10.1128/MCB.26.5.1679-1690.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 2011;1813:1619–33. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Nardozzi JD, Lott K, Cingolani G. Phosphorylation meets nuclear import: a review. Cell Commun Signal. 2010;8:32. doi: 10.1186/1478-811X-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu D, Farmer A, Chook YM. Recognition of nuclear targeting signals by Karyopherin-β proteins. Curr Opin Struct Biol. 2010;20:782–90. doi: 10.1016/j.sbi.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ström AC, Weis K. Importin-beta-like nuclear transport receptors. Genome Biol. 2001;2: doi: 10.1186/gb-2001-2-6-reviews3008. REVIEWS3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 14.Carlson SM, Chouinard CR, Labadorf A, Lam CJ, Schmelzle K, Fraenkel E, White FM. Large-scale discovery of ERK2 substrates identifies ERK-mediated transcriptional regulation by ETV3. Sci Signal. 2011;4:rs11. doi: 10.1126/scisignal.2002010. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/718113605
- 15.Kosako H, Yamaguchi N, Aranami C, Ushiyama M, Kose S, Imamoto N, Taniguchi H, Nishida E, Hattori S. Phosphoproteomics reveals new ERK MAP kinase targets and links ERK to nucleoporin-mediated nuclear transport. Nat Struct Mol Biol. 2009;16:1026–35. doi: 10.1038/nsmb.1656. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/1166137
- 16.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–26. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–12. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 18.Pimienta G, Pascual J. Canonical and alternative MAPK signaling. Cell Cycle. 2007;6:2628–32. doi: 10.4161/cc.6.21.4930. [DOI] [PubMed] [Google Scholar]
- 19.Zehorai E, Yao Z, Plotnikov A, Seger R. The subcellular localization of MEK and ERK--a novel nuclear translocation signal (NTS) paves a way to the nucleus. Mol Cell Endocrinol. 2010;314:213–20. doi: 10.1016/j.mce.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Wortzel I, Seger R. The ERK Cascade: Distinct Functions within Various Subcellular Organelles. Genes Cancer. 2011;2:195–209. doi: 10.1177/1947601911407328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harding A, Tian T, Westbury E, Frische E, Hancock JF. Subcellular localization determines MAP kinase signal output. Curr Biol. 2005;15:869–73. doi: 10.1016/j.cub.2005.04.020. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/1025812
- 22.Formstecher E, Ramos JW, Fauquet M, Calderwood DA, Hsieh JC, Canton B, Nguyen XT, Barnier JV, Camonis J, Ginsberg MH, Chneiweiss H. PEA-15 mediates cytoplasmic sequestration of ERK MAP kinase. Dev Cell. 2001;1:239–50. doi: 10.1016/S1534-5807(01)00035-1. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/718113606
- 23.Yao Z, Flash I, Raviv Z, Yung Y, Asscher Y, Pleban S, Seger R. Non-regulated and stimulated mechanisms cooperate in the nuclear accumulation of MEK1. Oncogene. 2001;20:7588–96. doi: 10.1038/sj.onc.1204963. [DOI] [PubMed] [Google Scholar]
- 24.Casar B, Pinto A, Crespo P. ERK dimers and scaffold proteins: unexpected partners for a forgotten (cytoplasmic) task. Cell Cycle. 2009;8:1007–13. doi: 10.4161/cc.8.7.8078. [DOI] [PubMed] [Google Scholar]
- 25.Reszka AA, Seger R, Diltz CD, Krebs EG, Fischer EH. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc Natl Acad Sci U S A. 1995;92:8881–5. doi: 10.1073/pnas.92.19.8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubinfeld H, Hanoch T, Seger R. Identification of a cytoplasmic-retention sequence in ERK2. J Biol Chem. 1999;274:30349–52. doi: 10.1074/jbc.274.43.30349. [DOI] [PubMed] [Google Scholar]
- 27.Zúñiga A, Torres J, Ubeda J, Pulido R. Interaction of mitogen-activated protein kinases with the kinase interaction motif of the tyrosine phosphatase PTP-SL provides substrate specificity and retains ERK2 in the cytoplasm. J Biol Chem. 1999;274:21900–7. doi: 10.1074/jbc.274.31.21900. [DOI] [PubMed] [Google Scholar]
- 28.Chuderland D, Seger R. Protein-protein interactions in the regulation of the extracellular signal-regulated kinase. Mol Biotechnol. 2005;29:57–74. doi: 10.1385/MB:29:1:57. [DOI] [PubMed] [Google Scholar]
- 29.Chuderland D, Konson A, Seger R. Identification and characterization of a general nuclear translocation signal in signaling proteins. Mol Cell. 2008;31:850–61. doi: 10.1016/j.molcel.2008.08.007. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/1122980
- 30.Plotnikov A, Chuderland D, Karamansha Y, Livnah O, Seger R. Nuclear extracellular signal-regulated kinase 1 and 2 translocation is mediated by casein kinase 2 and accelerated by autophosphorylation. Mol Cell Biol. 2011;31:3515–30. doi: 10.1128/MCB.05424-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Xu L, Massagué J. Nucleocytoplasmic shuttling of signal transducers. Nat Rev Mol Cell Biol. 2004;5:209–19. doi: 10.1038/nrm1331. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/718113611
- 32.Chuderland D, Marmor G, Shainskaya A, Seger R. Calcium-mediated interactions regulate the subcellular localization of extracellular signal-regulated kinases. J Biol Chem. 2008;283:11176–88. doi: 10.1074/jbc.M709030200. [DOI] [PubMed] [Google Scholar]
- 33.Chuderland D, Seger R. Calcium regulates ERK signaling by modulating its protein-protein interactions. Commun Integr Biol. 2008;1:4–5. doi: 10.4161/cib.1.1.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marenda DR, Vrailas AD, Rodrigues AB, Cook S, Powers MA, Lorenzen JA, Perkins LA, Moses K. MAP kinase subcellular localization controls both pattern and proliferation in the developing Drosophila wing. Development. 2006;133:43–51. doi: 10.1242/dev.02168. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/1029700
- 35.Lorenzen JA, Baker SE, Denhez F, Melnick MB, Brower DL, Perkins LA. Nuclear import of activated D-ERK by DIM-7, an importin family member encoded by the gene moleskin. Development. 2001;128:1403–14. doi: 10.1242/dev.128.8.1403. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/718113612
- 36.Vrailas AD, Marenda DR, Cook SE, Powers MA, Lorenzen JA, Perkins LA, Moses K. smoothened and thickveins regulate Moleskin/Importin 7-mediated MAP kinase signaling in the developing Drosophila eye. Development. 2006;133:1485–94. doi: 10.1242/dev.02334. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/718113615
- 37.Kumar JP, Hsiung F, Powers MA, Moses K. Nuclear translocation of activated MAP kinase is developmentally regulated in the developing Drosophila eye. Development. 2003;130:3703–14. doi: 10.1242/dev.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar JP, Tio M, Hsiung F, Akopyan S, Gabay L, Seger R, Shilo BZ, Moses K. Dissecting the roles of the Drosophila EGF receptor in eye development and MAP kinase activation. Development. 1998;125:3875–85. doi: 10.1242/dev.125.19.3875. [DOI] [PubMed] [Google Scholar]
- 39.Aitchison JD, Blobel G, Rout MP. Kap104p: a karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science. 1996;274:624–7. doi: 10.1126/science.274.5287.624. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/718113617
- 40.Fornerod M, van Deursen J, van Baal S, Reynolds A, Davis D, Murti KG, Fransen J, Grosveld G. The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J. 1997;16:807–16. doi: 10.1093/emboj/16.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/718113618
- 41.Yaseen NR, Blobel G. Cloning and characterization of human karyopherin beta3. Proc Natl Acad Sci U S A. 1997;94:4451–6. doi: 10.1073/pnas.94.9.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/718113620
- 42.Fridell RA, Truant R, Thorne L, Benson RE, Cullen BR. Nuclear import of hnRNP A1 is mediated by a novel cellular cofactor related to karyopherin-beta. J Cell Sci. 1997;110((Pt 11):1325–31. doi: 10.1242/jcs.110.11.1325. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/718113623
- 43.Rout MP, Blobel G, Aitchison JD. A distinct nuclear import pathway used by ribosomal proteins. Cell. 1997;89:715–25. doi: 10.1016/S0092-8674(00)80254-8. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/718113624
- 44.Görlich D, Dabrowski M, Bischoff FR, Kutay U, Bork P, Hartmann E, Prehn S, Izaurralde E. A novel class of RanGTP binding proteins. J Cell Biol. 1997;138:65–80. doi: 10.1083/jcb.138.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/718113625
- 45.Kutay U, Hartmann E, Treichel N, Calado A, Carmo-Fonseca M, Prehn S, Kraft R, Gorlich D, Bischoff FR. Identification of two novel RanGTP-binding proteins belonging to the importin beta superfamily. J Biol Chem. 2000;275:40163–8. doi: 10.1074/jbc.M006242200. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/718113626
- 46.Chook YM, Blobel G. Karyopherins and nuclear import. Curr Opin Struct Biol. 2001;11:703–15. doi: 10.1016/S0959-440X(01)00264-0. [DOI] [PubMed] [Google Scholar]
- 47.Görlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:607–60. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 48.Jäkel S, Albig W, Kutay U, Bischoff FR, Schwamborn K, Doenecke D, Görlich D. The importin beta/importin 7 heterodimer is a functional nuclear import receptor for histone H1. EMBO J. 1999;18:2411–23. doi: 10.1093/emboj/18.9.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/718113629
- 49.Gontan C, Güttler T, Engelen E, Demmers J, Fornerod M, Grosveld FG, Tibboel D, Görlich D, Poot RA, Rottier RJ. Exportin 4 mediates a novel nuclear import pathway for Sox family transcription factors. J Cell Biol. 2009;185:27–34. doi: 10.1083/jcb.200810106. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/718113631
- 50.Waldmann I, Wälde S, Kehlenbach RH. Nuclear import of c-Jun is mediated by multiple transport receptors. J Biol Chem. 2007;282:27685–92. doi: 10.1074/jbc.M703301200. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/718113632
- 51.Chachami G, Paraskeva E, Mingot J, Braliou GG, Görlich D, Simos G. Transport of hypoxia-inducible factor HIF-1alpha into the nucleus involves importins 4 and 7. Biochem Biophys Res Commun. 2009;390:235–40. doi: 10.1016/j.bbrc.2009.09.093. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/718113634
- 52.Yao X, Chen X, Cottonham C, Xu L. Preferential utilization of Imp7/8 in nuclear import of Smads. J Biol Chem. 2008;283:22867–74. doi: 10.1074/jbc.M801320200. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/718113635
- 53.Chen X, Xu L. Mechanism and regulation of nucleocytoplasmic trafficking of smad. Cell Biosci. 2011;1:40. doi: 10.1186/2045-3701-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/14274718
- 54.Freedman ND, Yamamoto KR. Importin 7 and importin alpha/importin beta are nuclear import receptors for the glucocorticoid receptor. Mol Biol Cell. 2004;15:2276–86. doi: 10.1091/mbc.E03-11-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/718113636
- 55.Fassati A, Görlich D, Harrison I, Zaytseva L, Mingot J. Nuclear import of HIV-1 intracellular reverse transcription complexes is mediated by importin 7. EMBO J. 2003;22:3675–85. doi: 10.1093/emboj/cdg357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J, Liu MY, Parish CR, Chong BH, Khachigian L. Nuclear import of early growth response-1 involves importin-7 and the novel nuclear localization signal serine-proline-serine. Int J Biochem Cell Biol. 2011;43:905–12. doi: 10.1016/j.biocel.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Wu F, Wang P, Young LC, Lai R, Li L. Proteome-wide identification of novel binding partners to the oncogenic fusion gene protein, NPM-ALK, using tandem affinity purification and mass spectrometry. Am J Pathol. 2009;174:361–70. doi: 10.2353/ajpath.2009.080521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinmann L, Höck J, Ivacevic T, Ohrt T, Mütze J, Schwille P, Kremmer E, Benes V, Urlaub H, Meister G. Importin 8 is a gene silencing factor that targets argonaute proteins to distinct mRNAs. Cell. 2009;136:496–507. doi: 10.1016/j.cell.2008.12.023. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/1158835
- 59.Arnold M, Nath A, Wohlwend D, Kehlenbach RH. Transportin is a major nuclear import receptor for c-Fos: a novel mode of cargo interaction. J Biol Chem. 2006;281:5492–9. doi: 10.1074/jbc.M513281200. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/718113637
- 60.Higashi N, Kunimoto H, Kaneko S, Sasaki T, Ishii M, Kojima H, Nakajima K. Cytoplasmic c-Fos induced by the YXXQ-derived STAT3 signal requires the co-operative MEK/ERK signal for its nuclear translocation. Genes Cells. 2004;9:233–42. doi: 10.1111/j.1356-9597.2004.00715.x. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/718113638
- 61.Miyauchi Y, Michigami T, Sakaguchi N, Sekimoto T, Yoneda Y, Pike JW, Yamagata M, Ozono K. Importin 4 is responsible for ligand-independent nuclear translocation of vitamin D receptor. J Biol Chem. 2005;280:40901–8. doi: 10.1074/jbc.M509347200. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/718113639
- 62.Lipowsky G, Bischoff FR, Schwarzmaier P, Kraft R, Kostka S, Hartmann E, Kutay U, Görlich D. Exportin 4: a mediator of a novel nuclear export pathway in higher eukaryotes. EMBO J. 2000;19:4362–71. doi: 10.1093/emboj/19.16.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/718113641
- 63.Harootunian AT, Adams SR, Wen W, Meinkoth JL, Taylor SS, Tsien RY. Movement of the free catalytic subunit of cAMP-dependent protein kinase into and out of the nucleus can be explained by diffusion. Mol Biol Cell. 1993;4:993–1002. doi: 10.1091/mbc.4.10.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adachi M, Fukuda M, Nishida E. Two co-existing mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. EMBO J. 1999;18:5347–58. doi: 10.1093/emboj/18.19.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melen K, Kinnunen L, Julkunen I. Arginine/lysine-rich structural element is involved in interferon-induced nuclear import of STATs. J Biol Chem. 2001;276:16447–55. doi: 10.1074/jbc.M008821200. [DOI] [PubMed] [Google Scholar]
- 66.Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb MH. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–15. doi: 10.1016/S0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- 67.Fagotto F, Glück U, Gumbiner BM. Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of beta-catenin. Curr Biol. 1998;8:181–90. doi: 10.1016/S0960-9822(98)70082-X. [DOI] [PubMed] [Google Scholar]
- 68.Xu L, Alarcón C, Cöl S, Massagué J. Distinct domain utilization by Smad3 and Smad4 for nucleoporin interaction and nuclear import. J Biol Chem. 2003;278:42569–77. doi: 10.1074/jbc.M307601200. [DOI] [PubMed] [Google Scholar]
- 69.Matsubayashi Y, Fukuda M, Nishida E. Evidence for existence of a nuclear pore complex-mediated, cytosol-independent pathway of nuclear translocation of ERK MAP kinase in permeabilized cells. J Biol Chem. 2001;276:41755–60. doi: 10.1074/jbc.M106012200. [DOI] [PubMed] [Google Scholar]
- 70.Whitehurst AW, Wilsbacher JL, You Y, Luby-Phelps K, Moore MS, Cobb MH. ERK2 enters the nucleus by a carrier-independent mechanism. Proc Natl Acad Sci U S A. 2002;99:7496–501. doi: 10.1073/pnas.112495999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawashima T, Bao YC, Nomura Y, Moon Y, Tonozuka Y, Minoshima Y, Hatori T, Tsuchiya A, Kiyono M, Nosaka T, Nakajima H, Williams DA, Kitamura T. Rac1 and a GTPase-activating protein, MgcRacGAP, are required for nuclear translocation of STAT transcription factors. J Cell Biol. 2006;175:937–46. doi: 10.1083/jcb.200604073. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/1056705
- 72.Williams CC, Allison JG, Vidal GA, Burow ME, Beckman BS, Marrero L, Jones FE. The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. J Cell Biol. 2004;167:469–78. doi: 10.1083/jcb.200403155. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/1023378
- 73.Wolf I, Rubinfeld H, Yoon S, Marmor G, Hanoch T, Seger R. Involvement of the activation loop of ERK in the detachment from cytosolic anchoring. J Biol Chem. 2001;276:24490–7. doi: 10.1074/jbc.M103352200. [DOI] [PubMed] [Google Scholar]
- 74.Lidke DS, Huang F, Post JN, Rieger B, Wilsbacher J, Thomas JL, Pouysségur J, Jovin TM, Lenormand P. ERK nuclear translocation is dimerization-independent but controlled by the rate of phosphorylation. J Biol Chem. 2010;285:3092–102. doi: 10.1074/jbc.M109.064972. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/1665956
- 75.Casar B, Pinto A, Crespo P. Essential role of ERK dimers in the activation of cytoplasmic but not nuclear substrates by ERK-scaffold complexes. Mol Cell. 2008;31:708–21. doi: 10.1016/j.molcel.2008.07.024. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/1124362
- 76.Ranganathan A, Yazicioglu MN, Cobb MH. The nuclear localization of ERK2 occurs by mechanisms both independent of and dependent on energy. J Biol Chem. 2006;281:15645–52. doi: 10.1074/jbc.M513866200. [DOI] [PubMed] [Google Scholar]
- 77.Kosugi S, Hasebe M, Entani T, Takayama S, Tomita M, Yanagawa H. Design of peptide inhibitors for the importin alpha/beta nuclear import pathway by activity-based profiling. Chem Biol. 2008;15:940–9. doi: 10.1016/j.chembiol.2008.07.019. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/1124119
- 78.Hintersteiner M, Ambrus G, Bednenko J, Schmied M, Knox AJS, Meisner N, Gstach H, Seifert J, Singer EL, Gerace L, Auer M. Identification of a small molecule inhibitor of importin β mediated nuclear import by confocal on-bead screening of tagged one-bead one-compound libraries. ACS Chem Biol. 2010;5:967–79. doi: 10.1021/cb100094k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ambrus G, Whitby LR, Singer EL, Trott O, Choi E, Olson AJ, Boger DL, Gerace L. Small molecule peptidomimetic inhibitors of importin α/β mediated nuclear transport. Bioorg Med Chem. 2010;18:7611–20. doi: 10.1016/j.bmc.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Soderholm JF, Bird SL, Kalab P, Sampathkumar Y, Hasegawa K, Uehara-Bingen M, Weis K, Heald R. Importazole, a small molecule inhibitor of the transport receptor importin-β. ACS Chem Biol. 2011;6:700–8. doi: 10.1021/cb2000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cansizoglu AE, Lee BJ, Zhang ZC, Fontoura BMA, Chook YM. Structure-based design of a pathway-specific nuclear import inhibitor. Nat Struct Mol Biol. 2007;14:452–4. doi: 10.1038/nsmb1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Süel KE, Gu H, Chook YM. Modular organization and combinatorial energetics of proline-tyrosine nuclear localization signals. PLoS Biol. 2008;6:e137. doi: 10.1371/journal.pbio.0060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chook YM, Jung A, Rosen MK, Blobel G. Uncoupling Kapbeta2 substrate dissociation and ran binding. Biochemistry. 2002;41:6955–66. doi: 10.1021/bi012122p. [DOI] [PubMed] [Google Scholar]
- 84.Siomi MC, Eder PS, Kataoka N, Wan L, Liu Q, Dreyfuss G. Transportin-mediated nuclear import of heterogeneous nuclear RNP proteins. J Cell Biol. 1997;138:1181–92. doi: 10.1083/jcb.138.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pollard VW, Michael WM, Nakielny S, Siomi MC, Wang F, Dreyfuss G. A novel receptor-mediated nuclear protein import pathway. Cell. 1996;86:985–94. doi: 10.1016/S0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 86.Bonifaci N, Moroianu J, Radu A, Blobel G. Karyopherin beta2 mediates nuclear import of a mRNA binding protein. Proc Natl Acad Sci U S A. 1997;94:5055–60. doi: 10.1073/pnas.94.10.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zakaryan RP, Gehring H. Identification and characterization of the nuclear localization/retention signal in the EWS proto-oncoprotein. J Mol Biol. 2006;363:27–38. doi: 10.1016/j.jmb.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 88.Güttinger S, Mühlhäusser P, Koller-Eichhorn R, Brennecke J, Kutay U. Transportin2 functions as importin and mediates nuclear import of HuR. Proc Natl Acad Sci U S A. 2004;101:2918–23. doi: 10.1073/pnas.0400342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jäkel S, Görlich D. Importin beta, transportin, RanBP5 and RanBP7 mediate nuclear import of ribosomal proteins in mammalian cells. EMBO J. 1998;17:4491–502. doi: 10.1093/emboj/17.15.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van der Giessen K, Gallouzi I. Involvement of transportin 2-mediated HuR import in muscle cell differentiation. Mol Biol Cell. 2007;18:2619–29. doi: 10.1091/mbc.E07-02-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rebane A, Aab A, Steitz JA. Transportins 1 and 2 are redundant nuclear import factors for hnRNP A1 and HuR. RNA. 2004;10:590–9. doi: 10.1261/rna.5224304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heese K, Yamada T, Akatsu H, Yamamoto T, Kosaka K, Nagai Y, Sawada T. Characterizing the new transcription regulator protein p60TRP. J Cell Biochem. 2004;91:1030–42. doi: 10.1002/jcb.20010. [DOI] [PubMed] [Google Scholar]
- 93.Ross AE, Vuica M, Desiderio S. Overlapping signals for protein degradation and nuclear localization define a role for intrinsic RAG-2 nuclear uptake in dividing cells. Mol Cell Biol. 2003;23:5308–19. doi: 10.1128/MCB.23.15.5308-5319.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/1015227
- 94.Lubert EJ, Sarge KD. Interaction between protein phosphatase 2A and members of the importin beta superfamily. Biochem Biophys Res Commun. 2003;303:908–13. doi: 10.1016/S0006-291X(03)00434-0. [DOI] [PubMed] [Google Scholar]
- 95.Lin W, Ye W, Cai L, Meng X, Ke G, Huang C, Peng Z, Yu Y, Golden JA, Tartakoff AM, Tao T. The roles of multiple importins for nuclear import of murine aristaless-related homeobox protein. J Biol Chem. 2009;284:20428–39. doi: 10.1074/jbc.M109.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Plafker SM, Macara IG. Importin-11, a nuclear import receptor for the ubiquitin-conjugating enzyme, UbcM2. EMBO J. 2000;19:5502–13. doi: 10.1093/emboj/19.20.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lai M, Kuo H, Chang W, Tarn W. A novel splicing regulator shares a nuclear import pathway with SR proteins. EMBO J. 2003;22:1359–69. doi: 10.1093/emboj/cdg126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tao T, Lan J, Lukacs GL, Haché RJG, Kaplan F. Importin 13 regulates nuclear import of the glucocorticoid receptor in airway epithelial cells. Am J Respir Cell Mol Biol. 2006;35:668–80. doi: 10.1165/rcmb.2006-0073OC. [DOI] [PubMed] [Google Scholar]
- 99.Bono F, Cook AG, Grünwald M, Ebert J, Conti E. Nuclear import mechanism of the EJC component Mago-Y14 revealed by structural studies of importin 13. Mol Cell. 2010;37:211–22. doi: 10.1016/j.molcel.2010.01.007. [DOI] [PubMed] [Google Scholar]


