Abstract
The loss of skeletal muscle size and function with aging, sarcopenia, may be related, in part, to an age-related muscle protein synthesis impairment. In this review, we discuss to what extent aging affects skeletal muscle protein synthesis and how nutrition and exercise can be strategically employed to overcome age-related protein synthesis impairments and slow the progression of sarcopenia.
Keywords: Aging, Sarcopenia, Exercise, Protein, Insulin, Muscle Protein Synthesis, mTORC1
INTRODUCTION
Aging is characterized by a gradual loss of skeletal muscle mass (22). The time course of skeletal muscle loss with aging was eloquently described in studies performed by Lexell et al. in which whole vastus lateralis muscle cross sections were examined from cadavers across a broad age range (22). Lexell et al. identified that the onset of muscle atrophy may begin as early as 25–30 years of age and that the rate of muscle atrophy accelerates with advancing age (22). While the intrinsic contractile properties of skeletal muscle appear to be resistant to aging (34), the gradual loss of muscle size does contribute to reductions in strength and function at the whole muscle level, which has debilitating consequences for older adults. Specifically, the collective loss of muscle mass and function with aging, commonly referred to as sarcopenia (5), is associated with impaired physical function and a reduced ability to perform activities of daily living, which substantially increases the risk for falls, frailty, and dependence in older adults (5).
Changes in skeletal muscle size are ultimately governed by the continuous and dynamic interplay between rates of muscle protein synthesis and muscle protein breakdown. In particular, changes in muscle size require a chronic imbalance favoring one process over the other. Results using current methodologies suggest that relative to muscle protein breakdown, the rate of muscle protein synthesis is more dynamic and responsive and therefore changes in muscle protein synthesis have largely been the focus of research examining the anabolic potential of a given stimulus. In particular, nutrition and exercise have been identified as powerful stimulators of skeletal muscle protein synthesis (2, 11, 16, 18, 25, 37, 39) and thus can be used acutely to tip the biological processes within skeletal muscle in favor of protein anabolism (i.e., net protein accretion). Over time, the summation of these acute increases in protein synthesis is thought to provide the necessary stimulus to preserve, or increase, skeletal muscle size and strength. Consequently, exercise and nutritional strategies represent promising and practical approaches that may be useful to slow or reverse the progression of sarcopenia. On the other hand, a chronic inability for these anabolic stimuli to routinely stimulate muscle protein synthesis would facilitate a gradual loss of skeletal muscle mass and function.
Although some discrepancies exist, the general consensus is the fractional synthesis and breakdown rate of muscle protein under basal conditions are similar between young and older adults (11, 38), indicating that sarcopenia is not facilitated through age-induced impairments in basal muscle protein metabolism. Instead, one of the primary factors thought to contribute to muscle loss with aging is an impaired ability for skeletal muscle of older adults to “respond” to anabolic stimuli, which has commonly been referred to as “anabolic resistance”. Numerous studies have been conducted to determine how aging affects the ability for nutrition and exercise to stimulate skeletal muscle protein synthesis, and to identify strategies to maximize the anabolic response of aging skeletal muscle to these important stimuli. The purpose of this review is to highlight the ability for nutrition and exercise to acutely stimulate protein synthesis/anabolism in skeletal muscle and to discuss to what extent anabolic impairments occur in aging skeletal muscle. We hypothesize that the strategic use of targeted nutritional and exercise therapies can attenuate protein synthesis impairments in aging skeletal muscle and slow the progression of sarcopenia and muscle wasting that occurs as a result of other clinical conditions (9, 11, 13).
PROTEIN ANABOLISM IN SKELETAL MUSCLE: EFFECTS OF NUTRITION AND AGING
Protein and Amino Acids
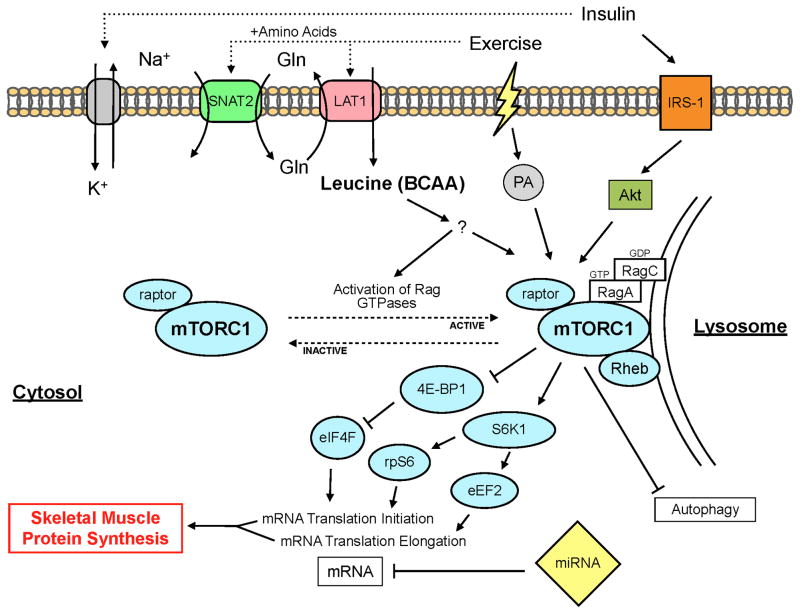
Several metabolic processes within skeletal muscle are sensitive to nutrients, and in particular the ability for increased circulating levels of amino acids to stimulate muscle protein synthesis is very well described (15, 18). The precise mechanisms through which increased amino acid availability stimulates skeletal muscle protein synthesis have yet to be completely understood, however, the stimulatory effect appears to require activation of the mechanistic/mammalian target of rapamycin complex 1 (mTORC1) signaling pathway (7). How amino acids precisely activate mTORC1 signaling remains at this time a very evolving area of research that requires further investigation and is beyond the scope of this review. In Figure 1 we provide an overview of how essential amino acids, insulin and exercise activate mTORC1 signaling and regulate skeletal muscle protein metabolism.
Figure 1.
Simplified schematic of the proposed cellular mechanisms regulating skeletal muscle protein synthesis in response to amino acids, insulin, and exercise. 4E-BP1, 4E binding protein 1; Akt, protein kinase B; BCAA, branch chain amino acids; eEF2, eukaryotic elongation factor 2; eIF4F, eukaryotic initiation factor 4F; Gln, glutamine; IRS-1, insulin receptor substrate 1; LAT1, L-type amino acid transporter 1; mTORC1, mammalian target of rapamycin complex 1; PA, phosphatidic acid; Rag, Ras-related GTPase; rpS6, ribosomal protein S6; S6K1, p70 ribosomal S6 kinase 1; SNAT2, sodium-coupled neutral amino acid transporter 2
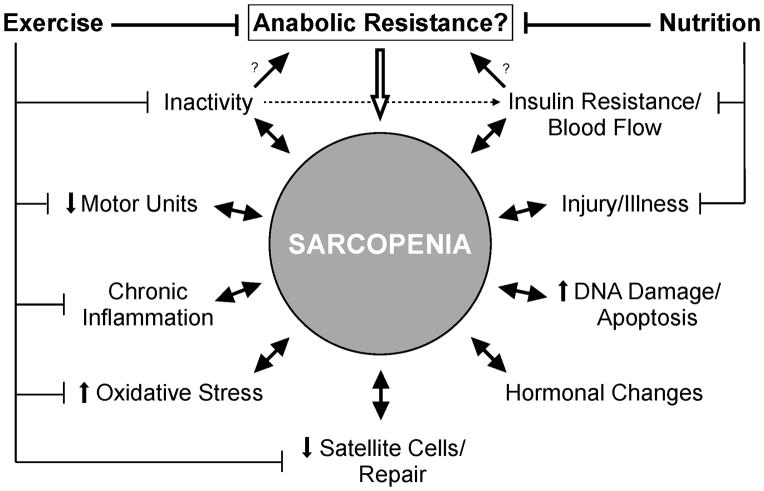
The progression of sarcopenia is likely a consequence of multiple factors that together manifest in a gradual loss of muscle mass and function (Figure 2). One issue that has received considerable attention as a contributing factor to the etiology of sarcopenia is the potential for age-related changes in the protein synthesis response to protein/amino acid ingestion. Several investigations have examined the ability for protein/amino acid ingestion to stimulate skeletal muscle protein synthesis in young and older adults and findings indicate an intricate interplay exists among amino acid availability, skeletal muscle protein synthesis, and aging. For instance, ingesting relatively small quantities of essential amino acids (~7–10g) is capable of stimulating skeletal muscle protein synthesis in young individuals, whereas in older adults this response is not observed (6, 18) indicating an impaired ability for relatively small quantities of amino acids to stimulate muscle protein synthesis in older adults. On the other hand, ingesting larger quantities of essential amino acids is able to stimulate skeletal muscle protein synthesis to a similar degree in young and older adults (37). Accordingly, while the sensitivity of skeletal muscle to amino acid availability appears to be reduced with age, at least when small quantities are ingested, the capacity for amino acid ingestion to stimulate skeletal muscle protein synthesis remains intact. Collectively, these data indicate that the “threshold” in which it is necessary for amino acids to stimulate skeletal muscle protein synthesis is increased with aging, and that any impaired muscle protein synthesis response of older adults to protein/amino acid ingestion can be overcome by ingesting proper quantities.
Figure 2.
Schematic representing many key factors thought to contribute to sarcopenia. These factors include: anabolic resistance (i.e., impairments in muscle protein synthesis), inactivity, insulin resistance, the loss of motor units, injury and illness (i.e., hospitalization), chronic inflammation, DNA damage, increased oxidative stress, changes in hormonal milieu, and a reduction in satellite cells. We hypothesize that strategic exercise and nutritional interventions can slow the progression of sarcopenia due to their ability to overcome anabolic impairments and attenuate the impact of several other contributing factors. Further, it is likely that sarcopenia itself contributes to, and/or accelerates the impact of these factors on skeletal muscle mass and function (represented by the double arrows).
The inability for relatively small amounts of protein/amino acids to stimulate protein synthesis in the skeletal muscle of older adults may be attributed to changes in the sensitivity of skeletal muscle to branched chain amino acids. In particular, leucine has been characterized as a potent stimulator of skeletal muscle protein synthesis, and aging appears to reduce the sensitivity of skeletal muscle to leucine. For instance, in young individuals muscle protein synthesis is stimulated to a similar extent following the ingestion of a 10g iso-nitrogenous essential amino acid mixture containing different quantities of leucine (1.8g vs. 3.5g of leucine) (15). In contrast, skeletal muscle protein synthesis in older adults appears unresponsive to ingesting ~7g of essential amino acids containing ~1.7g of leucine, whereas increasing the leucine content to ~2.8g in the same ~7g mixture restored the protein synthesis response of aging skeletal muscle, which was similar to that observed in younger individuals (18). This finding indicates that although the sensitivity to leucine is suppressed with age, increasing the content of leucine in a given meal may serve as a strategy to promote muscle protein synthesis in older adults (3). In particular, increasing leucine availability may be an effective treatment in those older adults who may routinely not ingest enough protein to reach the required “threshold” to stimulate skeletal muscle protein synthesis.
Collectively, these studies indicate that there may exist an age-related resistance of skeletal muscle protein synthesis when “small” quantities of protein/amino acids are ingested. This impaired response to small doses may contribute to the etiology of sarcopenia, and in particular, may accelerate the rate of sarcopenia in older adults whom routinely consume small portions of protein. However, studies indicate that ingesting 25–30g of high quality protein (24) or greater than 2g of leucine (18) can overcome this impairment to amino acid availability and stimulate skeletal muscle protein synthesis in older adults to a similar degree as young individuals. These observations support the notion that while the sensitivity to amino acids may be reduced with age, the capacity for skeletal muscle of older adults to respond to amino acids is preserved. In addition, it is important to note that aging is associated with reduced physical activity levels, and thus a common concern when performing cross-sectional aging studies is whether the findings are related to aging or inactivity? Indeed, immobilization has been shown to reduce the response of skeletal muscle protein synthesis to an amino acid infusion in young individuals (14), and 7 days of controlled inactivity (e.g., bed rest) in older adults substantially reduces the ability for essential amino acids to stimulate skeletal muscle protein synthesis (8). In contrast, in a recent study of healthy volunteers in the Netherlands, age-related muscle protein synthesis impairments were not observed after ingesting 20g of casein (25). Thus, it is interesting to speculate that perhaps societal discrepancies in daily physical activity and lifestyle between different cultures/countries could play a role in the development of muscle protein synthesis impairments to protein ingestion in the elderly. Nevertheless, while we cannot argue against the fact that aging is associated with a change in the ability for protein/amino acid ingestion to stimulate protein synthesis in skeletal muscle, more work is needed to better delineate between whether the observed changes in skeletal muscle protein synthesis in response to protein/amino acids observed with aging is truly a consequence of advancing age or rather an artifact from reduced physical activity.
The Role of Insulin
As discussed above, considerable attention has been given to whether age-related alterations in the metabolic response to protein/amino acid ingestion contribute to sarcopenia. This level of investigation is very logical given the robust anabolic stimulus that amino acid availability presents to skeletal muscle. However, from a practical standpoint many meals ingested throughout the day typically consist of a mixture of macronutrients. The physiological response to the ingestion of multiple macronutrients presents a very different stimulus to the muscle, in part by exposing skeletal muscle to increasing circulating levels of anabolic hormones (i.e., insulin) and other metabolic substrates (i.e., glucose, fatty acids). Interestingly, we have found that the physiological response elicited by ingesting a mixed meal interferes with the ability of nutrients to trigger a protein anabolic response in the skeletal muscle of older adults (36). Specifically, we have shown that the addition of carbohydrates (40g glucose) to a mixture of amino acids (40g) results in a blunted protein anabolic response in older adults. This blunted response in older adults seems to be the consequence of the inability for the mixed meal to stimulate skeletal muscle protein synthesis, as muscle protein breakdown was similarly reduced in both age groups. These discrepancies in protein metabolism between age groups occurred despite similar levels of circulating essential amino acids and insulin and a similar increase in glucose uptake. Further, this age-related protein anabolic impairment to a mixed meal occurred even in the presence of a relatively large dose of amino acids that when ingested by itself stimulates skeletal muscle protein synthesis to a similar degree in young and older adults (37). Therefore, these data indicate that aging may be associated with a blunted protein anabolic response to a mixed meal, and that this impairment is primarily due to a resistance of skeletal muscle protein synthesis, as we did not observe a differential age response in the ability for the mixed meal to reduce muscle protein breakdown. The chronic inability for a mixed meal to stimulate skeletal muscle protein synthesis in older adults may contribute to the etiology of sarcopenia.
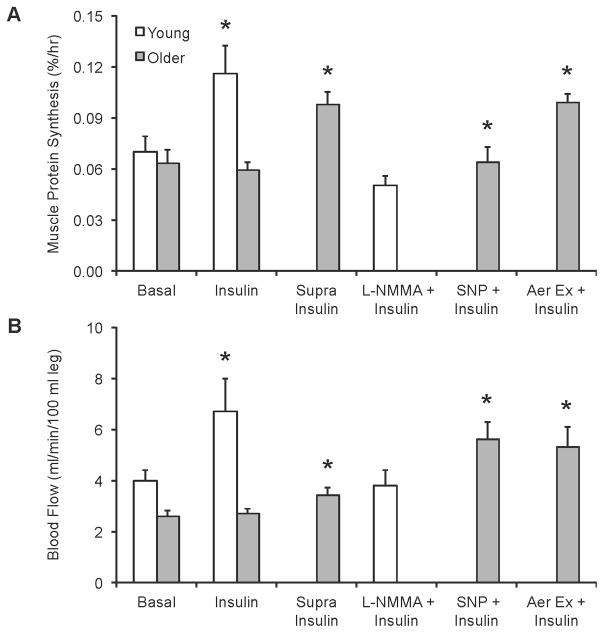
It is well understood that the addition of carbohydrates to a meal stimulates an increase in circulating insulin. Given the role of insulin as an anabolic agent for skeletal muscle, we have performed a variety of studies to determine how aging affects the response of skeletal muscle to insulin (Figure 3). For instance, using a local insulin infusion, in conjunction with a hyperinsulinemic-euglycemmic clamp, we have shown that postprandial insulin levels are capable of stimulating skeletal muscle protein synthesis in younger individuals, whereas in otherwise glucose tolerant older adults skeletal muscle protein synthesis is not increased in response to postprandial insulin (27). On the other hand, we have shown that skeletal muscle protein synthesis can be stimulated in older adults with supraphysiological levels of insulin (12). These data suggest that insulin is capable of triggering a protein synthesis response in the skeletal muscle of older adults, however, there appears to exist an age-related resistance of muscle protein synthesis to physiological levels of insulin. The resistance of skeletal muscle protein synthesis to the anabolic actions of insulin likely represents a key contributor to the inability for a mixed meal to stimulate muscle protein synthesis in older adults.
Figure 3.
The relationship between skeletal muscle protein synthesis (A) and blood flow (B) during a local insulin infusion. The ability for insulin to stimulate skeletal muscle protein synthesis requires an increase in muscle blood flow. The resistance of muscle protein synthesis to the anabolic actions of insulin in the elderly may be due to the inability for insulin to increase blood flow. This resistance to insulin in older adults can be overcome with prior aerobic exercise. Basal, fasted state (27); Insulin, postprandial insulin levels (27); Supra Insulin, supraphysiological insulin levels (12); Insulin + L-NMMA, simultaneous infusion of NG-monomethyl-L-arginine and insulin to inhibit the increase in blood flow (32); Insulin + SNP, simultaneous infusion of sodium nitroprusside and insulin to increase blood flow (33); Aer Ex + Insulin, aerobic exercise performed the night prior to an insulin infusion (postprandial level) (13). *Different from basal in respective study, p<0.05.
In addition to it’s many roles, insulin is a well described vasodilator through the activation of nitric oxide synthase (35). However, in older adults the ability for insulin to stimulate vasodilatation and increase muscle blood flow appears to be impaired (27), which may represent a mechanism to explain the resistance of muscle protein synthesis to insulin in older adults. To test this theory, we performed studies in which we pharmacologically manipulated the muscle blood flow response in young and older adults during a hyperinsulinemic-euglycemic clamp (Figure 3). Specifically, during these clamp studies we simultaneously infused young individuals with a pharmacological agent (NG-monomethyl-L-arginine [L-NMMA]) to inhibit the typical increase in muscle blood flow (32), whereas older adults were simultaneously infused with a pharmacological agent (sodium nitroprusside [SNP]) to increase muscle blood flow (33). Practically speaking, these models were used to “make the young subjects look old and the old subjects look young” with respect to the typical blood flow response to exogenously produced postprandial insulin levels. Under these circumstances, inhibiting the typical increase in muscle blood flow in younger individuals blocked the increase in skeletal muscle protein synthesis. On the other hand, elevating muscle blood flow, typical of the response of a younger individual, stimulated skeletal muscle protein synthesis in older adults. Further, the increase in skeletal muscle protein synthesis was similar to that observed in the young, indicating that the inability for insulin to stimulate an increase in muscle blood flow, and associated nutrient delivery to the muscle, represents a key mechanism contributing to the resistance of muscle protein synthesis to insulin in older adults.
These studies highlight that aging is associated with a resistance of skeletal muscle protein synthesis to insulin, which could represent a key contributor to the etiology of sarcopenia. This resistance to insulin is not only apparent when exogenous insulin is introduced to simulate postprandial levels of insulin, but this resistance also manifests in the presence of elevated endogenous insulin and circulating amino acids that occur in response to ingesting a mixed meal. The inability for older adults to increase muscle blood flow in response to insulin likely represents an important mechanism contributing to the resistance of muscle protein synthesis to insulin in older adults (Figure 3). Future studies are needed to more precisely uncover the mechanisms that contribute to this age-related resistance of skeletal muscle protein synthesis to insulin and to identify strategies to improve the ability for older adults to increase muscle blood flow in response to increased circulating insulin.
PROTEIN ANABOLISM IN SKELETAL MUSCLE: EFFECTS OF EXERCISE AND AGING
Resistance Exercise
Numerous laboratories have studied the impact of resistance exercise on skeletal muscle protein synthesis, and our understanding of this topic has grown tremendously over the past ~20 years. It is well accepted that resistance exercise stimulates skeletal muscle protein synthesis in young individuals, however, an acute bout of resistance exercise does not appear to elicit the same response in the skeletal muscle of older adults. For instance, we monitored rates of muscle protein synthesis for 24 hours following a bout of resistance exercise (8 sets of 10, ~70% 1RM) in a relatively large cohort of young and older adults (11). Protein synthesis was significantly elevated above resting levels only in the young individuals during the 24-hour time-course (Figure 4). The impaired response in older adults may have been related to the inability of resistance exercise to substantially increase mTORC1 signaling (11), which is a key component regulating the increase in human skeletal muscle protein synthesis following resistance exercise (10). Similarly, an age-related impairment in the ability for resistance exercise to stimulate muscle protein synthesis has also been observed over a range of exercise intensities (i.e., %1RM) (20), providing further support that protein synthesis in the skeletal muscle of older adults may be impaired in response to resistance exercise.
Figure 4.
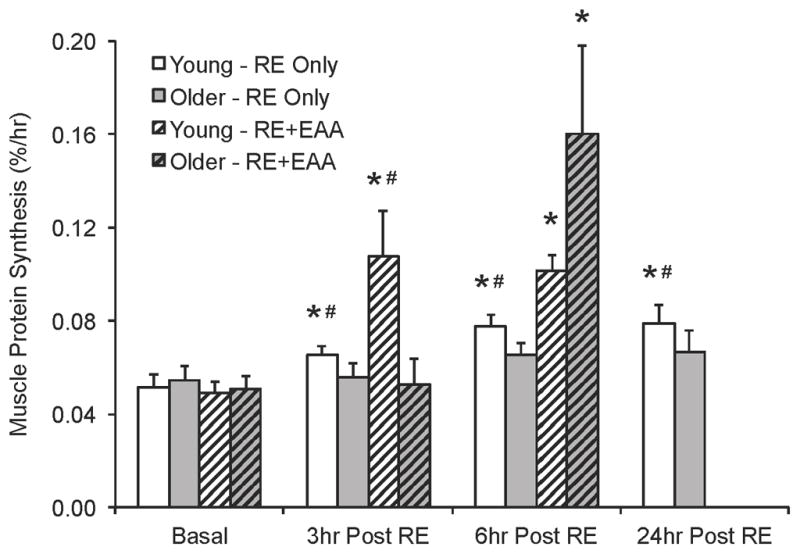
The response of skeletal muscle protein synthesis to resistance exercise without nutrition (RE Only) (11) and resistance exercise followed by essential amino acid (RE+EAA) ingestion 1 hour after exercise (9) in young and older adults. Aging is associated with an impaired skeletal muscle protein synthesis response to RE only, however, this impairment is overcome with post exercise nutrient ingesting. Further, nutrient ingestion following resistance exercise produces and additive increase in skeletal muscle protein synthesis in both young and older adults. *Different from basal in respective study, p<0.05, #Different from older in respective study, p<0.05.
Does this impaired protein synthesis response to an acute bout of resistance exercise imply that resistance exercise is not beneficial as a strategy to slow, or even counter, sarcopenia? Resistance exercise is certainly capable of increasing muscle size and strength in older adults (26), and thus does represent a strategy that, at the very least, can be used to slow the trajectory of muscle loss with aging. Although a recent meta-analysis did identify a negative association between age and gains in lean body mass with resistance exercise training (26), conclusions from resistance exercise training studies appear equivocal with respect to age-related impairments in whole muscle size and strength gains. On the other hand, a blunted response to resistance exercise training does appear evident in older adults when changes in muscle fiber size are examined (19, 23). Further, the existence of age-related impairments in skeletal muscle adaptation at the whole muscle level does appear to become more apparent in advanced age. In particular, adults over 80 years of age show only modest improvements in whole muscle size and strength following resistance exercise training, and almost no change in the size and contractile properties of slow and fast twitch muscle fibers (28, 31). This observation indicates the modest gains in whole muscle size and strength in this age group were likely the product of neural adaptations rather than substantial adaptive changes in muscle protein(s). Nevertheless, resistance exercise represents a promising strategy to improve muscle size and function in older adults, however, more work may be required to maximize the response of older adults and clinical populations, in particular those that may not be able to perform traditional resistance exercise.
Aerobic Exercise
Relative to the breadth of studies available investigating the ability for resistance exercise to stimulate skeletal muscle protein synthesis, the effect of aerobic exercise on muscle protein metabolism has received considerably less attention, although more focus has been given to this area in recent years. Indeed, acute aerobic exercise has been shown to stimulate skeletal muscle protein synthesis in the immediate hours following exercise in young individuals (2, 16). On the other hand, there are very limited data available on the effects of higher intensity aerobic exercise in older adults. Aerobic exercise has not historically received tremendous support as a countermeasure to sarcopenia, which is likely related to the general idea that aerobic exercise is thought to primarily produce metabolic adaptations in muscle rather than increasing muscle size and strength. However, recent work has highlighted a potential role for aerobic exercise training as a strategy to slow sarcopenia. In particular, 12 weeks of cycling has been shown to promote similar increases in muscle size and strength in young and older men (17). The ability for aerobic exercise to increase muscle size and strength, especially in older adults, may be manifested through increasing resting muscle protein synthesis rates (30), or through increasing the sensitivity of skeletal muscle to insulin or a subsequent meal (13). With respect to the latter, we have shown that prior aerobic exercise can overcome the age-related resistance of muscle protein synthesis to insulin and restore the ability for insulin to stimulate muscle protein synthesis in older adults (13), highlighting that routine aerobic exercise may represent a strategy to overcome the resistance of muscle protein synthesis to insulin with aging. Further, the ability for aerobic exercise to increase muscle size and strength in older adults may also work through indirect mechanisms. For instance, routine aerobic exercise has been shown to promote favorable effects on mitochondrial function and insulin sensitivity [see (21)]. These favorable adaptations are not only beneficial to metabolic health, but dysfunction of these metabolic processes is also associated with aging and muscle wasting. Collectively, routine aerobic exercise may represent a very promising and practical strategy to overcome the age-related protein anabolic resistance to a mixed meal and preserve and/or improve muscle health and function in older adults. However, further work is needed to more thoroughly identify and understand the ability for various modes of aerobic exercise to promote protein anabolism in the skeletal muscle of older adults.
CAN NUTRITIONAL STRATEGIES OVERCOME PROTEIN SYNTHESIS IMPAIRMENTS TO EXERCISE IN AGING MUSCLE?
Numerous studies have demonstrated that ingesting nutrients, particularly protein/amino acids, following a bout of resistance exercise produces an enhanced rate of muscle protein synthesis and a net protein anabolic environment within the exercised muscle. Consequently, extensive effort has been made to determine whether coupling exercise with nutritional strategies may serve as a means to overcome the protein synthesis impairments of aging skeletal muscle, particularly to resistance exercise. For instance, we have shown in both younger and older adults that ingesting 20g of essential amino acids shortly following a bout of resistance exercise stimulates skeletal muscle protein synthesis to a much greater degree than resistance exercise performed in the absence of nutrient ingestion (Figure 4) (9, 11). Further, the overall stimulation of muscle protein synthesis in the immediate hours following this combination was similar between young and older adults (9). It appears that this improved muscle protein synthesis response could be the result of an enhanced ability for the combination of resistance exercise and essential amino acid ingestion to stimulate skeletal muscle mTORC1 signaling (9, 11). These data support the notion that the age-related resistance of muscle protein synthesis to an acute bout of resistance exercise can be overcome with the ingestion of post-exercise nutrients. However, recent data indicate that relative to younger individuals, older adults may require a larger quantity of protein/amino acids following resistance exercise to maximally stimulate skeletal muscle protein synthesis (39). This latter finding suggests that the elevated threshold for protein/amino acids to stimulate muscle protein synthesis in older adults is also present even when preceded by exercise and that aging may require subtle alterations to exercise and nutritional strategies to maximize muscle growth and the ensuing improvements in muscle function.
A significant amount of work has been conducted by several laboratories to better understand the interaction among exercise, nutrition and protein synthesis in skeletal muscle. While it is well outside the scope of this review to summarize all this literature, much of this effort has recently focused on optimizing post exercise nutritional strategies to maximize the protein synthesis response of skeletal muscle, and in particular examining differences among varying amino acid compositions (i.e., leucine content) and/or among various protein sources (i.e., whey, casein, soy) that contain different intrinsic properties related to the stimulation of skeletal muscle protein synthesis. For example, in young individuals we recently examined whether ingesting a blend of whey, casein, and soy protein after a bout of resistance exercise differentially affected the protein synthesis response of skeletal muscle compared to the post-exercise ingestion of isolated whey protein matched for leucine content (29). Although skeletal muscle protein synthesis was stimulated by the post-exercise ingestion of both the protein blend and isolated whey protein, ingestion of the protein blend prolonged the increase in circulating amino acids, mTORC1 signaling, and skeletal muscle protein synthesis (29). These data suggest that post-exercise ingestion of a protein blend may take advantage of the intrinsic characteristics of each protein source (i.e., absorption rate, leucine content) and promote a longer lasting anabolic environment for skeletal muscle. How this prolonged response may translate into muscle mass and strength gains when performed chronically, and whether similar findings would be observed in older adults requires further investigation.
Despite the overwhelming amount of data supporting the benefits of post exercise nutrient ingestion to restore muscle protein synthesis following acute exercise in older adults, there is an extremely limited number of studies that have determined an added benefit of coupling protein/amino acid ingesting with resistance exercise training on muscle size and strength gains in older adults. However, several factors can influence the overall muscle protein anabolic response to exercise and nutrition and these factors should be considered when examining the findings in the current literature. For instance, is the quantity of protein or leucine content ingested following exercise meeting the specific age-related threshold (18, 39), is the daily distribution of protein or protein supplements being properly distributed to maximize the daily protein anabolic response (24) or the response to exercise (1), are the experimental and placebo groups already ingesting enough daily protein such that additional protein ingestion does not augment the response to exercise (24), and is the study duration long enough to detect an augmented growth and/or strength response to the training? These factors are likely important for determining muscle mass and strength gains with exercise training across a wide spectrum of ages, however, the relevance of each of these factors may become even more important for controlling the adaptation of muscle to exercise training in older adults.
To gain a broader perspective as to whether protein supplementation during resistance exercise training is beneficial for skeletal muscle size and strength adaptations, a recent meta-analysis was performed (4). The findings of this meta-analysis suggested that protein supplementation during resistance exercise training is indeed associated with greater increases in muscle strength (1RM) and lean body mass in both young and older adults. Although this type of analysis cannot confer cause and effect, the results further highlight the need for larger randomized controlled trials to determine whether concurrent protein supplementation can amplify the gains in muscle size and strength in older adults during resistance exercise. Of particular interest is whether or not protein supplementation during exercise and/or rehabilitation can improve anabolic outcomes particularly in older populations not ingesting proper quantities of daily protein, or older adults not properly distributing protein throughout the day (24). Similarly, it is intriguing to speculate that the therapeutic potential for exercise and protein supplementation may become even more critical for clinical populations and/or frail, undernourished older adults. There is certainly a need for more research to determine the translational capacity of this treatment strategy for use in clinical practice.
SUMMARY AND CONCLUSIONS
Aging is associated with a gradual reduction in skeletal muscle size and function termed sarcopenia. While the precise mechanisms remain to be fully understood, one of the underlying factors currently thought to contribute to sarcopenia is an age-related alteration in the ability for stimuli such as protein/amino acids, insulin, and exercise to substantially increase skeletal muscle protein synthesis in older adults. Indeed, we cannot deny there is ample support to indicate metabolic impairments are present in the skeletal muscle of older adults and these impairments in protein synthesis to anabolic stimuli, when sustained over time, likely contribute to, and may even accelerate, the loss of muscle size and function. However, we argue that the capacity to stimulate skeletal muscle protein synthesis is preserved with age, and when appropriate and targeted strategies are utilized, nutrition and exercise are capable catalysts for muscle protein anabolism in older adults and can serve to preserve/improve muscle size and function. In addition, distributing appropriate quantities of ingested protein throughout a given day and performing routine exercise may preserve the protein synthesis response of skeletal muscle and slow the progression of muscle atrophy. In particular, routine exercise serves to improve insulin sensitivity and the protein anabolic actions of insulin on skeletal muscle. Finally, the strategic coupling of exercise and nutrition also represents a practical strategy that has promise for restoring skeletal muscle size and function in those older adults who may already be at risk for the deleterious effects of sarcopenia, and this combination may be even more useful as a strategy to recover physical function in clinical aging populations.
Before exercise and nutritional strategies to slow or reverse the progression of sarcopenia can be maximized, there are several areas of research that still require further investigation. For instance, a better understanding of the precise cellular mechanisms (Figure 1) that contribute to sarcopenia and the observed age-related changes in the protein synthesis response of skeletal muscle would provide a basis to develop more promising therapeutic strategies, and in particular there is an overwhelming need for in vivo human studies in this area. Similarly, studies are needed to better differentiate between the effects of aging and those of inactivity on the progression of sarcopenia and in the ability for nutrition and exercise to stimulate skeletal muscle protein metabolism. There is also a definite need for well-controlled longitudinal studies in both healthy and clinical older populations to thoroughly examine the effectiveness for nutrition and exercise based strategies to preserve and/or recover muscle strength and function. Finally, given the overlap among various factors that are thought to contribute to sarcopenia (Figure 2), studies are needed to better define the precise role of muscle protein synthesis impairments on the etiology of sarcopenia relative to other contributing factors. These collective research areas will provide a more thorough understanding of the factors that contribute to the progression of sarcopenia and how aging may affect protein anabolism, and its regulation, in skeletal muscle. Consequently, these research areas will provide critical information necessary to maximize the effectiveness of strategies to protect and/or recover muscle size and function, and ultimately preserve independence and improve quality of life in the expanding aging population.
SUMMARY.
This review discusses how aging affects the protein synthesis response of skeletal muscle to nutrition and exercise.
Acknowledgments
The authors would like to acknowledge the wealth of outstanding research that has significantly increased our understanding of the topics discussed, and we apologize that the space limitations did not allow us to cite all this work.
The work from the authors’ laboratories described within this review was funded by NIH/NIAMS R01AR049877, NIH/NIA P30AG024832 and R01AG030070, Solae, LLC, and in part by NIH Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences
Footnotes
The authors do not have any conflicts of interest to declare.
Funding Disclosure: Supported by NIH/National Institute of Arthritis and Musculoskeletal and Skin Disease R01AR049877, NIH/National Institute on Aging P30AG024832 and R01AG030070, Solae, LLC, and NIH Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences.
References
- 1.Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, Jeacocke NA, Moore DR, Stellingwerff T, Phillips SM, Hawley J, Coffey VG. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol. 2013;591(9):2319–31. doi: 10.1113/jphysiol.2012.244897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carraro F, Stuart CA, Hartl WH, Rosenblatt J, Wolfe RR. Effect of exercise and recovery on muscle protein synthesis in human subjects. Am J Physiol. 1990;259(4 Pt 1):E470–6. doi: 10.1152/ajpendo.1990.259.4.E470. [DOI] [PubMed] [Google Scholar]
- 3.Casperson SL, Sheffield-Moore M, Hewlings SJ, Paddon-Jones D. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin Nutr. 2012;31(4):512–9. doi: 10.1016/j.clnu.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96(6):1454–64. doi: 10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M European Working Group on Sarcopenia in Older P. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19(3):422–4. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 7.Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141(5):856–62. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, Timmerman KL, Markofski MM, Paddon-Jones D, Rasmussen BB, Volpi E. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab. 2012;302(9):E1113–22. doi: 10.1152/ajpendo.00603.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104(5):1452–61. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587(Pt 7):1535–46. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skeletal Muscle. 2011;1:11. doi: 10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita S, Glynn EL, Timmerman KL, Rasmussen BB, Volpi E. Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia. 2009;52(9):1889–98. doi: 10.1007/s00125-009-1430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, Volpi E. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007;56(6):1615–22. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008;586(Pt 24):6049–61. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr. 2010;140(11):1970–6. doi: 10.3945/jn.110.127647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harber MP, Crane JD, Dickinson JM, Jemiolo B, Raue U, Trappe TA, Trappe SW. Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R708–14. doi: 10.1152/ajpregu.90906.2008. [DOI] [PubMed] [Google Scholar]
- 17.Harber MP, Konopka AR, Undem MK, Hinkley JM, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol. 2012;113(9):1495–504. doi: 10.1152/japplphysiol.00786.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291(2):E381–7. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 19.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006;101(2):531–44. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 20.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587(Pt 1):211–7. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanza IR, Nair KS. Muscle mitochondrial changes with aging and exercise. Am J Clin Nutr. 2009;89(1):467S–71S. doi: 10.3945/ajcn.2008.26717D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84(2–3):275–94. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 23.Mero AA, Hulmi JJ, Salmijarvi H, Katajavuori M, Haverinen M, Holviala J, Ridanpaa T, Hakkinen K, Kovanen V, Ahtiainen JP, Selanne H. Resistance training induced increase in muscle fiber size in young and older men. Eur J Appl Physiol. 2013;113(3):641–50. doi: 10.1007/s00421-012-2466-x. [DOI] [PubMed] [Google Scholar]
- 24.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12(1):86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr. 2011;93(2):322–31. doi: 10.3945/ajcn.2010.29649. [DOI] [PubMed] [Google Scholar]
- 26.Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc. 2011;43(2):249–58. doi: 10.1249/MSS.0b013e3181eb6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. Faseb J. 2006;20(6):768–9. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol. 2009;106(5):1611–7. doi: 10.1152/japplphysiol.91587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, Fry CS, Borack MS, Cope MB, Mukherjea R, Jennings K, Volpi E, Rasmussen BB. Protein Blend Ingestion Following Resistance Exercise Promotes Human Muscle Protein Synthesis. J Nutr. 2013;143(4):410–6. doi: 10.3945/jn.112.168021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286(1):E92–101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 31.Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R273–80. doi: 10.1152/ajpregu.00093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timmerman KL, Lee JL, Dreyer HC, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Sheffield-Moore M, Rasmussen BB, Volpi E. Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial-dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J Clin Endocrinol Metab. 2010;95(8):3848–57. doi: 10.1210/jc.2009-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timmerman KL, Lee JL, Fujita S, Dhanani S, Dreyer HC, Fry CS, Drummond MJ, Sheffield-Moore M, Rasmussen BB, Volpi E. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes. 2010;59(11):2764–71. doi: 10.2337/db10-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol. 2003;552(Pt 1):47–58. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Current Diabetes Reports. 2003;3(4):279–88. doi: 10.1007/s11892-003-0018-9. [DOI] [PubMed] [Google Scholar]
- 36.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85(12):4481–90. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277(3 Pt 1):E513–20. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 38.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. Jama. 2001;286(10):1206–12. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr. 2012;108(10):1780–8. doi: 10.1017/S0007114511007422. [DOI] [PubMed] [Google Scholar]