Abstract
Mitochondrial tRNAs are generally synthesized as part of polycistronic transcripts. Release of tRNAs from these precursors is thus not only required to produce functional adaptors for translation, but also responsible for the maturation of other mitochondrial RNA species. Cleavage of mitochondrial tRNAs appears to be exclusively accomplished by endonucleases. 5′-end maturation in the mitochondria of different Eukarya is achieved by various kinds of RNase P, representing the full range of diversity found in this enzyme family. While ribonucleoprotein enzymes with RNA components of bacterial-like appearance are found in a few unrelated protists, algae, and fungi, highly degenerate RNAs of dramatic size variability are found in the mitochondria of many fungi. The majority of mitochondrial RNase P enzymes, however, appear to be pure protein enzymes. Human mitochondrial RNase P, the first to be identified and possibly the prototype of all animal mitochondrial RNases P, is composed of three proteins. Homologs of its nuclease subunit MRPP3/PRORP, are also found in plants, algae and several protists, where they are apparently responsible for RNase P activity in mitochondria (and beyond) without the help of extra subunits. The diversity of RNase P enzymes is contrasted by the uniformity of mitochondrial RNases Z, which are responsible for 3′-end processing. Only the long form of RNase Z, which is restricted to eukarya, is found in mitochondria, even when an additional short form is present in the same organism. Mitochondrial tRNA processing thus appears dominated by new, eukaryal inventions rather than bacterial heritage. This article is part of a Special Issue entitled: Mitochondrial Gene Expression.
Keywords: RNase P, RNase Z, transfer RNA, RNA processing, Mitochondria
Highlights
► A great diversity of RNase P enzymes is found in mitochondria of different organisms. ► The RNase P of many fungi contains a highly degenerated RNA. ► Most mitochondrial RNase P enzymes, however, are composed of protein only. ► Mitochondrial 3′-end processing seems to rely exclusively on the long, eukaryal form of RNase Z.
1. Introduction
As the adaptors between genetic code and specific amino acids, transfer RNAs (tRNAs) are essential players in protein synthesis. Mitochondria thus require a set of tRNAs within their matrix compartment to translate the genes encoded by their genome. While in some cases these tRNAs are entirely derived from the cytosol by active transport through the two mitochondrial membranes [1], the mitochondrial genomes of many organisms encode a majority or all of the tRNAs required for mitochondrial protein synthesis.
Like those of any other genetic system, mitochondrial tRNAs are not transcribed in their mature form, but as precursors that require processing and modification for maturation [2]. Primary tRNA gene transcripts have extensions at both ends that have to be removed by specific nucleases [3]. Unlike nuclear tRNA transcripts, yet resembling their bacterial ancestors' tRNA gene expression, mitochondrial tRNAs are usually synthesized as part of polycistronic transcripts. Their 5′- and 3′-flanking sequences are thus not short non-coding sequences, but rather themselves (precursors of) mRNAs, rRNAs, or other tRNAs, and as a mitochondrial peculiarity often directly abutting the tRNA sequence, a phenomenon known as ‘tRNA punctuation’. Release of tRNAs from the polycistronic primary transcripts thus indirectly provides processed mRNAs and rRNAs as a consequence of tRNA recognition and cleavage. tRNA punctuation is widely used for transcript processing in the mitochondria of opisthokonts (fungi and animals) [4–6], and is best illustrated by the organization and processing of the human mitochondrial genome, where tRNA processing accounts for almost all primary transcript processing events [7]. Plant mitochondria do not use tRNA punctuation as the basic mechanism for transcript processing. Nevertheless, their tRNA genes are clustered and/or part of polycistronic primary transcripts [8]. Moreover, plant mitochondrial mRNA transcripts frequently contain tRNA-like structures called ‘t-elements’, which are coincident with the ends of the mature mRNA, suggesting that they too serve as recognition signals for mitochondrial tRNA processing enzymes [9].
The tRNA processing enzymes of mitochondria thus play a central role not only in the maturation of mitochondrial tRNAs, but for mitochondrial RNA maturation in general. In this review I will summarize what is currently known about the two responsible mitochondrial endonucleases RNase P and RNase Z, compare them to the respective enzymes active in the nucleus or in bacteria, and compare different eukaryal model systems to outline the differences or parallels.
Still, mitochondrial tRNA maturation is not complete after 5′- and 3′-end cleavage. Like nuclear tRNA genes, mitochondrial tRNA genes do generally not encode the CCA sequence universally found at the 3′ end of mature tRNAs. The CCA trinucleotide is added after 3′-end processing by a CCA-adding enzyme, an ATP(CTP):tRNA nucleotidyltransferase [10]. In yeast, and apparently also in humans, this enzyme is shared between compartments, i.e., derived from the same gene as that acting on nucleus-encoded tRNAs [11–14]. Mitochondrial tRNA genes lack introns and thus do not require splicing.
tRNAs are also the most extensively modified cellular RNA species, and so are mitochondrial tRNAs [15,16]. Nucleotides are modified posttranscriptionally by a variety of different modification enzymes some of which are shared and used in the modification of mitochondrial as well as cytosolic tRNAs, while others are specific for mitochondria, just as some modifications are only found in mitochondrial tRNAs [16–19]. However, due to the comparably low cellular abundance of mitochondrial tRNAs the study of their modifications and modification pathways lags far behind that of cytoplasmic or bacterial tRNAs.
Mitochondrial tRNAs of most Eukarya conform to the canonical type of tRNA structure found in Bacteria, Archaea, or the eukaryal cytosol. Metazoan mitochondrial tRNAs, however, generally deviate more or less from this structural consensus, are in general smaller, have highly reduced, or even lack, D or TΨC domains, and do not form many of the typical tertiary interactions stabilizing the tRNA L-shape; they are therefore in some cases referred to as bizarre [20–22]. These structural peculiarities appear to be reflected in the evolution of some of the enzymes interacting with animal mitochondrial tRNAs, including the enzyme involved in 5′ end processing.
2. tRNA 5′-end processing — RNase P
In all genetic systems analyzed so far 5′ extensions of tRNA precursors are removed by an endonuclease. This enzyme is called RNase P (EC 3.1.26.5); it is a metallonuclease that utilizes Mg2 + for phosphodiester hydrolysis [for review see refs. 3,23–26]. RNase P was first identified in Escherichia coli [27], and its subsequent characterization showed that it is composed of a small protein and an RNA [28]. The RNA was shown to be the actual catalytic part of the enzyme, capable of catalysis even in the absence of the protein subunit under specific in vitro conditions [29]. Historically, the trans-acting RNase P RNA was the first true RNA enzyme (ribozyme) identified.
RNA-based RNase P enzymes were subsequently identified in various forms of life (see Section 2.1) and the apparent omnipresence of the RNase P ribozyme not only led to the concept that this RNA enzyme is a universal relic of a hypothetical ‘RNA world’, but to the (still) prevailing view that tRNA 5′-end processing is inevitably linked to the action of a catalytic RNA. However, another form of RNase P, built of protein only, has recently been disclosed. First identified in human mitochondria [30], it was subsequently also demonstrated in plant mitochondria and chloroplasts [31] (see Sections 2.3 and 2.4). This enzyme appears typically composed of a single ~ 60 kDa polypeptide, but functionally equivalent to the RNA-based enzymes. In fact, the Arabidopsis mitochondrial/chloroplast enzyme is able to substitute for E. coli RNase P in vivo [31]. Its presence in the genomes of several eukaryal groups suggests that this proteinaceous RNase P is as frequent as the RNA-based enzyme in Eukarya and not restricted to mitochondria and chloroplasts (see Section 2.1).
2.1. The RNase P family in the three domains of life — a survey
All bacterial RNase P enzymes identified so far are composed of an RNA and a small protein subunit [3,32,33]. The RNAs of different bacteria fall into two structural classes, types A and B, and a small group that seems structurally intermediate [3,25,32,33]. An exceptionally small, structurally most reduced, quasi-minimal bacterial RNase P RNA is found in Mycoplasma fermentas [34], which lacks, e.g., the otherwise universally conserved helix P12 (Fig. 1). Despite this structural variation of the catalytic RNA moiety the protein subunits from even distant bacteria are structurally conserved and functionally exchangeable [35]. The protein is essential for RNase P function in vivo and plays an important role in substrate recognition, the stabilization of the RNA's tertiary structure, and increases the binding affinity for metal ions crucial for substrate positioning and catalysis [3,32]. Whereas structurally related RNase P RNAs are found in almost all Archaea and many Eukarya, the bacterial protein has no homologs in these two phylogenetic domains [36]. Neither a gene for RNase P RNA nor for an RNase P protein were identified in the genome of the hyperthermophilic bacterium Aquifex aeolicus, although an RNase P activity was detected in cell extracts [37–39].
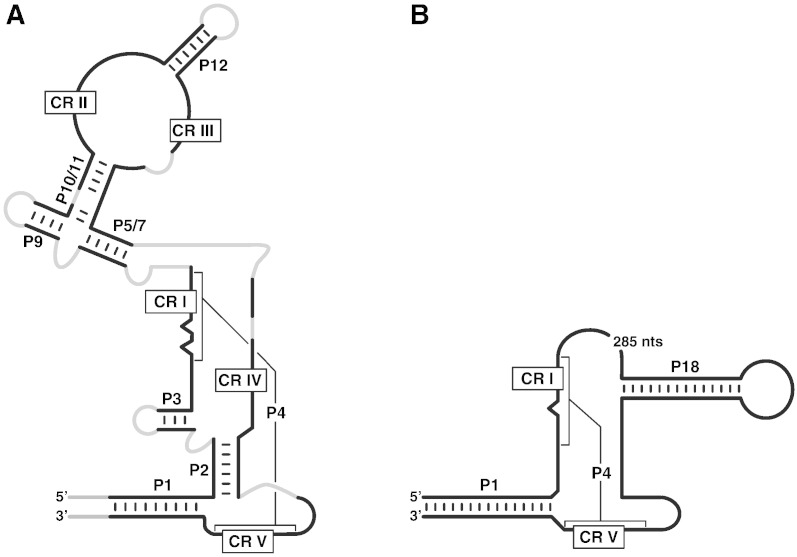
Fig. 1.
Schematic structures of RNase P RNAs. (A) Universal consensus secondary structure of bacterial, archaeal, and eukaryal nuclear RNase P RNAs [25]. Gray indicates variable structures connecting conserved regions. Helices P1–P12 [144] and conserved sequence regions CR I–V [145] are indicated. Structural elements absent only in single, minor phylogenetic groups are included: P12, absent in M. genitalium[34]; P11, P12, CR II, and CR III, absent in P. aerophilum[43]. (B) Secondary structure of S. cerevisiae mtRNase P RNA (Rpm1r) [65].
Archaeal RNase P is composed of a structurally related RNA and at least four proteins [40,41]. The catalytic competence of the RNA is not only suggested by the evolutionary relationship with bacterial RNase P RNA (Fig. 1), but was also verified experimentally, although more extreme ionic conditions were required to observe RNA alone activity [42]. The hyperthermophilic crenarchaeon Pyrobaculum aerophilum has another highly reduced, minimal form of RNase P RNA, and possibly also a reduced set of associated proteins [43]; still the RNA has retained catalytic activity. The archaeon Nanoarchaeum equitans might be the sole form of life having no RNase P at all [44]. The tRNAs of this parasitic archaeon are synthesized without 5′ extensions, resulting in mature tRNAs with a 5′ triphosphate.
Eukaryal nuclear RNase P is best characterized in the yeast Saccharomyces cerevisiae and in the human system [41,45,46]. It is composed of an RNA structurally related to that of Bacteria and Archaea (Fig. 1). Again, this RNA was shown to have some residual catalytic competence on its own [47]. The protein complement is further increased in number and mass relative to Archaea, and 9 protein subunits are found in yeast and 10 in humans, including a 100-kDa polypeptide (POP1) as the largest protein component. The majority of these proteins is also associated with the nuclear ribonucleoprotein RNase MRP [45,46]. Its RNA component is structurally related to that of RNase P, but has distinctive features too. RNase MRP does not act on the 5′ ends of tRNA precursors, but plays a role in rRNA processing and, by cleavage of cyclin B2 mRNA, in yeast cell cycle regulation [45,46].
Yeast and human nuclear RNase P are proposed to have many substrates in addition to tRNA precursors [45,48]. Moreover, human nuclear RNase P appears to play a role in nuclear transcription by RNA polymerases III and I [49]. This increased versatility is considered to have been a major evolutionary driving force for the increase in (protein) complexity of the eukaryal compared to the prokaryotic enzymes during evolution.
RNase P RNA sequences could not be identified in the genomes of major phylogenetic branches of Eukarya, such as land plants, green and red algae, and heterokonts (Stramenopiles) [50,51]. The same holds for homologs of proteins otherwise specifically associated with nuclear RNase P, although an RNase MRP RNA and its associated proteins were identified in the aforementioned groups [50,52]. Yet, neither RNase P nor RNase MRP RNA sequences, nor any of the typically associated proteins are found in the genomes of kinetoplastids (encompassing the parasitic Trypanosoma and Leishmania species) [50,52]. Interestingly, all these eukaryal groups encode one or more copies of the proteinaceous RNase P protein originally identified as the catalytic subunit of human mitochondrial RNase P [30]. Nuclear RNase P might thus be a pure protein enzyme in many Eukarya. Consistently, two of the three proteinaceous RNase P isoforms found in Arabidopsis thaliana indeed localize to the nucleus [31].
Apart from the nucleus and mitochondria, RNase P activity is also found in chloroplasts/plastids (in Eukarya). Consistent with the cyanobacterial origin, the plastid genomes of glaucophytes, red algae, and some prasinophytes (an early branching group of green algae) encode a bacterial type A RNase P RNA [53,54]. Other green algae and land plants have lost this gene from their plastid genomes. However, their nuclear genomes encode one or more proteinaceous RNase P enzymes (PRORP) [30,31]. We recently showed that A. thaliana chloroplast RNase P is indeed a protein and identical to the mitochondrial enzyme [31] (see Section 2.4). In fact, biochemical characterization of spinach chloroplast RNase P almost 25 years ago suggested that this enzyme does not contain RNA [55]. The protein, however, was identified only recently by homology to human mitochondrial RNase P (mtRNase P) [30]. Thus, chloroplasts of land plants and most green algae have apparently replaced their bacterial heritage with a protein enzyme, whereas more early branching photosynthetic Eukarya have at least partially kept the ancestral state in their plastids (the protein component(s) of these putative ribonucleoprotein RNase P enzymes have not been identified yet). It further appears that genomes of plastids originating from secondary endosymbiosis (e.g., in heterokonts, haptophytes, cryptomonads, apicomplexans, or euglenids) have generally lost their RNase P RNA gene, regardless if the plastids derived from red or green algae.
2.2. Mitochondrial RNase P in fungi
2.2.1. Yeast mitochondrial RNase P
Of all fungal mtRNases P that of the budding yeast S. cerevisiae is by far the best characterized [54]. Early genetic studies had revealed that the synthesis of yeast mitochondrial tRNAs depends on a locus mapping between the tRNAfMet and tRNAPro genes of the mitochondrial genome, which does not encode a protein [56,57]. Molecular studies of the expression of this region identified an A/U rich transcript of ~ 500 nucleotides in length [58], a mapping that was later refined to ~ 420 nucleotides [59]. This RNA cofractionated with mtRNase P activity upon purification [60], and 3′-matured tRNA with 5′ extension accumulated in deletion mutants of this mitochondrial locus [61]. Moreover, the activity was demonstrated to be sensitive to nuclease and protease treatment, indicating that it is a ribonucleoprotein that requires nucleus-encoded protein(s) for function, the latter inferred from the fact that yeast mitochondrial DNA (mtDNA) deletion mutants devoid of mitochondrial protein synthesis, but retaining the RNase P RNA locus, still have RNase P activity [60]. Surprisingly, the integrity of the full-length RNA is not required and two fragments from the 5′ and 3′ end (~ 70 nucleotides each), were shown to be sufficient to support the activity in vitro [62]; thus a major part of the RNA appears to be dispensable (Fig. 1).
RNase P RNA genes (RPM1, or rnpB in analogy to the bacterial gene) were also found in the mitochondrial genomes of many other budding yeasts (Saccharomycetales) [63–65]. All these RNAs are extremely A/U rich and vary considerably in size, down to the minimal rnpB of Saccharomycopsis fibuligera of only ~ 150 nucleotides. They all deviate considerably from the general, bacterial–archaeal–eukaryal nuclear, consensus structure or the bacterial minimal consensus structure (Fig. 1). Secondary structure predictions of these RNAs are further complicated by the low degree of primary sequence conservation even among rather closely related yeasts. Only the conserved regions CR I and CR V, forming helix P4, and helix P1 appear generally retained [65]. Also CR IV is found frequently, and S. cerevisiae and its close relatives harbor a stem-loop structure that may mimic helix P18 of bacterial RNase P RNAs [65]. Not surprisingly, none of these crippled RNase P RNAs gave rise to RNase P activity without protein in vitro, and it is assumed that the protein moiety of the enzyme has a more substantial, compensatory functional role in the holoenzyme.
The mtRNase P of S. cerevisiae is the only organellar RNA-based RNase P of which a protein component has been identified. Extensive purification of the enzyme revealed a 105-kDa protein encoded by a nuclear gene termed RPM2 [66]. Antibodies against Rpm2p immunoprecipitate mtRNase P activity as well as its RNA component (Rpm1r) [67], and a gene disruption producing a C-terminally truncated protein leads to the accumulation of mitochondrial tRNA precursors with 5′ extensions [66,68], though this effect is at least in part attributable to a role of this domain in Rpm1r maturation [69] (see also below). In vitro reconstitution of yeast mtRNase P from purified components has not been achieved so far. Thus, the contribution of Rpm2p to mtRNase P activity remains vague and it cannot be excluded that other proteins are required. Rpm2p has no homology to any other known RNase P protein and its gene is not found beyond closely related budding yeasts, not even in other ascomycete fungi [24,54].
Rpm2p has further functions not directly related to mtRNase P activity and even entirely unrelated ones. Complete deletion of RPM2 is lethal, even under conditions allowing fermentative growth (yeast requires mitochondrial protein synthesis for respiration, but not for fermentative growth on, e.g., glucose) [68]. Under fermentative conditions deletion of the C-terminal domain of Rpm2p leads to deletions of the mitochondrial genome (mitochondrial protein synthesis is required to maintain an intact mitochondrial genome in yeast [70]) and to the accumulation of 5′-unprocessed mitochondrial tRNAs even in case an intact RPM1 is retained [66,68]. However, if cell growth depends on respiration because a fermentable carbon source is lacking, the same partial rpm2ΔC deletion allele supports sufficient mtRNase P activity and the strains retain an intact mitochondrial genome [69]. Under both conditions, Rpm1r is not properly processed to its mature form, and large precursors of mtRNase P RNA accumulate [59,69]. Thus the C-terminal domain of Rpm2p seems to be strictly required for Rpm1r maturation, but not for pre-tRNA 5′-end processing. Rpm1r processing is restored if the C-terminal domain is provided in trans [69]. Yet, it is not known how Rpm2p contributes to Rpm1r precursor processing. Apparently, the Rpm1r precursor species support mtRNase P activity only insufficiently, leading to the mild accumulation of tRNA precursors under respiratory conditions. The C-terminal truncation possibly also impairs Rpm2p's functionality as an RNase P subunit and differences in rpm2ΔC expression might account for the more severe phenotype under fermentative conditions. Moreover, a fraction of Rpm2p localizes to the nucleus where it appears to upregulate the transcription of genes of the mitochondrial protein import machinery [71]. It is this role that, tied in with the protein's indirect effect on mtDNA maintenance, is believed to be essential for yeast viability [for discussion, see ref. 71]. Finally, Rpm2p seems to interact with cytoplasmic P bodies suggesting a function in mRNA metabolism [72].
Curiously, yeast mtRNase P function is impaired by disruption of the type II fatty acid synthesis (FAS II) pathway [73]. Deletion of any of the mitochondrial FAS II genes resulted in the accumulation of 5′-extended mitochondrial tRNA precursors and an Rpm1r precursor. MtRNase P itself, however, appears not modified by lipoic acid [73]. Recently, it was suggested that mtRNase P is part of a “supercomplex” together with RNase Z, the RNA degradosome, components of the mitochondrial translation machinery, the enzymes of the tricarboxylic acid cycle (TCA) cycle, and components of the FAS II pathway [74]. This “supercomplex” is apparently absent upon deletion of a single component of the FAS II pathway. The finding suggests physical association as the possible functional link between FAS II and mitochondrial tRNA processing. Still, it remains unclear how such apparent “supercomplex” association affects mtRNase P function. In the end, the biological rationale behind the apparent intersection of mitochondrial tRNA processing with fatty acid synthesis or the TCA cycle is currently mostly enigmatic.
2.2.2. Mitochondrial RNase P in other fungi
The occurrence of RNase P RNA genes in fungi appears highly variable. In ascomycetes, rnpB has a rather patchy distribution, being present in representatives of the different groups, but absent in other closely related members of the same groups [65]. An rnpB gene has not been identified in any of the basidiomycete and chytridiomycete mitochondrial genomes [65], but all zygomycete mitochondrial genomes sequenced so far contain an rnpB gene [75]. As in budding yeasts, length and structure variation appear to be general characteristics of fungal mtRNase P RNAs. Length variation is most extreme in the zygomycete lineage, ranging from 188 to 980 nucleotides and thereby including the longest RNase P RNA identified so far. The short mtRNase P RNA of Smittium culisetae appears structurally as reduced as the minimal yeast mtRNase P, essentially confined to the structural elements P1 and P4 [65,75]. Most of the longer zygomycete mtRNase P RNAs, however, have remained structurally close to the universal consensus [75]. Also some ascomycete mtRNase P RNAs, like that of Aspergillus nidulans, have a secondary structure similar to the universal consensus [65] (Fig. 1). A. nidulans is also the only other fungus beyond S. cerevisiae for which mtRNase P has also been studied biochemically [76]. Seven polypeptides, ranging from 16 to 55 kDa, were found in highly purified fractions, but no further characterization or identification of those has been reported. The knowledge about fungal mtRNase P proteins thus remains scarce and the identity and composition of mtRNase P in several clades, including model systems like Neurospora crassa, unknown.
2.3. Mitochondrial RNase P in animals
2.3.1. Human mitochondrial RNase P
A mtRNase P and its critical role for global mitochondrial transcript maturation were proposed when human mtDNA was first sequenced and the various encoded RNAs characterized [7,77,78]. Yet, despite early efforts to identify mitochondrial tRNA processing enzymes in human cells [79], it took almost 15 years until the processing of mitochondrial tRNA precursors by mitochondrial enzyme activities was first demonstrated [80]. The mtRNase P activity turned out to have properties that distinguished it from any other RNase P known at that time [80], including the apparent lack of an RNA component [81]. By combining classical enzyme purification with mass-spectrometry-based proteome analysis we more recently achieved to identify the components of human mtRNase P and to reconstitute its activity from three recombinantly produced proteins only, without an RNA component [30].
In 2008, an RNase P devoid of RNA was without precedent and violated the general paradigm of RNase P's ribozymal nature. Yet, despite the fundamentally different makeup of this new variant of RNase P, its basic enzymatic properties are similar to the RNA-based enzymes. Both recognize parts of the tRNA structure within the precursor, require Mg2 + for phosphodiester hydrolysis, and generate 5′-phosphate and 3′-hydroxyl products. The catalytic role of the RNA seems to be taken over by one of the three proteins, originally termed mitochondrial RNase P protein 3 (MRPP3), as it was the third component identified [30]. Homologs of MRPP3 were found in the genomes of various eukaryal lineages [30], and have meanwhile been shown to also localize to the other tRNA-coding cellular compartments, nucleus and chloroplasts, in the different eukaryal branches [ref. 31 and unpublished results]. In many cases, these MRPP3 homologs seem to be active on their own, not requiring any other proteins for RNase P activity [ref. 31 and unpublished results]. Animal mtRNase P in this regard seems to be the exception rather than the rule, and, though first identified, not the prototype of this form of protein-only RNase P. We have thus coined the more generally applicable name proteinaceous RNase P (PRORP) for this gene family, numbered only in cases of multiple isoforms in a genome [31].
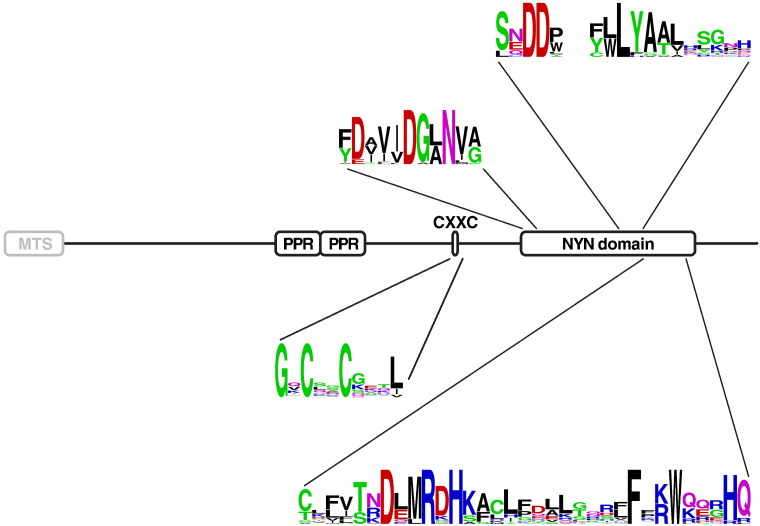
So far, MRPP3/PRORP homologs have been found in the genomes of animals, plants, green and red algae, heterokonts, haptophytes, and euglenids. The gene seems to be generally absent from the fungal lineage and possibly some protist groups. It has not been found in Archaea or Bacteria. The PRORP gene family is most conserved in its C-terminal part. This domain displays all the characteristics of a metallonuclease and places PRORP in a superfamily of predicted ribonucleases defined by the so-called NYN (N4BP1, YacP-like nuclease) domain [82] (Fig. 2). NYN domain proteins are found in all three main branches of life. The domain appears to be characterized by a common fold with conserved acidic residues presumably coordinating at least one catalytic metal ion in the active site. The NYN family seems related to the PIN and FLAP nuclease families, yet PRORP is the first NYN protein with a demonstrated ribonuclease function. Eukaryal NYN domains are found linked to various RNA binding domains/motifs and/or to the UBA ubiquitin-binding domain. In PRORP, the putative catalytic NYN domain is linked to an upstream zinc-finger-like (GX)CXXC signature and two pentatricopeptide repeats (PPR; Fig. 2). PPRs are motifs of 35 amino acids generally occurring in multiple consecutive copies and suggested to mediate RNA-binding activity [83]. PPR proteins are numerous in plants, but rare in animals. However, with only two PPRs in-tandem, PRORPs are not considered typical PPR proteins. The N-terminal part of PRORP is not well conserved and no motif apart from a facultative mitochondrial matrix-targeting signal has been recognized therein so far (Fig. 2).
Fig. 2.
Structure of proteinaceous RNase P (PRORP). MTS, mitochondrial targeting sequence (facultative); PPR, pentatricopeptide repeat; CXXC, zinc-finger-like motif; NYN domain, N4BP1-YacP-like metallonuclease domain [82]. Sequence logos were generated using WebLogo [146] and are based on a ClustalW alignment of PRORP sequences from the following species (number of PRORPs if more than one indicated in parentheses): man, macaque, dog, cow, mouse, platypus, chicken, Xenopus tropicalis, zebrafish, Tribolium castaneum, fruit fly, Aedes aegypti, Anopheles gambiae, Culex pipiens, Nasonia vitripennis, A. thaliana (3), Oryza sativa (3), Physcomitrella patens (3), T. brucei (2), Leishmania major (2).
In contrast to, e.g., A. thaliana PRORP1 [31], human MRPP3/PRORP requires two additional proteins for mtRNase P function: MRPP1 and MRPP2 [30]. MRPP1 is one of three vertebrate homologs of yeast TRM10 and has therefore also been named RNA (guanine-9-)methyltransferase domain containing 1 (RG9MTD1). Trm10p is responsible for the methylation of the N1 of guanosine at position 9 of a subset of yeast cytoplasmic tRNAs [84]. Mitochondrial homologs of TRM10 are apparently only found in animals and other eukarya seem to have no more than one gene. More recent work showed that MRPP1 is indeed involved in the methylation of mitochondrial tRNAs (manuscript in preparation). However, methylation and cleavage are neither coupled nor interdependent. This raises the question about the role of MRPP1 for tRNA processing by human mtRNase P. It seems reasonable to postulate that MRPP1 is involved in substrate recognition, but how this is achieved and why MRPP1 is required for mtRNase P function is enigmatic at present. Still, a consequence of this type of methyltransferase's involvement in mtRNase P may be an unusual way by which the MRPP3/PRORP nuclease subunit faces its substrate. The base of position 9 is buried in the core of an L-shaped tRNA structure and thereby rather inaccessible for an enzyme. Methylation would thus have to involve the transient unfolding of this part of the tRNA as well as interaction with parts of the tRNA structure not necessarily required to be normally “seen” by an RNase P enzyme [for a more thorough discussion see ref. [85]]. Thus, MRPP3 may at least partly recognize such transiently unfolded structural elements, which might explain the unique substrate specificity of human mtRNase P, differing from that of E. coli or human nuclear RNase P on numerous accounts [80,86–89]. Possibly, the requirement of the two extra components MRPP1 and MRPP2 may thereby represent an adaptation to the non-canonical structures of animal mitochondrial tRNAs.
The moonlighting of the third component, MRPP2, in human mitochondrial tRNA 5′-end processing is even more obscure. MRPP2, hydroxysteroid (17-β) dehydrogenase 10 (HSD17B10) according to current nomenclature, belongs to the short-chain dehydrogenase/reductase (SDR) family [90]. The protein has long been known as a dehydrogenase acting on a wide spectrum of substrates in vitro [91], but its best-documented role as a dehydrogenase in vivo is in branched-chain amino and fatty acid degradation [92]. While there is currently no clue as to the mechanism by which it contributes to mtRNase P, this moonlighting activity could be a key towards understanding the pathogenetic mechanism of 2-methyl-3-hydroxybutyryl-CoA dehydrogenase (MHBD) deficiency, a disease caused by mutations in HSD17B10 [92,93].
Human mtRNase P cleaves a wide range of mitochondrial tRNA precursors in vitro [30,80,86,89]. Its activity is readily detectable in even crude mitochondrial extracts and thereby appears to be relatively abundant [80]. Overexpression of MRPP1 boosts mtRNase P activity in mitochondrial extracts and mitochondrial tRNA precursors accumulate if the expression of any of its three components is knocked down by RNA interference [30]. These findings have firmly established the essential role of mtRNase P and its three subunits in the 5′-end maturation of mitochondrial tRNAs.
When we first identified mtRNase P activity we had also noticed the presence of small amounts of the RNA component of nuclear RNase P (H1 RNA) in mitochondrial preparations [80]. However, we were unable to detect any nuclear RNase P activity in mitochondrial extracts and H1 RNA was largely removed by applying more thorough mitochondrial purification regimens [80]. Moreover, we demonstrated that the remaining H1 RNA was neither required for nor associated with mtRNase P activity [80]. Using a bacterial tRNA precursor as a substrate (notably, a substrate not cleaved by mtRNase P [80]) others had before partially purified an RNase P activity from mitochondrial preparations [79] that later turned out to contain H1 RNA and to be apparently identical to the nuclear enzyme [94]. While the dispute about the possible identity of nuclear and mitochondrial RNase P in human cells was eventually settled after the distinct nature of human mtRNase P had been unraveled [30,88], the idea of nuclear RNase P being imported into mitochondria was recently revived. Polynucleotide phosphorylase (PNPase) was proposed to mediate the import of H1 and other RNAs into mitochondria, where H1 RNA was hypothesized to function in the processing of specific mitochondrial tRNA precursors as a quasi-additional mtRNase P [95]. Appreciation of all available data, however, gives rise to severe concerns that question the proposed scenario:
-
(1)
The number of H1 RNA molecules associated with mitochondria is extremely low, more than one order of magnitude lower than that of mtDNA [88,94]. It appears unlikely that such low amounts are sufficient to play an essential role in mitochondrial tRNA processing. Comparable or even higher amounts of other non-coding RNAs were found to be associated with mitochondria as well [88,94,96,97]. Might all these RNAs be imported into mitochondria by the PNPase-dependent import pathway? What should be the functional relevance of, e.g., spliceosomal snRNAs for mitochondrial biology?
-
(2)
The putative mitochondrial H1 RNA pool is apparently associated with the same proteins as the nuclear one; its sedimentation behavior mimics that of nuclear RNase P [94] and the RNA can be quantitatively immunoprecipitated by antibodies against nuclear RNase P [80]. However, none of the proteins associated with nuclear RNase P has a recognized mitochondrial targeting sequence or been found in mitochondrial proteomes [see ref. 30 and refs. therein].
-
(3)
PNPase was reported to interact with helix P9 of H1 RNA and to mediate the mitochondrial import of in vitro transcribed RNAs containing this short stem-loop structure [95]. However, in the nuclear RNase P ribonucleoprotein, P9 is covered by protein according to a recent footprinting analysis [98]. This raises the question how PNPase is able to access helix P9 in the context of the ribonucleoprotein.
-
(4)
A structurally distinct stem-loop of RNase MRP RNA (an extension of helix P12) was reported to mediate PNPase-dependent import as well [95]. Does this imply that any short stem-loop may be capable of mediating PNPase-dependent RNA import? How is selectivity maintained in such an import system of seemingly low specificity?
-
(5)
The localization of PNPase per se is controversial. Interaction with the mitochondrial matrix-helicase SUV3 in human cells [99,100] and the established role of PNPase in the mitochondrial RNA metabolism of plants [101] challenge the supposed localization in the mitochondrial intermembrane space and suggest a matrix localization instead.
-
(6)
Is the observed increase of some mitochondrial tRNA precursors in PNPase knockout cells cause or effect of the observed disturbance of mitochondrial structure and function? Is it at all due to a deficiency of tRNA processing activity, or does it originate from (or reflect) a lack of RNA degradation activity? In vitro 5′-end processing of mitochondrial tRNALys in mitochondrial extracts from PNPase knockout cells was unaltered, although the tRNALys precursor appeared to be enriched in mutant cells [95]. Only for the tRNAHis-flanked tRNASer(AGY) a direct processing deficiency was observed in PNPase knockout cells [95]. However, tRNASer(AGY) was previously shown to be released by tRNA punctuation via the 5′-abutting tRNAHis, thus circumventing 5′-end maturation by mtRNase P [86]. Owing to the conserved gene arrangement, the 5′ end of tRNASer(AGY) is generated by RNase Z acting on the 3′ terminus of tRNAHis. Mitochondrial extracts are unable to support 5′-end processing of a tRNASer(AGY) that is not flanked by an intact tRNAHis [86]. If the maturation of tRNASer(AGY) was independent of tRNAHis 3′-end processing, but instead depended on an RNase P able to process the 5′ end of tRNASer(AGY) directly, one would not expect the butt-jointed arrangement of the two tRNA genes to be conserved, but rather as variable as the boundaries of other vicinal mitochondrial tRNA genes, such as tRNASer(AGY)–tRNALeu(CUN) or tRNATyr–tRNACys, which are either found butt-jointed, separated by a spacer, or even overlapping in different mammalian mitochondrial genomes [86]. It seems possible that the apparent impairment of tRNASer(AGY) 5′ end processing in PNPase knockout cells is actually due to a reduction in RNase Z activity. Mitochondrial RNase Z seems to be regulated according to the functional status of mitochondria and its activity is virtually absent in cells lacking mitochondrial DNA due to the downregulation of its gene [81,102].
In conclusion, the concept of nuclear RNase P imported into mitochondria as a second mtRNase P activity, though not entirely excluded, appears quite speculative at present.
2.3.2. Mitochondrial RNase P in other animals
MtRNase P has not been studied in other animals. However, animal mitochondrial genomes are in general similar in organization and gene content, and all of them lack a mtDNA-encoded RNase P RNA [103,104]. Given that homologs of all three components of human mtRNase P are generally found in metazoan genomes, it is likely that a proteinaceous RNase P similar to that of human mitochondria is also active in the mitochondria of other animals. In some lineages, such as nematodes, homologs of MRPP3/PRORP and MRPP1 appear considerably more divergent [30], possibly the result of an accelerated adaptive evolution due to the bizarre mitochondrial tRNA structures found in these organisms [105].
2.4. Mitochondrial RNase P in plants and algae
A mtRNase P RNA gene has not been found in any mitochondrial genome from plants, green or red algae, with the exception of the two early branching prasinophyte algae Nephroselmis olivacea and Ostreococcus tauri [106,107]. Despite a secondary structure resembling that of α-proteobacterial RNase P RNAs, these algal RNAs are not catalytically active in vitro [54,108]. Even supplementation with E. coli RNase P protein did not activate the RNAs and the authentic protein component(s) of these putative mtRNase P enzymes have not been identified yet.
Early biochemical studies of mtRNase P in higher plants led to conflicting results with respect to the potential involvement of an RNA, but failed to identify any kind of enzyme component [109–111]. We recently showed that one of three A. thaliana homologs of human MRPP3/PRORP, PRORP1, localizes to mitochondria as well as chloroplasts [31]. A. thaliana PRORP1 is an essential gene and the purified recombinant protein removes extensions from plant mitochondrial or chloroplast tRNA precursors. In addition, it cleaves at tRNA-like structures (‘t-elements’) found in the untranslated regions of some mitochondrial mRNAs, at a site consistent with the in vivo mapped mRNA 3′ ends [31]. A. thaliana PRORP1 cleaves the precursor of mitochondrial tRNAHis preferentially, but unlike E. coli RNase P not exclusively, one nucleotide upstream of the canonical position to include an extra 5′ G residue characteristic of tRNAsHis [112]. The structure of A. thaliana PRORP1 is similar to human MRPP3/PRORP and has been described above (Section 2.3.1). The A. thaliana genome encodes two additional PRORP homologs that localize to the nucleus [31]. It appears as if plants have generally abandoned RNA-based RNase P and make use of the proteinaceous enzyme type in all their tRNA processing compartments (see also Section 2.1). Whereas land plant genomes encode three PRORP genes, algae seem to have only one. However, it is not unlikely that this protein acts as an RNase P in multiple compartments.
2.5. Mitochondrial RNase P in protists
Not much is known about mtRNase P in the evolutionary highly diverse “group” of protists. Genome information is still scarce and a biochemical study has only been carried out in Trypanosoma brucei [113]. Of the currently available mitochondrial genomes only those of the jakobids, like Reclinomonas americana, encode an RNase P RNA [108,114]. Notably, mtDNA of R. americana is regarded the mitochondrial genome most closely related to the ancestral proto-mitochondrial genome. Consistently, its mtRNase P RNA resembles α-proteobacterial RNase P RNAs [114]. Nonetheless, jakobid mtRNase P RNAs are apparently not active without their as yet unknown protein component(s) [108].
Two PRORP homologs have been found in the genomes of kinetoplastids [30,31]. In T. brucei, one of them localizes to the nucleus and one to the mitochondrion, and the recombinant proteins have RNase P activity on their own in vitro (unpublished results). A previous biochemical characterization of T. brucei mtRNase P also suggested that the enzyme lacks an RNA moiety [113]. The presence of RNase P in kinetoplastid mitochondria is indeed surprising, taking into account that their mitochondrial genome does not encode any tRNAs. Instead, nuclear-encoded tRNAs are imported into the mitochondrion from the cytosol [115,116]. Although in vitro import experiments suggested that T. brucei tRNAs are imported as precursors [117], tRNA import in vivo is independent of flanking sequences [116,118], suggesting that essentially only mature tRNAs are imported into the organelle. Thus, the natural substrates of T. brucei mtRNase P remain to be identified and kinetoplastid mtRNase P might be the first example of an RNase P activity not involved in tRNA processing in vivo.
3. tRNA 3′-end processing — RNase Z
While 5′ end processing of tRNAs is strictly endonucleolytic, 3′-end processing is more variable, and endonucleolytic, exonucleolytic, as well as combined pathways, involving both types of enzymes, are found in the different genetic systems [2,3,119,120]. In mitochondria, however, tRNA 3′-end processing seems to generally rely on an endonuclease, and the widespread use of polycistronic transcription, and in particular tRNA punctuation, where 3′ trailers are functional RNA species themselves, appears to preclude any exonucleolytic mechanisms of 3′-end maturation.
In contrast to RNase P enzymes, tRNA 3′-end processing endonucleases all seem to be related, belonging to the same family [for review see refs. [2,3,120–124]]. The functional role of this gene family was identified in 2002 and the enzyme named RNase Z (now also called tRNase Z; EC 3.1.26.11) [125]. The RNase Z family (also called ‘ElaC’ family according to the gene (elaC) encoding E. coli RNase Z) belongs to the β-lactamase superfamily, a group of related metallo-hydrolases with a characteristic fold of two parallel β-sheets flanked by α-helices (α–β/β–α) and the active site metal ion-coordinating motif HXHXDH [126]; in RNases Z as well as in most other members of the superfamily this metal ion is zinc. In addition to these common structural features a protruding flexible arm, the so-called ‘exosite’, characterizes the RNase Z family [3,122–124]. It is involved in the binding of the tRNA precursor substrate. There are two forms of RNase Z, one of 280 to 360 amino acids (RNase ZS) found in archaea and many, but not all, bacteria and eukarya, and another one more than twice this length (750–930 amino acids; RNase ZL), exclusively found in eukarya. The crystal structure of three bacterial RNases ZS was elucidated [127–129]. They are all homodimers of head-to-head arranged subunits. The dimer binds two tRNAs; the individual tRNAs seem like clamped between the ‘exosite’ of one subunit and an α-helix of the other [130], resembling “a ski boot in its bindings” [122]. Consistently, there is evidence for cooperativity in the action of RNase ZS enzymes [122]. Each enzyme subunit faces the stacked helices of the acceptor stem and the TΨC domain, whereas other elements of tRNA structure appear largely dispensable for substrate recognition [131,132]. For a more detailed discussion of RNase ZS structure and its implications for substrate recognition and cleavage I would like to refer the reader to more specific reviews [3,122–124].
The primary structure of RNase ZL suggests that it evolved from RNase ZS by duplication. The C-terminal half has retained a higher sequence similarity to the short forms and contains the presumed Zn-coordinating active site. In the N-terminal half the HXHXDH motif is highly degenerated, but this half contains the ‘exosite’ that was lost from the C-terminal part. No crystal structure of an RNase ZL is currently available. While all eukarya appear to have at least one RNase ZL gene, some also have an RNase ZS gene, or even two genes for one or both RNase Z isoforms [for review see refs. 3,121–124].
All mitochondrial RNase Z (mtRNase Z) enzymes identified so far are of the long form and in many cases the enzyme is derived from the same gene as nuclear RNase Z [14,133–138]. This may not seem surprising given that many Eukarya have only one gene encoding RNase ZL, but even in the presence of an additional RNase ZS, like e.g., in humans, still RNase ZL is shared between the nucleus and mitochondria, while RNase ZS is found in the cytosol and apparently not involved in the processing of primary tRNA transcripts [14]. Just in cases where there are two RNase ZL isoforms the dual targeting to nuclei and mitochondria seems dispensable. For example, four RNase Z isoenzymes are found in A. thaliana, two RNases ZL and two RNases ZS [135]. Interestingly, one RNase ZL is shared between the nucleus and mitochondria, whereas the other RNase ZL is confined to mitochondria. The two RNases ZS are found in the cytosol and in chloroplasts, respectively. In Schizosaccharomyces pombe, which has two RNase ZL genes, one RNase ZL isoform is found in the nucleus and the other one in mitochondria [138].
We have recently studied the mechanism underlying the dual nuclear/mitochondrial localization of human RNase ZL [14]. The first AUG of mammalian RNases ZL is consistently found in a poor context for initiation and is apparently frequently passed by scanning ribosomes. The second AUG more closely conforms to a consensus initiation site and appears to be used preferentially. This downstream initiation results in the loss of most of the mitochondrial targeting signal, whereby the protein translated from the second AUG is no longer imported into the mitochondrial matrix, but routed to the nucleus via a nuclear localization signal. The dual targeting mechanism of RNase Z in other organisms has not been studied.
Functional studies of mitochondrial RNase Z have been carried out in human cells [133], Drosophila cells [134], A. thaliana [135], S. pombe [138], and to a limited extent in S. cerevisiae [137] (in vitro studies of processing activities from crude mitochondrial extracts before the identification of the RNase Z family are not included in this list).
Recombinant human RNase ZL cleaves different mitochondrial tRNA precursors in vitro (unpublished results). In HeLa cells, silencing of ELAC2, the gene encoding human RNase ZL, by RNAi resulted in the accumulation of various 3′-unprocessed mitochondrial tRNA precursors [133]. Other processing sites, tRNA 5′ ends, tRNA antisense transcripts, or non-tRNA-abutted mRNA ends, were not effected by the RNase ZL knock-down. Nuclear-encoded, cytosolic tRNAs were not studied. In Drosophila S2 cells silencing of JhI-1 encoding RNase Z caused the accumulation of mitochondrial and nuclear tRNA precursors [134]. Consistently, the recombinant Drosophila enzyme catalyzed the 3′-end processing of tRNA precursors from both compartments in vitro [134]. All four RNases Z of A. thaliana have tRNA processing activity in vitro, and similar to mtRNase P, the two RNases ZL cleave ‘t-elements’ found in mitochondrial mRNA precursors [135]. Mutants of AthTrZL1 and AthTrZL2, encoding the two long, mitochondrial isoforms, are both viable, indicating that they functionally substitute for each other in mitochondrial tRNA 3′ end maturation [135]. The double mutant, however, seems to be lethal [135]. Taking into account that inactivation of AthTrZL1, encoding the only RNase Z isoform present in the nucleus, is not lethal, it appears that nuclear, in contrast to mitochondrial RNase Z is dispensable, most likely due to an exonucleolytic nuclear (backup) pathway similar to the one characterized in yeast [120]. In the fission yeast S. pombe the two genes encoding RNases ZL, sptrz1 and sptrz2, are both essential [139], but only the overexpression of the mitochondrial SpTrz2p resulted in as yet uncharacterized phenotypical abnormalities [138]. They both were shown to have RNase Z activity in vitro [138]. S. cerevisiae TRZ1 is an essential gene and decreasing its gene expression or a temperature sensitive allele showed mitochondrial deficiencies (petite phenotype), consistent with an essential role in mitochondrial tRNA biogenesis [137], although mitochondrial tRNAs or their precursors were not analyzed in these mutant strains.
The expression of human (mitochondrial) RNase ZL appears to be regulated in a peculiar way. In human cells lacking mitochondrial DNA (ρ0 cells) its mRNA was found down-regulated [102] and its activity, in contrast to that of mtRNase P, which was unchanged, strongly reduced in mitochondrial extracts [81]. The reasons for this apparent “adaption” in gene expression are not known.
4. Is there an “alphabetical” order in tRNA processing?
Does cleavage by mtRNase P generally precede that of mtRNase Z? Most in vitro processing studies and also some genetic data appear to favor such a scenario. In human mitochondrial extracts prepared from different types of cultured cells, cleavage by mtRNase P seems to generally occur first. The processing intermediates are 5′-matured tRNAs with 3′ extensions, and no 5′-extended, 3′-cleaved tRNAs were observed; this result was confirmed for a variety of mitochondrial tRNAs [30,81,86,87,89,140]. Consistently, knock-down of the MRPP1 subunit of human mtRNase P in HeLa cells inhibited both, 5′- and 3′-end maturation, whereas silencing of ELAC2, the gene encoding mtRNase Z, blocked 3′-end processing only [133]. Yet, based on the analysis of processing intermediates, it was also suggested that the order of processing would differ depending on the tissue: whereas a 3′-extended processing intermediate of mitochondrial tRNALeu(UUR) was reported for skeletal muscle, a 5′-extended intermediate of the same tRNA was observed in fibroblasts [141]. However, this apparent tissue specificity of the mitochondrial tRNA maturation pathway needs to be reproduced and should possibly be extended to other tRNAs and tissues to allow conclusions.
Silencing of RNase Z also provided direct evidence that 5′-end processing precedes 3′-end cleavage in Drosophila cell mitochondria [134]. No 5′-extended, 3′-processed tRNAs were detected in controls and mtRNase P continued to efficiently cleave tRNA precursors when 3′ end processing was impaired. In vitro studies in wheat mitochondrial extracts, in contrast, seemed to indicated that cleavages at the two tRNA ends are not ordered, as both possible forms of intermediates were reported [109]. Yet, processing apparently proceeded 5′ to 3′ in Oenothera mitochondrial extracts [110], and studies with partially purified potato RNase Z clearly indicated that 5′-end maturation has to occur first to allow 3′-end cleavage [142].
Mixed results were obtained for yeast mitochondrial tRNA processing. The 3′-end processing of yeast mitochondrial tRNAGlu was observed to depend on prior 5′ maturation in vitro [143], but a 5′-extended, 3′-processed tRNAfMet accumulated in deletion mutants of the mtRNase P RNA locus [61].
In summary, the order of processing seems to be flexible in some systems, and, depending on (i) the given tRNA precursor, (ii) the cell type, or (iii) possibly the relative abundance of RNase P versus RNase Z, one or the other pathway may predominate. In other systems 5′- before 3′-end processing clearly seems to be the favored, though possibly still not exclusive pathway.
Acknowledgements
I would like to thank Roland K. Hartmann for numerous detailed comments and suggestions on the manuscript, Elisa Vilardo for discussion and comments, Thomas Potuschak and Philippe Giegé for comments on the manuscript, and Christoph Weber for help with the preparation of Fig. 1. Related research in the Rossmanith lab was/is funded by the Austrian Science Fund FWF grants P17453 and I299, and the Vienna Science and Technology Fund WWTF grant LS09-032.
Footnotes
This article is part of a Special Issue entitled: Mitochondrial Gene Expression.
References
- 1.Schneider A. Mitochondrial tRNA import and its consequences for mitochondrial translation. Annu. Rev. Biochem. 2011;80:1033–1053. doi: 10.1146/annurev-biochem-060109-092838. [DOI] [PubMed] [Google Scholar]
- 2.Phizicky E.M., Hopper A.K. tRNA biology charges to the front. Genes Dev. 2010;24:1832–1860. doi: 10.1101/gad.1956510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartmann R.K., Gößringer M., Späth B., Fischer S., Marchfelder A. The making of tRNAs and more — RNase P and tRNase Z. Prog. Nucleic Acid Res. Mol. Biol. 2009;85:319–368. doi: 10.1016/S0079-6603(08)00808-8. [DOI] [PubMed] [Google Scholar]
- 4.Schäfer B. RNA maturation in mitochondria of S. cerevisiae and S. pombe. Gene. 2005;354:80–85. doi: 10.1016/j.gene.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Montoya J., López-Pérez M.J., Ruiz-Pesini E. Mitochondrial DNA transcription and diseases: past, present and future. Biochim. Biophys. Acta. 2006;1757:1179–1189. doi: 10.1016/j.bbabio.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Rossmanith W., Holzmann J. Processing mitochondrial (t)RNAs: new enzyme, old job. Cell Cycle. 2009;8:1650–1653. doi: 10.4161/cc.8.11.8502. [DOI] [PubMed] [Google Scholar]
- 7.Ojala D., Montoya J., Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290:470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- 8.Unseld M., Marienfeld J.R., Brandt P., Brennicke A. The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides. Nat. Genet. 1997;15:57–61. doi: 10.1038/ng0197-57. [DOI] [PubMed] [Google Scholar]
- 9.Forner J., Weber B., Thuss S., Wildum S., Binder S. Mapping of mitochondrial mRNA termini in Arabidopsis thaliana: t-elements contribute to 5′ and 3′ end formation. Nucleic Acids Res. 2007;35:3676–3692. doi: 10.1093/nar/gkm270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vörtler S., Mörl M. tRNA-nucleotidyltransferases: highly unusual RNA polymerases with vital functions. FEBS Lett. 2010;584:297–302. doi: 10.1016/j.febslet.2009.10.078. [DOI] [PubMed] [Google Scholar]
- 11.Chen J.-Y., Joyce P.B.M., Wolfe C.L., Steffen M.C., Martin N.C. Cytoplasmic and mitochondrial tRNA nucleotidyltransferase activities are derived from the same gene in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1992;267:14879–14883. [PubMed] [Google Scholar]
- 12.Nagaike T., Suzuki T., Tomari Y., Takemoto Hori C., Negayama F., Watanabe K., Ueda T. Identification and characterization of mammalian mitochondrial tRNA nucleotidyltransferases. J. Biol. Chem. 2001;276:40041–40049. doi: 10.1074/jbc.M106202200. [DOI] [PubMed] [Google Scholar]
- 13.Reichert A.S., Thurlow D.L., Mörl M. A eubacterial origin for the human tRNA nucleotidyltransferase? Biol. Chem. 2001;382:1431–1438. doi: 10.1515/BC.2001.176. [DOI] [PubMed] [Google Scholar]
- 14.Rossmanith W. Localization of human RNase Z isoforms: dual nuclear/mitochondrial targeting of the ELAC2 gene product by alternative translation initiation. PLoS One. 2011;6:e19152. doi: 10.1371/journal.pone.0019152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jühling F., Mörl M., Hartmann R.K., Sprinzl M., Stadler P.F., Pütz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–D162. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosjean H., editor. DNA and RNA modification enzymes: structure, mechanism, function and evolution. Landes Bioscience; Austin, Texas: 2009. [Google Scholar]
- 17.Helm M. Post-transcriptional nucleotide modification and alternative folding of RNA. Nucleic Acids Res. 2006;34:721–733. doi: 10.1093/nar/gkj471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czerwoniec A., Dunin-Horkawicz S., Purta E., Kaminska K.H., Kasprzak J.M., Bujnicki J.M., Grosjean H., Rother K. MODOMICS: a database of RNA modification pathways. 2008 update. Nucleic Acids Res. 2009;37:D118–121. doi: 10.1093/nar/gkn710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phizicky E.M., Alfonzo J.D. Do all modifications benefit all tRNAs? FEBS Lett. 2010;584:265–271. doi: 10.1016/j.febslet.2009.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dirheimer G., Keith G., Dumas P., Westhof E. Primary, secondary, and tertiary structures of tRNAs. In: Söll D., RajBhandary U.L., editors. tRNA: Structure, Biosynthesis, and Function. ASM Press; Washington, D.C.: 1995. pp. 93–126. [Google Scholar]
- 21.Helm M., Brulé H., Friede D., Giegé R., Pütz D., Florentz C. Search for characteristic structural features of mammalian mitochondrial tRNAs. RNA. 2000;6:1356–1379. doi: 10.1017/s1355838200001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki T., Nagao A., Suzuki T. Human mitochondrial tRNAs: biogenesis, function, structural aspects, and diseases. Annu. Rev. Genet. 2011;45:299–329. doi: 10.1146/annurev-genet-110410-132531. [DOI] [PubMed] [Google Scholar]
- 23.Liu F., Altman S., editors. Ribonuclease P. Springer; New York: 2010. [Google Scholar]
- 24.Lai L.B., Vioque A., Kirsebom L.A., Gopalan V. Unexpected diversity of RNase P, an ancient tRNA processing enzyme: challenges and prospects. FEBS Lett. 2010;584:287–296. doi: 10.1016/j.febslet.2009.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis J.C., Brown J.W. The RNase P family. RNA Biol. 2009;6:362–369. doi: 10.4161/rna.6.4.9241. [DOI] [PubMed] [Google Scholar]
- 26.Evans D., Marquez S.M., Pace N.R. RNase P: interface of the RNA and protein worlds. Trends Biochem. Sci. 2006;31:333–341. doi: 10.1016/j.tibs.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Robertson H.D., Altman S., Smith J.D. Purification and properties of a specific Escherichia coli ribonuclease which cleaves a tyrosine transfer ribonucleic acid precursor. J. Biol. Chem. 1972;247:5243–5251. [PubMed] [Google Scholar]
- 28.Stark B.C., Kole R., Bowman E.J., Altman S. Ribonuclease P: an enzyme with an essential RNA component. Proc. Natl. Acad. Sci. U. S. A. 1978;75:3717–3721. doi: 10.1073/pnas.75.8.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35:849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 30.Holzmann J., Frank P., Löffler E., Bennett K.L., Gerner C., Rossmanith W. RNase P without RNA: identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell. 2008;135:462–474. doi: 10.1016/j.cell.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Gobert A., Gutmann B., Taschner A., Gößringer M., Holzmann J., Hartmann R.K., Rossmanith W., Giegé P. A single Arabidopsis organellar protein has RNase P activity. Nat. Struct. Mol. Biol. 2010;17:740–744. doi: 10.1038/nsmb.1812. [DOI] [PubMed] [Google Scholar]
- 32.Ellis J.C., Brown J.W. The evolution of RNase P and its RNA. In: Liu F., Altman S., editors. Ribonuclease P. Springer; New York: 2010. pp. 17–40. [Google Scholar]
- 33.Kazantsev A.V., Pace N.R. Bacterial RNase P: a new view of an ancient enzyme. Nat. Rev. Microbiol. 2006;4:729–740. doi: 10.1038/nrmicro1491. [DOI] [PubMed] [Google Scholar]
- 34.Siegel R.W., Banta A.B., Haas E.S., Brown J.W., Pace N.R. Mycoplasma fermentans simplifies our view of the catalytic core of ribonuclease P RNA. RNA. 1996;2:452–462. [PMC free article] [PubMed] [Google Scholar]
- 35.Gößringer M., Hartmann R.K. Function of heterologous and truncated RNase P proteins in Bacillus subtilis. Mol. Microbiol. 2007;66:801–813. doi: 10.1111/j.1365-2958.2007.05962.x. [DOI] [PubMed] [Google Scholar]
- 36.Hartmann E., Hartmann R.K. The enigma of ribonuclease P evolution. Trends Genet. 2003;19:561–569. doi: 10.1016/j.tig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Swanson R.V. Genome of Aquifex aeolicus. Methods Enzymol. 2001;330:158–169. doi: 10.1016/s0076-6879(01)30373-7. [DOI] [PubMed] [Google Scholar]
- 38.Marszalkowski M., Willkomm D.K., Hartmann R.K. 5′-end maturation of tRNA in aquifex aeolicus. Biol. Chem. 2008;389:395–403. doi: 10.1515/BC.2008.042. [DOI] [PubMed] [Google Scholar]
- 39.Lombo T.B., Kaberdin V.R. RNA processing in Aquifex aeolicus involves RNase E/G and an RNase P-like activity. Biochem. Biophys. Res. Commun. 2008;366:457–463. doi: 10.1016/j.bbrc.2007.11.165. [DOI] [PubMed] [Google Scholar]
- 40.Lai L.B., Cho I.-M., Chen W.-Y., Gopalan V. Archaeal RNase P: a mosaic of its bacterial and eukaryal relatives. In: Liu F., Altman S., editors. Ribonuclease P. Springer; New York: 2010. pp. 153–172. [Google Scholar]
- 41.Jarrous N., Gopalan V. Archaeal/eukaryal RNase P: subunits, functions and RNA diversification. Nucleic Acids Res. 2010;38:7885–7894. doi: 10.1093/nar/gkq701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pannucci J.A., Haas E.S., Hall T.A., Harris J.K., Brown J.W. RNase P RNAs from some Archaea are catalytically active. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7803–7808. doi: 10.1073/pnas.96.14.7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai L.B., Chan P.P., Cozen A.E., Bernick D.L., Brown J.W., Gopalan V., Lowe T.M. Discovery of a minimal form of RNase P in Pyrobaculum. Proc. Natl. Acad. Sci. U. S. A. 2010;107:22493–22498. doi: 10.1073/pnas.1013969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randau L., Schröder I., Söll D. Life without RNase P. Nature. 2008;453:120–123. doi: 10.1038/nature06833. [DOI] [PubMed] [Google Scholar]
- 45.Walker S.C., Marvin M.C., Engelke D. Eukaryote RNase P and RNase MRP. In: Liu F., Altman S., editors. Ribonuclease P. Springer; New York: 2010. pp. 173–202. [Google Scholar]
- 46.Esakova O., Krasilnikov A.S. Of proteins and RNA: the RNase P/MRP family. RNA. 2010;16:1725–1747. doi: 10.1261/rna.2214510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kikovska E., Svärd S.G., Kirsebom L.A. Eukaryotic RNase P RNA mediates cleavage in the absence of protein. Proc. Natl. Acad. Sci. U. S. A. 2007;104:2062–2067. doi: 10.1073/pnas.0607326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marvin M.C., Clauder-Münster S., Walker S.C., Sarkeshik A., Yates J.R., III, Steinmetz L.M., Engelke D.R. Accumulation of noncoding RNA due to an RNase P defect in Saccharomyces cerevisiae. RNA. 2011;17:1441–1450. doi: 10.1261/rna.2737511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jarrous N., Reiner R., Dehtiar Y. Human RNase P and transcription. In: Liu F., Altman S., editors. Ribonuclease P. Springer; New York: 2010. pp. 223–234. [Google Scholar]
- 50.Piccinelli P., Rosenblad M.A., Samuelsson T. Identification and analysis of ribonuclease P and MRP RNA in a broad range of eukaryotes. Nucleic Acids Res. 2005;33:4485–4495. doi: 10.1093/nar/gki756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yusuf D., Marz M., Stadler P.F., Hofacker I.L. Bcheck: a wrapper tool for detecting RNase P RNA genes. BMC Genomics. 2010;11:432. doi: 10.1186/1471-2164-11-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenblad M.A., López M.D., Piccinelli P., Samuelsson T. Inventory and analysis of the protein subunits of the ribonucleases P and MRP provides further evidence of homology between the yeast and human enzymes. Nucleic Acids Res. 2006;34:5145–5156. doi: 10.1093/nar/gkl626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li D., Willkomm D.K., Schön A., Hartmann R.K. RNase P of the Cyanophora paradoxa cyanelle: a plastid ribozyme. Biochimie. 2007;89:1528–1538. doi: 10.1016/j.biochi.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Vioque A. RNase P from organelles. In: Liu F., Altman S., editors. Ribonuclease P. Springer; New York: 2010. pp. 203–222. [Google Scholar]
- 55.Wang M.J., Davis N.W., Gegenheimer P. Novel mechanisms for maturation of chloroplast transfer RNA precursors. EMBO J. 1988;7:1567–1574. doi: 10.1002/j.1460-2075.1988.tb02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin N.C., Underbrink-Lyon K. A mitochondrial locus is necessary for the synthesis of mitochondrial tRNA in the yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 1981;78:4743–4747. doi: 10.1073/pnas.78.8.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Underbrink-Lyon K., Miller D.L., Ross N.A., Fukuhara H., Martin N.C. Characterization of a yeast mitochondrial locus necessary for tRNA biosynthesis. Deletion mapping and restriction mapping studies. Mol. Gen. Genet. 1983;191:512–518. doi: 10.1007/BF00425771. [DOI] [PubMed] [Google Scholar]
- 58.Miller D.L., Martin N.C. Characterization of the yeast mitochondrial locus necessary for tRNA biosynthesis: DNA sequence analysis and identification of a new transcript. Cell. 1983;34:911–917. doi: 10.1016/0092-8674(83)90548-2. [DOI] [PubMed] [Google Scholar]
- 59.Stribinskis V., Gao G.-J., Sulo P., Dang Y.L., Martin N.C. Yeast mitochondrial RNase P RNA synthesis is altered in an RNase P protein subunit mutant: insights into the biogenesis of a mitochondrial RNA-processing enzyme. Mol. Cell. Biol. 1996;16:3429–3436. doi: 10.1128/mcb.16.7.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hollingsworth M.J., Martin N.C. RNase P activity in the mitochondria of Saccharomyces cerevisiae depends on both mitochondrion and nucleus-encoded components. Mol. Cell. Biol. 1986;6:1058–1064. doi: 10.1128/mcb.6.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin N.C., Miller D.L., Underbrink K., Ming X. Structure of a precursor to the yeast mitochondrial tRNAfMet. Implications for the function of the tRNA synthesis locus. J. Biol. Chem. 1985;260:1479–1483. [PubMed] [Google Scholar]
- 62.Morales M.J., Wise C.A., Hollingsworth M.J., Martin N.C. Characterization of yeast mitochondrial RNase P: an intact RNA subunit is not essential for activity in vitro. Nucleic Acids Res. 1989;17:6865–6881. doi: 10.1093/nar/17.17.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wise C.A., Martin N.C. Dramatic size variation of yeast mitochondrial RNAs suggests that RNase P RNAs can be quite small. J. Biol. Chem. 1991;266:19154–19157. [PubMed] [Google Scholar]
- 64.Sbisà E., Pesole G., Tullo A., Saccone C. The evolution of the RNase P- and RNase MRP-associated RNAs: phylogenetic analysis and nucleotide substitution rate. J. Mol. Evol. 1996;43:46–57. doi: 10.1007/BF02352299. [DOI] [PubMed] [Google Scholar]
- 65.Seif E.R., Forget L., Martin N.C., Lang B.F. Mitochondrial RNase P RNAs in ascomycete fungi: lineage-specific variations in RNA secondary structure. RNA. 2003;9:1073–1083. doi: 10.1261/rna.5880403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morales M.J., Dang Y.L., Lou Y.C., Sulo P., Martin N.C. A 105-kDa protein is required for yeast mitochondrial RNase P activity. Proc. Natl. Acad. Sci. U. S. A. 1992;89:9875–9879. doi: 10.1073/pnas.89.20.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dang Y.L., Martin N.C. Yeast mitochondrial RNase P. Sequence of the RPM2 gene and demonstration that its product is a protein subunit of the enzyme. J. Biol. Chem. 1993;268:19791–19796. [PubMed] [Google Scholar]
- 68.Kassenbrock C.K., Gao G.-J., Groom K.R., Sulo P., Douglas M.G., Martin N.C. RPM2, independently of its mitochondrial RNase P function, suppresses an ISP42 mutant defective in mitochondrial import and is essential for normal growth. Mol. Cell. Biol. 1995;15:4763–4770. doi: 10.1128/mcb.15.9.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stribinskis V., Gao G.-J., Sulo P., Ellis S.R., Martin N.C. Rpm2p: separate domains promote tRNA and Rpm1r maturation in Saccharomyces cerevisiae mitochondria. Nucleic Acids Res. 2001;29:3631–3637. doi: 10.1093/nar/29.17.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Myers A.M., Pape L.K., Tzagoloff A. Mitochondrial protein synthesis is required for maintenance of intact mitochondrial genomes in Saccharomyces cerevisiae. EMBO J. 1985;4:2087–2092. doi: 10.1002/j.1460-2075.1985.tb03896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stribinskis V., Heyman H.-C., Ellis S.R., Steffen M.C., Martin N.C. Rpm2p, a component of yeast mitochondrial RNase P, acts as a transcriptional activator in the nucleus. Mol. Cell. Biol. 2005;25:6546–6558. doi: 10.1128/MCB.25.15.6546-6558.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stribinskis V., Ramos K.S. Rpm2p, a protein subunit of mitochondrial RNase P, physically and genetically interacts with cytoplasmic processing bodies. Nucleic Acids Res. 2007;35:1301–1311. doi: 10.1093/nar/gkm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schonauer M.S., Kastaniotis A.J., Hiltunen J.K., Dieckmann C.L. Intersection of RNA processing and the type II fatty acid synthesis pathway in yeast mitochondria. Mol. Cell. Biol. 2008;28:6646–6657. doi: 10.1128/MCB.01162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Daoud R., Forget L., Franz Lang B. Yeast mitochondrial RNase P, RNase Z and the RNA degradosome are part of a stable supercomplex. Nucleic Acids Res. 2012;40:1728–1736. doi: 10.1093/nar/gkr941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seif E., Leigh J., Liu Y., Roewer I., Forget L., Lang B.F. Comparative mitochondrial genomics in zygomycetes: bacteria-like RNase P RNAs, mobile elements and a close source of the group I intron invasion in angiosperms. Nucleic Acids Res. 2005;33:734–744. doi: 10.1093/nar/gki199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee Y.C., Lee B.J., Hwang D.S., Kang H.S. Purification and characterization of mitochondrial ribonuclease P from Aspergillus nidulans. Eur. J. Biochem. 1996;235:289–296. doi: 10.1111/j.1432-1033.1996.00289.x. [DOI] [PubMed] [Google Scholar]
- 77.Anderson S., Bankier A.T., Barrell B.G., de Bruijn M.H., Coulson A.R., Drouin J., Eperon I.C., Nierlich D.P., Roe B.A., Sanger F., Schreier P.H., Smith A.J., Staden R., Young I.G. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 78.Ojala D., Merkel C., Gelfand R., Attardi G. The tRNA genes punctuate the reading of genetic information in human mitochondrial DNA. Cell. 1980;22:393–403. doi: 10.1016/0092-8674(80)90350-5. [DOI] [PubMed] [Google Scholar]
- 79.Doersen C.J., Guerrier-Takada C., Altman S., Attardi G. Characterization of an RNase P activity from HeLa cell mitochondria. J. Biol. Chem. 1985;260:5942–5949. [PubMed] [Google Scholar]
- 80.Rossmanith W., Tullo A., Potuschak T., Karwan R., Sbisà E. Human mitochondrial tRNA processing. J. Biol. Chem. 1995;270:12885–12891. doi: 10.1074/jbc.270.21.12885. [DOI] [PubMed] [Google Scholar]
- 81.Rossmanith W., Karwan R.M. Characterization of human mitochondrial RNase P: novel aspects in tRNA processing. Biochem. Biophys. Res. Commun. 1998;247:234–241. doi: 10.1006/bbrc.1998.8766. [DOI] [PubMed] [Google Scholar]
- 82.Anantharaman V., Aravind L. The NYN domains: novel predicted RNAses with a PIN domain-like fold. RNA Biol. 2006;3:18–27. doi: 10.4161/rna.3.1.2548. [DOI] [PubMed] [Google Scholar]
- 83.Schmitz-Linneweber C., Small I. Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends Plant Sci. 2008;13:663–670. doi: 10.1016/j.tplants.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 84.Jackman J.E., Montange R.K., Malik H.S., Phizicky E.M. Identification of the yeast gene encoding the tRNA m1G methyltransferase responsible for modification at position 9. RNA. 2003;9:574–585. doi: 10.1261/rna.5070303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holzmann J., Rossmanith W. tRNA recognition, processing, and disease: hypotheses around an unorthodox type of RNase P in human mitochondria. Mitochondrion. 2009;9:284–288. doi: 10.1016/j.mito.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Rossmanith W. Processing of human mitochondrial tRNASer(AGY): a novel pathway in tRNA biosynthesis. J. Mol. Biol. 1997;265:365–371. doi: 10.1006/jmbi.1996.0750. [DOI] [PubMed] [Google Scholar]
- 87.Rossmanith W., Karwan R.M. Impairment of tRNA processing by point mutations in mitochondrial tRNALeu(UUR) associated with mitochondrial diseases. FEBS Lett. 1998;433:269–274. doi: 10.1016/s0014-5793(98)00928-4. [DOI] [PubMed] [Google Scholar]
- 88.Rossmanith W., Potuschak T. Difference between mitochondrial RNase P and nuclear RNase P. Mol. Cell. Biol. 2001;21:8236–8237. doi: 10.1128/MCB.21.23.8236-8237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rossmanith W., Tullo A., Imre E.-M., Saccone C., Sbisà E., Karwan R. In: Nuclear-mitochondrial coevolution of RNA processing enzymes and cellular function. Palmieri F., Papa S., Saccone C., Gadaleta M.N., editors. vol. 5. Elsevier Science B.V; Amsterdam: 1995. pp. 143–147. (Progress in Cell Research). [Google Scholar]
- 90.Jörnvall H., Persson B., Krook M., Atrian S., Gonzàlez-Duarte R., Jeffery J., Ghosh D. Short-chain dehydrogenases/reductases (SDR) Biochemistry. 1995;34:6003–6013. doi: 10.1021/bi00018a001. [DOI] [PubMed] [Google Scholar]
- 91.Yang S.-Y., He X.-Y., Miller D. Hydroxysteroid (17β) dehydrogenase X in human health and disease. Mol. Cell. Endocrinol. 2011;343:1–6. doi: 10.1016/j.mce.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 92.Ofman R., Ruiter J.P.N., Feenstra M., Duran M., Poll-The B.T., Zschocke J., Ensenauer R., Lehnert W., Sass J.O., Sperl W., Wanders R.J.A. 2-Methyl-3-hydroxybutyryl-CoA dehydrogenase deficiency is caused by mutations in the HADH2 gene. Am. J. Hum. Genet. 2003;72:1300–1307. doi: 10.1086/375116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rauschenberger K., Schöler K., Sass J.O., Sauer S., Djuric Z., Rumig C., Wolf N.I., Okun J.G., Kölker S., Schwarz H., Fischer C., Grziwa B., Runz H., Nümann A., Shafqat N., Kavanagh K.L., Hämmerling G., Wanders R.J.A., Shield J.P.H., Wendel U., Stern D., Nawroth P., Hoffmann G.F., Bartram C.R., Arnold B., Bierhaus A., Oppermann U., Steinbeisser H., Zschocke J. A non-enzymatic function of 17β-hydroxysteroid dehydrogenase type 10 is required for mitochondrial integrity and cell survival. EMBO Mol. Med. 2010;2:51–62. doi: 10.1002/emmm.200900055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Puranam R.S., Attardi G. The RNase P associated with HeLa cell mitochondria contains an essential RNA component identical in sequence to that of the nuclear RNase P. Mol. Cell. Biol. 2001;21:548–561. doi: 10.1128/MCB.21.2.548-561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang G., Chen H.-W., Oktay Y., Zhang J., Allen E.L., Smith G.M., Fan K.C., Hong J.S., French S.W., McCaffery J.M., Lightowlers R.N., Morse H.C., III, Koehler C.M., Teitell M.A. PNPASE regulates RNA import into mitochondria. Cell. 2010;142:456–467. doi: 10.1016/j.cell.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kiss T., Filipowicz W. Evidence against a mitochondrial location of the 7-2/MRP RNA in mammalian cells. Cell. 1992;70:11–16. doi: 10.1016/0092-8674(92)90528-k. [DOI] [PubMed] [Google Scholar]
- 97.Mercer T.R., Neph S., Dinger M.E., Crawford J., Smith M.A., Shearwood A.-M.J., Haugen E., Bracken C.P., Rackham O., Stamatoyannopoulos J.A., Filipovska A., Mattick J.S. The human mitochondrial transcriptome. Cell. 2011;146:645–658. doi: 10.1016/j.cell.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Esakova O., Perederina A., Quan C., Schmitt M.E., Krasilnikov A.S. Footprinting analysis demonstrates extensive similarity between eukaryotic RNase P and RNase MRP holoenzymes. RNA. 2008;14:1558–1567. doi: 10.1261/rna.1106408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang D.D.-H., Shu Z., Lieser S.A., Chen P.-L., Lee W.-H. Human mitochondrial SUV3 and polynucleotide phosphorylase form a 330-kDa heteropentamer to cooperatively degrade double-stranded RNA with a 3′-to-5′ directionality. J. Biol. Chem. 2009;284:20812–20821. doi: 10.1074/jbc.M109.009605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Szczesny R.J., Borowski L.S., Brzezniak L.K., Dmochowska A., Gewartowski K., Bartnik E., Stepien P.P. Human mitochondrial RNA turnover caught in flagranti: involvement of hSuv3p helicase in RNA surveillance. Nucleic Acids Res. 2010;38:279–298. doi: 10.1093/nar/gkp903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Holec S., Lange H., Kühn K., Alioua M., Börner T., Gagliardi D. Relaxed transcription in Arabidopsis mitochondria is counterbalanced by RNA stability control mediated by polyadenylation and polynucleotide phosphorylase. Mol. Cell. Biol. 2006;26:2869–2876. doi: 10.1128/MCB.26.7.2869-2876.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mineri R., Pavelka N., Fernandez-Vizarra E., Ricciardi-Castagnoli P., Zeviani M., Tiranti V. How do human cells react to the absence of mitochondrial DNA? PLoS One. 2009;4:e5713. doi: 10.1371/journal.pone.0005713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boore J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999;27:1767–1780. doi: 10.1093/nar/27.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Burger G., Lang B.F. Parallels in genome evolution in mitochondria and bacterial symbionts. IUBMB Life. 2003;55:205–212. doi: 10.1080/1521654031000137380. [DOI] [PubMed] [Google Scholar]
- 105.Wolstenholme D.R., Macfarlane J.L., Okimoto R., Clary D.O., Wahleithner J.A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc. Natl. Acad. Sci. U. S. A. 1987;84:1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Turmel M., Lemieux C., Burger G., Lang B.F., Otis C., Plante I., Gray M.W. The complete mitochondrial DNA sequences of Nephroselmis olivacea and Pedinomonas minor. Two radically different evolutionary patterns within green algae. Plant Cell. 1999;11:1717–1730. doi: 10.1105/tpc.11.9.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Robbens S., Derelle E., Ferraz C., Wuyts J., Moreau H., Van de Peer Y. The complete chloroplast and mitochondrial DNA sequence of Ostreococcus tauri: organelle genomes of the smallest eukaryote are examples of compaction. Mol. Biol. Evol. 2007;24:956–968. doi: 10.1093/molbev/msm012. [DOI] [PubMed] [Google Scholar]
- 108.Seif E., Cadieux A., Lang B.F. Hybrid E. coli—mitochondrial ribonuclease P RNAs are catalytically active. RNA. 2006;12:1661–1670. doi: 10.1261/rna.52106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hanic-Joyce P.J., Gray M.W. Processing of transfer RNA precursors in a wheat mitochondrial extract. J. Biol. Chem. 1990;265:13782–13791. [PubMed] [Google Scholar]
- 110.Marchfelder A., Schuster W., Brennicke A. In vitro processing of mitochondrial and plastid derived tRNA precursors in a plant mitochondrial extract. Nucleic Acids Res. 1990;18:1401–1406. doi: 10.1093/nar/18.6.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marchfelder A., Brennicke A. Characterization and partial purification of tRNA processing activities from potato mitochondria. Plant Physiol. 1994;105:1247–1254. doi: 10.1104/pp.105.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Placido A., Sieber F., Gobert A., Gallerani R., Giegé P., Maréchal-Drouard L. Plant mitochondria use two pathways for the biogenesis of tRNAHis. Nucleic Acids Res. 2010;38:7711–7717. doi: 10.1093/nar/gkq646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Salavati R., Panigrahi A.K., Stuart K.D. Mitochondrial ribonuclease P activity of Trypanosoma brucei. Mol. Biochem. Parasitol. 2001;115:109–117. doi: 10.1016/s0166-6851(01)00273-0. [DOI] [PubMed] [Google Scholar]
- 114.Lang B.F., Burger G., O'Kelly C.J., Cedergren R., Golding G.B., Lemieux C., Sankoff D., Turmel M., Gray M.W. An ancestral mitochondrial DNA resembling a eubacterial genome in miniature. Nature. 1997;387:493–497. doi: 10.1038/387493a0. [DOI] [PubMed] [Google Scholar]
- 115.Hancock K., Hajduk S.L. The mitochondrial tRNAs of Trypanosoma brucei are nuclear encoded. J. Biol. Chem. 1990;265:19208–19215. [PubMed] [Google Scholar]
- 116.Tan T.H.P., Pach R., Crausaz A., Ivens A., Schneider A. tRNAs in Trypanosoma brucei: genomic organization, expression, and mitochondrial import. Mol. Cell. Biol. 2002;22:3707–3717. doi: 10.1128/MCB.22.11.3707-3716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hancock K., LeBlanc A.J., Donze D., Hajduk S.L. Identification of nuclear encoded precursor tRNAs within the mitochondrion of Trypanosoma brucei. J. Biol. Chem. 1992;267:23963–23971. [PubMed] [Google Scholar]
- 118.Hauser R., Schneider A. tRNAs are imported into mitochondria of Trypanosoma brucei independently of their genomic context and genetic origin. EMBO J. 1995;14:4212–4220. doi: 10.1002/j.1460-2075.1995.tb00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deutscher M.P. tRNA processing nucleases. In: Söll D., RajBhandary U.L., editors. tRNA: Structure, Biosynthesis, and Function. ASM Press; Washington, D.C.: 1995. pp. 51–65. [Google Scholar]
- 120.Maraia R.J., Lamichhane T.N. 3′ processing of eukaryotic precursor tRNAs. WIREs RNA. 2011;2:362–375. doi: 10.1002/wrna.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ceballos M., Vioque A. tRNase Z. Protein Pept. Lett. 2007;14:137–145. doi: 10.2174/092986607779816050. [DOI] [PubMed] [Google Scholar]
- 122.Redko Y., Li de la Sierra-Gallay I., Condon C. When all's zed and done: the structure and function of RNase Z in prokaryotes. Nat. Rev. Microbiol. 2007;5:278–286. doi: 10.1038/nrmicro1622. [DOI] [PubMed] [Google Scholar]
- 123.Späth B., Canino G., Marchfelder A. tRNase Z: the end is not in sight. Cell. Mol. Life Sci. 2007;64:2404–2412. doi: 10.1007/s00018-007-7160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vogel A., Schilling O., Späth B., Marchfelder A. The tRNase Z family of proteins: physiological functions, substrate specificity and structural properties. Biol. Chem. 2005;386:1253–1264. doi: 10.1515/BC.2005.142. [DOI] [PubMed] [Google Scholar]
- 125.Schiffer S., Rösch S., Marchfelder A. Assigning a function to a conserved group of proteins: the tRNA 3′-processing enzymes. EMBO J. 2002;21:2769–2777. doi: 10.1093/emboj/21.11.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aravind L. An evolutionary classification of the metallo-β-lactamase fold proteins. In Silico Biol. 1999;1:69–91. [PubMed] [Google Scholar]
- 127.Ishii R., Minagawa A., Takaku H., Takagi M., Nashimoto M., Yokoyama S. Crystal structure of the tRNA 3′ processing endoribonuclease tRNase Z from Thermotoga maritima. J. Biol. Chem. 2005;280:14138–14144. doi: 10.1074/jbc.M500355200. [DOI] [PubMed] [Google Scholar]
- 128.Kostelecky B., Pohl E., Vogel A., Schilling O., Meyer-Klaucke W. The crystal structure of the zinc phosphodiesterase from Escherichia coli provides insight into function and cooperativity of tRNase Z-family proteins. J. Bacteriol. 2006;188:1607–1614. doi: 10.1128/JB.188.4.1607-1614.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li de la Sierra-Gallay I., Pellegrini O., Condon C. Structural basis for substrate binding, cleavage and allostery in the tRNA maturase RNase Z. Nature. 2005;433:657–661. doi: 10.1038/nature03284. [DOI] [PubMed] [Google Scholar]
- 130.Li de la Sierra-Gallay I., Mathy N., Pellegrini O., Condon C. Structure of the ubiquitous 3′ processing enzyme RNase Z bound to transfer RNA. Nat. Struct. Mol. Biol. 2006;13:376–377. doi: 10.1038/nsmb1066. [DOI] [PubMed] [Google Scholar]
- 131.Nashimoto M., Tamura M., Kaspar R.L. Minimum requirements for substrates of mammalian tRNA 3′ processing endoribonuclease. Biochemistry. 1999;38:12089–12096. doi: 10.1021/bi9911942. [DOI] [PubMed] [Google Scholar]
- 132.Schiffer S., Helm M., Théobald-Dietrich A., Giegé R., Marchfelder A. The plant tRNA 3′ processing enzyme has a broad substrate spectrum. Biochemistry. 2001;40:8264–8272. doi: 10.1021/bi0101953. [DOI] [PubMed] [Google Scholar]
- 133.Brzezniak L.K., Bijata M., Szczesny R.J., Stepien P.P. Involvement of human ELAC2 gene product in 3′ end processing of mitochondrial tRNAs. RNA Biol. 2011;8 doi: 10.4161/rna.8.4.15393. [DOI] [PubMed] [Google Scholar]
- 134.Dubrovsky E.B., Dubrovskaya V.A., Levinger L., Schiffer S., Marchfelder A. Drosophila RNase Z processes mitochondrial and nuclear pre-tRNA 3′ ends in vivo. Nucleic Acids Res. 2004;32:255–262. doi: 10.1093/nar/gkh182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Canino G., Bocian E., Barbezier N., Echeverria M., Forner J., Binder S., Marchfelder A. Arabidopsis encodes four tRNase Z enzymes. Plant Physiol. 2009;150:1494–1502. doi: 10.1104/pp.109.137950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hazbun T.R., Malmström L., Anderson S., Graczyk B.J., Fox B., Riffle M., Sundin B.A., Aranda J.D., McDonald W.H., Chiu C.H., Snydsman B.E., Bradley P., Muller E.G., Fields S., Baker D., Yates J.R., III, Davis T.N. Assigning function to yeast proteins by integration of technologies. Mol. Cell. 2003;12:1353–1365. doi: 10.1016/s1097-2765(03)00476-3. [DOI] [PubMed] [Google Scholar]
- 137.Chen Y., Beck A., Davenport C., Chen Y., Shattuck D., Tavtigian S.V. Characterization of TRZ1, a yeast homolog of the human candidate prostate cancer susceptibility gene ELAC2 encoding tRNase Z. BMC Mol. Biol. 2005;6:12. doi: 10.1186/1471-2199-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gan X., Yang J., Li J., Yu H., Dai H., Liu J., Huang Y. The fission yeast Schizosaccharomyces pombe has two distinct tRNase ZLs encoded by two different genes and differentially targeted to the nucleus and mitochondria. Biochem. J. 2011;435:103–111. doi: 10.1042/BJ20101619. [DOI] [PubMed] [Google Scholar]
- 139.Zhao Z., Su W., Yuan S., Huang Y. Functional conservation of tRNase ZL among Saccharomyces cerevisiae, Schizosaccharomyces pombe and humans. Biochem. J. 2009;422:483–492. doi: 10.1042/BJ20090743. [DOI] [PubMed] [Google Scholar]
- 140.Rossmanith W. Institute of Microbiology and Genetics, University of Vienna, Vienna, Austria. 1996. Mammalian mitochondrial transcript processing; p. 145. [Google Scholar]
- 141.Bindoff L.A., Howell N., Poulton J., McCullough D.A., Morten K.J., Lightowlers R.N., Turnbull D.M., Weber K. Abnormal RNA processing associated with a novel tRNA mutation in mitochondrial DNA. J. Biol. Chem. 1993;268:19559–19564. [PubMed] [Google Scholar]
- 142.Kunzmann A., Brennicke A., Marchfelder A. 5′ end maturation and RNA editing have to precede tRNA 3′ processing in plant mitochondria. Proc. Natl. Acad. Sci. U. S. A. 1998;95:108–113. doi: 10.1073/pnas.95.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen J.-Y., Martin N.C. Biosynthesis of tRNA in yeast mitochondria. An endonuclease is responsible for the 3′-processing of tRNA precursors. J. Biol. Chem. 1988;263:13677–13682. [PubMed] [Google Scholar]
- 144.Haas E.S., Brown J.W., Pitulle C., Pace N.R. Further perspective on the catalytic core and secondary structure of ribonuclease P RNA. Proc. Natl. Acad. Sci. U. S. A. 1994;91:2527–2531. doi: 10.1073/pnas.91.7.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen J.L., Pace N.R. Identification of the universally conserved core of ribonuclease P RNA. RNA. 1997;3:557–560. [PMC free article] [PubMed] [Google Scholar]
- 146.Crooks G.E., Hon G., Chandonia J.-M., Brenner S.E. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]