Abstract
Importance
Smoking cessation reduces the risks of cardiovascular disease (CVD), but weight gain that follows quitting smoking may weaken the CVD benefit of quitting.
Objective
To test the hypothesis that weight gain following smoking cessation does not attenuate the benefits of smoking cessation among people with and without diabetes.
Design, Setting, and Participants
Prospective community-based cohort study using data from the Framingham Offspring Study collected from 1984 to 2011. At each 4-year exam, self-reported smoking status was assessed and categorized as smoker, recent quitter (≤ 4 years), long-term quitter (> 4 years), and non-smoker. Pooled Cox proportional hazards models were used to estimate the association between quitting smoking and 6-year CVD events and to test whether 4-year change in weight following smoking cessation modified the association between smoking cessation and CVD events.
Main outcome measure
Incidence over 6 years of total CVD events, comprising coronary heart disease, cerebrovascular events, peripheral artery disease, and congestive heart failure.
Results
After a mean follow-up of 25 years (SD, 9.6), 631 CVD events occurred among 3251 participants. Median 4-year weight gain was greater for recent quitters without diabetes (2.7 kg, Interquartile range [IQR] −0.5-6.4) and with diabetes (3.6 kg, IQR −1.4-8.2) than for long term quitters (0.9 kg, IQR −1.4-3.2 and 0.0 kg, IQR −3.2-3.2, respectively, p<0.001). Among people without diabetes, age and sex-adjusted incidence rate of CVD was 5.9/ 100 person-exams (95% confidence interval [CI] 4.9-7.1) in smokers, 3.2/ 100 person-exams (95% CI 2.1-4.5) in recent quitters, 3.1 /100 person-exams (95% CI 2.6-3.7) in long-term quitters, and 2.4 /100 person-exams (95% CI 2.0-3.0) in non-smokers. After adjustment for CVD risk factors, compared with smokers, recent quitters had a hazard ratio (HR) for CVD of 0.47 (95% CI, 0.23-0.94) and long-term quitters had an HR of 0.46 (95% CI, 0.34-0.63); these associations had only a minimal change after further adjustment for weight change. Among people with diabetes, there were similar point estimates that did not reach statistical significance.
Conclusions and Relevance
In this community based cohort, smoking cessation was associated with a lower risk of CVD events among participants without diabetes, and weight gain that occurred following smoking cessation did not modify this association. This supports a net cardiovascular benefit of smoking cessation despite subsequent weight gain.
Introduction
Cigarette smoking is the leading cause of preventable mortality in the United States1 and a major risk factor for cardiovascular disease (CVD). Smoking cessation substantially reduces the risks of CVD.2, However, quitting smoking is associated with a small number of adverse health consequences, weight gain being one of smokers’ major concerns3. The mean post-cessation weight gain varies between 3 and 6 kg in North America, happens within 6 months after smoking cessation, and persists over time3. Obesity is also a risk factor for CVD. Vascular mortality increases 40% for every 5 kg/m2 increase in body mass index (BMI) above 25 kg/m2 4. Weight gain following smoking cessation therefore might attenuate the benefits of quitting smoking on CVD outcomes. Among people with type 2 diabetes, weight gain following smoking cessation has potential to be of greater concern because it is a risk factor for poor diabetes control and increased risk of morbidity and mortality5. Weight control is a key factor in diabetes management to prevent microvascular and CVD complications6.
The effect on CVD of potential weight gain following smoking cessation is not well understood. One study indirectly assessed the association of weight gain following smoking cessation with CVD in Japanese men without diabetes and estimated that successful quitters had a 24% decreased risk of coronary heart disease (CHD) compared with smokers despite weight gain, but did not measure actual CHD events7. Among people with diabetes, studies have demonstrated the CVD benefits of quitting smoking8-10 but none have assessed the effect of weight change following smoking cessation on CVD.
The aim of this study was to assess the association between 4-year weight gain following smoking cessation and CVD event rate among people with and without diabetes. We tested the hypothesis that quitting smoking decreases CVD risk compared to continuing smoking regardless of any weight gain associated with smoking cessation, in people with and without diabetes.
Methods
Study population and study sample
We analyzed data from the Offspring cohort of the Framingham Heart Study. The Framingham Offspring cohort began in 1971 and enrolled 5124 children and spouses of children of the original Framingham Heart Study cohort. As previously described,11 participants of the Offspring cohort underwent repeated examinations approximately every 4 to 6 years. The present study sample comprised 3251 adult participants free of CVD at the beginning of examination 3.
Boston Medical Center’s institutional review board approved the study. All participants provided written informed consent.
Assessment of diabetes, smoking, weight, and weight change
At each examination, participants underwent a medical history, physical examination, and fasting blood sample collection for a lipid profile and blood glucose measurement. Participants were considered as having diabetes if they had fasting plasma glucose ≥ 7 mmol/l or if they were treated with insulin or an oral hypoglycemic agent. In the Offspring study 99% of the cases of diabetes are type 2 diabetes12. Type 1 diabetes was not excluded from our analyses but accounts for only 1% of diabetes cases.
Participants were classified as current smokers, former smokers, and non-smokers based on self-reported data at each examination. Current cigarette smoking was defined as regularly smoking cigarettes at any time during the prior year. For former smokers, information on the exact smoking cessation date and therefore time since quitting was not available. Therefore, we defined recent quitters as people who reported not smoking at one examination and had reported smoking at the examination 4 years earlier (ie, who had quit for ≤ 4 years). We defined long-term quitters as people who reported not smoking for 2 or more consecutive examinations after an examination in which they had been a smoker (ie, who had quit for > 4 years). For secondary analyses, we created another smoking category differentiating continuous smokers (participants who were smokers during the entire duration of the study), never smokers (participants who were never smokers during the entire study), quitters (smokers who had made a quit attempt and remained abstinent for the rest of the study), and relapsers (participants who alternated between smoking and smoking cessation during the study).
Participants were weighed in light street clothes per standard protocol using a calibrated scale, identically at each exam11. Height was measured at the baseline examination. BMI was calculated as weight in kilograms divided by the square of heights in meters. Weight change was calculated at each examination as weight at the current examination minus weight at the previous examination, reflecting 4-year weight change.
At each examination systolic blood pressure was measured twice after the participant had been sitting at least 5 minutes. The mean value was used for the analyses. Information about alcohol consumption, medication, and family history of diabetes were collected and updated at each examination.
CVD outcomes
The primary outcome was total CVD events. The Framingham Heart Study defined CVD events as a composite of CHD (coronary death, myocardial infarction, coronary insufficiency, and angina), cerebrovascular events (ischemic stroke, hemorrhagic stroke, and transient ischemic attack), peripheral arterial disease (intermittent claudication), and congestive heart failure13. In secondary analyses we considered a more restrictive outcome of hard CHD, defined as myocardial infarction and coronary death only. Surveillance for CVD consisted of regular examinations at the Framingham Heart Study clinic and review of medical records from outside physicians’ offices and hospitalizations. A panel of 3 experienced investigators evaluated all pertinent medical records, including prevalent cardiovascular disease risk factors, for suspected new events. More details regarding the CVD adjudication methods have been described14.
Follow-up time was defined by the time from the baseline examination until the first event date (for participants who had an event) or was censored at the last contact date (for participants who did not have any event or were lost to follow up) or the day of death (for participants who died of non-CVD causes). There were 3251 participants at examination 3 (1984-1987) and 2394 participants at examination 8 (2005-2008), meaning that 73.6% of participants had at least one period of follow up. We handled missing data as missing without data imputation.
Statistical analysis
The analyses began with the third examination (1984-1987) and extended through December 31, 2011 (end of the 8th examination). In order to have pools of similar lengths, we pooled exams 3 and 4 and exams 5 and 6 and kept exams 7 and 8 as separate pools; we thus obtained 4 pools of a mean duration of 6 years (ranging from 5.2 to 7.0 years). We examined each 6-year examination pool as a follow-up study and considered smoking status (independent variable) at the beginning of each examination and CVD event (dependent variable) during the 6-year follow-up. At the beginning of each examination, participants who had developed a CVD outcome were removed from the sample. We calculated mean 4-year weight change preceding the beginning of each examination in order to assess the association with weight gain concomitant with or shortly following smoking cessation.
We calculated age- and sex-adjusted 6-year incidence rates of CVD and corresponding 95% CIs. For each period we estimated the likelihood of a CVD event according to smoking status using Cox proportional hazard models and pooled the results of each model. At each exam, risk factors such as smoking status, weight, blood pressure, and cholesterol were updated based on new information. Pooling Cox models of each study period allowed consideration of changes in those risk factors over the next period of time with updated exposures.
Preplanned analyses were conducted separately for people with and without diabetes based on the hypothesis that weight change following smoking cessation might have a different association with CVD events depending on diabetes status. Hazard ratios (HRs) for CVD were calculated for recent quitters, long-term quitters, and non-smokers compared with smokers. Smokers were chosen as the reference group to assess the association between quitting smoking and CVD as recommended by the 1990 Surgeon General’s report15.
We built minimally adjusted models (adjusted only for age and sex) and CVD risk factor-adjusted models (adjusted for age, sex, alcohol consumption, self-reported family history of diabetes, HDL- and LDL-cholesterol, triglycerides, systolic blood pressure, baseline BMI, taking cholesterol lowering medication, and taking anti-hypertension medication). The choice of the covariates included in the CVD risk factor-adjusted model was based on a priori knowledge. To assess the modification of weight change following smoking cessation on CVD, we built a third model adding 4-year weight change prior to the index examination to the CVD risk factor-adjusted model. We verified the proportional hazards assumption using graphical methods and by including time dependent covariates in the models.
Secondary analyses used the more restrictive outcome of hard CHD. Minimally adjusted- and CVD risk factor-adjusted pooled Cox models assessed the association between weight gain following smoking cessation and hard CHD. We performed subgroup analyses by amount of weight gain. For these analyses, given the lack of interaction by diabetes, we pooled participants with and without diabetes together to have more power and avoid empty categories. We built 3 weight change categories: participants who lost weight, those who gained 0-5 kg and those who gained ≥5 kg.
Exploratory analyses assessed the association between smoking cessation, weight change, and the incidence of high blood pressure and hyperlipidemia. High blood pressure was defined as diastolic blood pressure ≥90 mmHg, systolic blood pressure ≥140 mmHg, or taking antihypertensive drugs. Hypercholesterolemia was defined as LDL-C > 4.1 mmol/l or taking cholesterol-lowering medications. For these analyses we used pooled logistic regression models with the same time intervals as the pooled Cox regression models. We examined each pool as a follow-up studies and considered smoking status at the beginning of each examination and incidence of high blood pressure or hyperlipidemia during the 6-year follow-up. Participants with high blood pressure, respectively hyperlipidemia, were removed from analyses at the beginning of each examination.
We considered a two-sided p-value < 0.05 as statistically significant. For the comparison of weight change between the 4 different smoking categories we used the Bonferroni method to adjust for multiple pairwise comparisons and defined a corrected p-value of 0.01 (4 pairwise comparisons). We tested if there was an interaction between smoking and diabetes by entering an interaction term in the Cox models. The test for trends was calculated by modeling smoking ordinal categories as continuous variables. Statistical analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
From the third examination, 3251 participants were followed over 4 examinations and contributed 11,148 person-exams. Baseline characteristics of participants at the beginning of each examination are given in Table 1. Smoking prevalence decreased from 31% at the third examination to 13% at the 8th examination.
Table 1.
Baseline characteristics of participants of the Framingham Offspring Cohort at each examination
| Exam 3 | Exam 5 | Exam 7 | Exam 8 | |
|---|---|---|---|---|
| 1984-1987 | 1991-95 | 1998-2001 | 2005-2008 | |
| N of participants, (%) | 3251 | 3061 | 2442 | 2394 |
| Follow-up (years), mean | ||||
| (SD) | 7.0 (1.0) | 6.8 (1.2) | 6.4 (1.2) | 5.2 (1.0) |
| Age, mean (SD) | 47.8 (10.0) | 54.1 (9.7) | 59.5 (9.0) | 65.5 (8.7) |
| Women, n (%) | 1679 (51.7) | 1666 (54.4) | 1392 (57.0) | 1373 (57.3) |
| Weight (kg), mean (SD) | 74.8 (15.8) | 77.5 (16.8) | 78.8 (17.7) | 78.8 (17.9) |
| BMI (kg/m2), mean (SD) | 26.1 (4.6) | 27.4 (5.0) | 28.0 (5.3) | 28.1 (5.4) |
| Systolic blood pressure | ||||
| (mmHg), mean (SD) | 123.3 (16.4) | 125.6 (18.5) | 125.4 (18.1) | 128.1 (16.9) |
| HDL cholesterol (mg/dL), | ||||
| mean (SD) | 51.5 (14.8) | 50.4 (15.1) | 54.9 (17.0) | 58.8 (18.3) |
| Triglyceride (mg/dL), mean | ||||
| (SD) | 119.7 (115.9) | 145.0 (105.4) | 131.6 (81.2) | 115.5 (66.1) |
| LDL cholesterol (mg/dL), | ||||
| mean (SD) | 132.9 (35.8) | 126.7 (33.0) | 121.8 (32.3) | 108.7 (30.3) |
| Fasting plasma glucose | ||||
| (mg/dl), mean (SD) | 94.3 (21.5) | 99.9 (27.7) | 101.7 (22.8) | 105.3 (22.0) |
| Family history of diabetes, n | ||||
| (%) | 567 (17.4) | 606 (19.8) | 470 (19.3) | 444 (18.5) |
| Alcohol consumption | ||||
| (oz/week), median (IQR) | 5.0 (0.0-11.0) | 2.0 (0.0-7.0) | 2.0 (0.0-8.0) | 1.0 (0.0-7.0) |
| Taking cholesterol lowering | ||||
| medication, n (%) | 23 (0.7) | 169 (5.5) | 370 (15.2) | 874 (36.6) |
| Taking anti-hypertension | ||||
| treatment, n (%) | 470 (14.5) | 492 (16.1) | 672 (27.5) | 1049 (43.8) |
| Diabetes prevalence, n (%) | 370 (11.4) | 349 (11.4) | 244 (10.0) | 320 (13.4) |
| Smoking status, n (%) | ||||
| Non smokers | 1077 (33.1) | 1070 (35.0) | 882 (36.1) | 869 (36.3) |
| Quit for > 4 years | 859 (26.4) | 1216 (39.7) | 1142 (46.8) | 1147 (47.9) |
| Quit for ≤ 4 years | 295 (9.1) | 220 (7.2) | 91 (3.7) | 77 (3.2) |
| Current smokers | 1020 (31.4) | 555 (18.1) | 327 (13.4) | 301 (12.6) |
N=number, SD=standard deviation, BMI= body-mass index, HDL = high density lipoprotein, LDL = low density lipoprotein, IQR = interquartile range
Weight gain occurred over 4 years in participants without and with diabetes (Table 2). Among participants without diabetes, recent quitters gained significantly more weight (median weight 2.7 kg, IQR −0.5-6.4) than long-term quitters (0.9 kg, IQR −1.4-3.2), smokers (0.9 kg, IQR −1.8-4.5) and non-smokers (1.4 kg, IQR −1.4-3.6) (p<0.001 for each pairwise comparison). Long-term quitters did not have a statistically significant difference in weight gain compared with nonsmokers and smokers, taking into account Bonferroni adjustment (p=0.02 for both comparisons). Among people with diabetes, recent quitters also gained significantly more weight (3.6 kg, IQR −1.4-8.2) than smokers (0.9 kg, IQR −3.2-4.1), long-term quitters (0.0 kg, IQR −3.2-3.2) and non-smokers (0.5 kg, IQR −2.7-3.6) (p<0.001 for each pairwise comparison).
Table 2.
Four-year weight change for participants
| No diabetes | ||||
|---|---|---|---|---|
| Smokers | Former smokers | Non smokers | ||
| Quit for ≤ 4 y | ||||
| Quit for > 4 y | ||||
| (n=978) | (n=205) | (n=676) | (n=920) | |
| BMI at exam 3 (kg/m2), mean (SD) | 25.4 (4.2) | 26.5 (4.6) | 25.9 (3.9) | 25.4 (4.3) |
|
| ||||
| Weight at exam 3 (kg), mean (SD) | 73.8 (15.7) | 77.3 (15.7) | 74.3 (14.3) | 71.4 (14.6) |
|
| ||||
| 1.2 (5.4) | 3.0 (7.3) | 0.9 (5.0) | 1.2 (5.2) | |
| 4-year weight change (kg), mean (SD) | ||||
| and (95% CI) * | (0.9 to 1.4) | (2.4 to 3.7) | (0.7 to 1.0) | (1.0 to 1.4) |
|
| ||||
| 4-year weight change (kg), median | ||||
| (IQR)* | 0.9 (−1.8-4.6) | 2.7 (−0.5-6.4) | 0.9 (−1.4-3.2) | 1.4 (−1.4-3.6) |
|
| ||||
| Diabetes | ||||
| Smokers | Former smokers | Non smokers | ||
| Quit for ≤ 4 y | Quit for > 4 y | |||
| (n=148) | (n=31) | (n=118) | (n=148) | |
|
| ||||
| BMI at exam 3 (kg/m2), mean (SD) | 28.7 (5.2) | 29.8 (5.1) | 29.1 (5.6) | 30.3 (5.8) |
|
| ||||
| Weight at exam 3 (kg), mean (SD) | 84.2 (17.7) | 88.3 (15.8) | 85.8 (17.1) | 84.2 (17.0) |
|
| ||||
| 4-year weight change (kg), mean (SD) | ||||
| and | 0.0 (7.4) | 3.8 (7.6) | 0.1 (6.4) | 0.5 (6.7) |
| (95% CI)* | (−0.1 to 1.1) | (2.1 to 5.4) | (−0.5 to 0.6) | (−0.1 to 1.1) |
| 4-year weight change (kg), median | ||||
| (IQR)* | 0.9 (−3.2-4.1) | 3.6 (−1.4-8.2) | 0.0 (−3.2-3.2) | 0.5 (−2.7-3.6) |
Mean 4-year weight change was concomitant or shortly following smoking cessation for smokers who had been quit for ≤ 4 years and was 4 years or more after cessation for smokers who had been quit for >4 years
Median 4-year weight change prior to each index examination according to smoking status is shown in eFigure 1 Among people without and with diabetes, there was no clear trend in weight change over time for recent quitters (p for trend = 0.97 and 0.32). In contrast, among long-term quitters, weight gain tended to decrease over time (p for trend <0.001 and 0.01). Diabetes incidence over time according to smoking status is shown in eFigure 2. Smokers had on average a higher incidence of diabetes compared with nonsmokers and long-term quitters. Recent quitters had a lower incidence at the beginning of the study; it became greater than that for smokers at examination 5 and 6 and decreased thereafter.
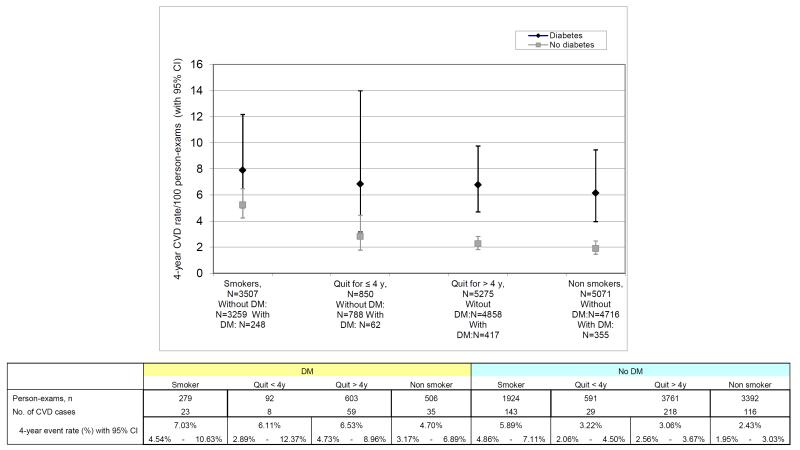
During follow-up, 631 CVD events occurred in 11148 person-exams. Of these, 53.4% were CHD events (Table 3). In people without diabetes, age- and sex- adjusted 4-year incidence rates of CVD were higher among smokers, followed by recent quitters, long term quitters, and non-smokers (Figure). The same pattern but with higher rates was observed among people with diabetes. There was no evidence of interaction between smoking and diabetes on the risk of CVD (p for interaction = 0.12).
Table 3.
CVD events and coronary heart disease events counts
| Outcome | Number (%) |
| Total CVD | 631 |
| Coronary heart disease | 337 (53.4%) |
| Cerebrovascular accident | 147 (23.3%) |
| Death from CVD | 7 (1.1%) |
| Peripheral artery disease | 73 (11.6%) |
| Heart failure | 67 (10.6%) |
| Hard coronary heart disease | 160 |
| Myocardial infarction | 155 (96.9%) |
| Coronary death | 5 (3.1%) |
Age- and sex-adjusted 6-year incidence rate of CVD for people with and without diabetes Black diamonds represent people with diabetes; grey squares represent people without diabetes.
The main results for the association between smoking cessation and CVD events are summarized in Table 4. Among participants without diabetes, the age and sex adjusted incidence rates were lower for non smokers (2.43 per 100 person-exams, 95% CI 1.95-3.03), recent quitters (3.22 per 100 person-exams, 95% CI 2.06-4.50) and long term quitters (3.06 per 100 person-exams, 95% CI 2.56-3.67) compared with smokers (5.89 per 100 person-exams, 95% CI 4.86-7.11). In the age- and sex-adjusted model, compared with smokers, HRs for CVD were 0.32 (95% CI 0.22-0.45) for non smokers, 0.50 (95% CI 0.25 to 1.00) for recent quitters and 0.50 (95% CI 0.37 to 0.68) for long-term quitters. Adjusting for CVD risk factors did not change this association significantly. Adding weight change to the CVD risk factor-adjusted model did not modify the HRs of CVD for recent and long term quitters. There was an apparent dose-response relationship with smoking and CVD risk (p<0.001 trend across smoking categories).
Table 4.
Association between smoking cessation, weight change, and CVD events
| No diabetes | ||||
|---|---|---|---|---|
| Smokers (ref) | Former smokers | Non smokers | ||
| Quit for ≤ 4 y | Quit for > 4 y | |||
| Person-exams | 1924 | 591 | 3761 | 3392 |
| No. of CVD events | 143 | 29 | 218 | 116 |
| Age-, sex-adjusted IR of | ||||
| CVD/100 person-exams | 3.06 (2.56- | |||
| (95% CI) | 5.89 (4.86-7.11) | 3.22 (2.06-4.50) | 3.67) | 2.43 (1.95-3.03) |
| Age-, sex-adjusted HR | 0.50 (0.37- | |||
| (95% CI) | 1 | 0.50 (0.25-1.00) | 0.68) | 0.32 (0.22-0.45) |
| CVD risk factors- | 0.46 (0.34- | |||
| adjusted† HR(95% CI) | 1 | 0.47 (0.23-0.94) | 0.63) | 0.30 (0.21-0.44) |
| CVD risk factors-+ weight | ||||
| change-adjusted HR (95% | 0.46 (0.34- | |||
| CI) | 1 | 0.49 (0.24-0.99) | 0.63) | 0.31 (0.21-0.44) |
|
| ||||
| Diabetes | ||||
| Smokers (ref) | Former smokers | Non smokers | ||
| Quit for ≤ 4 y | Quit for > 4 y | |||
|
| ||||
| Person-exams, n | 279 | 92 | 603 | 506 |
| No. of CVD events | 23 | 8 | 59 | 35 |
| Age-, sex-adjusted IR of | ||||
| CVD/100 person-exams | 7.03 (4.54- | 6.11 (2.89- | ||
| (95% CI) | 10.63) | 12.37) | 6.53 (4.73-8.96) | 4.70 (3.17-6.89) |
| Age-Sex-adjusted HR | ||||
| (95% CI) | 1 | 0.49 (0.11-2.16) | 0.53 (0.27-1.06) | 0.41 (0.19-0.86) |
| CVD risk factors- | ||||
| adjusted† HR (95% CI) | 1 | 0.49 (0.11-2.20) | 0.56 (0.28-1.14) | 0.49 (0.22-1.08) |
| CVD risk factors-+ weight | ||||
| change-adjusted HR (95% | ||||
| CI) | 1 | 0.49 (0.11-2.19) | 0.57 (0.28-1.15) | 0.49 (0.22-1.09) |
CVD=cardiovascular disease, IR= incidence rate, HR = Hazard Ratio, CI = Confidence Interval, BMI = body-mass index, ref = reference
Adjusted for age, sex, alcohol consumption, self-reported family history of diabetes, systolic, blood pressure, HDL-cholesterol, LDL-cholesterol, triglycerides, baseline BMI, cholesterol lowering treatment, anti-hypertension treatment
Among participants with diabetes, the age and sex adjusted incidence rates were lower for non smokers (4.70 per 100 person-exams, 95% CI 3.17-6.89), recent quitters (6.11 per 100 person-exams, 95% CI 2.89-12.37 and long term quitters (6.53 per100 person-exams, 95% CI 4.73-8.96) compared with smokers (7.03/100 person years, 95% CI 4.54-10.63). In the model adjusted for CVD risk factors, HR for CVD for non smokers was 0.41 (95% CI 0.19-0.86), for recent quitters 0.49 (95% CI 0.11-2.16) and for long term quitters 0.53 (95% CI 0.27-1.06) compared with smokers. Adjusting for CVD risk-factors and weight change did not significantly change these estimates.
In secondary analyses restricting the outcome to hard CHD, 160 events occurred during follow up (Table 3). Among participants without diabetes, the age and sex adjusted incidence rates were lower for recent quitters (3.93 per 100 person-exams, 95% CI 1.12-12.28) and long term quitters (2.32 per 100 person-exams, 95% CI 1.18-4.55) compared with smokers (5.12 per 100 person-exams, 95% CI 2.33-10.75). In the age- and sex-adjusted model, compared with smokers, HRs for CHD were 0.63 (95% CI 0.22 to 1.83) for recent quitters and 0.32 (95% CI 0.18 to 0.56) for long-term quitters (Table 5). Adjusting for CVD risk factors and weight change did not change this association significantly. Among participants with diabetes, the age and sex adjusted incidence rates were lower for recent quitters (5.49/100 person years, 95% CI 3.78-7.88) and long term quitters (4.84/100 person-years, 95% CI 4.06-5.77) compared with smokers (9.30/100 person years, 95% CI 7.67-11.22). In the model adjusted for CVD risk factors, HR for CHD for recent quitters was 0.40 (95% CI 0.05-3.17) and for long-term quitters 0.40 (95% CI 0.16-1.02) compared with smokers. Adjusting for CVD risk-factors and weight change did not significantly change these estimates.
Table 5.
Association between smoking cessation, weight change and coronary heart disease (CHD) events
| No diabetes | ||||
|---|---|---|---|---|
| Smokers (ref) | Former smokers | Non smokers | ||
| Quit for ≤ 4 y | Quit for > 4 y | |||
| Person-exams | 1924 | 591 | 3761 | 3392 |
| No. of CHD events | 48 | 10 | 45 | 21 |
| Age-, sex-adjusted IR of | 5.12 | 3.93 | 2.32 | 1.34 |
| CHD/100 person-exams | ||||
| (95% CI) | (2.33-10.75) | (1.12-12-28) | (1.18-4.55) | (0.06-3.24) |
| Age-, sex-adjusted HR | ||||
| (95% CI) | ||||
| Age-, sex-adjusted HR | ||||
| 1 | 0.63 (0.22-1.83) | 0.32 (0.18-0.56) | 0.19 (0.09-0.38) | |
| (95% CI) | ||||
| CVD risk factors- | ||||
| 1 | 0.58 (0.20-1.68) | 0.29 (0.16-0.52) | 0.17 (0.08-0.36) | |
| adjusted† HR(95% CI) | ||||
| CVD risk factors-+ | ||||
| weight change-adjusted | 1 | 0.61 (0.21-1.78) | 0.29 (0.16-0.52) | 0.17 (0.08-0.36) |
| HR (95% CI) | ||||
|
| ||||
| Diabetes | ||||
| Smokers (ref) | Former smokers | Non smokers | ||
| Quit for ≤ 4 y | Quit for > 4 y | |||
|
| ||||
| Person-exams, n | 279 | 92 | 603 | 506 |
| No. of CHD events | 10 | 3 | 16 | 7 |
| Age-, sex-adjusted IR of | 9.30 | 5.49 | 4.84 | 3.71 |
| CHD/100 person-exams | ||||
| (95% CI) | (7.67-11.22) | (3.78-7.88) | (4.06-5.77) | (2.99-4.60) |
| Age-Sex-adjusted OR | ||||
| 1 | 0.40 (0.05-3.17) | 0.40 (0.16-1.02) | 0.15 (0.04-0.52) | |
| (95% CI) | ||||
| CVD risk factors- | ||||
| 1 | 0.37 (0.05-3.01) | 0.42 (0.16-1.10) | 0.15 (0.04-0.57) | |
| adjusted† HR(95% CI) | ||||
| CVD risk factors-+ | ||||
| weight change-adjusted | 1 | 0.36 (0.04-2.97) | 0.42 (0.16-1.10) | 0.15 (0.04-0.57) |
| OR (95% CI) | ||||
No. = number, CHD = coronary heart disease = myocardial infarction + coronary death, HR = Hazard Ratio, CI = Confidence Interval, BMI = body-mass index, ref = reference
Adjusted for age, sex, alcohol consumption, self-reported family history of diabetes, systolic, blood pressure, HDL-cholesterol, LDL-cholesterol, Triglycerides, baseline BMI, cholesterol lowering treatment, anti-hypertension treatment
Using the alternate smoking definition, among people without diabetes quitters had a significantly decreased risk of CVD compared with sustained smokers (HR 0.46, 95% CI 0.33-0.63) in the CVD risk factor-adjusted model. (eTable 1). Among relapsers the point estimate for the association was weaker (HR 0.60, 95% CI 0.35-1.04) and not significant. Adjusting for weight change did not significantly modify these estimates. Among people with diabetes the CVD risk factor-adjusted HR of CVD events was 0.56 (95% CI 0.28-1.11) for quitters and 0.24 (95% CI 0.03-1.84) for relapsers compared with sustained smokers.
In subgroup analyses stratified by amount of weight gain (eTable 4), among participants who lost weight and those who gained 0-5 kg, the CVD risk factors-adjusted HRs of CVD were only significantly lower for long-term quitters compared with smokers (HR 0.41, 95% CI 0.27-0.63 for those who lost weight and HR 0.39, 95% CI 0.25-0.61 for those who gained 0-5 kg). Among participants who gained 5 kg or more we did not find any statistically significant association, although numbers of events in these categories were small.
Exploratory analyses were performed to assess the association between smoking cessation and weight gain with high blood pressure (eTable 2) and hyperlipidemia (eTable 3). No statistically significant associations were found for recent quitters or long-term quitters among people with or without diabetes.
Comment
Concerns have been raised about the potential risks of weight gain for CVD following smoking cessation16,17. However, in this study, 4-year weight gain associated with smoking cessation did not outweigh the benefits for CVD risk associated with smoking cessation. Among people without diabetes, compared with smokers recent quitters had an HR of 0.47 and long-term quitters an HR of 0.46 in CVD risk factor-adjusted models. Among people with diabetes, there were similar point estimates, although the CVD risk reduction associated with quitting smoking was not statistically significant. We observed similar benefits associated with smoking cessation for total CVD and for fatal and non-fatal CHD, with the cessation benefits not offset by weight gain. An alternate smoking definition that takes into account smoking exposure over time suggested the possibility of a dose-response relationship, with never smokers having the lowest risk of CVD compared with always smokers, followed by quitters and relapsers. Subgroup analyses by amount of weight gain had small numbers of events in many groups, limiting ability to draw unambiguous conclusions, but suggested that at least among participants who gained less than 5 kg there was a CVD benefit associated with smoking cessation.
The amount of weight gain following smoking cessation was comparable with other studies18. Recent analyses of 3 different US cohorts19 showed that within each 4-year period participants gained a mean of 1.52 kg, compared with a mean 4-year weight gain of 1.39 kg among people without diabetes in our study. Similarly to our study, weight gain following smoking cessation was observed in recent quitters (≤ 4 years) but decreased thereafter.
To our knowledge, only one study has indirectly assessed the effect of weight gain following smoking cessation on CVD in people without diabetes. The investigators followed 1995 Japanese male workers for 4 years7 and found that smokers who had successfully quit smoking for at least 6 months gained weight and had a significant worsening of their blood pressure, total cholesterol, triglycerides, and fasting blood glucose. In contrast, their HDL-cholesterollevels improved, and combined with cessation of smoking, successful quitters had a 24% decreased estimated risk of CHD (using a prediction rule based on CHD risk factors) compared with smokers despite weight gain. Numerous studies have shown the immediate benefits of smoking cessation on CHD, or on overall and CVD mortality, 20 but they did not take into account the effect of weight change following smoking cessation. Being able to quantify the association of weight gain after smoking cessation with actual CVD risk may allow for better counseling of patients.
There is scant literature on the effects of smoking cessation on CVD in populations with diabetes. Several studies have shown the benefits of quitting smoking for CHD and all-cause mortality in people with diabetes8,9,21 but none of these accounted for potential weight gain subsequent to smoking cessation in their analyses. In our study, we hypothesize that lack of power is one reason for not finding the same significant risk reductions in participants with diabetes as in participants without diabetes, since point estimates for participants with diabetes were similar to nondiabetics but not statistically significant.
There are multiple potential mechanisms of the decrease in risk in CVD associated with smoking cessation. Cigarette smoking has short and long term cardiovascular effects that are reversible shortly after cessation22,23. Cigarette smoking increases heart rate and myocardial contractility, induces arterial vasoconstriction, increases platelet aggregability, reduces oxygen delivery and on the long term induces endothelial injury and formation of atheroma24. The increase in CVD risk associated with smoking is also mediated through cardiovascular risk factors such as an increase in LDL-cholesterol and triglyceride, a decrease in HDL-cholesterol, or an increase in fasting blood glucose. Some of these cardiovascular risk factors, such as HDL-cholesterol or insulin sensitivity, improve after smoking cessation independent of potential weight gain25,26. People who manage to quit smoking are also often more health conscious than those continuing to smoke and might adopt a healthier lifestyle27.
Strengths of this study include the ability to investigate people with and without diabetes to assess the association between weight change following smoking cessation and CVD. Data on smoking, diabetes, and CVD were collected rigorously at periodic examinations. Weight change was measured, not self-reported, at each examination. We adjusted for many CVD risk factors that could act as confounders. Using pooled Cox models accounted for time-varying covariates such as smoking status, weight, and weight change.
Limitations should also be considered. First, smoking status was self-reported and there was no biochemical verification. Exact time since smoking cessation was not available in the dataset, and a variable for former smokers was created based on their report of smoking on previous exams. Smokers generally need several attempts before successfully quitting, so we might not have captured relapses between 2 examinations. Second, the choice of interval for the pooled Cox models might be too large to capture change in weight, but shorter intervals were not possible due to the design of the study. However, the similarity in weight gain following smoking cessation compared with other US cohorts suggests that we correctly captured weight change following smoking cessation. A longer interval might have allowed more CVD events to be observed, however the benefits of quitting smoking occur rapidly, especially in the first 2 years after smoking cessation15. Third, the Framingham Heart Study enrolled mostly white participants and these results might not be generalizable to a multiethnic population.
In conclusion, among people without diabetes, quitting smoking was associated with a lower risk of CVD compared with continuing smoking. There were qualitatively similar lower risks among participants with diabetes that did not reach statistical significance, possibly due to limited study power. Weight gain that occurred following smoking cessation was not associated with a reduction in the benefits of quitting smoking on CVD risk among adults without diabetes. This supports a net cardiovascular benefit of smoking cessation despite subsequent weight gain.
Supplementary Material
Acknowledgments
Peter Shrader, (Massachusetts General Hospital, General Medicine Division, Boston, Massachusetts) contributed to the statistical analyses. He was compensated for his work.
Parts of the data of this manuscript have been presented as an oral presentation at the 34th annual meeting of the Society of General Internal Medicine (SGIM) in May 2011.
Dr Clair had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial support: CC was supported by a grant from the Swiss National Science Foundation PBLAP3-127728/1 and by a grant from the SICPA foundation. NAR was supported by grant #5K24HL4440-10. JBM was supported by NIDDK K24 DK080140.
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
The Framingham Heart Study is supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (contract No. N01-HC-25195). The National Heart Lung and Blood Institute approved the manuscript.
Footnotes
Author’s contributions: CC led the analyses of the data and drafted the manuscript. NAR helped with drafting and reviewing the manuscript and in interpreting the data. BP performed the statistical analyses. CSF was involved in reviewing the manuscript. RBDA reviewed the manuscript. MP supervised the statistical analyses and reviewed the manuscript. JBM was involved in drafting and reviewing the manuscript and in interpreting the data. All authors read and approved the final manuscript.
Conflict of interest: CC does not report any conflict of interest
NAR reported having consulted without pay about smoking cessation for Pfizer and Allere Wellbeing, Inc. and having conducted research projects sponsored by Pfizer and Nabi Biopharmaceuticals.
JBM does not report any conflicts of interest.
References
- 1.Centers for Disease C, Prevention Smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 2000-2004. MMWR. Morb. Mortal. Wkly. Rep. 2008 Nov 14;57(45):1226–1228. [PubMed] [Google Scholar]
- 2.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N. Engl. J. Med. 2013 Jan 24;368(4):341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 3.Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A, Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A. Smoking cessation and weight gain. Obesity Reviews. 2004 May;5(2):95–103. doi: 10.1111/j.1467-789X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 4.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009 Mar 28;373(9669):1083–1096. [Google Scholar]
- 5.Feldstein AC, Nichols GA, Smith DH, et al. Weight change in diabetes and glycemic and blood pressure control. Diabetes Care. 2008;31(10):1960–1965. doi: 10.2337/dc08-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes A. Standards of medical care in diabetes--2012. Diabetes Care. 2012 Jan;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamura U, Tanaka T, Okamura T, et al. Changes in Weight, cardiovascular risk factors and estimated risk of coronary heart disease following smoking cessation in Japanese male workers: HIPOP-OHP study. Journal of atherosclerosis and thrombosis. 2010;17(1):12–20. doi: 10.5551/jat.1800. [DOI] [PubMed] [Google Scholar]
- 8.Al-Delaimy WK, Manson JE, Solomon CG, et al. Smoking and risk of coronary heart disease among women with type 2 diabetes mellitus. Arch. Intern. Med. 2002;162(3):273–279. doi: 10.1001/archinte.162.3.273. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care. 1997;20(8):1266–1272. doi: 10.2337/diacare.20.8.1266. [DOI] [PubMed] [Google Scholar]
- 10.Moy CS, LaPorte RE, Dorman JS, et al. Insulin-dependent diabetes mellitus mortality. The risk of cigarette smoking. Circulation. 1990;82(1):37–43. doi: 10.1161/01.cir.82.1.37. [DOI] [PubMed] [Google Scholar]
- 11.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev. Med. 1975;4(4):518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 12.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes. 2000;49(12):2201–2207. doi: 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- 13.D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 14.Cupples LA, D’Agostino RB. Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennal measurements: Framingham Study, 30-year follow-up. In: Kannel WB, Wolf PA, Garrison RJ, editors. The Framingham Heart Study: An Epidemiologic Investigation of cardiovascular Disease. National Institutes of Health; Washington, DC: 1987. pp. 87–203. [Google Scholar]
- 15.The Health Benefits of Smoking Cessation. A report of the Surgeon General. U.S. Department of Health and Human Services; Atlanta, GA: 1990. pp. 90–8416. DHHS Publication No.CDC. [PubMed] [Google Scholar]
- 16.Pisinger C, Jorgensen T. Waist circumference and weight following smoking cessation in a general population: the Inter99 study. Prev. Med. 2007;44(4):290–295. doi: 10.1016/j.ypmed.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Berlin I. Smoking-induced metabolic disorders: a review. Diabetes Metab. 2008;34(4 Pt 1):307–314. doi: 10.1016/j.diabet.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A. Smoking cessation and weight gain. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2004;5(2):95–103. doi: 10.1111/j.1467-789X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 19.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011;364(25):2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawachi I, Colditz GA, Stampfer MJ, et al. Smoking cessation in relation to total mortality rates in women. A prospective cohort study. Ann. Intern. Med. 1993;119(10):992–1000. doi: 10.7326/0003-4819-119-10-199311150-00005. [DOI] [PubMed] [Google Scholar]
- 21.Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316(7134):823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson HM, Gossett LK, Piper ME, et al. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J. Am. Coll. Cardiol. 2010;55(18):1988–1995. doi: 10.1016/j.jacc.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88(5 Pt 1):2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 24.Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog. Cardiovasc. Dis. Jul. 2003;46(1):91–111. doi: 10.1016/s0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 25.Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, Stein JH. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am. Heart J. 2011;161(1):145–151. doi: 10.1016/j.ahj.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eliasson B, Attvall S, Taskinen MR, Smith U. Smoking cessation improves insulin sensitivity in healthy middle-aged men. Eur. J. Clin. Invest. 1997;27(5):450–456. doi: 10.1046/j.1365-2362.1997.1330680.x. [DOI] [PubMed] [Google Scholar]
- 27.Chiolero A, Wietlisbach V, Ruffieux C, Paccaud F, Cornuz J. Clustering of risk behaviors with cigarette consumption: A population-based survey. Prev. Med. 2006;42(5):348–353. doi: 10.1016/j.ypmed.2006.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.