Abstract
Congenital myasthenic syndromes (CMSs) stem from genetic defects in endplate (EP)-specific presynaptic, synaptic, and postsynaptic proteins. The postsynaptic CMSs identified to date stem from a deficiency or kinetic abnormality of the acetylcholine receptor (AChR). All CMSs with a kinetic abnormality of AChR, as well as many CMSs with a deficiency of AChR, have been traced to mutations in AChR-subunit genes. However, in a subset of patients with EP AChR deficiency, the genetic defect has remained elusive. Rapsyn, a 43-kDa postsynaptic protein, plays an essential role in the clustering of AChR at the EP. Seven tetratricopeptide repeats (TPRs) of rapsyn subserve self-association, a coiled-coil domain binds to AChR, and a RING-H2 domain associates with β-dystroglycan and links rapsyn to the subsynaptic cytoskeleton. Rapsyn self-association precedes recruitment of AChR to rapsyn clusters. In four patients with EP AChR deficiency but with no mutations in AChR subunits, we identify three recessive rapsyn mutations: one patient carries L14P in TPR1 and N88K in TPR3; two are homozygous for N88K; and one carries N88K and 553ins5, which frameshifts in TPR5. EP studies in each case show decreased staining for rapsyn and AChR, as well as impaired postsynaptic morphological development. Expression studies in HEK cells indicate that none of the mutations hinders rapsyn self-association but that all three diminish coclustering of AChR with rapsyn.
Introduction
Rapsyn, under the influence of neural agrin, plays a critical role in concentrating the acetylcholine receptor (AChR) in the postsynaptic membrane of the motor endplate (EP) (Froehner et al. 1990). Rapsyn knockout mice cluster AChR poorly and fail to accumulate AChR at the EP (Gautam et al. 1995). Thus, rapsyn is an effector of agrin-induced clustering of AChR.
Rapsyn binds to the long cytoplasmic loop of each AChR subunit (Maimone and Merlie 1993, 1999) and links the receptor to the subsynaptic cytoskeleton via dystroglycan (Cartaud et al. 1998) and an actin-binding synaptic nebulin-related anchoring protein (S-NRAP) (Tseng et al. 2001). The primary structure of rapsyn predicts distinct structural domains: a myristoylation signal at the N-terminus, required for membrane association (Ramarao and Cohen 1998); seven tetratricopeptide repeats (TPRs; codons 6–279) that subserve rapsyn self-association (Ramarao and Cohen 1998; Ramarao et al. 2001); a coiled-coil domain (codons 298–331) whose hydrophobic surface can bind to determinants within the long cytoplasmic loop of each AChR subunit (Bartoli and Cohen 2001; Ramarao et al. 2001); a cysteine-rich RING-H2 domain (codons 363–402) that binds to the cytoplasmic domain of β-dystroglycan (Bartoli et al. 2001) and to S-NRAP (Tseng et al. 2001); and a serine phosphorylation site at codon 406.
Self-association of rapsyn is critical for linking AChR to the cytoskeleton. Rapsyn expressed in human embryonic kidney (HEK) cells or other nonmuscle cells self-associates into clusters on the cell surface. When AChR, β-dystroglycan, and S-NRAP are expressed individually in HEK cells, none aggregates into clusters, but when AChR (Froehner et al. 1990; Phillips et al. 1991; Ramarao et al. 2001), dystroglycan (Apel et al. 1995; Bartoli et al. 2001), or S-NRAP (Tseng et al. 2001) is coexpressed with rapsyn, each molecule is recruited to existing rapsyn clusters.
Congenital myasthenic syndromes (CMSs) result from genetic defects in EP-specific presynaptic, synaptic, or postsynaptic proteins. We and others have identified genetic defects in presynaptic, synaptic, and postsynaptic CMS (Engel et al. 1999). Mutations in the choline acetyltransferase gene (CHAT [MIM 118490]) result in decreased vesicular filling with acetylcholine (ACh) and in a presynaptic CMS (Ohno et al. 2001). Mutations in the gene encoding the collagenic tail subunit (COLQ [MIM 603033]) of acetylcholinesterase (AChE) cause a synaptic form of CMS (Donger et al. 1998; Ohno et al. 1998). Mutations in AChR-subunit genes that alter the kinetic properties (Ohno et al. 1995; Sine et al. 1995), the level of expression (Engel et al. 1996), or both (Ohno et al. 1997) of the receptor result in postsynaptic CMS. EP AChR deficiency is caused mostly by mutations in the AChR ε-subunit gene (CHRNE [MIM 100725]), probably because the fetal AChR γ subunit can partially substitute for the defective adult ε subunit and, thus, can rescue the phenotype (Engel et al. 1996; Ohno et al. 1997; Milone et al. 1998).
In the course of investigating CMS, we identified 37 patients with deficiency but no kinetic abnormality of AChR. A rigorous search for mutations in the AChR α-, β-, δ-, and ε-subunit genes revealed pathogenic mutations in 27 of these patients. To identify the cause of AChR deficiency in the other 10 patients, we sequenced the rapsyn gene (RAPSN [MIM 601592]) and identified a subset of 4 patients, each carrying two mutant alleles arising from three mutations. Expression studies of wild-type or mutant rapsyns cotransfected together with wild-type AChR subunits in HEK 293 cells reveal that none of the mutations hinders rapsyn self-association but that each mutation hinders recruitment of AChR to rapsyn clusters.
Patients and Methods
Patients
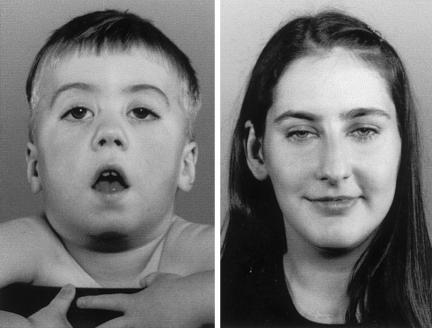
Patient (Pt) 1, a girl of age 2.5 years, and Pt 2, a boy of age 2 years (fig. 1, left), had weak fetal movements in utero. In the neonatal period they were hypotonic, with poor suck and cry and with ptosis of the eyelids. Pt 1 also required ventilatory assistance for 1 d and tube feeding for the first 5 mo of life. They walked at age 15 and 18 mo, respectively, but fell frequently. On examination, they are diffusely weak, have eyelid ptosis, fatigue easily, show a positive Gowers’ sign, and have a high-arched palate. Pt 3 is a woman of age 27 years. As an infant, she had a soft cry and fluctuating eyelid ptosis. She walked at age 18 mo but could never walk fast or run. On several occasions, respiratory infections or other intercurrent illnesses precipitated generalized weakness. On examination, the positive findings are limited to eyelid ptosis and the inability to rise from squatting (fig. 1, right). Pt 4 is a girl of age 11 years. Although her mother was unaware of decreased fetal movements in utero, she was born with multiple joint contractures. As a neonate, she had a weak suck and cry and gained weight poorly. At age 6 mo and several times in subsequent years, minor respiratory infections precipitated increased weakness and respiratory distress requiring assisted ventilation. On examination, she has mild eyelid ptosis and mild weakness of the cervical, torso, and limb muscles, and she fatigues easily on exertion. Pt 1 has a similarly affected brother. Pts 2, 3, and 4 have no similarly affected family members.
Figure 1.
Pt 2 (left) and Pt 3 (right). In Pt 2, note the bilateral eyelid ptosis, facial diplegia, and open mouth. Pt 3 shows only mild eyelid ptosis.
Electromyographic studies revealed a decremental response of the compound muscle-fiber action potential on 2-Hz stimulation in Pts 1 and 2. In Pt 3, single-fiber electromyography was required to uncover a defect in neuromuscular transmission. In Pt 4, a decremental response on 2-Hz stimulation appeared only after subtetanic stimulation for 5 min. All four patients have negative tests for anti-AChR antibodies and respond favorably, though incompletely, to anticholinesterase medications. Pt 3 derives additional benefit from the use of 3,4-diaminopyridine, a medication that increases the number of ACh quanta released by nerve impulse.
Muscle Specimens
Intercostal muscle specimens were obtained intact, from origin to insertion, from patients and from control subjects without muscle disease who were undergoing thoracic surgery. All human studies were in accord with the guidelines of the institutional review board of the Mayo Clinic.
AChR and AChE were colocalized in cryostat sections with rhodamine-labeled α-bungarotoxin (α-bgt) and a monoclonal anti-AChE antibody (Fambrough et al. 1982). Rapsyn and the vesicular ACh transporter (VAChT) were colocalized with a mouse anti-rapsyn monoclonal (mAb 1234, 2 μg/ml; a gift from S. Froehner) and a polyclonal rabbit anti-VAChT (1/500; a gift from J. D. Erickson) as primary antibodies, and with fluorescein-isothiocyanate (FITC)–labeled donkey anti-mouse IgG (5 μg/ml) and CY3-labeled donkey anti-rabbit IgG (1 μg/ml) as second antibodies (Jackson Laboratories).
EPs were localized for electron microscopy (Engel 1994a) and were quantitatively analyzed (Engel 1994b) by established methods. Peroxidase-labeled α-bgt was used for the ultrastructural localization of AChR (Engel et al. 1977). The number of AChRs per EP was measured with α-bgt labeled with 125I, as described by Engel (1993).
Electrophysiology of Muscle Specimens
Miniature EP (MEPP), miniature EP current (MEPC), and EP potential (EPP) recordings, as well as estimates of the number of transmitter quanta released by nerve impulse (Engel et al. 1993; Uchitel et al. 1993) and single-channel patch-clamp recordings from the EP in the cell-attached mode (Milone et al. 1997), were performed as described elsewhere.
Sequencing Procedures
PCR-amplified fragments were purified by the QIAquick 8 PCR Purification Kit (Qiagen). Plasmids were purified by the QIAprep Spin Miniprep Kit (Qiagen). PCR products and plasmids were sequenced with an ABI377 DNA sequencer (PE Biosystems), using fluorescently labeled dideoxy terminators.
Mutation Analysis
DNA was isolated from muscle and blood, as described by Ohno et al. (1995). We sequenced all exons of the AChR α-, β-, δ-, and ε-subunit genes, along with their flanking noncoding regions, as described elsewhere (Ohno et al. 1995). To sequence RAPSN, we used the genomic sequence of RAPSN (GenBank accession number AC074195) and synthesized five pairs of PCR primers to amplify and directly sequence eight exons and their flanking noncoding regions. After identifying mutations in RAPSN, we used allele-specific PCR to screen relatives of patients for the three observed mutations and to screen 400 normal alleles for the two missense mutations.
cDNA Cloning of Human RAPSN
We used nested RT-PCR to amplify the entire coding region of RAPSN from normal human muscle mRNA. The nested forward primer introduced the Kozak consensus sequence (5′-CCACC-3′; Kozak 1987) before the translational start site. The nested forward and reverse primers also carried the EcoRI- and BamHI-recognition sites, respectively, at their 5′ ends. Thus, the PCR product from the 5′ end included an EcoRI site; the Kozak consensus sequence; nucleotides 1–1239 of RAPSN, with the first nucleotide corresponding to the translational start site; and a BamHI site. The PCR product was then ligated into pEGFP-N1, a plasmid harboring the gene encoding enhanced green fluorescent protein (EGFP; Clontech), to attach EGFP to the C-terminal end of rapsyn (Ramarao and Cohen 1998).
The L14P and N88K missense mutations were introduced into pEGFP-RAPSN through use of the QuikChange Site Direct Mutagenesis Kit (Stratagene). Because direct introduction of 553ins5 into pEGFP-RAPSN abolishes EGFP translation, we eliminated a premature stop codon generated by 553ins5. We first PCR-amplified the wild-type RAPSN in pEGFP, from the Kozak consensus sequence to nucleotide 604. The forward and reverse primers carried the EcoRI- and BamHI-recognition sites, respectively, at their 5′ ends. After the PCR product was ligated into pEGFP-N1, 553ins5 was introduced using the QuikChange Site Direct Mutagenesis Kit. This construct encodes a truncated RAPSN (codons 1–184), 19 missense codons generated by 553ins5, and EGFP followed by a stop codon. For each construct, the presence of the introduced mutation and absence of unwanted artifacts was confirmed by sequencing of the entire insert.
Identification of Consensus cDNA Sequence of RAPSN
Compared to our clone, the published cDNA sequence (GenBank accession number Z33905; Buckel et al. 1996) harbors 13 discordant nucleotides in 12 codons, as well as 42 discordant nucleotides in its 5′ and 3′ noncoding regions. The substitutions are synonymous in eight codons and nonsynonymous in four. When 34 human alleles were analyzed, we found none that contained the discordant nucleotides in either their coding or noncoding regions. Also, the high-throughput genomic sequence of RAPSN, reported by the Human Genome Project (GenBank accession number AC074195), carries none of the discordant nucleotides in Z33905. Therefore, our clone likely has the human consensus sequence for RAPSN, and we posted it in the GenBank gene database (GenBank accession number AF449218).
Expression Studies in HEK Cells
Wild-type and mutant RAPSN-EGFP constructs were made, as described above. Human α- and δ-subunit cDNAs were a gift from J. Lindstrom (Schoepfer et al. 1988; Luther et al. 1989). The β- and ɛ-subunit cDNAs were cloned from normal human skeletal muscle (Ohno et al. 1996). All four cDNAs were subcloned into the CMV-based expression vector pRBG4 (Ohno et al. 1996).
HEK cells were maintained at 37°C in Dulbecco's minimal essential medium (DMEM) supplemented with 10% fetal bovine serum. Cells were grown on 22-mm cover slips coated with rat tail collagen type I (BD Biosciences), in six-well plates. Cells were transfected 48 h after plating, by the calcium phosphate method. Wild-type or mutant RAPSN-EGFP constructs (2.5 μg) were transfected with or without AChR-subunit cDNAs, at a ratio of 2:1:1:1 for α (2.5 μg), β, δ, and ɛ, respectively. Additional cells were transfected with AChR-subunit cDNAs only. To obtain optimal surface expression of rapsyn and AChR, the preparations were evaluated 30 h after transfection (Ramarao and Cohen 1998). Surface expression of AChR was visualized by incubation with 125 nM α-bgt labeled with rhodamine (Molecular Probes) in DMEM for 1 h at 37°C, followed by three rinses with DMEM and one with PBS over 30 min. Next, the cells were fixed with 2% paraformaldehyde in PBS for 20 minutes, rinsed with PBS, and then mounted under Vectashield (Vector Laboratories). Each transfection was repeated three or more times.
The preparations were analyzed with a Zeiss epifluorescence microscope, using a 100× Neofluar objective (numerical aperture [NA] 1.3), with FITC optics employed to visualize rapsyn-EGFP and rhodamine optics employed to visualize AChR. After transfections with wild-type or mutant rapsyn, each cover slip was systematically traversed until 100 cells expressing rapsyn, distributed either diffusely or in clusters, were encountered, and the proportion of cells displaying rapsyn in clusters was determined. After cotransfection of wild-type or mutant rapsyn along with AChR, each cover slip was systematically traversed until 100 cells expressing clustered rapsyn and diffusely distributed or clustered AChR were encountered, and the proportion of cells in which the receptor coclustered with rapsyn was determined. After transfection with AChR only, we examined 100 AChR-expressing cells and determined the number of cells expressing AChR diffusely or in clusters. Images for publication were obtained with a Zeiss LSD confocal microscope (green fluorescence: 488 nm excitation, 505–550 nm emission; red fluorescence: 543 nm excitation, >560 nm emission), using a 100× Planapochromat objective (NA 1.4).
Results
EP Studies
Histochemical studies of intercostal muscle specimens revealed a type 1–fiber preponderance in each patient. On fluorescence microscopy, AChE (fig. 2A and C) and VAChT (fig. 2E and G) were preserved, but AChR (fig. 2D) and rapsyn (fig. 2H) were markedly attenuated at all patient EPs. These findings do not establish whether deficiency of rapsyn or of AChR is the primary abnormality, for rapsyn expression is also reduced when EP AChR deficiency stems from low-expressor mutations in AChR-subunit genes (A.G.E., unpublished data).
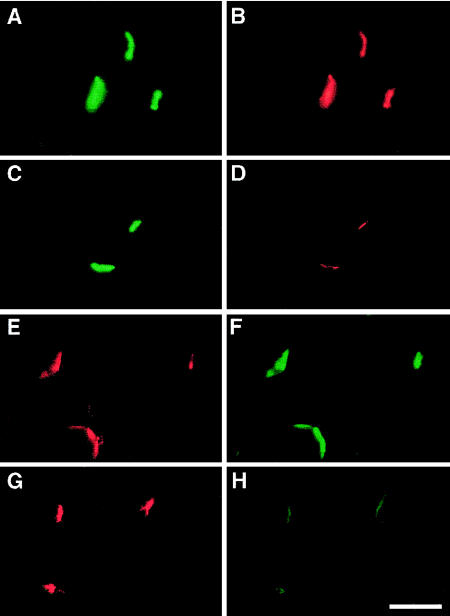
Figure 2.
Two-color fluorescence localization at EPs of AChE (A and C) with AChR (B and D) and of VAChT (E and G) with rapsyn (F and H), in a control subject (A, B, E, and F) and in Pt 1 (C, D, G, and H). Note the marked attenuation of the signal for AChR (D) and rapsyn (H) at the patient EP. Bar = 50 μm.
The configuration of the EPs, evaluated from the cytochemical reaction product for AChE on longitudinally oriented teased single muscle fibers, was abnormal, with an increased number of small EP regions (2–7 in Pt 1, 1–3 in Pt 2, and as many as 12 in Pts 3 and 4) distributed over an increased span of the muscle-fiber surface (fig. 3A and B).
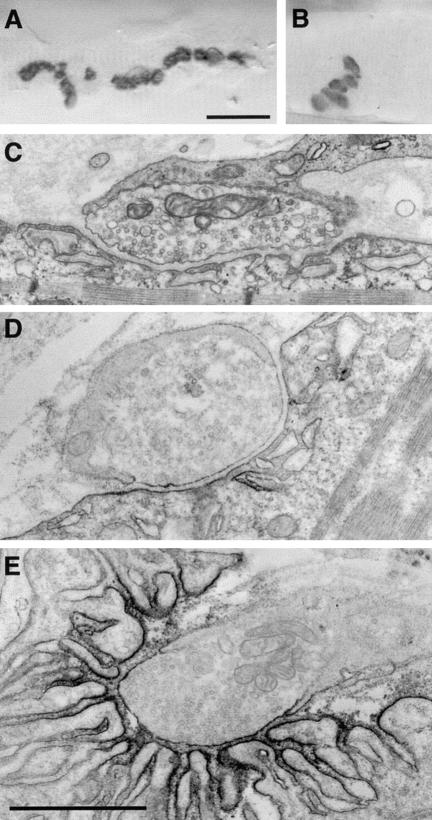
Figure 3.
Cholinesterase-reactive EP region in Pt 3 (A) and in a control subject (B). Note the dispersion of EP regions over an extended length of the muscle fiber in the patient. C, Typical EP regions in Pt 4. The postsynaptic area of folds and clefts is shallow and displays few secondary clefts. D and E, Ultrastructural localization of AChR with peroxidase-labeled α-bgt at an EP from Pt 3 (D) and at a control EP (E). At the patient EP, the reaction for AChR is attenuated and the length of the postsynaptic membrane reacting for AChR is reduced. Bar in A (which also applies to B) = 25 μm; bar in E (which also applies to C and D) = 1 μm.
On electron microscopy, the structural integrity of the nerve terminals and junctional folds was preserved, but the postsynaptic area of clefts and folds was only 17%–26% of normal, and the frequency of secondary clefts per unit length of the primary synaptic cleft was only 14%–36% of normal (table 1; fig. 3C and D), indicating impaired morphological development of the postsynaptic region. Ultrastructural localization of AChR with peroxidase-labeled α-bgt revealed a marked decrease in the density and distribution of AChR on the postsynaptic membrane (fig. 3D and E). The AChR index (defined as the length of the postsynaptic membrane reacting for AChR, normalized for the length of the primary synaptic cleft) was reduced to 35%–42% of normal (table 1). The structural abnormalities at the EPs were similar to those found in patients with low-expressor mutations in AChR-subunit genes (Engel et al. 1996; Ohno et al. 1997; Quiram et al. 1999).
Table 1.
Morphometric Analysis of EP Regions
|
Mean±Standard Errora |
||||
| Patientor Sample | Nerve Terminal Area(μm2) | Postsynaptic Area(μm2) | No. of Secondary Clefts perMicrometer of Primary Cleft | AChR Index |
| Pt 1 | 2.24±.21 (n = 39) | 2.24±.21 (n = 39) | .57±.078 (n = 39) | 1.12±.14 (n = 14) |
| Pt 2 | 3.04±.35 (n = 31) | 2.68±.20 (n = 31) | .48±.073 (n = 31) | 1.04±.080 (n = 37) |
| Pt 3 | 3.16±.46 (n = 27) | 2.80±.30 (n = 27) | .38±.069 (n = 27) | 1.27±.10 (n = 30) |
| Pt 4 | 2.57±.38 (n = 39) | 1.84±.13 (n = 39) | .22±.043 (n = 39) | NDb |
| Controls | 3.88±.39 (n = 63) | 10.60±.79 (n = 54) | 1.58±.056 (n = 88) | 3.01±.11 (n = 85) |
n represents the number of EP regions. More than one EP region can occur at one EP.
ND = not determined.
The numbers of AChRs per EP were 34%, 8%, 44%, and 48% of the control values (table 2). In Pts 1, 3, and 4, this decrease was less marked than suggested by visualization of AChR with rhodamine- and peroxidase-labeled α-bgt. A possible explanation for this discrepancy is that the increase in EP regions—and, hence, in the postsynaptic surface per EP—is greater in Pts 1, 3, and 4 than in Pt 2 (Engel 1993). Alternatively, unclustered AChR may go undetected on staining with rhodamine- or peroxidase-labeled α-bgt but is counted with 125I-α-bgt.
Table 2.
α-bgt Binding Sites per EP and Microelectrode Studies of Neuromuscular Transmission
|
Mean±Standard Errora |
|||||
| Patientor Sample | No. of [125I]α-bgtBinding Sites per EP(×106) | EPP QuantalContentb | MEPP Amplitudec(mV) | MEPC Amplituded(nA) | Burst Open Duratione(ms) |
| Pt 1 | 1.6 | 19±2 (n = 19) | .13±.024 (n = 17) | .81±.035 (n = 31) | 2.46±.31 (n = 7) |
| Pt 2 | .59 | 25±10 (n = 14) | .12±.0090 (n = 14) | 1.29±.068 (n = 22) | 2.50±.33 (n = 5) |
| Pt 3 | 5.7 | 60±7 (n = 23) | .47±.033 (n = 13) | 1.83±.15 (n = 10) | 3.21±.21 (n = 3) |
| Pt 4 | 6.1 | 39±8 (n = 11) | .41±.047 (n = 8) | 1.32±.098 (n = 14) | 2.68±.19 (n = 5) |
| Controls | 12.82±.79; 4.7f | 31±1; 18±1.5g | 1.00±.025 (n = 164) | 3.95±.10 (n = 79) | 2.99±.26 (n = 7) |
n represents the number of EPs. Temperatures were 29±.5°C for EPP and MEPP recordings and 22±.5°C for MEPC studies and patch-clamp studies.
Quantal content of EPP at 1-Hz stimulation, corrected for a resting membrane potential of −80 mV, nonlinear summation, and non-Poisson release.
Corrected for a resting membrane potential of −80 mV and a mean muscle-fiber diameter of 55 mm.
Membrane potential = −80 mV.
ACh = 1 μM for control individuals and 1–5 μM for patients; bandwidth = 12 kHz for control individuals and 10–12 kHz for patients; membrane potential = −80mV.
The first value is for 13 adults, and the second value is for one individual of age 3 years.
The first value is for 190 adult EPs, and the second value is for 18 EPs in one individual of age 3 years.
On electrophysiological studies, quantal release by nerve impulse was higher than normal in Pts 3 and 4, and in Pts 1 and 2 it was comparable to that in a control subject of age 3 years. Consistent with EP AChR deficiency, the MEPP amplitudes were reduced to 13%, 12%, 47%, and 33% of normal, and the MEPC amplitudes were reduced to 25%, 32%, 46%, and 33% of normal. Single-channel patch-clamp recordings obtained from each patient’s EPs showed that the AChR channels opened to a normal conductance of ∼60 picoSiemens and that the duration of the predominant component of channel-opening bursts was not significantly different from normal (table 2).
To summarize, EP studies demonstrate (1) AChR and rapsyn deficiency and (2) impaired morphological development of the postsynaptic region but (3) no decrease of ACh release by nerve impulse or kinetic abnormality of the AChR channel.
Mutation Analysis
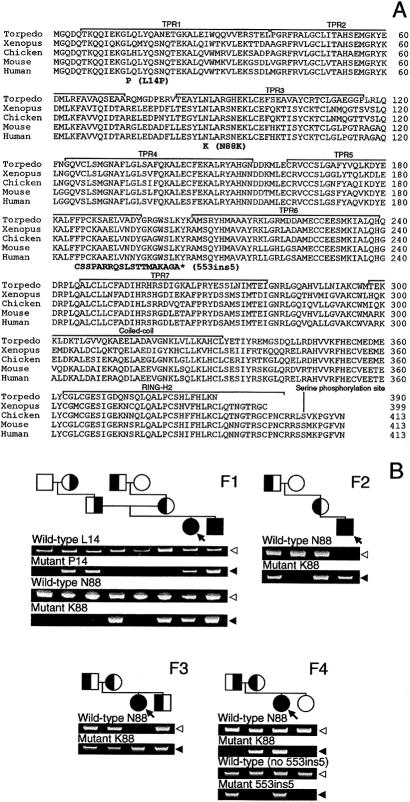
Because we detected no mutations on direct sequencing of AChR α-, β-, δ-, and ɛ-subunit genes, we next directly sequenced the eight exons of RAPSN with their flanking untranslated regions and identified three mutations in four patients (table 3). Pts 1 and 4 are heterozygous for two mutations, whereas Pts 2 and 3 are homozygous for a single mutation. Analysis of family members revealed that the two mutations in Pts 1 and 4 are heteroallelic and that all three mutations are recessive (fig. 4B). Neither L14P nor N88K was detected in 400 normal alleles. Both leucine at codon 14 and asparagine at codon 88 are conserved in Torpedo californica (Frail et al. 1987), Xenopus (Frail et al. 1987), chicken (Burns et al. 1996), and mouse (Frail et al. 1988; Froehner 1989) (fig. 4A).
Table 3.
Identified RAPSN Mutations
| Patient andNucleotide Change | Amino AcidChange | Domain |
| 1: | ||
| 41T→C | L14P | TPR1 |
| 264C→A | N88K | TPR3 |
| 2: | ||
| 264C→A | N88Ka | TPR3 |
| 3: | ||
| 264C→A | N88Ka | TPR3 |
| 4: | ||
| 264C→A | N88K | TPR3 |
| 553ins5 | Frameshift | TPR5 |
Homozygous mutation.
Figure 4.
A, Alignment of amino acid sequences of Torpedo californica, Xenopus, chicken, mouse, and human rapsyn. Identified mutations and functional domains are indicated. The 553ins5 mutation predicts 19 missense codons (boldface), followed by a stop codon (indicated by an asterisk [*]). The far-right column indicates amino acid numbers. B, Allele-specific PCR of family members. Affected members (shaded symbols) carry two mutant alleles. Unaffected members harbor no (unshaded symbols) or one (half-shaded symbols) mutant allele, indicating that each mutation is heteroallelic and recessive. Arrows indicate propositi. Unshaded arrowheads indicate normal fragments; shaded arrowheads point to mutant fragments. “F1”–“F4” indicate families 1–4.
Mutation analysis also revealed four polymorphisms in the RAPSN coding region: 172C/T, predicting R58C, and synonymous polymorphisms 456T/C, 855G/A, and 1143T/C; these four polymorphisms had respective allelic frequencies of 19/192, 25/34, 4/34, and 25/34. The 456T/C and 1143T/C polymorphisms are linked in all individuals.
Expression Studies in HEK Cells
To determine the mechanism by which the observed rapsyn mutations result in EP AChR deficiency, we engineered wild-type and mutant rapsyn-EGFP with or without AChR subunits, or AChR subunits alone, into HEK cells.
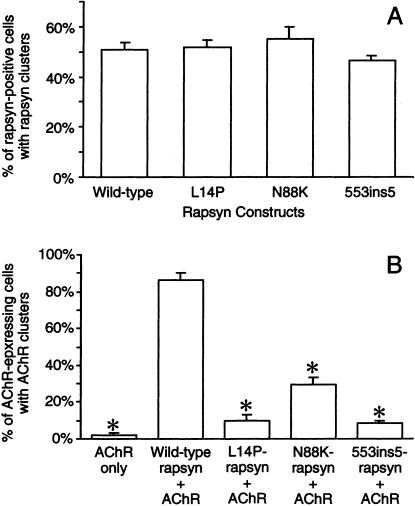
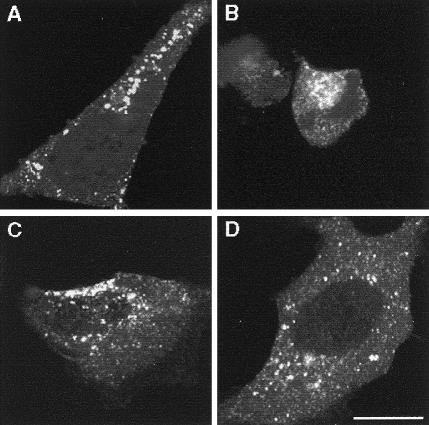
After transfection with the wild-type rapsyn-EGFP, 51% of rapsyn-expressing cells displayed <1- to ∼3-μm rapsyn granules (figs. 5A and 6A). After transfection with L14P-, N88K-, and 553ins5-rapsyn-EGFP, a similar proportion of rapsyn-positive cells displayed rapsyn granules (figs. 5A and 6B–D). Thus, none of the mutations hinders rapsyn self-aggregation in HEK cells.
Figure 5.
Expression studies in HEK cells. A, Percentage of rapsyn-positive cells with rapsyn clusters. B, Percentage of AChR-expressing cells with AChR clusters. The first bar indicates the percentage of AChR-positive cells with AChR clusters after transfection with AChR only; the next four bars indicate percentage of rapsyn- and AChR-positive cells in which AChR coclusters with wild-type or mutant rapsyns. Results are shown as mean±SD. Asterisks (*) indicate P<.001.
Figure 6.
Expression of (A) wild-type, (B) L14P-, (C) N88K-, and (D) 553ins5-rapsyn-EGFP, in HEK cells. Abundant rapsyn clusters appear in cells expressing wild-type or mutant rapsyns. Bar = 20 μm.
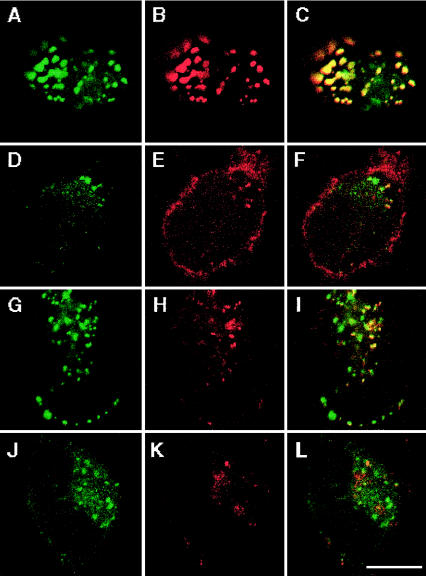
After transfection of HEK cells with AChR but without rapsyn, only 2% of cells expressing surface AChR displayed sparse AChR granules. After cotransfection of AChR with wild-type rapsyn-EGFP, AChR coclustered with rapsyn in 87% of cells that displayed rapsyn granules (figs. 5B and 7A–C). After transfection of HEK cells with AChR plus L14P-, N88K-, and 553ins5-rapsyn-EGFP, AChR coclustered with rapsyn in only 10%, 30%, and 8%, respectively, of cells with rapsyn granules (figs. 5B and 7D–F, G–I, and J–L). In those cells in which AChR did cocluster with mutant rapsyn, only a few of the rapsyn-positive granules were also AChR-positive (see fig. 7F, I, and L). Thus, although L14P, N88K, and 553ins5 do not affect self-aggregation of rapsyn-EGFP in HEK cells, each mutation significantly compromises recruitment of AChR to rapsyn clusters.
Figure 7.
Distribution of rapsyn (green signal, A–J), surface AChR (red signal, B–K), and superimposed images (C–L), in cells cotransfected with AChR and wild-type rapsyn (A–C), L14P-rapsyn (D–F), N88K-rapsyn (G–I), and 553ins5-rapsyn (J–L). The imaged cells were selected to show coclustering of AChR with rapsyn; however, whereas AChR coclustered with 87% of cells expressing both AChR and wild-type rapsyn, AChR coclustered with L14P-, N88K-, and 553ins5-rapsyn in only 10±2.8%, 30±3.4%, and 8±1.7% of cells, respectively, and, in these cells, only a few rapsyn-positive granules were also AChR-positive (yellow granules in F, I, and L). Bar = 10 μm.
Discussion
We here identify a subset of patients with CMS who have EP AChR deficiency caused not by mutations in an AChR-subunit gene but by mutations in rapsyn. It is important to note, however, that other EP-specific proteins involved in the regulation of AChR expression or in aggregation at the EP could also cause EP AChR deficiency. These proteins include neural agrin (Gautam et al. 1996; Burgess et al. 1999); MuSK (Glass et al. 1996); the predicted protein MASC, which enables agrin to bind to MuSK (Glass et al. 1996); the predicted protein RATL, which functionally couples MuSK to rapsyn (Apel et al. 1997); Src, Fyn, and Yes kinases signaling downstream from MuSK (Smith et al. 2001); β-dystroglycan (Cartaud et al. 1998); S-NRAP (Tseng et al. 2001); neuregulin and its signaling molecules (Si et al. 1996; Altiok et al. 1997; Sandrock et al. 1997); neurotrophin-4 (Belluardo et al. 2001) and its receptor, TrkB (Gonzales et al. 1999); as well as α-dystrobrevin (Newey et al. 2001), utrophin (Grady et al. 1997), and α-syntrophin (Adams et al. 2000). No mutations in these molecules in humans have been detected to date.
Phenotype Effects
In each patient, the safety margin of neuromuscular transmission is compromised by the EP AChR deficiency, which is secondary to the rapsyn deficiency. The consequences of EP AChR deficiency are abetted by the incompletely developed postsynaptic region, which is nearly devoid of junctional folds. The junctional folds increase the series resistance of the postsynaptic membrane (Martin 1998), and their troughs are enriched in Na+ channels, factors that augment the synaptic response to ACh (Wood and Slater 1997; Ruff 1998). Absence of junctional folds from the EP is estimated to result in a two- to fourfold decrease in the safety factor of neuromuscular transmission (Wood and Slater 1997).
We discerned no clear phenotype-genotype correlation in our patients. Pts 2 and 3 are homozygous for the same N88K mutation, but Pt 2 has severe myasthenic symptoms at age 2 years, whereas Pt 3 has only mild weakness at age 27 years. Pt 1, who is heterozygous for N88K and L14P, is as severely affected as Pt 2, and Pt 4, who harbors N88K and 553ins5 and was born with arthrogryposis, has only mild weakness at age 11 years. The variable phenotypic expressivity of the mutations may be caused by single-nucleotide polymorphisms in functionally related genes that govern (1) quantal release by nerve impulse, which was increased in Pts 3 and 4; (2) development of multiple small EP regions, which was most pronounced in Pts 3 and 4; or (3) other factors.
Structural Consequences of the Mutations
The 553ins5 mutation significantly decreases coclustering of rapsyn with AChR in HEK cells. This mutation causes a frameshift in TPR5 and thus prevents expression of TPRs 6 and 7 and of the coiled-coil and RING-H2 domains. Since the coiled-coil domain interacts with AChR (Ramarao et al. 2001), and since the RING-H2 domains interact with S-NRAP (Tseng et al. 2001) and β-dystroglycan (Bartoli et al. 2001), 553ins5 is predicted to abrogate the coclustering of AChR, dystroglycan, and S-NRAP with rapsyn.
L14P and N88K also decrease coclustering of rapsyn with AChR in HEK cells, which implies that the effects of L14P in TPR1 and of N88K in TPR3 propagate downstream to the coiled-coil domain. Possible explanations could be that L14P and N88K cause abnormal folding of rapsyn, hindering association with its binding partners, or that the mutations have an allosteric effect on the conformation of the coiled-coil and RING-H2 domains. One can also assume that similar mechanism(s) operate in a recently reported animal model of rapsyn deficiency caused by a point mutation in a TPR domain of the zebrafish, Danio rerio. These fish show reduced expression of AChR at the EP and have a myasthenic phenotype (Ono et al. 2001).
Rapsyn and AChR Expression at the EPs
Decreased recruitment of AChR to rapsyn clusters, as well as decreased rapsyn expression at the EPs, are likely to account for the EP AChR deficiency. A number of factors likely contribute to the reduced expression of rapsyn at the EPs. (1) Low-expressor mutations in AChR-subunit genes result in decreased rapsyn expression at the EPs. This implies that rapsyn clusters are stabilized by association with AChR. Conversely, a decreased association of the mutant rapsyns with AChR could result in EP rapsyn deficiency. (2) Accelerated degradation of abnormally folded nascent mutant peptides in the endoplasmic reticulum (Mori 2000) could also contribute to the rapsyn deficiency. (3) The expression of the 553ins5-rapsyn could also be diminished by nonsense-mediated mRNA decay (Frischmeyer and Dietz 1999) and by loss of anchorage to the subsynaptic cytoskeleton, due to absence of the RING-H2 domain.
Acknowledgments
This work was supported by the National Institutes of Health grant NS6277 and by an Muscular Dystrophy Association research grant to A.G.E. We thank Drs. Pierre Jacob, Robert T. Stone, Pierre R. Bourque, and Elizabeth C. Dooling, for patient referral.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for RAPSN genomic sequence [accession number AC074195], RAPSN cDNA sequence [accession numbers Z33905 and AF449218], and the high-throughput genomic sequence of RAPSN [accession number AC074195])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CHAT [MIM 118490], COLQ [MIM 603033], CHRNE [MIM 100725], and RAPSN [MIM 601592])
References
- Adams ME, Kramarcy M, Krall SP, Rossi SG, Rotundo RL, Sealock R, Froehner SC (2000) Absence of α-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J Cell Biol 150:1385–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altiok N, Altiok K, Changeux J-P (1997) Heregulin-stimulated acetylcholine receptor gene expression in muscle: requirement for MAP kinase and evidence for parallel inhibitory pathway independent electrical activity. EMBO J 16:717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel ED, Glass DJ, Moscosco LM, Yancopoulos GD, Sanes JR (1997) Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron 18:623–625 [DOI] [PubMed] [Google Scholar]
- Apel ED, Roberds SL, Campbell KP, Merlie JP (1995) Rapsyn may function as a link between the acetylcholine receptor and the agrin-binding dystrophin-associated glycoprotein complex. Neuron 15:115–126 [DOI] [PubMed] [Google Scholar]
- Bartoli M, Cohen JB (2001) Identification of the modular domains of rapsyn binding to nicotinic acetylcholine receptor (AChR) and to dystroglycan. Abstr Soc Neurosci 27:904.16 [Google Scholar]
- Bartoli M, Ramarao MK, Cohen JB (2001) Interactions of the rapsyn RING-H2 domain with dystroglycan. J Biol Chem 276:24911–24917 [DOI] [PubMed] [Google Scholar]
- Belluardo N, Westerblad H, Mudo G, Casabona A, Bruton J, Caniglia G, Pastoris O, Grassi F, Ibanez CF (2001) Neuromuscular junction disassembly and muscle fatigue in mice lacking neurotrophin-4. Mol Cell Neurosci 18:56–67 [DOI] [PubMed] [Google Scholar]
- Buckel A, Beeson D, James M, Vincent A (1996) Cloning of the cDNA encoding human rapsyn and mapping the RAPSN gene locus to chromosome 11p11.2-p11.1. Genomics 35:613–616 [DOI] [PubMed] [Google Scholar]
- Burgess RW, Nguyen QT, Son YJ, Lichtman JW, Sanes JR (1999) Alternatively spliced isoforms of nerve- and muscle-derived agrin: their roles at the neuromuscular junction. Neuron 23:33–44 [DOI] [PubMed] [Google Scholar]
- Burns AL, Benson D, Howard MJ, Margiotta JF (1997) Chick ciliary ganglion neurons contain transcripts coding for the acetylcholine receptor-associated protein at synapses (rapsyn). J Neurosci 17:5016–5026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartaud A, Coutant S, Petrucci TC, Cartaud J (1998) Evidence for in situ and in vitro association between β-dystroglycan and the subsynaptic 43K rapsyn protein. Consequence for acetylcholine receptor clustering at the synapse. J Biol Chem 273:11321–11326 [DOI] [PubMed] [Google Scholar]
- Donger C, Krejci E, Serradell P, Eymard B, Bon S, Nicole S, Chateau D, Gary F, Fardeau M, Massoulié J, Guicheney P (1998) Mutation in the human acetylcholinesterase-associated gene, COLQ, is responsible for congenital myasthenic syndrome with end-plate acetylcholinesterase deficiency. Am J Hum Genet 63:967–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AG (1993) The investigation of congenital myasthenic syndromes. Ann NY Acad Sci 681:425–434 [DOI] [PubMed] [Google Scholar]
- ——— (1994a) The muscle biopsy. In: Engel AG, Franzini-Armstrong C (eds) Myology. McGraw-Hill, New York, pp 822–831 [Google Scholar]
- ——— (1994b) Quantitative morphological studies of muscle. In: Engel AG, Franzini-Armstrong C (eds) Myology. McGraw-Hill, New York, pp 1018–1045 [Google Scholar]
- Engel AG, Lindstrom JM, Lambert EH, Lennon VA (1977) Ultrastructural localization of the acetylcholine receptor in myasthenia gravis and in its experimental autoimmune model. Neurology 27:307–315 [DOI] [PubMed] [Google Scholar]
- Engel AG, Nagel A, Walls TJ, Harper CM, Waisburg HA (1993) Congenital myasthenic syndromes. I. Deficiency and short open-time of the acetylcholine receptor. Muscle Nerve 16:1284–1292 [DOI] [PubMed] [Google Scholar]
- Engel AG, Ohno K, Bouzat C, Sine SM, Griggs RG (1996) End-plate acetylcholine receptor deficiency due to nonsense mutations in the ε subunit. Ann Neurol 40:810–817 [DOI] [PubMed] [Google Scholar]
- Engel AG, Ohno K, Sine SM. (1999) Congenital myasthenic syndromes. In: Engel AG (ed) Myasthenia gravis and myasthenic disorders. Oxford University Press, New York, pp 251–297 [Google Scholar]
- Fambrough DM, Engel AG, Rosenberry TL (1982) Acetylcholinesterase of human erythrocytes and neuromuscular junctions: homologies revealed by monoclonal antibodies. Proc Natl Acad Sci USA 79:1078–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frail DE, McLaughlin LL, Mudd J, Merlie JP (1988) Identification of the mouse muscle 43,000-dalton acetylcholine receptor-associated protein (RAPsyn) by cDNA cloning. J Biol Chem 263:15602–15607 [PubMed] [Google Scholar]
- Frail DE, Mudd J, Shah V, Carr C, Cohen JB , Merlie JP (1987) cDNAs for the postsynaptic 43-kDa protein of Torpedo electric organ encode two proteins with different carboxyl termini. Proc Natl Acad Sci USA 84:6302–6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischmeyer PA, Dietz HC (1999) Nonsense-mediated mRNA decay in health and disease. Hum Mol Genet 8:1893–1900 [DOI] [PubMed] [Google Scholar]
- Froehner SC (1989) Expression of RNA transcripts for the postsynaptic 43 kDa protein in denervated rat skeletal muscle. FEBS Lett 249:229–233 [DOI] [PubMed] [Google Scholar]
- Froehner SC, Luetje CW, Scotland PB, Patrick J (1990) The postsynaptic 43K protein clusters muscle nicotinic acetylcholine receptors in Xenopus oocytes. Neuron 5:403–410 [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie MP, Sanes JR (1996) Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 85:525–535 [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Mudd J, Nichol M, Chu GC, Sanes JR, Merlie JP (1995) Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature 377:232–236 [DOI] [PubMed] [Google Scholar]
- Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah H, Mattson K, Burden SJ, Distefano PS, Valenzuela DM, Dechiara TM, Yancopoulos GD (1996) Agrin acts via MuSK receptor complex. Cell 85:513–523 [DOI] [PubMed] [Google Scholar]
- Gonzales M, Ruggiero FP, Chang Q, Shi YJ, Rich MM, Kraner S, Balice-Gordon RJ (1999) Disruption of Trkb-mediated signaling induces disassembly of potsynaptic receptor clusters at neuromuscular junctions. Neuron 24:567–583 [DOI] [PubMed] [Google Scholar]
- Grady RM, Merlie JP, Sanes JR (1997) Subtle neuromuscular defects in utrophin-deficient mice. J Cell Biol 136:871–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. (1987) An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res 15:8125–8148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther MA, Schoepfer R, Whiting P, Casey B, Blatt Y, Montal MS, Montal M, Lindstrom J (1989) A muscle acetylcholine receptor is expressed in the human cerebellar medulloblastoma cell line TE671. J Neurosci 9:1082–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimone MM, Merlie JP (1993) Interaction of the 43 kd postsynaptic protein with all subunits of the muscle nicotinic acetylcholine receptor. Neuron 11:53–66 [DOI] [PubMed] [Google Scholar]
- ——— (1999) The intracellular domain of the nicotinic acetylcholine receptor a subunit mediates its coclustering with rapsyn. Mol Cell Neurosci 14:340–354 [DOI] [PubMed] [Google Scholar]
- Martin AR (1994) Amplification of neuromuscular transmission by postjunctional folds. Proc R Soc Lond B Biol Sci 258:321–326 [DOI] [PubMed] [Google Scholar]
- Milone M, Wang H-L, Ohno K, Fukudome T, Pruitt JN, Bren N, Sine SM, Engel AG (1997) Slow-channel syndrome caused by enhanced activation, desensitization, and agonist binding affinity due to mutation in the M2 domain of the acetylcholine receptor alpha subunit. J Neurosci 17:5651–5665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milone M, Wang H-L, Ohno K, Prince RJ, Shen X-M, Brengman JM, Griggs RC, Engel AG (1998) Mode switching kinetics produced by a naturally occurring mutation in the cytoplasmic loop of the human acetylcholine receptor ε subunit. Neuron 20:575–588 [DOI] [PubMed] [Google Scholar]
- Mori K (2000) Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell 101:451–454 [DOI] [PubMed] [Google Scholar]
- Newey SA, Gramolini AO, Wu J, lzfeind G, Smin BJ, Vies KE, Ake DJ (2001) A novel mechanism for modulating synaptic gene expression: differential localization of α-dystrobrevin transcripts in skeletal muscle. Mol Cell Neurosci 17:127–140 [DOI] [PubMed] [Google Scholar]
- Ohno K, Brengman JM, Tsujino A, Engel AG (1998) Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme. Proc Natl Acad Sci USA 95:9654–9659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K, Hutchinson DO, Milone M, Brengman JM, Bouzat C, Sine SM, Engel AG (1995) Congenital myasthenic syndrome caused by prolonged acetylcholine receptor channel openings due to a mutation in the M2 domain of the ε subunit. Proc Natl Acad Sci USA 92:758–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K, Quiram P, Milone M, Wang H-L, Harper CM, Pruitt JN, Brengman JM, Pao L, Fischbeck KH, Crawford TO, Sine SM, Engel AG (1997) Congenital myasthenic syndromes due to heteroallelic nonsense/missense mutations in the acetylcholine receptor ε subunit gene: identification and functional characterization of six new mutations. Hum Mol Genet 6:753–766 [DOI] [PubMed] [Google Scholar]
- Ohno K, Tsujino A, Brengman JM, Harper CM, Bajzer Z, Udd B, Beyring R, Robb S, Kirkham FJ, Engel AG (2001) Choline acetyltransferase mutations cause myasthenic syndrome associated with episodic apnea in humans. Proc Natl Acad Sci USA 98:2017–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K, Wang H-L, Milone M, Bren N, Brengman JM, Nakano S, Quiram P, Pruitt JN, Sine SM, Engel AG (1996) Congenital myasthenic syndrome caused by decreased agonist binding affinity due to a mutation in the acetylcholine receptor ε subunit. Neuron 17:157–170 [DOI] [PubMed] [Google Scholar]
- Ono F, Shcherbatko A, Mandel A, Brehm P (2001) A mutation in zebrafish rapsyn disrupts ACh receptor clustering and promotes synaptic depression. Abstr Soc Neurosci 27:918.1 [Google Scholar]
- Phillips WD, Kopta C, Blount P, Gardner PD, Steinbach JH, Merlie JP (1991) ACh-rich receptor membrane domains organized in fibroblasts by recombinant 43-kilodalton protein. Science 251:568–570 [DOI] [PubMed] [Google Scholar]
- Quiram P, Ohno K, Milone M, Patterson MC, Pruitt NJ, Brengman JM, Sine SM, Engel AG (1999) Mutation causing congenital myasthenia reveals acetylcholine receptor β/δ subunit interaction essential for assembly. J Clin Invest 104:1403–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramarao MK, Bianchetta MJ, Lanken J, Cohen JB (2001) Role of rapsyn tetratrichopeptide repeat and coiled-coil domains in self-association and nicotinic acetylcholine receptor clustering. J Biol Chem 276:7475–7483 [DOI] [PubMed] [Google Scholar]
- Ramarao MK, Cohen JB (1998) Mechanism of nicotinic acetylcholine receptor cluster formation by rapsyn. Proc Natl Acad Sci USA 95:4007–4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RL (1996) Effects of length changes on Na+ current amplitude and excitability near and far from the end-plate. Muscle Nerve 19:1084–1092 [DOI] [PubMed] [Google Scholar]
- Sandrock AW, Dryer SE, Rosen KM, Gozani SM, Kramer R, Theill LE, Fischbach GD (1997) Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction in vivo. Science 276:599–603 [DOI] [PubMed] [Google Scholar]
- Schoepfer R, Luther M, Lindstrom J (1988) The human medulloblastoma cell line TE671 expresses a muscle-like acetylcholine receptor. Cloning of the alpha-subunit cDNA. FEBS Lett 226:235–240 [DOI] [PubMed] [Google Scholar]
- Si J, Luo Z, Mei L (1996) Induction of acetylcholine receptor gene expression by ARIA requires activation of mitogen-activated protein kinase. J Biol Chem 271:19752–19759 [DOI] [PubMed] [Google Scholar]
- Sine SM, Ohno K, Bouzat C, Auerbach A, Milone M, Pruitt JN, Engel AG (1995) Mutation of the acetylcholine receptor a subunit causes a slow-channel myasthenic syndrome by enhancing agonist binding affinity. Neuron 15:229–239 [DOI] [PubMed] [Google Scholar]
- Smith CL, Mittaud P, Prescott ED, Fuhrer C, Burden SJ (2001) Src, Fyn, and Yes are not required for neuromuscular synapse formation but are necessary for stabilization of agrin-induced clusters of acetylcholine receptors. J Neurosci 21:3151–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CN, Yao Y, Wang JM, Viroonchatapan N, Rothe E, Wang ZZ (2001) A synaptic isoform of NRAP interacts with the postsynaptic 43K protein rapsyn and links it to the cytoskeleton at the neuromuscular junction. Abstr Soc Neurosci 27:694.6 [Google Scholar]
- Uchitel O, Engel AG, Walls TJ, Nagel A, Atassi ZM, Bril V (1993) Congenital myasthenic syndromes. II. A syndrome attributed to abnormal interaction of acetylcholine with its receptor. Muscle Nerve 16:1293–1301 [DOI] [PubMed] [Google Scholar]
- Wood SJ, Slater CR (1997) The contribution of the postsynaptic folds to the safety factor for neuromuscular transmission in rat fast- and slow-twitch fibers. J Physiol 500:165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]