Abstract
Several lines of evidence suggest that cognitive control deficits may be regarded as a connecting link between reported impairments in different cognitive domains of schizophrenia. However, the precise interplay within the fronto-cingulo-thalamic network known to be involved in cognitive control processes and its structural correlates has only been sparsely investigated in schizophrenia. The present multimodal study was therefore designed to model cognitive control processes within the fronto-cingulo-thalamic network. A disruption in effective connectivity in patients in association with abnormal white matter (WM) structure in this network was hypothesized. 36 patients with schizophrenia and 36 healthy subjects participated in the present study. Using functional magnetic resonance imaging (fMRI) a Stroop task was applied in an event-related design. For modeling effective connectivity dynamic causal modeling (DCM) was used. Voxel-based morphometry (VBM) was employed to study WM abnormalities. In the fMRI analysis, the patients demonstrated a significantly decreased BOLD signal in the fronto-cingulo-thalamic network. In the DCM analysis, a significantly decreased bilateral endogenous connectivity between the mediodorsal thalamus (MD) and the anterior cingulate cortex (ACC) was detected in patients in comparison to healthy controls, which was negatively correlated with the Stroop interference score. Furthermore, an increased endogenous connectivity between the right DLPFC and the right MD was observed in the patients. WM volume decreases were observed in the patients in the MD and the frontal cortex. The present results provide strong evidence for the notion that an abnormal fronto-cingulo-thalamic effective connectivity may represent the basis of cognitive control deficits in schizophrenia. Moreover, the data indicate that disrupted white matter connectivity in the mediodorsal thalamus and in the fronto-cingulo-thalamic network may constitute the determining cause of fronto-cingulo-thalamic dysconnectivity.
Keywords: Schizophrenia, FMRI, Cognitive control, Voxel-based morphometry, Thalamus
Highlights
-
•
Decreased BOLD signal in the fronto-thalamic network in the Stroop task in patients
-
•
Decreased endogenous connectivity between thalamus and the ACC in patients
-
•
Decreased WM volume in the thalamus and the frontal cortex in patients
-
•
Disrupted WM connectivity as potential cause of the fronto-thalamic dysconnectivity
1. Introduction
Schizophrenia is a serious debilitating disorder, with the cognitive dysfunction representing a core feature of this disease. Cognitive deficits may have strong impact on activities of daily living and they have been associated with poor clinical outcome (Green, 1996).
Neuropsychological studies reported a dysfunction in various cognitive domains like e.g. working memory (Glahn et al., 2003) or selective attention (Nuechterlein and Dawson, 1984). It was recently hypothesized that cognitive control deficits in schizophrenia may be regarded as a connecting link between reported impairments in different cognitive domains (Lesh et al., 2011). Defining “cognitive control” as an ability to maintain context information in the presence of interference and flexibly adapt behavior to reach the stated goal, it represents an integral part of a wide range of cognitive processes.
Neuroimaging studies in healthy subjects emphasized a pivotal role of the fronto-cingulate network in cognitive control functions (Mansouri et al., 2009). In particular, the dorsolateral prefrontal cortex (DLPFC) was associated with maintenance of context information (e.g. rules) and response selection (Egner and Hirsch, 2005), whereas the anterior cingulate cortex (ACC) was related to conflict detection, error and performance monitoring in order to signal need for behavioral adjustment (Kerns et al., 2004).
In a meta-analysis comprising 41 neuroimaging studies of the executive function (e.g. using Stroop task) in patients with schizophrenia, Minzenberg et al. (2009) reported a consistent pattern of reduced activation in bilateral DLPFC, ACC, and mediodorsal thalamus (MD). This result supports the notion that the brain network relevant for cognitive control is altered in schizophrenia. Furthermore, there is growing evidence, that apart from the fronto-cingulate regions the cerebellar (Schmahmann, 2010) and thalamic (Saalmann and Kastner, 2011) areas, commonly referred to as cortico–cerebellar–thalamic–cortical circuitry (CCTCC), are involved in higher cognitive functions.
In particular, it has been proposed that the thalamus plays a crucial role in synchronizing large-scale cortical oscillations within thalamo-cortical loops thus coordinating and facilitating information transfer (Siegel et al., 2012). For schizophrenia Andreasen et al. (1999) introduced the concept of “cognitive dysmetria” to characterize disintegration in the CCTCC leading to a defect in the timing or sequencing component of mental activity. Jones (1997) proposed that functional disturbances in the thalamo-cortical circuits in conjunction with loss of thalamic cells could lead to impaired thalamo-cortical oscillations in schizophrenia.
Electrophysiological studies provided strong evidence for the postulated abnormalities in synchronized oscillatory activity in schizophrenic patients, predominantly in the beta- and gamma-band range (Sun et al., 2011; Uhlhaas and Singer, 2010). Deficient synchronization of these oscillations is known to impair the integration of neural responses and may thus constitute the basis of the frequently reported alterations in functional connectivity of cortical networks resulting in deficient cognitive control processes in schizophrenia.
In the same vein, altered functional interactions were described predominantly on the basis of a correlational analysis in patients with schizophrenia with regard to intrafrontal, fronto-cingulate and fronto-thalamic connectivity (Pettersson-Yeo et al., 2011). Whereas measures of functional connectivity do not necessarily imply a causal link between correlated regions, studying effective connectivity, e.g. using dynamic causal modeling (DCM, Friston et al., 2003) enables causal inferences about the directionality of interactions between distinct brain regions together with their context-dependent modulation. Only very few studies have investigated the effective connectivity in the fronto-thalamic network so far. Using structural-equation modeling (SEM) our group observed abnormal effective connectivity in the fronto-thalamic circuitry during a working memory task (Schlösser et al., 2003). DCM is a powerful method for assessing effective connectivity and has been widely used across different imaging modalities, populations and tasks (Friston, 2009). The potential of DCM for modeling effective connectivity in schizophrenia has been demonstrated, e.g. with regard to the fronto-parietal network (Deserno et al., 2012). However, the fronto-thalamic connectivity during cognitive control has not been investigated using DCM in schizophrenia.
Furthermore, volume reductions of the thalamus and fronto-cingulate regions were consistently observed in schizophrenia (Honea et al., 2005; Wright et al., 2000). Volumetric studies of white matter (WM) often reported reduced volume of the anterior limb of the internal capsule (ALIC; Hulshoff Pol et al., 2004; Wobrock et al., 2008), a structure well known to connect the thalamus with ACC and DLPFC. A recent meta-analysis including 79 studies (Bora et al., 2011) revealed a significant WM volume bilateral reduction in the ALIC and in the right inferior longitudinal fasciculus. In the same meta-analysis of DTI studies the authors reported in agreement with volumetric studies reduced WM integrity in the ALIC in terms of decreased fractional anisotropy (FA). Further regions of reduced FA included, among others, the cingulum and the medial PFC (Bora et al., 2011). Thus, previous results clearly indicated abnormal white matter connectivity in the fronto-thalamic circuit in schizophrenia.
Multiple lines of evidence suggest that disrupted functional and structural connectivity within the cortico-thalamic network may represent the basis of cognitive control deficits in schizophrenia (Eisenberg and Berman, 2010; Stephan et al., 2009a). However, the precise interplay within the fronto-thalamic network during executive processing and its structural correlates has only been sparsely investigated.
Therefore, the present study was designed to further elucidate the functional and structural bases of cognitive control deficits in schizophrenia. Univariate fMRI data analysis was calculated to investigate the abnormal functional activation patterns in schizophrenia and to define the ROIs for the effective connectivity analysis. We hypothesized decreased BOLD signal in the fronto-cingulo-thalamic network in patients. The main focus of the present study was on modeling effective connectivity within the fronto-cingulo-thalamic network during performance of a cognitive control task by means of DCM. We hypothesized decreased effective connectivity in this network. Furthermore, voxel-based morphometry (VBM) of the white matter was used to uncover the hypothesized WM volume reductions in the fronto-cingulo-thalamic network and to relate WM volume to parameters of effective connectivity.
2. Materials and methods
2.1. Patients and controls
A total of 36 patients meeting the DSM-IV criteria for schizophrenia were recruited from the inpatient service of the Department of Psychiatry of the University Hospital Jena. Diagnosis was established by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and confirmed independently by two clinical psychiatrists (R.S. and C.S.). There was no history of drug and alcohol abuse or dependence in the patient or in the control group.
Two patients had to be excluded from the study due to brain abnormalities in the MRI scan. Thus, the final sample consisted of 34 patients (11 females and 23 males) and 36 healthy control subjects (11 females and 25 males) matched for age, gender and education. On average, patients were 35.9 ± 9.8 years old and had a mean education of 10.7 ± 1.2 years. In the healthy controls mean age was 32.4 ± 8.0 and mean education was 10.9 ± 1.2 years. There was no significant difference between the groups in terms of age, gender and education.
The patients were free of any other psychiatric diagnosis and had no interfering neurological conditions and were on long-term treatment with atypical antipsychotics, for 9.1 years on average. Regarding the type of substance class of atypical antipsychotics, 5 patients were treated with olanzapine, 7 with risperidone, 6 with clozapine, 5 with aripiprazole, 7 with quetiapine and 4 with amisulpride. Four patients were additionally treated with an SSRI.
The psychopathological status was assessed by the Scales for the Assessment of Positive and Negative Symptoms (Andreasen, 1990) (SAPS and SANS). The patients' scores were 40.03 ± 19.24 on SANS and 31.38 ± 25.79 on SAPS. The mean age at onset of schizophrenia was 26.3 years (SD = 9.38). The patients had on average 3.7 episodes with a range of 1 to 10 episodes.
A multiple choice vocabulary test (MWT-B, Lehrl et al., 1995) confirmed that none of the participants was mentally retarded (patients: M = 107.9, SD = 11.8; controls: M = 114.5, SD = 13.6).
Controls were recruited by local newspaper advertisement and screened for psychiatric or neurological diseases. The controls with past or current neurological or psychiatric diseases and/or first-degree relatives with axis I psychiatric disorders were excluded from the study. All participants were right-handed, according to the modified version of Annetts handedness inventory (Briggs and Nebes, 1975) and provided written informed consent prior to participating in the study. The study protocol was approved by the Ethics Committee of the University of Jena.
2.2. Experimental paradigm
The Stroop task was described in detail in our previous publication (Wagner et al., 2006). In brief, the Stroop task consisted of two conditions: a congruent and an incongruent condition. In the congruent condition, color words were presented in the color denoted by the corresponding word; in the incongruent condition, color words were displayed in one of three colors not denoted by the word. All subjects had to indicate the color type by pressing one of two buttons, which corresponded spatially to both possible answers.
2.3. MRI procedure
Functional data were collected on a 3 T whole body system equipped with a 12-element receive-only head matrix coil (MAGNETOM TIM Trio, Siemens). T2⁎-weighted images were obtained using a gradient-echo EPI sequence (TR = 2040 ms, TE = 26 ms, flip angle = 90°) with 40 contiguous transverse slices of 3.3 mm thickness covering the entire brain. Matrix size was 72 × 72 pixels with in-plane resolution of 2.67 × 2.67 mm2 corresponding to a field of view of 192 mm × 192 mm. A series of 220 whole-brain volume sets were acquired in one session.
High-resolution anatomical T1-weighted volume scans (MP-RAGE) were obtained in sagittal orientation (TR = 2300 ms, TE = 3.03 ms, TI = 900 ms, flip angle = 9°, FOV = 256 mm, matrix = 256 mm × 256 mm, number of sagittal slices = 192, acceleration factor (PAT) = 2, TA = 5:21 min) with an isotropic resolution of 1 × 1 × 1 mm3.
2.4. FMRI: univariate statistical analysis
For image processing and statistical analyses, we used the SPM8 software (http://www.fil.ion.ucl.ac.uk/spm). Data preprocessing was identical to our previous study (Wagner et al., 2006). In addition, individual movement parameters entered a fixed effects model at a single-subject level as covariates of no interest. None of the study participants showed excessive movements during the scanning session (exceeding 3 mm translation or 3° rotation). For the second level group comparison we set up an ANCOVA design with a between-subjects factor GROUP (patients vs. controls) and a within-subjects factor TASK (congruent vs. incongruent condition) controlling for the significant differences in behavioral performance between patients and control subjects. For this purpose we used the number of correct responses as well as the mean reaction time as covariates in the ANCOVA model. The main effect of GROUP as well as TASK × GROUP interaction was inclusively masked with the Stroop effect contrast (p < 0.05 uncorr.) and thresholded on the voxel-level at p < 0.001 (uncorrected) and on the cluster-level at p < 0.05, FWE corrected (equal to k = 227). The contrast used for masking was orthogonal to the contrast being tested for. The Stroop effect contrast, i.e. incongruent vs. congruent condition was computed in the ANOVA design (without covariates) and thresholded on the voxel-level at p < 0.05, FWE corrected.
2.5. Dynamic causal modeling (DCM)
DCM (Friston et al., 2003) as implemented in SPM8 was employed for the effective connectivity analysis. DCM is a hypothesis-driven approach, which aims to estimate and make inferences about the influence that one neural system exerts over another and how this is affected by the experimental context. For a given model, DCM estimates different sets of parameters: extrinsic parameters, which define the input of external stimuli on brain regions (“driving input”); endogenous parameters, which characterize context-independent connectivity between two regions; and modulatory parameters, which measure changes in effective connectivity modulated by the experimental task or by the activity in a region (Stephan et al., 2008). Parameter estimation is performed within a Bayesian framework using an empirical forward model that combines observed hemodynamic responses with the hidden neuronal dynamics.
2.6. DCM specification
Based on our hypothesis, the present univariate results (i.e. main effect of TASK) and previous cognitive control studies, we defined a model, which included the dorsal ACC, the DLPFC and the MD bilaterally as the key regions of the cognitive control network. In detail, the stimulation of the primary visual cortex (V1) was regarded as the driving input for the model. We assumed that the PFC receives visual stimulus material from the occipital cortex via the posterior parietal cortex (Kravitz et al., 2011).
However, to maintain a low complexity level of the model network, we directly modeled unidirectional input from V1 to DLPFC (analogous to Etkin et al., 2006; Schlösser et al., 2010). The DLPFC and the ventrolateral PFC (VLPFC) are integral parts of the fronto-cingulate network subserving the Stroop task. Previous studies have suggested that both the DLPFC and the VLPFC are involved in cognitive control (Blasi et al., 2006; Kondo et al., 2004). We restricted the included PFC areas to the DLPFC to keep the model complexity low. Another reason to restrict the analysis to DLPFC was its well described anatomical connectivity with the mediodorsal thalamus. The MD has dense anatomical connections with DLPFC and dACC and is strongly involved in cognitive control functions (Watanabe and Funahashi, 2012). For example, Tanibuchi and Goldman-Rakic (2003) observed during processing of a delayed response task that neurons in the MD, which are interconnected with neurons in the DLPFC, showed a similar activity as neurons in the DLPFC, indicating their important role in the maintenance of information. Furthermore, strong evidence points towards dense structural connectivity and close functional interplay between dACC and the DLPFC (Erickson et al., 2004; MacDonald et al., 2000). These two regions are considered as central components of most cognitive control models (Carter and van Veen, 2007). The ACC was modeled in the present study as a higher cognitive control unit responsible for error detection and conflict resolution (Mansouri et al., 2009). Consequently, we did not include direct interactions between V1 and dACC. This model follows previous conceptualizations of response competition tasks like the Stroop task where the stimulus material is received by units processing information about task demands (PFC) and cognitive control is exerted by nodes responsible for conflict monitoring (ACC) (Cohen et al., 2000).
With regard to the general pattern of endogenous connections, we were interested to figure out in which areas of the fronto-cingulo-thalamic network the existing connectivity was mainly located. In order to systematically investigate this aspect we compared different models with different architectures of endogenous connections using the Bayesian model selection (BMS) approach (Fig. 1).
Fig. 1.
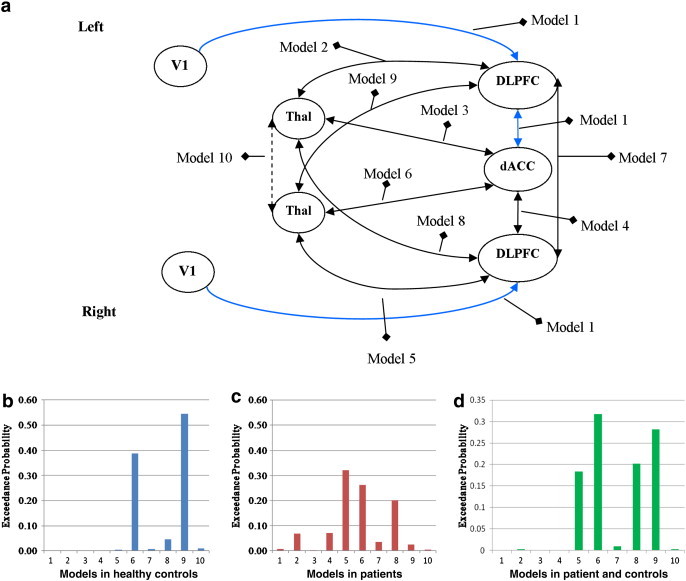
Bayesian model selection (BMS).
(a) Seven area DCM model of effective connectivity was constructed with bidirectional connections to/from dACC, DLPFC and mediodorsal thalamus. Models 1 to 10 specify different locations of endogenous connectivity with increasing model complexity (Model 2 = 1 + 2, Model 3 = 2 + 3, and so on). (b) Exceedance probabilities for models 1–10 in controls. (c) Exceedance probabilities for models 1–10 in schizophrenic patients. (d) Exceedance probabilities for models 1–10 in patients and controls. The exceedance probabilities in BMS give the probability that one model is more likely than another to generate the observed data. Model 9 was the optimal one in the group of healthy controls and model 5 in schizophrenia patients. In the next step the BMS was performed for all subjects together. The resulting optimal model was the model 6, which was used as the reference model of the cognitive control network. Endogenous and modulatory parameters were extracted from this model for further group comparison.
2.7. Model comparison
BMS was performed as part of a systematic comparison in which alternative models were evaluated (Penny et al., 2004). BMS was based upon a random effects model that accounts for between-subjects heterogeneity in terms of which model best explains the measured data. In the BMS procedure, the probability that the data are explained by the model, i.e. the model evidence, is approximated by the negative variational free-energy as an optimal compromise between accuracy and complexity of a model (Stephan et al., 2009b) and is used to compare between alternative models. The results of group specific BMS showed (Fig. 1) that in the control group the model that contains the full endogenous connectivity except interthalamic connection (model 9) is a winner model for the endogenous connection pattern, whereas in the schizophrenia group, model 5 with lower complexity is the winner.
Therefore, due to the fact that potential between-group differences in estimated parameters may be due to differences in model fit, BMS was conducted for all subjects together with no distinction by group. As illustrated in Fig. 1 the resulting optimal model for all subjects was model 6, which was used as the reference model of the cognitive control network.
2.8. Modulatory parameter
Modulatory connectivity parameters were specified to investigate the influence of the incongruent and congruent Stroop conditions on the defined connections within the modeled network. Assuming a specific task influence on all fronto-cingulo-thalamic connections as indicated by the univariate fMRI analysis, we examined the influence of induced conflict on all endogenous connections, determined by the previous model selection step (model 6)
2.9. Time series extraction
The ROI placement was based on the results of the univariate SPM analysis. To account for potential variability in the activation location at the individual subject level, we at first created a mask image as a 10 mm radius sphere around the maximum coordinates from all seven seed regions of interest as extracted from the contrast incongruent vs. congruent Stroop condition. Subsequently the individual local maximum within this mask image was extracted to build the individual seed ROI, for each subject, and average time courses were extracted from the seven seed regions of interest defined as a 4 mm sphere around coordinates derived from the individual local maximum coordinate within the ROIs. The first eigenvariate was calculated via singular value decomposition and used for further data processing. All time-series were adjusted for confounds (e.g. global mean, low-frequency components). Details of this approach have been described elsewhere (Schlösser et al., 2010). The final areas and mean coordinates of local maxima resulting from the described ROI definition strategy were: left (BA17/18, x = − 32, y = − 87, and z = − 9) and right primary visual cortex (BA17/18, x = 30, y = − 88, and z = − 6), dorsal ACC (BA24, x = 3, y = 29, and z = 31), left (BA9/46, x = − 46, y = 26, and z = 21) and right DLPFC (BA9/46, x = 47, y = 25, and z = 27), left (x = -8, y = − 16, and z = 8) and right thalamus (x = 9, y = − 12, and z = 9).
2.10. Group comparisons
Second level statistics were performed with connectivity parameters by means of ANOVA. This analysis included main effects of GROUP, TASK and TASK × GROUP interactions.
2.11. Voxel-based morphometric (VBM) analysis of the white matter
The preprocessing and statistical analyses were performed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm) as implemented in SPM8. The T1-weighted images were corrected for bias-field inhomogeneities, registered using a linear (12-parameter affine) and a nonlinear transformation, stripped of non-brain tissue, and tissue-classified into gray matter, white matter, and cerebrospinal fluid. A high-dimensional normalization (DARTEL) was performed using the DARTEL template in the MNI space that is provided with the VBM8 toolbox.
This VBM8 segmentation procedure contains partial volume estimation (PVE) to account for mixed voxels with two tissue types (Tohka et al., 2004). The algorithm is based on an adaptive maximum a posteriori (AMAP) approach (Rajapakse et al., 1997) with subsequent application of a hidden Markov random field model (Cuadra et al., 2005). This accounts for intensity inhomogeneities and other local variations of intensity.
Due to our primary hypothesis, we restricted the VBM analysis to only differences in white matter. Therefore, the resulting individual WM volume images were voxel-wise multiplied by the determinants of Jacobian matrices from nonlinear transformations before the statistical analysis on local WM volumes was performed. This modulation adjusts for local volume changes introduced by the nonlinear normalization. Finally, Gaussian smoothing was performed with a kernel of 8 mm FWHM.
Due to our strong a-priori hypothesis of differences in the fronto-cingulo-thalamic network regarding the WM volume between patients and healthy controls the conducted one-way ANOVA was thresholded on the voxel-level at p < 0.001, uncorrected and on the cluster-level according to the expected number of voxels per cluster.
Correlations between parameters of WM volume extracted from the significant clusters of the group comparison and connectivity parameters were finally performed to investigate a potential association between alterations in effective connectivity and WM volume in patients. All MNI coordinates were converted to Talairach coordinates using the mni2tal algorithm (Brett et al., 2001).
3. Results
3.1. Behavioral data
For the reaction times, the two-way ANOVA revealed a significant main effect of TASK (F(1, 68) = 82.65; p < 0.001), a significant main effect of GROUP (F(1, 68) = 16.99; p < 0.001) and a significant GROUP × TASK interaction (F(1, 68) = 8.10; p = 0.006), indicating significantly impaired performance in patients in the incongruent condition.
The patients showed 98.5% (SD = 3.4%) correct responses in the congruent and 84.6% (SD = 12.2%) correct responses in the incongruent condition. The control subjects showed 99.2% (SD = 1.9%) correct responses in the congruent and 93.5% (SD = 10.4%) correct responses in the incongruent condition. There was a significant difference in performance accuracy between groups for the incongruent (p < 0.001), but not for the congruent condition (p = 0.57) according to the Mann–Whitney Test.
3.2. FMRI — univariate statistical analysis
3.2.1. Main effect of TASK (Stroop effect)
In the second level random effects ANOVA a significant overall main effect of TASK (incongruent vs. congruent) was predominantly observed in the fronto-cingulo-thalamic network. We did not detect any significant activation differences in the opposite contrast (Table 1, Fig. 2a).
Table 1.
Main effect of TASK: incongruent vs. congruent Stroop condition (voxel-level p < 0.05, FWE corr.) for both groups together; main effect of GROUP controlling for differences in behavioral performance: healthy controls vs. schizophrenia patients for both Stroop conditions together (voxel-level: p < 0.001, cluster-level: p < 0.05, FWE corr.).
| Region of activation |
Right/Left |
Brodmann's area |
Cluster size |
Talairach coordinate |
T value |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Main effect of TASK: incongruent > congruent | |||||||
| Inferior frontal gyrus | L | 47 | 2049 | − 32 | 25 | 1 | 8.82 |
| Middle frontal gyrus | L | 46 | − 46 | 28 | 15 | 7.47 | |
| Inferior frontal gyrus | R | 47 | 652 | 34 | 25 | − 5 | 7.80 |
| Anterior cingulate | L | 32 | 1201 | − 2 | 14 | 42 | 7.78 |
| Middle frontal gyrus | R | 46 | 123 | 48 | 17 | 19 | 5.40 |
| Thalamus | R | 66 | − 4 | − 25 | 0 | 5.70 | |
| Thalamus | L | 6 | − 21 | 1 | 5.45 | ||
| Middle temporal gyrus | L | 22 | 38 | − 48 | − 44 | 11 | 5.34 |
| Cerebellum | R | 113 | 8 | − 71 | − 17 | 6.26 | |
| L | 13 | − 4 | − 47 | − 14 | 5.25 | ||
| Fusiform gyrus | L | 19 | 11 | − 36 | − 70 | − 7 | 5.28 |
| Caudate | R | 15 | 12 | 6 | 11 | 5.37 | |
| Main effect of GROUP, controlling for differences in Stroop task performance: controls > patients | |||||||
| + Middle frontal gyrus | R | 9/46 | 259 | 40 | 7 | 31 | 4.98 |
| Inferior frontal gyrus | R | 45 | 235 | 57 | 18 | 1 | 4.22 |
| Anterior cingulate | R | 32 | 398 | 6 | 43 | 9 | 3.78 |
| Thalamus | L | 771 | 12 | − 3 | 11 | 4.71 | |
| Thalamus | R | − 14 | − 3 | 13 | 4.17 | ||
| Cerebellum | L | 1101 | − 30 | − 71 | − 22 | 5.52 | |
| R | 24 | − 71 | − 20 | 4.48 | |||
Fig. 2.
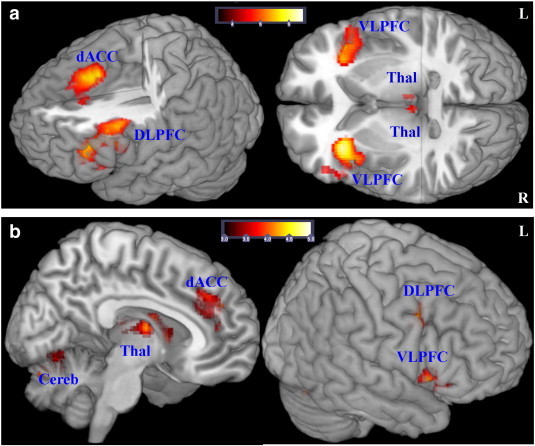
FMRI — univariate statistical analysis.
A: Significant (p < 0.05, FWE corr.) main effect of TASK, incongruent > congruent condition, for both groups together is detected in the dorsal ACC (BA 32), bilaterally in the DLPFC (BA 9/46), VLPFC (BA 47/45), and in the mediodorsal thalamus. Additionally, an overall main effect of TASK is also seen in the left middle temporal gyrus (BA 22), left cerebellum and the left fusiform gyrus (BA 19).
B: In the main effect of GROUP (congruent and incongruent conditions together, controlling for differences in behavioral performance) patients demonstrated significantly (voxel-level p < 0.001, cluster-level p < 0.05 FWE corrected) lower activation relative to healthy controls bilaterally in the cerebellum and the mediodorsal thalamus, in the dACC (BA 32), right DLPFC and VLPFC, right middle temporal gyrus, and bilaterally in the occipital lobe (BA 18/19).
3.2.2. Main effect of GROUP controlling for behavioral performance
In the ANCOVA main effect of GROUP (congruent and incongruent conditions together) patients demonstrated significantly lower activation relative to healthy controls in a number of regions of the CCTC circuitry (Table 1, Fig. 2b). We did not detect any significant activation differences in the opposite contrast, i.e. patients vs. controls.
3.2.3. Task by group interaction controlling for behavioral performance
There were no significant voxels in the TASK × GROUP interaction.
3.3. Dynamic causal modeling
3.3.1. Endogenous connectivity
Mean endogenous parameters for both groups are displayed in Table 2. As illustrated in Fig. 3, the patients showed lower endogenous parameters in the projections from the dACC to the left (p = 0.007) and right thalamus (p = 0.003), and stronger connectivity from the right DLPFC to the right thalamus (p = 0.003). All comparisons survived FDR correction for multiple comparisons. On the uncorrected level patients showed a lower connectivity between the dACC and the right DLPFC (p < 0.05).
Table 2.
DCM parameters of endogenous connectivity.
| Patients |
HC |
||||
|---|---|---|---|---|---|
| M | SD | M | SD | p | |
| L_DLPFC → L_Thal | 0.18 | 0.33 | 0.05 | 0.46 | n.s. |
| dACC → L_Thal_ | 0.15 | 0.61 | 0.57 | 0.64 | 0.007a |
| L_V1 → L_DLPFC | 0.28 | 0.24 | 0.24 | 0.32 | n.s. |
| L_Thal → L_DLPFC | 0.22 | 0.53 | -0.04 | 0.58 | n.s. |
| dACC → L_DLPFC | 0.15 | 0.72 | 0.47 | 0.70 | n.s. |
| R_DLPFC → R_Thal | 0.26 | 0.41 | -0.04 | 0.41 | 0.003a |
| dACC → R_Thal | 0.17 | 0.59 | 0.62 | 0.65 | 0.003a |
| R_V1 → R_DLPFC | 0.27 | 0.27 | 0.28 | 0.29 | n.s. |
| R_Thal → R_DLPFC | 0.17 | 0.46 | 0.02 | 0.52 | n.s. |
| dACC → R_DLPFC | 0.05 | 0.74 | 0.41 | 0.73 | 0.04 |
| L_Thal → dACC | 0.05 | 0.51 | 0.04 | 0.49 | n.s. |
| L_DLPFC → dACC | 0.05 | 0.22 | -0.01 | 0.40 | n.s. |
| R_Thal → dACC | 0.06 | 0.39 | 0.13 | 0.52 | n.s. |
| R_DLPFC → dACC | 0.06 | 0.28 | -0.10 | 0.38 | n.s. |
Survives FDR correction at p = 0.05.
Fig. 3.
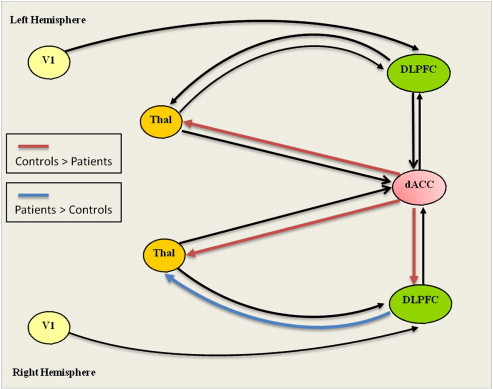
Effective connectivity analysis.
Dynamic causal modeling: significantly decreased parameters (red color) and increased (blue color) of endogenous connectivity in patients compared to healthy controls. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3.2. Modulatory connectivity
There were no significant differences between control subjects and patients with schizophrenia regarding the modulatory parameters.
3.3.3. Voxel-based morphometry
Testing differences in regional WM volume between the patients and healthy controls, we found three clusters of regional decreases in WM in the patients (Table 3, Fig. 4). The largest cluster comprised white matter voxels located bilaterally in the MD. The second cluster consisted of voxels lying in the right frontal lobe in close proximity to the cingulum bundle. On the left side, the patients showed decreased left frontal WM volume near BA 8. No significant differences were detected in the opposite contrast.
Table 3.
Voxel-based morphometry (VBM): healthy controls vs. schizophrenia patients (voxel-level: p < 0.001, cluster-level: according to expected voxels per cluster, ke = 116).
| Region of decreased white matter in patients |
Right/left |
Brodmann's area |
Cluster size |
Talairach coordinate |
T value |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Thalamus | L | 369 | − 2 | − 8 | 14 | 4.23 | |
| Thalamus | R | 3 | − 11 | 9 | 3.96 | ||
| Anterior cingulate | R | 8/32 | 261 | 9 | 16 | 32 | 3.81 |
| Superior frontal gyrus | L | 8/9 | 191 | − 15 | 36 | 42 | 4.04 |
Fig. 4.
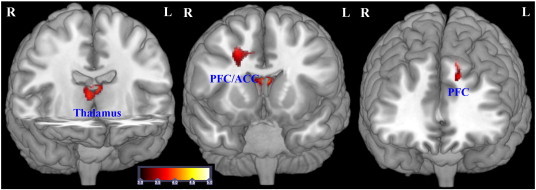
Morphometric analysis of the white matter.
Regions of significant white matter reduction in patients with schizophrenia compared to healthy controls (p < 0.001 uncorr., cluster size threshold according to the expected number of voxels per cluster, ke = 116).
3.4. Correlations
3.4.1. Task performance and endogenous connectivity
In patients with schizophrenia, a significant negative correlation between decreased endogenous parameter of the connectivity between dACC and left (r = − 0.38, p = 0.027) as well as right MD (r = − 0.42, p = 0.014) and the Stroop interference time was detected, indicating increased time demand to resolve the response conflict in the presence of decreased intrinsic cingulo-thalamic effective connectivity.
3.4.2. WM–DCM correlation
No significant correlations between the clusters of significantly decreased WM volume and decreased endogenous parameter could be detected in patients with schizophrenia.
4. Discussion
To our knowledge, this is the first study that examines effective connectivity within the cognitive control network in schizophrenia and relates connectivity parameters to brain abnormalities in the white matter volume. One major finding of the univariate analysis suggests a functional disruption of the fronto-cingulo-thalamic network in schizophrenia, which seems to constitute the neural basis of deficient cognitive control processes that were observable in terms of prolonged reaction times and increased number of errors in the Stroop task. This result confirms our initial hypothesis and is in line with a recent meta-analysis (Minzenberg et al., 2009) as well as with our own previous study (Schlösser et al., 2008). Furthermore, the lack of a significant TASK × GROUP interaction in the fMRI analysis in the presence of a significant main effect of GROUP might suggest that the patients and controls showed a comparable increase of the BOLD signal in the fronto-cingulo-thalamic network from the congruent to the incongruent condition. This indicates that the patients were able to recruit regions of the fronto-cingulo-thalamic network with increasing cognitive control demands, however, on a lower level compared to the healthy subjects.
The investigation of effective connectivity with DCM indicated that this altered fronto-cingulo-thalamic activation may be the consequence of an impaired interplay within these network areas as the endogenous connectivity – predominantly between the dACC and mediodorsal thalamus – turned out to be significantly decreased in patients with schizophrenia. It was further negatively correlated with the Stroop interferences time, indicating its important role in response inhibition and motor preparation processes (Schulz et al., 2011; Watanabe and Funahashi, 2012). On the other hand a stronger endogenous connectivity was observed between the right DLPFC and the right MD, potentially reflecting a compensatory hyperconnectivity in the light of the reduced predominantly right-lateralized BOLD signal in the DLPFC and thalamus. Furthermore, as revealed by the Bayesian model selection the information processing network of schizophrenia patients during cognitive control might be considerably different from that of the controls. The results of group specific BMS (as illustrated in Fig. 1) showed that in the control group the model that contains the full endogenous connectivity except interthalamic connection (model 9) is a winner for the endogenous connection pattern, whereas in the schizophrenia group, model 5 with lower complexity is the winner model. Thus, jointly activated ROIs in the controls and patients cannot be sufficiently fitted by the same model structure, suggesting that the complexity of the endogenous connectivity pattern in the cognitive control network was decreased in the patients with schizophrenia compared to healthy controls. This result may already account for the observed abnormal behavioral performance in the patients. Thus, the present results corroborate the hypothesis of altered fronto-cingulo-thalamic connectivity in schizophrenia with a method allowing causal inference about inter-regional interactions and emphasize the abnormal thalamic connectivity with anterior cingulate and DLPFC.
Reduced WM volumes were observed bilaterally in the mediodorsal thalamus, in close proximity to the right cingulum and in the left PFC. Even if we did not observe any significant correlations between decreased WM and decreased endogenous parameter, this structural finding provides strong evidence in favor of a structural deficit not only in the thalamus itself, but also in the fronto-thalamic structural connectivity in schizophrenia leading to its disordered effective connectivity. The structural deficits seem to be more pronounced in the right hemisphere, resulting therefore in stronger right-lateralized deficits in BOLD signal and connection strengths.
4.1. DCM results
Previous studies using DCM did not investigate the fronto-thalamic effective connectivity in schizophrenia. Instead, these studies aimed to investigate the fronto-temporal (Crossley et al., 2009), fronto-hippocampal (Benetti et al., 2009) or fronto-parietal (Deserno et al., 2012) connectivity during e.g. a working memory task and observed consistent disruption in terms of task-independent and task-dependent connectivities. Due to the strong connections of the thalamus with the prefrontal, temporo-limbic and parietal (Yeterian and Pandya, 1985) cortices we can speculate that these reported dysconnectivities may be the result of an aberrant thalamic input to the frontal and association cortices with abnormal modulation of their interaction. Assuming that cognitive control regulates a wide range of cognitive processes, a deficit in its neural basis, namely in the fronto-cingulo-thalamic circuitry, may result in deficits in different cognitive domains. This has been shown in earlier studies of our group in schizophrenia that revealed an activation deficit in a fronto-cingulo-thalamic network in association with a working memory task (Schlösser et al., 2008) as well as in a fronto-cingulate network in association with learning in the context of a probabilistic learning task (Koch et al., 2012). Another explanation for the observed deficits might be a primary deficit in stimulus encoding (Schlösser et al., 2008), which may result from the abnormal connectivity between primary sensory and higher-order cortical areas in schizophrenia (Dima et al., 2012). However, in the present study we did not observe any difference in the connectivity between V1 and DLPFC in the patients.
An abnormal role of the thalamus in a distributed network of the cortical–cerebellar–thalamic–cortical circuitry has been postulated within the framework of “cognitive dysmetria” (Andreasen, 1999), which accounts for cognitive deficits in various domains and clinical symptoms. Although we did not model the cerebellum to avoid further increase of complexity in the DCM model, the reduced BOLD fMRI signal in the cerebellum points to its involvement in deficient cognitive control processes in schizophrenia. Together with the present DCM results this finding provides further evidence for the concept of cognitive dysmetria and dysconnectivity in the CCTCC, with the thalamus being the key structure in this circuitry. Furthermore, negative correlation of reduced endogenous connectivity between dACC and thalamus with the Stroop interferences time in the patients points to the special relevance of cingulo-thalamic connections in the process of conflict resolution.
4.2. VBM results
Due to its prominent connections with the prefrontal cortex (Goldman-Rakic and Porrino, 1985) and the cerebellum (Middleton and Strick, 2001) a structural deficit in the mediodorsal nucleus of the thalamus might be strongly related to the often reported pathology of the PFC in schizophrenia, resulting in symptoms that potentially mirror prefrontal cortex functions, such as cognitive control processes.
In this vein, a large amount of literature of postmortem and neuroimaging studies has provided evidence for structural abnormalities of the thalamus. Smaller thalamic volume in schizophrenia was reported using structural MRI (Andreasen et al., 1990; Byne et al., 2009) and postmortem studies, the latter of which demonstrated reductions in neurons of specific thalamic nuclei, such as the MD (Pakkenberg, 1990) and the pulvinar (Byne et al., 2002). However, in a postmortem study Cullen et al. (2003) failed to find significant differences in the number of MD neurons. This may indicate that previously reported volume differences in the thalamus may not only be explained by the reduced size of neurons or neuropil in the thalamus, but also by the reduction of the thalamic white matter.
Although previous volumetric studies using ROI tracing methodology assessed both gray and white matter of the thalamus, direct evidence for decreased white matter volume has not been provided yet. However, indirect evidence for potential white matter pathology in the thalamus comes from studies using Magnetic Resonance Spectroscopy (MRS), which revealed a decrease of thalamic N-acetylaspartate (NAA)(Deicken et al., 2000) in the mediodorsal nucleus (Ende et al., 2001) in patients with schizophrenia indicating neuronal and especially axonal damage (Moffett et al., 2007). In a postmortem study Byne et al. (2008) suggested that the observed variations in the number of centromedian thalamic oligodendrocytes in schizophrenia may reflect variations in the myelination of axons originating from or passing through the thalamic nuclei. Furthermore, using diffusion-tensor imaging (DTI) Rose et al. (2006) demonstrated significantly increased mean diffusivity in PFC, ACC as well as in the mediodorsal thalamus, which potentially indicates an increase in the free bulk water due to altered gray and white matter cytoarchitecture (Kantarci et al., 2001). Mean diffusivity is greater in the CSF and smaller in the organized brain tissue, which potentially indicates differences in the intra- and extracellular space and a reduction in neuropil in schizophrenia (Selemon and Goldman-Rakic, 1999). Taken together, all these results fit well with our observation of a decreased white matter volume in the thalamus. Since the thalamus is a structure that contains a mixture of white and gray matter, correct classification of its voxels to a particular tissue class can be problematic. In the present study we used a segmentation approach implemented in the VBM-toolbox, which employs improved methods to estimate the parameters of the partial volume effect model to provide a more accurate segmentation (Tohka et al., 2004). Moreover, the registration between individuals was improved using the DARTEL algorithm (Ashburner, 2007), resulting in higher sensitivity to detect group differences.
Previous fronto-thalamic connectivity studies of the white matter often reported reduced volume or integrity of the anterior internal capsule (Hulshoff Pol et al., 2004; Wobrock et al., 2008), a structure that connects the thalamus with ACC and DLPFC and of the anterior thalamic radiation (Koch et al., 2010). DTI studies reported structural aberrations of prefrontal- and anterior cingulate–thalamic connections using tractography (Kunimatsu et al., 2008), fractional anisotropy (Zou et al., 2008) and mean diffusivity measurements (Rose et al., 2006). Moreover, in agreement with our findings the main result of a recent meta-analysis of DTI studies in schizophrenia was decreased structural integrity in that part of the frontal WM, which is traversed by white matter tracts interconnecting the PFC, thalamus and ACC (Ellison-Wright and Bullmore, 2009).
4.3. Thalamus as a critical structure for driving and modulating cortical activity
Converging lines of evidence from electrophysiological studies strengthen the notion of abnormal synchronized oscillatory activity of neurons playing a central role in the pathophysiology of schizophrenia (Uhlhaas and Singer, 2010). Abnormal gamma power was reported in the frontal regions during working memory and during cognitive control, which was related to impaired task performance in the patients (Cho et al., 2006; Uhlhaas and Singer, 2010). Synchronized neural oscillations are necessary for coordinated activity in the brain as they enable precise temporal correlations between distant regions and networks. The current hypotheses suggest that the vast majority of information flow between the cortical areas involves higher-order thalamic nuclei in the form of cortico-thalamo-cortical circuits (Saalmann and Kastner, 2011; Sherman and Guillery, 2002). Theyel et al. (2010) provided strong support for this notion observing that inactivation of the thalamic projection zone shared by two interconnected cortical areas resulted in a failure of cortico-cortical communication. Higher-order thalamic nuclei might therefore be crucial for synchronizing and facilitating oscillations between distant cortical regions (Saalmann and Kastner, 2011). Despite being speculative, due to its strong connectivity to DLPFC and ACC, a deficit in the MD and/or in the connecting white matter tracts may result in abnormally coordinated activity within the cognitive control network. The present results of fronto-cingulo-thalamic dysconnectivity point in this direction.
There are some potential limitations in the current study that should be considered. The lack of DTI data in the present data may be a potential limitation. However, even if potentially less sensitive in contrast to DTI, previous studies on WM volume, e.g. as summarized in the recent meta-analysis of Bora et al. (2011) were able to detect differences in WM volume in patients with schizophrenia in the same regions as revealed by studies using DTI and corresponding parameters of WM integrity such as fractional anisotropy (FA). Moreover, studies on healthy subjects provided evidence for an anatomical overlap between age-related reduction in VBM–WM volume and FA changes, in particular in the forceps minor, internal and external capsules, cerebral peduncle and temporal association fibers (Giorgio et al., 2010). Thus, volumetric analysis of WM using VBM has been shown to be suitable for detecting differences in WM volume. Furthermore, although parameters of DTI anisotropy were considered to reflect microscopic anatomy, spatial resolution obtained by MR–DTI remains at the macroscopic level (a typical voxel size is around 3 mm). In particular, in structures that contain a strong mixture of white and gray matter, like in the thalamus, a less precise measurement of WM integrity has to be expected. This may be not only due to relatively low resolution used in a typical DTI study, but also due to the fact, that the tensor model, used to estimate the anisotropy, cannot describe more than one dominant fiber orientation. Thus, for voxels containing crossing fibers such as the thalamus the tensor model may be not appropriate. As illustrated in the study of Jeurissen et al. (2012) crossing fibers have a clear impact on anisotropy analysis and therefore the authors consider the diffusion-tensor model as inadequate for regions with a high degree of crossing fibers. Using a relatively high resolution of 1 mm3 and an improved segmentation approach in regard to regions with a strong mixture of white and gray matter the present VBM analyses of WM are particularly suitable for the analysis of white matter changes in structures like the thalamus. Another potential limitation may be the potential negative effects of long-term treatment with antipsychotics, which are considered to constitute an important confounder of the brain volume changes in schizophrenia. With regard to the effect of atypical antipsychotics on brain structure and function, controlled animal studies have indicated that haloperidol- as well as olanzapine-treated animals have similar brain volume reductions, predominantly of the frontal and parietal regions (Dorph-Petersen et al., 2005; Konopaske et al., 2008). However, in patients with first-episode of schizophrenia, Lieberman et al. (2005) observed in a randomized, controlled and double-blind study, significant decreases in gray matter volume in haloperidol-treated, but not in olanzapine-treated patients. Moreover, higher dose of olanzapine and clozapine intake during a 5-year interval was associated with less decrease in gray matter volume in the dorsolateral prefrontal cortex (BA9/10) (van Haren et al., 2007). Dazzan et al. (2005), reported thalamic enlargement after treatment with atypical antipsychotics, whereas typically treated patients showed reduced volume in the frontal, anterior cingulate, temporal and parietal regions and increased volume in the basal ganglia. Furthermore, a recent meta-analysis of 77 studies on schizophrenia (Bora et al., 2011), in which most of the included patients were treated with atypical antipsychotics, revealed no significant effect of antipsychotic use and dose on volume of the white and gray matter.
Since most studies provide evidence for a neuroprotective effect of atypical antipsychotics, the potential negative effects (in terms of reduced volume or reduced BOLD signal in fronto-cingulo-thalamic network) of long-term treatment with atypical antipsychotics on brain structure and consequently on brain function can be regarded as negligible. Moreover, the available studies do not provide evidence for a clear differential effect of atypical antipsychotic drugs on brain structure in terms of potential neuroprotective effects.
5. Conclusions
Our results suggest that patients with schizophrenia have an abnormal fronto-cingulo-thalamic effective connectivity, which might be the basis of the cognitive control deficit. Moreover, the data indicate that disrupted white matter connectivity in the thalamus and fronto-thalamic networks may constitute the determining cause of the fronto-thalamic dysconnectivity. This aberrant neurophysiological circuitry may be related to a disruption in synchronized activity, which is essential for optimal and flexible behavioral performance.
In future studies, attempts should be made to more precisely identify the disrupted white matter tracts in this circuitry using high-resolution DTI tractography and to relate the extracted parameters to parameters of effective connectivity and electrophysiological data.
Acknowledgments
This study was supported by the German Research Foundation (DFG SCHL 400/2-1, DFG WA 3001/3-1).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Andreasen N.C. Methods for assessing positive and negative symptoms. Modern Problems of Pharmacopsychiatry. 1990;24:73–88. doi: 10.1159/000418013. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C. A unitary model of schizophrenia: Bleuler's “fragmented phrene” as schizencephaly. Archives of General Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., Ehrhardt J.C., Swayze V.W., II, Alliger R.J., Yuh W.T., Cohen G., Ziebell S. Magnetic resonance imaging of the brain in schizophrenia. The pathophysiologic significance of structural abnormalities. Archives of General Psychiatry. 1990;47:35–44. doi: 10.1001/archpsyc.1990.01810130037006. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Benetti S., Mechelli A., Picchioni M., Broome M., Williams S., McGuire P. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain. 2009;132:2426–2436. doi: 10.1093/brain/awp098. [DOI] [PubMed] [Google Scholar]
- Blasi G., Goldberg T.E., Weickert T., Das S., Kohn P., Zoltick B., Bertolino A., Callicott J.H., Weinberger D.R., Mattay V.S. Brain regions underlying response inhibition and interference monitoring and suppression. The European Journal of Neuroscience. 2006;23:1658–1664. doi: 10.1111/j.1460-9568.2006.04680.x. [DOI] [PubMed] [Google Scholar]
- Bora E., Fornito A., Radua J., Walterfang M., Seal M., Wood S.J., Yucel M., Velakoulis D., Pantelis C. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophrenia Research. 2011;127:46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Brett M., Christoff K., Lancaster J. Using Talairach atlas with the MNI template. Neuroimage. 2001;13:S85. [Google Scholar]
- Briggs G.G., Nebes R.D. Patterns of hand preference in a student population. Cortex. 1975;11:230–238. doi: 10.1016/s0010-9452(75)80005-0. [DOI] [PubMed] [Google Scholar]
- Byne W., Buchsbaum M.S., Mattiace L.A., Hazlett E.A., Kemether E., Elhakem S.L., Purohit D.P., Haroutunian V., Jones L. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. The American Journal of Psychiatry. 2002;159:59–65. doi: 10.1176/appi.ajp.159.1.59. [DOI] [PubMed] [Google Scholar]
- Byne W., Tatusov A., Yiannoulos G., Vong G.S., Marcus S. Effects of mental illness and aging in two thalamic nuclei. Schizophrenia Research. 2008;106:172–181. doi: 10.1016/j.schres.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byne W., Hazlett E.A., Buchsbaum M.S., Kemether E. The thalamus and schizophrenia: current status of research. Acta Neuropathologica. 2009;117:347–368. doi: 10.1007/s00401-008-0404-0. [DOI] [PubMed] [Google Scholar]
- Carter C.S., van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Cho R.Y., Konecky R.O., Carter C.S. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.D., Botvinick M., Carter C.S. Anterior cingulate and prefrontal cortex: who's in control? Nature Neuroscience. 2000;3:421–423. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- Crossley N.A., Mechelli A., Fusar-Poli P., Broome M.R., Matthiasson P., Johns L.C., Bramon E., Valmaggia L., Williams S.C., McGuire P.K. Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first-episode psychosis. Human Brain Mapping. 2009;30:4129–4137. doi: 10.1002/hbm.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadra M.B., Cammoun L., Butz T., Cuisenaire O., Thiran J.P. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Transactions on Medical Imaging. 2005;24:1548–1565. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- Cullen T.J., Walker M.A., Parkinson N., Craven R., Crow T.J., Esiri M.M., Harrison P.J. A postmortem study of the mediodorsal nucleus of the thalamus in schizophrenia. Schizophrenia Research. 2003;60:157–166. doi: 10.1016/s0920-9964(02)00297-9. [DOI] [PubMed] [Google Scholar]
- Dazzan P., Morgan K.D., Orr K., Hutchinson G., Chitnis X., Suckling J., Fearon P., McGuire P.K., Mallett R.M., Jones P.B., Leff J., Murray R.M. Different effects of typical and atypical antipsychotics on grey matter in first episode psychosis: the AESOP study. Neuropsychopharmacology. 2005;30:765–774. doi: 10.1038/sj.npp.1300603. [DOI] [PubMed] [Google Scholar]
- Deicken R.F., Johnson C., Eliaz Y., Schuff N. Reduced concentrations of thalamic N-acetylaspartate in male patients with schizophrenia. The American Journal of Psychiatry. 2000;157:644–647. doi: 10.1176/appi.ajp.157.4.644. [DOI] [PubMed] [Google Scholar]
- Deserno L., Sterzer P., Wustenberg T., Heinz A., Schlagenhauf F. Reduced prefrontal-parietal effective connectivity and working memory deficits in schizophrenia. The Journal of Neuroscience. 2012;32:12–20. doi: 10.1523/JNEUROSCI.3405-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima D., Frangou S., Burge L., Braeutigam S., James A.C. Abnormal intrinsic and extrinsic connectivity within the magnetic mismatch negativity brain network in schizophrenia: a preliminary study. Schizophrenia Research. 2012;135:23–27. doi: 10.1016/j.schres.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen K.A., Pierri J.N., Perel J.M., Sun Z., Sampson A.R., Lewis D.A. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- Egner T., Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature Neuroscience. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Eisenberg D.P., Berman K.F. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2010;35:258–277. doi: 10.1038/npp.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I., Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophrenia Research. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Ende G., Braus D.F., Walter S., Henn F.A. Lower concentration of thalamic n-acetylaspartate in patients with schizophrenia: a replication study. The American Journal of Psychiatry. 2001;158:1314–1316. doi: 10.1176/appi.ajp.158.8.1314. [DOI] [PubMed] [Google Scholar]
- Erickson K.I., Milham M.P., Colcombe S.J., Kramer A.F., Banich M.T., Webb A., Cohen N.J. Behavioral conflict, anterior cingulate cortex, and experiment duration: implications of diverging data. Human Brain Mapping. 2004;21:98–107. doi: 10.1002/hbm.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Friston K. Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biology. 2009;7:e33. doi: 10.1371/journal.pbio.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Harrison L., Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Giorgio A., Santelli L., Tomassini V., Bosnell R., Smith S., De Stefano N., Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51:943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn D.C., Therman S., Manninen M., Huttunen M., Kaprio J., Lonnqvist J., Cannon T.D. Spatial working memory as an endophenotype for schizophrenia. Biological Psychiatry. 2003;53:624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S., Porrino L.J. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. The Journal of Comparative Neurology. 1985;242:535–560. doi: 10.1002/cne.902420406. [DOI] [PubMed] [Google Scholar]
- Green M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? The American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Honea R., Crow T.J., Passingham D., Mackay C.E. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. The American Journal of Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol H.E., Schnack H.G., Mandl R.C., Cahn W., Collins D.L., Evans A.C., Kahn R.S. Focal white matter density changes in schizophrenia: reduced inter-hemispheric connectivity. Neuroimage. 2004;21:27–35. doi: 10.1016/j.neuroimage.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Jeurissen B., Leemans A., Tournier J.D., Jones D.K., Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Human Brain Mapping. 2012 doi: 10.1002/hbm.22099. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E.G. Cortical development and thalamic pathology in schizophrenia. Schizophrenia Bulletin. 1997;23:483–501. doi: 10.1093/schbul/23.3.483. [DOI] [PubMed] [Google Scholar]
- Kantarci K., Jack C.R., Jr., Xu Y.C., Campeau N.G., O'Brien P.C., Smith G.E., Ivnik R.J., Boeve B.F., Kokmen E., Tangalos E.G., Petersen R.C. Mild cognitive impairment and Alzheimer disease: regional diffusivity of water. Radiology. 2001;219:101–107. doi: 10.1148/radiology.219.1.r01ap14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns J.G., Cohen J.D., MacDonald A.W., 3rd, Cho R.Y., Stenger V.A., Carter C.S. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Koch K., Wagner G., Dahnke R., Schachtzabel C., Schultz C., Roebel M., Gullmar D., Reichenbach J.R., Sauer H., Schlosser R.G. Disrupted white matter integrity of corticopontine-cerebellar circuitry in schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2010;260:419–426. doi: 10.1007/s00406-009-0087-0. [DOI] [PubMed] [Google Scholar]
- Koch K., Schachtzabel C., Wagner G., Schikora J., Schultz C., Reichenbach J.R., Sauer H., Schlosser R.G. Altered activation in association with reward-related trial-and-error learning in patients with schizophrenia. Neuroimage. 2012;50:223–232. doi: 10.1016/j.neuroimage.2009.12.031. [DOI] [PubMed] [Google Scholar]
- Kondo H., Osaka N., Osaka M. Cooperation of the anterior cingulate cortex and dorsolateral prefrontal cortex for attention shifting. Neuroimage. 2004;23:670–679. doi: 10.1016/j.neuroimage.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Konopaske G.T., Dorph-Petersen K.A., Sweet R.A., Pierri J.N., Zhang W., Sampson A.R., Lewis D.A. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biological Psychiatry. 2008;63:759–765. doi: 10.1016/j.biopsych.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz D.J., Saleem K.S., Baker C.I., Mishkin M. A new neural framework for visuospatial processing. Nature Reviews. Neuroscience. 2011;12:217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimatsu N., Aoki S., Kunimatsu A., Yoshida M., Abe O., Yamada H., Masutani Y., Kasai K., Yamasue H., Ohtsu H., Ohtomo K. Tract-specific analysis of the superior occipitofrontal fasciculus in schizophrenia. Psychiatry Research. 2008;164:198–205. doi: 10.1016/j.pscychresns.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Lehrl S., Triebig G., Fischer B. Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurologica Scandinavica. 1995;91:335–345. doi: 10.1111/j.1600-0404.1995.tb07018.x. [DOI] [PubMed] [Google Scholar]
- Lesh T.A., Niendam T.A., Minzenberg M.J., Carter C.S. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36:316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J.A., Tollefson G.D., Charles C., Zipursky R., Sharma T., Kahn R.S., Keefe R.S., Green A.I., Gur R.E., McEvoy J., Perkins D., Hamer R.M., Gu H., Tohen M. Antipsychotic drug effects on brain morphology in first-episode psychosis. Archives of General Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- MacDonald A.W., III, Cohen J.D., Stenger V.A., Carter C.S. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mansouri F.A., Tanaka K., Buckley M.J. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nature Reviews. Neuroscience. 2009;10:141–152. doi: 10.1038/nrn2538. [DOI] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. Cerebellar projections to the prefrontal cortex of the primate. The Journal of Neuroscience. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg M.J., Laird A.R., Thelen S., Carter C.S., Glahn D.C. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett J.R., Ross B., Arun P., Madhavarao C.N., Namboodiri A.M. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Progress in Neurobiology. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein K.H., Dawson M.E. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophrenia Bulletin. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Archives of General Psychiatry. 1990;47:1023–1028. doi: 10.1001/archpsyc.1990.01810230039007. [DOI] [PubMed] [Google Scholar]
- Penny W.D., Stephan K.E., Mechelli A., Friston K.J. Comparing dynamic causal models. Neuroimage. 2004;22:1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Pettersson-Yeo W., Allen P., Benetti S., McGuire P., Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neuroscience and Biobehavioral Reviews. 2011;35:1110–1124. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Rajapakse J.C., Giedd J.N., Rapoport J.L. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Transactions on Medical Imaging. 1997;16:176–186. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- Rose S.E., Chalk J.B., Janke A.L., Strudwick M.W., Windus L.C., Hannah D.E., McGrath J.J., Pantelis C., Wood S.J., Mowry B.J. Evidence of altered prefrontal-thalamic circuitry in schizophrenia: an optimized diffusion MRI study. Neuroimage. 2006;32:16–22. doi: 10.1016/j.neuroimage.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Saalmann Y.B., Kastner S. Cognitive and perceptual functions of the visual thalamus. Neuron. 2011;71:209–223. doi: 10.1016/j.neuron.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlösser R., Gesierich T., Kaufmann B., Vucurevic G., Hunsche S., Gawehn J., Stoeter P. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage. 2003;19:751–763. doi: 10.1016/s1053-8119(03)00106-x. [DOI] [PubMed] [Google Scholar]
- Schlösser R.G., Koch K., Wagner G., Nenadic I., Roebel M., Schachtzabel C., Axer M., Schultz C., Reichenbach J.R., Sauer H. Inefficient executive cognitive control in schizophrenia is preceded by altered functional activation during information encoding: An fMRI study. Neuropsychologia. 2008;46:336–347. doi: 10.1016/j.neuropsychologia.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Schlösser R.G., Wagner G., Schachtzabel C., Peikert G., Koch K., Reichenbach J.R., Sauer H. Fronto-cingulate effective connectivity in obsessive compulsive disorder: a study with fMRI and dynamic causal modeling. Human Brain Mapping. 2010;31:1834–1850. doi: 10.1002/hbm.20980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J.D. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychology Review. 2010;20:236–260. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- Schulz K.P., Bedard A.C., Czarnecki R., Fan J. Preparatory activity and connectivity in dorsal anterior cingulate cortex for cognitive control. Neuroimage. 2011;57:242–250. doi: 10.1016/j.neuroimage.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon L.D., Goldman-Rakic P.S. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biological Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Sherman S.M., Guillery R.W. The role of the thalamus in the flow of information to the cortex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M., Donner T.H., Engel A.K. Spectral fingerprints of large-scale neuronal interactions. Nature Reviews. Neuroscience. 2012;13:121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- Stephan K.E., Kasper L., Harrison L.M., Daunizeau J., den Ouden H.E., Breakspear M., Friston K.J. Nonlinear dynamic causal models for fMRI. Neuroimage. 2008;42:649–662. doi: 10.1016/j.neuroimage.2008.04.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.E., Friston K.J., Frith C.D. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophrenia Bulletin. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.E., Penny W.D., Daunizeau J., Moran R.J., Friston K.J. Bayesian model selection for group studies. Neuroimage. 2009;46:1004–1017. doi: 10.1016/j.neuroimage.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Farzan F., Barr M.S., Kirihara K., Fitzgerald P.B., Light G.A., Daskalakis Z.J. Gamma oscillations in schizophrenia: mechanisms and clinical significance. Brain Research. 2011;1413:98–114. doi: 10.1016/j.brainres.2011.06.065. [DOI] [PubMed] [Google Scholar]
- Tanibuchi I., Goldman-Rakic P.S. Dissociation of spatial-, object-, and sound-coding neurons in the mediodorsal nucleus of the primate thalamus. Journal of Neurophysiology. 2003;89:1067–1077. doi: 10.1152/jn.00207.2002. [DOI] [PubMed] [Google Scholar]
- Theyel B.B., Llano D.A., Sherman S.M. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nature Neuroscience. 2010;13:84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohka J., Zijdenbos A., Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23:84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature Reviews. Neuroscience. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- van Haren N.E., Hulshoff Pol H.E., Schnack H.G., Cahn W., Mandl R.C., Collins D.L., Evans A.C., Kahn R.S. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32:2057–2066. doi: 10.1038/sj.npp.1301347. [DOI] [PubMed] [Google Scholar]
- Wagner G., Sinsel E., Sobanski T., Kohler S., Marinou V., Mentzel H.J., Sauer H., Schlosser R.G. Cortical inefficiency in patients with unipolar depression: an event-related FMRI study with the Stroop task. Biological Psychiatry. 2006;59:958–965. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Funahashi S. Thalamic mediodorsal nucleus and working memory. Neuroscience and Biobehavioral Reviews. 2012;36:134–142. doi: 10.1016/j.neubiorev.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Wobrock T., Kamer T., Roy A., Vogeley K., Schneider-Axmann T., Wagner M., Maier W., Rietschel M., Schulze T.G., Scherk H., Schild H.H., Block W., Traber F., Tepest R., Honer W.G., Falkai P. Reduction of the internal capsule in families affected with schizophrenia. Biological Psychiatry. 2008;63:65–71. doi: 10.1016/j.biopsych.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Wright I.C., Rabe-Hesketh S., Woodruff P.W., David A.S., Murray R.M., Bullmore E.T. Meta-analysis of regional brain volumes in schizophrenia. The American Journal of Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Yeterian E.H., Pandya D.N. Corticothalamic connections of the posterior parietal cortex in the rhesus monkey. The Journal of Comparative Neurology. 1985;237:408–426. doi: 10.1002/cne.902370309. [DOI] [PubMed] [Google Scholar]
- Zou L.Q., Xie J.X., Yuan H.S., Pei X.L., Dong W.T., Liu P.C. Diffusion tensor imaging study of the anterior limb of internal capsules in neuroleptic-naive schizophrenia. Academic Radiology. 2008;15:285–289. doi: 10.1016/j.acra.2007.09.026. [DOI] [PubMed] [Google Scholar]


