Abstract
Self-renewal of pluripotent mouse embryonic stem (ES) cells is sustained by the cytokine leukaemia inhibitory factor (LIF) acting through the transcription factor Stat3. Several targets of Stat3 have previously been identified, most notably the reprogramming factor Klf4. However, such factors are neither required nor sufficient for the potent effect of LIF. We took advantage of Stat3 null ES cells to confirm that Stat3 mediates the self-renewal response to LIF. Through comparative transcriptome analysis intersected with genome location data, we arrived at a set of candidate transcription factor effectors. Among these, Tfcp2l1 (also known as Crtr-1) was most abundant. Constitutive expression of Tfcp2l1 at levels similar to those induced by LIF effectively substituted for LIF or Stat3 in sustaining clonal self-renewal and pluripotency. Conversely, knockdown of Tfcp2l1 profoundly compromised responsiveness to LIF. We further found that Tfcp2l1 is both necessary and sufficient to direct molecular reprogramming of post-implantation epiblast stem cells to naïve pluripotency. These results establish Tfcp2l1 as the principal bridge between LIF/Stat3 input and the transcription factor core of naïve pluripotency.
Keywords: ES cell self-renewal, LIF, pluripotency, reprogramming
Introduction
Early mammalian embryos are characterized by the presence of a regulative population of cells each with the capability of giving rise to all somatic lineages and to germ cells. This property, pluripotency, first emerges in a naïve form in the epiblast in the pre-implantation blastocyst (Nichols and Smith, 2012). When exposed to an appropriate environment ex vivo, mouse naïve epiblast cells can be expanded indefinitely as embryonic stem (ES) cells (Evans and Kaufman, 1981; Martin, 1981; Nichols et al, 1990; Brook and Gardner, 1997). The cytokine leukaemia inhibitory factor (LIF) potently promotes ES cell self-renewal (Smith et al, 1988; Williams et al, 1988), and is routinely used in the derivation and culture of mouse ES cells (Smith, 2001). Binding of LIF to the gp130/LIF-R complex leads to activation of JAK kinases (Yoshida et al, 1994) that, in turn, phosphorylate the transcription factor Stat3 (Akira et al, 1994; Boeuf et al, 1997). Phosphorylated Stat3 dimerizes, enters the nucleus, and activates the expression of target genes (Zhong et al, 1994; Boeuf et al, 1997; Burdon et al, 2002; Bourillot et al, 2009). Artificial activation of Stat3 is sufficient to sustain ES cell self-renewal in the absence of LIF (Burdon et al, 1999; Matsuda et al, 1999), whereas antagonism of Stat3 leads to differentiation (Niwa et al, 1998; Bourillot et al, 2009). These findings indicate that Stat3 is the key mediator of LIF action in ES cells. However, LIF activates via JAK other signalling pathways that have also been proposed to play a role in ES cell maintenance (Welham et al, 2007; Niwa et al, 2009; Griffiths et al, 2011).
LIF/Stat3 signalling also plays a key role in the conversion of primordial germ cells into pluripotent EG cells (Matsui et al, 1992; Resnick et al, 1992; Leitch et al, 2013) and facilitates transcription factor directed reprogramming. Hyperactivation of Stat3 potently enhances reprogramming of somatic cells into induced pluripotent stem (iPS) cells (van Oosten et al, 2012), while blockade of the LIF/Stat3 pathway abolishes iPS cell generation (Tang et al, 2012). Furthermore, post-implantation epiblast stem cells, EpiSCs (Brons et al, 2007; Tesar et al, 2007), can be reprogrammed to a naïve pluripotent state simply by transient activation of Stat3 (Yang et al, 2010). These actions are separable from the self-renewal effect of LIF/Stat3 on established pluripotent stem cells (Yang et al, 2010).
Despite the central role ascribed to Stat3 in mouse pluripotency, its downstream effectors are incompletely described. LIF does not directly regulate core pluripotency factors Oct4, Sox2, Nanog, or Esrrb. Efforts to delineate the transcriptional programme stimulated by LIF have identified transcription factor targets of Stat3, such as Klf4, Pim1, and Gbx2 (Li et al, 2005; Hall et al, 2009; Niwa et al, 2009; Tai and Ying, 2013). However, none of these factors are indispensable for LIF responsiveness, nor can their forced expression fully recapitulate LIF activity. Notably, Klf4 is one of the four canonical Yamanaka factors that direct somatic cell reprogramming (Takahashi and Yamanaka, 2006), but LIF is required in addition (Tang et al, 2012). These observations suggest either, functional redundancy and additive effects between multiple Stat3 targets, or alternatively the existence of a pivotal unidentified target.
We previously showed that two selective small molecule inhibitors (2i) of Gsk3 and Mek kinases eliminate ES cell differentiation and can sustain self-renewal in the absence of LIF (Ying et al, 2008; Wray et al, 2010). Furthermore, 2i allows derivation and expansion of Stat3−/− ES cells. Here, we exploited these null cells in a refined search for critical effectors of the LIF/Stat3 pathway.
Results
Loss of Stat3 has no effect on potency of ES cells
Stat3 null ES cells can be derived and expanded when differentiation stimuli are blocked using 2i (Ying et al, 2008). We confirmed the identity and pluripotency of these cells by chimaera formation after blastocyst injection (Figure 1A). Consistent with this, when we examined the expression of genes associated with either pluripotency or germ layer specification we found no major differences between Stat3 null and wild-type cells maintained in 2i (Figure 1B). Furthermore, null cells did not exhibit any overt sign of spontaneous differentiation or appreciable cell death (Figure 1C) and were able to generate undifferentiated colonies at clonal density with efficiency equal to wild-type cells (Figure 1D). We therefore conclude that deletion of Stat3 does not impair ES cell self-renewal efficiency in 2i. In other culture conditions, however, the mutant cells cannot self-renew (Ying et al, 2008) because they are non-responsive to LIF, indicating that activation of Stat3 cannot be substituted by alternative mediators.
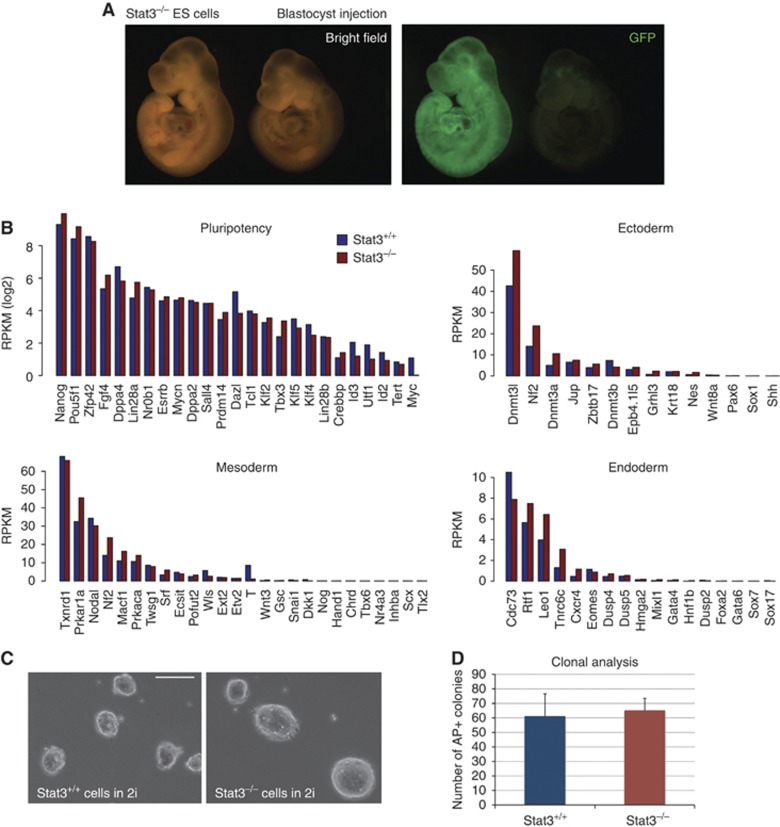
Figure 1.
Absence of Stat3 does not alter ES cell identity, pluripotency, or self-renewal in 2i. (A) GFP-labelled Stat3−/− ES cells were injected into blastocyst stage embryos; embryos were scored at E9.5 for the presence of GFP-positive cells and 15 embryos out of 19 showed widespread chimaerism. At mid-gestation (E12.5), 3 out of 3 embryos showed widespread chimaerism (not shown). A representative chimaeric embryo at E9.5 is shown. The embryo on the right showed no chimaerism and serves as a control for autofluorescence. See also Supplementary Figure S1A. (B) Absolute expression levels measured by RNA sequencing of genes associated with pluripotency or lineage priming, in Stat3+/+ and Stat3−/− cells. RPKM (Reads Per Kilobase per Million mapped reads) is a normalized unit of mRNA expression. (C) Morphology of Stat3+/+ and Stat3−/− cells in 2i culture. Both cell lines show homogeneous morphology with no sign of spontaneous differentiation. Scale bar, 100 μm. (D) Clonogenicity assay on Stat3+/+ and Stat3−/− cells. Three hundred cells per well were plated in 2i on laminin-coated plates and stained for alkaline phosphatase (AP) after 5 days. Bars show the number of AP-positive colonies obtained. Mean and s.d. of two independent experiments is shown.
Identification of Stat3 direct targets in mouse ES cells
Nonetheless, LIF is known to activate PI3 kinase and Erk signalling in addition to Stat3 (Burdon et al, 1999). It is also known that LIF enhances self-renewal efficiency in all ES cell culture conditions, including 2i (Wray et al, 2010). We therefore tested whether LIF has any additive effect on self-renewal of Stat3 null cells. As previously observed, LIF increased the colony-forming efficiency of wild-type cells (Figure 2A). However, Stat3 null cells showed no response, further verifying the primary role of Stat3 in mediating the contribution of LIF to ES cell self-renewal.
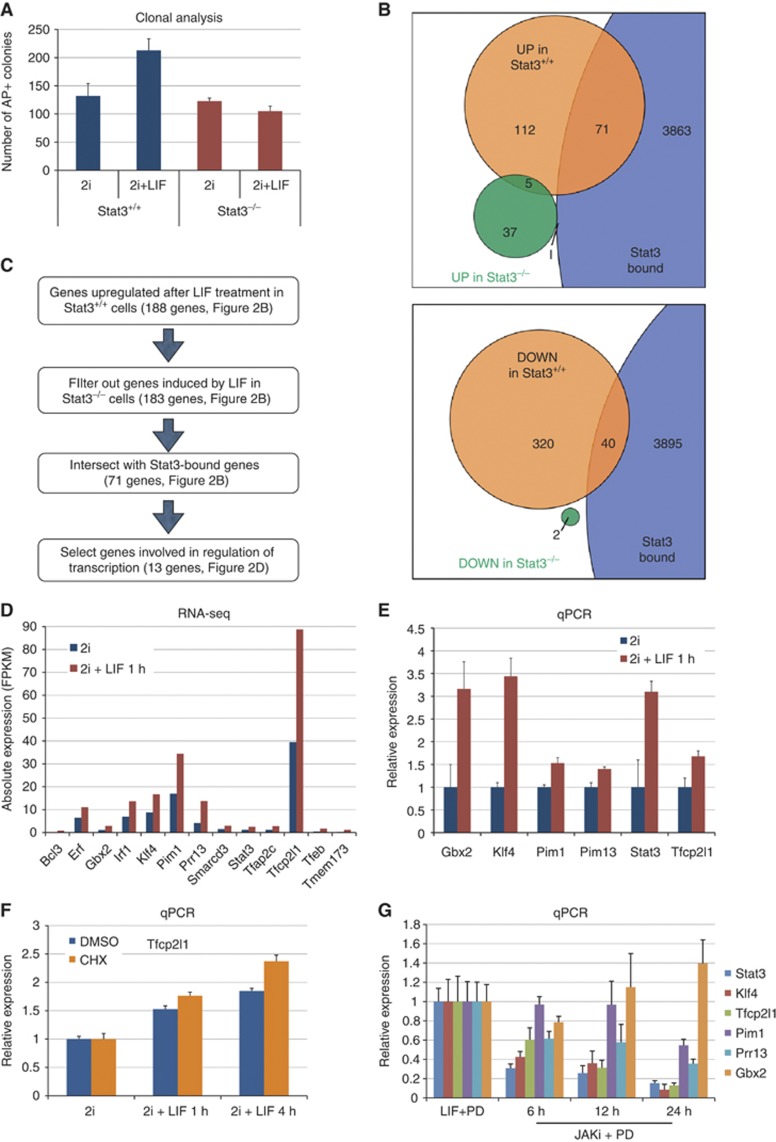
Figure 2.
Identification of Stat3 primary targets in mouse ES cells. (A) Clonogenicity assay. Six hundred cells per well were plated either in 2i or in 2i+LIF on laminin-coated plates and stained for alkaline phosphatase (AP) after 5 days. Bars show the number of AP-positive colonies obtained. Mean and s.d. of three independent experiments is shown. (B) Top: Venn diagram showing overlap between genes upregulated (P-value<0.05) after 1 h of LIF treatment in Stat3+/+ cells (orange), in Stat3−/− cells (green) and genes bound by Stat3 (see Materials and methods). Bottom: Venn diagram showing overlap between genes downregulated (P-value<0.05) after 1 h of LIF treatment in Stat3+/+ cells (orange), in Stat3−/− cells (green) and genes bound by Stat3. (C) Flow chart illustrating the approach used to identify candidate genes that mediate self-renewal downstream of Stat3. See also Supplementary Table S1. (D) Absolute expression of the 13 genes identified as potential mediators of Stat3 activity in ES cells. Blue bars indicate expression in 2i and red bars indicate expression after 1 h of LIF treatment in 2i. (E) Gene expression analysis of ES cells cultured in 2i and treated with LIF for 1 h. Beta-actin served as an internal control. Mean and s.d. of two independent experiments is shown. (F) Gene expression analysis of ES cells cultured in 2i after LIF stimulation for 1 and 4 h in the presence of the protein-synthesis inhibitor Cycloheximide (CHX). Cells were expanded in 2i without LIF and treated with LIF for 1 or 4 h, in the presence of either CHX (50 μm/ml, blue bars) or DMSO (vehicle, orange bars). CHX or DMSO was added to the medium 30 min before LIF treatment, and maintained throughout the experiment, to ensure effective inhibition of protein synthesis. Beta-actin served as an internal control and data are normalized to unstimulated 2i cultures. Mean and s.d. of four biological replicates is shown. (G) Gene expression analysis of ES cells cultured under serum-free conditions in the presence of LIF and the Mek inhibitor PD0325901 (PD). LIF was withdrawn and the Jak inhibitor (1 μM) was applied for the indicated time. Beta-actin served as an internal control. Mean and s.d. of two independent experiments is shown.
The existence and developmental potency of Stat3 null ES cells highlights the regulative nature of the naïve pluripotency network (Nichols and Smith, 2012). This flexibility creates the opportunity for manipulating the extrinsic environment to delineate the functional contributions of individual components (Martello et al, 2012). Accordingly, we exploited these mutant ES cells to define genes that are directly induced by activation of Stat3 rather than other signals downstream of LIF receptor. We exposed wild-type and Stat3 null cells to LIF for 1 h and prepared RNA for transcriptome analysis by deep sequencing. The short period of LIF stimulation is expected to enrich for primary transcriptional targets. We found that 188 genes were induced in Stat3 wild-type cells (Figure 2B, orange), and among these only 5 were induced in Stat3 null cells (Figure 2B, green). This indicates that the majority of genes acutely responsive to LIF require Stat3 for induction. We then used published Stat3 ChIP-seq (chromatin immunoprecipitation followed by massively parallel sequencing) data (Chen et al, 2008) to generate a list of genes (see Materials and methods) bound, and thus potentially directly regulated, by Stat3 (top panel of Figure 2B, purple). This yielded 3935 unique genes, representing ∼17% of all annotated genes. Significantly, a high proportion of genes induced by LIF in Stat3 wild-type cells were also bound by Stat3 (38.8%, P-value=3.4 × 10−14). In contrast, Stat3-bound genes were significantly underrepresented among those induced by LIF in Stat3 null cells (1.9%, P-value=0.0094). We performed a similar analysis on genes downregulated after LIF stimulation (bottom panel of Figure 2B), and found that Stat3-bound genes were underrepresented (11.2%, P-value=0.003). These results are consistent with the characterized role of Stat3 as a transcriptional activator (Boeuf et al, 1997; Darnell, 1997).
Accordingly, we focussed on genes induced by LIF in wild-type cells (Figure 2C and D), and selected those involved in the regulation of transcription, such as transcription factors or chromatin modifiers. We performed gene expression analysis by qRT-PCR on a set of 13 genes and validated 6 to be induced after LIF stimulation (Figure 2D). Induction of the remaining seven genes could not be confirmed because they are expressed at low levels and thus were difficult to quantify reliably by qRT-PCR. Among these six genes are Stat3 itself and other genes previously identified as Stat3 direct targets (Bourillot et al, 2009). Interestingly, however, the most highly expressed transcription factor in this group, Tfcp2l1 (also known as Crtr-1), has not previously been linked to LIF signalling. We therefore tested whether Tcfp2l1 is indeed directly responsive to LIF by stimulating cells in the presence of the protein synthesis inhibitor cycloheximide (Figure 2F). Induction was unaffected, confirming that Tfcp2l1 is a primary target of LIF signal transduction.
We then tested dependence on LIF for sustained expression. For this, we cultured ES cells under serum-free conditions in the presence of LIF and the Mek inhibitor PD0325901 (PD), then withdrew LIF and added a JAK kinase inhibitor (JAKi) to block LIF signalling completely. We analysed the expression of the six Stat3 targets over time and found that, with the exception of Gbx2, all genes were downregulated upon removal of the LIF signal (Figure 2G). Expression of Klf4, Pim1, Prr13, Stat3, and Tfcp2l1 therefore directly correlates with LIF stimulation, whereas Gbx2 expression is responsive to LIF (Figure 2D and E) but can be maintained by additional mechanisms.
Tfcp2l1 is the major effector of self-renewal downstream of Stat3
LIF, in combination with serum, maintains ES cell self-renewal. Therefore, omitting Stat3 itself, we tested whether the identified targets could confer self-renewal in serum without LIF. We used Rex1GFPd2 reporter cells to monitor uncommitted status (Marks et al, 2012; Martello et al, 2012) and transfected these with piggyBac expression vectors (Guo et al, 2009). Following hygromycin selection for two passages, we tested the response to LIF withdrawal in the presence of serum. Empty vector (PB-Vector)-transfected cells rapidly downregulated the GFP reporter and differentiated (Figure 3A; Supplementary Figure S1B). This was unchanged in Pim1 and Prr13 transfectants. Klf4 and Gbx2 expression maintained a proportion of ES cells in a morphologically undifferentiated state for several passages, and this was reflected in persistent expression of the Rex1-GFP reporter. However, Tfcp2l1 transfectants showed a stronger suppression of differentiation and maintenance of Rex1GFP, comparable to continuous culture in LIF (Figure 3A, compare top-left and bottom-right flow profiles). We also examined self-renewal capacity at the single-cell level by colony forming assay (Figure 3B). Gbx2 and Klf4 transfectants produced some undifferentiated colonies in the absence of LIF, consistent with previous reports (Hall et al, 2009; Niwa et al, 2009; Tai and Ying, 2013), but many more colonies were obtained from Tfcp2l1 transfectants.
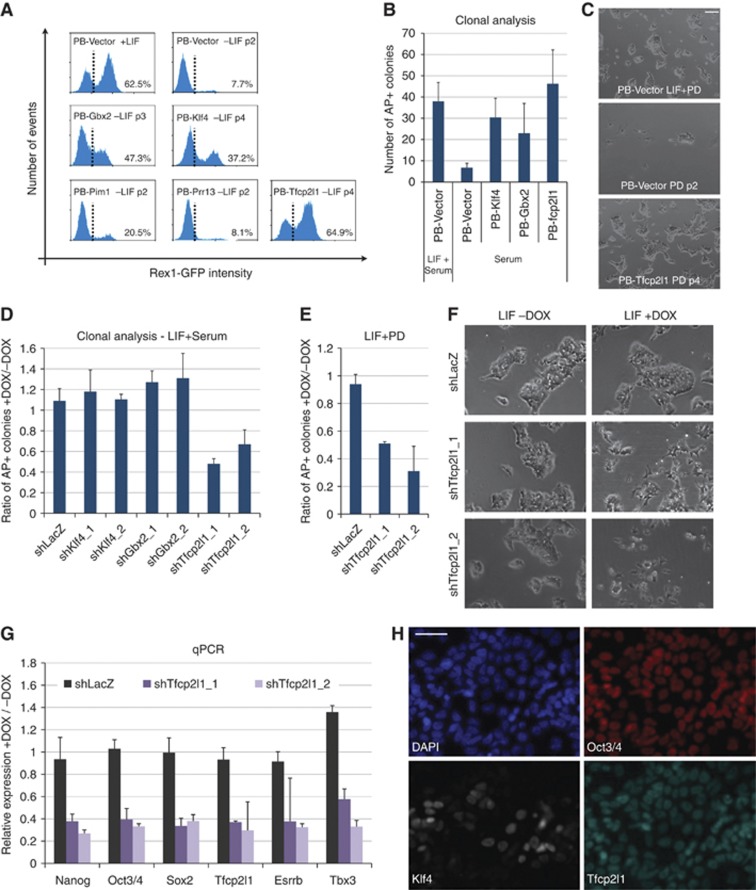
Figure 3.
Tfcp2l1 promotes ES cell self-renewal. (A) Flow cytometry analysis of Rex1-GFPd2 transfectants cultured in serum containing media either in the presence (+LIF) or in the absence (−LIF) of LIF. Rex1-GFPd2 cells were co-transfected with pBase helper plasmid and a piggyBac vector containing Gbx2, Klf4, Pim1, Prr13, and Tfcp2l1 or no cDNA (PB-Vector); transfected cells were selected for two passages with Hygromycin in LIF+serum. Only PB-Gbx2, PB-Klf4, and PB-Tfcp2l1 cells showed sustained self-renewal in the absence of LIF, expanding continuously for >10 passages. The dashed line separates GFP-positive and GFP-negative cells and the percentage of GFP-positive cells is indicated. See also Supplementary Figure S1B. (B) Clonogenicity assay in serum. The indicated cell lines were plated at clonal density in serum containing media either in the presence (LIF+Serum) or in the absence (Serum) of LIF, and stained for alkaline phosphatase (AP) after 5 days. Bars show the number of AP+ colonies obtained. Mean and s.d. of three independent experiments is shown. (C) Self-renewal in serum-free culture. PB-Vector and PB-Tfcp2l2 cells (described in Figure 3A) were cultured in the presence of LIF and the Mek inhibitor PD. After LIF withdrawal, PB-Vector cells rapidly differentiated or died (middle panel), whereas PB-Tfcp2l1 cells could be propagated with negligible differentiation for >12 passages (bottom panel, see also Figure 4). (D) Clonogenicity assay on inducible knockdown ES cell lines. DOX-inducible shRNA constructs targeting the indicated genes were stably transfected in Rex1-GFPd2 cells (see Materials and methods). An shRNA targeting LacZ mRNA served as a negative control. Cells were plated at clonal density in LIF+serum media either in the presence or in the absence of DOX and stained for AP after 5 days. Bars show the ratio between colonies obtained in the presence and absence of DOX for the indicated shRNA lines. Mean and s.d. of two independent experiments is shown. (E) Clonogenicity assay on inducible knockdown ES cell lines under serum-free conditions. Cells expressing the indicated shRNA constructs were plated at clonal density in LIF+PD media either in the presence or in the absence of DOX and stained for AP after 5 days. Bars show the ratio between colonies obtained in the presence and absence of DOX for the indicated shRNA lines. Mean and s.d. of two independent experiments is shown. See also Supplementary Figure S1D. (F) Morphology of the indicated shRNA lines cultured in LIF either in the absence or in the presence of DOX for 4 days. Knockdown of Tfcp2l1 resulted in differentiation. Scale bar, 100 μm. See also Supplementary Figure S1F. (G) Gene expression analysis of the indicated shRNA lines cultured in LIF+PD condition, either in the absence or in the presence of DOX for 4 days. Beta-actin served as an internal control and data are presented as ratios of +DOX and –DOX conditions. Mean and s.d. of two independent biological replicates is shown. (H) Fluorescence micrographs showing immunostaining for Oct3/4, Tfcp2l1, and Klf4 in wild-type ES cells cultured in LIF+serum. Scale bar, 100μm.
Klf4 and Gbx2 have previously been identified as factors promoting self-renewal downstream of LIF. However, the preceding data suggest that Tfcp2l1 has more potent self-renewal activity. LIF also supports self-renewal in serum-free conditions when combined with Mek inhibition (PD) (Wray et al, 2010). We found that Tfcp2l1 expression conferred long-term self-renewal capacity under serum-free conditions in the presence of PD without LIF (Figure 3C, see also Figure 4). Thus, overexpression of Tfcp2l1 largely recapitulates LIF stimulation in different culture conditions.
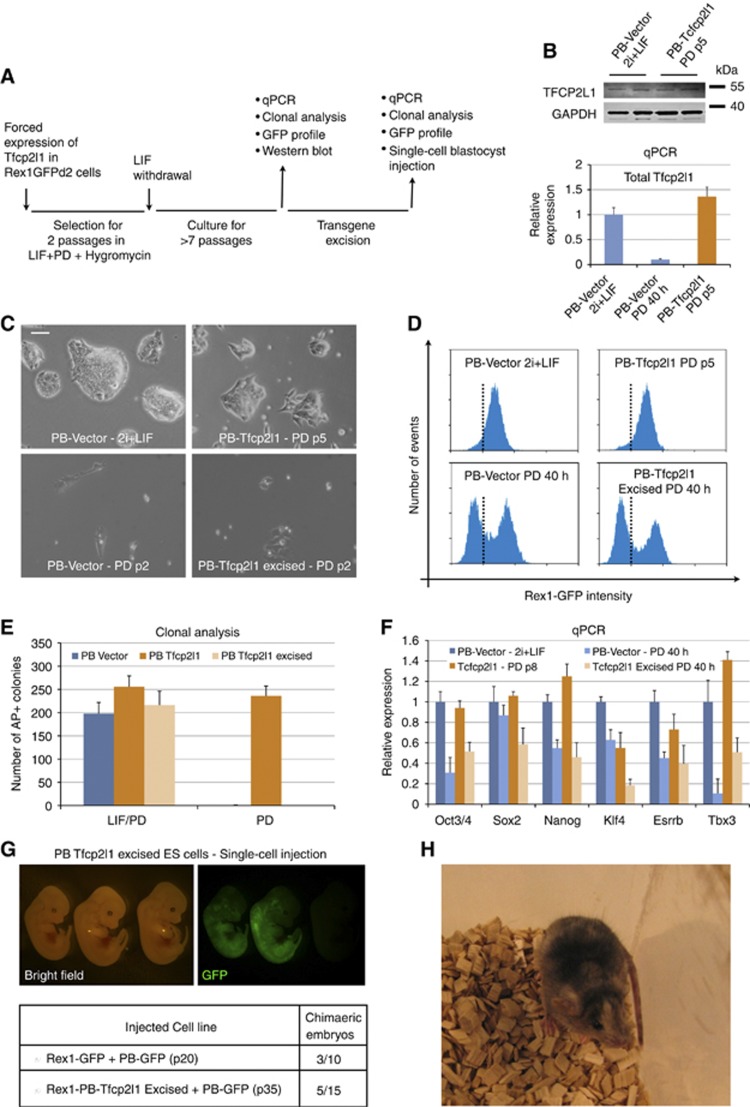
Figure 4.
Forced expression of Tfcp2l1 maintains pluripotency. (A) Experimental scheme for testing the sufficiency of Tfcp2l1 forced expression for the maintenance of ES cell pluripotency. (B) Top: western blot of Rex1GFPd2 cells transfected with an empty vector (PB-Vector) cultured in 2i+LIF, and PB-Tfcp2l1 cells cultured in PD for five passages. For each line, two biological replicates were loaded. GAPDH served as a loading control. Bottom: qRT-PCR analysis of total Tfcp2l1 expression of the indicated cell lines. Note that Tfcp2l1 is rapidly downregulated in PB-Vector cells cultured in PD alone. Beta-actin served as an internal control. (C) Representative pictures of PB-Vector and PB-Tfcp2l1 under the indicated culture conditions. Note that after excision of the transgene (PB-Tfcp2l1 excised) PB-Tfcp2l1 cells behave as PB-Vector cells. Scale bar, 100 μm. (D) Flow cytometry analysis of control (PB-Vector) and Tfcp2l1 expressing (PB-Tfcp2l1) cells under the indicated culture conditions. The dashed line separates GFP-positive and GFP-negative cells. (E) Clonogenicity assay of PB-Vector and PB-Tfcp2l1 cells before and after transgene excision. Note that PB-Tfcp2l1 cells behave as PB-Vector cells after excision of the Tfcp2l1 transgene (PB-Tfcp2l1 excised). Mean and s.d. of two independent biological replicates is shown. (F) Gene expression analysis of PB-Vector and PB-Tfcp2l1 cells cultured in the indicated conditions. Beta-actin was used as an endogenous control and data are normalized to PB-Vector cells cultured in 2i+LIF media. (G) Single-cell blastocyst injection. PB-Tfcp2l1 excised cells and Rex1GFPd2 cells were labelled with a constitutive GFP transgene (PB-GFP); single GFP-labelled cells were injected into blastocyst stage embryos; embryos were scored at mid-gestation (E12.5) for the presence of GFP-positive cells. Top: representative picture of chimaeric embryos derived after injection of single PB-Tfcp2l1 excised cells. The embryo on the right showed no chimaerism and serves as a control for GFP signal. Bottom: table summarizing the results obtained from two independent sections of injection. (H) Adult chimaeric animal showing widespread coat-colour contribution generated by single-cell blastocyst injection.
Source data for this figure is available on the online supplementary information page.
We then tested which target genes are required for LIF-responsive ES cell self-renewal by knockdown using a doxycycline (DOX)-inducible shRNA system (see Materials and methods). For each gene, we used two independent shRNA constructs with knockdown efficiencies >60% (Supplementary Figure S1C). When cultured in serum-containing media in the presence of LIF, ES cells transfected with a control shRNA (shLacZ) gave rise to the same number of colonies in the presence or absence of DOX (Figure 3D, first bar). Knockdown of Klf4 or Gbx2 had no effect in agreement with previous reports showing that suppression of either of these factors does not markedly impair ES cell self-renewal (Jiang et al, 2008; Tai and Ying, 2013). In contrast, Tfcp2l1 knockdown caused a marked decrease in the number of ES cell colonies (Figure 3D). Similar results were obtained in LIF/PD (Figure 3E). Furthermore, Tfcp2l1 depletion in bulk culture in presence of LIF precipitated differentiation (Figure 3F, middle and bottom panels, see also Supplementary Figure S1F). Consistent with this, gene expression analysis after 4 days of shTcfcp2l1 induction showed a reduction in mRNA levels of key pluripotency factors, including Nanog, Oct3/4, Sox2, and Esrrb (Figure 3G). Significantly, however, Tfcp2l1 knockdown was well tolerated by ES cells cultured in 2i+LIF (Supplementary Figure S1D and E), confirming that the phenotype is specific to LIF-stimulated self-renewal. These results suggest that Tfcp2l1 exerts a unique function downstream of Stat3 that is necessary for a full self-renewal response to LIF (Figure 3I).
Many naïve pluripotency factors such as Klf4 exhibit mosaic expression in ES cells cultured in LIF and serum (Wray et al, 2010). In contrast, we found that Tfcp2l1 protein is widely expressed, with >90% of Oct3/4 expressing cells being Tfcp2l1 positive (Figure 3H and data not shown). This broad expression may underlie the capacity of LIF to sustain self-renewal efficiently in serum.
As shown above, Tfcp2l1 overexpression can maintain self-renewal of ES cells in the absence of LIF (Figure 3A–C). In those experiments, however, Tfcp2l1 transgene mRNA may be up to three-fold higher than endogenous levels (Supplementary Figure S1B) which, given that endogenous Tfcp2l1 transcript is very abundant in ES cells (Figure 2D), raises the possibility of neomorphic effects. We therefore used low-dose Hygromycin selection to obtain ES cell transfectants in which Tfcp2l1 transgene expression was restricted at close to LIF-stimulated endogenous levels (Figure 4A), as measured by qRT–PCR and immunoblotting (Figure 4B). This constrained expression of Tfcp2l1 was fully sufficient to sustain self-renewal after LIF withdrawal, as shown by undifferentiated morphology (Figure 4C), expression of pluripotency markers (Figure 4D and F), and capacity to form AP-positive colonies (Figure 4E).
We then tested whether the effect of Tfcp2l1 on self-renewal in these cells was reversible by excising the floxed Tfcp2l1 transgene after expansion in the absence of LIF for seven passages (Figure 4A; Supplementary Figure S1G). After excision, cells differentiated unless maintained in the presence of LIF (Figure 4C–E). We carried out blastocyst injection with the excised cells to assay their developmental identify and potency. As a rigorous test, we injected single cells. We obtained chimaeras with an efficiency of ∼30%, similar to the parental ES cell line (Figure 4G, table). Mid-gestation chimaeras exhibited widespread contribution to all germ layers (Figure 4G, top panels). Single Tfcp2l1 excised cells could also give rise to healthy term chimaeras (Figure 4H). We conclude that constitutive expression of Tfcp2l1 is sufficient to replace LIF and sustains ES cell self-renewal without transformation or impairment of somatic differentiation potential. Collectively, these results indicate that Tfcp2l1 is the major effector of self-renewal downstream of Stat3.
Tfcp2l1 sustains self-renewal independently of Stat3
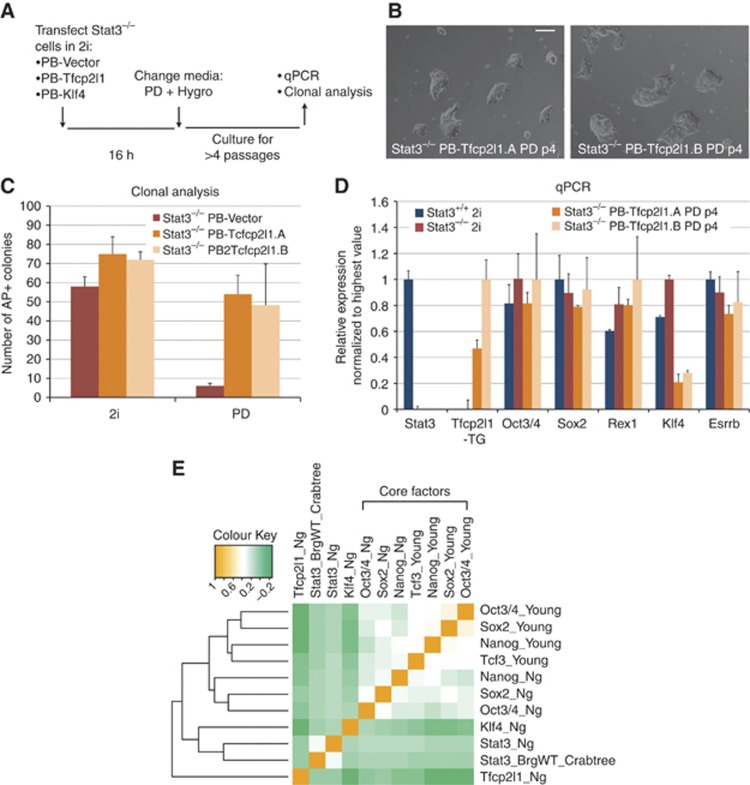
Next, we examined the epistatic relationship between Stat3 and Tfcp2l1. We transfected Stat3 null cells with expression vectors for Klf4 or Tfcp2l1. After transfection, cells were cultured in serum-free medium containing PD and the selection agent hygromycin (Figure 5A). PB-Vector cells rapidly differentiated or died, as did PB-Klf4 cells and no stable transfectants were obtained (data not shown). In contrast, Tfcp2l1 expressing cells expanded efficiently and could be maintained over several passages. They displayed undifferentiated morphology (Figure 5B) and were able to self-renew at clonal density (Figure 5C). We characterized the Stat3 null cells expressing Tfcp2l1 by qRT–PCR and found that they expressed Oct3/4, Sox2, Rex1, and Esrrb at levels similar to wild-type ES cells in 2i. Reduced Klf4 expression was observed, consistent with the absence of LIF/Stat3 signalling (Figure 5D). These data suggest that Tfcp2l1, but not Klf4, can replace Stat3 in ES cell self-renewal.
Figure 5.
Tfcp2l1 can replace Stat3 in ES cell self-renewal. (A) Experimental scheme for testing the ability of Tfcp2l1 and Klf4 to maintain self-renewal in Stat3−/− ES cells. (B) Representative pictures of PB-Tfcp2l1 transfected Stat3−/− ES cells cultured in PD for four passages. Two lines, generated in two independent experiments, are shown. Of note, neither PB-Vector nor PB-Klf4 transfected Stat3−/− cells could be recovered due to rapid differentiation and cell death. Scale bar, 100 μm. (C) Clonogenicity assay of PB-Vector and PB-Tfcp2l1 transfected Stat3−/− ES cells. The indicated cell lines were plated at clonal density either in 2i or in PD and stained for alkaline phosphatase (AP) after 5 days. Stat3−/− cells transfected with an empty vector (PB-Vector) were cultured in 2i before plating and serve as a control. Mean and s.d. of three independent experiments is shown. (D) Gene expression analysis of Stat3−/− cells expressing PB-Tfcp2l1 after prolonged culture in the presence of PD. Beta-actin was used as an endogenous control and data are normalized to the highest value. The Stat3 primers are designed to specifically recognize the wild-type allele of Stat3, confirming its absence in Stat3−/− lines. The Tfcp2l1-TG primers specifically recognize the exogenous Tfcp2l1 transcript. Mean and s.d. of three independent replicates is shown. (E) Hierarchical clustering of 11 genome-wide binding maps from the Mouse ES cell ChIP-seq Compendium. Hierarchical clustering and Pearson correlation coefficients were used to display all pairwise comparisons in a clustered heatmap. Colours in the heatmap show the level of correlation for all pairwise comparisons (orange=1; white=0.5, green=0). Factors have been clustered along both axes according to the level of correlation. Note that, as previously reported, the ‘core’ factors Oct3/4, Sox2, and Nanog cluster together; Klf4 clusters together with Stat3 whereas Tfcp2l1 has a distinct binding profile.
We then consulted the ES cell ChIP-seq compendium (http://bioinformatics.cscr.cam.ac.uk/ES_Cell_ChIP-seq_compendium.html) to interrogate available ChIP-seq data sets (Martello et al, 2012). Unsupervised hierarchical clustering showed that Stat3 and Klf4 genome-wide binding profiles are closely related (Figure 5E). In contrast, Tfcp2l1 has a distinct binding profile and clusters with neither the ‘core’ factors nor Stat3 (Figure 5E). This is consistent with Tfcp2l1 acting downstream of Stat3 and additive to the ‘core’ factors to sustain ES cell self-renewal (Figure 5B).
Tfcp2l1 mediates induction of pluripotency downstream of LIF/Stat3
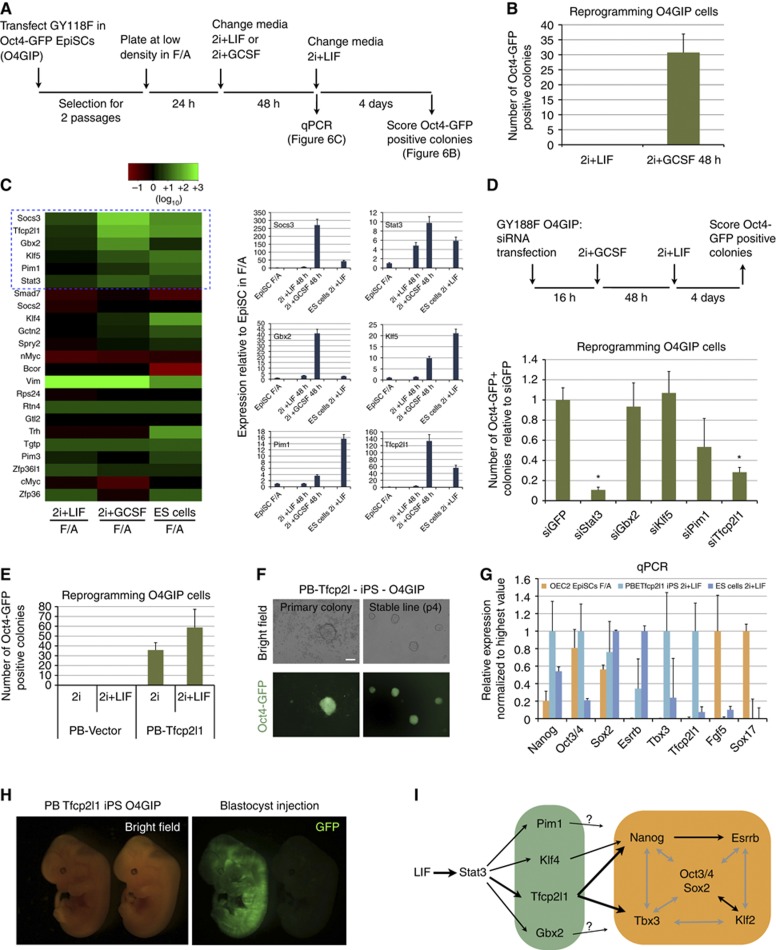
LIF signalling through Stat3 plays a key role in induction of pluripotency. Activation of LIF/Stat3 is required for iPS cell formation (Tang et al, 2012; van Oosten et al, 2012), and is sufficient to reprogram post-implantation EpiSCs to naïve pluripotency (Yang et al, 2010). It is not known, however, whether the target genes that sustain self-renewal are the same as those that mediate reprogramming. To identify Stat3 targets active during reprogramming, we used embryo-derived EpiSCs that stably express a chimaeric gp130 receptor, GY118F, which elicits hyperactivation of endogenous Stat3 in response to granulocyte colony stimulating factor (GCSF) (Burdon et al, 1999; Yang et al, 2010). Exposure of these EpiSCs to GCSF for 48 h in 2i is sufficient to induce formation of a modest number of reprogrammed colonies (Figure 6B, see also Yang et al, 2010). Importantly, exposing EpiSCs to LIF is not effective, suggesting that activation of Stat3 is limiting for induction of key target gene(s) to a level sufficient to drive reprogramming. We examined expression of a panel of candidate genes in EpiSCs exposed to either 2i+LIF or 2i+GCSF for 48 h in comparison with EpiSCs cultured in FGF2 and Activin A (F/A). We also included ES cells cultured in 2i+LIF as a reference. We chose 30 Stat3 targets, based on the present and previous studies (Bourillot et al, 2009), and determined their expression by qRT–PCR. We searched for genes that were induced in EpiSCs and, more specifically, were upregulated to higher levels in 2i/GCSF compared to 2i+LIF (Figure 6C, compare the second to the first column in the heatmap). Interestingly, we found that Klf4 and Myc, two reprogramming factors previously reported as Stat3 targets, were not induced by GCSF in EpiSCs, whereas four transcription factors, Klf5, Gbx2, Pim1, and Tfcp2l1, were upregulated to levels comparable to, or higher than, their expression in ES cells (Figure 6C, blue box in the heatmap and histograms). We tested whether induction of these four Stat3 targets was required for reprogramming by knockdown. We performed siRNA transfection followed by GCSF stimulation and scored the number of Oct4-GFP-positive colonies after 4 days in 2i+LIF (Figure 6D, top). Stat3 knockdown resulted in a ∼10-fold reduction, confirming that Stat3 mediates induction of pluripotency downstream of the chimaeric GCFS/gp130 receptor. We found that knockdown of Gbx2 and Klf5 had no effect, and knockdown of Pim1 resulted in a ∼2-fold decrease in colony number. However, Tfcp2l1 ablation substantially reduced the number of colonies recovered. This suggests that Tfcp2l1 plays a critical role in induction of naïve pluripotency downstream of Stat3.
Figure 6.
Tfcp2l1 mediates resetting of naive pluripotency. (A) Experimental scheme used to identify genes activated by the chimaeric GCSF/LIF-R receptor (GY118F) during EpiSC reprogramming. (B) Number of Oct4-GFP colonies obtained from O4GIP cells 6 days after induction of reprogramming. Note that activation for 48 h of the chimaeric GCSF/LIF-R receptor is sufficient to convert EpiSCs efficiently to ground-state pluripotency. (C) Left: heatmap showing the relative expression of the indicated genes in EpiSCs exposed to either 2i+LIF for 48 h (first column) or 2i+GCSF (second column); as a reference the expression in mouse ES cells is shown (third column). The fold change expression relative to EpiSCs cultured in bFGF and Activin A condition (F/A) is shown and beta-actin serves as an internal control. Right: histograms showing the relative expression of selected Stat3 targets. Stat3 itself and Socs3 served as positive controls, whereas Gbx2, Klf5, Pim1, and Tfcp2l1 were chosen for further functional validations. (D) Top: experimental scheme for testing the role of the indicated genes in the process of reprogramming. O4GIP EpiSCs expressing the chimaeric GCSF/LIF-R receptor were transfected with the indicated siRNAs in bFGF and Activin A media; after 16 h, they were exposed to GCSF in 2i media for 48 h, followed by 4 days of culture in 2i+LIF media. Bottom: Histogram showing the number of Oct4-GFP-positive colonies obtained relative to siGFP-transfected cells. Mean and s.d. of three independent experiments is shown. *P<0.01 (t-test) compared to siGFP control. (E) O4GIP EpiSCs were transfected with PB-Tfcp2l1 and an empty piggyBac vector control (PB-Vector) in bFGF and Activin A media; after 3 days of Hygro selection, transfectants were replated at low density and cultured in either 2i or 2i+LIF media for 6 days; the number of Oct4-GFP-positive colonies is shown as mean and s.d. of three independent experiments. Similar results were obtained by transient transfection of PB-Tfcp2l1 with no selection. (F) Representative pictures of primary Oct4-GFP colonies emerged after 5 days of reprogramming (left) and of a stable iPS line generated after PB-Tfcp2l1 transfection. Scale bar, 100 μm. (G) Gene expression analysis of parental O4GIP EpiSCs (orange), PB-Tfcp2l1 iPS cells (light blue), and wild-type ES cells (blue). Beta-actin was used as an endogenous control and data are normalized to the highest value. Mean and s.d. of two independent biological replicates is shown. (H) O4GIP iPS cells were transfected with a constitutive GFP expression plasmid and injected into blastocyst stage embryos after excision of the Tfcp2l1 transgene; embryos were scored at E12.5 for the presence of GFP-positive cells and two embryos out of six showed widespread chimaerism. One of these chimaeras is shown. The embryo on the right showed no chimaerism and served as a control for GFP signal. (I) Schematic diagram of LIF/Stat3 input to the pluripotency network. Among several transcription factor mediators Tfcp2l1 is of paramount importance and is uniquely required to promote self-renewal through integration into the core pluripotency gene regulatory network. Grey lines represent generic interconnectivity between core factors.
We then tested whether Tfcp2l1 expression is sufficient to convert EpiSCs into naïve pluripotency. We found that Oct4-GFP reporter EpiSCs (O4GIP) stably transfected with a Tfcp2l1 expression vector efficiently converted into naïve pluripotency when exposed to 2i (Figure 6E). Importantly, addition of LIF resulted in only a modest further increase in the number of colonies, suggesting that forced expression of Tfcp2l1 could largely recapitulate the contribution of LIF to EpiSC reprogramming. The reprogrammed colonies obtained could be passaged in 2i+LIF and gave rise to stable iPS cell lines (Figure 6F). Gene expression analysis confirmed activation of the naïve markers Tbx3 and Esrrb and downregulation of the EpiSC markers Fgf5 and Sox17 (Figure 6G).
EpiSCs are variable and one line (GOF18) derived on feeders in the presence of FGF2 has been reported to convert spontaneously to naïve pluripotency when exposed to 2i+LIF (Han et al, 2010). We found that GOF18 cells retained this ability even after prolonged culture under feeder-free conditions in the presence of FGF2 and Activin A. The conversion frequency in 2i alone was extremely low, but was greatly increased by addition of LIF (Supplementary Figure S2A). Moreover, we found that addition of LIF for only the first 48 h gave rise to the same number of colonies as continuous exposure (Supplementary Figure S2A, compare second and third bar). Therefore, we measured the expression of Stat3 targets by qPCR in GOF18 cells exposed to either 2i or 2i+LIF for 48 h. Both Gbx2 and Tfcp2l1 were expressed in these EpiSCs in 2i, but significantly only Tfcp2l1 was further upregulated by LIF (Supplementary Figure S2B). We transiently transfected GOF18 cells with a Tfcp2l1 expression vector and 24 h after transfection replated them in 2i without LIF. After 6 days, we scored the number of Oct4-GFP-positive colonies. We observed an ∼10-fold increase in colony formation after Tfcp2l1 transfection (Supplementary Figure S2C, left), indicating that a transient increase in Tfcp2l1 expression is sufficient to drive the reprogramming process. The iPS cells generated after Tfcp2l1 expression could be expanded in 2i+LIF over multiple passages, showing undifferentiated morphology, stable expression of the Oct4-GFP reporter (Supplementary Figure S2C, right panels) and of naïve pluripotency markers, and a concomitant reduction in EpiSC markers (Supplementary Figure S2D).
We analysed data on a panel of EpiSC lines (Bernemann et al, 2011) and found no significant differences in expression of LIF/Stat3 pathway components between lines that convert spontaneously and lines that do not (Supplementary Figure S2E, compare red and blue bars). We also found that both O4GIP and GOF18 cells express Tfcp2l1 mRNA at similar very low levels (Supplementary Figure S2F). Moreover, Tfcp2l1 protein 1 could not be detected by immunofluorescence in GOF18 cells, excluding the possibility of a rare sub-population of expressing cells (Supplementary Figure S2G). We conclude that spontaneous conversion into naïve pluripotency does not correlate with increased Tfcp2l1 expression in the EpiSC state, but rather with the ability to activate Tfcp2l1.
Finally, to confirm functional naïve pluripotent status after Tfcp2l1-mediated reprogramming, we transfected O4GIP-derived iPS cells with a constitutive GFP expression vector, excised the Tfcp2l1 transgene by Cre plasmid transfection, and carried out blastocyst injection. Two out of six transferred embryos showed widespread GFP expression at mid-gestation (Figure 6H). Taken together, these results suggest that Tfcp2l1 plays a major role downstream of LIF/Stat3 in the conversion of EpiSCs into authentic naïve pluripotency.
Discussion
The LIF/Stat3 pathway plays crucial facultative roles in mouse ES cell self-renewal (Smith et al, 1988; Williams et al, 1988; Niwa et al, 1998), maintenance of pluripotent epiblast during embryonic diapause (Nichols et al, 2001), generation of EG cells (Leitch et al, 2013), and molecular induction of pluripotency (Yang et al, 2010; Tang et al, 2012; van Oosten et al, 2012). How LIF input is integrated with the pluripotency gene regulatory network has remained unclear, however (Niwa et al, 1998, 2009; Nichols and Smith, 2012). cMyc has been proposed as a mediator (Cartwright et al, 2005), but neither cMyc nor NMyc are appreciably induced by LIF in ES cells (Hall et al, 2009). Klf4 has been shown to be a direct target of Stat3 (Li et al, 2005; Hall et al, 2009; Niwa et al, 2009), but is neither required nor sufficient to account for the potent effects of LIF stimulation. A significant role for alternative modes of LIF signalling has also been invoked, either through PI3 kinase (Welham et al, 2007; Niwa et al, 2009) or by JAK modification of chromatin (Griffiths et al, 2011).
To resolve this issue, we first confirmed that Stat3 null cells have functional ES cell identity and potency yet show no self-renewal response to LIF. We then sought the missing target(s) of Stat3 and identified the transcription factor Tfcp2l1. Tfcp2l1, also known as Crtr1, has previously been described in ES cells (Pelton et al, 2002; Ivanova et al, 2006; Chen et al, 2008), but its relationship to LIF/Stat3 has gone unnoticed. Tfcp2l1 is regulated directly by activated Stat3 and induced to high levels. Gain- and loss-of-function perturbations established that Tfcp2l1 is both necessary and largely sufficient to mediate the effects of LIF on ES cell self-renewal in either serum or serum-free culture conditions, in contrast to previously identified targets, Klf4, Gbx2, and Pim1. Strikingly, forcing expression of Tfcp2l1 at close to endogenous levels was sufficient to phenocopy LIF stimulation. Finally, we showed that Tcfcp2l1 is epistatic to Stat3 in sustaining ES cell self-renewal (Figure 5). From these results, we conclude that Tfcp2l1 is the major mediator of ES cell self-renewal downstream of LIF/Stat3, and that it exerts functions that cannot be compensated by other LIF targets. We previously noted that Tfcp2l1 is also induced by GSK3 inhibition (Martello et al, 2012). It may therefore constitute the postulated point of intersection between these two mechanisms for supporting ES cell self-renewal (Hao et al, 2006; Ogawa et al, 2006; Wray et al, 2010). Notably, however, while Tfcp2l1 appears sufficient to replace LIF/Stat3, it cannot reproduce the full effect of GSK3 inhibition (Figure 3A in Martello et al, 2012).
We also investigated mediators of LIF/Stat3 action during reprogramming. Klf4 is a canonical reprogramming factor (Takahashi and Yamanaka, 2006). However, LIF is required for efficient reprogramming of both somatic cells and EpiSCs even when Klf4 is overexpressed (Yang et al, 2010; Tang et al, 2012), suggesting a requirement for other LIF/Stat3 target(s). Analysis of the initial phase of EpiSC reprogramming did not show induction of known reprogramming factors such as Klf4 and cMyc, consistent with previous observations (Yang et al, 2010). In contrast, Gbx2, Klf5, Pim1, and Tfcp2l1 were induced (Figure 6C). Transient expression of Tfcp2l1 was sufficient to convert EpiSCs into naïve pluripotency without LIF stimulation, while Tfcp2l1 knockdown caused a dramatic reduction in reprogramming efficiency (Figure 6D). Importantly, when Tfcp2l1 is knocked down in ES cells in 2i+LIF there was no significant compromise to self-renewal (Supplementary Figure S1D and E). This suggests that Tfcp2l1 has a specific role in resetting of naive pluripotency in addition to its action in self-renewal.
Tfcp2l1 is a member of the CP2 family of transcription factors, which are generally considered to be activators of transcription (Lim et al, 1993; Bing et al, 1999). This has been demonstrated for Tcfp2l1 in ES cells (To et al, 2010), although in some contexts it may act as a repressor (Rodda et al, 2002). Interestingly, genome location data indicate that Tbx3, Esrrb, and Nanog are bound by Tfcp2l1 (data from Chen et al, 2008 and ES cell ChIP-seq Compendium) and upon Tfcp2l1 knockdown we noted downregulation of the three genes (Figure 3G). Similar effects of Tfcp2l1 depletion are also apparent in data from a recent microarray study (Nishiyama et al, 2013; Supplementary Figure S3A and B). Furthermore, we observed that upon overexpression of Tfcp2l1, Tbx3 and Nanog are significantly upregulated (Figure 6G; Supplementary Figures S2D, S3A and B and data not shown). These observations suggest that Tfcp2l1 sustains ES cell self-renewal in large part through transcriptional activation of Nanog and Tbx3, both of which have been proposed to lie genetically downstream of LIF (Niwa et al, 2009) but neither of which are direct targets (Bourillot et al, 2009; Hall et al, 2009).
Tfcp2l1 is expressed in the inner cell mass of the mouse blastocyst and is downregulated shortly after implantation (Pelton et al, 2002; Guo et al, 2010). It is rapidly downregulated when ES cells start to differentiate (Ivanova et al, 2006) and is absent from mouse EpiSCs (Figure 6) in common with other naïve pluripotency markers such as Esrrb, Rex1, and Klf2/4 (Tesar et al, 2007; Nichols and Smith, 2012). Human pluripotent ES cells are considered to be in a primed pluripotent state closer to mouse EpiSCs than ES cells. Tfcp2l1 is detected in the human ICM, but downregulated during derivation of human ES cells (O'Leary et al, 2012). Human ES cells have been reported to convert into a naïve-like state by overexpression of Klf2, Klf4, and Oct3/4 (Hanna et al, 2010). Interestingly, inspection of the microarray data from that study reveals that Tfcp2l1 is highly upregulated. It is possible therefore that Tfcp2l1 may play a role in generating and stabilizing a human naïve pluripotent state.
LIF is a stimulus that confers self-renewal and can promote emergence of the naïve pluripotent gene regulatory network, but is not itself integral to pluripotent identity (Nichols et al, 2001; Smith, 2001; Ying et al, 2008; Nichols and Smith, 2012). Consistent with this, Tfcp2l1 genome occupancy does not cluster with that of canonical pluripotency factors Oct4, Sox2, and Nanog. Instead, it binds to a largely distinct, and broad, set of genes. Significantly, however, these targets include key naïve pluripotency factors Nanog and Tbx3 previously shown to support ES cell self-renewal (Chambers et al, 2003; Ivanova et al, 2006; Niwa et al, 2009). Furthermore, the Tfcp2l1 promoter is bound by Oct4, Sox2, Nanog, Esrrb, and Klf4 (ES cell ChIP-seq compendium), and is targeted by the pluripotency repressor Tcf3 (Martello et al, 2012), indicating that Tfcp2l1 is hard-wired into the pluripotency gene regulatory network. We conclude that Tfcp2l1 is a previously overlooked transcriptional regulator that plays a pivotal role in the capability to generate, derive, and propagate naïve pluripotent stem cells. Although mutation of Tfcp2l1 is reported to be compatible with mouse embryo development, the characterized gene trap mutation may not create a null allele since residual full-length transcript is detectable (Yamaguchi et al, 2005). It will therefore be of interest to determine the effects of complete deletion of Tfcp2l1 on the emergence of pluripotency in the blastocyst, and epiblast maintenance during diapause when the Stat3 pathway is indispensable (Nichols et al, 2001).
Materials and methods
RNA-seq library construction
RNA was extracted using the TRIzol method (Invitrogen) followed by treatment with TURBO DNase (Ambion). Ribosomal RNA was depleted using RiboMinus (Invitrogen), and the remaining RNA was sheared by ultrasonication on a Covaris S2 for 90 s with the following parameter settings: Duty Cycle=10, Cycles per Burst=200, Intensity=5. Fragmented RNA was reverse-transcribed with SuperScript III (Invitrogen) at 50°C for 2 h using random hexamer and oligo-dT primers (10:1) in the presence of 6 μg/ml actinomycin D to inhibit the generation of second-strand products. Second-strand cDNA was synthesized by DNA Polymerase I for 2 h at 16°C in the presence of RNase H and with dUTPs substituted for dTTPs. End repair of double-strand cDNAs was carried out with T4 DNA polymerase and T4 polynucleotide kinase (New England Biolabs). Blunt-end, 3′-phosphorylated products were 3′-adenylated by exo-Klenow fragment in the presence of dATPs and ligated to sequencing adapters (Illumina) by T4 DNA ligase (New England Biolabs) at 20°C for 30 min. Following adapter ligation, the second strand of the library constructs was digested with uracil DNA glycosylase (UDG) and apurinic/apyrimidinic endonuclease 1 (APE 1) for 30 min at 37°C. PCR amplification of first-strand library constructs was carried out with Phusion DNA polymerase (Finnzymes) for 15 cycles. Purification of reaction products between each step was performed with Ampure XP paramagnetic beads (Beckman Coulter). The molarity and size distribution of the libraries was assessed by DNA 1000 microfluidic chips on the Agilent 2100 Bioanalyzer. Sequencing was performed on the Illumina GAIIx yielding 35–40 M 105 bp reads per sample. RNA-seq data are available in the ArrayExpress repository under accession E-MTAB-1796.
RNA-seq data analysis
Reads were aligned to the mouse reference genome (build mm10) using GSNAP version of 2012-05-24 (Wu and Nacu, 2010). The aligner was provided with known splice sites based on Ensembl version 68 (Flicek et al, 2012), but enabled to identify novel splice sites. A maximum of 10 mismatches were allowed for read alignment. Gene counts were calculated using the htseq-count utility (version 0.5.3p3, http://www-huber.embl.de/users/anders/HTSeq/doc/count.html) and used as an input for differential gene expression analysis with DESeq version 1.10.1 (Anders and Huber, 2010). Genes with a P-value of <0.05 (Benjamini–Hochberg adjusted) were selected for further analysis.
ChIP-seq data analysis
We obtained ChIP-seq data for Stat3 and a GFP control from a previous study (Chen et al, 2008). Reads were mapped to the mouse genome (build mm10) using bowtie version 0.12.8 (Langmead et al, 2009), discarding non-unique alignments. Peak detection was performed using MACS version 1.4.1 (Zhang et al, 2008). Each peak was associated with up to two genes as follows: the most proximal gene on each strand was identified by examining 50 kb upstream and downstream of the peak. Peaks were assigned to intersect genes unless an overlapping gene was found on the opposite strand. For the heatmap in Figure 5E a binary peak matrix was generated as described (Martello et al, 2012), analysed by unsupervised hierarchical clustering using Pearson’s correlation coefficients, and displayed using the heatmap function in R. The ChIP-seq data used for target gene intersection in Figure 5F are Esrrb—GSE11431 (GSM288355) and TFCP2L1—GSE11431. All raw and processed data used in this study are available at http://bioinformatics.cscr.cam.ac.uk/ES_Cell_ChIP-seq_compendium.html
ES cell culture
ES cells were cultured without feeders on plastic coated with 0.1% gelatine (Sigma, cat. G1890) and replated every 3 days at a split ratio of 1 in 10 following dissociation with Accutase (PAA, cat. L11-007). Cells were cultured either in the GMEM (Sigma, cat. G5154) supplemented with 10% FCS (Sigma, cat. F7524), 100 mM 2-mercaptoethanol (Sigma, cat. M7522), 1 × MEM non-essential amino acids (Invitrogen, cat. 1140-036), 2 mM L-glutamine, 1 mM sodium pyruvate (both from Invitrogen), and 100 units/ml LIF, or in the serum-free media N2B27 (NDiff N2B27 base medium, Stem Cell Sciences Ltd, cat. SCS-SF-NB-02) supplemented, as indicated, with small-molecule inhibitors PD (1 μM, PD0325901) and CH (3 μM, CHIR99021) and LIF prepared in-house. Colony forming assays were carried out by plating 60 ES cells/cm2 on plates coated with laminin (Sigma, cat. L2020). Plates were fixed and stained for alkaline phosphatase (Sigma, cat. 86 R-1KT) according to the manufacturer’s protocol. Plates were scanned using a CellCelector (Aviso) and scored manually. EpiSCs were cultured as previously described (Guo et al, 2009). O4GIP (OEC-2 line) was described in Betschinger et al (2013). GOF18 was described in (Han et al (2010). For DNA transfection, we used Lipofectamine 2000 (Invitrogen, cat. 11668030) and performed reverse transfection. For one well of a 6-well plate (10 cm2), we used 6 μl of transfection reagent, 2 μg of plasmid DNA, and 300 000 cells in 2 ml of N2B27 medium. The medium was changed after overnight incubation. The piggyBac vectors used for overexpression followed by transgene excision have been described in Guo et al (2009). The transgene is flanked by loxP sites, allowing efficient excision by transfection with Cre.
Gene expression analysis by quantitative RT–PCR with reverse transcription
Total RNA was isolated using the RNeasy kit (Qiagen) and 500 ng used for cDNA synthesis using SuperScript III (Invitrogen) and oligo-dT primers. Quantitative real-time PCR was carried out with SYBR green detection. Primers are detailed in Supplementary Table S2. Technical replicates were carried out for all reactions.
RNAi experiments
siRNAs were transfected at a final concentration of 40 nM using Dharmafect 1 (Dharmacon, cat. T-2001-01), following the protocol for reverse transfection. For a 12-well plate (4 cm2), we used 2 μl of transfection reagent, 2 μl of 20 μM siRNA solution, and 30 000 ES cells in 1 ml of N2B27 medium. The medium was changed after overnight incubation. siRNAs were purchased from Qiagen (Flexitube GeneSolution, see Supplementary Table S3).
Inducible shRNA
To generate a Dox-inducible shRNA system, we inserted into a piggyBac vector the murine pre-miR-155 hairpin (from the BLOCK-IT Pol2 miR RNAi system; Invitrogen) downstream of a third-generation Tet-responsive promoter (TRE3G). Sequences targeting the gene of interest (Supplementary Table S4) were cloned into the miR-155 hairpin by homologous recombination using the Infusion HD cloning kit (Clontech). The third-generation TET activator (TET3G) was cloned in a piggyBac vector under control of a constitutive CAG promoter. Cells were co-transfected with pBase helper plasmid and the two piggyBac vectors containing both the TRE3G-shRNA and the TET3G, and cultured in the presence of selection agent for 7 days prior to experimental assays.
Flow cytometry
After treatment with Accutase, live ES cells were resuspended in PBS with 3% FCS and 0.05 nM ToPro-3 (Invitrogen) was added at a concentration of 0.05 nM to detect dead cells. Flow cytometry analyses were performed using a Dako Cytomation CyAn ADP high-performance cytometer with Summit software.
Immunostaining
Cells were fixed for 10 min in 4% PFA at Room Temperature (RT), permeabilized for 5 min in PBS+0.2% Triton X at RT, and blocked for 30 min in PBS+3% donkey serum at RT. Cells were incubated overnight at 4°C with the primary antibodies (anti-Tcfcp2l1: rabbit IgG, Abcam 123354, 1:400; anti-Klf4: goat IgG, R&D Systems AF3158, 1:400; anti-Oct4: mouse IgG, Santa Cruz C-10, used at a 1:300 dilution). After washing in PBS, the cells were incubated with Alexa Fluor secondary antibodies (Invitrogen, 1:500 in PBS+3%serum), for 30 min at RT. After DAPI staining, images were acquired using a Zeiss AxioObserver D1 microscope. Automated single-cell image quantification was performed using CellProfile (Broad Institute—Carpenter et al, 2006).
Immunoblotting
Immunoblotting was performed as previously described in Yang et al (2010). Antibodies used are rabbit polyclonal anti-Tfcp2l1 (Abcam, AB123354, 1:500 dilution in 1% milk) and mouse monoclonal anti-GAPDH (Sigma-Aldrich, G8795, 1:1000 in 5% milk).
Supplementary Material
Acknowledgments
We thank Tüzer Kalkan, Jörg Betschinger, Ge Guo and Alison McGarvey for advice, reagents and help with experiments; Hans Schöler for providing GOF18 EpiSCs; William Mansfield, Charles-Etienne Dumeau, Peter Humphreys, and Andy Riddell for specialist technical support. We also thank the EMBL Genomics Core Facility for sequencing and Tamara Steijger for analysis of RNA-seq and ChIP-seq data. We are grateful to Qi Long Ying for sharing information prior to publication. This study was funded by the Biotechnology and Biological Sciences Research Council of the United Kingdom and the Swiss National Science Foundation Sinergia Programme. GM is recipient of a Human Frontier Science Program fellowship and AS is a Medical Research Council Professor.
Author contributions: GM and AS conceived the study and wrote the manuscript. GM performed and analysed the experiments. PB generated RNA-seq libraries and oversaw analysis.
Footnotes
The authors declare that they have no conflict of interest.
References
- Akira S, Nishio Y, Inoue M, Wang XJ, Wei S, Matsusaka T, Yoshida K, Sudo T, Naruto M, Kishimoto T (1994) Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell 77: 63–71 [DOI] [PubMed] [Google Scholar]
- Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernemann C, Greber B, Ko K, Sterneckert J, Han DW, Araúzo-Bravo MJ, Schöler HR (2011) Distinct developmental ground states of epiblast stem cell lines determine different pluripotency features. Stem Cells 29: 1496–1503 [DOI] [PubMed] [Google Scholar]
- Betschinger J, Nichols J, Dietmann S, Corrin PD, Paddison PJ, Smith A (2013) Exit from pluripotency is gated by intracellular redistribution of the bHLH transcription factor Tfe3. Cell 153: 335–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing Z, Reddy SA, Ren Y, Qin J, Liao WS (1999) Purification and characterization of the serum amyloid A3 enhancer factor. Cancer Res 274: 24649–24656 [DOI] [PubMed] [Google Scholar]
- Boeuf H, Hauss C, Graeve FD, Baran N, Kedinger C (1997) Leukemia inhibitory factor-dependent transcriptional activation in embryonic stem cells. J Cell Biol 138: 1207–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourillot P-Y, Aksoy I, Schreiber V, Wianny F, Schulz H, Hummel O, Hubner N, Savatier P (2009) Novel STAT3 target genes exert distinct roles in the inhibition of mesoderm and endoderm differentiation in cooperation with Nanog. Stem Cells 27: 1760–1771 [DOI] [PubMed] [Google Scholar]
- Brons IGM, Smithers LE, Trotter MWB, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448: 191–195 [DOI] [PubMed] [Google Scholar]
- Brook FA, Gardner RL (1997) The origin and efficient derivation of embryonic stem cells in the mouse. Proc Natl Acad Sci USA 94: 5709–5712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon T, Smith AG, Savatier P (2002) Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol 12: 432–438 [DOI] [PubMed] [Google Scholar]
- Burdon T, Stracey C, Chambers I, Nichols J, Smith AG (1999) Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev Biol 210: 30–43 [DOI] [PubMed] [Google Scholar]
- Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM (2006) CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 7: R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S (2005) LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 132: 885–896 [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith AG (2003) Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113: 643–655 [DOI] [PubMed] [Google Scholar]
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, Loh Y-H, Yeo HC, Yeo ZX, Narang V, Govindarajan KR, Leong B, Shahab A, Ruan Y, Bourque G, Sung W-K et al. (2008) Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133: 1106–1117 [DOI] [PubMed] [Google Scholar]
- Correa-Cerro LS, Piao Y, Sharov AA, Nishiyama A, Cadet JS, Yu H, Sharova LV, Xin L, Hoang HG, Thomas M, Qian Y, Dudekula DB, Meyers E, Binder BY, Mowrer G, Bassey U, Longo DL, Schlessinger D, Ko MSH (2011) Generation of mouse ES cell lines engineered for the forced induction of transcription factors. Sci Rep 1: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE (1997) STATs and gene regulation. Science 277: 1630–1635 [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292: 154–156 [DOI] [PubMed] [Google Scholar]
- Flicek P, Amode MR, Barrell D, Beal K, Brent S, Carvalho-Silva D, Clapham P, Coates G, Fairley S, Fitzgerald S, Gil L, Gordon L, Hendrix M, Hourlier T, Johnson N, Kähäri AK, Keefe D, Keenan S, Kinsella R, Komorowska M et al. (2012) Ensembl 2012. Nucleic Acids Res 40: D84–D90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths DS, Li J, Dawson MA, Trotter MWB, Cheng Y-H, Smith AM, Mansfield W, Liu P, Kouzarides T, Nichols J, Bannister AJ, Green AR, Göttgens B (2011) LIF-independent JAK signalling to chromatin in embryonic stem cells uncovered from an adult stem cell disease. Nat Cell Biol 13: 13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Huss M, Tong GQ, Wang C, Li Sun L, Clarke ND, Robson P (2010) Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev Cell 18: 675–685 [DOI] [PubMed] [Google Scholar]
- Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, Smith AG (2009) Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136: 1063–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Guo G, Wray J, Eyres I, Nichols J, Grotewold L, Morfopoulou S, Humphreys P, Mansfield W, Walker R, Tomlinson S, Smith A (2009) Oct4 and LIF/Stat3 additively induce Krüppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell 5: 597–609 [DOI] [PubMed] [Google Scholar]
- Han DW, Tapia N, Joo JY, Greber B, Araúzo-Bravo MJ, Bernemann C, Ko K, Wu G, Stehling M, Do JT, Schöler HR (2010) Epiblast stem cell subpopulations represent mouse embryos of distinct pregastrulation stages. Cell 143: 617–627 [DOI] [PubMed] [Google Scholar]
- Hanna JH, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R (2010) Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci USA 107: 9222–9227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Li T-G, Qi X, Zhao D-F, Zhao G-Q (2006) WNT/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev Biol 290: 81–91 [DOI] [PubMed] [Google Scholar]
- Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa A, Searle S, Barnes I, Bignell A, Boychenko V, Hunt T, Kay M, Mukherjee G, Rajan J, Despacio-Reyes G, Saunders G, Steward C et al. (2012) GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res 22: 1760–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C, Schafer X, Lun Y, Lemischka IR (2006) Dissecting self-renewal in stem cells with RNA interference. Nat Cell Biol 442: 533–538 [DOI] [PubMed] [Google Scholar]
- Jiang J, Chan Y-S, Loh Y-H, Cai J, Tong GQ, Lim C-A, Robson P, Zhong S, Ng H-H (2008) A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol 10: 353–360 [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch HG, Nichols J, Humphreys P, Mulas C, Martello G, Lee C, Jones K, Surami MA, Smith A (2013) Rebuilding Pluripotency from Primordial Germ Cells. Stem Cell Reports 1: 66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, McClintick J, Zhong L, Edenberg HJ, Yoder MC, Chan RJ (2005) Murine embryonic stem cell differentiation is promoted by SOCS-3 and inhibited by the zinc finger transcription factor Klf4. Blood 105: 635–637 [DOI] [PubMed] [Google Scholar]
- Lim LC, Fang L, Swendeman SL, Sheffery M (1993) Characterization of the molecularly cloned murine alpha-globin transcription factor CP2. Cancer Res 268: 18008–18017 [PubMed] [Google Scholar]
- Marks H, Kalkan T, Menafra R, Denissov S, Jones K, Hofemeister H, Nichols J, Kranz A, Stewart AF, Smith A, Stunnenberg HG (2012) The transcriptional and epigenomic foundations of ground state pluripotency. Cell 149: 590–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martello G, Sugimoto T, Diamanti E, Joshi A, Hannah R, Ohtsuka S, Göttgens B, Niwa H, Smith A (2012) Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell 11: 491–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 78: 7634–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, Yokota T (1999) STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J 18: 4261–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y, Zsebo K, Hogan BL (1992) Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell 70: 841–847 [DOI] [PubMed] [Google Scholar]
- Nichols J, Chambers I, Taga T, Smith A (2001) Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development 128: 2333–2339 [DOI] [PubMed] [Google Scholar]
- Nichols J, Evans EP, Smith AG (1990) Establishment of germ-line-competent embryonic stem (ES) cells using differentiation inhibiting activity. Development 110: 1341–1348 [DOI] [PubMed] [Google Scholar]
- Nichols J, Smith A (2012) Pluripotency in the embryo and in culture. Cold Spring Harb Perspect Biol 4: a008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Sharov AA, Piao Y, Amano M, Amano T, Hoang HG, Binder BY, Tapnio R, Bassey U, Malinou JN, Correa-Cerro LS, Yu H, Xin L, Meyers E, Zalzman M, Nakatake Y, Stagg C, Sharova L, Qian Y, Dudekula D et al. (2013) Systematic repression of transcription factors reveals limited patterns of gene expression changes in ES cells. Sci Rep 3: 1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Burdon T, Chambers I, Smith AG (1998) Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev 12: 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Ogawa K, Shimosato D, Adachi K (2009) A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature 460: 118–122 [DOI] [PubMed] [Google Scholar]
- O'Leary T, Heindryckx B, Lierman S, van Bruggen D, Goeman JJ, Vandewoestyne M, Deforce D, de Sousa Lopes SMC, De Sutter P (2012) Tracking the progression of the human inner cell mass during embryonic stem cell derivation. Nat Biotechnol 30: 278–282 [DOI] [PubMed] [Google Scholar]
- Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H (2006) Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun 343: 159–166 [DOI] [PubMed] [Google Scholar]
- Pelton TA, Sharma S, Schulz TC, Rathjen J, Rathjen PD (2002) Transient pluripotent cell populations during primitive ectoderm formation: correlation of in vivo and in vitro pluripotent cell development. J Cell Sci 115: 329–339 [DOI] [PubMed] [Google Scholar]
- Resnick JL, Bixler LS, Cheng L, Donovan PJ (1992) Long-term proliferation of mouse primordial germ cells in culture. Nature 359: 550–551 [DOI] [PubMed] [Google Scholar]
- Rodda SJ, Kavanagh SJ, Rathjen J, Rathjen PD (2002) Embryonic stem cell differentiation and the analysis of mammalian development. Int J Dev Biol 46: 449–458 [PubMed] [Google Scholar]
- Smith AG (2001) Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol 17: 435–462 [DOI] [PubMed] [Google Scholar]
- Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D (1988) Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 336: 688–690 [DOI] [PubMed] [Google Scholar]
- Tai C-I, Ying Q-L (2013) Gbx2, a LIF/Stat3 target, promotes reprogramming to and retention of the pluripotent ground state. J Cell Sci 126(Pt 5): 1093–1098 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Tang Y, Luo Y, Jiang Z, Ma Y, Lin C-J, Kim C, Carter MG, Amano T, Park J, Kish S, Tian XC (2012) Jak/Stat3 signaling promotes somatic cell reprogramming by epigenetic regulation. Stem Cells 30: 2645–2656 [DOI] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RDG (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448: 196–199 [DOI] [PubMed] [Google Scholar]
- To S, Rodda SJ, Rathjen PD, Keough RA (2010) Modulation of CP2 family transcriptional activity by CRTR-1 and sumoylation. PLoS One 5: e11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oosten AL, Costa Y, Smith A, Silva JCR (2012) JAK/STAT3 signalling is sufficient and dominant over antagonistic cues for the establishment of naive pluripotency. Nat Commun 3: 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welham MJ, Storm MP, Kingham E, Bone HK (2007) Phosphoinositide 3-kinases and regulation of embryonic stem cell fate. Biochem Soc Trans 35: 225–228 [DOI] [PubMed] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM (1988) Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 336: 684–687 [DOI] [PubMed] [Google Scholar]
- Wray J, Kalkan T, Smith AG (2010) The ground state of pluripotency. Biochem Soc Trans 38: 1027–1032 [DOI] [PubMed] [Google Scholar]
- Wu TD, Nacu S (2010) Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics 26: 873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, van Oosten AL, Theunissen TW, Guo G, Silva JCR, Smith A (2010) Stat3 activation is limiting for reprogramming to ground state pluripotency. Cell Stem Cell 7: 319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Ogura S, Ishida M, Karasawa M, Takada S (2005) Gene trap screening as an effective approach for identification of Wnt-responsive genes in the mouse embryo. Dev Dyn 233: 484–495 [DOI] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith AG (2008) The ground state of embryonic stem cell self-renewal. Nature 453: 519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Chambers I, Nichols J, Smith A, Saito M, Yasukawa K, Shoyab M, Taga T, Kishimoto T (1994) Maintenance of the pluripotential phenotype of embryonic stem cells through direct activation of gp130 signalling pathways. Mech Dev 45: 163–171 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biol 9: R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Wen Z, Darnell JE (1994) Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264: 95–98 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.