Abstract
Spt6 is a transcriptional elongation factor and histone chaperone that reassembles transcribed chromatin. Genome-wide H3 mapping showed that Spt6 preferentially maintains nucleosomes within the first 500 bases of genes and helps define nucleosome-depleted regions in 5′ and 3′ flanking sequences. In Spt6-depleted cells, H3 loss at 5′ ends correlates with reduced pol II density suggesting enhanced transcription elongation. Consistent with its ‘Suppressor of Ty’ (Spt) phenotype, Spt6 inactivation caused localized H3 eviction over 1–2 nucleosomes at 5′ ends of Ty elements. H3 displacement differed between genes driven by promoters with ‘open’/DPN and ‘closed’/OPN chromatin conformations with similar pol II densities. More eviction occurred on genes with ‘closed’ promoters, associated with ‘noisy’ transcription. Moreover, swapping of ‘open’ and ‘closed’ promoters showed that they can specify distinct downstream patterns of histone eviction/deposition. These observations suggest a novel function for promoters in dictating histone dynamics within genes possibly through effects on transcriptional bursting or elongation rate.
Keywords: chromatin, histone eviction/deposition, nucleosome occupancy, promoters, RNA pol II transcription, Spt6
Introduction
Nucleosome occupancy can affect transcription by governing the access of transcription factors to promoters and imposing an obstacle to elongating pol II within genes. Along each gene, chromatin structure is determined by the opposing forces of histone eviction and replacement that are coupled to transcription elongation (Kristjuhan and Svejstrup, 2004; Schwabisch and Struhl, 2004; Dion et al, 2007; Jamai et al, 2007; Rufiange et al, 2007; reviewed in Owen-Hughes and Gkikopoulos, 2012). Histone chaperones facilitate this replication-independent histone exchange and thereby help determine nucleosome occupancy (Schwabisch and Struhl, 2004, 2006; Adkins and Tyler, 2006; De Koning et al, 2007; Zaugg and Luscombe, 2012). Spt6 is a histone H3, H4 chaperone (Bortvin and Winston, 1996) that binds nucleosomes (Mcdonald et al, 2010) and is conserved between yeast and metazoans. Spt6 is localized throughout actively transcribed genes (Kim et al, 2004; Mayer et al, 2010) and binds directly to the phosphorylated C-terminal domain of pol II through its SH2 domains (Mayer et al, 2012). In seminal experiments, Spt6 inactivation was found to uncover transcription-dependent loss of nucleosomes and activation of cryptic promoters (Kaplan et al, 2003; Cheung et al, 2008). SPT6 was identified as a suppressor of the His− phenotype caused by insertion of a Ty δ element at the 5′ end of HIS4 (Winston et al, 1984). The basis for this suppression is not completely understood.
A study of 180 protein-coding genes on yeast ChrIII showed that Spt6 is most important for replacement of nucleosomes evicted from highly transcribed genes and that altered chromatin structure in the mutant does not always correlate with changes in mRNA abundance (Ivanovska et al, 2011). Spt6 inactivation also causes transcriptional derepression of some genes due to histone depletion at promoters (Adkins and Tyler, 2006; Jensen et al, 2008; Hainer et al, 2011; Ivanovska et al, 2011), but it is not known how generally Spt6 is required to maintain transcriptional repression. The fate of histones evicted from chromatin when Spt6 function is impaired is also not well understood; however, the pool of free histones increases (Morillo-Huesca et al, 2010).
Spt6 was first implicated as a transcription elongation factor by genetic interaction with TFIIS (Hartzog et al, 1998) and it can accelerate transcription of a naked DNA template (Endoh et al, 2004). On Drosophila heat-shock genes, Spt6 knock-down elevated pol II density consistent with slower elongation (Ardehali et al, 2009) but its effects on pol II occupancy have yet to be investigated genome-wide.
Replication-independent histone exchange is greatest at highly transcribed genes (Dion et al, 2007; Rufiange et al, 2007; Jamai et al, 2009) but in addition to pol II density within a gene, it has been suggested that there are additional unknown factors that govern rates of histone exchange (Gat-Viks and Vingron, 2009). One such factor may be elongation rate, since histone eviction in vitro is enhanced by faster transcription (Bintu et al, 2011). How Spt6 affects histone dynamics at fast and slow exchanging genes has not been determined. Moreover, it is unclear whether Spt6-mediated chromatin reassembly operates uniformly throughout transcribed genes or whether it is more active at specific locations.
In budding yeast, two major promoter classes are distinguished by their patterns of nucleosome occupancy (Tirosh and Barkai, 2008; Cairns, 2009; Zaugg and Luscombe, 2012). ‘Open’ promoters have a greater distance between the −1 and +1 nucleosomes (Zaugg and Luscombe, 2012) and they overlap extensively with the ‘depleted proximal nucleosome’ (DPN) class (Tirosh and Barkai, 2008). ‘Closed’ promoters have −1 and +1 nucleosomes closer together and they coincide extensively with the ‘occupied proximal nucleosome’ (OPN) class. ‘Open’/DPN promoters have fewer TATA boxes, more Htz1 in promoter nucleosomes, more constitutive transcription, and slower rates of histone turnover than ‘closed’/OPN promoters (Cairns, 2009). ‘Closed/OPN’ promoters are thought to drive ‘noisy’ expression associated with bursts of transcription initiation (Raser and O'Shea, 2004; Zenklusen et al, 2008; Chubb and Liverpool, 2010). It is not known whether transcribed chromatin downstream of ‘open’ and ‘closed’ promoters differs in its histone dynamics. In this report, we employed a new full-length ts degron mutant to investigate how Spt6 affects histone and pol II occupancy throughout the yeast genome. We found that while net histone eviction often occurs on highly expressed genes when Spt6 is inactivated, there is not always a correlation between histone loss and pol II density on a gene. Surprisingly, histone occupancy is affected differently within genes driven by ‘open’/DPN and ‘closed’/OPN promoters, suggesting a novel effect of promoters on histone eviction.
Results
Spt6 depletion in a degron mutant
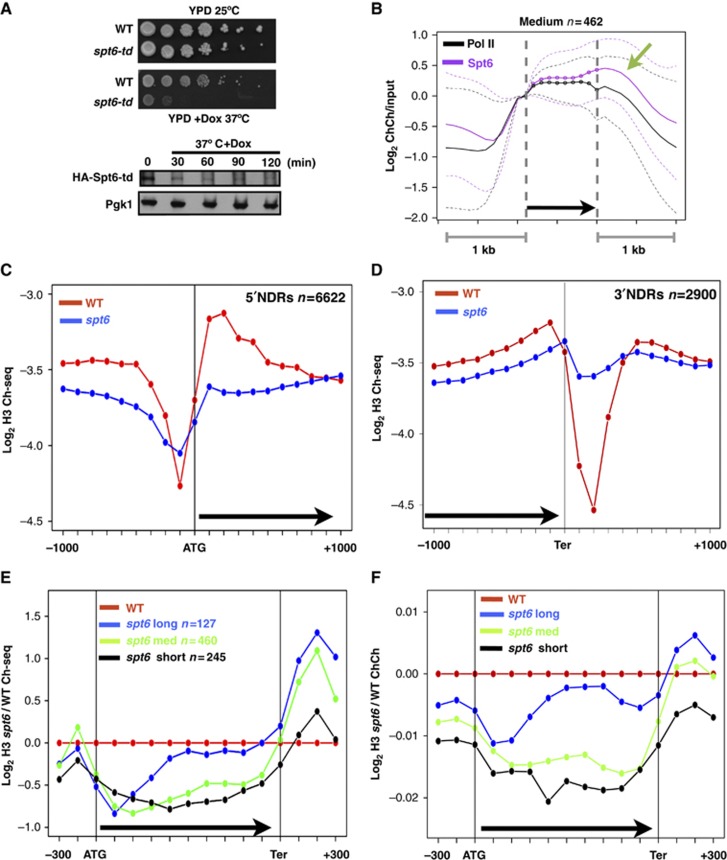
We investigated histone and pol II occupancy in a ts Spt6-degron mutant (spt6-td) under the control of a doxycycline (Dox) repressible promoter (Seward et al, 2007). This mutant expresses the full-length Spt6 fused to an N-terminal degron and grows normally at 25°C. Under non-permissive conditions (37°C+Dox), there is a severe growth defect and the protein is substantially depleted within 30 min (Figure 1A). Furthermore, Spt6 binding to genes as measured by ChIP is strongly diminished under non-permissive conditions (Supplementary Figure S1a and b, lanes 11 and 12). The Spt6-degron mutant has potential advantages over the spt6-1004 ts allele used previously (Kaplan et al, 2003; Adkins and Tyler, 2006; Jensen et al, 2008; Ivanovska et al, 2011) because the latter has an in-frame deletion of the helix-hairpin helix domain (residues 931–994). This deletion destabilizes the protein and causes transcriptional defects at the permissive temperature (Kaplan et al, 2005). Genome-wide ChIP-Chip of the HA-tagged Spt6-degron fusion protein showed that it was enriched throughout highly transcribed genes and is highest at 3′ ends (Figure 1B) in agreement with previous mapping of TAP-tagged Spt6 (Mayer et al, 2010). Spt6 was also enriched on non-coding genes for cryptic unstable transcripts (CUTs), stable unannotated transcripts (SUTs) and small nucleolar RNAs (snoRNAs) genes, but not on tRNA or rRNA genes (Supplementary Figure S2a–e).
Figure 1.
Spt6 degradation and histone eviction in the spt6-td ts degron mutant. (A) Upper panel: Growth defect of the spt6-td degron mutant (DBY875) relative to WT (DBY311) at 37°C+Doxycycline (Dox, 2 μg/ml). Lower panel: Western blot of HA-Spt6 degron fusion at time points after shifting to 37°C+Dox. Pgk1 is a loading control. (B) Average ChIP-Chip (ChCh) profiles of pol II and HA-Spt6 degron at 25°C normalized to the values at the start of the transcription unit on 462 highly transcribed genes 800–2000 bases long as described (Kim et al, 2010). Transcription units are divided into 10 equal intervals (dotted line) with 1 kb of 5′ and 3′ flanking sequences (smooth line). Note enrichment at the 3′ end (arrow). Dashed lines mark the central 80% of genes. (C, D) Average distributions of histone H3 ChIP-seq (Ch-seq) signals in the spt6-td and WT strains (37°C+Dox) at 5′ NDRs of all protein-coding genes and 3′ NDRs of 2900 genes as defined previously (Yadon et al, 2010). Note that within genes most of the H3 loss occurs within the first 500 bases. (E, F) Average distributions of H3 ChIP-seq and ChIP-Chip signals in the spt6-td degron normalized to WT (37°C+Dox) in highly transcribed short (<800 bp), medium (800–2000, bp) and long (>2000, bp) genes described previously (Kim et al, 2010). ORFs are divided into 10 equal bins. Note greater histone loss near 5′ ends and increased occupancy in 3′ flanking regions.
Spt6 maintains 5′ and 3′ nucleosome-depleted regions
To examine how Spt6 affects chromatin, we assayed H3 occupancy in independent ChIP-seq and ChIP-Chip experiments in isogenic WT and spt6-td strains shifted to 37°C+Dox for 1 h. Spt6 is required for nucleosome maintenance at several inducible promoters (Adkins and Tyler, 2006; Jensen et al, 2008; Ivanovska et al, 2011) and we examined how it affects histone occupancy at promoters generally (>6500 genes). Spt6 depletion caused the average dip in histone occupancy at promoter-associated nucleosome-depleted regions (NDRs) to flatten out and become less well defined (Figure 1C). A relative decrease in histone occupancy at the boundaries together with a small increase within the NDR are responsible for this effect. A similar flattening of the histone occupancy profile across the NDR was observed at ‘OPN’, ‘DPN’, ‘open’ and ‘closed’ promoters (Supplementary Figure S3a–d) (Tirosh and Barkai, 2008; Zaugg and Luscombe, 2012).
We also asked whether Spt6 affected 3′ NDRs near poly (A) sites (Mavrich et al, 2008; Fan et al, 2010; Yadon et al, 2010). Comparison of average H3 ChIP profiles around 2900 3′ ends with NDRs (Yadon et al, 2010) showed that removal of Spt6 strongly diminished the extent of nucleosome depletion at 3′ ends so that 3′ NDRs like 5′ NDRs became less well defined. Similar results were obtained in independent ChIP-Chip and ChIP-seq experiments (Figure 1D; Supplementary Figure S3e). Together, these observations show that Spt6 functions widely throughout the genome to maintain NDRs at 5′ and 3′ ends of genes.
Transcription and histone eviction in Spt6-depleted cells
As expected, degron-mediated depletion of Spt6 diminished histone occupancy within many protein-coding genes. Our results agreed well with those for the spt6-1004 ts mutant at 86 genes on chromosome III where histone loss occurred (Ivanovska et al, 2011) (Supplementary Figure S2f). Comparison of highly transcribed coding regions showed extensive H3 loss throughout the length of short genes (<800 bp), whereas on longer genes there is greater loss at 5′ ends than at 3′ ends in both ChIP-seq and ChIP-Chip experiments (Figure 1E and F). Most H3 displacement in Spt6-depleted cells occurred within the first 500 bases of the transcription unit (Figure 1C). Unexpectedly relative histone occupancy in the 3′ flanking regions was enhanced when Spt6 was depleted, especially for longer genes (Figure 1E and F). These results suggest that although Spt6 is most abundant at 3′ ends, the majority of Spt6-dependent nucleosome replacement occurs within the first 500 bases of most genes. They furthermore suggest the possibility that histones evicted from within genes when Spt6 is compromised may be deposited in intergenic regions downstream of genes.
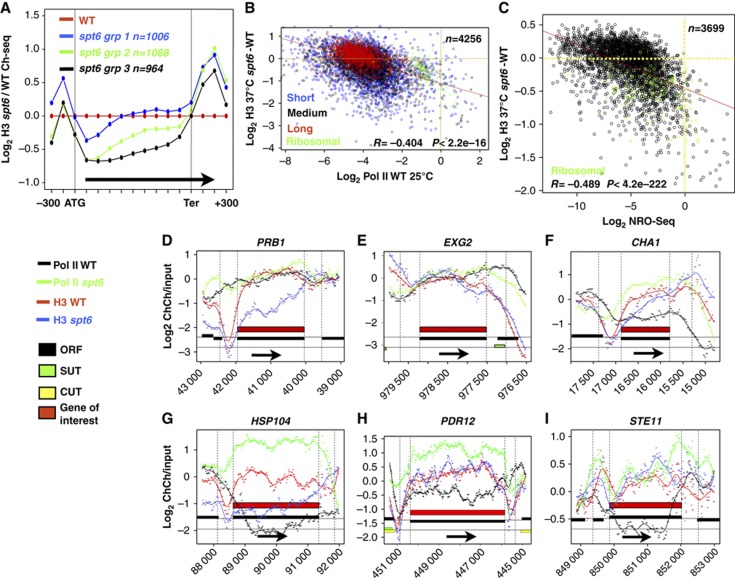
To investigate the relationship between Spt6-dependent histone deposition/eviction and transcription, we plotted H3 occupancy at 37°C in spt6-td relative to WT in three groups of genes (1–3) (Venters and Pugh, 2009) with low, intermediate and high expression, respectively. As expected, the greatest histone loss occurred among the most highly transcribed genes (group 3, Figure 2A; Supplementary Figure S3f). To examine this relationship in greater detail, we plotted the change in average H3 occupancy within genes caused by Spt6 depletion, against average pol II occupancy in WT cells at 25°C as determined by ChIP-seq, or transcriptional activity as determined by global nuclear runon sequencing (NRO-seq) (Mckinlay et al, 2011) (Figure 2B and C). The results show significantly greater histone loss in more highly transcribed genes with the strongest correlation between histone loss and nuclear runon signal. Average histone loss across genes is less on long genes (>2 kb, red dots in Figure 2B) probably because it occurs mostly near their 5′ ends (Figure 1E and F). We noted that among cytoplasmic ribosomal protein genes there is more heterogeneity of NRO-seq signals than for pol II ChIP signals (Figure 2B and C, green dots) consistent with the suggestion that not all polymerases have equivalent transcriptional activity (Pelechano et al, 2009). The discordance that can occur between pol II density and H3 loss in the spt6 mutant is exemplified by the PRB1 and EXG2 genes. PRB1 is strongly depleted of H3 in the absence of Spt6, whereas EXG2 is relatively unaffected, yet they have similar levels of pol II occupancy (Figure 2D and E).
Figure 2.
The relation of histone eviction to transcription in the spt6-td ts degron mutant. (A) Average distributions of histone H3 ChIP-seq signals in spt6-td normalized to WT as in Figure 1E for genes with low, intermediate and high expression (groups 1–3) (Venters and Pugh, 2009). (B, C) Plots of the change (spt6-td—WT) in H3 ChIP-ChIP signal (37°C+Dox) versus pol II ChIP-seq signal in W303 at 25°C (B) or NRO-seq signal (C) (Mckinlay et al, 2011) averaged over the length of each gene (see Supplementary Table S1). Short (<800 bp), medium (800–2000, bp), long genes (>2000, bp) and cytoplasmic ribosomal protein genes are marked. Gene numbers (n), regression lines and Pearson’s correlations are shown. (D–I) Diverse effects of Spt6 depletion on histone and pol II occupancy. Plots of log2 Chip signals relative to input DNA and smoothed curves for pol II and H3 in WT and spt6-td degron (37°C+Dox). Black arrows mark the direction of transcription. (D, E) PRB1 and EXG2 with similar pol II densities differ greatly in the extent of H3 loss in spt6-td. (F–I) Transcriptional derepression in spt6-td is associated with histone displacement at CHA1 and HSP104 as previously reported (Jensen et al, 2008; Ivanovska et al, 2011) but not at PDR12 or STE11 where cryptic transcripts are induced in spt6-1004 (Cheung et al, 2008).
Spt6 inactivation can either downregulate or upregulate transcription. We investigated the effects on transcription by comparing pol II ChIP-Chip in WT and spt6-td strains. We identified 553 genes where average pol II ChIP signals were reduced by >2.0-fold in spt6-td relative to two replicates of WT (DBY311 37°C, this paper and previously published Kim et al, 2010) (Supplementary Table S1) including the Dox-repressed SPT6 gene itself and CLN3, which is also downregulated in spt6-1004 (Morillo-Huesca et al, 2010) (Supplementary Figure S4a). We also found 109 genes whose average pol II ChIP signal was elevated by >2.0-fold in the spt6-td mutant relative to WT at 37°C (Supplementary Table S1) including CHA1 and HSP104 (Figure 2F and G) that are also derepressed in spt6-1004 (Jensen et al, 2008; Ivanovska et al, 2011) and the sporulation-specific genes SGA1, AMA1 and SPO20 (Supplementary Figure S4b; Supplementary Table S1). Among these upregulated genes are 18 that produce cryptic transcripts in spt6-1004 (Cheung et al, 2008) including STE11 (Figure 2I). The amount of histone H3 loss within the ORF varied widely among upregulated genes (Supplementary Table S1; Figure 2F–H) and we did not find that histone depletion within ORFs correlated with transcriptional derepression (Supplementary Figure S4c). In summary, though transcription is necessary for histone eviction when Spt6 function is impaired (Kaplan et al, 2003), these results show that the extent of histone loss is not always related in a simple way to average pol II density.
Spt6 and transcription elongation
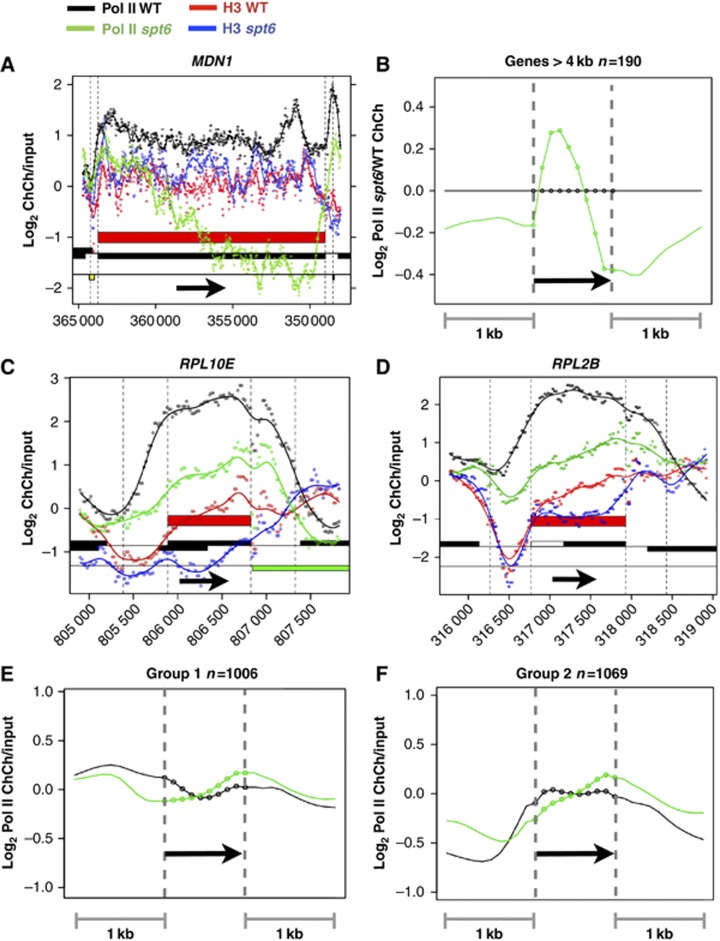
We examined how Spt6 affects transcription elongation by comparing profiles of pol II density along genes in the WT and Spt6-degron strains under non-permissive conditions. On exceptionally long genes (>4 kb) such as MDN1, Spt6 depletion reduced pol II density at the 3′ end relative to the 5′ end suggesting impaired transcriptional elongation, without much effect on histone H3 density (Figure 3A and B). This observation is consistent with a chromatin-independent stimulation of elongation by Spt6 (Endoh et al, 2004). The most common effect of Spt6 depletion on pol II localization, however, was to reduce its density at 5′ ends relative to 3′ ends. This shift in pol II distribution occurred on both poorly and highly transcribed genes including many ribosomal protein genes (Figure 3C–F) and is consistent with faster transcription elongation at 5′ ends where histones are preferentially lost.
Figure 3.
Effects of Spt6 depletion on transcription elongation. (A, B) On exceptionally long genes Spt6 inactivation causes an apparent processivity defect characterized by reduced pol II density at 3′ ends. (B) Average distributions of pol II in spt6-td normalized to WT (37°C+Dox) on protein-coding genes >4 kb long (Supplementary Table S1). (C–F) Reduced pol II occupancy at 5′ relative to 3′ ends in spt6-td compared to WT. (E, F) Average distributions of pol II in WT and spt6-td (37°C+Dox) at genes with low and intermediate levels of transcription (groups 1 and 2 as in Figure 2A) normalized to the average value in the body of the gene.
Highly localized histone maintenance by Spt6 at Ty elements
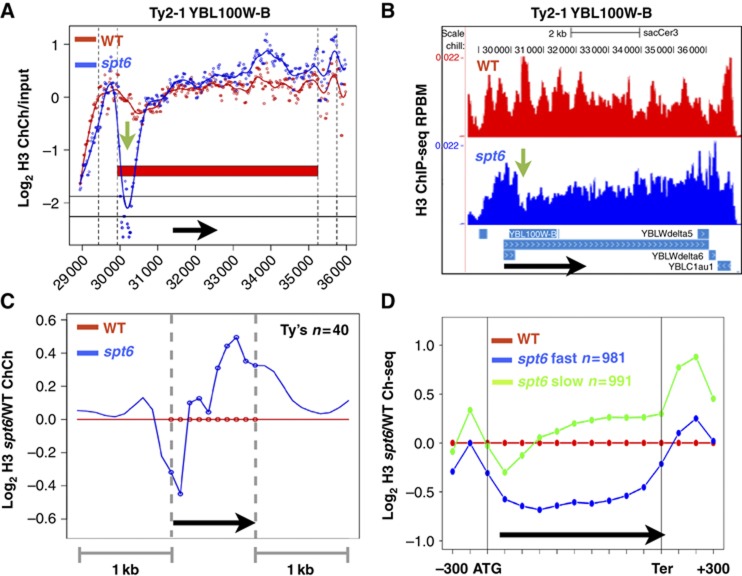
SPT6 mutants were discovered as suppressors of a δ insertion at the 5′ end of HIS4 (Winston et al, 1984) and we therefore examined H3 occupancy on Ty and δ elements. Although ChIP signals are not specific to individual Ty elements because of their repetitive nature, the results of independent ChIP-Chip and ChIP-seq experiments nevertheless established that on average there is localized displacement of H3 in a discrete region corresponding to 1–2 nucleosomes ∼200–500 bases downstream of the TSS when Spt6 is depleted (Figure 4A–C; Supplementary Figure S4d). These results suggest that Spt6 can act in a highly localized way to maintain histone occupancy within a transcription unit. The histone-depleted region that forms within Ty elements when Spt6 is compromised could have cryptic promoter function that would account for the Spt phenotype.
Figure 4.
(A, B) Localized loss of H3 over a region of 1–2 nucleosomes (green arrows) at the 5′ end of Ty2-1 in independent ChIP-Chip and ChIP-seq experiments. Note that ChIP-Chip signals at repetitive sequences will be affected by cross-hybridization. Reads per base pair per million mapped reads (RPBM). (C) Average distributions of histone H3 ChIP-Chip signals for the spt6-td degron relative to WT at 40 full-length Ty elements (Supplementary Table S1). (D) Average distributions of histone H3 ChIP-seq signals in the spt6-td degron and WT strains (37°C+Dox). The 1000 genes with the fastest and slowest exchange rates averaged over the ORF and 5′ and 3′ flanking regions (Rufiange et al, 2007) are analysed (Supplementary Table S1). Note greater eviction on fast exchanging genes when Spt6 is inactivated.
Distinct effects of Spt6 at genes with fast and slow histone exchange rates
Replication-independent histone exchange in yeast is pervasive at promoters and within highly transcribed genes (Dion et al, 2007; Rufiange et al, 2007). We compared H3 occupancy within groups of ∼1000 genes with the fastest and slowest rates of replication-independent histone exchange (Supplementary Table S1; Rufiange et al, 2007) in WT and Spt6-depleted cells and found a marked difference between them. At ‘fast’ exchanging genes relative H3 occupancy declined as expected, whereas at ‘slow’ exchanging genes, it was elevated (Figure 4D). Much the difference between how Spt6 inactivation affects chromatin on fast and slow exchanging genes is probably explained by the higher pol II occupancy on ‘fast’ exchanging genes (Dion et al, 2007; Rufiange et al, 2007) (data not shown). In summary, these results suggest that Spt6-mediated histone replacement is a major determinant of nucleosome occupancy within genes with high histone exchange rates where active displacement correlates with high pol II occupancy.
Promoter-specific effects on histone occupancy in spt6
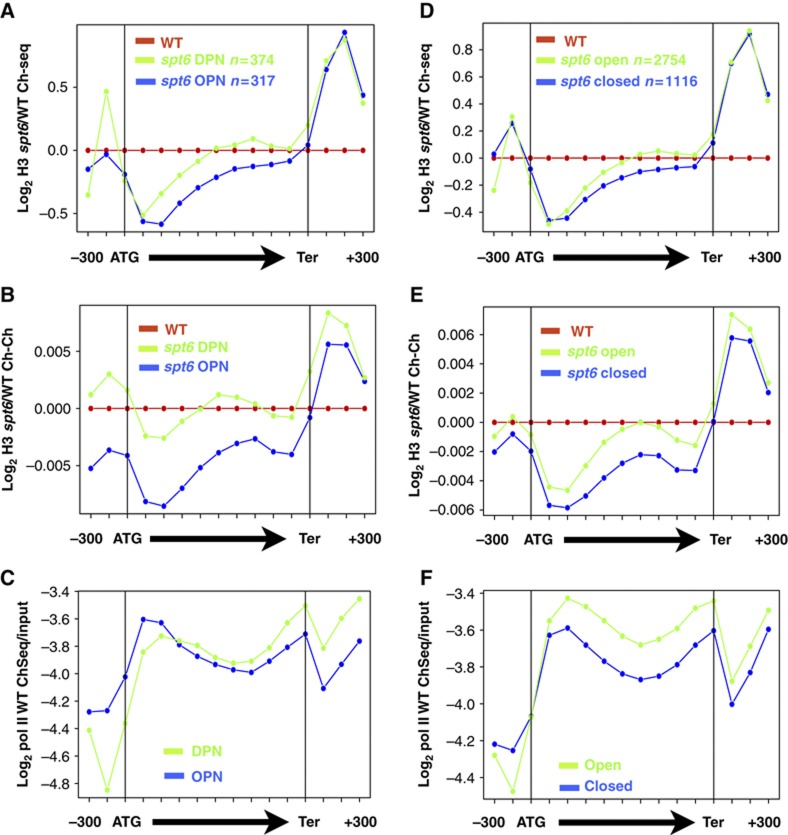
We asked whether promoter structure might influence histone eviction by comparing subsets of genes with open/DPN and closed/OPN promoters (Tirosh and Barkai, 2008; Zaugg and Luscombe, 2012) that have approximately equivalent average pol II occupancy. At DPN genes, there was modest histone loss in the ORF when Spt6 was depleted, whereas at OPN genes there was greater loss, particularly near their 5′ ends. Independent anti-H3 ChIP-Chip and ChIP-seq experiments yielded equivalent results (Figure 5A and B). Similarly, genes driven by ‘open’ promoters had less histone eviction that those driven by ‘closed’ promoters (Figure 5D and E). The distinct patterns of histone eviction on genes driven by different promoter classes are not explained by differences in their pol II density. In fact, there was slightly higher pol II density on the DPN/open genes that have less histone eviction (Figure 5C and F). Nor are the different histone eviction patterns correlated with differences in histone occupancy in WT cells (Supplementary Figure S5a and b) or gene length. (Median gene lengths for ‘open’, ‘closed’, ‘DPN’ and ‘OPN’ genes are 1552, 1480, 1378 and 1465 bases.) The OPN/Closed genes analysed had somewhat lower Spt6 occupancy that the DPN/Open genes (Supplementary Figure S5c and d), in accordance with their slightly lower pol II densities (Figure 5C and F). Together, the results suggest that histone eviction within coding regions is influenced by promoters in a way that is independent of average pol II density.
Figure 5.
Promoter-specific differences in histone eviction. Average histone H3 ChIP-seq (A, D) and ChIP-Chip (B, E) signals in spt6-td relative to WT (37°C+Dox) for genes driven by DPN versus OPN (Tirosh and Barkai, 2008) and ‘open’ versus ‘closed’ promoters (Zaugg and Luscombe, 2012). Subsets of OPN and DPN, open and closed genes >750 bp were selected with approximately equivalent average pol II densities as shown in (C, F) (see Supplementary Table S1). See Supplementary Figure S5a and b for relative H3 occupancy in WT. (C, F) Average pol II densities on selected DPN versus OPN and ‘open’ versus ‘closed’ promoters determined by pol II ChIP-seq in W303 at 25°C. Note greater histone eviction on ‘closed’/OPN genes does not correlate with greater pol II occupancy.
Promoters influence histone occupancy within genes
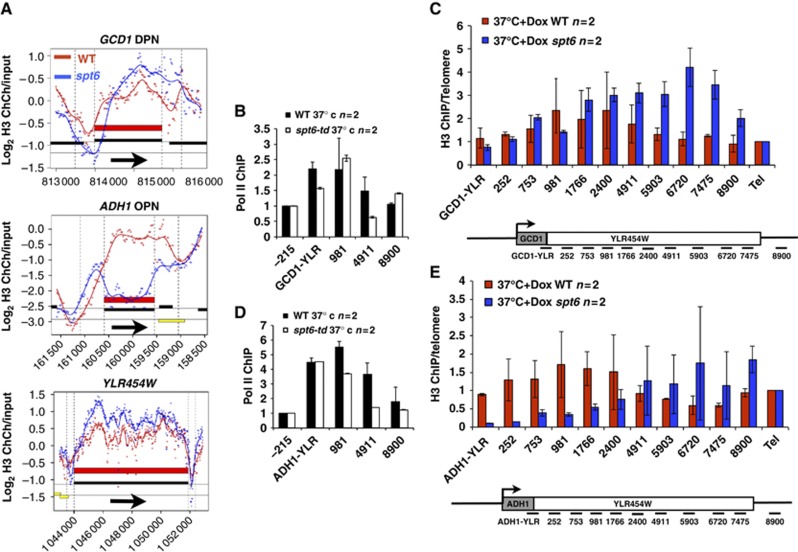
To test whether promoters can influence downstream histone occupancy, we constructed spt6-td strains in which the promoter of the chromosomal YLR454W gene was swapped for that of GCD1 or ADH1. GCD1 has an ‘open’ (DPN) promoter whereas ADH1 has a ‘closed’ (OPN) promoter. The YLR454W promoter that was replaced is of the ‘closed’ class but its response to Spt6 inactivation (Figure 6A bottom panel) is atypical. The effects of Spt6 depletion on H3 occupancy at the endogenous GCD1 and ADH1 genes (Figure 6A) are typical of their respective gene classes (Figure 5). Both GCD1 and ADH1 promoters drove higher levels of expression than the endogenous YLR454W promoter as shown by RT–PCR (Supplementary Figure S5e) and pol II ChIP signals were somewhat higher on the ADH1-YLR454W gene (Figure 6B and D) fragment. Transcription from both promoters was only modestly affected when Spt6 was inactivated (Figures 6B and D). We assayed H3 occupancy on the GCD1- and ADH1-YLR454W genes in isogenic WT and spt6-td strains at 37°C+doxycycline. ChIP results were quantified by Q-PCR with a panel of 11 amplicons relative to a telomere control (Figure 6C and E). Remarkably, Spt6 depletion had distinct effects on chromatin within the common YLR454W sequence in the two chimaeras. In the GCD1-YLR454W gene, H3 occupancy was little affected at the 5′ end, but increased at the 3′ end (amplicons 1766–8900) when Spt6 was depleted (Figure 6C). In contrast, at ADH1-YLR454W, H3 occupancy decreased markedly at the 5′ end (amplicons ADH1-YLR—2400) and increased at the 3′ end (amplicons 5903–8900, Figure 6E). These changes in histone occupancy resembled the changes within the ADH1 and GCD1 genes themselves in spt6-td. Thus, at GCD1, the major effect of Spt6 inactivation was an enrichment of H3 over the 3′ portion of the gene (Figure 6A) similar to the GCD1-YLR454W chimaera (Figure 6C). At ADH1, H3 was strongly depleted throughout the gene (Figure 6A) and at ADH1-YLR454W it was also depleted, but the effect was restricted to the 5′ end (amplicons ADH1-YLR—2400, Figure 6E). Similar results were obtained for chimaeras of YLR454W driven by the APA1 (YCL050C, DPN) and PDC1 (OPN) promoters (Supplementary Figure S6). Spt6 inactivation had only a small effect at APA1-YLR454W with notably little loss of H3 occupancy, similar to the GCD1 fusion and DPN genes as a whole. In contrast, both fusions driven by OPN promoters, PDC1 and ADH1, responded to Spt6 inactivation with H3 depletion at the 5′ end and enrichment at the 3′ end. While we cannot eliminate the possibility that differences in pol II occupancy within YLR454W fusion genes play some role, the promoter-swapping experiments are fully consistent with the idea that the differences in histone dynamics between closed/OPN and open/DPN genes (Figure 5) correlate with their promoters rather than their coding sequences.
Figure 6.
Promoters can influence histone eviction/deposition within genes. (A) Histone H3 occupancy at GCD1, ADH1 and YLR454W in the spt6-td degron and WT strains (B, D) Pol II ChIP for the integrated GCD1- and ADH1-YLR454W genes in isogenic spt6-td strains DBY1327, 1330 and WT strains DBY1369, 1370 at 37°C+Dox quantified by Q-PCR. Signals were normalized to the YLR454W −215 flanking region. Means of PCRs from two biological replicates (two PCRs of each) and standard deviations (s.d.) are shown. Values refer to positions of the centre of the amplicons relative to the ATG of YLR454W. GCD1-YLR and ADH1-YLR are amplicons that span the boundary of these sequences at the 5′ end. (C, E) Histone H3 ChIP Q-PCR normalized to telomere VIR (TEL) for the GCD1- and ADH1-YLR454W genes in WT and spt6-td strains at 37°C+Dox. Maps of the amplicons are not to scale. Means of PCRs from two biological replicates (three PCRs of each) with s.d. are shown. Note the distinct effects of Spt6 inactivation on H3 occupancy within YLR454W driven by different promoters.
Discussion
We investigated how the histone chaperone and transcriptional elongation factor, Spt6, affects histone H3 and pol II occupancy in budding yeast using a new ts degron mutant (Figure 1A). Our results show that Spt6 functions widely throughout the genome to maintain NDRs both at promoters and at 3′ ends. In Spt6-depleted cells, NDRs became less well defined as manifested by flatter H3 occupancy profiles with loss from their upstream and downstream boundary regions and some deposition within the depleted regions (Figure 1C and D). Together, the results suggest that Spt6 helps to deposit or stabilize nucleosomes that flank NDRs and to prevent nucleosome encroachment within NDRs. This function of Spt6 complements other chaperones and remodellers including Asf1, Isw2, RSC and SWI/SNF that prevent histone accumulation within NDRs (Whitehouse et al, 2007; Badis et al, 2008; Parnell et al, 2008; Hartley and Madhani, 2009; Tolkunov et al, 2011).
In agreement with previous work on selected genes (Kaplan et al, 2003; Jensen et al, 2008; Ivanovska et al, 2011), our genome-wide study revealed net histone eviction within highly transcribed genes in Spt6-depleted cells. In addition, we found that under these conditions, eviction occurs preferentially at genes with fast rates of histone exchange. Our results revealed a marked 5′-3′ bias with most histone eviction occurring within the first 500 bases of the transcription unit when Spt6 was depleted (Figures 1E, F and 4D). This 5′-3′ bias is consistent with the idea that transcription complexes that have elongated a short distance are more effective at displacing nucleosomes than those that have elongated a long distance. It will be of interest in future to examine whether the dynamics of elongation factor recruitment or CTD phosphorylation influence co-transcriptional nucleosome displacement.
We noted that relative histone occupancy was elevated at specific loci in Spt6-depleted cells particularly within genes with slow rates of histone exchange, and in 3′ flanking intergenic regions (Figure 1E, F, 4D and 6C; Supplementary Figures S2g, S4e and f). One possible explanation for this observation is that when Spt6 activity is low, histones evicted from actively transcribed regions could be re-deposited elsewhere by other chaperones such as Asf1 and FACT (De Koning et al, 2007).
Spt6 inactivation caused remarkably highly localized histone depletion over a region corresponding to 1–2 nucleosomes near the 5′ ends of Ty and δ elements about 200–500 bases from the TSS (Figure 4A–C; Supplementary Figure S4d). This phenomenon may explain why spt6 mutants were first isolated as suppressors of the transcriptional defect caused by insertion of a δ element at the 5′ end of HIS4 (Winston et al, 1984). We speculate that induction of such a discrete NDR at a δ element could rescue HIS4 transcription from a cryptic promoter by providing access to the pol II transcriptional apparatus. The DNA sequence of the H3-depleted region in Ty elements does not have a low predicted nucleosome occupancy score (Field et al, 2008). Why this region is so exquisitely dependent on Spt6 for nucleosome maintenance is an interesting open question.
We observed two effects of Spt6 depletion on pol II distribution that likely reflect changes in transcription elongation. On a few exceptionally long genes, pol II density declined 5′-3′ in the absence of Spt6 suggesting that it is required to maintain processivity over long distances (Figure 3A and B). More generally, Spt6 depletion depressed pol II density at 5′ ends relative to 3′ ends, a bias that coincides with greater histone loss at 5′ ends. Together, these results suggest that removal of 5′ nucleosomes permits faster transcriptional elongation and hence lower steady-state pol II occupancy. Co-transcriptional pre-mRNA splicing in yeast is thought to be coordinated with pol II pausing (Oesterreich et al, 2011). It is possible that disrupted pausing near 5′ ends contributes to the spt6-associated splicing defect (Burckin et al, 2005).
Histone loss in spt6 cells requires ongoing transcription (Kaplan et al, 2003); however, our results show that there is not a straightforward relationship between net histone eviction and the density of pol II on a gene. Histone loss correlates with pol II density and more strongly with nuclear runon signals (Figure 2B and C). However, there is considerable variation in histone eviction among genes with similar transcriptional activities (Figure 2), suggesting that additional factors influence this phenomenon (Gat-Viks and Vingron, 2009).
We investigated whether promoters help determine histone occupancy within genes when Spt6 is depleted. There are two major classes of promoters in yeast with distinct chromatin conformations and transcription characteristics (Tirosh and Barkai, 2008; Cairns, 2009; Zaugg and Luscombe, 2012). ‘Open’ promoters with ‘depleted proximal nucleosomes’ (DPN) are predominantly found at constitutively expressed genes lacking TATA boxes. ‘Closed’ promoters with ‘occupied proximal nucleosomes’ (OPN) often have TATA boxes, are more sensitive to regulation by chromatin remodellers, and drive ‘noisy’ expression associated with bursts of transcription initiation (Raser and O'Shea, 2004; Zenklusen et al, 2008; Chubb and Liverpool, 2010). A major conclusion of this report is that chromatin within genes driven by these two classes of promoter is affected differently by Spt6 depletion. Within genes driven by DPN/open promoters, there was relatively modest histone eviction when Spt6 was inactivated whereas at genes driven by OPN/closed promoters with equivalent or lower pol II density, eviction was greater and extended further towards 3′ ends (Figure 5). This connection between promoter class and downstream histone occupancy suggests that promoters in some way influence chromatin dynamics within their cognate transcription units. A second possibility is that sequence elements within the genes driven by DPN/‘open’ and OPN/‘closed’ promoters specify different levels of histone occupancy in the absence of the Spt6 chaperone. To distinguish between these possibilities, we performed a promoter swapping experiment. We compared two chromosomal reporter genes with a common transcribed sequence, the YLR454W ORF, driven by open/DPN (GCD1, APA1) or closed/OPN (ADH1, PDC1) promoters. This experiment revealed distinct outcomes for how histone occupancy was affected by Spt6 inactivation, strongly suggesting that promoters can influence chromatin dynamics within adjacent transcribed sequences (Figure 6; Supplementary Figure S6). Moreover, the changes in histone occupancy within YLR454W sequences in the chimaeric genes, approximated those in the cognate natural genes when Spt6 was depleted (Figure 6; Supplementary Figure S6 compare a, c and e). The latter result suggests that the nature of a promoter might exert a qualitative effect on histone dynamics within the adjacent transcription unit. This promoter effect on histone occupancy is not easily accounted for by differences in average pol II density within the downstream gene.
Promoters have been suggested to control aspects of gene expression beyond the amount of pol II that loads onto a gene including CTD phosphorylation (Kim et al, 2010), alternative splicing (Cramer et al, 1997; Kornblihtt, 2005) and mRNA degradation (Bregman et al, 2011; Trcek et al, 2011). Our results raise the question ‘How could promoters specify different patterns of chromatin dynamics within a common downstream sequence?’ One possibility is that they affect Spt6 recruitment or activity, however, we did not observe a marked difference between Spt6 levels relative to pol II on ‘closed’/OPN versus ‘open’/DPN genes (Supplementary Figure S5c and d). Promoters could also control recruitment of elongation factors that might affect histone eviction. Pol II transcription elongation in metazoans is influenced by transcription factors, promoters and enhancers (Bentley, 1995) and histone eviction in vitro is enhanced by faster elongation (Bintu et al, 2011). Alternatively, promoters could affect histone eviction by controlling the timing of initiation events which can occur as uncorrelated events or as bursts (Chubb and Liverpool, 2010). Transcription factors and promoter elements influence bursting activity in mammalian cells (Stavreva et al, 2009; Suter et al, 2011). In yeast, transcriptional bursting and consequent noisy expression is associated with TATA box-containing promoters (Raser and O'Shea, 2004; Zenklusen et al, 2008) with ‘closed’/OPN chromatin conformations where we observed the most extensive histone eviction (Figure 5). We suggest that clusters of pol II molecules at 5′ ends of genes resulting from bursts of initiation are more effective at evicting nucleosomes than single isolated polymerases. This idea is consistent with the strong 5′-3′ bias in histone depletion evident on long genes (Figure 1E and F) and with greater depletion on genes driven by ‘noisy’ promoters in spt6-td cells (Figure 5). Precedent for this model comes from the fact that pairs of E. coli RNA polymerase molecules cooperate to elongate through a nucleosome barrier better than single polymerases (Jin et al, 2010). It will be of interest in future to investigate how transcriptional bursting and elongation rate affect histone exchange within genes.
Materials and methods
Yeast strains
Yeast strains used in this study are described in Table I. The Spt6-td-3HA strain (DBY875) was constructed in two steps: first, the C-terminal HA3 C-terminal tag was added by integration of a PCR fragment amplified from pFA-3HA-kanMX6 into DBY311 to make DBY871. Second, the N-terminal ts degron Spt6 fusion plasmid (pRS306 Spt6-td) was integrated into DBY871 as described (Seward et al, 2007) at the endogenous SPT6 locus. We cannot exclude the possibility that substitution of the tet repressible promoter alters Spt6 expression under permissive conditions (25°C); however, we did not detect a large effect on HA-Spt6 ChIP signals relative to the globin recovery control (Supplementary Figure S1a and b compare lanes 9 and 11). YLR454W promoter swapping plasmids were constructed by insertion of PCR-amplified promoter regions of GCD1 (−154 to +150 relative to the ATG), ADH1 (−840 to +240), APA1 (YCL050C, −517 to +150) and PDC1 (−889 to +141) into the BamH1–SacI sites of pFL44S YLR454W-hygro that contains two fragments of YLR454W (−590 to −149 and +1 to +522) with flanking NotI sites situated on either side of the hygromycin resistance gene. NotI fragments excised from the plasmids were integrated into DBY875 and DBY311 (Table I).
Table 1. Yeast strains used in this study.
| Strain | Parent | Genotype | Reference |
|---|---|---|---|
| DBY311 | W303-1a | MATa ura3-1 his3-11 leu2-3,112 ade2-1 trp1-1 LEU2::pCM245 TetR-SSN6 HIS3::pRS403HIS3::pRS403-188 CMV tTA [pFL45SUBR1] | Seward et al (2007) |
| DBY871 | DBY311 | MATa ura3-1 his3-11 leu2-3,112 ade2-1 trp1-1 LEU2::pCM245 TetR-SSN6 HIS3::pRS403HIS3::pRS403-188 CMV tTA [pFL45SUBR1] SPT6::HA3Kan | This study |
| DBY875 | DBY871 | MATa ura3-1 his3-11 leu2-3,112 ade2-1 trp1-1 LEU2::pCM245 TetR-SSN6 HIS3::pRS403HIS3::pRS403-188 CMV tTA [pFL45SUBR1] spt6tet-degronHA3::URA3, Kan | This study |
| DBY1327 | DBY875 | MATa ura3-1 his3-11 leu2-3,112 ade2-1 trp1-1 LEU2::pCM245 TetR-SSN6 HIS3::pRS403HIS3::pRS403-188 CMV tTA [pFL45SUBR1] spt6tet-degronHA3::URA3, Kan GCD1-YLR454W::Hygro | This study |
| DBY1330 | DBY875 | MATa ura3-1 his3-11 leu2-3,112 ade2-1 trp1-1 LEU2::pCM245 TetR-SSN6 HIS3::pRS403HIS3::pRS403-188 CMV tTA [pFL45SUBR1] spt6tet-degronHA3::URA3, Kan ADH1-YLR454W::Hygro | This study |
| DBY1369 | DBY311 | MATa ura3-1 his3-11 leu2-3,112 ade2-1 trp1-1 LEU2::pCM245 TetR-SSN6 HIS3::pRS403HIS3::pRS403-188 CMV tTA [pFL45SUBR1] ADH1-YLR454W::Hygro | This study |
| DBY1370 | DBY311 | MATa ura3-1 his3-11 leu2-3,112 ade2-1 trp1-1 LEU2::pCM245 TetR-SSN6 HIS3::pRS403HIS3::pRS403-188 CMV tTA [pFL45SUBR1] GCD1-YLR454W::Hygro | This study |
| DBY1392 | DBY311 | MATa ura3-1 his3-11 leu2-3,112 ade2-1 trp1-1 LEU2::pCM245 TetR-SSN6 HIS3::pRS403HIS3::pRS403-188 CMV tTA [pFL45SUBR1] PDC1- YLR454W::Hygro | This study |
| DBY1396 | DBY311 | MATa ura3-1 his3-11 leu2-3,112 ade2-1 trp1-1 LEU2::pCM245 TetR-SSN6 HIS3::pRS403HIS3::pRS403-188 CMV tTA [pFL45SUBR1] APA1-YLR454W::Hygro | This study |
| DBY1398 | DBY875 | MATa ura3-1 his3-11 leu2-3,112 ade2-1 trp1-1 LEU2::pCM245 TetR-SSN6 HIS3::pRS403HIS3::pRS403-188 CMV tTA [pFL45SUBR1] spt6tet-degronHA3::URA3, Kan APA1-YLR454W::Hygro | This study |
| DBY1402 | DBY875 | MATa ura3-1 his3-11 leu2-3,112 ade2-1 trp1-1 LEU2::pCM245 TetR-SSN6 HIS3::pRS403HIS3::pRS403-188 CMV tTA [pFL45SUBR1] spt6tet-degronHA3::URA3, Kan PDC1- YLR454W::Hygro | This study |
ChIP
Cells were grown in YPD at 25°C to OD ∼0.8 and crosslinked for 15 min at room temperature in 1% formaldehyde for ChIP as described (Perales et al, 2011). Because we used total sheared chromatin rather than micrococcal nuclease-resistant fragments, our analysis does not permit localization of individual positioned nucleosomes. For inactivation of the Spt6 degron, cells were pelleted and resuspended in YPD at 37°C with 2–10 μg/ml doxycycline (Dox) and incubated at 37°C for 1 h. Q-PCR and primers have been described (Zhang et al, 2005; Perales et al, 2011) and are available on request.
ChIP-Chip and ChIP-seq
For anti-pol II and anti-H3 ChIP-Chip of DBY311 and DBY875, immunoprecipitated DNA was amplified by ligation-mediated PCR (23 cycles) and hybridized to Nimblegen whole genome arrays (cat. no. C4214-00-01 with 378 684 50mers tiled every 32 bases) and analysis with the ChIP-Viewer program using the Tk/Tcl platform implemented in the R statistics package as described (Kim et al, 2010). Log2 ratios of ChIP signal/input were scaled to be centred around zero as described (Nimblegen Epigenetics Userguide) and were averaged over 20 base intervals and smoothed using the locpoly Gaussian kernel algorithm in the R package. Note that in the figures Y axes for overlaid log2 ChIP signals may differ between genes. The H3 ChIP-Chip signals from overlapping sets of 50mers within the arrays were highly reproducible (Supplementary Figure S1e and f). For metagene analysis, data points within each gene were scaled and averaged over 10 equal intervals with fixed lengths of 5′ and 3′ flanking sequences. Normalization of ChIP-Chip results according to Peng et al (2007) did not alter any of the results reported here.
ChIP-seq libraries were made as described (Kim et al, 2011) in experiments independent of the ChIP-Chip. True-Seq indices were added in the final PCR amplification (18 cycles) and sequencing was done on the Illumina HiSeq platform at UCDenver. Single-end reads (after removing barcodes) were mapped to the SacCer3 genome (April 2011, GeneBank Assembly GCA_00146045.2) with Bowtie version 0.12.5 (Langmead et al, 2009). Using option –m2 in Bowtie, we obtained 1.33 × 107 (92% mapping) reads for anti-pol II in W303 (25°C), and 2.11 × 107 (90% mapping) and 1.01 × 107 (90% mapping) reads for anti-H3 ChIP-seq in DBY871 (WT, 37°C) and DBY1330 (spt6-td 37°C), respectively. Signals are expressed as reads per bin per million mapped reads (RPBM). For mapping to repetitive elements, we used the following bowtie options: ‘-m 2 --best –strata’. The numbers of read counts were weighted by the inverse of the number of multiple hits. Bedgraph profiles were made using 50 bp bins assuming a 200-bp fragment size shifting effect. There was good agreement between ChIP-Q-PCR, ChIP-ChIP and ChIP-seq results (Supplementary Figure S1a–d). For meta-analysis, genes were divided into bins as defined in the figures and for each bin the mean number of aligned reads per bp normalized to the total number of mapped reads for the genes analysed was calculated. In some cases, the number of genes included for meta-analysis of H3 ChIP-seq was slightly less than that for ChIP-Chip because those with extensive regions lacking reads were excluded. Data sets have been deposited at GEO accession GSE49928.
Antibodies
Rabbit anti-total pol II (pan-CTD) and H3 C-terminus were previously described (Zhang et al, 2005). Anti-HA was 12CA5 (Roche) and monoclonal anti-Pgk1 was from Molecular Probes.
Supplementary Material
Acknowledgments
This work was supported by NIH grant GM063873 to DB. HK was supported by ARRA award 3R01GM063873-06S1. We thank H Welsford for help with strain construction, J Tyler, J Hesselberth, P Megee and A Johnson for valuable discussions, A Yadon and T Tsukiyama for information on 3′ NDRs and C Araya and S Fields for NRO-seq data and the UC Denver sequencing facility.
Author contributions: RP, LZ and DB designed the experiments; RP, LZ, BE and EV performed ChIP and strain construction; RP, BE and HK performed data analysis; RP and DB wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adkins MW, Tyler JK (2006) Transcriptional activators are dispensable for transcription in the absence of Spt6-mediated chromatin reassembly of promoter regions. Mol Cell 21: 405–416 [DOI] [PubMed] [Google Scholar]
- Ardehali MB, Yao J, Adelman K, Fuda NJ, Petesch SJ, Webb WW, Lis JT (2009) Spt6 enhances the elongation rate of RNA polymerase II in vivo. EMBO J 28: 1067–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badis G, Chan ET, van Bakel H, Pena-Castillo L, Tillo D, Tsui K, Carlson CD, Gossett AJ, Hasinoff MJ, Warren CL, Gebbia M, Talukder S, Yang A, Mnaimneh S, Terterov D, Coburn D, Li Yeo A, Yeo ZX, Clarke ND, Lieb JD et al. (2008) A library of yeast transcription factor motifs reveals a widespread function for Rsc3 in targeting nucleosome exclusion at promoters. Mol Cell 32: 878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D (1995) Regulation of transcriptional elongation by RNA polymerase II. Curr Opin Genet Dev 5: 210–216 [DOI] [PubMed] [Google Scholar]
- Bintu L, Kopaczynska M, Hodges C, Lubkowska L, Kashlev M, Bustamante C (2011) The elongation rate of RNA polymerase determines the fate of transcribed nucleosomes. Nat Struct Mol Biol 18: 1394–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortvin A, Winston F (1996) Evidence that Spt6p controls chromatin structure by a direct interaction with histones. Science 272: 1473–1476 [DOI] [PubMed] [Google Scholar]
- Bregman A, Avraham-Kelbert M, Barkai O, Duek L, Guterman A, Choder M (2011) Promoter elements regulate cytoplasmic mRNA decay. Cell 147: 1473–1483 [DOI] [PubMed] [Google Scholar]
- Burckin T, Nagel R, Mandel-Gutfreund Y, Shiue L, Clark TA, Chong JL, Chang TH, Squazzo S, Hartzog G, Ares M Jr (2005) Exploring functional relationships between components of the gene expression machinery. Nat Struct Mol Biol 12: 175–182 [DOI] [PubMed] [Google Scholar]
- Cairns BR (2009) The logic of chromatin architecture and remodelling at promoters. Nature 461: 193–198 [DOI] [PubMed] [Google Scholar]
- Cheung V, Chua G, Batada NN, Landry CR, Michnick SW, Hughes TR, Winston F (2008) Chromatin- and transcription-related factors repress transcription from within coding regions throughout the Saccharomyces cerevisiae genome. PLoS Biol 6: e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb JR, Liverpool TB (2010) Bursts and pulses: insights from single cell studies into transcriptional mechanisms. Curr Opin Genet Dev 20: 478–484 [DOI] [PubMed] [Google Scholar]
- Cramer P, Pesce C, Baralle F, Kornblihtt A (1997) Functional association between promoter structure and transcripts alternative splicing. Proc Natl Acad Sci USA 94: 11456–11460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koning L, Corpet A, Haber JE, Almouzni G (2007) Histone chaperones: an escort network regulating histone traffic. Nat Struct Mol Biol 14: 997–1007 [DOI] [PubMed] [Google Scholar]
- Dion MF, Kaplan T, Kim M, Buratowski S, Friedman N, Rando OJ (2007) Dynamics of replication-independent histone turnover in budding yeast. Science 315: 1405–1408 [DOI] [PubMed] [Google Scholar]
- Endoh M, Zhu W, Hasegawa J, Watanabe H, Kim DK, Aida M, Inukai N, Narita T, Yamada T, Furuya A, Sato H, Yamaguchi Y, Mandal SS, Reinberg D, Wada T, Handa H (2004) Human Spt6 stimulates transcription elongation by RNA polymerase II in vitro. Mol Cell Biol 24: 3324–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Moqtaderi Z, Jin Y, Zhang Y, Liu XS, Struhl K (2010) Nucleosome depletion at yeast terminators is not intrinsic and can occur by a transcriptional mechanism linked to 3′-end formation. Proc Natl Acad Sci USA 107: 17945–17950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field Y, Kaplan N, Fondufe-Mittendorf Y, Moore IK, Sharon E, Lubling Y, Widom J, Segal E (2008) Distinct modes of regulation by chromatin encoded through nucleosome positioning signals. PLoS Comput Biol 4: e1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat-Viks I, Vingron M (2009) Evidence for gene-specific rather than transcription rate-dependent histone H3 exchange in yeast coding regions. PLoS Comput Biol 5: e1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainer SJ, Pruneski JA, Mitchell RD, Monteverde RM, Martens JA (2011) Intergenic transcription causes repression by directing nucleosome assembly. Genes Dev 25: 29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley PD, Madhani HD (2009) Mechanisms that specify promoter nucleosome location and identity. Cell 137: 445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzog GA, Wada T, Handa H, Winston F (1998) Evidence that Spt4, Spt5, and Spt6, control transcription elongation by RNA polymerase II in Saccharomyces cerevisiae. Genes Dev 12: 357–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovska I, Jacques PE, Rando OJ, Robert F, Winston F (2011) Control of chromatin structure by spt6: different consequences in coding and regulatory regions. Mol Cell Biol 31: 531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamai A, Imoberdorf RM, Strubin M (2007) Continuous histone H2B and transcription-dependent histone H3 exchange in yeast cells outside of replication. Mol Cell 25: 345–355 [DOI] [PubMed] [Google Scholar]
- Jamai A, Puglisi A, Strubin M (2009) Histone chaperone spt16 promotes redeposition of the original h3-h4 histones evicted by elongating RNA polymerase. Mol Cell 35: 377–383 [DOI] [PubMed] [Google Scholar]
- Jensen MM, Christensen MS, Bonven B, Jensen TH (2008) Requirements for chromatin reassembly during transcriptional downregulation of a heat shock gene in Saccharomyces cerevisiae. FEBS J 275: 2956–2964 [DOI] [PubMed] [Google Scholar]
- Jin J, Bai L, Johnson DS, Fulbright RM, Kireeva ML, Kashlev M, Wang MD (2010) Synergistic action of RNA polymerases in overcoming the nucleosomal barrier. Nat Struct Mol Biol 17: 745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan CD, Holland MJ, Winston F (2005) Interaction between transcription elongation factors and mRNA 3′-end formation at the Saccharomyces cerevisiae GAL10-GAL7 locus. J Biol Chem 280: 913–922 [DOI] [PubMed] [Google Scholar]
- Kaplan CD, Laprade L, Winston F (2003) Transcription elongation factors repress transcription initiation from cryptic sites. Science 301: 1096–1099 [DOI] [PubMed] [Google Scholar]
- Kim H, Erickson B, Luo W, Seward D, Graber JH, Pollock DD, Megee PC, Bentley DL (2010) Gene-specific RNA polymerase II phosphorylation and the CTD code. Nat Struct Mol Biol 17: 1279–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Ahn SH, Krogan NJ, Greenblatt JF, Buratowski S (2004) Transitions in RNA polymerase II elongation complexes at the 3′ ends of genes. EMBO J 23: 354–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim H, Fong N, Erickson B, Bentley DL (2011) Pre-mRNA splicing is a determinant of histone H3K36 methylation. Proc Natl Acad Sci USA 108: 13564–13569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt AR (2005) Promoter usage and alternative splicing. Curr Opin Cell Biol 17: 262–268 [DOI] [PubMed] [Google Scholar]
- Kristjuhan A, Svejstrup JQ (2004) Evidence for distinct mechanisms facilitating transcript elongation through chromatin in vivo. EMBO J 23: 4243–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrich TN, Ioshikhes IP, Venters BJ, Jiang C, Tomsho LP, Qi J, Schuster SC, Albert I, Pugh BF (2008) A barrier nucleosome model for statistical positioning of nucleosomes throughout the yeast genome. Genome Res 18: 1073–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Heidemann M, Lidschreiber M, Schreieck A, Sun M, Hintermair C, Kremmer E, Eick D, Cramer P (2012) CTD tyrosine phosphorylation impairs termination factor recruitment to RNA polymerase II. Science 336: 1723–1725 [DOI] [PubMed] [Google Scholar]
- Mayer A, Lidschreiber M, Siebert M, Leike K, Söding J, Cramer P (2010) Uniform transitions of the general RNA polymerase II transcription complex. Nat Struct Mol Biol 17: 1272–1278 [DOI] [PubMed] [Google Scholar]
- Mcdonald SM, Close D, Xin H, Formosa T, Hill CP (2010) Structure and Biological Importance of the Spn1-Spt6 Interaction, and Its Regulatory Role in Nucleosome Binding. Mol Cell 40: 725–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckinlay A, Araya CL, Fields S, Gasch A (2011) Genome-wide analysis of nascent transcription in Saccharomyces cerevisiae. G3 1: 549–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillo-Huesca M, Maya D, Muñoz-Centeno MC, Singh RK, Oreal V, Reddy GU, Liang D, Géli V, Gunjan A, Chávez S (2010) FACT prevents the accumulation of free histones evicted from transcribed chromatin and a subsequent cell cycle delay in G1. PLoS Genet 6: e1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterreich FC, Bieberstein N, Neugebauer KM (2011) Pause locally, splice globally. Trends Cell Biol 21: 328–335 [DOI] [PubMed] [Google Scholar]
- Owen-Hughes T, Gkikopoulos T (2012) Making sense of transcribing chromatin. Curr Opin Cell Biol 24: 296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell TJ, Huff JT, Cairns BR (2008) RSC regulates nucleosome positioning at Pol II genes and density at Pol III genes. EMBO J 27: 100–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelechano V, Jimeno-González S, Rodríguez-Gil A, García-Martínez J, Pérez-Ortín JE, Chávez S (2009) Regulon-specific control of transcription elongation across the yeast genome. PLoS Genet 5: e1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Alekseyenko AA, Larschan E, Kuroda MI, Park PJ (2007) Normalization and experimental design for ChIP-chip data. BMC Bioinformatics 8: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales R, Zhang L, Bentley D (2011) Histone occupancy in vivo at the 601 nucleosome binding element is determined by transcriptional history. Mol Cell Biol 31: 3485–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raser JM, O'Shea EK (2004) Control of stochasticity in eukaryotic gene expression. Science 304: 1811–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufiange A, Jacques P-E, Bhat W, Robert F, Nourani A (2007) Genome-wide replication-independent histone H3 exchange occurs predominantly at promoters and implicates H3 K56 acetylation and Asf1. Mol Cell 27: 393–405 [DOI] [PubMed] [Google Scholar]
- Schwabisch M, Struhl K (2004) Evidence for eviction and rapid deposition of histones upon transcriptional elongation by RNA polymerase II. Mol Cell Biol 24: 10111–10117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabish MA, Struhl K (2006) Asf1 mediates histone eviction and deposition during elongation by RNA polymerase II. Mol Cell 22: 415–422 [DOI] [PubMed] [Google Scholar]
- Seward DJ, Cubberley G, Kim S, Schonewald M, Zhang L, Tripet B, Bentley DL (2007) Demethylation of trimethylated histone H3 Lys4 in vivo by JARID1 JmjC proteins. Nat Struct Mol Biol 14: 240–242 [DOI] [PubMed] [Google Scholar]
- Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA, Pooley JR, Johnson TA, Voss TC, Lightman SL, Hager GL (2009) Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol 11: 1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter DM, Molina N, Gatfield D, Schneider K, Schibler U, Naef F (2011) Mammalian genes are transcribed with widely different bursting kinetics. Science 332: 472–474 [DOI] [PubMed] [Google Scholar]
- Tirosh I, Barkai N (2008) Two strategies for gene regulation by promoter nucleosomes. Genome Res 18: 1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolkunov D, Zawadzki KA, Singer C, Elfving N, Morozov AV, Broach JR (2011) Chromatin remodelers clear nucleosomes from intrinsically unfavorable sites to establish nucleosome-depleted regions at promoters. Mol Biol Cell 22: 2106–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trcek T, Larson Daniel R, Moldón A, Query Charles C, Singer Robert H (2011) Single-Molecule mRNA decay measurements reveal promoter- regulated mRNA stability in yeast. Cell 147: 1484–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venters BJ, Pugh BF (2009) A canonical promoter organization of the transcription machinery and its regulators in the Saccharomyces genome. Genome Res 19: 360–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse I, Rando OJ, Delrow J, Tsukiyama T (2007) Chromatin remodelling at promoters suppresses antisense transcription. Nature 450: 1031–1035 [DOI] [PubMed] [Google Scholar]
- Winston F, Chaleff DT, Valent B, Fink GR (1984) Mutations affecting Ty-mediated expression of the HIS4 gene of Saccharomyces cerevisiae. Genetics 107: 179–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadon AN, Van de Mark D, Basom R, Delrow J, Whitehouse I, Tsukiyama T (2010) Chromatin remodeling around nucleosome-free regions leads to repression of noncoding RNA transcription. Mol Cell Biol 30: 5110–5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg JB, Luscombe NM (2012) A genomic model of condition-specific nucleosome behavior explains transcriptional activity in yeast. Genome Res 22: 84–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenklusen D, Larson DR, Singer RH. (2008) Single-RNA counting reveals alternative modes of gene expression in yeast. Nat Struct Mol Biol 15: 1263–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Schroeder S, Fong N, Bentley DL. (2005) Altered nucleosome occupancy and histone H3K4 methylation in response to 'transcriptional stress'. EMBO J 24: 2379–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.