Significance
Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which codes for a chloride/bicarbonate channel whose absence leads to dehydration and acidification of CF airways. A contributing factor to CF lung disease is dysregulation of the epithelial Na+ channel (ENaC), which exacerbates mucus dehydration. Here, we show that ENaC hyperactivity in CF airways is direct consequence of acidic airway surface liquid (ASL) and that ASL hydration is restored by raising ASL pH. Additionally, we show that short palate lung and nasal epithelial clone 1, the most abundant gene in airway epithelia, is the extracellular pH-sensitive factor that inhibits ENaC in normal but not CF airways. We suggest that future CF therapy be directed toward raising the pH of CF airways.
Keywords: bacterial permeability-increasing protein, ion channels, COPD innate defense
Abstract
The ability to maintain proper airway surface liquid (ASL) volume homeostasis is vital for mucus hydration and clearance, which are essential aspects of the mammalian lung’s innate defense system. In cystic fibrosis (CF), one of the most common life-threatening genetic disorders, ASL dehydration leads to mucus accumulation and chronic infection. In normal airways, the secreted protein short palate lung and nasal epithelial clone 1 (SPLUNC1) effectively inhibits epithelial Na+ channel (ENaC)-dependent Na+ absorption and preserves ASL volume. In CF airways, it has been hypothesized that increased ENaC-dependent Na+ absorption contributes to ASL depletion, and hence increased disease. However, this theory is controversial, and the mechanism for abnormal ENaC regulation in CF airways has remained elusive. Here, we show that SPLUNC1 is a pH-sensitive regulator of ENaC and is unable to inhibit ENaC in the acidic CF airway environment. Alkalinization of CF airway cultures prevented CF ASL hyperabsorption, and this effect was abolished when SPLUNC1 was stably knocked down. Accordingly, we resolved the crystal structure of SPLUNC1 to 2.8 Å. Notably, this structure revealed two pH-sensitive salt bridges that, when removed, rendered SPLUNC1 pH-insensitive and able to regulate ASL volume in acidic ASL. Thus, we conclude that ENaC hyperactivity is secondary to reduced CF ASL pH. Together, these data provide molecular insights into the mucosal dehydration associated with a range of pulmonary diseases, including CF, and suggest that future therapy be directed toward alkalinizing the pH of CF airways.
The epithelial Na+ channel (ENaC) is the rate-determining step for Na+ absorption across the colon, kidney, and lung (1). ENaC is a heterotrimer consisting of α-, β-, and γ-subunits (2). The extracellular domains of the α- and γ-ENaC subunits must be proteolytically cleaved by serine proteases, such as trypsin or neutrophil elastase, or by intracellular furin-type convertases in order for the channel to become active and to conduct Na+ (2, 3). In contrast, the β-subunit is highly glycosylated and not cleaved but may form a regulatory subunit that governs ENaC surface densities (1, 4). In some cases, ENaC may bypass the steps required for proteolysis and be inserted into the plasma membrane as near-silent, inactive channels (5). Abnormal ENaC activity has been linked with the pathogenesis of several diseases, including cystic fibrosis (CF), Liddle syndrome, and salt-sensitive hypertension (6). In CF airways, the absence of functional cystic fibrosis transmembrane conductance regulator (CFTR) in the apical plasma membrane causes ENaC hyperactivity, and the resulting excessive Na+ absorption contributes to airway surface liquid (ASL) dehydration, mucus stasis, and bacterial infections (7). Similar lung disorders have been observed in transgenic mice either overexpressing β-ENaC or exhibiting altered regulation of ENaC by the ubiquitin protein ligase NEDD4L, thus linking Na+ hyperabsorption and ASL volume depletion to the development of pulmonary disease (4, 8). However, whether or not ENaC activity is up-regulated in CF airways is currently controversial (9). Part of this problem may lie in the lack of an identified mechanism for ENaC hyperactivity in CF airways. Thus, although ENaC has been shown to be up-regulated in vivo, in freshly isolated human airway tissues, and in cell culture, no mechanism for this up-regulation has been discovered (10–13).
Normal but not CF airway cultures autoregulate ASL volume by coordinating CFTR and ENaC activity via soluble “reporter molecules” that, by virtue of their dilution and concentration, transmit information on ASL volume to the epithelia (14). The short palate lung and nasal epithelial clone 1 (SPLUNC1), the most abundant secreted protein in the airways, is one such reporter molecule and is absolutely required for limiting ENaC activity and maintaining normal ASL homeostasis (14, 15). We have previously shown that SPLUNC1 binds to ENaC, causing it to be internalized, thus protecting ENaC from proteolytic cleavage and activation (15). SPLUNC1 is also a secreted protein that has been proposed to share homology with the N-terminal domain of bacterial permeability-increasing protein (BPI) (16).
Despite the presence of SPLUNC1 in CF ASL (17, 18), CF airway cultures are unable to regulate ENaC (19). Importantly, CF ENaC is not fully dysfunctional and is sensitive to exogenous protease inhibitors, suggesting that the defect lies elsewhere (19). CFTR conducts HCO3−, which maintains ASL at near-neutral pH, and when CFTR is defective (20), the ASL becomes more acidic (21). Because SPLUNC1–ENaC interactions are extracellular, we hypothesized that an acidified ASL prevented SPLUNC1 from inhibiting ENaC. To test this hypothesis, we examined the relationship between SPLUNC1-dependent regulation of ENaC and the ASL pH. To explore this interaction further, we resolved the crystal structure of SPLUNC1 to ∼2.8 Å and used this structure to understand better how ENaC is regulated in CF airway cultures.
Results
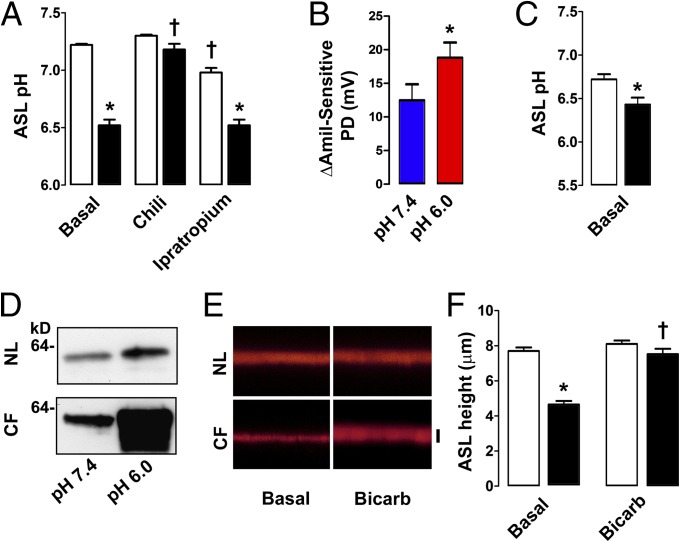
As previously described, we found that SPLUNC1 was present in CF ASL (17, 19) (Fig. S1). We then verified that CF ASL pH was abnormally low in CF subjects. In agreement with previous studies (21, 22), CF ASL pH was ∼0.5 units lower than normal ASL pH in vivo (Fig. 1A). Stimulation of glandular secretion with chili peppers abolished this difference, which is consistent with a previous report in which the pH of glandular secretions derived from normal subjects and subjects with CF was not different (23), and inhibition of glands with ipratropium preserved it (Fig. 1A). Together, these data indicate that the surface epithelial cells are responsible for the observed pH difference.
Fig. 1.
Bicarbonate addition increases ASL height in CF airways. (A) CF ASL pH is reduced in vivo. Nasal secretions obtained under basal conditions (n = 18 normal subjects and 7 subjects with CF) or after chili pepper alone (n = 7 normal subjects and 5 subjects with CF) or ipratropium alone (n = 7 normal subjects and 5 subjects with CF) exposure. (B) Amiloride (Amil)-sensitive PD was measured following nostril exposure to pH 7.4 or pH 6.0 Ringer solution (n = 7 normal subjects). (C) ASL pH was measured in normal and CF airway cultures (n = 12 for both). (D) Representative Western blots showing cleaved surface γ-ENaC 1 h postexposure to pH 6.0 or pH 7.4 solution. NL, normal. (E) Representative x–z confocal images showing preservation of ASL (red) on CF airway cultures following exposure to 20 μL of Ringer solution or modified Ringer solution with 100 mM NaHCO3− instead of 100 mM NaCl. Bicarb, bicarbonate. (F) Mean data from E (n = 8 for all). Open bars represent normal subjects, and closed bars represent subjects with CF. *P < 0.05, difference between normal subjects and subjects with CF; †P < 0.05, difference from control subjects. (Scale bar: 7 μm.) Error bars represent SE.
To determine if ASL pH has functional effects on ENaC activity in vivo, we measured the amiloride-sensitive potential difference (PD) at pH 7.4 vs. 6.0 as a marker of ENaC activity. Lowering the pH significantly raised the amiloride-sensitive PD in normal subjects, indicating that ENaC is sensitive to changes in extracellular pH in vivo (Fig. 1B). Normal and CF airway cultures replicate the in vivo pH difference (Fig. 1C). CF airway cultures show significantly increased ENaC proteolysis (12). To test if ENaC proteolysis was pH-dependent, we clamped normal and CF ASL pH at 6.0 or 7.4 for 1 h (Fig. S2 A and B) and then performed apical surface biotinylation followed by Western blot analysis using an antibody that recognizes cleaved γ-ENaC. In both normal and CF airway cultures, ENaC cleavage was greater at acidic pH (Fig. 1D and Fig. S3). Raising ASL HCO3− levels has been proposed as a therapy for the treatment of acidic CF ASL (24). To test whether alkalization prevented CF ASL hyperabsorption, we added 20 μL of high-bicarbonate, isotonic Ringer solution mucosally. This maneuver had no effect on normal ASL height, which remained at ∼7 μm (Fig. 1 E and F). However, the addition of bicarbonate Ringer solution slowed ASL absorption on CF airway culture surfaces (i.e., height was kept in the range that enables cilia to beat and clear mucus), suggesting that ASL pH has functional consequences for ENaC activity and ASL volume regulation.
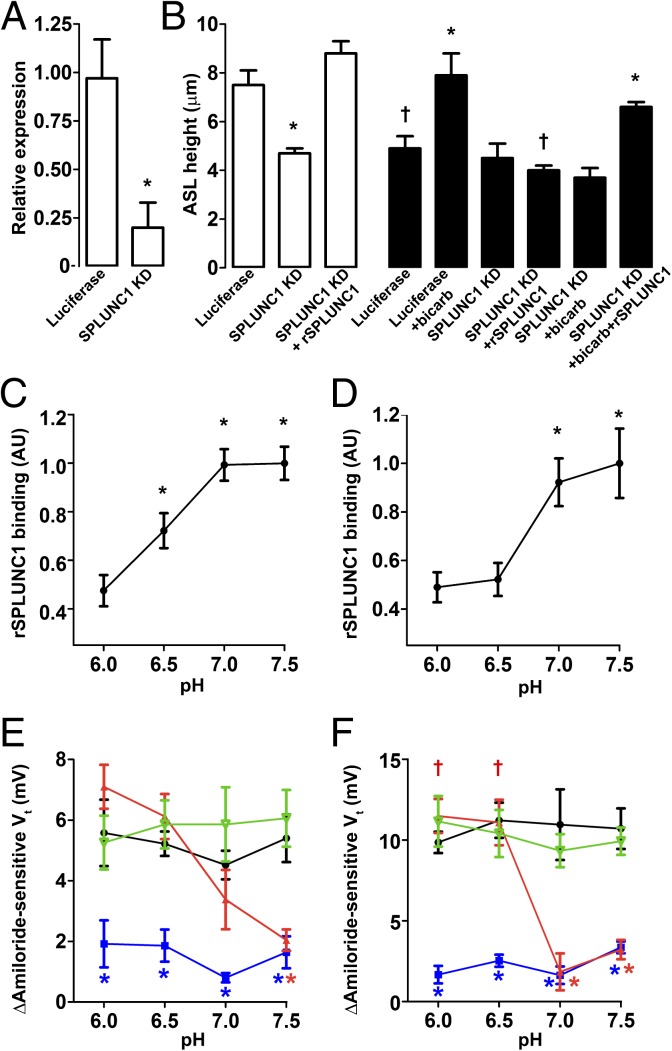
Because ENaC cleavage and ASL height regulation were pH-sensitive, we next tested whether SPLUNC1 regulated ENaC in a pH-dependent manner. As described (15), stable knockdown of SPLUNC1 abolished ASL height regulation in normal airway cultures and was restored with recombinant SPLUNC1 (rSPLUNC1; Fig. 2 A and B). In contrast, neither SPLUNC1 knockdown nor rSPLUNC1 addition had any effect on CF ASL height, suggesting that SPLUNC1 cannot function properly in CF airways (Fig. 2B). Furthermore, the positive effect of bicarbonate addition on CF ASL height was lost when SPLUNC1 was knocked down. Crucially, addition of both rSPLUNC1 and bicarbonate was required to increase CF ASL height, indicating that the ability of SPLUNC1 to regulate ENaC/ASL height is pH-dependent (Fig. 2B).
Fig. 2.
SPLUNC1 is a pH-dependent regulator of ENaC. (A) SPLUNC1 expression relative to GAPDH following stable shRNA knockdown of luciferase (control) or SPLUNC1 knockdown. KD, knockdown. (B) Mean ASL height following luciferase or SPLUNC1 KD in normal (open bars; n = 9) and CF (closed bars; n = 6) airway cultures and following addition of rSPLUNC1, high-bicarbonate Ringer solution, or both. Normal (C) and CF (D) airway cultures bind Alexa 488–labeled rSPLUNC1 in a pH-dependent manner (n ≥ 9). AU, arbitrary units. Normal (E) and CF (F) airway cultures exhibit changes in Vt (n > 6 for all). rSPLUNC1, red; vehicle, black; S18, blue; rSPLUNC1Δ44, green. *P < 0.05, difference between vehicle/control/pH 6.0 as appropriate; †P < 0.05, difference between normal and CF. Error bars represent SE. For E vs. F, data for control/vehicle and rSPLUNC1Δ44 were all significantly different between normal and CF cultures at corresponding pH; statistical symbols were omitted for clarity.
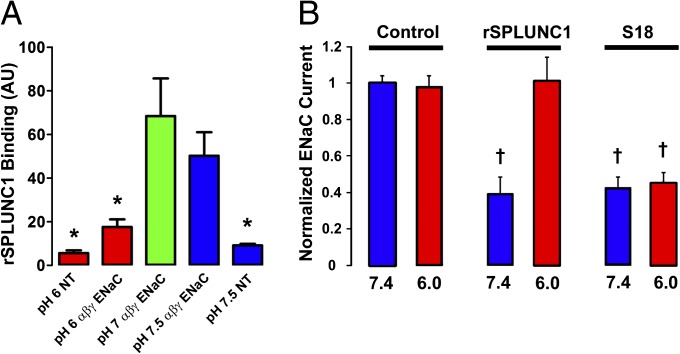
To test whether the SPLUNC1–ENaC interaction was pH-dependent, we measured fluorescent rSPLUNC1 binding to the apical surface of normal and CF airway cultures when the pH was clamped over the range of 6.0–7.5 by mucosal addition of a modified, isotonic Ringer solution, where pH was set with 100 mM of either MES (pH 6.0 or pH 6.5) or HEPES (pH 7.0 or pH 7.5). Binding was pH-dependent in both normal and CF airway cultures, with significantly more binding at pH ≥7.0 (Fig. 2 C and D). Inhibition of the amiloride-sensitive transepithelial PD (Vt), as a marker of ENaC activity, was also pH-dependent in both normal and CF airway cultures (Fig. 2 E and F), as was ASL height (Fig. S2 C–F). However, a peptide corresponding to the SPLUNC1 region important for ENaC inhibition (S18; amino acids G22–A39) inhibited ENaC in a pH-independent fashion (Fig. 2 E and F). This S18 peptide also bound to ENaC and prevented its cleavage in a pH-independent fashion (Fig. S4). As a control, we added SPLUNC1Δ44, which lacks the S18 region, and this had no effect on ENaC activity (Fig. 2 E and F). rSPLUNC1 did not bind to HEK 293T cells unless they were transfected with αβγ-ENaC, and the pH dependency was preserved in this system, indicating that binding was ENaC-dependent (Fig. 3A). The pH-dependent inhibition of ENaC by SPLUNC1 was preserved in Xenopus oocytes expressing αβγ-ENaC, providing further evidence that this is an ENaC-mediated effect (Fig. 3B).
Fig. 3.
SPLUNC1–ENaC interactions are pH-dependent. (A) Fluorescence intensity of Alexa 488-rSPLUNC1 binding to nontransfected (NT) or αβγ-ENaC–transfected HEK 293T cells over the pH range of 6.0–7.5 (n ≥ 9 for all). (B) Effect of rSPLUNC1 and S18 on the amiloride-sensitive ENaC current. αβγ-ENaC subunits were injected into Xenopus oocytes and incubated for 1 h with 30 μM of either rSPLUNC1 or S18, and the relative ENaC current was measured by a two-electrode voltage clamp (n = 9–12 for all). *P < 0.05, significant difference from rSPLUNC1; †P < 0.05, significant difference from rSPLUNC1, pH 6. Data are plotted as mean ± SE.
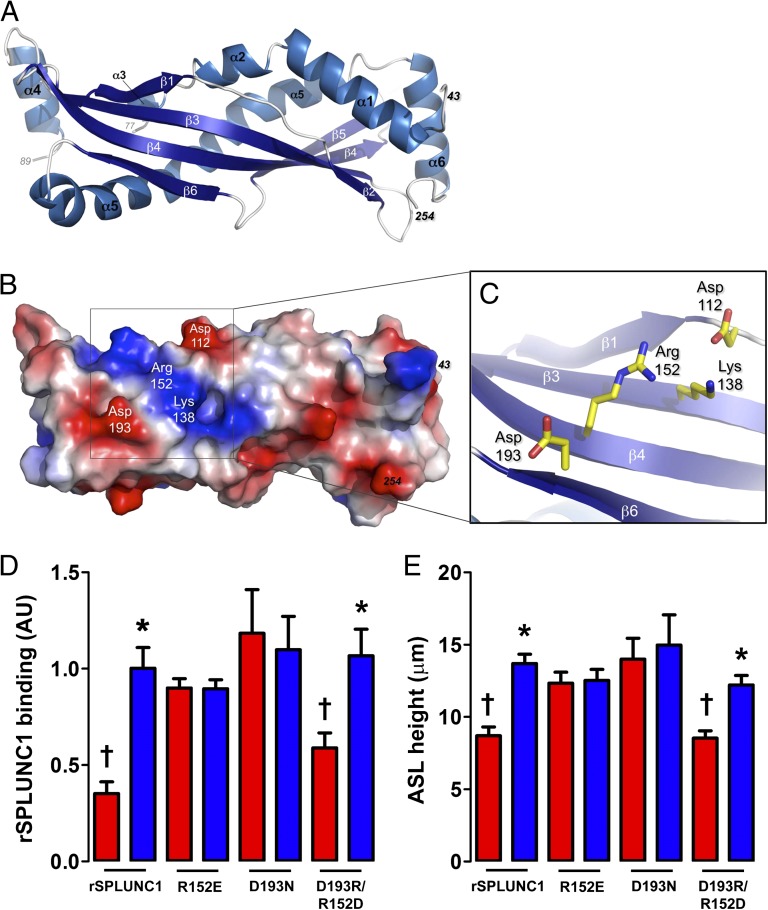
To unravel the molecular basis of this activity, we determined crystal structures of human rSPLUNC1 in both low and high salt (Fig. 4A, Fig. S5A, and Tables S1 and S2). SPLUNC1 is a monomer composed of a central six-stranded, antiparallel β-sheet flanked by six α-helices (Fig. 4A and Fig. S5 B and C), and it exhibits structural similarity to the N-terminal half of BPI (Fig. 5). Unfortunately, the S18 region of SPLUNC1 that is required for ENaC inhibition was not ordered in our structures. Furthermore, although intact SPLUNC1 functions in a pH-dependent manner, the S18 peptide alone is pH-independent, which could suggest a conformational change in SPLUNC1 (Figs. 2 E and F and 3 A and B and Fig. S4). However, we did not detect any changes in SPLUNC1 secondary structure when examined by CD spectropolarimetry at different pH (Fig. S6). Thus, we hypothesized that structural features of the surface of SPLUNC1 are involved in presenting the S18 region to ENaC.
Fig. 4.
Human SPLUNC1 crystal structure. (A) X-ray crystal structure (resolution of 2.8 Å) of residues 43–254 of human rSPLUNC1 with secondary structural elements indicated. (B) Electrostatic surface representation of human SPLUNC1 showing positively (blue) and negatively (red) charged regions. (C) Close-up of Asp-112, Lys-138, Arg-152, and Asp-193 on the surface of human SPLUNC1 shown in the same orientation as in A and B. (D) Fluorescence intensity of Alexa 488-labeled rSPLUNC1 mutant proteins (30 μM) binding to the apical surface of normal airway cultures (n ≥ 5). (E) Mean ASL height in normal airway cultures 2 h after addition of 30 μM SPLUNC1 mutant proteins in modified Ringer solution at pH 6.0 or pH 7.5 (n ≥ 9). *P < 0.05, significant difference between pH 6.0 (red bars) and pH 7.5 (blue bars) for a given mutant; †P < 0.05, significance vs. rSPLUNC1, pH 7.5. Error bars represent SE.
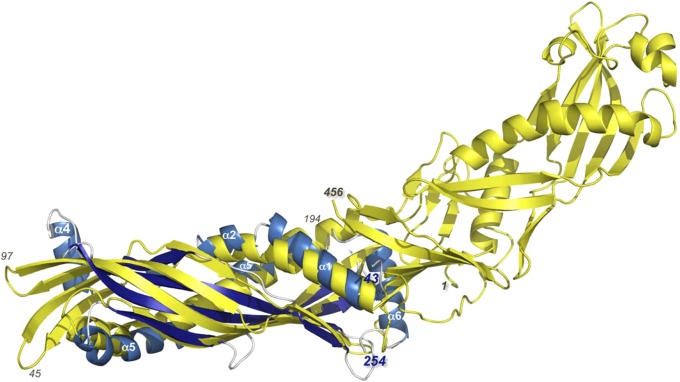
Fig. 5.
Human SPLUNC1 (blue) shares structural similarity with the N-terminal half of human BPI (yellow). Residues 43–254 of SPLUNC1 were superimposed on residues 1–194 of BPI, and the two structures were found to share an rmsd of 3.8 Å over 157 Ca positions with only 12% sequence identity. Thus, SPLUNC1 appears to represent half of the complete BPI. Amino acids 1, 45, 97, 194, and 456 of the BPI are labeled for clarity, as are amino acids 43 and 254 and α-helices 1, 2, 4, 5, and 6 of SPLUNC1. It is interesting to note that helix 6 of SPLUNC1 in the structure presented here is located in a position that occludes the lipid-binding pocket of BPI; however, one can envision a conformational change that may open an analogous pocket in SPLUNC1.
The electrostatic surface of SPLUNC1 features a cluster of charged residues (D112, K138, R152, and D193) in close proximity to residue 43, onto which the S18 peptide (amino acids G22–A39) is fused (Fig. 4 B and C). We reasoned that this relatively conserved cluster of amino acids (Fig. S7) is important for SPLUNC1 function. To examine this prediction, we created rSPLUNC1 proteins with either D193 mutated to N or R152 replaced with E. Unlike WT rSPLUNC1, these mutant proteins functioned in a pH-independent manner; they were able to maintain proper ASL hydration (Fig. 4D) and bind the surface of airway cultures (Fig. 4E) at both pH 6.0 and pH 7.5. Our data indicate that these residues form a salt bridge (Fig. S8), which, when disrupted, causes SPLUNC1 to regulate ENaC in a pH-insensitive manner. Strikingly, when the potential salt bridge was “restored” in a double mutant in which the positions of the residues were swapped, D193R/R152D, ASL hydration, and airway culture binding were again pH-sensitive. A second pair of surface residues, D112 and K138, gave similar results (Fig. S8). Although it is possible that ENaC contributes to this pH sensitivity, taken together, our data pinpoint a site on SPLUNC1 that controls the presentation of S18 to ENaC and governs the pH-dependent regulation of ASL volume in normal but not CF airways.
Discussion
The etiology of CF lung disease is controversial and has been ascribed to ASL volume depletion, reduced ASL pH, altered mucus rheology, decreased bacterial killing, and more (22, 25–28). These differences may not be mutually exclusive; for example, impaired bacterial killing has recently been attributed to reduced ASL pH (22) and impaired mucus clearance due to either low ASL volume or altered mucus rheology would increase the chance of bacterial colonization (29). Here, we found that acidity in CF ASL is causal for increased ENaC cleavage/activity and CF ASL volume depletion, suggesting that many of the defects in CF lung innate defense have a similar etiology, namely, reduced ASL pH. Gastric reflux is common in subjects with CF and has also been linked with lung disease in patients who do not have CF (30, 31). Thus, in addition to the reduced pH caused by CFTR’s absence, exposure of very low pH due to gastric reflux may further exacerbate this issue in patients with CF and contribute to a failure of mucus clearance in patients who do not have CF and are prone to gastric reflux.
Consistent with previous observations that CF ASL pH is reduced due to the lack of bicarbonate secretion through CFTR and associated proteins (21, 32), we detected a decrease in CF ASL pH that was associated with increased ENaC activity in vivo and in vitro (Fig. 1). Airway epithelia are leaky, and there is a continual backflux of ions through the paracellular pathway that appears to limit this difference to ∼0.5 units (Fig. 1). Due to the large paracellular permeability for physiological ions and a large serosal reservoir of Cl−, the concentration of Cl− is not different between normal and CF ASL (27). Because there is a large submucosal reservoir of HCO3−, it is perhaps surprising that there is reduced CF ASL pH, especially because there is a diffusion gradient for HCO3− into the ASL. However, an H+/K+ ATPase has previously been reported in the apical membrane of airway epithelia (21). Thus, the difference in ASL pH between normal and CF ASL may reflect active cellular H+ secretion that outstrips passive paracellular HCO3− diffusion. Surprisingly, our data indicated that glandular secretions did not contribute to the observed pH difference seen in CF nasal secretions. This is in agreement with previous studies of secretions from isolated normal vs. CF human airway glands, where the pH was not altered in CF (23), and suggests that CFTR is not involved in pH regulation in human submucosal glands.
As previously described, knockdown of SPLUNC1 abolished the natural restraint on ENaC, and standard ASL height was restored only with rSPLUNC1 addition in normal airway cultures (15). Despite the presence of SPLUNC1 in CF ASL (Fig. S1), SPLUNC1 knockdown was without effect in CF airway cultures and both bicarbonate and rSPLUNC1 were required to restore ASL homeostasis (Fig. 2). Thus, our data strongly suggest that Na+ hyperabsorption was secondary to altered ASL pH and that altered SPLUNC1–ENaC interactions are the physiological mechanism of Na+ hyperabsorption in CF airways. In earlier studies, it was proposed that altered ENaC sensitivity to cAMP in CF airways was the cause of Na+ hyperabsorption (11, 33). However, these studies were performed under “thick-film” conditions, where the endogenous ASL was washed away and epithelial pH was clamped at pH 7.4. Conversely, our experiments were performed with a “thin film” of ASL that more accurately reprises the situation in the lung. Despite this, we cannot exclude the possibility that cAMP metabolism is pH-sensitive and different between normal and CF airway cultures or that cAMP affects SPLUNC1–ENaC interactions. Importantly, the pH dependency of SPLUNC1 in vitro matched the normal vs. CF pH differences in vivo, and binding was increased by 50% from pH 6.5 to pH 7.0 (Fig. 2 C and D), which was mirrored by an equal or greater gain in functionality (Fig. 2 E and F). Although the pH dependency was broadly similar between normal and CF cultures, we consistently saw more binding/function in normal HBECs than in CF HBECs at pH 6.5 and the slope of the curves was also different (Fig. 2 and Fig. S2 C and D). As a result, we hypothesize that CFTR confers a change in ENaC conformation that facilitates increased SPLUNC1 binding at higher pH in normal airways. Furthermore, although our data with heterologous expression systems suggest that the pH dependency is largely due to ENaC–SPLUNC1 interactions, it is also possible that SPLUNC1 binds to other proteins either in the ASL or on the cell surface. Indeed, SPLUNC1 is a multifunctional protein that can regulate ENaC, facilitate bacterial killing, and affect ASL surface tension. However, the effects of SPLUNC1 binding to other proteins remain to be explored (15, 34, 35).
As predicted (16), SPLUNC1 shares significant structural similarity with human BPI (Fig. 5). The most striking difference between the two proteins lay at SPLUNC1’s C-terminal region, where, in the structure presented here, a unique α6-helix in SPLUNC1 occludes the equivalent lipid-binding site in BPI (Fig. 5). This unique region may be responsible for the additional roles that SPLUNC1 plays in lung defense, such as S18-mediated regulation of ENaC. Whereas full-length SPLUNC1 is strongly pH-dependent, S18 can bind to and inhibit ENaC in a pH-independent fashion (Fig. 2 and Figs. S2 and S4). Despite this, the secondary structure of SPLUNC1 was not altered between the range of pH 6.0 and pH 7.5 (Fig. S5A), and further investigation revealed that the S18 region of SPLUNC1 (i.e., residues G22–A39) was disordered, and hence not visible, on the SPLUNC1 crystal structure. However, the structure revealed surface features essential to the pH-dependent regulation of ENaC, such as an electrostatic region (Fig. 4). This surface electrostatic patch is essentially conserved in the primate SPLUNC1s, as is the sequence of S18, and we note that as the sequence of S18 diverges from that of the human protein, so does the identity of the residues in the electrostatic surface patch examined here. Thus, SPLUNC1 proteins from species more distantly related to humans, like mouse and rat, do not completely maintain the surface residues and also contain an S18 region that is different (i.e., 16–24 residues longer) than the human S18. Thus, we speculate that SPLUNC1 function, and pH-sensitivity, will vary from species to species.
In conclusion, we posit that the region encompassing R152 and D193 is required for the proper availability of S18 and for SPLUNC1 regulation of ENaC/ASL homeostasis. We propose that the failure of SPLUNC1 to regulate CF ASL height is due to inappropriate interactions between the S18 and R152/D193 regions at an acidic pH that precludes SPLUNC1 binding to ENaC. Taken together, our data establish key molecular features essential for controlling ENaC activity and provide detailed insights into the pH-dependent misregulation of ENaC associated with CF lung disease. Furthermore, our data indicate that normalization of CF ASL pH may be a treatment option that can restore SPLUNC1-dependent regulation of ENaC and help prevent the mucus dehydration seen in CF airways.
Materials and Methods
This section is a summary of the most important techniques used. A detailed description of all methods can be found in SI Materials and Methods.
Expression and Purification of Human SPLUNC1.
SPLUNC1 cDNA was kindly provided by Colin Bingle (University of Sheffield, Sheffield, United Kingdom). BL21-CodonPlus competent cells (Agilent Technologies) were transformed with the expression plasmid and cultured in the presence of antifoam (50 μL), ampicillin (100 μg/mL), and chloramphenicol (34 μg/mL) in LB with vigorous shaking at 37 °C until an OD600 of 0.6 was attained. Expression was induced with the addition of 0.1 mM isopropyl-1-thio-D-galactopyranoside (IPTG), temperature was decreased to 18°C, and bacteria were cultured overnight. Cell pellets were resuspended in buffer A [20 mM potassium phosphate (pH 7.4), 50 mM imidazole, 500 mM NaCl, 0.02% NaAzide], along with lysozyme, DNase1, and protease inhibitor tablets. Cells were sonicated, and cell lysate was separated into insoluble and soluble fractions, which were filtered and flowed over an Ni-NTA His-Trap gravity column and washed with buffer A. The bound protein was eluted with buffer B [20 mM potassium phosphate (pH 7.4), 500 mM imidazole, 500 mM NaCl, 0.02% NaAzide] and separated using an S200 gel filtration column on an ÄKTAxpress (GE Healthcare Life Sciences). Nickel and size exclusion chromatography, and tobacco etch virus protease removed the tag from purified SPLUNC1. We confirmed that SPLUNC1 purified in this fashion inhibited ENaC in normal airway cultures (n = 6).
Study Subjects.
The study protocol was approved by the University of North Carolina Committee on the Protection of Rights of Human Subjects, and written informed consent was obtained. All normal subjects had no history of chronic rhinitis and no acute nasal symptoms for at least 2 wk before sampling. None took any chronic medication. The mean age of normal subjects was 25.4 ± 1.06 y. All subjects with CF were homozygous for ΔF508, and their mean age was 28.2 ± 2.67 y.
In Vivo Nasal PDs and Nasal Secretions.
Nasal PD was measured between an s.c. reference electrode and an exploring electrode placed against the inferior turbinate as described (36).
ASL pH Measurement and pH Clamping.
ASL pH was measured with pH-sensitive microelectrodes (Microelectrodes) in small-volume samples that could be quickly temperature-, water vapor-, and gas-equilibrated as described (21). When indicated, apical pH was clamped by adding 20 μL of modified Ringer solution containing either 100 mM HEPES (pH 7.0 and pH 7.5) or 100 mM MES (pH 6.0 and pH 6.5) at 37 °C with 5% CO2 gas in air. To verify that pH was clamped appropriately, pH-sensitive pHrodo dextran (10 μM) and pH-insensitive Alexa 647 dextran (10 μM) were added to the Ringer solution and measured in situ on airway cultures at timed intervals at 585 nm and 668 nm, respectively, using a Tecan Infinite M1000 plate reader at 37 °C.
Cell Culture.
Human donor lungs and excised recipient lungs were obtained from main stem/lobar bronchi of CF or normal lungs, using protocols approved by the University of North Carolina Committee on the Protection of the Rights of Human Subjects (15). shRNA-induced knockdown of SPLUNC1 was performed using retrovirus as described (15).
ASL Height Measurements.
ASL was labeled by adding 20 μL of Ringer solution containing 10 kDa of Texas red or FITC dextran at 1 mg/mL to the apical surface and measured using a Leica SP5 confocal microscope with a 63× glycerol immersion objective, as described previously (15).
Fluorescence-Based Binding Assay.
SPLUNC1 and peptides were labeled on their N terminus with Dylight-488 amine-reactive dye (Thermo Scientific) per kit instructions. Fluorescent proteins/peptides were then added apically to airway cultures in 20 μL of modified Ringer solution, incubated for 1 h, and imaged with a Nikon Ti-S microscope using a 60× water immersion objective lens at 484 nM (emission >517 nm). Total fluorescence was quantified using ImageJ software (National Institutes of Health).
Vt Studies.
A single-barreled Vt-sensing electrode was positioned in the ASL by micromanipulator and used in conjunction with a macroelectrode in the serosal solution to measure Vt using a voltmeter (15).
Crystallization of rSPLUNC1.
Initial crystals of purified rSPLUNC1 (residues 20–256) were grown in low salt using hanging drop vapor diffusion in 8% (vol/vol) PEG-400, 0.2 M trisodium citrate, and 0.1 M Tris⋅HCL (pH 8.5). These crystals were cryoprotected in 20% PEG-400 and were used for initial data collection and determination of a preliminary model using single-wavelength anomalous dispersion (SAD) methods to a resolution of 3.2 Å. A variant form of human rSPLUNC1 (L68M/L157M) was generated to produce signal sufficient for SAD phasing. Improved crystals of WT human rSPLUNC1 (residues 20–256) were grown in high-salt 6 M ammonium nitrate and 0.1 M Tris⋅HCL (pH 8.5) and cryoprotected in 15% glycerol. A crystal from these higher salt conditions was used to collect the data used for final structure determination and refinement at a resolution of 2.8 Å (Table S1).
Structure Determination and Refinement.
Initial human rSPLUNC1 crystals diffracted X-rays to a resolution of only 3.2 Å but were sufficient to determine initial phases using SAD methods. rSPLUNC1 (L68M/L157M) was created using PCR mutagenesis. This created a form of human SPLUNC1 that contained three total methionines, and this recombinant protein was labeled with selenomethionine using standard Escherichia coli methods. Data were collected at the peak wavelength for selenium anomalous scattering; the exact wavelength, as well as f′ and f′′, was calculated from a fluorescence scan obtained at Advance Photon Source National Institute of General Medical Sciences and the National Cancer Institute Collaborative Access Team beamline 23. SPLUNC1 low-salt crystals displayed anisotropic diffraction and a very high Wilson B-factor of 104 Å2. These data, which exhibited a resolution of 100-3.2 Å (Table S2), were sufficient to locate the selenium sites from the selenomethionines using Python-Based Hierarchical Environment for Integrated Xtallography (PHENIX) (www.phenix-online.org). The anomalous signal was calculated to be 0.07–0.08 to a resolution of 4.5 Å. There were six selenium sites per asymmetrical unit in the P4 unit cell. Electron density maps calculated from the selenium phases, including density modification in PHENIX (RESOLVE), had mostly continuous electron density with both α-helices and β-sheets easily discerned. Using the selenomethionine sites as sequence markers, a model with two human rSPLUNC1 monomers in the asymmetrical unit was built that encompassed the final six β-sheets and six α-helices, as well as some loop regions. Refinement was initiated to improve the electron density in all areas; some of the loops, however, were not visualized, and the average thermal displacement parameter for the protein model was 103 Å2 (Table S2). Large cavities were evident in the crystal packing, and the solvent content of the crystals was 70%, which may partially explain the low diffraction resolution and high thermal motions observed.
At this point, a splunc1 crystal form was discovered in 6 M ammonium nitrate that diffracted to 2.8 Å with space group C2221 (see Table S1). We then used molecular replacement with a single monomer of our preliminary SPLUNC1 model from the low-salt data as a search model. Molecular replacement revealed two clear monomers in the asymmetric unit using the data from the high-salt crystals. Refinement was completed with PHENIX and manual adjustments in the Crystallographic Object-Oriented Toolkit using standard methods, and produced a highly improved final model with reasonable Wilson B-factors and average thermal displacement parameters of 70 Å2 and 74 Å2, respectively (Table S1).
Statistical Analyses.
Data were analyzed with the Student t test or ANOVA followed by the Tukey test, where the variances were homogeneously distributed. All values are expressed as mean ± SEM, and P < 0.05 was considered statistically significant. Airway cultures derived from three or more donors were used per experiment, and experiments using cell lines were repeated on at least three separate occasions. All analyses were conducted using Instat (GraphPad) or Prism 5 (GraphPad) software.
Supplementary Material
Acknowledgments
We thank Hong He and Yan Dang for technical assistance, and we thank the University of North Carolina Cystic Fibrosis Center Molecular and Tissue Cores. This study was funded by National Institutes of Health Grants PPG P01HL034322, R01HL103940, R01HL108927, R01AI78924 and CFF R026-CR02.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The preliminary and final models have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4KGO and 4KGH, respectively).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311999110/-/DCSupplemental.
References
- 1.Eaton DC, Malik B, Bao HF, Yu L, Jain L. Regulation of epithelial sodium channel trafficking by ubiquitination. Proc Am Thorac Soc. 2010;7(1):54–64. doi: 10.1513/pats.200909-096JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol. 2009;71:361–379. doi: 10.1146/annurev.physiol.010908.163108. [DOI] [PubMed] [Google Scholar]
- 3.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: Regulation of epithelial sodium channels by proteases. J Biol Chem. 2009;284(31):20447–20451. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10(5):487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol. 2004;286(1):C190–C194. doi: 10.1152/ajpcell.00342.2003. [DOI] [PubMed] [Google Scholar]
- 6.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: Interaction between genetic and environmental factors. Annu Rev Physiol. 2002;64:877–897. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- 7.Althaus M. ENaC inhibitors and airway re-hydration in cystic fibrosis: State of the art. Curr Mol Pharmacol. 2013;6(1):3–12. doi: 10.2174/18744672112059990025. [DOI] [PubMed] [Google Scholar]
- 8.Kimura T, et al. Deletion of the ubiquitin ligase Nedd4L in lung epithelia causes cystic fibrosis-like disease. Proc Natl Acad Sci USA. 2011;108(8):3216–3221. doi: 10.1073/pnas.1010334108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collawn JF, Lazrak A, Bebok Z, Matalon S. The CFTR and ENaC debate: How important is ENaC in CF lung disease? Am J Physiol Lung Cell Mol Physiol. 2012;302(11):L1141–L1146. doi: 10.1152/ajplung.00036.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles M, Gatzy J, Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981;305(25):1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- 11.Boucher RC, Stutts MJ, Knowles MR, Cantley L, Gatzy JT. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest. 1986;78(5):1245–1252. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentzsch M, et al. The cystic fibrosis transmembrane conductance regulator impedes proteolytic stimulation of the epithelial Na+ channel. J Biol Chem. 2010;285(42):32227–32232. doi: 10.1074/jbc.M110.155259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berdiev BK, Qadri YJ, Benos DJ. Assessment of the CFTR and ENaC association. Mol Biosyst. 2009;5(2):123–127. doi: 10.1039/b810471a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaillard EA, et al. Regulation of the epithelial Na+ channel and airway surface liquid volume by serine proteases. Pflugers Arch. 2010;460(1):1–17. doi: 10.1007/s00424-010-0827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Caballero A, et al. SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proc Natl Acad Sci USA. 2009;106(27):11412–11417. doi: 10.1073/pnas.0903609106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bingle CD, Craven CJ. PLUNC: A novel family of candidate host defence proteins expressed in the upper airways and nasopharynx. Hum Mol Genet. 2002;11(8):937–943. doi: 10.1093/hmg/11.8.937. [DOI] [PubMed] [Google Scholar]
- 17.Bingle L, et al. Differential epithelial expression of the putative innate immune molecule SPLUNC1 in cystic fibrosis. Respir Res. 2007;8:79. doi: 10.1186/1465-9921-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roxo-Rosa M, et al. Proteomic analysis of nasal cells from cystic fibrosis patients and non-cystic fibrosis control individuals: Search for novel biomarkers of cystic fibrosis lung disease. Proteomics. 2006;6(7):2314–2325. doi: 10.1002/pmic.200500273. [DOI] [PubMed] [Google Scholar]
- 19.Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol. 2006;127(5):591–604. doi: 10.1085/jgp.200509468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 1994;91(12):5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coakley RD, et al. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci USA. 2003;100(26):16083–16088. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pezzulo AA, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487(7405):109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayaraman S, Joo NS, Reitz B, Wine JJ, Verkman AS. Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na(+)] and pH but elevated viscosity. Proc Natl Acad Sci USA. 2001;98(14):8119–8123. doi: 10.1073/pnas.131087598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinton PM. Cystic fibrosis: Impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008;372(9636):415–417. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 25.Chmiel JF, Davis PB. State of the art: Why do the lungs of patients with cystic fibrosis become infected and why can’t they clear the infection? Respir Res. 2003;4:8. doi: 10.1186/1465-9921-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derichs N, Jin BJ, Song Y, Finkbeiner WE, Verkman AS. Hyperviscous airway periciliary and mucous liquid layers in cystic fibrosis measured by confocal fluorescence photobleaching. FASEB J. 2011;25(7):2325–2332. doi: 10.1096/fj.10-179549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsui H, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95(7):1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 28.Quinton PM. Role of epithelial HCO3⁻ transport in mucin secretion: Lessons from cystic fibrosis. Am J Physiol Cell Physiol. 2010;299(6):C1222–C1233. doi: 10.1152/ajpcell.00362.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109(5):571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boren EJ, Teuber SS, Gershwin ME. A review of non-cystic fibrosis pediatric bronchiectasis. Clin Rev Allergy Immunol. 2008;34(2):260–273. doi: 10.1007/s12016-007-8036-z. [DOI] [PubMed] [Google Scholar]
- 31.Mousa HM, Woodley FW. Gastroesophageal reflux in cystic fibrosis: Current understandings of mechanisms and management. Curr Gastroenterol Rep. 2012;14(3):226–235. doi: 10.1007/s11894-012-0261-9. [DOI] [PubMed] [Google Scholar]
- 32.Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4(2):107–116. doi: 10.1007/s12551-012-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stutts MJ, et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269(5225):847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 34.Bartlett JA, et al. PLUNC: A multifunctional surfactant of the airways. Biochem Soc Trans. 2011;39(4):1012–1016. doi: 10.1042/BST0391012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, et al. SPLUNC1/BPIFA1 contributes to pulmonary host defense against Klebsiella pneumoniae respiratory infection. Am J Pathol. 2013;182(5):1519–1531. doi: 10.1016/j.ajpath.2013.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coakley RD, et al. 17beta-Estradiol inhibits Ca2+-dependent homeostasis of airway surface liquid volume in human cystic fibrosis airway epithelia. J Clin Invest. 2008;118(12):4025–4035. doi: 10.1172/JCI33893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.