Abstract
Although fixation of the stapes is usually progressive and secondary to otosclerosis, it may present congenitally, with other skeletal manifestations, as an autosomal dominant syndrome—such as proximal symphalangism (SYM1) or multiple-synostoses syndrome (SYNS1), both of which are caused by mutations in NOG, the gene encoding noggin. We describe a family that was ascertained to have nonsyndromic otosclerosis but was subsequently found to have a congenital stapes ankylosis syndrome that included hyperopia, a hemicylindrical nose, broad thumbs and great toes, and other minor skeletal anomalies but lacked symphalangism. A heterozygous nonsense NOG mutation—c.328C→T (Q110X), predicted to truncate the latter half of the protein—was identified, and a heterozygous insertion in NOG—c.252-253insC, in which the frameshift is predicted to result in 96 novel amino acids before premature truncation—was identified in a previously described second family with a similar phenotype. In contrast to most NOG mutations that have been reported in kindreds with SYM1 and SYNS1, the mutations observed in these families with stapes ankylosis without symphalangism are predicted to disrupt the cysteine-rich C-terminal domain. These clinical and molecular findings suggest that (1) a broader range of conductive hearing-loss phenotypes are associated with NOG mutations than had previously been recognized, (2) patients with sporadic or familial nonsyndromic otosclerosis should be evaluated for mild features of this syndrome, and (3) NOG alterations should be considered in conductive hearing loss with subtle clinical and skeletal features, even in the absence of symphalangism.
Stapes ankylosis is characterized by conductive hearing loss due to congenital or acquired fixation of the stapes. Conductive hearing loss results from impairment of the sound-conduction mechanism (the external auditory canal, tympanic membrane, and/or middle-ear ossicles). In contrast, sensorineural hearing loss may be caused by disorders of the cochlea, acoustic nerve, or brainstem. Otosclerosis (MIM 166800), the most common cause of progressive conductive hearing loss in adults, is generally manifested as nonsyndromic, delayed-onset, conductive hearing loss, but it may also affect the inner ear to cause sensorineural loss. Congenital stapes ankylosis may be difficult to differentiate from otosclerosis when the diagnosis of conductive hearing loss is delayed. Stapes ankylosis may be associated with skeletal dysplasias, such as osteogenesis imperfecta type I (MIM 166200), or may be present as an isolated temporal bone anomaly, such as X-linked stapes fixation with perilymphatic gusher (DFN3 [MIM 304400]). Furthermore, skeletal anomalies associated with stapes ankylosis may be subtle, such that a syndrome is not recognized.
Mutations in NOG (MIM 602991), the gene encoding noggin, have been identified in two such stapes ankylosis syndromes—namely, proximal symphalangism (SYM1 [MIM 185800]) and multiple-synostoses syndrome (SYNS1 [MIM 186500]) (Gong et al. 1999; Takahashi et al. 2001; Mangino et al. 2002). NOG encodes a secreted protein, noggin, that is essential for normal bone and joint development in both humans and mice (Zimmerman et al. 1996). Noggin binds and inactivates bone morphogenetic proteins (BMPs), which are specific signaling proteins belonging to the transforming growth factor–β superfamily. SYM1, also known as “Cushing symphalangism,” consists of stapes ankylosis, proximal interphalangeal joint fusion, and skeletal anomalies. SYNS1, also known as “facio-audio-symphalangism,” is similar to SYM1 but has the additional feature of a broad hemicylindrical nose.
In this study, we ascertained a family of Italian descent (family 16) that had conductive hearing loss that was inherited as an autosomal dominant trait with complete penetrance (fig. 1). Each affected individual was thought to have had nonsyndromic otosclerosis before participation in this study. The University of Michigan institutional review board approved the study, and each family member gave informed consent and completed a questionnaire. Venous-blood samples were obtained from 11 family members (8 affected members, 1 unaffected member, and 2 spouses). DNA was extracted from the blood samples by use of standard methodology (PureGene DNA Isolation Kit; Gentra).
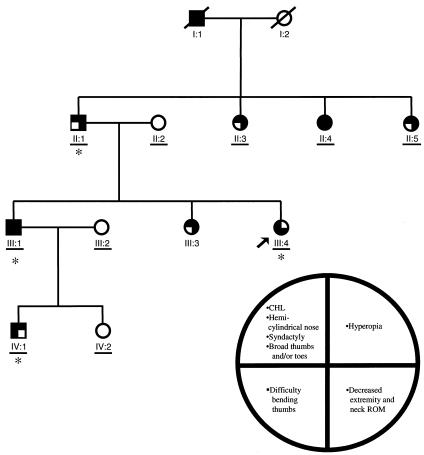
Figure 1.
Pedigree of family 16. (A key to phenotypes is given in the circle. CHL = conductive hearing loss; ROM = range of motion.) The arrow (↗) denotes the proband. Square symbols denote male patients, and circles denote female patients; blackened symbols denote affected individuals, and unblackened symbols denote unaffected individuals; each quadrant defines a phenotypic element or a set of phenotypic elements, and blackened quadrants indicate the presence of the corresponding phenotypic element(s). Asterisks (*) indicate family members who underwent complete genetic, otolaryngological, ophthalmologic, and radiological evaluations. Horizontal bars (—) indicate family members who were personally examined by an investigator (D.J.B.).
The NOG coding region was first amplified from genomic DNA by PCR with forward (5′-GGACGCGGGACGAAGCAGCAG-3′) and reverse (5′-GAGGATCAAGTGTCCGGGTGC-3′) primers that were designed from the human NOG cDNA sequence, by use of conditions described elsewhere (Gong et al. 1999), and was then bidirectionally sequenced with an ABI 3700 automated DNA sequencer (Applied Biosystems) at the University of Michigan DNA Sequencing Core. Each PCR product was eluted on a 1% low-melting-point agarose (UltraPURE; GibcoBRL) gel and then was purified using standard methodology (QIAquick Gel Extraction Kit). In addition, the NOG coding region was sequenced in 100 control DNA samples, of which 90 were from the DNA Polymorphism Discovery Resource (Collins et al. 1998) and 10 were from the Italian Human Variation Panel (Coriell Cell Repositories).
Conductive hearing loss was documented at age ⩽4 years in two family members and remained stable through subsequent years—consistent with congenital stapes ankylosis, rather than otosclerosis. All affected family members except III:4 have hyperopia, or farsightedness, and the age at which corrective lenses were required varied from 2 to 22 years. The median spherical equivalent for all affected eyes was +4.75 diopters (D), with a mean of +5.50 D (range +1.25 D to +10.00 D). Keratometry readings, which provide a measure of corneal curvature, were in the normal range for II:1 and III:4 and were slightly steeper for III:1 and IV:1. Biomicroscopy showed a cataract change in II:1 that was age appropriate. Both II:1 and III:1 had Brushfield spots, a normal iris variant without known clinical significance. The three affected family members who were examined were all found to have shorter-than-average axial lengths: 21.19 mm oculus dexter (OD) (right eye) and 22.52 mm oculus sinister (OS) (left eye) for II:1, 20.40 mm OD and 20.42 mm OS for III:1, and 22.02 mm OD and 22.52 mm OS for III:4.
The phenotype in family 16 is characterized by autosomal dominant stapes ankylosis with broad thumbs and toes and hyperopia, as well as other minor skeletal anomalies (fig. 1 and table 1). Radiological findings are summarized in table 2. Symmetrically short distal thumb phalanges were noted in each family member. No evidence of symphalangism was evident in hand radiographs of any of the four family members who were examined. Review of hand photographs of III:3 revealed broad and dysmorphic middle fingers and great toes but normal thumb morphology.
Table 1.
Physical Examination Findings for the Four Members of Family 16 Who Were Examined at the University of Michigan[Note]
| Feature | No.Affected |
| Posteriorly sloping forehead | 4 |
| Prominent supraorbital ridges | 3a |
| Broad, hemicylindrical nose with bulbous tip and short philtrum | 4 |
| Nasal tip cleft | 3a |
| Mild malar flattening | 4 |
| Chin cleft | 2 |
| Mild synophrys | 3a |
| Limited neck range of motion | 3a |
| Limited elbow flexion, extension, and supination | 3a |
| Limited wrist dorsiflexion | 3a |
| Broad thumbs with foreshortened nails | 4 |
| Limited hip range of motion | 3a |
| Soft-tissue syndactyly of toes 2 and 3 | 4 |
| Broad and short great toes | 4 |
| Pes planus | 2 |
Note.— II:1, III:1, III:4, and IV:1 were examined at the University of Michigan.
Absent in IV:1 (at age 12 years).
Table 2.
Plain-Film Radiology Findings for the Four Members of Family 16 Who Were Examined at the University of Michigan[Note]
| Feature | No.Affected |
| Degenerative spine changes | 4 |
| Abnormal humero-radial joint morphology | 4 |
| Broad and short thumbs and toes | 4 |
| Slightly broad metacarpals | 2 |
| Premature fusion of thumb distal phalangeal growth plates | 1a |
| Slight inferior narrowing of pelvis | 3 |
| Premature fusion of the growth plates of great-toe proximal phalanges | 1a |
| Partial fusion of right-5th-toe distal interphalangeal joint | 1b |
Note.— II:1, III:1, III:4, and IV:1 were examined at the University of Michigan.
Found only in IV:1 (at age 12 years).
Normal variant.
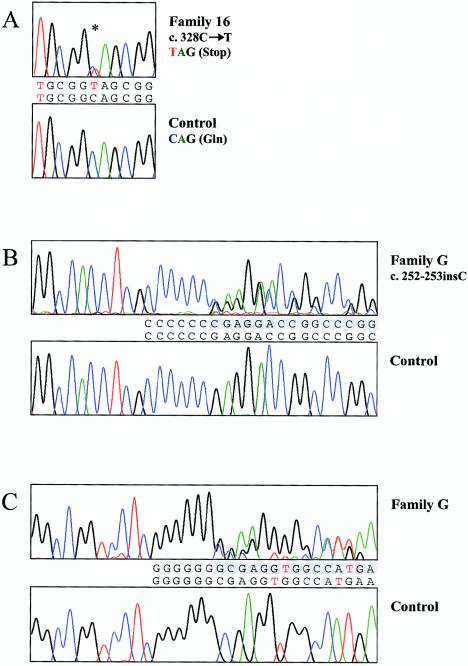
We then analyzed NOG in family 16 and in three members of a family that was described by Milunsky et al. (1999) (family G) and that had a similar phenotype. In family 16, a heterozygous nonsense mutation—1139C→T, or c.328C→T (Q110X)—was found in all eight affected family members (fig. 2A), but not present in the reference sequence (GenBank accession numbers U31202 and NM_005450). In the two affected members of family G, a 1-bp insertion, 1063-1064insC (c.252-253insC), caused a frameshift that, before encountering a premature stop codon, leads to a mutant peptide with 96 novel amino acids (figs. 2B and 2C). Neither mutation was found in unaffected family members, spouses, or 100 control samples. In addition, NOG sequencing data from the 100 control samples revealed no variations, as compared to the reference sequence. A comparison of the wild-type and mutant proteins is presented in figure 3. To confirm the 252-253insC mutation, we cloned the PCR products into pGEM-T Easy vectors (Promega), used ligation products to transform DH5α-competent Escherichia coli cells, and isolated the plasmid DNA (Wizard Plus SV Minipreps; Promega). Through sequencing, we confirmed the presence of both the mutant and wild-type alleles in the population of subclones.
Figure 2.
Sequence chromatograms. A, Chromatogram from sequencing of forward strand, demonstrating NOG mutations in affected members of family 16 (top) versus control samples (bottom). The asterisk (*) denotes overlapping peaks—indicating heterozygous mutation c.328C→T (Q110X), not present in control samples. B, Chromatogram from sequencing of forward strand in affected member of family G (top)—demonstrating heterozygous mutation c.252-253insC—as compared to control samples (bottom). Text between chromatograms indicate the sequence of the mutant strand (line 1) and the sequence of the wild-type strand (line 2), aligned with corresponding heterozygous peaks on the top chromatogram. C, Chromatogram from sequencing of reverse strand in the affected member of family G (top) and control samples (bottom). A heterozygous 1-bp insertion leads to a frameshift (shaded sequence), as compared to the wild-type sequence.
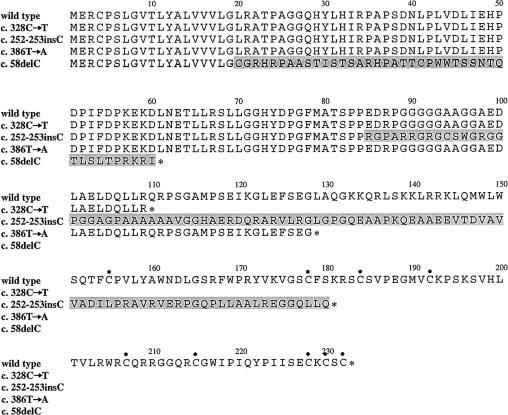
Figure 3.
Comparison of noggin sequences among wild-type human noggin (line 1), among c.328C→T and c.252-253insC mutations (lines 2 and 3, respectively) that cause stapes ankylosis with broad thumbs and toes and hyperopia, and among previously reported mutations that cause SYM1 (c.386T→A [line 4]) and SYNS1 (c.58delC [line 5]). Shading indicates novel amino acids caused by frameshift mutations. Asterisks (*) indicate stop codons. Bullets (•) indicate conserved cysteine residues.
Two other families that had stapes ankylosis with broad thumbs and toes (MIM 184460) and hyperopia have been clinically described elsewhere (Teunissen and Cremers 1990; Hilhorst-Hofstee et al. 1997) without detailed description of the ophthalmological findings. The syndromes diagnosed in families G and 16 share several features with these syndromes, as well as with SYM1 and SYNS1 (table 3). The key features that differentiate stapes ankylosis with broad thumbs and toes and hyperopia from SYM1 and SYNS1 include a characteristic physiognomy, hyperopia, and the absence of cervical vertebral fusion and symphalangism. In contrast to SYM1 and SYNS1, the affected members of family 16 have dysmorphic digits and limitation of joint movement that is not attributable to bony joint fixation.
Table 3.
Comparison between Phenotypes Observed in Family 16 and Those in Families Described Elsewhere[Note]
|
Family Described by |
||||||
| Family 16 [No.Affected/Total] | Milunsky et al.(1999) (Family G) | Teunissen andCremers (1990) | Hilhorst-Hofsteeet al. (1997) | SYM1a | SYNS1a | |
| Autosomal dominant | + | + | + | + | + | + |
| Stapes ankylosis | + [8/8] | + | + | + | + | + |
| Hyperopia | + [7/8] | + | + | + | +b | + |
| Fused cervical vertebrae | − [0/8] | − | + | + | +b | + |
| Hemicylindrical nose | + [8/8] | + | − | + | +b | + |
| Broad thumbs and toes | + [8/8]c | + | + | + | +d | + |
| Symphalangism | − [0/8] | − | +b | +b | + | + |
| Syndactyly | + [8/8] | + | + | − | + | + |
| Carpal/tarsal fusions | − [0/4] | − | − | − | + | + |
| Tall stature (⩾90th percentile) | + [8/8] | − | − | − | − | − |
| NOG mutation | + | + | U | U | + | + |
Note.— + = Present; − = absent; U = unknown.
Based on aggregate data from Hilhorst-Hofstee et al. (1997).
Individual case reported.
One individual had normal thumbs but broad toes and middle fingers.
Rarely reported.
NOG was first discovered in an expression screen for signaling molecules that are produced by the Spemann organizer, which is important in the development of the dorsal-ventral axis (Smith and Harland 1992). In early development, the noggin/BMP pathways play an important role in both determination of retinal-cell fate in chick (Moore and Moody 1999; Belecky-Adams and Adler 2001) and ocular growth and lens induction in chick (Trousse et al. 2001) and mouse (Furuta and Hogan 1998). Direct implications for the noggin/BMP pathways in human ocular development have yet to be elucidated.
Numerous skeletal features found in all affected adults in family 16 were absent in the affected child, suggesting that NOG mutations also have effects in postnatal growth and development. Premature fusion of growth plates may contribute to the joint dysfunction that was observed. Characteristic facial features may also be associated with NOG mutations, since noggin, BMPs, and retinoic acid together play a role in development of the frontonasal and the maxillary facial structure in some species (Lee et al. 2001). Family G is of Mexican descent, and the differences in stature between family 16 (⩾90th percentile) and family G (25th–50th percentile) may result from allelic differences in modifier genes, rather than from specific effects of different NOG mutations. Tall stature has not been reported with other NOG mutations and, overall, is uncommon in skeletal dysplasias.
NOG consists of a single exon without introns and encodes the protein noggin, which is 232 amino acids long. NOG is highly conserved, with 97% identity between the human and mouse cDNA sequences, and the human and mouse proteins are identical. Consistent with this finding, we observed no variations, among 100 control samples, in the NOG sequence. Noggin is posttranslationally modified and is secreted as a homodimer that is linked by disulfide bonds (Smith et al. 1993). After secretion of the protein, the N-terminus (amino acids 1–19) is proteolytically cleaved from the mature noggin peptide, and the remaining peptide is glycosylated. The sequence of the signal peptide region is somewhat variable across species (Valenzuela et al. 1995). The C-terminal portion (amino acids 155–232) of noggin contains a series of nine conserved cysteine residues that are thought to be important in disulfide-bond formation (fig. 3). Alternatively, these cysteine residues may form a cysteine-knot motif, as described in other BMP antagonists (Vitt et al. 2001).
Noggin knockout mice have grossly dysmorphic skeletal development and die prenatally, whereas heterozygous noggin null mutants have a phenotype that is indistinguishable from wild type (Brunet et al. 1998). In contrast, both genetic and biochemical studies demonstrate that heterozygous missense or nonsense NOG mutations can cause human disease. Mutant noggin proteins that contain single-amino-acid substitutions underlying SYM1 and SYNS1 had normal function in Xenopus, in terms of BMP binding and dorsalizing activity (Marcelino et al. 2001). In COS-7 cells, the mutants were secreted and dimerized with varying efficiency, sometimes in a monomeric and/or non–disulfide-bonded form, but presence of the mutant protein did not appear to affect disulfide dimerization of the wild-type protein. Marcelino et al. (2001) suggest that noggin has species-specific and joint-specific effects, dependent on the amount of functional protein.
The c.328C→T and c.252-253insC mutations both result in a polypeptide that lacks the cysteine-rich C-terminal domain. In contrast, most NOG mutations that are associated with SYM1 and SYNS1 are heterozygous missense mutations (Gong et al. 1999; Takahashi et al. 2001; Mangino et al. 2002). Two nonsense mutations—c.386T→A (L129X) and c.58delC, which have been reported in a family with SYM1 and a family with SYNS1, respectively (Takahashi et al. 2001)—also result in the deletion of the C-terminal domain (fig. 3). Differences in genetic background control the expression of exencephaly in noggin knockout mice (McMahon et al. 1998) and may also explain why similar genetic mutations in humans result in variable phenotypes. Alternatively, there may be other, as-yet-unknown noggin subdomains, such as BMP-binding sites, that explain the different phenotypic effects of these mutations.
The phenotype of stapes ankylosis with broad thumbs and toes and hyperopia that is associated with these mutations is consistent with the role that noggin plays in the development and maintenance of normal joint function, in conjunction with normal skeletal growth. Further studies will be necessary to determine if the mutant polypeptides that result from these mutations are properly transcribed, translated, modified, dimerized, and secreted. The association between stapes ankylosis and other physical features that are subtle or otherwise common in the general population may sometimes remain unrecognized. Families with apparent autosomal dominant otosclerosis or other types of conductive hearing loss should have a complete physical and radiological examination, to exclude subtle skeletal features found in the spectrum of stapes ankylosis syndromes that are caused by NOG mutations. Finally, NOG and/or other genes that are involved in the noggin/BMP pathway should be considered as candidate genes for these disorders.
Acknowledgments
We thank the family, for their participation, and Drs. Michael DiPietro, Margaret Lomax, Margit Burmeister, and Thomas Glover. This work was supported by the Research Fund of the American Otological Society (grant to T.B.K.), National Institute on Deafness and Other Communication Disorders grants K23 DC00161 (to M.M.L.) and T32 DC00024 (to D.J.B.), the Margaret G. Bertsch Endowment Fund, and University of Michigan General Clinical Research Center grant M01 RR00042 (D.J.B.).
Footnotes
Previously presented, in part, at the 135th annual meeting of the American Otological Society.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for complete coding sequence [accession number U31202] and mRNA [accession number NM_005450])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for DFN3 [MIM 304400], NOG [MIM 602991], osteogenesis imperfecta type I [MIM 166200], otosclerosis [MIM 166800], stapes ankylosis with broad thumbs and toes [MIM 184460], SYM1 [MIM 185800], and SYNS1 [MIM 186500])
References
- Belecky-Adams T, Adler R (2001) Developmental expression patterns of bone morphogenetic proteins, receptors, and binding proteins in the chick retina. J Comp Neurol 430:562–572 [PubMed] [Google Scholar]
- Brunet LJ, McMahon JA, McMahon AP, Harland RM (1998) Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science 280:1455–1457 [DOI] [PubMed] [Google Scholar]
- Collins FS, Brooks LD, Chakravarti A (1998) A DNA polymorphism discovery resource for research on human genetic variation. Genome Res 8:1229–1231 [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL (1998) BMP4 is essential for lens induction in the mouse embryo. Genes Dev 12:3764–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Krakow D, Marcelino J, Wilkin D, Chitayat D, Babul-Hirji R, Hudgins L, Cremers CW, Cremers FP, Brunner HG, Reinker K, Rimoin DL, Cohn DH, Goodman FR, Reardon W, Patton M, Francomano CA, Warman ML (1999) Heterozygous mutations in the gene encoding noggin affect human joint morphogenesis. Nat Genet 21:302–304 [DOI] [PubMed] [Google Scholar]
- Hilhorst-Hofstee Y, Watkin PM, Hall CM, Baraitser M (1997) The autosomal dominant syndrome with congenital stapes ankylosis, broad thumbs and hyperopia. Clin Dysmorphol 6:195–203 [DOI] [PubMed] [Google Scholar]
- Lee SH, Fu KK, Hui JN, Richman JM (2001) Noggin and retinoic acid transform the identity of avian facial prominences. Nature 414:909–912 [DOI] [PubMed] [Google Scholar]
- Mangino M, Flex E, Digilio MC, Giannotti A, Dallapiccola B (2002) Identification of a novel NOG gene mutation (P35S) in an Italian family with symphalangism. Hum Mutat 19:308 [DOI] [PubMed] [Google Scholar]
- Marcelino J, Sciortino CM, Romero MF, Ulatowski LM, Ballock RT, Economides AN, Eimon PM, Harland RM, Warman ML (2001) Human disease-causing NOG missense mutations: effects on noggin secretion, dimer formation, and bone morphogenetic protein binding. Proc Natl Acad Sci USA 98:11353–11358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan C-M, Harland RM, McMahon AP (1998) Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev 12:1438–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milunsky J, Suntra C, MacDonald CB (1999) Congenital stapes ankylosis, broad thumbs, and hyperopia: report of a family and refinement of a syndrome. Am J Med Genet 82:404–408 [PubMed] [Google Scholar]
- Moore KB, Moody SA (1999) Animal-vegetal asymmetries influence the earliest steps in retina fate commitment in Xenopus. Dev Biol 212:25–41 [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM (1992) Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell 70:829–840 [DOI] [PubMed] [Google Scholar]
- Smith WC, Knecht AK, Wu M, Harland RM (1993) Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature 361:547–549 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Takahashi I, Komatsu M, Sawaishi Y, Higashi K, Nishimura G, Saito H, Takada G (2001) Mutations of the NOG gene in individuals with proximal symphalangism and multiple synostosis syndrome. Clin Genet 60:447–451 [DOI] [PubMed] [Google Scholar]
- Teunissen B, Cremers WR (1990) An autosomal dominant inherited syndrome with congenital stapes ankylosis. Laryngoscope 100:380–384 [DOI] [PubMed] [Google Scholar]
- Trousse F, Esteve P, Bovolenta P (2001) BMP4 mediates apoptotic cell death in the developing chick eye. J Neurosci 21:1292–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela DM, Economides AN, Rojas E, Lamb TM, Nuñez L, Jones P, Ip NY, Espinosa R 3rd, Brannan CI, Gilbert DJ, Copeland NG, Jenkins NA, Le Beau MM, Harland RM, Yancopoulos GD (1995) Identification of mammalian noggin and its expression in the adult nervous system. J Neurosci 15:6077–6084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitt UA, Hsu SY, Hsueh AJ (2001) Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Mol Endocrinol 15:681–694 [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM (1996) The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 86:599–606 [DOI] [PubMed] [Google Scholar]