Abstract
Malformations of cortical development (MCDs) are commonly complicated by intractable focal epilepsy. Epileptogenesis in these disorders is not well understood and may depend on the type of MCD. The cellular mechanisms involved in interictal and ictal events are notably different, and could be influenced independently by the type of pathology. We evaluated the relationship between interictal and ictal zones in eight patients with different types of MCD in order to better understand the generation of these activities: four had nodular heterotopia, two focal cortical dysplasia and two subcortical band heterotopia (double-cortex). We used the non-invasive EEG-fMRI technique to record simultaneously all cerebral structures with a high spatio-temporal resolution. We recorded interictal and ictal events during the same session. Ictal events were either electrical only or clinical with minimal motion. BOLD changes were found in the focal cortical dysplasia during interictal and ictal epileptiform events in the two patients with this disorder. Heterotopic and normal cortices were involved in BOLD changes during interictal and ictal events in the two patients with double cortex, but the maximum BOLD response was in the heterotopic band in both patients. Only two of the four patients with nodular heterotopia showed involvement of a nodule during interictal activity. During seizures, although BOLD changes affected the lesion in two patients, the maximum was always in the overlying cortex and never in the heterotopia. For two patients intracranial recordings were available and confirm our findings. The dysplastic cortex and the heterotopic cortex of band heterotopia were involved in interictal and seizure processes. Even if the nodular gray matter heterotopia may have the cellular substrate to produce interictal events, the often abnormal overlying cortex is more likely to be involved during the seizures. The non-invasive BOLD study of interictal and ictal events in MCD patients may help to understand the role of the lesion in epileptogenesis and also determine the potential surgical target.
Keywords: malformation of cortical development, EEG, functional MRI, epileptogenesis, seizure
Introduction
Malformations of cortical development (MCDs) are a frequent cause of intractable epilepsy (Palmini et al., 1991; Raymond et al., 1995). Patients with MCD can present a large variability in clinical signs with either symptomatic generalized, multifocal or focal seizures. The main MCD classification is based principally on anatomical findings. Three groups are usually distinguished (Barkovich et al., 2001): (i) abnormalities of neuronal and glial proliferation and differentiation (e.g. focal cortical dysplasia, hemimegalencephaly); (ii) abnormalities of neuroblast migration (e.g. subcortical band heterotopia or the double-cortex syndrome, periventricular and subcortical nodular heterotopia); (iii) abnormalities of cortical organization (e.g. polymycrogyria). Mechanisms of epileptogenesis of these MCDs are not fully understood. Intrinsic epileptogenesis is characteristic of focal cortical dysplasia with the occurrence of a typical rhythmic epileptiform discharge (Chassoux et al., 2000; Palmini et al., 2000; Rosenow et al., 2001), but this is not the case with the other types of MCD. Surgical decision may be difficult and surgical outcome remains poor for many patients with intractable epilepsy due to MCD (Palmini et al., 1991; Hirabayashi et al., 1993; Dubeau et al., 1995; Raymond et al., 1995; Li et al., 1997; Wyllie et al., 1998; Aghakhani et al., 2005). The challenge in these patients is to define accurately the epileptogenic zone. Because the usual non-invasive methods are often not sufficient to define it, intracerebral EEG recordings are then performed to specify which structures are involved in the seizure onset. However, invasive techniques have major limitations. They expose the patients to a surgical risk, they are costly and time-consuming and they may be inaccurate or blind to the epileptic focus because of the limited amount of brain that can be investigated.
Combined EEG and functional magnetic resonance imaging (EEG-fMRI) recording has shown the ability to identify generators of interictal epileptiform events that define the irritative zone (Gotman et al., 2006). This tool is especially efficient in the case of cortical lesions (Krakow et al., 2001) and previous studies on MCD patients have shown consistent results (Krakow et al., 2001; Diehl et al., 2003; Salek Haddadi et al., 2003b, 2006; Federico et al., 2005a; Kobayashi et al., 2005, 2006a). The lesion was often involved in the process of spike generation in focal cortical dysplasia and double-cortex patients. On the other hand, for patients with gray matter nodular heterotopia the findings were more inconsistent, the nodule being involved or not, in association with the overlying cortex, in the generation of epileptic activity. Interictal and ictal events have different mechanisms of generation (de Curtis and Avanzini, 2001) and irritative and seizure onset zones could be different. EEG-fMRI has only been used in a few cases during ictal events (Salek-Haddadi et al., 2002, 2003a; Federico et al., 2005b; Bonaventura et al., 2006; Di Bonaventura et al., 2006; Kobayashi et al., 2006b). It presents the advantage of being a safe tool to explore the whole brain and to investigate the irritative zone but also the epileptogenic zone.
In order to better understand mechanisms of epileptogenicity in MCDs, we compared BOLD signal changes induced by interictal and ictal epileptiform discharges in a series of epileptic patients with focal cortical dysplasia, gray matter nodular and band heterotopia.
Methods
Subjects
From the EEG-fMRI database of the Montreal Neurological Institute (November 2003–December 2007, including 159 patients), we selected the eight epileptic patients with MCD and both interictal and ictal events recorded during the same EEG-fMRI: four had a nodular gray matter heterotopia, two a focal cortical dysplasia and two a double cortex (Tables 1 and 2). One patient was recorded twice (pt 1) and results have already been published (Kobayashi et al., 2006b). Patients had a low-average intelligence quotient and no predisposing factor such as febrile convulsion, and no history of head trauma, encephalitis or meningitis. One patient (pt 3) had surgery prior to the study. Patients recruited for this database had focal epilepsy with clear EEG interictal epileptiform activity. The EEG-fMRI was done for research purposes and patients were not necessary surgical candidates. Only two of them, Patients 4 and 5, were recorded at the time of presurgical exploration before intracranial study. Patients participated in the research study after giving their written informed consent, according to a protocol approved by the ethics committee of the Montreal Neurological Institute and Hospital. All EEG-fMRI recordings were done following the same protocol. We did not try to induce seizures and especially no sleep deprivation or drug reduction was performed specifically for the test.
Table 1.
Characteristics of the patients: clinical and electrographic features recorded outside the MRI
| Pts | Age (year) | Epilepsy type | Seizure semiology | Age of first seizure (year) | Scalp EEG recordings outside the scanner | Antiepileptic drugs | Seizure frequency |
|---|---|---|---|---|---|---|---|
| 1 | 25 | R TLE | Rising epigastric aura followed by out-of-breath sensation and loss of consciousness | 24 | Interictal: RTor RFT spike or polyspike-slow waves Ictal: none |
None | Two events in 1 year |
| 2 | 39 | R TOLE | Stroboscopic black and white light in the L visual field, or forced eye-deviation to the L with loss of consciousness | 12 | Interictal: spikes and spike-slow waves over the R posterior quadrant Ictal: rhythmic burst of spikes over the R T region sometimes spreading over the L side |
Carbamazepine 2400 mg, Valproic acid 1000 mg, Clobazam 10 mg | 1/month |
| 3 | 52 | TOLE | Before surgery visual distortion of objects and lights with language difficulty and now also associated with auditive distorsion | 17 | Interictal: independent R and L temporal spikes Ictal: none |
Pregabalin 450 mg, Topiramate 300 mg | 1/day (at least) |
| 4 | 23 | L TLE | Feeling of familiarity, ‘déjá vu’ or ‘deja vecu’ sensation then loss of contact with staring, lip smacking and swallowing movements (lower limbs movements sometimes) | 14 | Interictal: L T and R T independent irregular sharp waves or spikes Ictal: not recorded in scalp EEG |
Carbamazepine 600 mg, Topiramate 300, Clobazam 20 mg | 3–4/day in clusters |
| 5 | 11 | R FLE | Sensation of fear followed by a deep breath movement. He could sometimes scream and turns his head to the left | 4 | Interictal: R F spike-waves isolated or organized in short burst Ictal: Prolonged bursts of rhythmic bilateral F spike-waves with a clear R predominance |
Phenytoin 100 mg, Valproic acid 250 mg | 20–30/day |
| 6 | 33 | L CPLE | Somesthesic sensation in the R elbow followed by a slow non-tonic R arm elevation | ? | Interictal: polyspikes or poly-sharp waves over the L CP area. Ictal: rhythmic fast activity starting over the L CP regions followed by a spreading over the FT areas |
Carbamazepine 1200 mg, Topiramate 75 mg, Clobazam 20 mg | 1–3/day |
| 7 | 26 | Bil TOLE | Chest pressure feeling followed by loss of contact, staring, chewing and sometimes R eyes-deviation | 9 | Interictal: spike and slow waves complexes over TO electrodes bilateral or predominant over the R side Ictal: bilateral slow waves followed by rhythmic fast activity seen synchronously over the posterior temporal regions with a predominance over the R side. Irregular generalized slow waves followed |
Carbamazepine 1000 mg, Clobazam 40 mg | 1/week |
| 8 | 23 | Bil TOLE | Start to see things smaller and far away from her, then she becomes blind or sees lights forming faces | 13 | Interictal: synchronous and rhythmic slow wave discharges with intermixed sharp components over both posterior head regions Ictal: prolonged electrographic attack, maximal over both posterior temporal regions with clear predominance over the R side |
Clobazam 40 mg, Lamotrigine 500 mg, Phenytoin 300 mg | 1–2/week |
| Mean ± SD | 29 ±12.3 | 13,3 ± 6.3 |
R = right; L = left; Bil = bilateral; T = temporal; O = occipital; C = central; P = parietal.
Table 2.
Characteristics of patients: anatomical MRI features and EEG abnormalities
| Pts | Anatomical MRI | Interictal events
|
Ictal events
|
|||
|---|---|---|---|---|---|---|
| Types | Number | Types | Number | Duration (mean; range) | ||
| 1 | R TP nodular heterotopia (trigone, occipital horn and subcortical region). Thick overlying cortex | R T spike and waves | 13 | R FT sharp waves followed by polyspikes Electrical events during sleep |
24 | 3.5 s; 2.1–6.2 s |
| 2 | R OT nodular heterotopia (occipital horn). Abnormal overlying cortex over the whole posterior quadrant with increase of thickness | R post. T spikes | 30 | R post. T sharp rhythmic activity Electrical event |
1 | 22.3 s |
| 3 | Bil. nodular heterotopia (trigone). L anterior T resection | R T spikes L T spikes |
41 40 |
R centroT 10Hz activity Clinical event |
1 | 65 s |
| 4 | Bil. nodular heterotopia (trigones, temporal and occipital horns). Thick overlying occipital cortex | R T spikes L T spikes |
23 8 |
L Trhythmic sharp waves Electrical events |
3 | 2.5 s; 1.6–3.5 s |
| 5 | R ant. F focal cortical dysplasia | R F spike and waves | 31 | R F rhythmic spike and waves Clinical events |
15 | 21.3 s; 7.2– 49.7 s |
| 6 | L centro P focal cortical dysplasia | L centro P spikes | 42 | Rhythmic L centro P sharp fast activity Clinical events |
1 | 9.2 s |
| 7 | Band heterotopia predominating over parietal, occipital and temporal areas, R 4L | L TO polyspikes R TO polyspikes |
100 74 |
R TPO discharges propagating to L homologous regions Electrical events during sleep |
11 | 6.2; 3.8–13 s |
| 8 | Band heterotopia predominating over the frontal and parietal regions, bilaterally | Bil. TPO rhythmic slow waves | 15 | Rhythmic bil. occipital and R TPO discharges Electrical events during sleep |
2 | 12 s; 10.5–13.5 |
| Mean ± SD | 38 ± 27.5 | 7.2 ± 8.6 | 17.7 ± 20.5 s | |||
R = right; L = left; Bil = bilateral; T = temporal; F = frontal; C = central; P = parietal; O = occipital.
Detailed medical history for Patients 4 and 5
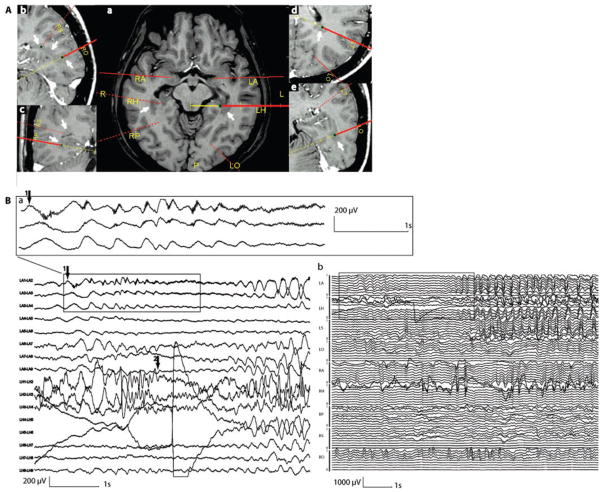
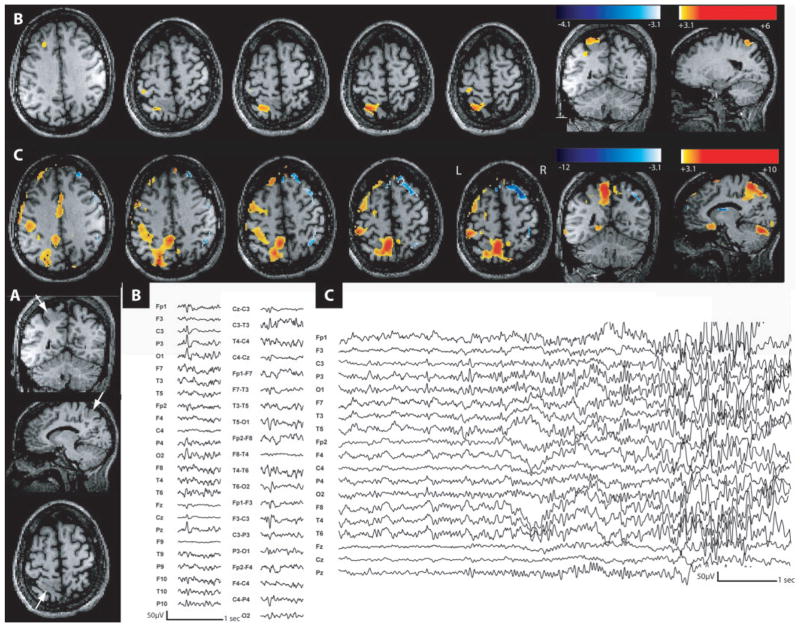
Patient 4 was a 23-year-old right-handed man investigated for a refractory seizure disorder starting at age 14 years. His typical attacks were preceded by an aura consisting in a ‘déjá vu’ and ‘déja vécu’ sensation. Then he lost contact and stared with chewing movements. He was usually pale and mute, could also rub both hands together. He could deambulate if he was standing up. After the event, he had no recollection of the event and no speech deficit. The events lasted in average 1 min. On scalp EEG, independent interictal epileptiform abnormalities were seen in both temporal regions (maximum over F8–T4 and F7–T3). The MRI showed a gray matter heterotopia, bilaterally extending from the anterior temporal horn toward the atrium and posterior to the occipital lobe. The neuropsychological evaluation did not show evidence for temporal lobe impairment. This patient underwent an intracranial exploration with four depth electrodes on the left side and five on the right side (Fig. 1). We targeted the amygdala (LA, RA), the hippocampus (LH, RH), the posterior hippocampus (RP), the trigonal area and supramarginal gyrus (LT, RS) and the occipital lobe (LO, RO). LH (contacts 2–3), RH (contacts 3–4), RP (1–2), LT (contacts 1–2), RS (1–2), LO (contacts 1–2) and RO (1–2) reached a nodule. Interictal discharges were recorded independently in both hippocampal formations and amygdalae. The right-sided interictal events sometimes spread to the overlying temporal neocortex. None of the contacts placed in or in close proximity to nodular heterotopia showed interictal events. We recorded 18 seizures (9 clinical). Electrical seizures originated as often from the right hippocampus as from the left amygdala with propagation to the ipsilateral hippocampus. Clinical seizures were also recorded independently from both mesial temporal structures with predominance on the right side. Seizures from the right hippocampus propagated to the ipsilateral amygdala, the temporal neocortex and more posteriorly over the trigone, and eventually to the left side. Clinical seizures from the left amygdala propagated to the ipsilateral hippocampus (Fig. 1) and in some cases posteriorly to the trigone. The nodular heterotopia were involved late and during the propagation only (over the trigone). In this case of severe bitemporal epilepsy, the right mesial temporal structures were removed (non-dominant side and more active) to reduce the number of seizures (palliative surgery). After 1 year the patient experienced a reduction of 40% in his seizures.
Fig. 1.
Intracranial recording for Patient 4. (A) Electrodes positioning planned with a neuronavigation system (yellow lines indicate the planned direction of the electrodes path, the red lines represent the electrodes). On axial (a and d), sagittal (b and e) and coronal (c) MRI, heterotopic nodules (white arrows) were targeted by RH, LH, RP, RS, LO, RO and LT. RA and LA did not reach any nodule. (B) One example of a clinical seizure starting from the left amygdala (arrow 1) recorded during intracranial study (a). Rhythmic short bursts of high-frequency oscillations started over LA1 and LA2 followed by an attenuation of the EEG signal dominated by this high-frequency activity. In LH1, LH2 and LH3 (2–3 probably in the nodule), a later attenuation of the background activity (arrow 2) followed by a 8 Hz spiking activity is observed. A regional 4 Hz discharge involved then all LA contacts and to a lesser extent the last LH contacts and later LT contacts. The seizure remained only on the left side with involvement of all the contacts (b). LA = left amygdala; RA = right amygdala; LH = left hippocampus; RH = right hippocampus; RP = right parahippocampus; LT = left trigonal area; RS = right supra marginal gyrus, LO = left occipital; RO = right occipital.
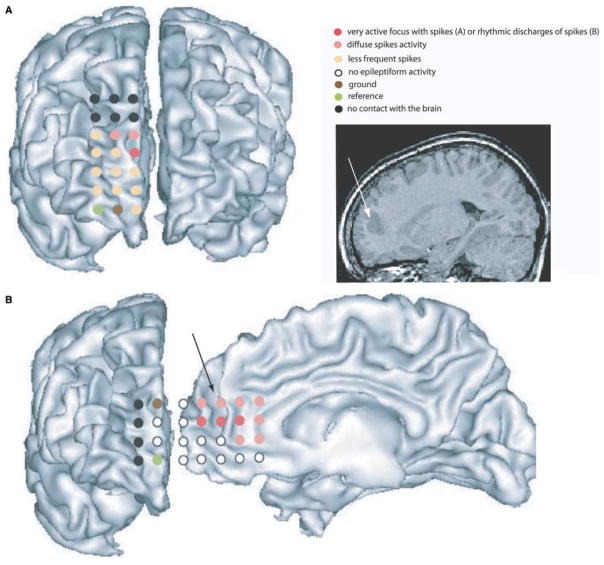
Patient 5 was an 11-year-old right-handed boy with intractable epilepsy starting at age 4 years. He presented daily seizures (20–30 per day in clusters) characterized by an aura of fear followed by vocalization. In some cases we noticed a left eye and head deviation. The events lasted for 5–30 s. On scalp EEG we observed isolated or short bursts of spike and waves over the right frontal area, maximum at F4. Simultaneously with clinical events, a prolonged rhythmic discharge of spikes and waves was seen first over the right frontal region then propagating to the left frontal area. On MRI, an increase of grey matter thickness without abnormal MRI signal was observed at the bottom of the right frontal sulcus in the anterior cingulate gyrus. A dysplastic lesion was suspected. An ictal 99mTc-ECD SPECT (injection 1 s after seizure onset) showed a large activation in the right superior and antero-lateral frontal and parasagittal areas. A right frontal hypometabolism was seen in FDG-PET. The neuropsychological exploration reported language and attention deficits. An electrocorticography (ECoG) was performed during surgery (Fig. 2) defining the seizure onset zone (short seizures were frequent during this 20 min recording) in the right anterior cingulate gyrus with propagation over the right frontopolar lobe. The data were collected using a 3 × 8 and a 4 × 8 grid placed respectively over the right frontopolar region and over the right side of the interhemispheric space. Contacts of the 3 × 8 grid in front of the area of the anterior cingulate gyrus showed focal spikes. Contacts of the 4 × 8 grid covering the right anterior cingulate gyrus showed prolonged rhythmic discharges. The patient underwent a right frontopolar resection including the suspicious sulcus. An ECoG was done around the scar and demonstrated the absence of epileptiform activity. Pathological analysis confirmed the dysplastic nature of the lesion. After 15 months the patient is still seizure-free.
Fig. 2.
Electrocorticographic recording for Patient 5. The white arrow on theT1 MRI and the black arrow on the reconstructed cortical surface show the dysplastic sulcus in the right anterior cingulate gyrus. (A) A 3 × 8 grid was positioned over the right frontopolar area. The two upper rows of electrodes did not touch the brain (contacts in black). A spiking activity was clearly observed on the red contact, more diffuse over the contacts in pink. Rare spikes were noticed over the orange contacts. Pink and red contacts were in front or in close vicinity of the dysplastic sulcus. (B) A 4 × 8 grid was inserted in the interhemispheric space in contact with the right anterior cingulate area. The last two rows of electrodes were curved and only one was in contact with the frontopolar area. Contacts in red showed prolonged rhythmic discharges of spike activity and were in front of the bottom of the dysplastic sulcus. In pink, contacts showed diffuse spiking activity.
EEG acquisition
The EEG acquisition was always performed with 25 MR compatible electrodes (Ag/AgCl) placed on the scalp using the 10–20 (21 usual electrodes without Fpz and Oz) and 10–10 (F9, T9, P9, F10, T10 and P10) electrode placement systems. The reference electrode was at FCz. Two electrodes located on the back recorded the electrocardiogram (EKG). To minimize movement artefacts and for patient’s comfort, the head was immobilized with a pillow filled with foam microspheres (Siemens, Germany). Data were transmitted from a BrainAmp amplifier (Brain Products, Munich, Germany, 5 kHz sampling rate) via an optic fibre cable to the EEG monitor located outside the scanner room.
fMRI acquisition
Functional images were acquired using a 1.5 T MR scanner (Siemens, Sonata, Germany) for two patients (pts 1 and 2) and a 3 T MR scanner (Siemens, Trio, Germany) for the other six. A T1-weighted anatomical acquisition was first done (1 mm slice thickness, 256 × 256 matrix; for the 1.5 T: TE = 9.2 ms and TR = 22 ms; for the 3 T: TE = 7.4 ms and TR = 23 ms; flip angle 30°) and used for superposition with the functional images. The functional data were acquired in series of 6–14 runs of 6 min each using a T2*-weighted EPI sequence (voxel dimensions 5 × 5 × 5 mm3, 25 slices, 64 × 64 matrix; for the 1.5 T: TE = 50 ms and TR = 3 s; for the 3 T: TE = 30 ms and TR = 1750 ms; flip angle 90°). The patients were at rest during the 2 h of recording session. No sedation was given.
EEG processing
Brain Vision Analyser software (Brain Products, Munich, Germany) was used for off-line correction of the gradient artefact and filtering of the EEG signal. This software uses the method described by Allen et al. (2000). It is based on the subtraction of the average signal obtained during the interval of the scanner artefact. A 50 Hz low-pass filter was also applied to remove the remaining artefact. The ballistocardiogram (BKG) artefact was removed by independent component analysis (ICA) (Bénar et al., 2003).
A neurologist reviewed the entire EEG recording and selected the epileptiform events. We defined as ictal each electrical event with clinical changes similar to those observed during typical clinical seizures. When clinical changes were absent or too subtle to be seen inside the scanner, we characterized as ictal each electrical discharge obtained inside the scanner similar (duration, propagation and morphological pattern) to those recorded outside the scanner. These events presented a longer duration and a clear electrical propagation pattern compared to the very brief and localized epileptiform abnormalities (interictal events). In the absence of a seizure outside the scanner for comparison, these last features were used as criteria to define the seizures recorded inside the scanner. The expert put markers corresponding to interictal and ictal events (timing and duration).
fMRI processing
The EPI images were motion-corrected and smoothed (6 mm full width at half maximum) using the software package from the Brain Imaging Center of the Montreal Neurological Institute (http://www.bic.mni.mcgill.ca/software/). Temporal autocorrelations were accounted for by fitting an AR model of order 1 according to the methods of Worsley et al. (2002), and low-frequency drifts in the signal were modelled with a third-order polynomial fitted to each run. A regressor for each type of interictal spike and a regressor for ictal events (ictal events were grouped if they were similar) were built using the timing and duration of each event and convolved with four haemodynamic response functions (HFRs) with peaks at 3, 5, 7 and 9 s (Bagshaw et al., 2004). All these regressors were included in the same general linear model. A statistic t-map was obtained for each regressor using the other regressors as confounds (a study was performed for each type of interictal event and for the group of similar ictal discharges) in the fMRI analysis (fMRIstat, Worsley et al., 2002). At each voxel, the maximum t-value was taken from four individual t-maps created with the four HRFs. We compared in particular the location of the BOLD response due to interictal and ictal events in relation to the lesion.
The EPI frames were realigned together using a linear six-parameter rigid-body transformation (three translations and three rotations) to correct for movement effects. However, residual artefacts may still contaminate the fMRI data even after motion correction (Friston et al., 1996). Therefore, the six parameters used for the realignment were also integrated in the analysis as confound regressors in the general linear model for the patients with clinical seizures.
EEG-fMRI analysis
To be significant, a response should have a minimum of five contiguous voxels with a t = 3.1, corresponding to P<0.05 corrected for the multiple comparisons resulting from the number of voxels in the brain and the use of four HRFs. The t-map results were represented using the same types of scale: red–yellow scale corresponding to positive BOLD changes (activation) and blue–white scale to negative BOLD changes (deactivation).
Results
Anatomical MRI findings
On the anatomical MRI obtained at the time of the fMRI study we confirmed the MCD lesions (Table 1) already observed after a complete MRI exploration (T1, T2, T2*, flair, apparent diffusion coefficient) and analysed by a radiologist expert in epilepsy. In the four patients with nodular heterotopia (pts 1–4), two had bilateral and two unilateral nodules. The heterotopic gray matter was periventricular along the trigone, temporal and occipital horns and in one case (pt 1) was also subcortical. In three patients, the cortex overlying the heterotopia was also abnormal with increased thickness. In the two patients with focal cortical dysplasia (pts 5 and 6), one had only a discrete dysplastic lesion in the right anterior cingulate gyrus while the second had a lesion extending to half the left parietal lobe. Anatomopathology confirmed this latter abnormality after resection of the abnormal sulcus and of the overlying right frontopolar cortex. In the two patients with band heterotopia (pts 7 and 8), one showed an anterior and the other a posterior preponderance of the malformation.
EEG recordings: interictal and ictal epileptiform activity
For every patient we recorded interictal and ictal epileptiform events (Table 2) during the same recording. Five patients presented only one type of spikes, and three patients with bilateral lesions (pts 3, 4 and 7) had two types. The number of spikes recorded during the fMRI session ranged from 8 to 100 (average ± SD: 38 ± 27.5). Compared to the EEG recorded outside the scanner, the interictal events selected during scanning were similar for every patient (Tables 1 and 2).
Three patients had a single seizure and the others had several ictal events (Table 2). The seizures were either pure EEG events (41) or clinical-EEG events with minimal clinical manifestations (17). Because video was not always available inside the scanner we could have missed some of the clinical signs. Patient 3 reported an auditory distortion associated with his usual seizures (he never experienced seizures induced by sound, even during earlier MRI sessions). Patient 5 presented 15 similar events with a feeling of fear and the necessity to take a deep breath (visible on the online video). Patient 6 reported his typical semiology (no video available) with somesthesic sensations in the right elbow followed by right arm movements. Patients 1 and 3 did not have previous EEG recording of seizures outside the scanner and a comparison of the EEG signal could not be done. We recorded seizures for Patient 4 only during an intracranial study that was subsequent to scanning (see later). For the others, they showed during scanning the same EEG pattern as outside the scanner during their typical seizures (Tables 1 and 2). Patient 7 had only electrical seizures recorded in the scanner and they were shorter than the clinical ones recorded outside, but the EEG changes were similar at the onset.
BOLD responses
A BOLD response was observed in each study (100%). The maximum t-value, reflecting the highest correlation between BOLD changes and epileptic events, was larger for ictal (positive BOLD: 12.4 ± 5.3; negative BOLD: −11 ± 2.7) than for interictal events (positive BOLD: 6.3 ± 1.8; negative BOLD: −5.3 ± 2.5). We evaluated quantitatively this difference by performing a non-parametric comparison, which confirmed that maximum positive BOLD responses were significantly higher for ictal than interictal events (P = 0.012); similarly for negative BOLD responses (P = 0.018). This suggests a better correlation between the BOLD signal changes and the seizure time course and also a BOLD response with higher amplitude at the time of the seizure compared to the interictal events.
We analysed separately patients with nodular heterotopia, focal cortical dysplasia and band heterotopia. We compared in each the location of BOLD responses during interictal (Table 3 and Supplementary Table 1) and ictal events (Table 4 and Supplementary Table 2).
Table 3.
BOLD responses (activation and deactivation) during interictal epileptiform events
| Lesion | Pt | Activation
|
Deactivation
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Areas involved in BOLD response
|
Areas of the max BOLD response
|
Areas involved in BOLD response
|
Areas of the max BOLD response
|
||||||||||||
| Lesion | Overl Cx | Dist | Max t-value | Lesion | Overl Cx | Dist | Lesion | Overl Cx | Dist | Max t-value | Lesion | Overl Cx | Dist | ||
| Nodular gray matter heterotopia | 1 | Y | – | – | 5.6 | Y | – | – | – | – | Y | −4 | – | – | Y |
| 2 | Y | Y | – | 6.3 | Y | – | – | – | Y | – | −7 | – | Y | – | |
| 3 | – | – | Y | 5.2 | – | – | Y | – | – | Y | −4.5 | – | – | Y | |
| – | – | Y | 4.8 | – | – | Y | – | – | Y | −5 | – | – | Y | ||
| 4 | – | Y | – | 4.3 | – | Y | – | – | – | Y | −4.2 | – | – | Y | |
| Y | Y | Y | 8.2 | – | – | Y | – | Y | Y | −5.2 | – | – | Y | ||
| Focal cortical dysplasia | 5 | Y | Y | – | 10.8 | Y | – | – | – | – | Y | −4.7 | – | – | Y |
| 6 | Y | – | Y | 5.8 | Y | – | – | – | – | – | – | – | – | – | |
| Band gray matter heterotopia | 7 | Y | Y | – | 5.4 | Y | – | – | – | Y | – | −8.8 | – | Y | – |
| Y | Y | – | 5.3 | Y | – | – | – | Y | – | −9.2 | – | Y | – | ||
| 8 | – | – | Y | 7.4 | – | – | Y | Y | Y | – | −6.3 | Y | – | – | |
| Mean ± SD | 6.3 ±1.2 | −5.9 ±1.6 | |||||||||||||
The lesion in focal cortical dysplasia and in band heterotopia is always involved by BOLD changes during interictal events generation. Results for patients with nodular heterotopia were more variable. Overl Cx = overlying cortex; Dist. = distant area; Y = significant BOLD changes observed in this area.
Table 4.
BOLD responses (activation and deactivation) during ictal epileptiform events
| Lesion | Pt | Activation
|
Deactivation
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Areas involved in BOLD response
|
Areas of the max BOLD response
|
Areas involved in BOLD response
|
Areas of the max BOLD response
|
||||||||||||
| Lesion | Overl Cx | Dist | Max t-value | Lesion | Overl Cx | Dist | Lesion | Overl Cx | Dist | Max t-value | Lesion | Overl Cx | Dist | ||
| Nodular gray matter heterotopia | 1 | Y | Y | – | 22.8 | – | Y | – | – | – | Y | −7.6 | – | – | Y |
| 2 | – | Y | Y | 6.8 | – | Y | – | – | Y | Y | −8.8 | – | – | Y | |
| 3 | – | Y | Y | 10.6 | – | Y | – | – | – | Y | −8.6 | – | – | Y | |
| 4 | Y | Y | Y | 10.6 | – | Y | – | – | Y | Y | −12 | – | Y | – | |
| Focal cortical dysplasia | 5 | Y | – | Y | 13.9 | Y | – | – | – | – | Y | −15 | – | – | Y |
| 6 | Y | – | Y | 9.9 | Y | – | – | – | – | Y | −11.5 | – | – | Y | |
| Band heterotopia | 7 | Y | Y | – | 16.8 | Y | – | – | – | Y | Y | −11.5 | – | Y | – |
| 8 | Y | Y | Y | 7.8 | Y | – | – | – | Y | – | −10.4 | – | Y | – | |
| Mean ± SD | 12.4 ±5.3 | −7.8 ± 8.1 | |||||||||||||
The lesion in focal cortical dysplasia and in band heterotopia is always involved by BOLD changes during ictal events generation. Nodules were never involved by the larger significant BOLD changes (max t-value). Overl Cx = overlying cortex; Dist. = distant area; Y = significant BOLD changes observed in this area.
Nodular heterotopia
Interictal events
Six studies were performed considering that two of the four patients had two types of interictal events. Every patient had a positive and a negative BOLD response. Activation was seen in the heterotopia in three studies, in two this activation also involved the overlying cortex (pt 2 and pt 4, type 2) and in one only the heterotopia (pt 1). For patient 3 (Fig. 3), the activation involved the overlying cortex and distant cortical areas in the two types of events. The largest correlation between the BOLD signal and interictal events (maximum t-value) was obtained in the heterotopic area in only two of the six studies (pts 1 and 2) (Fig. 4). Deactivation was found in regions adjacent or distant from the lesion, but never in the heterotopia.
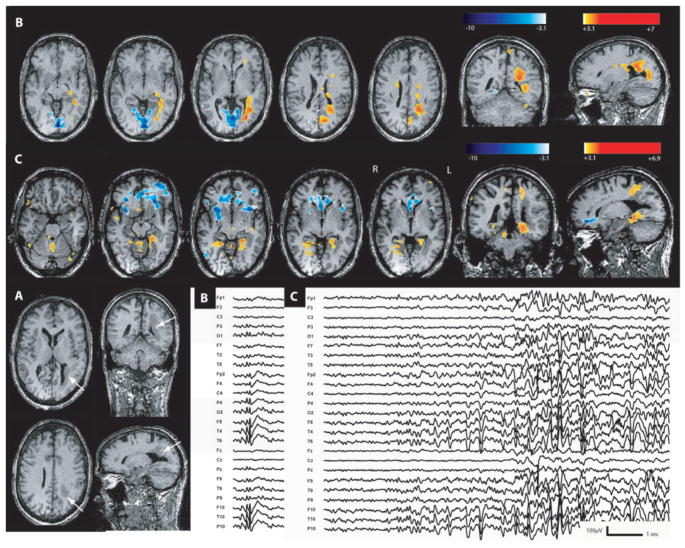
Fig. 3.
Nodular heterotopia (Patient 3). (A) Anatomical MRI showed a nodular heterotopia along both occipital horns (head of the white arrows). (B) Increased BOLD signal in the right temporal and parietal cortex and bilateral medial occipital gyrus during right temporal spikes. (C) Activation in the left temporoparietal cortex and bilateral middle frontal gyri during left temporal spikes. (D) Maximum activation during a single seizure in right occipitotemporal region. On EEG, the seizure started with a right centroparietotemporal fast activity. Nodular heterotopia were never involved during these different types of epileptic events.
Fig. 4.
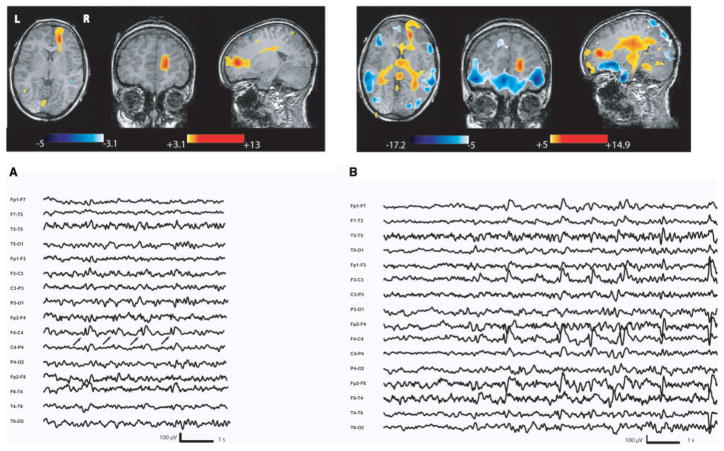
Nodular heterotopia (Patient 2). (A) Anatomical MRI showed a right occipitotemporal nodular heterotopia over the occipital horn (head of white arrows). Abnormal overlying cortex over the whole posterior quadrant. (B) Increase of BOLD signal involved essentially the nodular heterotopia during right posterior temporoparietal spikes. Deactivation was confined in the cuneus. (C) Activation during the seizure involved only the overlying cortex; the nodular heterotopia was not involved. On the EEG, the seizure started by a right posterior temporoparietal sharp rhythmic activity.
Ictal events
BOLD signal changes were also seen in every patient but were more widespread. A positive BOLD response involved the nodular heterotopia in two studies (pts 1 and 4). For every patient, activation was also or only observed in the overlying cortex (Figs 3 and 4). The maximum t-value was always located in structures surrounding the nodular heterotopia, outside the lesion. Negative BOLD responses were always outside the heterotopia. They involved areas surrounding the previous described activation (pts 1 and 4) or distant from the lesion (pts 2, 3 and 4).
Focal cortical dysplasia
Interictal events
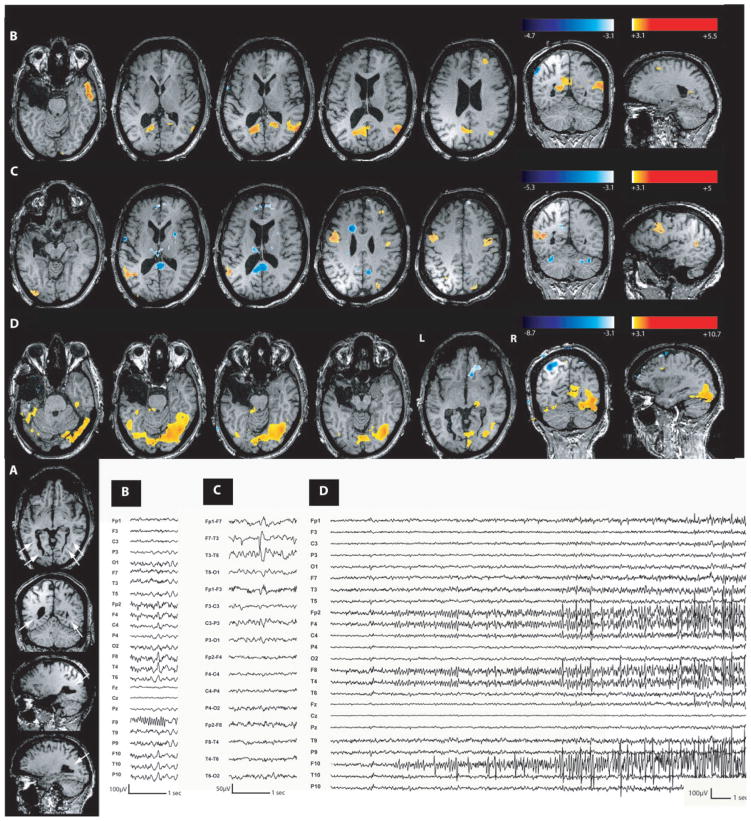
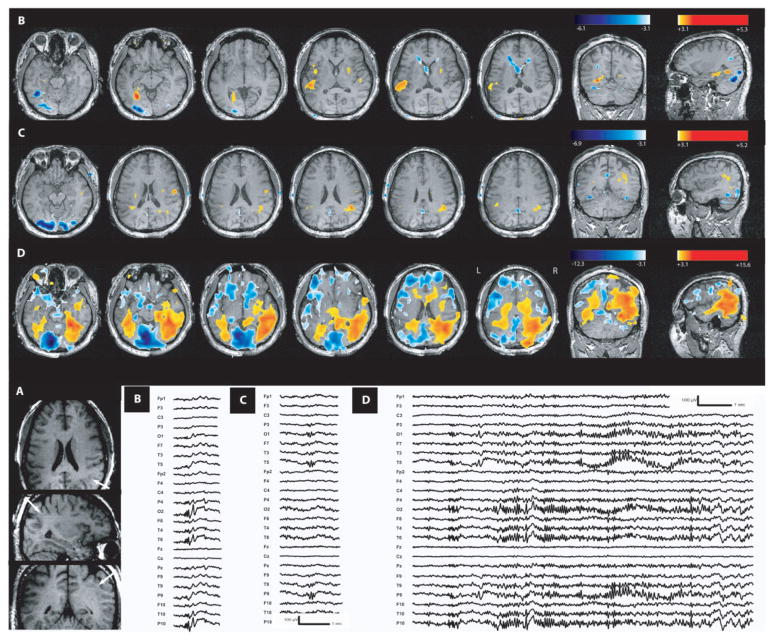
Two studies were done for this group (two patients, each having one type of event). A positive response, including the maximum t-value, involved the dysplasia in both cases. Patient 5 with a lesion in the anterior cingulate gyrus also presented an activation in the right frontopolar cortex (Fig. 5), close to the lesion and connected with it. Patient 6 with a centroparietal dysplasia (Fig. 6) had, in addition to the activation in the lesion, an activation at a distant area in the left inferior frontal lobe, not directly connected with the lesion. Deactivation was obtained only for Patient 5 and in both cuneus, far from the right frontal lobe lesion.
Fig. 5.
Focal cortical dysplasia (Patient 5). Figure 2 showed in the anatomical MRI a right dysplastic sulcus in the anterior cingulate gyrus (white arrow). (A) Activation in the dysplasia during interictal right frontal spike and waves. (B) BOLD signal changes observed during clinical seizures with prolonged rhythmic discharge of right frontal spike and waves with synchronous contralateral activity. Maximum activation is again in the lesion with propagation over the right frontopolar area, thalami and the midbrain (left frontopolar area, left anterior cingulate area were also involved but not shown on this picture).
Fig. 6.
Focal cortical dysplasia (Patient 6). (A) Anatomical MRI showed a left centroparietal dysplasia (head of the white arrows). (B) Activation in the dysplasia during C3–P3 spikes. (C) BOLD signal changes induced by single seizure. Maximum activation is again in the lesion. Ictal event starts by a C3–P3– O1 spike activity followed by left parietal fast activity. For both types of events, the activation involved the dysplasia. Concerning the ictal event, BOLD increase is more diffuse and involved the entire dysplasia and connected areas.
Ictal events
An activation, including the maximum t-value, was seen in the lesion for both patients. In Patient 5 (Fig. 5), activation was also observed in adjacent areas (R fronto-polar and anterior cingulate areas). In Patient 6 (Fig. 6), two other structures distant from the lesion were also involved (left rolandic and frontobasal areas). Deactivation was found in both patients but never in the lesion. In Patient 5, a negative response was observed in the adjacent orbitofrontal area and in the distant posterior cingulate, superior parietal and frontal areas and occipital regions. In Patient 6 a single zone of deactivation was seen distant from the lesion in the right frontopolar area.
Band heterotopia
Interictal events
Three studies were performed in this group (one patient had two types of interictal events). An activation was observed in the two patients. In Patient 7 (Fig. 7), we observed an activation in the heterotopic band and in the overlying cortex for the two types of interictal events. Patient 8 (Fig. 8) presented an activation in the thalami and in a small area of the normotopic cortex, in the posterior central gyrus, bilaterally. Deactivation was also found in both patients. For Patient 7 the deactivation involved the lesion and spread over the normotopic cortex in the posterior areas. Patient 8 presented a large area of deactivation over the lesion, including the maximum t-value. The normotopic cortex was also involved, with a smaller t-value.
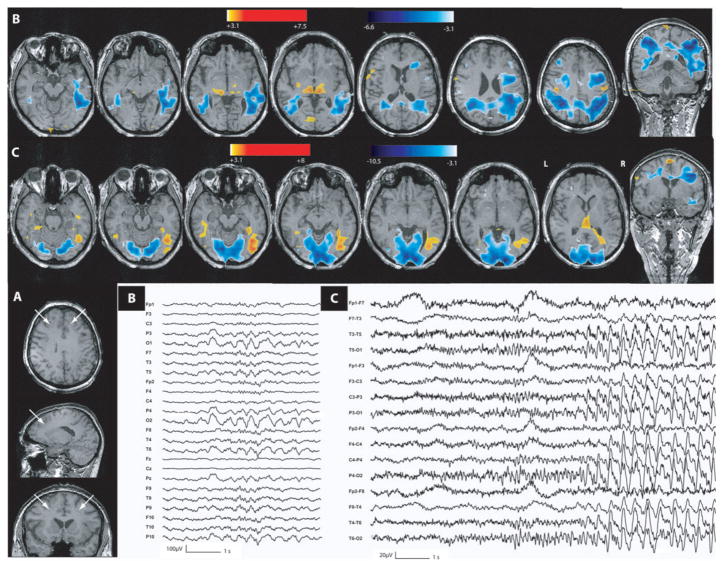
Fig. 7.
Band heterotopia (Patient 7). (A) Anatomical MRI showed a bilateral posterior band heterotopia (head of white arrows). (B) Activation in the left band heterotopia and overlying cortex duringT5–P9– O1polyspikes. (C) Activation of the right band heterotopia duringT6–P10– O2 polyspikes (deactivation, blue, in both occipital lobes). (D) Maximum activation during seizures seen in right and left band heterotopia and overlying cortex (deactivation in blue). Ictal EEG onset showed right posterior temporal fast activity followed by left posterior temporal fast activity. In interictal and ictal events, heterotopic and normotopic cortices are involved by BOLD changes. These BOLD changes are predominant in the heterotopic cortex.
Fig. 8.
Band heterotopia (Patient 8). (A) Anatomical MRI showed a double cortex predominant in frontoparietal areas (head of white arrows). (B) BOLD decreased bilaterally in the heterotopic and normal cortices and increased in the thalami during short burst of temporoparietooccipital rhythmic slow waves. (C) During seizures, activation in the heterotopic and normal cortices mostly between the right occipitotemporal gyrus and the right inferior temporal lobe and in the left inferior temporal lobule. Ictal onset showed a rhythmic bilateral occipital and right temporoparietooccipital discharges. BOLD changes involved the normotopic and the heterotopic cortices during interictal and ictal epileptiform events. A deactivation was observed in the case of interictal events. These changes were predominant in the heterotopic cortex.
Ictal events
A positive response was again obtained for both patients. Activation was found mostly in the heterotopic cortex in both cases (maximum t-value). While Patient 7 (Fig. 7) presented a large activation covering almost the entire lesion, activation in Patient 8 (Fig. 8) involved only a restricted part of the heterotopic band, posteriorly. Activation was also seen in the overlying normotopic cortex in both cases, but compared to the normotopic cortex activation, this involved a smaller area and had a low t-value. Patient 8 showed a thalamic activation, as for interictal events. Deactivation was found in both patients outside the lesion and in normotopic cortex adjacent to activation.
Discussion
Two main points stand out from our study. First, we demonstrated that we could record and analyse electrical or small clinical seizures using the EEG/fMRI method in several epileptic patients non-specifically selected for their seizure frequency. We obtained relevant results for all the patients studied. We showed that the EEG-fMRI technique provides additional information about the generators and structures involved in interictal and ictal epileptiform events compared to the usual exploration in epilepsy (invasive intracranial study limited by a low spatial sampling, interictal study not possible in SPECT). Secondly we could compare in these MCD patients, the structures involved in the interictal and ictal events recorded during the same MRI session. We found that BOLD changes in response to epileptiform activity varies with the type of MCD, supporting the notion that defects occurring at different time of embryogenesis lead to a variety of neuronal organizations and electrophysiological changes. In focal cortical dysplasia and band heterotopia, interictal and ictal epileptic activities involved the lesion and apparently the same neuronal groups. In nodular heterotopia, however, we found a discrepancy between the structures involved during interictal and ictal discharges, as seizures seemed to originate in the cortex overlying the malformation, whereas spikes appear generated in heterotopia and cortex, and not overlapping with the epileptogenic area, supporting previous findings in the same type of patient, but studied with invasive electrophysiological methods.
The EEG-fMRI technique in the study of epilepsy is still mostly based on BOLD changes related to interictal epileptiform events. However, the definition of the epileptogenic zone requires ictal studies. One aim of this study was to provide some information about the location of the irritative and epileptogenic zones. The interest of the EEG-fMRI in the localization of interictal events generators has already been validated by several studies demonstrating the predominance of the BOLD increase at the source of interictal activity (Lemieux et al., 2001; Al-Asmi et al., 2003; Bagshaw et al., 2004, 2006; Bénar et al., 2006). This ability to localize these generators opened additional prospects in presurgical exploration (Zijlmans et al., 2007). For ictal events, interpretation of the results is limited by the ability to discriminate clearly the ictal onset zone from the structures involved during the propagation. Statistical considerations are such that temporal analysis could only separate with confidence focal from propagated activity if propagation was consistently more than one TR (between 2 and 3 s in our study). Despite this limitation, we argue that the highest t-statistic is most likely to represent the focus rather than a region of propagation. Indeed, first the highest change in metabolism during a seizure is most likely to take place at the focus. Since the first stimulation studies of Penfield (1933, 1937), it has been shown that during a seizure a local hyperperfusion occurs at the seizure onset zone. This hyperperfusion reflects the highest neuronal activity located at the seizure onset zone. Ictal SPECT studies used this hyperperfusion analysis to localize the seizure onset zone (Stefan et al., 1990; Duncan et al., 1996, 1997; Van Paesschen, 2004). FMRI technique measures the BOLD changes related to an event, reflecting indirectly the neuronal activity through blood flow modification (Logothetis and Wandell, 2004). Ictal SPECT and EEG-fMRI analysing similar pathophysiological phenomena, the fMRI method should provide equivalent results as ictal SPECT. Compared to ictal SPECT, the simultaneous recording of EEG and fMRI analyses ictal events according to their duration and therefore increases the specificity of the results. This technique allows also the study of brief discharges for which a post-ictal injection is usually obtained during SPECT (Van Paesschen, 2004). Secondly, the statistical analysis (Friston et al., 1995; Worsley and Friston, 1995; Worsley et al., 2002) is performed in order to compare the signal at each voxel with a model (regressor) including a rectangular shape corresponding to the whole seizure convolved with the haemodynamic function. The region best fitting this model (highest t-value) is a region for which metabolism changes from beginning to end of the seizure. And in this best fitting region, the higher is the BOLD signal amplitude, the higher would be the t-value. Thus, voxels with the same BOLD signal time course as the seizures and with the highest BOLD signal amplitude (corresponding to the hyperperfusion) should be in the seizure onset zone. Then, we suggest that the area with the highest t-value is likely to reflect the generator of the epileptiform event. Single case studies reported this correlation (Salek Haddadi et al., 2002, Di Bonaventura et al., 2006; Kobayashi et al., 2006b). In two of our patients (pts 4 and 5), intracranial exploration confirmed the epileptogenic foci suggested by the maximum t-value. For Patient 4 we found a maximum activation over the left amygdala concordant with the seizure onset in the left amygdala recorded with depth electrodes. For Patient 5, ECoG showed the seizure onset zone in the right anterior cingulate gyrus at the same location as the maximum BOLD activation. For patients without intracranial study, BOLD findings were similar to those of the literature using intracerebral exploration (see later). The number of ictal events did not affect the reliability of our results. However in case of a presurgical exploration in order to confirm the seizure onset zone location, additional recording can be performed safely (as is currently performed in ictal SPECT study). Finally, interictal and ictal EEG-fMRI provide valuable information about the structures involved during those events and could suggest the location of their generator. To confirm the interest of EEG-fMRI in defining the epileptogenic zone, further studies with systematic comparisons between fMRI and intracerebral results should be conducted in larger groups of patients and in other types of focal epilepsy.
Analysis of seizures in EEG-fMRI is complicated by the difficulty to predict seizure occurrence during the short time of recording and also by the artefacts generated by movements of the subject. Only single cases were so far reported (Salek Haddadi et al., 2002, 2003a; Federico et al., 2005b; Di Bonaventura et al., 2006; Kobayashi et al., 2006b), and one study analysed only the pre-ictal state (Federico et al., 2005b). We were able to record eight patients with ictal events during the MRI session. These patients were only selected because of the frequent occurrence of interictal events. We could not predict the occurrence of seizures before the recording. The 2 h session of EEG-fMRI recording increases the likelihood to record such events because of its prolonged duration and also because of alertness fluctuations. It is also not unusual for patients with MCDs to have very active epilepsy and frequent seizures. This explains the number of patients with ictal events during EEG-fMRI recording in our study. Ictal events were either electrical or clinical with no or small motion. For patients with small clinical seizures, we applied an additional motion correction in the general linear model using the six parameters of the EPI frames realignment as confounds. This decreased the risk of contamination by motion. The long recording is necessary to increase the likelihood to record epileptiform events but this creates some problems in interpreting the results. Indeed, the patients had to stay at rest during the recording but some of them fell asleep. The ‘baseline’ or residual BOLD signal after removing the other confounds (events, drift and motion) used in the analysis of the events could be different for each subject. The effect of baseline fluctuation is not well known, but statistically it could reduce the number of voxels with a significant BOLD response related to the event. Deactivation instead of activation during spiking activity has been observed in the focus in some patients recorded during sleep (Pasley et al., 2007; Shulman et al., 2007). Further studies should be conducted in order to control these baseline fluctuations during prolonged fMRI recording (for epilepsy, cognitive task, sleep…).
These EEG-fMRI results of the first series of patients with ictal events are promising. For this study, eight patients were selected in a database of 159 patients. We kept all the patients with MCDs and with interictal and ictal events recorded during the same recording. Our study provided relevant results for each of those patients. This small number does not reduce the impact of our finding concerning the interest of the EEG-fMRI technique. Indeed, the database was obtained by selecting patients according to interictal activity occurrence and not to seizure frequency. We think that in a population of patients specifically selected for its high rate of seizures (sleep deprivation, drug reduction, presurgical exploration…) we could record both interictal and ictal events in a good proportion of patients with probably the same reliability that we showed in this study. In such a context, we would recommend to watch closely the patient during the recording, as we did. In our current protocol, a neurologist is always watching the EEG and the patient with video, and is ready to intervene rapidly in case of a clinical seizure to avoid secondary injury.
The recording of interictal and ictal events in the same MRI session gave us the opportunity to compare non-invasively, electrophysiological and haemodynamic changes in the irritative and epileptogenic zones in three different types of MCD.
Nodular heterotopia
In patients with gray matter nodular heterotopia, BOLD changes inconsistently involved the lesion during interictal discharges. Two patients presented a clear involvement of the heterotopia but in the other two patients the overlying and distant cortices were mostly involved. The patients without heterotopia involvement were recorded with the 3 T scanner. Because the 3 T scanner has a better sensitivity than the 1.5 T scanner (Krasnow et al., 2003), these discrepancies cannot be explained by the power of the magnetic field. Patient 3 underwent a left temporal resection several years prior to the recording. The effects of surgery on BOLD studies are not known. However, BOLD changes obtained for this patient were in accordance with the electrical field of the interictal and ictal abnormalities; the effects on these results of surgery contralateral to the epileptic events are most likely minimal. In a previous study of patients with grey matter heterotopia, Kobayashi et al. (2006a) showed in patients with nodules that BOLD changes were observed inside the nodules in six studies and in the overlying or distant cortex in 10 studies. Ictal BOLD responses were more consistent and the maximum t-value always involved the overlying cortex or distant areas and never the heterotopia. For our Patient 1, Kobayashi et al. (2006a) demonstrated in a previous study that BOLD changes in the heterotopia had a much lower level of significance than that of the overlying cortex, indicating that the heterotopic activation could reflect a spread more than the real focus of the seizure.
The ability of the nodular heterotopia to generate epileptic activity is still debated. Our findings suggest that interictal epileptiform activity can be generated by the nodules, but seizures are more likely generated by the cortex overlying the malformation. The intracerebral study of Patient 4 confirmed a seizure onset zone outside the lesion, but nodules were sometimes involved during seizure propagation, suggesting interconnections. Previous intracerebral studies in patients with gray matter heterotopia also showed variable results. Interictal activity was generated independently in the heterotopia or in the overlying cortex (Preul et al., 1997) or in both structures simultaneously (Dubeau et al., 1995; Aghakhani et al., 2005; Battaglia et al., 2006). Different ictal patterns were also described in the literature: (i) seizure onset in the overlying cortex with or without simultaneous activity in the heterotopia (Tassi et al., 2005; Battaglia et al., 2006; Stefan et al., 2007); (ii) independent seizure onsets in heterotopia (usually without clinical signs) and in the overlying cortex (Aghakhani et al., 2005); (iii) electrical stimulation of overlying cortex triggers usual aura or clinical seizures, but is ineffective in nodules (Tassi et al., 2005). Most of these studies minimized the role of the nodular heterotopia in seizure generation. Only one study suggested that the seizure onset zone could be in the malformation itself (Kothare et al., 1998), but because of the implantation protocol, the overlying cortex was not sampled simultaneously during the recordings. The immaturity of the GABA receptors in heterotopia neurons could explain the spontaneous hyperexcitability necessary to generate at least interictal discharges (Hannan et al., 1999; Ben-Ari, 2006). Clearly, however, those electrophysiological studies and the haemodynamic responses observed in our EEG-fMRI study, provide little evidence for an important role of the nodules in seizure generation (even if we cannot exclude with standard MRI sequences very subtle gray matter heterotopia in our BOLD responses area). Neuronal connections between nodules and between nodules and cortex (Hannan et al., 1999; Kakita et al., 2002) may have a role in synchronization and amplification of the cortical neuronal activity. Heterotopia are commonly associated with a structural disorganization of the overlying cortex (pachygyria, polymycrogyria, dysplasia and even atrophy seen in histological specimens) or in a distant area (e.g. hippocampus atrophy and developmental defects of the hippocampal formation) (Baulac et al., 1998; Hannan et al., 1999; Aghakhani et al., 2005; Bernasconi et al., 2005; Tassi et al., 2005). All these developmental changes are known to generate seizures.
Focal cortical dysplasia
In the two patients with focal cortical dysplasia, BOLD changes (activations) overlapped in the lesion during interictal and ictal events, suggesting common neuronal generators. Intrinsic epileptogenicity of the dysplastic lesion was confirmed during per-operative ECoG in Patient 5 (15 months after surgery, the patient is still seizure-free). Interictal activity induced more confined BOLD changes (only part of the lesion involved) compared to the haemodynamic changes seen during seizures. In Patient 6, with a much larger dysplasia, there was again an overlap of the two zones, but the irritative zone was much smaller and located at the periphery of the lesion. In both cases, BOLD changes also involved distant areas suggesting some connecting pathways with the lesion.
Similar patterns of BOLD response were commonly found in the lesions and distant areas involved in the epileptic network in previous interictal EEG-fMRI studies of focal cortical dysplasia (Krakow et al., 2001; Federico et al., 2005a; Kobayashi et al., 2006a). All these studies showed that a part of the lesion is able to generate interictal activity. During ictal discharges, our study showed that a similar group of neurons was involved. The larger activation in the lesion suggests a more intense neuronal activity with higher blood flow changes during the seizure. Moreover, during interictal and ictal discharges, activated distant areas were similar, implying the involvement of a common epileptic network.
Intracerebral recording showed that the typical rhythmic spike discharges (Palmini et al., 1995; Dubeau et al., 1998) observed in scalp EEG (Raymond et al., 1995, Gambardella et al., 1996) and similar to what was seen in our patients, is clearly generated in the lesion itself. Intracerebral recordings demonstrated that the seizures are also generated in the dysplastic cortex (Palmini et al., 1995; Rosenow et al., 1998; Chassoux et al., 2000; Boonyapisit et al., 2003). Moreover, epileptogenic and irritative zones have been shown to overlap; the first being defined in the area of maximal interictal activity (Chassoux et al., 2000) (irritative zone). Our EEG-fMRI findings are therefore in full agreement with intracerebral studies.
Band heterotopia
In patients with band heterotopia, both the normo- and heterotopic cortices showed haemodynamic changes during ictal and interictal epileptiform events. Interictal discharges involved a smaller part of the network while seizures were associated with large and widespread BOLD responses. During ictal events, the two patients presented a positive BOLD response in the band and in the normal cortex with a higher t-value than during interictal events. We found a BOLD change in the heterotopic band during interictal events, but Patient 7 had an increase of BOLD in the band while Patient 8 presented a large deactivation in the entire double cortex. This difference could be explained by two mechanisms. First, the patients had a different baseline (Patient 8 was sleeping). Deactivation or activation could both reflect an active process and the presence of a positive or a negative response could depend on the level of the BOLD baseline for the same neuronal activity (Pasley et al., 2007; Shulman et al., 2007). Thus, deactivation can represent an active process during sleep (Jacobs et al., 2007). Second, the type of interictal events was different for the two patients: one had bursts of polyspikes and the other bursts of slow waves. Paroxysmal slow-wave activity has been correlated with decrease of neuronal activity, in normal slow-wave sleep and in epileptic disorders during the post-ictal period or in spike and wave discharges (Gloor, 1978). In addition, Kobayashi et al. (2006c) observed a decreased BOLD response when spikes were associated with slow waves.
We showed that in both types of epileptic events, BOLD changes involved simultaneously the heterotopic and normal cortices. The role of the heterotopic band in generating the interictal and ictal discharges is not well understood. Rat models of band heterotopia (tish rat model) showed that the overlying cortex is sufficient to generate seizures (Schottler et al., 1998; Chen et al., 2000). Very few intracerebral EEG recordings of the heterotopic band were reported in humans. Interictal epileptiform activity has been recorded acutely directly from the heterotopic cortex, synchronously or not with the normal cortex (Morrell et al., 1992). Also, spontaneous rhythmic discharges reflecting electrical seizures in the heterotopic cortex (Bernasconi et al., 2001) or synchronous ictal discharges in both normo- and heterotopic cortices were observed (Mai et al., 2003). At the moment, we do not know which part of the double cortex is responsible for seizure generation. This distinction is especially hard to make because activity of the heterotopic cortex seems highly correlated with that of the normal cortex, which is consistent with the strong neuronal interconnections existing between the two (De Volder et al., 1994; Iannetti et al., 2001). The BOLD responses were always larger in the heterotopic cortex, indicating that both the interictal and ictal events could be generated in the heterotopia. This difference of BOLD response between the two cortices probably reflects a difference of the neuronal activity and not of the neurovascular coupling. Previous studies in double cortex patients with fMRI during motor or cognitive tasks (Pinard et al., 2000; Iannetti et al., 2001; Spreer et al., 2001; Keene et al., 2004; Briellmann et al., 2006) never demonstrated neurovascular coupling disturbance in the heterotopic cortex compared to the normal cortex.
The particular cellular organization of the double cortex could facilitate the generation of ictal discharges in the heterotopic band. Close neuronal interconnections with the normal cortex allow a focal and fast spread of the discharge that can then be seen on the scalp EEG because the normal cortex is more superficial. The double cortex is a diffuse cortical developmental malformation, but surprisingly patients with this anomaly often have multiple focal epileptic generators as reflected by their clinical and EEG patterns. The important and extended BOLD changes seen in our two patients and mainly in the heterotopic lesion during ictal events, suggest that there is more than a single focus or that the generator involves an extended portion of the malformation in this type of MCD. Indeed, patients with a double cortex have a poor surgical outcome after attempts to remove a single epileptic focus and limited portion of the epileptogenic lesion (Bernasconi et al., 2001).
Ictal imaging is a challenging but essential step for the understanding of epileptic discharge generation, particularly in the context of a presurgical evaluation. Ictal SPECT plays a major role in this context, but it is rarely successful when seizures are short and it often does not provide a very precise localization. It also does not allow the comparison of ictal and interictal discharges (the interictal SPECT is not specific to the time of EEG spikes but reflects the background EEG state). Even if this technique needs further methodological improvement to correct some issues such as the control of the BOLD baseline, we demonstrated that the analysis of BOLD changes during interictal and ictal events in eight MCD patients with difficult-to-treat epilepsies could bring relevant information about the structures involved. The haemodynamic or metabolic changes observed during interictal and ictal epileptiform events corroborate previous studies done with EEG-fMRI and with intracerebral recording. Moreover, BOLD changes obtained in two of our patients were consistent with intracranial EEG recordings done after our exploration. In MCD patients, we demonstrated that the neuronal networks involved in the generation of the epileptic activity are different for each type of lesion reflecting different neuronal organizations. We showed that the EEG-fMRI technique can be used safely in selected patients with epilepsy to define the irritative and epileptogenic zones. It is also conceivable that fMRI could be used to determine if epileptogenic lesions are involved in cognitive processes, as has been done with intracerebral EEG (Kirschstein et al., 2003).
Supplementary Material
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (CIHR) grant MOP-38079. Tyvaert L. was supported by the Rotary International and is presently supported by the Savoy Foundation for epilepsy. Kobayashi E. is supported Early Career Physician Scientist Award from AES. LeVan P. is supported by the Natural Sciences and Engineering Research Council. We are grateful to Dr Nguyen Dang (Notre Dame Hospital, Montreal) for his help with patient recruitment and for providing useful clinical information. We wish to thank Dr Christophe Grova (Montreal Neurological Institute) for his help with the illustrations.
Abbreviations
- MCD
malformation of cortical development
- ICA
independent component analysis
- HFR
haemodynamic response functions
Footnotes
Supplementary material is available at Brain online.
For Permissions, please journals.permissions@oxfordjournals.org
References
- Aghakhani Y, Kinay D, Gotman J, Soualmi L, Andermann F, Olivier A, et al. The role of periventricular nodular heterotopia in epileptogenesis. Brain. 2005;128:641–51. doi: 10.1093/brain/awh388. [DOI] [PubMed] [Google Scholar]
- Al-Asmi A, Bénar CG, Gross DW, Khani YA, Andermann F, Pike B, et al. fMRI activation in continuous and spike-triggered EEG-fMRI studies of epileptic spikes. Epilepsia. 2003;44:1328–39. doi: 10.1046/j.1528-1157.2003.01003.x. [DOI] [PubMed] [Google Scholar]
- Allen PJ, Josephs O, Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage. 2000;12:230–39. doi: 10.1006/nimg.2000.0599. [DOI] [PubMed] [Google Scholar]
- Bagshaw AP, Aghakhani Y, Bénar CG, Kobayashi E, Hawco C, Dubeau F, et al. EEG-fMRI of focal epileptic spikes: analysis with multiple haemodynamic functions and comparison with gadolinium-enhanced MR angiograms. Hum Brain Mapp. 2004;22:179–92. doi: 10.1002/hbm.20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw AP, Kobayashi E, Dubeau F, Pike GB, Gotman J. Correspondence between EEG-fMRI and EEG dipole localisation of interictal discharges in focal epilepsy. Neuroimage. 2006;30:417–25. doi: 10.1016/j.neuroimage.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. Classification system for malformations of cortical development: update 2001. Neurology. 2001;57:2168–78. doi: 10.1212/wnl.57.12.2168. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Chiapparini L, Franceschetti S, Freri E, Tassi L, Bassanini S, et al. Periventricular nodular heterotopia: classification, epileptic history, and genesis of epileptic discharges. Epilepsia. 2006;47:86–97. doi: 10.1111/j.1528-1167.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- Baulac M, De Grissac N, Hasboun D, Oppenheim C, Adam C, Arzimanoglou A, et al. Hippocampal developmental changes in patients with partial epilepsy: magnetic resonance imaging and clinical aspects. Ann Neurol. 1998;44:223–33. doi: 10.1002/ana.410440213. [DOI] [PubMed] [Google Scholar]
- Bénar C, Aghakhani Y, Wang Y, Izenberg A, Al-Asmi A, Dubeau F, Gotman J. Quality of EEG in simultaneous EEG-fMRI for epilepsy. Clin Neurophysiol. 2003;114:569–80. doi: 10.1016/s1388-2457(02)00383-8. [DOI] [PubMed] [Google Scholar]
- Bénar CG, Grova C, Kobayashi E, Bagshaw AP, Aghakhani Y, Dubeau F, et al. EEG-fMRI of epileptic spikes: concordance with EEG source localization and intracranial EEG. Neuroimage. 2006;30:1161–70. doi: 10.1016/j.neuroimage.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Basic developmental rules and their implications for epilepsy in the immature brain. Epileptic Disord. 2006;8:91–102. [PubMed] [Google Scholar]
- Bernasconi N, Kinay D, Andermann F, Antel S, Bernasconi A. Analysis of shape and positioning of the hippocampal formation: an MRI study in patients with partial epilepsy and healthy controls. Brain. 2005;128:2442–52. doi: 10.1093/brain/awh599. [DOI] [PubMed] [Google Scholar]
- Bernasconi A, Martinez V, Rosa-Neto P, D’Agostino D, Bernasconi N, Berkovic S, et al. Surgical resection for intractable epilepsy in “double cortex” syndrome yields inadequate results. Epilepsia. 2001;42:1124–9. doi: 10.1046/j.1528-1157.2001.39900.x. [DOI] [PubMed] [Google Scholar]
- Bonaventura CD, Vaudano AE, Carnì M, Pantano P, Nucciarelli V, Garreffa G, et al. EEG/fMRI study of ictal and interictal epileptic activity: methodological issues and future perspectives in clinical practice. Epilepsia. 2006;47:52–8. doi: 10.1111/j.1528-1167.2006.00878.x. [DOI] [PubMed] [Google Scholar]
- Boonyapisit K, Najm I, Klem G, Ying Z, Burrier C, LaPresto E, et al. Epileptogenicity of focal malformations due to abnormal cortical development: direct electrocorticographic-histopathologic correlations. Epilepsia. 2003;44:69–76. doi: 10.1046/j.1528-1157.2003.08102.x. [DOI] [PubMed] [Google Scholar]
- Briellmann RS, Little T, Harvey AS, Abbott DF, Jacobs R, Waites AB, et al. Pathologic and physiologic function in the subcortical band of double cortex. Neurology. 2006;67:1090–3. doi: 10.1212/01.wnl.0000237554.39283.6b. [DOI] [PubMed] [Google Scholar]
- Chassoux F, Devaux B, Landré E, Turak B, Nataf F, Varlet P, et al. Stereoelectroencephalography in focal cortical dysplasia: a 3D approach to delineating the dysplastic cortex. Brain. 2000;123:1733–51. doi: 10.1093/brain/123.8.1733. [DOI] [PubMed] [Google Scholar]
- Chen ZF, Schottler F, Bertram E, Gall CM, Anzivino MJ, Lee KS. Distribution and initiation of seizure activity in a rat brain with subcortical band heterotopia. Epilepsia. 2000;41:493–501. doi: 10.1111/j.1528-1157.2000.tb00201.x. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63:541–67. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- De Volder AG, Gadisseux JF, Michel CJ, Maloteaux JM, Bol AC, Grandin CB, et al. Brain glucose utilization in band heterotopia: synaptic activity of “double cortex”. Pediatr Neurol. 1994;11:290–4. doi: 10.1016/0887-8994(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Di Bonaventura C, Carnfi M, Vaudano AE, Pantano P, Garreffa G, Le Piane E, et al. Ictal hemodynamic changes in late-onset Rasmussen encephalitis. Ann Neurol. 2006;59:432–3. doi: 10.1002/ana.20752. [DOI] [PubMed] [Google Scholar]
- Diehl B, Salek-haddadi A, Fish DR, Lemieux L. Mapping of spikes, slow waves, and motor tasks in a patient with malformation of cortical development using simultaneous EEG and fMRI. Magn Reson Imaging. 2003;21:1167–73. doi: 10.1016/j.mri.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Dubeau F, Palmini A, Fish D, Avoli M, Gambardella A, Spreafico R, Andermann F. The significance of electrocorticographic findings in focal cortical dysplasia: a review of their clinical, electrophysiological and neurochemical characteristics. Electroencephalogr Clin Neurophysiol Suppl. 1998;48:77–96. [PubMed] [Google Scholar]
- Dubeau F, Tampieri D, Lee N, Andermann E, Carpenter S, Leblanc R, et al. Periventricular and subcortical nodular heterotopia. A study of 33 patients. Brain. 1995;118:1273–87. doi: 10.1093/brain/118.5.1273. [DOI] [PubMed] [Google Scholar]
- Duncan R, Biraben A, Patterson J, Hadley D, Bernard AM, Lecloirec J, et al. Ictal single photon emission computed tomography in occipital lobe seizures. Epilepsia. 1997;38:839–43. doi: 10.1111/j.1528-1157.1997.tb01472.x. [DOI] [PubMed] [Google Scholar]
- Duncan R, Rahi S, Bernard AM, Biraben A, Devillers A, Lecloirec J, et al. Ictal cerebral blood flow in seizures originating in the posterolateral cortex. J Nucl Med. 1996;37:1946–51. [PubMed] [Google Scholar]
- Federico P, Abbott DF, Briellmann RS, Harvey AS, Jackson GD. Functional MRI of the pre-ictal state. Brain. 2005b;128:1811–7. doi: 10.1093/brain/awh533. [DOI] [PubMed] [Google Scholar]
- Federico P, Archer JS, Abbott DF, Jackson GD. Cortical/subcortical BOLD changes associated with epileptic discharges: an EEG-fMRI study at 3 T. Neurology. 2005a;64:1125–30. doi: 10.1212/01.WNL.0000156358.72670.AD. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, et al. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–55. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gambardella A, Palmini A, Andermann F, Dubeau F, Da Costa JC, Quesney LF, et al. Usefulness of focal rhythmic discharges on scalp EEG of patients with focal cortical dysplasia and intractable epilepsy. Electroencephalogr Clin Neurophysiol. 1996;98:243–9. doi: 10.1016/0013-4694(95)00266-9. [DOI] [PubMed] [Google Scholar]
- Gloor P. Generalized epilepsy with bilateral synchronous spike and wave discharge. New findings concerning its physiological mechanisms. Electroencephalogr Clin Neurophysiol Suppl. 1978;34:245–9. [PubMed] [Google Scholar]
- Gotman J, Kobayashi E, Bagshaw AP, Bénar CG, Dubeau F. Combining EEG and fMRI: a multimodal tool for epilepsy research. J Magn Reson Imaging. 2006;23:906–20. doi: 10.1002/jmri.20577. [DOI] [PubMed] [Google Scholar]
- Hannan AJ, Servotte S, Katsnelson A, Sisodiya S, Blakemore C, Squier M, et al. Characterization of nodular neuronal heterotopia in children. Brain. 1999;122:219–38. doi: 10.1093/brain/122.2.219. [DOI] [PubMed] [Google Scholar]
- Hirabayashi S, Binnie CD, Janota I, Polkey CE. Surgical treatment of epilepsy due to cortical dysplasia: clinical and EEG findings. J Neurol Neurosurg Psychiatry. 1993;56:765–70. doi: 10.1136/jnnp.56.7.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti P, Spalice A, Raucci U, Perla FM. Functional neuroradiologic investigations in band heterotopia. Pediatr Neurol. 2001;24:159–63. doi: 10.1016/s0887-8994(00)00247-2. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Kobayashi E, Boor R, Muhle H, Stephan W, Hawco C, et al. Hemodynamic responses to interictal epileptiform discharges in children with symptomatic epilepsy. Epilepsia. 2007;48:2068–78. doi: 10.1111/j.1528-1167.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- Kakita A, Hayashi S, Moro F, Guerrini R, Ozawa T, Ono K, et al. Bilateral periventricular nodular heterotopia due to filamin 1 gene mutation: widespread glomeruloid microvascular anomaly and dysplastic cytoarchitecture in the cerebral cortex. Acta Neuropathol. 2002;104:649–57. doi: 10.1007/s00401-002-0594-9. [DOI] [PubMed] [Google Scholar]
- Keene DL, Olds J, Logan WJ. Functional MRI study of verbal fluency in a patient with subcortical laminar heterotopia. Can J Neurol Sci. 2004;31:261–4. doi: 10.1017/s0317167100053920. [DOI] [PubMed] [Google Scholar]
- Kirschstein T, Fernández G, Grunwald T, Pezer N, Urbach H, Blümcke I, et al. Heterotopias, cortical dysplasias and glioneural tumors participate in cognitive processing in patients with temporal lobe epilepsy. Neurosci Lett. 2003;338:237–41. doi: 10.1016/s0304-3940(02)01398-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw AP, Grova C, Gotman J, Dubeau F. Grey matter heterotopia: what EEG-fMRI can tell us about epileptogenicity of neuronal migration disorders. Brain. 2006a;129:366–74. doi: 10.1093/brain/awh710. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Hawco CS, Grova C, Dubeau F, Gotman J. Widespread and intense BOLD changes during brief focal electrographic seizures. Neurology. 2006b;66:1049–55. doi: 10.1212/01.wnl.0000204232.37720.a4. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw AP, Grova C, Dubeau F, Gotman J. Negative BOLD responses to epileptic spikes. Hum Brain Mapp. 2006c;27:488–97. doi: 10.1002/hbm.20193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw AP, Jansen A, Andermann F, Andermann E, Gotman J, et al. Intrinsic epileptogenicity in polymicrogyric cortex suggested by EEG-fMRI BOLD responses. Neurology. 2005;64:1263–66. doi: 10.1212/01.WNL.0000154640.23656.A3. [DOI] [PubMed] [Google Scholar]
- Kothare SV, VanLandingham K, Armon C, Luther JS, Friedman A, Radtke RA. Seizure onset from periventricular nodular heterotopias: depth-electrode study. Neurology. 1998;51:1723–7. doi: 10.1212/wnl.51.6.1723. [DOI] [PubMed] [Google Scholar]
- Krakow K, Lemieux L, Messina D, Scott CA, Symms MR, Duncan JS, et al. Spatio-temporal imaging of focal interictal epileptiform activity using EEG-triggered functional MRI. Epileptic Disord. 2001;3:67–74. [PubMed] [Google Scholar]
- Krasnow B, Tamm L, Greicius MD, Yang TT, Glover GH, Reiss AL, et al. Comparison of fMRI activation at 3 and 1. 5 T during perceptual, cognitive, and affective processing. Neuroimage. 2003;18:813–26. doi: 10.1016/s1053-8119(03)00002-8. [DOI] [PubMed] [Google Scholar]
- Lemieux L, Krakow K, Fish DR. Comparison of spike-triggered functional MRI BOLD activation and EEG dipole model localization. Neuroimage. 2001;14:1097–104. doi: 10.1006/nimg.2001.0896. [DOI] [PubMed] [Google Scholar]
- Li LM, Dubeau F, Andermann F, Fish DR, Watson C, Cascino GD, et al. Periventricular nodular heterotopia and intractable temporal lobe epilepsy: poor outcome after temporal lobe resection. Ann Neurol. 1997;41:662–8. doi: 10.1002/ana.410410516. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–69. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Mai R, Tassi L, Cossu M, Francione S, Lo Russo G, Garbelli R, et al. A neuropathological, stereo-EEG, and MRI study of subcortical band heterotopia. Neurology. 2003;60:1834–8. doi: 10.1212/01.wnl.0000065884.61237.24. [DOI] [PubMed] [Google Scholar]
- Morrell F, Whistler WW, Hoeppner TJ, Smith MC, Kanner AM, Pierre-Louis C, et al. Electrophysiology of heterotopic gray matter in the double cortex syndrome. Epilepsia. 1992;33 (Suppl 3):76. [Google Scholar]
- Palmini A. Disorders of cortical development. Curr Opin Neurol. 2000;13:183–92. doi: 10.1097/00019052-200004000-00012. [DOI] [PubMed] [Google Scholar]
- Palmini A, Andermann F, Olivier A, Tampieri D, Robitaille Y, Andermann E, et al. Focal neuronal migration disorders and intractable partial epilepsy: a study of 30 patients. Ann Neurol. 1991;30:741–9. doi: 10.1002/ana.410300602. [DOI] [PubMed] [Google Scholar]
- Palmini A, Gambardella A, Andermann F, Dubeau F, da Costa JC, Olivier A, et al. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann Neurol. 1995;37:476–87. doi: 10.1002/ana.410370410. [DOI] [PubMed] [Google Scholar]
- Pasley BN, Inglis BA, Freeman RD. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage. 2007;36:269–76. doi: 10.1016/j.neuroimage.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W. The evidence for a cerebral vascular mechanism in epilepsy. Ann Intern Med. 1933;7:303–10. [Google Scholar]
- Penfield W. The circulation of the epileptic brain. Res Publ Assoc Nerv Ment Dis. 1937;18:605–737. [Google Scholar]
- Pinard J, Feydy A, Carlier R, Perez N, Pierot L, Burnod Y. Functional MRI in double cortex: functionality of heterotopia. Neurology. 2000;54:1531–3. doi: 10.1212/wnl.54.7.1531. [DOI] [PubMed] [Google Scholar]
- Preul MC, Leblanc R, Cendes F, Dubeau F, Reutens D, Spreafico R, et al. Function and organization in dysgenic cortex. Case report J Neurosurg. 1997;87:113–21. doi: 10.3171/jns.1997.87.1.0113. [DOI] [PubMed] [Google Scholar]
- Raymond AA, Fish DR, Sisodiya SM, Alsanjari N, Stevens JM, Shorvon SD. Abnormalities of gyration, heterotopias, tuberous sclerosis, focal cortical dysplasia, microdysgenesis, dysembryoplastic neuroepithelial tumour and dysgenesis of the archicortex in epilepsy. Clinical, EEG and neuroimaging features in 100 adult patients. Brain. 1995;118:629–60. doi: 10.1093/brain/118.3.629. [DOI] [PubMed] [Google Scholar]
- Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- Rosenow F, Lüders HO, Dinner DS, Prayson RA, Mascha E, Wolgamuth BR, et al. Histopathological correlates of epileptogenicity as expressed by electrocorticographic spiking and seizure frequency. Epilepsia. 1998;39:850–6. doi: 10.1111/j.1528-1157.1998.tb01179.x. [DOI] [PubMed] [Google Scholar]
- Salek-Haddadi A, Diehl B, Hamandi K, Merschhemke M, Liston A, Friston K, et al. Hemodynamic correlates of epileptiform discharges: an EEG-fMRI study of 63 patients with focal epilepsy. Brain Res. 2006;1088:148–66. doi: 10.1016/j.brainres.2006.02.098. [DOI] [PubMed] [Google Scholar]
- Salek-Haddadi A, Lemieux L, Merschhemke M, Diehl B, Allen PJ, Fish DR. EEG quality during simultaneous functional MRI of interictal epilepti-form discharges. Magn Reson Imaging. 2003b;21:1159–66. doi: 10.1016/j.mri.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Salek-Haddadi A, Lemieux L, Merschhemke M, Friston KJ, Duncan JS, Fish DR. Functional magnetic resonance imaging of human absence seizures. Ann Neurol. 2003a;53:663–7. doi: 10.1002/ana.10586. [DOI] [PubMed] [Google Scholar]
- Salek-Haddadi A, Merschhemke M, Lemieux L, Fish DR. Simultaneous EEG-correlated ictal fMRI. Neuroimage. 2002;16:32–40. doi: 10.1006/nimg.2002.1073. [DOI] [PubMed] [Google Scholar]
- Schottler F, Couture D, Rao A, Kahn H, Lee KS. Subcortical connections of normotopic and heterotopic neurons in sensory and motor cortices of the tish mutant rat. J Comp Neurol. 1998;395:29–42. [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Hyder F. A BOLD search for baseline. Neuroimage. 2007;36:277–81. doi: 10.1016/j.neuroimage.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreer J, Martin P, Greenlee MW, Wohlfarth R, Hammen A, Arnold SM, et al. Functional MRI in patients with band heterotopia. Neuroimage. 2001;14:357–65. doi: 10.1006/nimg.2001.0813. [DOI] [PubMed] [Google Scholar]
- Stefan H, Bauer J, Feistel H, Schulemann H, Neubauer U, Wenzel B, et al. Regional cerebral blood flow during focal seizures of temporal and frontocentral onset. Ann Neurol. 1990;27:162–66. doi: 10.1002/ana.410270211. [DOI] [PubMed] [Google Scholar]
- Stefan H, Nimsky C, Scheler G, Rampp S, Hopfengärtner R, Hammen T, et al. Periventricular nodular heterotopia: a challenge for epilepsy surgery. Seizure. 2007;16:81–6. doi: 10.1016/j.seizure.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Tassi L, Colombo N, Cossu M, Mai R, Francione S, Lo Russo G, et al. Electroclinical, MRI and neuropathological study of 10 patients with nodular heterotopia, with surgical outcomes. Brain. 2005;128:321–37. doi: 10.1093/brain/awh357. [DOI] [PubMed] [Google Scholar]
- Van Paesschen W. Ictal SPECT. Epilepsia. 2004;45 (Suppl 4):35–40. doi: 10.1111/j.0013-9580.2004.04008.x. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited–again. Neuroimage. 1995;2:173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, et al. A general statistical analysis for fMRI data. Neuroimage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
- Wyllie E, Comair YG, Kotagal P, Bulacio J, Bingaman W, Ruggieri P. Seizure outcome after epilepsy surgery in children and adolescents. Ann Neurol. 1998;44:740–8. doi: 10.1002/ana.410440507. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Huiskamp G, Hersevoort M, Seppenwoolde JH, van Huffelen AC, Leijten FS. EEG-fMRI in the preoperative work-up for epilepsy surgery. Brain. 2007;130:2343–53. doi: 10.1093/brain/awm141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.