Abstract
Parasitic plants can adversely influence the growth of their hosts by removing resources and by affecting photosynthesis. Such negative effects depend on resource availability. However, at varied resource levels, to what extent the negative effects on growth are attributed to the effects on photosynthesis has not been well elucidated. Here, we examined the influence of nitrogen supply on the growth and photosynthesis responses of the host plant Mikania micrantha to infection by the holoparasite Cuscuta campestris by focusing on the interaction of nitrogen and infection. Mikania micrantha plants fertilized at 0.2, 1 and 5 mM nitrate were grown with and without C. campestris infection. We observed that the infection significantly reduced M. micrantha growth at each nitrate fertilization and more severely at low than at high nitrate. Such alleviation at high nitrate was largely attributed to a stronger influence of infection on root biomass at low than at high nitrate fertilization. However, although C. campestris altered allometry and inhibited host photosynthesis, the magnitude of the effects was independent of nitrate fertilizations. The infection reduced light saturation point, net photosynthesis at saturating irradiances, apparent quantum yield, CO2 saturated rate of photosynthesis, carboxylation efficiency, the maximum carboxylation rate of Rubisco, and maximum light-saturated rate of electron transport, and increased light compensation point in host leaves similarly across nitrate levels, corresponding to a similar magnitude of negative effects of the parasite on host leaf soluble protein and Rubisco concentrations, photosynthetic nitrogen use efficiency and stomatal conductance across nitrate concentrations. Thus, the more severe inhibition in host growth at low than at high nitrate supplies cannot be attributed to a greater parasite-induced reduction in host photosynthesis, but the result of a higher proportion of host resources transferred to the parasite at low than at high nitrate levels.
Introduction
Parasitic plants are a taxonomically diverse group of organisms that obtain some or all of their nutrients and other resources, such as water, carbon and phytohormones, from their host plants via haustoria [1]. Interactions between them and their hosts are one of the key research topics in parasitic plant biology [2], [3]. Press et al. [1] indicated that the extent to which parasites compete with their hosts for nutrients depends on the relative sink strength and the degree of autotrophy of the parasites. In hemiparasitic plants, nutrient transfer and resource acquisition from the hosts are facilitated by the parasite maintaining high transpiration rates, high leaf conductance and low water potentials, and in holoparasitic plants, by high osmotic potentials [3]. Furthermore, parasitic plants can affect the photosynthesis of their hosts at the leaf and/or whole plant level [4]. These processes can adversely affect the hosts, and such negative effects depend on resource availability: they might be negligible when resources are abundant but when resources are limiting they can be severe, ranging from reduction of growth and development to death of the hosts [3].
The influence of nitrogen on host-parasite associations has been investigated in the economically important root hemiparasite Striga hermonthica [5], [6] and the stem holoparasite Cuscuta reflexa [7], [8]. Striga hermonthica-infected C4 sorghum had lower rates of photosynthesis than uninfected plants, but the difference in both growth and photosynthesis between uninfected and infected sorghum plants was lower or even negligible when high nitrogen concentrations were supplied [5]. In contrast, high nitrogen supply did not result in an alleviation of the effects of the parasite on the host C3 rice to the same degree that S. hermonthica did on the sorghum host, as reflected by similar growth and photosynthesis in uninfected and infected plants at high nitrogen supply [6].
Among the species in Cuscuta (Convolvulaceae), nitrogen relations in the parasitic associations of C. reflexa and its leguminous or non-leguminous hosts have been studied [7]–[9]. Modelling the solute transfer between C. reflexa and its leguminous host Lupinus albus [9] indicated that the massive demand of the parasite led to resource losses of the host, particularly nitrogen from leaves and roots. As a result of such highly competitive sink activity of the parasite, net photosynthesis of L. albus appeared to be stimulated. However, C. reflexa infection increased tissue nitrogen levels in the non-N2-fixing hosts Ricinus communis [8] and Coleus blumei [7]. Growth and development of C. reflexa were restricted similarly with those of the hosts when fed with different concentrations of nitrate, suggesting a fine tuning of the parasite sink strength with the source capacity of both hosts [7], [8]. In these associations, C. reflexa led to a substantial sink-dependent stimulation of the host’s photosynthesis and, under N-limiting conditions, to an increase in the host’s tissue nitrogen concentrations. The reason for the different effects of C. reflexa on the symbiotically N-fed L. albus and on nitrate-fed R. communis and C. blumei was attributed to the overriding competition between C. reflexa and L. albus in the tripartite association L. albus-Rhizobium-C. reflexa, whilst this additional factor was absent in the associations with R. communis and C. blumei [7], [8]. Although the holoparasite C. reflexa substantially decreased growth of both R. communis [8] and C. blumei [7] regardless of nitrate supply, the inhibition in growth of infected R. communis was exacerbated at low N supply, but in contrast, the inhibition in growth of infected C. blumei was similar at low and high N supply.
In our previous studies, we investigated the influence of another Cuscuta species, C. campestris, on growth, biomass allocation and photosynthesis of an invasive weed, Mikania micrantha H.B.K. (Asteraceae). We found different growth and photosynthesis influence patterns from those of C. reflexa. Cuscuta campestris significantly reduced the total biomass, changed the biomass allocation patterns and completely inhibited the flowering of M. micrantha plants [10]. In addition to direct resource capture by C. campestris, the parasite also reduced the stomatal conductance, and carboxylation and light use efficiencies of the host, resulting in reduced growth of infected plants [11]. We also observed that the total biomass of the parasite plus its host was significantly less than that of uninfected hosts [10], and the parasite suppressed photosynthesis of the hosts [11]. However, Jeschke and Hilpert [8] and Jeschke et al. [7] observed that the total biomass of C. reflexa plus its hosts was similar to that of the uninfected and C. reflexa led to a sink-dependent stimulation of host photosynthesis. Thus, it is of interest to study if the nitrogen relations are also different between C. campestris-host and C. reflexa-host associations.
In the present project we investigated the nitrogen relations in the M. micrantha-C. campestris host-parasite association by focusing on the interaction of nitrogen and infection. We hypothesized that both growth and photosynthesis responses in M. micrantha to C. campestris infection would be more affected by parasitism at low than high nitrogen supply.
Materials and Methods
Study Species
Mikania micrantha H.B.K. is a fast-growing climbing perennial vine of the family Asteraceae, native to Central and South America [12]. In its palaeotropic exotic range, it is a notorious invasive weed, severely damaging forestry and plantation crops [13]. In South China, it grows in poor to fertile soils with total nitrogen 0.14–1.62 g kg–1 [13]. In the field, the generalist stem parasite Cuscuta campestris Yuncker infects M. micrantha and it has been one of the most effective means of biologically controlling M. micrantha in South China [10], [13]. Cuscuta campestris is the most widespread species in the genus and the only parasitic weed of North America that has spread to the Old World [14]. It is a holoparasite and draws all nutrients from its host. It is a very powerful sink for host photosynthates, severely suppressing host growth, preventing flowering and fruiting, and even resulting in host death [10], [14]. It can infect many herbaceous plants and results in damage to horticultural and agricultural crops, and it is the worst pest of alfalfa and other legumes [14].
Plant Culture and Growth Conditions
The experiment was carried out during the July 2011–January 2012 growing season in an unheated greenhouse with natural light at the same field station of South China Botanical Garden as in our previous study [10]. On 26 July 2011, whole M. micrantha plants were collected from a M. micrantha population near the station. Two-node segments, similar in size, were obtained from the middle of the stems. The segments were planted in containers filled with washed moist sand, with the low nodes buried below and the upper about 5 cm above the sand surface. The upper nodes began to sprout 5 days later. On 20 August, 90 healthy sprouts about 20 cm long were transplanted into 8.36 L pots filled with washed moist sand, one per pot, and the pots were placed in the glasshouse with a temperatures range 12–28°C, mean 17.8°C, and relative humidity range 50–90%, mean 70% during August 2011–January 2012. The plants were watered twice daily at 06∶00 h and 18∶00 with distilled water during the first week after transplanting. From then on to the end of the experiment, they were watered at 06∶00 h with distilled water and at 18∶00 h with modified Hoagland solutions containing 0.2, 1 or 5 mM nitrate with 200 ml per pot and 30 pots per nitrate concentration. The pots were thoroughly rinsed with water once a week.
On 7 October when the M. micrantha plants had been treated with nitrate for 41 days, half of them within each nitrate treatment were randomly chosen and inoculated with C. campestris filaments about 5 cm in length, one per plant, and the rest were left as control. To ensure simultaneous attachment, excised and previously twined shoot cuttings of C. campestris were allowed to attach to the lowest two M. micrantha stem internodes. By 14 October, all the inoculated M. micrantha plants had become infected with C. campestris stems as indicated by renewed vigorous growth of the filaments. Thus, this day was considered day 0 after parasitization (DAP). To prevent M. micrantha from climbing from one pot to another, a bamboo cane was placed vertically in each pot for M. micrantha to climb on. The experiment ended on 14 January 2012, 90 DAP or 172 days after planting, when the uninfected M. micrantha plants fertilized at 5 mM nitrate were in full bloom.
Growth Measurements and Observations
During the experiment, both destructive and nondestructive measurements of growth were made. Height from the base of the stem to the apex of the shoot and number of visible leaves per M. micrantha plant were recorded on 0, 15, 40, 60, 90 DAP. Flowering times of M. micrantha and C. campestris plants were also recorded.
Mikania micrantha plants on 0 DAP, and the uninfected and infected and parasite plants on 90 DAP were randomly sampled and harvested, five per treatment. We measured the leaf area using a LI-3000C portable laser area meter (LI-COR Inc., Lincoln, NE, USA), removed the dead material and counted the number of dead leaves of the sampled M. micrantha plants, but the number of dead leaves was not used in the growth analyses. We separated the living parts of the sampled plants into stems, leaves, reproductive organs (if present) and roots. Roots were soaked in tap water, washed and separated carefully in running water over a 2-mm mesh sieve. Stems, tendrils and reproductive organs of C. campestris were carefully dissected from stems and leaves of M. micrantha plants.
All plant material was oven dried at 70°C until constant weights were achieved, and they were used to obtain tissue C and N concentrations and dry weights. For the M. micrantha plants harvested on 90 DAP, specific leaf area (SLA, the ratio of leaf area to dry mass) and shoot-to-root dry weight ratio (S/R), relative growth rate (RGR, the dry weight increase per plant per day), leaf area ratio (LAR, the ratio of leaf area to dry weight per plant) and unit leaf rate (ULR, dry weight production per m2 leaf area per day) were calculated according to Hunt and Parsons [15].
Measurements of Photosynthesis
In situ gas exchange measurements were made on M. micrantha leaves using a LI-6400 portable photosynthesis system with a standard 6 cm2 leaf chamber (LI-COR Inc., Lincoln, NE, USA) on 30 and 80 DAP, at around 10∶00 h, and photosynthetic parameters were calculated based on von Caemmerer and Farquhar [16]. To ensure that leaves measured were similar in age and developmental stage, only the youngest fully expanded mature sun leaves were sampled, one leaf per plant, from five randomly selected M. micrantha plants per treatment. Conditions inside the leaf chamber during the measurements were controlled as follows. Irradiance was provided by an integrated red-blue light-emitting diode source (model 6400-02B, LI-COR, Inc.) at photosynthetic photon flux density (PPFD) of 1000 µmol photons m−2 s−1 except for the light response study, CO2 concentration (C a) was controlled at 360 µmol mol−1 with a CO2 mixer except for the leaf internal CO2 concentration (C i) response study, flow rate was set at 500 µmol s−1, and leaf temperature (T l) was controlled at 20°C on 80 DAP and at 30°C on 30 DAP. Net photosynthetic rate (P n), stomatal conductance (g s, mol H2O m–2 s–1), rate of transpiration (E, mmol H2O m−2 s−1), intercellular CO2 concentration (C i), C a, air temperature (T a), T l, air relative humidity (RH), and PPFD were recorded after equilibration to a steady state with a coefficient of variation ≤1% at each measurement had been reached. Water use efficiency (WUE, µmol CO2 mmol H2O–1) was calculated as P n/E for each measurement. Methods and conditions used to obtain photosynthesis light and C i response curves were the same as described in the above paragraph unless specified otherwise.
Determination of Chlorophyll and Carotenoid Concentrations
Leaf chlorophyll concentrations were measured on the leaves used for the measurements of photosynthesis. Leaf pigments were extracted from about 70 mg of leaf sample put in 10 mL 80% acetone for 72 hours in the dark, and carotenoid and chlorophyll a and b concentrations were determined spectrophotometrically at 663, 645 and 470 nm according to Arnon [17].
Light Response Curves
To construct light response curves, on two clear days, 80–81 DAP, photosynthesis measurements were made between 08∶00 h and 11∶00 h. Leaf temperature in the leaf chamber was maintained at 20°C. When a leaf in the chamber had acclimated to a PPFD of 500 µmol photons m−2s−1 for 20 min, photosynthesis measurements were taken at PPFD in the following order: 500, 800, 1000, 1500, 1800, 2000, 200, 100, 50, 20, 0 µmol photons m−2s−1. For each measurement, apparent quantum yield (Φ, mol CO2 mol−1 photons), dark respiration rate (R d, µmol CO2 m−2s−1) and light compensation point (LCP) were obtained by linear regression using data obtained at PPFD of 0, 20 and 50 µmol photons m−2s−1 [18]. The entire photosynthetic light response curves were fitted using Photosynthesis Work Bench (LI-COR Inc., Lincoln, NE, USA). Maximum leaf light-saturated photosynthetic rate (P max) and light saturating point (LSP) were estimated.
Ci Response Curves
To study the relationship between P n and leaf internal CO2 concentration C i, photosynthesis was measured on two clear days, 75–76 DAP. Leaf temperature inside the leaf chamber was maintained at 20°C, and PPFD, at 1000 µmol photons m–2s–1. P n was measured at C a in the following order: 400, 250, 150, 100, 50, 0, 400, 400, 600, 800, 1000 and 1200 µmol mol–1 provided by a CO2 mixer. Sigma Plot for Windows 10.0 was used to fit the P n/C i response curves using an exponential function [19]:
where P n is leaf net photosynthetic rate and x is C i. Using this equation, the CO2 saturated rate of photosynthesis (P sat) was calculated as a+c, and the carboxylation efficiency (CE), as the slope at P n = 0 or b(a+c).
Maximum carboxylation rate of Rubisco (V cmax) and maximum light-saturated rate of electron transport (J max) were determined using Photosynthesis Assistant software (Version 1.1, Dundee Scientific, Dundee, UK) according to Farquhar et al. [20], modified by Harley and Sharkey [21] and Harley et al. [22].
Soluble Protein and Rubisco Contents
The leaves used for light and C i response curves were collected to determine soluble protein and Rubisco content. Approximately 0.5 g of fresh leaf material per sample with the mid-vein removed was ground in liquid nitrogen to a fine powder with 10 mg of PVPP. Extraction buffer [50 mM sodium phosphate buffer pH 7.8, 10% (v/v) glycerol, 1% (v/v) β-mercaptoethanol] was added at 3 ml g–1 fresh weight. The homogenate was centrifuged at 16,000 g for 15 min at 4°C. Protein concentration of the supernatant was estimated by the protein dye-binding method of Bradford [23] using bovine serum albumin (BSA) as the standard.
Rubisco content was determined following the protocol of Makino et al. [24] modified by Irving and Robinson [25]. Briefly, equal amounts of protein and extraction buffer were mixed and boiled for 2 min. Proteins in the extracts together with bovine serum albumin standards were separated using SDS-PAGE following the method of Laemmli [26] using 12% acrylamide resolving and 5% acrylamide stacking gels and the Mini-PROTEAN 3 System (Bio-Rad Laboratories, Richmond, CA, USA). Gels were stained using 1% (w/v) Coomassie Brilliant Blue R250 for 3 hours, the Rubisco containing band was excised, and the protein concentration was determined spectrophotometrically at 595 nm after elution of the stain in formamide at 50°C for 12 hours.
Carbon and Nitrogen Analysis
Tissue C and N concentrations in M. micrantha and in stems and flowers of C. campestris plants harvested on 90 DAP were assayed by GC using a Vario EL CHNS elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). They were also determined for the leaves used to measure photosynthesis. Photosynthetic nitrogen use efficiency (PNUE) was calculated as P max/N area.
Data Analysis
All statistical tests were carried out at α = 0.05 level using SPSS (version 11.5, SPSS Inc., Chicago, IL, USA). Two-way analysis of variance (ANOVA) was performed to evaluate the effects of nitrate supply, C. campestris infection, and their interaction on the growth and physiological traits. Repeated measures ANOVA was conducted to test the main effects, their interactions and measuring times (0, 15, 40, 60 and 90 DAP) on the number of leaves. One-way ANOVA was performed to test the effects of nitrate treatments on parasite biomass. Treatment means of the significant ANOVA effects were compared at α = 0.05 level using the least significant difference (LSD) analysis. Correlation analysis was conducted to test the relationships between P sat or CE and leaf nitrogen concentrations for M. micrantha plants. To satisfy the assumptions of ANOVA, some data were square-root transformed; however, untransformed data are presented in tables and figures.
Results
There were no differences in the flowering initiation of the uninfected or infected M. micrantha among the three nitrate treatments. In both uninfected and infected plants, compared with 5 mM nitrate fertilization, the other two delayed the further development of inflorescence after the inflorescence had formed and such delay was more at 0.2 than at 1 mM nitrate (data not shown), and they also reduced the number of florets. At all nitrate levels, the uninfected started to develop terminal inflorescences on 15 DAP, but the infected, on 40 DAP. From 15 to 60 DAP, C. campestris grew vigorously with a lot of branching. It started flowering on 20 DAP at 0.2 mM, and on 25 DAP at 1 or 5 mM nitrate treatments.
Number of Leaves
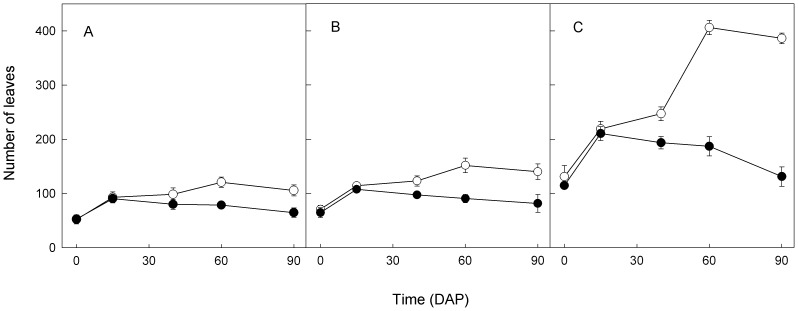
Repeated measures ANOVA indicated there were significant (P<0.001) infection, nitrate and their interaction effects on the number of leaves of M. micrantha over the measurement times. From 0 to 15 DAP, the number of leaves increased regardless of infection and fertilization treatments (Figure 1). From 15 to 90 DAP, the number of leaves of the infected M. micrantha was smaller than that of the control, and the differences between them became greater as nitrate fertilization levels increased from 0.2 to 1 to 5 mM. The number of leaves of the infected decreased continuously from 15 to 90 DAP, and that of the control increased from 0 to 60 DAP and then decreased slightly from 60 to 90 DAP. At harvest on 90 DAP, infected plants had 61%, 58% and 34% of the number of leaves of uninfected plants at 0.2, 1 and 5 mM nitrate supplies, respectively.
Figure 1. Means (±SE, n = 5) of number of leaves of uninfected (○) and infected (•) M. micrantha plants by C. campestris on different days after parasitization (DAP) at 0.2 (A), 1 (B) and 5 (C) mM nitrate fertilizations.
Plant Biomass Components
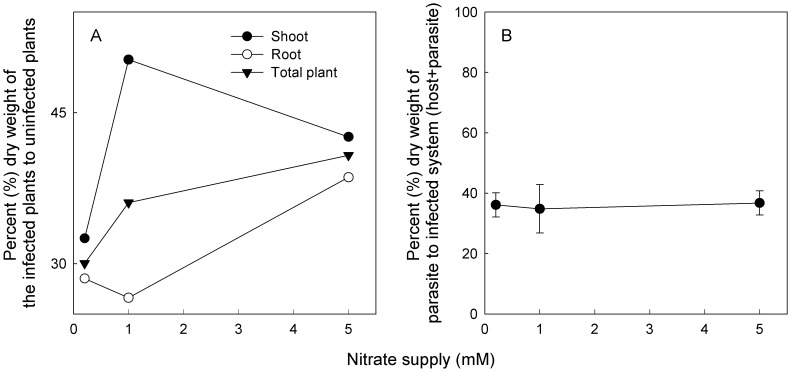
By 90 DAP, the dry mass of the infected system (host plus parasite) was significantly less than that of uninfected M. micrantha across all nitrate treatments (Table 1). Mikania micrantha total biomass and its components were significantly reduced by C. campestris infection at all nitrate treatments, and the magnitude of the reduction was dependent on nitrate fertilization levels as indicated by significant nitrate × Cuscuta interaction (Table 1). The infection reduced M. micrantha root biomass by about 71%, 73% and 61%, flower biomass by about 91%, 79% and 71% and total biomass by about 70%, 64% and 59% at 0.2, 1 and 5 mM nitrate fertilizations, respectively. These proportional decreases in biomass with the increases in nitrate supply occurred although the infected plants supported significantly higher parasite biomass at high than at low nitrate fertilizations (Figure 2A; Table 1). However, the parasite was always a similar proportion of the infected system (host plus parasite) at all nitrate levels (Figure 2B).
Table 1. ANOVA results and means (±SE, n = 5) of plant dry biomass (g) components based on data collected on 90 DAP for uninfected and infected M. micrantha plants by C. campestris at 0.2, 1 and 5 mM nitrate fertilization concentrations.
| M. micrantha | |||||||
| Treatments | Roots | Stem | Leaves | Flowers | Total | Infected system (host+parasite) | C. campestris |
| 0.2 mM nitrate | |||||||
| Uninfected | 22.64±2.17c | 8.50±0.76de | 3.86±0.49c | 1.17±0.50bd | 36.16±3.25c | 36.16±3.25d | |
| Infected | 6.46±1.91d | 2.66±0.41c | 1.63±0.14d | 0.11±0.11c | 10.86±2.28d | 16.44±2.12e | 5.58±0.22b |
| 1 mM nitrate | |||||||
| Uninfected | 32.95±3.37b | 12.70±0.75bd | 6.44±0.64b | 2.88±0.53b | 54.97±4.20b | 54.97±4.20c | |
| Infected | 8.77±1.78d | 7.12±1.69ce | 3.35±0.65cd | 0.60±0.30cd | 19.83±4.27d | 29.04±3.23d | 9.21±1.33b |
| 5 mM nitrate | |||||||
| Uninfected | 53.10±3.51a | 35.35±5.22a | 14.94±0.86a | 13.87±3.25a | 117.26±5.61a | 117.26±5.61a | |
| Infected | 20.49±2.96c | 17.06±2.69b | 6.31±1.01b | 3.96±1.32b | 47.81±4.63bc | 75.39±4.55b | 27.57±2.82a |
| Source of variation | F values from ANOVA | ||||||
| Nitrate (N) | 35.89*** | 36.77*** | 70.12*** | 18.72*** | 109.36*** | 170.00*** | 42.76***† |
| Infection (I) | 121.16*** | 22.86*** | 68.59*** | 13.55** | 161.39*** | 80.36*** | |
| N × I | 4.61* | 4.11* | 12.77*** | 5.33* | 15.42*** | 4.11* | |
Figure 2. Percent (%) dry weight of the infected to the uninfected M. micrantha plants (A) and of the parasite to infected system (host plus parasite) (B) in the association M. micrantha-C. campestris fertilized at 0.2, 1 and 5 mM nitrate fertilizations.
RGR and Leaf Area
RGR was affected significantly by nitrate and infection, but not by their interaction (Table 2). Significant decreases in RGR occurred in the infected M. micrantha plants at each nitrate fertilization level, and generally as nitrate supplies increased, RGR increased within each infection treatment. Infection significantly reduced leaf area of M. micrantha, and this negative effect was greater at 5 than at 0.2 or 1 mM nitrate (Table 2).
Table 2. ANOVA results and mean (±SE, n = 5) of relative growth rate (RGR, g g–1d–1), leaf area (m2), leaf area ratio (LAR, m2 kg–1 of plant, specific leaf area (SLA, m2 kg–1 of leaves), unit leaf rate (ULR, g m–2 d–1), shoot:root dry weight ratio (S:R, g g–1), and (host shoot+Cuscuta):host root ((H+C)/HR, g g–1) for M. micrantha plants infected and uninfected by C. campestris and fertilized at 0.2, 1 and 5 mM nitrate.
| Treatments | RGR | Leaf area | LAR | SLA | ULR | S:R | (H+C)/HR |
| 0.2 mM nitrate | |||||||
| Uninfected | 0.045±0.001b | 0.12±0.02c | 4.12±0.28c | 32.00±0.93ab | 11.22±0.78a | 0.61±0.05c | 0.61±0.05b |
| Infected | 0.031±0.002d | 0.06±0.01d | 6.05±0.88ab | 33.63±2.57ab | 5.79±1.10b | 0.87±0.20bc | 2.03±0.47a |
| 1 mM nitrate | |||||||
| Uninfected | 0.052±0.001a | 0.18±0.02b | 4.16±0.13c | 29.04±1.78b | 12.55±0.46a | 0.69±0.05c | 0.69±0.05b |
| Infected | 0.040±0.002c | 0.12±0.02c | 6.65±0.67a | 34.54±2.63ab | 6.24±0.82b | 1.24±0.11ab | 2.63±0.44a |
| 5 mM nitrate | |||||||
| Uninfected | 0.056±0.001a | 0.45±0.03a | 4.89±0.15bc | 30.27±1.92ab | 11.42±0.36a | 1.22±0.05ab | 1.22±.0.05b |
| Infected | 0.046±0.001b | 0.22±0.04b | 5.80±0.48ab | 35.06±1.88a | 8.07±0.66b | 1.41±0.22a | 2.84±0.32a |
| Source of variation | F values from ANOVA | ||||||
| Nitrate (N) | 32.79*** | 50.31*** | 0.22 ns | 0.15 ns | 1.51 ns | 9.28** | 2.90 ns |
| Infection (I) | 94.98*** | 34.23*** | 18.02*** | 5.75* | 69.48*** | 9.27** | 47.48*** |
| N × I | 0.80 ns | 6.63** | 1.24 ns | 0.51 ns | 2.11 ns | 1.01 ns | 0.39 ns |
Biomass Allocation
Biomass allocation parameters, except the percentage of total biomass allocated to flowers, of M. micrantha were all significantly affected by infection, but not by the interaction of nitrate and infection (Tables 2, 3).
Table 3. ANOVA results and means (±SE, n = 5) of the percentages (%) of total biomass allocated to roots, stems, leaves and flowers of the uninfected and infected M. micrantha plants by C. campestris on 90 DAP under 0.2, 1 and 5 mM nitrate fertilization treatments.
| Treatments | Roots | Stems | Leaves | Flowers |
| 0.2 mM nitrate | ||||
| Uninfected | 62.55±2.34a | 23.69±1.36b | 10.66±0.74b | 3.10±1.08b |
| Infected | 55.93±5.58a | 25.44±1.57b | 17.11±3.18a | 1.53±1.53b |
| 1 mM nitrate | ||||
| Uninfected | 59.47±1.82a | 23.35±1.34b | 11.73±0.84b | 5.44±1.14bc |
| Infected | 45.00±2.18b | 35.27±0.82a | 17.25±1.11a | 2.48±1.19b |
| 5 mM nitrate | ||||
| Uninfected | 45.16±1.16b | 29.86±3.24ab | 12.74±0.45b | 12.23±2.92a |
| Infected | 42.81±3.85b | 35.87±4.04a | 12.93±1.08ab | 8.38±1.93ac |
| Source of variation | F values from ANOVA | |||
| Nitrate (N) | 11.58*** | 6.21** | 0.60 ns | 9.14** |
| Infection (I) | 9.10** | 11.53** | 10.57** | 3.00 ns |
| N × I | 1.88 ns | 2.33 ns | 2.45 ns | 0.17 ns |
Generally, C. campestris infection significantly increased LAR, SLA and shoot:root ratios (S:R), but it reduced ULR of M. micrantha plants, and its effects on these traits were independent of nitrate treatments (Table 2). Within each nitrate treatment, the infection effects were more negative on root than on shoot growth (Figure 2A), resulting in higher S:R in infected plants than in control plants. The ratio of above to below-ground biomass in the host-parasite system was 2.3–3.8 times that of the uninfected plants among the nitrate treatments (Table 2).
Cuscuta campestris infection increased biomass allocation to stems and leaves and reduced them to roots and flowers of M. micrantha plants although the effects within all nitrate levels were not always significant (Table 3). The interaction of nitrate and infection was not significant in the allocations to these biomass components of M. micrantha plants.
Pn of M. micrantha Leaves
The interaction of nitrate and infection had no significant effects on Pn and related parameters of M. micrantha leaves on 30 and 80 DAP (Table 4). The infected plants had lower leaf Pn, g s, E and WUE, but higher C i than the uninfected plants at each nitrate fertilization level. Mostly, nitrate treatment did not result in significant changes in P n, g s, E, C i and WUE measured on 80 DAP but led to no consistent changes in these traits on 30 DAP within infection treatments (Table 4).
Table 4. ANOVA results and means (±SE, n = 5) of the net photosynthetic rate (P n), stomatal conductance (g s), transpiration rate (E), water use efficiency (WUE), intercellular CO2 concentration (C i) of the youngest fully expanded mature leaves of the uninfected and infected M. micrantha plants by C. campestris on different days after parasitization (DAP) under different nitrate fertilization treatments.
| P n (µmol CO2 m–2 s–1) | g s (mol H2O m–2s–1) | E (mmol H2O m–2s–1) | C i (µmol mol–1) | WUE (µmol CO2 mmol −1 H2O) | ||||||
| Treatments | 30 DAP | 80 DAP | 30 DAP | 80 DAP | 30 DAP | 80 DAP | 30 DAP | 80 DAP | 30 DAP | 80 DAP |
| 0.2 mM nitrate | ||||||||||
| Uninfected | 5.15±0.42bd | 4.47±0.42a | 0.11±0.01bc | 0.09±0.02a | 1.64±0.15b | 1.10±0.21ab | 278.52±4.67ab | 259.68±6.96bc | 3.16±0.14b | 4.38±0.45b |
| Infected | 2.23±0.28c | 1.75±0.34b | 0.09±0.02c | 0.07±0.02ab | 1.53±0.37b | 0.71±0.13bc | 303.14±8.16a | 316.51±8.26a | 1.65±0.30ac | 2.48±0.27c |
| 1 mM nitrate | ||||||||||
| Uninfected | 6.22±0.85b | 4.07±0.54a | 0.16±0.03a | 0.06±0.01ab | 1.48±0.19b | 0.71±0.13ac | 287.72±8.21a | 240.37±8.87c | 4.30±0.45a | 5.99±0.43ab |
| Infected | 4.08±0.35cd | 1.08±0.27b | 0.12±0.01ac | 0.04±0.01b | 1.46±0.17b | 0.46±0.11c | 290.68±12.95a | 289.95±21.27ab | 3.04±0.55bc | 2.50±0.72c |
| 5 mM nitrate | ||||||||||
| Uninfected | 8.44±0.97a | 4.48±0.66a | 0.15±0.03ab | 0.07±0.01ab | 2.71±0.42a | 0.73±0.14ac | 255.01±12.06b | 231.18±13.66c | 3.28±0.38ab | 6.41±0.84a |
| Infected | 3.51±0.85cd | 1.21±0.58b | 0.09±0.02c | 0.04±0.01b | 1.66±0.30b | 0.40±0.11c | 289.19±6.47a | 295.72±11.37ab | 2.09±0.24c | 2.59±0.53c |
| Source of variation | F values from ANOVA | |||||||||
| Nitrate (N) | 5.80** | 0.60 ns | 3.57* | 2.71 ns | 3.59* | 3.55* | 2.53 ns | 2.36 ns | 6.51** | 1.92 ns |
| Infection (I) | 36.11*** | 56.44*** | 7.85* | 4.51* | 2.83 ns | 7.79* | 7.46* | 30.33*** | 19.31*** | 43.23*** |
| N × I | 2.26 ns | 0.16 ns | 0.43 ns | 0.11 ns | 1.96 ns | 0.14 ns | 1.50 ns | 0.17 ns | 0.10 ns | 1.62 ns |
Photosynthesis in Response to Light
Cuscuta campestris infection had significant effects on LSP, P max, LCP and Φ, but not on R d of M. micrantha leaves in response to light (Figure S1; Table 5). Leaves of uninfected plants had higher LSP, P max and Φ but lower LCP than infected plants at each nitrate treatment. However, nitrate and its interaction with infection had no significant effects on these parameters (Table 5).
Table 5. ANOVA results and means (±SE, n = 5) of photosynthesis parameter estimates from the light response curves for the youngest fully expanded mature leaves of the uninfected and infected M. micrantha by C. campestris under different nitrate fertilization concentrations.
| Parameter estimates of photosynthesis | ||||||
| Treatments | LSP (µmol photonsm–2 s–1) | P max (µmol CO2m–2 s–1) | LCP (µmol photonsm–2 s–1) | Φ (mol CO2 mol–1 photons) | R d (µmol m–2 s–1) | |
| 0.2 mM nitrate | ||||||
| Uninfected | 376.00±53.11bc | 4.96±0.41a | 9.73±0.73c | 0.042±0.002a | 0.40±0.03 | |
| Infected | 293.60±23.38c | 2.19±0.43b | 21.09±3.84ab | 0.021±0.004b | 0.40±0.07 | |
| 1 mM nitrate | ||||||
| Uninfected | 516.00±65.62ab | 5.17±0.70a | 16.16±3.43abc | 0.042±0.005a | 0.62±0.07 | |
| Infected | 361.40±38.89bc | 2.79±0.48b | 25.39±2.80ab | 0.015±0.001b | 0.39±0.06 | |
| 5 mM nitrate | ||||||
| Uninfected | 586.40±75.82a | 5.33±0.77a | 14.07±2.66bc | 0.034±0.001a | 0.46±0.08 | |
| Infected | 273.40±33.00c | 2.24±0.52b | 27.96±5.45a | 0.019±0.004b | 0.42±0.04 | |
| Source of variation | F values from ANOVA | |||||
| Nitrate (N) | 2.49 ns | 0.26 ns | 1.68 ns | 0.83 ns | 1.43 ns | |
| Infection (I) | 18.88*** | 35.10*** | 16.61*** | 45.17*** | 2.99 ns | |
| N × I | 2.61 ns | 0.20 ns | 0.23 ns | 1.20 ns | 1.83 ns | |
LSP, light saturation point; P max, net photosynthesis at LSP; LCP, light compensation point; Φ, apparent quantum yield; R d, dark respiration rate.
Photosynthesis in Response to Ci
Leaves of uninfected plants had significantly higher CE, P sat, V cmax and J max than infected plants at each nitrate treatment (Figure S2; Table 6). CE and P sat were higher at 5 than at 0.2 and 1.0 mM nitrate in both the infected and uninfected.
Table 6. ANOVA results and means (±SE, n = 5) of the photosynthesis parameter estimates from the P n-C i response curves for the youngest fully expanded mature leaves of the uninfected and infected M. micrantha by C. campestris under different concentrations of nitrate fertilization.
| Parameter estimates of photosynthesis | ||||
| Treatments | CE | P sat (µmol CO2 m–2 s–1) | V cmax (µmol m–2 s–1) | J max (µmol m–2 s–1) |
| 0.2 mM nitrate | ||||
| Uninfected | 0.038±0.005b | 8.22±0.47a | 18.8±1.08ab | 78.64±4.97ab |
| Infected | 0.010±0.001cd | 3.24±0.38bc | 8.63±0.87c | 36.02±3.54cd |
| 1 mM nitrate | ||||
| Uninfected | 0.034±0.006b | 8.66±1.02a | 19.6±2.71ab | 87.84±13.98ab |
| Infected | 0.006±0.001c | 2.15±0.13c | 6.76±0.78c | 28.78±2.70d |
| 5 mM nitrate | ||||
| Uninfected | 0.061±0.008a | 10.52±1.18a | 23.5±2.14a | 104.26±10.64a |
| Infected | 0.025±0.009bd | 4.87±1.05b | 13.39±4.21bc | 58.94±18.79bcd |
| Source of variation | F values from ANOVA | |||
| Nitrate (N) | 8.06** | 4.70* | 3.13 ns | 3.21 ns |
| Infection (I) | 38.53*** | 74.95*** | 34.07*** | 30.60*** |
| N × I | 0.27 ns | 0.45 ns | 0.23 ns | 0.33 ns |
CE, carboxylation efficiency; P sat, CO2 saturated rate of photosynthesis; V cmax, maximum rate of Rubisco carboxylase activity; J max, maximum rate of photosynthetic electron transport.
Chlorophyll and Carotenoid
The concentrations of total chlorophyll, chlorophyll a and b, and carotenoid of M. micrantha leaves were significantly affected by nitrate, infection and their interaction (Table 7). There was a greater reduction in chlorophyll concentration of infected plants at 5 mM nitrate than at 0.2 or 1 mM nitrate (Table 7). The chlorophyll a:b ratio was not significantly affected by infection at each nitrate treatment.
Table 7. ANOVA results and mean (±SE, n = 5) concentrations (mg g–1) of total chlorophyll (Chl), Chl a, Chl b, carotenoid (mg g–1), Chl a:b ratio, soluble protein, Rubisco and nitrogen (g m–2) of the youngest fully expanded mature leaves of uninfected and infected M. micrantha plants fertilized at 0.2, 1 and 5 mM nitrate concentrations.
| Treatments | Total Chl | Chl a | Chl b | Chl a/b ratio | Carotenoid | Soluble protein | Rubisco | Nitrogen | PNUE |
| 0.2 mM nitrate | |||||||||
| Uninfected | 0.73±0.04b | 0.52±0.03b | 0.21±0.01b | 2.51±0.05b | 0.057±0.003b | 3.39±0.22b | 1.08±0.14a | 0.36±0.03c | 12.73±1.56a |
| Infected | 0.29±0.05c | 0.20±0.04c | 0.09±0.01c | 2.25±0.20b | 0.038±0.005c | 1.27±0.17c | 0.59±0.14b | 0.36±0.06c | 5.21±1.01bcd |
| 1 mM nitrate | |||||||||
| Uninfected | 0.70±0.12b | 0.50±0.08b | 0.20±0.03b | 2.44±0.12b | 0.055±0.009b | 4.50±0.19a | 1.42±0.13a | 0.55±0.07b | 7.58±1.01b |
| Infected | 0.22±0.04c | 0.15±0.03c | 0.07±0.01c | 2.25±0.17b | 0.031±0.006c | 1.41±0.41c | 0.52±0.13b | 0.31±0.01c | 3.54±0.89cd |
| 5 mM nitrate | |||||||||
| Uninfected | 1.47±0.15a | 1.10±0.11a | 0.37±0.040a | 2.97±0.04a | 0.105±0.008a | 5.12±0.42a | 1.53±0.14a | 0.76±0.06a | 6.00±1.09bc |
| Infected | 0.35±0.07c | 0.26±0.05c | 0.09±0.02c | 3.10±0.23a | 0.046±0.007bc | 2.08±0.44c | 0.50±0.24b | 0.49±0.03b | 2.52±0.82d |
| Source of variation | F values from ANOVA | ||||||||
| Nitrate (N) | 15.61*** | 18.02*** | 9.33** | 12.92*** | 13.67*** | 7.38** | 0.71 ns | 16.55*** | 9.94** |
| Infection (I) | 88.26*** | 87.35*** | 87.06*** | 0.75 ns | 37.70*** | 104.26*** | 38.34*** | 19.58*** | 31.65*** |
| N × I | 9.38** | 9.98** | 7.48** | 0.93 ns | 5.28* | 1.35 ns | 1.61 ns | 5.07* | 2.01 ns |
Proteins and Rubisco Contents
Nitrate treatment had a significant influence on total soluble protein content, but not on Rubisco content (Table 7). Higher nitrate supply resulted in higher soluble protein content. Infection significantly reduced both total soluble protein and Rubisco contents, and the response to infection was similar across nitrate levels (Table 7).
Leaf Nitrogen, Nitrogen Partitioning and PNUE
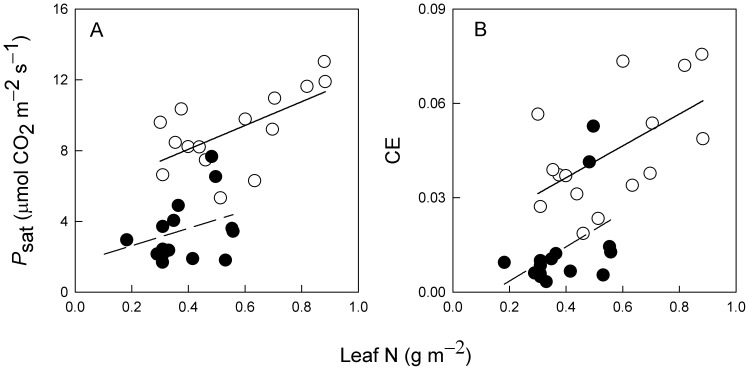
Cuscuta campestris infection significantly reduced M. micrantha plant leaf nitrogen content and its effect depended on nitrate supply (Table 7). The infected plants had significantly reduced leaf nitrogen contents at 1 and 5 mM nitrate fertilizations, but not at 0.2 mM. The nitrogen concentrations in C. campestris were not significantly different among the three nitrate fertilizations; 14.4±0.66, 15.2±0.76 and 16.9±0.60 mg g–1 at 0.2, 1 and 5 mM nitrate, respectively. There was a significant positive linear correlation between P sat or CE and leaf nitrogen content for uninfected M. micrantha plants (Figure 3). Cuscuta campestris infection significantly reduced photosynthetic nitrogen use efficiency (PNUE) of M. micrantha plants but the negative effects did not differ across the three nitrate treatments (Table 7).
Figure 3. The relationships between leaf nitrogen concentrations (Leaf N) and P sat (A) and CE (B) for M. micrantha plants either uninfected (○; solid line) or infected (•; broken line) with C. campestris (Data from all nitrate treatments are included).
The correlation coefficients are 0.62 (P<0.05) and 0.40 (P>0.05) in (a), and 0.56 (P<0.05) and 0.44 (P>0.05) in (b) for the uninfected and infected plants, respectively.
Discussion
The present study shows that C. campestris infection had significant effects on most of the traits related to growth (biomass traits, number of leaves and leaf area), biomass allocation, photosynthesis and biochemical parameters of M. micrantha host plants. The extent of the negative effects of the parasite on host growth, and chlorophyll and leaf nitrogen content varied with the concentration of nitrate supplied to the host plants, as indicated by the significant nitrate × infection effects on these variables. However, the effects of infection on biomass allocation and leaf photosynthesis related traits of M. micrantha were independent of nitrate supply.
Growth
In the present study, C. campestris infection reduced the number of leaves, leaf area, and biomass traits (total, root, stem, leaf and flower dry weights) of M. micrantha plants at each level of nitrate fertilization, and the negative impacts of the parasite on host growth were generally less severe at high than at low nitrate supplies. Such an alleviation of the impacts at high nitrogen was attributable to a more negative influence of the infection on root biomass at low than at high nitrogen fertilization. Alleviation of growth inhibition at high N supply has also been observed in R. communis infected by C. reflexa [8] and in sorghum [5] or rice [6] infected by S. hermonthica. However, as N supply increased, inhibition in the growth of C. blumei infected by C. reflexa increased [7], which might be due to the strangling effect exerted by the haustorial coil of C. reflexa. Such strangling was not found in C. reflexa-infected R. communis [8] or in C. campestris-infected M. micrantha in this study.
Inhibitions in the reproductive growth of infected plants also became more severe at lower nitrate supply with fewer resources for reproduction as growth became severely inhibited. It has been reported that flowering was delayed and the number of florets was reduced at high N supply in C. reflexa-infected C. blumei [7], R. communis [8], Vicia faba [27] and L. albus [9], and flowering of M. micrantha was completely inhibited by low N supply and by C. campestris infection [10]. We did not find complete flowering inhibition in the present study which contradicts results of our earlier study [10]. However, although the plants in both studies were of similar age at the time of infection treatments, the treatments were applied about 45 days later in the growing season in the present than in the previous study.
In this study, the total biomass of the infected system (host+parasite) was less than uninfected M. micrantha, with greater proportional reductions as nitrate concentration decreased. Similar results were observed in the same system [10] and in S. hermonthica-sorghum association [5]. In these cases, the reduced growth of infected plants resulted from resource capture by the parasite and the negative effects of the parasite on host photosynthesis. As infection reduced host photosynthesis in our present study at each level of nitrate supplies, and in our previous study [11], the response of the host to infection cannot be explained by a simple source-sink relation regardless of nitrogen treatment [1], [7], [8]. As nitrate supply increased, the biomass of infected hosts increased, as did the corresponding biomass of the parasite. However, the percentage of total biomass allocated to the parasite did not differ among the three nitrate treatments, indicating the growth of the parasite is dependent on or tuned to the size or carrying capacity of the host. Therefore, C. campestris growth on its host may be resource-dependent: resource uptake is not linear but eventually reaches a plateau. This is possible as C. campestris obtains all its resources from its host M. micrantha, and its host’s physiological conditions would change directly with parasite densities; in turn, C. campestris somehow ‘senses’ these changes and then regulates its growth accordingly. A fine tuning of the sink power of the parasite in the association R. communis–C. reflexa [8] and the adaptation of C. campestris life cycle completion to the resource availability of its host M. micrantha [10] has been observed. Such sensing or tuning strategies can ensure the survival of the hosts for the normal growth and development of the parasites, and the biochemical and physiological mechanisms underlying them are unknown and merit future studies.
Biomass Allocation
Cuscuta campestris had more negative effects on host root than shoot growth. Cuscuta campestris infection resulted in greater biomass allocation to stems and leaves but lesser allocation to roots, thus resulting in increased shoot:root ratios of infected M. micrantha plants. Similar results were found in our previous studies [10], [28]. Cuscuta campestris is a shoot parasite and competes for the resources that the host allocates to shoot and root growth. The host may allocate relatively more resources to shoots to compensate for the resources directly captured by the parasite, or transfer relatively fewer resources to roots, or a higher competitive demand and a stronger source demand from the shoot system resulting in greater transfer of resources to shoots. In root parasites, reduced shoot:root ratios have been reported in S. hermonthica-infected rice and sorghum [5], [6] and Orobanche aegyptiaca-infected tomato [29]. Therefore, the negative effects of shoot parasites may be more severe on the roots than on the shoots of their hosts, and the opposite may apply to root parasites. This requires further study.
Photosynthesis
Our previous studies [11], [30] showed that C. campestris infection reduced leaf P n of M. micrantha and speculated that this was due to the parasite’s indirect adverse impacts on g s and direct negative effects on the photosynthetic metabolism of M. micrantha. Our present study shows similar negative effects resulting from the lower light and CO2 use efficiencies of leaves of infected plants than uninfected plants. Infection reduced LSP, P max, Φ, P sat, CE, V cmax J max and PNUE and increased LCP across all nitrate levels. Lower photosynthetic efficiency of infected plants was also caused by lower leaf nitrogen, chlorophyll a and b, soluble protein and Rubisco concentrations, and g s than uninfected plants. Low nitrogen concentrations in infected leaves could accelerate leaf senescence, reducing leaf photosynthesis and the number of leaves. This chain of effects in infected plants explains the lower photosynthesis we observed at leaf level, resulting in lower total photosynthesis and growth at the plant level in comparison to uninfected plants.
The magnitude of the negative effects of C. campestris on M. micrantha photosynthesis was similar across all nitrate fertilization levels. Thus, the less severe inhibition in host growth at high than at low nitrate levels is not attributable to inhibition of host photosynthesis and hence leaf production. It has been shown that Cuscuta can form a strong sink to redirect the flow of host resources to itself [8], [27], and Cuscuta species alter host physiology by acting as a stronger sink for photosynthates than any host organs [31]. Redirection of more resources by C. campestris at low than at high nitrate levels resulted in a greater reduction in infected M. micrantha total biomass and root biomass.
Uninfected M. micrantha plants made greater use of the extra nitrogen to produce ‘greener’ leaves than infected plants, as shown by the higher leaf chlorophyll content at 5 mM than at 1 and 0.2 mM nitrate in uninfected plants. However, greener leaves did not have increased P max although there was a good relationship between N concentration and P sat. The significant effect of nitrate and infection interaction on leaf chlorophyll content (per leaf area or leaf mass) resulted from the chlorophyll content of the leaves of infected plants being more reduced at 5 mM than at 0.2 or 1 mM nitrate. This was consistent with the variation pattern for leaf nitrogen content per unit leaf area. The reason for this might be that the total nitrogen absorption and supply capacities of the roots of infected plants were more reduced at high than at low nitrate level.
Infection reduced transpiration and g s on 30 and 80 DAP at each nitrate treatment, which may have induced stomatal closure or reduced stomatal opening. Parallel reductions in leaf nitrogen and P n were observed, and P n decreases would reduce carbon production. It has been suggested that low leaf nitrogen often leads to high leaf abscisic acid (ABA) levels and increases in xylem translocation of ABA from root to shoot [32] and high leaf ABA induces stomatal closure [30]. Cuscuta campestris infection lowered leaf nitrogen in M. micrantha, which may increase leaf ABA and thus contribute to the stomatal closure of infected plants [30].
In this study, as in our previous studies, C. campestris reduced the number of leaves of M. micrantha, through a host response of reducing new leaf initiation and/or accelerating leaf senescence and abscission [10], [11]. Leaf senescence is characterized by a decline in photosynthesis accompanied by the loss of Rubisco and chlorophyll/protein complexes and the decline in stomatal conductance [33]–[35]. Leaf chlorophyll and protein contents are often used as indicators of leaf senescence [36]. In the present study, the lower leaf P n and g s, leaf nitrogen, total soluble protein and chlorophyll concentrations in infected plants than in uninfected plants suggest that host leaf senescence is a response to C. campestris infection.
In summary, the results indicate that the negative effects of the holoparasite C. campestris on the growth of M. micrantha were dependent on nitrate supply to the host, and they were more severe at low than at high nitrate levels. The more severe inhibition in host growth at low than at high nitrate supplies is largely attributable to the transfer of more host resources to C. campestris at low than at high nitrate levels as the magnitude of inhibition in host photosynthesis was similar across nitrate levels. In addition, C. campestris seems able to sense the carrying capacity of the host and regulates its growth accordingly, indicating a synchronicity in growth and development between the parasite and its host.
Supporting Information
Mean net photosynthetic rates (P n, ±SE, n = 5) at different photosynthetic photon flux densities (PPFD) for the youngest fully expanded mature leaves of the uninfected (○) and infected (•) M. micrantha plants by C. campestris at (a) 0.2, (b) 1 and (c) 5 mM nitrate fertilizations.
(TIF)
Response of net photosynthetic rates (P n) to intercellular CO2 concentrations (C i) in the youngest fully expanded mature leaves of the uninfected (○) and infected (•) M. micrantha plants by C. campestris at (a) 0.2, (b) 1 and (c) 5 mM nitrate fertilizations. Data points are means ±SE (n = 5).
(TIF)
Acknowledgments
We thank Dr. Jane Prider for her constructive comments on the manuscript.
Funding Statement
This work was funded by the National Key Technologies R&D Program of China (2012BAC07B04). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Press MC, Scholes JD, Watling JR (1999) Parasitic plants: physiological and ecological interactions with their hosts. In: Press MC, Scholes JD, Barker MG, editors. Physiological plant ecology: the 39th Symposium of the British Ecological Society. York: University of York. 175–197.
- 2. Press MC, Graves JD, Stewart GR (1990) Physiology of the interaction of angiosperm parasites and their higher plant hosts. Plant Cell and Environment 13: 91–104. [Google Scholar]
- 3. Shen H, Ye W, Hong L, Huang H, Wang Z, et al. (2006) Progress in parasitic plant biology: Host selection and nutrient transfer. Plant Biology 8: 175–185. [DOI] [PubMed] [Google Scholar]
- 4. Watling JR, Press MC (2001) Impacts of infection by parasitic angiosperms on host photosynthesis. Plant Biology 3: 244–250. [Google Scholar]
- 5. Cechin I, Press MC (1993) Nitrogen relations of the sorghum-Striga hermonthica host-parasite association: growth and photosynthesis. Plant, Cell and Environment 16: 237–247. [DOI] [PubMed] [Google Scholar]
- 6. Cechin I, Press MC (1994) Influence of nitrogen on growth and photosynthesis of a C3 cereal, Oryza sativa, infected with the root hemiparasite Striga hermonthica . Journal of Experimental Botany 45: 925–930. [Google Scholar]
- 7. Jeschke WD, Baig A, Hilpert A (1997) Sink-stimulated photosynthesis, increased transpiration and increased demand-dependent stimulation of nitrate uptake: nitrogen and carbon relations in the parasitic association Cuscuta reflexa-Coleus blumei . Journal of Experimental Botany 48: 915–925. [Google Scholar]
- 8. Jeschke WD, Hilpert A (1997) Sink-stimulated photosynthesis and sink-dependent increase in nitrate uptake: nitrogen and carbon relations of the parasitic association Cuscuta reflexa-Ricinus communis . Plant, Cell and Environment 20: 47–56. [Google Scholar]
- 9. Jeschke WD, Bäumel P, Räth N, Czygan FC, Proksch P (1994) Modelling of the flows and partitioning of carbon and nitrogen in the holoparasite Cuscuta reflexa Roxb. and its host Lupinus albus L. II. Flows between host and parasite and within the parasitized host. Journal of Experimental Botany 45: 801–812. [Google Scholar]
- 10. Shen H, Ye WH, Hong L, Cao HL, Wang ZM (2005) Influence of the obligate parasite Cuscuta campestris on growth and biomass allocation of its host Mikania micrantha . Journal of Experimental Botany 56: 1277–1284. [DOI] [PubMed] [Google Scholar]
- 11. Shen H, Hong L, Ye WH, Cao HL, Wang ZM (2007) The influence of the holoparasitic plant Cuscuta campestris on the growth and photosynthesis of its host Mikania micrantha . Journal of Experimental Botany 58: 2929–2937. [DOI] [PubMed] [Google Scholar]
- 12.Holm LG, Plucknett DL, Pancho JV, Herberger JP (1977) The world’s worst weeds: distribution and biology. Honolulu: University Press of Hawaii. 320–327 p. [Google Scholar]
- 13. Zhang LY, Ye WH, Cao HL, Feng HL (2004) Mikania micrantha H.B.K. in China - an overview. Weed Research 44: 42–49. [Google Scholar]
- 14. Dawson JH, Musselman LJ, Wolswinkel P, Dörr I (1994) Biology and control of Cuscuta . Review of Weed Science 6: 265–317. [Google Scholar]
- 15. Hunt R, Parsons IT (1974) A computer program for deriving growth-functions in plant growth-analysis. Journal of Applied Ecology 11: 297–307. [Google Scholar]
- 16. von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387. [DOI] [PubMed] [Google Scholar]
- 17. Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris . Plant Physiology 24: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hieke S, Menzel CM, Lüdders P (2002) Effects of light availability on leaf gas exchange and expansion in lychee (Litchi chinensis). Tree Physiology 22: 1249–1256. [DOI] [PubMed] [Google Scholar]
- 19. Watling JR, Press MC (2000) Infection with the parasitic angiosperm Striga hermonthica influences the response of the C3 cereal Oryza sativa to elevated CO2 . Global Change Biology 6: 919–930. [Google Scholar]
- 20. Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90. [DOI] [PubMed] [Google Scholar]
- 21. Harley PC, Sharkey TD (1991) An improved model of C3 photosynthesis at high CO2: reversed O2 sensitivity explained by lack of glycerate reentry into the chloroplast. Photosynthesis Research 27: 169–178. [DOI] [PubMed] [Google Scholar]
- 22. Harley PC, Thomas RB, Reynolds JF, Strain BR (1992) Modeling photosynthesis of cotton grown in elevated CO2 . Plant Cell and Environment 15: 271–282. [Google Scholar]
- 23. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye-binding. Analytical Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 24. Makino A, Mae T, Ohira K (1986) Colorimetric measurement of protein stained with Coomassie Brilliant Blue R on sodium dodecyl sulfate-polyacrylamide gel electrophoresis by eluting with formamide. Agricultural and Biological Chemistry 50: 1911–1912. [Google Scholar]
- 25. Irving LJ, Robinson D (2006) A dynamic model of Rubisco turnover in cereal leaves. New Phytologist 169: 493–504. [DOI] [PubMed] [Google Scholar]
- 26. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 27. Wolswinkel P (1974) Complete inhibition of setting and growth of fruits of Vicia faba L., resulting from the draining of the phloem system by Cuscuta species. Acta Botanica Neerlandica 23: 48–60. [Google Scholar]
- 28. Shen H, Hong L, Chen H, Ye WH, Cao HL, et al. (2011) The response of the invasive weed Mikania micrantha to infection density of the obligate parasite Cuscuta campestris and its implications for biological control of M. micrantha . Botanical Studies 52: 89–97. [Google Scholar]
- 29. Barker ER, Press MC, Scholes JD, Quick WP (1996) Interactions between the parasitic angiosperm Orobanche aegyptiaca and its tomato host: growth and biomass allocation. New Phytologist 133: 637–642. [Google Scholar]
- 30. Chen H, Shen H, Ye W, Cao H, Wang Z (2011) Involvement of ABA in reduced photosynthesis and stomatal conductance in Cuscuta campestris - Mikania micrantha association. Biologia Plantarum 55: 545–548. [Google Scholar]
- 31.Parker C, Riches CR (1993) Parasitic weeds of the world: biology and control. Wallingford: CAB International. 332 p. [Google Scholar]
- 32. Peuke AD, Jeschke WD, Hartung W (1994) The uptake and flow of C, N and ions between roots and shoots in Ricinus communis L. III. Long-distance transport of abscisic acid depending on nitrogen nutrition and salt stress. Journal of Experimental Botany 45: 741–747. [Google Scholar]
- 34. Murchie EH, Chen YZ, Hubbart S, Peng SB, Horton P (1999) Interactions between senescence and leaf orientation determine in situ patterns of photosynthesis and photoinhibition in field-grown rice. Plant Physiology 119: 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang CZ, Rodermel SR, Shibles RM (1993) Photosynthesis, Rubisco activity and amount, and their Regulation by transcription in senescing soybean leaves. Plant Physiology 101: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vanacker H, Sandalio LM, Jimenez A, Palma JM, Corpas FJ, et al. (2006) Roles for redox regulation in leaf senescence of pea plants grown on different sources of nitrogen nutrition. Journal of Experimental Botany 57: 1735–1745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean net photosynthetic rates (P n, ±SE, n = 5) at different photosynthetic photon flux densities (PPFD) for the youngest fully expanded mature leaves of the uninfected (○) and infected (•) M. micrantha plants by C. campestris at (a) 0.2, (b) 1 and (c) 5 mM nitrate fertilizations.
(TIF)
Response of net photosynthetic rates (P n) to intercellular CO2 concentrations (C i) in the youngest fully expanded mature leaves of the uninfected (○) and infected (•) M. micrantha plants by C. campestris at (a) 0.2, (b) 1 and (c) 5 mM nitrate fertilizations. Data points are means ±SE (n = 5).
(TIF)