Abstract
WHIM-syndrome is an inherited immunodeficiency disorder with abnormal susceptibility to human papillomavirus (HPV) infection and diseases. We determined safety and immunogenicity to a quadrivalent HPV vaccine in WHIM-syndrome by detection of HPV-specific antibodies and lymphoproliferation. In virus-like-particle (VLP)-ELISA, a WHIM patient showed antibody titers up to 400 for HPV-6/11/16/18, whereas immuno-competent controls developed titers of 6,400-25,600. In pseudovirion assays, the patient’s neutralization titers ranged from 20-400 to the four HPV vaccine types, while titers of 1,600-25,600 were detected in healthy vaccinees. Specific proliferation of PBMC of the WHIM patient to the HPV vaccine was demonstrated. This first report on response to HPV vaccination in WHIM-immunodeficiency highlights that patients with WHIM-syndrome, and probably other immunodeficiencies, may benefit from HPV immunoprophylaxis.
Keywords: human papillomavirus, vaccination, immunodeficiency, WHIM syndrome
1. Introduction
WHIM syndrome is a rare primary immunodeficiency disorder characterized by human papillomavirus (HPV)-induced warts, hypogammaglobulinemia, recurrent bacterial infections and myelokathexis [1,2]. Most patients carry autosomal dominant inherited heterozygous mutations in the gene encoding for the CX chemokine receptor 4 (CXCR4) [3], resulting in truncations of the receptor protein. Upon exposure to the unique natural ligand, CXCL12, also known as stromal cell-derived-factor-1 (SDF-1), the mutated CXCR4 receptor displays impaired internalization and desensitization properties [4]. Enhanced and prolonged receptor activation [5,6] appears to impair leukocyte trafficking and to account for the clinical manifestations of WHIM syndrome. [7]The clinical presentation of WHIM patients is heterogeneous and severity and onset of disease vary [2]. The WHIM phenotype includes peripheral neutropenia, T- and B-cell lymphopenia as well as hypogammaglobulinemia [8,9]. The pathognomonic finding in the bone marrow is termed myelokathexis [10], which represents a form of myeloid hypercellularity caused by retention of adult myeloid cells with morphologic abnormalities consistent with apoptosis [11]. The increased number of apoptotic neutrophils in the bone marrow is associated with severe neutropenia in the peripheral blood. Moreover, quantitative as well as functional T-cell abnormalities have been observed in a subset of WHIM patients, although the composition of the main T-cell subsets appears normal [5]. B-cell lymphopenia was shown in several cases, and in particular circulating CD27+ memory B-cells were significantly reduced [5]. Disturbed B-cell function results in hypogammaglobulinemia that may range from moderate to mild and is either restricted to immunoglobulin (Ig) G or also involves IgM or IgA [2,9]. Ig substitution therapy is regarded as one of the backbones of therapy, in order to minimize the frequency of infections.
WHIM patients generally have difficulties to cope with bacterial infections and typically present with recurrent pyogenic infections from early childhood [2]. Susceptibility to opportunistic or mycobacterial infections has not been observed, even in patients with low absolute T-cell counts. There is no increased incidence of viral diseases, with the exception of a specific susceptibility to papillomavirus infections [9].
Human papillomaviruses (HPV) are small, double-stranded DNA tumor viruses that infect epithelia of the skin and mucosa, causing papillomas or warts [12,13]. Most HPV predominantly infect either non-genital cutaneous or mucosal/genital tissue, leading to their designation as cutaneous or genital-mucosal HPV types, respectively. Mucosal types are further divided into low-risk or high-risk types, according to their association with benign genital warts or cervical cancer [14]. Low-risk HPV 6 and 11 cause about 90% of anogenital warts (condylomata acuminata), laryngeal papillomas and rarely giant condylomata acuminata (Buschke Löwenstein tumor). Persistent anogenital infection with high-risk HPV may cause intraepithelial dysplasia and progression to invasive cancer. HPV 16 and 18 are the high-risk types responsible for approximately 70% of cervical cancers and their precursor lesions, and a subset of vaginal, vulvar, penile, anal and oropharyngeal cancers.
The reason for the specific susceptibility of WHIM patients for infections with both cutaneous and mucosal HPV is still unknown. Individuals with WHIM syndrome may be afflicted with extensive cutaneous and/or anogenital warts. Female patients are at higher risk to develop cervical and vulvar dysplasia, which in several cases have progressed to carcinoma [8,15].
In recent years two HPV vaccines consisting of the major capsid protein L1 assembled into virus-like particles (VLP) have been introduced [16,17]. VLP morphologically and immunologically resemble native infectious virions [18], but lack the potentially oncogenic viral genome and the ability to replicate and thus may be safely applied to immuno-compromised individuals. The available vaccines comprise VLP of high-risk mucosal HPV 16 and 18 (bivalent HPV vaccine), or additionally VLP of low-risk mucosal HPV 6 and 11 (quadrivalent HPV vaccine). Vaccinations elicit a strong, long-lasting and type-restricted antibody response capable of neutralizing infectious virions. In previously uninfected women these vaccines have shown up to 100% efficacy in preventing genital HPV infection and associated disease caused by the types included in the vaccine formulation [16,19]. Protective vaccine efficacy in immuno-competent individuals was shown to last a minimum of 5 years after the initial administration [20]. To our knowledge, there are no studies published on the vaccine efficiency in immuno-compromised individuals.
The aim of the present study was to investigate safety and immunogenicity of the HPV vaccine in the setting of immunodeficiency with high HPV susceptibility. A 12 year old female patient suffering from WHIM syndrome was immunized with the quadrivalent HPV vaccine and the humoral and cellular immune responses were determined and compared to those of immuno-competent controls.
2. Material and methods
2.1. Characteristics of the WHIM patient
In a 12 year old female patient the diagnosis of WHIM syndrome was established by detection of the R334X mutation in the CXCR4 gene, which had been described previously to result in a 19 residues truncation from the cytoplasmic tail [3]. No CXCR4 mutations were detected in the patient’s two healthy siblings and her parents. The patient presented with neutropenia with absolute counts of <1000/mm3, T-cell lymphopenia (CD4+ T cells <300/mm3), B-cell lymphopenia (CD19+ B cells <50/mm3), myelokathexis, and suffers from recurrent bacterial infections including pneumonia and urinary tract infections. As described in other WHIM patients, no opportunistic infections were observed and at the time of presentation the patient was not suffering from cutaneous or anogenital warts. The patient is maintained on weekly administered Ig substitution therapy. The patient was immunized intramuscularly with the quadrivalent HPV vaccine (Gardasil®, Merck & Co, Inc.) at months 0, 2 and 6 according to the licensed vaccination protocol. Blood samples were collected prior to immunization, at each vaccination timepoint and 8 weeks after the third immunization. Sera were stored at −20°C for further analysis. Peripheral blood mononuclear cells (PBMC) were separated by density centrifugation and kept cryopreserved until further testing.
2.2. Control populations
The positive and negative control populations consisted of three immuno-competent individuals (age range: 28-49 years; mean 37,3 years), who received the HPV vaccine according to the same vaccination protocol, and three age-matched, non-vaccinated immuno-competent individuals (age ranging from 27-47 years; mean 37 years), respectively. Control participants had no previous history of HPV-associated diseases or immune deficiencies. Serum samples and PBMC were collected in a similar manner to the WHIM patient.
2.3. Generation and purification of HPV VLP
Recombinant baculoviruses expressing the major capsid protein L1 of HPV 6, 11, 16 and 18 were described previously [21,22]. VLP of HPV types 6, 11, 16 and 18 were generated by infection of Sf9 insect cells and purified by density gradient ultra-centrifugation [18,23]. VLP of bovine papillomavirus type 1 (BPV-1) served as controls [18].
Purified VLP were analyzed by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie staining for the presence of HPV-L1 proteins. Transmission electron microscopy was used to determine integrity of the self-assembled particles, following negative staining with 1 % uranylacetate and visualization in a JEOL 1010 electron microscope at 80 kV and × 30,000 magnification [23,24].
2.4. VLP ELISA
Native, intact VLP of HPV 6, 11, 16 or 18 were used as antigen in a direct ELISA as described [23,25]. Serial dilutions of patients’ sera ranging from 1:100 to 1:102,400 were investigated in triplicates for type-specific antibodies to the vaccine VLP types. Polyclonal rabbit sera obtained after VLP immunization served as appropriate controls. Specific optical density (OD) values at 405 nm were calculated by subtracting mean values obtained in antigen-coated wells without first and second step antibodies (PBS only). Replication variation in the assays was less than 5%. Results are given as increase in mean OD values 2 months after the third immunization, over mean values of preimmune sera.
2.5. Pseudovirion (PsV) neutralization assays
Pseudovirions (PsV) of HPV types 6, 11, 16 and 18 were generated by co-transfection of the human embryonic kidney line 293 TT [26,27] with plasmids encoding for the respective L1 and L2 capsid genes and secreted alkaline phosphatase (SEAP) [23,28,29]. Following high-level amplification and expression, PsV encapsidating the SEAP reporter plasmid were purified on density gradients.
For neutralization assays, sera were serially diluted (1:20 to 1:102,400) and tested for their ability to inhibit infection of 293 TT cells by PsV of HPV types 6, 11, 16 or 18 according to previously published methods [27]. Release of SEAP into culture supernatant inversely correlates with prevention of PsV infection. Immune serum dilutions showing at least 50 % reduction in SEAP activity compared to preimmune serum at the same dilutions were considered neutralizing. Data shown are the mean OD +/− standard deviation (SD) of triplicate wells determined at 405 nm of a representative experiment.
2.5. Lymphoproliferation Assays
Cryopreserved PBMC were thawed and plated in triplicates at 2×105/well in 96-well round-bottom plates in RPMI-1640 medium supplemented with 10 % fetal calf serum, penicillin-streptomycin (100 μg/ml-100 U/ml) and glutamine (2 mM). Cells were cultured in the presence of Gardasil® (0.375 μg of total L1 VLP per well) or were left unstimulated. Phytohemagglutinin (PHA) (Sigma) at end dilution of 1 μg/ml was used as positive control. To determine type-specificity of the induced responses, VLP preparations of BPV-1, corresponding to the same amount of total HPV L1 protein in cultures with Gardasil®, were included as control antigen in the experiment. After 5 days of culture at 37°C, cells were pulsed with 1 μCi [3H]-thymidine (Sigma) for additional 18 hours. Cells were harvested onto glass fibre filters and [3H]-thymidine incorporation was measured in a beta-scintillation counter. Results are expressed in mean counts per minute (cpm), determined by subtraction of mean background cpm of unstimulated PBMC from mean cpm of cultures in the presence of Gardasil®.
3. Results
3.1 Gardasil vaccination according to the licensed protocol induces HPV-specific antibodies in WHIM immunodeficiency
The quadrivalent HPV vaccine Gardasil was administered intramuscularly at time points 0, 2, 6 month to the WHIM patient and three immuno-competent volunteers according to the licensed vaccination protocol. Vaccination was well tolerated in all immunized individuals and no adverse effects have been observed during the vaccination period and the follow-up period of 16 months. Three additional immuno-competent volunteers, who did not receive the vaccine, served as controls. Blood samples were drawn prior to each vaccination timepoint and 2 months after the third and final injection.
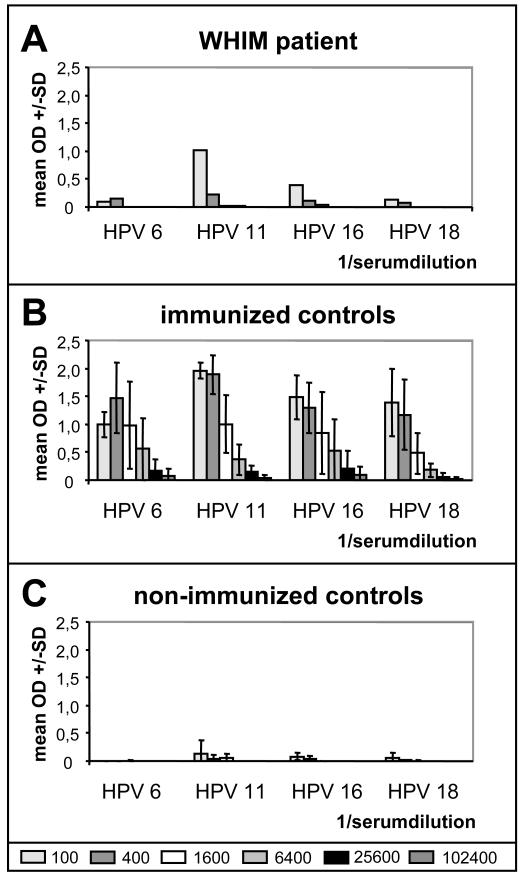
HPV-specific antibody titers were measured by ELISA using VLP of HPV 6, 11, 16 or 18 as the antigen. Immunization elicited HPV-specific antibodies in the WHIM patient with a titer of 400 for HPV 6, HPV 11, HPV 16, and a titer of 100 for HPV 18 (Figure 1A).
Fig. 1.
Serum antibodies elicited by HPV vaccination were determined by type-specific VLP-ELISA. A. In the WHIM patient titers of 400 against HPV 6, 11, 16 and of 100 against HPV 18 were measured. B. Immuno-competent individuals mounted antibody titers from 6,400-102,400 against all four HPV types. Results are given as the mean type-specific OD values +/− SD for sera of three vaccinated controls. C. Non-vaccinated immuno-competents showed no increase in type-specific antibodies.
As expected, vaccination of immuno-competent controls induced HPV-specific antibodies against all four HPV vaccine types with antibody titers from 6,400-102,400. Figure 1B gives the mean of HPV specific antibody titers of three immuno-competent individuals. Non-vaccinated immuno-competent individuals did not develop detectable HPV vaccine type-specific antibodies during the eight months study period (Figure 1C).
3.2 Immune sera are neutralizing in HPV PsV infection assays
Native HPV virions are notoriously difficult to propagate in vitro, thus neutralization assays have recently been developed based on PsV vectors. Similar to authentic papillomaviruses, PsV are composed of the viral capsid proteins, but instead of the viral genome encapsidate a plasmid encoding for a reporter gene. PsV display properties similar to native, infectious virions and have been used to develop neutralization assays. Detection of neutralizing antibodies has been correlated with protection against natural and experimental papillomavirus infection [30].
To determine, whether neutralizing antibodies were elicited by HPV vaccination, sera of the WHIM patient as well as three immunized controls were tested for their neutralization capacity in HPV 6, 11, 16 and 18 PsV assays (Table 1).
Table 1. HPV-specific neutralization titers.
| HPV 6 | HPV 11 | HPV 16 | HPV 18 | |
|---|---|---|---|---|
| Whim patient | 50 | 400 | 200 | 20 |
| Control 1 | 1600 | 1600 | 1600 | 1600 |
| Control 2 | 25600 | 6400 | 6400 | 1600 |
| Control 3 | 6400 | 6400 | 1600 | 1600 |
Preimmune sera of the WHIM patient, despite Ig replacement therapy, and the immunized controls did not display neutralization of HPV 6 PsV (data not shown). Immune serum of the WHIM patient, drawn 2 months after the final vaccination, was capable of neutralizing HPV 6 PsV infection with a titer of 50. In comparison, immune sera of immuno-competent controls neutralized HPV 6 PsV at titers ranging from 1,600 to 25,600 (Table 1).
Similarly, none of the vaccinated individuals had preexisting neutralizing antibodies to PsV of HPV 11 (data not shown). Immune serum of the WHIM patient displayed neutralization at a titer of 400, whereas immunized controls readily neutralized HPV 11 PsV with titers of 1,600 to 6,400 (Table 1).
High-risk mucosal HPV type 16 is responsible for at least half of the cervical carcinomas and their precursors. Addition of immune serum of the WHIM patient to HPV 16 PsV resulted in neutralization titers of 200 (Table 1), compared to preimmune serum which did not neutralize HPV 16 PsV (data not shown). As expected, neutralizing capacity of the immuno-competent control sera was higher with titers of 1,600-6,400. One of the immuno-competent vaccinated control persons had neutralizing anti-HPV 16 antibodies prior to vaccination, as preimmune serum neutralized HPV 16 PsV with titers of 1,600 (data not shown). After the first administration of the HPV vaccine this individuals titers were boosted to 6,400 and this titer was maintained irrespective of additional vaccinations.
High-risk HPV 18 is detected in approximately 20% of cervical dysplasias and cancers and is the predominant cause of adenocarcinoma of the cervix. HPV 18 PsV infection was not neutralized by addition of preimmune serum of either the WHIM patient nor vaccinated controls (data not shown). After completion of the vaccination schedule, neutralization of HPV 18 PsV was detected at a titer of 20 in the WHIM patient, whereas immuno-competent individuals displayed neutralization titers of 1,600 (Table 1).
3.3. HPV vaccination using Gardasil® induces a cellular immune response in WHIM syndrome
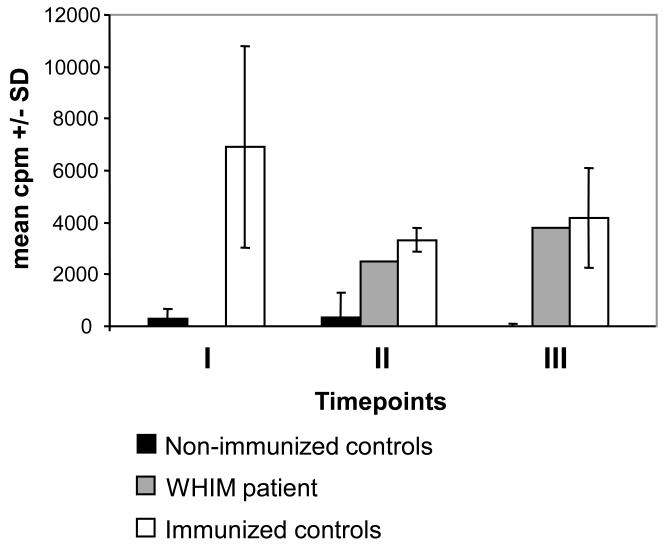
Moreover, we determined whether a cellular immune response to the HPV vaccine types is induced in the WHIM patient as compared to immuno-competent controls. PBMC were isolated and tested for proliferation in response to HPV antigens. Due to peripheral neutropenia, only limited numbers of PBMC were available from the WHIM patient for analysis. Therefore stimulation of PBMC was not possible to be performed with vaccine VLP individually, but was induced by addition of Gardasil®. PBMC were cultured in the presence or absence of Gardasil®. Unrelated BPV-1 VLP and PHA were used as negative or positive controls, respectively. Cryopreserved PBMC were tested for proliferation responses 2 months after the initial injection (timepoint I at month 2), 4 months after the second injection (timepoint II at month 6) and 2 months after the third and final immunization (timepoint III at month 8) (Figure 2). Vaccinated and non-vaccinated controls were grouped and results are shown as the mean cpm.
Fig. 2.
Lymphoproliferative response of PBMC 2 months after the initial injection (timepoint I at month 2), 4 months after the second injection (timepoint II at month 6) and 2 months after the third and final immunization (timepoint III at month 8). Vaccinated and non-vaccinated controls were grouped and results are shown as the mean cpm +/−SD. Vaccinated immuno-competents (white) showed significantly lymphoproliferative responses already 2 months after the first injection at timepoint I, whereas PBMC of non-vaccinated immuno-competent controls (black) did not proliferate in response to addition of the HPV vaccine. In the WHIM patient (grey) proliferation was detectable at timepoints II and III.
For all tested individuals including the WHIM patient, PBMC proliferation was detected in response to PHA (not shown), confirming that the proliferative response to mitogens is conserved in WHIM syndrome [2,5].
In the vaccinated immuno-competent control group significantly increased lymphoproliferative responses against Gardasil® as antigen were observed already 2 months after the first injection at timepoint I (Figure 2). Surprisingly following HPV vaccination, PBMC of the WHIM patient displayed proliferation in response to addition of the VLP vaccine. However, in contrast to immuno-competent controls lymphoproliferative responses were delayed in the WHIM patient, being detectable 4 months after having received two injections (timepoint II). In contrast, PBMC of the non-vaccinated immuno-competent controls showed no proliferation over the whole study period (data not shown).
Discussion
This study shows for the first time, that a VLP-based HPV vaccine can induce humoral and cellular immune responses in a severe primary immunodeficiency disorder, termed WHIM syndrome, which is characterized by an exceptional susceptibility to HPV infections, neutropenia, T-cell and/or B-cell lymphopenia and hypogammaglobulinemia. Female WHIM patients carry a high risk for development of HPV-associated high-grade dysplasia and carcinoma of the cervix and vulva and thus may particularly benefit from prophylactic HPV vaccination. [15]
Whereas application of live-attenuated vaccines is not feasible, inactivated or subunit vaccines such as HPV vaccine do not represent a danger to immuno-compromised persons and in general can be administered as recommended for immuno-competents [31,32,33]. However, in most B-cell disorders the application of vaccines will not result in production of specific antibodies and with ongoing therapy with immunoglobulins might be regarded as dispensable, as long as protective antibodies are present in the Ig preparation. Neutralizing HPV antibodies are not necessarily present in plasma preparations in levels sufficient to confer protection, as not every individual does seroconvert following natural HPV infection, and titers are on average 40 times lower than those following HPV vaccination.
Even if vaccines can be safely applied, immune responses in immuno-compromised patients are often diminished and robust responses might be hard to achieve. In WHIM syndrome, antibody responses to immunizations with tetanus or diphtheria toxoid have been detected in spite of lymphopenia [1,5,9]. However, evidence suggests a defect in the development or maintenance of memory B-cells, resulting in loss of detectable titers over time [5].
Papillomavirus VLP vaccines induce close to 100% seroconversion [35] and high titer neutralizing antibodies, which represent the primary effectors of protection as observed in HPV vaccine studies and experimental animal models [30,36-38]. In addition specific CD4+ and CD8+ T-cell responses have been observed after HPV vaccination in immuno-competent individuals [39-41], which may provide further help to B-cells for efficient antibody induction. The WHIM patient was able to mount HPV-specific antibody titers to the vaccine types. More importantly, the patient’s antisera were able to neutralize PsV infection in vitro, indicating that protection against HPV infection and associated disease appears achievable even in severely immuno-compromised individuals. Although antibody titers were significantly lower in comparison to the immuno-competent controls, it is unknown whether higher antibody levels correlate with longer protection.
HPV 18 causes adenocarcinoma of the cervix, which is often situated in the cervical canal where the anatomy makes detection difficult during routine (Pap) screening. Probably for that reason HPV 18 positive cancers have been reported to progress more rapidly and carry a poorer prognosis than HPV 16 positive carcinomas. In the WHIM patient the observed antibody titers against HPV 18 were very low. In HPV vaccine trials, 5 years after Gardasil® vaccination, 35% of vaccinated women have lost detectable antibodies to HPV 18 yet have remained protected against infection [38].
Additional HPV vaccination after 5 years has boosted antibody titers in immuno-competents [43], suggesting the induction of a memory B-cell response. However, in WHIM patients evidence suggests a defect in the development or maintenance of memory B-cells, in particular circulating CD27+ cells, resulting in loss of detectable antibody titers to other vaccines over time. Additional booster vaccination may represent a reasonable approach to further augment HPV antibody titers.
Cell-mediated immune responses, especially CD4+ T-cells, participate in the generation and maintenance of protective B-cell responses. Furthermore, cellular immune responses may play an adjunct role in disease prevention. Although we were limited in the amount of PBMC that could be isolated from the lymphopenic WHIM patient, lymphoproliferative responses suggested the induction of a memory response in the vaccine recipient.
In summary, immunization of a WHIM patient with the quadrivalent HPV vaccine Gardasil induces neutralizing antibodies as well as a cellular immune response, indicating the vaccine’s potential of generating protective immunity in this high-risk group of patients, and eventually in other immunodeficiency disorders.
References
- 1.Wetzler M, Talpaz M, Kleinerman ES, King A, Huh YO, Gutterman JU, et al. A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. Am J Med. 1990;89:663–672. doi: 10.1016/0002-9343(90)90187-i. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Malech HL. WHIM syndrome: congenital immune deficiency disease. Curr Opin Hematol. 2009;16:20–26. doi: 10.1097/MOH.0b013e32831ac557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34:70–74. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- 4.Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105:2449–2457. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- 5.Gulino AV, Moratto D, Sozzani S, Cavadini P, Otero K, Tassone L, et al. Altered leukocyte response to CXCL12 in patients with warts hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome. Blood. 2004;104:444–452. doi: 10.1182/blood-2003-10-3532. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Choi U, Whiting-Theobald NL, Linton GF, Brenner S, Sechler JM, et al. Enhanced function with decreased internalization of carboxy-terminus truncated CXCR4 responsible for WHIM syndrome. Exp Hematol. 2005;33:460–468. doi: 10.1016/j.exphem.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Balabanian K, Levoye A, Klemm L, Lagane B, Hermine O, Harriague J, et al. Leukocyte analysis from WHIM syndrome patients reveals a pivotal role for GRK3 in CXCR4 signaling. J Clin Invest. 2008;118:1074–1084. doi: 10.1172/JCI33187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorlin RJ, Gelb B, Diaz GA, Lofsness KG, Pittelkow MR, Fenyk JR., Jr. WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am J Med Genet. 2000;91:368–376. [PubMed] [Google Scholar]
- 9.Diaz GA, Gulino AV. WHIM syndrome: a defect in CXCR4 signaling. Curr Allergy Asthma Rep. 2005;5:350–355. doi: 10.1007/s11882-005-0005-0. [DOI] [PubMed] [Google Scholar]
- 10.Zuelzer WW. “Myelokathexis”--a New Form of Chronic Granulocytopenia. Report of a Case. N Engl J Med. 1964;270:699–704. doi: 10.1056/NEJM196404022701402. [DOI] [PubMed] [Google Scholar]
- 11.Aprikyan AA, Liles WC, Park JR, Jonas M, Chi EY, Dale DC. Myelokathexis, a congenital disorder of severe neutropenia characterized by accelerated apoptosis and defective expression of bcl-x in neutrophil precursors. Blood. 2000;95:320–327. [PubMed] [Google Scholar]
- 12.Kirnbauer R, Lenz P, Okun MM. Human Papillomavirus. In: Bolognia J, Jorizzo J, Rapini R, editors. Dermatology. Vol. 1. Mosby; London: 2008. pp. 1183–1198. [Google Scholar]
- 13.Handisurya A, Schellenbacher C, Kirnbauer R. Diseases caused by human papillomaviruses (HPV) J Dtsch Dermatol Ges. 2009;7:453–466. doi: 10.1111/j.1610-0387.2009.06988.x. quiz 466, 467. [DOI] [PubMed] [Google Scholar]
- 14.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 15.Tarzi MD, Jenner M, Hattotuwa K, Faruqi AZ, Diaz GA, Longhurst HJ. Sporadic case of warts, hypogammaglobulinemia, immunodeficiency, and myelokathexis syndrome. J Allergy Clin Immunol. 2005;116:1101–1105. doi: 10.1016/j.jaci.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 16.Schiller JT, Castellsague X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine. 2008;26(Suppl 10):K53–61. doi: 10.1016/j.vaccine.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J Clin Invest. 2006;116:1167–1173. doi: 10.1172/JCI28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn JA. HPV vaccination for the prevention of cervical intraepithelial neoplasia. N Engl J Med. 2009;361:271–278. doi: 10.1056/NEJMct0806938. [DOI] [PubMed] [Google Scholar]
- 20.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459–1466. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rose RC, Bonnez W, Da Rin C, McCance DJ, Reichman RC. Serological differentiation of human papillomavirus types 11, 16 and 18 using recombinant virus-like particles. J Gen Virol. 1994;75( Pt 9):2445–2449. doi: 10.1099/0022-1317-75-9-2445. [DOI] [PubMed] [Google Scholar]
- 22.Kirnbauer R, Taub J, Greenstone H, Roden R, Durst M, Gissmann L, et al. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J Virol. 1993;67:6929–6936. doi: 10.1128/jvi.67.12.6929-6936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Handisurya A, Gambhira R, Schellenbacher C, Shafti-Keramat S, Forslund O, Favre M, et al. Serological relationship between cutaneous human papillomavirus types 5, 8 and 92. J Gen Virol. 2009;90:136–143. doi: 10.1099/vir.0.006189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamora E, Handisurya A, Shafti-Keramat S, Borchelt D, Rudow G, Conant K, et al. Papillomavirus-like particles are an effective platform for amyloid-beta immunization in rabbits and transgenic mice. J Immunol. 2006;177:2662–2670. doi: 10.4049/jimmunol.177.4.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirnbauer R, Hubbert NL, Wheeler CM, Becker TM, Lowy DR, Schiller JT. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J Natl Cancer Inst. 1994;86:494–499. doi: 10.1093/jnci/86.7.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78:751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med. 2005;119:445–462. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- 28.Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 29.Schellenbacher C, Roden R, Kirnbauer R. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. J Virol. 2009;83:10085–10095. doi: 10.1128/JVI.01088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, et al. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroger AT, Atkinson WL, Marcuse EK, Pickering LK. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–48. [PubMed] [Google Scholar]
- 32.Davis LE, Bodian D, Price D, Butler IJ, Vickers JH. Chronic progressive poliomyelitis secondary to vaccination of an immunodeficient child. N Engl J Med. 1977;297:241–245. doi: 10.1056/NEJM197708042970503. [DOI] [PubMed] [Google Scholar]
- 33.Sankano T, Kittaka E, Tanaka Y, Yamaoka H, Kobayashi Y, Usui T. Vaccine-associated poliomyelitis in an infant with agammaglobulinemia. Acta Paediatr Scand. 1980;69:549–551. doi: 10.1111/j.1651-2227.1980.tb07131.x. [DOI] [PubMed] [Google Scholar]
- 34.Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. Jama. 2007;297:813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 35.Schiller JT, Lowy DR. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. J Infect Dis. 2009;200:166–171. doi: 10.1086/599988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 37.Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res (Phila Pa) 2009;2:868–878. doi: 10.1158/1940-6207.CAPR-09-0031. [DOI] [PubMed] [Google Scholar]
- 38.Joura EA, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine. 2008;26:6844–6851. doi: 10.1016/j.vaccine.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 39.Emeny RT, Wheeler CM, Jansen KU, Hunt WC, Fu TM, Smith JF, et al. Priming of human papillomavirus type 11-specific humoral and cellular immune responses in college-aged women with a virus-like particle vaccine. J Virol. 2002;76:7832–7842. doi: 10.1128/JVI.76.15.7832-7842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans TG, Bonnez W, Rose RC, Koenig S, Demeter L, Suzich JA, et al. A Phase 1 study of a recombinant viruslike particle vaccine against human papillomavirus type 11 in healthy adult volunteers. J Infect Dis. 2001;183:1485–1493. doi: 10.1086/320190. [DOI] [PubMed] [Google Scholar]
- 41.Pinto LA, Edwards J, Castle PE, Harro CD, Lowy DR, Schiller JT, et al. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J Infect Dis. 2003;188:327–338. doi: 10.1086/376505. [DOI] [PubMed] [Google Scholar]
- 42.Fraser C, Tomassini JE, Xi L, Golm G, Watson M, Giuliano AR, et al. Modeling the long-term antibody response of a human papillomavirus (HPV) viruslike particle (VLP) type 16 prophylactic vaccine. Vaccine. 2007;25:4324–4333. doi: 10.1016/j.vaccine.2007.02.069. [DOI] [PubMed] [Google Scholar]
- 43.Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007;25:4931–4939. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]