Abstract
Recently, mutations in ZFHX1B, the gene that encodes Smad-interacting protein-1 (SIP1), were found to be implicated in the etiology of a dominant form of Hirschsprung disease–mental retardation syndrome in humans. To clarify the molecular mechanisms underlying the clinical features of SIP1 deficiency, we generated mice that bear a mutation comparable to those found in several human patients. Here, we show that Zfhx1b-knockout mice do not develop postotic vagal neural crest cells, the precursors of the enteric nervous system that is affected in patients with Hirschsprung disease, and they display a delamination arrest of cranial neural crest cells, which form the skeletomuscular elements of the vertebrate head. This suggests that Sip1 is essential for the development of vagal neural crest precursors and the migratory behavior of cranial neural crest in the mouse. Furthermore, we show that Sip1 is involved in the specification of neuroepithelium.
Hirschsprung disease (aganglionic megacolon) is the most common (1/4,500 live births) human congenital neurocristopathy affecting the innervation of the gut. Heterozygous deletions or mutations in ZFHX1B (GenBank accession number NM_015753, for the mouse homologue), which encodes Smad-interacting protein-1 (SIP1), are implicated in the etiology of Hirschsprung disease–mental retardation syndrome (MIM 235730) (Amiel et al. 2001; Cacheux et al. 2001; Wakamatsu et al. 2001; Yamada et al. 2001; Zweier et al. 2002), a syndromic form of Hirschsprung disease associated with multiple congenital anomalies, such as mental retardation, facial dysmorphology, and heart disease. SIP1, like δEF1 (Funahashi et al. 1993), is a zinc finger and homeodomain containing transcription factor that exerts a repressive activity by binding by binding to CACCT sequences in regulatory elements of candidate target genes (Remacle et al. 1999; Verschueren et al. 1999) (fig. 1A). Accordingly, the E-cadherin gene has recently been shown to be a direct target of SIP1, at least in cultured epithelial cells (Comijn et al. 2001). To study, in an animal model system, the role of ZFHX1B in the etiology of the anomalies in patients with Hirschsprung disease–mental retardation syndrome, we generated mice deficient in Sip1 (Higashi et al. 2002).
Figure 1.
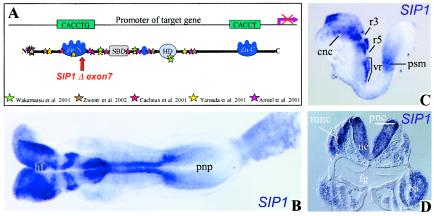
Expression of Sip1 mRNA in the mouse embryo at E8.5. A, The proposed model for transcriptional repression of target genes by SIP1 whereby each target CACCT(G) sequence in a spaced bipartite binding site would be bound by one zinc finger cluster of the repressor monomer (Remacle et al. 1999). The mutations in human ZFHX1B that are so far identified as causes of Hirschsprung disease–mental retardation syndrome are represented by colored stars and are compared with the mouse Zfhx1b Δexon 7 mutation described here (Higashi et al. 2002). B, Dorsal view, at E8.5, of an embryo hybridized with the Sip1 probe (NM_015753) (bp 335–2950), according to the procedure of Wilkinson (1992). Sip1 mRNA is expressed in the neuroepithelium of headfolds, neural tube, and, to a lesser extent, in the prospective neural plate. C, Lateral view of an embryo at E8.5. High levels of Sip1 mRNA are observed in premigratory and migrating neural crest cells of cranial and postotic vagal origin. Cranial neural crest cells show a segmentally restricted pattern, with absence of Sip1 transcripts in r3 and r5, known to be depleted from neural crest cells (Smith and Graham 2001). A distinct domain of Sip1 expression is seen at the anterior end of the presomitic mesoderm. D, Transverse section of a Sip1 in situ hybridization at the level of the first branchial arch. Note expression in the neuroepithelium, premigratory and migrating neural crest cells, and the branchial arches. ba = branchial arches; cnc = cranial neural crest cells; fg = foregut; HD = homeodomain; hf = ; ne = neuroepithelium; pnc = premigratory neural crest; pnp = prospective neural plate; psm = presomitic mesoderm; SBD = Smad-binding domain; vr = vagal region; Zn-C = C-terminal zinc finger cluster C; Zn-N = N-terminal zinc finger.
At embryonic day (E) 8.5, two major populations of cells express Sip1 mRNA. One is the neuroepithelium, where expression levels closely follow the maturation of the neural plate (fig. 1B and 1D). Sip1 transcripts are lacking from rhombomeres 3 (r3) and 5 (r5), as analyzed by double in situ hybridization with the r3- and r5-specific transcription factor Krox20 (data not shown). Another embryonic tissue expressing Sip1 mRNA is the neural crest. High levels of Sip1 transcripts are seen in premigratory and migrating neural crest cells of cranial (fig. 1C and 1D) and postotic vagal origin (fig. 1C) and in the branchial arch mesenchyme (fig. 1D).
Constitutive deletion of exon 7 from Zfhx1b generates a null allele in the mouse and is conceptually comparable to nearly all mutations found in human patients in whom frame-shift mutations lead to truncation of the protein (see fig. 1A) (Higashi et al. 2002). Homozygous mutant embryos exhibited defects from E8.5 on. The neural tube failed to close, a sharp boundary between the neural plate and the rest of the ectoderm was absent, and the first branchial arch was missing (fig. 2A and 2B). Homozygous mutant embryos were severely retarded in their growth by E9.5, did not undergo embryonic turning, and then died.
Figure 2.
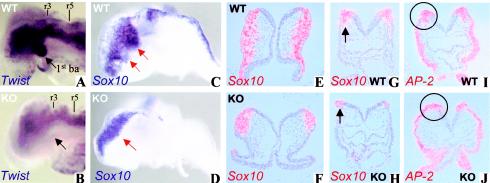
Analysis of cranial neural crest cells in wild-type and Zfhx1b-knockout embryos. A and B, Lateral view of the head region of E8.5 embryos hybridized with the Twist probe (Fuchtbauer 1995). Note the reduced Twist mRNA expression in the head mesenchyme and the absence of the first branchial arch (black arrow) in the knockout embryos (B). C and D, Sox10 mRNA expression at E8.5 (lateral view, probe [MMU66141]; bp 1194–2064). In wild-type embryos, Sox10-expressing trigeminal or facial neural crest cells are migrating (red arrow) from the brain plate toward the maxillary component of the first and second branchial arches, respectively, whereas in the knockout embryo the Sox10-positive cranial neural crest cells do not delaminate at the junctional zone between the neural and surface ectoderm. E–H, Radioactive in situ hybridization (according to Dewulf et al. [1995]) for Sox10 in transverse sections taken at the level of the headfolds (E and F) and the heart (at r4) (G and H). The sections show Sox10-positive crest cells that initiated migration in the morphologically wild-type embryos (black arrow in G). In the knockout embryos, Sox10-positive neural crest cells do not delaminate nor do they initiate migration (black arrow in H). I and J, Radioactive in situ hybridization for AP-2 (Zhang et al. 1996) on sections of the midbrain. In wild-type embryos, AP-2 is expressed in ectoderm and in neural crest cells migrating from the cranial neural fold (circle in I). In the Zfhx1b-knockout littermates, AP-2-positive migrating neural crest cells are never seen in this area (circle in J). ba = branchial arches; cnc = cranial neural crest; KO = knockout; WT = wild type.
Neural crest cells emerge at the dorsal part of the closing neural plate, and their developmental potential and fate have initially been studied intensively by using quail neural crest transplantation into chick embryos. On the basis of their area of origin along the anteroposterior axis and the structure to which these cells give rise, four functional segments are often discriminated in avian embryos, and this division has been extrapolated to mouse embryos. The cranial or cephalic segment extends from the diencephalon to the first somite and gives rise to craniofacial mesenchyme that forms the cartilage, bone, cranial neurons, glia, and connective tissues of the face. Cells from this segment also enter the pharyngeal arches to give rise to thymic cells, odontoblasts of the tooth primordia, and the cartilage of the inner ear and jaw. The vagal neural crest cells originate, in the mouse embryo, at the level of somites 1–7 and form the muscle and connective tissue of the large arteries and the heart septum and, together with neural crest cells from the sacral segment (which is located posterior to somite 28), the parasympathetic (enteric) ganglia of the gut. The trunk neural crest cells (at the level of somites 8–28) give rise to the sensory and sympathetic ganglia, Schwann cells, the adrenal medulla, aortic nerve clusters, and the melanocytes of the skin.
Visualization of neural crest cells at E8.5, by use of Sox10 in situ hybridization, showed that in normal embryos the cranial neural crest cells emigrated from the neural ridge and followed ventrolateral routes (fig. 2C, 2E, and 2G). In homozygous Zfhx1b mutants, the cranial crest cells did form, but the cells remained clustered at the junctional zone between the neural and surface ectoderm, without significant migration (fig. 2D, 2F, and 2H). This early arrest in cranial neural crest cell migration was confirmed by use of the cranial crest marker gene AP-2 (fig. 2I and 2J) and the neural crest cell marker Hfh2 (Labosky and Kaestner 1998) (data not shown). Thus, we exclude a direct transcriptional effect of Sip1 on Sox10 in migrating neural crest cells, the latter gene being implicated in Hirschsprung disease as well (Pingault et al. 1998). Accordingly, a strong reduction of the Twist-labeled neural crest population, which is abundant in the first branchial arch region (fig. 2A and 2B), was seen in the mutant embryos.
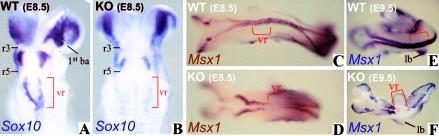
Another significant defect of the homozygous mutant mouse embryos was the lack of Sox10-expressing neural crest cells at the postotic vagal level (fig. 3A and 3B). The loss of the vagal level neural crest was also confirmed by the lack of Msx1 expression from this region in homozygous mutant mice (fig. 3C–3F). Msx1 is expressed in neural crest cells (Houzelstein et al. 1997) and is indicative of functional bone morphogenetic protein signaling, which has been shown elsewhere to be important in the production of neural crest cells (Liem et al. 1995).
Figure 3.
Vagal neural crest cell development in wild-type and Zfhx1b-knockout embryos. A and B, Dorsal view of the head region of E8.5 embryos hybridized for Sox10. Sox10 expression is lost from the postotic vagal region in the Zfhx1b-knockout embryos (B). C–F, Dorsal view of wild-type and Zfhx1b-knockout embryos hybridized with the Msx1 probe (Hill et al. 1989). Msx1 is expressed in cranial and trunk neural crest precursors and tail bud. Zfhx1b-knockout embryos are devoid of Msx1 expression in the postotic vagal region. ba = branchial arches; KO = knockout; lb = limb bud; vr = vagal region; WT = wild type.
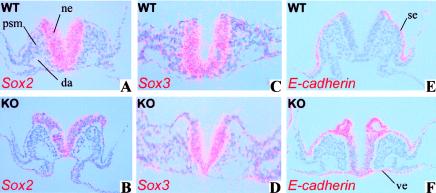
Since the neural tube never closes in the homozygous mutant mouse embryos and since neural crest cell development depends on interaction between neural plate and ectoderm (Selleck and Bronner-Fraser 1995), it is possible that the neural plate or the neuroepithelium is affected in mutant embryos, which may result in the impaired development of neural crest cells. Hence, we investigated the authenticity of the neuroepithelium of homozygous mutant embryos, using a number of marker genes. One of these, Sox2, which is an early neural ectoderm marker known to be involved in early neurogenesis (Rex et al. 1997; Kishi et al. 2000), was severely reduced in homozygous mutant embryos at E8.5 (fig. 4A and 4B). Sox3 expression was unaffected (fig. 4C and 4D). E-cadherin gene expression, which is normally down-regulated when the neuroepithelium differentiates from the ectoderm (Hatta and Takeichi 1986), persisted in the homozygous mutant neuroepithelium (fig. 4E and 4F). These observations suggest that the neural plate or neuroepithelium of homozygous mutant mice is already affected prior to dorsal closure or formation of neural crest cells. This failure of generation (or maintenance) of intact neural ectoderm may interfere with the formation of the neural crest cells, as well as with the separation of the neural tube from the surface ectoderm. The unprecedented finding that in vivo E-cadherin gene expression is not appropriately repressed in the neuroepithelium by candidate repressors (i.e., Sip1, in this case) in these mutant Zfhx1b-knockout mice is consistent with the proposed role of SIP1 in active repression of E-cadherin, which was based on DNA-binding studies of SIP1 and documentation of SIP1-induced loss of E-cadherin in cultured epithelial cells (Remacle et al. 1999; Comijn et al. 2001).
Figure 4.
Altered expression of Sox2 and E-cadherin in homozygous Zfhx1b-knockout embryos at E8.5. A and B, Radioactive in situ hybridization in transverse sections for Sox2 (Wood and Episkopou 1999). Sox2 is significantly down-regulated in the neural epithelium of knockout embryos, compared with wild-type littermates. The sections shown are at the level of the presomitic mesoderm. C and D, Sox3 expression (Wood and Episkopou 1999) is unaffected in the Zfhx1b-knockout embryos. E and F, E-cadherin (Shimamura et al. 1994) is expressed in the neural ectoderm of Zfhx1b-knockout embryos, although it is turned off in the neuroepithelium of wild-type embryos. The ectopic E-cadherin expression also occurs in the visceral endoderm, which normally expresses Sip1 (data not shown). Knockout embryos often display tubules or small spheres, which may reflect epithelial blisters at the border between the surface ectoderm and the neuroepithelium. da = dorsal aorta; KO = knockout; ne = neuroepithelium; psm = presomitic mesoderm; se = surface ectoderm; ve = visceral endoderm; vr = vagal region; WT = wild type.
Heterozygous mutant animals (in the predominantly CD1 genetic background used in the present study) so far do not develop aganglionic phenotypes analogous to those in the majority of patients with Hirschsprung disease–mental retardation syndrome, although the latter syndrome is caused by haploinsufficiency of ZFHX1B. This lack of phenotype is consistent with other mouse models for Hirschsprung disease–causing genes that, unlike in humans, fail to give an aganglionic phenotype when heterozygous (Wartiovaara et al. 1998). Nonetheless, the embryonic phenotype of homozygous Sip1 mutant embryos, as documented here, turns out to be instrumental to the clarification of many aspects of the Hirschsprung disease–mental retardation syndrome. The complete lack of vagal neural crest precursor cells in homozygous Sip1 mutant embryos reflects the dependence of these precursors on Sip1 activity for their normal development. It is tempting to speculate that SIP1 haploinsufficiency in humans leads to the generation of far fewer vagal neural crest cell precursors and, as a consequence, to varying degrees of failure to fully colonize the gastrointestinal tract from its anterior end. Developmental defects in the neural crest from this region may explain the high frequency of heart defects in patients with Hirschsprung disease–mental retardation syndrome (Amiel et al. 2001; Yamada et al. 2001). Hirschsprung disease–mental retardation syndrome is not always associated with an aganglionic colon phenotype (Yamada et al. 2001). The discrepancy between our observation showing the complete lack of vagal neural crest precursors in homozygous Zfhx1b Δexon 7 CD1 mice and the incomplete penetrance of the megacolon phenotype associated with ZFHX1B haploinsufficiency in humans may be due to the nature or size of the gene deletion in the individual patients. Also, the variability of (and resistance to) some clinical consequences of SIP1 haploinsufficiency observed in human patients may depend on nonallelic association with low-penetrance modifier alleles that might contribute to the modification of the expression or function of ZFHX1B or on noncoding polymorphisms in the human population (e.g., in the regulatory elements of ZFHX1B or of direct targets of SIP1). To dissect the genetic determinants that contribute to the variable penetrance and expressivity of the aganglionosis observed in patients with Hirschsprung disease, it may be interesting, in future studies, to analyze the heterozygous Zfhx1b mouse mutation on genetic backgrounds with an increased susceptibility to aganglionosis (Southard-Smith et al. 1999).
Another important aspect of the Zfhx1b-knockout mice is the attenuation of the migration of cranial neural crest cells. Expression of the α4-integrin gene (Pinco et al. 2001), a putative target gene for proteins of the ZFHX1 family (Postigo et al. 1997; Remacle et al. 1999), was not affected in the mutant neural crest cells (data not shown). It remains to be determined whether the dysregulation of E-cadherin gene expression is also involved in this migration defect. Cranial neural crest forms craniofacial cartilage, bone, and facial connective tissue. Therefore, this migration defect may provide additional clues to the etiology of the distinctive facial features and wide nasal bridge routinely observed in patients with Hirschsprung disease–mental retardation syndrome. Finally, the aberrant regulation of E-cadherin gene expression in the neural ectoderm and the decreased expression of the neural transcription factor Sox2 may be relevant to neurological problems in patients with Hirschsprung disease–mental retardation syndrome, because these genes, as well as SIP1 (Yamada et al. 2001), are also highly expressed in the adult CNS (Gure et al. 2000) and because E-cadherin mRNA is known to have a specific architectural expression pattern in the brain (Shimamura and Takeichi 1992).
Acknowledgments
We thank the “Celgen Mouse” group and Marc Missoul, for their help; An Zwijsen, for expert discussion; and Herman Van den Berghe, for uninterrupted encouragement since the start of our laboratory and the expansion of its major interest in developmental biology to the field of human genetics. We also thank R. Behringer, K. Kaestner, R. Lovell-Badge, B. Robert, M. Takeichi, T. Williams, and J. Yang for supplying probes. This work was supported by the Japanese Ministry of Education, Culture, Sports, Science, and Technology; the Japan Society for the Promotion of Science; Flanders Interuniversity Institute for Biotechnology (VIB7); Fund for Scientific Research–Flanders (Belgium) grant G.0105.02; and University of Leuven grant 0T/00/41.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for Hirschsprung disease–mental retardation syndrome [MIM 235730])
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for Mus musculus zinc finger homeobox 1b [Zfhx1b] [accession number NM_015753] and Mus musculus transcription factor Sox-M [accession number MMU66141])
References
- Amiel J, Espinosa-Parrilla Y, Steffann J, Gosset P, Pelet A, Prieur M, Boute O, Choiset A, Lacombe D, Philip N, Le Merrer M, Tanaka H, Till M, Touraine R, Toutain A, Vekemans M, Munnich A, Lyonnet S (2001) Large-scale deletions and SMADIP1 truncating mutations in syndromic Hirschsprung disease with involvement of midline structures. Am J Hum Genet 69:1370–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacheux V, Dastot-Le Moal F, Kaariainen H, Bondurand N, Rintala R, Boissier B, Wilson M, Mowat D, Goossens M (2001) Loss-of-function mutations in SIP1 Smad interacting protein 1 result in a syndromic Hirschsprung disease. Hum Mol Genet 10:1503–1510 [DOI] [PubMed] [Google Scholar]
- Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, Van Roy F (2001) The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell 7:1267–1278 [DOI] [PubMed] [Google Scholar]
- Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, ten Dijke P (1995) Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology 136:2652–2663 [DOI] [PubMed] [Google Scholar]
- Fuchtbauer EM (1995) Expression of M-twist during postimplantation development of the mouse. Dev Dyn 204:316–322 [DOI] [PubMed] [Google Scholar]
- Funahashi J, Sekido R, Murai K, Kamachi Y, Kondoh H (1993) δ-crystallin enhancer binding protein δEF1 is a zinc finger–homeodomain protein implicated in postgastrulation embryogenesis. Development 119:433–446 [DOI] [PubMed] [Google Scholar]
- Gure AO, Stockert E, Scanlan MJ, Keresztes RS, Jager D, Altorki NK, Old LJ, Chen YT (2000) Serological identification of embryonic neural proteins as highly immunogenic tumor antigens in small cell lung cancer. Proc Natl Acad Sci USA 97:4198–4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K, Takeichi M (1986) Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature 320:447–449 [DOI] [PubMed] [Google Scholar]
- Higashi Y, Maruhashi M, Nelles L, Van De Putte T, Verschueren K, Miyoshi T, Yoshimoto A, Kondoh H, Huylebroeck D (2002) Generation of the floxed allele of the SIP1 (Smad-interacting protein 1) gene for Cre-mediated conditional knockout in the mouse. Genesis 32:82–84 [DOI] [PubMed] [Google Scholar]
- Hill RE, Jones PF, Rees AR, Sime CM, Justice MJ, Copeland NG, Jenkins NA, Graham E, Davidson DR (1989) A new family of mouse homeobox-containing genes: molecular structure, chromosomal location, and developmental expression of Hox-71. Genes Dev 3:26–37 [DOI] [PubMed] [Google Scholar]
- Houzelstein D, Cohen A, Buckingham ME, Robert B (1997) Insertional mutation of the mouse Msx1 homeobox gene by an nlacZ reporter gene. Mech Dev 65:123–133 [DOI] [PubMed] [Google Scholar]
- Kishi M, Mizuseki K, Sasai N, Yamazaki H, Shiota K, Nakanishi S, Sasai Y (2000) Requirement of Sox2-mediated signaling for differentiation of early Xenopus neuroectoderm. Development 127:791–800 [DOI] [PubMed] [Google Scholar]
- Labosky PA, Kaestner KH (1998) The winged helix transcription factor Hfh2 is expressed in neural crest and spinal cord during mouse development. Mech Dev 76:185–190 [DOI] [PubMed] [Google Scholar]
- Liem KF Jr, Tremml G, Roelink H, Jessell TM (1995) Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell 82:969–979 [DOI] [PubMed] [Google Scholar]
- Pinco KA, Liu S, Yang JT (2001) α4 integrin is expressed in a subset of cranial neural crest cells and in epicardial progenitor cells during early mouse development. Mech Dev 100:99–103 [DOI] [PubMed] [Google Scholar]
- Pingault V, Bondurand N, Kuhlbrodt K, Goerich DE, Prehu MO, Puliti A, Herbarth B, Hermans-Borgmeyer I, Legius E, Matthijs G, Amiel J, Lyonnet S, Ceccherini I, Romeo G, Smith JC, Read AP, Wegner M, Goossens M (1998) SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat Genet 18:171–173 [DOI] [PubMed] [Google Scholar]
- Postigo AA, Sheppard AM, Mucenski ML, Dean DC (1997) c-Myb and Ets proteins synergize to overcome transcriptional repression by ZEB. EMBO J 16:3924–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacle JE, Kraft H, Lerchner W, Wuytens G, Collart C, Verschueren K, Smith JC, Huylebroeck D (1999) New mode of DNA binding of multi-zinc finger transcription factors: δEF1 family members bind with two hands to two target sites. EMBO J 18:5073–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex M, Orme A, Uwanogho D, Tointon K, Wigmore PM, Sharpe PT, Scotting PJ (1997) Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Dev Dyn 209:323–332 [DOI] [PubMed] [Google Scholar]
- Selleck MA, Bronner-Fraser M (1995) Origins of the avian neural crest: the role of neural plate-epidermal interactions. Development 121:525–538 [DOI] [PubMed] [Google Scholar]
- Shimamura K, Hirano S, McMahon AP, Takeichi M (1994) Wnt-1–dependent regulation of local E-cadherin and α N-catenin expression in the embryonic mouse brain. Development 120:2225–2234 [DOI] [PubMed] [Google Scholar]
- Shimamura K, Takeichi M (1992) Local and transient expression of E-cadherin involved in mouse embryonic brain morphogenesis. Development 116:1011–1019 [DOI] [PubMed] [Google Scholar]
- Smith A, Graham A (2001) Restricting Bmp-4 mediated apoptosis in hindbrain neural crest. Dev Dyn 220:276–283 [DOI] [PubMed] [Google Scholar]
- Southard-Smith EM, Angrist M, Ellison JS, Agarwala R, Baxevanis AD, Chakravarti A, Pavan WJ (1999) The Sox10(Dom) mouse: modeling the genetic variation of Waardenburg-Shah (WS4) syndrome. Genome Res 9:215–225 [PubMed] [Google Scholar]
- Verschueren K, Remacle JE, Collart C, Kraft H, Baker BS, Tylzanowski P, Nelles L, Wuytens G, Su MT, Bodmer R, Smith JC, Huylebroeck D (1999) SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5′-CACCT sequences in candidate target genes. J Biol Chem 274:20489–20498 [DOI] [PubMed] [Google Scholar]
- Wakamatsu N, Yamada Y, Yamada K, Ono T, Nomura N, Taniguchi H, Kitoh H, Mutoh N, Yamanaka T, Mushiake K, Kato K, Sonta S, Nagaya M (2001) Mutations in SIP1, encoding Smad interacting protein-1, cause a form of Hirschsprung disease. Nat Genet 27:369–370 [DOI] [PubMed] [Google Scholar]
- Wartiovaara K, Salo M, Sariola H (1998) Hirschsprung's disease genes and the development of the enteric nervous system. Ann Med 30:66–74 [DOI] [PubMed] [Google Scholar]
- Wilkinson DG (1992) Whole mount in-situ hybridization of vertebrate embryos. In: Wilkinson DG (ed) In situ hybridization: a practical approach. IRL Press, Oxford, pp 75–84 [Google Scholar]
- Wood HB, Episkopou V (1999) Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre-gastrulation to early somite stages. Mech Dev 86:197–201 [DOI] [PubMed] [Google Scholar]
- Yamada K, Yamada Y, Nomura N, Miura K, Wakako R, Hayakawa C, Matsumoto A, Kumagai T, Yoshimura I, Miyazaki S, Kato K, Sonta S, Ono H, Yamanaka T, Nagaya M, Wakamatsu N (2001) Nonsense and frameshift mutations in ZFHX1B, encoding Smad-interacting protein 1, cause a complex developmental disorder with a great variety of clinical features. Am J Hum Genet 69:1178–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hagopian-Donaldson S, Serbedzija G, Elsemore J, Plehn-Dujowich D, McMahon AP, Flavell RA, Williams T (1996) Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature 381:238–241 [DOI] [PubMed] [Google Scholar]
- Zweier C, Albrecht B, Mitulla B, Behrens R, Beese M, Gillessen-Kaesbach G, Rott HD, Rauch A (2002) “Mowat-Wilson” syndrome with and without Hirschsprung disease is a distinct, recognizable multiple congenital anomalies-mental retardation syndrome caused by mutations in the zinc finger homeobox 1B gene. Am J Med Genet 108:177–181 [PubMed] [Google Scholar]