Abstract
Genetic and biochemical evidence has established that a SNARE complex consisting of syntaxin 5 (Sed5)-mYkt6 (Ykt6)-GOS28 (Gos1)-GS15 (Sft1) is required for transport of proteins across the Golgi stack in animals (yeast). We have utilized quantitative immunogold labeling to establish the cis-trans distribution of the v-SNARE GS15 and the t-SNARE subunits GOS28 and syntaxin 5. Whereas the distribution of the t-SNARE is nearly even across the Golgi stack from the cis to the trans side, the v-SNARE GS15 is present in a gradient of increasing concentration toward the trans face of the stack. This contrasts with a second distinct SNARE complex, also required for intra-Golgi transport, consisting of syntaxin 5 (Sed5)-membrin (Bos1)-ERS24 (Sec22)-rBet1 (Bet1), whose v-(rBet1) and t-SNARE subunits (membrin and ERS24), progressively decrease in concentration toward the trans face. Transport within the stack therefore appears to utilize countercurrent gradients of two Golgi SNAREpins and may involve a mechanism akin to homotypic fusion.
INTRODUCTION
Intracellular membrane fusion results from the assembly of a target membrane t-SNARE, composed of one heavy chain (syntaxin) and two distinct light chains, with a cognate vesicle membrane v-SNARE (Sollner et al., 1993; Weber et al., 1998; Hu et al., 2003). The resulting SNARE-pin, held together by a bundle of four α-helices (Sutton et al., 1998), forcefully perturbs the apposing lipid bilayers, thereby triggering fusion (McNew et al., 2000b). Cognate SNARE pairing between bilayers is the immediate determinant of the specificity of membrane fusion (Fukuda et al., 2000; McNew et al., 2000a; Parlati et al., 2000, 2002; Paumet et al., 2001). The v-SNARE is functionally distinguished from the other subunits of the SNAREpin because fusion can only occur when the v-SNARE resides in the opposite bilayer from the remaining subunits, which comprise the t-SNARE (Fukuda et al., 2000).
In particular, fusion of vesicles derived from the ER with the Golgi requires the pairing of the v-SNARE Bet1 (in yeast; rBet1 in animals) with the t-SNARE comprised of the syntaxin heavy chain Sed5 (syntaxin 5) and light chains Bos1 (membrin) and Sec22 (ERS24, also named Sec22b; Parlati et al., 2000), abbreviated t-Sed5/Bos1, Sec22. The genes encoding these four “ER-Golgi” SNAREs are required for ER-to-Golgi transport in yeast in vivo (Newman and Ferro-Novick, 1987; Newman et al., 1990; Shim et al., 1991; Hardwick and Pelham, 1992; Cao and Barlowe, 2000). Liposomes bearing the v-SNARE Bet1 only fuse with liposomes bearing the cognate t-SNARE Sed5/Bos1, Sec22 (Parlati et al., 2000, 2002; Paumet et al., 2001).
The intracellular distribution of these SNAREs determined by immunoelectron microscopy fits well with their roles in ER-to-Golgi transport in animal cells (Paek et al., 1997; Hay et al., 1998; Orci et al., 2000; Martinez-Menarguez et al., 2001). The “short” Golgi-localized form of the t-SNARE heavy chain syntaxin 5 (Hui et al., 1997), which lacks the ER-retrieval signal encoded in the additional exon of the “long” form, is located in every cisternae across the Golgi stack including the trans-Golgi network (TGN). By contrast, the t-SNARE light chains membrin, ERS24 and the v-SNARE rBet1 are located in the intermediate compartment (IC) and largely within the first two cisternae of the Golgi stack on the cis side. The presence of syntaxin 5 throughout the stack, well beyond its light chains membrin and ERS24 suggested that it could play a dual role, also serving as the heavy chain of a second, distinct intra-Golgi t-SNARE within later cisternae. Consistent with this, syntaxin 5/Sed5 coimmunoprecipitates with several additional SNAREs in extracts from animal and yeast cells (Sogaard et al., 1994; Banfield et al., 1995; Fischer von Mollard et al., 1997; Hay et al., 1997, 1998; Lupashin et al., 1997; Xu et al., 1998, 2002; Zhang and Hong, 2001; Parlati et al., 2002; Shorter et al., 2002).
Recently, this postulated second tetrameric fusogenic Sed5/syntaxin 5–based SNARE complex has been established (Parlati et al., 2002). The t-SNARE consists of Sed5 (syntaxin 5) as the heavy chain together with Ykt6 (mYkt6) and Gos1 (GOS28) as the light chains. The cognate v-SNARE is Sft1 (GS15). The two Golgi SNAREpins are completely distinct functionally, as v-Sft1 will only fuse with t-Sed5/Ykt6, Gos1 and not with t-Sed5/Bos1, Sec22 and v-Bet1 will only fuse with t-Sed5/Bos1, Secc22 and not with t-Sed5/Ykt6, Gos1 (Parlati et al., 2002).
Where within the Golgi stack does this novel t-SNARE (Sed5/Ykt6, Gos1) and its cognate v-SNARE (Sft1) reside? Which of these SNAREs are enriched in peri-Golgi vesicles compared with the cisternae? What are the relative molar abundances of Golgi SNAREs? Answering these questions is central to understanding how proteins flow in the Golgi stack because the pattern of localization and abundance of these SNARE proteins will pinpoint the fusion events that are required for transport of cargo within the Golgi stack.
MATERIALS AND METHODS
Antibodies
Monoclonal antibodies against GOS28, GS15, and GM130 were from Transduction Laboratories (Lexington, KY) and against membrin and rBet1 (clone 16G6) from StressGen Biotechnology (Victoria, British Columbia, Canada). Monoclonal anti-mannosidase II and anti-TGN38 antibodies were purchased from Covance Inc. (Richmond, CA) and BD Biosciences (San Diego, CA), respectively. Rabbit polyclonal antibodies against membrin and GS15 were obtained from Dr. F. Wieland (Biochemie-Zentrum, Heidelberg) and Dr. W. Hong (Institute of Molecular and Cell Biology, Singapore), respectively. A goat anti-Ykt6 antibody was generated commercially (Covance Inc.) to his6-tagged human Ykt6 according to the company's standard protocol. An anti-GS15 peptide antibody was generated commercially (SynPep Inc., Dublin, CA) by immunizing rabbits with a GS15 peptide (aa27–41) coupled through a C-terminal cysteine to KLH. These polyclonal antibodies were affinity-purified by Sulfolink (Pierce Inc., Rockford, IL) columns coupled with either GS15 peptide (aa27–41+cysteine) or his6-tagged human Ykt6. Purified antibodies were eluted with 0.1 M glycine, pH 2.8, and neutralized with 2 M Tris-Cl, pH 8.0. Rabbit polyclonal antibodies against GOS28, ERS24, and Syntaxin 5 were generated and affinity-purified as described previously (Nagahama et al., 1996; Paek et al., 1997; Orci et al., 2000). Rabbit polyclonal antibodies against rBet1 and Vti1b were generated by immunizing rabbits with recombinant rBet1 (aa1-95) and Vti1b (aa1–206). These antibodies were affinity-purified as described previously for our other SNARE antibodies (Nagahama et al., 1996; Paek et al., 1997; Orci et al., 2000).
Secondary antibodies were from Molecular Probes (Eugene, OR; Alexa Fluor 488 goat anti-rabbit and Alexa Fluor 546 goat anti-mouse), Jackson ImmunoResearch (West Grove, PA; FITC and Cy5 donkey anti-rabbit and anti-mouse) and British BioCell International (Cardiff, United Kingdom; gold-conjugated goat anti-mouse and goat anti-rabbit IgG). Whole rabbit IgG was purchased from Sigma (St. Louis, MO). Fab fragments were prepared using an ImmunoPure Fab Kit (Pierce Inc.) according to the manufacturer's instructions. The Fab fragments were then dialyzed against (25 mM Tris-Cl, pH 7.4; 25 mM KCl, 10% glycerol) and concentrated using 20% PEG in the same buffer.
Immunofluorescence Microscopy
NRK or Hela cells were seeded onto 12-mm glass coverslips in 12-well dishes and treated as described in the figure legends. Cell fixation, permeabilization, and subsequent antibody labeling was performed as described in reference (Volchuk et al., 2000). For the labeling in Figure 1, rabbit anti-GS15 (1:50) and either mouse mannosidase II (1:2000) or mouse TGN38 (1:30) were used as the primary antibodies, followed by detection with Alexa Fluor 488–conjugated goat anti-rabbit and Alexa Fluor 546–conjugated goat anti-mouse (both at 1:400 dilution) secondary antibodies. Rabbit polyclonal anti-GS15 antibodies from W. Hong (Xu et al., 1997, 2002) and our anti-GS15 peptide antibody were used and both gave similar results. For the labeling in Figure 2A, goat anti-Ykt6 (1:20) and rabbit anti-GOS28 (1:100) were used as the primary antibodies followed by detection with FITC-conjugated donkey anti-goat and Cy5-conjugated donkey anti-rabbit (both at 1:1000 dilution) secondary antibodies. Immunofluorescence images were obtained with a Zeiss LSM 510 confocal microscope (Thornwood, NY).
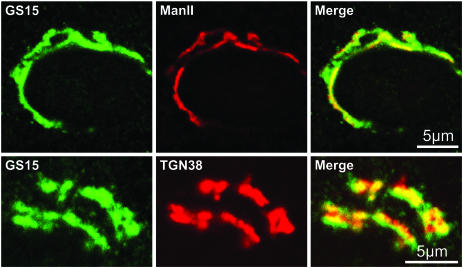
Figure 1.
Localization of GS15 in NRK cells by immunofluorescence microscopy. Rat NRK cells were fixed and immunostained for GS15 and Mannosidase II (top panels) or GS15 and TGN38 (bottom panels) and analyzed by laser confocal microscopy as described in MATERIALS AND METHODS. Areas of colocalization of the two proteins in the merged images appear yellow.
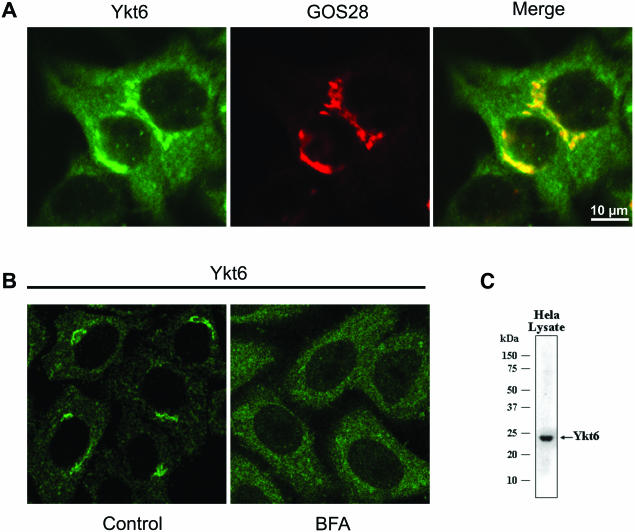
Figure 2.
Localization of Ykt6 in Hela cells by immunofluorescence microscopy. (A) Human Hela cells were fixed and immunostained with a goat anti-Ykt6 antibody and a rabbit anti-GOS28 antibody as described in MATERIALS AND METHODS. (B) Human Hela cells were stained for Ykt6 before (Control) and after treatment with brefeldin A (10 μg/ml for 30 min; BFA). (C) Hela cell total lysate (60 μg) was resolved by SDS-PAGE and immunoblotted with a goat anti-Ykt6 antibody.
Electron Microscopy and Quantitative Evaluation
Rat NRK cells were used for the immunolocalization of endogenous GS15, rBet1, membrin, ERS24, GOS28, syntaxin 5, and GM130. The cells were washed two times in PBS before fixation for 30 min at room temperature with 2% formaldehyde and 0.2% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.4). The cells were washed three times in PBS, scraped gently with a plastic cell scraper, and pelleted by centrifugation in a microfuge (12,000 rpm, 2 min). Cell pellets were embedded in 12% gelatin and cooled on ice. Small blocs of gelatin-embedded cells were infused with 2.3 M sucrose, frozen in liquid nitrogen, and sectioned with a cryoultramicrotome. Thin sections were collected and immunogold labeled as described in Orci et al. (1997). Antibody dilutions were as follows: anti-GS15 from Dr. W. Hong (1:20), anti-membrin (1:70), anti-syntaxin 5 and anti-ERS24 (1:20), anti-rBet1 monoclonal (1:20) and anti-GOS28 (1:3). Double-label immunocytochemistry was performed by combined labeling of GM130 with ERS24, membrin, or GS15. GM130 antibody was revealed by goat anti-mouse IgG conjugated with 10-nm gold, and ERS24, membrin, or GS15 antibodies were revealed by goat anti-rabbit IgG conjugated to 15-nm gold particles.
The subcellular localization of the different antigens was analyzed quantitatively on thin cryosections incubated with the appropriate antibodies followed by protein A-gold. Mouse monoclonal anti-rBet1 was labeled with gold-conjugated goat anti-mouse IgG. Sections of immunolabeled Golgi areas with a clear cis-trans orientation were photographed and printed at a final magnification of 96,000×. For each antigen, 40 different Golgi areas were quantified (50 different Golgi areas were used for the rBet1 analysis). The compartments evaluated were as follows: a) intermediate compartment (IC), defined as the tubulo-vesicular membrane profiles located in the area limited by the cis-most Golgi cisternae and the transitional elements of the rough endoplasmic reticulum (RER); b) cisternae, defined as elongated stacked membrane profiles enclosing a lumen (lateral cisternal rims and buds were included in this compartment); cisternae were numbered C1 to C5 from cis to trans; c) peri-Golgi lateral vesicles, defined as circular ∼70-nm membrane profiles not connected to cisternae on the sections and located within 200 nm of the lateral rim of the cisternae; d) TGN, defined as the tubulo-vesicular membrane profiles at the trans-side of the Golgi stack. The distribution of gold particles over the compartments defined above was expressed as a percentage of the total number of gold particles. For the cisternal cis-trans distribution of the labeling (Table 1, right side), the number of gold on each cisterna was expressed as the percentage of the total gold present over the stack of cisternae. The linear density was quantified as follows: the length of linear profiles (such as cisternae or tubulovesicles) was measured with an electronic pen. The length of peri-Golgi vesicles was estimated by multiplying the circumference of a 70-nm circle times the total number of vesicles counted. The number of gold particles on each compartment was recorded with the same pen and the labeling densities were expressed as the number of gold per μm of membrane. The data represent the mean of the values obtained on 40 different Golgi areas (or 50 different Golgi areas for rBet1), ± SEM. For unknown reasons, the rBet1 antibody also elicited a gold labeling over the nucleoplasm.
Table 1.
Distribution of SNAREs in the Golgi area of NRK cells
| Percentage of total gold particles
|
Percentage of total Golgi stack gold particles over cis to trans cisternae
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| IC | Golgi stack | Peri-Golgi vesicles | TGN | Cisterna 1 | Cisterna 2 | Cisterna 3 | Cisterna 4 | Cisterna 5 | |
| GS15 | 5 ± 1 | 71 ± 2 | 6 ± 1 | 18 ± 2 | 4 ± 1 | 10 ± 2 | 18 ± 2 | 33 ± 2 | 35 ± 3 |
| GOS28 | 8 ± 2 | 66 ± 4 | 16 ± 3 | 10 ± 2 | 16 ± 3 | 25 ± 3 | 27 ± 3 | 18 ± 3 | 14 ± 2 |
| rBet1 | 72 ± 3 | 23 ± 3 | 3 ± 1 | 2 ± 1 | 60 ± 6 | 20 ± 5 | 6 ± 3 | 10 ± 4 | 3 ± 2 |
| Membrin | 20 ± 3 | 68 ± 4 | 9 ± 2 | 3 ± 1 | 70 ± 4 | 23 ± 4 | 7 ± 2 | 0.2 ± 0.2 | 0.5 ± 0.4 |
| ERS24 | 35 ± 4 | 47 ± 3 | 6 ± 1 | 12 ± 2 | 64 ± 4 | 14 ± 3 | 10 ± 3 | 4 ± 1 | 8 ± 2 |
| Syntaxin 5 | 11.5 ± 2 | 73 ± 2 | 3 ± 1 | 12 ± 2 | 23 ± 2 | 20 ± 2 | 20 ± 2 | 24 ± 3 | 13 ± 2 |
Refer to MATERIALS AND METHODS in the Electron Microscopy and Quantitative Evaluation section for the details of the quantitations. Data are the mean ± SEM.
35S-Methionine Cell Labeling, SNARE Immunoprecipitation, and Molar Ratio Calculation
Rat NRK cells were labeled for 24 h with 100 μCi/ml 35S-methionine (in vivo cell labeling grade, Amersham, Piscataway, NJ) in methionine-free DME containing 10% dialyzed FCS and 100 μM cold methionine. The cells were subsequently washed, lysed in Triton-X 100 lysis buffer (1% TX-100, 100 mM KCl, 20 mM HEPES, pH 7.3, 2 mM EDTA, 1 mM dithiothreitol (DTT), 230 μM PMSF, supplemented with protease inhibitor tablets; Roche, Nutley, NJ), and incubated for 1 h at 4°C. The lysate was clarified by centrifugation (14,000 rpm, 10 min, 4°C). The protein concentration of the lysate was determined using the BCA Protein Assay (Pierce Inc). SNARE proteins were quantitatively immunoprecipitated from 100 μg of lysate by adding 5–10 μg of antibodies specific for each SNARE for 1 h at 4°C under constant rotation. A protein A-Agarose slurry (20 μl; Roche) was added to the mixture and incubated for an additional 2 h at 4°C. The beads were subsequently washed three times 5 min each with lysis buffer and once for 5 min with lysis buffer containing 0.1% TX-100. The washed beads were boiled (5 min) in 2× NuPage LDS sample buffer (Invitrogen, Carlsbad, CA) supplemented with 10% (vol/vol) β-mercaptoethanol. The radiolabeled SNARE immunoprecipitates were resolved by SDS-PAGE, and the gel was dried and exposed to a Molecular Dynamics (Sunnyvale, CA) Storage Phosphor screen and processed by a PhosphorImager. The molar ratios of the various SNARE proteins were determined as follows: Band intensities in arbitrary units of the individual SNAREs were quantified by ImageQuant software. To account for the fact that different SNAREs have different amounts of methionine, which causes the band intensity, the intensity values for each SNARE were normalized to the number of methionines in GOS28. GOS28 with 11 methionines based on its cDNA sequence (the highest number of methionines for the various SNAREs) was assigned a normalization factor of 1 (i.e., ERS24 with 7 methionines has a normalization factor of 1.57, etc.). In this way the relative levels of each SNARE in arbitrary units present in 100 μg of starting protein was determined.
Similarly, the collective band intensities of 1% (1 μg) of all 35S-labeled proteins in the starting material was quantified using a PhosphorImager and ImageQuant software. This value was multiplied by 100 to give the relative intensity of proteins in arbitrary units in 100 μg total protein. This value was then normalized for the average methionine content of human proteins (2.29%, determined from analysis of the Human Genome Project). The individual SNARE values (in arbitrary units) were divided by this number to obtain the amount of each SNARE (in grams) in the starting material. By taking into account the true molecular weights of each SNARE (g/mol) the moles/mg cell protein of each SNARE was obtained yielding the molar ratios of the SNARE proteins within cells (see values presented in the Figure 7 legend).
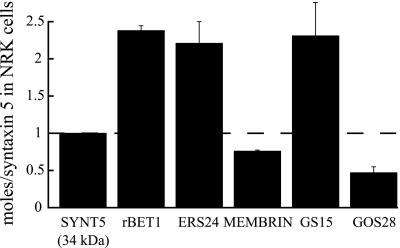
Figure 7.
Relative molar amounts of Golgi SNAREs in cells. Rat NRK cells were uniformly labeled with 35[S]methionine as described in MATERIALS AND METHODS. Individual SNARE proteins were immunoprecipitated and resolved by SDS-PAGE. The amount of each 35S-labeled SNARE in arbitrary units was determined using a Molecular Dynamics PhosphorImager. The absolute abundance (pmol/mg cell protein) of the individual SNAREs were calculated as stated in MATERIALS AND METHODS and are as follows: rBet1, 317 ± 9; GS15, 308 ± 61; ERS24, 295 ± 39; syntaxin 5 (34 kDa), 133 ± 28; membrin, 101 ± 2; GOS28, 63 ± 10. Values were obtained from three independent experiments and shown are the mean ± SD normalized to syntaxin 5. SYNT5, syntaxin 5 (34-kDa Golgi form).
Cell-free Transport Assay
Wild-type CHO cells and a mutant clone 15B (defective in UDP-GlcNAC glycosyltransferase I) were cultured as described (Balch et al., 1984a). The 15B cells were infected with VSV and Golgi membranes from the infected 15B cells (donor Golgi) and wild-type CHO cells (acceptor Golgi) were prepared as described previously (Balch et al., 1984a). Cytosol was prepared from wild-type CHO cells by a modified protocol originally used for bovine brain cytosol preparation (Malhotra et al., 1989). Briefly, 3 L of CHO cells were grown to a density of 3–5 × 105 cells/ml. The cells were washed with PBS, resuspended in homogenization buffer (250 mM sucrose, 10 mM Tris-Cl, 50 mM KCl, pH 7.4) and homogenized with a ball bearing homogenizer. The homogenate was centrifuged in a SW55 rotor for 1 h at 55K rpm at 4°C. The supernatant was removed and dialyzed against 25 mM Tris-Cl, 50 mM KCl, 0.5 mM DTT, pH 7.4. The dialyzed supernatant was incubated at 37°C for 30 min and centrifuged as above. The supernatant (cytosol) was decanted, the protein concentration was assayed (Bio-Rad, Hercules, CA), and aliquots were frozen in liquid nitrogen and stored at -80°C.
Transport assays were conducted according to a previously published protocol (Balch et al., 1984a). Briefly, 50 μl reactions contained the following (final concentrations): distilled water (20 μl), buffer (25 mM KCl, 25 mM HEPES, pH 7.0, 2.5 mM magnesium acetate; 5 μl), 0.5 μCi UDP-GlcNAC (New England Nuclear, Boston, MA) dried before resuspension, ATP-regenerating system (0.2 mM ATP, 1.0 mM UTP, 5.0 mM creatine phosphate, 18.2 IU/ml creatine kinase; 5 μl), and 75 μg cytosol (10 μl) and ∼1.5 μg donor (5 μl) and acceptor Golgi (5 μl). Fab fragments (dialyzed in 25 mM Tris-Cl, pH 7.4, 25 mM KCl, 10% glycerol) or dialysis buffer alone were substituted for the water in reactions containing antibodies. Premixes of all ingredients except the ATP-regenerating system were incubated on ice for 10 min, before the ATP-regeneration system was added, and samples were further incubated at 4°C for 15 min. The reactions were then transferred to a 37°C water bath for 60 min. The samples were then placed on ice for 5 min and lysed by buffer containing 1% Triton X-100, and VSV-G was immunoprecipitated as described (Balch et al., 1984a). The immune precipitate was recovered on glass filters (Whatman, Clifton, NJ) and washed, and incorporated radioactivity was determined by scintillation counting. A sample without cytosol addition and/or a complete reaction incubated on ice and processed as the other samples was used to determine the background signal. This value was subtracted from all samples. For each antibody the assay was performed at least twice on different days with triplicate samples for each condition.
RESULTS
Subcellular Localization of Golgi SNAREs in Mammalian Cells
In this study we examine in detail the distribution of Golgi SNAREs. All localization studies were performed exclusively on endogenous SNAREs to avoid potential artifacts, which can result from the use of tagged or overexpressed proteins. Double-label immunofluorescence localization of GS15 and the medial Golgi enzyme mannosidase II in rat NRK cells revealed a partial colocalization, in agreement with previous reports (Figure 1, top panels; Xu et al., 1997, 2002). In addition, we observed significant colocalization with a TGN marker TGN38 (Figure 1, bottom panels), indicating that this SNARE distributes across the Golgi stack and is also present in the trans-Golgi face.
Ykt6 lacks a transmembrane domain and consequently only a fraction (<50%) of the endogenous protein is associated with membranes (McNew et al., 1997; Zhang and Hong, 2001). A recent study however has reported that Ykt6 is not present in the Golgi of mammalian cells, but in an unidentified specialized compartment primarily in neuronal cells (Hasegawa et al., 2003). This contrasts with the results of Zhang and Hong (2001), who showed that endogenous Ykt6 is present in Golgi-enriched membranes from liver by Western blotting and in the Golgi area of NRK cells by immunofluorescence microscopy. To test whether Ykt6 is indeed a Golgi-localized SNARE, we generated a goat antibody to the human Ykt6 protein. The antibody recognizes a single band by Western blotting of Hela cell total lysate (Figure 2C). By immunofluorescence microscopy in Hela cells (Figure 2A), this antibody gave a perinuclear Golgi pattern that colocalized to a large extent with GOS28, a Golgi SNARE localized throughout the Golgi stack (Nagahama et al., 1996; Orci et al., 2000; and Figure 6). As a control, the immunofluorescence signal was completely blocked by preincubation of the Ykt6 antibody with recombinant Ykt6 protein (unpublished data). This is consistent with the observation that immunoprecipitation of GOS28 from rat liver Golgi extracts coimmunoprecipitates Ykt6 (Xu et al., 2002, and our unpublished observations). In addition, treating the cells with brefeldin A caused a complete dispersal of the Golgi staining for Ykt6 (Figure 2B), indicating that the majority of the Ykt6 is localized in the Golgi stack (Klausner et al., 1992). Unfortunately, despite testing several antibodies to Ykt6, none have proven to be useful for immunogold localization by EM, precluding a more precise localization at the cisternae level. Although Ykt6 is indeed a bona fide Golgi SNARE protein, it is not exclusively localized there (Hasegawa et al., 2003). The yeast Ykt6 protein has been implicated in post-Golgi transport steps (Ungermann et al., 1999), and we did observe significant punctate staining that could be of endosomal origin or other organelles (Figure 2A and 2B).
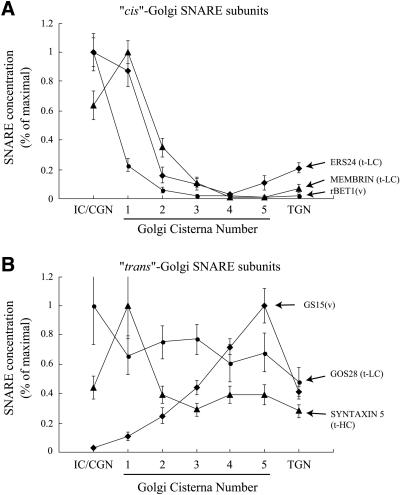
Figure 6.
Distribution of SNAREs within the IC, Golgi stack, and TGN. The distribution of the indicated SNAREs within the IC, Golgi stack (cisterna 1 (cis) to cisterna 5 (trans)), and TGN, were obtained as outlined in MATERIALS AND METHODS. Shown is the linear density of labeling (gold particles/μm membrane) within these compartments. For each SNARE its distribution was normalized to the value in the compartment having the highest labeling density. (A) Data for the subunits of the cis-SNAREpin, v-rBet1, and light chains membrin and ERS24. t-LC, t-SNARE light chain; v, v-SNARE. (B) Data for the subunits of the trans-SNAREpin, v-GS15, and t-SNARE heavy chain syntaxin 5 and t-light chain GOS28 (t-HC, t-SNARE heavy chain). Error bars, SEM
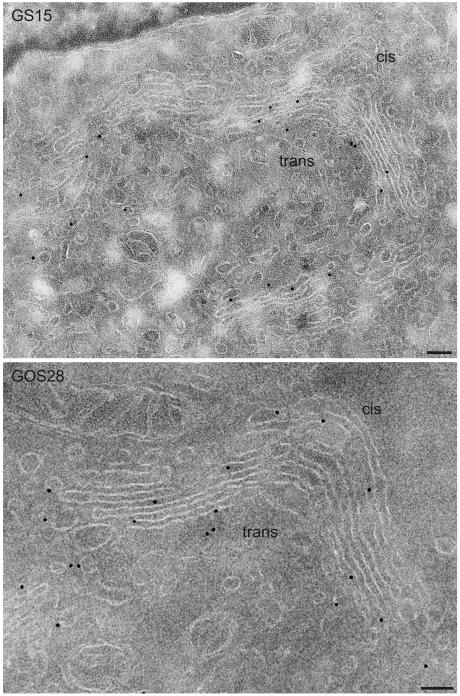
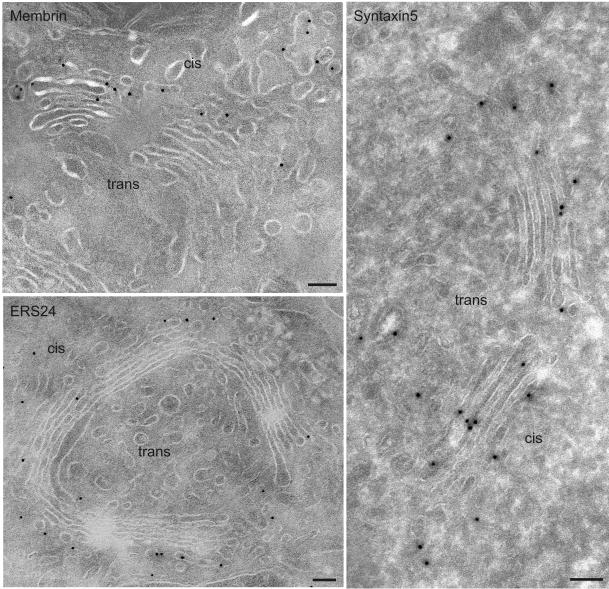
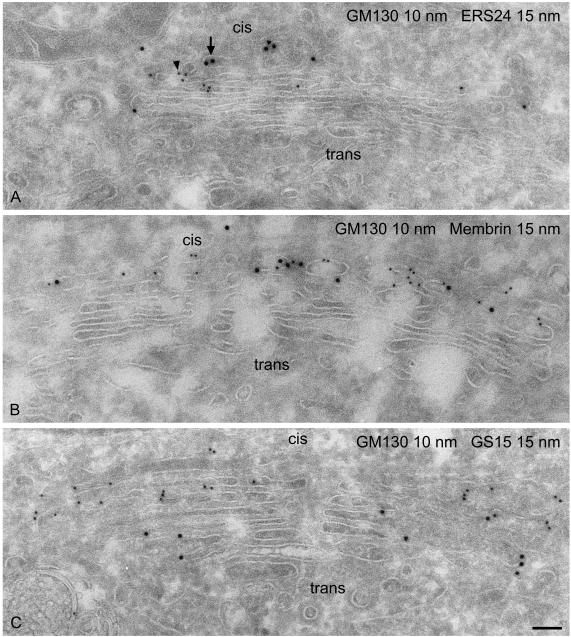
Representative examples of the ultrastructural localization of GS15 as well as several other Golgi SNAREs are shown in Figures 3, 4, 5. GS15 is most prominent in the medial and trans portion of the stack (Figure 3). Figure 5C shows double labeling with the cis Golgi marker GM130 (Nakamura et al., 1995). This result agrees well with the immunofluorescence localization observed with medial and trans-Golgi markers (Figure 1). It is only in partial agreement with the electron microscopic distribution reported by Xu et al. (2002), who observed a primarily medial distribution of endogenous GS15 in CHO and 3T3-L1 cells. However, no quantitative data for the distribution were presented in this study. Syntaxin 5 and GOS28 are localized throughout the Golgi stack, whereas ERS24, rBet1, and membrin reside primarily in the cis face (Figures 3, 4, 5; Tables 1 and 2).
Figure 3.
Ultrathin cryosections of NRK cells showing immunogold labeling of GS15 and GOS28. GS15 is preferentially localized over the medial and trans cisternae of the Golgi stack, whereas GOS28 is distributed across the stack. The quantitative evaluation of the labeling is shown in Tables 1 and 2. Bar, 100 nm.
Figure 4.
Ultrathin cryosections of NRK cells showing immunogold labeling of membrin, ERS24, and syntaxin 5. Membrin and ERS24 distribute mostly to the cis side of the Golgi, including elements of the intermediate compartment, whereas syntaxin 5 is present throughout the entire Golgi stack. The quantitative evaluation of the labeling is shown in Tables 1 and 2. Bar, 100 nm.
Figure 5.
Assessment of the cis-trans polarity of the Golgi stack. Double labeling of GM130 and ERS24 (A), GM130 and membrin (B), GM130 and GS15 (C). GM130, a cis-Golgi marker, colocalizes with ERS24 and membrin but not with GS15, a SNARE localized to the medial/trans-Golgi. GM130 was visualized with small (10 nm) gold particles (arrowhead), whereas ERS24, membrin, and GS15 were revealed by large (15 nm) gold particles (arrow). Bar, 100 nm.
Table 2.
Distribution of SNAREs in the Golgi area of NRK cells
| IC | Golgi stack | Peri-Golgi vesicles | TGN | |
|---|---|---|---|---|
| GS15 | 0.04 ± 0.01 | 0.65 ± 0.04 | 0.60 ± 0.14 | 0.55 ± 0.06 |
| GOS28 | 0.79 ± 0.21 | 0.52 ± 0.04 | 1.15 ± 0.18 | 0.38 ± 0.08 |
| rBet1 | 4.45 ± 0.44 | 0.29 ± 0.04 | 0.15 ± 0.07 | 0.08 ± 0.03 |
| Membrin | 1.08 ± 0.18 | 0.51 ± 0.03 | 0.91 ± 0.19 | 0.11 ± 0.05 |
| ERS24 | 3.02 ± 0.39 | 0.54 ± 0.07 | 0.85 ± 0.22 | 0.64 ± 0.10 |
| Syntaxin 5 | 1.10 ± 0.19 | 1.01 ± 0.09 | 0.51 ± 0.17 | 0.69 ± 0.10 |
Refer to the MATERIALS AND METHODS section in the Electron Microscopy and Quantitative Evaluation section for the details of the quantitations. Data are the mean ± SEM. Values are concentration (gold particles/μm membrane).
Tables 1 and 2 provide quantitative results regarding the intra-Golgi distribution of GS15, syntaxin 5, ERS24, membrin, rBet1, and GOS28 in NRK cells. The data presented here for these SNAREs are in good agreement with previously published results in other cell types (Paek et al., 1997; Hay et al., 1998; Orci et al., 2000; Martinez-Menarguez et al., 2001). We also provide extensive data on the cisterna-by-cisterna concentrations of these SNAREs based on the linear density of labeling (Figure 6) and on the cisternal vs. peri-Golgi vesicle abundance and concentration of each SNARE (Table 1 and 2; see also Figure 8). ERS24, membrin, and rBet1 are concentrated most in the IC/cis-Golgi network (CGN) and the cis-Golgi (cisternae 1 and 2) and are greatly reduced in concentration at the trans end of the stack (Figure 6A). Syntaxin 5 is most abundant in the cis-most cisterna and is evenly distributed at ∼40% of its peak concentration in the Golgi stack (Figure 6B). The high level of syntaxin 5 observed in cisterna 1 compared with the rest of the stack may likely be due to the fact that the polyclonal antibody used for localization recognizes both the 34-kDa Golgi form and the 41-kDa ER/IC form of syntaxin 5 (Hui et al., 1997). The 41-kDa form is also likely present in cisterna 1 (Hui et al., 1997); thus, the signal observed is a combination of the two splice forms. GOS28, like syntaxin 5, also distributes approximately equally throughout the stack, declining to ∼50% of its peak concentration in the TGN. GS15 exhibits the opposite distribution, with progressively higher concentrations toward the trans side of the stack, resulting in a gradient of a factor of ∼9 in concentration from IC/CGN to the last Golgi cisternae (Figure 6B).
Figure 8.
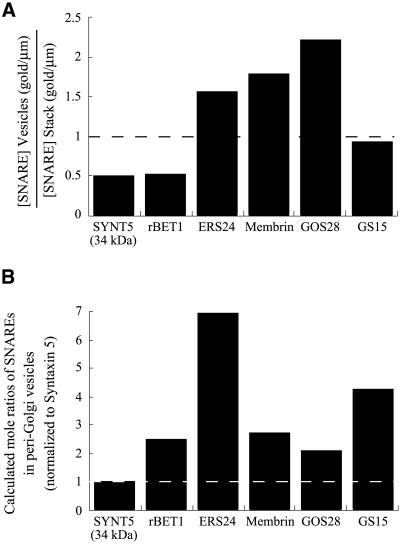
Concentration of SNAREs in peri-Golgi vesicles relative to the common Golgi t-SNARE heavy chain, syntaxin 5. (A) Shown for each SNARE is the ratio of the linear density (gold particles/μm) within lateral peri-Golgi vesicles (from Table 2) relative to the linear density in the Golgi stack (from Table 2). SYNT5, syntaxin 5 (34-kDa Golgi form). (B) Shown is the calculated mole ratio of the various SNAREs to syntaxin 5 in peri-Golgi vesicles. The values were obtained by multiplying the values in Figure 7 (mole ratios of the SNAREs in cells) by the values in panel A (linear density of gold particles in peri-Golgi vesicles/Golgi stack), and renormalizing to syntaxin 5.
Relative Molar Concentrations of SNAREs across the Stack and in Vesicles
It is evident from the localization data that each cisterna contains a mixture of all seven of the subunits comprising the two Golgi-localized SNAREpins, v-rBet1: t-syntaxin 5/membrin, ERS24 and v-GS15: t-syntaxin 5/mYkt6, GOS28, but their ratios in the mixture vary across the stack. In particular every Golgi cisterna contains varying ratios of two alternative sets of t-SNARE light chains (membrin+ERS24 vs. mYkt6+GOS28) that will compete for binding to a common heavy chain (syntaxin 5), to form one or the other t-SNARE. The data in Tables 1 and 2 and Figure 6 speak to how each SNARE (with the exception of Ykt6) distributes across the stack, but not to the actual number of copies of each SNARE in each compartment, the more fundamental quantity in establishing which t-SNARE prevails in this competition. Therefore, we wished to assess the relative molar abundance of the various SNAREs throughout the cisternae and in the transport vesicles.
To measure the molar abundance of SNAREs in whole cells, rat NRK cells were labeled with 35S-methionine to a steady-state (24 h) and SNARE proteins were quantitatively immunoprecipitated from the lysate. In each case the precipitated SNARE was evident as a major band in SDS-PAGE of the expected molecular weight (unpublished data). The bands were quantified and normalized according to the known methionine content of each SNARE to yield data on the relative molar abundance of the SNAREs, which are presented in Figure 7, normalized to the common heavy chain syntaxin 5 (34-kDa Golgi-localized form). Because only a small fraction of these SNAREs reside outside the IC/Golgi (12% of rBet1, 11% of ERS24, 9% of membrin, and 7% of syntaxin 5 are in the ER; Hay et al., 1998), the total cellular ratios are, within experimental error, representative of the Golgi area. Lack of a suitable antibody precluded results for mYkt6.
Several notable features emerge from this analysis. First, the various light chains are present in broadly similar molar amounts relative to each other and to their common heavy chain, syntaxin 5, consistent with a dynamic competition to form one or the other t-SNARE throughout the stack.
Second, and surprisingly, the two v-SNAREs rBet1 and GS15 are present in molar excess (∼2.3-fold) over syntaxin 5. The actual molar ratio of each of these v-SNAREs to its cognate t-SNARE will be even greater than this for two reasons. First, the syntaxin 5 will be partitioned between two t-SNAREs, only one of which can bind rBet1 or GS15. Second, the v-SNAREs are heavily concentrated toward the cis (rBet1) or trans (GS15) face, whereas syntaxin 5 evenly distributes in the stack. Therefore, for both SNAREpins the concentration of v-SNARE is much greater than the concentration of t-SNARE in the Golgi complex.
Does this same condition hold in the peri-Golgi transport vesicles? Figure 8A shows the concentration of each SNARE in the vesicles relative to its concentration in cisternae, calculated from the data in Table 2 by dividing (for each SNARE) the linear density of labeling (gold particles/μm) in the peri-Golgi vesicles by the linear density of labeling in the Golgi stack. Evidently, all of the SNAREs (data lacking for Ykt6) are also present in the vesicles, where they are neither dramatically concentrated or excluded. In the case of GOS28, this SNARE is about twofold concentrated in vesicles compared with cisternae, consistent with an earlier study (Nagahama et al., 1996).
Combining the data in Figure 8A with the data in Figure 7 (molar abundance of SNAREs in the cell), it is evident that the concentration of the v-SNAREs GS15 and rBet1 are also in excess of syntaxin 5 in Golgi-associated vesicles (Figure 8B). Therefore, the morphological and biochemical analysis implies that [v-SNARE] >> [t-SNARE] both in peri-Golgi vesicles and in the Golgi cisternae. This in turn suggests a fusion mechanism akin to homotypic fusion, as will be explored in the DISCUSSION.
Both Golgi SNAREpins Are Required for Cell-free Golgi Transport
GOS28 has previously been shown to be functionally required for intra-Golgi transport in the cell-free system (Nagahama et al., 1996). GOS28 and other SNAREs are also required in a more complex permeabilized cell assay measuring the transport of VSV-G protein starting in the ER to the medial Golgi (endoglycosidase H-resistance). Unfortunately, this assay does not distinguish between requirements for ER-to-Golgi from intra-Golgi transport, because both must occur for the assay to register (endoglycosidase H resistance is not conferred until the removal of mannose from the N-linked oligosaccharide chain by mannosidase II, after the activity of NAGTI, which is concentrated in the medial Golgi; Dunphy et al., 1985). Essentially complete inhibition was observed in this assay with antibodies to syntaxin 5 (Rowe et al., 1998), ERS24 (Zhang et al., 1999), rBet1 (Zhang et al., 1997), mYkt6 (Zhang and Hong, 2001), and marked inhibition was reported for GOS28 (Subramaniam et al., 1996).
To directly establish requirements for intra-Golgi transport, we have tested the effects of Fab fragments targeted to the subunits of the two alternative Golgi SNAREpins on reconstituted transport using isolated Golgi membranes (Balch et al., 1984a). Polyclonal antibodies to SNARE proteins were affinity purified and Fab fragments were generated. The specificity of each Fab was confirmed as only a single expected band was observed in Western blots of whole cell lysates and/or Golgi membranes (unpublished data).
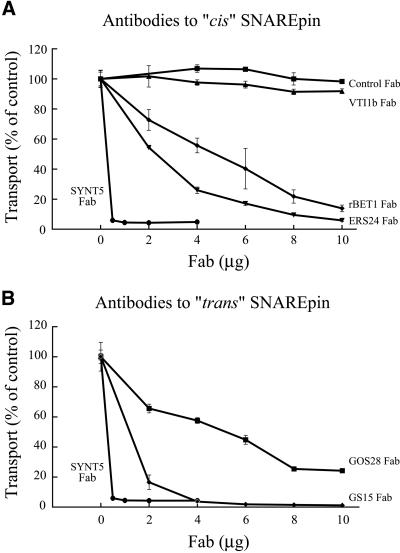
The Fab directed to syntaxin 5, the common Golgi t-SNARE heavy chain, completely inhibited transport (Figure 9). Fab fragments against ERS24, rBet1, GOS28, and GS15 also profoundly inhibited transport in a dose-dependent manner. The inhibition was specific based on the following data: 1) control rabbit Fab fragments had no effect, nor did Fab fragments against another SNARE, Vti1b (Figure 9A), 2) the inhibitory effects were completely neutralized by prior denaturation of the antibodies by boiling, and 3) preincubating the antibodies with recombinant soluble SNAREs lacking transmembrane domains (rBet1 and GOS28) or the GS15 immunizing peptide, neutralized the inhibitory effects (unpublished data). Unfortunately, suitable antibodies directed to membrin and mYkt6 were not available for these experiments. In conclusion, subunits of both Golgi SNAREpins, v-rBet1: t-syntaxin 5/membrin, ERS24 and v-GS15: t-syntaxin 5/mYkt6, GOS28 are functionally required for intra-Golgi transport, which must therefore require fusion processes mediated by each SNAREpin.
Figure 9.
Requirement for SNAREs in cell-free reconstituted Golgi transport. Transport-coupled glycosylation of VSV-G protein was measured. Purified Golgi membranes from VSV-infected 15B cells (donor) and wild-type CHO cells (acceptor), were incubated with cytosol and ATP as described in MATERIALS AND METHODS. The assay was performed with increasing concentrations of the indicated Fab fragments or (as controls) nonspecific rabbit Fab or a Fab directed against Vti1b, as indicated. Triplicate samples were used for each condition generating the SDs shown. SYNT5, syntaxin 5.
DISCUSSION
Opposing cis-trans Gradients of the Two SNAREpins within the Golgi Stack
That transport in the Golgi stack relies upon membrane fusion has long been suggested by its morphology in the cell (Palade, 1975) and its intrinsic capacity to bud and fuse vesicles (Balch et al., 1984b) and most recently is evident from the specific requirement for individual SNARE proteins found within this organelle. The localization of these SNAREs along the cis-trans axis within the stack can therefore provide answers to important questions concerning where fusion occurs and the pattern of membrane flow, motivating this comprehensive study.
The nearly even distribution of the heavy chain (syntaxin 5) and one light chain (GOS28; and likely the other light chain mYkt6 from Figure 2 at the immunofluorescence level) suggest that the combination of the three, i.e., the functional t-SNARE syntaxin 5/mYkt6, GOS28, is present throughout the Golgi stack. On the other hand the cognate v-SNARE GS15 is localized in a cis < trans gradient (Figure 6B), suggesting on the face of it that the concentration of this Golgi SNAREpin and accordingly its ability to function in fusion, may progressively increase toward the trans face of the Golgi stack. This contrasts with the cis-Golgi SNAREpin whose t-SNARE (a compound of the distributions of syntaxin 5 [cis = trans], membrin [cis > trans], and ERS24 [cis > trans]) and whose v-SNARE rBet1 (cis > trans), each progressively decrease toward the trans face of the stack (Figure 6A). The simultaneous requirement for both SNAREpins for reconstituted Golgi transport (Figure 9) implies that they function coordinately in membrane fusion processes that maintain the ongoing transport activity of the Golgi apparatus.
The cis > trans distribution of the cis-Golgi SNAREpin suggests that it functions in membrane fusion events that are progressively directed toward the cis face within the stack, i.e., retrograde vesicle transport within the Golgi. This suggests, of course, that the cis-SNAREpin does double duty, because it also fuses ER-derived COPII vesicles with the Golgi at its cis face (Newman and Ferro-Novick, 1987; Newman et al., 1990; Shim et al., 1991; Hardwick and Pelham, 1992; Cao and Barlowe, 2000).
By the same reasoning, the present work suggests as the simplest possibility that the oppositely oriented trans-SNAREpin should support anterograde flow, i.e., transport across the Golgi that depends on membrane fusion within the stack that is progressively directed toward the trans face, presumably involving transport vesicles. The alternative, that membrane fusion supports transport by direct fusion of adjacent cisternae without an intervening transfer vesicle, seems remote because three-dimensional reconstruction fails to reveal continuities between nonequivalent cisternae (Rambourg et al., 1987; Ladinsky et al., 1999).
Having said this, the trans > cis distribution of the trans-Golgi SNAREpin does not speak against the possibility of a concurrent cisternal progression/maturation process, which likely occurs in parallel with vesicular transport (Pelham and Rothman, 2000).
Homotypic Fusion between Vesicles and Cisternae in the Golgi?
The concentration of the v-SNAREs (rBet1 and GS15) exceeds that of their cognate t-SNAREs (limited by the concentration of their common syntaxin heavy chain, syntaxin 5) in Golgi cisternae and in the vesicles forming from them. This condition is reminiscent of what occurs during homotypic fusion between closely related or identical membranes in which both the v-SNARE and its cognate t-SNARE reside in both partners (Mellman and Warren, 2000). For example, in the yeast vacuole almost all of the cognate SNAREs exist in v-t SNARE complexes residing within each membrane partner wherein the v-SNARE and the t-SNARE are bound to each other and therefore unavailable to pair between membranes to form the SNAREpins that bridge the two membranes and are required for fusion (Nichols et al., 1997; Ungermann et al., 1998). For homotypic fusion to occur, the v- and t-SNAREs bound up within each partner first need to be separated by the ATPase of NSF so they are then free to partner between membranes (Ungermann et al., 1998). In the case of Golgi cisternae and vesicles, because [v] >> [t], separation of SNAREs by NSF/SNAP need only occur in one of the partners to free up t-SNARE there; excess free v-SNARE will always be available in the other partner.
The activation of v-t SNAREs for fusion is ordinarily linked to the assembly of the COPI coat preventing the direct fusion of cisternae, but cisternal fusion can be triggered by “uncoupling” membrane fusion from transport vesicle budding, either by brefeldin A (Klausner et al., 1992) or by removing ARF or coatomer (Orci et al., 1993; Elazar et al., 1994). Molecular structure studies are now revealing the subtle mechanisms used by coats to regulate fusion and couple it to budding (Miller et al., 2003; Mossessova et al., 2003).
How may the cis-Golgi and trans-Golgi SNAREpins distribute between the two compositionally distinct populations of COPI vesicles that have previously been identified (Orci et al., 1997)? The “retrograde” population of COPI vesicles contain retrograde-directed KDEL salvage receptor and the cis-Golgi v-SNARE rBet1 (Martinez-Menarguez et al., 2001). The “anterograde” population of COPI peri-Golgi vesicles contain the trans-Golgi t-SNARE light-chain GOS28 and anterograde-directed cargo (VSV-G protein).1 Although the data are limited, so far they are consistent with the simple idea that the vesicles carrying anterograde-directed cargo have the trans-Golgi v- and t-SNAREs, whereas the vesicles carrying retrograde-directed cargo have the cis-Golgi SNAREs. With this in mind, it is our current hypothesis that the oppositely distributing cis- and trans-Golgi SNARE complexes are separately packaged into the two distinct populations of COPI vesicles. The needed segregation could occur during budding from a single bilayer phase, perhaps using isotypes of coatomer (Blagitko et al., 1999; Futatsumori et al., 2000; Hahn et al., 2000; Lee et al., 2000; Wegmann et al., 2003) or before budding by segregation into distinct lateral membrane domains, as is the case for different rab proteins in the same endosomes (Sonnichsen et al., 2000; Barbero et al., 2002).
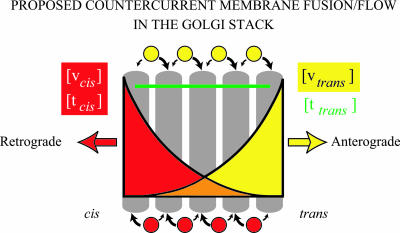
In summary, the countercurrent distribution of SNAREs (Figure 10) naturally suggests opposing streams of vesicles whose cargo would be subject to repeated rounds of purification in successive cisternae (the countercurrent distribution; Craig, 1948; Rothman, 1981).
Figure 10.
Countercurrent distribution of SNAREpins and its implication for vesicle transport patterns in the Golgi stack. Distinct cis- and trans-Golgi SNAREpins are needed for the operation of the Golgi stack. They distribute in countercurrent concentration gradients across the stack. Two types of COPI vesicles bud at every level of the Golgi stack (Orci et al., 1997). One contains retrograde-directed cargo and the cis-Golgi v- and t-SNAREs (vcis and tcis). The other contains anterograde-directed cargo and the trans-Golgi v- and t-SNAREs (vtrans and ttrans). Both vesicles fuse homotypically with cognate SNAREs. They are restrained from dissociating by a carpet of tethers (Orci et al., 1998; Sonnichsen et al., 1998; Seemann et al., 2000) and therefore are restricted to fusion with adjacent cisternae on either side. With these conditions, at each level of the stack a vesicle will have a higher probability of fusing to the neighboring cisternae that contains the higher—rather than the lower—concentration of its cognate (homotypic) SNAREs. The opposing cis-trans distribution of the two cognate SNARE pairs would mean that the “retrograde” vesicles carrying cis-Golgi SNAREs should have a systematically higher probability of fusing up their gradient toward the cis face, whereas the “anterograde” vesicles carrying trans-Golgi SNAREs would, to the contrary, fuse preferentially up their gradient toward the trans face. In other words, the two types of vesicles would engage in systematically biased random walks toward the opposite ends of the stack, as individual vesicles essentially chromatograph up their gradients. This mechanism would naturally create a net flow of the cargo contained in the “anterograde” vesicles across the stack in the cis-to-trans direction, and a net flow of the cargo contained in the “retrograde” vesicles in the opposite trans-to-cis direction (this model is a refinement of the “percolating vesicle” model which envisioned an unbiased random walk; Pelham and Rothman, 2000). The countercurrent of cargo could of course be fine-tuned by additional layers of specificity involving the distributions of regulatory proteins like rabs and tethers. This model for vesicular transport in the stack does not speak to the cisternal progression/maturation process that likely occurs concurrently (Pelham and Rothman, 2000).
Acknowledgments
We thank Dr. B. Jagla for the calculation of the average methionine content of human proteins, Dr. P. Cosson for his helpful comments on the manuscript, and Dr. F. Wieland (Biochemie-Zentrum, Heidelberg) and Dr. W. Hong (Institute of Molecular and Cell Biology, Singapore) for generously providing antibodies. This work was supported by grants from the Swiss National Science Foundation (to L.O.) and from the National Institutes of Health (to J.E.R.). A.V. was supported in part by a postdoctoral fellowship from the Medical Research Council of Canada (now the Canadian Institutes of Health Research).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–08–0625. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–08–0625.
Abbreviations used: CHO, Chinese hamster ovary; CGN, cis-Golgi network; IC, intermediate compartment; GFP, green fluorescent protein; NRK, normal rat kidney cells; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; TGN, trans-Golgi network; VSV, vesicular stomatitis virus; VSV-G, vesicular stomatitis virus glycoprotein.
Footnotes
While Klumperman and colleagues (Martinez-Menarguez et al., 2001) have confirmed the two types of COPI vesicles identified by Orci et al. (1997), i.e. VSV-G-positive and KDEL receptor-positive vesicle populations, the VSV-G population is less abundant in their studies than in ours (Orci et al., 1997; Orci et al., 2000). It should be noted that, whereas Orci and colleagues studied VSV-viral infected cells, Martinez-Menárguez and colleagues employed cells transfected with plasmid encoding GFP-tagged VSV-G. VSV-G is much more abundantly expressed in infected cells (where all cell protein synthesis is replaced by only five major viral proteins) than in transfected cells. Therefore, the load of protein needing transport in the Golgi is much larger in our studies.
References
- Balch, W.E., Dunphy, D.G., Braell, W.A., and Rothman, J.E. (1984a). Reconstitution of the transport of protein between successive compartments of the Golgi measured by the incorporation of N-acetyl glucosamine. Cell 39, 405-416. [DOI] [PubMed] [Google Scholar]
- Balch, W.E., Glick, B.S., and Rothman, J.E. (1984b). Sequential intermediates in the pathway of intercompartmental transport in a cell-free system. Cell 39, 535-536. [DOI] [PubMed] [Google Scholar]
- Banfield, D.K., Lewis, M.J., and Pelham, H.R. (1995). A SNARE-like protein required for traffic through the Golgi complex. Nature 375, 806-809. [DOI] [PubMed] [Google Scholar]
- Barbero, P., Bittova, L., and Pfeffer, S.R. (2002). Visualization of Rab9-mediated vesicle transport from endosomes to the trans-Golgi in living cells. J. Cell Biol. 156, 511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagitko, N., Schulz, U., Schinzel, A.A., Ropers, H.H., and Kalscheuer, V.M. (1999). γ2-COP, a novel imprinted gene on chromosome 7q32, defines a new imprinting cluster in the human genome. Human Mol. Genet. 8, 2387-2396. [DOI] [PubMed] [Google Scholar]
- Cao, X., and Barlowe, C. (2000). Asymmetric requirements for a Rab GTPase and SNARE proteins in fusion of COPII vesicles with acceptor membranes. J. Cell Biol. 149, 55-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, L.C. (1948). Counter-current distribution. Fed. Proc. 7, 469-473. [PubMed] [Google Scholar]
- Dunphy, W.G., Brands, R., and Rothman, J.E. (1985). Attachment of terminal N-acetylglucosamine to asparagine-linked oligosaccharides occurs in central cisternae of the Golgi stack. Cell 40, 463-472. [DOI] [PubMed] [Google Scholar]
- Elazar, Z., Orci, L., Ostermann, J., Amherdt, M., Tanigawa, G., and Rothman, J.E. (1994). ADP-ribosylation factor and coatomer couple fusion to vesicle budding. J. Cell Biol. 124, 415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard, G., Nothwehr, S.F., and Stevens, T.H. (1997). The yeast v-SNARE Vti1p mediates two vesicle transport pathways through interactions with the t-SNAREs Sed5p and Pep12p. J. Cell Biol. 137, 1511-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, R., Weber, T., Parlati, F., Engel, T., Nickel, W., Rothman, J.E., and Sollner, T. (2000). Functional architecture of an intracellular-membrane t-SNARE. Nature 407, 198-202. [DOI] [PubMed] [Google Scholar]
- Futatsumori, M., Kasai, K., Takatsu, H., Shin, H.W., and Nakayama, K. (2000). Identification and characterization of novel isoforms of COPI subunits. J. Biochem. 128, 793-801. [DOI] [PubMed] [Google Scholar]
- Hahn, Y., Lee, Y.J., Yun, J.H., Yang, S.K., Park, C.W., Mita, K., Huh, T.L., Rhee, M., and Chung, J.H. (2000). Duplication of genes encoding non-clathrin coat protein gamma-COP in vertebrate, insect and plant evolution. FEBS Lett. 482, 31-36. [DOI] [PubMed] [Google Scholar]
- Hardwick, K.G., and Pelham, H.R. (1992). SED5 encodes a 39-kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J. Cell Biol. 119, 513-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa, H., Zinsser, S., Rhee, Y., Vik-Mo, E.O., Davanger, S., and Hay, J.C. (2003). Mammalian Ykt6 is a neuronal SNARE targeted to a specialized compartment by its profilin-like amino terminal domain. Mol. Biol. Cell 14, 698-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, J.C., Chao, D.S., Kuo, C.S., and Scheller, R.H. (1997). Protein interactions regulating vesicle transport between the endoplasmic reticulum and Golgi apparatus in mammalian cells. Cell 89, 149-158. [DOI] [PubMed] [Google Scholar]
- Hay, J.C., Klumperman, J., Oorschot, V., Steegmaier, M., Kuo, C.S., and Scheller, R.H. (1998). Localization, dynamics, and protein interactions reveal distinct role for ER and Golgi SNAREs. J. Cell Biol. 141, 1489-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, C., Ahmed, M., Melia, T.J., Sollner, T.H., Mayer, T., and Rothman, J.E. (2003). Fusion of cells by flipped SNAREs. Science 300, 1745-1749. [DOI] [PubMed] [Google Scholar]
- Hui, N., Nakamura, N., Sonnichsen, B., Shima, D.T., Nilsson, T., and Warren, G. (1997). An isoform of the Golgi t-SNARE, syntaxin 5, with an endoplasmic reticulum retrieval signal. Mol. Biol. Cell 8, 1777-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner, R.D., Donaldson, J.G., and Lippincott-Schwartz, J. (1992). Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116, 1071-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladinsky, M.S., Matronarde, D.N., McIntosh, J.R., Howell, K.E., and Staehelin, L.A. (1999). Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J. Cell Biol. 144, 1135-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.J., Park, C.W., Hahn, Y., Park, J., Lee, J., Yun, J.H., Hyun, B., and Chung, J.H. (2000). Mit1/Lb9 and Copg2, new members of mouse imprinted genes closely linked to Peg1/Mest(1). FEBS Lett. 472, 230-234. [DOI] [PubMed] [Google Scholar]
- Lupashin, V.V., Podrovskaya, I.D., McNew, J.A., and Waters, M.G. (1997). Characterization of a novel yeast SNARE protein implicated in Golgi retrograde traffic. Mol. Biol. Cell 8, 2659-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra, V., Serafini, T., Orci, L., Shepherd, J.C., and Rothman, J.E. (1989). Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell 58, 329-336. [DOI] [PubMed] [Google Scholar]
- Martinez-Menarguez, J.A., Prekeris, R., Oorschot, V.M.J., Scheller, R., Slot, J.W., Geuze, H.J., and Klumperman, J. (2001). Peri-Golgi vesicles contain retrograde but not anterograde proteins consistent with the cisternal progression model of intra-Golgi transport. J. Cell Biol. 155, 1213-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNew, J.A., Fukuda, R., Parlati, F., Johnston, R.J., Paz, K., Paumet, F., Sollner, T.H., and Rothman, J.E. (2000a). Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407, 153-159. [DOI] [PubMed] [Google Scholar]
- McNew, J.A., Sogaard, M., Lampen, N.M., Machida, S., Ye, R.R., Lacomis, L., Tempst, P., Rothman, J.E., and Sollner, T.H. (1997). Ykt6, a prenylated SNARE essential for endoplasmic reticulum-Golgi transport. J. Biol. Chem. 272, 17776-17783. [DOI] [PubMed] [Google Scholar]
- McNew, J.A., Weber, T., Parlati, F., Johnston, R.J., Melia, T.J., Sollner, T.H., and Rothman, J.E. (2000b). Close is not enough: SNARE-dependent membrane fusion requires an active mechanism that transduces force to membrane anchors. J. Cell Biol. 150, 105-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman, I., and Warren, G. (2000). The road taken: past and future foundations of membrane traffic. Cell 100, 99-112. [DOI] [PubMed] [Google Scholar]
- Miller, E.A., Beilharz, T.H., Malkus, P.N., Lee, M.C.S., Hamamoto, S., Orci, L., and Schekman, R. (2003). Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell 114, 497-509. [DOI] [PubMed] [Google Scholar]
- Mossessova, E., Bickford, L.C., and Goldberg, J. (2003). SNARE selectivity of the COPII coat. Cell 114, 483-495. [DOI] [PubMed] [Google Scholar]
- Nagahama, M., Orci, L., Ravazzola, M., Amherdt, M., Lacomis, L., Tempst, P., Rothman, J.E., and Sollner, T.H. (1996). A v-SNARE implicated in intra-Golgi transport. J. Cell Biol. 133, 507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, N., Rabouille, C., Watson, R., Nilsson, T., Hui, N., Slusarewics, P., Kries, T.E., and Warren, G. (1995). Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 131, 1715-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, A.P., and Ferro-Novick, S. (1987). Characterization of new mutants in the early part of the yeast secretory pathway isolated by a [3H]mannose suicide selection. J. Cell Biol. 105, 1587-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, A.P., Shim, J., and Ferro-Novick, S. (1990). BET1, BOS1, and SEC22 are members of a group of interacting yeast genes required for transport from the endoplasmic reticulum to the Golgi complex. Mol. Cell. Biol. 10, 3405-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, B.J., Ungermann, C., Pelham, H.R., Wickner, W.T., and Haas, A. (1997). Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature 387, 199-202. [DOI] [PubMed] [Google Scholar]
- Orci, L., Palmer, D.J., Ravazzola, M., Perrelet, A., Amherdt, M., and Rothman, J.E. (1993). Budding from Golgi membranes requires the coatomer complex of non-clathrin coat proteins. Nature 362, 648-652. [DOI] [PubMed] [Google Scholar]
- Orci, L., Perrelet, A., and Rothman, J.E. (1998). Vesicles on strings: morphological evidence for processive transport within the Golgi stack. Proc. Natl. Acad. Sci. USA 95, 2279-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci, L., Ravazzola, M., Volchuk, A., Engel, T., Gmachl, M., Amherdt, M., Perrelet, A., Sollner, T.H., and Rothman, J.E. (2000). Anterograde flow of cargo across the Golgi stack potentially mediated via bidirectional “percolating” COPI vesicles. Proc. Natl. Acad. Sci. USA 97, 10400-10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci, L., Stamnes, M., Ravazzola, M., Amherdt, M., Perrelet, A., Sollner, T.H., and Rothman, J.E. (1997). Bidirectional transport by distinct populations of COP I-coated vesicles. Cell 90, 335-349. [DOI] [PubMed] [Google Scholar]
- Paek, I., Orci, L., Ravazzola, M., Erdjument-Bromage, H., Amherdt, M., Tempst, P., Sollner, T.H., and Rothman, J.E. (1997). ERS-24, a mammalian v-SNARE implicated in vesicle traffic between the ER and the Golgi. J. Cell Biol. 137, 1017-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade, G. (1975). Intracellular aspects of the process of protein synthesis. Science 189, 347-358. [DOI] [PubMed] [Google Scholar]
- Parlati, F., McNew, J.A., Fukuda, R., Miller, R., Sollner, T.H., and Rothman, J.E. (2000). Topological restriction of SNARE-dependent membrane fusion. Nature 407, 194-198. [DOI] [PubMed] [Google Scholar]
- Parlati, F., Varlamov, O., Paz, K., McNew, J.A., Hurtado, D., Sollner, T.H., and Rothman, J.E. (2002). Distinct SNARE complexes mediating membrane fusion in Golgi transport based on combinatorial specificity. Proc. Natl. Acad. Sci. USA 99, 5424-5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumet, F., Brugger, B., Parlati, F., Sollner, T., and Rothman, J.E. (2001). A t-SNARE of the endocytic pathway must be activated for fusion. J. Cell Biol. 155, 961-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham, H.R.B., and Rothman, J.E. (2000). The dabate about transport in the Golgi-two sides of the same coin? Cell 102, 713-719. [DOI] [PubMed] [Google Scholar]
- Rambourg, A., Clermont, Y., Hermo, L., and Segretain, D. (1987). Tridimensional structure of the Golgi apparatus of non-ciliated epithelial cells of the ductuli efferentes in rat: an electron microscopic stereoscopic study. Biol. Cell 60, 103-116. [DOI] [PubMed] [Google Scholar]
- Rothman, J.E. (1981). The Golgi apparatus: two organelles in tandem. Science 213, 1212-1219. [DOI] [PubMed] [Google Scholar]
- Rowe, T., Dascher, C., Bannykh, S., Plutner, H., and Balch, W.E. (1998). Role of vesicle-associated syntaxin 5 in the assembly of pre-Golgi intermediates. Science 279, 696-700. [DOI] [PubMed] [Google Scholar]
- Seemann, J., Jokitalo, E.J., and Warren, G. (2000). The role of the tethering proteins p115 and GM130 in transport through the Golgi apparatus in vivo. Mol. Biol. Cell 11, 635-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim, J., Newman, A.P., and Ferro-Novick, S. (1991). The BOS1 gene encodes an essential 27-kD putative membrane protein that is required for vesicular transport from the ER to the Golgi complex in yeast. J. Cell Biol. 113, 55-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter, J., Beard, M.B., Seemann, J., Dirac-Svejstrup, A.B., and Warren, G. (2002). Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J. Cell Biol. 157, 45-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard, M., Tani, K., Ye, R.R., Geromanos, S., Tempst, P., Kirchausen, T., Rothman, J.E., and Sollner, T. (1994). A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell 78, 937-948. [DOI] [PubMed] [Google Scholar]
- Sollner, T., Whiteheart, S.W., Brunner, M., Erdjument-Bromage, H., Geromanos, S., Tempst, P., and Rothman, J.E. (1993). SNAP receptors implicated in vesicle targeting and fusion. Nature 362, 318-324. [DOI] [PubMed] [Google Scholar]
- Sonnichsen, B., De Renzis, S., Nielson, E., Rietdorf, J., and Zerial, M. (2000). Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149, 901-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnichsen, B., Lowe, M., Levine, T., Jamsa, E., Dirac-Svejstrup, B., and Warren, G. (1998). A role for giantin in docking COPI vesicles to Golgi membranes. J. Cell Biol. 140, 1013-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam, V.N., Peter, F., Philp, R., Wong, S.H., and Hong, W. (1996). GS28, a 28-kilodalton Golgi SNARE that participates in ER-Golgi transport. Science 272, 1161-1163. [DOI] [PubMed] [Google Scholar]
- Sutton, R.B., Fasshauer, D., Jahn, R., and Brunger, A.T. (1998). Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347-353. [DOI] [PubMed] [Google Scholar]
- Ungermann, C., Fischer von Mollard, G., Jensen, O.N., Margolis, N., Stevens, T.H., and Wickner, W. (1999). Three v-SNAREs and Two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion. J. Cell Biol. 145, 1435-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann, C., Nichols, B.J., Pelham, H.R., and Wickner, W. (1998). A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J. Cell Biol. 140, 61-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volchuk, A., Amherdt, M., Ravazzola, M., Brugger, B., Rivera, V.M., Clackson, T., Perrelet, A., Söllner, T.H., Rothman, J.E., and Orci, L. (2000). Megavesicles implicated in the rapid transport of intracisternal aggregates across the Golgi stack. Cell 102, 335-348. [DOI] [PubMed] [Google Scholar]
- Weber, T., Zemelman, B.V., McNew, J.A., Westermann, B., Gmachl, M., Parlati, F., Sollner, T.H., and Rothman, J.E. (1998). SNAREpins: minimal machinery for membrane fusion. Cell 92, 759-772. [DOI] [PubMed] [Google Scholar]
- Wegmann, D., Hess, P., Baier, C., Wieland, F.T., and Reinhard, C. (2003). Novel isotypic g/z-subunits reveal three coatomer complexes in mammals. Mol. Cell. Biol. 24, 1070-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y., Martin, S., James, D.E., and Hong, W. (2002). GS15 forms a SNARE complex with syntaxin 5, GS28, and Ykt6 and is implicated in traffic in the early cisternae of the Golgi apparatus. Mol. Biol. Cell 13, 3493-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y., Wong, S.H., Tang, B.L., Subramaniam, V.N., Zhang, T., and Hong, W. (1998). A 29-kilodalton Golgi soluble N-ethylmaleimide-sensitive factor attachment protein receptor (Vti1-rp2) implicated in protein trafficking in the secretory pathway. J. Biol. Chem. 273, 21783-21789. [DOI] [PubMed] [Google Scholar]
- Xu, Y., Wong, S.H., Zhang, T., Subramaniam, V.N., and Hong, W. (1997). GS15, a 15-kilodalton Golgi soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) homologous to rbet1. J. Biol. Chem. 272, 20162-20166. [DOI] [PubMed] [Google Scholar]
- Zhang, T., and Hong, W. (2001). Ykt6 forms a SNARE complex with syntaxin 5, GS28, and Bet1 and participates in a late stage in endoplasmic reticulum-Golgi transport. J. Biol. Chem. 276, 27480-27487. [DOI] [PubMed] [Google Scholar]
- Zhang, T., Wong, S.H., Tang, B.L., Xu, Y., and Hong, W. (1999). Morphological and functional association of Sec22b/ERS-24 with the pre-Golgi intermediate compartment. Mol. Biol. Cell 10, 435-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T., Wong, S.H., Tang, B.L., Xu, Y., Peter, F., Subramaniam, V.N., and Hong, W. (1997). The mammalian protein (rbet1) homologous to yeast Bet1p is primarily associated with the pre-Golgi intermediate compartment and is involved in vesicular transport from the endoplasmic reticulum to the Golgi apparatus. J. Cell Biol. 139, 1157-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]