Abstract
This study tested the hypothesis that heightened bacterial colonization and delayed wound closure in aged mice could be attenuated by granulocyte-colony stimulating factor (G-CSF) treatment. Previously, we reported that aged mice had elevated bacterial levels, protracted wound closure and reduced wound neutrophil accumulation following Staphylococcus aureus wound infection relative to young mice. In aseptic wound models, G-CSF treatment improved wound closure in aged mice to rates observed in young mice. Given these data, our objective was to determine if G-CSF could restore age-associated differences in wound bacterial burden and closure by increasing wound neutrophil recruitment. Young (3–4 month) and aged (18–20 month) BALB/c mice received three dorsal, subcutaneous injections of G-CSF (250 ng/50 μl/injection) or saline control (50 μl/injection) 30 minutes after wound infection. Mice were sacrificed at days 3 and 7 post wound infection and bacterial colonization, wound size, wound leukocyte accumulation and peripheral blood were evaluated. At days 3 and 7 after wound infection, bacterial colonization was significantly reduced in G-CSF-treated aged mice to levels observed in saline-treated young animals. Wound size was reduced in G-CSF-treated aged animals, with no affect on wound size in G-CSF-treated young mice. Local G-CSF treatment significantly enhanced neutrophil wound accumulation in aged mice, whereas there was no G-CSF-induced change in young mice. These data demonstrate that G-CSF enhances bacterial clearance and wound closure in an age-dependent manner. Moreover, G-CSF may be of therapeutic potential in the setting of post-operative wound infection or chronic, non-healing wounds in elderly patients.
Keywords: Aging, neutrophils, macrophages, wound healing, innate immune, chronic wounds
Introduction
Chronic, non-healing wounds are estimated to account for over 25 billion in US health care costs (1). The failure to effectively heal wounds is often compounded by co-morbidities, such as diabetes or obesity. Another major patient population afflicted with chronic wounds are the elderly (1–3). With advanced age, a decline in immune function elevates rates of wound infection and further impairs wound closure in these patients (4, 5). In particular, Staphylococcus aureus (S. aureus) infection accounts for 30% of surgical site infections in healthy, non-aged patients (2). In the elderly, S. aureus infection jumps to 50%, suggesting a proclivity of this Gram-positive bacterium for the immunocompromised (2). This increased predilection for infectious complications raises additional public health concerns, such as prolonged hospital stays, risk for septic complications or infectious spread in long-term care accommodations. As we continue to see a precipitous rise in the aged population, the number of total patients affected by non-healing wounds is expected to rise from the estimated 6.5 million (1). Moreover, despite investment in antibiotic development for multi-drug resistant bacterial strains, these bacteria continually evolve to evade our treatment options (6). New therapeutic targets that harness the host immune system to help eradicate infection and promote efficient wound healing will be necessary to decrease the public health cost due to chronic wounds and wound infection (7).
Despite the knowledge that aging impairs wound healing, little has been done to determine how the early innate immune response is altered with age in the setting of wound infection. Models of aging and wound healing have shown alteration in the innate immune response during the early inflammatory phased of wound healing, as well as perturbations in mediators of the proliferative and remodeling stages (8–12). To evaluate how age negatively impacts the innate immune response to cutaneous wound infection, we previously developed a model of cutaneous wound injury and S. aureus infection in young and aged BALB/c mice (13). In this model, aged mice had heightened levels of bacterial colonization and delayed wound closure as compared to young animals. These findings were associated with reduced neutrophil recruitment to the wound site, despite elevated chemokine levels and adequate peripheral blood neutrophil CXCR2 expression (13). Moreover, we saw diminished neutrophil chemotaxis to the skin following subcutaneous injections of the murine neutrophil chemoattractant KC (13). Others have demonstrated that addition of granulocyte-colony stimulating factor (G-CSF) to their model of aseptic wound healing enhanced wound closure in aged animals (14), and that G-CSF can restore defects in age-associated neutrophil chemotaxis (15). Herein, we are the first to demonstrate that local administration of G-CSF reduced bacterial colonization and enhanced wound closure in aged animals via increased neutrophil recruitment at early time points. These data suggest that local G-CSF treatment may serve as a potential therapeutic intervention in elderly patients with wound infections.
Materials and Methods
Animal Model of Cutaneous Injury and Infection
Young (3–4 month) and aged (18–20 month) BALB/c mice (Charles River/NIA, Kingston Facility, Stony Ridge, NY) were used to determine if local, subcutaneous (s.c.) G-CSF treatment could restore age-dependent differences to S. aureus wound infection. All animal studies were approved and performed with strict accordance to the regulations established by the Loyola University Chicago Animal Care and Use Committee. Following acclimation at Loyola’s Animal Care Facility, young and aged mice were subjected to dorsal excisional cutaneous injury as previously described (13, 16). Briefly, mice were given 10 mg/kg xylazine and 100 mg/kg ketamine intraperitoneal (i.p.) followed by i.p. saline to ensure systemic distribution of the anesthetic. The dorsal skin of the mice was placed over a hockey puck, and mice were subjected to 3 sets of 2, 3 mm dermal punch wounds (Acuderm, Ft. Lauderdale, FL) for a total of 6 dorsal full-thickness cutaneous wounds. Immediately after injury, mice received ~103 colony forming units (CFU) of S. aureus/10 μL of saline and were returned to their cages on heating pads. At 30 minutes after injury and infection, animals received three s.c. G-CSF injections (250 ng/50 μl/injection; R&D Systems, Minneapolis, MN) or saline vehicle injections (50 μl/injection) in the dorsal midline in between each set of wounds. As sepsis is known to negative impact wound healing, a low bacterial inoculum was used to limit the risk of systemic dissemination of bacteria (17). S. aureus Newman strain was grown overnight in tryptic soy broth (TSB) at 37°C under constant agitation. The next day, 1 mL of the overnight culture was resuspended in 2mL fresh TSB and incubated at 37°C for 2 hours to ensure mid-logarithmic growth at the time of application to cutaneous wounds. Absorbance at 600 nm was used to determine the bacterial concentration (CFU/mL) and the final inoculum was confirmed by back-plating on mannitol salt agar (MSA; BD Diagnostics, Sparks, MD).
Mice were sacrificed at days 3 and 7 after injury and infection. The pelt was removed and photographed to measure wound size as described below. A larger 5 mm punch biopsy was used to remove the 3 mm wounds. Wounds were examined for bacterial colonization and flow cytometric analysis of wound immune infiltrate (13). Heparinized whole blood was obtained via cardiac puncture for flow cytometric analysis (13).
Bacterial Colonization
One skin wound was homogenized in 1 mL sterile phosphate buffered saline (PBS; Gibco, Grand Island, NY) and 10-fold serial dilutions to 106 were plated on MSA plates (BD Diagnostics, Sparks, MD). Plates were incubated for 24–48 hours at 37°C and colonies were counted to determine levels of bacterial colonization (13).
Wound Size
Wound size was evaluated by digital photography and image analysis as previously described (13, 16). At days 3 and 7 wounds were photographed with a Canon EOS SLR digital camera. Each pelt was placed flat on a hockey puck and photographed at a fixed distance of 20 cm with a ruler placed within the frame of each photograph. Photoshop 7.0 (Adobe Systems Inc., San Jose, CA) was used to determine the number of pixels in the open wound area using the magic wand tool, with a tolerance setting of 60 and zoom at 100%. Separate animals were sacrificed immediately following wound injury and wound size was determined to represent day 0. Wound areas at each time point were compared with day 0 wounds: (pixels at days1–10/pixels at day 0)×100 were used to determine the percent open wound area at each time point.
Skin Cell Isolation and Flow Cytometry
Single cell suspensions of wound cells for flow cytometry were generated as previously described (13, 18). At days 3 and 7 after injury and infection, animals were euthanized, pelts removed and wounds excised using a 5 mm punch biopsy. Two diced wounds were incubated overnight at 4°C in RPMI 1640 culture media (Gibco, Grand Island, NY) containing 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), 2 mM L-glutamine (Gibco, Grand Island, NY), 1% penicillin/streptomycin (Gibco, Grand Island, NY), 2 mg gentamycin sulfate (Mediatech Inc, Manassas, VA) and 0.3 mg dispase II (Roche Diagnostics, Indianapolis, IN). Tissue pieces were then removed and subjected to further enzymatic digestion with 1 mg collagenase from Clostridium histolyticum type 1A (Sigma-Aldrich, St. Louis, MO), 1.2 mg DNAse I from bovine pancreas Grade II (Roche Diagnostics, Indianapolis, IN), 1 mg hyaluronidase from bovine testes type 1-S (Sigma-Aldrich, St. Louis, MO), in RPMI 1640 with 5% FBS, 2 mM L-glutamine, 1% penicillin/streptomycin, gentamycin and magnesium chloride hexahydtrate for 2 hours at 37°C. After two hours, these solutions were combined and adherent cells were treated with Accutase (eBioscience, San Diego, CA) for 8 minutes at 37°C followed by vigorous pipetting. Single cells suspensions were filtered through at 70 μm filter to remove debris. Cells were washed and adjusted to 1×106/mL. Wound cells were blocked for 20 minutes with FcBlock (anti-CD16/CD32, eBioscience) and rat IgG (Jackson ImmunoResearch, West Grove, PA), and then stained with PE-Cy7-F4/80 (eBioscience, San Diego, CA), FITC-Gr-1 (eBioscience, San Diego, CA) and APC-CD3 (eBioscience, San Diego, CA). Cells were washed twice and then resuspended in flow buffer (1% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO), 0.1% sodium azide and 2mM EDTA in PBS). Events were collected on the FACS LSR Fortessa (BD Biosciences, San Jose, CA) and data were analyzed by FlowJo Software (Tree Star Inc, Ashland, OR). Fluorescence minus one control staining and single color controls were used to determine positive staining. F4/80+Gr-1− cells were considered macrophages, F4/80−Gr-1+ cells were considered neutrophils and CD3+ cells were considered T cells.
Blood Flow Cytometry
Heparinized whole blood was collected and stained per the manufacture’s protocol (Leinco Technologies, St. Louis, MO). 100 μL whole blood was blocked for 20 minutes with FcBlock and rat IgG and then stained with PE-Cy7-F4/80, FITC-Gr-1 and PE-CXCR2 (R&D Systems, Minneapolis, MN). Erythrocytes were then lysed with 2 mL Easy Lyse Solution (Leinco Technologies, St. Louis, MO) for 11.5 minutes. Lysis was stopped by addition of 2 mL ice cold Wash Buffer (Leinco Technologies, St. Louis, MO) and placement on ice. Cells were washed twice and resuspended in 500 μL flow buffer. Events were collected on the FACS LSR Fortessa (BD Biosciences, San Jose, CA) and data were analyzed by FlowJo Software (Tree Star Inc, Ashland, OR). Fluorescence minus one control staining and single color controls were used to determine positive staining. F4/80−Gr-1+ cells were considered peripheral blood neutrophils.
Statistical analysis
Data are shown as mean ± SEM of each group. Data were tested for normality and were analyzed by one-way ANOVA with Tukey’s post-hoc tests or Mann-Whitney U tests where appropriate using GraphPad Instat3 (GraphPad, La Jolla, CA). A value of p ≤0.05 was considered significant.
Results
Local G-CSF treatment improves resolution of bacterial infection and wound closure in aged mice
Previously, we demonstrated that aged mice exhibited elevated bacterial colonization following cutaneous S. aureus wound infection that correlated with reduced neutrophil recruitment (13). G-CSF has been shown to enhance neutrophil recruitment and improve wound closure in aged animals (14), as well as restore age-associated chemotatic defects (15). Thus, we chose to investigate if G-CSF could restore bacterial clearance in aged mice by enhancing wound leukocyte recruitment. Following G-CSF administration, we saw a 75% decrease in bacterial colonization at day 3 in aged mice as compared to aged, saline control animals (Figure 1A, p<0.05). At day 3, G-CSF did not affect wound bacterial levels in young mice. These differences in wound S. aureus colonization persisted out to day 7 with a 97% reduction in bacterial growth in aged animals administered G-CSF compared to aged-saline control (Figure 1B, p<0.05). While a mild elevation in day 7 wound bacterial levels in G-CSF-treated young mice was observed, this difference was not statistically significant from young saline. In concert with these data, we observed that local injection of G-CSF enhanced wound closure in aged animals at day 3 compared to aged, saline controls (Figure 2A+B, p<0.05). At day 7, differences in wound size observed between saline treated young and aged mice was ameliorated following G-CSF treatment (Figure 2A+C, p<0.05). G-CSF treatment did not affect that rate of wound closure in young mice at day 3 or 7.
Figure 1. Local G-CSF treatment reduces bacterial colonization in aged mice.
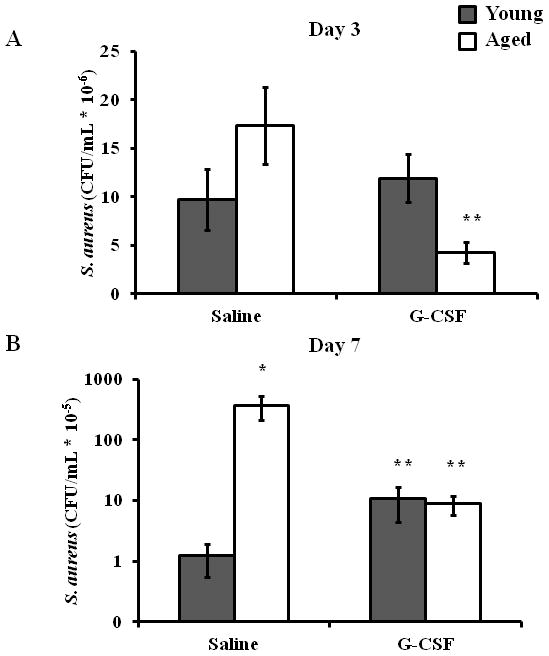
Young (3–4 month, gray bars) and aged (18–20 month, white bars) mice received six, 3 mm dorsal cutaneous wounds followed by ~103 CFU S. aureus/wound. 30 minutes after injury and infection, mice received either s.c. G-CSF injections (250 ng/50 μl/injection) or saline vehicle injections (50 μl/injection). At days 3 (A) and 7 (B), mice were sacrificed and bacterial colonization was determined by growth on MSA plates. Data are shown as mean ± SEM, *p<0.01 compared to young saline and **p<0.05 compared to aged saline by one-way ANOVA (A) or Mann-Whitney U test (B); N=7–14 per group.
Figure 2. Treatment with G-CSF abrogates age-associated deficits in wound closure.
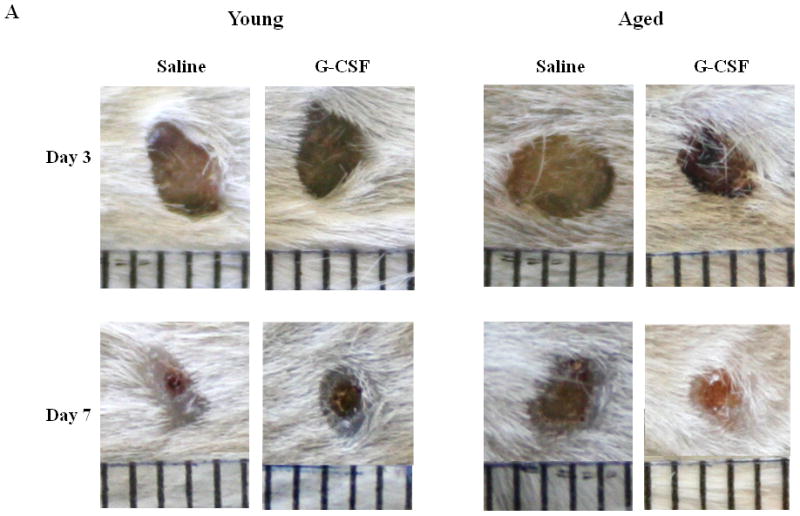
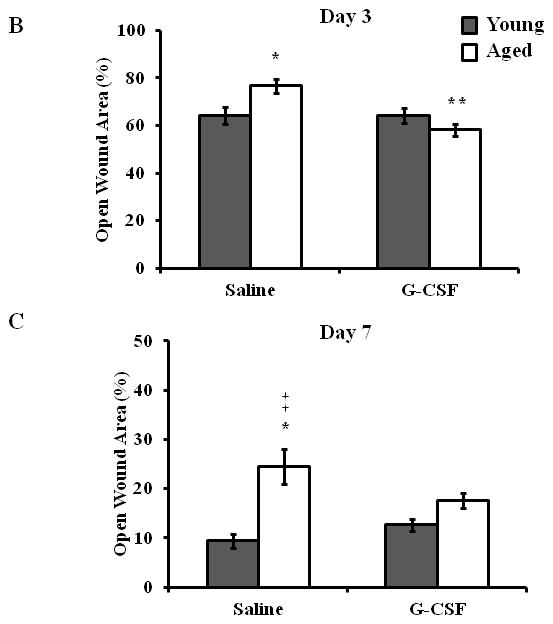
(A) Representative images of wound from young (left panel) and aged (right panel) mice at days 3 (top panel) and 7 (bottom panel) after S. aureus wound infection with or without G-CSF treatment. Wound size expressed as percent open wound area relative to time zero at days 3 (B) and 7 (C) after cutaneous wound infection in young (gray bars) and aged (white bars) mice. Data are shown as mean ± SEM, *p<0.05 compared to young saline, **p<0.01 compared to aged saline and ‡p<0.05 compared to young G-CSF by one-way ANOVA; N=7–14 per group.
Wound neutrophil recruitment in aged animals in enhanced by local G-CSF treatment
Given that G-CSF restored bacterial clearance and wound closure in aged mice, we sought to examine if this correlated with enhanced leukocyte recruitment to the wound bed (Figure 3A). At day 3 following injury and infection, saline-treated aged mice had reduced absolute numbers of neutrophil and macrophage accumulation compared to young, saline treated mice (Figure 3B+C, p<0.5). Treatment with G-CSF increased neutrophil numbers in aged mice in respect to aged, saline treated animals (Figure 3B, p<0.05) and neutrophil recruitment was no longer different from young mice. No differences in neutrophil accumulation in young mice administered saline or G-CSF were observed. G-CSF treatment did not impact macrophage recruitment at day 3 in aged animals (Figure 3C). While a mild reduction in macrophage recruitment in young animals given G-CSF was observed, these differences were not significant.
Figure 3. Neutrophil accumulation is enhanced in aged mice following G-CSF treatment.

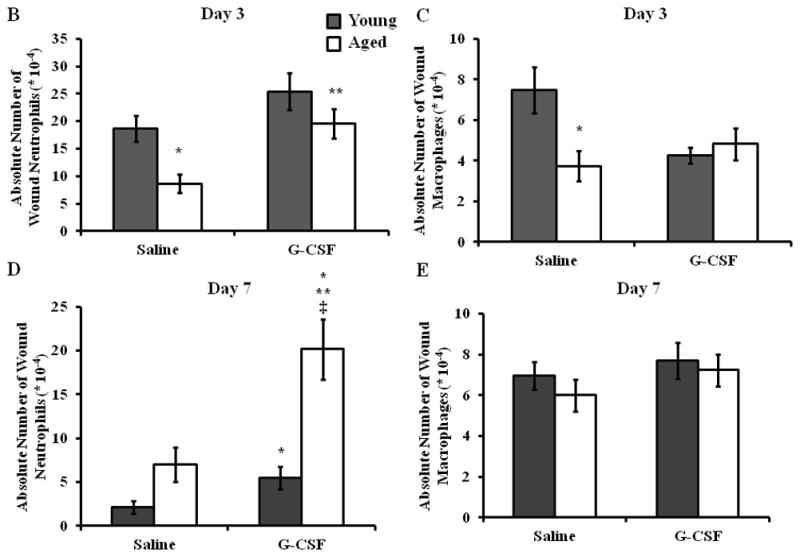
Leukocyte infiltration to the wound and surrounding tissue area was assessed by flow cytometry at days 3 and 7 following cutaneous wound infection and G-CSF treatment. (A) Gating strategy for wound neutrophils (F4/80−/Gr-1+) and wound macrophages (F4/80+/Gr-1−). Absolute number of wound neutrophils and macrophages at day 3 (B and C, respectively) and day 7 (D and E, respectively). Data are shown as mean ± SEM, *p<0.05 compared to young saline, **p<0.05 compared to aged saline and ‡p<0.01 compared to young G-CSF by one-way ANOVA (B, C, E) or Mann-Whitney U-test (D); N=7–14 per group.
No-age dependent effects of local G-CSF treatment on circulating leukocytes
To eliminate the possibility that local, s.c. G-CSF was inducing changes in the peripheral blood pool, we evaluated the frequency of circulating neutrophils in young and aged mice given saline or G-CSF injections (Figure 4). As reported previously (13), aged mice exhibited heightened levels of circulating neutrophils at day 3 compared young mice (Figure 4B, p<0.5), and this was not altered by G-CSF administration. This age-dependent elevation in the frequency of peripheral blood neutrophils persisted out to day 7 (Figure 4C, p<0.05). The frequency of CXCR2+ neutrophils in circulation was mildly increased in aged mice in both treatment groups at days 3 and 7 compared to young animals (Figure 4D–E, p<0.05). Again, G-CSF treatment did not impact the age-dependent difference in the frequency of CXCR2+ neutrophils. Similar to our previous study, aged animals exhibited elevated expression of CXCR2 at day 3 compared to young animals (Figure 4F, p<0.05), and G-CSF did not impact the MFI of CXCR2 in either young or aged mice. No age- or G-CSF-dependent differences in CXCR2 expression on circulating neutrophils were observed at day 7 (Figure 4G).
Figure 4. Local G-CSF does not alter the circulating neutrophil pool in aged mice.
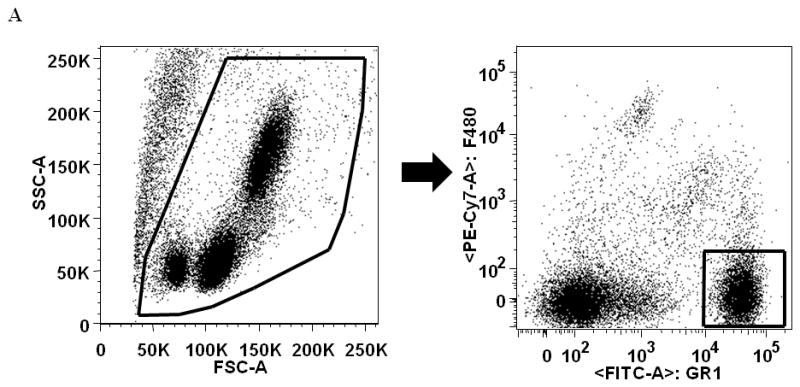
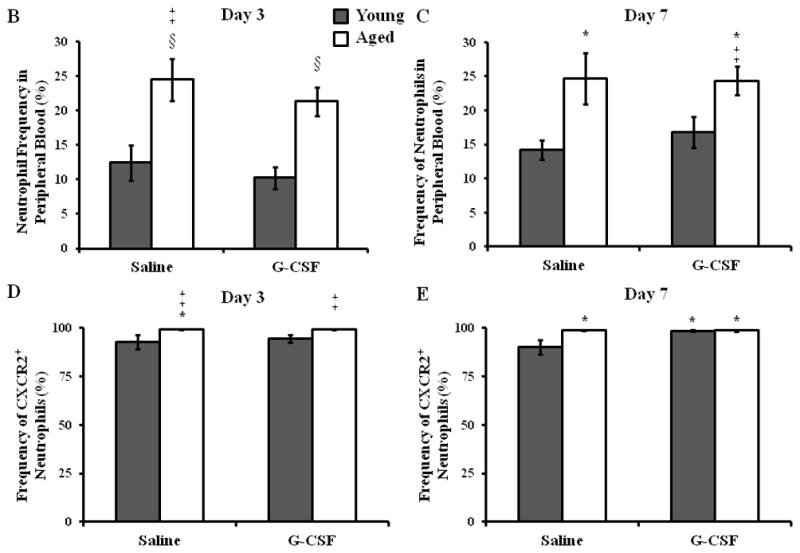
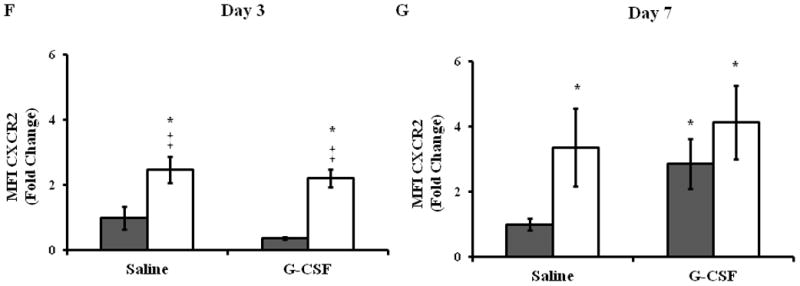
Whole blood was analyzed by flow cytometry to determine the percentage of circulating neutrophils and expression of the chemotatic receptor CXCR2. (A) Gating strategy for neutrophils (F4/80−Gr-1+) in whole blood by flow cytometry. Percentage of circulating neutrophils in peripheral blood in young (gray bars) and aged (white bars) mice at day 3 (B) and day 7 (C). Frequency of CXCR2+ neutrophils in peripheral blood at day 3 (D) and day 7 (E). Expression of CXCR2 on peripheral blood neutrophils at day 3 (F) and day 7 (G). Data are shown as mean ± SEM, *p<0.05 compared to young saline, ‡p<0.05 compared to young G-CSF and §p<0.05 compared to young with the same treatment by one-way ANOVA (B) or Mann-Whitney U-test (C–G); N=7–14 per group.
Discussion
Herein, we demonstrate a beneficial effect of G-CSF treatment on resolution of infection and wound healing in aged mice following S. aureus wound infection. These reductions in our primary outcomes were associated with enhanced neutrophil recruitment to the wound site in aged mice following treatment with G-CSF. To our knowledge, this is the first study to demonstrate the differential effects of local G-CSF as a therapeutic modality in the setting of wound infection with advanced aged.
Aging studies in aseptic wound models have shown that systemic G-CSF enhances neutrophil recruitment and reduces time to wound resolution in aged animals (14). While the role of neutrophils in aseptic wound healing has been questioned (19), patient populations that are at increased risk for infectious complications, such as aging or immunocompromised patients, demonstrate a necessity for neutrophil recruitment during wound repair (13). Previously, we demonstrated that neutrophils from aged mice exhibit a migratory defect to the murine neutrophil CXCR2 ligand KC in vitro and in vivo (13, 20). Moreover, we have observed neutrophil chemokine levels to be similar or elevated in wound homogenates and enhanced expression of CXCR2 on circulating neutrophils in aged mice (13). Together, these data suggested that age-associated alterations in the downstream CXCR2 signaling cascade may contribute to reduced neutrophil recruitment. G-CSF has been shown to mediate neutrophil migration to macrophage-inflammatory protein-2 (MIP-2), another CXCR2 ligand, via induction of CXCR2 expression and regulation of CXCR2 signaling (21). Additionally, G-CSF has been show to restore neutrophil chemotaxis in aged rats (15) and following systemic trauma (22). In our study, we did not observe a difference in CXCR2 expression pre- or post- G-CSF treatment, irrespective of age. Previously, we demonstrated that aging was associated with reduced ICAM-1 expression in wound homogenates in aged animals as compared to young (13). In addition to the effects on CXCR2 signaling, G-CSF has been reported to promote endothelial ICAM-1 expression (23) and adherence of neutrophils to endothelial ICAM-1(24, 25). We postulate that restoration of neutrophil recruitment in our model may be through a G-CSF mediated restitution of CXCR2-directed chemotaxis in aged mice, without direct effects on CXCR2 expression, or may be related to the effects of G-CSF on endothelial adhesion proteins. Future studies from our laboratory will focus on these possible mechanisms for age-dependent G-CSF mediated restoration of bacterial clearance and wound closure.
G-CSF is a known modulator of granulopoiesis and hematopoietic stem cell mobilization as well as a regulator of neutrophil bone marrow emigration, survival, chemotaxis and phagocytosis (26–28). Currently, G-CSF (Filagrastim/PEG-Filagrastim) is indicated for patients with congenital or iatrogenic neutropenias (29) and for donors prior to hematopoietic stem cell transplantation (30). Pre-clinical and clinical trials are underway to investigate the therapeutic potential of G-CSF in amyotrophic lateral sclerosis (31, 32), acute liver failure/alcoholic hepatitis (33), myocardial infarction (34), peripheral arterial disease (35) and diabetic foot infections (36). Alongside our study, these reports provided novel indications for the expanded use of G-CSF as therapeutic interventions.
The studies using G-CSF in the setting of diabetic wound infection provide an interesting point of comparison to the data presented in this manuscript. Cruiciana et al. reviewed five randomized controlled trials with a total of 167 patients that added G-CSF to treatment regimens for patients with diabetic foot infections. Though one study reported a shorter time to resolution of infection and intravenous antibiotic use (37), the meta-analysis revealed that addition of G-CSF did not decrease the risk of infectious complications or enhance wound closure, but it did reduce the relative risk of lower limb surgical interventions and the hospital length of stay (36). Similar to advanced aged, neutrophil dysfunction and elevated risk of S. aureus infection are associated with diabetes (38). However, the studies above do not suggest a strong therapeutic benefit for G-CSF in diabetic patients. In contrast to our model, diabetes is also associated with peripheral vascular disease that may inhibit additional facets of wound healing which may not be modulated by G-CSF. Moreover, studies in db/db mouse models have actually demonstrated elevated neutrophil recruitment to sites of S. aureus infection in db/db mice (38). This is in contrast to our model, in which reduced neutrophil accumulation in aged mice was associated with delayed bacterial clearance and wound closure.
Other studies do support a therapeutic benefit for G-CSF in prevention of infectious complications (39, 40). In patients undergoing esophagectomy for esophageal cancer, perioperative, s.c. G-CSF improved neutrophil effector functions and elevated circulating leukocyte counts (39). Postoperative mortality and infection rates were also reduced in the treatment groups as compared to historical controls (39). In a case report of a neutropenic patient that developed a non-healing pharyngocutaneous fistula following radical laryngectomy, daily G-CSF treatment promoted fistula closure where previous aggressive wound therapy failed (40). These papers support the use of G-CSF in infectious wound care settings in neutropenic patients. While no peripheral neutropenia was noted in our aged animals prior to G-CSF treatment, we did observe a local reduction in neutrophil numbers at the wound infection site in saline-treated aged mice that was restored following G-CSF administration.
The goal of this current study was to evaluate the potential role for G-CSF in improved wound closure and resolution of infection in aged animals. We do recognize that our study represents an acute wound infection setting, and its clinical applications to chronic wound states are limited as our model does not replicate chronic wound healing observed clinically in aging or neutropenic patients. Our aim was to identify early perturbations in wound healing with advanced age that may offer therapeutic targets to prevent development of chronic wound states. Further work to evaluate the role of G-CSF in chronic wound models is warranted. Additionally, while our group and others have consistently reported the used of 3mm punch wound models in aging murine studies (8, 13, 14, 16), concerns about the role of contracture given the small wound size are realistic. However, studies controlling for contracture with the use of a silicon ring continue to demonstrate similar age-dependent difference in wound healing, suggesting that age-dependent differences in wound healing remain independent of contractile forces in murine models (9, 41). We do understand that while some animal models fail to recapitulate clinical findings, our primary outcome of delayed would closure and impaired bacterial clearance were used to design this model in a best effort to parallel clinical observations (2, 3).
While future work needs to be conducted to elucidate the mechanism by which G-CSF exerts these age-dependent effects, our data highlights a putative role for expanded indications of G-CSF treatment within the wound care setting to the elderly patient population as well as patients with local or systemic neutropenia.
Acknowledgments
The authors would like to thank Juan L. Rendon, Sara Hlavin, Stewart R. Carter and Patricia Simms for their technical assistance and thoughtful critique of this manuscript. The authors would also like to thank Pamela L. Witte, PhD as head of the Immunology and Aging Program at Loyola University Medical Center and for her support on the Institutional training grant. This work was supported by NIH/NIA R01 AG018859 (EJK), T32 AG031780 (PLW), the Ralph and Marian C. Falk Medical Research Trust (EJK) and the Loyola University Chicago Stritch School of Medicine MD/PhD Program.
Footnotes
Disclosures:
The authors have no financial conflict of interest.
References
- 1.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaye KS, Anderson DJ, Sloane R, Chen LF, Choi Y, Link K, Sexton DJ, Schmader KE. The effect of surgical site infection on older operative patients. J Am Geriatr Soc. 2009;57:46–54. doi: 10.1111/j.1532-5415.2008.02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vowden KR, Vowden P. The prevalence, management and outcome for patients with lower limb ulceration identified in a wound care survey within one English health care district. J Tissue Viability. 2009;18:13–19. doi: 10.1016/j.jtv.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Gosain A, DiPietro LA. Aging and wound healing. World J Surg. 2004;28:321–6. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- 5.Brubaker AL, Palmer JL, Kovacs EJ. Age-related Dysregulation of Inflammation and Innate Immunity: Lessons Learned from Rodent Models. Aging Dis. 2011;2:346–360. [PMC free article] [PubMed] [Google Scholar]
- 6.Ug A, Ceylan O. Occurrence of resistance to antibiotics, metals, and plasmids in clinical strains of Staphylococcus spp. Arch Med Res. 2003;34:130–6. doi: 10.1016/S0188-4409(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 7.Harrison CJ. Innate immunity as a key element in host defense against methicillin resistant Staphylococcus aureus. Minerva Pediatr. 2009;61:503–514. [PubMed] [Google Scholar]
- 8.Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 2001;117:1027–35. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- 9.Loh SA, Chang EI, Galvez MG, Thangarajah H, El-ftesi S, Vial IN, Lin DA, Gurtner GC. SDF-1 alpha expression during wound healing in the aged is HIF dependent. Plast Reconstr Surg. 2009;123:65S–75S. doi: 10.1097/PRS.0b013e318191bdf4. [DOI] [PubMed] [Google Scholar]
- 10.Ashcroft GS, Horan MA, Ferguson MW. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab Invest. 1998;78:47–58. [PubMed] [Google Scholar]
- 11.Komi-Kuramochi A, Kawano M, Oda Y, Asada M, Suzuki M, Oki J, Imamura T. Expression of fibroblast growth factors and their receptors during full-thickness skin wound healing in young and aged mice. J Endocrinol. 2005;186:273–89. doi: 10.1677/joe.1.06055. [DOI] [PubMed] [Google Scholar]
- 12.Ashcroft GS, Horan MA, Ferguson MW. Aging is associated with reduced deposition of specific extracellular matrix components, an upregulation of angiogenesis, and an altered inflammatory response in a murine incisional wound healing model. J Invest Dermatol. 1997;108:430–7. doi: 10.1111/1523-1747.ep12289705. [DOI] [PubMed] [Google Scholar]
- 13.Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ. Reduced Neutrophil Chemotaxis and Infiltration Contributes to Delayed Resolution of Cutaneous Wound Infection with Advanced Age. J Immunol. 2013;190(4):1746–57. doi: 10.4049/jimmunol.1201213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishio N, Okawa Y, Sakurai H, Isobe K. Neutrophil depletion delays wound repair in aged mice. Age (Dordr) 2008;30:11–19. doi: 10.1007/s11357-007-9043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshino T, Tamura M, Kawabe M, Nomura H, Imai N, Ono M. Effects of recombinant human granulocyte colony-stimulating factor on neutrophil functions in aged animals. Br J Haematol. 1992;82:664–670. doi: 10.1111/j.1365-2141.1992.tb06941.x. [DOI] [PubMed] [Google Scholar]
- 16.Schneider DF, Palmer JL, Tulley JM, Kovacs EJ, Gamelli RL, Faunce DE. Prevention of NKT Cell Activation Accelerates Cutaneous Wound Closure and Alters Local Inflammatory Signals. J Surg Res. 2010;171(1):361–73. doi: 10.1016/j.jss.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rico RM, Ripamonti R, Burns AL, Gamelli RL, DiPietro LA. The effect of sepsis on wound healing. J Surg Res. 2002;102:193–7. doi: 10.1006/jsre.2001.6316. [DOI] [PubMed] [Google Scholar]
- 18.Brubaker AL, Schneider DF, Palmer JL, Faunce DE, Kovacs EJ. An improved cell isolation method for flow cytometric and functional analyses of cutaneous wound leukocytes. J Immunol Methods. 2011;373:161–166. doi: 10.1016/j.jim.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73:448–55. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 20.Nomellini V, Brubaker AL, Mahbub S, Palmer JL, Gomez CR, Kovacs EJ. Dysregulation of Neutrophil CXCR2 and Pulmonary Endothelial ICAM-1 Promotes Age-Related Pulmonary Inflammation. Aging and disease. 2012;3:234–47. [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen-Jackson H, Panopoulos AD, Zhang H, Li HS, Watowich SS. STAT3 controls the neutrophil migratory response to CXCR2 ligands by direct activation of G-CSF-induced CXCR2 expression and via modulation of CXCR2 signal transduction. Blood. 2010;115:3354–63. doi: 10.1182/blood-2009-08-240317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sartorelli KH, Silver GM, Gamelli RL. The effect of granulocyte colony-stimulating factor (G-CSF) upon burn-induced defective neutrophil chemotaxis. J Trauma. 1991;31:523–9. doi: 10.1097/00005373-199104000-00012. discussion 529–30. [DOI] [PubMed] [Google Scholar]
- 23.Fuste B, Mazzara R, Escolar G, Merino A, Ordinas A, Diaz-Ricart M. Granulocyte colony-stimulating factor increases expression of adhesion receptors on endothelial cells through activation of p38 MAPK. Haematologica. 2004;89:578–585. [PubMed] [Google Scholar]
- 24.Hakansson L, Hoglund M, Jonsson UB, Torsteinsdottir I, Xu X, Venge P. Effects of in vivo administration of G-CSF on neutrophil and eosinophil adhesion. Br J Haematol. 1997;98:603–611. doi: 10.1046/j.1365-2141.1997.2723093.x. [DOI] [PubMed] [Google Scholar]
- 25.Chakraborty A, Hentzen ER, Seo SM, Smith CW. Granulocyte colony-stimulating factor promotes adhesion of neutrophils. Am J Physiol Cell Physiol. 2003;284:C103–10. doi: 10.1152/ajpcell.00165.2002. [DOI] [PubMed] [Google Scholar]
- 26.Roberts AW. G-CSF: a key regulator of neutrophil production, but that’s not all! Growth Factors. 2005;23:33–41. doi: 10.1080/08977190500055836. [DOI] [PubMed] [Google Scholar]
- 27.Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100:854–61. doi: 10.1182/blood.v100.3.854. [DOI] [PubMed] [Google Scholar]
- 28.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 29.Morishita M, Leonard RC. Pegfilgrastim; a neutrophil mediated granulocyte colony stimulating factor-expanding uses in cancer chemotherapy. Expert Opin Biol Ther. 2008;8:993–1001. doi: 10.1517/14712598.8.7.993. [DOI] [PubMed] [Google Scholar]
- 30.Martino M, Fedele R, Massara E, Recchia AG, Irrera G, Morabito F. Long-term safety of granulocyte colony-stimulating factor in normal donors: is it all clear? Expert Opin Biol Ther. 2012;12:609–621. doi: 10.1517/14712598.2012.674937. [DOI] [PubMed] [Google Scholar]
- 31.Chio A, Mora G, La Bella V, Caponnetto C, Mancardi G, Sabatelli M, Siciliano G, Silani V, Corbo M, Moglia C, et al. Repeated courses of granulocyte colony-stimulating factor in amyotrophic lateral sclerosis: clinical and biological results from a prospective multicenter study. Muscle Nerve. 2011;43:189–195. doi: 10.1002/mus.21851. [DOI] [PubMed] [Google Scholar]
- 32.Duning T, Schiffbauer H, Warnecke T, Mohammadi S, Floel A, Kolpatzik K, Kugel H, Schneider A, Knecht S, Deppe M, et al. G-CSF prevents the progression of structural disintegration of white matter tracts in amyotrophic lateral sclerosis: a pilot trial. PLoS One. 2011;6:e17770. doi: 10.1371/journal.pone.0017770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, Sakhuja P, Sarin SK. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:505–512.e1. doi: 10.1053/j.gastro.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 34.Kang HJ, Yoon EJ, Lee EJ, Kim MK, Seo JW, Park KW, Lee HY, Park KU, Cho YS, Koo BK, et al. Co-treatment with darbepoetin and granulocyte-colony stimulating factor is efficient to recruit pro-angiogenic cell populations in patients with acute myocardial infarction. Cell Transplant. 2012;21(5):1055–61. doi: 10.3727/096368911X627499. [DOI] [PubMed] [Google Scholar]
- 35.Kawamura I, Takemura G, Tsujimoto A, Watanabe T, Kanamori H, Esaki M, Kobayashi H, Takeyama T, Kawaguchi T, Goto K, et al. Treatment of leg ischemia with biodegradable gelatin hydrogel microspheres incorporating granulocyte colony-stimulating factor. J Cardiovasc Pharmacol. 2011;57:416–423. doi: 10.1097/FJC.0b013e31820c9776. [DOI] [PubMed] [Google Scholar]
- 36.Cruciani M, Lipsky BA, Mengoli C, de Lalla F. Granulocyte-colony stimulating factors as adjunctive therapy for diabetic foot infections. Cochrane Database Syst Rev. 2009;(3):CD006810. doi: 10.1002/14651858.CD006810.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Gough A, Clapperton M, Rolando N, Foster AV, Philpott-Howard J, Edmonds ME. Randomised placebo-controlled trial of granulocyte-colony stimulating factor in diabetic foot infection. Lancet. 1997;350:855–859. doi: 10.1016/S0140-6736(97)04495-4. [DOI] [PubMed] [Google Scholar]
- 38.Park S, Rich J, Hanses F, Lee JC. Defects in innate immunity predispose C57BL/6J-Leprdb/Leprdb mice to infection by Staphylococcus aureus. Infect Immun. 2009;77:1008–1014. doi: 10.1128/IAI.00976-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schafer H, Hubel K, Bohlen H, Mansmann G, Hegener K, Richarz B, Oberhauser F, Wassmer G, Holscher AH, Pichlmaier H, et al. Perioperative treatment with filgrastim stimulates granulocyte function and reduces infectious complications after esophagectomy. Ann Hematol. 2000;79:143–151. doi: 10.1007/s002770050570. [DOI] [PubMed] [Google Scholar]
- 40.Cody DT, 2nd, Funk GF, Wagner D, Gidley PW, Graham SM, Hoffman HT. The use of granulocyte colony stimulating factor to promote wound healing in a neutropenic patient after head and neck surgery. Head Neck. 1999;21:172–175. doi: 10.1002/(sici)1097-0347(199903)21:2<172::aid-hed13>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 41.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon B, Le AD. Human Gingiva-Derived Mesenchymal Stem Cells Elicit Polarization of M2 Macrophages and Enhance Cutaneous Wound Healing. Stem Cells. 2010;28(10):1856–68. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]


