Abstract
Schizophrenia is a highly debilitating mental disorder which afflicts approximately 1% of the global population. Cognitive and negative deficits account for the lifelong disability associated with schizophrenia, whose symptoms are not effectively addressed by current treatments. New medicines are needed to treat these aspects of the disease. Neurodevelopmental, neuropathological, genetic, and behavioral pharmacological data indicate that schizophrenia stems from a dysfunction of glutamate synaptic transmission, particularly in frontal cortical networks. A number of novel pre- and postsynaptic mechanisms affecting glutamatergic synaptic transmission have emerged as viable targets for schizophrenia. While developing orthosteric glutamatergic agents for these targets has proven extremely difficult, targeting allosteric sites of these targets has emerged as a promising alternative. From a medicinal chemistry perspective, allosteric sites provide an opportunity of finding agents with better drug-like properties and greater target specificity. Furthermore, allosteric modulators are better suited to maintaining the highly precise temporal and spatial aspects of glutamatergic synaptic transmission. Herein, we review neuropathological and genomic/genetic evidence underscoring the importance of glutamate synaptic dysfunction in the etiology of schizophrenia and make a case for allosteric targets for therapeutic intervention. We review progress in identifying allosteric modulators of AMPA receptors, NMDA receptors, and metabotropic glutamate receptors, all with the aim of restoring physiological glutamatergic synaptic transmission. Challenges remain given the complexity of schizophrenia and the difficulty in studying cognition in animals and humans. Nonetheless, important compounds have emerged from these efforts and promising preclinical and variable clinical validation has been achieved.
Keywords: Allosterism, AMPA, glycine, glutamate, NAMS, NMDA, PAMS, schizophrenia
INTRODUCTION
Schizophrenia is a debilitating mental disorder afflicting ~1% of the global population [1]. The disorder has three clinical symptom domains: episodic psychosis (hallucination, delusions), chronic withdrawal (negative symptoms) and pervasive cognitive deficits. There are also psychophysical abnormalities that may be the underpinnings of these clinical symptoms [2]. Schizophrenia is typically diagnosed at the first episode of psychosis that results in hospitalization. Psychosis is treated with the broad class of antipsychotic medications that act by inhibiting the dopamine D2 receptor. However, these drugs are only partially effective and cause severe motor, behavioral, and metabolic side effects [3]. Significantly, current treatment emphasis is now shifting to address the chronic cognitive deficits and withdrawal associated with the disease since it is these aspects of schizophrenia that prevent patients from resuming normal activities after the initial hospitalization and largely account for the lifetime disability associated with the disease [4]. These symptoms are not adequately treated by current antipsychotics nor by any other available therapies [5]. Thus, treating the cognitive and negative symptoms of schizophrenia, in addition to more effectively treating psychosis, is now the priority.
Neurodevelopmental, neuropathological, genetic, and behavioral pharmacological data indicate that schizophrenia stems from a dysfunction of glutamate synaptic transmission, particularly in frontal cortical networks [6, 7]. Glutamate neurotransmission is mediated by four classes of glutamate receptors, AMPA, Kainate (KA), NMDA, and metabotropic [8–11], and these receptors have emerged as prime targets to remediate the synaptic deficits that underlie schizophrenia. Given that dysfunctional glutamate synaptic transmission is putatively the core pathology of schizophrenia [12, 13], these receptor targets offer the possibility of developing drugs with a comprehensive therapeutic benefit beyond that afforded by the currently used antipsychotic agents [14, 15]. Herein, we first review the evidence for glutamate dysfunction in schizophrenia. We then make the case that allosteric modulation of the different glutamate receptor classes offers the best approach for the successful development of new drugs to target these receptors and to significantly improve outcomes for patients with schizophrenia.
BIOLOGY AND PATHOLOGY OF SCHIZOPHRENIA
Schizophrenia is a developmental disorder [16, 17]. Although the first psychotic break typically occurs in late adolescence or early adulthood, it is increasingly recognized that diagnosis is preceded by a prolonged prodromal phase. The prodrome is notable in that it presents as a decline in cognitive performance as often as it presents behavioral symptoms related to psychosis, reinforcing the characterization of schizophrenia as a disorder of cognitive function [18]. The prodromal period corresponds to a significant epoch in the development of cortical glutamatergic networks. During this period, there is a significant pruning of glutamate synapses between principal neurons, particularly in frontal cortical areas, paralleled by an increase in inhibitory synapses [16]. These changes in synaptic densities are thought to reflect a final step towards an adult cortical network organization. Schizophrenia appears to arise from a failure in successfully traversing this final cortical developmental stage.
Characterizing schizophrenia as a failure in cortical development is supported in neuropathological findings from autopsy samples taken from patients who have expressed schizophrenia [19]. At the macroscopic level, thinning of cortex in frontal regions is consistently observed, attributable to a profound reduction in the density of dendritic spines on the glutamatergic principal neurons, particularly in layers II/III. Abnormalities in the distribution of glutamatergic neurons within the cortex are also present, suggesting defects in glutamatergic neuron migration. A second, well established pathological hallmark of the disease is loss of parvalbumin (PV) expression, again notably in layers II/III of frontal cortex [20]. The loss of PV has been traced to fast spiking basket interneurons, and is accompanied by a significant reduction in GAD67, the principal enzyme for GABA synthesis [21]. Reduction in GABAA receptors at synapses of FS basket neuron terminals onto principal neuron cell bodies is also observed [22]. These findings are interpreted to suggest that the GABAergic input from the FS basket neurons is down-regulated at the cell bodies of the glutamatergic principal neurons. Given that glutamatergic synaptic transmission between principal neuron terminals and GABAergic interneurons drive the interneuron network, it is believed that the loss of glutamate synapses and down regulation of GABAergic inhibition are interrelated [23], summing to aberrant cortical network architecture and function.
The hypothesis that these network abnormalities are the result of a developmental defect is supported in findings from gene co-expression network analyses. One pertinent study compared microarray-based brain gene expression in prefrontal cortex obtained post-mortem from normal and schizophrenic subjects. These data indicated that the normal age-related decreases in expression of genes related to central nervous system developmental processes does not occur in subjects with schizophrenia [24]. Extrapolating these findings back to the earlier stage of development supports the concept that schizophrenia pathogenesis is associated with a failure of normal developmental-related gene expression. As reviewed below, detailed analyses of gene expression patterns indicate that glutamate receptor genes are prominently represented among those that are aberrantly regulated.
THE IMPORTANCE OF GLUTAMATE PATHWAYS AND NETWORKS IN THE BIOLOGY OF SCHIZOPHRENIA
Genetic evidence
Based on a variety of analyses, it is estimated that as much as ~80% of the vulnerability to schizophrenia has a genetic underpinning [25]. Genetically-linked susceptibilities interact with environmental factors to induce symptomatic expression of the disorder [26, 27]. Significant effort has been expended in the past decade to identify genes influencing schizophrenia susceptibility, including more than 2400 association studies. Significantly, these efforts have yet to identify causative genetic factors [28]. From the traditional perspective of causal mutations in protein coding regions, single gene mutations that specifically cause schizophrenia have not been identified and few of the genes or markers associated with the disorder have been reliably replicated. Instead, most of the identified genetic variation predisposing subjects to schizophrenia lie in regions of the genome outside those directly coding for proteins. Our understanding of the functional organization of the genome, and particularly the non-coding regions, is increasing rapidly [29]. Recently published findings from the ENCODE consortium [30] indicates that the vast majority of the genome is involved in the intricate regulation of gene expression in a cell-type and developmental stage specific manner. In light of this emerging knowledge, it seems apparent that the genetic vulnerability to schizophrenia relates to the unfolding of the complex genetic program that orchestrates late adolescent cortical developmental. Thus, mapping the genetic variations associated with schizophrenia risk onto the normal developmental program of gene expression will be critical in elucidating the etiology of schizophrenia. Progress is beginning to be made in this regard with the integration of information on putative schizophrenia genes [25, 31–35] with that of pathways and networks [36–42].
One large study (4673 schizophrenics & 4965 controls) independently identified a group of 1026 synaptic genes that are significantly associated with the risk of schizophrenia [31]. Analysis of synaptic subgroups suggested that the strongest association signals are derived from three synaptic gene groups: intracellular signal transduction, excitability, and cell adhesion and trans-synaptic signaling. Given that ~80% of cortical synapses utilize glutamate as excitatory neurotransmitter, these results are consistent with a role of glutamate synaptic dysfunction in schizophrenia [41]. Additional studies specifically highlight glutamate receptors and signaling pathways. In one example, data from genome-wide association studies (GWAS) was integrated with gene expression data (human post mortem brain & human blood) and relevant animal model data to identify and prioritize genes involved in schizophrenia [25]. The top genes identified from this study are shown in Fig. (1). Through a polyevidence scoring mechanism and pathway analyses, schizophrenia susceptibility gene networks were identified that involve brain development, myelination, cell adhesion, glutamate receptor signaling, G-protein-coupled receptor signaling, and cAMP-mediated signaling. Among the top candidate genes identified for schizophrenia in this investigation were a number of glutamate receptor genes (GRIA1, GRIA4, GRIN2B and GRM5), as well as GAD1, an enzyme involved in glutamate metabolism, and SLC1A2, a glutamate transporter. Other genes involved in glutamate signaling identified with lower scores were GRIN2A, SLC1A3, GRIA3, GRIK4, GRM1, GRM4 and GRM7.
Fig. 1. Biology of schizophrenia.
Top thirty-five candidate genes from the convergent functional genomics study of Ayalew et al. [25] are listed. Glutamate genes are shown in bold. In parentheses in bold are seven additional glutamate genes identified with lower scores.
Another approach considered the functional unit conferring risk for schizophrenia as pathways per se, as opposed to single pathway nodes [36]. In this investigation, one hundred sixty schizophrenia genes were prioritized based on a multidimensional evidence-based gene ranking system and twenty-four pathways were identified that were significantly enriched for schizophrenia genes. Among these pathways, nine were directly related to neurodevelopment, further supporting the notion of neurodevelopmental abnormalities in schizophrenia. Four neurotransmitter-related pathways stood out at the top of the list ranked by the significance level: glutamate receptor signaling (ranked 1st), serotonin receptor signaling (2nd), GABA receptor signaling (5th) and dopamine receptor signaling (7th). By combining these schizophrenia-related pathways with protein-protein interaction networks and a literature survey, a schizophrenic molecular network was constructed [36]. The network highlights glutamate, GABA, dopamine and serotonin, their transmembrane receptors and their downstream interactions such as activations, inhibitions, and feedback regulations in the cellular system. Fig. (2) highlights the part of this network that is associated with glutamatergic signaling.
Fig. 2. Schizophrenia molecular network (SMN).
This SMN was modified from Zhao et al. [36] who constructed it using schizophrenia related pathways, protein-protein interactions and literature survey. Schizophrenia genes are underlined. The enriched pathways are highlighted in white font within the dark colored boxes. Several feedback loops are identified.
In summary, glutamate receptor signaling emerges as one of the top canonical pathways represented in schizophrenia-focused genetic analyses and this information can begin to be interpreted within the larger context of schizophrenia genetics, which implicates dysregulation of gene expression networks over mutations in coding sequences for specific proteins. Thus, it is reasonable to speculate that there may be a disruption in the deployment of glutamate signaling components in late adolescence that contributes to the deranged cortical development that underlies schizophrenia.
NMDA receptor hypofunction in the expression of schizophrenia
There is also compelling evidence from human behavioral pharmacological studies that implicates glutamate signaling dysfunction in the expression of schizophrenia. It is now extremely well established that treatment of healthy humans with drugs which block NMDA receptor ion channels induces behavioral symptoms that can be indistinguishable from those associated with an acute psychotic episode of schizophrenia [12, 43]. NMDA receptor channel blockade also causes an acute exacerbation of psychotic symptoms in patients with schizophrenia [44, 45]. Such effects in healthy humans were first documented for phencyclidine by Domino and Luby in the 1950’s, who used the term ‘schizophrenomimetic’ in describing the effects of this compound on humans [46]. Subsequently, it was determined that phencyclidine is an NMDA receptor channel blocker and that this blockade was the mechanism of schizophrenomimetic action [47] This conclusion was unequivocally confirmed in findings which demonstrated that multiple high potency, high specificity NMDA channel blockers produce similar effects in clinical trials [48–50]. The effect of NMDA receptor channel blockade in humans has been studied systematically through the use of ketamine, the only approved drug in this class [45, 51–57]. These studies indicate a remarkably complete overlap between the behavioral effects of ketamine and the symptoms of schizophrenia [58]. Ketamine reproduces not only psychosis but also the cardinal chronic negative symptoms and cognitive disruption associated with schizophrenia. Furthermore, recent evidence indicates that the drug reproduces many of the psychophysical abnormalities evidenced by patients with schizophrenia, including sensory gating deficits and abnormalities in brain network recruitment during behavioral tasks [2, 59]. Thus, using the terminology of genetics, a single, ketamine-induced ‘mutation’ in the NMDA receptor channel reproduces the full spectrum of symptoms of the disorder. These observations have given rise to the hypothesis that NMDA receptor hypofunction is a key element in the expression of schizophrenia [12, 13, 60]. Specifically, it appears that NMDA receptor channel blockade induces a defect in glutamate receptor signaling that closely mimics the one resulting from the derangement of cortical development that gives rise to the symptoms of schizophrenia in adulthood.
There are additional data from clinical trials of other types of NMDA receptor antagonists that may be relevant to the nature of the putative NMDA receptor dysfunction in schizophrenia. One intriguing finding is the apparent lack of schizophrenomimetic side effects reported in trials of glycine-site NMDA receptor antagonists, although the interpretation of these data is tempered by the fact that the glycine-site antagonists have poor brain exposure [61, 62]. In contrast, a negative allosteric modulator selective for the GluN2B subtype of NMDA receptor was reported to cause cognitive disruption and dissociative effects in several trials [63–65], although it has not been systematically analyzed as to what degree such effects mirror those induced by ketamine. Nonetheless, the suggestion of differences in schizophrenomimetic effect based on the mechanism of NMDA receptor inhibition has implications for the NMDA receptor hypofunction model of schizophrenia. There are a number of systematic studies in rodents that indicate that different classes of NMDA receptor antagonists produce distinct behavioral effects [66–68] and subjective effects in cross-discrimination paradigms [69–71]. Further analysis of the physiological basis for these differences may provide important clues to understanding the mechanisms by which NMDA receptor hypofunction contributes to the appearance of schizophrenia symptoms.
Glutamate/dopamine interactions
Emphasis on the glutamate system in schizophrenia does not obviate the involvement of dopaminergic dysfunction, particularly in the development and expression of psychosis (Fig. (3)) [72, 73]. In fact, these two signaling systems are highly interrelated and the deregulation of glutamate signaling may underlie the disruption within the dopamine system, contributing to both positive and negative symptoms. In the normal brain, (left panels, Fig. (3) the mesolimbic dopaminergic pathway is regulated by cortical glutamatergic input via NMDA receptors to set the basal tone. In schizophrenia, (right panels) hypofunction of the descending glutamatergic pathway results in lack of stimulation of GABAergic interneurons. This, in turn, disinhibits the mesolimbic dopaminergic pathway, contributing to positive symptoms like delusions and hallucinations. With regard to negative symptoms, the descending glutamatergic pathway acting via NMDA receptors drives the mesocortical dopaminergic pathway to regulate the liberation of dopamine in PFC. In schizophrenia, hypofunction of this descending glutamatergic pathway results in a deficit in the liberation of dopamine on PFC contributing to negative symptoms include blunted affect and lack of motivation as well as cognitive disorders. Ameliorating the primary glutamatergic synaptic deficit in schizophrenia is predicted to re-establish balance in the dopaminergic system through multiple network interaction. Thus, glutamate-targeted drugs are predicted to impact all of the symptoms of schizophrenia, including the positive and negative symptoms highly sensitive to dopaminergic deregulation.
Fig. 3.
Contribution of mesocorticolimbic glutamatergic hypofunction to positive (A) and negative (B) symptoms of schizophrenia (modified from López-Muñoz and Álamo [74]). N. Acc. indicates nucleus accumbens; VTA, ventral tegmental area; PFC, prefrontal cortex; DA, dopamine; GLU, glutamate; NMDA, NMDA receptor; dlPFC, dorsolateral prefrontal cortex; vmPFC, ventromedial prefrontal cortex.
Glutamate synaptic dysfunction: treatment implications
The neuropathological and genetic data strongly implicate glutamate synaptic dysfunction as contributing to the cause of schizophrenia. Understanding the role of glutamate receptor signaling in cortical developmental, and its derangement in schizophrenia, will be the key element in solving the mystery of this disorder. Such knowledge may provide true biological diagnostic criteria for schizophrenia and insight into whether there are meaningful variants of the disorder. This understanding will, in turn, guide and perhaps enable individualized therapy and the prediction of treatment responses. Indeed, it may one day be possible to detect and prevent the disorder in genetically susceptible individuals. However, we are only just beginning to understand our genetic code in a way that will enable us to interpret the genetic variance that underlies susceptibility to schizophrenia. Thus, these diagnostic and early intervention goals are aspirational and treatment of symptoms after expression of the developmental defect remains the only present option. On the other hand, the human pharmacology data also implicates glutamate synaptic dysfunction in the expression of schizophrenia. It is remarkable that specific inhibition of the NMDA receptor, and possibly even a single NMDA receptor subtype, reproduces the complex symptoms of the disorder. This observation implies that a deficit in glutamate synaptic transmission may be causing the symptoms of schizophrenia and underwrites interest in drugs targeting glutamate receptors to ameliorate this deficit. Thus, glutamate receptors are of immediate interest in developing new drugs to better treat the symptoms of schizophrenia post-diagnosis (Fig. (4)).
Fig. 4. Glutamatergic neurotransmission (reprinted with permission from Schoepp [75]).
Primary localization of mGlu and ionotropic glutamate receptors with respect to presynaptic glutamatergic nerve terminals, postsynaptic cells, presynaptic GABAergic nerve terminals, and glia are shown. A primary function of mGlu2 receptors is as an autoreceptor for glutamatergic nerve terminals. The mGlu3 receptor is present as a heteroceptor on a number of GABAergic interneurons throughout the brain. This receptor is one of two primary mGlu receptors, along with mGlu5 receptors, that modulate the function of glia. The mGlu3 receptor is also present as a postsynaptic receptor as well. The mGlu5 receptor has been intensely studied with respect to functional positive coupling with NMDA receptors. A portion of these receptors also appear to be located on glutamatergic axons/nerve endings in addition to the glial localization noted above.
There has been considerable work in recent years laying the foundation for developing new classes of agents that modulate both the ionotropic and metabotropic glutamate receptors. This includes detailed insight into the structural basis for the activity of these receptors and how this activity is modulated by physiological ligands and protein interactions [8]. This work has been particularly informative in identifying allosteric mechanisms of modulation and binding sites for such modulators. In the next sections, we make the case that allosteric modulation of glutamate receptors in the treatment of schizophrenia is particularly attractive from both a physiological and a medicinal chemistry perspective.
IMPORTANCE OF ALLOSTERIC TARGETS IN GLUTAMATE-CENTERED SCHIZOPHRENIA DRUG DISCOVERY
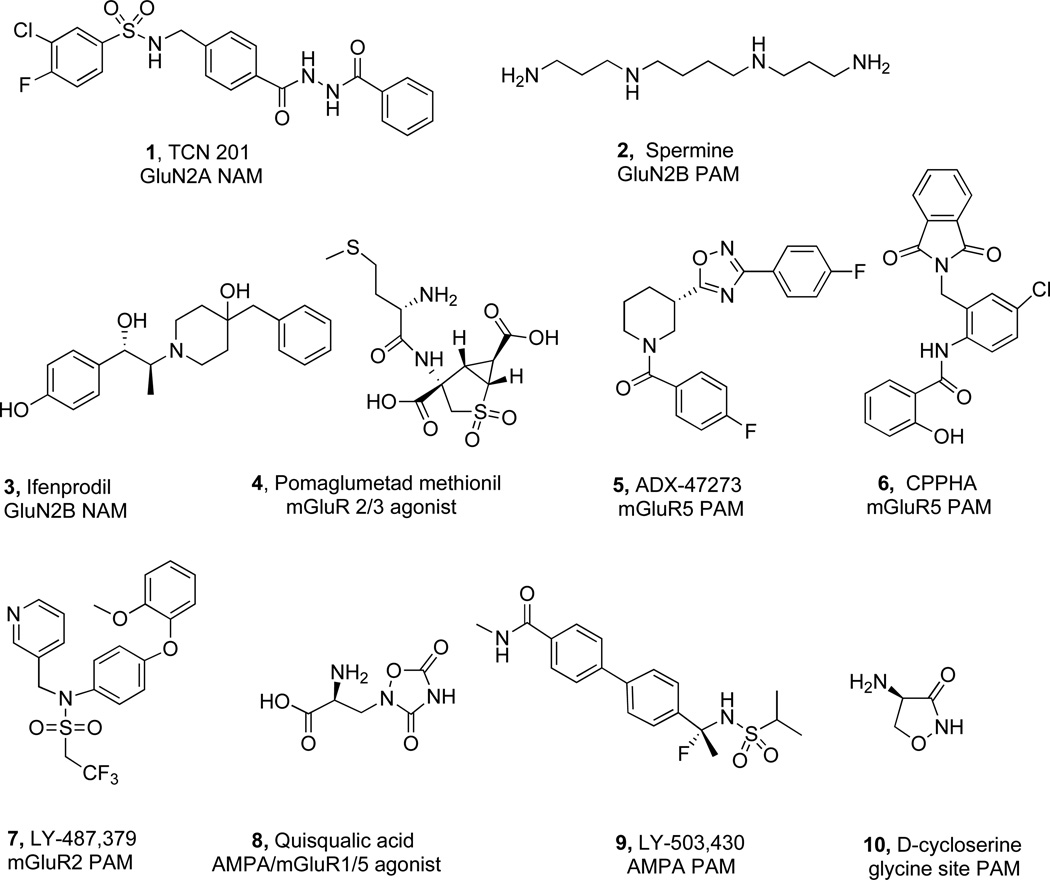
Designing orthosteric ligands for glutamate receptors has been challenging. Low molecular weight agonists and antagonists are typically amino acid derivatives with limited oral bioavailability and brain permeability (Fig. (5)). These compounds have a comparatively high number of H-bond donor groups, a low cLogP and a high polar surface area (PSA) (see Table 1). Each of these properties contributes to the significant hurdles associated with converting orthosteric glutamate modulators into CNS drugs [76]. Considerable effort has been focused on addressing the problems associated with orthosteric receptor modulation, with limited success. At the same time, allosteric receptor modulation has emerged as an attractive alternative to overcoming many of the inherent challenges of orthosteric target-centered approaches. Allosteric modulators can function as antagonists (negative allosteric modulators, NAMs), potentiators, (positive allosteric modulators, PAMs), agonists (ago-allosterics or allo-agonists, ago-PAMs) and silent modulators (SAMs). The power of targeting sites topographically distinct (”allosteric”) from the recognition (“orthosteric”) site for the natural or exogenous agonists or competitive antagonists emanates from the ability of the target protein to change its three dimensional conformation and thereby alter the affinities and/or efficacies of orthosteric ligands [77, 78].
Fig. 5.
Selected allosteric and orthosteric modulators affecting glutamatergic synaptic transmission.
Table 1.
Properties of Allosteric and Orthosteric Modulators Shown in Fig. (5).
| Site | Ligands | Orthosteric | NAM | PAM | EC50 µM | Selective | HBD | LogP | PSA | Drug-like |
|---|---|---|---|---|---|---|---|---|---|---|
| GluN1/GluN2A | TCN 201 (1) | X | 0.16 | X | 3 | 3.3 | 113 | |||
| GluN1/GluN2B | Spermine (2) | X | 37 | 6 | −0.54 | 76.1 | ||||
| GluN1/GluN2B | Ifenprodil (3) | X | 0.150 | X | 3 | 1.92 | 63.9 | X | ||
| mGluR 2/3 | Pomaglumetad methionil (4) | X | 0.045 | 5 | −1.11 | 197.5 | ||||
| mGluR5 | ADX-47273 (5) | X | 0.168 | 0 | 3.64 | 59.2 | X | |||
| mGluR5 | CPPHA (6) | X | 0.250 | 2 | 2.84 | 86.7 | X | |||
| mGluR2 | LY-487379 (7) | X | 0.270 | 0 | 3.53 | 77.1 | X | |||
| AMPA | Quisqualic acid (8) | X | 4 | −1.26 | 122 | |||||
| AMPA | LY-503,430 (9) | X | 0.48 | X | 2 | 2.26 | 83.7 | X | ||
| glycine | D-cycloserine (10) | X | 3 | −2.99 | 64.4 | |||||
| Average CNS medicine | 0–2 | 1.5–2.7 | 20–80 |
There are a number of potential physiological advantages of allosteric glutamate receptor modulators, particularly with regard to schizophrenia. Information processing in the glutamate/GABA cortical networks requires high temporal resolution [79–82]. AMPA receptors mediate fast excitatory signaling, responding with msec resolution to synaptic glutamate release. KA and metabotropic receptors modulate these rapid responses through effects on presynaptic glutamate release, postsynaptic membrane excitability, and downstream signal transduction. NMDA receptors also contribute directly to postsynaptic neuronal excitation, albeit on a time scale of 10s to 100s of msec. Furthermore, a key role of the NMDA receptor is to regulate plasticity at the glutamate synapses and such plasticity is very tightly tied to the timing of pre- and post-synaptic events. The requirement for very high temporal resolution within the glutamatergic system differs qualitatively and quantitatively from other modulatory systems such as the monoaminergic and peptidergic systems that are more traditional drug targets. A key advantage of allosteric modulators is that their modulation is in concert with the temporal and spatial organization of physiological receptor activation [78]. For example, positive allosteric modulators (PAMs) do not stimulate the receptor directly (no on-off receptor activation), but enhance the function of receptors on the timescale activated by endogenous agonist. Another advantage stemming from such use-dependence is less propensity for receptor desensitization as is often seen with persistent orthosteric agonist treatment. Together, these properties may reduce the side effect potential relative to orthosteric agonists, which stimulate a given receptor independently of its physiological state. The possibility of limiting side effects in the treatment of schizophrenia is obviously of significant importance given the current therapies.
From a medicinal chemistry perspective, allosteric modulators of glutamate receptors have afforded greater selectivity among the four classes of glutamate receptors and, significantly, greater selectivity for a given receptor subtype within a class compared to orthosteric ligands. This stems from the fact that allosteric binding sites are typically more structurally diverse, since they have had less stringent demands for evolutionary conservation than the orthosteric glutamate binding sites [8]. Furthermore, some allosteric sites, such as those within the NMDA receptor amino terminal domain, reside on the cell surface away from the active site, making such sites more accessible to drug-like molecules compared to orthosteric sites. There has been considerable progress made in the development of new allosteric modulators of glutamate receptors. Current schizophrenia drug discovery efforts affecting glutamatergic synaptic transmission have centered on identifying drug-like mGluR1/5, mGluR2/3, AMPA, glycine and GluN1/GluN2B PAMs and GluN1/ GluN2A NAMs. These areas of research are reviewed in the next sections.
ALLOSTERIC APPROACHES TARGETING GLUTAMATERGIC SYNAPTIC TRANSMISSION
AMPA receptor modulation
AMPA (2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid) receptors mediate fast excitatory synaptic transmission at ~70–80 % of brain synapses. AMPA receptors are permeable to Na+ and activation results in postsynaptic depolarization [8]. The density of AMPA receptors in the post-synaptic active zone determines the current density in response to a packet of glutamate release, i.e., synaptic strength. The rapid insertion or removal of AMPA receptors within the active zone is a principal mechanism for regulating synaptic strength [83]. In fact, dynamic regulation of AMPA receptor trafficking is the basis of NMDA receptor-regulated long term potentiation and depression (LTP and LTD) which are widely held to be the molecular basis for some forms of learning and memory [84–86]. A minority of AMPA receptors are permeable to both Na+ and Ca2+ [87]. These Ca2+ permeable AMPA receptors play an additional role in some synaptic locations by directly regulating intracellular Ca2+ signaling cascades.
Potentiating the activity of AMPA receptors is proposed as a promising approach for improving cognitive deficits in disorders such as schizophrenia in which glutamatergic transmission is weakened and/or impaired [88, 89]. AMPA receptor positive allosteric modulators may have at least three beneficial actions in this regard: 1) direct facilitation of AMPA receptor mediated depolarization, mimicking NMDA receptor-mediated AMPA receptor insertion in LTP, 2) increasing depolarization-induced removal of the Mg2+ block of the NMDA receptor to facilitate the induction of LTP, and 3) induction of activity-dependent BDNF synthesis to augment activation of signaling pathways downstream to this neurotrophic factor [90]. The AMPA PAMs described to date bind to a site on the ligand binding domain of AMPA receptor; however, they have no agonist or antagonist effect of their own. Instead they stabilize the receptor in its channel open state following the binding of released glutamate, thereby prolonging current flow through the receptor [91]. Direct activation of AMPA receptors theoretically carries the risk of producing seizures, excitotoxicity and loss of efficacy due to desensitization. Importantly, AMPA PAMs are able to enhance receptor activity while avoiding these issues [92].
AMPA receptors, like all of the ionotropic glutamate receptors, have four subunits organized as a dimer of dimers [8, 93]. The extracellular portion of each subunit contains two domains - an amino-terminal domain (ATD) and a ligand-binding domain (LBD), while the transmembrane portion is made up of three helices. There are four subtypes of AMPA receptor subunit, GluR1-GluR4, that functionalize as hetero- or homo-tetramers [8, 93]. Additional structural diversity is conferred by alternate splicing of RNA for each subunit [94]. Alternative splicing in the extracellular ligand binding domain of the AMPA receptors generates two variants, i.e., flip and flop [95]. The flop sequence of GluR2 promotes the channel to close more rapidly and consequently to desensitize with a faster rate than the flip sequence. For GluR1, the alternative splicing does not seem to affect the channel kinetics. The flip/flop sequence cassette of AMPA receptors, in a sequence-dependent manner, regulates the rate of the channel closing process, in the microsecond time domain, through which it further regulates the channel desensitization in the millisecond time region [96].
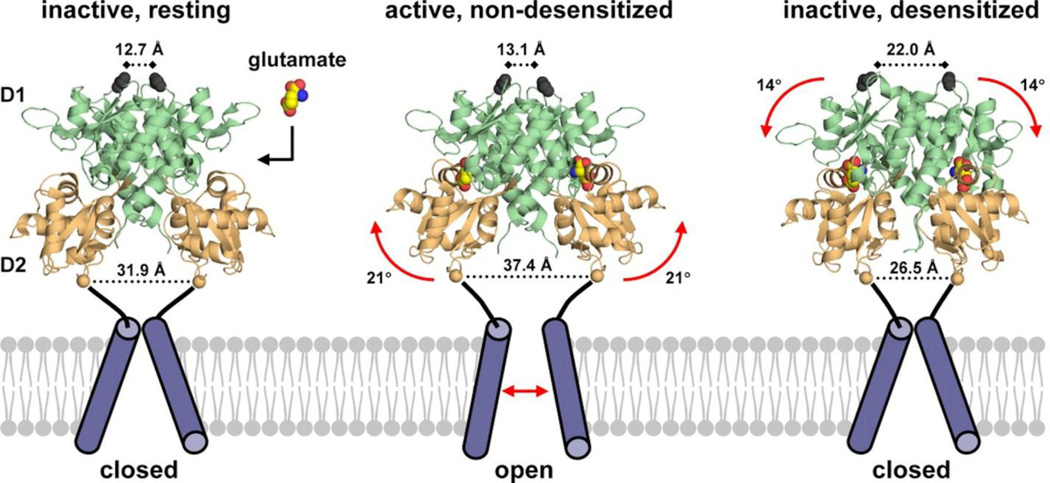
The structure of the AMPA receptor is the best-characterized of the ionotropic glutamate receptors [97–99]. A rapidly growing number of high-resolution crystal structures of the extracellular domains, and recently the first structure of a full-length AMPA receptor [99], have begun to reveal the molecular basis of receptor action. The structure of the full-length AMPA receptor reveals an unexpected crossover of the dimer pairs in the ATD and LBD layers, such that the subunit pairs that dimerize in the ATD are different from the pairs that form an LBD dimer (Fig. (6)). The membrane-proximal LBD has a bilobate structure which has been shown to adopt ‘open’ and ‘closed’ conformations depending on whether it is bound to antagonists or agonists, respectively [98, 101, 102]. Glutamate binding to the LBD triggers lobe closure to initiate the gating cascade. The ATDs of AMPA receptors have relatively rigid structure and only loose coupling to the LBD, suggests that these ATDs may not possess sites of allosteric modulation.
Fig. 6.
A. The structure of the full-length AMPA receptor [99] reveals an unexpected crossover of the dimer pairs in the ATD and LBD layers, such that the subunit pairs that dimerize in the ATD (e.g., magenta and yellow) are different from the pairs that form an LBD (e.g., magenta and blue) dimer. The membrane-proximal LBD has a bilobate structure which has been shown to adopt ‘open’ and ‘closed’ conformations depending on whether it is bound to antagonists or agonists. L-glutamate binding to the LBD triggers lobe closure which initiates the gating cascade. Positive allosteric modulators of AMPA receptors bind at the LBD dimer interface and preferentially stabilize the ‘closed’ conformation, thus strengthening AMPA receptor transmission. Crystal structures of multiple AMPA LBD – modulator complexes have been solved and used to guide the design of novel classes of positive allosteric modulators of the AMPA receptor. There are no known ligands for the ATDs of AMPA receptors, and their relatively rigid structure and loose coupling to the LBD suggests that these ATDs may not possess sites of allosteric modulation. B. Two molecules of Cyclothiazide (CTZ) (16) bind at the dimer interface. C. CTZ (16) binds at the dimer interface [103], stabilizing the agonist-bound, ‘closed clamshell’ conformation of the ligand-binding domain of the AMPA receptor. L-glutamate is shown in spheres, bound at the orthosteric site. D. LY451646 (shown in green), the active enantiomer of LY404187 (17), binds very differently from cyclothiazide (16) (shown in orange and white) [100], with LY451646 having a 2:1 receptor: PAM stoichiometry as opposed to a 1:1 stoichiometry for CTZ (16).
There have now been many co-crystal structures solved for the AMPA ligand binding domain (LBD) with bound positive allosteric modulators. AMPA PAMs bind at the LBD dimer interface (Fig. (7)) and preferentially stabilize the LBD in its active, agonist bound conformation, thus strengthening AMPA receptor transmission [91]. The presence of multiple allosteric sites is indicated by experiments demonstrating that some potentiators affect receptor deactivation to prolong signal duration while others slow desensitization and enhance signal amplitude [104]. Crystal structures of multiple LBD/modulator complexes have been solved and used to guide the design of novel classes of positive allosteric modulators of the AMPA receptor.
Fig. 7.
Shown are two subunits of the tetramer, with the ATD omitted and the transmembrane domains represented by a single cylinder. The ligand-binding cores are labeled D1 and D2, respectively. Glutamate binds to the receptor and domain closure occurs, with two possible outcomes: either the D1–D1 interface remains fixed and the domain closure is translated into ion activation, or the D1–D1 interface ruptures and the ion channel closes, leading to the desensitized state. Perturbations that destabilize the interface enhance desensitization. Stabilization of the intradimer interface by allosteric modulators reduces desensitization [91]. Reprinted from Traynelis et. al. [8].
Three broad structural classes of AMPA PAMs (Fig. (8)) have been reported: 1) benzamides (CX-516 (13), CX-691 (14) and pyrrolidones (piracetam (11), aniracetam (12)); 2) benzothiadiazines (S18986) (15), cyclothiazide (CTZ) (16), & 3) biarylpropylsulfonamides (LY404187 (17), LY451395 (18)). A crystal structure of the ligand binding domain of pyrrolidone allosteric modulators and the AMPA receptor subtypes GluA2 and GluA3 has been obtained [105]. While aniracetam (12) binds to a symmetrical site at the center of the dimer interface, piracetam (11) binds to multiple sites along the dimer interface with low occupation, one of which is a novel PAM binding site. Sulfonamide LY404187 (17), a selective, potent and centrally active PAM of AMPA receptors, preferentially acts at recombinant human homomeric GluR2 and GluR4 versus GluR1 and GluR3 AMPA receptors [106]. In addition, LY404187 (17) potentiates the flip splice variant of these AMPA receptors to a greater degree than the flop splice variant. In both recombinant and native AMPA receptors, potentiation by LY404187 (17) displays a unique time-dependent action that appears to involve a suppression of the desensitization process of these ion channels. Cyclothiazide (CTZ (16) is known to produce a fast inhibition of AMPA receptor desensitization and a much slower potentiation of the AMPA current [107]. Effects of CTZ (16) were studied in HEK 293 cells stably transfected with the rat flip GluR1 subunit [108, 109]. Upon CTZ treatment, a slow whole-cell current potentiation, a fast inhibition of desensitization, and a lengthening of single-channel openings occurred. The authors speculate that CTZ binds to AMPA receptors and “freezes” them in their existing state, i.e., nondesensitized or desensitized. Upon agonist application, the nondesensitized receptors shift to their open states, with a very small probability of undergoing desensitization, whereas the desensitized receptors slowly become available for activation, yielding a delayed potentiation.
Fig. 8.
Structure of AMPA and AMPA PAMS. EC50’s are approximate and represent selectively enhanced glutamate-evoked currents through AMPA receptor/channels of acutely isolated pyramidal neurons.
The allosteric sites on AMPA receptors provide a target for amplifying receptor activity without eliminating either signal content or the homeostatic processes that maintain excitatory neurotransmission in the physiological range. There is a wealth of data characterizing the effects of AMPA PAMs in preclinical model systems that largely support the therapeutic value of this approach [90, 91, 113]. In the first reported schizophrenia trial, piracetam (11), an AMPA PAM and marketed nootropic, was coadministered with haloperidol in an 8 week trial [110]. Piracetam improved psychotic symptoms but did not improve the PANSS (positive & negative symptoms) scores. In human studies involving CX-516 (13), the good in vitro activity and positive results seen in animal tests [111] have not translated to efficacy in human trials. CX-516 (13)) did not produce an antipsychotic effect when administered as monotherapy in a small sample of schizophrenic patients withdrawn from antipsychotic treatment [112]. In a double-blind trial on 19 schizophrenic patients, CX-516 (13) in add-on to the atypical antipsychotic clozapine was reported to produce an improvement in cognitive and memory tasks and in negative symptoms compared to patients treated with clozapine only [113]. However, in a study involving 105 stable schizophrenia patients treated with clozapine, olanzapine, or risperidone, who were randomly assigned to add-on treatment with CX-516 (13) ( 900 mg three times daily for 4 weeks), CX-516 (13) was generally well tolerated but was not effective for cognition or for symptoms of schizophrenia when added to any of the three antipsychotics [114].
In conclusion, it appears that studies on humans for AMPA PAMs are still very limited and do not provide a clear verdict on the future of these agents. At this juncture, AMPA PAMs do not appear to be effective antipsychotics when given in monotherapy, while they may have a moderate efficacy on negative and cognitive symptoms when co-administered with conventional antipsychotics. One might argue that clinical investigations of AMPA PAMs with better potency and bioavailability than CX-516 (13) are needed to more definitively test the potential of this class in schizophrenia.
NMDA receptor modulation
NMDA (N-methyl-D-aspartic acid) receptors co-localize with AMPA receptors in synapses throughout the brain [8, 115–117]. NMDA receptors are permeable to both Na+ and Ca2+ and also mediate excitatory synaptic transmission, albeit on a considerably slower time scale (10s to 100s of msec) than AMPA receptors. However, the unique role of NMDA receptors is to regulate the strength of glutamate synapses by transducing the intensity and temporal coincidence of pre- and post-synaptic activation [85, 118]. At resting membrane potentials, the NMDA channel is blocked by Mg2+ and block is relieved when the postsynaptic membrane is depolarized. NMDA receptors become activated with intense presynaptic glutamate release that both depolarizes the postsynaptic membrane (via AMPA receptor activity) and gates the NMDA receptor ion channel. NMDA receptor activation may also occur when presynaptic glutamate release precisely coincides with postsynaptic depolarization from, for example, back-propagating action potentials [119]. In both cases, NMDA channel activation results in Ca2+ influx that triggers biochemical cascades to strengthen the coincidentally active synapses. Conversely, if presynaptic glutamate release is decreased or mis-timed with postsynaptic depolarization, NMDA receptor-regulated biochemical signals arise that weaken these poorly synchronized synapses. In these ways, the NMDA receptor serves as Hebbian coincidence detector, strengthening or weakening synapses in response to the timing and strength of synaptic activity [120, 121]. The constant adjustment of synaptic strength is a key molecular basis for the adaptive function of the brain, particularly the cognitive functions of learning and memory. Thus, the NMDA receptor may be considered the ‘molecular switch’ for learning and memory.
NMDA receptors share the overall topology of the ionotropic glutamate receptor super-family, including the tetrameric subunit composition and the modular topology of the individual subunits [8]. However, there are several key points of divergence that contribute to the unique functions of these receptors that are important to the development of allosteric modulators. The NMDA receptor tetramer is formed from two distinct types of subunits, GluN1 and GluN2. An GluN3 subunit may also form functional receptors with GluN1 and such receptors are expressed primarily in early development. However, the focus of drug discovery has so far remained on the much more prevalent GluN1/GluN2 receptors. It is the GluN2 subunits that bind glutamate and play the principal role in regulating channel activity in response to synaptic release of the neurotransmitter. There are 4 GluN2 subtypes, GluN2A-D, with the distinct subtypes imparting unique biophysical characteristics to the receptor [117, 122]. Receptors composed of different combinations of GluN2 subunits are differentially expressed in brain circuits and neuronal subtypes. Expression and assembly of receptors with different GluN2 subunits is a principal means to fine-tune to purpose the characteristics of NMDA receptors throughout the brain.
Another unique aspect of NMDA receptor physiology is a requirement for a second ligand, glycine or D-serine, to gate the channel. These bind to the ligand binding domain of the GluN1 subunits and receptor activation by synaptic release of glutamate occurs only with occupation of the GluN1 glycine binding site. Mechanistically, glycine or D-serine binding allosterically influences the NMDA receptor to increase the recovery rate from receptor desensitization during synaptic activation. The resulting lengthening of the decay time-constant of the NMDA EPSP provides a longer depolarizing time window most suitable for temporal summation of EPSPs [123, 124]. Significantly, glycine and D-serine are not released synaptically [125]. Instead, levels are controlled by neuronal and glial glycine transporters (Fig. (10)). From a functional perspective, glycine and D-serine appear to regulate the availability of NMDA receptors that may be activated upon synaptic glutamate release [126]. It is noteworthy that the time scale for this function is vastly different than that for regulation by glutamate. Thus, glycine and D-serine are perhaps better viewed as setting the tone for NMDA receptor activity, whereas glutamate regulates temporal activity and intensity of response. This formulation notwithstanding, there is considerable complexity to the regulation of NMDA receptor tone by glycine and D-serine. NMDA receptor affinity for glycine and D-serine (25) depends on what combination of the GluN1/GluN2 subunits that the NMDA receptor possesses. Glycine and D-serine (25) generally have a ~10-fold higher affinity for GluN2B, GluN2C or GluN2D over GluN2A. Moreover, while GluN2A and GluN2B subunits are mostly distributed in pyramidal neurons, GluN2C and GluN2D subunits are more concentrated in interneurons, as shown in the hippocampus [127, 128]. This suggests that neuromodulation of the synaptic network by glycine and D-serine is dependent on the subunit- and cell-type specific distribution of GluN2 subunits. A further level of complexity is suggested by a recent study indicating that glycine and D-serine may play distinct modulatory roles for different pools of NMDA receptors on individual nerve terminals [129].
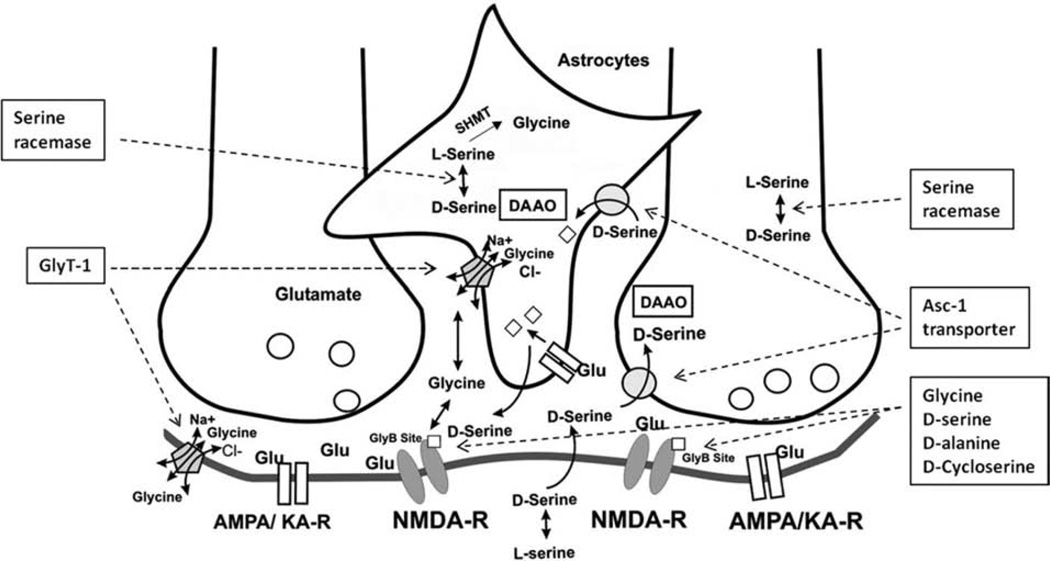
Fig. 10.
A schematic model showing a NMDA synapse and the inhibitory glycinergic synapse side-by-side (modified from Yang and Svensson [125]). Under physiological condition, glycine is actively and bidirectionally transported into or out of the glial cell by a Na+/Cl− dependent GlyT-1. The concentration of glycine is regulated at less than 1 uM in the synaptic region of glutamatergic nerves despite much higher (ca. 2 mM) in the surrounding glia. When GlyT-1 is blocked, glycine transport into the glial cell is impaired and extracellular glycine levels near the NMDA receptor are elevated. Therefore, when synaptic glutamate release occurs, more glycine is available for co-activation of the NMDA receptor. In addition, L-serine is converted to D-serine (25) by serine racemase in neurons and glia of the brain. Activation of AMPA/kainate receptor on glia can also lead to D-serine (25) release from glia. Synaptic D-serine (25) can be transported back to neuronal compartment, e.g. presynaptically via the Na+-independent alanine-serine-cysteine (Asc-1) transporter. D-serine (25) can be degraded by D-amino acid oxidase neuronally. Since both synaptic glycine and D-serine (25) act as NMDA receptor coagonists, a selective inhibition of glycine uptake (e.g. by GlyT-1) or D-serine (25) breakdown (e.g. by DAAO) will elevate synaptic glycine and/or D-serine (25), thus providing more of the coagonist to exert a therapeutic potentiation of the hypofunctional NMDA receptor system.
There is evidence that homeostasis of the physiological glycine-site ligands may be disrupted in psychiatric disorders. The plasma levels of total serine (L- and D-serine (25)) and glycine are higher in patients with schizophrenia than those of controls [130], and the levels of serine and glycine in the brains of schizophrenic patients are higher than those of controls [130, 131], suggesting a possible abnormality in serine hydroxymethyltransferase, which is involved in the conversion of glycine to L-serine [132]. Interestingly, it has been reported that serum D-serine (25) levels and the D-serine (25)/total serine ratio in patients with schizophrenia are significantly lower than those of healthy control subjects, supporting the hypothesis of NMDA receptor hypofunction in schizophrenia [133].
Given the role of the NMDA receptor in regulating synaptic strength in relation to cognition, and the fact that NMDA receptor channel block induces schizophrenia-like behavioral responses in humans, there is very high interest in the NMDA receptor as a target for new schizophrenia therapies. The majority of effort to date has focused on potentiating NMDA receptor activity by increasing the availability of glycine or glycine-site ligands. However, recent insights into the structure/function of the ATDs have opened a path to subtype-specific allosteric modulators and a number of new compounds have recently been disclosed. These two areas will be reviewed in turn.
Glycine-site modulation
There is ample evidence to indicate that NMDA receptor activity may be increased by pharmacologically modulating the levels of glycine/D-serine in the brain and there are several such approaches under consideration in the treatment of schizophrenia [134]. The administration of glycine or analogs has been most extensively investigated and glycine-site full agonists (glycine, D-serine (25)), partial agonists (D-cycloserine (10), D-alanine (26)) and antagonists have been examined for the treatment of a variety of neuropsychiatric disorders (Fig. (9)) [135]. While clinical reports describing the efficacy of glycine in treating schizophrenia are numerous, the success rate for patients in these trials has varied. Glycine is extensively metabolized in the liver, and only poorly crosses the blood-brain barrier. Therefore, a large amount of glycine is needed for the treatment of schizophrenia [136]. One of the most convincing pieces of data for the antipsychotic effects of glycine is from a small double-blind, placebo-controlled study which showed that high doses of glycine (30 g/day) improved negative symptoms in all of the neuroleptic-treated schizophrenics in the study [137]. In a subsequent trial, glycine (at a dose of approximately 60 g/day) was reported to improve negative, positive and general psychopathology, with increasing efficacy during a treatment period of 6 weeks [136]. In contrast, in studies in which glycine has been co-administered with clozapine, glycine addition has failed to ameliorate clinical symptoms and in some cases, symptoms worsened [138]. Overall however, clinical data suggests that glycine may have efficacy, albeit limited, on negative, and perhaps cognitive, symptoms when added to most antipsychotics, with the exception of clozapine. Positive clinical results, however, have primarily been obtained in small samples size and have not been confirmed in larger multi-center trials. Glycine may represent a rationale augmentation strategy in refractory patients exhibiting prominent negative and cognitive symptoms. It remains to be conclusively demonstrated that co-administration of glycine (or other glycine agonists) with antipsychotics is more efficacious than clozapine treatment alone on these symptom domains.
Fig. 9. Molecular architecture of NMDA receptors.
(a) Topology of a generic glutamatergic receptor subunit (top) and modular organization of GluN1 and GluN2 subunits (bottom). (b) Structures of glycine, D-serine (25), D-alanine (26), D-cycloserine (10) and sarcosine (24).
D-serine (25) administration has also been investigated in schizophrenic patients [135]. D-serine (25) is synthesized and stored in astrocytes and acts as a co-agonist on the glycine binding site of NMDA receptors (Fig. (10)). It is generated from its enantiomer via the D-serine (25)-synthesizing enzyme, serine racemase, which is localized within pyramidal neurons of the cortex and hippocampus and GABAergic neurons of the striatum [139]. Interestingly, mutant mice lacking serine racemase were found to exhibit behaviors relevant to schizophrenia, which were reverted by D-serine (25) [140]. When orally administered, D-serine (25) is substantially catabolized by D-amino acid oxidase (DAAO), contributing to its low bioavailability. Although DAAO activity is lower in the forebrain than in the cerebellum, it is detectable in the prefrontal cortex where it has been reported to be higher in post-mortem schizophrenic brains than in healthy comparators [141]. In animal models, mice lacking D-amino-acid oxidase activity have a higher D-amino-acid concentration in the brain compared to the wild-type mice [142] and exhibited better performance in spatial memory task test and hippocampal LTP than WT mice [143] as well as attenuated hyperactivity, stereotypy and ataxia induced by the NMDA receptor antagonist, MK-801 [144]. The elevation of synaptic D-serine (25) levels has been attempted by means of inhibitors of DAAO and this strategy is considered a viable option for improving the clinical efficacy of D-serine (25) (and D-alanine (26)) [145]. The efficacy of D-serine (25) in clinical trials has been mixed and its current utility has been as an add-on strategy to improve antipsychotic drugs’ effects. It has been found to be safe and effective in multiple schizophrenia symptom domains, mostly for negative and cognitive ones, as add-on to antipsychotics, with the exclusion of clozapine [146]. Not surprisingly, large doses are required due to its poor pharmacokinetics (PK) and CNS penetration. These results support the viability of alternative approaches for increasing synaptic glycine levels. To date, D-serine is mainly used in antipsychotic augmentation therapy, most likely for refractory patients (25).
There has also been a more limited investigation of non-physiological glycine/D-serine analogs. D-alanine (26) is analogous to D-serine (25) in acting as a glycine site agonist and it also suffers from poor oral bioavailability. Preclinical studies report a possibility for improving D-alanine (26) and D-serine (25) bioavailabilities. Co-administration of CBIO, a DAAO inhibitor, with D-alanine (26) (as well as D-serine (25)) reverts drug-induced PPI deficits in rats, while D-alanine (26) (as well as 25) treatment alone was ineffective in this task [145]. Furthermore, co-administration of CBIO and D-serine (25) or D-alanine (26) was associated with an increase in frontal cortex extracellular levels of these compounds. D-cycloserine (10), an antibacterial used in the treatment of tuberculosis, has been shown to be a glycine site partial agonist. It has also has primarily been used to augment antipsychotic therapy (with activity ranges within 40–60% of glycine) and may preferentially activate NMDA receptors containing the GluN2C subunit [147, 148] expressed on interneurons and cerebellar granule cells. Positive effects in clinical trials have been variable. As is the case with glycine, the positive action on antipsychotic efficacy is completely abolished when D-cycloserine (10) is added to clozapine. To date, D-cycloserine (10) use is as an augmentation therapy. The partial agonist action of 10 may explain its dose-dependent effect. Speculation is that high doses may exert a functional antagonism rather than agonism on the glycine binding site and thus on NMDA receptor activity, highlighting the complexity of interpreting D-cycloserine (10) clinical results.
In summary, the clinical benefits of glycine and glycine-analogs in schizophrenia are modest, primarily yielding improvement in negative symptoms with less impact on cognitive dysfunction or psychosis. The caveat to interpreting these results has been the extremely poor pharmaceutical properties of these compounds and the difficulty in their administration. An alternative approach to increasing the availability of glycine to recruit NMDA receptors has been through inhibition of glycine uptake in the perisynaptic region. This approach has been pursued through the development of glycine transporter 1 inhibitors, as reviewed in the next section.
Glycine transporter-1 (GlyT) modulation
Synaptic levels of glycine are regulated by specific high affinity, sodium/chloride-dependent, glycine transporters, GlyT-1 and GlyT-2, which possess 12 putative transmembrane-spanning domains, and share approximately 50% amino acid sequence identity [126, 149, 150]. GlyT-2 has a limited, predominantly neuronal distribution in spinal cord, brainstem, and cerebellum. This transporter is primarily involved in regulating glycine neurotransmission at strychnine-sensitive glycine receptors in these regions [151]. In contrast, GlyT-1 is widely expressed in the CNS, where it is predominantly present on glial cells and is responsible for glycine reuptake in forebrain areas [151, 152] (Fig. (10)). Evidence suggests that GlyT-1 maintains local synaptic glycine at very low levels (the concentration of glycine is regulated at less than 1 uM in the synaptic region of glutamatergic nerves despite much higher levels (ca. 2 mM) in the surrounding glia) and in this way plays an important role in regulating glutamatergic neurotransmission at NMDA receptors [153]. Support for such a functional role comes from knockout mice deficient in the GlyT-1 gene [154, 155]. Studies reveal that a homozygous GlyT-1 (−/−) knockout in mice is neonatally lethal. However heterozygous GlyT-1 (+/−) mice survive to adulthood and display enhanced NMDA receptor function in the hippocampus, better memory retention, and no disruption in sensory gating when dosed with amphetamine [155]. Furthermore, in a small placebo-controlled study, schizophrenia patients, stabilized on antipsychotic medication, who were administered 2 g/day N-methyl glycine (sarcosine (24)), a weak (IC50 = 50 uM) but selective GlyT-1 inhibitor, exhibited improvement in negative and cognitive symptoms [156]. These data, in conjunction with the results from the glycine and D-serine (25) trials, provide evidence that increasing synaptic levels of glycine by inhibition of its uptake will lead to enhanced NMDA receptor activation. Such results have fueled considerable interest in the discovery of GlyT-1 inhibitors with drug-like, CNS-penetrant properties as a novel treatment for schizophrenia.
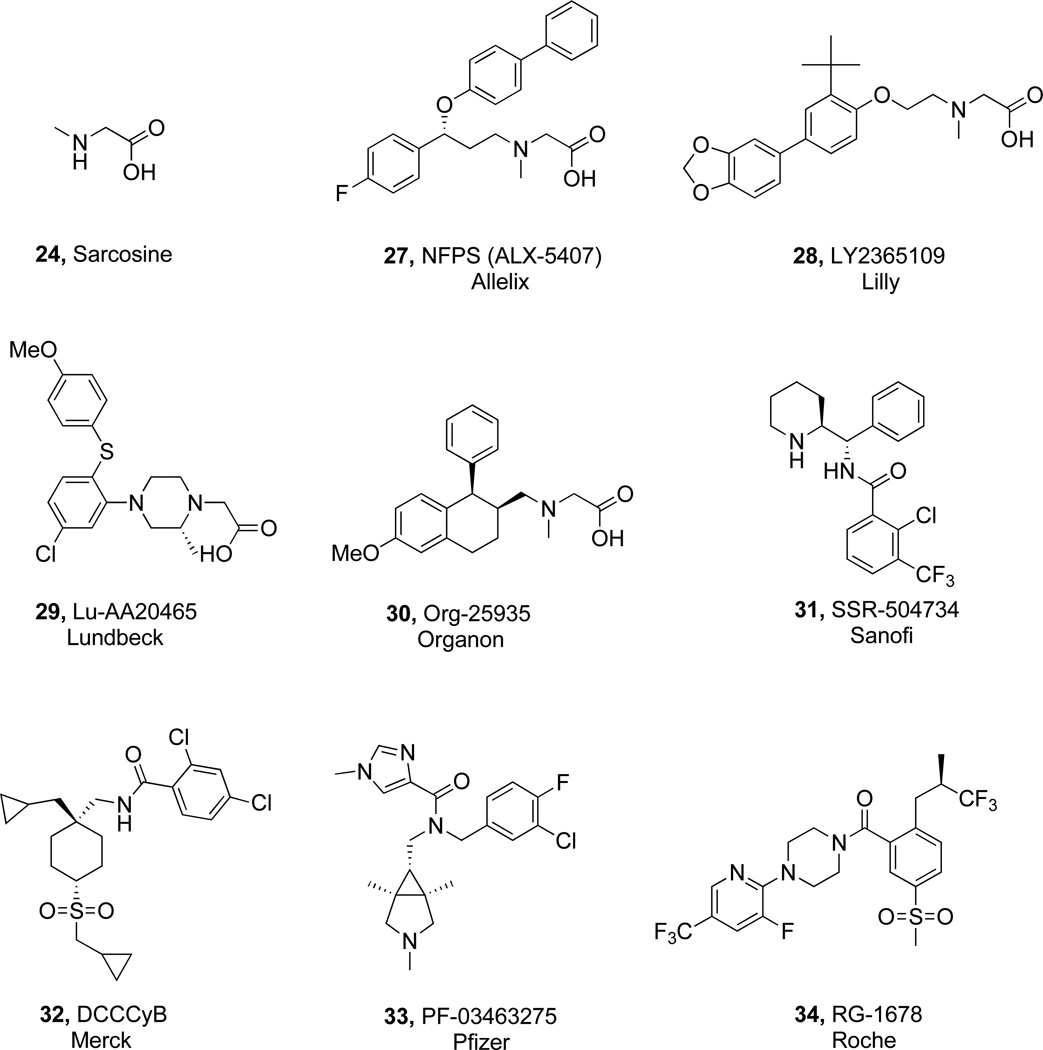
The first reported high affinity GlyT-1 inhibitors were sarcosine (24) based. Given that GlyT-1 and GlyT-2 belong to the SLC6 family of transporters which include dopamine (DA), serotonin (5-HT), norepinephrine (NE), GABA, leucine, proline and taurine transporters, it is not surprising that GlyT-1 inhibitors (Fig. (11)) bear structural similarity with inhibitors of other members in this broad family which are currently on the market. ALX-5407 (NFPS (27)) [157, 158] and LY2365109 (28) [159] are two members of the sarcosine (24) series which have been investigated in great detail. They are potent (IC50 = 7 & 16 nM respectively) and are highly selective for glycine transporters (likely not for all glycine isoforms). ALX-5407 (27) has been used extensively in the field as a pharmacological tool for gaining a better understanding of the role of GlyT-1 in the CNS and for providing a proof of mechanism in several animal models predictive of antipsychotic activity. Despite the progress reported for sarcosine (24)-based inhibitors [160], the class has come under scrutiny due to reported toxicity associated with ALX-5407 (27) and LY2365109 (28). Both induce hypoactivity, impaired respiration, and ataxia in rodents [159], and ALX-5407 (27) exhibits slow dissociation kinetics, rendering binding to GlyT-1 essentially irreversible and leading to elevated glycine levels in the rat prefrontal cortex (PFC) for periods >24 h [157]. Furthermore, ALX-5407 (27) is non-competitive with glycine [161], and its binding profile is thought to contribute to excessive and prolonged elevated glycine levels in rodents, which could lead to overstimulation of strychnine sensitive glycine receptors [161, 162]. Whether the toxicity associated with ALX-5407 (27) and LY2365109 (28) is mechanism based or compound specific has not been established. Another sarcosine (24)-like structure, piperazine acetic acid, Lu-AA20465 (29) (IC50 = 150 nM), has also been investigated [163]. The most advanced sarcosine (24)-based inhibitor reported to date is Org-25935 (30) which doesn’t appear to have the toxicity liabilities of ALX-5407 (27) and LY2365109 (28) [164]. In a recent study, 12 healthy subjects were administered Org-25935 (30) or placebo in random order followed by the drug ketamine, which is known to induce schizophrenia-like psychotic symptoms [165]. The schizophrenia-like effects of ketamine as well as perceptual alterations were significantly reduced by treatment with Org-25935 (30) while some aspects of learning and delayed recall worsened. This study provides compelling evidence that in humans, a GlyT1 inhibitor reduces the effects induced by NMDA receptor antagonism and further supports the antipsychotic potential of GlyT1 inhibitors.
Fig. 11.
Structures of nine GlyT-1 inhibitors.
In recent years, considerable attention has been focused on the identification of non sarcosine -based inhibitors. SSR-504734 (31) is a potent (IC50 = 18 nM) reversible inhibitor of GlyT-1 that is competitive with glycine [161, 166]. SSR-504734 (31) exhibits ameliorative effects in animal models of schizophrenia, suggesting that it might not only be efficacious in treating positive symptoms, but also negative symptoms (i.e., cognitive deficits) of schizophrenia [166]. The inhibitor rapidly and reversibly blocks the uptake of [14C]glycine in mouse cortical homogenates, which was sustained for up to 7 h. Complete cessation of blockade and return to glycine basal levels occurs prior to 24 h, which is in stark contrast to ALX-5407 (27) (>24 h). SSR-504734 (31) potentiates a nearly twofold increase of NMDA receptor-mediated excitatory postsynaptic currents (EPSCs) in rat hippocampal slices and produces an increase in contralateral rotations in mice when microinjected into the striatum. Microdialysis experiments indicate that the inhibitor induced a rapid and sustained increase in extracellular glycine levels in the PFC of freely moving rats [166]. SSR-504734 (31) has been in clinical trials for schizophrenia.
The most advanced of the GlyT-1 inhibitors is RG-1678 (34) from Roche and the results of a large Phase II proof of concept study has recently been made public (http://www.roche.com/investors/ir_update/inv-update-2010-12-06b.htm). This multicenter 323-patient study investigated RG-1678 (34) compared to placebo in patients with pre-dominantly negative symptoms of schizophrenia. Patients were treated for eight weeks with different doses of RG-1678 (34) or placebo in combination with antipsychotics. RG-1678 (34) had a modest, partially significant effect on negative symptoms in the low dose group. The compound was generally well tolerated at all doses. Thus, based on this first public disclosure, it would appear that the Roche GlyT1 inhibitor offers efficacy similar to glycine and glycine analogs against the negative symptoms of schizophrenia. Roche has announced that RG-1678 (34) will be advanced to Phase III with a primary indication of negative symptoms in schizophrenia.
In summary, the clinical experience with glycine and analogs, and now with RG-1678 (34), suggests that targeting the glycine site of the NMDA receptor activity may be of utility in treating the negative symptoms of schizophrenia, but of less value against the broad spectrum of symptoms. These results are in contrast to the ‘efficacy’ of the NMDA receptor channel blockers in producing a full spectrum of schizophrenia-like symptoms. A simple, perhaps obvious, insight from this analysis is that the mechanism of NMDA receptor modulation fundamentally impacts therapeutic efficacy. In this regard, there is a growing understanding of allosteric regulation of NMDA receptor activity that is opening the way for the development of new classes of modulators, including agents with NMDA receptor subtype selectivity. This area of research is reviewed in the next section.
Subtype selective NMDA receptor modulation
As stated above, there are 4 GluN2 subtypes, GluN2A-D, and individual receptors may be configured with the same or two different subtypes in combination with the two GluN1 subunits (Fig. (12)) [8]. The composition of the GluN2 compliment imparts unique biophysical characteristics to the NMDA receptor channel behavior [117, 167] and the ATDs of the GluN2 subunits appear to be the source of these unique regulatory functions [128,129]. This was elegantly demonstrated in studies by the Traynelis lab in which the biophysical characteristics of receptors containing GluN2A or GluN2D subunits were compared after manipulation of their ATDs [168]. These two subtypes differ most significantly in terms of channel gating kinetics and open probabilities. Removal of the GluN2 ATD from either subtype rendered channels with kinetics and open probabilities intermediate between native GluN2A and GluN2D receptors and no longer significantly different. Furthermore, when ATDs were swapped between the two subtypes, the biophysical properties of the chimeras resembled those corresponding to the source of the ATD. These experiments indicate that the GluN2 ATDs function as physiological allosteric regulators of NMDA channel properties.
Fig. 12. NMDA receptor architecture and ligand binding sites (adapted from Ogden et al. [169]).
NMDA receptors are tetrameric ion channels canonically comprising two GluN1 (labeled N1) subunits and two GluN2 subunits (2A, 2B, 2C or 2D) (a). Receptors containing different GluN1 splice variants and/or a GluN3 subunit have distinct functional properties. Additionally, much of the diversity among receptor subtypes arises from the GluN2 subunits, which are crucial in determining biophysical and pharmacological properties of the receptor. (b) Key ligands of individual GluN2-containing receptors are shown below the receptor subtypes. (c) Major ligand binding sites on the NMDA receptor are depicted on a GluN1/GluN2D homology model (Ifenprodil (3) and related GluN2B–selective molecules bind to the amino terminal domain (ATD) as do polyamines). Competitive antagonists of glycine and glutamate bind to the ligand binding domain (LBD) of GluN1 and GluN2, respectively. Channel blockers bind in the transmembrane domain (TMD). An individual GluN2 subunit from a tetrameric complex is shown with the sites for QNZ46, DQP-1105 and CIQ highlighted. (d) Model for conformational changes in the ATD with spermine (2) (PAM) [185]. (e) Model for conformational changes in the ATD with Ifenprodil (3) (NAM) [185].
The regulatory function of the GluN2 ATDs may be modified by subtype selective binding of several physiological ligands and a number of different classes of xenobiotics. Zinc binds with high affinity to the GluN2A or GluN2B ATD to inhibit receptor activity [170, 171], whereas the polyamines spermine (2) and spermidine bind to the GluN2B ATD to potentiate channel activity [172]. Multiple classes of xenobiotic GluN2B negative allosteric modulators have been identified and a number of these compounds have progressed to clinical trials [173–177]. More recently, modulators of GluN2C and GluN2D receptors have been identified that appear to interact with sequences linking the ATD and ligand binding domains [178–182]. Insight into conformational changes in the GluN2 ATDs that are associated with allosteric modulation of channel activity has been gained through a variety of experimental approaches, including recent X-ray protein crystallography studies of isolated GluN2A and GluN2B ATDs and complexes of GluN1/GluN2B dimers [183, 184]. Functional analyses of mutated GluN2A and GluN2B receptors were used to localize the binding site of zinc, deep within the hinge of the ATD clamshell. Analysis of the X-ray crystal structure of zinc bound to the GluN2B ATD indicated that zinc binding induces a closure of the R1 and R2 lobes of the clamshell around the ligand. These and other observations lead to a model suggesting the degree of opening or closing of the GluN2A/B ATD allosterically increases or decreases channel open probabilities, respectively [167, 170, 185]. This model is similar to that proposed for the AMPA receptor glutamate binding domain to account for the functional activity of ligands that vary from full agonists to full antagonists [98, 101]. It was initially proposed that the GluN2B xenobiotic NAMs similarly bind within the GluN2B clamshell pocket to promote a closed conformation [167, 170]. However, a recent X-ray crystal structure of a GluN1/GluN2B heterodimer revealed that such compounds may actually bind at the ATD dimer interface to induce a more complex rearrangement of the R1 and R2 domains of both the GluN1 and GluN2 ATDs [186,187]. One potential model for this rearrangement based on the x-ray crystallographic data is shown in Fig. (12). In this model, the GluN1R and GluN2R subunits are brought in close proximity upon binding with ifenprodil (3), a GluN2B NAM; this binding then results in a conformational change that is transmitted through the LBD resulting in closing of the ion channel (Fig. (12E)). Conversely, spermine (2) which is a known physiologic GluN2B PAM is believed to bind to the ATD resulting in the separation of the GluN1R and GluN2R domains (Fig. (12D)); this binding interaction results in a different conformational change that is transmitted through the LBD resulting in an opening of the ion channel [185].
Receptors with different GluN2 subunits are differentially localized to different brain circuits and in the subsynaptic space, representing distinct drug targets [182]. The GluN2B subunit is highly expressed in the adult cortex, hippocampus, thalamus, and striatum, forebrain regions that support higher cognitive and emotional functions. In fact, there is a wealth of experimental data that indicate that GluN2B receptors are particularly involved in mediating the forms of synaptic plasticity (e.g., LTP) that underlie new learning and memory formation. Functional data supporting this contention comes from genetic manipulation of GluN2B expression in rodents. Both transgenic mice and rats engineered to over-express the GluN2B subunit in forebrain demonstrate enhanced LTP in hippocampus and prefrontal cortex as well as improved performance in various learning and memory tasks [188–191]. Conversely, biasing away from GluN2B impedes the induction of LTP and degrades learning and memory. Conventional knock out (KO) of the GluN2B subunit is embryonic lethal; however, there are a number of conditional models that circumvent this issue. These include mice with conditional GluN2B KO in principal glutamatergic neurons in forebrain [192, 193], hippocampal CA1 and dentate gyrus [193], and hippocampal CA3 [194]. In each case, the KOs displayed a defect in the induction or expression of LTP at relevant synapses as well as degraded performance in hippocampal-dependent learning and memory tasks. This pattern of results was also observed in aged Fischer 344 rats that have reduced expression of the GluN2B subunit in hippocampus and in young rats in which the GluN2B subunit was knocked down with intrahippocampal siRNA infusion [195].
The data summarized above support a hypothesis that pharmacological agents that increase the activity of GluN2B subtype receptors may bias synapses toward an increase in synaptic efficacy to facilitate learning and memory processes and that such drugs may be useful to treat cognitive dysfunction in a variety of neuropsychiatric conditions. Significantly, GluN2B potentiators may be particularly useful in the treatment of schizophrenia in light of the clinical data of GluN2B negative allosteric modulators which have shown that GluN2B antagonism induces the full spectrum of schizophrenia symptoms. There are a number of compounds that are known negative allosteric modulators of GluN2B receptors [176, 182, 196–198]. GluN2B antagonists cross-discriminate with NMDA channel blockers in rat and primate drug discrimination studies [199, 200]. This indicates that the GluN2B NAMs share discriminative stimulus properties with pan-NMDA receptor channel blockers. One GluN2B NAM, CP-101,606 [201, 202], has been studied in several Phase II clinical trials. In these trials, CP-101,606 was reported to cause dose-dependent disruption of cognitive function and memory impairment and also to produce dissociative sensory experiences [63, 203–205]. These preclinical and clinical data indicate that allosteric inhibition of the GluN2B receptor induces schizophrenomimetic effects similar to the effects caused by the pan-NMDA channel blockers. These channel blocker effects provide the support for the NMDA receptor hypofunction hypothesis of schizophrenia. We suggest that this hypothesis may be refined to a GluN2B hypofunction hypothesis. This refined hypothesis is supported by the association of genetic polymorphisms in the GluN2B gene (GRIN2B) with schizophrenia [206, 207]. Given that allosteric inhibition of GluN2B receptors is putatively schizophrenomimetic, positive allosteric modulation of these receptors becomes a highly compelling molecular mechanism to pursue for the comprehensive treatment of schizophrenia.
Drug-like, selective GluN2B PAMS are currently not available (Fig. (12)). The neurosteroid, pregnenolone sulfate (PS) (21) potentiates responses at GluN2B but is not selective [208]. Chimera and point mutation studies have identified a putative binding site for PS (21) potentiation [208]. Modeling of receptor kinetics suggests that 21 increases the open probability of NMDA receptor responses. Naphthoic and phenanthroic acid NMDA receptor PAMs having NMDA receptor potentiating activity have also been identified [209]. In common with the neurosteroids, some of the naphthoic and phenanthroic acid compounds discriminate between GluN2A/GluN2B vs. GluN2C/ GluN2D receptors. The GluN2B potentiator investigated the most is the polyamine spermine (2) which is selective but neither potent nor drug-like (Table 1). It potentiates by two mechanisms; by increasing glycine affinity (glycine-dependent potentiation) and by an allosteric interaction that can be seen in saturating glycine conditions (glycine-independent potentiation). This latter action of spermine (2) has the same subunit specificity as that displayed by potentiation due to histamine and Mg2+. Potentiation is specific to the combination of GluN1 subunits that lack exon 5 and GluN2B subunits [210, 211].
There is strong evidence for a dynamic interaction between GluN2B and GluN2A subunits as a key element in the continual network reorganization that is the basis for learning and memory [212]. Synapses with more GluN2B subunits undergo activity-dependent strengthening, which subsequently results in the insertion of GluN2A to stabilize the strengthened connection [213]. Reduced use of a synapse over time is accompanied by a reversion to higher GluN2B content and the synapse again becomes primed for activity-driven potentiation [213]. Such activity-driven, GluN2B-mediated potentiation of the synapse is a basic element in new learning and the formation of memories [212]. It may be possible to create a more plastic developmental state by pharmacological inhibition of GluN2A receptors with GluN2A negative allosteric modulators (GluN2A NAMs). Such compounds should shift the balance towards GluN2B-dependent activity and this will facilitate remapping of neuronal networks. Accordingly, inhibition of GluN2A receptors should increase synaptic plasticity to facilitate cortical remapping. This hypothesis is supported by studies in a model of synaptic plasticity[214]. Recently, a selective GluN2A NAM, TCN 201 (1) (Fig. (12)) has been identified and having this agent will assist in assessing the promise of GluN2A as a target [215].
In summary, enthusiasm for GluN2B PAMS and GluN2A NAMS for neuropsychiatric disorders is tempered by the current unavailability of potent, selective, drug-like agents and potential challenges inherent in targeting subtype selective GluN2 targets. Careful analysis of NMDA receptor-mediated synaptic currents in the well-studied hippocampal CA3–CA1 synapse, for example, suggests that GluN1/GluN2A/GluN2B receptors provide the majority of the NMDA synaptic response [216]. The presence of heterotrimeric NMDA receptors, such as those containing GluN1/GluN2A/GluN2B subunits, significantly increases the difficulty in resolving the function of different NMDA receptor subtypes. For instance, the highly GluN2B-selective ifenprodil (3) displays a high affinity but lowered efficacy at GluN1/GluN2A/GluN2B receptors [217]. Thus, if the relative contribution of the different di- and tri-heteromeric receptor complexes is unknown, it will be very challenging to make quantitative conclusions regarding subunit contribution to receptor function. Nonetheless, many agents targeting the GluN2A-D receptors have recently been identified and GluN2B PAMS and GluN2A NAMs provide considerable promise for cognitive enhancement in schizophrenia treatment [182].
Metabotropic glutamate receptor modulation
Over the past twenty years, the ‘glutamate’ or ‘NMDA hypofunction’ hypothesis of schizophrenia has rapidly progressed, with a promise of a multitude of novel pre- and postsynaptic as well as glial mechanisms and targets to ameliorate NMDA dysfunction [60, 218, 219]. Of these, metabotropic glutamate receptors (mGluRs), Class C GPCRs, have garnered a great deal of interest, and robust preclinical and variable clinical validation has been achieved [60, 218–220]. The Class C GPCRs are distinguished by two unique structural features: 1) a large extracellular, venus fly-trap amino-terminal agonist binding site linked to the heptahelical transmembrane (7TM) domain via a cysteine-rich region and 2) the formation of constitutive homo- and heterodimers that engender diverse activation modes. There has been some controversy surrounding mGluR activation, and whether the binding of glutamate to only one promoter within the dimer is sufficient to elicit activation of the complex [77, 78, 221–232].
The orthosteric agonist glutamate, along with the other known agonists and competitive antagonists of the mGlu receptors, bind within the extracellular N-terminal region; in contrast, all of the positive allosteric modulators (PAMs) and negative allosteric modulators (NAMs) described thus far bind within the 7TM domain. To date, eight mGluRs have been cloned and sequenced, and have been assigned to three groups based on their structure, preferred coupling to effector mechanisms and pharmacology. Group I receptors (mGlu1 and mGlu5) are predominantly located post-synaptically and couple to Gaq, resulting in increases in intra-cellular calcium. Group II (mGlu2 and mGlu3) and Group III (mGlu4, mGlu6, mGlu7 and mGlu8) receptors are coupled through Gi/Go resulting in decreases in cAMP levels, are located presynaptically and generally have inhibitory effects on neurotransmitter release [77, 78, 221–232]. Of these, post-synaptic mGlu1 and mGlu5 and presynaptic mGlu2/3 are relevant to schizophrenia and the glutamate hypothesis [60, 218–220].
Of the three mGluRs of interest in schizophrenia, mGlu1 is the least advanced, but recent genetic studies are generating renewed enthusiasm for the target [233, 234]. The mGlu1 receptor controls the postsynaptic release of the key neurotransmitters GABA and glutamate, and also interacts with the NMDA receptor [231]. As anticipated, mGlu1 knock-out mice display motor dysfunction and diminished learning and memory capabilities. Recent genetic data indicates loss of function mutation in the GRM1 gene, which encodes mGlu1, in patients suffering from schizophrenia and bipolar disorder [233, 234]. Thus, mGlu1 PAMs may represent novel treatment approaches for a subset of schizophrenic patients with the GRM1 mutation; however, few mGlu1 PAMs have been described, and the known ligands represent two distinct scaffolds (Fig. (13)). In contrast, a large number of mGlu1 NAMs with tremendous structural diversity have been developed (Fig. (13)) [235].
Fig. 13. Representative mGlu1 allosteric modulator ligands.
mGlu1 PAMs 35–37 and mGlu1 NAMs 38 and 39. Limited mGlu1 PAM tool compounds exist, in contrast to mGlu1 NAMs.
Group I (mGlu1 and mGlu5) agonists, such as DHPG, are excitotoxic and lead to pronounced epileptiform activity as well as the classic issues with receptor desensitization and down regulation [78, 220, 221]. Thus, most drug discovery efforts have focused on positive allosteric modulation of mGlu5, to maintain temporal and spatial control of receptor signaling. Allosteric modulation of mGlu5 affords opportunities for the treatment of a wide range of CNS disorders [78, 220, 221, 236, 237]. With respect to mGlu5 PAMs (Fig. (14)), the major therapeutic interests are focused on schizophrenia and cognition, via potentiation of the N-methyl-D-aspartate (NMDA) receptor and the NMDA hypofunction hypothesis of schizophrenia [60, 78, 218–221]. mGlu5 physically, via scaffolding proteins, and functionally interacts with NMDA receptors, through a reciprocal positive-feedback loop, to indirectly regulate NMDA receptor function in critical forebrain regions. mGlu5 PAMs also enhance hippocampal synaptic plasticity (LTP and LTD), processes crucial for cognitive function, and reverse cognitive deficits induced by NMDA antagonists [60, 78, 218–221]. Multiple mGlu5 PAMs have been shown to potentiate NMDA currents, afford robust efficacy in preclinical models predictive of antipsychotic activity and demonstrated pro-cognitive profiles in a variety of preclinical cognition assays, suggesting mGlu5 PAMs can address all three symptom clusters in schizophrenia [60, 78, 218–221]. However, no clinical data has yet to be reported, but the patent literature is growing rapidly and many companies are rumored to be in IND-enabling studies.
Fig. 14. Representative mGlu5 PAMs 5, 6, 41–48, highlighting a wide diversity of chemotypes.
All bind at the MPEP (40) site except 6, 43 and 44, which bind at unique, not fully characterized allosteric site(s) on mGlu5.
For mGlu5 PAMs, ligands have been developed that bind at one of three distinct allosteric sites, further adding complexity, as the MPEP (40) binding site is the only characterized allosteric site on mGlu5 [60, 78, 218–221]. While the majority of PAMs bind at the MPEP (40) site, CPPHA (6) [238, 239], VU0034403 (43) [240] and VU0357121 (44) [241], have been shown to potentiate mGlu5 at a site distinct from the MPEP allosteric site. It is not yet clear if the unique conformations stabilized by ligands at the other allosteric sites offer advantages or disadvantages over modulation via the MPEP (40) site, but it is known that CPPHA (6) displays ligand-biased signaling [242]. Regardless, pure mGlu5 PAMs are essential, as ago-PAMs, may suffer the same adverse events as orthosteric Group I agonists [60, 78, 218–221]. Moreover, MPEP-site mGlu5 PAMs possess a greater propensity for displaying ‘molecular switches’ with subtle structural modifications during the lead optimization campaign, and in vivo through oxidative metabolism [243, 244] This latter point is of critical importance, as metabolites may become ago-PAMs, NAMs or switch mGlu subtype selectivity, requiring extensive metabolite identification, synthesis and pharmacological evaluation. The best practice is to quickly assess the propensity for a series to display ‘molecular switches’, and pursue series that possess ‘molecular locks’ to avoid this complication, while still taking advantage of the radio-ligands and PET tracers available for MPEP-site PAMs [60, 78, 218–221].
The most advanced of the mGluRs as a novel treatment strategy for schizophrenia are the Group II mGluRs (mGlu2 and mGlu3) [60, 78, 218–221]. By normalizing excessive glutamatergic tone, due to disinhibition of glutamate neurons as a result of reduced NMDA activity, the Lilly mGlu2/3 agonist LY354740 (49) represents the first non-dopaminergic agent with comparable efficacy to atypical antipsychotics in treating the positive and negative symptom clusters of schizophrenia (Fig. (15)) [219, 245]. This efficacy has been demonstrated in multiple preclinical animal studies as well as in human clinical trials. In the first clinical trial, 49 reduced the cognitive impairing effects of ketamine in healthy volunteers, and in the first published Phase II study, 49 was comparable in efficacy to olanzopine, but lacked the metabolic and motor-related side effects [219, 246–249]. A subsequent larger trial was inconclusive, as both 49 and olanzapine did not separate from placebo, and a later safety trial witnessed a number of subjects withdrawing due to reduced efficacy. These data suggest that an orthosteric agonist may lead to receptor desensitization over time, leading to a lack of efficacy. In addition, 49 is a glutamate analog and must be given as a pro-drug for optimal exposure [215, 246–249]. Finally, studies in mGlu2 and mGlu3 knock-out mice indicate that the antipsychotic efficacy of 49 is due to activation of only mGlu2 [60, 78, 218–221].
Fig. 15.
Representative mGlu2 PAMs 50–57, highlighting a wide diversity of chemotypes that are distinct from the Lilly mGlu2/3 agonist LY354740 (49).
Therefore, many companies (Merck, Lilly, GSK, Pfizer, etc…) have pursued the development of mGlu2 PAMs to avoid the necessity of pro-drugs, avoid receptor desensitization and to only activate the relevant mGluR, mGlu2 [60, 78, 218–221, 250]. A vast multitude of chemotypes have been developed as mGlu2 PAMs (Fig. (15)), that have recapitulated the efficacy of 49 in multiple preclinical models of schizophrenia. Beyond schizophrenia, mGlu2 PAMs have also displayed efficacy in anxiety assays, drug dependence (cocaine) and sleep-wake architecture (normalizing REM states characteristic of depression). Unlike mGlu5, little is known about the allosteric binding site for mGlu2, or if allosteric ligands occupy the same or physically distinct allosteric binding sites [250]. This is due to the lack of selective mGlu2 NAMs from which radioligands can be developed. In general, due to high cooperativity with glutamate, mGlu PAMs typically bind the receptor with weak affinity, yet provide potent functional activity – undesirable for radioligand and PET tracer development; however, within series, one may identify a PAM with low cooperativity, where functional potency approximates affinity [60, 78, 218–221, 250]. Despite the lack of PET tracers for target engagement/occupancy studies, several companies are believed to have mGlu2 PAMs in either late stage IND-enabling studies or are initiating Phase I trials [250]. Phase II data with an mGlu2 PAM in schizophrenic patients is eagerly awaited, and it will be important to compare to the data obtained with 49. As of this writing, no selective mGlu3 PAMs have been reported; therefore the therapeutic potential of selective mGlu3 activation remains unclear.
PERSPECTIVE AND FUTURE DIRECTIONS
Drug discovery for schizophrenia is evolving. Through the 80’s and 90’s, drug discovery efforts were focused almost exclusively on developing new antipsychotic agents of the D2/5HT2A antagonist class. However, this era is being brought to a close because of two movements. The first was the realignment of treatment goals brought on by the seminal work of Green [4, 251] indicating that the profound, chronic cognitive dysfunction largely accounts for the poor prognosis and outcomes for patients diagnosed with schizophrenia. This realignment ultimately gave rise to the MATRICS initiative (www.matrics.ucla.edu), a combined effort of academia, NIH and FDA, and industry to chart a path for the development and approval of new drugs that ameliorate the cognitive deficits of the disorder. The second precipitating event is the loss of patent exclusivity through 2013 of the blockbuster D2/5HT2A antagonists. This is occurring in the face of definitive clinical data from the CATIE and CUt-LASS trials [252, 253] which show that these agents do not differentiate from one another and that they do not meaningfully impact cognitive dysfunction [5], despite numerous claims to the contrary [254]. Thus, there is a lack of incentive for further development of D2-based antipsychotics.
The realignment of treatment goals towards cognitive dysfunction is also prompting a ‘new reassessment’ of the biological underpinnings of schizophrenia and the landscape of molecular targets for drug intervention. Schizophrenia is a neurodevelopmental disease that reflects a failure to successfully traverse a late adolescent phase of cortical development [16]. The susceptibility to schizophrenia has a very high but diffuse genetic component. In fact, it appears that schizophrenia arises from derailment of the complex genetic program that transitions the cortex to a final adult organization, not from defects in individual proteins. Deciphering this genetic program will undoubtedly give tremendous insight into the biological basis of schizophrenia. We can forecast that this will lead to biologically-based diagnoses, meaningful differentiation of subcategories, better assessment of prognoses, and much better utilization of treatment options. However, given the complexity of the cortical developmental process and of the orchestrating genetic code, these forecasts remain to be realized well in the future. For the present, treatment remains focused on ameliorating the aftermath of the cortical developmental failure. This focus has largely turned to the glutamate neurotransmitter system, stemming from multiple lines of evidence. Genetic analyses consistently highlight glutamate receptor genes in the susceptibility to schizophrenia. Neuropathological findings indicate severe disruption of cortical glutamate synapses, as well as in the interconnected GABAergic system. Furthermore, disruption of glutamate synaptic transmission in healthy individuals with NMDA receptor channel blockers reproduces to a striking degree the clinical and neurocognitive symptoms of schizophrenia. This vast array of evidence converges to strongly implicate disruption of glutamate synaptic transmission in the cause of schizophrenia as well as in its expression.
In the present article, we highlight the potential benefits of allosteric modulators of the glutamate receptors for the treatment of schizophrenia. The cortical glutamatergic/GABAergic networks that are disrupted in schizophrenia operate with very high temporal precision. We argue that, in principal, such temporal precision is maintained only through allosteric modulation of signaling, as this approach maintains the temporal aspects of signaling. However, implementing and prosecuting such drug discovery strategies also present unique hurdles. Finding the optimal profile of cooperative and/or agonistic activity of an allosteric drug remains a challenge. Determining what magnitude of allosteric modulation, for example, is required to best treat a disease like schizophrenia is unknown and animal models are not capable of providing a solution to this problem. To further complicate the complexity of the allosteric drug discovery process is an appreciation that allosteric signal propagation is poorly understood. Just as the topology and dynamics of cellular networks (e.g. protein-protein, gene transcription networks, metabolic networks, signaling networks) are increasingly used to understand cellular function in overall health and disease, so too will they be used to understand allosteric drug structure-function. Recently the concept of allosteric drugs has been broadened to include allo-network drugs to reflect a more realistic (and more complex) portrayal of dynamic signaling from a systems-based perspective [255]. In the case of schizophrenia, such an analysis will be required as we recognize that reductionist approaches targeting specific proteins have not been successful and aiming at networks as opposed to target proteins will eventually be part of future drug discovery strategy [28]. Thus, the focus is on the network architecture of the glutamate synapse and the ways in which this network may be modulated as a new treatment approach to schizophrenia.
There are also challenges to the development of allosteric modulators from a medicinal chemistry standpoint. The factors that offer the possibility of identifying highly specific molecules for glutamate receptor families, and even subtypes of receptors within a family, also present potential risks in the development of new glutamate receptor allosteric modulators. The lack of evolutionary pressure on allosteric binding sites which is so important for allosteric receptor subtype selectivity, has a down side in that this very diversity can lead to significant species differences in response to allosteric modulators. This is particularly problematic when rodent animal models are used and translation to clinical studies is required. Another frequently encountered challenge involves the existence of limited structure-activity relationships (SAR) within allosteric modulator series. Allosteric GPCR modulators can have “flat,” nontractable SAR, which creates challenges for improving on the activity of lead molecules with micromolar affinities [239]. There are also a number of cases where compound metabolism or even slight structural modifications in series result in important changes in pharmacological responses, from positive to negative allosteric modulation [78]. Despite these challenges, we high-light the tremendous progress that has been made in identifying new molecular probes of glutamate receptor function.
In summary, there is a tremendous interest in targeting glutamate receptors as new therapeutic approaches to schizophrenia. At present, glutamate receptor allosteric modulators will be directed at ameliorating the glutamate synaptic dysfunction that is a core aspect of the expression of the symptoms of the disorder. As we gain a better understanding of the role of glutamate synaptic dysfunction in the development of the disorder, it is hoped that these drugs may also have a place in advancing treatment into the prodromal stage, and even into prevention.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the contribution of Hans Rollema to the organization of this manuscript. Research in the laboratories of CWL and PJC that is relevant to this review is supported by funding from the National Institutes of Health (NIMH, NINDS, NIDA, and MLPCN) and the Janssen Pharmaceutica.
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Mueser K, McGurk S. Schizophrenia. The Lancet. 2004;363:2063–2072. doi: 10.1016/S0140-6736(04)16458-1. [DOI] [PubMed] [Google Scholar]
- 2.Javitt DC. When doors of perception close: Bottom-up models of disrupted cognition in schizophrenia. Annu. Rev. Clin. Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS drugs. 2005;19(Suppl 1):1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 4.Green M. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Davis CE, Hsiao JK, Lieberman JA. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch. Gen. Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 6.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am. J. Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 7.Ross C, Margolis R, Reading S, Pletnikov M, Coyle J. Neurobiology of Schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 10.Conn PJ. Physiological roles and therapeutic potential of metabotropic glutamate receptors. Ann. N Y Acad. Sci. 2003;1003:12–21. doi: 10.1196/annals.1300.002. [DOI] [PubMed] [Google Scholar]
- 11.Kew JNC, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology. 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- 12.Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology. 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 13.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J. Psychiatr. Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 14.Kantrowitz ER, Javitt D. Glutamate: New hope for schizophrenia therapy. Current Psychiatry. 2011;10:69–74. [Google Scholar]
- 15.Field JR, Walker AG, Conn PJ. Targeting glutamate synapses in schizophrenia. Trends Mol. Med. 2011;17:689–698. doi: 10.1016/j.molmed.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol. Psychiatry. 2012;17:1228–1238. doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owen MJ, O'Donovan MC, Thapar A, Craddock N. Neurodevelopmental hypothesis of schizophrenia. Br. J. Psychiatry. 2011;198:173–175. doi: 10.1192/bjp.bp.110.084384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niendam T, Bearden C, Johnson J, McKinley M, Loewy R, Obrien M, Nuechterlein K, Green M, Cannon T. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophr. Res. 2006;84:100–111. doi: 10.1016/j.schres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat. Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- 20.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat. Rev.Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 21.Curley AA, Lewis DA. Cortical basket cell dysfunction in schizophrenia. J. Physiol. 2012;590:715–724. doi: 10.1113/jphysiol.2011.224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glausier JR, Lewis DA. Selective Pyramidal Cell Reduction of GABA(A) Receptor alpha1 Subunit Messenger RNA Expression in Schizophrenia. Neuropsychopharmacology. 2011;36:2103–2110. doi: 10.1038/npp.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Burgos G, Lewis DA. NMDA Receptor Hypofunction, Parvalbumin-Positive Neurons and Cortical Gamma Oscillations in Schizophrenia. Schizophr. Bull. 2012;38:950–957. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torkamani A, Dean B, Schork NJ, Thomas EA. Coexpression network analysis of neural tissue reveals perturbations in developmental processes in schizophrenia. Genome Res. 2010;20:403–412. doi: 10.1101/gr.101956.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayalew M, Le-Niculescu H, Levey DF, Jain N, Changala B, Patel SD, Winiger E, Breier A, Shekhar A, Amdur R, Koller D, Nurnberger JI, Corvin A, Geyer M, Tsuang MT, Salomon D, Schork NJ, Fanous AH, O'Donovan MC, Niculescu AB. Convergent functional genomics of schizophrenia: from comprehensive understanding to genetic risk prediction. Mol. Psychiatry. 2012;17:887–905. doi: 10.1038/mp.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mann JJ, Haghighi F. Genes and environment: multiple pathways to psychopathology. Biol. Psychiatry. 2010;68:403–404. doi: 10.1016/j.biopsych.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Tsuang M. Schizophrenia: genes and environment. Biol. Psychiatry. 2000;47:210–220. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan PF. Puzzling over schizophrenia: schizophrenia as a pathway disease. Nat. Med. 2012;18:210–211. doi: 10.1038/nm.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurman RE, Rynes E, Humbert R, Vierstra J, Maurano MT, Haugen E, Sheffield NC, Stergachis AB, Wang H, Vernot B, Garg K, John S, Sandstrom R, Bates D, Boatman L, Canfield TK, Diegel M, Dunn D, Ebersol AK, Frum T, Giste E, Johnson AK, Johnson EM, Kutyavin T, Lajoie B, Lee BK, Lee K, London D, Lotakis D, Neph S, Neri F, Nguyen ED, Qu H, Reynolds AP, Roach V, Safi A, Sanchez ME, Sanyal A, Shafer A, Simon JM, Song L, Vong S, Weaver M, Yan Y, Zhang Z, Lenhard B, Tewari M, Dorschner MO, Hansen RS, Navas PA, Stamatoyannopoulos G, Iyer VR, Lieb JD, Sunyaev SR, Akey JM, Sabo PJ, Kaul R, Furey TS, Dekker J, Crawford GE, Stamatoyannopoulos JA. The accessible chromatin landscape of the human genome. Nature. 2012;489:75–82. doi: 10.1038/nature11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lips ES, Cornelisse LN, Toonen RF, Min JL, Hultman CM, Holmans PA, O'Donovan MC, Purcell SM, Smit AB, Verhage M, Sullivan PF, Visscher PM, Posthuma D. Functional gene group analysis identifies synaptic gene groups as risk factor for schizophrenia. Mol. Psychiatry. 2012;17:996–1006. doi: 10.1038/mp.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J, Han L, Zhao Z. Gene- and evidence-based candidate gene selection for schizophrenia and gene feature analysis. Artif. Intell. Med. 2010;48:99–106. doi: 10.1016/j.artmed.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, Jia P, Fanous AH, Webb BT, van den Oord EJ, Chen X, Bukszar J, Kendler KS, Zhao Z. A multidimensional evidence-based candidate gene prioritization approach for complex diseases-schizophrenia as a case. Bioinformatics. 2009;25:2595–6602. doi: 10.1093/bioinformatics/btp428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia P, Sun J, Guo AY, Zhao Z. SZGR: a comprehensive schizophrenia gene resource. Mol. Psychiatry. 2010;15:453–462. doi: 10.1038/mp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vawter MP, Mamdani F, Macciardi F. An integrative functional genomics approach for discovering biomarkers in schizophrenia. Brief Funct. Genomics. 2011;10:387–399. doi: 10.1093/bfgp/elr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun J, Jia P, Fanous AH, van den Oord E, Chen X, Riley BP, Amdur RL, Kendler KS, Zhao Z. Schizophrenia gene networks and pathways and their applications for novel candidate gene selection. PLoS One. 2010;5:e11351. doi: 10.1371/journal.pone.0011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SA, Tsao TT, Yang KC, Lin H, Kuo YL, Hsu CH, Lee WK, Huang KC, Kao CY. Construction and analysis of the protein-protein interaction networks for schizophrenia, bipolar disorder, and major depression. BMC Bioinformatics. 2011;12(Suppl 13):S20. doi: 10.1186/1471-2105-12-S13-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Jong S, Boks MP, Fuller TF, Strengman E, Janson E, de Kovel CG, Ori AP, Vi N, Mulder F, Blom JD, Glenthøj B, Schubart CD, Cahn W, Kahn RS, Horvath S, Ophoff RA. A gene co-expression network in whole blood of schizophrenia patients is independent of antipsychotic-use and enriched for brain-expressed genes. PLoS One. 2012;7:e39498. doi: 10.1371/journal.pone.0039498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Hirvonen J, Hietala J. Dysfunctional brain networks and genetic risk for schizophrenia: specific neurotransmitter systems. CNS Neurosci. Ther. 2011;17:89–96. doi: 10.1111/j.1755-5949.2010.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balu DT, Coyle JT. Neuroplasticity signaling pathways linked to the pathophysiology of schizophrenia. Neurosci. Biobehav. Rev. 2011;35:848–870. doi: 10.1016/j.neubiorev.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buonanno A. The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. Brain Res. Bull. 2010;83:122–131. doi: 10.1016/j.brainresbull.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res. Bull. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 45.Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 46.Domino EF, Luby ED. Phencyclidine/Schizophrenia: One View Toward the Past, The Other to the Future. Schizophr. Bull. 2012;38:914–919. doi: 10.1093/schbul/sbs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muir KW. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr. Opin. Pharmacol. 2006;6:53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Dyker AG, Edwards KR, Fayad PB, Hormes JT, Lees KR. Safety and tolerability study of aptiganel hydrochloride in patients with an acute ischemic stroke. Stroke. 1999;30:2038–2042. doi: 10.1161/01.str.30.10.2038. [DOI] [PubMed] [Google Scholar]
- 49.Muir KW, Grosset DG, Lees KR. Clinical pharmacology of CNS 1102 in volunteers. Ann. N Y Acad. Sci. 1995;765:279–289. doi: 10.1111/j.1749-6632.1995.tb16585.x. discussion 298. [DOI] [PubMed] [Google Scholar]
- 50.Muir KW, Lees KR. Excitatory amino acid antagonists for acute stroke. Cochrane Database Syst. Rev. 2003:CD001244. doi: 10.1002/14651858.CD001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adler CM, Goldberg TE, Malhotra AK, Pickar D, Breier A. Effects of ketamine on thought disorder, working memory, and semantic memory in healthy volunteers. Biol. Psychiatry. 1998;43:811–816. doi: 10.1016/s0006-3223(97)00556-8. [DOI] [PubMed] [Google Scholar]
- 52.Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am. J. Psychiatry. 1999;156:1646–1649. doi: 10.1176/ajp.156.10.1646. [DOI] [PubMed] [Google Scholar]
- 53.Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am. J. Psychiatry. 1997;154:805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- 54.Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14:301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- 55.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 56.Honey GD, Corlett PR, Absalom AR, Lee M, Pomarol-Clotet E, Murray GK, McKenna PJ, Bullmore ET, Menon DK, Fletcher PC. Individual Differences in Psychotic Effects of Ketamine Are Predicted by Brain Function Measured under Placebo. J. Neurosci. 2008;28:6295–6303. doi: 10.1523/JNEUROSCI.0910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Honey GD, O'Loughlin C, Turner DC, Pomarol-Clotet E, Corlett PR, Fletcher PC. The Effects of a Subpsychotic Dose of Ketamine on Recognition and Source Memory for Agency: Implications for Pharmacological Modelling of Core Symptoms of Schizophrenia. Neuropsychopharmacology. 2005;31:413–423. doi: 10.1038/sj.npp.1300846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Javitt DC, Zukin SR, Heresco-Levy U, Umbricht D. Has an Angel Shown the Way? Etiological and Therapeutic Implications of the PCP/NMDA Model of Schizophrenia. Schizophr. Bull. 2012;38:958–966. doi: 10.1093/schbul/sbs069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Javitt DC. Sensory processing in schizophrenia: neither simple nor intact. Schizophr. Bull. 2009;35:1059–1064. doi: 10.1093/schbul/sbp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marek GJ, Behl B, Bespalov AY, Gross G, Lee Y, Schoemaker H. Glutamatergic (N-Methyl-D-aspartate Receptor) Hypofrontality in Schizophrenia: Too Little Juice or a Miswired Brain? Mol. Pharmacol. 2009;77:317–326. doi: 10.1124/mol.109.059865. [DOI] [PubMed] [Google Scholar]
- 61.Lees KR, Asplund K, Carolei A, Davis SM, Diener HC, Kaste M, Orgogozo JM, Whitehead J. Glycine antagonist (gavestinel) in neuroprotection (GAIN International) in patients with acute stroke: a randomised controlled trial. GAIN International Investigators. Lancet. 2000;355:1949–1954. doi: 10.1016/s0140-6736(00)02326-6. [DOI] [PubMed] [Google Scholar]
- 62.Sacco RL, DeRosa JT, Haley EC, Jr, Levin B, Ordronneau P, Phillips SJ, Rundek T, Snipes RG, Thompson JL. Glycine antagonist in neuroprotection for patients with acute stroke: GAIN Americas: a randomized controlled trial. JAMA. 2001;285:1719–1728. doi: 10.1001/jama.285.13.1719. [DOI] [PubMed] [Google Scholar]
- 63.Nutt JG, Gunzler SA, Kirchhoff T, Hogarth P, Weaver JL, Krams M, Jamerson B, Menniti FS, Landen JW. Effects of a GLUN2B selective NMDA glutamate antagonist, CP-101,606, on dyskinesia and Parkinsonism. Mov. Disord. 2008;23:1860–1866. doi: 10.1002/mds.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Preskorn SH, Baker BR, Kolluri S, Menniti FS, Krams M, Landen JW. An Innovative Design to Establish Proof of Concept of the Antidepressant Effects of the NR2B Subunit Selective N-Methyl-D-Aspartate Antagonist, CP-101,606, in Patients With Treatment-Refractory Major Depressive Disorder. J. Clin. Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 65.Sang CN, Weaver JJ, Jinga L, Wouden J, Saltarelli MD. Society for Neuroscience. New Orleans, LA: 2003. The GLUN2B subunit-selective NMDA receptor antagonist CP-101,606 reduces pain intensity in patients with central and peripheral neuropathic pain. 814.819. [Google Scholar]
- 66.Smith JW, Gastambide F, Gilmour G, Dix S, Foss J, Lloyd K, Malik N, Tricklebank M. A comparison of the effects of ketamine and phencyclidine with other antagonists of the NMDA receptor in rodent assays of attention and working memory. Psychopharmacology. 2011;217:255–269. doi: 10.1007/s00213-011-2277-5. [DOI] [PubMed] [Google Scholar]
- 67.Dix S, Gilmour G, Potts S, Smith JW, Tricklebank M. A within-subject cognitive battery in the rat: differential effects of NMDA receptor antagonists. Psychopharmacology. 2010;212:227–242. doi: 10.1007/s00213-010-1945-1. [DOI] [PubMed] [Google Scholar]
- 68.Gilmour G, Pioli EY, Dix SL, Smith JW, Conway MW, Jones WT, Loomis S, Mason R, Shahabi S, Tricklebank MD. Diverse and often opposite behavioural effects of NMDA receptor antagonists in rats: implications for “NMDA antagonist modelling” of schizophrenia. Psychopharmacology. 2009;205:203–216. doi: 10.1007/s00213-009-1530-7. [DOI] [PubMed] [Google Scholar]
- 69.Chaperon F, Muller W, Auberson YP, Tricklebank MD, Neijt HC. Substitution for PCP, disruption of prepulse inhibition and hyperactivity induced by N-methyl-D-aspartate receptor antagonists: preferential involvement of the NR2B rather than NR2A subunit. Behav. Pharmacol. 2003;14:477–487. doi: 10.1097/01.fbp.0000091471.79060.ed. [DOI] [PubMed] [Google Scholar]
- 70.Nicholson KL, Balster RL. The discriminative stimulus effects of N-methyl-d-aspartate glycine-site ligands in NMDA antagonist-trained rats. Psychopharmacology. 2009;203:441–451. doi: 10.1007/s00213-009-1469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nicholson KL, Mansbach RS, Menniti FS, Balster RL. The phencyclidine-like discriminative stimulus effects and reinforcing properties of the NR2B-selective N-methyl-D-aspartate antagonist CP-101,606 in rats and rhesus monkeys. Behav. Pharmacol. 2007;18:731–743. doi: 10.1097/FBP.0b013e3282f14ed6. [DOI] [PubMed] [Google Scholar]
- 72.Coyle JT. Glutamate and Schizophrenia: Beyond the Dopamine Hypothesis. Cell. Mol. Neurobiol. 2006;26:363–382. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- 73.Lisman J, Coyle J, Green R, Javitt D, Benes F, Heckers S, Grace A. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lopez-Munoz F, Alamo C. Neurobiological background for the development of new drugs in schizophrenia. Clin. Neuropharmacol. 2011;34:111–126. doi: 10.1097/WNF.0b013e318215c2f7. [DOI] [PubMed] [Google Scholar]
- 75.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- 76.Schwartz TW, Holst B. Allosteric enhancers, allosteric agonists and ago-allosteric modulators: where do they bind and how do they act? Trends Pharmacol. Sci. 2007;28:366–373. doi: 10.1016/j.tips.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 77.Urwyler S. Allosteric modulation of family C G-protein-coupled receptors: from molecular insights to therapeutic perspectives. Pharmacol. Rev. 2011;63:59–126. doi: 10.1124/pr.109.002501. [DOI] [PubMed] [Google Scholar]
- 78.Conn JP, Christopoulus A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Engel AK, Fries P, Singer W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 80.Hajos M. Targeting information-processing deficit in schizophrenia: a novel approach to psychotherapeutic drug discovery. Trends Pharmacol. Sci. 2006;27:391–398. doi: 10.1016/j.tips.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 81.Lisman J, Buzsaki G. A Neural Coding Scheme Formed by the Combined Function of Gamma and Theta Oscillations. Schizophr. Bull. 2008;34:974–980. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uhlhaas PJ, Singer W. Neuronal Dynamics and Neuropsychiatric Disorders: Toward a Translational Paradigm for Dysfunctional Large-Scale Networks. Neuron. 2012;75:963–980. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 83.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 84.Malenka RC, Bear MF. LTP and LTD: An Embarrassment of Riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 85.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 86.Shepherd JD, Huganir RL. The Cell Biology of Synaptic Plasticity: AMPA Receptor Trafficking. Annu. Rev. Cell De.v Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 87.Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 88.Bleakman D, Lodge D. Neuropharmacology of AMPA and Kainate Receptors. Neuropharmacology. 1998;37:1187–1204. doi: 10.1016/s0028-3908(98)00139-7. [DOI] [PubMed] [Google Scholar]
- 89.Black MD. Therapeutic Potential of Positive AMPA Modulators and Their Relationship to AMPA Receptor Subunits. A Review of Preclinical Data. Psychopharmacology. 2005;179:154–163. doi: 10.1007/s00213-004-2065-6. [DOI] [PubMed] [Google Scholar]
- 90.Lynch G, Gall C. Ampakines and the threefold path to cognitive enhancement. Trends Neurosci. 2006;29:554–562. doi: 10.1016/j.tins.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 91.Jin R, Clark S, Weeks AM, Dudman JT, Gouaux E, Partin KM. Mechanism of Positive Allosteric Modulators Acting on AMPA Receptors. J. Neurosci. 2005;25:9027–9036. doi: 10.1523/JNEUROSCI.2567-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Staubli U, Rogers G, Lynch G. Facilitation of Glutamate Receptors Enhances Memory. Proc. Natl. Acad. Sci. U S A. 1994;91:777–781. doi: 10.1073/pnas.91.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wisden W, Seeburg PH. Mammalian ionotropic glutamate receptors. Curr. Opin. Neurobiol. 1993;3:291–298. doi: 10.1016/0959-4388(93)90120-n. [DOI] [PubMed] [Google Scholar]
- 94.Monyer H, Seeburg PH, Wisden W. Glutamate-operated channels: developmentally early and mature forms arise by alternative splicing. Neuron. 1991;6:799–810. doi: 10.1016/0896-6273(91)90176-z. [DOI] [PubMed] [Google Scholar]
- 95.Sommer B, Keinanen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg PH. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990;249:1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- 96.Pei W, Huang Z, Wang C, Han Y, Park JS, Niu L. Flip and flop: a molecular determinant for AMPA receptor channel opening. Biochemistry. 2009;48:3767–3777. doi: 10.1021/bi8015907. [DOI] [PubMed] [Google Scholar]
- 97.Mayer ML, Armstrong N. Structure and Function of Glutamate Receptor Ion Channels1. Annu. Rev. Physiol. 2004;66:161–181. doi: 10.1146/annurev.physiol.66.050802.084104. [DOI] [PubMed] [Google Scholar]
- 98.Mayer ML. Glutamate receptors at atomic resolution. Nature. 2006;440:456–462. doi: 10.1038/nature04709. [DOI] [PubMed] [Google Scholar]
- 99.Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gouaux E, Song L, Xie Y, Landry DW. GluR2 Receptor Modulators. WO 2007/101116 A2. Patent. 2007 Sep 7;
- 101.Jin R, Banke TG, Mayer ML, Traynelis SF, Gouaux E. Structural basis for partial agonist action at ionotropic glutamate receptors. Nat Neurosci. 2003;6:803–810. doi: 10.1038/nn1091. [DOI] [PubMed] [Google Scholar]
- 102.Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–18. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- 103.Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- 104.Partin KM, Fleck MW, Mayer ML. AMPA receptor flip/flop mutants affecting deactivation, desensitization, and modulation by cyclothiazide, aniracetam, and thiocyanate. J. Neurosci. 1996;16:6634–6647. doi: 10.1523/JNEUROSCI.16-21-06634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ahmed AH, Oswald RE. Piracetam defines a new binding site for allosteric modulators of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors. J. Med. Chem. 2010;53:2197–2203. doi: 10.1021/jm901905j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quirk JC, Nisenbaum ES. LY404187: a novel positive allosteric modulator of AMPA receptors. CNS Drug Reviews. 2002;8:255–282. doi: 10.1111/j.1527-3458.2002.tb00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Partin KM, Patneau DK, Mayer ML. Cyclothiazide differentially modulates desensitization of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor splice variants. Mol. Pharmacol. 1994;46:129–138. [PubMed] [Google Scholar]
- 108.Ptak CP, Ahmed AH, Oswald RE. Probing the allosteric modulator binding site of GluR2 with thiazide derivatives. Biochemistry. 2009;48:8594–8602. doi: 10.1021/bi901127s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fucile S, Miledi R, Eusebi F. Effects of cyclothiazide on GluR1/AMPA receptors. Proc. Natl. Acad. Sci. U S A. 2006;103:2943–2947. doi: 10.1073/pnas.0511063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Noorbala AA, Akhondzadeh S, Davari-Ashtiani R, Amini-Nooshabadi H. Piracetam in the treatment of schizophrenia: implications for the glutamate hypothesis of schizophrenia. J. Clin. Pharm. Ther. 1999;24:369–374. doi: 10.1046/j.1365-2710.1999.00238.x. [DOI] [PubMed] [Google Scholar]
- 111.Danysz W. CX-516 Cortex pharmaceuticals. Curr. Opin. Investig. Drugs. 2002;3:1081–1088. [PubMed] [Google Scholar]
- 112.Marenco S, Egan MF, Goldberg TE, Knable MB, McClure RK, Winterer G, Weinberger DR. Preliminary experience with an ampakine (CX516) as a single agent for the treatment of schizophrenia: a case series. Schizophr. Res. 2002;57:221–226. doi: 10.1016/s0920-9964(01)00311-5. [DOI] [PubMed] [Google Scholar]
- 113.Goff DC, Leahy L, Berman I, Posever T, Herz L, Leon AC, Johnson SA, Lynch G. A placebo-controlled pilot study of the ampakine CX516 added to clozapine in schizophrenia. J. Clin. Psychopharmacol. 2001;21:484–487. doi: 10.1097/00004714-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 114.Goff DC, Lamberti JS, Leon AC, Green MF, Miller AL, Patel J, Manschreck T, Freudenreich O, Johnson SA. A placebo-controlled add-on trial of the Ampakine, CX516, for cognitive deficits in schizophrenia. Neuropsychopharmacologyy. 2008;33:465–472. doi: 10.1038/sj.npp.1301444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 116.Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- 117.Cull-Candy SG, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 118.Bliss TVP, Collingridge GL. Memories of NMDA receptors and LTP. Trends Neurosci. 1993;18:54. [PubMed] [Google Scholar]
- 119.Markram H. A history of spike-timing-dependent plasticity. Front. Synaptic Neurosci. 2011;3:1–24. doi: 10.3389/fnsyn.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cotman CW, Monaghan DT, Ganong AH. Excitatory amino acid neurotransmission: NMDA receptors and Hebb-type synaptic plasticity. Annu. Rev. Neurosci. 1988;11:61–80. doi: 10.1146/annurev.ne.11.030188.000425. [DOI] [PubMed] [Google Scholar]
- 121.Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- 122.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Science STKE. 2004;16:1–9. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 123.Lester RA, Tong G, Jahr CE. Interactions between the glycine and glutamate binding sites of the NMDA receptor. J. Neurosci. 1993;13:1088–1096. doi: 10.1523/JNEUROSCI.13-03-01088.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Parsons CG, Zong X, Lux HD. Whole cell and single channel analysis of the kinetics of glycine-sensitive N-methyl-D-aspartate receptor desensitization. Br. J. Pharmacol. 1993;109:213–221. doi: 10.1111/j.1476-5381.1993.tb13556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang CR, Svensson KA. Allosteric modulation of NMDA receptor via elevation of brain glycine and D-serine: the therapeutic potentials for schizophrenia. Pharmacol. Ther. 2008;120:317–332. doi: 10.1016/j.pharmthera.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 126.Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends Biochem. Sci. 2005;30:325–333. doi: 10.1016/j.tibs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 127.Priestley T, Laughton P, Myers J, Le Bourdelles B, Kerby J, Whiting PJ. Pharmacological properties of recombinant human N-methyl-D-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol. Pharmacol. 1995;48:841–848. [PubMed] [Google Scholar]
- 128.Laurie DJ, Seeburg PH. Ligand affinities at recombinant N-methyl-D-aspartate receptors depend on subunit composition. Eur. J. Pharmacol. 1994;268:335–345. doi: 10.1016/0922-4106(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 129.Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, Oliet SH. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150:633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 130.Waziri R, Baruah S, Hegwood TS, Sherman AD. Abnormal serine hydroxymethyl transferase activity in the temporal lobes of schizophrenics. Neurosci. Lett. 1990;120:237–240. doi: 10.1016/0304-3940(90)90048-e. [DOI] [PubMed] [Google Scholar]
- 131.Waziri R, Baruah S, Sherman AD. Abnormal serine-glycine metabolism in the brains of schizophrenics. Schizophr. Res. 1993;8:233–243. doi: 10.1016/0920-9964(93)90021-a. [DOI] [PubMed] [Google Scholar]
- 132.Hashimoto K, Shimuzu E, Iyo M. Dysfunction of glia-neuron communication in pathophysiology of schizophrenia. Curr. Psychiatry Rev. 2005;1:151–163. [Google Scholar]
- 133.Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Hasegawa H, Imai K, Iyo M. Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch. Gen. Psychiatry. 2003;60:572–576. doi: 10.1001/archpsyc.60.6.572. [DOI] [PubMed] [Google Scholar]
- 134.Coyle JT, Tsai G. The NMDA receptor glycine modulatory site: a therapeutic target for improving cognition and reducing negative symptoms in schizophrenia. Psychopharmacology (Berl) 2004;174:32–38. doi: 10.1007/s00213-003-1709-2. [DOI] [PubMed] [Google Scholar]
- 135.de Bartolomeis A, Sarappa C, Magara S, Iasevoli F. Targeting glutamate system for novel antipsychotic approaches: relevance for residual psychotic symptoms and treatment resistant schizophrenia. Eur. J. Pharmacol. 2012;682:1–11. doi: 10.1016/j.ejphar.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 136.Heresco-Levy U, Javitt DC. Comparative effects of glycine and D-cycloserine on persistent negative symptoms in schizophrenia: a retrospective analysis. Schizophr. Res. 2004;66:89–96. doi: 10.1016/S0920-9964(03)00129-4. [DOI] [PubMed] [Google Scholar]
- 137.Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch. Gen. Psychiatry. 1999;56:29–36. doi: 10.1001/archpsyc.56.1.29. [DOI] [PubMed] [Google Scholar]
- 138.Diaz P, Bhaskara S, Dursun SM, Deakin B. Double-blind, placebo-controlled, crossover trial of clozapine plus glycine in refractory schizophrenia negative results. J. Clin. Psychopharmacol. 2005;25:277–278. doi: 10.1097/01.jcp.0000165740.22377.6d. [DOI] [PubMed] [Google Scholar]
- 139.Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, Hongou K, Miyawaki T, Mori H. Serine racemase is predominantly localized in neurons in mouse brain. J. Comp. Neurol. 2008;510:641–654. doi: 10.1002/cne.21822. [DOI] [PubMed] [Google Scholar]
- 140.Labrie V, Clapcote SJ, Roder JC. Mutant mice with reduced NMDA-NR1 glycine affinity or lack of D-amino acid oxidase function exhibit altered anxiety-like behaviors. Pharmacol. Biochem. Behav. 2009;91:610–620. doi: 10.1016/j.pbb.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 141.Madeira C, Freitas ME, Vargas-Lopes C, Wolosker H, Panizzutti R. Increased brain D-amino acid oxidase (DAAO) activity in schizophrenia. Schizophr. Res. 2008;101:76–83. doi: 10.1016/j.schres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 142.Hashimoto A, Nishikawa T, Konno R, Niwa A, Yasumura Y, Oka T, Takahashi K. Free D-serine, D-aspartate and D-alanine in central nervous system and serum in mutant mice lacking D-amino acid oxidase. Neurosci. Lett. 1993;152:33–36. doi: 10.1016/0304-3940(93)90476-2. [DOI] [PubMed] [Google Scholar]
- 143.Maekawa M, Watanabe M, Yamaguchi S, Konno R, Hori Y. Spatial learning and long-term potentiation of mutant mice lacking d-amino-acid oxidase. Neurosci. Res. 2005;53:34–38. doi: 10.1016/j.neures.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 144.Hashimoto A, Yoshikawa M, Niwa A, Konno R. Mice lacking D-amino acid oxidase activity display marked attenuation of stereotypy and ataxia induced by MK-801. Brain Res. 2005;1033:210–215. doi: 10.1016/j.brainres.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 145.Hashimoto K, Fujita Y, Horio M, Kunitachi S, Iyo M, Ferraris D, Tsukamoto T. Co-administration of a D-amino acid oxidase inhibitor potentiates the efficacy of D-serine in attenuating prepulse inhibition deficits after administration of dizocilpine. Biol. Psychiatry. 2009;65:1103–1106. doi: 10.1016/j.biopsych.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 146.Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr. Pharm. Des. 2010;16:522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- 147.Sheinin A, Shavit S, Benveniste M. Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology. 2001;41:151–158. doi: 10.1016/s0028-3908(01)00073-9. [DOI] [PubMed] [Google Scholar]
- 148.Dravid SM, Burger PB, Prakash A, Geballe MT, Yadav R, Le P, Vellano K, Snyder JP, Traynelis SF. Structural determinants of D-cycloserine efficacy at the NR1/NR2C NMDA receptors. J. Neurosci. 2010;30:2741–2754. doi: 10.1523/JNEUROSCI.5390-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Borowsky B, Mezey E, Hoffman BJ. Two glycine transporter variants with distinct localization in the CNS and peripheral tissues are encoded by a common gene. Neuron. 1993;10:851–863. doi: 10.1016/0896-6273(93)90201-2. [DOI] [PubMed] [Google Scholar]
- 150.Aragon C, Lopez-Corcuera B. Glycine transporters: crucial roles of pharmacological interest revealed by gene deletion. Trends Pharmacol. Sci. 2005;26:283–286. doi: 10.1016/j.tips.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 151.Cubelos B, Gimenez C, Zafra F. Localization of the GLYT1 glycine transporter at glutamatergic synapses in the rat brain. Cereb. Cortex. 2005;15:448–459. doi: 10.1093/cercor/bhh147. [DOI] [PubMed] [Google Scholar]
- 152.Zafra F, Gomeza J, Olivares L, Aragon C, Gimenez C. Regional distribution and developmental variation of the glycine transporters GLYT1 and GLYT2 in the rat CNS. Eur. J. Neurosci. 1995;7:1342–1352. doi: 10.1111/j.1460-9568.1995.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 153.Bergeron R, Meyer TM, Coyle JT, Greene RW. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc. Natl. Acad. Sci. U S A. 1998;95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gomeza J, Hulsmann S, Ohno K, Eulenburg V, Szoke K, Richter D, Betz H. Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron. 2003;40:785–796. doi: 10.1016/s0896-6273(03)00672-x. [DOI] [PubMed] [Google Scholar]
- 155.Tsai G, Ralph-Williams RJ, Martina M, Bergeron R, Berger-Sweeney J, Dunham KS, Jiang Z, Caine SB, Coyle JT. Gene knockout of glycine transporter 1: characterization of the behavioral phenotype. Proc. Natl. Acad. Sci. U S A. 2004;101:8485–8490. doi: 10.1073/pnas.0402662101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lane HY, Liu YC, Huang CL, Chang YC, Liau CH, Perng CH, Tsai GE. Sarcosine (N-methylglycine) treatment for acute schizophrenia: a randomized, double-blind study. Biol Ps.ychiatry. 2008;63:9–12. doi: 10.1016/j.biopsych.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 157.Atkinson BN, Bell SC, De Vivo M, Kowalski LR, Lechner SM, Ognyanov VI, Tham CS, Tsai C, Jia J, Ashton D, Klitenick MA. ALX 5407: a potent, selective inhibitor of the hGlyT1 glycine transporter. Mol. Pharmacol. 2001;60:1414–1420. doi: 10.1124/mol.60.6.1414. [DOI] [PubMed] [Google Scholar]
- 158.Harsing LG, Jr, Juranyi Z, Gacsalyi I, Tapolcsanyi P, Czompa A, Matyus P. Glycine transporter type-1 and its inhibitors. Curr. Med. Chem. 2006;13:1017–1044. doi: 10.2174/092986706776360932. [DOI] [PubMed] [Google Scholar]
- 159.Perry KW, Falcone JF, Fell MJ, Ryder JW, Yu H, Love PL, Katner J, Gordon KD, Wade MR, Man T, Nomikos GG, Phebus LA, Cauvin AJ, Johnson KW, Jones CK, Hoffmann BJ, Sandusky GE, Walter MW, Porter WJ, Yang L, Merchant KM, Shannon HE, Svensson KA. Neurochemical and behavioral profiling of the selective GlyT1 inhibitors ALX5407 and LY2365109 indicate a preferential action in caudal vs. cortical brain areas. Neuropharmacology. 2008;55:743–754. doi: 10.1016/j.neuropharm.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 160.Cioffi CL, Liu S, Wolf MA. Annual Reports in Medicinal Chemistry. Vol. 45. Amsterdam: Academic Press (Elsevier); 2010. pp. 19–35. [Google Scholar]
- 161.Mezler M, Hornberger W, Mueller R, Schmidt M, Amberg W, Braje W, Ochse M, Schoemaker H, Behl B. Inhibitors of GlyT1 affect glycine transport via discrete binding sites. Mol. Pharmacol. 2008;74:1705–1715. doi: 10.1124/mol.108.049312. [DOI] [PubMed] [Google Scholar]
- 162.Bridges TM, Williams R, Lindsley CW. Design of potent GlyT1 inhibitors: in vitro and in vivo profiles. Curr. Opin. Mol. Ther. 2008;10:591–601. [PubMed] [Google Scholar]
- 163.Smith G, Ruhland T, Mikkelsen G, Andersen K, Christoffersen CT, Alifrangis LH, Mork A, Wren SP, Harris N, Wyman BM, Brandt G. The synthesis and SAR of 2-arylsulfanyl-phenyl piperazinyl acetic acids as glyT-1 inhibitors. Bioorg. Med. Chem. Lett. 2004;14:4027–4030. doi: 10.1016/j.bmcl.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 164.Andrews N, Ge J, Walker G, Schipper J, marston HM. Effect of the selective glycine reuptake (GlyT-1) inhibitor Org 25935 on glycine levels in CSF and dialysates. Neuropsychopharmacology. 2007;17:S497. [Google Scholar]
- 165.D'Souza DC, Singh N, Elander J, Carbuto M, Pittman B, de Haes JU, Sjogren M, Peeters P, Ranganathan M, Schipper J. Glycine transporter inhibitor attenuates the psychotomimetic effects of ketamine in healthy males: preliminary evidence. Neuropsychopharmacology. 2012;37:1036–1046. doi: 10.1038/npp.2011.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Depoortere R, Dargazanli G, Estenne-Bouhtou G, Coste A, Lanneau C, Desvignes C, Poncelet M, Heaulme M, Santucci V, Decobert M, Cudennec A, Voltz C, Boulay D, Terranova JP, Stemmelin J, Roger P, Marabout B, Sevrin M, Vige X, Biton B, Steinberg R, Francon D, Alonso R, Avenet P, Oury-Donat F, Perrault G, Griebel G, George P, Soubrie P, Scatton B. Neurochemical, electrophysiological and pharmacological profiles of the selective inhibitor of the glycine transporter-1 SSR504734, a potential new type of antipsychotic. Neuropsychopharmacology. 2005;30:1963–1985. doi: 10.1038/sj.npp.1300772. [DOI] [PubMed] [Google Scholar]
- 167.Gielen M, Retchless BS, Mony L, Johnson JW, Paoletti P. Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature. 2009;459:703–707. doi: 10.1038/nature07993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yuan H, Hansen KB, Vance KM, Ogden KK, Traynelis SF. Control of NMDA Receptor Function by the NR2 Subunit Amino-Terminal Domain. J. Neurosci. 2009;29:12045–12058. doi: 10.1523/JNEUROSCI.1365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ogden KK, Traynelis SF. New advances in NMDA receptor pharmacology. Trends Pharmacol. Sci. 2011;32:726–733. doi: 10.1016/j.tips.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mony L, Krzaczkowski L, Leonetti M, Le Goff A, Alarcon K, Neyton J, Bertrand HO, Acher F, Paoletti P. Structural Basis of NR2B-Selective Antagonist Recognition by N-Methyl-D-aspartate Receptors. Mol. Pharmacol. 2009;75:60–74. doi: 10.1124/mol.108.050971. [DOI] [PubMed] [Google Scholar]
- 171.Erreger K, Traynelis SF. Zinc inhibition of rat NR1/NR2A N-methyl-D-aspartate receptors. J. Physiol. 2008;586:763–778. doi: 10.1113/jphysiol.2007.143941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mony L, Zhu S, Carvalho S, Paoletti P. Molecular basis of positive allosteric modulation of GluN2B NMDA receptors by polyamines. Embo J. 2011;30:3134–3146. doi: 10.1038/emboj.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chenard BL, Menniti FS. Antagonists selective for NMDA receptors containing the GLUN2B subunit. Curr. Pharm. Des. 1999;5:381–404. [PubMed] [Google Scholar]
- 174.Nikam SS, Meltzer LT. NR2B selective NMDA receptor antagonists. Curr. Pharm. Des. 2002;8:845–855. doi: 10.2174/1381612024607072. [DOI] [PubMed] [Google Scholar]
- 175.McCauley JA. NR2B subtype-selective NMDA receptor antagonists: 2001–2004. Expert Opin. Ther. Pat. 2005;15:389–407. [Google Scholar]
- 176.Layton ME, Kelly MJ, 3rd, Rodzinak KJ. Recent advances in the development of NR2B subtype-selective NMDA receptor antagonists. Curr. Top. Med. Chem. 2006;6:697–709. doi: 10.2174/156802606776894447. [DOI] [PubMed] [Google Scholar]
- 177.Beinat C, Banister S, Moussa I, Reynolds AJ, McErlean CSP, Kassiou M. Insights into Structure-Activity Relationships and CNS Therapeutic Applications of NR2B Selective Antagonists. Curr. Med. Chem. 2010;17:4166–4190. doi: 10.2174/092986710793348572. [DOI] [PubMed] [Google Scholar]
- 178.Acker TM, Yuan H, Hansen KB, Vance KM, Ogden KK, Jensen HS, Burger PB, Mullasseril P, Snyder JP, Liotta DC, Traynelis SF. Mechanism for noncompetitive inhibition by novel GluN2C/D N-methyl-D-aspartate receptor subunit-selective modulators. Mol. Pharmacol. 2011;80:782–795. doi: 10.1124/mol.111.073239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hansen KB, Traynelis SF. Structural and mechanistic determinants of a novel site for noncompetitive inhibition of GluN2D-containing NMDA receptors. J. Neurosci. 2011;31:3650–3661. doi: 10.1523/JNEUROSCI.5565-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mosley CA, Acker TM, Hansen KB, Mullasseril P, Andersen KT, Le P, Vellano KM, Brauner-Osborne H, Liotta DC, Traynelis SF. Quinazolin-4-one derivatives: A novel class of noncompetitive NR2C/D subunit-selective N-methyl-D-aspartate receptor antagonists. J. Med. Chem. 2010;53:5476–5490. doi: 10.1021/jm100027p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mullasseril P, Hansen KB, Vance KM, Ogden KK, Yuan H, Kurtkaya NL, Santangelo R, Orr AG, Le P, Vellano KM, Liotta DC, Traynelis SF. A subunit-selective potentiator of NR2C- and NR2D-containing NMDA receptors. Nat. Commun. 2010;1:1–8. doi: 10.1038/ncomms1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Monaghan DT, Irvine MW, Costa BM, Fang G, Jane DE. Pharmacological modulation of NMDA receptor activity and the advent of negative and positive allosteric modulators. Neurochem. Int. 2012;61:581–592. doi: 10.1016/j.neuint.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Karakas E, Simorowski N, Furukawa H. Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors. Nature. 2011;475:249–253. doi: 10.1038/nature10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Karakas E, Simorowski N, Furukawa H. Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. Embo J. 2009;28:3910–3920. doi: 10.1038/emboj.2009.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tomitori H, Suganami A, Saiki R, Mizuno S, Yoshizawa Y, Masuko T, Tamura Y, Nishimura K, Toida T, Williams K, Kashiwagi K, Igarashi K. Structural Change of R Domain Heterodimer of NMDA Receptor GluN1 and GluN2B through Binding of Spermine and Ifenprodil. J. Pharmacol. Exp. Ther. 2012;343:82–90. doi: 10.1124/jpet.112.192286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Marinelli L, Cosconati S, Steinbrecher T, Limongelli V, Bertamino A, Novellino E, Case DA. Homology modeling of NR2B modulatory domain of NMDA receptor and analysis of ifenprodil binding. ChemMedChem. 2007;2:1498–1510. doi: 10.1002/cmdc.200700091. [DOI] [PubMed] [Google Scholar]
- 187.Hansen KB, Mullasseril P, Dawit S, Kurtkaya NL, Yuan H, Vance KM, Orr AG, Kvist T, Ogden KK, Le P, Vellano KM, Lewis I, Kurtkaya S, Du Y, Qui M, Murphy TJ, Snyder JP, Brauner-Osborne H, Traynelis SF. Implementation of a fluorescence-based screening assay identifies histamine H3 receptor antagonists clobenpropit and iodophenpropit as subunit-selective N-methyl-D-aspartate receptor antagonists. J. Pharmacol. Exp. Ther. 2010;333:650–662. doi: 10.1124/jpet.110.166256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cao X, Cui Z, Feng R, Tang YP, Qin Z, Mei B, Tsien JZ. Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur. J. Neurosci. 2007;25:1815–1822. doi: 10.1111/j.1460-9568.2007.05431.x. [DOI] [PubMed] [Google Scholar]
- 189.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 190.Tang YP, Wang H, Feng R, Kyin M, Tsien JZ. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology. 2001;41:779–790. doi: 10.1016/s0028-3908(01)00122-8. [DOI] [PubMed] [Google Scholar]
- 191.Wang D, Cui Z, Zeng Q, Kuang H, Wang LP, Tsien JZ, Cao X. Genetic enhancement of memory and long-term potentiation but not CA1 long-term depression in NR2B transgenic rats. PLoS One. 2009;4:e7486. doi: 10.1371/journal.pone.0007486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, Mathur P, Davis MI, Bock R, Gustin RM, Colbran RJ, Alvarez VA, Nakazawa K, Delpire E, Lovinger DM, Holmes A. Loss of GluN2B-Containing NMDA Receptors in CA1 Hippocampus and Cortex Impairs Long-Term Depression, Reduces Dendritic Spine Density, and Disrupts Learning. J. Neurosci. 2010;30:4590–4600. doi: 10.1523/JNEUROSCI.0640-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.von Engelhardt J, Doganci B, Jensen V, Hvalby Ø, Göngrich C, Taylor A, Barkus C, Sanderson DJ, Rawlins JNP, Seeburg PH, Bannerman DM, Monyer H. Contribution of Hippocampal and Extra-Hippocampal NR2B-Containing NMDA Receptors to Performance on Spatial Learning Tasks. Neuron. 2008;60:846–860. doi: 10.1016/j.neuron.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 194.Akashi K, Kakizaki T, Kamiya H, Fukaya M, Yamasaki M, Abe M, Natsume R, Watanabe M, Sakimura K. NMDA receptor GluN2B (GluR epsilon 2/NR2B) subunit is crucial for channel function, postsynaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses. J. Neurosci. 2009;29:10869–10882. doi: 10.1523/JNEUROSCI.5531-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. A Hippocampal NR2B Deficit Can Mimic Age-Related Changes in Long-Term Potentiation and Spatial Learning in the Fischer 344 Rat. J. Neurosci. 2002;22:3628–3637. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chazot PL. The NMDA receptor NR2B subunit: a valid therapeutic target for multiple CNS pathologies. Curr. Med. Chem. 2004;11:389–396. doi: 10.2174/0929867043456061. [DOI] [PubMed] [Google Scholar]
- 197.Gogas KR. Glutamate-based therapeutic approaches: NR2B receptor antagonists. Curr Opin. Pharmacol. 2006;6:68–74. doi: 10.1016/j.coph.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 198.Mony L, Kew JN, Gunthorpe MJ, Paoletti P. Allosteric modulators of NR2B-containing NMDA receptors: molecular. mechanisms and therapeutic potential. Br. J. Pharmacol. 2009;157:1301–1317. doi: 10.1111/j.1476-5381.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nicholson KL, Mansbach RS, Menniti FS, Balster RL. The phencyclidine-like discriminative stimulus effects and reinforcing properties of the NR2B-selective N-methyl-D-aspartate antagonist CP-101606 in rats and rhesus monkeys. Behav. Pharmacol. 2007;18:731–743. doi: 10.1097/FBP.0b013e3282f14ed6. [DOI] [PubMed] [Google Scholar]
- 200.Chaperon F, Muller W, Auberson YP, Tricklebank MD, Neijt HC. Substitution for PCP, disruption of prepulse inhibition and hyperactivity induced by N-methyl-D-aspartate receptor antagonists: preferential involvement of the NR2B rather than NR2A subunit. Behav. Pharmacol. 2003;14:477–487. doi: 10.1097/01.fbp.0000091471.79060.ed. [DOI] [PubMed] [Google Scholar]
- 201.Chenard BL, Bordner J, Butler TW, Chambers LK, Collins MA, De Costa DL, Ducat MF, Dumont ML, Fox CB, Mena EE. (1S,2S)-1-(4-hydroxyphenyl)-2-(4-hydroxy-4-phenylpiperidino)-1-propanol: a potent new neuroprotectant which blocks N-methyl-D-aspartate responses. J. Med. Chem. 1995;38:3138–3145. doi: 10.1021/jm00016a017. [DOI] [PubMed] [Google Scholar]
- 202.Menniti F, Chenard B, Collins M, Ducat M, Shalaby I, White F. CP-101,606, a potent neuroprotectant selective for forebrain neurons - I. Evidence for efficacy in models of focal cerebral ischemia. Eur. J. Pharmacol. 1997;331:117–126. doi: 10.1016/s0014-2999(97)10092-9. [DOI] [PubMed] [Google Scholar]
- 203.Bullock MR, Merchant RE, Carmack CA, Doppenberg E, Shah AK, Wilner KD, Ko G, Williams SA. An open-label study of CP-101,606 in subjects with a severe traumatic head injury or spontaneous intracerebral hemorrhage. Ann. N Y Acad. Sci. 1999;890:51–58. doi: 10.1111/j.1749-6632.1999.tb07980.x. [DOI] [PubMed] [Google Scholar]
- 204.Merchant RE, Bullock MR, Carmack CA, Shah AK, Wilner KD, Ko G, Williams SA. A double-blind, placebo-controlled study of the safety, tolerability and pharmacokinetics of CP-101,606 in patients with a mild or moderate traumatic brain injury. Ann. N Y Acad. Sci. 1999;890:42–50. doi: 10.1111/j.1749-6632.1999.tb07979.x. [DOI] [PubMed] [Google Scholar]
- 205.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J. Clin. Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 206.Endele S, Rosenberger G, Geider K, Popp B, Tamer C, Stefanova I, Milh M, Kortüm F, Fritsch A, Pientka FK, Hellenbroich Y, Kalscheuer VM, Kohlhase J, Moog U, Rappold G, Rauch A, Ropers H-H, von Spiczak S, Tönnies H, Villeneuve N, Villard L, Zabel B, Zenker M, Laube B, Reis A, Wieczorek D, Van Maldergem L, Kutsche K. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat. Genet. 2010;42:1021–1026. doi: 10.1038/ng.677. [DOI] [PubMed] [Google Scholar]
- 207.Li D, He L. Association study between the NMDA receptor 2B subunit gene (GRIN2B) and schizophrenia: a HuGE review and meta-analysis. Genet. Med. 2007;9:4–8. doi: 10.1097/01.gim.0000250507.96760.4b. [DOI] [PubMed] [Google Scholar]
- 208.Horak M, Vlcek K, Petrovic M, Chodounska H, Vyklicky L., Jr Molecular mechanism of pregnenolone sulfate action at NR1/NR2B receptors. J. Neurosci. 2004;24:10318–10325. doi: 10.1523/JNEUROSCI.2099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Costa BM, Irvine MW, Fang G, Eaves RJ, Mayo-Martin MB, Laube B, Jane DE, Monaghan DT. Structure-activity relationships for allosteric NMDA receptor inhibitors based on 2-naphthoic acid. Neuropharmacology. 2012;62:1730–1736. doi: 10.1016/j.neuropharm.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Paoletti P, Neyton J, Ascher P. Glycine-independent and subunit-specific potentiation of NMDA responses by extracellular Mg2+ Neuron. 1995;15:1109–1120. doi: 10.1016/0896-6273(95)90099-3. [DOI] [PubMed] [Google Scholar]
- 211.Williams K, Zappia AM, Pritchett DB, Shen YM, Molinoff PB. Sensitivity of the N-methyl-D-aspartate receptor to polyamines is controlled by NR2 subunits. Mol. Pharmacol. 1994;45:803–809. [PubMed] [Google Scholar]
- 212.Quinlan EM, Lebel D, Brosh I, Barkai E. A molecular mechanism for stabilization of learning-induced synaptic modifications. Neuron. 2004;41:185–192. doi: 10.1016/s0896-6273(03)00874-2. [DOI] [PubMed] [Google Scholar]
- 213.Lee MC, Yasuda R, Ehlers MD. Metaplasticity at single glutamatergic synapses. Neuron. 2010;66:859–870. doi: 10.1016/j.neuron.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cho KK, Bear MF. Promoting neurological recovery of function via metaplasticity. Future Neurol. 2010;5:21–26. doi: 10.2217/fnl.09.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bettini E, Sava A, Griffante C, Carignani C, Buson A, Capelli AM, Negri M, Andreetta F, Senar-Sancho SA, Guiral L, Cardullo F. Identification and characterization of novel NMDA receptor antagonists selective for NR2A- over NR2B-containing receptors. J. Pharmacol. Exp. Ther. 2010;335:636–644. doi: 10.1124/jpet.110.172544. [DOI] [PubMed] [Google Scholar]
- 216.Rauner C, Kohr G. Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J. Biol. Chem. 2011;286:7558–7566. doi: 10.1074/jbc.M110.182600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hatton CJ, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron. 2005;46(2):261–274. doi: 10.1016/j.neuron.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 218.Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams DL, Jr, Sur C, Kinney GG. Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr. Top. Med. Chem. 2006;6:771–785. doi: 10.2174/156802606777057599. [DOI] [PubMed] [Google Scholar]
- 219.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol. Sci. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Melancon BJ, Hopkins CR, Wood MR, Emmitte KA, Niswender CM, Christopoulos A, Conn PJ, Lindsley CW. Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery. J. Med. Chem. 2012;55:1445–1464. doi: 10.1021/jm201139r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bridges TM, Lindsley CW. G-protein-coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chem. Biol. 2008;3:530–541. doi: 10.1021/cb800116f. [DOI] [PubMed] [Google Scholar]
- 223.Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 224.Chun L, Zhang WH, Liu JF. Structure and ligand recognition of class C GPCRs. Acta Pharmacol. Sin. 2012;33:312–323. doi: 10.1038/aps.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 226.Kenakin TP. 7TM receptor allostery: putting numbers to shapeshifting proteins. Trends Pharmacol. Sci. 2009;30:460–469. doi: 10.1016/j.tips.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 227.Langmead CJ, Christopoulos A. Allosteric agonists of 7TM receptors: expanding the pharmacological toolbox. Trends Pharmacol. Sci. 2006;27:475–481. doi: 10.1016/j.tips.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 228.May LT, Leach K, Sexton PM, Christopoulos A. Allosteric modulation of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2007;47:1–51. doi: 10.1146/annurev.pharmtox.47.120505.105159. [DOI] [PubMed] [Google Scholar]
- 229.Lewis JA, Lebois EP, Lindsley CW. Allosteric modulation of kinases and GPCRs: design principles and structural diversity. Curr. Opin. Chem. Biol. 2008;12:269–280. doi: 10.1016/j.cbpa.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 230.Christopoulos A. Allosteric binding sites on cell surface receptors: novel targets for drug discovery. Nat. Rev. Drug Discov. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- 231.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rocheville M, Garland SL. An industrial perspective on positive allosteric modulation as a means to discover safe and selective drugs. Drug Discov. Today Technol. 2010;7:e87–e94. doi: 10.1016/j.ddtec.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 233.Frank RA. Clustered coding variants in the glutamate receptor complexes of individuals with schizophrenia and bipolar disorder. PLoS One. 2011;6:e19011. doi: 10.1371/journal.pone.0019011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ayoub MA. Deleterious GRM1mutations n schizophrenia. PLoS One. 2012;7:e32849. doi: 10.1371/journal.pone.0032849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Owen DR. Recent advances in the medicinal chemistry of the metabotropic glutamate receptor 1 (mGlu(1)) ACS Chem. Neurosci. 2011;2:394–401. doi: 10.1021/cn2000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Williams DL, Jr, Lindsley CW. Discovery of positive allosteric modulators of metabotropic glutamate receptor subtype 5 (mGluR5) Curr. Top. Med. Chem. 2005;5:825–846. doi: 10.2174/1568026054750290. [DOI] [PubMed] [Google Scholar]
- 237.Stauffer SR. Progress toward positive allosteric modulators of the metabotropic glutamate receptor subtype 5 (mGlu(5)) ACS Chem. Neurosci. 2011;2:450–470. doi: 10.1021/cn2000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.O'Brien JA, Lemaire W, Wittmann M, Jacobson MA, Ha SN, Wisnoski DD, Lindsley CW, Schaffhauser HJ, Rowe B, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL., Jr A novel selective allosteric modulator potentiates the activity of native metabotropic glutamate receptor subtype 5 in rat forebrain. J. Pharmacol. Exp. Ther. 2004;309:568–577. doi: 10.1124/jpet.103.061747. [DOI] [PubMed] [Google Scholar]
- 239.Zhao Z, Wisnoski DD, O'Brien JA, Lemaire W, Williams DL, Jr, Jacobson MA, Wittman M, Ha SN, Schaffhauser H, Sur C, Pettibone DJ, Duggan ME, Conn PJ, Hartman GD, Lindsley CW. Challenges in the development of mGluR5 positive allosteric modulators: the discovery of CPPHA. Bioorg. Med. Chem. Lett. 2007;17:1386–1391. doi: 10.1016/j.bmcl.2006.11.081. [DOI] [PubMed] [Google Scholar]
- 240.Rodriguez AL, Zhou Y, Williams R, David Weaver C, Vinson PN, Dawson ES, Steckler T, Lavreysen H, Mackie C, Bartolomé JM, Macdonald GJ, Scott Daniels J, Niswender CM, Jones CK, Jeffrey Conn P, Lindsley CW, Stauffer SR. Discovery and SAR of a novel series of non-MPEP site mGlu(5) PAMs based on an aryl glycine sulfonamide scaffold. Bioorg. Med. Chem. Lett. 2012;22:7388–7392. doi: 10.1016/j.bmcl.2012.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hammond AS, Rodriguez AL, Townsend SD, Niswender CM, Gregory KJ, Lindsley CW, Conn PJ. Discovery of a novel chemical class of mGlu(5) allosteric ligands with distinct modes of pharmacology. ACS Chem. Neurosci. 2010;1:702–716. doi: 10.1021/cn100051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhang Y, Rodriguez AL, Conn PJ. Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J. Pharmacol. Exp. Ther. 2005;315:1212–1219. doi: 10.1124/jpet.105.090308. [DOI] [PubMed] [Google Scholar]
- 243.Sharma S, Rodriguez AL, Conn PJ, Lindsley CW. Synthesis and SAR of a mGluR5 allosteric partial antagonist lead: unexpected modulation of pharmacology with slight structural modifications to a 5-(phenylethynyl)pyrimidine scaffold. Bioorg. Med. Chem. Lett. 2008;18:4098–4101. doi: 10.1016/j.bmcl.2008.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wood MR, Hopkins CR, Brogan JT, Conn PJ, Lindsley CW. "Molecular switches" on mGluR allosteric ligands that modulate modes of pharmacology. Biochemistry. 2011;50:2403–2410. doi: 10.1021/bi200129s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Schoepp DD, Johnson BG, Wright RA, Salhoff CR, Mayne NG, Wu S, Cockerman SL, Burnett JP, Belegaje R, Bleakman D, Monn JA. LY354740 is a potent and highly selective group II metabotropic glutamate receptor agonist in cells expressing human glutamate receptors. Neuropharmacology. 1997;36:1–11. doi: 10.1016/s0028-3908(96)00160-8. [DOI] [PubMed] [Google Scholar]
- 246.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 247.Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat. Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 248.Krystal JH, Abi-Saab W, Perry E, D'Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, Breier A. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology. 2005;179:303–309. doi: 10.1007/s00213-004-1982-8. [DOI] [PubMed] [Google Scholar]
- 249.Kinon BJ, Zhang L, Millen BA, Osuntokun OO, Williams JE, Kollack-Walker S, Jackson K, Kryzhanovskaya L, Jarkova N. A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J. Clin. Psychopharmacol. 2011;31:349–355. doi: 10.1097/JCP.0b013e318218dcd5. [DOI] [PubMed] [Google Scholar]
- 250.Sheffler DJ, Pinkerton AB, Dahl R, Markou A, Cosford ND. Recent progress in the synthesis and characterization of group II metabotropic glutamate receptor allosteric modulators. ACS Chem. Neurosci. 2011;2:382–393. doi: 10.1021/cn200008d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the "right stuff"? Schizophr. Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 252.Lieberman JA, Stroup TS. The NIMH-CATIE Schizophrenia Study: What Did We Learn? Am. J. Psychiatry. 2011;168:770–775. doi: 10.1176/appi.ajp.2011.11010039. [DOI] [PubMed] [Google Scholar]
- 253.Jones PB, Barnes TR, Davies L, Dunn G, Lloyd H, Hayhurst KP, Murray RM, Markwick A, Lewis SW. Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1) Arch. Gen. Psychiatry. 2006;63:1079–1087. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- 254.Heres S, Davis J, Maino K, Jetzinger E, Kissling W, Leucht S. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: an exploratory analysis of head-to-head comparison studies of second-generation antipsychotics. Am. J. Psychiatry. 2006;163:185–194. doi: 10.1176/appi.ajp.163.2.185. [DOI] [PubMed] [Google Scholar]
- 255.Nussinov R, Tsai CJ, Csermely P. Allo-network drugs: harnessing allostery in cellular networks. Trends Pharmacol. Sci. 2011;32:686–693. doi: 10.1016/j.tips.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]