Abstract
Previous studies have established a role for cytoplasmic phospholipase A2 (PLA2) activity in tubule-mediated retrograde trafficking between the Golgi complex and the endoplasmic reticulum (ER). However, little else is known about how membrane tubule formation is regulated. This study demonstrates that isotetrandrine (ITD), a biscoclaurine alkaloid known to inhibit PLA2 enzyme activation by heterotrimeric G-proteins, effectively prevented brefeldin A (BFA)-induced tubule formation from the Golgi complex and retrograde trafficking to the ER. In addition, ITD inhibited BFA-stimulated tubule formation from the trans-Golgi network and endosomes. ITD inhibition of the BFA response was potent (IC50 ∼10-20 μM) and rapid (complete inhibition with a 10-15-min preincubation). ITD also inhibited normal retrograde trafficking as revealed by the formation of nocodazole-induced Golgi mini-stacks at ER exit sites. Treatment of cells with ITD alone caused the normally interconnected Golgi ribbons to become fragmented and dilated, but cisternae were still stacked and located in a juxtanuclear position. These results suggest that a G-protein-binding PLA2 enzyme plays a pivotal role in tubule mediated trafficking between the Golgi and the ER, the maintenance of the interconnected ribbons of Golgi stacks, and tubule formation from endosomes.
INTRODUCTION
Recent studies have provided evidence that cytoplasmic Ca+-independent phospholipase A2 (PLA2) enzymes play roles in tubule-mediated trafficking in the secretory and endocytic pathways (Brown et al., 2003). For example, a wide spectrum of PLA2 antagonists, including those specific for cytoplasmic Ca+-independent PLA2 enzymes, are potent inhibitors of brefeldin A (BFA)-stimulated tubule formation from the Golgi complex, trans-Golgi network (TGN), and endosomes, and tubule-mediated trafficking pathways from these organelles (de Figueiredo et al., 1998, 1999, 2000, 2001; Drecktrah and Brown, 1999; Polizotto et al., 1999). The mechanisms responsible for PLA2-mediated tubule formation likely include the localized accumulation of inverted coneshaped lysophospholipids (LPLs) that are generated by PLA2 hydrolysis. In particular, LPLs on the cytoplasmic surfaces of organelle membranes could contribute to the generation of outward bending, thus initiating tubule formation (Burger, 2000; Huijbregts et al., 2000; Huttner and Schmidt, 2002; Brown et al., 2003). Support for this idea was provided by studies showing that inhibition of LPL reacylation by a Golgi-associated lysophospholipid acyltransferase (LPAT) also leads to increased tubule formation and retrograde trafficking (Drecktrah et al., 2003). These studies suggest that LPATs function to negatively influence membrane tubulation by limiting the accumulation of LPLs. Thus, PLA2 and LPAT enzymes provide for direct positive and negative effects on membrane tubule formation, respectively.
The exact identities of the Ca+-independent PLA2 and LPAT enzymes involved in tubule formation are unknown, and, moreover, little is known generally about how tubule formation is regulated. Although no direct role for GTP or GTP-binding proteins in tubule formation per se has been uncovered (Banta et al., 1995), there are intriguing hints that tubule formation could be indirectly connected to GTP-binding proteins (Kano et al., 2000).
One level of connection could involve monomeric GTP-binding proteins. BFA inhibits several guanine nucleotide exchange factors (GEFs) that catalyze the GDP/GTP exchange on ADP-ribosylation factor (ARF), a GTP-binding protein that is required for COPI and AP-1 clathrin-coated vesicle production (Jackson and Casanova, 2000; Scales et al., 2000). Thus, in vivo, BFA inhibits COPI and AP-1 clathrin-coated vesicle formation while also inducing tubule formation. Although several BFA-sensitive GEFs clearly play a role in regulating Golgi morphology and function (Donaldson and Jackson, 2000; Jackson and Casanova, 2000), a longstanding, unresolved question in the field is: why does BFA stimulate tubule formation when ARF GDP/GTP exchange is inhibited? One idea is that there might be a link between the tubulation machinery (PLA2?) and the regulatory proteins (GEFs and GAPs) that control the ARF GDP/GTP cycle.
In addition to monomeric G-proteins, heterotrimeric G-proteins might also be linked to regulation of membrane tubule formation (Stow et al., 1991; De Vries et al., 1995, 2000). One example might be Galpha interacting protein (GAIP), which participates in vesicle production from the TGN by binding to Gαi-3 (De Vries et al., 1995; Wylie et al., 1999). Interestingly, when the N-terminal membrane-binding domain of GAIP (lacking the G-protein-binding domain) is expressed in cells, the TGN forms extensive membrane tubules (Wylie et al., 2003). This result suggests that in the absence of binding to Gαi-3 and inducing vesicle formation, GAIP may positively signal to tubulation machinery. Finally, other studies have shown that protein kinase D (PKD) may also be indirectly involved in tubule formation (Van Lint et al., 2002). PKD binds to and is activated by Gβγ before its recruitment to membranes containing diacylglycerol, and similar to GAIP, activated PKD is involved in the formation of vesicles that bud from the TGN and transport cargo to the plasma membrane (Jamora et al., 1999; Baron and Malhotra, 2002). However, overexpression of kinase-defective PKD induces extensive TGN tubule formation (Liljedahl et al., 2001). Thus, GAIP and PKD share a common property: expression of forms that are defective in vesicle production induce tubule formation. These combined results strongly suggest a link between G-proteins that regulate vesicle production and molecules that are directly involved in tubule formation, e.g., PLA2 enzymes.
The goal of the present study was to gain further insight into this possible regulatory connection. To do this we have taken advantage of the unique properties of the biscoclaurine alkaloid, isotetrandrine (ITD). ITD is a small molecule inhibitor that specifically disrupts Gβ subunit activation of cytoplasmic PLA2 enzyme activities (but not PLC or PLD) that are involved in signal-mediated secretory responses (Hashizume et al., 1991; Akiba et al., 1992; Akiba et al., 1995; Tsunoda and Owyang, 1995). We reasoned that if PLA2-mediated tubulation is somehow linked to G-proteins, then ITD might also disrupt intracellular membrane trafficking that involves tubules. We report here that ITD potently inhibited tubulation from the Golgi complex, TGN, and endosomes, and trafficking events associated with these tubules. These results provide evidence that tubulation is directly or indirectly regulated by G-proteins and show that ITD can be added to the arsenal of small molecule inhibitors that are useful for dissecting intracellular membrane trafficking events.
MATERIALS AND METHODS
Reagents and Antibodies
BFA and ITD were obtained from Biomol (Plymouth Meeting, PA), and nocodazole was purchased from Sigma Chemical Co. (St. Louis, MO). Stock solutions of nocodazole (6 mg/ml) were stored in DMSO and BFA (10 mg/ml) in 100% ethanol at -20°C. The stock solution of ITD in DMSO (25 mM) was stored at -20°C and had a shelf-life of ∼1 month under these conditions. Polyclonal anti-α-mannosidase II (ManII) antibodies were kindly supplied by Dr. Kelley Moremen (University of Georgia), monoclonal anti-human transferrin (Tf) receptor antibodies were from Boehringer-Mannheim (Indianapolis, IN), and polyclonal anti-cation independent mannose 6-phosphate receptor (CI-M6PR) antibodies were those previously described (Wood et al., 1991). Monoclonal anti-ARF1 antibodies were from Affinity Bioreagents (Golden, CO), and monoclonal anti-β-COP (M3A5) antibodies were kindly provided by Dr. William Balch (Scripps Research Institute). The secondary fluorescent antibodies goat anti-rabbit-TRITC and goat anti-mouse-FITC were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Sheep anti-rabbit Fab-HRP-conjugated fragments were from Biosys (Compiegne, France).
Cell Culture and Immunocytochemistry
Rat Clone 9 hepatocytes and HeLa cells were maintained in modified Eagle's minimal essential medium (MEM) with 10% fetal bovine serum, 50 U/ml penicillin, and 50 μg/ml streptomycin from Life Technologies (Grand Island, NY) at 37°C in a humidified atmosphere of 95% air and 5% CO2. For immunofluorescence labeling, both cell types were grown on glass coverslips for 2 days before experimental manipulation. After experimental manipulation, cells were fixed and processed for immunofluorescence as described (de Figueiredo et al., 1998). Polyclonal α-mannosidase II antibody was used at a 1:1000 dilution, and monoclonal anti-Tf receptor antibody at 1:200. In most cases, cells were visualized with a Zeiss Axioskop II (Thornwood, NY) using a Hamamatsu Orca II (Bridgewater, NJ) digital camera and OpenLab software by Improvision (Coventry, UK). In a few cases, cells were imaged using a Perkin Elmer-Cetus UltraView spinning disk confocal microscope (Norwalk, CT). For immunoperoxidase localization of ManII at the electron microscopic level, Clone 9 cells were fixed and processed exactly as described (Brown, 2003). Images were obtained with a FEI/Philips Morgagni transmission EM using Advantage HR digital imaging system (Mahwah, NJ). All digital images were processed for slight contrast and brightness adjustments with Adobe Photoshop 7 (San Jose, CA).
ITD and BFA Studies
Working solutions of ITD and BFA were freshly prepared on the day of the experiment by diluting stock solutions with serum-free MEM. Cells were washed three times with serum-free MEM and then pretreated with or without ITD in MEM for various periods of time at 37°C. The cells were then incubated with BFA at the working concentration of 5 μg/ml with or without ITD as appropriate at 37°C for the times indicated below.
ITD and Nocodazole Studies
Clone 9 cells were briefly washed three times with MEM and then incubated with MEM in the presence or absence of ITD (concentrations indicated below) for 10 min at 4°C. The media was then replaced with 37°C MEM containing nocodazole (6 μg/ml), with or without ITD as appropriate. Cells were then incubated for various periods of time at 37°C.
RESULTS
BFA stimulates the formation of Golgi membrane tubules and their movement to, and fusion with, the ER (Lippincott-Schwartz et al., 1989, 1990). This tubule-mediated process has previously been shown to be inhibited by well-characterized PLA2 antagonists (de Figueiredo et al., 1998), so here we ask if ITD, a compound known to inhibit G-protein-activated PLA2 enzymes, has a similar effect on BFA-stimulated tubulation and retrograde trafficking.
ITD Inhibits BFA-induced Golgi Membrane Tubulation and Retrograde Trafficking
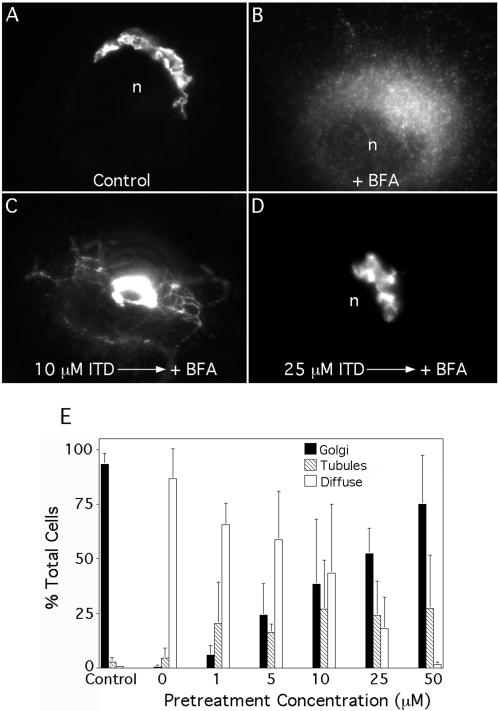
In untreated Clone 9 cells, the Golgi complex is seen as a smooth, interconnected reticulum in the juxtanuclear region, as shown by immunofluorescence with anti-ManII antibodies (Figure 1A). Treatment with BFA alone (5 μg/ml for 5 min) caused the anti-αManII staining to become diffuse, indicative of the movement and retention of Golgi membranes in the ER (Figure 1B). However, when cells were pretreated (10 min) with increasing concentrations of ITD before addition of BFA, tubulation and retrograde movement were significantly inhibited (Figure 1, C and D). Quantitation of these results showed that ITD inhibited BFA-stimulated retrograde trafficking with an IC50 ∼10-20 μM (Figure 1E). Interestingly, at intermediate concentrations of ITD, BFA-treated cells often displayed Golgi membrane tubules (Figure 1C), when normally the entire Golgi would have already moved to the ER. Finally, we note that ITD did not inhibit tubule formation when added to MEM containing fetal bovine serum. These results suggest that lower amounts of ITD had a partial inhibitory effect, sufficient to slow down the retrograde process such that tubules were revealed when they normally would have already formed and fused with the ER.
Figure 1.
ITD inhibits BFA-stimulated formation of membrane tubules from the Golgi complex and retrograde trafficking to the ER in a dose-dependent manner. Clone 9 cells were washed three times with MEM (without serum), pretreated with various concentrations of ITD for 10 min, and then incubated with BFA (5 μg/ml) in the continuous presence of ITD (as appropriate) at 37°C. Cells were then fixed and stained for immunofluorescence of ManII. (A-D) Immunofluorescence micrographs: (A) Untreated cells with a typical juxtanuclear Golgi complex; (B) BFA treatment induced the redistribution of ManII to a diffuse, ER pattern and the complete loss of the central Golgi complex; (C) cell pretreated with 10 μM ITD shows an intermediate effect with membrane tubules and some central Golgi staining; (D) cell pretreated with 25 μM ITD in which BFA-induced redistribution to the ER was completely inhibited. (E) Quantitation of immunofluorescence results in which cells staining for ManII were scored as having a typical Golgi complex (as in A and D), diffuse ER staining (B), or tubule formation (C). Results show the mean ± 1 SD (n ≥ 3 for all data points). n, nucleus.
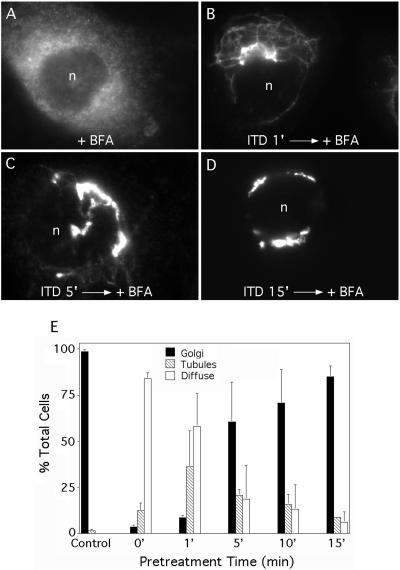
The above studies indicate that ITD rapidly inhibits its target, so to determine the minimum time needed for inhibition, cells were pretreated for various periods of time with a fixed concentration of ITD (25 μM) before addition of BFA. The results showed that pretreatment for just 1-5 min had a significant inhibitory effect on BFA-stimulated retrograde trafficking as tubular intermediates were frequently seen and diffuse staining was reduced (Figure 2, A-D). By 15 min of pretreatment, BFA-stimulated retrograde trafficking was almost completely inhibited (Figure 2, D and E). These results demonstrate that ITD rapidly inactivates a target that is required for BFA-stimulated, tubule-mediated retrograde trafficking to the ER.
Figure 2.
ITD requires only a short preincubation period to inhibit BFA-stimulated Golgi tubulation. Clone 9 cells were washed, pretreated with ITD (25 μM) for various periods of time, and then incubated with BFA (5 μg/ml) in the continuous presence of ITD for 5 min. Cells were fixed and stained for immunofluorescence localization of ManII. (A-D) Immunofluorescence micrographs of cells pretreated with ITD for the times indicated before addition of BFA. (E) Quantitation of immunofluorescence results. Cells were scored as in Figure 1E. Results show the mean ± 1 SD (n ≥ 3 for all data points). n, nucleus.
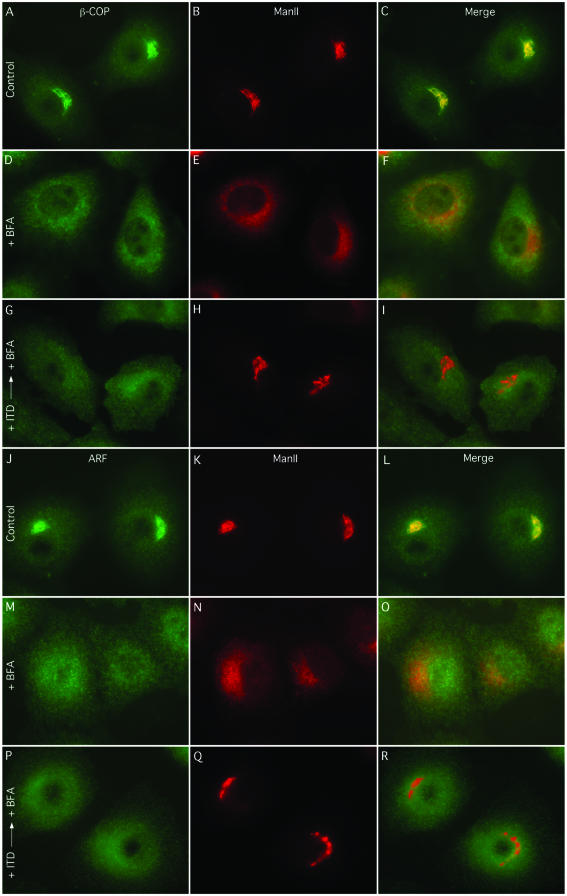
One possible reason that ManII did not redistribute to the ER in BFA- and ITD-treated cells is because ITD did not allow the loss of COPI components from Golgi membranes. To test this possibility, we examined the effect of ITD on the distribution of ARF1, β-COP, and ManII by double-label immunofluorescence. In control cells, both ARF1 (Figure 3, A-C) and β-COP (Figure 3, J-L) colocalized to a large extent with ManII in the Golgi complex, and treatment with BFA alone caused all three proteins to redistribute to a diffuse pattern (Figure 3, D-F, and M-O), as previously shown (Klausner et al., 1992). Conversely, pretreatment with ITD (25 μM for 5 min) before BFA, although preventing ManII redistribution, did not inhibit redistribution of either ARF1 (Figures 3, G-I) or β-COP (Figure 3, P-R) to the cytoplasm. These results are consistent with previous studies that used other PLA2 antagonists and reemphasize that loss of ARF and/or COPI components from Golgi membranes alone is not sufficient to induce tubule-mediated retrograde trafficking to the ER (de Figueiredo et al., 1998; Kano et al., 2000).
Figure 3.
BFA-induced redistribution of ARF1 and β-COP is not inhibited by ITD. Clone 9 cells were kept in MEM and treated with solvents only (Control), BFA alone (5 μg/ml) for 5 min (+ BFA), or pretreated with ITD (25 μM for 5 min) before addition of BFA (+ ITD → + BFA), as indicated. Staining is as follows: ARF1 and ManII double-immunofluorescence (A-I); β-COP and ManII double-immunofluorescence (J-R).
ITD Inhibits Normal Retrograde Trafficking as Revealed by Nocodazole-induced Mini-Stack Formation
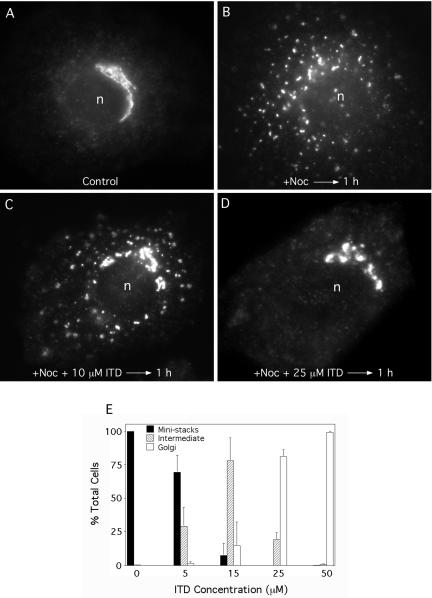
Although Golgi membrane proteins predominantly reside in the Golgi complex, most also slowly cycle between the Golgi complex and the ER (Lippincott-Schwartz et al., 2000). This constitutive cycling can be revealed by treating cells with the microtubule-depolymerizing drug nocodazole, which inhibits anterograde trafficking of ER-derived tubulo-vesicular elements to the Golgi complex, but not retrograde trafficking from the Golgi to the ER (Cole et al., 1996; Storrie et al., 1998). As a consequence, Golgi proteins and membranes recycle to and through the ER, and they reform small Golgi “mini-stacks” at peripheral ER exit sites. After 1-2 h of nocodazole treatment, the central Golgi complex is completely consumed by retrograde carriers and replaced by peripheral mini-stacks. Although the exact nature of the carriers involved in this constitutive retrograde trafficking pathway have not been determined, previous studies have shown that PLA2 antagonists inhibit this retrograde process (Drecktrah and Brown, 1999). Therefore, we asked if ITD has a similar effect on nocodazole-induced mini-stack formation.
As seen by immunofluorescence staining of ManII, treatment of Clone 9 hepatocytes with nocodazole (6 μg/ml) for 1 h resulted in the loss of the typical juxtanuclear ribbon (Figure 4A) and its replacement with small puncta (representing mini-stacks) located throughout the cytoplasm (Figure 4B). Inclusion of 10 μM ITD during the nocodazole treatment period retarded the loss of central Golgi elements (Figure 4C), and 25 μM ITD significantly inhibited mini-stack formation and loss of the central Golgi (Figure 4D). Quantitation of these results revealed that mini-stack formation and loss of the central Golgi complex was almost completely inhibited by 25 μM ITD (Figure 4E).
Figure 4.
ITD inhibits constitutive retrograde trafficking of ManII as revealed by nocodazole-induced Golgi mini-stack formation at ER exit sites. Clone 9 cells were incubated with various concentrations of ITD for 10 min at 4°C, medium was replaced with 37°C MEM containing nocodazole (plus or minus ITD as appropriate), and cells were further incubated for 1 h at 37°C. Cells were then fixed and stained by immunofluorescence for ManII. (A-D) Immunofluorescence micrographs: (A) control, untreated cells with a central interconnected Golgi complex; (B) cells treated with nocodazole for 1 h reveal the complete loss of a central, intact Golgi complex, which was replaced by small mini-stacks at ER exit sites throughout the cytoplasm. (C) Addition of 10 μM ITD partially inhibited min-stack formation and the loss of the central Golgi complex; (D) addition of 25 μM ITD substantially inhibited mini-stack formation and loss of the Golgi complex. (E) Quantitation of immunofluorescence results in which cells staining for ManII were scored as having widely distributed mini-stacks (as in B), an intermediate amount of inhibition with some mini-stacks and partial retention of a central Golgi complex (as in C), or a more typical juxtanuclear Golgi complex and no mini-stacks (as in A and D). Results show the mean ± 1 SD (n ≥ 3 for all data points). n, nucleus.
ITD Inhibits BFA-stimulated Tubule Formation From the TGN
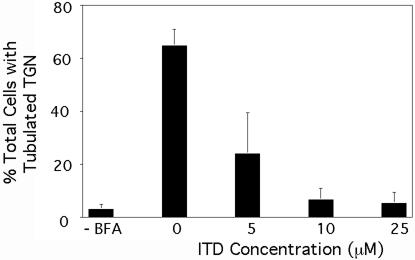
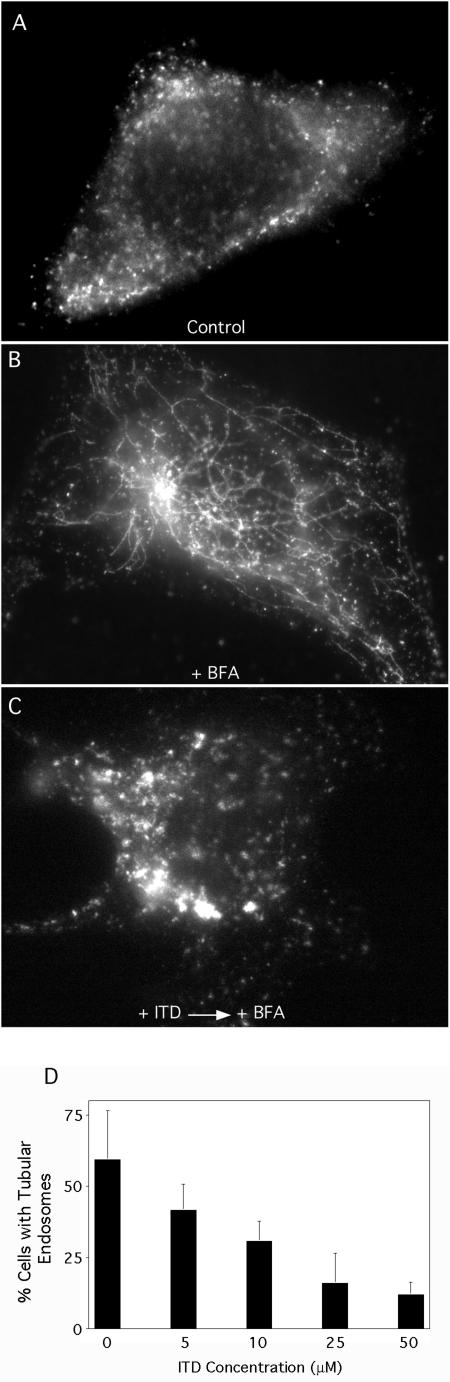
BFA-stimulated tubule formation from the TGN is also inhibited by PLA2 antagonists (de Figueiredo et al., 1998). To see if ITD exhibited a similar inhibitory effect on the TGN, Clone 9 cells were pretreated with varying concentrations of ITD, subsequently incubated with BFA, and then fixed and stained to visualize the CI-M6PR, which serves as a useful marker for TGN tubule formation (Wood et al., 1991). Quantitation of these results shows that untreated cells contain very few long membrane tubules, but BFA treatment induced extensive tubule formation from the TGN (Figure 5). ITD inhibited BFA-stimulated TGN tubulation with an IC50 ∼5 μM, which was slightly lower than for inhibition of Golgi membrane tubulation.
Figure 5.
BFA-induced tubule formation from the TGN is inhibited by ITD. Clone 9 cells were washed in MEM (without serum), pretreated with varying concentrations of ITD, and then subsequently incubated with BFA (5 μg/ml) in the continuous presence or absence of ITD as appropriate for 15 min at 37°C. Cells were then fixed and processed for immunofluorescence localization of the CI-M6PR. Quantitative results show the percentage of cells with extensive TGN tubules. Results show the mean ± 1 SD (n = 3).
ITD Causes Fragmentation of the Golgi Complex and Swelling of Cisternal Elements
In the experiments above showing that ITD inhibited the loss of the Golgi complex in nocodazole-treated cells, we observed that the Golgi complex became increasingly fragmented in longer ITD incubations, e.g., compare control cell in Figure 4A with those treated with nocodazole and ITD for 1 h in Figure 4D. This observation is interesting because previous studies found that PLA2 antagonists by themselves caused interconnected Golgi ribbons to become separated into large fragments, which were nevertheless composed of normal, stacked cisternal elements (de Figueiredo et al., 1999). Those and other experiments led to the conclusion that the normal steady-state architecture of the interphase Golgi complex requires the continuous formation of PLA2-mediated membrane tubules.
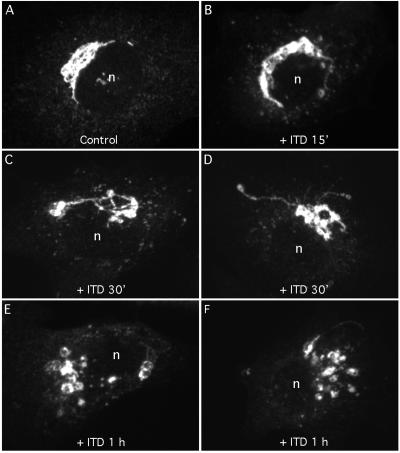
To determine if ITD also fragments the Golgi complex, cells were treated with ITD alone for various periods of time and then examined by immunofluorescence and immunoelectron microscopy using anti-ManII antibodies. For approximately the first 15 min in ITD (25 μM), the Golgi complex appeared normal by immunofluorescence and confocal microscopy (Figure 6A and B). However, by 30 min in ITD the Golgi complex became slightly fragmented and misshapen (Figure 6C and D). After 1 h in ITD, the Golgi was often clearly fragmented and the normally thin ribbons became dilated (Figure 6, E and F). Although misshapen and fragmented, the large Golgi fragments remained in the juxtanuclear region even after 2 h in ITD (our unpublished data). When treated with 25 μM ITD for 1 h and then washed free of the drug with MEM containing 10% fetal bovine serum, the Golgi complex recovered its typical morphology after ∼1 h (our unpublished data). Finally, although the morphology of the Golgi complex is critically dependent on microtubules, and BFA-induced, tubule-mediated retrograde trafficking is greatly facilitated by microtubules (Lippincott-Schwartz, 1998), we found that ITD treatment alone (25 μM for 1 h) had no obvious effects on microtubules as seen by immunofluorescence (our unpublished data).
Figure 6.
ITD alone causes fragmentation of the juxtanuclear Golgi ribbon. Clone 9 cells were washed with MEM (minus serum) and then incubated for various periods of time with 25 μM ITD. Cells were then fixed and stained with anti-ManII antibody and visualized by immunofluorescence and confocal microscopy. (A) Untreated cells; (B) cells treated for 15 min; (C and D) cells treated for 30 min; and (E and F) cells treated for 1 h. Each micrograph represents the composite image obtained from ∼20, 0.2-μm confocal slices.
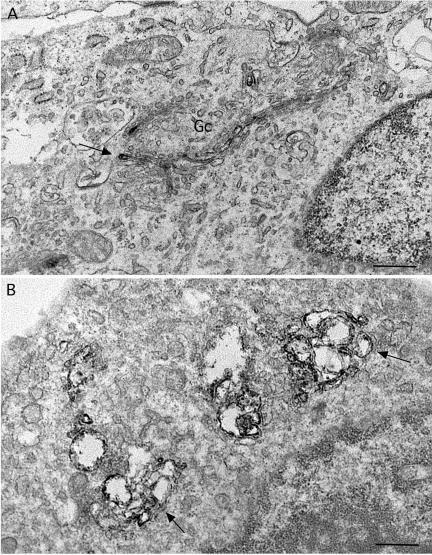
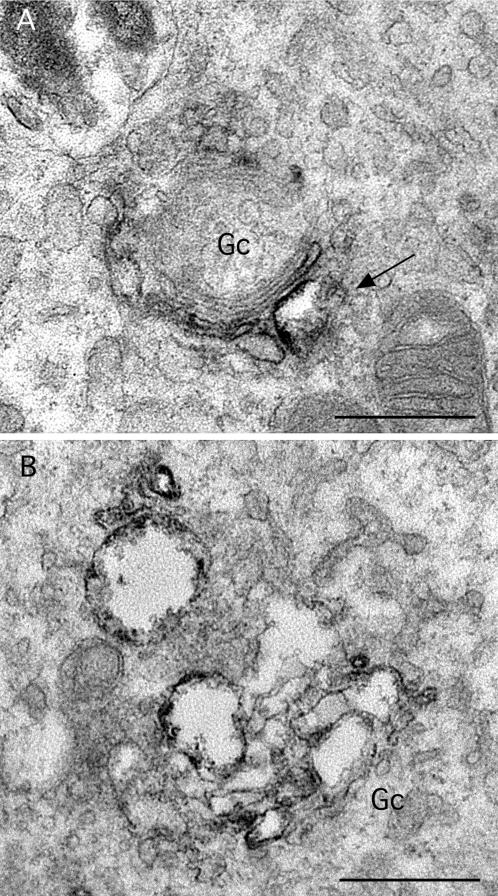
Electron microscopic observations of immunoperoxidase-stained cells confirmed and extended these findings. Control cells displayed typical stacks of Golgi cisternae, with one or two of the cisternae containing immunoperoxidase reaction product (Figure 7A). Consistent with the immunofluorescence images, treatment with ITD (25 μM) for 1 h caused a marked dilation of apparently all cisternae, which still appeared to be connected into bunches of stacks (Figures 7B and 8B). After 15 min of treatment, a time at which changes were less obvious by immunofluorescence, cisternae were often slightly dilated (Figure 8A). However, we could not tell from this analysis if any particular region, i.e., cis, medial, or trans, was more sensitive to ITD than others.
Figure 7.
Immunoperoxidase localization of ManII at the electron microscopic level in ITD-treated cells reveals dilated Golgi cisternae. (A) Control cells with a Golgi complex (Gc) consisting of stacks of flattened cisternae. The electron-dense immunoreaction product of ManII staining is typically found in one or two medial cisternae (arrow). (B) Cells treated with ITD (25 μM) for 1 h show extensively dilated cisternae that contain immunoreaction product (arrows). The dilated cisternae still appear to be stacked together in clumps. Bars, 0.5 μm.
Figure 8.
Higher magnification views of Golgi complexes in ITD-treated cells. (A) In cells treated with ITD (25 μM) for 15 min Golgi complexes (Gc) were often fairly normal but with some dilated cisternae (arrow), in this case, one that contains ManII immunoreaction product. (B) In cells treated for 1 h, all cisternae appeared to be swollen, although they are still found within a stacked unit. Bars, 0.5 μm.
ITD Inhibits BFA-stimulated Tubulation of Endosomes
Previous studies have demonstrated that PLA2 antagonists also inhibit BFA-stimulated tubule formation from endosomes and the normal exit of transferrin (Tf) and Tf receptors, i.e., endocytic recycling, from endosomes (de Figueiredo et al., 2001). These studies suggest that PLA2 activity is a general requirement for at least certain types of membrane tubule formation. Therefore, we investigated the effects of ITD on BFA-stimulated tubule formation from endosomes. In untreated HeLa cells, Tf receptors are seen in numerous punctate structures throughout the cytoplasm, representing various early and recycling endosomes (Figure 9A). Treatment of cells with BFA (5 μg/ml for 15 min) resulted in extensive tubule formation of Tf receptor-positive endosomes (Figure 8B), which was inhibited by pretreatment with ITD (Figure 9C). Quantitation of dose-response experiments showed that ITD inhibited BFA-stimulated endosome tubule formation with an IC50 ∼10 μM (Figure 9D). Finally, it appeared that in ITD-treated cells, the Tf receptor-positive endosomes were larger than their control counterparts (compare Figures 9, A and C).
Figure 9.
BFA-stimulated tubule formation from endosomes is inhibited by ITD in a dose-dependent manner. HeLa cells were washed with MEM (without serum) and preincubated with various concentrations of ITD in MEM for 10 min, BFA (5 μg/μl) was added (in the continuous presence or absence of ITD as appropriate), and cells were further incubated for 15 min at 37°C. Cells were fixed and stained by immunofluorescence to visualize Tf receptors. (A-C) Immunofluorescence micrographs: (A) control, untreated cells; (B) cells treated with BFA showing extensive membrane tubules; (C) cells pretreated with 50 μM ITD before BFA treatment. (D) Quantitation of immunofluorescence experiments. Results show the mean ± 1 SD (n = 3 for all data points).
DISCUSSION
Previous studies have provided evidence that membrane tubule formation from the Golgi complex and endosomes requires the generation of curve-inducing LPLs by the direct action of a cytoplasmic Ca2+-independent PLA2 enzyme on membrane phospholipids (de Figueiredo et al., 1998, 1999; Polizotto et al., 1999; Drecktrah et al., 2003). Here we provide evidence for a previously unsuspected role for heterotrimeric G-proteins in the regulation of PLA2-mediated membrane tubule formation. These studies demonstrate that the biscoclaurine (bisbenzyltetrahydroisoquinoline) alkaloid ITD, which specifically inhibits heterotrimeric G-protein-activated PLA2 enzyme activities (Hashizume et al., 1991; Akiba et al., 1995; Tsunoda and Owyang, 1995), is a general inhibitor of BFA-stimulated tubule formation from the Golgi complex, TGN, and endosomes. ITD also inhibits BFA-stimulated retrograde trafficking from the Golgi to the ER and normal constitutive retrograde trafficking from the Golgi to the ER, as revealed by nocodazole-induced mini-stack formation. Finally, ITD causes a reversible loss in the interconnected architecture of the Golgi complex, in addition to causing cisternal dilation.
ITD inhibition of Golgi, TGN, and endosome tubule formation is similar to that exhibited by well-characterized PLA2 antagonists (de Figueiredo et al., 1998, 2001). An additional similarity is that ITD led to the fragmentation of Golgi ribbons into physically separate stacks, consistent with previous studies which concluded that the steady-state architecture of the interconnected Golgi complex requires the dynamic formation of PLA2-mediated membrane tubules (de Figueiredo et al., 1999). However, ITD differs from PLA2 antagonists because it also caused noticeable cisternal dilation. Why ITD should cause dilation is unclear, but ITD could affect unknown G-protein-dependent processes in the Golgi that influence cisternal structure. Keep in mind, however, that ITD significantly inhibited tubule formation (within 10 min) well before the dilation effect was obvious (∼30 min). Therefore, it seems unlikely that ITD's inhibitory effects on tubulation are related to cisternal dilation.
ITD also inhibited endosome tubule formation with a resultant enlargement of Tf receptor-positive endosomes, similar to that previously observed with PLA2 antagonists (de Figueiredo et al., 2001). In the case of PLA2 antagonists, endosome enlargement appeared to result from the inhibition of receptor and membrane export from endosomes. Thus, we suspect that the enlargement of endosomes by ITD is different from the dilation of Golgi cisternae (seen in long-term ITD treatment). Indeed, preliminary studies strongly suggest that ITD inhibits endocytic recycling of Tf and TfR receptors from both early sorting and later recycling endosomes (our unpublished data).
ITD (1R,1′S-isotetrandrine) and its isomer, tetrandrine (1S,1′S-tetrandrine) are naturally occurring plant alkaloids (ITD from Stephania tetrandra and Berberis vulgaris) that have been reported to have numerous effects on animals and cells, including immunomodulatory and vasculature effects (Li et al., 2001; Lai, 2002). Tetrandrine has been much more widely studied than ITD and their effects are not identical, so making general conclusions about the actions of these two compounds is difficult. But, for example, tetrandrine has been reported to induce cell-cycle arrest and to inhibit the acquisition of multidrug resistance in tumor cells (Fu et al., 2002; Oh and Lee, 2003). On the other hand, both ITD and tetrandrine have been reported to inhibit agonist-induced Ca2+ transport (Takemura et al., 1995; Shuttleworth, 1996; Chen et al., 2000). More directly relevant to our studies, both ITD and tetrandrine appear to prevent Gβ activation of PLA2 activity (Hashizume et al., 1991; Akiba et al., 1992). If fact, we found that like ITD, tetrandrine inhibited BFA-stimulated tubule formation and retrograde trafficking (our unpublished data). Despite the various effects reported for tetrandrine and ITD, their exact molecular target(s) are unknown. Therefore, caution should be exercised when interpreting results obtained with these compounds. Nevertheless, compounds that have multiple effects can still point to new avenues of investigation. Also, we should point out that ITD was used to examine relatively short-term effects, i.e., those exhibited within 10-60 min of treatment, that were completely reversible, thus avoiding any possible long-term effects brought on toxicity.
Finally, we should emphasize that ITD differs from PLA2 antagonists because ITD is not an enzyme inhibitor per se; rather, it inhibits Gβ activation of PLA2 activities (Akiba et al., 1992; Tsunoda and Owyang, 1995). In these studies the exact Gβ isoform was not identified; however, it may be derived from a Gq complex. Moreover, these molecules were identified in the context of PLA2-stimulated amylase secretion from permeabilized pancreatic acini; therefore it seems likely that a different set of ITD-inhibited G-proteins will be used for Golgi tubulation events.
Our results extend previous studies on membrane tubulation in an important way by suggesting a novel connection between PLA2-mediated membrane tubule formation and regulation by heterotrimeric G-proteins. Moreover, our results are consistent with previous studies which found that GTPγS stimulates BFA-induced Golgi tubule formation in a permeabilized cell assay (Kano et al., 2000). Thus, as with coated vesicles generated at the Golgi, TGN, and endosomes, membrane tubules may also be regulated by G-proteins. As discussed in the Introduction, these results may suggest new ways to think about the relationship between vesicle and tubule formation. Whether or not regulation of PLA2-mediated tubule formation by heterotrimeric G-proteins can be generalized to situations other than BFA-stimulation, e.g., those tubulation events stimulated by mutant forms of GAIP or PKD, remains to be determined.
Acknowledgments
We thank Drs. Bill Balch and Kelley Moremen for providing antibodies. D.C. was supported in part as a Cornell Hughes Scholar. This work was supported by National Institutes of Health Grant DK 51596 (to W.J.B.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-09-0644. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-09-0644.
References
- Akiba, S., Kato, E., Sato, T., and Fujii, T. (1992). Biscoclaurine alkaloids inhibit receptor-mediated phospholipase A2 activation probably through uncoupling of a GTP-binding protein from the enzyme in rat peritoneal mast cells. Biochem. Pharmacol. 44, 45-50. [DOI] [PubMed] [Google Scholar]
- Akiba, S., Nagatomo, R., Ishimoto, T., and Sato, T. (1995). Effect of berbamine on cytosolic phospholipase A2 activation in rabbit platelets. Eur. J. Pharmacol. 291, 343-350. [DOI] [PubMed] [Google Scholar]
- Banta, M., Polizotto, R.S., Wood, S.A., de Figueiredo, P., and Brown, W.J. (1995). Characterization of a cytosolic activity that induces the formation of Golgi membrane tubules in a cell-free reconstitution system. Biochemistry 34, 13359-13366. [DOI] [PubMed] [Google Scholar]
- Baron, C.L., and Malhotra, V. (2002). Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 295, 325-328. [DOI] [PubMed] [Google Scholar]
- Brown, W.J. (2003). Immunoperoxidase methods for localization of antigens in cultured cells and tissues. Curr. Protocols Cell Biol. 1, 4.6.1-4.6.17. [DOI] [PubMed] [Google Scholar]
- Brown, W.J., Chambers, K., and Doody, A. (2003). Phospholipase A2 (PLA2) Enzymes in membrane trafficking: mediators of membrane shape and function. Traffic 4, 214-221. [DOI] [PubMed] [Google Scholar]
- Burger, K.N. (2000). Greasing membrane fusion and fission machineries. Traffic 1, 605-613. [DOI] [PubMed] [Google Scholar]
- Chen, L.Y., Chen, X., Tian, X.L., and Yu, X.H. (2000). Effects of tetrandrine on calcium transport, protein fluorescences and membrane fluidity of sarcoplasmic reticulum. Br. J. Pharmacol. 131, 530-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, N.B., Sciaky, N., Marotta, A., Song, J., and Lippincott-Schwartz, J. (1996). Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol. Biol. Cell 7, 631-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Figueiredo, P., Doody, A., Polizotto, R.S., Drecktrah, D., Wood, S., Banta, M., Strang, M., and Brown, W.J. (2001). Inhibition of transferrin recycling and endosome tubulation by phospholipase A2 antagonists. J. Biol. Chem. 276, 47361-47370. [DOI] [PubMed] [Google Scholar]
- de Figueiredo, P., Drecktrah, D., Katzenellenbogen, J.A., Strang, M., and Brown, W.J. (1998). Evidence that phospholipase A2 activity is required for Golgi complex and trans Golgi network membrane tubulation. Proc. Natl. Acad. Sci. USA 95, 8642-8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Figueiredo, P., Drecktrah, D., Polizotto, R.S., Cole, N.B., Lippincott-Schwartz, J., and Brown, W.J. (2000). Phospholipase A2 antagonists inhibit constitutive retrograde membrane traffic to the endoplasmic reticulum. Traffic 1, 504-511. [DOI] [PubMed] [Google Scholar]
- de Figueiredo, P., Polizotto, R.S., Drecktrah, D., and Brown, W.J. (1999). Membrane tubule-mediated reassembly and maintenance of the Golgi complex is disrupted by phospholipase A2 antagonists. Mol. Biol. Cell 10, 1763-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries, L., Mousli, M., Wurmser, A., and Farquhar, M.G. (1995). GAIP, a protein that specifically interacts with the trimeric G protein G alpha i3, is a member of a protein family with a highly conserved core domain. Proc. Natl. Acad. Sci. USA 92, 11916-11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries, L., Zheng, B., Fischer, T., Elenko, E., and Farquhar, M.G. (2000). The regulator of G protein signaling family. Annu. Rev. Pharmacol. Toxicol. 40, 235-271. [DOI] [PubMed] [Google Scholar]
- Donaldson, J.G., and Jackson, C.L. (2000). Regulators and effectors of the ARF GTPases. Curr. Opin. Cell Biol. 12, 475-482. [DOI] [PubMed] [Google Scholar]
- Drecktrah, D., and Brown, W.J. (1999). Phospholipase A2 antagonists inhibit nocodazole-induced Golgi ministack formation: evidence of an ER intermediate and constitutive cycling. Mol. Biol. Cell 10, 4021-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drecktrah, D., Chambers, K., Racoosin, E.L., Cluett, E.B., Gucwa, A., Jackson, B., and Brown, W.J. (2003). Inhibition of a Golgi complex lysophospholipid acyltransferase induces membrane tubule formation and retrograde trafficking. Mol. Biol. Cell 14, 3459-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, L.W., Zhang, Y.M., Liang, Y.J., Yang, X.P., and Pan, Q.C. (2002). The multidrug resistance of tumour cells was reversed by tetrandrine in vitro and in xenografts derived from human breast adenocarcinoma MCF-7/adr cells. Eur. J. Cancer 38, 418-426. [DOI] [PubMed] [Google Scholar]
- Hashizume, T., Yamaguchi, H., Sato, T., and Fujii, T. (1991). Suppressive effect of biscoclaurine alkaloids on agonist-induced activation of phospholipase A2 in rabbit platelets. Biochem. Pharmacol. 41, 419-423. [DOI] [PubMed] [Google Scholar]
- Huijbregts, R.P.H., Topalof, L., and Bankaitis, V.A. (2000). Lipid metabolism and regulation of membrane trafficking. Traffic 1, 195-202. [DOI] [PubMed] [Google Scholar]
- Huttner, W.B., and Schmidt, A.A. (2002). Membrane curvature: a case of endofeelin'. Trends Cell Biol. 12, 155-158. [DOI] [PubMed] [Google Scholar]
- Jackson, C.L., and Casanova, J.E. (2000). Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol. 10, 60-67. [DOI] [PubMed] [Google Scholar]
- Jamora, C., Yamanouye, N., Van Lint, J., Laudenslager, J., Vandenheede, J.R., Faulkner, D.J., and Malhotra, V. (1999). Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell 98, 59-68. [DOI] [PubMed] [Google Scholar]
- Kano, F., Sako, Y., Tagaya, M., Yanagida, T., and Murata, M. (2000). Reconstitution of brefeldin A-induced Golgi tubulation and fusion with the endoplasmic reticulum in semi-intact Chinese hamster ovary cells. Mol. Biol. Cell 11, 3073-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner, R.D., Donaldson, J.G., and Lippincott-Schwartz, J. (1992). Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116, 1071-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, J.H. (2002). Immunomodulatory effects and mechanisms of plant alkaloid tetrandrine in autoimmune diseases. Acta Pharmacol. Sin. 23, 1093-1101. [PubMed] [Google Scholar]
- Li, D.G., Wang, Z.R., and Lu, H.M. (2001). Pharmacology of tetrandrine and its therapeutic use in digestive diseases. World J. Gastroenterol. 7, 627-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljedahl, M., Maeda, Y., Colanzi, A., Ayala, I., Van Lint, J., and Malhotra, V. (2001). Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell 104, 409-420. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J. (1998). Cytoskeletal proteins and Golgi dynamics. Curr. Opin. Cell Biol. 10, 52-59. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Donaldson, J.G., Schweizer, A., Berger, E.G., Hauri, H.P., Yuan, L.C., and Klausner, R.D. (1990). Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell 60, 821-836. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Roberts, T.H., and Hirschberg, K. (2000). Secretory protein trafficking and organelle dynamics in living cells. Annu. Rev. Cell. Dev. Biol. 16, 557-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Yuan, L.C., Bonifacino, J.S., and Klausner, R.D. (1989). Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56, 801-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, S.H., and Lee, B.H. (2003). Induction of apoptosis in human hepatoblastoma cells by tetrandrine via caspase-dependent Bid cleavage and cytochrome c release. Biochem. Pharmacol. 66, 725-731. [DOI] [PubMed] [Google Scholar]
- Polizotto, R.S., de Figueiredo, P., and Brown, W.J. (1999). Stimulation of Golgi membrane tubulation and retrograde trafficking to the ER by phospholipase A2 activating protein (PLAP) peptide. J. Cell. Biochem. 74, 670-683. [PubMed] [Google Scholar]
- Scales, S.J., Gomez, M., and Kreis, T.E. (2000). Coat proteins regulating membrane traffic. Int. Rev. Cytol. 195, 67-144. [DOI] [PubMed] [Google Scholar]
- Shuttleworth, T.J. (1996). Arachidonic acid activates the noncapacitative entry of Ca2+ during [Ca2+]i oscillations. J. Biol. Chem. 271, 21720-21725. [DOI] [PubMed] [Google Scholar]
- Storrie, B., White, J., Rottger, S., Stelzer, E.H., Suganuma, T., and Nilsson, T. (1998). Recycling of Golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J. Cell Biol. 143, 1505-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow, J.L., de Almeida, J.B., Narula, N., Holtzman, E.J., Ercolani, L., and Ausiello, D.A. (1991). A heterotrimeric G protein, G alpha i-3, on Golgi membranes regulates the secretion of a heparan sulfate proteoglycan in LLC-PK1 epithelial cells. J. Cell Biol. 114, 1113-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura, H., Kwan, C.Y., and Ohshika, H. (1995). Calcium antagonistic actions of tetrandrine depend on cell types. Res. Commun. Mol. Pathol. Pharmacol. 90, 59-68. [PubMed] [Google Scholar]
- Tsunoda, Y., and Owyang, C. (1995). The regulatory site of functional GTP binding protein coupled to the high affinity cholecystokinin receptor and phospholipase A2 pathway is on the G beta subunit of Gq protein in pancreatic acini. Biochem. Biophys. Res. Commun. 211, 648-655. [DOI] [PubMed] [Google Scholar]
- Van Lint, J., Rykx, A., Maeda, Y., Vantus, T., Sturany, S., Malhotra, V., Vandenheede, J.R., and Seufferlein, T. (2002). Protein kinase D: an intracellular traffic regulator on the move. Trends Cell Biol. 12, 193-200. [DOI] [PubMed] [Google Scholar]
- Wood, S.A., Park, J.E., and Brown, W.J. (1991). Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell 67, 591-600. [DOI] [PubMed] [Google Scholar]
- Wylie, F., Heimann, K., Le, T.L., Brown, D., Rabnott, G., and Stow, J.L. (1999). GAIP, a Galphai-3-binding protein, is associated with Golgi-derived vesicles and protein trafficking. Am. J. Physiol. 276, C497-C506. [DOI] [PubMed] [Google Scholar]
- Wylie, F. G., Lock, J. G., Jamriska, L., Khromykh, T., D, L. B., and Stow, J. L. (2003). GAIP participates in budding of membrane carriers at the trans-Golgi network. Traffic 4, 175-189. [DOI] [PubMed] [Google Scholar]