Abstract
Guard cells use compensatory feedback controls to adapt to conditions that produce excessively open stomata.
In the past 15 years or more, many mutants that are impaired in stimulus-induced stomatal closing and opening have been identified and functionally characterized in Arabidopsis (Arabidopsis thaliana), leading to a mechanistic understanding of the guard cell signal transduction network. However, evidence has only recently emerged that mutations impairing stomatal closure, in particular those in slow anion channel SLOW ANION CHANNEL-ASSOCIATED1 (SLAC1), unexpectedly also exhibit slowed stomatal opening responses. Results suggest that this compensatory slowing of stomatal opening can be attributed to a calcium-dependent posttranslational down-regulation of stomatal opening mechanisms, including down-regulation of inward K+ channel activity. Here, we discuss this newly emerging stomatal compensatory feedback control model mediated via constitutive enhancement (priming) of intracellular Ca2+ sensitivity of ion channel activity. The CALCIUM-DEPENDENT PROTEIN KINASE6 (CPK6) is strongly activated by physiological Ca2+ elevations and a model is discussed and open questions are raised for cross talk among Ca2+-dependent and Ca2+-independent guard cell signal transduction pathways and Ca2+ sensitivity priming mechanisms.
Stomatal pores formed by two guard cells enable CO2 uptake from the atmosphere, but also ensure leaf cooling and provide a pulling force for nutrient uptake from the soil via transpiration. These vitally important processes are inevitably accompanied by water loss through stomata. Stomatal opening and closure is caused by the uptake and release of osmotically active substances and is tightly regulated by signaling pathways that lead to the activation or inactivation of guard cell ion channels and pumps. Potassium ions enter guard cells through the inward-rectifying K+ channels (K+in) during stomatal opening and are released via outward-rectifying K+ channels during stomatal closure (Schroeder et al., 1987; Hosy et al., 2003; Roelfsema and Hedrich 2005). Cytosolic Ca2+, an important second messenger in plants, mediates ion channel regulation, particularly down-regulation of inward-conducting K+in channels and activation of S-type anion channels, thus mediating stomatal closure and inhibiting stomatal opening (Schroeder and Hagiwara, 1989; Dodd et al., 2010; Kim et al., 2010). Stomatal closure is initiated by anion efflux via the slow S-type anion channel SLAC1 (Negi et al., 2008; Vahisalu et al., 2008; Kollist et al., 2011) and the voltage-dependent rapid R-type anion channel QUICK-ACTIVATING ANION CHANNEL1 (Meyer et al. 2010; Sasaki et al., 2010).
In recent years, advances have been made toward understanding mechanisms mediating abscisic acid (ABA)-induced stomatal closure (Cutler et al., 2010; Kim et al., 2010; Raghavendra et al., 2010). The core ABA signaling module, consisting of PYR/RCAR (for pyrabactin resistance 1/regulatory components of ABA receptors) receptors, clade A protein phosphatases (PP2Cs), SNF-related protein kinase OPEN STOMATA1 (OST1), and downstream targets, is Ca2+-independent (Ma et al., 2009; Park et al., 2009; Hubbard et al., 2010). However, ABA-induced stomatal closure was reduced to only 30% of the normal stomatal closure response under conditions that inhibited intracellular cytosolic free calcium ([Ca2+]cyt) elevations in Arabidopsis (Siegel et al., 2009), consistent with previous findings in other plants (De Silva et al., 1985; Schwartz, 1985; McAinsh et al., 1991; MacRobbie, 2000). Together these and other studies show the importance of [Ca2+]cyt for a robust ABA-induced stomatal closure. Here, we discuss Ca2+-dependent and Ca2+-independent signaling pathways in guard cells and open questions on how these may work together.
Plants carrying mutations in the SLAC1 anion channel have innately more open stomata, and exhibit clear impairments in ABA-, elevated CO2-, Ca2+-, ozone-, air humidity-, darkness-, and hydrogen peroxide-induced stomatal closure (Negi et al., 2008; Vahisalu et al., 2008; Merilo et al., 2013). Recent research, however, unexpectedly revealed that mutations in SLAC1 also down-regulate stomatal opening mechanisms and slow down stomatal opening (Laanemets et al., 2013).
UNEXPECTED SLOWING OF STOMATAL OPENING IN SLAC1 MUTANT ALLELES
Stomatal opening in plants is mediated by increased light intensity or enhanced air humidity and by decreased CO2 concentrations inside the leaf (Ci) that occur as a result of photosynthesis. During light-induced stomatal opening, phototropin-related blue-light signaling leads to the activation of H+-ATPases, resulting in H+ efflux and plasma membrane hyperpolarization (for review, see Shimazaki et al., 2007), which in turn leads to the uptake of K+ via K+ channels (Schroeder et al., 1984). Simultaneously, due to active photosynthesis, Ci is reduced and S-type anion channels are inactivated, which further favors stomatal opening (Roelfsema et al., 2002). Mutations in the SLAC1 gene result in impaired anion efflux, and would therefore be expected to accelerate stomatal opening in response to opening stimuli. Unexpectedly, the opposite was detected: Stomatal opening of intact whole rosettes induced by three independent biological stimuli (light, low Ci, and high humidity) was slower in slac1 mutants (Laanemets et al., 2013). Independent research showed that slac1 mutant guard cells show a greatly reduced activity of K+in channels (Laanemets et al., 2013), which contribute to stomatal opening (Kwak et al., 2001; Figs. 1 and 2). These independent findings suggest that plants possess a system that counteracts the impaired stomatal closing of S-type anion channels in slac1 mutants by down-regulating stomatal opening mechanisms to prevent excessive water loss.
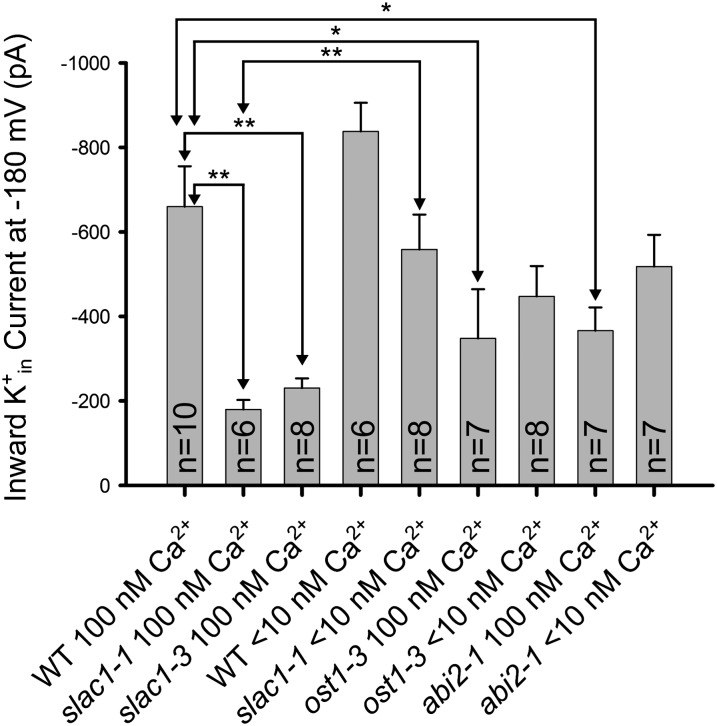
Figure 1.
K+in channel current activity is reduced in the stomatal closing impaired mutants slac1, ost1, and abi2-1 and this K+ channel down-regulation is rapidly reversed in slac1 guard cells by lowering [Ca2+]cyt to less than 10 nm. Average K+in channel current magnitudes at −180 mV are shown for wild-type Columbia-0 (WT) and the slac1, ost1, and abi2-1 alleles. The concentrations of buffered [Ca2+]cyt concentrations are indicated. Whole-cell patch clamp recordings were performed on guard cell protoplasts at the indicated cytosolic free Ca2+ concentrations. Note that an abi2-1 allele in the Columbia-0 accession was analyzed (Nishimura et al., 2004). Error bars (sem) for the indicated number of guard cells analyzed are depicted. K+in channel current magnitudes in slac1 recovered at less than 10 nm [Ca2+]cyt compared with 100 nm [Ca2+]cyt (P < 0.005). Statistical analyses showed significant down-regulation of K+in channel current magnitudes in the slac1-1, slac1-3 (P < 0.001), ost1 (P < 0.04), and abi2-1 (Columbia-0; P < 0.02) mutants compared with wild-type guard cells at 100 nm free [Ca2+] in the cytosol. Small but statistically nonsignificant differences for comparisons of K+in channel current magnitudes in response to lowering [Ca2+] from 100 nm to less than 10 nm for ost1-3 (P value = 0.525) and abi2-1 (P value = 0.109) were found. *P < 0.05; **P < 0.01. Unpaired Student’s t tests were applied to assess significance. Data from WT < 10 nm and slac1-1 < 10 nm are from Laanemets et al., 2013. Methods were as described in Laanemets et al., 2013 (see Supplemental Text S1).
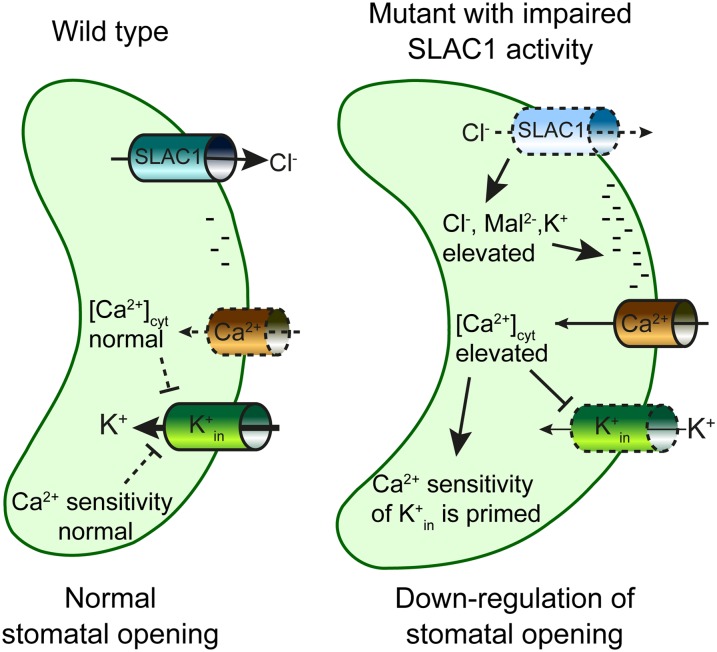
Figure 2.
Schematic model for K+in down-regulation in mutants with impaired SLAC1 activity. Without SLAC1, ions accumulate in guard cells, and the plasma membrane is charged more negatively (hyperpolarization) due to reduced anion efflux, which leads to activation of hyperpolarization-dependent Ca2+-permeable influx channels and elevated levels of [Ca2+]cyt. The increased [Ca2+]cyt concentration down-regulates K+in channel activity. Furthermore, K+in channels exhibit an enhanced (primed) Ca2+ sensitivity, thus enhancing K+in channel down-regulation. The negative charges shown at the inner side of the plasma membrane (right model) indicate the more negative (hyperpolarized) membrane potential expected for slac1 mutant alleles.
IMPAIRED ANION EFFLUX LEADS TO A CHANGED IONOMIC PROFILE IN SLAC1 GUARD CELLS
Severely reduced S-type anion channel activity and reduced anion efflux in slac1 guard cells change the entire ionomic profile of guard cells. Elevated accumulation of anions such as chloride, malate, and fumarate, but also potassium was observed (Negi et al., 2008). Hyperaccumulation of chloride and malate can suppress H+-coupled anion transport (Sanders et al., 1989). Accordingly, the cytosolic pH (pHcyt) of slac1 guard cells was slightly more alkaline (Wang et al., 2012). Furthermore, the removal of S-type anion channel activity in slac1 mutant guard cells (Vahisalu et al., 2008) is expected to cause a more negative (“hyperpolarized”) membrane potential due to the reduced anion efflux from guard cells. This is predicted to enhance the activity of hyperpolarization-activated Ca2+ influx channels resulting in a slightly elevated [Ca2+]cyt in slac1 guard cells (Grabov and Blatt, 1998; Hamilton et al., 2000; Pei et al., 2000). Slightly elevated [Ca2+]cyt in slac1 guard cells was experimentally observed in two studies (Wang et al., 2012; Laanemets et al., 2013) and causes down-regulation of K+in activity (Schroeder and Hagiwara, 1989; Siegel et al., 2009), thereby slowing stomatal opening in slac1.
DOWN-REGULATION OF GUARD CELL K+ UPTAKE CHANNEL ACTIVITY BY ENHANCED [Ca2+]CYT SENSITIVITY IN SLAC1
Analyses of guard cell ion channel transcript levels showed only partially reduced expression of K+in and H+-ATPase gene transcripts in slac1 guard cells, suggesting that posttranslational mechanisms may down-regulate K+in (Laanemets et al., 2013). The patch clamp method enables clamping of defined [Ca2+]cyt and pHcyt conditions by rapidly equilibrating the cytosol with the patch pipette solution. The first experiments showing dramatic down-regulation of K+in channel activity in plants lacking SLAC1 (slac1-1 and slac1-3) were performed at 250 nm free [Ca2+]cyt. Interestingly, the reduction of K+in activity was rapidly reversed by lowering [Ca2+]cyt to a subphysiological [Ca2+]cyt concentration of less than 10 nm (Laanemets et al., 2013), indicating that Ca2+-induced inhibition of K+in is more sensitive to [Ca2+]cyt in slac1 than in wild-type plants (Fig. 1). However, whether K+in channel activity in slac1 guard cells is also affected at resting [Ca2+]cyt levels had not yet been investigated. Additional patch clamp experiments show that K+in activity of slac1-1 and slac1-3 is also greatly reduced at physiological resting [Ca2+]cyt of 100 nm (Fig. 1; Supplemental Fig. S1), indicating a down-regulation of K+in activity in slac1 plants even at resting [Ca2+]cyt. These results demonstrate that the sensitivity of K+in channels to physiological [Ca2+]cyt is constitutively enhanced (primed) in slac1 guard cells. These findings support the hypothesis that the sensitivity of Ca2+ signaling mechanisms in guard cells can be enhanced such that guard cells respond to resting [Ca2+]cyt levels, thus resulting in possible residual [Ca2+]cyt signaling (Siegel et al., 2009).
The priming of K+in channel sensitivity to [Ca2+]cyt leads to reduced K+ influx representing a mechanism to counteract the potential adverse effect of more open stomata in slac1 plants (Laanemets et al., 2013). Guard cell [Ca2+]cyt elevation alone is not sufficient to explain the slowed stomatal opening of slac1 mutants. As mentioned above, anion accumulation in the slac1 mutant resulted in elevated pHcyt (Wang et al., 2012), which might also slow stomatal opening. In sum, the primed Ca2+ sensitivity of K+in channels, together with higher [Ca2+]cyt and more alkaline pHcyt, provide a feedback mechanism helping to prevent excessive water loss in slac1 mutant plants that are defective in stomatal closure (Fig. 2).
The next question was whether slac1 plants always show higher Ca2+ sensitivity of K+in channels or whether this is reversible. Patch clamp experiments showed that the reduction of K+in activity was reversed by lowering [Ca2+]cyt to a subphysiological Ca2+ concentration (Laanemets et al., 2013). If stomata of slac1 plants are in a more closed state, does this provide feedback to [Ca2+]cyt, pHcyt, and most importantly to Ca2+-priming of K+in channels, resulting in a wild-type-like stomatal opening rate? The answer to this question requires further research, but results so far indicate that the Ca2+-priming of K+in channels is indeed reversible and depends on initial stomatal “openness”. When slac1 and wild-type plants showed nearly similar steady-state starting stomatal conductances (Laanemets et al., 2013), the differences in half-times for stomatal opening between slac1 and wild-type plants were only moderate (Table I). However, when the starting stomatal apertures of slac1 plants were considerably larger than those of the wild type, the differences in half-times for stomatal opening were also larger, about 2-fold (Wang et al., 2012), indicating that in the latter experiments the compensatory feedback control of stomatal opening functioned to counteract further stomatal opening in this already open state.
Table I. Increase in stomatal conductance is slower in slac1-1, slac1-3, and ost1-3 mutants (background Columbia-0) and in abi2-1 mutant (background Ler).
*P < 0.1; **P < 0.05 (statistical difference from the wild type, one-way ANOVA, n = 5–18).
| Genotype | Half-Times for Stomatal Openinga |
|
|---|---|---|
| Low CO2 | Light | |
| min | ||
| Wild type (Columbia-0) | 18.8 ± 0.9 | 15.4 ± 0.6 |
| slac1-1 | 22.8 ± 2.0* | 21.1 ± 1.1** |
| slac1-3 | 26.3 ± 2.3** | 19.7 ± 1.4** |
| ost1-3 | 24.3 ± 1.2** | 22.1 ± 1.3** |
| Wild type (Ler) | 19.8 ± 0.8 | 10.1 ± 0.8 |
| abi2-1 | 24.9 ± 3.3 | 16.6 ± 3.9** |
In light experiments, plants were kept in the measurement cuvettes (Kollist et al., 2007) overnight and stomatal opening was measured during the onset of the light period in the morning. In CO2 experiments, plants were kept at ambient CO2 (400 mmol mol−1) for 2 h, then the CO2 was decreased to 40 mmol mol−1The stomatal opening response, within first 45 min, was scaled to the range from 0% to 100%, directly yielding the half-times for stomatal opening. Plant growth conditions were as described in Laanemets et al., 2013. See Supplemental Text S1 for further experimental details.
SLOWED STOMATAL OPENING AND DOWN-REGULATION OF GUARD CELL K+ UPTAKE CHANNEL ACTIVITY IS ALSO OBSERVED IN OTHER MUTANTS WITH MORE OPEN STOMATA
If down-regulation of K+in channel activity via Ca2+ priming is caused by the open stomata phenotype of slac1 mutants, the question arises whether the same phenotype is also present in other mutants with constitutively more open stomata. To address this point, experiments were performed with ost1-3 (Mustilli et al., 2002; Yoshida et al., 2002) and the dominant mutant aba insensitive2 (abi2-1; Koornneef et al., 1984). OST1 is a protein kinase that activates SLAC1 anion channels via phosphorylation (Geiger et al., 2009; Lee et al., 2009; Vahisalu et al., 2010) and functional ABA activation of SLAC1 channels via OST1 was reconstituted in oocytes (Brandt et al., 2012). An unexpected reduced K+in channel activity in abi2-1 mutant guard cells was shown in an earlier study (Pei et al., 1997). The dominant abi2-1 mutation generates a mutant ABI2 protein phosphatase that is refractory to ABA-induced inhibition by PYR/RCAR receptors and suppresses OST1 activation (Ma et al., 2009; Park et al., 2009; Umezawa et al., 2009). Guard cells lacking functional OST1 or having a dominant active ABI2, are likely to hyperaccumulate ions and exhibit more negative plasma membrane potential, which would lead to an increase in [Ca2+]cyt (Grabov and Blatt, 1998; Hamilton et al., 2000; Pei et al., 2000). Our gas-exchange experiments showed that both light- and low-CO2-induced stomatal opening responses were slower in ost1-3 and abi2-1 plants compared with corresponding wild types (Table I). Additional patch clamp experiments with abi2-1 and ost1-3 guard cells were performed and K+in channel activity was found to be reduced in ost1-3 guard cells and confirmed to be reduced in abi2-1 guard cells (Pei et al., 1997; Fig. 1; Supplemental Figs. S2 and S3). However, reducing [Ca2+]cyt to a subphysiological Ca2+ concentration (less than 10 nm) only slightly improved K+in activity in ost1-3 and in abi2-1 guard cells compared with slac1 (Fig. 1; Supplemental Figs. S2 and S3; Laanemets et al., 2013). These recent studies also highlight that K+in channel activities in guard cells do not always correlate with the predominant phenotype of a given mutant, as illustrated for the slac1, abi2-1, and ost1 mutants (Pei et al., 1997; Laanemets et al., 2013; Fig. 1; Supplemental Figs. S1–S3).
Taken together, elevated [Ca2+]cyt, combined with an increased sensitivity of Ca2+-mediated K+in inhibition in slac1 plants (Wang et al., 2012; Laanemets et al., 2013), leads to the down-regulation of K+in channel activity, even at physiological resting [Ca2+]cyt concentrations (Fig. 1; Supplemental Fig. S1). This results in slowed stomatal opening of intact slac1 plants in response to several stimuli such as air humidity, CO2, and light. Reduced K+in activity and slowed stomatal opening of ost1-3 and abi2-1 mutants further suggests that this may be a general characteristic of plants with more open stomata or of plants with impaired S-type anion channel activation. Further research of mutants with an enhanced open stomata phenotype independent of S-type anion channels is needed to address this point. Importantly, in slac1 mutants the down-regulation of K+in channel activity was reversible at low [Ca2+]cyt, whereas it was not clearly reversible in ost1-3 and only partly reversible in abi2-1 mutants, indicating that either active OST1 is involved in the increase of K+in at low [Ca2+]cyt or this type of reversible compensatory regulation of ion channel activity is a unique characteristic related to the impaired SLAC1 anion channel.
PHYSIOLOGICAL STIMULI RAPIDLY ENHANCE [Ca2+]CYT SENSITIVITY
Considering that the Arabidopsis genome encodes over 200 calcium binding (EF-hand containing) proteins (Day et al., 2002), understanding the mechanisms that mediate specific responses to Ca2+ is a subject of current research interest in plants and in eukaryotes in general (Berridge, 2012). Several mechanisms have been proposed to mediate specificity in Ca2+ signaling in plants, all of which may contribute to this phenomenon (Dodd et al., 2010; Kudla et al., 2010). However, strong cellular and biochemical evidence for any given model is missing and needed in plants, as well as in other systems (Berridge, 2012). Research on guard cell signal transduction has led to a new model that can contribute a mechanism for specificity in Ca2+ signaling. Studies in different plant species have shown that calcium is required for both ABA- and CO2-induced stomatal closing (De Silva et al., 1985; Schwartz, 1985; Webb et al., 1996; Grabov and Blatt, 1998; Staxén et al., 1999; MacRobbie, 2000; Mori et al., 2006; Young et al., 2006; Siegel et al., 2009). Several independent findings support the model that the stomatal closing signals, ABA and elevated CO2, “prime” specific early Ca2+ sensing mechanisms, switching them from a relatively inactivated state to a Ca2+-responsive “primed” state, and therefore tightly controlling Ca2+ responsiveness. Here, we briefly review evidence supporting this Ca2+ sensitivity priming model (Table II).
Table II. Evidence for stimulus-induced Ca2+ sensitivity enhancement (priming) in guard cells.
| Experimental Observations | Reference |
|---|---|
| Spontaneous calcium transients found in guard cells | Grabov and Blatt, 1998; Allen et al., 1999; Staxén et al., 1999; Klüsener et al., 2002; Young et al., 2006 |
| Spontaneous calcium transients found in guard cells even when stomatal opening stimulus is applied | Young et al., 2006 |
| Rapid Ca2+ reactive stomatal closing occurs for any Ca2+ elevation pattern above a threshold level | Allen et al., 2001; Supplemental Fig. S4: http://www.nature.com/nature/journal/v411/n6841/extref/4111053a0_S1.htm |
| Calcium is required for both ABA and CO2 induced stomatal closing | e.g De Silva et al., 1985; Schwartz, 1985; Webb et al., 1996; Staxén et al., 1999; MacRobbie, 2000; Mori et al., 2006; Young et al., 2006; Zhu et al., 2007; Siegel et al., 2009 |
| Priming (enhancement) of [Ca2+]cyt sensitivity of S-type anion and K+in channel regulation by ABA, elevated CO2 and high external Ca2+ | Allen et al., 2002; Siegel et al., 2009; Chen et al., 2010; Xue et al., 2011 |
| Constitutive priming of Ca2+ sensitivity of K+in channel down-regulation in slac1 guard cells | Laanemets et al., 2013 |
Ca2+ imaging in guard cells resolved “spontaneous” repetitive [Ca2+]cyt transients that are more likely to occur at increasingly negative membrane potentials (Grabov and Blatt, 1998; Allen et al., 1999; Klüsener et al., 2002; Young et al., 2006; Siegel et al., 2009; Table II). Surprisingly, repetitive [Ca2+]cyt elevations even occurred when the stomatal opening stimulus low CO2 was applied (Young et al., 2006). The following question arose: How can [Ca2+]cyt be required for stomatal closing if [Ca2+]cyt elevations are also observed while applying stomatal opening stimuli (Young et al., 2006)? Previous research showed that any imposed [Ca2+]cyt elevation above a threshold value can cause a rapid Ca2+-reactive stomatal closure (Allen et al., 2001; Table II). Moreover, the [Ca2+]cyt oscillation frequency and pattern did not affect this rapid “Ca2+-reactive” stomatal closure response (Allen et al., 2001). (Note that the Ca2+ elevation pattern does affect the ability of closed stomata to reopen later, a response called “Ca2+-programmed” stomatal response [Allen et al., 2001; Cho et al., 2009; Eisenach et al., 2012].) The above findings together led to the hypothesis that stomatal closing stimuli may modulate and thus enhance the Ca2+ sensitivity of specific Ca2+-activated stomatal closing mechanisms (Young et al., 2006).
Further studies are consistent with the stimulus-induced Ca2+ sensitivity priming hypothesis (Table II). In brief, an early study showed that raising [Ca2+]cyt alone does not trigger S-type anion channel activation in Arabidopsis guard cells (Allen et al., 2002). However, if the guard cell protoplasts were preexposed to high external Ca2+ during isolations prior to recordings, then elevated [Ca2+]cyt rapidly activated S-type anion currents (figure 3 in Allen et al., 2002). A similar and physiologically more relevant effect was found for ABA: when guard cells were preexposed to ABA, elevated [Ca2+]cyt strongly activated S-type anion currents by shifting the [Ca2+]cyt sensitivity to lower [Ca2+]cyt levels (Siegel et al., 2009; Chen et al., 2010). Interestingly, ABA preincubation also primed K+in down-regulation by [Ca2+]cyt (Siegel et al., 2009). An increase in the Ca2+ sensitivity of S-type anion channel activation was also triggered by elevated CO2 (Xue et al., 2011). Intracellular bicarbonate and CO2 levels lead to strong S-type anion channel activation in the presence of 2 µm [Ca2+]cyt, but not at 0.1 µm [Ca2+]cyt, already 3 to 5 min after achieving the patch clamp whole-cell configuration, which allows equilibration of the pipette solution with the cytosol (Xue et al., 2011). This rapid Ca2+ sensitivity priming indicates that the underlying processes are less likely mediated by transcriptional changes. Early ABA signaling mechanisms were determined to indirectly or partially affect CO2 control of stomatal closing (Merilo et al., 2013), which could be explained by the finding that both pathways require S-type anion channels and the OST1 protein kinase (Roelfsema et al., 2004; Hu et al., 2010; Xue et al., 2011; Merilo et al., 2013). Furthermore, basal ABA signaling in guard cells may partially prime guard cells to respond stronger to other stimuli such as CO2 elevation (Merilo et al., 2013).
In preliminary experiments we have observed that simply continuously increasing the extracellular Ca2+ concentration appears to show a weaker Ca2+ reactive stomatal closure response than when oscillations in extracellular Ca2+ are imposed. As hyperpolarization of guard cells causes Ca2+ oscillations (Grabov and Blatt, 1998; Staxén et al., 1999; Klüsener et al., 2002; Siegel et al., 2009), slac1 mutants may enhance (prime) the cytosolic Ca2+ sensitivity via this pathway. Thus, prior Ca2+ exposure itself can play a role in Ca2+ sensitivity priming (see figure 3 in Allen et al., 2002). More work is needed, however, to identify the underlying mechanisms.
The result showing that the compensatory down-regulation of K+in channels in slac1 guard cells can be rapidly reversed by lowering [Ca2+]cyt provides additional strong evidence that the [Ca2+]cyt sensitivity of mechanisms leading to stomatal movements can be primed (Laanemets et al., 2013). Interestingly, in slac1 mutants, [Ca2+]cyt down-regulation of K+in channels is constitutively primed under these conditions (Laanemets et al., 2013; Fig. 1). Moreover, it was reported that ABA-induced stomatal closure does not require preceding [Ca2+]cyt signaling (Levchenko et al., 2005; but see De Silva et al., 1985; Schwartz, 1985; Grabov and Blatt, 1998; Staxén et al., 1999; MacRobbie, 2000; Mori et al., 2006; Young et al., 2006; Siegel et al., 2009; Chen et al., 2010). Ca2+ sensitivity priming, such that physiological resting [Ca2+]cyt levels enable Ca2+ signaling, may explain this (Levchenko et al., 2005).
Modulation of the sensitivity of calcium sensors provides a mechanism which could contribute to the specificity in Ca2+ signaling in other plant responses and might help to resolve the crucial question of how Ca2+ elevations are “translated” into specific responses with numerous Ca2+ binding proteins expressed in individual cells. Further research is needed to determine whether this mechanism might also occur in other cell types and represent a more broadly used option to achieve specificity in responses to [Ca2+]cyt in plants.
PUTATIVE BIOCHEMICAL MECHANISMS THAT MAY MEDIATE Ca2+ SENSITIVITY PRIMING
In vivo research has shown that CPKs are important mediators of Ca2+-dependent stomatal closing and S-type anion channel activation (Mori et al., 2006; Zhu et al., 2007; Zou et al., 2010). The CPKs that are presently known to function in Ca2+-induced stomatal closing in vivo are CPK6, CPK3, CPK4, CPK10, and CPK11 (Mori et al., 2006; Zhu et al., 2007; Zou et al., 2010; Hubbard et al., 2012). In addition, CPK23 and CPK21 mutants were reported to show enhanced drought resistance (Ma and Wu, 2007; Franz et al., 2011), whereas recent data showed slightly impaired stomatal closing phenotypes in response to environmental stimuli for a CPK23 mutant (Merilo et al., 2013). However, the cellular and molecular signaling mechanisms mediating Ca2+ sensitivity priming remain unknown. Research in Xenopus laevis oocytes and in vitro biochemistry are providing insights into how CPKs may mediate stomatal closing. Expression of the Ca2+-dependent protein kinases CPK23, CPK21, and CPK6 showed that these CPKs activate SLAC1 anion channels in oocytes (Geiger et al., 2010; Brandt et al., 2012). Furthermore, expression of a truncated and constitutively active CPK3 also resulted in SLAC1 activation (Scherzer et al., 2012).
Although CPK6 functions in Ca2+-, ABA-, and methyl jasmonate-induced activation of S-type anion channels in vivo (Mori et al., 2006; Munemasa et al., 2011), CPK6 was reported to interact with SLAC1 only weakly (Geiger et al., 2010) and not to show physiologically relevant Ca2+-activated protein kinase activity in vitro (Scherzer et al., 2012). However, quantitative phosphorylation analyses showed a strong preference for CPK6 to phosphorylate the N terminus of SLAC1 in a Ca2+-dependent manner (Brandt et al., 2012). A stringent biochemical analysis (modified after Hastie et al., 2006) of CPK6 protein kinase activity reveals that CPK6 is strongly activated by elevation in [Ca2+] in the physiological range of [Ca2+]cyt increases from baseline levels of approximately 100 to 150 nm to concentrations greater than or equal to 300 nm (Fig. 3). Taken together, CPK6 is activated by physiological [Ca2+] increases and interacts with SLAC1 to phosphorylate SLAC1 Ca2+ dependently (Fig. 3; Brandt et al., 2012). One hypothesis for a mechanism mediating Ca2+ sensitivity priming is that the clade A PP2Cs directly down-regulate CPK activity (see Fig. 4), similar to PP2C-mediated down-regulation of OST1 activity (Belin et al., 2006; Yoshida et al., 2006; Umezawa et al., 2009; Vlad et al., 2009). However, to date no study has shown the down-regulation of CPK activity by PP2Cs, neither in vivo nor in vitro, and thus more research is needed to address this or other hypotheses.
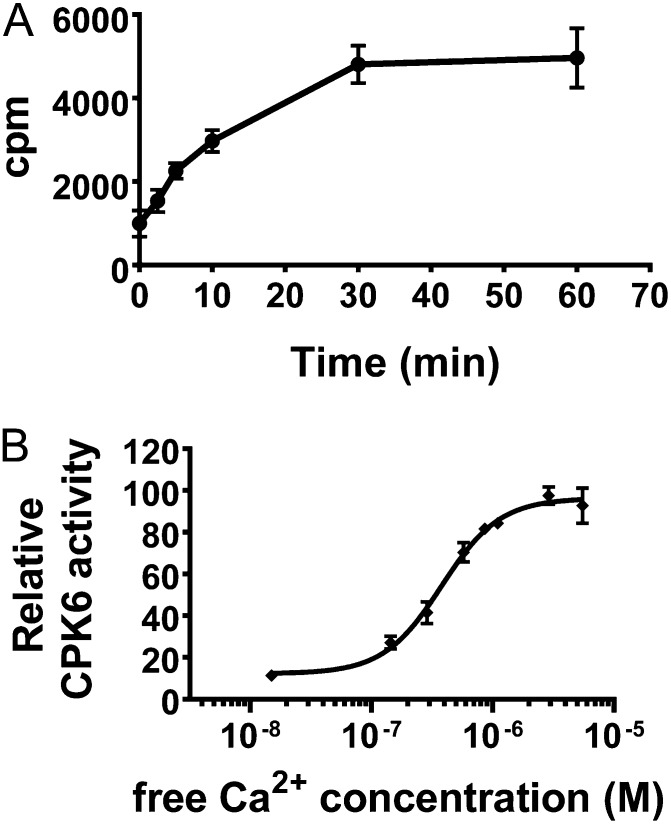
Figure 3.
Quantitative phosphorylation assays show a strong Ca2+ activation of CPK6 activity for physiological [Ca2+] elevations. A, To determine proper conditions, the time-dependent phosphorylation of Syntide-2 (400 µm) by CPK6 (75 nm) was analyzed by measuring the incorporation of 32P into the substrate (in counts per minute [cpm]). B, A time point in the linear range of product phosphorylation in A (4.5 min) was chosen to determine CPK6 activities at defined free Ca2+ concentrations. CPK6 activity is strongly dependent on the free Ca2+ concentration (fit parameters: KA = 508 nm; Hill coefficient = 1.8 ± 0.2 se; R2 = 0.98). Error bars represent sd (n = 3 experiments). See Supplemental Text S1 for a detailed description of the method used.
Figure 4.
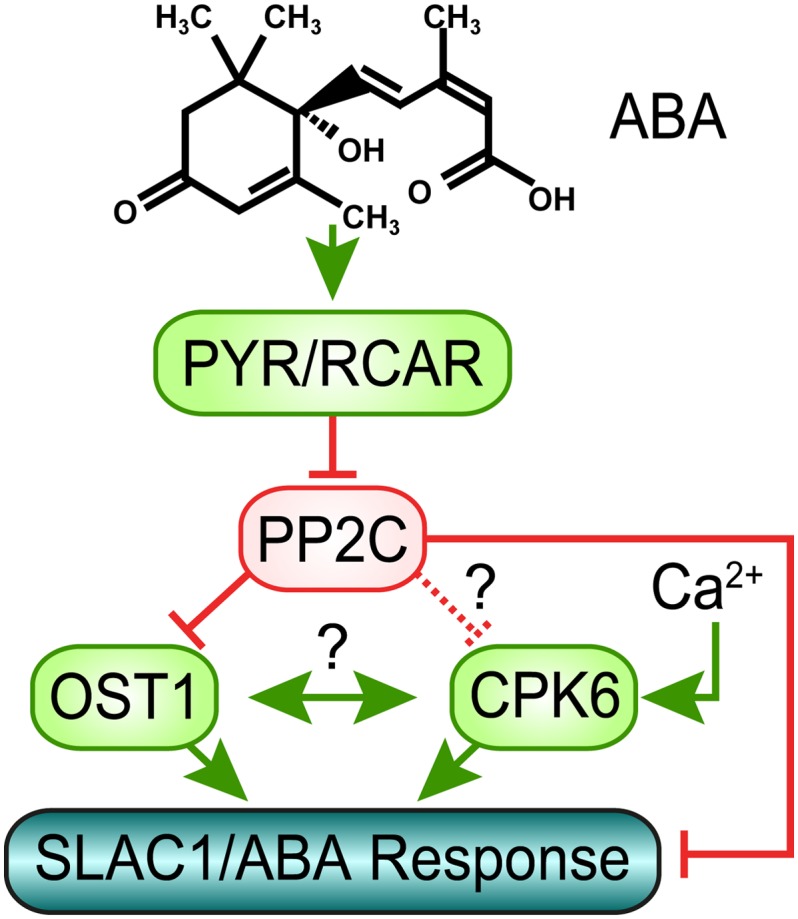
Simplified model of abscisic acid signaling in guard cells. In the presence of ABA, PYR/RCAR proteins inhibit PP2C phosphatases. This enables activation of the protein kinase OST1, which in turn phosphorylates and activates SLAC1, representing the Ca2+-independent branch of SLAC1 activation. Furthermore, the ABI1 PP2C directly dephosphorylates SLAC1 leading to deactivation of SLAC1. Decreased PP2C activity induced by ABA also leads to decreased negative regulation of SLAC1 activation by calcium dependent protein kinases. Whether this regulation solely occurs by dephosphorylation of SLAC1 or whether in addition PP2Cs directly regulate CPKs remains to be determined (see text). A potential mechanism of cross talk between Ca2+-dependent and -independent SLAC1 activation may occur by cross regulation of OST1 and CPKs, which is indicated by ?, but is hypothetical and remains to be investigated.
Studies showed that the Ca2+-independent protein kinase OST1 can activate SLAC1 in X. laevis oocytes and that this is inhibited by the presence of clade A PP2C phosphatases (Geiger et al., 2009; Lee et al., 2009). Moreover, a recent study demonstrated that functional ABA-activation of SLAC1 channels can be reconstituted in X. laevis oocytes by coexpression of ABA receptors, PP2Cs, protein kinase, and SLAC1 (Brandt et al., 2012). Either the Ca2+-dependent protein kinase CPK6 (Fig. 3) or the Ca2+-independent protein kinase OST1 was sufficient for functional reconstitution of ABA activation of SLAC1 (Brandt et al., 2012). Further research is needed to determine the genetic and cell signaling mechanisms that mediate stimulus-induced enhancement (priming) of [Ca2+]cyt -dependent signal transduction.
COMMUNICATION AMONG Ca2+-DEPENDENT AND Ca2+-INDEPENDENT MECHANISMS
It remains unknown how the above-described Ca2+-dependent and Ca2+-independent pathways communicate with one another in guard cells in vivo. Several nonexclusive models can be envisioned as discussed below, although other mechanisms may also mediate this communication. A hypothesis in which PP2Cs may down-regulate CPKs (Fig. 4) remains to be investigated, as discussed above. Given that Ca2+-dependent and Ca2+-independent stomatal closing appear to depend on one another quantitatively (Mustilli et al., 2002; Siegel et al., 2009), an additional hypothesis is that CPKs and OST1 (cross) regulate each other (Fig. 4). However, no biochemical evidence for such cross regulation or protein-protein interaction has presently been reported, in vivo or in vitro, and this hypothesis would need to be investigated. In addition to these models, recent research demonstrated that the ABI1 PP2C phosphatase directly dephosphorylates the N terminus of SLAC1 (Fig. 4; Brandt et al., 2012). (Note that PP2Cs are Mg2+-requiring protein phosphatases and millimolar Mg2+ concentrations are best included at all times, including during all PP2C protein purification steps, to assess their roles in target dephosphorylation.) The dephosphorylation of SLAC1 by ABI1 provides a mechanism for the required tight regulation of S-type anion channel activity in guard cells (Fig. 4; Pei et al., 1997). Direct regulation of ion channels by protein phosphatases has been reported for other plant and animal ion channels (Westphal et al., 1999; Chérel et al., 2002; Lee et al., 2007; Zhou et al., 2010). Furthermore, OST1 may regulate [Ca2+]cyt levels via the NADPH oxidases respiratory burst oxidase homolog D and F and subsequent reactive oxygen species bursts (Sirichandra et al., 2009). Through this pathway, OST1 could control [Ca2+]cyt (Kwak et al., 2003) and regulate CPK activities. A fourth hypothesis, which does not exclude the above models, is that SLAC1 serves as a coincidence detector for phosphorylation and activation by OST1 and CPKs (Fig. 4). OST1 has been shown to phosphorylate residues including Ser 120 (S120) in the N terminus of SLAC1 and S120 phosphorylation is essential for the SLAC1 activation by OST1 in oocytes (Geiger et al., 2009) and for stomatal closing (Vahisalu et al., 2010). However, recent experiments showed that stomatal closure induced by environmental factors were clearly less impaired in slac1-7 plants that carry S120F mutation than those observed for SLAC1 knockout plants, further suggesting that SLAC1 activation is a process that involves phosphorylation of multiple amino acids by multiple protein kinases (Merilo et al., 2013). In line with this assumption, S120A mutation did not disrupt activation of SLAC1 by CPK23 (Geiger et al., 2010). Moreover, CPK6 phosphorylated Ser 59 (S59) in the SLAC1 N terminus and S59 phosphorylation is essential for SLAC1 activation by CPK6 (Brandt et al., 2012). Data show that S59 can also be phosphorylated by OST1 in vitro (Vahisalu et al., 2010). However, whether this is required for OST1 activation of SLAC1 remains unknown. Thus, a combination of the above options and/or additional mechanisms may mediate Ca2+ specificity and sensitivity priming and synergistic effects of Ca2+-dependent and Ca2+-independent signal transduction. These models await investigation and could lead to a detailed mechanistic understanding of a network that mediates specificity in plant calcium signal transduction.
SUMMARY
In conclusion, recent findings show that stomata compensate for excessively open apertures by mechanisms that include constitutive priming (enhancement) of Ca2+ sensitivity, as found in slac1 guard cells (Laanemets et al., 2013; Figs. 1 and 2; Supplemental Fig. S1). The finding that stomatal regulation can adapt to and compensate for impaired stomatal responses (Laanemets et al., 2013) could be of broader relevance for plant-environment interactions. A precise biochemical and cellular understanding of the mechanisms that ensure compensatory regulation of stomatal movements and detailed mechanisms mediating specificity in Ca2+ signaling remain to be elucidated in plants. ABA- and CO2-induced Ca2+ sensitivity priming in guard cells (Young et al., 2006; Siegel et al., 2009; Chen et al., 2010; Xue et al., 2011) provides a system that can explain calcium signaling specificity in guard cells and adds to other (nonexclusive) models for Ca2+ signaling specificity in plants (Kudla et al., 2010). An in depth biochemical and cellular understanding of mechanisms mediating specificity in Ca2+ signaling is also a present goal in animal cell signaling research (Berridge, 2012). The hypotheses and models proposed here (Fig. 4) could enable the underlying specificity mechanisms to be characterized at an in depth mechanistic level in a plant cell system.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers supplied in Supplemental Text S1.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. slac1 shows enhanced Ca2+ sensitivity at resting [Ca2+]cyt.
Supplemental Figure S2. K+in channels are down-regulated in ost1-3 mutant plants.
Supplemental Figure S3. K+in channels are down-regulated in abi2-1 mutant plants.
Glossary
- K+in
inward-rectifying K+
- [Ca2+]cyt
cytosolic free calcium
- ABA
abscisic acid
- Ci
CO2 concentration in the leaf
- pHcyt
cytosolic pH
References
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI. (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI. (1999) Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J 19: 735–747 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Murata Y, Chu SP, Nafisi M, Schroeder JI. (2002) Hypersensitivity of abscisic acid-induced cytosolic calcium increases in the Arabidopsis farnesyltransferase mutant era1-2. Plant Cell 14: 1649–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin C, de Franco P-O, Bourbousse C, Chaignepain S, Schmitter J-M, Vavasseur A, Giraudat J, Barbier-Brygoo H, Thomine S. (2006) Identification of features regulating OST1 kinase activity and OST1 function in guard cells. Plant Physiol 141: 1316–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. (2012) Calcium signalling remodelling and disease. Biochem Soc Trans 40: 297–309 [DOI] [PubMed] [Google Scholar]
- Brandt B, Brodsky DE, Xue S, Negi J, Iba K, Kangasjärvi J, Ghassemian M, Stephan AB, Hu H, Schroeder JI. (2012) Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc Natl Acad Sci USA 109: 10593–10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Hills A, Lim CK, Blatt MR. (2010) Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant J 61: 816–825 [DOI] [PubMed] [Google Scholar]
- Chérel I, Michard E, Platet N, Mouline K, Alcon C, Sentenac H, Thibaud J-B. (2002) Physical and functional interaction of the Arabidopsis K+ channel AKT2 and phosphatase AtPP2CA. Plant Cell 14: 1133–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D, Kim SA, Murata Y, Lee S, Jae SK, Nam HG, Kwak JM. (2009) De-regulated expression of the plant glutamate receptor homolog AtGLR3.1 impairs long-term Ca2+-programmed stomatal closure. Plant J 58: 437–449 [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61: 651–679 [DOI] [PubMed] [Google Scholar]
- Day IS, Reddy VS, Shad Ali G, Reddy AS. (2002) Analysis of EF-hand-containing proteins in Arabidopsis. Genome Biol 3: H0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva DLR, Cox RC, Hetherington AM, Mansfield TA. (1985) Suggested involvement of calcium and calmodulin in the responses of stomata to abscisic acid. New Phytol 101: 555–563 [Google Scholar]
- Dodd AN, Kudla J, Sanders D. (2010) The language of calcium signaling. Annu Rev Plant Biol 61: 593–620 [DOI] [PubMed] [Google Scholar]
- Eisenach C, Chen ZH, Grefen C, Blatt MR. (2012) The trafficking protein SYP121 of Arabidopsis connects programmed stomatal closure and K+ channel activity with vegetative growth. Plant J 69: 241–251 [DOI] [PubMed] [Google Scholar]
- Franz S, Ehlert B, Liese A, Kurth J, Cazalé A-C, Romeis T. (2011) Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol Plant 4: 83–96 [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KAS, et al. (2009) Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci USA 106: 21425–21430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, Liese A, Wellmann C, Al-Rasheid KAS, Grill E, et al. (2010) Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci USA 107: 8023–8028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Blatt MR. (1998) Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc Natl Acad Sci USA 95: 4778–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DWA, Hills A, Kohler B, Blatt MR. (2000) Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc Natl Acad Sci USA 97: 4967–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie CJ, McLauchlan HJ, Cohen P. (2006) Assay of protein kinases using radiolabeled ATP: a protocol. Nat Protoc 1: 968–971 [DOI] [PubMed] [Google Scholar]
- Hosy E, Vavasseur A, Mouline K, Dreyer I, Gaymard F, Porée F, Boucherez J, Lebaudy A, Bouchez D, Very AA, et al. (2003) The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc Natl Acad Sci USA 100: 5549–5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Boisson-Dernier A, Israelsson-Nordstroem M, Boehmer M, Xue S, Ries A, Godoski J, Kuhn JM, Schroeder JI. (2010) Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat Cell Biol 12: 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. (2010) Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev 24: 1695–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard KE, Siegel RS, Valerio G, Brandt B, Schroeder JI. (2012) Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses. Ann Bot (Lond) 109: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI. (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61: 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüsener B, Young JJ, Murata Y, Allen GJ, Mori IC, Hugouvieux V, Schroeder JI. (2002) Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol 130: 2152–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H, Jossier M, Laanemets K, Thomine S. (2011) Anion channels in plant cells. FEBS J 278: 4277–4292 [DOI] [PubMed] [Google Scholar]
- Kollist T, Moldau H, Rasulov B, Oja V, Rämma H, Hüve K, Jaspers P, Kangasjärvi J, Kollist H. (2007) A novel device detects a rapid ozone-induced transient stomatal closure in intact Arabidopsis and its absence in abi2 mutant. Physiol Plant 129: 796–803 [Google Scholar]
- Koornneef M, Reuling G, Karssen CM. (1984) The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant 61: 377–383 [Google Scholar]
- Kudla J, Batistič O, Hashimoto K. (2010) Calcium signals: the lead currency of plant information processing. Plant Cell 22: 541–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Murata Y, Baizabal-Aguirre VM, Merrill J, Wang M, Kemper A, Hawke SD, Tallman G, Schroeder JI. (2001) Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis. Plant Physiol 127: 473–485 [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI. (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanemets K, Wang YF, Lindgren O, Wu J, Nishimura N, Lee S, Caddell D, Merilo E, Brosche M, Kilk K, et al. (2013) Mutations in the SLAC1 anion channel slow stomatal opening and severely reduce K+ uptake channel activity via enhanced cytosolic [Ca2+] and increased Ca2+ sensitivity of K+ uptake channels. New Phytol 197: 88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan WZ, Kim BG, Li LG, Cheong YH, Pandey GK, Lu GH, Buchanan BB, Luan S. (2007) A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. Proc Natl Acad Sci USA 104: 15959–15964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S. (2009) A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA 106: 21419–21424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenko V, Konrad KR, Dietrich P, Roelfsema MR, Hedrich R. (2005) Cytosolic abscisic acid activates guard cell anion channels without preceding Ca2+ signals. Proc Natl Acad Sci USA 102: 4203–4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S-Y, Wu W-H. (2007) AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Mol Biol 65: 511–518 [DOI] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- MacRobbie EAC. (2000) ABA activates multiple Ca2+ fluxes in stomatal guard cells, triggering vacuolar K+ (Rb+) release. Proc Natl Acad Sci USA 97: 12361–12368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. (1991) Partial inhibition of ABA-induced stomatal closure by calcium-channel blockers. Proc R Soc Lond, Ser B Biol Sci 243: 195–201 [Google Scholar]
- Merilo E, Laanemets K, Hu H, Xue S, Jakobson L, Tulva I, Gonzalez-Guzman M, Rodriguez PL, Schroeder JI, Broschè M, et al. (May 28, 2013) PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness- and CO2-induced stomatal regulation. Plant Physiol http://dx.doi.org/10.1104/pp.113.220608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Mumm P, Imes D, Endler A, Weder B, Al-Rasheid KA, Geiger D, Marten I, Martinoia E, Hedrich R. (2010) AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J 63: 1054–1062 [DOI] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang Y, Munemasa S, Wang Y-F, Andreoli S, Tiriac H, Alonso JM, Harper JF, Ecker JR, et al. (2006) CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol 4: e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y. (2011) The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiol 155: 553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli A-C, Merlot S, Vavasseur A, Fenzi F, Giraudat J. (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K. (2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486 [DOI] [PubMed] [Google Scholar]
- Nishimura N, Yoshida T, Murayama M, Asami T, Shinozaki K, Hirayama T. (2004) Isolation and characterization of novel mutants affecting the abscisic acid sensitivity of Arabidopsis germination and seedling growth. Plant Cell Physiol 45: 1485–1499 [DOI] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. (1997) Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9: 409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z-M, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Gonugunta VK, Christmann A, Grill E. (2010) ABA perception and signalling. Trends Plant Sci 15: 395–401 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hanstein S, Felle HH, Hedrich R. (2002) CO2 provides an intermediate link in the red light response of guard cells. Plant J 32: 65–75 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R. (2005) In the light of stomatal opening: new insights into ‘the Watergate’. New Phytol 167: 665–691 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Levchenko V, Hedrich R. (2004) ABA depolarizes guard cells in intact plants, through a transient activation of R- and S-type anion channels. Plant J 37: 578–588 [DOI] [PubMed] [Google Scholar]
- Sanders D, Hopgood M, Jennings IR. (1989) Kinetic response of H+-coupled transport to extracellular pH: critical role of cytosolic pH as a regulator. J Membr Biol 108: 253–261 [Google Scholar]
- Sasaki T, Mori IC, Furuichi T, Munemasa S, Toyooka K, Matsuoka K, Murata Y, Yamamoto Y. (2010) Closing plant stomata requires a homolog of an aluminum-activated malate transporter. Plant Cell Physiol 51: 354–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer S, Maierhofer T, Al-Rasheid KA, Geiger D, Hedrich R. (2012) Multiple calcium-dependent kinases modulate ABA-activated guard cell anion channels. Mol Plant 5: 1409–1412 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Raschke K, Neher E. (1987) Voltage dependence of K+ channels in guard-cell protoplasts. Proc Natl Acad Sci USA 84: 4108–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Hedrich R, Fernandez JM. (1984) Potassium-selective single channels in guard cell protoplasts of Vicia faba. Nature 312: 361–362 [Google Scholar]
- Schroeder JI, Hagiwara S. (1989) Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338: 427–430 [Google Scholar]
- Schwartz A. (1985) Role of Ca2+ and EGTA on stomatal movements in Commelina communis L. Plant Physiol 79: 1003–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki KI, Doi M, Assmann SM, Kinoshita T. (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58: 219–247 [DOI] [PubMed] [Google Scholar]
- Siegel RS, Xue S, Murata Y, Yang Y, Nishimura N, Wang A, Schroeder JI. (2009) Calcium elevation-dependent and attenuated resting calcium-dependent abscisic acid induction of stomatal closure and abscisic acid-induced enhancement of calcium sensitivities of S-type anion and inward-rectifying K+ channels in Arabidopsis guard cells. Plant J 59: 207–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichandra C, Gu D, Hu HC, Davanture M, Lee S, Djaoui M, Valot B, Zivy M, Leung J, Merlot S, et al. (2009) Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett 583: 2982–2986 [DOI] [PubMed] [Google Scholar]
- Staxén I, Pical C, Montgomery LT, Gray JE, Hetherington AM, McAinsh MR. (1999) Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc Natl Acad Sci USA 96: 1779–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K. (2009) Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci USA 106: 17588–17593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmäki A, Brosché M, Moldau H, Desikan R, et al. (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Puzõrjova I, Brosché M, Valk E, Lepiku M, Moldau H, Pechter P, Wang Y-S, Lindgren O, Salojärvi J, et al. (2010) Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J 62: 442–453 [DOI] [PubMed] [Google Scholar]
- Vlad F, Rubio S, Rodrigues A, Sirichandra C, Belin C, Robert N, Leung J, Rodriguez PL, Laurière C, Merlot S. (2009) Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell 21: 3170–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Papanatsiou M, Eisenach C, Karnik R, Williams M, Hills A, Lew VL, Blatt MR. (2012) Systems dynamic modeling of a guard cell Cl− channel mutant uncovers an emergent homeostatic network regulating stomatal transpiration. Plant Physiol 160: 1956–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AAR, McAinsh MR, Mansfield TA, Hetherington AM. (1996) Carbon dioxide induces increases in guard cell cytosolic free calcium. Plant J 9: 297–304 [Google Scholar]
- Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser IDC, Langeberg LK, Sheng M, Scott JD. (1999) Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science 285: 93–96 [DOI] [PubMed] [Google Scholar]
- Xue S, Hu H, Ries A, Merilo E, Kollist H, Schroeder JI. (2011) Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J 30: 1645–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K. (2002) ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol 43: 1473–1483 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K. (2006) The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J Biol Chem 281: 5310–5318 [DOI] [PubMed] [Google Scholar]
- Young JJ, Mehta S, Israelsson M, Godoski J, Grill E, Schroeder JI. (2006) CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proc Natl Acad Sci USA 103: 7506–7511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X-B, Wulfsen I, Utku E, Sausbier U, Sausbier M, Wieland T, Ruth P, Korth M. (2010) Dual role of protein kinase C on BK channel regulation. Proc Natl Acad Sci USA 107: 8005–8010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S-Y, Yu X-C, Wang X-J, Zhao R, Li Y, Fan R-C, Shang Y, Du S-Y, Wang X-F, Wu F-Q, et al. (2007) Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 19: 3019–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JJ, Wei FJ, Wang C, Wu JJ, Ratnasekera D, Liu WX, Wu WH. (2010) Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol 154: 1232–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]