Abstract
Experimental and modeling breakthroughs will help establish the genetic identities of plant calcium channels.
The transient elevation of free calcium ion concentration in the cytosol and organelles of plant cells is a well-established phenomenon. As discussed in this Focus issue on calcium signaling, these elevations occur in response to a range of biotic and abiotic stimuli (McAinsh and Pittman, 2009; Dodd et al., 2010). Recently, transcriptomic analyses coupled with calcium determinations have demonstrated that a specific stimulus (membrane voltage or ozone) can cause a specific free calcium ion transient, leading to a specific transcriptional response (Whalley et al., 2011; Short et al., 2012; Whalley and Knight, 2013). It is also clear that free cytosolic Ca2+ ([Ca2+]cyt) has a role to play in regulating the polar growth of root hairs and pollen tubes (Monshausen et al., 2008; Michard et al., 2011). What has yet to be determined fully is the mechanistic basis of these calcium signaling “signatures” and the oscillations of [Ca2+]cyt that occur during polar growth.
There would be sufficient energy in transmembrane Ca2+ gradients to drive passive Ca2+ flux into the cytosol from the apoplast or internal stores (such as the vacuole or endoplasmic reticulum). Pharmacological intervention has implicated such Ca2+ influx in elevating free cytosolic [Ca2+]cyt in a variety of Ca2+ signatures (response to environment, stress, hormones, immunity) and during polar growth. With the prediction of passive Ca2+ transport comes the hypothesis that Ca2+-permeable channel proteins in plasma membranes and endomembranes would be responsible for [Ca2+]cyt elevation. This, in turn, has driven the electrophysiological characterization of these channel proteins and the search for the encoding genes (for review, see Demidchik and Maathuis, 2007; Wheeler and Brownlee, 2008; Dietrich et al., 2010; Dodd et al., 2010; Jammes et al., 2011; Hedrich, 2012; Kurusu et al., 2013). The electrophysiological approach has seen a relatively small band of laboratories overcome the technical barriers presented by plant cells to reveal distribution and regulatory properties of plasma membrane and endomembrane channels. The search for genes has been stimulated, but also to some extent stymied, by the advent of genome sequencing. For while plant genomes harbor two families of putative channel subunit genes that bear some relation to animal counterparts (the cyclic nucleotide-gated channels [CNGCs] and glutamate receptor-like [GLR]), they reveal no compelling candidates for the majority of channels or ionotropic receptors discovered by electrophysiology. For example, at this point, genes coding for the receptor-like channel activated by extracellular nucleotides and for the endomembrane inositol 1,4,5-trisphosphate- and cyclic ADP ribose-activated channels remain unknown (Allen et al., 1995; Demidchik et al., 2011). With a limited pharmacological arsenal with which to capture channel proteins (despite some early advances with a verapamil-binding protein from maize [Zea mays]; Harvey et al., 1989) and difficulties with the characterization of recombinant channel proteins in heterologous expression systems, the Ca2+ channel field has understandably lagged behind that of K+ and Cl− channels and other transporters in terms of gene identification and characterization of the gene product (Hedrich, 2012).
There have been several excellent reviews recently that have summarized gene families, evolution, channel activity, and possible functions (Demidchik and Maathuis, 2007; Wheeler and Brownlee, 2008; Dietrich et al., 2010; Dodd et al., 2010; Matzke et al., 2010; Jammes et al., 2011; Peiter, 2011; Hedrich, 2012; Stael et al., 2012; Kurusu et al., 2013). Beyond a brief introduction to the various channels for the benefit of readers new to the area, this Update will focus on the latest breakthroughs not addressed by those reviews, their implications, and the renewed efforts now evident in Ca2+ channel research to find those genes. Before going on, a note on terminology. The relevant channels characterized in plant membranes so far do a really good job of transporting K+ and other cations in addition to Ca2+. The permeability ratio of Ca2+ to K+ can be as low as 0.5 or as high as 15 (Véry and Davies, 2000; Demidchik and Maathuis, 2007), and this has led many researchers to use terms such as “Ca2+-permeable (nonselective) cation channel.” Here, the term “calcium channel” will be used liberally, because in the signaling context of this Focus, even a spark or puff of Ca2+ emanating from these proteins could be enough to trigger the next event. The active transporters involved in terminating Ca2+ signals will not be addressed.
THIS MUCH WE KNOW
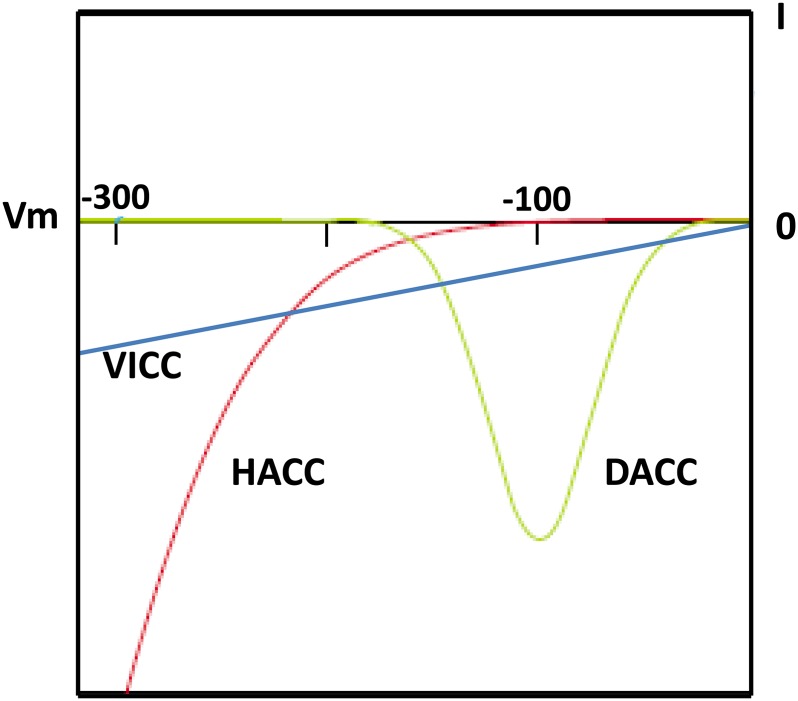
Biophysical studies have clearly demonstrated the existence of voltage-regulated and voltage-independent Ca2+ channels in the plant plasma membrane from a variety of cell types, most notably guard and root cells. Hyperpolarization-activated calcium channels (HACCs) can coexist with depolarization-activated calcium channels (DACCs) and voltage-independent calcium channels (VICCs). As can be inferred from the current-voltage relationships in Figure 1, coexistence means that a cell would be competent for Ca2+ influx across a very wide voltage range. Therefore, regulation of the transporters generating or recovering a given voltage (primarily H+-ATPases, K+, and anion channels) and the possibility of channel sequestration in specific membrane areas are of paramount importance in generating specific changes in [Ca2+]cyt (Miedema et al., 2001; Chen et al., 2012). HACCs operate in guard cell closure and polar growth and are downstream of light, hormones (notably abscisic acid in guard cells; Hamilton et al., 2000), and elicitors. Modeling studies point to the ability of HACCs to stabilize guard cell [Ca2+]cyt and stomatal aperture (Chen et al., 2012; Hills et al., 2012). Whether regulators act directly or indirectly is often poorly understood, but they are responsive to (for example) actin, [Ca2+]cyt, cAMP/cyclic GMP (cGMP), heterotrimeric G proteins, phosphorylation, and reactive oxygen species (ROS; for review, see Demidchik and Maathuis, 2007; Dodd et al., 2010; Jammes et al., 2011). Defining the exact channel responding to signaling intermediates and so to specific receptors or sensors is one the great challenges facing the field.
Figure 1.
Coresidence of different Ca2+ channels in the plasma membrane affords variable Ca2+ influx from the apoplast, controlled by membrane voltage. The schematic illustrates the effect of plasma membrane voltage (Vm) on net Ca2+ current measured using the “whole cell” patch-clamp configuration (Miedema et al., 2001; Demidchik and Maathuis, 2007). Negative current is generated by Ca2+ entry into the cytosol. DACC-mediated current is maximal at less negative plasma membrane voltage than HACC, while VICC-mediated current is effectively ohmic. Together, these channels would have the potential to vary [Ca2+]cyt as plasma membrane voltage changes.
DACC activity is quickly lost in patch-clamp recordings and may not always be stabilized by microtubule disruption. DACCs have received scant experimental attention, and the inability to resolve depolarization-activated [Ca2+]cyt elevation has brought their significance into question. VICCs are a notable feature of the root cell plasma membrane, where their potential ability to let Na+ into cells is of significant agronomic importance (Maathuis and Sanders, 2001; Demidchik and Maathuis, 2007). Finally, mechanosensitive (MS) Ca2+ channels have been characterized in leaf, root, and pollen plasma membrane (for review, see Hedrich, 2012; Kurusu et al., 2013), where they may be responsible for reporting turgor and topography. Despite the technical challenges, endomembrane Ca2+ channels have been characterized at the vacuole, endoplasmic reticulum, chloroplast, and nucleus (Enz et al., 1993; Grygorczyk and Grygorczyk, 1998; for review, see Matzke et al., 2010; Peiter, 2011; Hedrich, 2012; Stael et al., 2012).
Attention has focused on the GLR and CNGC gene families as the most likely sources of genes encoding HACCs, DACCs, and VICCs. Members of both of these families (each comprising 20 members in Arabidopsis [Arabidopsis thaliana]) are predicted to form conventional transmembrane-spanning proteins that would function as channels when assembled into tetramers. Analysis of mutants has implicated members of both families in calcium signaling and [Ca2+]cyt-dependent polar growth (for review, see Dietrich et al., 2010; Dodd et al., 2010; Jammes et al., 2011; Hedrich, 2012). The rice (Oryza sativa) HKT2.4 potassium transporter was proposed to be a plasma membrane Ca2+-permeable nonselective cation channel on the basis of expression studies in Xenopus laevis oocytes (Lan et al., 2010), but recently, Sassi et al. (2012) reported contradictory data. Recent reports have shed light on two gene families (MSL, for MscS-like, and MCA, for Mid1-complementing activity) encoding transmembrane proteins with potential for MS Ca2+ channel activity (for review, see Kurusu et al., 2013). At the vacuole, TWO PORE CHANNEL1 (TPC1) is the slow vacuolar (SV) Ca2+-release channel in Arabidopsis (Peiter et al., 2005), but its role in signaling remains obscure. This is Ca2+ channel research: find the gene that encodes the channel, find what the impact is on Ca2+ handling, and find a phenotype.
GLRs: MORE EVIDENCE FOR CALCIUM CHANNELS
The tobacco (Nicotiana tabacum) pollen tube contains a d-Ser-activated Ca2+ influx channel in the apical plasma membrane that is firmly implicated in growth control (Michard et al., 2011). Leading on from this, the Arabidopsis Atglr1.2 mutant was found to have both aberrant apical [Ca2+]cyt oscillations and morphology. The slower pollen tube growth rate of the Atglr3.7 mutant is suggestive of a role in [Ca2+]cyt regulation (Michard et al., 2011). Analysis of function by electrophysiology has shown that the Atglr3.4 mutant has impaired plasma membrane amino acid-regulated Ca2+ channel activity (Stephens et al., 2008), but the acid test is whether a recombinant protein can reconstitute that activity. Otherwise, the phenotype could be just a pleiotropic effect of the mutation. Heterologous expression of GLRs has proved to be problematic, but replacing the pore region of an animal ionotropic Glu receptor with that of AtGLR1.1 or AtGLR1.4 and then expressing the chimera in X. laevis oocytes delivered compelling evidence for Ca2+ transport by plant GLRs, despite the dissimilarity of their pore regions to those of the ionotropic Glu receptors (Tapken and Hollmann, 2008). Expression of AtGLR3.7 and AtGLR3.4 (but not AtGLR2.1) in X. laevis oocytes resulted in nonendogenous Ca2+ influx currents, but these were not activated by amino acids (Roy et al., 2008). A recent breakthrough from the Spalding laboratory involved successful expression of AtGLR3.4 in human embryonic kidney (HEK) cells. GFP tagging clearly showed that AtGLR3.4 localized in the plasma membrane, while electrophysiological analysis demonstrated a high level of Ca2+ permeability facilitated by AtGLR3.4 (Vincill et al., 2012). This Ca2+ influx route was activated by the same amino acids (Asn, Ser, Gly) that caused AtGLR3.4-dependent [Ca2+]cyt elevation in HEK cells. Moreover, a previous study on Arabidopsis hypocotyls had shown that Asn- and Ser-dependent Ca2+ influx current required AtGLR3.4 (Stephens et al., 2008). So why didn’t amino acid activation of AtGLR3.4 occur in Roy’s study using oocytes (Roy et al., 2008)? Is this a protein-processing problem or an indication of the host cell’s capacity to provide interacting proteins or regulators?
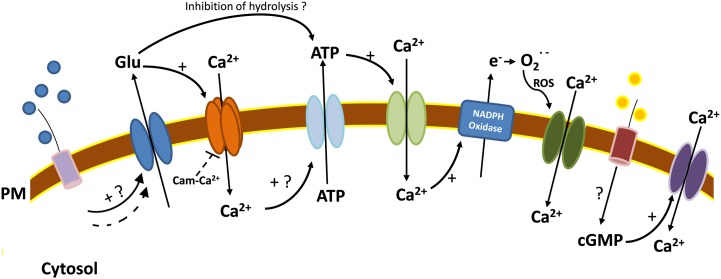
This year, Vincill et al. (2013) identified AtGLR3.2 as an interacting protein for AtGLR3.4, but one with as yet no clear effect on the latter’s channel activity in HEK cells. Although both of these plasma membrane proteins reside at the sieve plate in roots, their activities ultimately manifest in the pericycle with a regulatory effect on the production of lateral root primordia (Vincill et al., 2013). The finding that AtGLR3.4 can reside in the chloroplast (Teardo et al., 2011), although not confirmed by Vincill et al. (2012), raises further opportunities and challenges for the GLR community. The question remains whether the concentrations of amino acids used in assays would occur in planta (Vincill et al., 2012); many studies use concentrations of ligand higher than would be expected outside of cells. Tomato (Solanum lycopersicum) may be an interesting study area in this respect, as it harbors 15 GLR genes and its fruits contain high Glu levels (Aouini et al., 2012). Finally, Kwaaitaal et al. (2011) have recently implicated GLRs in the Arabidopsis [Ca2+]cyt signature caused by the microbial epitopes flg22, elf18, and chitin. The [Ca2+]cyt increase was perturbed by antagonists of Glu binding to animal ionotropic Glu receptors and modulated by exogenous Glu. Pharmacological block also implicates GLRs in the response of tobacco to cryptogein, which also caused Glu efflux (Vatsa et al., 2011). It will be interesting to see whether GLR mutants are defective in this important component of immunity and how they relate to extracellular nucleotide-induced [Ca2+]cyt signaling. In Arabidopsis roots, exogenous Glu causes an accumulation of extracellular ATP (Dark et al., 2011) that, in turn, can activate plasma membrane Ca2+ influx channels via ROS production by a NADPH oxidase (Demidchik et al., 2009). A schematic of this possible relationship is shown in Figure 2.
Figure 2.
Microbial epitopes could activate a relay of plasma membrane (PM) Ca2+ channels. Binding of microbial epitopes to cognate receptors could cause Glu efflux, which can then activate Ca2+ influx across the plasma membrane, probably mediated by GLRs (Kwaaitaal et al., 2011; Vatsa et al., 2011). Extracellular Glu stimulates extracellular ATP accumulation (Dark et al., 2011), but it is unknown whether this is by the activation of efflux (by an unknown transport pathway) or the inhibition of hydrolysis. Extracellular nucleotides trigger Ca2+-stimulated NADPH oxidase activity, possibly by binding a receptor-like Ca2+ channel (Demidchik et al., 2009, 2011). The resultant ROS activate a Ca2+ channel of unknown molecular identity that could amplify the signal (Demidchik et al., 2009). Binding of extracellular peptides to their cognate AtPepR1 receptor in damage-associated molecular pattern signaling activates AtCNGC2, with the receptor itself possibly generating cGMP (Qi et al., 2010; Ma et al., 2012).
CNGCs: FROM RECEPTOR TO CHANNEL, A NEW ROLE IN THERMOSENSING AND ADVANCES IN UNDERSTANDING STRUCTURE-FUNCTION RELATIONSHIPS
Although the mechanisms of cyclic nucleotide elevation remain poorly understood, CNGCs as downstream targets have been studied in the greatest detail in Arabidopsis. AtCNGC2 forms a cyclic nucleotide-regulated HACC when heterologously expressed, and loss of function results in loss of a native plasma membrane cyclic nucleotide-regulated HACC (for review, see Dietrich et al., 2010). AtCNGC2 has now been shown to be a component in the perception of peptides generated as damage-associated molecular patterns of the immune response. Binding of an extracellular peptide to its cognate AtPepR1 receptor elevates cGMP levels (possibly by the receptor’s guanylyl cyclase domain), which then causes AtCNGC2-dependent elevation of [Ca2+]cyt (Qi et al., 2010; Ma et al., 2012). This is summarized in Figure 2. Analysis of mutants has implicated CNGCs in immunity, senescence, salt and heavy metal stress responses, and (polar) growth (for review, see Dietrich et al., 2010; Jammes et al., 2011; Hedrich, 2012). In Arabidopsis, AtCNGC7 has now been found localized to the plasma membrane at the flank of the pollen tube (Tunc-Ozdemir et al., 2013a). The Atcngc7/8 double mutant is male sterile, with mutant pollen bursting at germination (Tunc-Ozdemir et al., 2013a), suggesting that AtCNGC7 and AtCNGC8 could function as Ca2+ channels to coordinate pollen growth. AtCNGC16 is also implicated in pollen tube growth (Tunc-Ozdemir et al., 2013b). A role in nutritional stress is now likely, as boron deficiency increases the expression of AtCNGC19 in roots (Quiles-Pando et al., 2013). This raises an interesting possibility of vacuolar involvement in boron stress, as AtCNGC19 and AtCNGC20 have now been found localized at the tonoplast (Quiles-Pando et al., 2013; Yuen and Christopher, 2013).
The recent findings that CNGCs participate in heat stress signaling in Arabidopsis and Physcomitrella patens provide a mechanistic basis for the well-documented heat-induced [Ca2+]cyt elevation (Finka et al., 2012; Gao et al., 2012). Heat stress raises cAMP in Arabidopsis and activates a root cell plasma membrane HACC that is also activated by membrane-permeant cAMP (Gao et al., 2012). This HACC is absent from the Atcngc6 mutant, leading to aberrant heat shock protein expression and lowered thermotolerance. In contrast, the Atcngc2 mutant and P. patens cngcb mutant (orthologous genes) were found to be hyperthermosensitive (Finka et al., 2012). Loss of CNGCB activity in moss plasma membrane caused the loss of one of three heat-activated Ca2+ channels and increased the likelihood of the other two opening. Accordingly, the heat-induced [Ca2+]cyt signature was altered, and it is envisaged that these CNGCs act as heteromers in thermosensing (Finka et al., 2012). This year, Tunc-Ozdemir et al. (2013b) demonstrated that heat increases cGMP levels and that the Atcngc16 mutant has heat- and drought-sensitive pollen germination and pollen tube outgrowth. Use of such mutants will help elucidate the role of [Ca2+]cyt in thermosensing. In addition to heat, phytohormones also elevate cGMP, resulting in a suite of phosphorylated proteins (Isner et al., 2012). More proteins responsible for cAMP/cGMP elevation now need to be identified.
One of the many puzzles presented by the CNGCs is how they respond to cAMP/cGMP, an issue relevant to salinity stress and K+ nutrition. Although several papers report channel activation in native membranes (for review, see Dietrich et al., 2010), Maathuis and Sanders (2001) found inhibition by cAMP and cGMP of both Na+ influx into Arabidopsis roots (tracer) and Na+ influx into root protoplasts (patch clamp). AtCNGC3 has since been implicated in Na+ transport into roots (Gobert et al., 2006). cAMP/cGMP could play a further role in salinity tolerance by regulating the activity of the plasma membrane SOS1 Na+ extruder through a putative cyclic nucleotide-binding domain (Oh et al., 2007). More recently, Caballero et al. (2012) found that a membrane-permeant cyclic nucleotide inhibited K+ uptake by roots of the Atakt1/Athak5 mutant that lacks key K+ uptake transporters and suggested that a CNGC could provide a compensatory uptake pathway. Structural studies on CNGCs are now gathering pace, with this year’s finding that Phe-to-Trp substitutions between the cytosolic C-terminal cyclic nucleotide-binding domain and the calmodulin (CaM)-binding site prevent mutant rescue by AtCNGC7 (Tunc-Ozdemir et al., 2013a). Previous studies (for review, see Dietrich et al., 2010) have established Ca2+-dependent CaM binding of the CNGC C terminus, and the current model is that [Ca2+]cyt increases promote CaM binding with subsequent displacement of the cyclic nucleotide, resulting in channel closure. Fischer et al. (2013) showed that AtCNGC20 can bind all five Arabidopsis CaM isoforms (but not CaM-like proteins) and have demonstrated the existence of a conserved Ile-Gln motif that constitutes an additional CaM-binding site. Intriguingly, in contrast to Yuen and Christopher (2013), AtCNGC20 localized to the plasma membrane in the study by Fischer et al. (2013).
MS CHANNELS: Ca2+ PERMEABILITY, A NEW GENE, AND A POTENTIAL ROLE IN [Ca2+]cyt OSCILLATIONS
Ca2+ channels are central to mechanosensing in plants, which is key to growth and development as well as the response to changes in environmental conditions. Of the 10 AtMSL proteins that were identified through similarity to bacterial MS channels, AtMSL2 and AtMSL3 are plastidic, and although channel activity has not been determined, they are involved in osmoregulation and plastid division (Veley et al., 2012; for review, see Stael et al., 2012; Kurusu et al., 2013). Electrophysiological characterization of single Arabidopsis mutants of MSL9 and MSL10 suggested that these are root plasma membrane MS channels with some permeability for Ca2+ (Haswell et al., 2008). Further analysis established that heterologously expressed AtMSL10 can provide a MS channel activity with a moderate preference for anions, but Ca2+ selectivity was not addressed (Maksaev and Haswell, 2012). AtMSL9 and AtMSL10 are now depicted in the literature as anion channels (Kurusu et al., 2013), but it is premature to dismiss them as Ca2+-permeable channels. In the original study by Haswell et al. (2008), current was still generated when external Ca2+ was lowered from 50 to 1 mm in the presence of a K+ channel blocker. This would still provide a good driving force for Ca2+ influx, and Ca2+ was not removed completely to see if that abolished current. While AtMSL9 and AtMSL10 primarily but not exclusively localize to the plasma membrane of root cells, their roles in [Ca2+]cyt signaling remain to be established, and single mutants, the double mutant msl9-1;msl10-1, and even the quintuple mutant msl4-1;msl5-2;msl6-1;msl9-1;msl10-1 have shown no clear phenotype (Haswell et al., 2008).
MCAs were first identified in Arabidopsis because of their ability to complement the Mid1 yeast (Saccharomyces cerevisiae) mutant (Nakagawa et al., 2007). MCAs comprise two transmembrane domains, contain an EF hand, but show no significant similarity to known ion channels (for review, see Kurusu et al., 2013). All MCAs characterized thus far have localized to the plasma membrane and mediate Ca2+ uptake, with the N-terminal and EF hand regions as necessary structures for transport (for review, see Kurusu et al., 2013). Electrophysiological analyses of AtMCA1 expressed in X. laevis oocytes demonstrated that AtMCA1 can provide a MS cation channel activity of small conductance (34 pS; Furuichi et al., 2012). While a MCA gene family typically holds two homologs in dicots, only one homolog could be found in the Poaceae (Kurusu et al., 2013). In Arabidopsis, MCA1 and MCA2 seem to play functionally different roles, with mca1-null showing an impaired touch response in the primary root while mca2-null showed lower Ca2+ uptake in the roots (Nakagawa et al., 2007; Kurusu et al., 2013). A role for MCA1 in Ca2+ influx in response to mechanical stimulation at the plasma membrane was also supported by studies reporting the effect of hypoosmotic shock on rice OsMCA1 suppression lines and tobacco overexpressor cells. Induced transcript levels during the G1 phase suggested an additional role for NtMCA2 and AtMCA1 (for review, see Kurusu et al., 2013) during the cell cycle. Studies on plant MS channels now need to extend to piezo proteins; these are animal and insect MS channel subunits, and a single possible gene has been identified in Arabidopsis (At2g48060; Hedrich, 2012). These together with the MSL and MCA proteins are the clear candidates for testing a new model of [Ca2+]cyt oscillations, in which Ca2+ released from periplasmic arabinogalactan glycoproteins enters the cytosol through plasma membrane MS Ca2+ influx channels (Lamport and Várnai, 2013).
ROS-RESPONSIVE CHANNELS: SKOR AND ANNEXINS
ROS continue to enjoy significant research interest due to their participation in the life and death of plants, ranging from germination and growth to immunity and death. Plasma membrane Ca2+ channels can be responsive to ROS, depending on the cell type, which ROS is present, and at which membrane face (Demidchik et al., 2003, 2007). Only recently have these channels started to be linked with genes. The Shaker-like STELAR K+ OUTWARD RECTIFIER (SKOR) is a member of the K+ channel superfamily but has an experimentally determined Ca2+ permeability that could result in Ca2+ influx at the plasma membrane (Gaymard et al., 1998). The Atskor mutant accumulates less Ca2+ in its leaves than the wild type, consistent with SKOR-dependent retrieval of Ca2+ from the transpiration stream in the roots (Gaymard et al., 1998). K+ transport by the heterologously expressed AtSKOR responds positively to exogenous hydrogen peroxide, and although Ca2+ permeation was not tested, such results beg the question of whether AtSKOR is acting as a de facto ROS-activated calcium channel in vivo (Garcia-Mata et al., 2010). A well-executed study on wheat (Triticum aestivum) root protoplasts that combined patch clamping with flux determination delivered “no consistent indication” of Ca2+ permeation through the K+ efflux pathway (Gilliham et al., 2006). It may now be timely to reexamine this question, perhaps by combining patch clamp with Ca2+ imaging (Gradogna et al., 2009) and by exploiting the ability to mutate AtSKOR for functional analysis (Porée et al., 2005).
Plant annexins have been mooted to form Ca2+ channels for some time, but there is now firm evidence from in vitro and in vivo studies that they have some form of transport capacity (Hofmann et al., 2000; Laohavisit et al., 2009, 2010, 2012; Laohavisit and Davies, 2011a). Plant annexins are small (32–36 kD) amphipathic proteins that are capable of Ca2+-dependent and -independent binding to anionic phospholipids. They are present throughout the plant kingdom, and an excellent recent review by Clark et al. (2012) charts their phylogeny from green algal ancestors onward. Annexins are seemingly ubiquitously distributed throughout the angiosperm body, from pollen and egg cells to the mature vegetative organs (for review, see Laohavisit and Davies 2011b; Clark et al., 2012). They also appear free to be distributed throughout the plant from their site of production via phloem sap (Laohavisit and Davies 2011b; Clark et al., 2012). In stark contrast to conventional Ca2+ channels, which will be transported from the Golgi to reside probably exclusively in specific membranes, plant annexins appear able to occupy more than one cellular location simultaneously and may also occupy lipid rafts (for review, see Laohavisit and Davies, 2011b; Clark et al., 2012). This provides scope for rapid, stimulus-driven recruitment to a membrane region not necessarily reliant on vesicle delivery.
Purified maize annexins conduct Ca2+ across planar lipid bilayers (PLB), changing from being voltage independent to hyperpolarization activated (Fig. 1), when malondialdehyde is incorporated into the PLB to mimic lipid peroxidation (Laohavisit et al., 2009, 2010). Thus, in vivo, interaction with malondialdehyde (which forms in membranes during stress responses known to involve ROS and [Ca2+]cyt elevation; for review, see Laohavisit et al., 2010) could restrict annexin-mediated Ca2+ transport to more negative voltages, and this may have an impact on a Ca2+ signature. Greater progress on in vivo function has been made with Arabidopsis ANNEXIN1 (AtANN1), one of eight in this plant. In addition to a presence in the cytosol, proteomic, immunolocalization, and GFP studies have identified AtANN1 at the plasma membrane, endoplasmic reticulum, vacuole, mitochondria, chloroplast, and cell wall (for review, see Laohavisit and Davies, 2011b). Its localization and expression in roots correspond well with the presence of a plasma membrane Ca2+ conductance that is involved in root cell elongation and is activated by hyperpolarization and extracellular hydroxyl radicals (OH•; Demidchik et al., 2003; Foreman et al., 2003; Laohavisit et al., 2012). Accordingly, the Atann1 knockout mutant was found to lack this Ca2+ channel in epidermal and root hair apical plasma membrane, with mutant root hairs found to be shorter than wild-type root hairs (Laohavisit et al., 2012). Mutant root hairs still retained the constitutive (i.e. not ROS-activated) HACC first described by Véry and Davies (2000), showing that this channel is encoded by a different gene and helping to explain why hairs could still grow.
Annexins appear as potentially multifunctional proteins in vitro, so their lipid- and actin-binding properties could mean that they are involved in regulating the trafficking of a more conventional Ca2+ channel to a membrane, and care needs to be taken in interpreting the loss of channel activity in an annexin mutant. However, recombinant AtANN1 formed an OH•- and hyperpolarization-activated Ca2+ conductance in PLB that very strongly resembled the OH•-activated Ca2+ channel conductance of the wild-type plasma membrane (Laohavisit et al., 2012). Despite conservation of the salt bridges thought to be involved in selectivity, the estimated permeability ratio of calcium to potassium PCa:PK of 0.6 is much lower than that of animal annexins (Laohavisit et al., 2012), and the mechanism through which AtANN1 forms a transport route now needs to be elucidated. Animal counterparts form transport routes in vitro by destabilizing or inserting into bilayers, promoted by voltage, pH, GTP, and ROS, with annexin concentration determining voltage dependence (for review, see Laohavisit and Davies, 2011b). The structural differences between plant and animal annexins that could be relevant to functional diversification have been reviewed recently by Clark et al. (2012). Posttranslational modification may alter membrane association and the S-glutathionylation of AtANN1 severely limits membrane interaction (Konopka-Postupolska et al., 2009), so this could help restrict OH•-activated Ca2+ influx by this annexin. Interacting protein partners have been considered recently by Laohavisit et al. (2011b) and Clark et al. (2012). The position of an annexin is likely to be central to its contribution to Ca2+ signaling. How plant annexins can become extracellular remains to be determined (Laohavisit et al., 2011b; Clark et al., 2012), but their ability to increase [Ca2+]cyt by acting at the apoplast face of the plasma membrane has now been demonstrated (Laohavisit et al., 2009). The maize annexins found to form a Ca2+ conductance in PLB were also capable of transiently increasing the [Ca2+]cyt of Arabidopsis protoplasts, but whether this was by directly forming a Ca2+ influx pathway or through the activation of other channels was not determined (Laohavisit et al., 2009).
ENDOMEMBRANES: TPC1 REMAINS CONTROVERSIAL, AND NEW INSIGHT FROM MODELING
After years of controversy on whether the SV channel was Ca2+ permeable, the debate now is about its function. Peiter et al., (2005) showed unequivocally that SV current magnitude varied with the expression of TPC1 in Arabidopsis, while Gradogna et al. (2009) combined patch clamping with Ca2+ imaging to show TPC1-dependent Ca2+ transport. Hamada et al. (2012) have found that rice OsTPC1 forms a voltage-regulated Ca2+ conductance in the HEK cell plasma membrane. A Ca2+-binding site that confers sensitivity to vacuolar Ca2+ has now been identified, and the finding of an N-terminal di-Leu motif (EDPLI) that directs AtTPC1 to the tonoplast rather than the plasma membrane helps resolve the debate about the localization of that protein (Peiter, 2011; Hedrich, 2012; Larisch et al., 2012). In contrast, immunoblotting of rice plasma membrane still suggests that OsTPC1 resides there, and further analysis reveals a role in [Ca2+]cyt response to a fungal xylanase linking to phytoalexin synthesis (Hamada et al., 2012). Given the large magnitude of SV currents (which should permit vacuolar Ca2+ efflux), the lack of effect of AtTPC1 loss of function on [Ca2+]cyt in a wide range of signaling scenarios has been surprising (Ranf et al., 2008; Islam et al., 2010). However, whole seedling or organ studies may mask cell-specific responses; for example, the Atann1 root [Ca2+]cyt response to ROS was identical to that in the wild type, but genotypic differences were apparent at the epidermis (Laohavisit et al., 2012). Perhaps the finding that polyunsaturated fatty acids drastically inhibit SV activity (Gutla et al., 2012) may help explain why Attpc1 presents as the wild type in some [Ca2+]cyt studies. The polyunsaturated fatty acid α-linoleic acid is a potent SV inhibitor and is synthesized during wounding and pathogen attack (Gutla et al., 2012). Is experimental handling in signaling studies enough to trigger polyunsaturated fatty acid synthesis and inhibit the SV, leaving Attpc1 to phenocopy the wild type?
The expression of AtTPC1 is greater in guard cells than the mesophyll, and patch clamping has shown that SV characteristics differ between these two cell types, probably due to activities of regulatory proteins or cell-specific posttranslational modifications (Rienmüller et al., 2010). Analysis of the impact of loss of AtTPC1 function on guard cell [Ca2+]cyt dynamics could implicate AtTPC1 only in the priming of the Ca2+ sensitivity of the plasma membrane S-type anion channel (Islam et al., 2010). Recent predictive modeling studies of guard cell [Ca2+]cyt dynamics could not secure a clear role for TPC1 and, rather, emphasized the potential of other voltage-regulated vacuolar Ca2+ channels that now need to be identified at the genetic level (Chen et al., 2012; Hills et al., 2012). TPC1 is unsuited to a role in calcium release induced by an increase in [Ca2+]cyt because it is not self-regulating with regard to [Ca2+]cyt (Chen et al., 2012; Hills et al., 2012). The significance of TPC1 may lie, rather, in an ability to regulate vacuolar Ca2+ accumulation. AtTPC1 expression correlates positively with leaf Ca2+ content (Conn et al., 2012), but analysis of epidermal and mesophyll vacuolar Ca2+ revealed a negative relationship between that parameter and AtTPC1 expression (Gilliham et al., 2011). An attractive possibility is that TPC1’s function is to release vacuolar Ca2+ to prevent accumulation in specific cell types (Gilliham et al., 2011; Conn et al., 2012).
As mentioned earlier, there are now indications of CNGC residence at the tonoplast, GLR residence at the chloroplast, and MSL residence in chloroplasts (Teardo et al., 2011; Veley et al., 2012; Yuen and Christopher, 2013). The chloroplast protein translocon TIC complex conducts K+ in PLB, and its capacity for Ca2+ conduction now needs to be explored (Kikuchi et al., 2013). Mitochondria still present as a black box with regard to Ca2+ channels. It will be interesting to see whether the Arabidopsis genes identified as encoding possible homologs of the animal mitochondrial calcium uniporter Ca2+ channel can conduct Ca2+ (De Stefani et al., 2011; Stael et al., 2012).
FUTURE DIRECTIONS
Recent advances have made it clear that there are now even more genes to identify, consider, and even reconsider as encoding Ca2+-permeable channels. The successful reconstitution of AtGLR3.4 in HEK cells should prove to be a turning point in Ca2+ channel research, permitting structure-function relationships to be explored further. Cellular expression systems may prove to be a useful tool in finding regulatory proteins. Protein-protein interaction studies may help reduce the number of theoretically possible heteromeric GLR and CNGC subunit combinations to be tested. A key challenge for annexin research is establishing their mode of Ca2+ transport. Modeling channel activity in Ca2+-handling scenarios looks set to be a powerful tool in helping to predict and identify participating channels, particularly for specific signaling pathways. Identifying and characterizing Ca2+ signaling mutants holds much promise for future discoveries (Pan et al., 2012; Ranf et al., 2012), and it is hoped that these will include the identities of more HACCs, the DACCs, and the Ca2+ channels of the organelles.
Glossary
- [Ca2+]cyt
cytosolic Ca2
- CNGC
cyclic nucleotide-gated channel
- GLR
glutamate receptor-like
- HACC
hyperpolarization-activated calcium channel
- DACC
depolarization-activated calcium channel
- VICC
voltage-independent calcium channel
- ROS
reactive oxygen species
- cGMP
cyclic GMP
- MS
mechanosensitive
- SV
slow vacuolar
- HEK
human embryonic kidney
- CaM
calmodulin
- PLB
planar lipid bilayers
- OH•
hydroxyl radicals
References
- Allen GJ, Muir SR, Sanders D. (1995) Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science 268: 735–737 [DOI] [PubMed] [Google Scholar]
- Aouini A, Matsukura C, Ezura H, Asamizu E. (2012) Characterisation of 13 glutamate receptor-like genes encoded in the tomato genome by structure, phylogeny and expression profiles. Gene 493: 36–43 [DOI] [PubMed] [Google Scholar]
- Caballero F, Botella MA, Rubio L, Fernández JA, Martínez V, Rubio F. (2012) A Ca2+-sensitive system mediates low-affinity K+ uptake in the absence of AKT1 in Arabidopsis plants. Plant Cell Physiol 53: 2047–2059 [DOI] [PubMed] [Google Scholar]
- Chen Z-H, Hills A, Bätz U, Amtmann A, Lew VL, Blatt MR. (2012) Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol 159: 1235–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark GB, Morgan RO, Fernandez M-P, Roux SJ. (2012) Evolutionary adaptation of plant annexins has diversified their molecular structures, interactions and functional roles. New Phytol 196: 695–712 [DOI] [PubMed] [Google Scholar]
- Conn SJ, Berninger P, Broadley MR, Gilliham M. (2012) Exploiting natural variation to uncover candidate genes that control element accumulation in Arabidopsis thaliana. New Phytol 193: 859–866 [DOI] [PubMed] [Google Scholar]
- Dark A, Demidchik V, Richards SL, Shabala S, Davies JM. (2011) Release of extracellular purines from plant roots and effect on ion fluxes. Plant Signal Behav 6: 1855–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Maathuis FJM. (2007) Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytol 175: 387–404 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM. (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J Cell Sci 116: 81–88 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Davies JM. (2007) Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant J 49: 377–386 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shang Z, Shin R, Colaço R, Laohavisit A, Shabala S, Davies JM. (2011) Receptor-like activity evoked by extracellular ADP in Arabidopsis root epidermal plasma membrane. Plant Physiol 156: 1375–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Shang Z, Shin R, Thompson E, Rubio L, Laohavisit A, Mortimer JC, Chivasa S, Slabas AR, Glover BJ, et al. (2009) Plant extracellular ATP signalling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J 58: 903–913 [DOI] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich P, Anschütz U, Kugler A, Becker D. (2010) Physiology and biophysics of plant ligand-gated ion channels. Plant Biol (Stuttg) (Suppl 1) 12: 80–93 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D. (2010) The language of calcium signaling. Annu Rev Plant Biol 61: 593–620 [DOI] [PubMed] [Google Scholar]
- Enz C, Steinkamp T, Wagner R. (1993) Ion channels in the thylakoid membrane (a patch clamp study). Biochim Biophys Acta 1143: 67–76 [Google Scholar]
- Finka A, Cuendet AFH, Maathuis FJM, Saidi Y, Goloubinoff P. (2012) Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 24: 3333–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C, Kugler A, Hoth S, Dietrich P. (2013) An IQ domain mediates the interaction with calmodulin in a plant cyclic nucleotide-gated channel. Plant Cell Physiol 54: 573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al. (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Furuichi T, Iida H, Sokabe M, Tatsumi H. (2012) Expression of Arabidopsis MCA1 enhanced mechanosensitive channel activity in the Xenopus laevis oocyte plasma membrane. Plant Signal Behav 7: 1022–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Han X, Wu J, Zheng S, Shang Z, Sun D, Zhou R, Li B. (2012) A heat-activated calcium-permeable channel—Arabidopsis cyclic nucleotide-gated ion channel 6—is involved in heat shock responses. Plant J 70: 1056–1069 [DOI] [PubMed] [Google Scholar]
- Garcia-Mata C, Wang J, Gajdanowicz P, Gonzalez W, Hills A, Donald N, Riedelsberger J, Amtmann A, Dreyer I, Blatt MR. (2010) A minimal cysteine motif required to activate the SKOR K+ channel of Arabidopsis by the reactive oxygen species H2O2. J Biol Chem 285: 29286–29294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferrière N, Thibaud JB, Sentenac H. (1998) Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94: 647–655 [DOI] [PubMed] [Google Scholar]
- Gilliham M, Athman A, Tyerman SD, Conn SJ. (2011) Cell-specific compartmentation of mineral nutrients is an essential mechanism for optimal plant productivity: another role for TPC1? Plant Signal Behav 6: 1656–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliham M, Sullivan W, Tester M, Tyerman SD. (2006) Simultaneous flux and current measurement from single plant protoplasts reveals a strong link between K+ fluxes and current, but no link between Ca2+ fluxes and current. Plant J 46: 134–144 [DOI] [PubMed] [Google Scholar]
- Gobert A, Park G, Amtmann A, Sanders D, Maathuis FJM. (2006) Arabidopsis thaliana cyclic nucleotide gated channel 3 forms a non-selective ion transporter involved in germination and cation transport. J Exp Bot 57: 791–800 [DOI] [PubMed] [Google Scholar]
- Gradogna A, Scholz-Starke J, Gutla PVK, Carpaneto A. (2009) Fluorescence combined with excised patch: measuring calcium currents in plant cation channels. Plant J 58: 175–182 [DOI] [PubMed] [Google Scholar]
- Grygorczyk C, Grygorczyk R. (1998) A Ca2+- and voltage-dependent cation channel in the nuclear envelope of red beet. Biochim Biophys Acta 1375: 117–130 [DOI] [PubMed] [Google Scholar]
- Gutla PVK, Boccaccio A, De Angeli A, Gambale F, Carpaneto A. (2012) Modulation of plant TPC channels by polyunsaturated fatty acids. J Exp Bot 63: 6187–6197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Kurusu T, Okuma E, Nokajima H, Kiyoduka M, Koyano T, Sugiyama Y, Okada K, Koga J, Saji H, et al. (2012) Regulation of a proteinaceous elicitor-induced Ca2+ influx and production of phytoalexins by a putative voltage-gated cation channel, OsTPC1, in cultured rice cells. J Biol Chem 287: 9931–9939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DWA, Hills A, Köhler B, Blatt MR. (2000) Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc Natl Acad Sci USA 97: 4967–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey HJ, Venis MA, Trewavas AJ. (1989) Partial purification of a protein from maize (Zea mays) coleoptile membranes binding the Ca2+-channel antagonist verapamil. Biochem J 257: 95–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM. (2008) Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr Biol 18: 730–734 [DOI] [PubMed] [Google Scholar]
- Hedrich R. (2012) Ion channels in plants. Physiol Rev 92: 1777–1811 [DOI] [PubMed] [Google Scholar]
- Hills A, Chen Z-H, Amtmann A, Blatt MR, Lew VL. (2012) OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant Physiol 159: 1026–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A, Proust J, Dorowski A, Schantz R, Huber R. (2000) Annexin 24 from Capsicum annuum: x-ray structure and biochemical characterization. J Biol Chem 275: 8072–8082 [DOI] [PubMed] [Google Scholar]
- Islam MM, Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y. (2010) Roles of AtTPC1, vacuolar two pore channel 1, in Arabidopsis stomatal closure. Plant Cell Physiol 51: 302–311 [DOI] [PubMed] [Google Scholar]
- Isner JC, Nühse T, Maathuis FJ. (2012) The cyclic nucleotide cGMP is involved in plant hormone signalling and alters phosphorylation of Arabidopsis thaliana root proteins. J Exp Bot 63: 3199–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammes F, Hu H-C, Villiers F, Bouten R, Kwak JM. (2011) Calcium-permeable channels in plant cells. FEBS J 278: 4262–4276 [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Bédard J, Hirano M, Hirabayashi Y, Oishi M, Imai M, Takase M, Ide T, Nakai M. (2013) Uncovering the protein translocon at the chloroplast inner envelope membrane. Science 339: 571–574 [DOI] [PubMed] [Google Scholar]
- Konopka-Postupolska D, Clark G, Goch G, Debski J, Floras K, Cantero A, Fijolek B, Roux S, Hennig J. (2009) The role of annexin 1 in drought stress in Arabidopsis. Plant Physiol 150: 1394–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu T, Kuchitsu K, Nakano M, Nakayama Y, Iida H. (2013) Plant mechanosensing and Ca2+ transport. Trends Plant Sci 18: 227–233 [DOI] [PubMed] [Google Scholar]
- Kwaaitaal M, Huisman R, Maintz J, Reinstädler A, Panstruga R. (2011) Ionotropic glutamate receptor (iGluR)-like channels mediate MAMP-induced calcium influx in Arabidopsis thaliana. Biochem J 440: 355–365 [DOI] [PubMed] [Google Scholar]
- Lamport DTA, Várnai P. (2013) Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytol 197: 58–64 [DOI] [PubMed] [Google Scholar]
- Lan WZ, Wang W, Wang SM, Li LG, Buchanan BB, Lin HX, Gao JP, Luan S. (2010) A rice high-affinity potassium transporter (HKT) conceals a calcium-permeable cation channel. Proc Natl Acad Sci USA 107: 7089–7094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Brown AT, Cicuta P, Davies JM. (2010) Annexins: components of the calcium and reactive oxygen signaling network. Plant Physiol 152: 1824–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Davies JM (2011a) Annexins. In Coding and Decoding of Calcium Signals in Plants. Springer-Verlag, Heidelberg, pp 111–128 [Google Scholar]
- Laohavisit A, Davies JM. (2011b) Annexins. New Phytol 189: 40–53 [DOI] [PubMed] [Google Scholar]
- Laohavisit A, Mortimer JC, Demidchik V, Coxon KM, Stancombe MA, Macpherson N, Brownlee C, Hofmann A, Webb AAR, Miedema H, et al. (2009) Zea mays annexins modulate cytosolic free Ca2+ and generate a Ca2+-permeable conductance. Plant Cell 21: 479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Shang Z, Rubio L, Cuin TA, Véry AA, Wang A, Mortimer JC, Macpherson N, Coxon KM, Battey NH, et al. (2012) Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca2+- and K+-permeable conductance in root cells. Plant Cell 24: 1522–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larisch N, Schulze C, Galione A, Dietrich P. (2012) An N-terminal dileucine motif directs two-pore channels to the tonoplast of plant cells. Traffic 13: 1012–1022 [DOI] [PubMed] [Google Scholar]
- Ma Y, Walker RK, Zhao Y, Berkowitz GA. (2012) Linking ligand perception by PEPR pattern recognition receptors to cytosolic Ca2+ calcium elevation and downstream immune signaling in plants. Proc Natl Acad Sci USA 109: 19852–19857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D. (2001) Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiol 127: 1617–1625 [PMC free article] [PubMed] [Google Scholar]
- Maksaev G, Haswell ES. (2012) MscS-Like10 is a stretch-activated ion channel from Arabidopsis thaliana with a preference for anions. Proc Natl Acad Sci USA 109: 19015–19020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke AJM, Weiger TM, Matzke M. (2010) Ion channels at the nucleus: electrophysiology meets the genome. Mol Plant 3: 642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Pittman JK. (2009) Shaping the calcium signature. New Phytol 181: 275–294 [DOI] [PubMed] [Google Scholar]
- Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, Gilliham M, Liu LH, Obermeyer G, Feijó JA. (2011) Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 332: 434–437 [DOI] [PubMed] [Google Scholar]
- Miedema H, Bothwell JHF, Brownlee C, Davies JM. (2001) Calcium uptake by plant cells: channels and pumps acting in concert. Trends Plant Sci 6: 514–519 [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Messerli MA, Gilroy S. (2008) Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol 147: 1690–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, Kishigami A, Sokabe M, Kojima I, Sato S, et al. (2007) Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci USA 104: 3639–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DH, Zahir A, Yun DJ, Bressan RA, Bohnert HJ. (2007) SOS1 and halophytism. Plant Signal Behav 4: 1081–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Zhao Y, Zheng Y, Liu J, Jiang X, Guo Y. (2012) A high-throughput method for screening Arabidopsis mutants with disordered abiotic stress-induced calcium signal. J Genet Genomics 39: 225–235 [DOI] [PubMed] [Google Scholar]
- Peiter E. (2011) The plant vacuole: emitter and receiver of calcium signals. Cell Calcium 50: 120–128 [DOI] [PubMed] [Google Scholar]
- Peiter E, Maathuis FJM, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D. (2005) The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434: 404–408 [DOI] [PubMed] [Google Scholar]
- Porée F, Wulfetange K, Naso A, Carpaneto A, Roller A, Natura G, Bertl A, Sentenac H, Thibaud J-B, Dreyer I. (2005) Plant K(in) and K(out) channels: approaching the trait of opposite rectification by analyzing more than 250 KAT1-SKOR chimeras. Biochem Biophys Res Commun 332: 465–473 [DOI] [PubMed] [Google Scholar]
- Qi Z, Verma R, Gehring C, Yamaguchi Y, Zhao Y, Ryan CA, Berkowitz GA. (2010) Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc Natl Acad Sci USA 107: 21193–21198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiles-Pando C, Rexach J, Navarro-Gochicoa MT, Camacho-Cristóbal JJ, Herrera-Rodríguez MB, González-Fontes A. (2013) Boron deficiency increases the levels of cytosolic Ca2+ and expression of Ca2+-related genes in Arabidopsis thaliana roots. Plant Physiol Biochem 65: 55–60 [DOI] [PubMed] [Google Scholar]
- Ranf S, Grimmer J, Pöschl Y, Pecher P, Chinchilla D, Scheel D, Lee J. (2012) Defense-related calcium signaling mutants uncovered via a quantitative high-throughput screen in Arabidopsis thaliana. Mol Plant 5: 115–130 [DOI] [PubMed] [Google Scholar]
- Ranf S, Wünnenberg P, Lee J, Becker D, Dunkel M, Hedrich R, Scheel D, Dietrich P. (2008) Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. Plant J 53: 287–299 [DOI] [PubMed] [Google Scholar]
- Rienmüller F, Beyhl D, Lautner S, Fromm J, Al-Rasheid KAS, Ache P, Farmer EE, Marten I, Hedrich R. (2010) Guard cell-specific calcium sensitivity of high density and activity SV/TPC1 channels. Plant Cell Physiol 51: 1548–1554 [DOI] [PubMed] [Google Scholar]
- Roy SJ, Gilliham M, Berger B, Essah PA, Cheffings C, Miller AJ, Davenport RJ, Liu L-H, Skynner MJ, Davies JM, et al. (2008) Investigating glutamate receptor-like gene co-expression in Arabidopsis thaliana. Plant Cell Environ 31: 861–871 [DOI] [PubMed] [Google Scholar]
- Sassi A, Mieulet D, Khan I, Moreau B, Gaillard I, Sentenac H, Véry A-A. (2012) The rice monovalent cation transporter OsHKT2;4: revisited ionic selectivity. Plant Physiol 160: 498–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short EF, North KA, Roberts MR, Hetherington AM, Shirras AD, McAinsh MR. (2012) A stress-specific calcium signature regulating an ozone-responsive gene expression network in Arabidopsis. Plant J 71: 948–961 [DOI] [PubMed] [Google Scholar]
- Stael S, Wurzinger B, Mair A, Mehlmer N, Vothknecht UC, Teige M. (2012) Plant organellar calcium signalling: an emerging field. J Exp Bot 63: 1525–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens NR, Qi Z, Spalding EP. (2008) Glutamate receptor subtypes evidenced by differences in desensitization and dependence on the GLR3.3 and GLR3.4 genes. Plant Physiol 146: 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapken D, Hollmann M. (2008) Arabidopsis thaliana glutamate receptor ion channel function demonstrated by ion pore transplantation. J Mol Biol 383: 36–48 [DOI] [PubMed] [Google Scholar]
- Teardo E, Formentin E, Segalla A, Giacometti GM, Marin O, Zanetti M, Lo Schiavo F, Zoratti M, Szabò I. (2011) Dual localization of plant glutamate receptor AtGLR3.4 to plastids and plasmamembrane. Biochim Biophys Acta 1807: 359–367 [DOI] [PubMed] [Google Scholar]
- Tunc-Ozdemir M, Rato C, Brown E, Rogers S, Mooneyham A, Frietsch S, Myers CT, Poulsen LR, Malhó R, Harper JF. (2013a) Cyclic nucleotide gated channels 7 and 8 are essential for male reproductive fertility. PLoS ONE 8: e55277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunc-Ozdemir M, Tang C, Ishka MR, Brown E, Groves NR, Myers CT, Rato C, Poulsen LR, McDowell S, Miller G, et al. (2013b) A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol 161: 1010–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatsa P, Chiltz A, Bourque S, Wendehenne D, Garcia-Brugger A, Pugin A. (2011) Involvement of putative glutamate receptors in plant defence signaling and NO production. Biochimie 93: 2095–2101 [DOI] [PubMed] [Google Scholar]
- Veley KM, Marshburn S, Clure CE, Haswell ES. (2012) Mechanosensitive channels protect plastids from hypoosmotic stress during normal plant growth. Curr Biol 22: 408–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry A-A, Davies JM. (2000) Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc Natl Acad Sci USA 97: 9801–9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincill ED, Bieck AM, Spalding EP. (2012) Ca2+ conduction by an amino acid-gated ion channel related to glutamate receptors. Plant Physiol 159: 40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincill ED, Clarin AE, Molenda JN, Spalding EP. (2013) Interacting glutamate receptor-like proteins in phloem regulate lateral root initiation in Arabidopsis. Plant Cell 25: 1304–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley HJ, Knight MR. (2013) Calcium signatures are decoded by plants to give specific gene responses. New Phytol 197: 690–693 [DOI] [PubMed] [Google Scholar]
- Whalley HJ, Sargeant AW, Steele JF, Lacoere T, Lamb R, Saunders NJ, Knight H, Knight MR. (2011) Transcriptomic analysis reveals calcium regulation of specific promoter motifs in Arabidopsis. Plant Cell 23: 4079–4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler GL, Brownlee C. (2008) Ca2+ signalling in plants and green algae: changing channels. Trends Plant Sci 13: 506–514 [DOI] [PubMed] [Google Scholar]
- Yuen CCY, Christopher DA. (2013) The group IV-A cyclic nucleotide-gated channels, CNGC19 and CNGC 20, localize to the vacuole membrane in Arabidopsis thaliana. AoB Plants 5: plt012 [Google Scholar]