Cyclic GMP activates Ca2+-permeable cation channels in the plasma membrane of Arabidopsis guard cells.
Abstract
Cytosolic Ca2+ in guard cells plays an important role in stomatal movement responses to environmental stimuli. These cytosolic Ca2+ increases result from Ca2+ influx through Ca2+-permeable channels in the plasma membrane and Ca2+ release from intracellular organelles in guard cells. However, the genes encoding defined plasma membrane Ca2+-permeable channel activity remain unknown in guard cells and, with some exceptions, largely unknown in higher plant cells. Here, we report the identification of two Arabidopsis (Arabidopsis thaliana) cation channel genes, CNGC5 and CNGC6, that are highly expressed in guard cells. Cytosolic application of cyclic GMP (cGMP) and extracellularly applied membrane-permeable 8-Bromoguanosine 3′,5′-cyclic monophosphate-cGMP both activated hyperpolarization-induced inward-conducting currents in wild-type guard cells using Mg2+ as the main charge carrier. The cGMP-activated currents were strongly blocked by lanthanum and gadolinium and also conducted Ba2+, Ca2+, and Na+ ions. cngc5 cngc6 double mutant guard cells exhibited dramatically impaired cGMP-activated currents. In contrast, mutations in CNGC1, CNGC2, and CNGC20 did not disrupt these cGMP-activated currents. The yellow fluorescent protein-CNGC5 and yellow fluorescent protein-CNGC6 proteins localize in the cell periphery. Cyclic AMP activated modest inward currents in both wild-type and cngc5cngc6 mutant guard cells. Moreover, cngc5 cngc6 double mutant guard cells exhibited functional abscisic acid (ABA)-activated hyperpolarization-dependent Ca2+-permeable cation channel currents, intact ABA-induced stomatal closing responses, and whole-plant stomatal conductance responses to darkness and changes in CO2 concentration. Furthermore, cGMP-activated currents remained intact in the growth controlled by abscisic acid2 and abscisic acid insensitive1 mutants. This research demonstrates that the CNGC5 and CNGC6 genes encode unique cGMP-activated nonselective Ca2+-permeable cation channels in the plasma membrane of Arabidopsis guard cells.
Plants lose water via transpiration and take in CO2 for photosynthesis through stomatal pores. Each stomatal pore is surrounded by two guard cells, and stomatal movements are driven by the change of turgor pressure in guard cells. The intracellular second messenger Ca2+ functions in guard cell signal transduction (Schroeder and Hagiwara, 1989; McAinsh et al., 1990; Webb et al., 1996; Grabov and Blatt, 1998; Allen et al., 1999; MacRobbie, 2000; Mori et al., 2006; Young et al., 2006; Siegel et al., 2009; Chen et al., 2010; Hubbard et al., 2012). Plasma membrane ion channel activity and gene expression in guard cells are finely regulated by the intracellular free calcium concentration ([Ca2+]cyt; Schroeder and Hagiwara, 1989; Webb et al., 2001; Allen et al., 2002; Siegel et al., 2009; Kim et al., 2010; Stange et al., 2010). Ca2+-dependent protein kinases (CPKs) function as targets of the cytosolic Ca2+ signal, and several members of the CPK family have been shown to function in stimulus-induced stomatal closing, including the Arabidopsis (Arabidopsis thaliana) CPK3, CPK4, CPK6, CPK10, and CPK11 proteins (Mori et al., 2006; Zhu et al., 2007; Zou et al., 2010; Brandt et al., 2012; Hubbard et al., 2012). Further research found that several CPKs could activate the S-type anion channel SLAC1 in Xenopus laevis oocytes, including CPK21, CPK23, and CPK6 (Geiger et al., 2010; Brandt et al., 2012). At the same time, the Ca2+-independent protein kinase Open Stomata1 mediates stomatal closing and activates the S-type anion channel SLAC1 (Mustilli et al., 2002; Yoshida et al., 2002; Geiger et al., 2009; Lee et al., 2009; Xue et al., 2011), indicating that both Ca2+-dependent and Ca2+-independent pathways function in guard cells.
Multiple essential factors of guard cell abscisic acid (ABA) signal transduction function in the regulation of Ca2+-permeable channels and [Ca2+]cyt elevations, including Abscisic Acid Insensitive1 (ABI1), ABI2, Enhanced Response to Abscisic Acid1 (ERA1), the NADPH oxidases AtrbohD and AtrbohF, the Guard Cell Hydrogen Peroxide-Resistant1 (GHR1) receptor kinase, as well as the Ca2+-activated CPK6 protein kinase (Pei et al., 1998; Allen et al., 1999, 2002; Kwak et al., 2003; Miao et al., 2006; Mori et al., 2006; Hua et al., 2012). [Ca2+]cyt increases result from both Ca2+ release from intracellular Ca2+ stores (McAinsh et al., 1992) and Ca2+ influx across the plasma membrane (Hamilton et al., 2000; Pei et al., 2000; Murata et al., 2001; Kwak et al., 2003; Hua et al., 2012). Electrophysiological analyses have characterized nonselective Ca2+-permeable channel activity in the plasma membrane of guard cells (Schroeder and Hagiwara, 1990; Hamilton et al., 2000; Pei et al., 2000; Murata et al., 2001; Köhler and Blatt, 2002; Miao et al., 2006; Mori et al., 2006; Suh et al., 2007; Vahisalu et al., 2008; Hua et al., 2012). However, the genetic identities of Ca2+-permeable channels in the plasma membrane of guard cells have remained unknown despite over two decades of research on these channel activities.
The Arabidopsis genome includes 20 genes encoding cyclic nucleotide-gated channel (CNGC) homologs and 20 genes encoding homologs to animal Glu receptor channels (Lacombe et al., 2001; Kaplan et al., 2007; Ward et al., 2009), which have been proposed to function in plant cells as cation channels (Schuurink et al., 1998; Arazi et al., 1999; Köhler et al., 1999). Recent research has demonstrated functions of specific Glu receptor channels in mediating Ca2+ channel activity (Michard et al., 2011; Vincill et al., 2012). Previous studies have shown cAMP activation of nonselective cation currents in guard cells (Lemtiri-Chlieh and Berkowitz, 2004; Ali et al., 2007). However, only a few studies have shown the disappearance of a defined plasma membrane Ca2+ channel activity in plants upon mutation of candidate Ca2+ channel genes (Ali et al., 2007; Michard et al., 2011; Laohavisit et al., 2012; Vincill et al., 2012). Some CNGCs have been found to be involved in cation nutrient intake, including monovalent cation intake (Guo et al., 2010; Caballero et al., 2012), salt tolerance (Guo et al., 2008; Kugler et al., 2009), programmed cell death and pathogen responses (Clough et al., 2000; Balagué et al., 2003; Urquhart et al., 2007; Abdel-Hamid et al., 2013), thermal sensing (Finka et al., 2012; Gao et al., 2012), and pollen tube growth (Chang et al., 2007; Frietsch et al., 2007; Tunc-Ozdemir et al., 2013a, 2013b). Direct in vivo disappearance of Ca2+ channel activity in cngc disruption mutants has been demonstrated in only a few cases thus far (Ali et al., 2007; Gao et al., 2012). In this research, we show that CNGC5 and CNGC6 are required for a cyclic GMP (cGMP)-activated nonselective Ca2+-permeable cation channel activity in the plasma membrane of Arabidopsis guard cells.
RESULTS
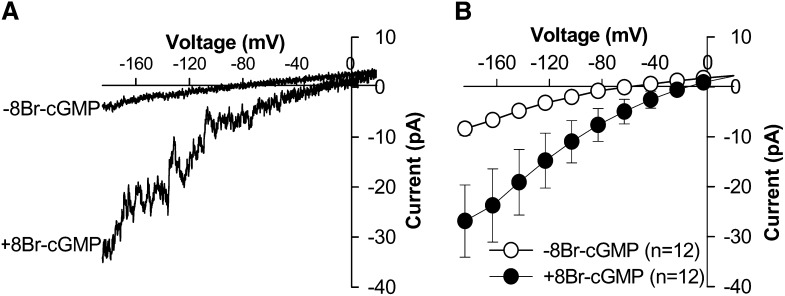
8Br-cGMP Activates Inward Nonselective Cation Currents in Arabidopsis Guard Cell Protoplasts
Cyclic nucleotide-activated ion channels function as a type of cyclic nucleotide-gated Ca2+-permeable channel in mammalian cells, and some Arabidopsis CNGCs have been reported to encode functional cyclic nucleotide-gated Ca2+-permeable channels (Leng et al., 2002; Ali et al., 2007; Gao et al., 2012). We performed experiments to test whether cyclic nucleotides could trigger currents in Arabidopsis guard cells. We found that inward currents were activated in Columbia wild-type guard cells upon extracellular application of 500 μm membrane-permeable 8-Bromoguanosine 3′,5′-cyclic monophosphate (8Br)-cGMP (Fig. 1). We used Mg2+ as the main divalent cation in the bath solution because it has been established that Ca2+-permeable channels in plants cells are often permeable to multiple divalent cations, including Ca2+, Ba2+, and Mg2+, and Mg2+ would be unlikely to interfere with Ca2+-dependent responses (Thuleau et al., 1994; Pei et al., 2000; Véry and Davies, 2000; Wang et al., 2004; Finka et al., 2012).
Figure 1.
Whole-cell currents recorded in Columbia wild-type guard cell protoplasts of Arabidopsis. A, Typical whole-cell recording in a Columbia wild-type guard cell protoplast before (−) and after (+) application of the membrane-permeable cGMP analog 8Br-cGMP (500 μm) to the bath solution. B, Average current-voltage curves of steady-state whole-cell currents recorded in Columbia wild-type guard cell protoplasts before (−) and after (+) application of 500 μm 8Br-cGMP (n = 12). Values depict means ± se.
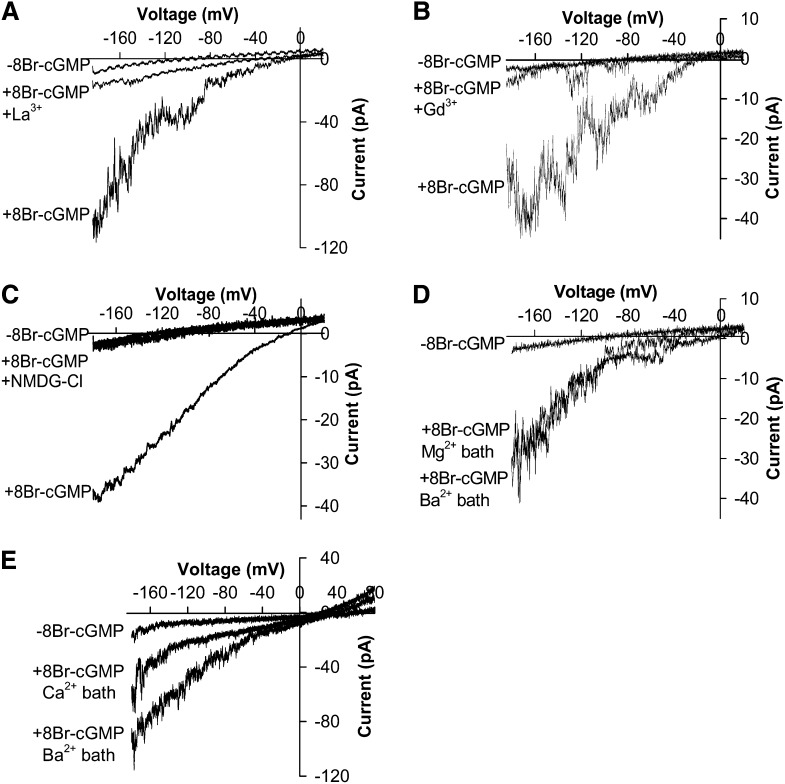
Since Mg2+ and Cl− were the main ions in the bath and pipette solutions under the imposed conditions, it was possible that the 8Br-cGMP-activated inward currents result from either the influx of Mg2+ ions or the efflux of Cl− across the plasma membrane of Arabidopsis guard cells. Therefore, we analyzed the reversal potential of the 8Br-cGMP-activated currents. Due to the voltage dependence of the 8Br-cGMP-activated currents, the reversal potential could be approximately extrapolated as the voltage where the average 8Br-cGMP-activated currents merged with the average control currents in wild-type Arabidopsis guard cells, which was approximately +21 mV (Fig. 1B) after correction of the liquid junction potential of −4 mV measured as described previously (Ward and Schroeder, 1994). Under the imposed conditions, equilibrium potentials of Mg2+ and Cl− were +23 and −81 mV after correction of ionic activities, respectively. The reversal potential of the 8Br-cGMP-activated currents at +21 mV was close to the equilibrium potential of Mg2+ (+23 mV) but far from that of Cl− (−81 mV). These data show a Mg2+ permeability of the 8Br-cGMP-activated currents. We tested the effects of La3+ and Gd3+, two well-known plant Ca2+ channel blockers (Allen and Sanders, 1994; Grabov and Blatt, 1999; Lemtiri-Chlieh and Berkowitz, 2004; Wang et al., 2004; Gao et al., 2012), on the 8Br-cGMP-activated inward currents in guard cells. We found that the 8Br-cGMP-activated inward currents were strongly inhibited by 100 μm La3+ (n = 3) or 100 μm Gd3+ (n = 3; Fig. 2, A and B). We further tested the selectivity of 8Br-cGMP-induced currents to cations by replacing Mg2+ with the large cation, N-methyl-d-glucamine (NMDG), in the bath solution and found that the 8Br-cGMP-activated inward currents were abolished (Fig. 2C). In contrast, similar current amplitudes were observed after MgCl2 was replaced by BaCl2 at the same concentration in the bath solution (Fig. 2D), showing that the 8Br-cGMP-activated inward currents recorded in guard cells were carried mainly by these divalent cations. We also analyzed the 8Br-cGMP-activated currents using simple BaCl2 solutions, as described previously (Pei et al., 2000), and found the 8Br-cGMP-activated Ba2+ currents (Fig. 2E). The reversal potential of the recorded 8Br-cGMP-activated currents was +19 mV (Fig. 2E), close to the equilibrium potential of Ba2+ (+20 mV) but distant from the equilibrium potential of Cl− (−53 mV). 8Br-cGMP-activated Ba2+ currents were also recorded using step-wise voltage pulses, indicating that the currents are hyperpolarization activated, but the activation is not time dependent (Supplemental Fig. S1). The “spiky” nature of these 8Br-cGMP-activated currents is similar to Ca2+-permeable cation channel currents recorded previously in guard cells (Schroeder and Hagiwara, 1990; Hamilton et al., 2000; Pei et al., 2000). These inward currents were observed after BaCl2 was replaced by CaCl2 in the bath solution with BaCl2 in the pipette solution (n = 9) and the reversal potential was +17 mV, far from the equilibrium potential of Cl− (−53 mV; Fig. 2E). The permeability ratio for Ba2+ relative to Ca2+ was 1.46, as determined according to the Goldman-Hodgkin-Katz equation (Hille, 1992). These results show that 8Br-cGMP activates nonselective Ca2+-permeable cation currents. Further experiments showed that the 8Br-cGMP-activated currents can also be carried by the monovalent cation Na+ (Supplemental Fig. S2), similar to ABA-activated Ca2+-permeable cation (ICa) channels in guard cells (Kwak et al., 2003). Taken together, these results showed that cGMP activates nonselective Ca2+-permeable cation channels in the plasma membrane of guard cells. Therefore, we named the 8Br-cGMP-activated nonselective Ca2+-permeable cation currents Icat-cGMP.
Figure 2.
Ion selectivity and blocker sensitivity of 500 μm 8Br-cGMP-activated channels recorded in Arabidopsis Columbia wild-type guard cell protoplasts. A, Whole-cell recording in a Columbia wild-type guard cell protoplast showing the inhibition of 8Br-cGMP (500 μm)-activated currents by the Ca2+ channel blocker La3+ (100 μm). B, Whole-cell recordings in a Columbia wild-type guard cell protoplast showing 8Br-cGMP-activated channel inhibition by the Ca2+ channel blocker Gd3+ (100 μm). Two overlapping traces after Gd3+ application are depicted. C, Whole-cell recording of a Columbia wild-type guard cell protoplast showing the inhibition of 8Br-cGMP-activated currents by the replacement of Mg2+ in the bath solution by 100 mm NMDG-Cl. Two overlapping traces before 8Br-cGMP application and after replacement of Mg2+ in the bath solution by 100 mm NMDG-Cl are depicted. D, Whole-cell recording of 8Br-cGMP-activated currents in a Columbia wild-type guard cell protoplast showing similar current amplitudes when MgGlu and MgCl2 in the bath solution were replaced with 100 mm BaCl2. E, 8Br-cGMP-activated currents in a Columbia wild-type protoplast recorded using BaCl2 pipette and bath solutions without NADPH in the pipette solution (see “Materials and Methods”). 8Br-cGMP-activated currents were recorded in a 100 mm BaCl2 bath solution first, and then 100 mm BaCl2 in the bath solution was replaced with 100 mm CaCl2.
Guard Cell-Expressed Putative CNGC Genes
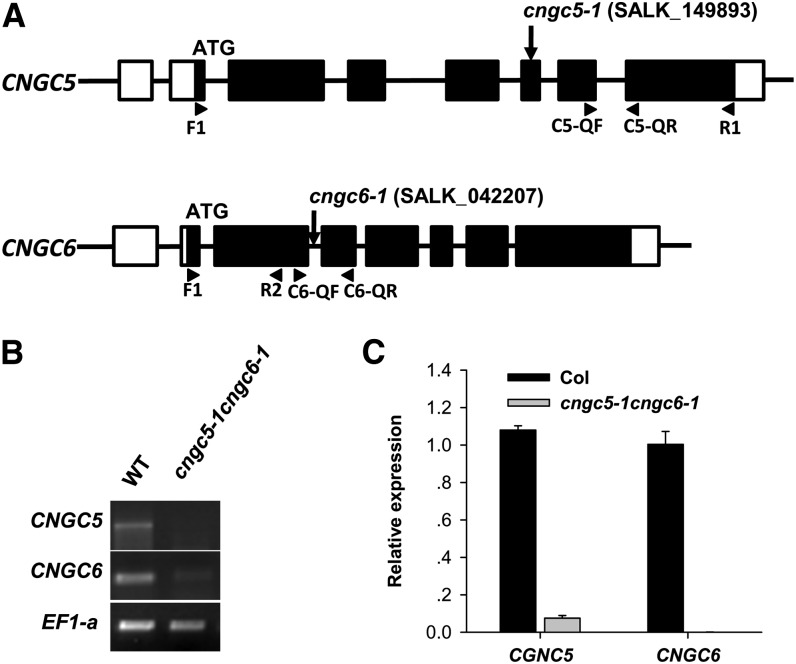
Genes encoding defined plasma membrane Ca2+-permeable channels remain poorly understood in guard cells. To identify putative Ca2+-permeable channel genes underlying Icat-cGMP, we analyzed guard cell-specific microarray sets of Arabidopsis (Yang et al., 2008; Pandey et al., 2010) and found that CNGC1 (At5g53130), CNGC2 (At5g15410), CNGC5 (At5g57940), CNGC6 (At2g23980), CNGC15 (At2g28260), and CNGC20 (At3g17700) genes were highly expressed in guard cells (Supplemental Table S1). We obtained cngc transfer DNA (T-DNA) insertion mutant lines for these CNGC genes. As CNGC5 and CNGC6 are among the two most closely related of the 20 CNGC genes, we generated cngc5 cngc6 double mutants by crossing the single mutants for further experiments (Fig. 3). Reverse transcription PCR and quantitative real-time (RT)-PCR analyses showed that the mRNA levels of CNGC5 and CNGC6 were greatly reduced or abolished in cngc5-1cngc6-1 (Fig. 3, B and C).
Figure 3.
CNGC5 and CNGC6 transcript analysis in cngc5-1 and cngc6-1 mutants. A, Cartoon showing the genomic structures of CNGC5 and CNGC6 and the T-DNA insertion sites of the cngc5-1 cngc6-1 double mutant. To amplify each CNGC-specific band, RT-PCR was performed with primer sets as indicated by the arrowheads. B, RT-PCR analysis of the cngc5-1 cngc6-1 double mutant and the Columbia wild type (WT) showing that no clear CNGC5 transcripts were detected in the cngc5-1 cngc6-1 mutant, but a possible low level of CNGC6 transcripts was detected in the cngc5-1 cngc6-1 mutant after 35 PCR cycles of amplification. C, Quantitative RT-PCR analysis of the cngc5-1 cngc6-1 double mutant shows that the transcript levels of CNGC5 and CNGC6 were greatly reduced.
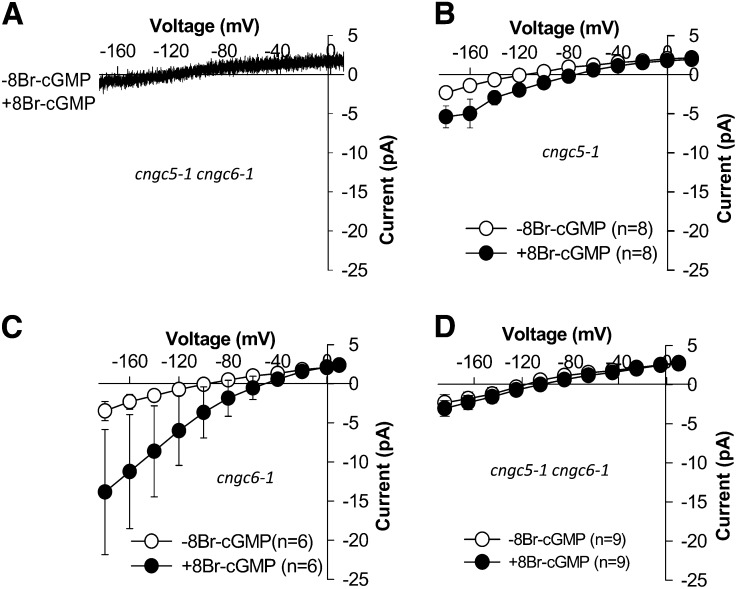
Mutations in CNGC5 and CNGC6 Impaired Icat-cGMP
To pursue the identification of genes encoding ion channels that mediate Icat-cGMP in Arabidopsis guard cells, we performed patch-clamp experiments on cngc5-1 and cngc6-1 single mutant and cngc5-1 cngc6-1 double mutant guard cells as well as on cngc2 and cngc1 cngc20 double mutants. Patch-clamp experiments showed that average current magnitudes of Icat-cGMP were only partially reduced in the two single mutants, including an insignificant average reduction in the cngc6-1 single mutant (Fig. 4, B and C; P ≤ 0.005 for cngc5-1 versus the wild type, P = 0.585 for cngc6-1 versus the wild type at −184 mV). Note that all data were included in these analyses, and the data from cngc6-1 guard cells included one guard cell with a large leak-like current after 8Br-cGMP addition, thus adding to a larger degree of error (Fig. 4C; for an independent cngc6-1 data set, see Fig. 7C below). We analyzed cngc5-1 cngc6-1 double mutant guard cells, which showed strongly impaired 8Br-cGMP-activated currents (Fig. 4, A and D), compared with the Columbia wild type (Fig. 1; P < 0.001 at –184 mV). These data indicate that CNGC5 and CNGC6 contribute to the activity of the Icat-cGMP channel currents in Arabidopsis guard cells.
Figure 4.
Mutations in CNGC5 and CNGC6 impair 500 μm 8Br-cGMP-activated inward currents in Arabidopsis guard cells. A, Typical whole-cell recording in a cngc5-1 cngc6-1 double mutant guard cell protoplast before (−) and after (+) application of the membrane-permeable cGMP analog 8Br-cGMP (500 μm) to the bath solution. B to D, Average current-voltage curves of steady-state whole-cell currents recorded in cngc5-1 single mutant (B; n = 8), cngc6-1 single mutant (C; n = 6), and cngc5-1 cngc6-1 double mutant (D; n = 9) guard cell protoplasts. Values depict means ± se.
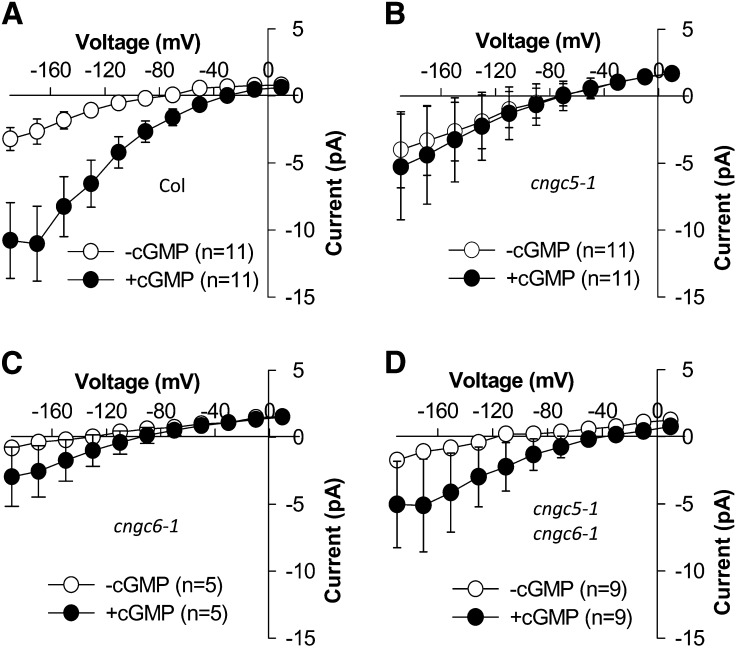
Figure 7.
cGMP (20 μm) added to the pipette solution activated obvious currents in Columbia wild-type guard cells, but currents activated by 20 μm cGMP were dramatically impaired in cngc5-1, cngc6-1, and cngc5-1 cngc6-1 guard cells. Average current-voltage curves of whole-cell recordings represent the Columbia (Col) wild type (A; n = 11), the cngc5-1 single mutant (B; n = 11), the cngc6-1 single mutant (C; n = 5), and the cngc5-1 cngc6-1 double mutant (D; n = 9). Values depict means ± se.
To further test the effects of mutations in CNGC5 and CNGC6 on Icat-cGMP, we generated a second double mutant allele, cngc5-2 cngc6-2, in the Wassilewskija (Ws) accession. CNGC6 mRNA levels in the cncg5-2 cngc6-2 mutant were greatly reduced (Supplemental Fig. S3). The CNGC5 transcript has two splice isoforms in Ws (Supplemental Fig. S3). We thus amplified several independent CNGC5 transcripts in cngc5-2. The 5′ untranslated region insertion in cngc5-2 showed either greatly reduced transcripts for two different primer pairs, including primers that amplified transcript starting 490 bp 5′ of the CNGC start site (Supplemental Fig. S3), or an increased transcript level with primers amplifying from the predicted start (ATG) site of the CNGC5 transcript (Supplemental Fig. S3, B and C). These data indicate that the CNGC6 gene was clearly reduced, whereas the CNGC5 gene was less clearly affected in the cngc5-2 cngc6-2 double mutant. We confirmed significant activation of Icat-cGMP by 20 μm cGMP applied in the pipette solution in Ws wild-type guard cells (Supplemental Fig. S4). In contrast, impaired activation was observed in cngc6-2 mutant guard cells (cngc5-2 cngc6-2 double mutant allele; Supplemental Fig. S4), providing evidence for an important function of CNGC6 in mediating the cGMP-activated current in the Ws accession. The effect of the T-DNA insertion in CNGC5 and aberrant CNGC5 transcripts amplified in cngc5-2 cngc6-2 cannot, at present, unequivocally define or exclude a contribution of CNGC5 to this phenotype in the Ws accession.
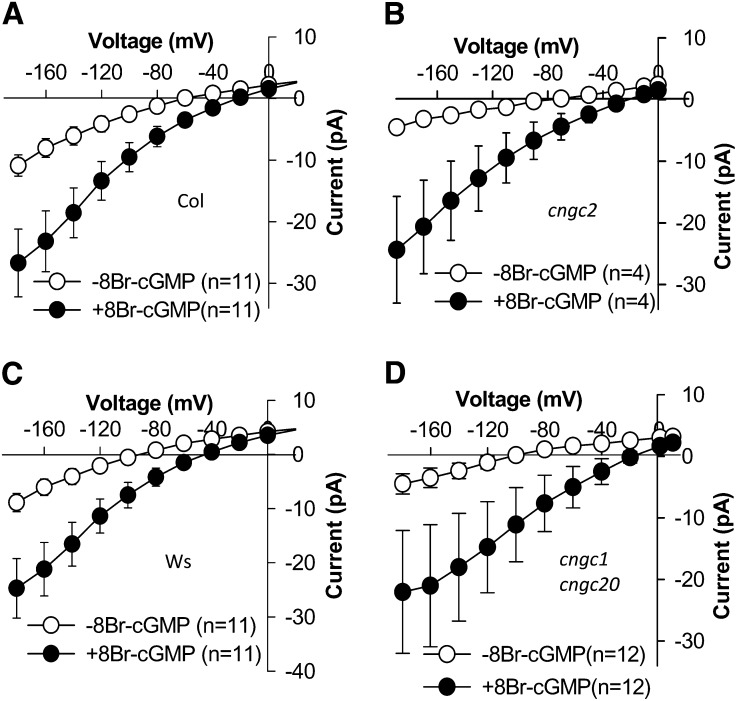
Microarray data sets suggest that several other CNGC genes are expressed in guard cells, including CNGC1, CNGC2, CNGC15, and CNGC20 (Leonhardt et al., 2004; Yang et al., 2008; Pandey et al., 2010; Bauer et al., 2013; Supplemental Table S1), and it has been reported that CNGC2 functions as a cAMP-activated Ca2+-permeable channel in Arabidopsis guard cells (Ali et al., 2007). Therefore, we isolated cngc1, cngc2, and cngc20 T-DNA insertion mutants and generated a cngc1 cngc20 double mutant line in the Ws ecotype background. The cngc15 insertion mutant line (SALK_017995) had a T-DNA insertion in the 5′ untranslated region of CNGC15 and did not exhibit a reduction in CNGC15 transcript level; thus, CNGC15 could not be analyzed in this study. We performed further patch-clamp experiments on cngc2 and cngc1 cngc20 mutant guard cells and found that there was no significant difference of Icat-cGMP in cngc2 mutant guard cells (Fig. 5B) and its Columbia wild type (Fig. 5A; P = 0.474 at −184 mV) as well as between the cngc1 cngc20 double mutant (Fig. 5D) and its Ws wild type (Fig. 5C; P = 0.245 at −184 mV). These results indicate that 8Br-cGMP mainly activated CNGC5 and CNGC6 in the wild type relative to CNGC1, CNGC2, and CNGC20 in Arabidopsis guard cells under the imposed conditions.
Figure 5.
Mutations in CNGC1, CNGC20, and CNGC2 did not disrupt the currents activated by 8Br-cGMP added to the bath solution. Average current-voltage curves of whole-cell recordings show 8Br-cGMP (500 μm)-activated inward currents recorded in guard cell protoplasts of the Columbia (Col) wild type (A; n = 11), the cngc2 mutant (B; n = 4), the Ws wild type (C; n = 11), and the cngc1 cngc20 double mutant (D; n = 12). Values depict means ± se.
cAMP has been shown to activate Ca2+-permeable currents in Arabidopsis guard cells using Ba2+ as the main divalent cation in both bath and pipette solutions (Lemtiri-Chlieh and Berkowitz, 2004). As reported above, 8Br-cGMP-activated inward currents could be carried by Ba2+ (Fig. 2, D and E). We analyzed cAMP-activated inward currents using Mg2+ as the main charge carrier. The results showed that only modest cAMP-activated inward currents were observed in both Columbia wild-type (Supplemental Fig. S5A) and cngc5-1 cngc6-1 double mutant (Supplemental Fig. S5B) guard cells with 100 μm cAMP applied into the pipette solution. There was no significant difference between Columbia wild-type and cngc5-1 cngc6-1 double mutant guard cells (Supplemental Fig. S5; P = 0.151 at −184 mV). These data and the lack of impairment of cGMP activation in cngc2 guard cells (Fig. 5B), compared with cAMP response impairment in cngc2 (Ali et al., 2007), suggest that the cGMP-activated currents in the Mg2+ solutions analyzed here differ from the cAMP-activated currents.
YFP-CNGC5 and YFP-CNGC6 Localize to the Periphery of Nicotiana benthamiana Protoplasts
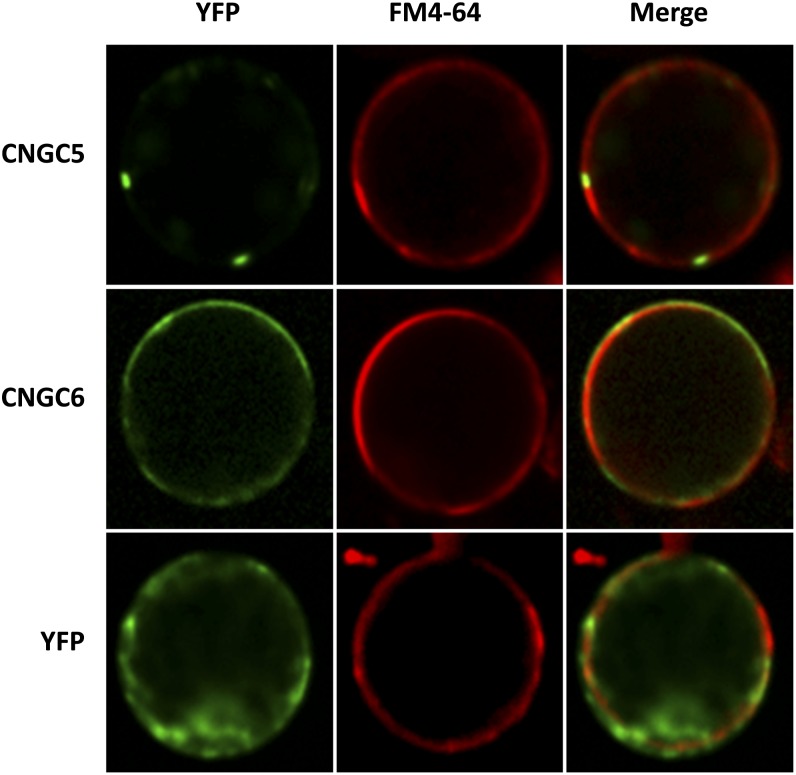
To gain insight into the cellular targeting of yellow fluorescent protein (YFP)-tagged CNGC5 and CNGC6 proteins, we transiently expressed CNGC5 and CNGC6 fused to enhanced-YFP (EYFP) in N. benthamiana protoplasts and conducted EYFP fluorescence imaging experiments using confocal microscopy. The results showed localization of YFP-CNGC5 in microdomains in the periphery of N. benthamiana protoplasts (Fig. 6). Similar microdomain localizations of plant GFP-CNGC fusions have been observed previously, including for CNGC1, CNGC11, and CNGC12 (Ali et al., 2006; Urquhart et al., 2007). YFP-CNGC6 also localized to the periphery of N. benthamiana protoplasts. YFP-CNGC6 did not show a strong concentration in microdomains (Fig. 6) and overlapped partially with the plasma membrane stain FM4-64 (Bolte et al., 2004; Fig. 6). In contrast, control YFP-expressing protoplasts showed more broadly distributed YFP fluorescence signals, including in the cytoplasm and nuclei (Fig. 6). These results provide initial evidence that CNGC5 and CNGC6 are targeted to the periphery of plant cells at the plasma membrane, which correlates with the strong reductions in Icat-cGMP activity in the plasma membrane of cngc5-1 cngc6-1 double mutant guard cells and is consistent with recent YFP fusion localization analysis of CNGC6 (Gao et al., 2012).
Figure 6.
Subcellular localization analysis of YFP-CNGC5 and YFP-CNGC6 in N. benthamiana protoplasts. Image columns depict YFP, plasma membrane label FM4-64, and merged images of YFP and FM4-64 fluorescence of N. benthamiana protoplasts expressing YFP-CNGC5, YFP-CNGC6, and YFP control.
Application of Intracellular cGMP Activates Currents in Wild-Type Arabidopsis Guard Cells
We used membrane-permeable 8Br-cGMP to activate large currents in guard cells, allowing facile analyses before and after application (Figs. 1, 2, 4, and 5). To test whether intracellular cGMP can activate Icat-cGMP, we conducted further patch-clamp experiments using cGMP at a lower concentration (20 μm) added to the pipette solution in whole-cell recordings. We observed obvious Icat-cGMP currents in Columbia wild-type guard cells (Fig. 7A). Icat-cGMP was reduced in cngc5-1 (Fig. 7B), cngc6-1 (Fig. 7C), and cngc5-1 cngc6-1 (Fig. 7D) mutant guard cells compared with the Columbia wild type (Fig. 7A; P = 0.013, 0.044, and 0.032 for cngc5-1, cngc6-1, and cngc5-1 cngc6-1 mutants, respectively, compared with the wild type at −184 mV), indicating that intracellular cGMP is capable of activating Icat-cGMP and CNGC5 and CNGC6 function in the intracellular cGMP response. Note that we cannot exclude that an additional CNGC gene(s) may be up-regulated in these cngc insertion mutant lines or that additional CNGC genes contribute to the small average residual currents in cncg5 cngc6 double mutant guard cells. However, clearly, both CNGC5 and CNGC6 function in the establishment of the cGMP-activated currents. We also tested the effects of 20 μm cAMP added in the pipette solution. We did not observe obvious activation of inward currents in Columbia wild-type guard cells (Supplemental Fig. S6), indicating that cAMP activation may occur through other channels or at higher cAMP concentrations.
CNGC5 and CNGC6 Are Not Solely Essential for ABA, CO2, and Light/Dark Transition-Induced Stomatal Signaling
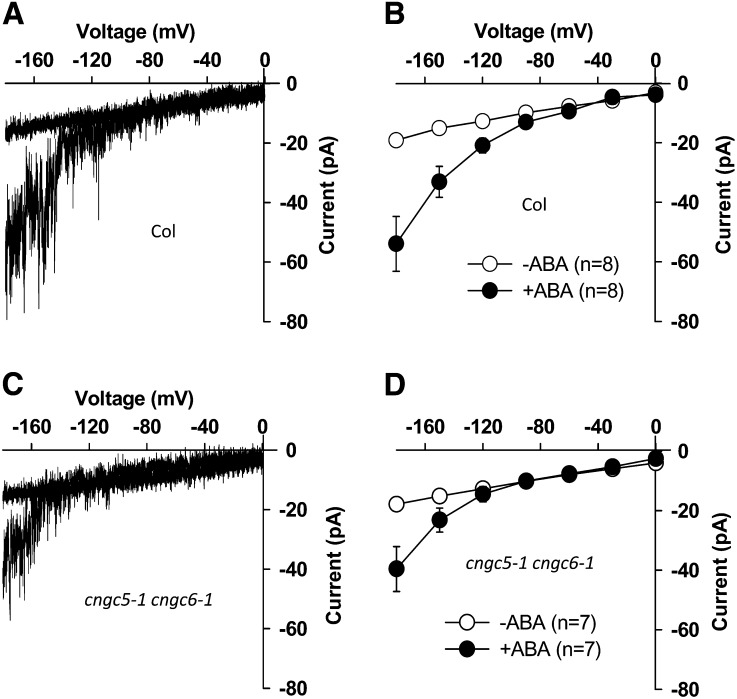
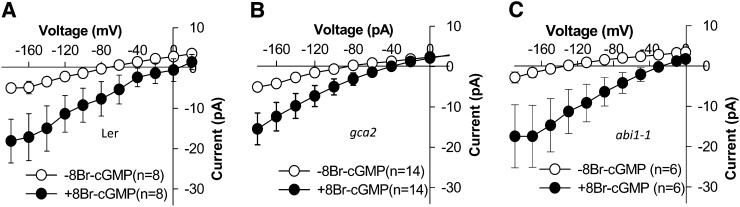
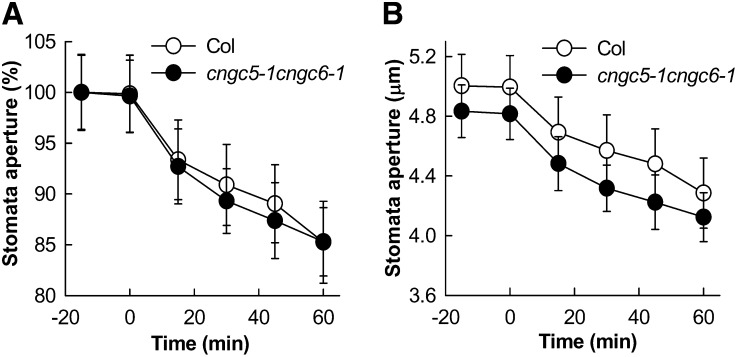
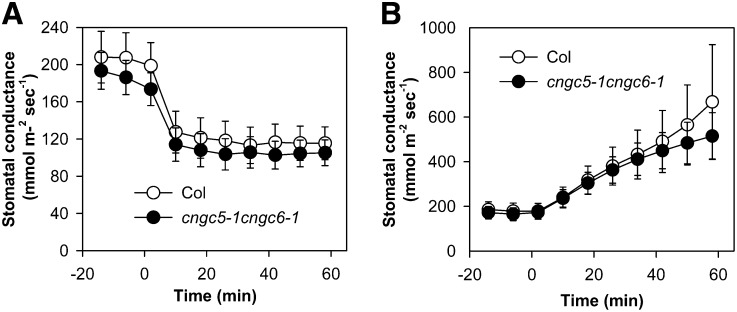
ABA triggers cytosolic Ca2+ increases and activates nonselective Ca2+-permeable cation channels in Arabidopsis guard cells (Schroeder and Hagiwara, 1990; Hamilton et al., 2000; Pei et al., 2000; Murata et al., 2001; Kwak et al., 2003; Miao et al., 2006; Hua et al., 2012). Similar to Icat-cGMP, ABA-activated ICa channels are permeable to Ba2+ (Pei et al., 2000; Murata et al., 2001), Na+ (Kwak et al., 2003), and Mg2+ (Supplemental Fig. S7). Therefore, we performed patch-clamp experiments to test whether CNGC5 and CNGC6 function as ABA-activated ICa channels using Ba2+ as the main divalent cation in both bath and pipette solutions, as reported previously (Pei et al., 2000; Murata et al., 2001). Genotype-blind patch-clamp experiments showed that measurable ABA activation of ICa currents was observed in cngc5-1 cngc6-1 guard cells (P < 0.037; Fig. 8, C and D) as well as in Columbia wild-type guard cells (P < 0.018; Fig. 8, A and B). A small effect of the cngc5 cngc6 double mutation on ABA-activated ICa channel currents could not be excluded, based on slightly smaller average currents and an apparent slight shift in the activation potential. The ABA-insensitive mutants growth controlled by abscisic acid2 (gca2) and abscisic acid insensitive1 (abi1-1) show impaired ABA activation of ICa channel currents (Pei et al., 2000; Murata et al., 2001). We next analyzed Icat-cGMP in gca2 and abi1-1 mutant guard cells as well as in Landsberg erecta wild-type guard cells and found that 8Br-cGMP-activated Icat-cGMP currents were not disrupted in gca2 (Fig. 9B) and abi1-1 (Fig. 9C) guard cells compared with the Landsberg erecta wild type (Fig. 9A). These observations together strongly suggest that CNGC5 and CNGC6 alone are not essential for ABA-activated ICa channels in Arabidopsis guard cells. To further test whether CNGC5 and CNGC6 function in ABA signaling, we pursued ABA-induced stomatal closure analyses and found that both the Columbia wild type and the cngc5-1 cngc6-1 double mutant showed functional ABA-induced responses (Fig. 10), in line with patch-clamp analyses (Figs. 8 and 9). Moreover, cytosolic Ca2+ has also been shown to play a role in CO2-induced stomatal movements (Schwartz et al., 1988; Webb et al., 1996; Young et al., 2006; Xue et al., 2011). Therefore we also addressed CO2-induced changes in whole-plant stomatal conductance of CNGC5 and CNGC6 double mutants. The results show that mutation of CNGC5 and CNGC6 did not disrupt CO2-induced stomatal closure and opening (Fig. 11). Moreover, light/dark transitions showed similar intact plant stomatal conductances in wild-type and cngc5-1 cngc6-1 double mutant plants (Supplemental Fig. S8).
Figure 8.
Mutations in CNGC5 and CNGC6 did not impair ABA (50 μm) activation of ICa channels in Arabidopsis guard cells. A, Typical whole-cell recording of ABA-activated ICa in a Columbia (Col) wild-type guard cell protoplast. B, Average current-voltage curves of whole-cell ICa recordings in the Columbia wild type (n = 8). C, Typical whole-cell recording of ABA-activated ICa in a cngc5-1 cngc6-1 double mutant guard cell protoplast. D, Average current-voltage curves of whole-cell ICa recordings in the cngc5-1 cngc6-1 double mutant (n = 7). Values depict means ± se.
Figure 9.
gca2 and abi1-1 mutants showed intact cGMP-activated currents in guard cells. Average current-voltage curves of whole-cell recordings showing 8Br-cGMP-activated inward currents were recorded in guard cell protoplasts of the Landsberg erecta (Ler) wild type (A; n = 8), the gca2 mutant (B; n = 14), and the abi1-1 mutant (C; n = 6). A concentration of 500 μm 8Br-cGMP was added to the bath solution by bath perfusion after establishing whole-cell recordings and analysis of background currents in guard cells. Values depict means ± se.
Figure 10.
ABA-induced stomatal closure in the Columbia wild type and the cngc5-1 cngc6-1 double mutant. A, Percentage changes in stomatal apertures relative to the stomatal apertures prior to 1 μm ABA incubation. B, Stomatal apertures from the same experiments shown in micrometers. Time-course experiments were pursued for ABA-induced stomatal closing in the Columbia (Col) wild type and the cngc5-1 cngc6-1 double mutant (genotype-blind experiments). Stomatal apertures were individually mapped, and images were captured and measured before and after the addition of 1 μm ABA (Siegel et al., 2009). Average stomatal apertures at time −15 min were 5.0 ± 0.2 μm (Columbia wild type) and 4.83 ± 0.18 μm (cngc5-1 cngc6-1); n = 24 individually mapped stomata each for the Columbia wild type and the cngc5-1 cngc6-1 double mutant. Values depict means ± se.
Figure 11.
Time-resolved patterns of intact whole-plant rosette stomatal conductances in response to elevated (A) and reduced (B) CO2 concentration in cngc5-1 cngc6-1 double mutant and Columbia (Col) wild-type plants. To analyze elevated CO2-induced stomatal closure, the ambient CO2 concentration was increased from 400 to 800 μL L−1 at time zero (A). To analyze low-CO2-induced stomatal opening, CO2 was decreased from 400 to 0 μL L−1 at time zero (B). Data represent averages of 12 individual plants ± se.
DISCUSSION
Intracellular Ca2+ plays an essential role in the regulation of guard cell ion channels and stomatal movements (Schroeder and Hagiwara, 1989; McAinsh et al., 1990; Webb et al., 1996; Grabov and Blatt, 1998; MacRobbie, 2000; Fan et al., 2004; Mori et al., 2006; Young et al., 2006; Marten et al., 2007; Siegel et al., 2009; Chen et al., 2010). Stimulus-triggered Ca2+ influx across the plasma membrane of plant cells plays essential roles in many Ca2+ signaling responses (Dodd et al., 2010). Two gene families have been proposed to encode Ca2+ channels in plants, the Glu receptor-like gene family (Lacombe et al., 2001) and the CNGC gene family (Kaplan et al., 2007). However, there are only a few studies that have shown disruption of a defined Ca2+-permeable channel activity in mutant plants in defined candidate plant Ca2+ channel genes (Ali et al., 2007; Gao et al., 2012; Laohavisit et al., 2012).
Activation mechanisms of plasma membrane Ca2+-permeable ICa currents have been characterized in Arabidopsis guard cells (Hamilton et al., 2000; Pei et al., 2000; Murata et al., 2001; Köhler and Blatt, 2002; Kwak et al., 2003; Miao et al., 2006; Mori et al., 2006; Hua et al., 2012). However, the identities of genes encoding defined plasma membrane Ca2+ channels in guard cells remain unknown. CNGCs have been found to be key players as cation channels in mammalian cells, and the Arabidopsis genome includes 20 CNGC genes (Kaplan et al., 2007; Ward et al., 2009). Some of these Arabidopsis CNGCs were found to function in pollen tube growth, plant immune responses, and heat responses (Yu et al., 1998; Frietsch et al., 2007; Ma and Berkowitz, 2011; Finka et al., 2012; Mach, 2012; Abdel-Hamid et al., 2013; Fischer et al., 2013; Tunc-Ozdemir et al., 2013a, 2013b). But direct voltage-clamp recordings of a cngc mutant in planta has been reported for two cngc mutants to date (Ali et al., 2007; Gao et al., 2012). In this research, we identified CNGC5 and CNGC6, which are required for cGMP-activated nonselective Ca2+-permeable cation channel activity in Arabidopsis guard cells (Figs. 2, 4, and 7). T-DNA insertion mutants in these CNGC genes show that CNGC5 and CNGC6 encode a new type of nonselective cation channel in the plasma membrane of Arabidopsis guard cells.
A previous study showed cAMP-activated Ca2+-permeable currents in Arabidopsis guard cells using Ba2+ in the bath and pipette solutions by adding membrane-permeable 1 mm dibutylyl-cAMP to the bath solution (Lemtiri-Chlieh and Berkowitz, 2004; Ali et al., 2007). In addition, it was recently reported that 50 μm dibutylyl-cAMP together with the animal phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine activated significant inward Ca2+ currents in Arabidopsis root protoplasts using Ca2+ as the main charge carrier, but effects of cGMP were not analyzed (Gao et al., 2012). Under our experimental conditions, we added cyclic nucleotides in the pipette solution at a lower concentration (20 and 100 μm for cAMP, 20 μm for cGMP) and observed limited cAMP activation of cation currents in guard cells (Supplemental Figs. S5 and S6) but a significant activation of the cation currents by 20 μm cGMP (Fig. 7) using solutions in which Mg2+ was the main divalent cation. Our findings do not exclude that higher cAMP concentrations may activate such channels in a Mg2+-containing bath solution. In addition, cAMP might have weaker effects on these CNGCs compared with cGMP in guard cells. Indeed, it has been reported that mammalian CNGCs expressed in X. laevis oocytes could be activated by cGMP approximately at a 100-fold lower concentration than cAMP (Gordon and Zagotta, 1995). Further research will be needed to determine whether different interacting proteins, including possible different CNGC heteromeric complexes, or conditions affect channel activation properties in these different plant cell types, and a direct comparison of cAMP- or cGMP-activated currents in the same type of cells (root cells or guard cells) would require further experiments.
Note that, although cGMP and cAMP can activate plant ion channels, it remains unknown whether these small molecules act as second messengers in cells of terrestrial plants. Indeed, whether cAMP and cGMP are produced in response to stimuli in higher plants is controversial and remains a matter of debate. Nevertheless, the ability of cGMP to activate CNGC5- and CNGC6-dependent ion channel activity in guard cells, with little effect of cAMP, indicates a preference in nucleotide activation mechanisms for CNGC5 and CNGC6. Furthermore, proteins with possible guanylate cyclase activity in plants have been proposed and debated (Ludidi and Gehring, 2003; Qi et al., 2010; Ashton, 2011; Berkowitz et al., 2011). Further research is needed to determine whether this animal paradigm can be applied to plant ion channel regulation. It is conceivable that CNGC5 and CNGC6 are activated in vivo in guard cells by other natural stimuli than cGMP, and more research will be needed to investigate this question. For example, ABA activates Ca2+-permeable channels with properties very similar to these currents, via distinct signaling mechanisms (Murata et al., 2001; Pei et al., 2000; Köhler and Blatt, 2002; Kwak et al., 2003; Miao et al., 2006; Mori et al., 2006; Hua et al., 2012). These data indicate that other or higher order cngc mutants might well function as the ABA-activated ICa channels.
In previous studies of reactive oxygen species and ABA activation of Ca2+-permeable channels, no ATP was added in the pipette solutions for electrophysiological analyses (Pei et al., 2000; Mori et al., 2006). In this study, we used ATP-free solutions containing 0.1 mm dithiothreitol (DTT) for both ABA-activated ICa and cGMP-activated Icat-cGMP recordings, indicating that ATP was not strictly required for the activation of these channels. NADPH was included for ABA activation. Similarly, ATP was not required for ligand-activated Ca2+-permeable cation channels in pollen (Wu et al., 2011). Note, however, that these results do not exclude additional regulation of Ca2+-permeable channels by ATP-dependent mechanisms or protein kinases, as demonstrated previously for guard cell ICa channels (Köhler and Blatt, 2002; Mori et al., 2006), given that conditions prior to whole-cell patch clamping did not preclude ATP-dependent reactions; thus, the phosphorylation state of these channels may be preset prior to patch clamping. For example, ABA activation of ICa channels is disrupted in the Ca2+-dependent protein kinase cpk6 and cpk3 mutant guard cells (Mori et al., 2006).
This study and a recent study (Gao et al., 2012) provide evidence that YFP fusions of CNGC6 are localized in the vicinity of the plasma membrane, consistent with patch-clamp analyses of T-DNA insertion mutants. YFP fusions of the close homolog YFP-CNGC5 showed localization in microdomains in the cell periphery. Similar results were reported previously for other plant membrane proteins, including CNGC1, CNGC11, and CNGC12 channels (Brady et al., 2004; Ali et al., 2006; Sutter et al., 2006; Urquhart et al., 2007; Gutierrez et al., 2010). Further research is needed to determine the relevance of such microdomain accumulation of membrane proteins.
Gene chip data show that several CNGCs are expressed in Arabidopsis guard cells, including CNGC1, CNGC2, CNGC5, CNGC6, CNGC15, and CNGC20, and the expression level of CNGC5 and CNGC6 is relatively high compared with other homologs (Yang et al., 2008; Supplemental Table S1). Patch-clamp results show that mutations in CNGC5 and CNGC6 impaired 8Br-cGMP-activated currents, but T-DNA insertion mutations in CNGC1, CNGC2, and CNGC20 did not, indicating that CNGC5 and CNGC6 are important targets for guard cell cGMP-activated channel activity in the plasma membrane of Arabidopsis guard cells under the imposed conditions. Additional CNGCs may be involved in the overlapping, partially redundant formation of regulated ion channels in guard cells. Therefore, our findings do not exclude that other CNGCs in guard cells, in addition to or together with CNGC5 and CNGC6, form regulated ion channels in vivo.
ABA-activated ICa channel activity was not disrupted by mutations in CNGC5 and CNGC6 (Fig. 8). In addition, we found that the ABA-insensitive gca2 and abi1-1 mutants, for which ABA activation of ICa channels is impaired (Pei et al., 2000; Murata et al., 2001; Miao et al., 2006), showed Icat-cGMP similar to the wild type (Fig. 9). Taken together, we conclude that CNGC5- and CNGC6-mediated ion channels alone are not essential for these ABA responses. It has been suggested that cGMP is required, but not sufficient, for ABA- and nitric oxide-induced stomatal closure (Dubovskaya et al., 2011). Analyses of additional guard cell-expressed cGMP-dependent proteins, in particular CNGCs, in addition to CNGC5 and CNGC6 are needed for understanding a proposed link between cGMP and ABA signaling. This includes analyses of higher order cngc mutants, including expansion of the cngc5-1 cngc6-1 double mutants analyzed here, as higher order (partial) redundancy cannot be excluded at this time.
In this study, we identify two genes, CNGC5 and CNGC6, that are required for the function of a new type of nonselective Ca2+-permeable cation-permeable channel activity in the plasma membrane of plants. CNGC5 and CNGC6 are required for the cGMP activation of Icat-cGMP in the guard cell plasma membrane.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) plants (Columbia, Landsberg erecta, and Ws ecotypes) were grown in soil (Sunrise) in a growth chamber (Conviron) under a 16-h-light/8-h-dark cycle at a photon fluence rate of approximately 75 μmol m−2 s−1 during the day, a humidity of approximately 75%, and a temperature of 21°C ± 0.5°C. The cngc2 (SALK_129133), cngc5-1 (SALK_149893), and cngc6-1 (SALK_042207) mutants were obtained from the Arabidopsis Biological Resource Center. cngc5-2 (FLAG_295E04) and cngc6-2 (FLAG418_D11) were in the Ws background and obtained from the INRA. cngc1 and cngc20 were in the Ws background and kindly provided by Dr. Hillel Fromm (INRA) and the Arabidopsis knockout facility at the University of Wisconsin Biotechnology Center (Krysan et al., 1999; Sunkar et al., 2000).
Patch-Clamp Experiments
Guard cell protoplasts of Arabidopsis were isolated enzymatically as described previously (Vahisalu et al., 2008). For patch-clamp experiments of Icat-cGMP, the bath solution contained 92.5 mm MgGlu, 7.5 mm MgCl2, and 10 mm MES-HCl (5 mm HCl), pH 5.6, and osmolarity was adjusted to 485 mmol L−1 using d-sorbitol. The pipette solutions contained 9.75 mm MgGlu, 0.25 mm MgCl2, 4 mm EGTA, and 10 mm HEPES-Tris, pH 7.1, and osmolarity was adjusted to 500 mmol L−1. Each day, 0.1 mm DTT was freshly added in the bath and pipette solutions, and no NADPH was added in the pipette solution for all cGMP-dependent Icat-cGMP recordings. Control experiments performed in the absence of DTT showed that 8Br-cGMP continued to activate Icat-cGMP (n = 4 guard cells). A voltage ramp protocol from −180 to +20 mV (holding potential, 0 mV; ramp speed, 200 mV s−1) was applied for Icat-cGMP recordings. Whole-cell currents were recorded every minute for 10 min after accessing whole-cell configurations with patch-clamp seal resistances of no less than 10 GΩ. Data prior to 8Br-cGMP exposure were used as preexposure baseline control conditions. Subsequently, cyclic nucleotide-activated currents were recorded 30 times per minute after cyclic nucleotide (8Br-cGMP) was added to the bath solution. For experiments analyzing the effects of cAMP and cGMP added to the pipette solution, the first trace recorded after accessing the whole-cell configuration was used as a baseline control, and the traces recorded subsequently were analyzed for effects of cyclic nucleotides. Liquid junction potential was −4 ± 1 mV, measured as described previously (Ward and Schroeder, 1994) and corrected in Figure 1B. No leak subtraction was applied to the depicted data. A 1 m KCl agar bridge was used as a bath electrode to stabilize bath electrode potentials, which is needed in particular when bath Cl− concentrations are changed during recordings.
Whole-cell patch-clamp recordings of ABA activation of ICa were performed as described previously (Munemasa et al., 2007; Vahisalu et al., 2008). The pipette solution contained 10 mm BaCl2, 4 mm EGTA, and 10 mm HEPES-Tris, pH 7.1, osmolarity was adjusted to 500 mmol L−1 using d-sorbitol, and 0.1 mm DTT and 5 mm NADPH were freshly added each day. The bath solution contained 100 mm BaCl2 and 10 mm MES-Tris, pH 5.6, osmolarity was adjusted to 485 mmol L−1 using d-sorbitol, and 0.1 mm DTT was freshly added each day. A ramp voltage protocol from +20 to −180 mV (holding potential, 0 mV; ramp speed, 200 mV s−1) was used for ICa recordings. Initial control whole-cell currents were recorded once each minute for 16 times starting 1 to 3 min after accessing the whole-cell configurations. The seal resistance was no less than 10 GΩ. Then, ABA was added to the bath solution by perfusion, and guard cell protoplasts were exposed to ABA in the bath solution for 3 min. Subsequently, ICa was recorded for another 16 min. Liquid junction potential of −18 mV was not corrected in the figures, and leak currents were not subtracted.
RNA Isolation, RT-PCR, and Quantitative RT-PCR Experiments
Total RNA was isolated with an RNeasy Plant Mini Kit (Qiagen). After treatment with RNase-free DNase I (Qiagen), first-strand complementary DNA (cDNA) was synthesized from 1 μg of total RNA using a first-strand cDNA synthesis kit (GE Healthcare) according to the manufacturer’s instructions. DNA fragments for CNGC5 and CNGC6 were amplified by 35 PCR cycles using specific primers (Supplemental Table S2). The EF-1α transcript was amplified for 22 PCR cycles.
For real-time quantitative RT-PCR analysis, total RNA was extracted from 3-week-old plants using Trizol reagent (Invitrogen). cDNA was synthesized from RNA using Moloney murine leukemia virus reverse transcriptase (Promega) with oligo(dT)15 primers (Promega). Real-time quantitative PCR was performed using TransStart Green qPCR SuperMix on a Bio-Rad CFX Connect Real Time PCR system according to the manufacturer’s protocols with specific primers (Supplemental Table S2). Quantification of relative gene expression was achieved by normalization to 18S ribosomal RNA.
YFP Fusion Protein Expression Analyses
The coding regions of the CNGC5 and CNGC6 cDNAs were cloned into pENTR/D-TOPO vector using specific primers (Supplemental Table S2), sequenced, and then transferred to the N-terminal fusions to YFP destination vector pH35YG by Gateway LR recombination reaction (Invitrogen). The Agrobacterium tumefaciens strain GV3101 carrying the gene of interest was used and infiltrated at an optical density at 600 nm of 0.5 together with the p19 strain in Nicotiana benthamiana. Mesophyll protoplasts were isolated from the leaves after 5 d of infiltration according to instructions (Asai et al., 2002; Cheng et al., 2002) and then treated with FM4-64 (Invitrogen). Fluorescence imaging was analyzed by confocal microscopy (Nikon Eclipse TE2000-U) using 488-nm excitation and 500- to 550-nm emission filters for YFP or 568-nm excitation and 580- to 650-nm emission filters for FM4-64.
Stomatal Movement Imaging
All experiments were conducted as genotype-blind experiments. ABA-dependent stomatal apertures were analyzed as described previously (Vahisalu et al., 2008; Siegel et al., 2009).
Whole-Rosette Stomatal Conductance Measurements
To analyze CO2- and light/dark transition-induced changes in whole-plant stomatal conductance, we used 25- to 28-d-old plants and a custom-made gas-exchange device. The device and plant growth conditions have been described previously (Kollist et al., 2007; Vahisalu et al., 2008).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers CNGC1 (At5g53130), CNGC2 (At5g15410), CNGC5 (At5g57940), CNGC6 (At2g23980), CNGC15 (At2g28260), and CNGC20 (At3g17700).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Barium currents activated by 8Br-cGMP in guard cells.
Supplemental Figure S2. Na+ permeability of the 8Br-cGMP-activated currents in guard cells.
Supplemental Figure S3. CNGC5 and CNGC6 transcript analysis in cngc5-2 cngc6-2 mutant.
Supplemental Figure S4. Inward currents activated by 20 mm cGMP.
Supplemental Figure S5. Modest inward currents activated by 100 μm cAMP.
Supplemental Figure S6. Twenty millimeters of cAMP failed to activate obvious inward currents in Columbia wild-type guard cells.
Supplemental Figure S7. ABA-activated ICa currents are Mg2+ permeable.
Supplemental Figure S8. Whole plant stomatal conductances in response to light/dark transition.
Supplemental Table S1. Transcriptome-derived raw expression data of CNGC transcripts in guard cells.
Supplemental Table S2. Oligonucleotides used in this work.
Acknowledgments
We thank A. Boisson-Dernier for technical advice and T. Demura for the pH35YG plasmid.
AUTHOR CONTRIBUTIONS
The cGMP-activation of ionic currents in guard cells was initially found by I.M. and impairment in these currents in cngc5, cngc6 and cngc5 cngc6 mutants were found by Y.-F.W. both at UCSD. N.R. and N.N. isolated and genotyped cngc mutant alleles. S.M. and Y.-F.W. independently confirmed functional ABA-activation of ICa channels in mutant guard cells. N.N. analyzed the membrane localizations of YFP-CNGC proteins, and with S.L. and M.H. analyzed ABA-induced stomatal closing in cngc double mutant alleles, and H.-M.R. conducted additional stomatal response analyses. Most experiments were conducted at UCSD with exception of patch clamp experiments in Figures 2E and 8 (S.M.), qPCR experiments in Figures 3C and S3C (H.-M.R., Y.-F.W.) and intact plant stomatal conductance analyses (H.K., I.P.). This project was proposed by J.I.S. Y.-F.W., J.I.S., N.N. and S.M. wrote the manuscript.
Glossary
- [Ca2+]cyt
intracellular free calcium concentration
- CPK
Ca2+-dependent protein kinase
- ABA
abscisic acid
- cGMP
cyclic GMP
- CNGC
cyclic nucleotide-gated channel
- NMDG
N-methyl-d-glucamine
- T-DNA
transfer DNA
- RT
reverse transcription
- Ws
Wassilewskija
- YFP
yellow fluorescent protein
- DTT
dithiothreitol
- cDNA
complementary DNA
- RT-PCR
real-time PCR
References
- Abdel-Hamid H, Chin K, Moeder W, Shahinas D, Gupta D, Yoshioka K. (2013) A suppressor screen of the chimeric AtCNGC11/12 reveals residues important for intersubunit interactions of cyclic nucleotide-gated ion channels. Plant Physiol 162: 1681–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, von Bodman S, Berkowitz GA. (2007) Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell 19: 1081–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali R, Zielinski RE, Berkowitz GA. (2006) Expression of plant cyclic nucleotide-gated cation channels in yeast. J Exp Bot 57: 125–138 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI. (1999) Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell 11: 1785–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GJ, Murata Y, Chu SP, Nafisi M, Schroeder JI. (2002) Hypersensitivity of abscisic acid-induced cytosolic calcium increases in the Arabidopsis farnesyltransferase mutant era1-2. Plant Cell 14: 1649–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GJ, Sanders D. (1994) Two voltage-gated, calcium release channels coreside in the vacuolar membrane of broad bean guard cells. Plant Cell 6: 685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arazi T, Sunkar R, Kaplan B, Fromm H. (1999) A tobacco plasma membrane calmodulin-binding transporter confers Ni2+ tolerance and Pb2+ hypersensitivity in transgenic plants. Plant J 20: 171–182 [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Ashton AR. (2011) Guanylyl cyclase activity in plants? Proc Natl Acad Sci USA 108: E96, author reply [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagué C, Lin B, Alcon C, Flottes G, Malmström S, Köhler C, Neuhaus G, Pelletier G, Gaymard F, Roby D. (2003) HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel ion channel family. Plant Cell 15: 365–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KA, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N, et al. (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol 23: 53–57 [DOI] [PubMed] [Google Scholar]
- Berkowitz GA, Gehring C, Irving HR, Kwezi L. (2011) Reply to Ashton. The putative guanylyl cyclase domain of AtPepR1 and similar plant receptors. Proc Natl Acad Sci USA 108: E97–E98 [Google Scholar]
- Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B. (2004) FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J Microsc 214: 159–173 [DOI] [PubMed] [Google Scholar]
- Brady JD, Rich TC, Le X, Stafford K, Fowler CJ, Lynch L, Karpen JW, Brown RL, Martens JR. (2004) Functional role of lipid raft microdomains in cyclic nucleotide-gated channel activation. Mol Pharmacol 65: 503–511 [DOI] [PubMed] [Google Scholar]
- Brandt B, Brodsky DE, Xue S, Negi J, Iba K, Kangasjärvi J, Ghassemian M, Stephan AB, Hu H, Schroeder JI. (2012) Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc Natl Acad Sci USA 109: 10593–10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero F, Botella MA, Rubio L, Fernández JA, Martínez V, Rubio F. (2012) A Ca2+-sensitive system mediates low-affinity K+ uptake in the absence of AKT1 in Arabidopsis plants. Plant Cell Physiol 53: 2047–2059 [DOI] [PubMed] [Google Scholar]
- Chang F, Yan A, Zhao L-N, Wu WH, Yang Z. (2007) A putative calcium-permeable cyclic nucleotide-gated channel, CNGC18, regulates polarized pollen tube growth. J Integr Plant Biol 49: 1261–1270 [Google Scholar]
- Chen ZH, Hills A, Lim CK, Blatt MR. (2010) Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant J 61: 816–825 [DOI] [PubMed] [Google Scholar]
- Cheng S-H, Willmann MR, Chen HC, Sheen J. (2002) Calcium signaling through protein kinases: the Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol 129: 469–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Fengler KA, Yu IC, Lippok B, Smith RK, Jr, Bent AF. (2000) The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc Natl Acad Sci USA 97: 9323–9328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D. (2010) The language of calcium signaling. Annu Rev Plant Biol 61: 593–620 [DOI] [PubMed] [Google Scholar]
- Dubovskaya LV, Bakakina YS, Kolesneva EV, Sodel DL, McAinsh MR, Hetherington AM, Volotovski ID. (2011) cGMP-dependent ABA-induced stomatal closure in the ABA-insensitive Arabidopsis mutant abi1-1. New Phytol 191: 57–69 [DOI] [PubMed] [Google Scholar]
- Fan LM, Zhao Z, Assmann SM. (2004) Guard cells: a dynamic signaling model. Curr Opin Plant Biol 7: 537–546 [DOI] [PubMed] [Google Scholar]
- Finka A, Cuendet AF, Maathuis FJ, Saidi Y, Goloubinoff P. (2012) Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 24: 3333–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C, Kugler A, Hoth S, Dietrich P. (2013) An IQ domain mediates the interaction with calmodulin in a plant cyclic nucleotide-gated channel. Plant Cell Physiol 54: 573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, Harper JF. (2007) A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci USA 104: 14531–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Han X, Wu J, Zheng S, Shang Z, Sun D, Zhou R, Li B. (2012) A heat-activated calcium-permeable channel—Arabidopsis cyclic nucleotide-gated ion channel 6—is involved in heat shock responses. Plant J 70: 1056–1069 [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, Liese A, Wellmann C, Al-Rasheid KAS, Grill E, et al. (2010) Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci USA 107: 8023–8028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KA, et al. (2009) Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci USA 106: 21425–21430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SE, Zagotta WN. (1995) A histidine residue associated with the gate of the cyclic nucleotide-activated channels in rod photoreceptors. Neuron 14: 177–183 [DOI] [PubMed] [Google Scholar]
- Grabov A, Blatt MR. (1998) Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc Natl Acad Sci USA 95: 4778–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Blatt MR. (1999) A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiol 119: 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo KM, Babourina O, Christopher DA, Borsics T, Rengel Z. (2008) The cyclic nucleotide-gated channel, AtCNGC10, influences salt tolerance in Arabidopsis. Physiol Plant 134: 499–507 [DOI] [PubMed] [Google Scholar]
- Guo KM, Babourina O, Christopher DA, Borsics T, Rengel Z. (2010) The cyclic nucleotide-gated channel AtCNGC10 transports Ca2+ and Mg2+ in Arabidopsis. Physiol Plant 139: 303–312 [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Grossmann G, Frommer WB, Ehrhardt DW. (2010) Opportunities to explore plant membrane organization with super-resolution microscopy. Plant Physiol 154: 463–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DW, Hills A, Kohler B, Blatt MR. (2000) Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc Natl Acad Sci USA 97: 4967–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B (1992) Ionic Channels of Excitable Membrane, Ed 2. Sinauer Associates, Sunderland, MA [Google Scholar]
- Hua D, Wang C, He J, Liao H, Duan Y, Zhu Z, Guo Y, Chen Z, Gong Z. (2012) A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard KE, Siegel RS, Valerio G, Brandt B, Schroeder JI. (2012) Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses. Ann Bot (Lond) 109: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan B, Sherman T, Fromm H. (2007) Cyclic nucleotide-gated channels in plants. FEBS Lett 581: 2237–2246 [DOI] [PubMed] [Google Scholar]
- Kim T-H, Böhmer M, Hu H, Nishimura N, Schroeder JI. (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61: 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler B, Blatt MR. (2002) Protein phosphorylation activates the guard cell Ca2+ channel and is a prerequisite for gating by abscisic acid. Plant J 32: 185–194 [DOI] [PubMed] [Google Scholar]
- Köhler C, Merkle T, Neuhaus G. (1999) Characterisation of a novel gene family of putative cyclic nucleotide- and calmodulin-regulated ion channels in Arabidopsis thaliana. Plant J 18: 97–104 [DOI] [PubMed] [Google Scholar]
- Kollist T, Moldau H, Rasulov B, Oja V, Rämma H, Hüve K, Jaspers P, Kangasjärvi J, Kollist H. (2007) A novel device detects a rapid ozone-induced transient stomatal closure in intact Arabidopsis and its absence in abi2 mutant. Physiol Plant 129: 796–803 [Google Scholar]
- Krysan PJ, Young JC, Sussman MR. (1999) T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 11: 2283–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler A, Köhler B, Palme K, Wolff P, Dietrich P. (2009) Salt-dependent regulation of a CNG channel subfamily in Arabidopsis. BMC Plant Biol 9: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe B, Becker D, Hedrich R, DeSalle R, Hollmann M, Kwak JM, Schroeder JI, Le Novère N, Nam HG, Spalding EP, et al. (2001) The identity of plant glutamate receptors. Science 292: 1486–1487 [DOI] [PubMed] [Google Scholar]
- Laohavisit A, Shang Z, Rubio L, Cuin TA, Véry AA, Wang A, Mortimer JC, Macpherson N, Coxon KM, Battey NH, et al. (2012) Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca2+- and K+-permeable conductance in root cells. Plant Cell 24: 1522–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S. (2009) A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA 106: 21419–21424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, Berkowitz GA. (2004) Cyclic adenosine monophosphate regulates calcium channels in the plasma membrane of Arabidopsis leaf guard and mesophyll cells. J Biol Chem 279: 35306–35312 [DOI] [PubMed] [Google Scholar]
- Leng Q, Mercier RW, Hua BG, Fromm H, Berkowitz GA. (2002) Electrophysiological analysis of cloned cyclic nucleotide-gated ion channels. Plant Physiol 128: 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt N, Kwak JM, Robert N, Waner D, Leonhardt G, Schroeder JI. (2004) Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell 16: 596–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludidi N, Gehring C. (2003) Identification of a novel protein with guanylyl cyclase activity in Arabidopsis thaliana. J Biol Chem 278: 6490–6494 [DOI] [PubMed] [Google Scholar]
- Ma W, Berkowitz GA. (2011) Cyclic nucleotide gated channel and Ca2+-mediated signal transduction during plant senescence signaling. Plant Signal Behav 6: 413–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach J. (2012) Calcium channels and acquired thermotolerance: here comes the sun and it’s all right. Plant Cell 24: 3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie EA. (2000) ABA activates multiple Ca2+ fluxes in stomatal guard cells, triggering vacuolar K+ (Rb+) release. Proc Natl Acad Sci USA 97: 12361–12368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten H, Konrad KR, Dietrich P, Roelfsema MR, Hedrich R. (2007) Ca2+-dependent and -independent abscisic acid activation of plasma membrane anion channels in guard cells of Nicotiana tabacum. Plant Physiol 143: 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. (1990) Abscisic acid-induced elevation of guard cell cytosolic Ca2+ precedes stomatal closure. Nature 343: 186–188 [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. (1992) Visualizing changes in cytosolic-free Ca2+ during the response of stomatal guard cells to abscisic acid. Plant Cell 4: 1113–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Lv D, Wang P, Wang XC, Chen J, Miao C, Song CP. (2006) An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18: 2749–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, Gilliham M, Liu LH, Obermeyer G, Feijó JA. (2011) Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 332: 434–437 [DOI] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang Y, Munemasa S, Wang YF, Andreoli S, Tiriac H, Alonso JM, Harper JF, Ecker JR, et al. (2006) CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol 4: e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Oda K, Watanabe-Sugimoto M, Nakamura Y, Shimoishi Y, Murata Y. (2007) The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells: specific impairment of ion channel activation and second messenger production. Plant Physiol 143: 1398–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder J. (2001) Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J. (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Wang RS, Wilson L, Li S, Zhao Z, Gookin TE, Assmann SM, Albert R. (2010) Boolean modeling of transcriptome data reveals novel modes of heterotrimeric G-protein action. Mol Syst Biol 6: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schroeder JI. (1998) Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282: 287–290 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Qi Z, Verma R, Gehring C, Yamaguchi Y, Zhao Y, Ryan CA, Berkowitz GA. (2010) Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc Natl Acad Sci USA 107: 21193–21198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S. (1989) Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338: 427–430 [Google Scholar]
- Schroeder JI, Hagiwara S. (1990) Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels. Proc Natl Acad Sci USA 87: 9305–9309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurink RC, Shartzer SF, Fath A, Jones RL. (1998) Characterization of a calmodulin-binding transporter from the plasma membrane of barley aleurone. Proc Natl Acad Sci USA 95: 1944–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A, Ilan N, Grantz DA. (1988) Calcium effects on stomatal movement in Commelina communis L.: use of EGTA to modulate stomatal response to light, KCl and CO2. Plant Physiol 87: 583–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RS, Xue S, Murata Y, Yang Y, Nishimura N, Wang A, Schroeder JI. (2009) Calcium elevation-dependent and attenuated resting calcium-dependent abscisic acid induction of stomatal closure and abscisic acid-induced enhancement of calcium sensitivities of S-type anion and inward-rectifying K channels in Arabidopsis guard cells. Plant J 59: 207–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange A, Hedrich R, Roelfsema MRG. (2010) Ca2+-dependent activation of guard cell anion channels, triggered by hyperpolarization, is promoted by prolonged depolarization. Plant J 62: 265–276 [DOI] [PubMed] [Google Scholar]
- Suh SJ, Wang YF, Frelet A, Leonhardt N, Klein M, Forestier C, Mueller-Roeber B, Cho MH, Martinoia E, Schroeder JI. (2007) The ATP binding cassette transporter AtMRP5 modulates anion and calcium channel activities in Arabidopsis guard cells. J Biol Chem 282: 1916–1924 [DOI] [PubMed] [Google Scholar]
- Sunkar R, Kaplan B, Bouché N, Arazi T, Dolev D, Talke IN, Maathuis FJ, Sanders D, Bouchez D, Fromm H. (2000) Expression of a truncated tobacco NtCBP4 channel in transgenic plants and disruption of the homologous Arabidopsis CNGC1 gene confer Pb2+ tolerance. Plant J 24: 533–542 [DOI] [PubMed] [Google Scholar]
- Sutter JU, Campanoni P, Tyrrell M, Blatt MR. (2006) Selective mobility and sensitivity to SNAREs is exhibited by the Arabidopsis KAT1 K+ channel at the plasma membrane. Plant Cell 18: 935–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuleau P, Ward JM, Ranjeva R, Schroeder JI. (1994) Voltage-dependent calcium-permeable channels in the plasma membrane of a higher plant cell. EMBO J 13: 2970–2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunc-Ozdemir M, Rato C, Brown E, Rogers S, Mooneyham A, Frietsch S, Myers CT, Poulsen LR, Malhó R, Harper JF. (2013a) Cyclic nucleotide gated channels 7 and 8 are essential for male reproductive fertility. PLoS ONE 8: e55277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunc-Ozdemir M, Tang C, Ishka MR, Brown E, Groves NR, Myers CT, Rato C, Poulsen LR, McDowell S, Miller G, et al. (2013b) A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol 161: 1010–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart W, Gunawardena AH, Moeder W, Ali R, Berkowitz GA, Yoshioka K. (2007) The chimeric cyclic nucleotide-gated ion channel ATCNGC11/12 constitutively induces programmed cell death in a Ca2+ dependent manner. Plant Mol Biol 65: 747–761 [DOI] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmäki A, Brosché M, Moldau H, Desikan R, et al. (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry AA, Davies JM. (2000) Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc Natl Acad Sci USA 97: 9801–9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincill ED, Bieck AM, Spalding EP. (2012) Ca2+ conduction by an amino acid-gated ion channel related to glutamate receptors. Plant Physiol 159: 40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Fan LM, Zhang WZ, Zhang W, Wu WH. (2004) Ca2+-permeable channels in the plasma membrane of Arabidopsis pollen are regulated by actin microfilaments. Plant Physiol 136: 3892–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Mäser P, Schroeder JI. (2009) Plant ion channels: gene families, physiology, and functional genomics analyses. Annu Rev Physiol 71: 59–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Schroeder JI. (1994) Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell 6: 669–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AAR, Larman MG, Montgomery LT, Taylor JE, Hetherington AM. (2001) The role of calcium in ABA-induced gene expression and stomatal movements. Plant J 26: 351–362 [DOI] [PubMed] [Google Scholar]
- Webb AAR, McAinsh MR, Mansfield TA, Hetherington AM. (1996) Carbon dioxide induces increases in guard cell cytosolic free calcium. Plant J 9: 297–304 [Google Scholar]
- Wu J, Wang S, Gu Y, Zhang S, Publicover SJ, Franklin-Tong VE. (2011) Self-incompatibility in Papaver rhoeas activates nonspecific cation conductance permeable to Ca2+ and K+. Plant Physiol 155: 963–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, Hu H, Ries A, Merilo E, Kollist H, Schroeder JI. (2011) Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J 30: 1645–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Costa A, Leonhardt N, Siegel RS, Schroeder JI. (2008) Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K. (2002) ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol 43: 1473–1483 [DOI] [PubMed] [Google Scholar]
- Young JJ, Mehta S, Israelsson M, Godoski J, Grill E, Schroeder JI. (2006) CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proc Natl Acad Sci USA 103: 7506–7511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IC, Parker J, Bent AF. (1998) Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc Natl Acad Sci USA 95: 7819–7824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S-Y, Yu X-C, Wang X-J, Zhao R, Li Y, Fan R-C, Shang Y, Du SY, Wang XF, Wu FQ, et al. (2007) Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 19: 3019–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JJ, Wei FJ, Wang C, Wu JJ, Ratnasekera D, Liu WX, Wu WH. (2010) Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol 154: 1232–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]