Calcium-dependent protein kinase CPK6 positively functions in induction by yeast elicitor of stomatal closure and inhibition by yeast elicitor of light-induced stomatal opening in Arabidopsis.
Abstract
Yeast elicitor (YEL) induces stomatal closure that is mediated by a Ca2+-dependent signaling pathway. A Ca2+-dependent protein kinase, CPK6, positively regulates activation of ion channels in abscisic acid and methyl jasmonate signaling, leading to stomatal closure in Arabidopsis (Arabidopsis thaliana). YEL also inhibits light-induced stomatal opening. However, it remains unknown whether CPK6 is involved in induction by YEL of stomatal closure or in inhibition by YEL of light-induced stomatal opening. In this study, we investigated the roles of CPK6 in induction by YEL of stomatal closure and inhibition by YEL of light-induced stomatal opening in Arabidopsis. Disruption of CPK6 gene impaired induction by YEL of stomatal closure and inhibition by YEL of light-induced stomatal opening. Activation by YEL of nonselective Ca2+-permeable cation channels was impaired in cpk6-2 guard cells, and transient elevations elicited by YEL in cytosolic-free Ca2+ concentration were suppressed in cpk6-2 and cpk6-1 guard cells. YEL activated slow anion channels in wild-type guard cells but not in cpk6-2 or cpk6-1 and inhibited inward-rectifying K+ channels in wild-type guard cells but not in cpk6-2 or cpk6-1. The cpk6-2 and cpk6-1 mutations inhibited YEL-induced hydrogen peroxide accumulation in guard cells and apoplast of rosette leaves but did not affect YEL-induced hydrogen peroxide production in the apoplast of rosette leaves. These results suggest that CPK6 positively functions in induction by YEL of stomatal closure and inhibition by YEL of light-induced stomatal opening in Arabidopsis and is a convergent point of signaling pathways for stomatal closure in response to abiotic and biotic stress.
Stomata, formed by pairs of guard cells, play a critical role in regulation of plant CO2 uptake and water loss, thus critically influencing plant growth and water stress responsiveness. Guard cells respond to a variety of abiotic and biotic stimuli, such as light, drought, and pathogen attack (Israelsson et al., 2006; Shimazaki et al., 2007; Melotto et al., 2008).
Elicitors derived from microbial surface mimic pathogen attack and induce stomatal closure in various plant species such as Solanum lycopersicum (Lee et al., 1999), Commelina communis (Lee et al., 1999), Hordeum vulgare (Koers et al., 2011), and Arabidopsis (Arabidopsis thaliana; Melotto et al., 2006; Khokon et al., 2010). Yeast elicitor (YEL) induces stomatal closure in Arabidopsis (Klüsener et al., 2002; Khokon et al., 2010; Salam et al., 2013). Our recent studies showed that YEL inhibits light-induced stomatal opening and that protein phosphorylation is involved in induction by YEL of stomatal closure and inhibition by YEL of light-induced stomatal opening (Salam et al., 2013).
Cytosolic Ca2+ has long been recognized as a conserved second messenger in stomatal movement (Shimazaki et al., 2007; Roelfsema and Hedrich 2010; Hubbard et al., 2012). Elevation of cytosolic free Ca2+ concentration ([Ca2+]cyt) is triggered by influx of Ca2+ from apoplast and release of Ca2+ from intracellular stores in guard cell signaling (Leckie et al., 1998; Hamilton et al., 2000; Pei et al., 2000; Garcia-Mata et al., 2003; Lemtiri-Chlieh et al., 2003). The influx of Ca2+ is carried by nonselective Ca2+-permeable cation (ICa) channels that are activated by plasma membrane hyperpolarization and H2O2 (Pei et al., 2000; Murata et al., 2001; Kwak et al., 2003). Elevation of [Ca2+]cyt activates slow anion (S-type) channels and down-regulates inward-rectifying potassium (Kin) channels in guard cells (Schroeder and Hagiwara, 1989; Grabov and Blatt, 1999). The activation of S-type channels is a hallmark of stomatal closure, and the suppression of Kin channels is favorable to stomatal closure but not to stomatal opening (Pei et al., 1997; Kwak et al., 2001; Xue et al., 2011; Uraji et al., 2012).
YEL induces stomatal closure with extracellular H2O2 production, intracellular H2O2 accumulation, activation of ICa channels, and transient [Ca2+]cyt elevations (Klüsener et al., 2002; Khokon et al., 2010). However, it remains to be clarified whether YEL activates S-type channels and inhibits Kin channels in guard cells.
Calcium-dependent protein kinases (CDPKs) are regulators in Ca2+-dependent guard cell signaling (Mori et al., 2006; Zhu et al., 2007; Geiger et al., 2010, 2011; Zou et al., 2010; Munemasa et al., 2011; Brandt et al., 2012; Scherzer et al., 2012). In guard cells, CDPKs regulate activation of S-type and ICa channels and inhibition of Kin channels (Mori et al., 2006; Zou et al., 2010; Munemasa et al., 2011). A CDPK, CPK6, positively regulates activation of S-type channels and ICa channels without affecting H2O2 production in abscisic acid (ABA)- and methyl jasmonate (MeJA)-induced stomatal closure (Mori et al., 2006; Munemasa et al., 2011). CPK6 phosphorylates and activates SLOW ANION CHANNEL-ASSOCIATED1 expressed in Xenopus spp. oocyte (Brandt et al., 2012; Scherzer et al., 2012). These findings underline the role of CPK6 in regulation of ion channel activation and stomatal movement, leading us to test whether CPK6 regulates the induction by YEL of stomatal closure and inhibition by YEL of light-induced stomatal opening.
In this study, we investigated activation of S-type channels and inhibition of Kin channels by YEL and roles of CPK6 in induction by YEL of stomatal closure and inhibition by YEL of light-induced stomatal opening. For this purpose, we examined the effects of mutation of CPK6 on induction by YEL of stomatal closure and inhibition by YEL of light-induced stomatal opening, activation of ICa channels, transient [Ca2+]cyt elevations, activation of S-type channels, inhibition of Kin channels, H2O2 production in leaves, and H2O2 accumulation in leaves and guard cells.
RESULTS
Impairment of Induction by YEL of Stomatal Closure and Inhibition by YEL of Light-Induced Stomatal Opening in cpk6 Mutants
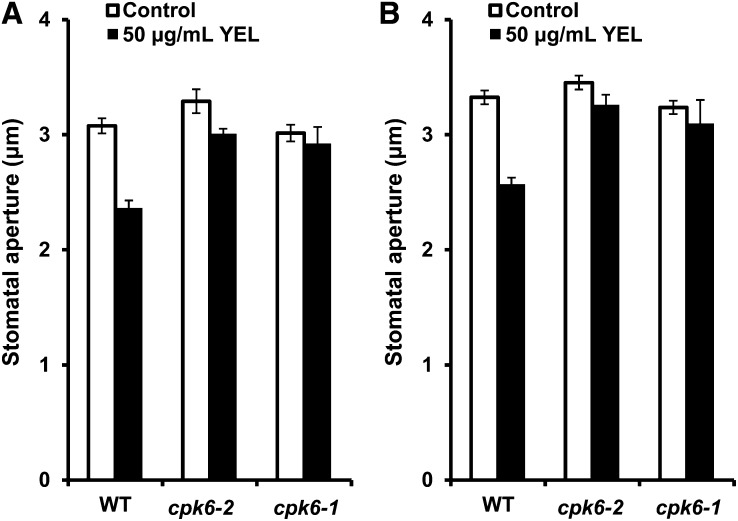
Application of 50 µg mL–1 YEL induced stomatal closure (P < 0.001; Fig. 1A) and inhibited light-induced stomatal opening (P < 0.001; Fig. 1B). YEL-induced stomatal closure was impaired in cpk6-1 (P = 0.60) and cpk6-2 (P = 0.12) mutants (Fig. 1A). Inhibition by YEL of light-induced stomatal opening was impaired in the cpk6-1 (P = 0.55) and the cpk6-2 (P = 0.14) mutants (Fig. 1B). These results suggest that CPK6 is involved in induction by YEL of stomatal closure and inhibition by YEL of light-induced stomatal opening.
Figure 1.
Induction by YEL of stomatal closure and inhibition by YEL of light-induced stomatal opening in the wild type and cpk6-1 and cpk6-2 mutants. A, YEL-induced stomatal closure in the wild type (WT) and cpk6-1 and cpk6-2 mutants. B, Inhibition by YEL of light-induced stomatal opening in the wild type and cpk6-1 and cpk6-2 mutants. Averages from three independent experiments (90 total stomata per bar) are shown. Error bars represent ses (n = 3).
Impairment of Activation of ICa Currents and Induction of Transient [Ca2+]cyt Elevations by YEL in cpk6 Guard Cells
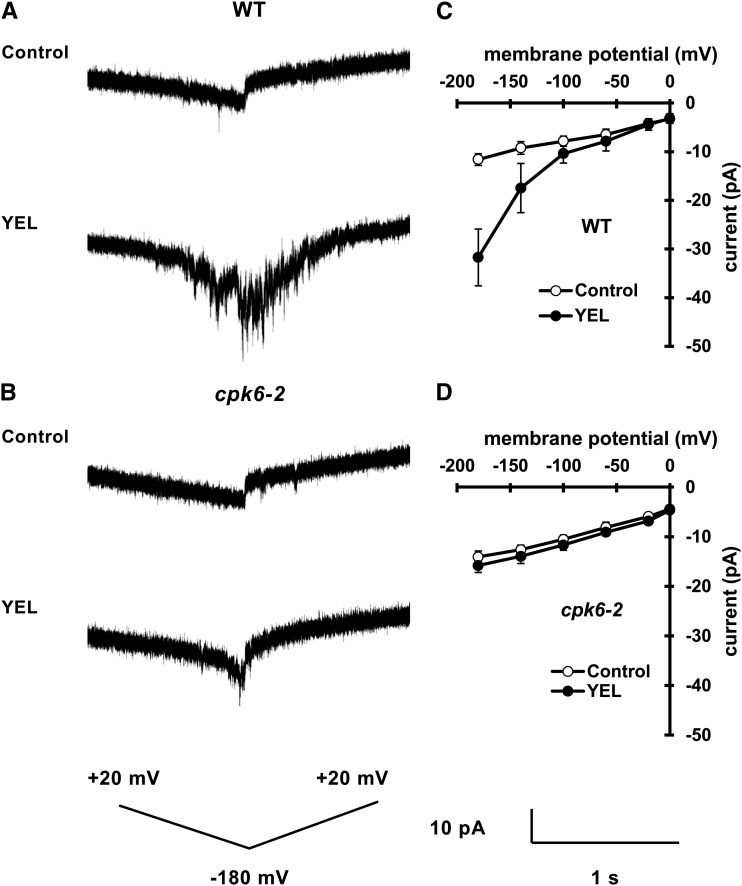
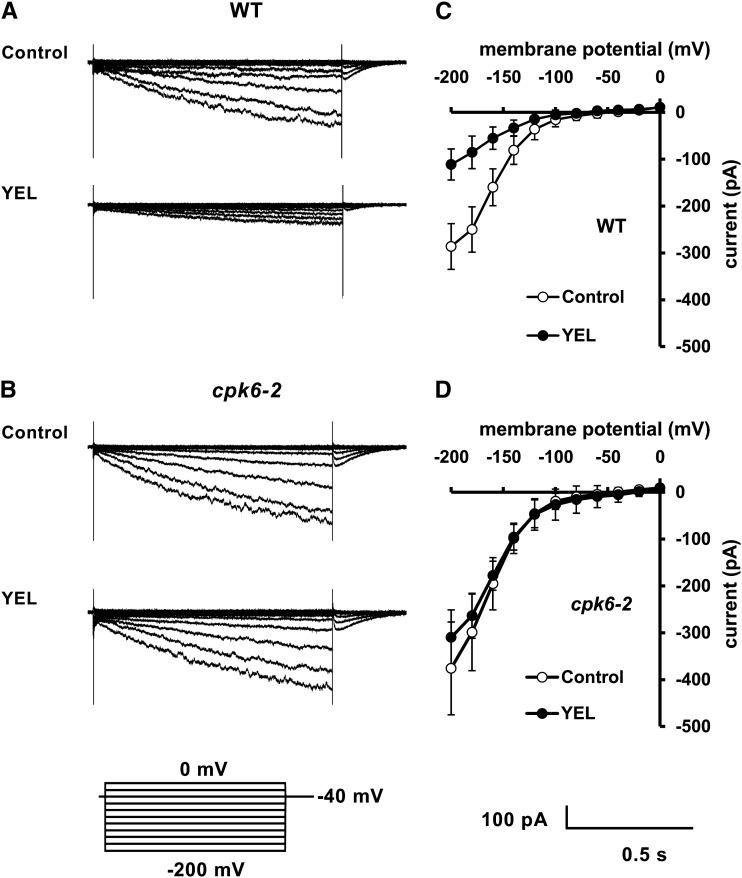
Application of 50 µg mL–1 YEL activated ICa currents in wild-type guard cell protoplasts (GCPs) (P < 0.01 at –180 mV; Fig. 2, A and C) but did not in the cpk6-2 GCPs (P = 0.37 at –180 mV; Fig. 2, B and D). These results suggest that CPK6 is involved in activation of ICa channels by YEL.
Figure 2.
YEL activation of ICa currents in wild-type and cpk6-2 GCPs. A, ICa currents in wild-type (WT) GCPs treated without YEL (top trace) or with 50 µg mL–1 YEL (bottom trace). B, ICa currents in cpk6-2 GCPs treated without YEL (top trace) or with 50 µg mL–1 YEL (bottom trace). C, Current-voltage relationship for YEL activation of ICa currents in wild-type GCPs (n = 5) as recorded in A (white circles, control; black circles, 50 µg mL–1 YEL). D, Current-voltage relationship for YEL activation of ICa currents in cpk6-2 GCPs (n = 5) as recorded in B (white circles, control; black circles, 50 µg mL–1 YEL). A ramp voltage protocol from +20 to –180 mV (holding potential, 0 mV; ramp speed, 200 mV s–1) was used. After making whole-cell configuration, GCPs were recorded 16 times to get averages for control. After adding YEL extracellularly, the GCPs were recorded for 16 times to get averages for the YEL treatment. The interpulse period was 1 min.
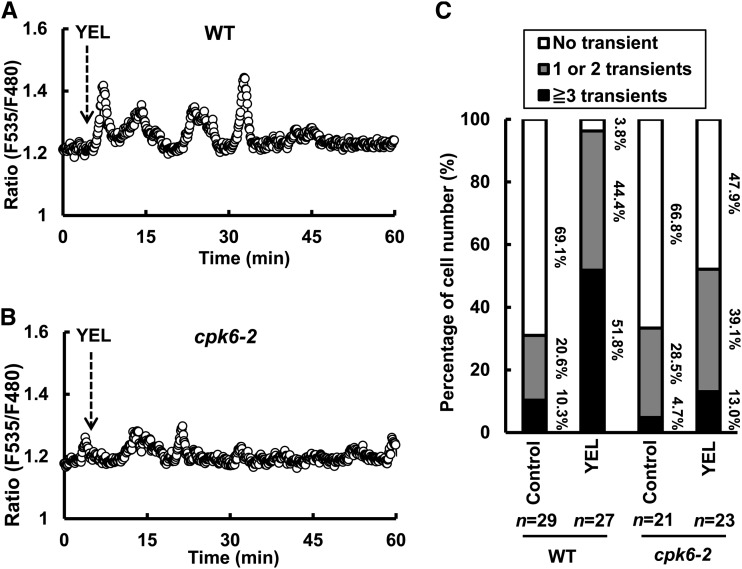
In untreated wild-type plants, 30.9% of guard cells showed [Ca2+]cyt elevations (Fig. 3C). Application of 50 µg mL–1 YEL induced [Ca2+]cyt elevations in 96.2% of wild-type guard cells (Fig. 3, A and C). In untreated cpk6-2 plants, 33.2% of guard cells showed [Ca2+]cyt elevations (Fig. 3C). Application of 50 µg mL–1 YEL induced [Ca2+]cyt elevations in 52.1% of cpk6-2 mutant guard cells (Fig. 3, B and C), which is significantly lower than the percentage of number of guard cells showing [Ca2+]cyt elevations in the wild type (P < 0.01). In untreated cpk6-1 plants, 36.8% of guard cells showed [Ca2+]cyt elevations (Supplemental Fig. S1). Application of 50 µg mL–1 YEL induced [Ca2+]cyt elevations in 44.4% of cpk6-1 mutant guard cells (Supplemental Fig. S1), which is significantly lower than the percentage of number of guard cells showing [Ca2+]cyt elevations in the wild type (P < 0.01). These results suggest that CPK6 is involved in YEL-induced transient [Ca2+]cyt elevations.
Figure 3.
YEL-induced transient [Ca2+]cyt elevations in wild-type and cpk6-2 guard cells expressing YC3.6. A, A representative trace of fluorescence emission ratios (535/480 nm) showing 50 µg mL–1 YEL-induced transient [Ca2+]cyt elevations in wild-type (WT) guard cells. B, A representative trace of fluorescence emission ratios (535/480 nm) showing 50 µg mL–1 YEL-induced transient [Ca2+]cyt elevations in cpk6-2 guard. C, Percentage of number of guard cells showing different number of transient [Ca2+]cyt elevations in wild-type and cpk6-2 guard cells. [Ca2+]cyt elevations were counted when changes in fluorescence emission ratios were more than or equal to 0.1 from the baseline.
Impairment of Activation of S-Type Currents and Suppression of Kin Currents by YEL in cpk6 Guard Cells
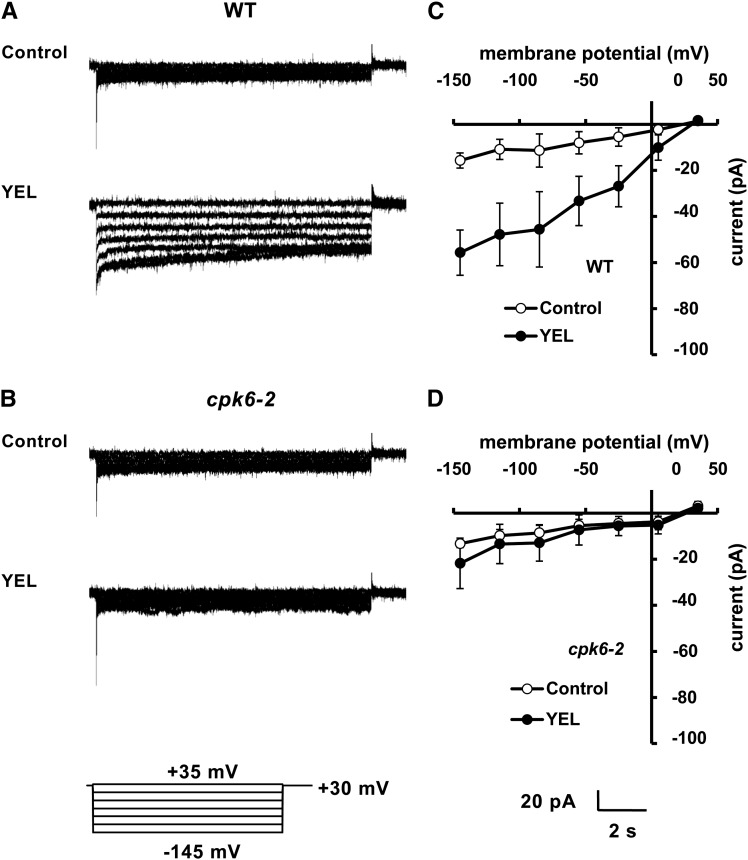
Application of 50 µg mL–1 YEL activated S-type currents in wild-type GCPs (P < 0.0001 at –145 mV; Fig. 4, A and C) but did not in cpk6-2 GCPs (P = 0.12 at –145 mV; Fig. 4, B and D) or cpk6-1 GCPs (current = –16.26 ± 5.43 pA at –145 mV, n = 3; data not shown). YEL at 50 µg mL–1 suppressed Kin currents in wild-type GCPs (P < 0.0001 at –200 mV; Fig. 5, A and C) but did not in cpk6-2 GCPs (P = 0.15 at –200 mV; Fig. 5, B and D) or cpk6-1 GCPs (current = –290.75 ± 25.68 pA at –200 mV, n = 4; data not shown). These results suggest that CPK6 is involved in activation of S-type channels by YEL and suppression of Kin channels by YEL.
Figure 4.
YEL activation of S-type currents in wild-type (WT) and cpk6-2 GCPs. A, S-type currents in wild-type GCPs treated without (top trace) or with 50 µg mL–1 YEL (bottom trace). B, S-type currents in cpk6-2 GCPs treated without (top trace) or with 50 µg mL–1 YEL (bottom trace). C, Steady-state current-voltage relationship for YEL activation of S-type currents in wild-type GCPs as recorded in A (white circles, control; black circles, YEL). D, Steady-state current-voltage relationship for YEL activation of S-type currents in cpk6-2 GCPs as recorded in B (white circles, control; black circles, YEL). The voltage protocol was stepped up from +35 mV to –145 mV in 30-mV decrements (holding potential, +30 mV). GCPs were treated with YEL for 2 h before recordings. Each datum point was obtained from five GCPs. Error bars represent ses.
Figure 5.
YEL inhibition of Kin currents in wild-type (WT) and cpk6-2 GCPs. A, Kin currents in wild-type GCPs treated without (top trace) or with 50 µg mL–1 YEL (bottom trace). B, Kin currents in cpk6-2 GCPs treated without (top trace) or with 50 µg mL–1 YEL (bottom trace). C, Steady-state current-voltage relationship for YEL inhibition of Kin currents in wild-type GCPs as recorded in A (white circles, control; black circles, YEL). D, Steady-state current-voltage relationship for YEL inhibition of Kin currents in cpk6-2 GCPs as recorded in B (white circles, control; black circles, YEL). The voltage protocol was stepped up from 0 to –200 mV in 20-mV decrements (holding potential, –40 mV). GCPs were treated with YEL for 2 h before recordings. Each datum point was obtained from at least seven GCPs. Error bars represent ses.
Effects of cpk6 Mutations on YEL-Induced H2O2 Production and Accumulation
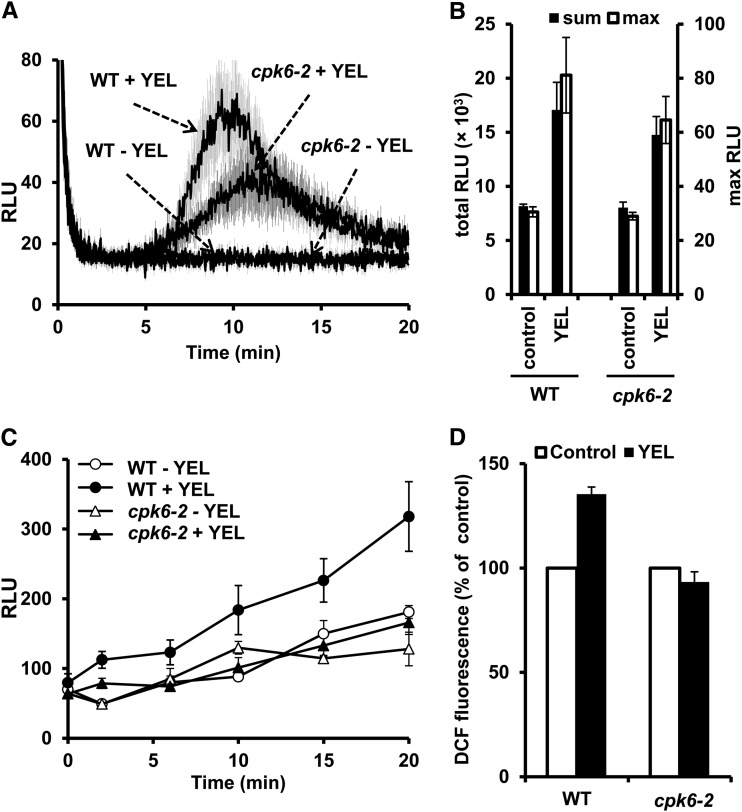
Application of 50 µg mL–1 YEL significantly induced H2O2 production in apoplast of wild-type, cpk6-2, and cpk6-1 leaf tissues. There is no significant difference in YEL-induced apoplastic H2O2 production between the wild type and the cpk6-2 mutant (P = 0.46 for total relative light units (RLU); P = 0.33 for max RLU; Fig. 6, A and B) and between the wild type and the cpk6-1 mutant (P = 0.81 for total RLU; P = 0.36 for max RLU; Supplemental Fig. S2, A and B).
Figure 6.
YEL-induced H2O2 production in apoplast of leaf tissues and H2O2 accumulation in apoplast of leaf tissues and guard cells. A, H2O2 production induced by 50 µg mL–1 YEL in apoplast of leaf tissues of the wild type (WT) and cpk6-2 mutant. B, Total and maximal H2O2 production induced by YEL as recorded in A. The luminescence was measured between 1 and 20 min after adding YEL, where the luminescence at each sampling point was integrated for 2 s. “Total RLU” is the sum of luminescence between 1 and 20 min, and “max RLU” is the highest luminescence. C, H2O2 accumulation induced by 50 µg mL–1 YEL in the apoplast of leaf tissues of the wild type and cpk6-2 mutant. D, H2O2 accumulation induced by 50 µg mL–1 YEL in guard cells of the wild type and cpk6-2 mutant. H2O2 accumulation was expressed as the percentage of 2',7'-dichlorofluorescein (DCF) fluorescence levels. Averages from three independent experiments (more than 150 total guard cells per bar in total) are shown.
Application of 50 µg mL–1 YEL induced H2O2 accumulation in the apoplast of the wild-type leaf tissues (P < 0.05 at 20 min, Fig. 6C; P < 0.05 at 20 min, Supplemental Fig. S2C) but did not in the cpk6-2 leaf tissues (P = 0.24 at 20 min, Fig. 6C) or the cpk6-1 leaf tissues (P = 0.90 at 20 min, Supplemental Fig. S2C). Application of 50 µg mL–1 YEL induced H2O2 accumulation in wild-type guard cells (P < 0.01, Fig. 6D; P < 0.05, Supplemental Fig. S2D) but did not in the cpk6-2 guard cells (P = 0.24, Fig. 6D) or the cpk6-1 guard cells (P = 0.78, Supplemental Fig. S2D). These results suggest that CPK6 is involved in YEL-induced H2O2 accumulation in the apoplast of leaf tissues and in the cytoplasm of guard cells but not in H2O2 production.
DISCUSSION
CPK6 Is Involved in Induction by YEL of Stomatal Closure and Inhibition by YEL of Light-Induced Stomatal Opening
Our previous studies using the cpk6-1 and cpk6-2 mutants reveal that CPK6 positively regulates ABA- and MeJA-induced stomatal closure in Arabidopsis (Mori et al., 2006; Munemasa et al., 2011). In this study, the mutations of CPK6 impaired the YEL-induced stomatal closure (Fig. 1). These results suggest that CPK6 is involved in YEL-induced stomatal closure and a key component shared with ABA- and MeJA-induced stomatal closure. Moreover, CPK3 functions in ABA-induced stomatal closure but not in MeJA- or YEL-induced stomatal closure (Mori et al., 2006; Munemasa et al., 2011; data not shown). Hence, CPK3 may function in stomatal response to drought stress rather than biotic stress, and regulation of CPK3 activation may be different from that of CPK6 in guard cells.
It has been reported that CPK4, CPK10, and CPK11 function as positive regulators in inhibition by ABA of light-induced stomatal opening (Zhu et al., 2007; Zou et al., 2010). In this study, the mutations of CPK6 impaired the inhibition by YEL of light-induced stomatal opening (Fig. 1), which suggests that CPK6 functions in YEL-induced inhibition of stomatal opening. Taken together, CPK6 may function as a key regulator in induction by YEL of stomatal closure and inhibition by YEL of light-induced stomatal opening.
CPK6 Is Involved in YEL-Induced H2O2 Accumulation But Not in H2O2 Production
H2O2 functions as an important second messenger during stomatal closure (Pei et al., 2000; Zhang et al., 2001; Khokon et al., 2011; Salam et al., 2013). H2O2 activates ICa channels in guard cells, which triggers influx of Ca2+ through ICa channels from apoplast. Consequently, the influx of Ca2+ initiates transient elevations of [Ca2+]cyt in guard cells (Pei et al., 2000; Allen et al., 2001; Murata et al., 2001). Releasing of Ca2+ from internal stores contributes to the following transient elevations of [Ca2+]cyt (Garcia-Mata et al., 2003; Lemtiri-Chlieh et al., 2003).
H2O2 is mainly formed in the apoplast from superoxide produced by two NADPH oxidase catalytic subunits, AtrbohD and AtrbohF, in ABA and MeJA signaling (Kwak et al., 2003; Suhita et al., 2004; Jannat et al., 2011, 2012), and H2O2 is produced by apoplastic peroxidases in YEL signaling (Khokon et al., 2010), leading to the accumulation of H2O2 in guard cells. CPK6 is not involved in ABA- and MeJA-induced H2O2 accumulation in guard cells (Munemasa et al., 2011), whereas CPK6 is involved in YEL-induced H2O2 accumulation in guard cells but not in YEL-induced H2O2 production (Fig. 6, A and B; Supplemental Fig. S2, A and B). It appears that CPK6 is not involved in ABA-, MeJA-, or YEL-induced H2O2 production, but there is a difference in involvement of CPK6 between ABA-induced H2O2 accumulation and YEL-induced H2O2 accumulation, suggesting that there is a difference in the H2O2 scavenging system between ABA signaling and YEL signaling during stomatal closure.
In catalase mutants and catalase inhibitor-treated wild-type plants, ABA- and MeJA-induced stomatal closures are significantly enhanced, suggesting that H2O2 scavenging mechanisms are involved in stomatal closure (Jannat et al., 2011, 2012). An alcoholic yeast (Saccharomyces cerevisiae) extract does not have catalase activity but can accelerate activity of catalases and/or protect catalases from inactivation by H2O2 (Kreke et al., 1945; Sumner and Sisler, 1946; Kreke and Maloney, 1948). Therefore, CPK6 may negatively regulate the YEL-activated H2O2 scavenging system, including catalases. However, ABA does not significantly affect catalase activities (Jannat et al., 2011). This is why ABA induces H2O2 accumulation in the cpk6 mutant as well as in the wild type.
CPK6 Positively Functions in Activation of ICa Channels and Transient [Ca2+]cyt Elevations Induced by YEL
ICa channels are activated by H2O2, ABA, MeJA, and elicitors (Hamilton et al., 2000; Pei et al., 2000; Klüsener et al., 2002; Munemasa et al., 2007). The regulation of ICa channels also involves phosphorylation and [Ca2+]cyt (Hamilton et al., 2000; Köhler and Blatt, 2002). CPK6 positively regulates the activation of ICa channels in ABA and MeJA signaling (Mori et al., 2006; Munemasa et al., 2011). In this study, YEL-induced ICa currents were impaired in the cpk6-2 guard cells (Fig. 2). These results indicate that CPK6 is involved in activation of ICa channels induced by abiotic and biotic stimuli.
Activation by YEL of ICa channels required NADPH in the pipette solution (Klüsener et al., 2002; data not shown), and YEL-induced stomatal closure was not inhibited by an NADPH oxidase inhibitor, diphenylene iodonium chloride, or atrbohD atrbohF double mutation (Khokon et al., 2010). These results suggest that activation by YEL of ICa channels is modulated by redox status but not by activation of NADPH oxidases.
YEL-induced stomatal closure is accompanied by transient [Ca2+]cyt elevations (Klüsener et al., 2002; Khokon et al., 2010; Salam et al., 2013; Fig. 3; Supplemental Fig. S1). In this study, mutations of CPK6 impaired the YEL-induced [Ca2+]cyt elevations (Fig. 3; Supplemental Fig. S1). The YEL-induced ICa currents were abolished, and the YEL-induced [Ca2+]cyt elevations were impaired in the cpk6-2 mutant. Hence, these results indicate that the impaired influx of Ca2+ from extracellular space contributes to the impaired [Ca2+]cyt elevations.
CPK6 Positively Functions in Activation of S-Type Channels and Inhibition of Kin Channels Induced by YEL
Activation of S-type channels is essential for stomatal closure induced by various stimuli, such as H2O2, Ca2+, ABA, MeJA, CO2, and elicitors (Schroeder and Hagiwara, 1989; Pei et al., 1997; Munemasa et al., 2007; Koers et al., 2011; Xue et al., 2011; Hua et al., 2012). YEL was reported to activate anion channel in root cells (Wu et al., 2011). This study shows that YEL activated S-type channels in guard cells (Fig. 4). CPK6 positively regulates the activation of S-type channels in ABA and MeJA signaling (Mori et al., 2006; Munemasa et al., 2011). In this study, the activation by YEL of S-type currents was impaired by the cpk6-2 mutation (Fig. 4) and cpk6-1 mutation (data not shown), suggesting that CPK6 is a key component in activation of S-type channels induced by abiotic and biotic stimuli in Arabidopsis guard cells.
Inhibition of Kin channels is preferred in stomatal closure and impairs light-induced stomatal opening (Schroeder et al., 1987; Kwak et al., 2001; Khokon et al., 2011; Uraji et al., 2012). A 22-amino acid sequence of the conserved N-terminal part of flagellin, flg22, inhibits Kin channels in Arabidopsis guard cells (Zhang et al., 2008). In this study, YEL inhibited the Kin currents (Fig. 5). It was reported that CPK10 positively regulates the inhibition by ABA of Kin channels (Zou et al., 2010). The presented results showed that the cpk6-2 (Fig. 5) and cpk6-1 (data not shown) mutations impaired the inhibition by YEL of the Kin currents, suggesting CPK6 is a negative regulator of Kin channel activation.
ABA signaling is mediated by a calcium-dependent pathway and a calcium-independent pathway (Levchenko et al., 2005; Lee et al., 2009; Hubbard et al., 2012). YEL-induced stomatal closure is accompanied by transient [Ca2+]cyt elevations (Klüsener et al., 2002; Khokon et al., 2010; Salam et al., 2013; Fig. 3; Supplemental Fig. S1), whereas flg22 induces stomatal closure, in which a Ca2+-independent protein kinase, Open Stomata1, is involved (Melotto et al., 2006). Hence, YEL signaling, like ABA signaling, may employ a calcium-independent pathway.
CONCLUSION
The presented results suggest that CPK6 positively functions in induction by YEL of stomatal closure and inhibition by YEL of light-induced stomatal opening and is a convergent point of signaling pathways for stomatal closure in response to abiotic and biotic stress.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) wild type (ecotype Columbia), cpk6-1 (SALK_093308), and cpk6-2 (SALK_033392) were grown in pots containing a mixture of 70% (v/v) vermiculite (Asahi-Kogyo) and 30% (v/v) kureha soil (Kureha Chemical) in a growth chamber (photon flux density of 80 µmol m–2 s–1 under a 16-h-light/8-h-dark regime). The temperature and relative humidity in the growth chamber were 22°C ± 2°C and 60% ± 10%. Twice or three times a week, 0.1% (v/v) Hyponex solution was provided to the plants. [Ca2+]cyt in guard cells was measured using a Ca2+-sensing fluorescent protein, yellow cameleon3.6 (YC3.6) (Nagai et al., 2004). To obtain YC3.6-expressing cpk6-2 mutant, cpk6-2 mutants were crossed with wild-type plants that had previously been transformed with YC3.6.
Elicitor Preparation
YEL was prepared as described previously (Schumacher et al., 1987). Briefly, 200 g of commercial baker’s yeast (Saccharomyces cerevisiae) was dissolved in 300 mL of 20 mm sodium citrate buffer (pH 7.0) and autoclaved at 121°C and 110,000 Pa for 60 min. The autoclaved suspension was centrifuged at 10,000g for 20 min, and the supernatant and ethanol were mixed in equal volume and stirred gently overnight. The mixture was centrifuged at 10,000g for 20 min, and the supernatant was mixed with 3 times of its volume of ethanol gently overnight. The precipitate obtained by decantation was lyophilized and stored at –80°C until use. Mass spectrometry analysis revealed that YEL at 50 µg mL–1 does not contain ABA, MeJA, or jasmonic acid at concentration higher than 1 µm (data not shown), and transcription analysis revealed that YEL at 50 µg mL–1 did not increase transcript levels of ABA-responsive gene Responsive To Dessication29B and jasmonic acid-responsive gene Vegetative Storage Protein1 (data not shown). Note that YEL contains yeast mannan as a major biologically active compound (Schumacher et al., 1987).
Stomatal Aperture Measurement
Fully expanded young leaves from 4- to 5-week-old plants were excised for stomatal aperture measurements as described previously (Uraji et al., 2012). For assays of light-induced stomatal opening, leaves were floated on assay solution containing 5 mm KCl, 50 μm CaCl2, and 10 mm MES-Tris (pH 6.15) with their adaxial surface upward in the dark for 2 h to close the stomata. After adding YEL (50 µg mL–1), the leaves were kept in the light (80 μmol m–2 s–1) for 3 h before measurement. For assays of stomatal closure, leaves were floated on the assay solution in the light for 2 h to open the stomata. Then YEL (50 µg mL–1) was added, and the leaves were kept in the light for 2 h before measurement. For measurement of stomatal apertures, the leaves were shredded for 30 s, and epidermal tissues were collected using nylon mesh. Thirty stomatal apertures were measured for each sample.
Patch-Clamp Measurement
Current measurements of Kin, ICa, and S-type channels in Arabidopsis guard cells were performed as described previously (Mori et al., 2006; Uraji et al., 2012). Arabidopsis GCPs were prepared from rosette leaves of 4- to 6-week-old plants with the digestion solution containing 1.0% (w/v) Cellulase R10, 0.5% (w/v) Macerozyme R10, 0.5% (w/v) bovine serum albumin, 0.1% (w/v) kanamycin, 10 mm ascorbic acid, 0.1 mm KCl, 0.1 mm CaCl2, and 500 mm d-mannitol (pH 5.5) with KOH. Whole-cell currents were recorded using a CEZ-2200 patch-clamp amplifier (Nihon Kohden). No leak subtraction was applied for all current-voltage curves. For data analysis, pCLAMP 10.3 software (Molecular Devices) was used.
For Kin channel current measurement, pipette solution contained 30 mm KCl, 70 mm K-Glu, 2 mm MgCl2, 3.35 mm CaCl2, 6.7 mm EGTA, and 10 mm HEPES-Tris (pH 7.1). Bath solution contained 30 mm KCl, 2 mm MgCl2, 40 mm CaCl2, and 10 mm MES-Tris (pH 5.5). For ICa channel current measurement, pipette solution contained 10 mm BaCl2, 0.1 mm dithiothreitol, 5 mm NADPH, 4 mm EGTA, and 10 mm HEPES-Tris (pH 7.1). Bath solution contained 100 mm BaCl2, 0.1 mm dithiothreitol, 1 mm CaCl2, and 10 mm MES-Tris (pH 5.6). For S-type channel current measurement, pipette solution contained 150 mm CsCl, 2 mm MgCl2, 6.7 mm EGTA, 5.58 mm CaCl2 (free Ca2+ concentration, 2 µm), 5 mm ATP, and 10 mm HEPES-Tris (pH 7.1). Bath solution contained 30 mm CsCl, 2 mm MgCl2, 1 mm CaCl2, and 10 mm MES-Tris (pH 5.6). In all cases, osmolarity was adjusted to 500 mmol kg–1 (pipette solutions) and 485 mmol kg–1 (bath solutions) with d-sorbitol.
Imaging of [Ca2+]cyt in Guard Cells
Four- to 6-week-old wild-type and cpk6-2 plants expressing YC3.6 were used for the measurement of [Ca2+]cyt in guard cells as described previously (Uraji et al., 2012). The abaxial side of an excised leaf was gently mounted on a glass slide with a medical adhesive (stock no. 7730; Hollister), followed by removal of the adaxial epidermis and the mesophyll tissue with a razor blade to keep the lower epidermis intact on the slide. The remaining abaxial epidermis was incubated in solution containing 5 mm KCl, 50 μm CaCl2, and 10 mm MES-Tris (pH 6.15) in the light for 2 h at 22°C to promote stomatal opening. Turgid guard cells were used to measure [Ca2+]cyt. Guard cells were treated with 50 µg mL–1 YEL using a peristaltic pump at 5 min after monitoring. For dual-emission ratio imaging of YC3.6, we used a 440AF21 excitation filter, a 445DRLP dichroic mirror, a 480DF30 emission filter for cyan fluorescent protein (CFP), and a 535DF25 emission filter for yellow fluorescent protein (YFP). The CFP and YFP fluorescence intensity of guard cells were imaged and analyzed using the W-View system and AQUA COSMOS software (Hamamatsu Photonics). CFP and YFP fluorescence were simultaneously monitored.
Detection of H2O2 in Apoplast of Leaf Tissues and Guard Cells
H2O2 produced in apoplast of leaf tissues was measured by a luminol-based assay (Trujillo et al., 2008). Leaf discs (3 mm in diameter) of 4- to 6-week-old plants were incubated in water for 16 h. H2O2 production was triggered by 100 µL reaction solution (100 µm luminol, 1 µg mL–1 horseradish peroxidase, and 50 µg mL–1 YEL). The luminescence was measured between 1 and 20 min after adding YEL, where the luminescence at each sampling point was integrated for 2 s, using a luminometer (AB2200, Atto). The experiments were repeated for seven times. Note that the assay using luminol monitors time course of production of H2O2 in extracellular space including cell wall.
We measured apoplastic H2O2 accumulation as the amount of H2O2 leaked from leaf tissues to 100 µL of a bathing solution. After incubated in water for 16 h, leaf discs were incubated in 100 µL water with or without YEL (50 µg mL–1). At indicated time of treatment, H2O2 in the bathing solution, leaked from the leaf discs, was measured by a luminol-based assay. Briefly, 60 µL of the bathing solution was reacted with 40 µL of a reaction solution containing 50 µg mL–1 YEL, 250 µm luminol, and 2.5 µg mL–1 horseradish peroxidase. The luminescence was measured for 6 s. The experiments were repeated six times.
H2O2 accumulation in guard cells was evaluated using 2',7'-dichlorodihydrofluorescein diacetate (H2DCF-DA; Uraji et al., 2012). Epidermal tissues were isolated after leaves of 4- to 6-week-old plants shredded with a commercial blender. The epidermal tissues were incubated in medium containing 5 mm KCl, 50 µm CaCl2, and 10 mm MES-Tris (pH 6.15) in the light at room temperature for 3 h. After incubation, 50 µm H2DCF-DA was added to the medium. The epidermal tissues were incubated in the dark at room temperature for 30 min, and then the excess dye was washed out with the same medium. The dye-loaded tissues were treated with 50 µg mL–1 YEL in the dark at room temperature for 20 min. The stained guard cells were imaged using a fluorescence microscope (Biozero BZ-8000, Keyence) with an OP-66835 BZ GFP filter (excitation wavelength, 480/30 nm; absorption wavelength, 510 nm; and dichroic mirror wavelength, 505 nm). Fluorescence pictures of the guard cells were taken at optical magnification of ×40 with exposure time of 1.5 s. Excited light intensity was attenuated by a FM20 filter (transmission rate of 20%). The fluorescence levels of the guard cells were determined using ImageJ software (National Institutes of Health) and expressed as percentage of the control. Note that H2DCF-DA permeates plasma membrane and is hydrolyzed by cytosolic esterases to yield a free form 2',7'-dichlorodihydrofluorescein in cytosol and that 2',7'-dichlorodihydrofluorescein can react with H2O2 in the presence of cytosolic peroxidases to produce the fluorescent oxidized form.
Statistical Analysis
The significance of differences between data sets was assessed by Student’s t test. The response of [Ca2+]cyt was assessed by χ2 test. Differences were considered significant for P < 0.05.
Arabidopsis Genome Initiative number for the gene discussed in this article is as follows: CPK6 (AT2G17290).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. YEL-induced transient [Ca2+]cyt elevations in cpk6-1 guard cells expressing YC3.6.
Supplemental Figure S2. YEL-induced H2O2 production in apoplast of leaf tissues and H2O2 accumulation in apoplast of leaf tissues and guard cells.
Acknowledgments
We thank Teruhiko Nitoda (Okayama University) and Kohei Asao (Okayama University) for their technical help.
Glossary
- YEL
yeast elicitor
- [Ca2+]cyt
cytosolic free Ca2+ concentration
- ICa
Ca2+-permeable cation
- Kin
inward-rectifying potassium
- ABA
abscisic acid
- MeJA
methyl jasmonate
- GCP
guard cell protoplast
- RLU
relative light units
- H2DCF-DA
2',7'-dichlorodihydrofluorescein diacetate
References
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI. (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Brandt B, Brodsky DE, Xue S, Negi J, Iba K, Kangasjärvi J, Ghassemian M, Stephan AB, Hu H, Schroeder JI. (2012) Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc Natl Acad Sci USA 109: 10593–10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR. (2003) Nitric oxide regulates K+ and Cl– channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci USA 100: 11116–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Maierhofer T, Al-Rasheid KAS, Scherzer S, Mumm P, Liese A, Ache P, Wellmann C, Marten I, Grill E, et al. (2011) Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci Signal 4: ra32. [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, Liese A, Wellmann C, Al-Rasheid KA, Grill E, et al. (2010) Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci USA 107: 8023–8028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Blatt MR. (1999) A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiol 119: 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DWA, Hills A, Kohler B, Blatt MR. (2000) Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc Natl Acad Sci USA 97: 4967–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua D, Wang C, He J, Liao H, Duan Y, Zhu Z, Guo Y, Chen Z, Gong Z. (2012) A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard KE, Siegel RS, Valerio G, Brandt B, Schroeder JI. (2012) Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses. Ann Bot (Lond) 109: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelsson M, Siegel RS, Young J, Hashimoto M, Iba K, Schroeder JI. (2006) Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr Opin Plant Biol 9: 654–663 [DOI] [PubMed] [Google Scholar]
- Jannat R, Uraji M, Hossain MA, Islam MM, Nakamura Y, Mori IC, Murata Y. (2012) Catalases negatively regulate methyl jasmonate signaling in guard cells. J Plant Physiol 169: 1012–1016 [DOI] [PubMed] [Google Scholar]
- Jannat R, Uraji M, Morofuji M, Islam MM, Bloom RE, Nakamura Y, McClung CR, Schroeder JI, Mori IC, Murata Y. (2011) Roles of intracellular hydrogen peroxide accumulation in abscisic acid signaling in Arabidopsis guard cells. J Plant Physiol 168: 1919–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokon AR, Okuma E, Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y. (2011) Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ 34: 434–443 [DOI] [PubMed] [Google Scholar]
- Khokon MAR, Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y. (2010) Yeast elicitor-induced stomatal closure and peroxidase-mediated ROS production in Arabidopsis. Plant Cell Physiol 51: 1915–1921 [DOI] [PubMed] [Google Scholar]
- Klüsener B, Young JJ, Murata Y, Allen GJ, Mori IC, Hugouvieux V, Schroeder JI. (2002) Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol 130: 2152–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koers S, Guzel-Deger A, Marten I, Roelfsema MRG. (2011) Barley mildew and its elicitor chitosan promote closed stomata by stimulating guard-cell S-type anion channels. Plant J 68: 670–680 [DOI] [PubMed] [Google Scholar]
- Köhler B, Blatt MR. (2002) Protein phosphorylation activates the guard cell Ca2+ channel and is a prerequisite for gating by abscisic acid. Plant J 32: 185–194 [DOI] [PubMed] [Google Scholar]
- Kreke CW, Bartlett SMD, Smalt SMA. (1945) An accelerator of catalase activity. J Biol Chem 158: 469–474 [Google Scholar]
- Kreke CW, Maloney P. (1948) Acceleration of catalase action. J Biol Chem 172: 317–324 [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI. (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Murata Y, Baizabal-Aguirre VM, Merrill J, Wang M, Kemper A, Hawke SD, Tallman G, Schroeder JI. (2001) Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis. Plant Physiol 127: 473–485 [PMC free article] [PubMed] [Google Scholar]
- Leckie CP, McAinsh MR, Allen GJ, Sanders D, Hetherington AM. (1998) Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose. Proc Natl Acad Sci USA 95: 15837–15842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Choi H, Suh S, Doo IS, Oh KY, Choi EJ, Schroeder Taylor AT, Low PS, Lee Y. (1999) Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol 121: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S. (2009) A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA 106: 21419–21424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, MacRobbie EA, Webb AA, Manison NF, Brownlee C, Skepper JN, Chen J, Prestwich GD, Brearley CA. (2003) Inositol hexakisphosphate mobilizes an endomembrane store of calcium in guard cells. Proc Natl Acad Sci USA 100: 10091–10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenko V, Konrad KR, Dietrich P, Roelfsema MRG, Hedrich R. (2005) Cytosolic abscisic acid activates guard cell anion channels without preceding Ca2+ signals. Proc Natl Acad Sci USA 102: 4203–4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Underwood W, He SY. (2008) Role of stomata in plant innate immunity and foliar bacterial diseases. Annu Rev Phytopathol 46: 101–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980 [DOI] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang Y, Munemasa S, Wang YF, Andreoli S, Tiriac H, Alonso JM, Harper JF, Ecker JR, et al. (2006) CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol 4: e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y. (2011) The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiol 155: 553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Oda K, Watanabe-Sugimoto M, Nakamura Y, Shimoishi Y, Murata Y. (2007) The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiol 143: 1398–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder J. (2001) Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. (2004) Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci USA 101: 10554–10559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. (1997) Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9: 409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Roelfsema MR, Hedrich R. (2010) Making sense out of Ca2+ signals: their role in regulating stomatal movements. Plant Cell Environ 33: 305–321 [DOI] [PubMed] [Google Scholar]
- Salam MA, Jammes F, Hossain MA, Ye W, Nakamura Y, Mori IC, Kwak JM, Murata Y. (2013) Two guard cell-preferential MAPKs, MPK9 and MPK12, regulate YEL signalling in Arabidopsis guard cells. Plant Biol (Stuttg) 15: 436–442 [DOI] [PubMed] [Google Scholar]
- Scherzer S, Maierhofer T, Al-Rasheid KAS, Geiger D, Hedrich R. (2012) Multiple calcium-dependent kinases modulate ABA-activated guard cell anion channels. Mol Plant 5: 1409–1412 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S. (1989) Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338: 427–430 [Google Scholar]
- Schroeder JI, Raschke K, Neher E. (1987) Voltage dependence of K channels in guard-cell protoplasts. Proc Natl Acad Sci USA 84: 4108–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher HM, Gundlach H, Fiedler F, Zenk MH. (1987) Elicitation of benzophenanthridine alkaloid synthesis in Eschscholtzia cell cultures. Plant Cell Rep 6: 410–413 [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T. (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58: 219–247 [DOI] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A. (2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol 134: 1536–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JB, Sisler EB. (1946) On the activation of catalase. J Biol Chem 165: 7–9 [PubMed] [Google Scholar]
- Trujillo M, Ichimura K, Casais C, Shirasu K. (2008) Negative regulation of PAMP-triggered immunity by an E3 ubiquitin ligase triplet in Arabidopsis. Curr Biol 18: 1396–1401 [DOI] [PubMed] [Google Scholar]
- Uraji M, Katagiri T, Okuma E, Ye W, Hossain MA, Masuda C, Miura A, Nakamura Y, Mori IC, Shinozaki K, et al. (2012) Cooperative function of PLDδ and PLDα1 in abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol 159: 450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SJ, Siu KC, Wu JY. (2011) Involvement of anion channels in mediating elicitor-induced ATP efflux in Salvia miltiorrhiza hairy roots. J Plant Physiol 168: 128–132 [DOI] [PubMed] [Google Scholar]
- Xue S, Hu H, Ries A, Merilo E, Kollist H, Schroeder JI. (2011) Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J 30: 1645–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, He SY, Assmann SM. (2008) The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J 56: 984–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP. (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126: 1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SY, Yu XC, Wang XJ, Zhao R, Li Y, Fan RC, Shang Y, Du SY, Wang XF, Wu FQ, et al. (2007) Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 19: 3019–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JJ, Wei FJ, Wang C, Wu JJ, Ratnasekera D, Liu WX, Wu WH. (2010) Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol 154: 1232–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]